آلپرازولام در درمان لرزش اساسی (essential tremor)
چکیده
پیشینه
لرزش اساسی (essential tremor; ET) یکی از شایعترین اختلالات حرکتی به حساب میآید. درمان آن در درجه اول شامل استفاده از عوامل دارویی است. بر این اساس، اگرچه درمان با پروپرانولول (propranolol) و پریمیدون (primidone) در طبابت بالینی به خوبی تثبیت شدهاند، ممکن است در 25 تا 55 درصد از بیماران بیاثر باشند و در درصد زیادی از آنها میتوانند عوارض جانبی (AEs) جدی ایجاد کنند. به همین دلایل، ارزیابی گزینههای درمانی جایگزین برای ET ممکن است یک اقدام ارزشمند باشد. آلپرازولام به عنوان یک عامل بالقوه مفید برای درمان افراد مبتلا به ET پیشنهاد شده، اما اثربخشی و ایمنی آن نامشخص است.
اهداف
اهداف اولیه
ارزیابی اثربخشی و ایمنی آلپرازولام در درمان افراد مبتلا به ET.
اهداف ثانویه
بررسی تأثیرات درمان با آلپرازولام بر کیفیت زندگی مبتلایان به ET.
روشهای جستوجو
ما یک جستوجوی سیستماتیک را بدون اعمال محدودیتهای زبان مقاله، برای شناسایی همه کارآزماییهای مرتبط انجام دادیم. ما پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL)؛ MEDLINE (ژانویه 1966 تا سپتامبر 2019)؛ EMBASE (ژانویه 1988 تا سپتامبر 2019)، انستیتوی ملی سلامت و تعالی مراقبت (NICE) (1999 تا سپتامبر 2019)، ClinicalTrials.gov (1997 تا سپتامبر 2019) و پلتفرم بینالمللی پایگاه ثبت کارآزماییهای بالینی (ICTRP) سازمان بهداشت جهانی (WHO) (2004 تا سپتامبر 2019) را جستوجو کردیم. ما منابع علمی خاکستری را به صورت دستی جستوجو کرده و فهرست منابع مطالعات و مرورهای شناسایی شده را بررسی کردیم.
معیارهای انتخاب
ما همه کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) را در رابطه با مقایسه آلپرازولام در مقابل دارونما (placebo) یا هر نوع درمان دیگری وارد کردیم. ما مطالعاتی را در نظر گرفتیم که تشخیص ET در آنها طبق معیارهای تشخیصی پذیرفته شده و معتبر انجام شده بود. مطالعاتی را که روی بیمارانی با اشکال ثانویه لرزش انجام شده یا فقط پارامترهای نوروفیزیولوژیکی را برای هدف ارزیابی پیامدها گزارش کرده بودند، حذف کردیم.
گردآوری و تجزیهوتحلیل دادهها
دو مرورگر به طور مستقل از هم دادهها را با استفاده از فرم جمعآوری دادهها، گردآوری و استخراج کردند. ما خطر سوگیری (bias) و بدنه شواهد را ارزیابی کردیم. ما از روشهای واریانس معکوس برای پیامدهای پیوسته و مقیاسهای اندازهگیری استفاده کردیم. ما تفاوتهای میان گروههای درمانی را در قالب تفاوتهای میانگین مقایسه کردیم. برای مدیریت و تجزیهوتحلیل دادهها از نرمافزار Review Manager استفاده کردیم.
نتایج اصلی
ما در این مرور یک کارآزمایی را وارد کردیم که آلپرازولام را با دارونما مقایسه کرد (24 شرکتکننده). این کارآزمایی خطر سوگیری (bias) کلی بالایی داشت. کیفیت کلی شواهد را در سطح بسیار پائین درجهبندی کردیم. در مقایسه با گروه دارونما، شرکتکنندگان تحت درمان با آلپرازولام کاهش معنیداری را در شدت لرزش نشان دادند (تفاوت میانگین (MD): 0.75‐؛ 95% فاصله اطمینان (CI): 0.83‐ تا 0.67‐). 9 شرکتکننده تحت درمان با آلپرازولام (75%) دچار AE شدند که عمدتا سداسیون (50%)، یبوست (17%) و خشکی دهان (9%) بودند. هیچ شرکتکنندهای در گروه آلپرازولام و هیچ شرکتکنندهای در گروه دارونما درمان را قطع نکرده و از مطالعه خارج نشدند.
نتیجهگیریهای نویسندگان
در حال حاضر، اطلاعات موجود شواهد کافی را برای ارزیابی اثربخشی و ایمنی درمان با آلپرازولام برای افراد مبتلا به ET نشان نمیدهد.
PICO
خلاصه به زبان ساده
استفاده از آلپرازولام برای درمان لرزش اساسی (essential tremor)
لرزش اساسی (essential tremor; ET) شایعترین اختلال حرکتی به حساب میآید. اگرچه از نظر تأثیر آن بر امید به زندگی خوشخیم به حساب میآید، معمولا پیشرونده بوده و بالقوه ناتوان کننده در نظر گرفته میشود. درمان در درجه اول شامل عوامل دارویی (پروپرانولول (propranolol) و پریمیدون (primidone) به عنوان درمان خط اول) است، که میتواند در 25 تا 55 درصد بیماران بیاثر باشد. آلپرازولام (alprazolam) به عنوان یک درمان بالقوه مفید در ET پیشنهاد شده است. نویسندگان این بررسی سعی کردند اثربخشی و ایمنی آن را در افراد مبتلا به ET ارزیابی کنند. یک مطالعه تصادفیسازی شده وارد شد که آلپرازولام و دارونما (placebo) را در 24 فرد مبتلا به ET سر و/يا اندام مقايسه کرد. آلپرازولام منجر به کاهش در شدت لرزش همراه با فراوانی بالای حوادث جانبی شد. با این حال، تعداد اندک مطالعات و وجود شواهدی از محدودیتهای زیاد روششناسی در مطالعه وارد شده، مانع از نتیجهگیریهای محکم در زمینه پروفایل مزیت‐خطر این درمان شد. برای تأیید اثربخشی و ارزیابی ایمنی طولانیمدت آلپرازولام در درمان بیماران مبتلا به ET، انجام تحقیقات بیشتری لازم است.
Authors' conclusions
Summary of findings
| Alprazolam for essential tremor | ||||||
| Patient or population: patients with essential tremor | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Control | Alprazolam | |||||
| Tremor severity | Mean tremor severity in control groups was | Mean tremor severity in intervention group was | 24 | ⊕⊝⊝⊝ | ||
| Withdrawals due to adverse events Follow‐up: 2 weeks | None | None | 24 (1 study) | ⊕⊝⊝⊝ | ||
| Quality of life Investigator global assessment Scale from 1 to 7 Follow‐up: 2 weeks | Mean score in the control group was 3.83 points | Mean score in the intervention group was 1.16 lower (0.17 to 2.15 lower) | 24 (1 study) | ⊕⊝⊝⊝ | ||
| Quality of life Investigator global assessment Scale from 1 to 7 Follow‐up: 2 weeks | Mean score in the control group was 3.5points | Mean score in the intervention group was 0.67 lower (0.27 to 1.61 lower) | 24 (1 study) | ⊕⊝⊝⊝ | ||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by 2 levels for very serious risk of bias: allocation and blinding methods not described (selection bias); number of adverse events in the placebo group not reported (reporting bias); essential tremor diagnostic criteria applied to participants not reported; patient exclusion and inclusion criteria not specified. An arbitrary, not validated, clinical rating scale for tremor severity was used for assessment of tremor. | ||||||
Background
Description of the condition
Essential tremor (ET) is one of the most common movement disorders, presenting an overall estimated prevalence ranging from 0.9% to 2.2%, with a higher rate reported among people over 65 years of age (4.6%) (Louis 2010).
Essential tremor is characterised by postural and kinetic tremor involving the arms and less commonly the head, lower limbs and voice, frequently accompanied by a family history of a similar tremor (Louis 2005). However, ET is a heterogeneous disorder, and little agreement among neurologists can be found regarding both the clinical definition and the diagnostic criteria (Jankovic 2002). Although benign in term of its effect on life expectancy, ET often causes embarrassment and, in a small percentage of patients, serious disability (Koller 1986; Busenbark 1991). Moreover, symptoms are typically progressive and are potentially disabling, often forcing patients to change jobs or to seek early retirement (Deuschl 2000). Treatment is based primarily on pharmacological agents, although surgical intervention may be an option in the most disabling cases. Pharmacotherapy may be used to improve function or to reduce the embarrassment associated with this condition, but treatment should be tailored to the patient's level of disability. Although primidone and propranolol are well‐established agents for the treatment of ET, additional medicaments may be useful in reducing tremor (Sullivan 2004). In fact, even though both propranolol and primidone have been reported to improve tremor in about two‐thirds of patients (Koller 1989; Wasielewski 1998), these agents tend to lose efficacy over time (Louis 2001a). In addition, their use is limited, particularly in the elderly (> 70 years) (Zesiewicz 2002), because of interactions with commonly used medications (e.g. digoxin, calcium channel blockers, anti‐arrhythmics) (Hansten 2004). Benzodiazepines have been suggested as potentially useful agents for the treatment of patients with ET, and they are usually well tolerated.
Description of the intervention
Alprazolam is a triazolo 1,4‐benzodiazepine that binds to a specific area of the gamma‐aminobutyric acid (GABA)‐A receptor, modulating transmission of the inhibitory neurotransmitter GABA as allosteric agonists and facilitating opening of the receptor’s chloride channel. Following oral administration, alprazolam is rapidly absorbed with nearly complete bioavailability. Peak concentrations in the plasma occur within 0.3 to 3 hours after administration, and plasma mean half‐life ranges from 10 to 14 hours. Alprazolam is excreted primarily in the urine ‐ 50% of the dose within 24 hours, and 94% after 72 hours.
How the intervention might work
Essential tremor may be caused by a deficiency in the α1‐subunit of the GABA‐A receptor, as demonstrated in a knockout model in mice (Kralic 2005). This mechanism suggests that the GABAergic system could serve as a potential target for pharmacotherapy, and that GABA‐A receptor agonists may be effective in ET (Pahwa 2003; Kralic 2005). In fact, in the light of their mechanisms of action, agents that enhance GABAergic neurotransmission have shown some effectiveness in the treatment of patients with ET. A positive effect of 1,4‐benzodiazepine (alprazolam, clonazepam) on ET has been reported (Thompson 1984; Huber 1988a) and should be related to allosteric modulation and enhancement of GABA‐A receptor function. Moreover, since the time of their discovery, 1,4‐benzodiazepines have been demonstrated to present an anxiolytic effect that might be helpful for patients with ET, whose condition is known to be worsened by anxiety.
Why it is important to do this review
In 2005 the American Academy of Neurology published the Practice Parameter for ET (Zesiewicz 2005) and recommended propranolol and primidone as first‐line therapy. The quality of evidence was assessed via an arbitrary four‐tiered scheme. Alprazolam was considered probably effective in reducing tremor and was given a level B recommendation in a recent update of this work (Zesiewicz 2011). The GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system was used to assess the quality of evidence and the strength of recommendations in another recent work (Zappia 2013), in which alprazolam was indicated as second‐line treatment for ET. Given that serious adverse events (AEs) limit the use of primidone or propranolol, and that these agents tend to lose efficacy in long‐term therapies, evaluating other treatment alternatives for ET may be a worthwhile pursuit. As uncertainty about the efficacy of alprazolam is ongoing, a systematic review aimed at evaluating whether this agent could be an effective alternative for patients with ET requiring additional drugs may generate clinically useful information.
Objectives
Primary
-
To assess the efficacy and safety of alprazolam in the treatment of individuals with ET.
Secondary
-
To examine effects of alprazolam treatment on the quality of life of people with ET.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) based on both parallel‐group and cross‐over designs.
Types of participants
Adults (aged ≥ 16 years) with ET meeting criteria proposed by the Tremor Investigation Group (Bain 2000a), the Consensus Statement of the Movement Disorder Society on Tremor (Deuschl 1998) or previously accepted and validated clinical criteria (Rejput 1984; Snow 1989; Haerer 1992; Salemi 1994; Chouinard 1997; Louis 1998).
We excluded from our review participants with a secondary form of tremor (e.g. tremor in parkinsonian disorders, dystonia, thyroid disease).
Types of interventions
Alprazolam for ET compared with any other pharmacological treatment or placebo.
We will not exclude trials on the basis of dose or route of administration.
Types of outcome measures
We excluded studies that reported only neurophysiological parameters (e.g. electromyographic recordings, accelerometry, spirography, digitising tablets) when assessing outcomes. These instrumental tests have important limitations, as their accuracy and reproducibility are not well established. Moreover, neurophysiological measures can lead to fallacious assessment of the benefit of treatment, as cross‐sectional studies showed weak correlation between those measures and functional disability (Bain 1997; Bain 2000b).
Primary outcomes
-
Tremor severity, as measured by the Fahn‐Tolosa‐Marin Tremor Rating Scale (TRS) (Fahn 1993), at the end of follow‐up.
-
-
The TRS assesses rest and postural and action tremor. The TRS score is derived from three TRS subscales.
-
-
Examiner‐reported upper limb postural and action tremor severity (amplitude), four elements.
-
Examiner‐reported ability to perform specific motor tasks (writing, drawing and pouring with dominant and non‐dominant hands), nine elements.
-
Patient‐reported functional disabilities due to tremor (eating, speaking, drinking, performing hygiene, dressing, writing, working and social activities), eight elements.
-
-
-
Withdrawals due to adverse events (AEs) as defined in a standard manner in studies, and the number of participants with any AE associated with treatment.
Secondary outcomes
-
Quality of life as measured by a validated scale or questionnaire (e.g. Short Form (SF)‐36, EuroQoL Quality of Life Questionnaire) or by a patient self evaluation rating scale (e.g. Beck Depression Inventory (BDI), Patient Global Impression (PGI), Clinical Global Impression (CGI)).
Search methods for identification of studies
We carried out a systematic search without language restrictions to identify all relevant published and unpublished RCTs.
Electronic searches
We searched the following databases for relevant trials.
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (up to Issue 10).
-
MEDLINE (January 1966 to September 2015).
-
EMBASE (January 1988 to September 2015).
-
National Institute for Health and Care Excellence (NICE) (1999 to September 2015).
-
ClinicalTrials.gov (1997 to September 2015).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (2004 to September 2015).
We based search strategies for each database on the strategy developed for MEDLINE, revising it appropriately for each database to take into account differences in controlled vocabulary and syntax rules. See Appendix 1 and Appendix 2.
Searching other resources
In addition to conducting the electronic searches above, we:
-
screened the reference lists of all available review articles and primary studies;
-
handsearched the references quoted in recent abstract books of European Federation of Neurological Societies (2005 to 2015), American Academy of Neurology (2003 to 2015), American Neurological Association (2006 to 2015), World Federation of Neurology (2008 to 2014) and Movement Disorder Society (2003 to 2015);
-
contacted the corresponding authors of relevant trials; and
-
contacted drug manufacturers to ask for information on ongoing trials.
Data collection and analysis
Two review authors (EB and GQ) independently assessed the titles and abstracts of all studies identified by electronic searching or by handsearching. We obtained the full text of potentially relevant trials.
Selection of studies
After reading the abstracts, EB and GQ independently selected eligible articles, independently scrutinised the full texts of selected studies and decided which trials met the inclusion criteria for this review. We resolved disagreements concerning inclusion and exclusion of trials by discussion.
Data extraction and management
EB and GQ extracted the following data independently, using a data collecting form.
-
Trial design.
-
Randomization methods.
-
Allocation concealment.
-
Blinding of treatments and assessments.
-
Comparability of treatment groups in terms of demographic and clinical characteristics.
-
Inclusion and exclusion criteria.
-
Duration of treatment.
-
Length of follow‐up.
-
Outcome measures (use of validated scales).
-
Number of withdrawals and respective causes.
-
Descriptions of AEs.
We resolved disagreements on extracted data by discussion.
Assessment of risk of bias in included studies
Review authors independently judged trial quality according to the methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We considered seven specific domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective reporting.
-
Other sources of bias.
Two review authors (EB, GQ) independently assessed the risk of bias of each included study and resolved disagreements by discussion to reach consensus. The overall assessment of risk of bias was based on recommendations reported in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If one or more domains were assessed as having high risk of bias, we rated the overall score as high. If all domains were rated as having low risk of bias, we considered the overall score as low. We rated all other combinations as having unclear overall risk of bias.
During interpretation of primary outcome results, we considered the risk of bias of included studies by using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. We rated the overall quality of evidence as 'high', 'moderate', 'low' or 'very low'. Through the GRADE approach, we assigned RCTs an initially high rating that may be subsequently modified by sequential judgement of limitations, inconsistency of results, indirectness of evidence, imprecision of data and presence of publication bias. Primary outcomes considered were tremor severity, withdrawals and numbers of AEs. We have reported and summarised results of this assessment by preparing a 'Summary of findings' table.
Measures of treatment effect
We analysed as continuous variables measurement scales used to assess ET. We calculated and expressed intervention effects as mean differences (MDs) and standard deviations (SDs). We used changes from baseline for continuous variables and frequencies, and percentages for categorical variables (numbers of withdrawals and numbers of AEs).
Unit of analysis issues
To avoid the 'carry‐over' effect that can induce alteration of response to subsequent treatment (Sibbald 1998), we considered only data from the first treatment phase after randomisation for cross‐over studies.
Dealing with missing data
To estimate effects of participant withdrawals or loss to follow‐up on primary outcomes, we planned to extract available information about incomplete data and about the intention‐to‐treat analysis performed. We considered the impact of missing data during assessment of risk of bias.
Assessment of reporting biases
We planned to use funnel plots to assess reporting biases.
Data synthesis
We calculated MDs and SDs to assess efficacy, frequency and percentage for withdrawals and AEs. Provided that an outcome of interest was reported by at least two included studies, we combined data in a meta‐analysis. We planned to use, in the presence of between‐trial homogeneity, a fixed‐effect model, and, in cases of heterogeneity, a random‐effects model. We used inverse variance methods for continuous outcomes and measurement scales. We compared differences between treatment groups as MDs; we combined results for dichotomous outcomes (withdrawals, AEs) by using Mantel‐Haenszel methods and obtained risk differences (RDs) to compare treatment groups. We used Review Manager software for management and analysis of data (RevMan 2012).
Subgroup analysis and investigation of heterogeneity
We planned to investigate effects of potential positive or negative interactions between alprazolam and other anti‐tremor medications on primary outcomes by performing a subgroup analysis of trials in which only the experimental anti‐tremor medication was allowed (alprazolam or placebo), and of trials including participants using other anti‐tremor medications during the study period. For trials in which treatment effects are reported for more than one dose, we planned to investigate effects of different doses reported separately.
For heterogeneity assessment, we planned to use the I2 statistic (Higgins 2003).
Sensitivity analysis
We performed no sensitivity analysis.
Results
Description of studies
Results of the search
Electronic databases revealed a total of 11 references, six of which we excluded because they were published as review articles or protocols; two were duplicate references. We selected three citations aimed at evaluating alprazolam treatment for ET and obtained the full text. A flowchart presents results of the electronic search in Figure 1. We identified no additional records by searching other resources.

Flowchart of the literature search on alprazolam and essential tremor.
Included studies
We considered one study as eligible for this review (Huber 1988) (Characteristics of included studies). This study was a double‐blind parallel RCT comparing alprazolam versus placebo. Duration of follow‐up was two weeks. The study included participants with upper limb and head ET (diagnostic criteria were not reported). A total of 24 participants with a mean age of 61.2 years (range 27 to 73 years) were included. Treatment with other anti‐tremor medications was stopped one week before entry into the study. A total of 12 participants were randomly assigned to alprazolam treatment, and a second group of 12 participants received placebo. The therapeutic scheme for alprazolam ranged between 0.75 and 3 mg per day, divided into three daily doses. The primary outcome measure used was an arbitrary clinical scale for tremor severity, ranging from 0 (no tremor) to 4 (severe tremor). Clinical ratings at baseline and at study endpoint were reported. A global impression scale was completed by both investigator and participant, and the score ranged from 1 (very much improved) to 7 (very much worse). The number of participants experiencing AEs was reported only for the alprazolam group. No participants dropped out of the study.
Excluded studies
Ibanez 2014 was an open, non‐randomised study that included eight participants with a diagnosis of ET and assessed hand tremor and contralateral cortical activity before and after a single dose of alprazolam. Investigators measured outcomes using neurophysiological parameters only: Tremor on the most affected side was recorded with solid‐state gyroscope and surface electromyography (EMG).
Gunal 2000 was a double‐blind, cross‐over, placebo‐controlled trial. Participants with ET received, in random order, alprazolam, acetazolamide, primidone and placebo for four weeks, with a two‐week washout period between treatments. This study did not specify, for each participant, the first treatment received and the subsequent order of other treatments. Moreover, data from the first treatment phase after randomisation were not available for analysis. Study authors reported only final scores; therefore the study presented a very high risk of carry‐over effect. We contacted the corresponding author of this paper in the attempt to obtain further information but are still awaiting a reply. This study was considered among Studies awaiting classification.
Risk of bias in included studies
We reported the results in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The trial included did not report methods for sequence generation and allocation concealment and was considered at high risk of bias.
Blinding
The trial included did not report methods for blinding personnel, participants and outcome assessors to treatment allocation and was considered at high risk of bias.
Incomplete outcome data
All participants completed the study and were assessed at study endpoint. We considered the study to have low risk of attrition bias.
Selective reporting
The primary outcome (tremor severity) was reported. Scores of investigator global assessment and participant global assessment were reported. Numbers of AEs in the placebo group were not reported, and the trial was considered overall to have unclear risk of reporting bias.
Other potential sources of bias
Diagnostic criteria for ET as applied to participants were not reported, nor were patient exclusion and inclusion criteria; thus, this was considered an additional source of bias. Moreover, information concerning the validation process for the clinical scale used to assess the primary outcome (tremor severity) was not reported. Finally, the short duration of follow‐up was considered another source of bias for the well‐known influence of treatment duration and treatment effect related to use of benzodiazepine (long‐term tolerance).
Effects of interventions
See: Summary of findings 1 Alprazolam for essential tremor
See summary of findings Table 1, which reports the comparison of alprazolam versus placebo and GRADE assessment results.
The study compared alprazolam and placebo and involved a total of 24 participants (12 alprazolam, 12 placebo). We rated the overall risk of bias as high. We considered the overall quality of evidence as very low.
Primary outcomes
Investigators reported tremor severity and number of withdrawals in the included study. They reported numbers of AEs only for the alprazolam group.
At study end (two weeks), researchers reported mean reduction from baseline on the overall clinical tremor rating scale of 0.79 points for alprazolam and 0.04 for placebo (P value < 0.01). Data analysis showed statistically significant differences between alprazolam and placebo in terms of efficacy measured by the clinical tremor rating scale score (MD ‐0.75, 95% CI ‐0.83 to ‐0.67; Analysis 1.1).
No participants in the alprazolam group and no participants in the placebo group discontinued treatment and dropped out of the study.
In terms of AEs, investigators reported their occurrence only for the alprazolam group, hampering any comparison between groups. Nine alprazolam‐treated participants (75%) developed AEs. The most common AE was sedation or drowsiness (50%), followed by constipation (17%) and dry mouth (9%).
We did not perform meta‐analysis, as only one study was included. We did not perform subgroup analysis to assess differences in efficacy and safety due to the interaction between combined anti‐tremor treatments, as data and the number of trials included were insufficient.
Secondary outcomes
At the study endpoint, investigator global assessment and participant global assessment were completed. The investigator reported better improvement for the alprazolam group, indicated by a mean score of 2.67 (SD 1.5), and less improvement for the placebo group, with a mean score of 3.83 (SD 0.9). These results were similar to those reported by participant global assessment, indicating a mean score of 2.83 (SD 1.4) for alprazolam and 3.5 (SD 1.1) for placebo.
Discussion
Summary of main results
We included in this review one randomised controlled trial (RCT) comparing alprazolam versus placebo for treatment of essential tremor (ET) (Huber 1988). This study involved a group of 24 participants presenting with head and/or limb ET. Investigators reported a positive effect of alprazolam on tremor severity at study endpoint, with a statistically significant difference in terms of efficacy favoring alprazolam versus placebo. A consistent number of participants treated with alprazolam reported adverse events (AEs). However, comparison between groups of the risk to develop AEs was impossible, as no data were reported for participants receiving placebo. The risk of study dropout was negligible in both groups after two weeks of follow‐up. These data should be interpreted cautiously because of the small number of trials included, high risk of bias and very low strength of provided evidence.
Overall completeness and applicability of evidence
Important factors limited the validity of results reported in the study. The sample size was very small, and the duration of follow‐up was short. This last point should be particularly regarded before alprazolam efficacy and use in patients with ET are considered. As ET is a chronic disease requiring long‐term treatment, long‐term use of alprazolam should be adequately assessed through studies considering longer duration of follow‐up. Tolerance, manifesting with decreased effectiveness, and dependence are well‐known effects following long‐term use of benzodiazepine and, even in intermittent therapies, could lead to important limitations in use and management of these medications. The short duration of the included study hindered any possible consideration of these issues.
An additional remark pertains to the incomplete presentation of study results and absolute omission of reporting of AEs for the placebo group.
All of these factors represent a limitation in the overall completeness of assessment and hamper the possibility of balancing benefit and risk linked to alprazolam treatment.
Quality of the evidence
Risk of bias evaluation and strength of evidence assessment disclosed a large range of limitations of the study analysed. The global quality of the evidence provided was judged 'very low' and thus insufficient to permit adequate conclusions.
Potential biases in the review process
To minimize biases, we performed a comprehensive systematic review by searching different databases, without language restrictions, to identify all relevant studies. Two review authors performed data management.
Agreements and disagreements with other studies or reviews
Two reviews of the literature analysed alprazolam treatment for ET (Zesiewicz 2005; Zappia 2013). These reviews applied no inclusion/exclusion criteria for considering studies in the review process, and included both of the studies found in the present work.
The paucity of studies evaluating alprazolam for ET, the low quality of studies already under way on this topic and some uncertainty concerning alprazolam use for ET were further discussed in these manuscripts.
The Practice Parameter for Essential Tremor (Zesiewicz 2005) used a four‐tired classification scheme based mainly on study design and including uncontrolled studies, case series and case reports. Neurophysiological parameters (electromyographic recordings, accelerometry, spirography, digitising tablets) were considered among outcome measures. Recommendations on alprazolam were formulated on the basis of one class I study (Gunal 2000a) and one class II study (Huber 1988).
The systematic review of evidence and recommendations from the Italian Movement Disorders Association (DISMOV‐SIN) (Zappia 2013) was based on the use of GRADE (Grades of Recommendation, Assessment, Development and Evaluation) to assign the level of evidence. Besides RCTs, the review also included case series, case reports and studies using neurophysiological assessment of tremor. Review authors' conclusions on alprazolam are based on evaluation of two studies (Huber 1988; Gunal 2000a) and highlight the very low quality of evidence provided by the literature, attributing a weak recommendation with very low quality of evidence.
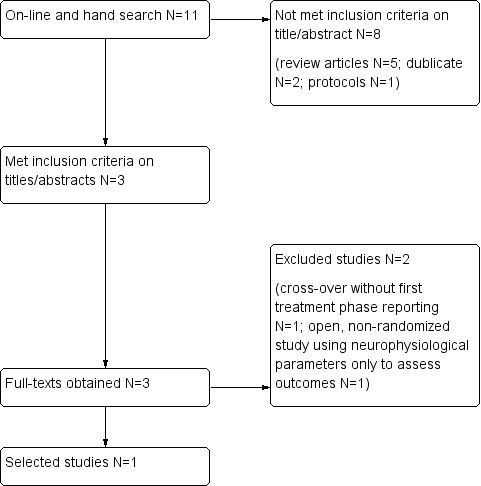
Flowchart of the literature search on alprazolam and essential tremor.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Comparison for efficacy, Outcome 1: Clinical rating scale
| Alprazolam for essential tremor | ||||||
| Patient or population: patients with essential tremor | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Control | Alprazolam | |||||
| Tremor severity | Mean tremor severity in control groups was | Mean tremor severity in intervention group was | 24 | ⊕⊝⊝⊝ | ||
| Withdrawals due to adverse events Follow‐up: 2 weeks | None | None | 24 (1 study) | ⊕⊝⊝⊝ | ||
| Quality of life Investigator global assessment Scale from 1 to 7 Follow‐up: 2 weeks | Mean score in the control group was 3.83 points | Mean score in the intervention group was 1.16 lower (0.17 to 2.15 lower) | 24 (1 study) | ⊕⊝⊝⊝ | ||
| Quality of life Investigator global assessment Scale from 1 to 7 Follow‐up: 2 weeks | Mean score in the control group was 3.5points | Mean score in the intervention group was 0.67 lower (0.27 to 1.61 lower) | 24 (1 study) | ⊕⊝⊝⊝ | ||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by 2 levels for very serious risk of bias: allocation and blinding methods not described (selection bias); number of adverse events in the placebo group not reported (reporting bias); essential tremor diagnostic criteria applied to participants not reported; patient exclusion and inclusion criteria not specified. An arbitrary, not validated, clinical rating scale for tremor severity was used for assessment of tremor. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Clinical rating scale Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐0.83, ‐0.67] |

