கடுமையான பக்கவாதத்திற்கு காமா அமினோ‐பியூட்ரிக் அமிலம் வாங்கி முதன்மை இயக்கிகள்
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | A multicenter, randomized, stratified, double‐blind, placebo‐controlled clinical trial to examine the efficacy and safety of diazepam in acute stroke | |
| Participants | Adult males and females were included within 12 hours after stroke onset. CT or MRI within 7 days was mandatory. People with a clear indication for, or contraindication to benzodiazepines (at the discretion of the attending physician) were excluded, as were people with unresponsive coma. 879 eligible people from 35 hospitals in 5 European countries were randomized into the trial | |
| Interventions | Diazepam 10 mg or placebo by rectiole, as soon as possible, followed by 10 mg tablets twice daily for 3 days versus placebo | |
| Outcomes | Independence (mRS < 3); complete recovery (BI ≧ 95 or mRS ≦ 1); adverse events; mortality | |
| Notes | Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized using a computer‐generated random listing of the 2 treatment assignments, blocked in groups of 4 and stratified for center |
| Allocation concealment (selection bias) | Low risk | Trial medication was packed and labeled by the hospital's pharmacist according to a medication code schedule generated before the trial, and sent to the participating centers in boxes of 20 treatment packs |
| Blinding of participants and personnel (performance bias) | Low risk | All the participants were blinded to trial medication |
| Blinding of outcome assessment (detection bias) | Low risk | All the investigators, treating physicians and nurses were blinded to trial medication |
| Incomplete outcome data (attrition bias) | Low risk | 31 participants (3.5%) discontinued the study after randomization, with explicit reasons |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | All efficacy and safety outcomes were analyzed by intention‐to‐treat |
| Methods | The safety of chlormethiazole versus placebo in hemorrhagic stroke patients was evaluated in a randomized, double‐blind trial | |
| Participants | Conscious participants aged 18 to 90 years were included within 12 hours after stroke onset. 201 eligible participants were recruited and randomized into the trial | |
| Interventions | Chlormethiazole (68 mg/kg) or placebo was given as an intravenous infusion over a 24‐hour period | |
| Outcomes | Adverse events; mortality; independence (BI ≧ 60 or mRS < 3); NIHSS; SSS‐48 | |
| Notes | Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | The allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Chlormethiazole and placebo were supplied in identical bottles to keep the treatment assignment blinded |
| Blinding of outcome assessment (detection bias) | Low risk | All the measurements were made by an assessor who was not involved during the administration of the study drug, to maintain blinding of treatment assignment |
| Incomplete outcome data (attrition bias) | Low risk | The study drug was not administered to 1 participant in the chlormethiazole group and to 2 participants in the placebo group. Therefore, 3/201 (1%) participants were not included in the analysis of safety or efficacy |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was found |
| Methods | A randomized, double‐blind, multicenter, placebo‐controlled study to explore the safety of t‐PA combined with chlormethiazole | |
| Participants | There were 101 participants randomized to the chlormethiazole group and 99 to the placebo group by 76 of the 142 hospitals involved in the study | |
| Interventions | All participants received 0.9 mg/kg t‐PA, beginning within 3 hours of stroke onset and then either 68 mg/kg chlormethiazole (N = 97) iv over 24 hours or placebo (N = 93) beginning within 12 hours of stroke onset | |
| Outcomes | Adverse events; mortality; independence (BI ≧ 60) | |
| Notes | Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The stratified randomization was implemented but the method of random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | The allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Chlormethiazole and placebo were supplied in identical bottles to keep the treatment assignment blinded |
| Blinding of outcome assessment (detection bias) | Low risk | All the measurements were made by an assessor who was not involved during the administration of the study drug, to maintain blinding of treatment assignment |
| Incomplete outcome data (attrition bias) | Low risk | After randomization, 10/200 (5%) participants (4 in the chlormethiazole group and 6 in the placebo group) did not receive the study drug and thus were not included in the safety analysis. All 10 participants showed signs of clinical deterioration after randomization before the study drug could be initiated |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was found |
| Methods | A randomized, double‐blind, multinational, placebo‐controlled investigation of the efficacy and safety of chlormethiazole for acute ischemic stroke | |
| Participants | Conscious participants aged 18 to 90 years were included within 12 hours after stroke onset. NIHSS score ≧ 3. 1198 eligible participants were recruited from 139 US and 14 Canadian centers and randomized into the trial | |
| Interventions | Chlormethiazole (68 mg/kg) or placebo was given as an intravenous infusion over a 24‐hour period | |
| Outcomes | Independence (BI ≧ 60 or mRS < 3); NIHSS; SSS‐48; adverse events; mortality | |
| Notes | Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation was not described |
| Allocation concealment (selection bias) | Low risk | The allocation was conducted by a central randomization scheme via telephone |
| Blinding of participants and personnel (performance bias) | Low risk | Chlormethiazole and placebo were supplied in identical bottles to keep the treatment assignment blinded |
| Blinding of outcome assessment (detection bias) | Low risk | All the measurements were made by an assessor who was not involved during the administration of the study drug, to maintain blinding of treatment assignment |
| Incomplete outcome data (attrition bias) | Low risk | Data from 29/1198 (2%) participants were not available for the efficacy analysis. Treatment was never started in 27 participants: 12 in the chlormethiazole group and 15 in the placebo group. In addition, 2 participants (1 per group) provided no efficacy data but were included in the safety analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was found |
| Methods | Randomized, double‐blind, multicenter, placebo‐controlled study to test the efficacy and safety of the neuroprotective drug chlormethiazole for acute stroke | |
| Participants | Participants aged 40 to 90 years with full consciousness before treatment were included. The symptoms should have lasted more than 1 hour and less than 12 hours. SSS‐48 of ≦ 40, with a sum of scores on arm, hand and leg motor items of ≦ 14. 1360 eligible participants from 85 clinical centers in 7 European countries and Canada were randomized; 546 participants had TACS and 95 participants had hemorrhagic stroke | |
| Interventions | Chlormethiazole (75 mg/kg) or placebo were given as an intravenous infusion over a 24‐hour period | |
| Outcomes | Independence (BI ≧ 60); SSS‐48; SSS‐MP; adverse events; mortality | |
| Notes | Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization was stratified by center, but the method of random sequence generation was not described |
| Allocation concealment (selection bias) | Low risk | All validations were made with the treatment allocation blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Only the independent data monitoring committee had access to unblinded data during the course of the study |
| Blinding of outcome assessment (detection bias) | Low risk | Only the independent data monitoring committee had access to unblinded data during the course of the study |
| Incomplete outcome data (attrition bias) | Low risk | 16/1360 (1%) randomized participants did not complete the study. 4 participants did not receive treatment (1 randomized to chlormethiazole, 3 to placebo). 4/1360 (0.3%) randomized participants were not available for the safety analysis. In subgroup analyses, data from 1/95 (1%) randomized hemorrhagic stroke participants and 6/546 (1%) randomized TACS participants were not available for analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was found |
BI: Barthel Index score
CT: computerized tomography
iv: intravenous
MRI: magnetic resonance imaging
mRS: modified Rankin Scale
NIHSS: National Institutes of Health Stroke Scale
SSS‐48: 48‐point Scandinavian Stroke Scale
SSS‐MP: Scandinavian Stroke Scale motor power score
t‐PA: tissue‐type plasminogen activator
TACS: total anterior circulation syndrome
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Neurological outcome of patients was not addressed | |
| Not an RCT | |
| Not an RCT | |
| The participants were not eligible |
RCT: randomized controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency Show forest plot | 5 | 3758 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.08] |
| Analysis 1.1  Comparison 1 Efficacy and safety for acute stroke, Outcome 1 Death or dependency. | ||||
| 1.1 Chlormethiazole versus placebo | 4 | 2909 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.11] |
| 1.2 Diazepam versus placebo | 1 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.07] |
| 2 Somnolence Show forest plot | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [3.50, 5.95] |
| Analysis 1.2 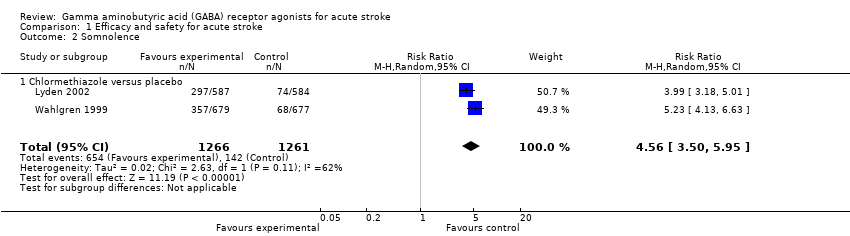 Comparison 1 Efficacy and safety for acute stroke, Outcome 2 Somnolence. | ||||
| 2.1 Chlormethiazole versus placebo | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [3.50, 5.95] |
| 3 Rhinitis Show forest plot | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [2.67, 8.46] |
| Analysis 1.3 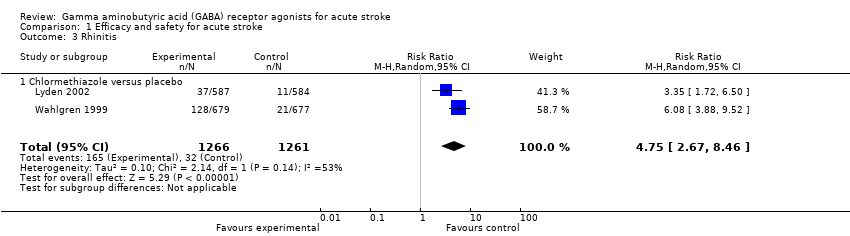 Comparison 1 Efficacy and safety for acute stroke, Outcome 3 Rhinitis. | ||||
| 3.1 Chlormethiazole versus placebo | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [2.67, 8.46] |
| 4 Functional independence Show forest plot | 5 | 3758 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| Analysis 1.4 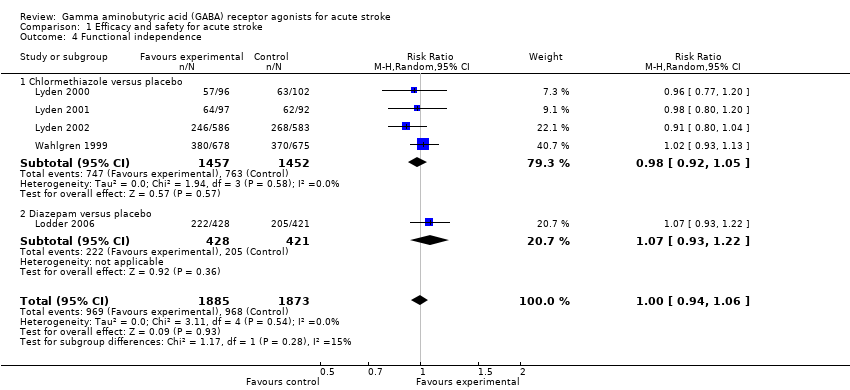 Comparison 1 Efficacy and safety for acute stroke, Outcome 4 Functional independence. | ||||
| 4.1 Chlormethiazole versus placebo | 4 | 2909 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 4.2 Diazepam versus placebo | 1 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.93, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency Show forest plot | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.12] |
| Analysis 2.1 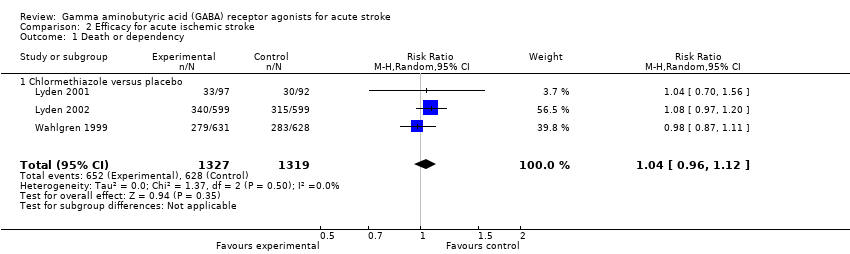 Comparison 2 Efficacy for acute ischemic stroke, Outcome 1 Death or dependency. | ||||
| 1.1 Chlormethiazole versus placebo | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.12] |
| 2 Functional independence Show forest plot | 4 | 3394 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.93, 1.08] |
| Analysis 2.2  Comparison 2 Efficacy for acute ischemic stroke, Outcome 2 Functional independence. | ||||
| 2.1 Chlormethiazole versus placebo | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.05] |
| 2.2 Diazepam versus placebo | 1 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.96, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency Show forest plot | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| Analysis 3.1 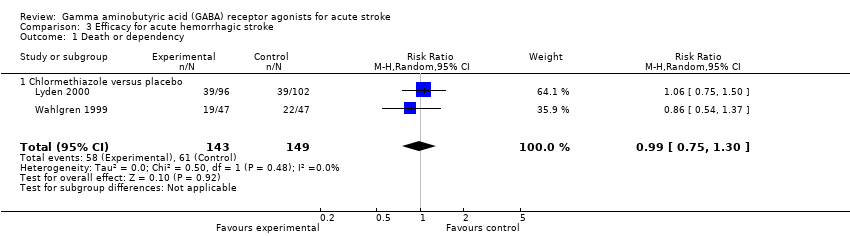 Comparison 3 Efficacy for acute hemorrhagic stroke, Outcome 1 Death or dependency. | ||||
| 1.1 Chlormethiazole versus placebo | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| 2 Functional independence Show forest plot | 3 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.16] |
| Analysis 3.2  Comparison 3 Efficacy for acute hemorrhagic stroke, Outcome 2 Functional independence. | ||||
| 2.1 Chlormethiazole versus placebo | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.21] |
| 2.2 Diazepam versus placebo | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional independence Show forest plot | 2 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.08, 1.63] |
| Analysis 4.1  Comparison 4 Efficacy for TACS, Outcome 1 Functional independence. | ||||
| 1.1 Chlormethiazole versus placebo | 2 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.08, 1.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional independence Show forest plot | 3 | 1314 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.80, 1.21] |
| Analysis 5.1 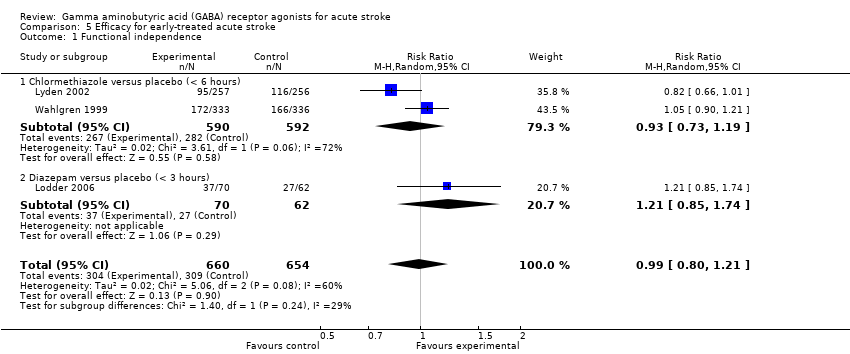 Comparison 5 Efficacy for early‐treated acute stroke, Outcome 1 Functional independence. | ||||
| 1.1 Chlormethiazole versus placebo (< 6 hours) | 2 | 1182 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| 1.2 Diazepam versus placebo (< 3 hours) | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.85, 1.74] |
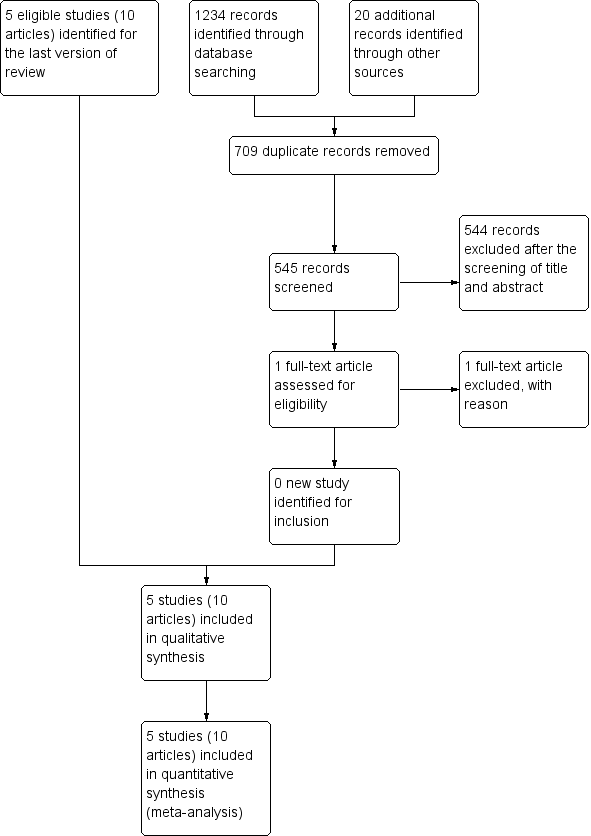
Study flow diagram.
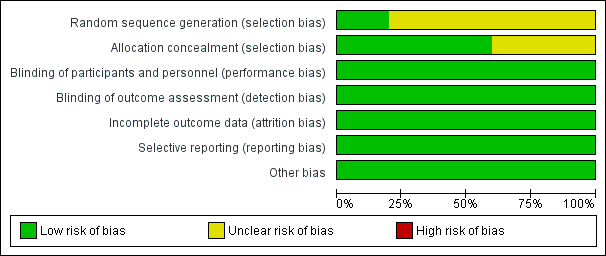
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
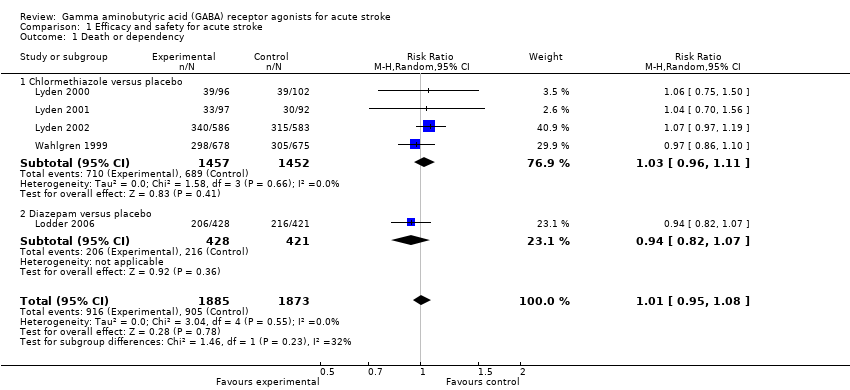
Comparison 1 Efficacy and safety for acute stroke, Outcome 1 Death or dependency.
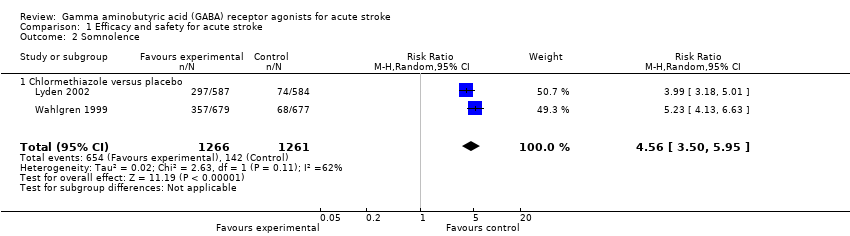
Comparison 1 Efficacy and safety for acute stroke, Outcome 2 Somnolence.
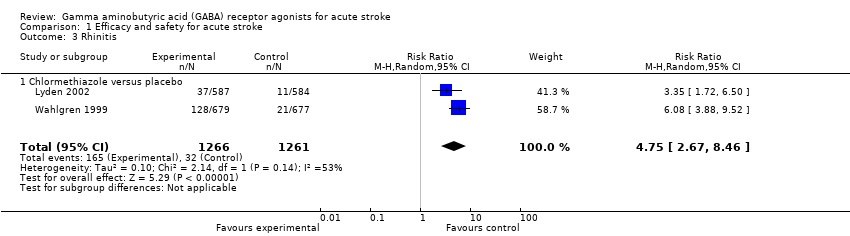
Comparison 1 Efficacy and safety for acute stroke, Outcome 3 Rhinitis.
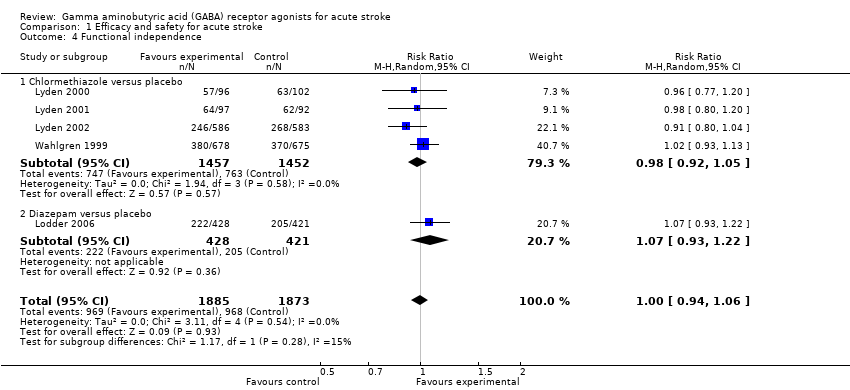
Comparison 1 Efficacy and safety for acute stroke, Outcome 4 Functional independence.
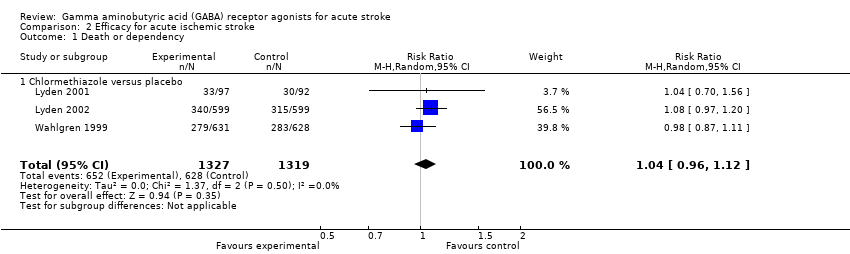
Comparison 2 Efficacy for acute ischemic stroke, Outcome 1 Death or dependency.

Comparison 2 Efficacy for acute ischemic stroke, Outcome 2 Functional independence.
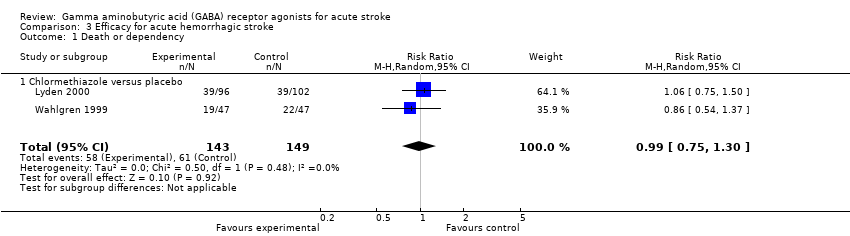
Comparison 3 Efficacy for acute hemorrhagic stroke, Outcome 1 Death or dependency.

Comparison 3 Efficacy for acute hemorrhagic stroke, Outcome 2 Functional independence.

Comparison 4 Efficacy for TACS, Outcome 1 Functional independence.
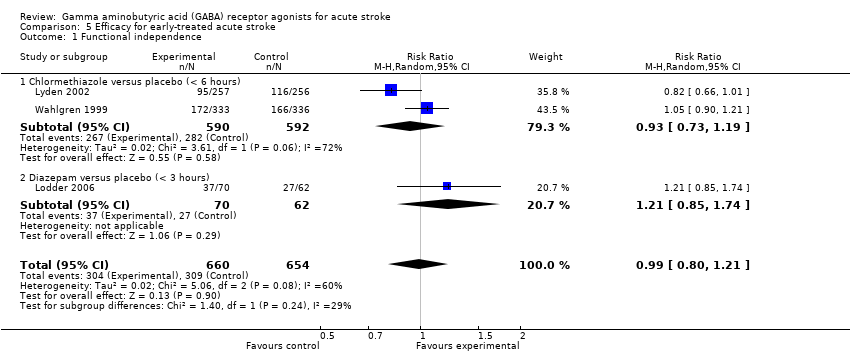
Comparison 5 Efficacy for early‐treated acute stroke, Outcome 1 Functional independence.
| Chlormethiazole compared with placebo for acute stroke | ||||||
| Patient or population: people with acute stroke Settings: inpatients Intervention: chlormethiazole Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Chlormethiazole | |||||
| Death or dependency | 475 per 1000 | 487 per 1000 | RR 1.03 (0.96 to 1.11) | 2909 (4) | ⊕⊕⊕⊝ | — |
| Adverse events | Somnolence 113 per 1000 Rhinitis 25 per 1000 | Somnolence 517 per 1000 Rhinitis 130 per 1000 | RR 4.56 (3.50 to 5.95) RR 4.75 (2.67 to 8.46) | 2527 (2) | ⊕⊕⊕⊝ | — |
| Functional independence | 525 per 1000 | 513 per 1000 | RR 0.98 (0.92 to 1.05) | 2909 (4) | ⊕⊕⊕⊝ | — |
| Other stroke scales | — | — | — | NIHSS 1367 (2) SSS 2727 (3) | ⊕⊕⊕⊝ | In Lyden 2000, the mean change of the NIHSS score was ‐4.5 in the chlormethiazole group (N = 96) and ‐4.0 in the placebo group (N = 102; P = 0.36). In Lyden 2002, the change of NIHSS score (median (quartiles)) was ‐5.5 (‐11, 17) in the chlormethiazole group (N = 586) and ‐6.0 (‐10, 16) in the placebo group (N = 583; P = 0.68). In Wahlgren 1999, no significant difference was found between the placebo and chlormethiazole groups for the change in score in the SSS 48‐point (P = 0.56) and SSS motor power score (P = 0.96). In Lyden 2000 and Lyden 2002, the change in score in the SSS was not significant in the two groups (P = 0.06 and P = 0.23, respectively). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to unclear risk of selection bias Functional independence, defined as a BI score higher than 60 or a mRS score less than 3 | ||||||
| Diazepam compared with placebo for acute stroke | ||||||
| Patient or population: people with acute stroke Settings: inpatients Intervention: diazepam Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Diazepam | |||||
| Death or dependency | 513 per 1000 | 481 per 1000 | RR 0.94 (0.82 to 1.07) | 849 (1) | ⊕⊕⊕⊝ | — |
| Adverse events | 357 per 1000 | 355 per 1000 | RR 0.99 (0.75 to 1.31) | 865 (1) | ⊕⊕⊕⊝ | — |
| Functional independence | 487 per 1000 | 519 per 1000 | RR 1.07 (0.93 to 1.22) | 849 (1) | ⊕⊕⊕⊝ | — |
| Other stroke scales | Not reported | Not reported | — | — | — | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level: one study with small sample size Functional independence, defined as a BI score higher than 60 or a mRS score less than 3 | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency Show forest plot | 5 | 3758 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.08] |
| 1.1 Chlormethiazole versus placebo | 4 | 2909 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.11] |
| 1.2 Diazepam versus placebo | 1 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.07] |
| 2 Somnolence Show forest plot | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [3.50, 5.95] |
| 2.1 Chlormethiazole versus placebo | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [3.50, 5.95] |
| 3 Rhinitis Show forest plot | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [2.67, 8.46] |
| 3.1 Chlormethiazole versus placebo | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [2.67, 8.46] |
| 4 Functional independence Show forest plot | 5 | 3758 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 4.1 Chlormethiazole versus placebo | 4 | 2909 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 4.2 Diazepam versus placebo | 1 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.93, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency Show forest plot | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.12] |
| 1.1 Chlormethiazole versus placebo | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.12] |
| 2 Functional independence Show forest plot | 4 | 3394 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.93, 1.08] |
| 2.1 Chlormethiazole versus placebo | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.05] |
| 2.2 Diazepam versus placebo | 1 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.96, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency Show forest plot | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| 1.1 Chlormethiazole versus placebo | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| 2 Functional independence Show forest plot | 3 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.16] |
| 2.1 Chlormethiazole versus placebo | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.21] |
| 2.2 Diazepam versus placebo | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional independence Show forest plot | 2 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.08, 1.63] |
| 1.1 Chlormethiazole versus placebo | 2 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.08, 1.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional independence Show forest plot | 3 | 1314 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.80, 1.21] |
| 1.1 Chlormethiazole versus placebo (< 6 hours) | 2 | 1182 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| 1.2 Diazepam versus placebo (< 3 hours) | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.85, 1.74] |

