Bloqueos nerviosos para el tratamiento inicial del dolor de la fractura femoral en niños
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009587.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 17 diciembre 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Lesiones óseas, articulares y musculares
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Karen Black conceived the review. Karen Black and Jason Howard designed the protocol with input at various stages from the other three authors. Karen Black and Nancy Murphy reviewed titles, abstracts and full text articles for inclusion in the review. Karen Black and Jason Howard extracted data from each study and discussed the analysis and conclusions. Karen Black and Catherine Bevan performed the risk of bias assessments and graded the quality of the studies.
Karen Black secured funding for the review and is the guarantor. Funding was provided by the Nova Scotia Health Research Foundation (NSHRF) through a Knowledge Synthesis grant. The NSHRF had no input or vested interested in any part of this review.
Sources of support
Internal sources
-
IWK Health Centre Library, Librarian Darlene Chapman, Canada.
Search strategy support
External sources
-
Nova Scotia Health Research Foundation, Canada.
Research Grant: the NSHRF has no vested interest in the outcome of this review.
Declarations of interest
Karen JL Black: a research grant was awarded by the Nova Scotia Health Research Foundation to support a Knowledge Synthesis project in the form of a Cochrane Systematic Review. The granting agency had no involvement in the development of the protocol. The grant did not support the researchers' salaries.
Catherine A Bevan: none known
Nancy G Murphy: none known
Jason J Howard: none known
Acknowledgements
We thank Darlene Chapman and Joanne Elliott for their assistance in designing the search strategies and for performing the searches. We thank Jill Hayden, Janet Curran and Amy Plint for their advice at all stages of protocol development and review.
Lindsey Elstub, Joanne Elliott, Vrisha Madhuri and Helen Handoll all provided constructive guidance and editing at the protocol stage for which we are grateful. Thanks, too, to Tracy Daley for her help as research assistant in this review.
We thank Xavier Griffin and Martyn Parker for their thoughtful suggestions for this review and Laura MacDonald for editorial processing. Helen Handoll provided feedback and final editing for which we thank her.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Dec 17 | Nerve blocks for initial pain management of femoral fractures in children | Review | Karen JL Black, Catherine A Bevan, Nancy G Murphy, Jason J Howard | |
| 2012 Jan 18 | Nerve blocks for initial pain management of femoral fractures in children | Protocol | Karen JL Black, Nancy G Murphy, Kara Thompson, Catherine A Bevan, Jason J Howard | |
Differences between protocol and review
Specific cut‐off values for pain scores were specified after the protocol but prior to analysing the results of the included study.
We used methods provided in Hozo 2005 to calculate the mean and standard deviations from median and 95% confidence interval data for an exploratory analysis of duration of analgesia.
The protocol planned for meta‐analysis of studies and related statistical analyses. The inclusion of only one study precluded these.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Child; Child, Preschool; Humans; Infant;

Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Fascia iliaca compartment block versus intravenous morphine, Outcome 1 Failure of analgesia at 30 minutes.

Comparison 1 Fascia iliaca compartment block versus intravenous morphine, Outcome 2 Adverse events up to 12 hours.
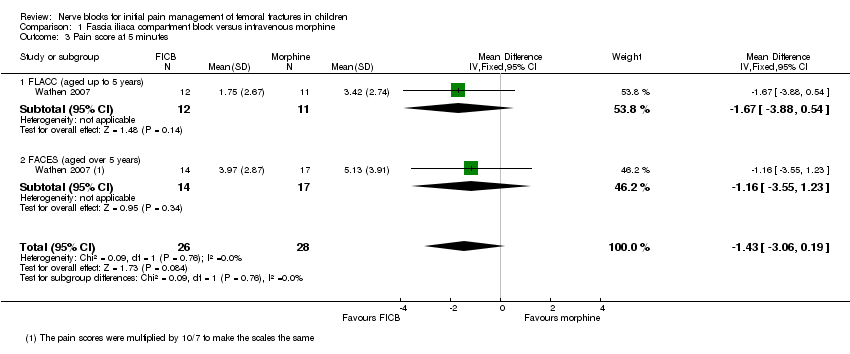
Comparison 1 Fascia iliaca compartment block versus intravenous morphine, Outcome 3 Pain score at 5 minutes.

Comparison 1 Fascia iliaca compartment block versus intravenous morphine, Outcome 4 Duration of analgesia (minutes).

Comparison 1 Fascia iliaca compartment block versus intravenous morphine, Outcome 5 Child and parental satisfaction with provided analgesia (highly satisfied).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure of analgesia at 30 minutes Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.20] |
| 1.1 Children aged up to 5 years | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.47] |
| 1.2 Children aged over 5 years | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.70] |
| 2 Adverse events up to 12 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Respiratory depression | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 CNS (obtundation) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Other (pain or redness at nerve block site) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pain score at 5 minutes Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐3.06, 0.19] |
| 3.1 FLACC (aged up to 5 years) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐1.67 [‐3.88, 0.54] |
| 3.2 FACES (aged over 5 years) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐3.55, 1.23] |
| 4 Duration of analgesia (minutes) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Child and parental satisfaction with provided analgesia (highly satisfied) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Patient satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Parental satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

