Interventions pour le traitement de la maladie aiguë de haute altitude
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Two‐group parallel RCT, 1 centre ITT: no Unit of randomization: assignment of gas composition was randomized in blocks of 9 Follow‐up period: 24 h "all investigations were carried out within a day after arrival at 4559 m" Diagnosis of AMS
Scale used for assessing AMS
| |
| Participants | Number of participants randomized: 20 Sex: men = 19 (95%) Age: median 32 years (range 22 to 51) Baseline data
Inclusion criteria
Exclusion criteria
| |
| Interventions | Intervention group 1 (n = 6)
Intervention group 2 (n = 7)
Control group (n = 7)
Co‐intervention
| |
| Outcomes | Not pre‐fixed as 'primary' or 'secondary'
Outcomes of interest in the review
| |
| Notes | Country: Swiss‒Italian border, Capanna "Regina Margherita" in the Alps Valais Altitude setting: 4559 m (barometric pressure 430 mmHg to 440 mmHg) Study dates: not reported Identifier number: not reported A priori sample estimation: no Conflicts of interest: not reported Funding/Support
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "assignment of gas composition was randomised in blocks of nine" (page 773) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "the person responsible for the gas supply, the gas bottles, and the reservoir‐balloon were hidden behind a curtain from the subjects and the examiners" (page 773) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "four investigators carried out one each of four different measurements throughout the study‐clinical examinations, ventilation, blood gas analysis, and transcranial doppler ultrasound examination. They were not aware of each other’s results during treatment of any particular patient" (page 773) |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes: no withdrawals. Outcome data was available for all participants |
| Selective reporting (reporting bias) | High risk | Results are reported in a figure. Exact numbers could not be retrieved. In the text, authors reported interpretation of data and P values |
| Other bias | Unclear risk | Bias in the presentation data: baseline characteristics by groups was not shown |
| Methods | Three‐group parallel RCT, 1 centre ITT: no Unit of randomization: participants Follow‐up period: 12 h Diagnosis of AMS
Scales used for assessing acute mountain sickness
| |
| Participants | Number of participants randomized: 64 Sex: men = 49 (77%) Age : mean 31 years (range 18 to 52) Baseline data
Inclusion criteria
Exclusion criteria
| |
| Interventions | Intervention group (n = 31)
Control group 1 (n = 23)
Control group 2 (n = 10)
Cointervention
**Characteristics of the chamber: fabric hyperbaric chamber made by Certec (F‐692 10 Sourcieux‐les‐Mines, France) | |
| Outcomes | Do the authors define outcomes as 'primary' or 'secondary'?: yes Primary
Secondary
Outcomes of interest in the review
| |
| Notes | Country: Swiss‒Italian border, Capanna "Regina Margherita" in the Alps Valais Altitude setting 4559 m (barometric pressure 430 mmHg to 440 mmHg) Identifier number: not reported Study dates: 1990 to 1991 A priori sample estimation: no Conflicts of Interest: not reported Funding/Support
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was performed in blocks of six (in 1990) and nine (in 1991)." Page 1098 |
| Allocation concealment (selection bias) | High risk | Quote: "the investigator assigned the treatment by drawing a lot from an envelope containing the assignments of one block. When the remaining lots could be predicted they were added to the envelope containing the next randomisation block." Page 1099 |
| Blinding of participants and personnel (performance bias) | High risk | No blinding method reported. Hyperbaric chamber compared to bed rest has not been masked. Outcomes are dependent on subjective assessment |
| Blinding of outcome assessment (detection bias) | High risk | No blinding method reported; however, hyperbaric chamber compared to bed rest has not been masked and the outcome is dependent on subjective assessment |
| Incomplete outcome data (attrition bias) | High risk | Quote: "in 1990 the first seven subjects assigned to low pressure were unintentionally treated with 39 mbar (equivalent to a descent of 500 m) until the inaccuracy of the built in manometer in the low pressure range was discovered. Their results were excluded from analysis, although they were not significantly different from those obtained in subjects treated with 16 or 23 mbar." Page 1099. Outcome data was not available for seven participants in the intervention group (unbalanced attrition) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Protocol not available |
| Other bias | Unclear risk | The use of analgesics and antiemetics was permitted during the study period as an option in the three groups. Authors found no significant statistical difference among groups in the use of these drugs |
| Methods | Two‐group parallel RCT, 1 centre ITT: no Unit of randomization: participants Follow‐up period
Diagnosis of AMS
Scale used for assessing acute mountain sickness
| |
| Participants | Number of participants randomized: 25 Sex: not reported Age: adults, details about age were not reported Baseline data
Inclusion criteria
Exclusion criteria
| |
| Interventions | Intervention group (n = 12) Control group (n = 13) Cointerventions
| |
| Outcomes | Primary
Secondary
Adverse effects
| |
| Notes | Country: Swiss‐Italian border, Capanna "Regina Margherita" in the Alps Valais Altitude setting: 4559 m (barometric pressure 430 mmHg to 440 mmHg) Identifier number: not reported Study dates: not reported A priori sample estimation: yes Conflicts of interest: not reported Funding/Support Study was supported by
M.R.T.
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized, placebo‐controlled, double‐blind trial". "The Geneva University Hospital Pharmacy was responsible for randomization (table of random numbers) and preparation of the study drugs" Page 270 |
| Allocation concealment (selection bias) | Low risk | Quote: "the Geneva University Hospital Pharmacy was responsible for randomization (table of random numbers) and preparation of the study drugs." Comment: Central allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "randomized, placebo‐controlled, double‐blind trial". Page 270. Quote: "study drugs were provided in identical, numbered 20 ml ampoules" Page 271 |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "randomized, placebo‐controlled, double‐blind trial". Page 270. Quote: "study drugs were provided in identical, numbered 20 ml ampoules" Page 271 |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes: no withdrawals. Outcome data was available for all participants |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Protocol not available. |
| Other bias | High risk | 20 patients (80%) received magnesium as prophylaxis, in a prevention trial, hours or few days before the treatment trial. This may be a confusion variable. It is not clearly stated the timing of rescue medication or the reason (either treatment failure or volunteers' wish) |
| Methods | Two‐group parallel RCT, 1 centre ITT: no Unit of randomization: patients Follow‐up period: unclear, apparently 12 to 16 h Diagnosis of AMS
Scale used for assessing Acute Mountain Sickness
| |
| Participants | Number of participants randomized: 35 Sex: men = 28 (80%) Age: "the two groups were comparable in age" Baseline data (mean symptom score per group)
Inclusion criteria
Exclusion criteria
| |
| Interventions | Intervention group 1 (n = 17)
Control group (n = 18)
Cointervention
| |
| Outcomes | Not pre‐fixed as 'primary' or 'secondary'
Outcomes of interest in the review
| |
| Notes | Country: Swiss‒Italian border, Capanna "Regina Margherita" in the Alps Valais Altitude setting: 4559 m (barometric pressure 430 mmHg to 440 mmHg) Identifier number: not reported Study dates: not reported Priori sample estimation: no Conflicts of Interest: not reported. Funding/Support
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Quote: "patients were randomly assigned". Page 1381 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Quote: "patients were randomly assigned". Page 1381 |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Quote: "A double blind, randomised, placebo controlled trial". Page 1380 |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Quote: "A double blind, randomised, placebo controlled trial". Page 1380 |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes: no withdrawals. Outcome data was available for all participants |
| Selective reporting (reporting bias) | High risk | Protocol not available. Data presented graphically for individuals. No data available for each group for symptomatic scores |
| Other bias | Unclear risk | Baseline characteristics poorly presented |
| Methods | Two‐group parallel RCT, 1 centre ITT: yes Unit of randomization: participants Follow‐up period: not clearly specified. Probably 24 h after intervention Diagnosis of AMS
Scale used for assessing Acute Mountain Sickness
| |
| Participants | Number of participants randomized: 12 Sex: men = 10 (91%) Age: median 32 years (range 25 to 46) Baseline data Mean symptom scores
Inclusion criteria
Exclusion criteria
| |
| Interventions | Intervention group (n = 6)
Control group (n = 6)
Co‐intervention
| |
| Outcomes | Main outcome measures
Secondary outcomes
Outcomes of interest in the review
| |
| Notes | Country: Alaska. Denali Medical Research Project high altitude research station, McKinley Altitude setting: 4200 m Study dates: June 1989 A priori sample estimation: no Conflicts of Interest: not reported Funding/Support
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomly assigned to receive either acetazolamide or placebo in a double‐blind fashion". Quote: "randomization was done in blocks of four to ensure equivalent numbers in each group". Page 462 |
| Allocation concealment (selection bias) | High risk | Quote: "one participant reported a history of sulfa‐drug allergy and was assigned (non‐randomly) to the placebo group" Page 462 |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "participants were randomly assigned to receive either acetazolamide or placebo in a double‐blind fashion" Page 462. However, authors reported "several participants reported increased urination and suspected that they were receiving acetazolamide" Page 463, this situation may have influenced results like acute mountain sickness score |
| Blinding of outcome assessment (detection bias) | High risk | Not reported. However, authors reported "several participants reported increased urination and suspected that they were receiving acetazolamide" Page 463, this situation may have influenced results like the acute mountain sickness score |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes: no withdrawals. Outcome data was available for all participants |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk'. Protocol not available |
| Other bias | Unclear risk | Design bias: not sample size calculation |
| Methods | Two‐group parallel RCT, 1 centre ITT: yes Unit of randomization: climbers Follow‐up period: 2 h Scale used for assessing acute mountain sickness score
Scale used for assessing high altitude headache
| |
| Participants | Number of participants randomized: 74 Sex: men = 30 (40%) Age: mean 33 years (range 13 to 61) Baseline data
Inclusion criteria
Exclusion criteria
No cases of HAPE or HACE were noted during the study period | |
| Interventions | Intervention group 1 (n = 39)
Intervention group 2 (n = 35)
Cointerventions
| |
| Outcomes | Not pre‐fixed as 'primary' or 'secondary'
Outcomes of interest in the review
| |
| Notes | Country: Nepal Altitude setting: 4243 m Identifier number: not reported Study dates: not reported A priori sample estimation: no Conflicts of Interest: not reported Funding/Support
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Quote: "randomly assigned rapid‐release capsules" Page 384 |
| Allocation concealment (selection bias) | Unclear risk | Quote: "each was given an envelope containing a detailed history questionnaire, followed by four separate, identical pages containing 10 cm visual analogue scales (VAS). The envelope also contained identical, randomly assigned rapid‐release capsules..." Page 384. Comments: it is not stated whether the envelope was opaque or not |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information about the blinding of participants and personnel to permit judgment of 'Low risk' or High risk' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information about the blinding of participants and personnel to permit judgment of 'Low risk' or High risk' |
| Incomplete outcome data (attrition bias) | Low risk | Lost after randomization: 1 (1%) ("after choosing to leave the study area (decided to hike further during the day"), study group not reported. Outcome data (all outcomes) were available for the rest of the participants |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk'. Protocol not available |
| Other bias | Unclear risk | Design bias: not sample size calculation |
| Methods | Two‐group parallel RCT, 1 centre ITT: no Follow‐up period: 18 h Scale used for assessing acute mountain sickness score
| |
| Participants | Number of participants randomized: 24 Sex: men = 14 (58%) Age: mean 29.1 years (SD = 1.7, range 18 to 50 years) Inclusion criteria
Exclusion criteria
| |
| Interventions | Intervention group (n = 12)
Control group (n = 12)
Co‐intervention
| |
| Outcomes | Primary endpoints
Secondary endpoints
Outcomes of interest in the review
| |
| Notes | Country: Iran (Tochal Hotel) Altitude setting: 3500 m Study dates: 1 to 7 January and 10 to 20 February 2007 A priori sample estimation: no Financial disclosures: not reported Funding/Support
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the computer‐generated randomisation codes" (page 1275) |
| Allocation concealment (selection bias) | Low risk | Quote: "only the pharmacist who provided the drugs knew the details of the computer‐generated randomisation codes" (page 1275) Quote: "medications were in identical opaque boxes labelled with randomisation codes that were not disclosed to investigators or assessor." (page 1275) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "medications were in identical opaque boxes labelled with randomisation codes that were not disclosed to investigators or assessor." (page 1275) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "medications were in identical opaque boxes labelled with randomisation codes that were not disclosed to investigators or assessor." (page 1275) |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes: no withdrawals. Outcome data was available for all participants |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk'. Protocol not available. |
| Other bias | High risk | "We acknowledge Dr Alireza Madjd, managing director of Darou Darman Pars Pharmaceuticals, for providing gabapentin and placebo". There was no statement addressing the independence of authors with regard to those providing funding (source of industry bias) |
| Methods | Two‐group parallel RCT, 1 centre ITT: no Unit of randomization: participants Follow‐up period: "patients were monitored for only one hour after treatment" Diagnosis of AMS
Scale used for assessing acute mountain sickness
| |
| Participants | Number of participants randomized: 29; "because of mechanical and technical errors, complete data were available in only 24 of the subjects" Sex: men = 17 (71%) Age: mean 37 years Baseline data
Inclusion criteria
Exclusion criteria
| |
| Interventions | Intervention group (n = 13)
Control group (n = 11)
Co‐intervention
| |
| Outcomes | Not pre‐fixed as 'primary' or 'secondary'
Outcomes of interest in the review
| |
| Notes | Country: USA (Snake River Health Clinic, Keystone, Colorado) Altitude setting: 2850 m Identifier number: not reported Study dates: not reported A priori sample estimation: no Conflicts of interest: not reported Funding/Support
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Quote: "patients agreeing to participate signed informed consent and then were randomly assigned to oxygen or hyperbaric treatment protocols." Page 1110 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Quote: "patients agreeing to participate signed informed consent and then were randomly assigned to oxygen or hyperbaric treatment protocols." Page 1110 |
| Blinding of participants and personnel (performance bias) | High risk | The study was not blinded. Quote: "we did not attempt to blind either the oxygen or the hyperbaric therapy." Page 1111 |
| Blinding of outcome assessment (detection bias) | High risk | The study was not blinded. Quote: "we did not attempt to blind either the oxygen or the hyperbaric therapy." Page 1111 |
| Incomplete outcome data (attrition bias) | High risk | Outcome data was missing from: hyperbaric chamber group = 2 out of 13 (15%) participants; oxygen group = 3 out of 11 (27%) participants. Reason: Quote: "because of mechanical and technical errors, complete data were available in only 24 of the subjects, and the remainder was excluded from data analysis." "These errors occurred in the monitoring equipment, not with the hyperbaric chamber" Page 1111 |
| Selective reporting (reporting bias) | High risk | Protocol not available. Data presented graphically for individuals. No data available for each group for symptomatic scores |
| Other bias | High risk | Design bias: not sample size calculation There was no statement considering the independence of authors with respect to those providing funding (source of industry bias) |
| Methods | Two‐group parallel RCT, 1 centre ITT: yes Unit of randomization: patients Follow‐up period: at least 11 h Scales for assessing acute mountain sickness score
| |
| Participants | Number of participants randomized: 31 climbers with symptoms of acute mountain sickness Sex: men = 22 (71%) Age: mean 31.5 years Inclusion criteria
Exclusion criteria
Quote: "most subjects had ascended to high altitude without prior acclimatisation... by using a cable car to an altitude of 3200." (page 1232) | |
| Interventions | Intervention group (n = 15)
Control group (n = 16)
Co‐intervention
| |
| Outcomes | Not pre‐fixed as 'primary' or 'secondary'
Outcomes of interest in the review
| |
| Notes | Country: Swiss‒Italian border. Capanna "Regina Margherita" located at an altitude of in the Alps Valais Altitude setting: 4559 m (barometric pressure 430 mmHg to 440 mmHg) Study dates: not reported A priori sample estimation: no Financial disclosures: not reported Funding/Support
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was performed in blocks of eight by drawing lots from an envelope containing the assignments of one block." Page 310 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Quote: "randomisation was performed in blocks of eight by drawing lots from an envelope containing the assignments of one block." Page 310 |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' |
| Blinding of outcome assessment (detection bias) | Unclear risk | No blinding. Quote: "the volunteers completed a questionnaire on environmental symptoms' and the Lake Louise self assessment questionnaire directed towards the symptoms of acute mountain sickness. The responses were checked with the investigator, and subsequently a clinical examination for peripheral oedema, pulmonary rales, and ataxia (Romberg test and heel to toe walking test) was performed." "Interviews and clinical examinations were always performed by the same investigator" Page 1233 |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes: no withdrawals. Outcome data was available for all participants |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk'. Protocol not available |
| Other bias | Unclear risk | Design bias: not sample size calculation |
| Methods | Two‐group parallel RCT, 1 centre ITT: no Unit of randomization: participants Follow‐up period: not clearly reported Diagnosis of AMS
Scale used for assessing acute mountain sickness
| |
| Participants | Number of participants randomized: 47 Sex: men = 47 (100%) Age: mean 18 (range 16 to 21) Baseline data
Inclusion criteria
Exclusion criteria
| |
| Interventions | Intervention group (n = 24)
Control group (n = 23)
Co‐intervention
| |
| Outcomes | Not pre‐fixed as 'primary' or 'secondary'
Outcomes of interest in the review
| |
| Notes | Country: Tibet, China Altitude setting: 3658 m Identifier number: not reported Study dates: not reported A priori sample estimation: no Conflicts of Interest: not reported Funding/Support
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Quote: “47 male participants were randomised into 2 groups”. Page 1631. Authors did not specify if a random sequence generation was used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes: no withdrawals. Outcome data was available for all participants |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk'. Protocol not available |
| Other bias | Unclear risk | Design bias: not sample size calculation |
| Methods | Two‐group parallel RCT, 1 centre ITT: yes Unit of randomization: participants Follow‐up period: up to 12 h after medication Diagnosis of AMS
Scale used for assessing Acute Mountain Sickness
| |
| Participants | Number of participants randomized: 29 Sex: men = 23 (79%)
Age: mean 34.5 years (18 to 56 years) Baseline data
Inclusion criteria
Exclusion criteria
| |
| Interventions | Intervention group (n = 14)
Co‐intervention
| |
| Outcomes | Not pre‐fixed as 'primary' or 'secondary'
Outcomes of interest in the review
| |
| Notes | Country: Italy, Capanna "Regina Margherita" in the Alps Valais Altitude setting: 4559 m (barometric pressure 430 mmHg to 440 mmHg) Identifier number: not reported Study dates: not reported A priori sample estimation: no Conflicts of interest: not reported Funding/Support: the Sezione Varallo del Club Alpino Italiano and of the Glaxo‐Wellcome Company. Study drug was supplied by Glaxo‐Wellcome company (Bad Oldesole, Germany) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization, without stratification, was performed in blocks of 4 subjects." Page 389 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgment of 'Low risk' or High risk' |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...sumatriptan and placebo had identical appearance..." Page 389 |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information about blinding of outcome assessment to permit judgment of 'Low risk' or High risk' |
| Incomplete outcome data (attrition bias) | High risk | Outcome data was not available in:
Reason:
|
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk'. Protocol not available |
| Other bias | High risk | Baseline differences: Quote: # 1: "despite strict randomisation in blocks of 4 subjects, all 6 women participating in the study were assigned to the placebo group, resulting in a significant difference of gender distribution between treatment groups" Page 389 There was no statement considering the independence of authors with respect to those providing funding (source of industry bias) |
| Methods | Three‐group parallel RCT, 1 centre ITT: yes Unit of randomization: participants Follow‐up period: not clearly reported, apparently until recovery Diagnosis of AMS
Scale used for assessing Acute Mountain Sickness
| |
| Participants | Number of participants randomized: 65 soldiers and railway workers Sex: men = 65 (100%) Age: mean 25 years Baseline data Symptom duration before recruitment: nifedipine: 9 days ± 3; nitric oxide: 8 days ± 3; conventional therapy: 8 days ± 3 Inclusion criteria
Exclusion criteria
| |
| Interventions | Intervention group 1 (n = 24) Name: nifedipine in addition to conventional therapy; nifedipine route: oral; dose: set at 20 mg at the first time, then 10 mg; frequency: every 8 h; duration of the intervention: until fully recovered Intervention group 2 (n = 22) Nitric oxide In addition to oral nifedipine. Nitric oxide (BG‐951, co‐developed by Guangzhou General Hospital and Beijing Factory of Analytical Machinery): dose: 10 ppm; route: inhalation, balanced with oxygen at 80% concentration level, inhalation rate was set at 8 L/min to 10 L/min; frequency and duration of the intervention: during 30 min Control group (n = 19) Conventional therapy: oxygen, intravenous furosemide, aminophylline and dexamethasone. Dose: not reported; route: inhalation in the case of oxygen; intravenous injection for furosemide, aminophylline and dexamethasone; frequency and duration: not reported Co‐intervention
| |
| Outcomes | Not pre‐fixed as 'primary' or 'secondary'
Outcomes of interest in the review: none | |
| Notes | Country: China (military hospital at Kunlun Mountain at Sinkiang province) Altitude setting: 3700 m Identifier number: not reported Study dates: not reported A priori sample estimation: no Conflicts of interest: not reported Funding/Support: Military Medical and Health Research Fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Quote: “65 participants were randomised into 3 groups” Page 212, without specifying how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes: no withdrawals. Outcome data was available for all participants In the Results section, authors summarized, “All of the 65 participants were fully recovered”. Comment: outcome data were available for all participants |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Protocol not available |
| Other bias | Low risk | No other sources of bias identified |
| Methods | *Results presented here correspond to the second of three experiments carried out by the researchers Experiment 1
Experiment 2
Experiment 3
Two‐group parallel RCT, 1 centre ITT: yes Unit of randomization: participants Follow‐up period: five days Diagnosis of AMS
Scale used for assessing Acute Mountain Sickness
| |
| Participants | Number of participants randomized: 13 Sex: not reported for experiment 2 Age: not reported for experiment 2. They reported subjects aged 22 to 58 for the three experiments (see methods above) Baseline data
Inclusion criteria
Exclusion criteria
| |
| Interventions | Intervention group (n = 6)
Control group (n = 7)
Co‐intervention
| |
| Outcomes | Not pre‐fixed as 'primary' or 'secondary'
Outcomes of interest in the review
| |
| Notes | Country: Karakoram mountains, located in the borders between Pakistan, India and China Altitude setting: 3200 to 5486 m Identifier number: not reported Study dates: not reported A priori sample estimation: no Conflicts of Interest: not reported Funding/Support
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Quote: "subjects were randomly allocated on a double‐blind basis" Page 51 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk' Quote: "subjects were randomly allocated on a double‐blind basis" Page 51 |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "all drugs and placebo were prepared in identical gelatin capsules" Page 51 However, blinding was discontinued "Six of the placebo group were given 1.5 grams oral acetazolamide 24 hours after entry into the trial because AMS symptoms had persisted. At this point all subjects were aware of their treatment status" Page 51 |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "all drugs and placebo were prepared in identical gelatin capsules" Page 51 However, blinding was discontinued "Six of the placebo group were given 1.5 grams oral acetazolamide 24 hours after entry into the trial because AMS symptoms had persisted. At this point all subjects were aware of their treatment status" Page 51 |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes: no withdrawals. Outcome data was available for all participants |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Low risk' or 'High risk'. Protocol not available |
| Other bias | High risk | Some participants in experiment 2 could have participated in the other two experiments (see methods above). Quote: "23 were studied during one of the three expeditions, six in two expeditions and three subjects in all three expeditions." Page 50. There is not enough information regarding how many participants from the second expedition were involved in the other two, and how much time passed between one expedition and another to identify a carry‐over effect. There was no statement considering the independence of authors with respect to those providing funding (source of industry bias) |
List of acronyms and abbreviations used in these tables
RCT: randomized controlled trial; ITT: intention‐to‐treat analysis; AMS: acute mountain sickness; AMS‐C: acute mountain sickness‐cerebral; h: hour(s); HACE: high altitude cerebral oedema; HAH: high altitude headache; HAPE: high altitude pulmonary oedema; LL: Lake Louise; mbar: millibar (millibars, a derived unit of the metric unit of pressure bars); MCA: median cerebral artery; min: minute; mmol: millimoles; n: number; NSAID: nonsteroidal anti‐inflammatory drugs; SD: standard deviation; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Cross‐over trial. The study design was considered inappropriate to the review question | |
| It is not a randomized trial | |
| Study intervention used for the prevention of HAI | |
| It is not a randomized trial | |
| Cross‐over trial. The study design was considered inappropriate to the review question: outcomes were reported after the patients received both intervention and control treatment | |
| Study intervention used for the prevention of HAI | |
| Cross‐over trial. The study design was considered inappropriate to the review question. | |
| Narrative review | |
| It is not a randomized trial | |
| It is not a randomized trial | |
| It is a case series study | |
| Study intervention used for the prevention of HAI | |
| Study intervention used for the prevention of HAI | |
| Cross‐over trial. The study design was considered inappropriate to the review question: outcomes were reported after the patients received both intervention and control treatment | |
| Quasi‐randomized study (randomization was based on the participants’ hospitalization registration number) | |
| Narrative review | |
| The study population were healthy male volunteers | |
| It is not a randomized trial | |
| It is not a randomized trial | |
| Study intervention used for studying AMS pathophysiology | |
| We wrote to [email protected] in 2014 in order to contact the main author: Dr Wright ([email protected]) replied saying that the study was not randomized | |
| Quasi‐randomized study (randomization was based on the participants’ hospitalization registration number) | |
| Quasi‐randomized study (randomization was based on the participants’ first name's starting initial) | |
| The study Intervention does not meet the review eligibility criteria (Bundle treatment) |
Acronyms and abbreviations used in these tables
AMS: acute mountain sickness; HAI: high altitude illness
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Oral trimetazidine for reducing the symptoms of acute mountain sickness and improving exercise performance |
| Methods | Single centre randomized, parallel, double‐blind, controlled, prospective trial |
| Participants | Shapingba District and Tibetan Autonomous Prefecture of Garzê (Chongqing, and Sichuan), China Inclusion criteria
Exclusion criteria
|
| Interventions | Interventions
Control
|
| Outcomes | Lake Louise Score |
| Starting date | According to the Chinese Clinical Trial Registry, the study is currently recruiting (last update February 2016); however the reported study completion time is from 30 June 2013 to 30 December 2013 |
| Contact information | Qin Jun; Huang Lan |
| Notes | Approved by ethic committee: yes. Institute of Cardiovascular Diseases of PLA, Xinqiao Hospital, Third Military Medical University, Chongqing, China Primary sponsor: Institute of Cardiovascular Diseases of PLA, Xinqiao Hospital, Third Military Medical University, Chongqing, China We have contacted the study leader, and the applicant by e‐mail in order to obtain more information (February 2017); answer is pending. |
| Trial name or title | Comparison of metoclopramide and ibuprofen for the treatment of acute mountain sickness |
| Methods | Allocation: randomized Endpoint classification: efficacy study Intervention model: parallel assignment Primary purpose: treatment Masking: double blind (subject, caregiver, investigator) |
| Participants | Trekkers travelling through the Annapurna Circuit in Nepal during the 3‐month time period of March to May 2012 Acute mountain sickness/high altitude headache Age group: adult/senior Sex: male and female Enrolment: 300 Inclusion criteria
Exclusion criteria
|
| Interventions | Drug: ibuprofen
Drug: metoclopramide
|
| Outcomes | Headache and nausea (visual analogue scales)
Lake Louise acute mountain sickness Score
|
| Starting date | March 2012 Currently recruiting, according to ClinicalTrials.gov registry (last verified February 2017) Estimated study completion date: March 2017 |
| Contact information | John B Tanner, MD [email protected] Principal Investigator: Norman S Harris, MD, MFA Massachusetts General Hospital |
| Notes | International study Sponsor/collaborators: Massachusetts General Hospital URL: ClinicalTrials.gov/show/NCT01522326 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AMS symptoms (standardized) Show forest plot | 2 | 25 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐2.56, 0.27] |
| Analysis 1.1 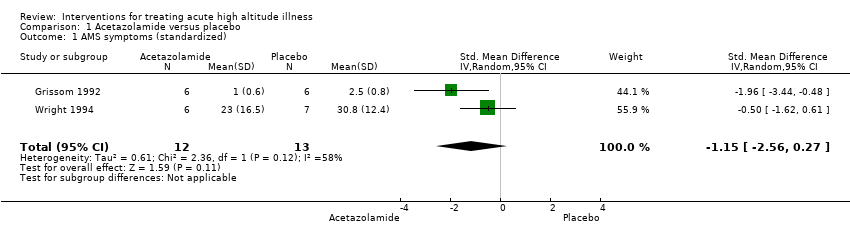 Comparison 1 Acetazolamide versus placebo, Outcome 1 AMS symptoms (standardized). | ||||
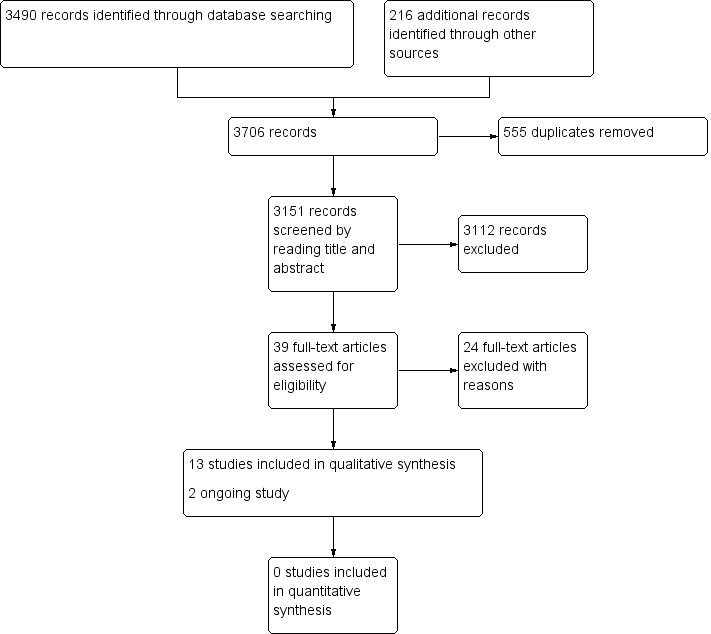
Study flow diagram.
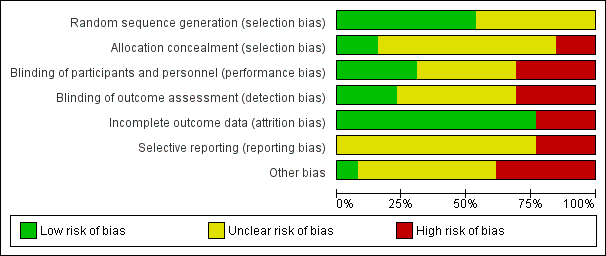
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
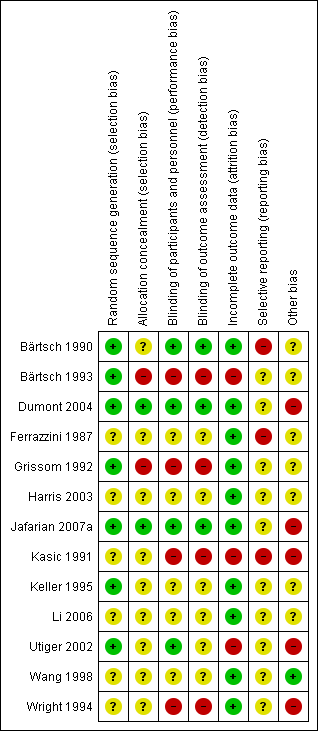
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Acetazolamide versus placebo, Outcome 1 AMS symptoms (standardized).
| Non‐pharmacological interventions for treating acute high altitude illness | ||||||
| Patient or population: people suffering from high altitude illness | ||||||
| Outcomes and intervention | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with various interventions | Risk with non‐pharmacological interventions | |||||
| All‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Complete relief of AMS symptoms | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Reduction in symptom score severity at 12 hours (Clinical score: ranged from 0 to 11 (worse)) Intervention: Simulated descent of 193 millibars versus 20 millibars | The mean score in the control group was 3.1 | The mean score in the intervention group was 2.5 | 0.6 points lower with intervention | 64 (1 RCT) | ⊕⊕⊝⊝ | |
| Adverse effects during treatment Intervention: Hyperbaric chamber/ 160 millibars versus supplementary oxygen | 0 per 1000 | 0 per 1000 | Nil | 29 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Quality of evidence downgraded by two levels due to serious risk of bias (performance bias (blinding was not specified), attrition bias and selective reporting bias) and serious imprecision (optimal information size criteria not achieved) | ||||||
| Pharmacological interventions for treating acute high altitude illness | |||||||
| Patient or population: people suffering from high altitude illness | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | ||
| Risk with various interventions | Risk with pharmacological interventions | ||||||
| All‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported | |
| Complete relief of AMS symptoms (12 to 16 hours after treatment) Scale used: Acute Mountain Sickness score (ranged from 0 to 9 (worse)) | Dexamethasone versus placebo | 0 per 1000 | 471 per 1000 | No estimable | 35 | ⊕⊕⊝⊝ | |
| Reduction in symptom score severity Time of measurement: 1 to 48 hours after treatment, end of treatment Scale of measurement: Self‐administered AMS questionnaires (ranged from 0 to 90 (worse)), AMS Symptom Questionnaire (ranged from 0 to 22 (worse)), Acute Mountain Sickness score (ranged from 0 to 9 (worse)), HAH Visual analogue score (VAS) (range no stated), Lake Louise Score (from 0 to 15 (worse)), | Acetazolamide versus placebo | Standardized Mean Difference 1.15 lower | 25 | ⊕⊕⊝⊝ | |||
| Dexamethasone versus placebo | Mean change from baseline: 0.4 units | Mean change from baseline: 4.1 units | Difference of 3.7 units (reported by trial authors) | 35 | ⊕⊕⊕⊝ | ||
| Gabapentin versus placebo | Mean VAS score: 4.75 | Mean VAS score: 2.92 | Not stated | 24 | ⊕⊕⊝⊝ | ||
| Magnesium versus placebo | Mean score: 10.3 units | Mean score: 9 units | Not stated | 25 | ⊕⊕⊝⊝ | ||
| Adverse effects Time of measurement: 1 to 48 hours after treatment, end of treatment Scale of measurement:not stated | Acetazolamide versus placebo | No reported | 0 per 1000 | Not estimable | 25 | ⊕⊕⊝⊝ | |
| Gabapentin versus placebo | 0 per 1000 | 0 per 1000 | Not stated | 24 | ⊕⊕⊝⊝ | ||
| Magnesium sulphate versus placebo | 77 per 1000 | 750 per 1000 | Not stated | 25 | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 Quality of evidence downgraded by two levels due to very serious risk of bias (multiple unclear biases and high risk of selective reporting bias) 2 Quality of evidence downgraded by two levels due to serious risk of bias (selection bias) and serious inconsistency (I² = 58%). 3 Quality of evidence downgraded by one level due to serious risk of bias (selection, performance and detection bias). 4 Quality of evidence downgraded by two levels due to serious risk of bias and serious imprecision. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AMS symptoms (standardized) Show forest plot | 2 | 25 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐2.56, 0.27] |

