Intervensi untuk merawat penyakit altitud tinggi
Abstract
Background
Acute high altitude illness is defined as a group of cerebral and pulmonary syndromes that can occur during travel to high altitudes. It is more common above 2500 metres, but can be seen at lower elevations, especially in susceptible people. Acute high altitude illness includes a wide spectrum of syndromes defined under the terms 'acute mountain sickness' (AMS), 'high altitude cerebral oedema' and 'high altitude pulmonary oedema'. There are several interventions available to treat this condition, both pharmacological and non‐pharmacological; however, there is a great uncertainty regarding their benefits and harms.
Objectives
To assess the clinical effectiveness, and safety of interventions (non‐pharmacological and pharmacological), as monotherapy or in any combination, for treating acute high altitude illness.
Search methods
We searched CENTRAL, MEDLINE, Embase, LILACS, ISI Web of Science, CINAHL, Wanfang database and the World Health Organization International Clinical Trials Registry Platform for ongoing studies on 10 August 2017. We did not apply any language restriction.
Selection criteria
We included randomized controlled trials evaluating the effects of pharmacological and non‐pharmacological interventions for individuals suffering from acute high altitude illness: acute mountain sickness, high altitude pulmonary oedema or high altitude cerebral oedema.
Data collection and analysis
Two review authors independently assessed the eligibility of study reports, the risk of bias for each and performed the data extraction. We resolved disagreements through discussion with a third author. We assessed the quality of evidence with GRADE.
Main results
We included 13 studies enrolling a total of 468 participants. We identified two ongoing studies. All studies included adults, and two studies included both teenagers and adults. The 13 studies took place in high altitude areas, mostly in the European Alps. Twelve studies included participants with acute mountain sickness, and one study included participants with high altitude pulmonary oedema. Follow‐up was usually less than one day. We downgraded the quality of the evidence in most cases due to risk of bias and imprecision. We report results for the main comparisons as follows.
Non‐pharmacological interventions (3 studies, 124 participants)
All‐cause mortality and complete relief of AMS symptoms were not reported in the three included trials. One study in 64 participants found that a simulated descent of 193 millibars versus 20 millibars may reduce the average of symptoms to 2.5 vs 3.1 units after 12 hours of treatment (clinical score ranged from 0 to 11 ‒ worse; reduction of 0.6 points on average with the intervention; low quality of evidence). In addition, no complications were found with use of hyperbaric chambers versus supplementary oxygen (one study; 29 participants; low‐quality evidence).
Pharmacological interventions (11 trials, 375 participants)
All‐cause mortality was not reported in the 11 included trials. One trial found a greater proportion of participants with complete relief of AMS symptoms after 12 and 16 hours when dexamethasone was administered in comparison with placebo (47.1% versus 0%, respectively; one study; 35 participants; low quality of evidence). Likewise, when acetazolamide was compared with placebo, the effects on symptom severity was uncertain (standardized mean difference (SMD) −1.15, 95% CI −2.56 to 0.27; 2 studies, 25 participants; low‐quality evidence). One trial of dexamethasone in comparison with placebo in 35 participants found a reduction in symptom severity (difference on change in the AMS score: 3.7 units reported by authors; moderate quality of evidence). The effects from two additional trials comparing gabapentin with placebo and magnesium with placebo on symptom severity at the end of treatment were uncertain. For gabapentin versus placebo: mean visual analogue scale (VAS) score of 2.92 versus 4.75, respectively; 24 participants; low quality of evidence. For magnesium versus placebo: mean scores of 9 and 10.3 units, respectively; 25 participants; low quality of evidence). The trials did not find adverse events from either treatment (low quality of evidence). One trial comparing magnesium sulphate versus placebo found that flushing was a frequent event in the magnesium sulphate arm (percentage of flushing: 75% versus 7.7%, respectively; one study; 25 participants; low quality of evidence).
Authors' conclusions
There is limited available evidence to determine the effects of non‐pharmacological and pharmacological interventions in treating acute high altitude illness. Low‐quality evidence suggests that dexamethasone and acetazolamide might reduce AMS score compared to placebo. However, the clinical benefits and harms related to these potential interventions remain unclear. Overall, the evidence is of limited practical significance in the clinical field. High‐quality research in this field is needed, since most trials were poorly conducted and reported.
PICO
Ringkasan bahasa mudah
Rawatan untuk penyakit altitud tinggi
Latar belakang
Penyakit altitud tinggiyang akut, yang juga dikenali sebagai penyakit gunung akut, boleh terjadi dengan pelbagai gejala. Ia disebabkan oleh penurunan tahap oksigen pada gunung yang semakin tinggi; dan ia boleh dialami semasa mencapai gunung yang tinggi apabila melancong, mendaki atau memanjat gunung atau kawasan lain yang tinggi. Orang ramai pergi ke ketinggian lebih dari 4000 meter, perempuan, orang yang lebih muda dari pertengahan dewasa, dan orang yang mempunyai sejarah migrain berisiko tinggi mendapat penyakit altitud tinggi. Gejala yang paling biasa adalah sakit kepala, kehilangan selera makan, insomnia, dan loya. Walau bagaimanapun, bentuk yang teruk boleh merangkumi kekeliruan, kesukaran berjalan, batuk progresif, sesak nafas, dan juga kematian.
Soalan ulasan
Apakah manfaat dan risiko rawatan yang berbeza untuk orang yang menderita daripada mabuk gunung?
Ciri‐ciri kajian
Kami memasukkan 13 kajian dengan sejumlah 468 peserta. Kebanyakan kajian melibatkan peserta dengan bentuk mabuk gunung yang ringan atau sederhana, dan hanya satu kajian termasuk bentuk neurologi yang teruk (gangguan sistem saraf). Susulan biasanya kurang dari satu hari. Kami juga mengenal pasti dua kajian yang sedang berterusan.
Keputusan Utama
Kami menjumpai kajian yang menilai intervensi berikut: keturunan simulasi dengan kebuk hiperbarik (penggunaan perubatan oksigen dalam ruang khas lebih besar daripada tekanan atmosfera untuk meningkatkan ketersediaan oksigen dalam badan); oksigen; ubat‐ubatan: acetazolamide, dexamethasone, ibuprofen, paracetamol, gabapentin, sumatriptan, nitric oksida, dan magnesium sulfat. Tiada kajian melaporkan kesan intervensi‐intervensi ini terhadap kematian semua punca. Laporan kelegaan lengkap dari simptom mabuk gunung akut, dan kejadian buruk jarang berlaku. Kajian yang berkaitan dengan penurunan simulasi dengan penggunaan kebuk hiperbarik tidak mendapat faedah tambahan atau bahaya yang berkaitan dengan intervensi ini (3 kajian, 124 peserta). Di samping itu, kajian yang berkaitan dengan pemberian ubat‐ubatan mendapati beberapa manfaat dari segi pengurangan gejala dengan penggunaan acetazolamide (2 kajian, 25 peserta), dan dexamethasone (1 kajian, 35 peserta), tanpa peningkatan kesan sampingan.
Kualiti bukti
Kualiti bukti yang kami dapati adalah rendah, dan dengan itu kepastian kami dalam penemuan adalah terhad. Terdapat maklumat yang tidak mencukupi tentang cara kajian dijalankan, dan dalam beberapa kes terdapat bukti yang mengganggu pada beberapa peringkat kajian. Tambahan pula, bilangan orang dalam setiap kajian adalah sangat kecil (<30 peserta), dan oleh itu hasilnya tidak jelas (tidak tepat). Sesetengah kajian tidak dibutakan (iaitu peserta tahu apa rawatan eksperimen yang mereka terima), dan ini mungkin memberi kesan cara peserta menilai gejala mereka sendiri.
Authors' conclusions
Summary of findings
| Non‐pharmacological interventions for treating acute high altitude illness | ||||||
| Patient or population: people suffering from high altitude illness | ||||||
| Outcomes and intervention | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with various interventions | Risk with non‐pharmacological interventions | |||||
| All‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Complete relief of AMS symptoms | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Reduction in symptom score severity at 12 hours (Clinical score: ranged from 0 to 11 (worse)) Intervention: Simulated descent of 193 millibars versus 20 millibars | The mean score in the control group was 3.1 | The mean score in the intervention group was 2.5 | 0.6 points lower with intervention | 64 (1 RCT) | ⊕⊕⊝⊝ | |
| Adverse effects during treatment Intervention: Hyperbaric chamber/ 160 millibars versus supplementary oxygen | 0 per 1000 | 0 per 1000 | Nil | 29 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Quality of evidence downgraded by two levels due to serious risk of bias (performance bias (blinding was not specified), attrition bias and selective reporting bias) and serious imprecision (optimal information size criteria not achieved) | ||||||
| Pharmacological interventions for treating acute high altitude illness | |||||||
| Patient or population: people suffering from high altitude illness | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | ||
| Risk with various interventions | Risk with pharmacological interventions | ||||||
| All‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported | |
| Complete relief of AMS symptoms (12 to 16 hours after treatment) Scale used: Acute Mountain Sickness score (ranged from 0 to 9 (worse)) | Dexamethasone versus placebo | 0 per 1000 | 471 per 1000 | No estimable | 35 | ⊕⊕⊝⊝ | |
| Reduction in symptom score severity Time of measurement: 1 to 48 hours after treatment, end of treatment Scale of measurement: Self‐administered AMS questionnaires (ranged from 0 to 90 (worse)), AMS Symptom Questionnaire (ranged from 0 to 22 (worse)), Acute Mountain Sickness score (ranged from 0 to 9 (worse)), HAH Visual analogue score (VAS) (range no stated), Lake Louise Score (from 0 to 15 (worse)), | Acetazolamide versus placebo | Standardized Mean Difference 1.15 lower | 25 | ⊕⊕⊝⊝ | |||
| Dexamethasone versus placebo | Mean change from baseline: 0.4 units | Mean change from baseline: 4.1 units | Difference of 3.7 units (reported by trial authors) | 35 | ⊕⊕⊕⊝ | ||
| Gabapentin versus placebo | Mean VAS score: 4.75 | Mean VAS score: 2.92 | Not stated | 24 | ⊕⊕⊝⊝ | ||
| Magnesium versus placebo | Mean score: 10.3 units | Mean score: 9 units | Not stated | 25 | ⊕⊕⊝⊝ | ||
| Adverse effects Time of measurement: 1 to 48 hours after treatment, end of treatment Scale of measurement:not stated | Acetazolamide versus placebo | No reported | 0 per 1000 | Not estimable | 25 | ⊕⊕⊝⊝ | |
| Gabapentin versus placebo | 0 per 1000 | 0 per 1000 | Not stated | 24 | ⊕⊕⊝⊝ | ||
| Magnesium sulphate versus placebo | 77 per 1000 | 750 per 1000 | Not stated | 25 | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 Quality of evidence downgraded by two levels due to very serious risk of bias (multiple unclear biases and high risk of selective reporting bias) 2 Quality of evidence downgraded by two levels due to serious risk of bias (selection bias) and serious inconsistency (I² = 58%). 3 Quality of evidence downgraded by one level due to serious risk of bias (selection, performance and detection bias). 4 Quality of evidence downgraded by two levels due to serious risk of bias and serious imprecision. | |||||||
Background
High altitude is arbitrarily classified as high (1500 to 3500 metres), very high (3500 to 5500 metres), and extreme (above 5500 metres) (Paralikar 2010). At high altitude there is a drop in barometric pressure, which causes a decrease in the partial pressure of oxygen. In most cases, this hypobaric hypoxia triggers physiological responses that help the individual tolerate and adapt to the low oxygen conditions. However in other cases there are abnormal responses, that in turn cause one of three forms of acute altitude illness: acute mountain sickness (AMS), high altitude cerebral oedema (HACE) and high altitude pulmonary oedema (HAPE) (Luks 2017).
Acute high altitude illness is more common above 2500 metres, but can be seen at lower elevations, especially in susceptible people. Factors such as the rate of ascent, the absolute change in altitude and individual physiology are the primary determinants as to whether HAI will develop or not (Palmer 2010). People going to altitudes over 4000 metres, females, people younger than mid‐adulthood, and people with a history of migraine are at greater risk of suffering from altitude sickness (Bärtsch 2013; Canoui‐Poitrine 2014).
Description of the condition
High altitude illness (HAI)
The potential medical problems associated with a high altitude excursion are many, and terminology has sometimes confused their classification. For the purposes of this review, high altitude illness (HAI) is defined as a group of cerebral and pulmonary syndromes that can occur during travel to elevations above 2500 metres (Luks 2014). This includes syndromes covered by the terms 'acute mountain sickness' (AMS), 'high altitude cerebral oedema' (HACE),and 'high altitude pulmonary oedema' (HAPE). The risk categories for acute mountain sickness are shown in Appendix 1 (Luks 2010; Luks 2014). HAI is considered as an important cause of mountain mortality (Windsor 2009).
Other medical problems that may be encountered at high altitudes include acute hypoxia, cerebrovascular syndromes, peripheral oedema, retinopathy, retinal haemorrhage, thromboembolism, sleep disorders and periodic breathing, high altitude pharyngitis and bronchitis, ultraviolet exposure and keratitis (snow blindness) and exacerbation of pre‐existing illness (CATMAT 2007; Palmer 2010; Schoene 2008); however these will not be considered in this review.
Acute mountain sickness (AMS) and high altitude cerebral oedema (HACE)
AMS is a neurological disorder characterized by headache, anorexia, nausea and sometimes vomiting, light‐headedness, insomnia, and fatigue or loss of energy (Palmer 2010). Headache is the most prevalent symptom (Luks 2017). In contrast, HACE is a potentially fatal neurologic disorder that is characterized by altered consciousness or ataxia (Imray 2010), or both. If left untreated, HACE can result in death subsequent to brain herniation (Bailey 2009). HACE is widely viewed as the end stage of AMS, and is normally preceded by symptoms of AMS (Basnyat 2003), which suggests that they result from a similar pathophysiologic process (Palmer 2010). Both syndromes are characterized by oedematous brain swelling, and intracranial hypertension (Luks 2017). The severity of AMS can be graded using the Lake Louise Questionnaire, Environmental Symptoms Questionnaire, or by the use of a simple analogue scale (Imray 2010).
The pathophysiology apparently involves an interaction of multiple physiological responses to hypoxia (ventilation, cerebral vasculature, autonomic nervous system and nociceptive thresholds), and anatomical factors such as the compensatory capacity for cerebrospinal fluid, and the capacity of venous outflow (Luks 2017).
High altitude pulmonary oedema (HAPE)
HAPE is a non‐cardiogenic pulmonary oedema (Smedley 2013). It is characterized by cough, progressive dyspnoea with exertion, and decreased exercise tolerance, generally developing within two to four days after arrival at high altitude (Hall 2011). HAPE is rare after one week of acclimatization at a particular altitude (Maggiorini 2010; Palmer 2010). Hypoxia is the trigger that results in a complex cascade of events leading to HAPE (Stream 2008). Essentially, HAPE is due to a "persistent imbalance between the forces that drive water into the airspace and the biologic mechanisms for its removal" (Scherrer 2010). The hallmark of this condition is hypoxic pulmonary hypertension, which may be mediated via at least three potential mechanisms: defective pulmonary nitric oxide synthesis; exaggerated endothelin‐1 synthesis; and exaggerated sympathetic activation (Scherrer 2010). A defect in alveolar transepithelial sodium transport has also been suggested (Scherrer 2010). An extensive review of pulmonary hypertension induced by high altitude is reported by Pasha 2010.
Epidemiology of acute high altitude illness (HAI)
It has been estimated that 25% of people at moderate altitude are affected by acute mountain sickness (AMS), and 50% to 85% of travellers at 4000 meters or more (Eide 2012). The incidence of high altitude cerebral oedema and high altitude pulmonary oedema is much lower than for AMS, with estimates in the range of 0.1% to 4.0% (Luks 2010). Rapid ascent, poor acclimatization, physical exertion at altitude, young age, and history of prior altitude illness are major risk factors to develop HAI (Eide 2012). Other risk factors are permanent residence lower than 900 metres; obesity (Ri‐Li 2003); and coronary heart disease (Dehnert 2010).
(See Appendix 2 for a glossary of medical terms.)
Description of the intervention
Interventions for treating HAI can be broadly classified as pharmacological and non‐pharmacological. Several consensus statements and guidelines have been published in this area. Some of them have been published by the Wilderness Medical Society (Luks 2014); the Committee to Advise on Tropical Medicine and Travel statement (CATMAT 2007); and the Centers for Disease Control and Prevention (CDC; CDC Yellow Book 2016).
A) Non‐pharmacological interventions
-
Descent (Hackett 2004)
-
Hyperbaric chamber (Bärtsch 1993; Kasic 1991; Keller 1995)
-
Portable pressure bag or Gamow bag (Austin 1998; Freeman 2004; Zafren 1998)
-
Breathing system designed to conserve oxygen supplies at high altitude (Pattinson 2005)
-
Positive airway pressure and other therapies (Koch 2009; Schoene 1985)
B) Pharmacological interventions
-
Oxygen (Hill 1909; Zafren 1996)
-
Carbonic anhydrase inhibitors: acetazolamide (Grissom 1992)
-
Glucocorticosteroids: dexamethasone (Ferrazzini 1987; Hackett 1988; Hackett 2004; Levine 1989; Wright 2008); medroxyprogesterone (Wright 2008)
-
Non‐steroidal anti‐inflammatory drugs (NSAIDs): ibuprofen (Broome 1994; Harris 2003); paracetamol (Harris 2003); and aspirin (Burtscher 2001)
-
Selective 5‐hydroxytryptamine (1) receptor agonist: sumatriptan (Utiger 2002)
-
Inhaled nitric oxide (Scherrer 1996; Schoene 2004)
-
Anticonvulsant drugs: gabapentin (Jafarian 2007a)
-
Diuretics: furosemide (Hultgren 1975)
-
Non‐selective phosphodiesterase inhibitor (theophylline or aminophylline) (Fisher 2000)
-
Magnesium (Dumont 2004)
How the intervention might work
Both pharmacological and non‐pharmacological interventions are used to treat acute high altitude illness; however, immediate descent or evacuation to a lower altitude is lifesaving and the treatment of choice for patients with fully developed severe high altitude illness (Luks 2014). Treatments other than descent are considered when descent is not possible due to bad weather, terrain or other logistical factors.
Some of the ways the pharmacological and non‐pharmacological treatments might work are as follows.
A) Acute mountain sickness (AMS) and high altitude cerebral oedema (HACE)
-
Carbonic anhydrase inhibitors (acetazolamide, methazolamide) inhibit carbonic anhydrase in the kidneys, resulting in bicarbonaturia and metabolic acidosis. This results in hyperventilation in order to compensate through a respiratory alkalosis and thus this drug causes improvements in ventilation in order to respond more fully to hypoxic stimuli at altitude (Leaf 2007). Acetazolamide can also cause pulmonary vasodilation unrelated to carbonic anhydrase inhibition (Höhne 2007).
-
Steroids (dexamethasone and medroxyprogesterone): dexamethasone blocks hypoxia‐induced endothelial dysfunction (Murata 2004; Murata 2005); and medroxyprogesterone acts as a respiratory stimulant (Wright 2004).
-
Furosemide: this diuretic drug would reduce pulmonary extravascular fluid accumulation; however, diuretics have no role in high altitude pulmonary oedema (HAPE) treatment particularly because many HAPE patients have concurrent intravascular volume depletion (Luks 2010).
-
Non‐steroidal anti‐inflammatory drugs (NSAIDs) (ibuprofen, paracetamol, aspirin): a prostaglandin‐mediated increase in cerebral microvascular permeability may contribute to the pathophysiology of AMS, and treatment with prostaglandin synthetase inhibitors may reduce this response (CATMAT 2007).
-
Selective 5‐hydroxytryptamine (1) receptor agonists (sumatriptan) are selective cerebral vasoconstrictors (Jafarian 2007b).
-
Anticonvulsant drugs (gabapentin): gabapentin is an anticonvulsant drug with analgesic properties (Cheng 2006; Maneuf 2006).
-
Hyperbaric therapy (chambers, manual air pump, fabric pressure bags or Gamow bags) simulate descent and gives symptomatic improvement within a few hours as a temporary measure while awaiting descent (CATMAT 2007).
B) High altitude pulmonary oedema (HAPE)
-
Calcium channel blockers (e.g. nifedipine) reduce pulmonary vascular resistance (Hackett 1992).
-
Nitric oxide is an endothelium‐derived relaxing factor which attenuates the pulmonary vasoconstriction produced by hypoxia (Blitzer 1996; Scherrer 1996; Schoene 2004; Wang 2003).
-
Non‐selective phosphodiesterase inhibitor (theophylline or aminophylline): the antihypoxia and antioxidation effects of aminophylline may reduce periodic breathing, cerebral and pulmonary microvascular permeability (Yang 2007), and also pulmonary artery pressure (Wright 2008).
-
Positive airway pressure and other therapies: breathing against a positive expiratory pressure improves arterial oxygen saturation (Bärtsch 1992; Larson 1992; Schoene 1985).
(See Appendix 3 for reported adverse effects of the pharmacological interventions).
Why it is important to do this review
It is important to conduct this systematic review for a number of reasons. First, many people travel to recreational areas located at high altitude, and with rapidly increasing levels of world travel, this trend is increasing (CATMAT 2007). Second, there is considerable uncertainty about the true effectiveness of the many approaches to treating acute HAI (Adams 2004; Bärtsch 2004; CATMAT 2007; Elphick 2004), and their clinical effectiveness and safety must be assessed. This is especially important, considering that travellers may be falsely reassured that they will be safe going to high altitudes, as they believe they have an effective remedy in their rucksacks in case they get ill.
A systematic review, including a rigorous assessment of the risk of bias, of the most up‐to‐date evidence will help clinicians make informed decisions on the use of non‐pharmacological and pharmacological interventions for treating acute HAI.
Objectives
To assess the clinical effectiveness, and safety of interventions (non‐pharmacological and pharmacological), as monotherapy or in any combination, for treating acute high altitude illness.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) irrespective of publication status (trials may be unpublished or published as an article, an abstract or a letter). We applied no language and no country limitation. We did not apply restrictions with respect to periods of follow‐up. We excluded studies about chronic mountain sickness or Monge’s syndrome (Leissner 2009; León‐Velarde 2010; Monge 1942). We excluded quasi‐randomized studies, and prospective observational studies for evaluating clinical effectiveness.
Types of participants
We included trials involving people with high altitude Illness (acute mountain sickness/high altitude cerebral oedema, or high altitude pulmonary oedema, or both), with or without a history of high altitude Illness. We did not apply any restriction by age and gender.
Types of interventions
Interventions
A) Non‐pharmacological interventions
-
Descent
-
Hyperbaric chamber
-
Portable pressure bag or Gamow bag
-
Breathing system designed to conserve oxygen supplies at high altitude
-
Positive airway pressure
B) Pharmacological interventions
-
Oxygen
-
Carbonic anhydrase inhibitors (e.g. acetazolamide)
-
Glucocorticosteroids: dexamethasone and medroxyprogesterone
-
Non‐steroidal anti‐inflammatory drugs (NSAIDs) and paracetamol: ibuprofen, aspirin and paracetamol
-
Selective 5‐hydroxytryptamine (1) receptor agonist: sumatriptan
-
Inhaled nitric oxide
-
Anticonvulsant drugs (e.g. gabapentin)
-
Diuretics (e.g. furosemide)
-
Calcium channel blockers: nifedipine
-
Magnesium
Comparisons
Placebo, monotherapy or any combination (non‐pharmacological plus pharmacological; pharmacological interventions).
Types of outcome measures
Primary outcomes
-
All‐cause mortality: we assessed this outcome through three approaches.
-
The number of deaths from any cause divided by the number of the participants in each group.
-
To determine how many deaths were caused by HAPE or HACE: the number of deaths from high altitude pulmonary oedema (HAPE) or high altitude cerebral oedema (HACE) divided by the number of participants in each group.
-
To determine how lethal HAPE or HACE were: the number of deaths by HAPE or HACE divided by the number of participants affected by HAPE or HACE in each group.
-
-
Complete relief of acute mountain sickness symptoms: defined as the complete absence of acute mountain sickness symptoms by the end of the study.
Secondary outcomes
-
Reduction in illness severity scores of acute mountain syndrome (headache, nausea, insomnia and dizziness; alone or in any combination) evaluated by the Lake Louise Questionnaire (Roach 1993), Environmental Symptoms Questionnaire (Sampson 1983), or any other validated scale. Because these different scales are not directly comparable, we analysed the results for each scale separately.
-
Adverse events
-
Adverse events: total adverse events and total serious adverse events. We defined adverse events as "any untoward medical occurrence that may present during treatment with a pharmaceutical product but which does not necessarily have a causal relationship with this treatment" (Nebeker 2004). Adverse drug reaction was defined as "a response to a drug which is noxious and uninitiated, and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiologic functions" (Nebeker 2004).
-
(See Appendix 3 for commonly described adverse events of the pharmacological approaches).
Search methods for identification of studies
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies specifically designed to identify relevant trials without restrictions to language or publication status.
We searched the following databases for relevant trials.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 7)
-
MEDLINE (Ovid SP, 1966 to 10 August 2017)
-
Embase (www.embase.com, 1988 to 10 August 2017)
-
LILACS (BIREME interface, 1982 to 10 August 2017)
-
ISI Web of Science (1973 to 10 August 2017)
-
CINAHL (EBSCO host, 1982 to 10 August 2017)
-
Wanfang (Wanfangdata.com to 10 August 2017)
We developed a subject‐specific search strategy in MEDLINE, and used that as the basis for the search strategies in the other databases listed. Where appropriate, the search strategy was expanded with search terms for identifying RCTs. Our search strategies can be found in Appendix 4.
Searching other resources
We scanned the World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch) for ongoing and unpublished trials on 19 August 2017; (see Appendix 5).
We developed the search strategy in consultation with the Information Specialist.
We scanned the reference lists and citations of included trials and any relevant systematic reviews identified for further references to additional trials.
Where necessary we contacted trial authors for additional information (February and March 2018 ).
Data collection and analysis
Selection of studies
Two review authors (DSR and IAR) independently assessed each reference identified by the search against the inclusion criteria. We resolved any disagreements by discussion. We consulted a third author (DO) as an arbiter if we could not reach agreement. We retrieved text in full for those references which appeared to meet the inclusion criteria, for further independent assessment by the same three review authors.
Data extraction and management
We used a predefined form to extract data (Appendix 6). We extracted the following data: eligibility criteria; demographics (age, gender, and country); rate of ascent (metres/hour); final altitude reached (metres); Acute Mountain Syndrome scale; study design; history of high altitude illness (HAI); type of HAI; intervention; and outcomes. For eligible studies, four review authors in two groups (DSR‒IAR and DO‒YX) extracted the data using the form. We resolved discrepancies through discussion or, when required, we consulted a fifth author (RH). We entered data into Review Manager 5 (RevMan 5) software (Review Manager 2014), and checked for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to obtain further details.
Assessment of risk of bias in included studies
We used Cochrane’s tool for assessing risk of bias, a two‐part tool that addresses six specific domains: random sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective reporting; and other bias (Higgins 2011). The first part describes the risk of bias, the second part provides criteria for making judgements about the risk of bias from each of the six domains in the tool. Based on this tool, we implemented a 'Risk of bias' worksheet to be completed for included studies. We used bias definitions from Porta 2008 for coding the "Other sources of bias" domain. The risk of bias was assessed by four review authors in two groups (DSR‒IAR and DO‒YX). We resolved any disagreement through consultation with an additional author (RH or JVAF). We displayed the results by creating a 'Risk of bias' graph, and a 'Risk of bias' summary figure using Review Manager 5 software (Review Manager 2014). We present the risk of bias in the Results section. We also provide summary assessments of the risk of bias for each outcome within and across studies (see Characteristics of included studies and Risk of bias in included studies).
Measures of treatment effect
We reviewed the evidence separately for the different interventions. For the binary outcomes (all‐cause mortality, complete relief of AMS symptoms, and adverse events), we presented results as summary risk ratios with 95% confidence intervals (95% CI). For continuous outcomes (reduction in illness severity scores) we reported the results as standardized mean difference with 95% CI instead of a mean difference as planned in the published protocol. This is a change from the protocol (Martí‐Carvajal 2012), and is explained in the Differences between protocol and review section.
Unit of analysis issues
The unit of analysis was the patient. We collected and analysed a single measurement for each outcome from each participant.
Dealing with missing data
In the case of missing data on participants or missing statistics (such as standard deviations (SD)) we planned to contact the trial authors. If unsuccessful, we planned to base our main analysis on the number reaching follow‐up, but we planned to perform a sensitivity analysis for worst and best case scenarios. For all outcomes we carried out analyses, as far as possible, on an intention‐to‐treat basis; that is we planned to include all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We planned to evaluate the extent of heterogeneity by visual inspection of the forest plot, and to use the I² statistic to quantify it (Higgins 2003; Higgins 2011), investigating possible causes of heterogeneity through subgroup analysis. If pre‐specified subgroup analyses did not explain the statistical heterogeneity, we planned to perform a sensitivity analysis in which small studies would be excluded. However, due to the scarcity of information we were not able to perform the subgroup analysis. This is a change from the protocol (Martí‐Carvajal 2012), and is explained in the Differences between protocol and review section.
Assessment of reporting biases
Where we suspected reporting bias, we planned to contact study authors asking them to provide missing outcome data. When this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis. We also planned to assess whether the review was subject to publication bias by using a funnel plot to graphically illustrate variability between trials. If asymmetry was detected, we planned to explore causes other than publication bias. We planned to conduct a funnel plot if 10 or more RCTs were included in the review. However, due to the scarcity of information we were not able to perform these analyses. This is a change from the protocol (Martí‐Carvajal 2012), and is explained in the Differences between protocol and review section.
Data synthesis
We planned to summarize the findings using both fixed‐effect and random‐effects models. In the presence of statistical heterogeneity, and an absence of small‐study effects, we expected the 95% CI from the random‐effects model to include the 95% CI from the fixed‐effect model. In this case, we planned to report only the data using the random‐effects model as it appropriately conveys heterogeneity. If a substantial difference was observed between both models, we planned to investigate this further as it can be due to an association between effect size and sample size. However, due to the scarcity of information we were not able to perform this analysis. This is a change from the protocol (Martí‐Carvajal 2012), and is explained in the Differences between protocol and review section.
Subgroup analysis and investigation of heterogeneity
We anticipated clinical heterogeneity in the effect of the intervention and we intended to conduct the following subgroup analyses, if the data were available.
-
Final altitude (metres)
-
High altitude illness history
-
The state of pre‐acclimatization
-
The regular intake of medication
-
Pre‐existing disease
We planned to perform subgroup analysis only for primary outcomes. However, due to the scarcity of information, we were not able to perform this analysis. This is a change from the protocol (Martí‐Carvajal 2012), and is explained in the Differences between protocol and review section.
Sensitivity analysis
We planned to conduct a sensitivity analysis comparing the results using all trials as follows.
-
For those RCTs with high methodological quality (studies classified as having a 'low risk of bias' (Higgins 2011)), we planned to choose three core domains instead of all: generation of allocation sequence, incomplete outcome data, and selective reporting bias.
-
For dichotomous outcomes, we planned to conduct ‘best‐case’ and ‘worst‐case’ scenarios. The ‘best‐case’ scenario is that all participants with missing outcomes in the experimental intervention group had good outcomes and all those with missing outcomes in the control intervention group had poor outcomes; the ‘worst‐case’ scenario is the converse (Higgins 2011).
We also planned to evaluate the risk of attrition bias, as estimated by the percentage of participants lost to follow‐up. Those studies with a total attrition of more than 20% or where differences between the groups exceed 10%, or both, would be included in the review but excluded from the meta‐analysis trials. However, due to the scarcity of information we were not able to perform this analysis. This is a change from the protocol (Martí‐Carvajal 2012), and is explained in the Differences between protocol and review section.
'Summary of findings' tables and GRADE
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes (Guyatt 2008): all‐cause mortality, by high altitude pulmonary oedema (HAPE), or by high altitude cerebral oedema (HACE); complete relief of acute mountain syndrome (AMS) symptoms; reduction in illness severity scores; and adverse events (safety). We developed 'Summary of findings’ (SoF) tables using GRADE software (GRADEpro GDT) for the comparisons.
-
Non‐pharmacological interventions for treating acute high altitude illness (summary of findings Table for the main comparison).
-
Pharmacological interventions for treating acute high altitude illness (summary of findings Table 2).
The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias. We downgraded the evidence one level or two taking into account these criteria. When imprecision was one of the reasons to downgrade the evidence, we provide the corresponding optimal information size calculations in Appendix 7.
We generated a 'Summary of findings' table for each of the interventions stated in the protocol where we found studies reporting the primary outcome: all‐cause mortality and complete relief of AMS symptoms. The 'Summary of findings' tables provide outcome‐specific information concerning the overall quality of evidence, the magnitude of effect of the interventions examined, and the amount of available data on the outcomes we considered.
Results
Description of studies
Results of the search
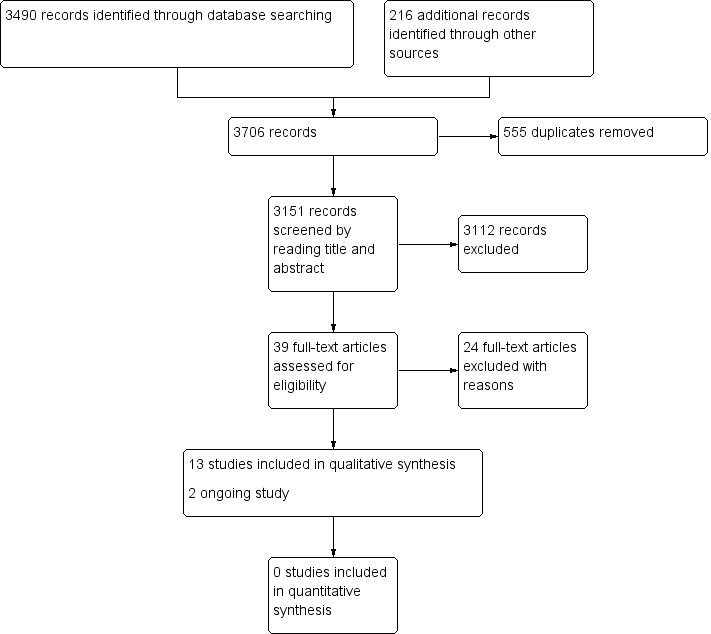
Figure 1 shows the review flow chart.

Study flow diagram.
We ran the search in August 2017 and it yielded 3706 records. We identified 3490 records through database searching, and retrieved 216 references from other sources. We excluded 555 duplicated references, leaving 3151 unique references. We screened the unique references by reading titles and abstracts. From these 3151 references, we identified 39 potentially eligible citations that we reviewed in full text, from which we excluded 24. Of the remaining 15 references, we identified 13 studies of high altitude illness (HAI) which met the inclusion criteria (published in 13 articles), and two ongoing studies (ChiCTR‐TRC‐13003298; NCT01522326).
Included studies
We included 13 studies (468 participants) in the review (Bärtsch 1990; Bärtsch 1993; Dumont 2004; Ferrazzini 1987; Grissom 1992; Harris 2003; Jafarian 2007a; Kasic 1991; Keller 1995; Li 2006; Utiger 2002; Wang 1998; Wright 1994). See also Characteristics of included studies tables.
Study design
All studies were parallel RCTs. The number of participants varied between 12 (Grissom 1992), and 74 (Harris 2003).
Participants
The proportion of men in the studies ranged from 40% (Harris 2003), to 95% (Bärtsch 1990), except for two studies that included only men (Li 2006; Wang 1998). Distribution by sex was not reported in two studies (Dumont 2004; Wright 1994). In most of the studies the participants were adults aged 18 years old or more. However, two studies also included teenagers (Harris 2003; Li 2006). The age of participants ranged from 13 to 61 years old. Three studies did not report the age distribution (Dumont 2004; Ferrazzini 1987; Wright 1994).
Studies included participants with mild to more severe symptoms of acute high altitude illness, and used different scores to define HAI as inclusion criteria (see Appendix 8). For instance, Wang 1998 recruited participants with HAPE using the definition of high altitude illnesses set forth by the Ad Hoc Committee on High Altitude Illnesses of Chinese Medical Association (Chinese Medical Association 1996). This score is different to the Lake Louise score.
Setting
Six studies took place in the Swiss‒Italian border region (Bärtsch 1990; Bärtsch 1993; Dumont 2004; Ferrazzini 1987; Keller 1995; Utiger 2002). The remaining studies were carried out in Alaska (Grissom 1992), the USA (Kasic 1991), Nepal (Harris 2003), Iran (Jafarian 2007a), Tibet (Li 2006), and China (Wang 1998). One study took place in the border areas between China, India and Pakistan (Wright 1994).
Two studies were carried out at high altitude (1500 to 3500 metres; Kasic 1991; Wright 1994), and the remaining in very high altitude (3500 to 5500 metres). No studies were done at extreme altitude (above 5500 metres).
Interventions
A variety of interventions were assessed in the studies. Non‐pharmacological intervention studies were limited to the hyperbaric chamber (Bärtsch 1993; Kasic 1991; Keller 1995), while pharmacological interventions were: oxygen (Bärtsch 1990), acetazolamide (Grissom 1992; Wright 1994), dexamethasone (Ferrazzini 1987; Keller 1995; Li 2006; Wang 1998), ibuprofen (Harris 2003), paracetamol (Harris 2003), sumatriptan (Utiger 2002), inhaled nitric oxide (Li 2006; Wang 1998), gabapentin (Jafarian 2007a), nifedipine (Wang 1998), and magnesium (Dumont 2004). Other drugs were included as part of the control group, such as aminophylline (Li 2006; Wang 1998), and furosemide (Li 2006; Wang 1998). We found no studies assessing descent, portable pressure bags or breathing systems.
Six studies were placebo controlled (Dumont 2004; Ferrazzini 1987; Grissom 1992; Jafarian 2007a; Utiger 2002; Wright 1994). The remaining seven studies used a treatment control group. The control group was described as standard care in two studies. The standard care was a combination of aminophylline and dexamethasone plus furosemide (Li 2006), or plus furosemide and oxygen (Wang 1998).
Funding sources
The majority of studies were funded by medical societies, universities or grants from governments or hospitals. In four studies, the private companies that developed the evaluated technologies provided financial support for the study (Harris 2003; Jafarian 2007a; Utiger 2002; Wright 1994). Only in Harris 2003 was there a statement about the independent control of the study by the researchers.
Outcomes
From the four outcomes predefined in the protocol, none of the included studies reported all‐cause mortality. Only two studies reported the proportion of participants who experienced a complete relief of symptoms (Ferrazzini 1987; Grissom 1992). All of the studies bar Wang 1998 evaluated reduction in illness severity scores. Utiger 2002 also used a headache score (0 = none, 1 = mild, 2 = moderate, 3 = severe headache), while Harris 2003 and Jafarian 2007a used a standard visual analogue scale (VAS). Four studies reported whether or not participants experienced adverse events (Dumont 2004; Grissom 1992; Jafarian 2007a; Kasic 1991).
In most of the RCTs the follow‐up was of 24 hours or less. The exceptions were Li 2006 and Wright 1994, who reported a follow‐up of three and five days, respectively; and Wang 1998, where follow‐up was until recovery.
Excluded studies
We excluded 24 studies for the following reasons: non‐randomized trials, narrative review, preventive studies or did not meet other eligibility criteria (Anand 1998; Bärtsch 1992; Bärtsch 1994; Bates 2007; Benedetti 2015; Bradwell 1988; Broome 1994; Brown 1977; Burtscher 1995; Deshwal 2012; Fagenholz 2007; Forster 1982; Forwand 1968; Levine 1989; Li 2010; Maggiorini 1995; Meehan 1986; Oelz 1989; Oelz 1992; Roggla 2001; Wright 1988; Yan 2010; Yanamandra 2016; Zhang 2012).
See the table Characteristics of excluded studies for further details.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
We identified two ongoing studies. ChiCTR‐TRC‐13003298 aims to assess the effect of oral trimetazidine for reducing the symptoms of acute mountain sickness and improving exercise performance. However, the information provided in the World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch), is not clear enough to allow us to define eligibility and we have not found any related publications. The second study is taking place in Nepal, and compares ibuprofen with metoclopramide (NCT01522326) (see Characteristics of ongoing studies).
Risk of bias in included studies
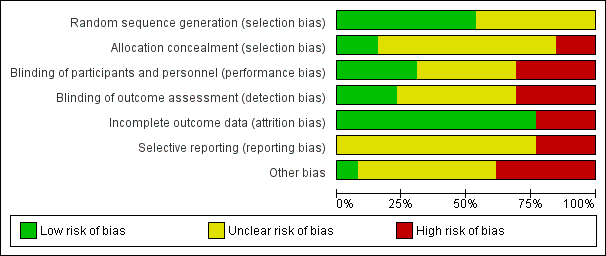
The risk of bias in terms of allocation, blinding, outcome, reporting, and other criteria is summarized in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
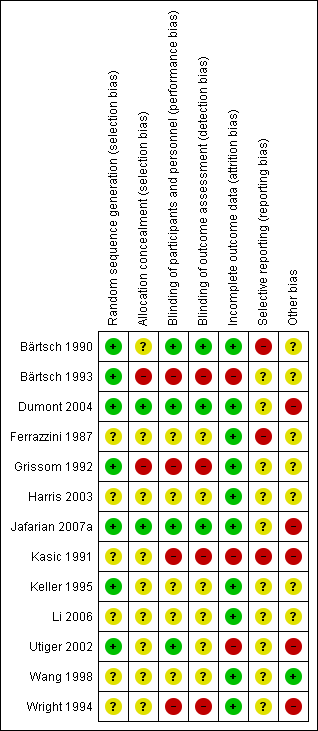
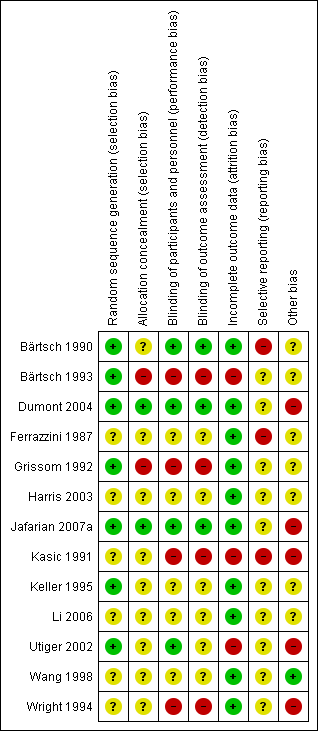
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Seven studies had low risk of selection bias. Two studies used random sequence generation to minimize selection bias, either by using a random number table (Dumont 2004), or computer‐generated randomization codes (Jafarian 2007a). In five studies randomization was performed in blocks (Bärtsch 1990; Bärtsch 1993; Grissom 1992; Keller 1995; Utiger 2002).
Six studies did not provide enough information to assess the sequence generation (Ferrazzini 1987; Harris 2003; Kasic 1991; Li 2006; Wang 1998; Wright 1994).
Allocation concealment
Two studies explicitly reported how the allocation was concealed: in Jafarian 2007a the computer‐generated randomization codes were exclusively kept by the pharmacist; and in Dumont 2004 the random numbers table was used centrally by the Hospital's Pharmacy. Two studies seem to have compromised allocation concealment: in Grissom 1992, a participant allergic to sulfa‐drug was manually assigned to the placebo group; and in Bärtsch 1993, the researcher manually adjusted the order of to‐be‐assigned blocks. The remaining studies did not provide enough information regarding allocation concealment to assess the risk of bias.
Blinding
Performance bias
Four studies had appropriate blinding methods for participants and personnel (Bärtsch 1990; Dumont 2004; Jafarian 2007aUtiger 2002). Four studies had high risk of bias for this domain considering the subjectivity of the outcomes assessed: two were not blinded (Bärtsch 1993; Kasic 1991); and authors from two studies stated that blinding was compromised during the study (Grissom 1992; Wright 1994). The remaining studies did not provide adequate data to allow assessment of blinding.
Detection bias
Three studies had appropriate methods of blinding assessment outcome (Bärtsch 1990; Dumont 2004; Jafarian 2007a). Four studies had high risk of bias for this domain: two were not blinded in spite of evidently different interventions (Bärtsch 1993; Kasic 1991); and in two studies blinding was compromised (Grissom 1992; Wright 1994). The remaining studies did not provide adequate data to allow assessment of blinding.
Incomplete outcome data
Most of the studies had low risk of attrition bias. The studies provided detailed characteristics of the recruited participants and followed up throughout their trials. Three studies had high risk of bias for this domain: one study, though the authors did not report any withdrawal, reported a small number of participants at the end of the study (Utiger 2002). In Bärtsch 1993, a subgroup of participants were excluded after randomization. And in the third study there was incomplete outcome data due to errors that occurred in the monitoring equipment (Kasic 1991).
Selective reporting
We had not enough information to determine if there was a high risk of bias from selective reporting, since the protocol was not available for any of them. Moreover, we considered three studies to be at high risk of selective reporting because outcome data was presented only graphically (Bärtsch 1990; Ferrazzini 1987; Kasic 1991).
Other potential sources of bias
One study had low risk of other potential bias (Wang 1998). However, seven studies inadequately reported the research design, such as sample size calculation, or had not reported sources of funding; therefore we considered them as having an unclear risk of bias (Bärtsch 1990; Bärtsch 1993; Ferrazzini 1987; Grissom 1992; Harris 2003; Keller 1995; Li 2006).
Five studies were judged to have high risk of bias (Dumont 2004; Jafarian 2007a; Kasic 1991; Utiger 2002; Wright 1994). In Utiger 2002, there were baseline differences after randomization (all females were in the placebo group). In two studies, the potential carry‐over effect was a source of bias since participants in the reported study had sequentially participated in a previous trial (Dumont 2004; Wright 1994).
Effects of interventions
See: Summary of findings for the main comparison Non‐pharmacological interventions for treating acute high altitude illness; Summary of findings 2 Pharmacological interventions for treating acute high altitude illness
See summary of findings Table for the main comparison; summary of findings Table 2.
We have obtained some of the numerical results below from graphs in the included papers rather than numerical results given in the text. We have indicated in the results below when this has been done.
Group 1: non‐pharmacological interventions
Primary outcomes
All‐cause mortality (comparison 1, outcome 1.1)
We found no trials reporting this outcome when using the following non‐pharmacological interventions: descent from altitude; use of a portable pressure bag (Gamow Bag); breathing systems for oxygen delivery; or the use of positive airway pressure. In addition, we identified three studies which compared the use of a hyperbaric chamber to simulate descent (Bärtsch 1993; Kasic 1991; Keller 1995), enrolling a total of 124 participants (26% of the total in this review), and none specifically stated that mortality was an outcome of interest. No deaths were reported.
Complete relief of acute mountain sickness symptoms (comparison 1, outcome 2.1)
We found no trials reporting this outcome when using the following non‐pharmacological interventions: descent from altitude; use of a portable pressure bag (Gamow Bag); use of a hyperbaric chamber; breathing systems for oxygen delivery; or the use of positive airway pressure.
Secondary outcomes
Reduction in illness severity scores of acute mountain syndrome (comparison 1, outcome 3.1)
We found no trials reporting this outcome when using the following non‐pharmacological interventions: descent from altitude; use of a portable pressure bag (Gamow Bag); breathing systems for oxygen delivery; or the use of positive airway pressure.
3.1 Hyperbaric chamber simulated descent
Three studies reported this outcome, enrolling a total of 124 participants (26% of the total in this review). No pooling of data was possible however, due to clinical heterogeneity arising from the use of different comparators in each trial.
Kasic 1991 included 29 participants, and compared a pressurization of 120 mmHg (equivalent to 160 millibars) versus supplementary oxygen. Clinical outcome data was only presented graphically. For the pressurization group, the estimated score mean is near to 0.7; and for the oxygen group it is near to 0.8 (data estimated from Kasic 1991, Figure 2). The authors stated that both groups had a reduction in symptom scores compared to baseline but there were no important differences between groups.
Bärtsch 1993 included 64 participants, and compared simulated descent using a pressure of 193 millibars versus a pressure of 20 millibars, with a third group in which participants had bed rest. This trial reported both a clinical score, and the Acute Mountain Syndrome ‐ Cerebral (AMS‐C) score, a subscore of the Environmental Symptoms Questionnaire developed by Sampson 1983. The AMS‐C score was measured after one hour and 12 hours of treatment, as well as at rest. There were no clear differences in the clinical severity scores between the three trial groups 12 hours after treatment (pressure increases of 193 millibar group (mean = 2.5), 20 millibar group (mean = 3.1), and rest only (mean = 2.3); estimated reduction of 0.6 points); or in terms of the AMS‐C score (pressure increases of 193 millibar group (mean = 1.02), 20 millibar group (mean = 1.36), and rest only (mean = 0.92)). We downgraded the quality of evidence from high to low due to risk‐of‐bias issues as well as imprecision (summary of findings Table for the main comparison).
Keller 1995 included 31 participants, and compared simulated descent using a pressure of 193 millibars with dexamethasone. This trial reported a reduction in clinical score at one hour when a hyperbaric chamber was compared with dexamethasone (mean of −4.0 points and −1.5 points, respectively). Similar results were found when Lake Louise Score, and AMS‐C score were analysed. However, after 11 hours the clinical scores in the simulated descent group were higher than in those who had received dexamethasone (mean of −1.0 and −4.1, respectively; higher results mean worse symptoms).
Adverse events (comparison 1, outcome 4.1)
We found no trials reporting this outcome when using the following non‐pharmacological interventions: descent from altitude; use of a portable pressure bag (Gamow Bag); breathing systems for oxygen delivery; or the use of positive airway pressure.
4.1. Hyperbaric chamber simulated descent
Kasic 1991 included 29 participants, and stated there were no complications associated with the use of the hyperbaric chamber (no events in either arm). We downgraded the quality of evidence from high to low due to risk of bias and imprecision issues (summary of findings Table for the main comparison).
Group 2: pharmacological interventions
Primary outcomes
All‐cause mortality (comparison 2, outcome 2.1)
We found no trials specifically reporting this outcome when using the following pharmacological interventions: oxygen; carbonic anhydrase inhibitors; glucocorticosteroids; non‐steroidal anti‐inflammatory drugs and acetaminophen; selective 5‐HT(1) antagonists; inhaled nitric oxide; anticonvulsant drugs; diuretics; calcium channel blockers; phosphodiesterase inhibitors; or magnesium.
Complete relief of acute mountain sickness symptoms (comparison 2, outcome 2.2 and 2.3)
We found no trials specifically reporting this outcome when using the following pharmacological interventions: oxygen; carbonic anhydrase inhibitors; selective 5‐HT(1) antagonists; inhaled nitric oxide; anticonvulsant drugs; diuretics; calcium channel blockers; phosphodiesterase inhibitors; or magnesium.
2.2. Non‐steroidal anti‐inflammatories and paracetamol
Grissom 1992 enrolled 12 participants (3% of the total in this Cochrane Review), and compared the NSAID ibuprofen 400 mg with paracetamol 1000 mg (six participants to each). At 24 hours five out of six (83%) participants in the ibuprofen group were healthy, compared to none (0%) of the six participants in the paracetamol group (estimated RR 11, CI 95% 0.74 to 163.4).
2.3. Glucocorticosteroids
Ferrazzini 1987 enrolled 35 participants (3% of the total in this Cochrane Review), 17 (49%) allocated to dexamethasone and 18 (51%) to a placebo. Eight out of 17 (47%) participants treated with dexamethasone had all symptoms and signs of acute mountain sickness resolved (score 0) after 12 and 16 hours, compared to none of the 18 (0%) participants who had received placebo (RR not estimable). We downgraded the quality of evidence from high to low due to risk of bias and imprecision issues (summary of findings Table 2).
Secondary outcomes
Reduction in illness severity scores of acute mountain syndrome (comparison 2, outcomes 2.4 to 2.11)
We found no trials specifically reporting this outcome when using the following pharmacological interventions: carbonic anhydrase inhibitors; diuretics; calcium channel blockers; or phosphodiesterase inhibitors.
2.4. Oxygen
Bärtsch 1990 enrolled 13 participants (3% of the total in this Cochrane Review) in the comparison of 33% oxygen (six participants, 46%) and a control group breathing normal compressed air (seven participants, 54%). This trial reported that the oxygen group had a greater decrease in the AMS‐C score compared with the normal air group (estimated mean score after treatment = 1.1 versus 1.0; data estimated from Bärtsch 1990, figure 1).
2.5. Carbonic anhydrase inhibitors
Two studies reported this outcome, enrolling a total of 25 participants, 5% of the total number of participants included in this Cochrane Review (Grissom 1992; Wright 1994). There was no clear benefit from the use of acetazolamide compared to placebo (SMD 1.15 lower with acetazolamide, 95% CI 2.56 lower to 0.27 higher; I² = 58%; Analysis 1.1). We downgraded the quality of evidence from high to low due to risk of bias, and inconsistency issues (summary of findings Table 2).
2.6. Glucocorticosteroids
Ferrazzini 1987 enrolled 35 participants (7.5% of the total in this Cochrane Review), 17 (49%) allocated to dexamethasone and 18 (51%) to a placebo. The mean AMS score dropped from 5.4 (SD 1.7) to 1.3 in the dexamethasone group, and from 4.8 (SD 1) to 4.2 (SD 2.2). Authors reported that the change in the acute mountain sickness score was 4.1 in the dexamethasone group, and 0.4 in the placebo group, a difference of 3.7 units between these groups (SD for each group not reported; confidence interval of the mean difference reported by authors = −5.3 to −2.2). We downgraded the quality of evidence from high to moderate due to the risk of bias (summary of findings Table 2).
2.7. Non‐steroidal anti‐inflammatory drugs (NSAIDs) and paracetamol
Harris 2003 enrolled 74 participants (16% of the total in this Cochrane Review), and compared the NSAID ibuprofen 400 mg (39 (53%) participants) with paracetamol 1000 mg (35 (47%) participants). At one hour, there were no differences in the mean score between the ibuprofen group (mean = 1.8; SD = 1.69), and the paracetamol group (mean = 2.1; SD = 2.18). Within two hours of treatment, the mean of headache intensity was lower in both groups, but there were no differences between the ibuprofen (mean = 0.8; SD = 1.38), and the paracetamol group (mean = 0.9; SD = 1.6).
2.8. Selective 5‐hydroxytryptamine (1) receptor agonist
Utiger 2002 enrolled 29 participants (6% of the total in this Cochrane Review), and compared sumatriptan in 14 participants (48%) with a placebo in 15 participants (52%). This trial reported that the headache score decreased significantly in both study groups at one, three and 12 hours after medication. However, there were no significant differences between sumatriptan and placebo at any particular moment of the trial: within three hours the mean score in sumatriptan group was 1.5 (SD = 0.9) versus 1.7 (SD = 1.1) in the placebo group. Within 12 hours (n = 20) sumatriptan mean was 1.5 (SD = 1.1) versus 1.7 for the placebo group (SD = 0.9).
2.9. Inhaled nitric oxide
Li 2006 enrolled 47 participants (10% of the total participants in this Cochrane Review) with 24 (51%) allocated to receive nitric oxide compared to 23 (49%) allocated to a control treatment. Authors reported that both groups had a reduction in symptom scores using the Lake Louise Score, with a mean of 1.78 for the nitric oxide group (SD 1.31) versus a mean of 2.43 for the standard care group (SD 1.56).
2.10. Anticonvulsant drugs
Jafarian 2007a enrolled 24 participants (5% of the total participants in this Cochrane Review), 12 to each of a gabapentin group and a placebo group. Within one hour of treatment there were no differences in the mean VAS score between the gabapentin group (mean = 2.92; SD = 2.91), and the placebo group (mean = 4.75; SD = 3.11. Mean difference not reported by trial authors). We downgraded the quality of evidence from high to low due to risk of bias, and imprecision issues (summary of findings Table 2).
2.11. Magnesium
Dumont 2004 enrolled 25 participants (5% of the total in this Cochrane Review) with 12 (48%) allocated to receive magnesium and 13 (52%) to receive a placebo preparation. Authors reported that the mean scores of both groups at two hours were comparable (magnesium sulphate mean score = 9; SD = 3.5; placebo mean score = 10.3; SD = 2.8. Mean difference not reported by trial authors). We downgraded the quality of evidence from high to low due to risk of bias, and imprecision issues (summary of findings Table 2).
Adverse events (outcome 2 and outcomes 2.12)
We found no trials specifically reporting this outcome when using the following pharmacological interventions: oxygen; glucocorticosteroids; non‐steroidal anti‐inflammatory drugs and acetaminophen; selective 5‐HT(1) antagonists; inhaled nitric oxide; diuretics; calcium channel blockers; or phosphodiesterase inhibitors.
2.12. Carbonic anhydrase inhibitors
Grissom 1992 stated that no significant adverse events of acetazolamide were found (0% for acetazolamide arm; data not reported for placebo arm; RR not estimable). We downgraded the quality of evidence from high to low due to risk of bias and imprecision issues (summary of findings Table 2).
2.13. Anticonvulsant drugs
Jafarian 2007a enrolled 24 participants (5% of the total participants in this Cochrane Review), 12 to each assessed group. The authors reported no adverse events (0% for both arms; RR not reported by trial authors). We downgraded the quality of evidence from high to low due to risk of bias, and imprecision issues (summary of findings Table 2).
2.14. Magnesium
Dumont 2004 enrolled 25 participants (5% of the total in this Cochrane Review) with 12 (48%) allocated to receive magnesium and 13 (52%) to receive a placebo preparation. Authors reported that 9 out of 12 participants who had received intravenous magnesium sulphate had flushing, compared to 1 out of 13 participants who had received placebo (75% versus 7%, respectively; RR not reported). We downgraded the quality of evidence from high to low due to risk of bias and imprecision issues (summary of findings Table 2).
Discussion
Summary of main results
We retrieved 3706 articles through our search strategy. After applying the eligibility criteria, we included 13 studies and 468 participants in the review, and classified two studies as ongoing. We found sparse evidence from small trials evaluating a wide variety of treatments for high altitude illness (HAI). All studies included adults, and two studies included both teenagers and adults. The 13 studies took place in high altitude areas, mostly in the European Alps. Twelve studies included participants with acute mountain sickness, and one study included participants with high altitude pulmonary oedema. Follow‐up was usually less than one day. We report results for the main comparisons as follows.
Non‐pharmacological interventions (3 studies, 124 participants)
All‐cause mortality, and complete relief of AMS symptoms were not reported for included trials. Regarding reduction in symptom score severity, we found for simulated descent of 193 millibars versus 20 millibars mean scores (read from graphs) of 2.5 and 3.1 after 12 hours of treatment, respectively (one study; 64 participants; low quality of evidence). In addition, no complications were found with use of hyperbaric chambers versus supplementary oxygen (one study; 29 participants; low‐quality evidence).
Pharmacological interventions (11 trials, 375 participants)
All‐cause mortality was not reported for included trials. One trial found a greater proportion of participants with complete relief of AMS symptoms after 12 and 16 hours when dexamethasone was administered in comparison with placebo (47.1% versus 0%, respectively; RR not estimable; one study; 35 participants; low quality of evidence). Likewise, data on acetazolamide versus placebo did not show differences in terms of reduction in symptom score severity (standardized mean difference (SMD) −1.15, 95% CI −2.56 to 0.27; 2 studies, 25 participants; low‐quality evidence). One trial found benefits, in terms of reduction in symptom score severity, when dexamethasone is compared to placebo (difference on change in the AMS score: 3.7 units, reported by authors; one study; 35 patients; moderate quality of evidence). Two additional trials on gabapentin versus placebo, and magnesium versus placebo did not find reductions in symptom score severity at the end of the treatment. (For gabapentin versus placebo: mean VAS score of 2.92 versus 4.75, respectively; one study; 24 participants; low quality of evidence. For magnesium versus placebo: mean scores of 9 and 10.3 units, respectively; one study; 25 participants; low quality of evidence). Regarding adverse effects after treatment, trials comparing acetazolamide versus placebo and gabapentin versus placebo did not find adverse events. (For acetazolamide trial: one study; 25 participants; low quality of evidence; for gabapentin trial: one study; 24 participants; low quality of evidence). One trial comparing magnesium sulphate versus placebo found that flushing was a frequent event in the magnesium arm. (Percentage of flushing: 75% versus 7.7%, respectively; one study; 25 participants; low quality of evidence).
We found no studies addressing interventions such as descent, portable pressure bag or Gamow bag, breathing system designed to conserve oxygen supplies at high altitude, positive airway pressure, aspirin or medroxyprogesterone.
Overall completeness and applicability of evidence
The evidence supporting or refuting the usefulness of a wide range of approaches to treating HAI is incomplete. We identified a limited number of studies addressing the effectiveness and safety of potential interventions to management of acute high altitude illness (13 studies, and 468 participants). Most of the studies did not include participants suffering from high altitude pulmonary oedema (HAPE), and none of the included studies assessed the treatment of high altitude cerebral oedema (HACE). HAPE and HACE are the most severe forms of high altitude illness (HAI). Therefore we have insufficient evidence of the effects of interventions for these conditions. Furthermore, the only study which included participants suffering from HAPE did not report the most severe outcome — mortality. Likewise, the identification of only one study for several assessed comparisons was a common scenario, which limited the ability to address the objectives of this review.
Few included studies reported our primary and secondary outcomes of interest. In addition, we found a variable definition of "standard care" across the included studies. In some cases, the control "standard care" included the use of oxygen, furosemide and aminophylline. This fact may lead to challenges when extrapolating the evidence to practice, since they may not reflect the standard care provided in other settings or countries. We also found the report of outcomes was not complete in many studies. Some studies reported composite outcomes for "cure" which included radiographic findings, and clinical findings. These results are difficult to interpret since we cannot ascertain how much of this definition was based on radiographic or clinical findings.
Quality of the evidence
We used the GRADE system to assess the quality of the body of evidence associated with primary and secondary outcomes. See summary of findings Table for the main comparison and summary of findings Table 2 for complete assessments and the rationale for ratings. We downgraded the quality of evidence in most cases due to risk of bias as well as imprecision (optimal information size (OIS) was not met due to insufficient sample sizes). In addition, most of the included studies were poorly reported in methodology and outcome data. The poor reporting may be due to the fact that more than half of the studies (8 out of 13) were conducted in the 1980s and 1990s when standards for reporting had not yet been proposed. This explains that a great number of domains in the risk of bias assessment had an "unclear" judgment. Blinding in most cases was not clear or reported as not possible; this fact may limit the interpretation of the study findings, since most of the outcomes were measured with symptomatic scores reported by participants. In addition, funding was a source of bias in a group of studies, and the independence of the research teams was not guaranteed. For further details on the risk of bias, see the Risk of bias in included studies. Finally we could not address the risk of publication bias with a statistical approach, since we did not find enough studies to perform a statistical analysis. However we found no evidence supporting the suspicion of publication bias (e.g. completed clinical trials in registries not published).
Potential biases in the review process
We followed the methodology for systematic reviews outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We performed a comprehensive search of the evidence for high altitude illness (HAI). Nevertheless, the reports were often incomplete and the attempts at requesting clarification were unsuccessful. Additionally, a single author performed data extraction and risk of bias assessment for the studies reported in Chinese; nevertheless, the results were discussed with the whole review team.
When considering study results, most of the results were narrative as supplied in the paper in question, since meta‐analysis was not possible due to clinical heterogeneity. Finally, we did not include observational studies for the assessment of the incidence of adverse events (See Differences between protocol and review). The report of adverse events in the included randomized controlled trials was limited, and therefore this review might not adequately assess this outcome comprehensively.
Agreements and disagreements with other studies or reviews
We found other systematic reviews addressing pharmacological interventions for high altitude illness (Murdoch 2010; Seupaul 2012; Tang 2014; Xu 2014); but all these systematic reviews included randomized controlled trials which evaluated preventive measures, but not treatment, for HAI. Considering the underlying common pathophysiological pathway, many interventions used for prevention are also used for treatment (See Nieto Estrada 2017 for an assessment of pharmacological interventions commonly used for prevention of high altitude illness).
Study flow diagram.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Acetazolamide versus placebo, Outcome 1 AMS symptoms (standardized).
| Non‐pharmacological interventions for treating acute high altitude illness | ||||||
| Patient or population: people suffering from high altitude illness | ||||||
| Outcomes and intervention | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with various interventions | Risk with non‐pharmacological interventions | |||||
| All‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Complete relief of AMS symptoms | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Reduction in symptom score severity at 12 hours (Clinical score: ranged from 0 to 11 (worse)) Intervention: Simulated descent of 193 millibars versus 20 millibars | The mean score in the control group was 3.1 | The mean score in the intervention group was 2.5 | 0.6 points lower with intervention | 64 (1 RCT) | ⊕⊕⊝⊝ | |
| Adverse effects during treatment Intervention: Hyperbaric chamber/ 160 millibars versus supplementary oxygen | 0 per 1000 | 0 per 1000 | Nil | 29 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Quality of evidence downgraded by two levels due to serious risk of bias (performance bias (blinding was not specified), attrition bias and selective reporting bias) and serious imprecision (optimal information size criteria not achieved) | ||||||
| Pharmacological interventions for treating acute high altitude illness | |||||||
| Patient or population: people suffering from high altitude illness | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | ||
| Risk with various interventions | Risk with pharmacological interventions | ||||||
| All‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported | |
| Complete relief of AMS symptoms (12 to 16 hours after treatment) Scale used: Acute Mountain Sickness score (ranged from 0 to 9 (worse)) | Dexamethasone versus placebo | 0 per 1000 | 471 per 1000 | No estimable | 35 | ⊕⊕⊝⊝ | |
| Reduction in symptom score severity Time of measurement: 1 to 48 hours after treatment, end of treatment Scale of measurement: Self‐administered AMS questionnaires (ranged from 0 to 90 (worse)), AMS Symptom Questionnaire (ranged from 0 to 22 (worse)), Acute Mountain Sickness score (ranged from 0 to 9 (worse)), HAH Visual analogue score (VAS) (range no stated), Lake Louise Score (from 0 to 15 (worse)), | Acetazolamide versus placebo | Standardized Mean Difference 1.15 lower | 25 | ⊕⊕⊝⊝ | |||
| Dexamethasone versus placebo | Mean change from baseline: 0.4 units | Mean change from baseline: 4.1 units | Difference of 3.7 units (reported by trial authors) | 35 | ⊕⊕⊕⊝ | ||
| Gabapentin versus placebo | Mean VAS score: 4.75 | Mean VAS score: 2.92 | Not stated | 24 | ⊕⊕⊝⊝ | ||
| Magnesium versus placebo | Mean score: 10.3 units | Mean score: 9 units | Not stated | 25 | ⊕⊕⊝⊝ | ||
| Adverse effects Time of measurement: 1 to 48 hours after treatment, end of treatment Scale of measurement:not stated | Acetazolamide versus placebo | No reported | 0 per 1000 | Not estimable | 25 | ⊕⊕⊝⊝ | |
| Gabapentin versus placebo | 0 per 1000 | 0 per 1000 | Not stated | 24 | ⊕⊕⊝⊝ | ||
| Magnesium sulphate versus placebo | 77 per 1000 | 750 per 1000 | Not stated | 25 | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 Quality of evidence downgraded by two levels due to very serious risk of bias (multiple unclear biases and high risk of selective reporting bias) 2 Quality of evidence downgraded by two levels due to serious risk of bias (selection bias) and serious inconsistency (I² = 58%). 3 Quality of evidence downgraded by one level due to serious risk of bias (selection, performance and detection bias). 4 Quality of evidence downgraded by two levels due to serious risk of bias and serious imprecision. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AMS symptoms (standardized) Show forest plot | 2 | 25 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐2.56, 0.27] |

