Córneas artificiales versus córneas de donantes para los trasplantes corneales repetidos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009561.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 05 noviembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud ocular y de la visión
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Conceiving and designing the review: EKA
Coordinating the review: KL
Data collection for the review
Designing electronic search strategies: Iris Gordon at the CEVG editorial base
Undertaking manual searches: EKA, KL
Screening search results: MA, FSH, SMN, KL
Organising retrieval of papers: SMN, KL
Screening retrieved papers against inclusion criteria: MA, FSH, SMN, KL
Appraising quality of papers: MA, FSH, SMN, KL
Extracting data from papers: MA, FSH, SMN, KL
Data management for the review: KL, SMN
Entering data into RevMan: KL, SMN
Interpretation of data
Providing a methodological perspective: KL, SMN
Providing a clinical perspective: EKA, MA, FSH
Providing a policy perspective: EKA, MA, FSH
Providing a consumer perspective: EKA, MA, FSH
Writing the review: EKA, KL, MA, FSH, SMN
Providing general advice on the review: EKA, KL, MA, FSH, SMN
Performing previous work that was the foundation of the current study: EKA
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
-
National Institute for Health Research (NIHR), UK.
-
Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
-
Declarations of interest
None known.
Acknowledgements
We acknowledge Iris Gordon, Trials Search Co‐ordinator for the Cochrane Eyes and Vision Group (CEVG), for devising and running electronic search strategies. We acknowledge Anthony Aldave, Roy Chuck, and Anupa Shah (Managing Editor for CEVG) for commenting on previous versions of this review. We thank Irmgard Behlau, Maria Cortina, Francis Price, Marie‐Claude Robert and Barbara Hawkins for reviewing and providing feedback for this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 May 13 | Artificial corneas versus donor corneas for repeat corneal transplants | Review | Masako Chen, Sueko M Ng, Esen K Akpek, Sumayya Ahmad | |
| 2014 Nov 05 | Artificial corneas versus donor corneas for repeat corneal transplants | Review | Esen K Akpek, Majed Alkharashi, Frank S Hwang, Sueko M Ng, Kristina Lindsley | |
| 2012 Jan 18 | Artificial corneas versus donor corneas for repeat corneal transplants | Protocol | Esen K Akpek, Majed Alkharashi, Kristina Lindsley | |
Differences between protocol and review
Because no eligible trials were identified after screening titles and abstracts, we revised the classification of studies for the full‐text screening stage. Rather than classifications of (a) include, (b) unclear, or (c) exclude, we assessed study reports as (a) include for discussion, (b) exclude from discussion, or (c) exclude, not relevant. Studies assessed as (a) include for discussion are listed in the review as Excluded studies.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO
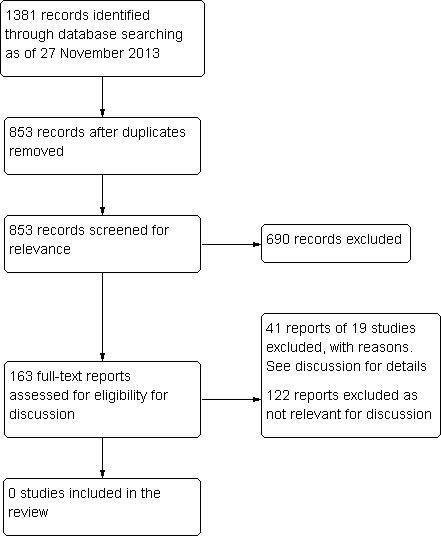
Results for searching for studies for inclusion in the review.
| Study | Study design | Study dates | Country | Follow‐up | Number of participants | Number with repeat PK | Funding source and declarations of interest |
| Multicenter retrospective and prospective case | 2003‐2008 | 18 sites in the USA | Mean 17 (range 1 week to 6.1 years) | 300 (300 eyes) | 244/300 (81.3%) | "No surgeons in the study group have any proprietary interest in the Boston Type 1 keratoprosthesis." | |
| Retrospective case series | 2004‐2011 | USA (Jules Stein Eye Institute) | Mean 24 months (range 0 to 84 months) | 94 (98 eyes, 110 devices) | 82/98 (83.7%) eyes | "The author(s) have no proprietary or commercial interest in any materials discussed in this article." | |
| Retrospective case series | 2004‐2011 | Armenia, India, Indonesia, Nepal, Philippines, Russia, and Saudi Arabia | Mean 14.2 months (range 0 to 48 months) | 100 (107 eyes, 113 devices) | 74/107 (69.2%) eyes | "The author(s) have no proprietary or commercial interest in any materials discussed in this article." | |
| Retrospective case series | 2005‐2007 | USA (Wills Eye Institute) | Mean 16 months (range 6 to 28 months) | 37 (37 eyes) | 29/37 (78.4%) | "Dr. H. F. Chew was supported by the E. A. Baker Fellowship Fund Grant from the Canadian National Institute for the Blind." | |
| Retrospective case series | 2004‐2008 | USA (Wilmer Eye Institute and University of Rochester Eye Institute) | 6 months | 122 (126 eyes) | 112/126 (88.9%) eyes | "The authors have no proprietary or commercial interest in any of the materials discussed in this article." | |
| Retrospective case series | 2004‐2008 | USA (University of California, Davis) | Mean 19 months (range 1 to 48 months) | 28 (30 eyes) | 26/30 (86.7%) eyes | "The author(s) have no proprietary or commercial interest in any materials discussed in this article." | |
| Retrospective case series | 2006‐2011 | Spain (Instituto Microcirugia Ocular of Barcelona) | Mean 20 months (range 1 to 56 months) | 53 (54 eyes) | 49/54 (90.7%) eyes | "This work has been done with the help of the Ophthalmological Society of the Valencian Community, Valencia, Spain. The first author of the work has been awarded a fellowship for further study of residents at the Ocular Microsurgery Institute of Barcelona, sponsored by Pfizer."; "The authors have no financial or proprietary interest in the materials presented herein." | |
| Retrospective case series | 2003‐2009 | USA (Kellogg Eye Center, University of Michigan) | Mean 17 months (range 3 to 67 months) | 29 (30 eyes) | 23/30 (76.7%) eyes | "Supported in part by a departmental grant from the Research to Prevent Blindness (RPB), the RPB Lew R.Wasserman Merit Award, and the National Eye Institute K23 Mentored Clinician Scientist Award." | |
| Retrospective case series | 2009‐2011 | Germany (Neuhann & Colleagues) | Mean 9.1 months (range 1 to 21 months) | 14 (14 eyes) | 13/14 (92.9%) | "The corresponding author indicates no conflict of interests." | |
| Retrospective case series | 2006‐2010 | USA (New York Eye and Ear Infirmary) | Mean 21.5 months (range 3 to 47 months) | 51 (58 eyes) | 47/58 (81.0%) eyes | "The authors declare no conflict of interest." | |
| Retrospective case series | 2007‐2010 | Jordan (King Abdullah University Hospital) | Mean 18 months (range 3 to 36 months) | 19 (20 eyes) | 19/20 (95%) eyes | "Source of Support: Nil, Conflict of Interest: None declared." | |
| Retrospective case series | 2008‐2009 | Canada (Centre Hospitalier de l’Université de Montréal, Hôpital Notre‐Dame), | Mean 16.5 months | 38 (38 eyes) | 25/38 (65.8%) eyes | "Supported by a research grant from the Fonds de Recherche en Ophthalmologie de l’Université de Montréal, Montreal, Canada; and a Resident Research Grant from Pfizer Canada (Kirkland, Canada)"; "The authors indicate no financial conflict of interest." | |
| Early model of Boston keratoprosthesis (known as Dohlman‐Doane keratoprosthesis) | |||||||
| Retrospective case series | 2003‐2005 | USA (University of Rochester Eye Institute) | 12 months | 25 (25 eyes) | 22/25 (88%) | "The authors have no proprietary interest in any products mentioned in this article." | |
| Retrospective case series | 1990‐2004 | USA (Massachusetts Eye and Ear Infirmary) | Mean 35 months (range 1 to 108 months) | 128 (157 eyes) | 157/157 (100%) eyes | "Supported by a Massachusetts Eye and Ear Infirmary fund and the Alcon Research Institute award." | |
| PK: penetrating keratoplasty | |||||||
| Study | Number with BCVA ≥ 20/100 | Other visual acuity outcomes | Proportion of graft failures* | Number of device extrusions | ||||
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |||
| 29 of 62 (46.8%) | NR | NR | "The number of patients with best‐corrected VA (BCVA) 20/200 or better went from 3.6% preoperatively to 57% postoperatively. Nineteen percent had postoperative vision of 20/40 or better." | 21/300 (7.0%) (12/244, 4.9% in participants with previous failed graft); failure rate 6% (n = 161 eyes) | NR; failure rate 11% (n = 91 eyes) | NR | 4/300 (1.3%) | |
| 42 of 77 (54.5%) | 22 of 47 (46.8%) | 5 of 7 (71.4%) | NR | 22/110 (20.0%) devices failed at final follow‐up; failure rate 8.3% (n = 74) | NR; failure rate 21.6% (n = 45) | NR; failure rate 38.4% (n = 5) | NR | |
| 33 of 65 (50.8%) | 18 of 34 (52.9%) | 0 of 1 (0%) | "In 82.2% (74/90) of the eyes in the international series in which the keratoprosthesis was retained at the final follow‐up visit, the final postoperative CDVA was better than the preoperative CDVA, and in 13.3% (12/90) of eyes, the preoperative and postoperative CDVAs were the same." | 22/113 (19.5%) devices failed at final follow‐up; failure rate 20.8% (n = 58) | NR; failure rate 25.4% (n = 18) | NA | NR | |
| NR | NR | NA | The mean BCVA at any point postoperatively and at last follow‐up were 20/50 (range: 20/400 to 20/20; P < 0.001) and 20/90 (range: light perception to 20/25; P < 0.001), respectively. The mean BCVA over time at 6 ,12, and 18 months showed significant visual improvement (P < 0.001) compared with BCVA preoperatively.16 patients (43%) achieved a BCVA better than or equal to 20/50 at last follow‐up (time not specified); 23 of 30 patients (76.7%) with minimum follow‐up of 12 months had a BCVA better than or equal to 20/200) | 1/30 (3%) (the type 2 model) | NA | NA | 1/30 (3%) (the type 2 model) | |
| NA | NA | NA | "Of 126 eyes, 104 (82.5%) achieved improved vision within the first 6 months postoperatively."; "At the 3‐month follow‐up, 54% of eyes had 20/200 vision or better, and 18% were 20/40 or better. Twenty‐two of the eyes (22/126; 17.4%) did not have improved vision. Eight eyes lost vision" | NA | NA | NA | 3/126 (2.4%) extrusion/corneal melt at 6 months | |
| NR | NR | NA | In the subgroup of 16 eyes followed for at least 1 year after keratoprosthesis implantation (mean follow‐up, 28 months; range 12 to 48 months; SD 12.8 months; median 24 months), vision was ≥ 20/200 in 75% of eyes and ≥ 20/40 in 25% of eyes | 5/30 (16.7%) at mean of 19 months follow‐up | NR | NR | NR | |
| 12/54 eyes at final follow‐up | NR | NR | "The postoperative BCVA was 0.097 (SD 0.18). Thirty‐three (33%) achieved a BCVA ≥ 0.1 (18 eyes) and 7.4% achieved ≥ 0.4 (4 eyes). Rapid improvement in the BCVA was observed. The measurements obtained 3 months after the operation do not differ significantly from the final BCVA." | 2/54 (3.7%); failure rate 4% | NR | NR | NR | |
| NR | NR | NR | Mean postoperative BCVA 20/390 (range 20/40 to LP; n = 16) at one year; "When comparing preoperative BCVA with final BCVA, vision improved in 19 of 30 eyes (63%) and was unchanged in 7 eyes (23%). Vision worsened in 4 eyes (13%) because of glaucoma in 3 eyes, 2 of which became no light perception, and retinal detachment occurred in 1 eye” | 6/20 (30%) | NR | NR | NR | |
| 2 of 4 (50%) | NA | NA | 10 of 14 had BCVA ≥ 0.03, 1 had CF, and 3 had HM at the last follow‐up (mean 9; range 1 to 21 months) | failure rate 0% (n = 4) | NA | NA | None | |
| NR | NR | NA | "At the last follow‐up, 43.1% of eyes attained BCVA ≥ 20/200. By follow‐up, the percentage of eyes with BCVA ≥ 20/200 showed a decreasing trend with 74.5% (35/47) at 1 year, 50.0% (16/32) at 2 years, and 36.3% (4/11) at 3 years"; "At the last follow‐up, BCVA improved in 55.2% of eyes (32/58), remained the same in 27.6% of eyes (16/58), and worsened in 17.2% of eyes (10/58) because of postoperative complications." | 7/58 (12.1%) | None | NA | 4/58 (6.9%) | |
| NR | NR | NA | "In the subgroup of 15 eyes followed for at least 1 year (median follow‐up: 20 months; range: 12–36 months) after KPro implantation, vision improved in 86.7% of eyes; it was 20/200 in 66.7% of eyes and 20/50 in 26.7% of eyes." | 0/15; failure rate 0% (n = 15) | 2 had extrusion (12 and 15 months) | NA | 2/20 (10%) | |
| 11 of 32 (34.4%) | NR | NA | Thirty‐one eyes (82%) that underwent KPro surgery displayed postoperative BCVA improvement. | 0/32; failure rate 0% (n = 32) with a mean follow‐up time of 16.5 (SD 4.7) months | NR | NA | None | |
| Early model of Boston keratoprosthesis (known as Dohlman‐Doane keratoprosthesis) | ||||||||
| NR | NA | NA | 12/25 with 20/200 or better; 3/25 with 20/40 or better | NR | NA | NA | None at one year | |
| NR | NR | NR | NR | NR | NR | NR | NR | |
| *graft failure as reported by individual studies BCVA: best‐corrected visual acuity | ||||||||
| Study | Study design | Study dates | Country | Follow‐up | Number of participants | Number with repeat PK | Funding source and declarations of interest | |||
| Multicenter surveillance data | 1998‐2006 | 11 countries, including Australia, Singapore, and USA | Mean 15.5 months (range 0.5 to 7.4 years) | 302 (304 eyes, 322 devices implanted) | 302 (304 eyes, 322 devices implanted) | "Hicks and Crawford have a financial interest with the manufacturer of AlphaCor, CooperVision Surgical, through support of departmental funding, travel and research." | ||||
| Retrospective case series | NR | Germany/Czech Republic | Mean 38 months (range 12 to 67 months) | 15 (15 eyes) | 12/15 (80%) | "Supported in part by research project MZO 00179906 "The authors declare no conflict of interest." | ||||
| Retrospective case series | 2009‐2011 | France | Mean 16 months (range 2 to 24 months) | 14 (14 eyes) | 10/14 (71%) | "Conference invitations as a speaker for Addition Technology, Inc." | ||||
| Study | Number with BCVA ≥ 20/100 | Other visual acuity outcomes | Number of graft failures | Number of device extrusions | ||||||
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |||||
| "41.4% of all post–stage 2 cases achieved 20/200 or better", timing not specified by year | VA achieved postoperatively was LP to 20/20, mean 20/200; "mean improvement of 2 lines"; "6 eyes permanently lost vision over a summed 416 years of follow‐up. This finding equates to an annual risk per eye of 0.014." | NR; failure rate 20% for on‐label use | NR; failure rate 38% for on‐label use | 110/322 devices at final follow‐up; failure rate 34.2% | NR | |||||
| 2 of 13 (15%) | 2 of 7 (29%) | NR | NR | NR | NR | NR | NR | |||
| NR | NR | NA | "Postoperative mean visual acuity gain was 2.5 +/‐ 3.1 lines (from 0 to +11 lines). Visual acuity was superior or equal to 20/200 in 21% of cases." | NR; failure rate 28.6% with mean follow‐up of 15.6 months | NR | NA | 1/14 (7%) | |||
| BCVA: best‐corrected visual acuity | ||||||||||
| Study | Study design | Study dates | Country | Follow‐up | Number of participants | Number with repeat PK | Funding source and declarations of interest | |||
| Retrospective case series of the Fyodorov‐Zuev KPro | 2003‐2007 | Iran (Dr. Khodadoust Eye Hospital) | Mean 52 months (range 28 to 84 months) | 10 (10 eyes) | 10/10 (100%) | "None of the authors have any financial or proprietary interest in any material or method mentioned." | ||||
| Retrospective case series the OOKP | 1993‐2004 | Germany (University of Saarland) | Median 2.9 years | 25 (25 eyes) | 18/25 (72%) | NR | ||||
| Study | Number with BCVA ≥ 20/100 | Other visual acuity outcomes | Number of graft failures | Number of device extrusions | ||||||
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |||||
| 8/10 | 8/10 | 2/4 | "Patients retained BUVA of 20/200–20/50 in 50%, 20/60‐20/ 100 in 30%, 20/200 in 10% and 20/400 in 10% of cases. Overall, 90% had 1 year postoperative vision of 20/200 or better. Eyes had 20/200 or better in 70% of cases at the last follow‐up (average 52 months)." | 1/10 (10%) | 2/10 (20%) | 3/10 (30%) at 3 years; failure rate 30% during follow‐up | 3/10 (30%) during follow‐up | |||
| 11/20 (55%) | 7/14 (50%) | 2/5 (40%) | 79% achieved ambulatory vision of 20/400 at last follow up | NR | NR | NR | None reported | |||
| BCVA: best‐corrected visual acuity | ||||||||||

