Diferentes tipos de dispositivos de compresión neumática intermitente para la prevención de la tromboembolia venosa en pacientes después del reemplazo total de cadera
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009543.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 diciembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vascular
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Zhao JM selected relevant trials, assessed trial quality, extracted data and wrote the review.
He ML conceived the idea for this systematic review and coordinated its development. He screened search results, selected articles for inclusion, assessed trial eligibility, extracted data and assessed trial quality.
Xiao ZM supervised all stages of the review and resolved disagreements in trial selection, assessment of trial quality and data extraction.
Li TS, Wu H and Jiang H contacted the original authors for missing data, performed data analysis, interpreted the data and wrote the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government., UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
Declarations of interest
JMZ: None known
MLH: None known
ZMX: None known
TSL: None known
HW: None known
HJ: None known
Acknowledgements
Warm thanks to the Peripheral Vascular Diseases Group editorial team for their prompt advice and help with the protocol and review.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Dec 22 | Different types of intermittent pneumatic compression devices for preventing venous thromboembolism in patients after total hip replacement | Review | Jin Min Zhao, Mao Lin He, Zeng Ming Xiao, Ting Song Li, Hao Wu, Hua Jiang | |
| 2012 Nov 14 | Different types of intermittent pneumatic compression devices for preventing venous thromboembolism in patients after total hip replacement | Review | Jin Min Zhao, Mao Lin He, Zeng Ming Xiao, Ting Song Li, Hao Wu, Hua Jiang | |
| 2012 Jan 18 | Different types of intermittent pneumatic compression devices for preventing venous thromboembolism in patients after total hip replacement | Protocol | Ming Lei, Mao Lin He, Zeng Ming Xiao, Ting Song Li, Hao Wu, Jun Liao, Hua Jiang | |
Differences between protocol and review
In the review, three clinical trial databases (http://apps.who.int/trialsearch/; http://clinicaltrials.gov/; http://www.controlled‐trials.com/) were searched by the TSC for details of ongoing and unpublished studies, which was not mentioned in the protocol.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.
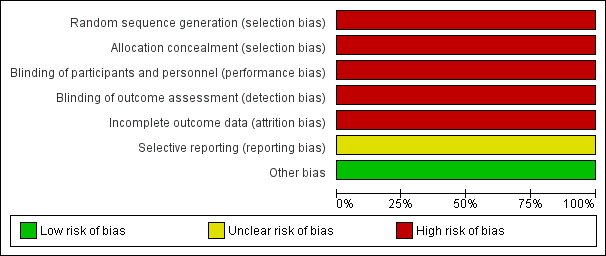
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

