Сроки внутривенного введения профилактических антибиотиков для предотвращения послеродовой инфекционной заболеваемости у женщин, подвергающихся кесаревому сечению.
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized controlled trial. | |
| Participants | 953 pregnant women, more than 34 weeks of gestation, requiring cesarean deliveries. Excluded:obstetric complications (pre‐eclampsia, antepartum hemorrhage, etc), renal disease, heart disease, diabetes mellitus, febrile during or prior to screening, ruptured membranes with or without antibiotic prophylaxis, any exposure to antibiotic during past 1 week, obstetrical indication for emergency cesarean delivery during labor, penicillin or cephalosporin allergy. | |
| Interventions | Group A received prophylactic single‐dose intravenous antibiotic (2 g ceftriaxone mixed with 10 mL) prior to incision and intravenous placebo (10 mL water) after cord clamp (n = 476). Group B received intravenous placebo (10 mL water) prior to incision and intravenous ceftriaxone 2 g in 10 mL after cord clamp (n = 477). | |
| Outcomes | Primary outcome: postoperative maternal infectious morbidity. Secondary outcomes: neonatal complications, postoperative hospital stay of mother and stay of neonates at NICU (days). | |
| Notes | July 2010 to December 2011, at 2 teaching hospitals in West Bengal India. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomisation sequence" was used. |
| Allocation concealment (selection bias) | Low risk | "The allocation was concealed in sealed, sequentially numbered, brown envelopes (opaque), which had been prepared by the statistician of each centre and handed over to the sister‐in‐charge of the operation theatre" was used. |
| Blinding of participants and personnel (performance bias) | Low risk | "The drugs were supplied in small sealed bags", "Both vials were identical", conducted double‐blinded manner. |
| Blinding of outcome assessment (detection bias) | Low risk | "Postoperative follow‐up was done by resident doctors who were blinded to the patients' and babies' identity." Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | All the women randomized included in the analysis (intention‐to‐treat analysis), however, the number of women who completed the intervention group was n = 458 (96.2%), and in the control group was n = 456 (95.6%). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Other bias | Low risk | No other bias evident. |
| Methods | Randomized controlled trial. | |
| Participants | 896 women undergoing non‐emergent cesarean delivery including women with ruptured membranes and experiencing labor, and scheduled cesarean deliveries. Excluded:fever > 38°C, age < 18 years old, diagnosed as chorioamnionitis before delivery, allergy to cefazolin and clindamycin, any exposure to antibiotic within 1 week before delivery. 95 women lost to follow‐ up: 39 from Group 1; 56 from Group 2 (cord clamp). | |
| Interventions | Prophylactic single‐dose antibiotic (2 g ceftriaxone or clindamycin 900 mg if women allergic to penicillin) within 30 to 60 minutes of expected skin incision (n = 410) and placebo after umbilical cord clamping or placebo within 30 to 60 minutes of expected incision and the antibiotic (2 g ceftriaxone or clindamycin 900 mg if women allergic to penicillin) after umbilical cord clamping (n = 391). | |
| Outcomes | Primary outcomes: maternal infectious morbidity including wound infection, UTI, endometritis and pneumonia. Secondary outcomes: neonatal antibiotic administration, admission to the NICU, and hospital readmission rates. | |
| Notes | Single site: St Vincent Hospital in Indianapolis, Indiana, USA, September 2006 to January 2011. Underpowered as noted in article. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomisation sequence was generated by the hospital statistician.” “The randomisation list was e‐mailed to the research pharmacist.” Comments: unclear technique used to generate randomization list, but we suspect that method was appropriate as it was done by a statistician. |
| Allocation concealment (selection bias) | Low risk | “The randomisation list was e‐mailed to the research pharmacist, who was the only person with access to the randomisation information for the duration of the study.” |
| Blinding of participants and personnel (performance bias) | Low risk | “Treatment assignment was performed in the pharmacy and all physicians and patients were blinded to which bag contained the antibiotic and which one had saline.” |
| Blinding of outcome assessment (detection bias) | Low risk | Only pharmacy was aware of timing of antibiotic. |
| Incomplete outcome data (attrition bias) | Low risk | The 95 women lost to follow‐ up (10.6%), 39 (4.4%) were in the skin incision group and 56 (6.2%) were in the cord clamp group. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Other bias | Low risk | No other bias evident. |
| Methods | Randomized controlled trial. | |
| Participants | 874 women undergoing elective cesarean delivery. 437 women in each group. Excluded: history of diabetes mellitus, severe anemia, obese women (BMI >= 25) ruptured membranes, retro positive women, immune‐suppressant drugs, history of allergy to ceftriaxone No women lost to follow‐up. | |
| Interventions | Single dose of ceftriaxone 1 g intravenously 15‐45 minutes before skin incision (n = 437) versus the same medication after cord clamping (n = 437). | |
| Outcomes | Primary outcome: maternal postoperative infectious morbidities such as surgical site wound infection, febrile morbidity, endometritis, UTIs and neonatal sepsis. | |
| Notes | Single site: conducted from October 2010 to July 2012. 1 hospital in Puducherry, India. About 27 women (17 in before skin incision and 10 in cord clamping) continued receiving antibiotics after surgery due to various infections. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The patients were randomly categorized into two groups using serially numbered opaque sealed envelope (SNOPE) technique.” |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelope was used. |
| Blinding of participants and personnel (performance bias) | High risk | This was not specified, but we suspect that no blinding occurred. |
| Blinding of outcome assessment (detection bias) | High risk | This was not specified, but we suspect that no blinding occurred. |
| Incomplete outcome data (attrition bias) | Low risk | All women accounted for, no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were stated. |
| Other bias | Low risk | No other bias evident. |
| Methods | Randomized controlled trial. | |
| Participants | 100 primigravid women with singleton pregnancy at term undergoing elective cesarean. Excluded: age < 20 or > 30 years; BMI < 19 or >= 25; exposure to antibiotics within 1 week of delivery; premature rupture of membranes; indication for emergency cesarean; hypersensitivity to cephalosporins; temperature > 37.8 degrees celsius. | |
| Interventions | 2 g cefazolin administered 30 minutes preoperatively (n = 50) versus after cord clamp (n = 50). | |
| Outcomes | Endometritis, wound infection, UTI. | |
| Notes | 1 hospital in Egypt. June 2011‐December 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 50 cards were prepared for each intervention. The cards were placed into opaque envelopes and "shuffled to produce a form of random assignment". |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | This was not specified, but we suspect that no blinding occurred. |
| Blinding of outcome assessment (detection bias) | High risk | This was not specified, but we suspect that no blinding occurred. |
| Incomplete outcome data (attrition bias) | Low risk | None reported. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Other bias | Low risk | No other bias evident. |
| Methods | Randomized controlled trial. | |
| Participants | 434 women undergoing non‐emergency cesarean deliveries >= 36 weeks excluding women with known fetal anomaly, antibiotics within 7 days of admission, overt intrapartum infection, or ruptured membranes > 18 hours. | |
| Interventions | 1 g cefazolin administered < 30 minutes preoperatively (n = 217) versus after cord clamp (n = 217). They did not specify intravenous treatment, but we included this trial as it was assumed that cefazolin was administered intravenously. | |
| Outcomes | Primary: postoperative fever, wound infection, endomyometritis, UTI. Secondary: NICU admission, proven or suspected neonatal sepsis (with resistant bacteria), neonatal length of stay (days). | |
| Notes | 2 centers in USA. Study period not defined. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted blocks. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) | Low risk | Adminstered by anesthesiologist to maintain blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | This was not specified. |
| Incomplete outcome data (attrition bias) | Low risk | Not specifically mentioned, but it appears that outcomes are available for all enrolled women. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Other bias | Low risk | No other bias evident. |
| Methods | Randomized controlled trial. | |
| Participants | 367 women >= 24 weeks who were undergoing cesarean delivery excluding women with a cephalosporin allergy, age < 18 years, exposure to antibiotics within 7 days, need for emergency surgery. | |
| Interventions | 1 g cefazolin administered at 15‐60 minutes pre‐incision and normal saline after cord clamp (n = 175) versus normal saline at 15‐60 minutes pre‐incision and 1 g cefazolin after cord clamp (n = 182). They did not specify intravenous treatment, but we included this trial as it was assumed that cefazolin was administered intravenously. | |
| Outcomes | Primary: total infectious morbidity. | |
| Notes | Abstract and paper; study commenced January 2003. 1 hospital in the United States of America. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomization using random number tables. |
| Allocation concealment (selection bias) | Low risk | Investigational pharmacy staff delivered both antibiotics and placebo. |
| Blinding of participants and personnel (performance bias) | Low risk | Intraoperative labeled bag given by anesthesia. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind trial but does not describe blinding method. |
| Incomplete outcome data (attrition bias) | Low risk | The preoperative group lost 3 women and the cord clamp group lost 5 women to attrition. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Other bias | Low risk | No other bias evident. |
| Methods | Randomized controlled trial. | |
| Participants | 346 women in active labor excluding women with chorioamnionitis, cephalosporin allergy, antibiotics within 2 weeks of labor. | |
| Interventions | 2 g cefazolin administered pre‐incision and normal saline after cord clamp (n = 153) versus normal saline pre‐incision and 2 g cefazolin after cord clamp (n = 149). | |
| Outcomes | Postoperative infection, neonatal infections and sepsis. | |
| Notes | 1 center in the United States of America from November 2000 until April 2003. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study only states that randomization was performed by pharmacy personnel. |
| Allocation concealment (selection bias) | Low risk | Allocated by pharmacy personnel. |
| Blinding of participants and personnel (performance bias) | Low risk | Care providers were unaware of which bag contained antibiotic versus placebo. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) | Low risk | Not specifically mentioned, but it appears that outcomes are available for all enrolled women who were not excluded following randomization. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Other bias | Low risk | No other bias evident. |
| Methods | Randomized controlled trial. | |
| Participants | 90 women undergoing cesarean in labor with a single fetus ≥ 37 weeks' gestation excluding women with an allergy to penicillin or cephalosporin, antibiotic use within 2 weeks of delivery, temperature ≥ 37.8°C in labor, administration of group B streptococcal or subacute bacterial endocarditis prophylaxis during labor, insulin‐dependent diabetes mellitus, human immunodeficiency virus infection, chronic glucocorticoid use, or multiple gestation. | |
| Interventions | 1 g cefazolin administered over approximately 5 minutes upon decision for cesarean delivery and normal saline after cord clamp, each intervention in identical 50 mL infusions (n = 49) versus normal saline over approximately 5 minutes upon decision for cesarean delivery and 1 g cefazolin after cord clamp (n = 41). | |
| Outcomes | Primary maternal outcomes: endometritis and wound infection. Secondary maternal outcomes: intra‐abdominal abscess, septic pelvic thrombophlebitis, or symptomatic UTI. Neonatal outcomes: sepsis screen, sepsis, pneumonia, and meningitis. | |
| Notes | 1 center in the United States of America over a 12‐month period (exact dates not provided). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization. |
| Allocation concealment (selection bias) | Low risk | Randomization code was produced by, and known only by pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | Each participants received 2 bags, effectively blinding to timing of antibiotic. |
| Blinding of outcome assessment (detection bias) | Low risk | Only pharmacy was aware of timing of antibiotic. |
| Incomplete outcome data (attrition bias) | Low risk | 7 participants were lost to 2 week follow‐up. 14 participants were lost to 6 week follow‐up. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes. |
| Other bias | Low risk | No other bias evident. |
| Methods | Randomized controlled trial. | |
| Participants | 1112 women with a fetus ≥ 37 weeks' gestation undergoing elective cesarean delivery of a fetus with reassuring fetal heart rate tracing. | |
| Interventions | Group A: 2 g cefazolin in 100 mL saline 20‐30 minutes before incision (n = 370). Group B: 2 g cefazolin in 100 mL saline immediately after cord clamp (n = 371). Group C: 100 mL saline 20‐30 minutes before incision (n = 371). | |
| Outcomes | Total postoperative infectious morbidity (endometritis, wound infection, UTI). | |
| Notes | 1 hospital in Vienna, Austria. 3/1/04 ‐ 1/31/10. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated permuted blocks of 5. |
| Allocation concealment (selection bias) | Low risk | Only the study nurse was not blinded and handed appropriate infusion bag to anesthesiologist. |
| Blinding of participants and personnel (performance bias) | Low risk | Women and surgeons masked to administration schedule. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Infectious morbidity was evaluated by 2 residents who were masked to group assignments. |
| Incomplete outcome data (attrition bias) | Low risk | Protocol violations in Groups 1, 2, and 3 respectively: 12, 7, and 13. 32 women lost to follow‐ up/protocol violations/withdrawal. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes. |
| Other bias | Low risk | No other bias evident. |
| Methods | Randomized controlled trial. | |
| Participants | 400 women undergoing elective cesarean delivery prior to labor initially included with 11 excluded due to blood transfusion leaving 389 participants. Additional exclusion criteria included the use of antibiotics in the last 24 hours, pathology that should be treated with antibiotics, pre‐existing maternal disease (such as diabetes, collagen vascular disease, immune system problems), chorioamnionitis, fever on admission, need of transfusion before or during cesarean delivery, ruptured membranes, emergency cesarean delivery and preterm cesarean delivery. | |
| Interventions | 1 g of cefazolin ≤ 45 minutes pre‐incision (n = 194) versus 1 g cefazolin after cord clamp (n = 195). They did not specify intravenous treatment, but we included this trial as it was assumed that cefazolin was administered intravenously. | |
| Outcomes | Rates of postoperative infectious morbidity (endometritis, wound infection, febrile morbidity, UTI), estimated blood loss and operative time. Neonatal outcomes: NICU admission, Apgar score less than 7 at 5 minutes, neonatal sepsis and sepsis workup. | |
| Notes | Conducted June 2007‐December 2007 at 1 hospital inTurkey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 2 parts, blocked randomization. |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially distributed envelopes indicating group A (pre‐incision) or group B (following cord camp) chosen by the participants, opened by the investigator. |
| Blinding of participants and personnel (performance bias) | High risk | This was not specified, but we suspect that no blinding occurred. |
| Blinding of outcome assessment (detection bias) | High risk | This was not specified, but we suspect that no blinding occurred. |
| Incomplete outcome data (attrition bias) | Low risk | None lost to follow‐ up. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes. |
| Other bias | Low risk | No other bias evident. |
BMI: body mass index
NICU: neonatal intensive care unit
UTI: urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomized controlled trial. | |
| Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Women continued to receive antibiotics postoperatively. | |
| Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Women continued to receive antibiotics postoperatively. | |
| Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Women continued to receive antibiotics postoperatively. | |
| Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Women continued to receive antibiotics postoperatively. | |
| There is insufficient information provided in the abstract. There was no information on randomization (sequence generation and allocation concealment were not described). There was no blinding. The group sizes were very different 55 versus 84. It was not clear whether this was due to chance. There was no information on attrition. There were no usable data (most results were not reported according to randomization group but according to risk), the results that were reported by randomization group did not present the data (P values only)). | |
| There is insufficient information provided in the abstract. This was described as a small pilot study. There was no information on sequence generation or allocation concealment although it was described as a double blind‐trial. It was not clear whether the placebo was identical. There was no information on attrition or missing data (it was not stated how many were randomized to each group although percentages in the results suggest 20 women were randomized to each arm). There were no SDs reported for continuous data. The intervention was not clear. The women were randomized to receive antibiotics before or after cord clamping; but the interval was not described (it was not clear whether the antibiotic was administered immediately before cord clamping, after skin incision or immediately after or a long period after). | |
| Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Women continued to receive antibiotics postoperatively. | |
| Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Comparison was made between single dose and multiple day regimens. | |
| Antibiotic regimens were not limited to preoperative versus post‐cord clamp. Comparison was made between single dose and multiple day regimens. | |
| Antibiotic regimens were limited to preoperative versus postoperative but were not given at cord clamp. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomized controlled trial. |
| Participants | 50 women undergoing scheduled cesarean delivery. |
| Interventions | 2 g cefazolin preoperatively versus after cord clamp (number randomized to each group not specified). |
| Outcomes | Primary: endometritis and wound infection. Secondary: neonatal sepsis work‐up, sepsis, pneumonia, and meningitis. Followed 6 weeks postoperatively for late complications. |
| Notes | Abstract pilot study, in USA. Does not currently provide enough information to make an assessment of methodologic quality. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Timing of perioperative antibiotics for cesarean: a multicenter randomized controlled study. |
| Methods | Randomized control trial. |
| Participants | Women were eligible for the trial if they were > 37 weeks' gestation and undergoing cesarean delivery. Exclusion criteria: allergy to cefathiamidine, exposure to any antibiotic agent within 1 week of delivery, > 37.5°C before cesarean or age < 18 years or > 40 years. |
| Interventions | Cefethiamidine administered 30 minutes prior to skin incision but no more than 120 minutes before versus after cord clamping. |
| Outcomes | Endomyometritis, wound infection, urinary tract infection, neonatal sepsis, neonatal sepsis workup, neonatal admission, neonatal dysbacteriosis, temperature after cesarean, number of white blood count and neutrophile granulocyte after cesarean, ratio of neonatal stool coccus and bacillus. |
| Starting date | December 2011‐June 2012. |
| Contact information | Zhang Chuan, Department of Pharmacy West China Second University Hospital, Sichuan University (email: [email protected]) |
| Notes | Settings ‐ 3 facilities (West China Second University Hospital Sichuan University, Sichuan Women and Children's Health, Nanchong Central Hospital). |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Composite morbidity (as defined by trials) Show forest plot | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.45, 0.72] |
| Analysis 1.1  Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 1 Composite morbidity (as defined by trials). | ||||
| 1.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.35, 0.86] |
| 1.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.43, 0.76] |
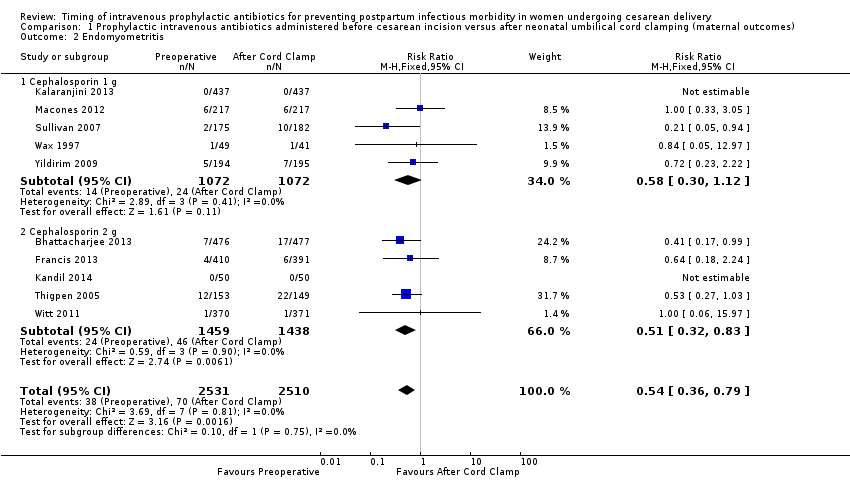
| 2 Endomyometritis Show forest plot | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.36, 0.79] |
| Analysis 1.2  Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 2 Endomyometritis. | ||||
| 2.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.12] |
| 2.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.32, 0.83] |
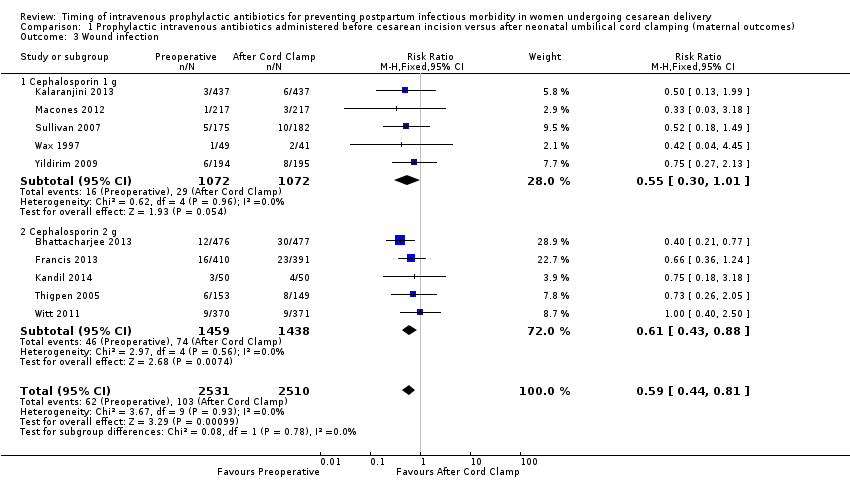
| 3 Wound infection Show forest plot | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.44, 0.81] |
| Analysis 1.3 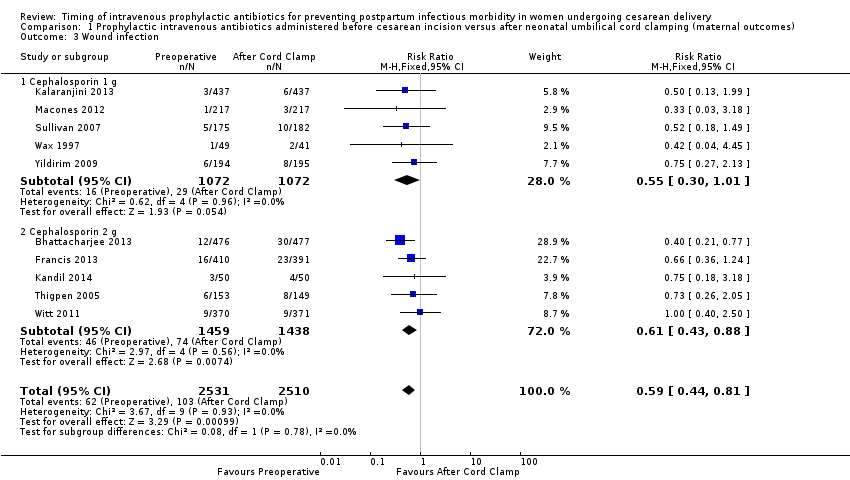 Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 3 Wound infection. | ||||
| 3.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| 3.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.43, 0.88] |
| 4 UTI/cystitis/pyelonephritis Show forest plot | 8 | 4001 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.65, 1.59] |
| Analysis 1.4 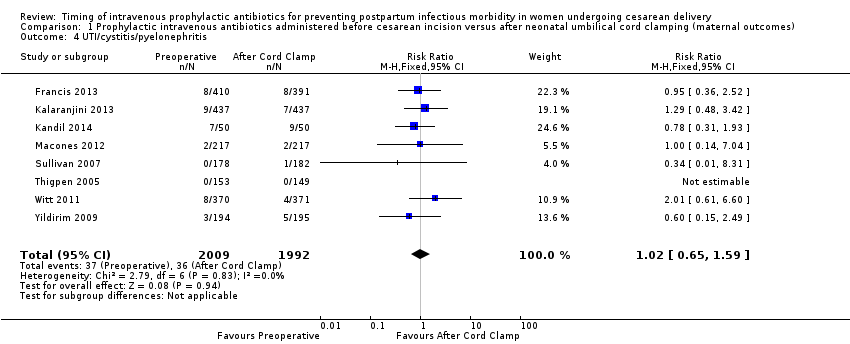 Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 4 UTI/cystitis/pyelonephritis. | ||||
| 5 Pelvic abscess Show forest plot | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.97] |
| Analysis 1.5  Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 5 Pelvic abscess. | ||||
| 6 Respiratory infection (pneumonia) Show forest plot | 4 | 1849 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.34, 15.45] |
| Analysis 1.6  Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 6 Respiratory infection (pneumonia). | ||||
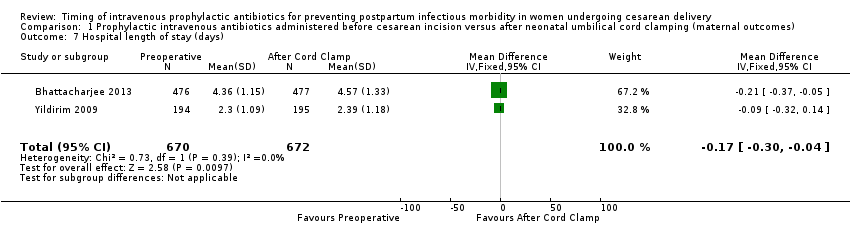
| 7 Hospital length of stay (days) Show forest plot | 2 | 1342 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.30, ‐0.04] |
| Analysis 1.7 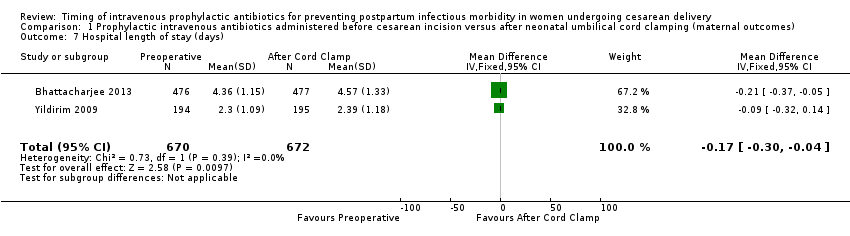 Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 7 Hospital length of stay (days). | ||||
| 8 Febrile Illness Show forest plot | 4 | 2650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.63, 1.35] |
| Analysis 1.8  Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 8 Febrile Illness. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
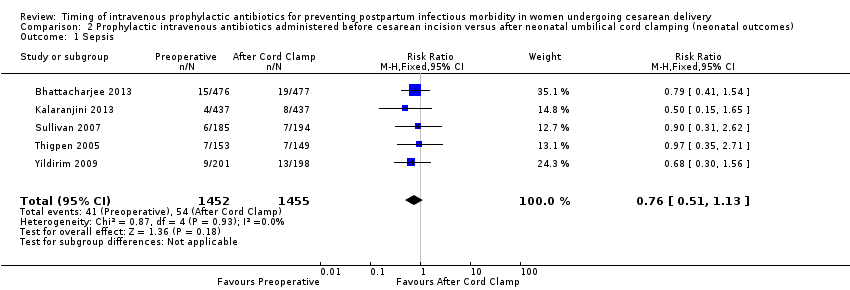
| 1 Sepsis Show forest plot | 5 | 2907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.51, 1.13] |
| Analysis 2.1  Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 1 Sepsis. | ||||
| 2 Neonatal sepsis work up Show forest plot | 4 | 1170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.23] |
| Analysis 2.2 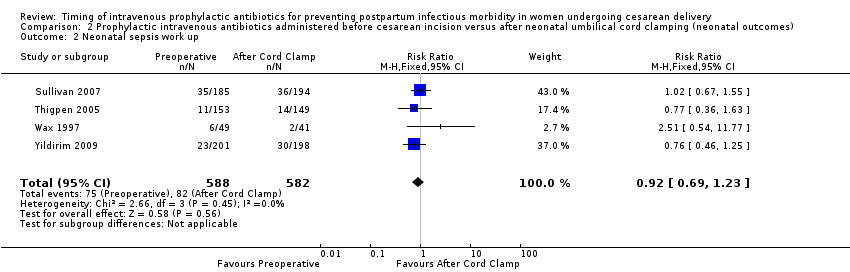 Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 2 Neonatal sepsis work up. | ||||
| 3 Infection with a resistant organism Show forest plot | 1 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.12, 4.14] |
| Analysis 2.3  Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 3 Infection with a resistant organism. | ||||
| 4 Infection (other) Show forest plot | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.52, 1.64] |
| Analysis 2.4 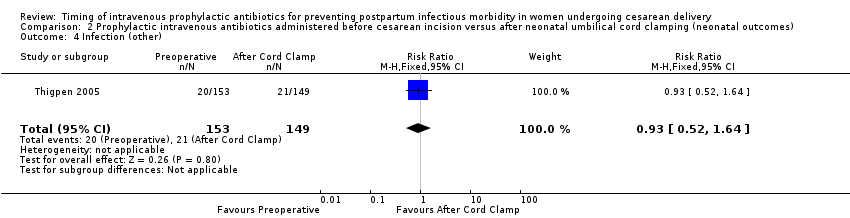 Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 4 Infection (other). | ||||
| 5 ICU admission Show forest plot | 6 | 3708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| Analysis 2.5  Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 5 ICU admission. | ||||
| 6 ICU length of stay (days) Show forest plot | 3 | 1731 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐2.60, 2.46] |
| Analysis 2.6 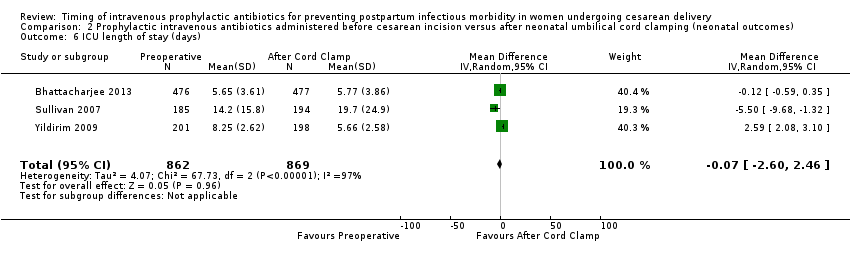 Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 6 ICU length of stay (days). | ||||
| 7 Neonatal antibiotic treatment Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.12, 5.68] |
| Analysis 2.7  Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 7 Neonatal antibiotic treatment. | ||||
| 8 Fever Show forest plot | 1 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.28, 1.62] |
| Analysis 2.8  Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 8 Fever. | ||||

Study flow diagram.

'Risk of bias. graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
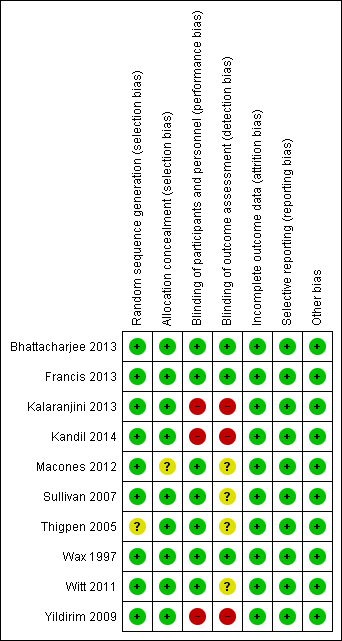
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Maternal postpartum infectious morbidity, outcome: 1.1 Composite morbidity (as defined by trials).
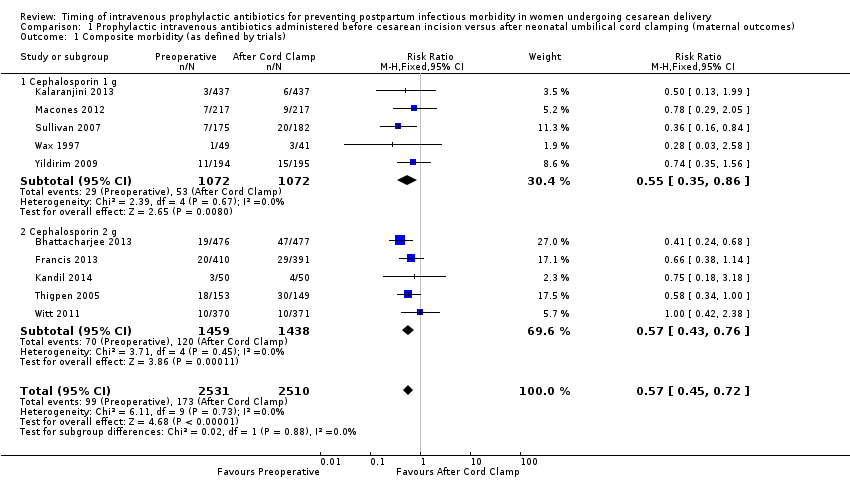
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 1 Composite morbidity (as defined by trials).
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 2 Endomyometritis.
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 3 Wound infection.

Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 4 UTI/cystitis/pyelonephritis.

Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 5 Pelvic abscess.

Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 6 Respiratory infection (pneumonia).
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 7 Hospital length of stay (days).

Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 8 Febrile Illness.
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 1 Sepsis.

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 2 Neonatal sepsis work up.
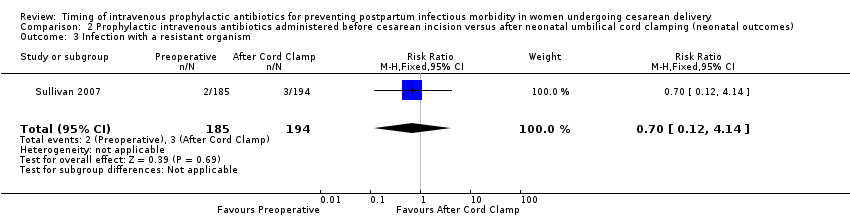
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 3 Infection with a resistant organism.

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 4 Infection (other).

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 5 ICU admission.

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 6 ICU length of stay (days).

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 7 Neonatal antibiotic treatment.

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 8 Fever.
| Prophylactic antibiotics for preventing postpartum infectious morbidity in women and infants after cesarean delivery | ||||||
| Population: women undergoing cesarean delivery. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Maternal and neonatal postpartum infectious morbidity | |||||
| Maternal composite morbidity | Study population | RR 0.67 | 5041 | ⊕⊕⊕⊕ | ||
| 85 per 1000 | 57 per 1000 | |||||
| Moderate | ||||||
| 97 per 1000 | 65 per 1000 | |||||
| Maternal endomyometritis | Study population | RR 0.54 | 5041 | ⊕⊕⊕⊕ | ||
| 28 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 26 per 1000 | 14 per 1000 | |||||
| Maternal wound Infection | Study population | RR 0.59 | 5041 | ⊕⊕⊕⊕ | ||
| 41 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 51 per 1000 | 30 per 1000 | |||||
| Maternal UTI/cystitis/pyelonephritis | Study population | RR 1.02 | 4001 | ⊕⊕⊕⊝ | ||
| 18 per 1000 | 18 per 1000 | |||||
| Moderate | ||||||
| 13 per 1000 | 13 per 1000 | |||||
| Maternal respiratory infection (pneumonia) | Study population | RR 2.3 | 1158 | ⊕⊕⊝⊝ | ||
| 2 per 1000 | 4 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Neonatal sepsis | Study population | RR 0.76 | 2907 | ⊕⊕⊕⊝ | ||
| 37 per 1000 | 28 per 1000 | |||||
| Moderate | ||||||
| 40 per 1000 | 30 per 1000 | |||||
| Neonatal ICU admission | Study population | RR 0.91 | 3708 | ⊕⊕⊕⊕ | ||
| 86 per 1000 | 78 per 1000 | |||||
| Moderate | ||||||
| 71 per 1000 | 65 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide confidence interval crossing the line of no effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Composite morbidity (as defined by trials) Show forest plot | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.45, 0.72] |
| 1.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.35, 0.86] |
| 1.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.43, 0.76] |
| 2 Endomyometritis Show forest plot | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.36, 0.79] |
| 2.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.12] |
| 2.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.32, 0.83] |
| 3 Wound infection Show forest plot | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.44, 0.81] |
| 3.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| 3.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.43, 0.88] |
| 4 UTI/cystitis/pyelonephritis Show forest plot | 8 | 4001 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.65, 1.59] |
| 5 Pelvic abscess Show forest plot | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.97] |
| 6 Respiratory infection (pneumonia) Show forest plot | 4 | 1849 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.34, 15.45] |
| 7 Hospital length of stay (days) Show forest plot | 2 | 1342 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.30, ‐0.04] |
| 8 Febrile Illness Show forest plot | 4 | 2650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.63, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sepsis Show forest plot | 5 | 2907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.51, 1.13] |
| 2 Neonatal sepsis work up Show forest plot | 4 | 1170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.23] |
| 3 Infection with a resistant organism Show forest plot | 1 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.12, 4.14] |
| 4 Infection (other) Show forest plot | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.52, 1.64] |
| 5 ICU admission Show forest plot | 6 | 3708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| 6 ICU length of stay (days) Show forest plot | 3 | 1731 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐2.60, 2.46] |
| 7 Neonatal antibiotic treatment Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.12, 5.68] |
| 8 Fever Show forest plot | 1 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.28, 1.62] |

