Momento de administración de los antibióticos profilácticos intravenosos para la prevención de la morbilidad infecciosa posparto en pacientes sometidas a cesárea
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009516.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 05 diciembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
A. Dhanya Mackeen, Roger Packard and Erika Ota abstracted data from the studies and entered data into RevMan. A. Dhanya Mackeen, Roger Packard, Vincenzo Berghella, and Erika Ota prepared the first draft. Erika Ota prepared the 'Summary of findings' tables. All authors approved the final version of the update for publication. A. Dhanya Mackeen is guarantor for the review.
Sources of support
Internal sources
-
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization.
External sources
-
No sources of support supplied
Declarations of interest
None known.
Acknowledgements
Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
The authors would like to thank Tanya Ohly and Samantha Weed for helping develop the protocol (Baxter 2011).
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Dec 05 | Timing of intravenous prophylactic antibiotics for preventing postpartum infectious morbidity in women undergoing cesarean delivery | Review | A. Dhanya Mackeen, Roger E Packard, Erika Ota, Vincenzo Berghella, Jason K Baxter | |
| 2011 Dec 07 | Timing of prophylactic antibiotics for preventing postpartum infectious morbidity in women undergoing cesarean delivery | Protocol | Jason K Baxter, Vincenzo Berghella, A Dhanya Mackeen, N. Tanya Ohly, Samantha Weed | |
Differences between protocol and review
We have assessed the quality of the body of evidence using the GRADE approach (Schunemann 2009) ‐ this was not pre‐specified in our published protocol (Baxter 2011).
We added a subgroup analysis based on dose of cephalosporin (1 g versus 2 g).
We changed the definition of our primary outcome of composite morbidity to include sepsis (including septic shock), endomyometritis, wound infection, or death attributed to infection. We removed necrotizing fasciitis and bacteremia as these would have been included in infection and sepsis.
In addition, some sections of the methods have been updated in accordance with the Cochrane Pregnancy and Childbirth Group's standard methods text:
-
assessment of reporting biases;
-
subgroup analysis and investigation of heterogeneity.
We have also made minor edits to the list of planned outcomes:
Secondary maternal outcomes
-
'Urinary tract infection' has been edited to 'Urinary tract infection, cystitis and pyelonephritis'
-
'Upper respiratory infection (pneumonia) has been edited to 'Respiratory infection (e.g. pneumonia)'
-
'Fever' has been changed to 'Febrile illness'
-
'Sepsis' has been changed to 'Sepsis, including septic shock'
-
Clarified that the unit for hospital length of stay is in days
Secondary infant outcomes
-
'Neonatal workup for infection' has been edited to 'Neonatal sepsis workup'
-
Length of ICU stay has been clarified as 'Length of ICU stay (days)'
-
'Antibiotic treatment' has been edited to 'Neonatal antibiotic treatment'
-
Febrile illness has been added as a new secondary outcome
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anti-Bacterial Agents [*administration & dosage];
- Antibiotic Prophylaxis [*standards];
- Cesarean Section [*adverse effects];
- Drug Administration Schedule;
- Endometritis [prevention & control];
- Injections, Intravenous;
- Randomized Controlled Trials as Topic;
- Surgical Wound Infection [*prevention & control];
- Urinary Tract Infections [prevention & control];
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

Study flow diagram.

'Risk of bias. graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
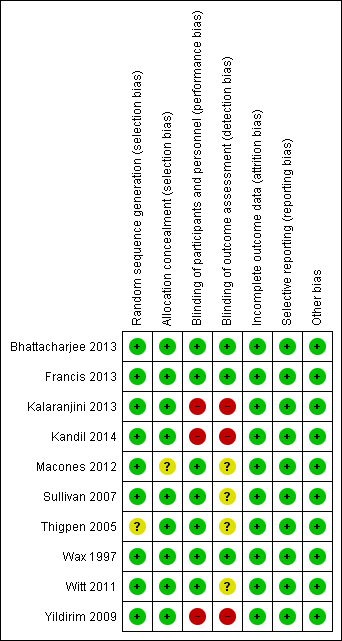
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Maternal postpartum infectious morbidity, outcome: 1.1 Composite morbidity (as defined by trials).
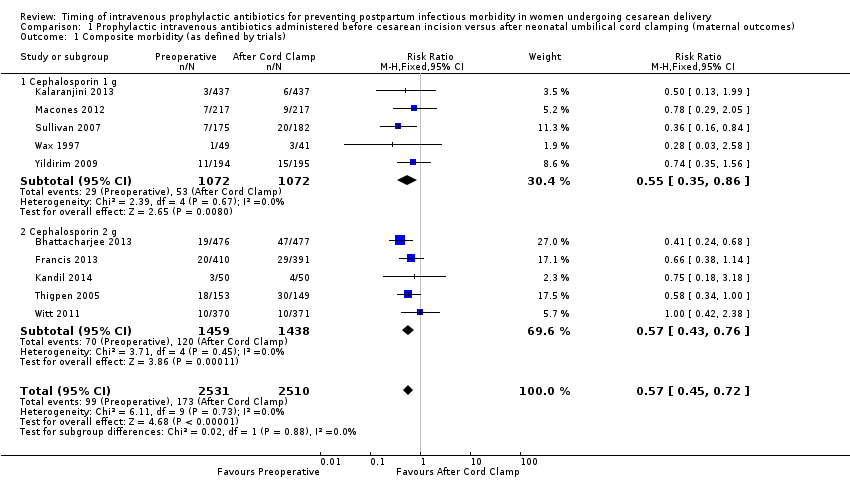
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 1 Composite morbidity (as defined by trials).
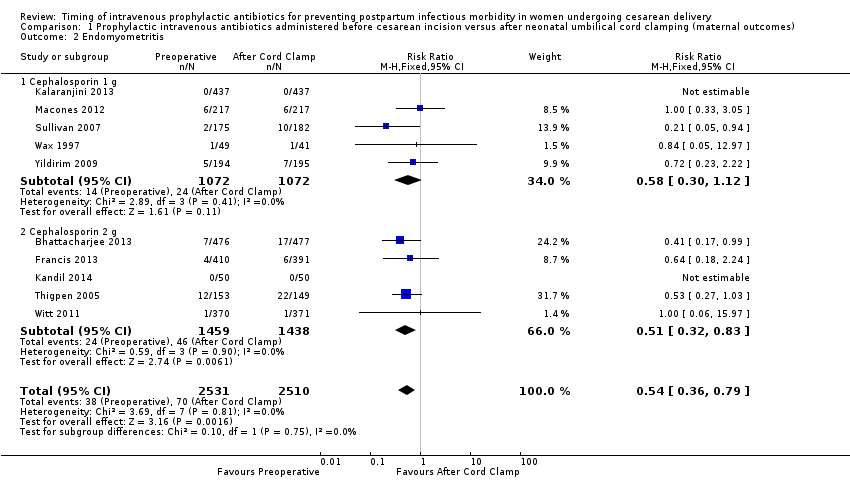
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 2 Endomyometritis.
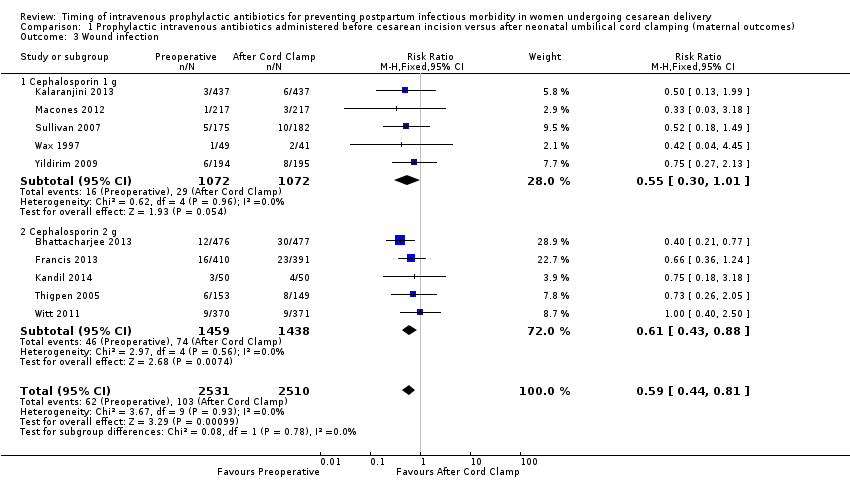
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 3 Wound infection.

Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 4 UTI/cystitis/pyelonephritis.

Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 5 Pelvic abscess.

Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 6 Respiratory infection (pneumonia).
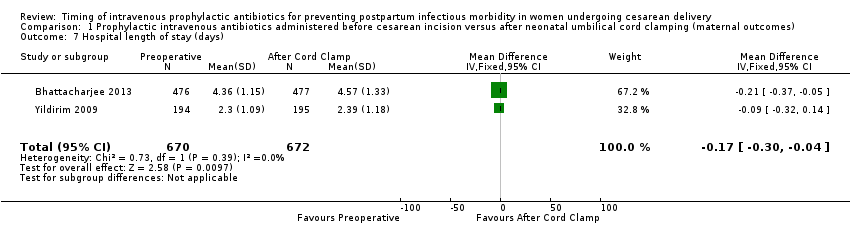
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 7 Hospital length of stay (days).

Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 8 Febrile Illness.
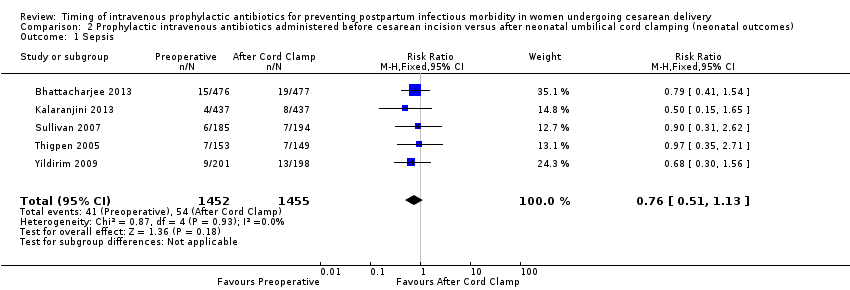
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 1 Sepsis.

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 2 Neonatal sepsis work up.
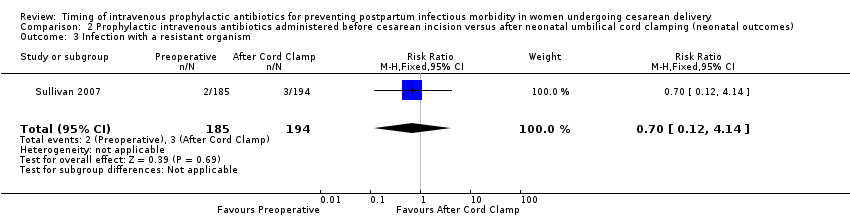
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 3 Infection with a resistant organism.

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 4 Infection (other).

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 5 ICU admission.

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 6 ICU length of stay (days).

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 7 Neonatal antibiotic treatment.

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 8 Fever.
| Prophylactic antibiotics for preventing postpartum infectious morbidity in women and infants after cesarean delivery | ||||||
| Population: women undergoing cesarean delivery. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Maternal and neonatal postpartum infectious morbidity | |||||
| Maternal composite morbidity | Study population | RR 0.67 | 5041 | ⊕⊕⊕⊕ | ||
| 85 per 1000 | 57 per 1000 | |||||
| Moderate | ||||||
| 97 per 1000 | 65 per 1000 | |||||
| Maternal endomyometritis | Study population | RR 0.54 | 5041 | ⊕⊕⊕⊕ | ||
| 28 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 26 per 1000 | 14 per 1000 | |||||
| Maternal wound Infection | Study population | RR 0.59 | 5041 | ⊕⊕⊕⊕ | ||
| 41 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 51 per 1000 | 30 per 1000 | |||||
| Maternal UTI/cystitis/pyelonephritis | Study population | RR 1.02 | 4001 | ⊕⊕⊕⊝ | ||
| 18 per 1000 | 18 per 1000 | |||||
| Moderate | ||||||
| 13 per 1000 | 13 per 1000 | |||||
| Maternal respiratory infection (pneumonia) | Study population | RR 2.3 | 1158 | ⊕⊕⊝⊝ | ||
| 2 per 1000 | 4 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Neonatal sepsis | Study population | RR 0.76 | 2907 | ⊕⊕⊕⊝ | ||
| 37 per 1000 | 28 per 1000 | |||||
| Moderate | ||||||
| 40 per 1000 | 30 per 1000 | |||||
| Neonatal ICU admission | Study population | RR 0.91 | 3708 | ⊕⊕⊕⊕ | ||
| 86 per 1000 | 78 per 1000 | |||||
| Moderate | ||||||
| 71 per 1000 | 65 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide confidence interval crossing the line of no effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Composite morbidity (as defined by trials) Show forest plot | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.45, 0.72] |
| 1.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.35, 0.86] |
| 1.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.43, 0.76] |
| 2 Endomyometritis Show forest plot | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.36, 0.79] |
| 2.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.12] |
| 2.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.32, 0.83] |
| 3 Wound infection Show forest plot | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.44, 0.81] |
| 3.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| 3.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.43, 0.88] |
| 4 UTI/cystitis/pyelonephritis Show forest plot | 8 | 4001 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.65, 1.59] |
| 5 Pelvic abscess Show forest plot | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.97] |
| 6 Respiratory infection (pneumonia) Show forest plot | 4 | 1849 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.34, 15.45] |
| 7 Hospital length of stay (days) Show forest plot | 2 | 1342 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.30, ‐0.04] |
| 8 Febrile Illness Show forest plot | 4 | 2650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.63, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sepsis Show forest plot | 5 | 2907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.51, 1.13] |
| 2 Neonatal sepsis work up Show forest plot | 4 | 1170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.23] |
| 3 Infection with a resistant organism Show forest plot | 1 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.12, 4.14] |
| 4 Infection (other) Show forest plot | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.52, 1.64] |
| 5 ICU admission Show forest plot | 6 | 3708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| 6 ICU length of stay (days) Show forest plot | 3 | 1731 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐2.60, 2.46] |
| 7 Neonatal antibiotic treatment Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.12, 5.68] |
| 8 Fever Show forest plot | 1 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.28, 1.62] |

