Optimalno vrijeme davanja intravenskih profilaktičkih antibiotika za sprječavanje pospartalnih infekcija u žena podvrgnutih carskom rezu
Abstract
Background
Given the continued rise in cesarean birth rate and the increased risk of surgical site infections after cesarean birth compared with vaginal birth, effective interventions must be established for prevention of surgical site infections. Prophylactic intravenous (IV) antibiotic administration 60 minutes prior to skin incision is recommended for abdominal gynecologic surgery; however, administration of prophylactic antibiotics has traditionally been withheld until after neonatal umbilical cord clamping during cesarean delivery due to the concern for potential transfer of antibiotics to the neonate.
Objectives
To compare the effects of cesarean antibiotic prophylaxis administered preoperatively versus after neonatal cord clamp on postoperative infectious complications for both the mother and the neonate.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (1 March 2014) and reference lists of retrieved papers.
Selection criteria
Randomized controlled trials (RCTs) comparing maternal and neonatal outcomes following prophylactic antibiotics administered prior to skin incision versus after neonatal cord clamping during cesarean delivery. Cluster‐RCTs were eligible for inclusion but none were identified. Quasi‐RCT and trials using a cross‐over design were not eligible for inclusion in this review. Studies published in abstract form only were eligible for inclusion if sufficient information was available in the report.
Data collection and analysis
At least two review authors independently assessed the studies for inclusion, assessed risk of bias, abstracted data and checked entries for accuracy. We assessed the quality of evidence using the GRADE approach.
Main results
We included 10 studies (12 trial reports) from which 5041 women contributed data for the primary outcome. The overall risk of bias was low.
When comparing prophylactic intravenous (IV) antibiotic administration in women undergoing cesarean delivery, there was a reduction in composite maternal infectious morbidity (risk ratio (RR) 0.57, 95% confidence interval (CI) 0.45 to 0.72, high quality evidence), which was specifically due to the reduction in endometritis (RR 0.54, 95% CI 0.36 to 0.79, high quality evidence) and wound infection (RR 0.59, 95% CI 0.44 to 0.81, high quality evidence) in those that received antibiotics preoperatively as compared to those who received antibiotics after neonatal cord clamping. There were no clear differences in neonatal sepsis (RR 0.76, 95% CI 0.51 to 1.13, moderate quality evidence).
There were no clear differences for other maternal outcomes such as urinary tract infection (UTI), cystitis and pyelonephritis (moderate quality evidence), respiratory infection (low quality evidence), or any neonatal outcomes. Maternal side effects were not reported in the included studies.
The quality of the evidence using GRADE was high for composite morbidity, endomyometritis, wound infection and neonatal intensive care unit admission, moderate for UTI/cystitis/pyelonephritis and neonatal sepsis, and low for maternal respiratory infection.
Authors' conclusions
Based on high quality evidence from studies whose overall risk of bias is low, intravenous prophylactic antibiotics for cesarean administered preoperatively significantly decreases the incidence of composite maternal postpartum infectious morbidity as compared with administration after cord clamp. There were no clear differences in adverse neonatal outcomes reported. Women undergoing cesarean delivery should receive antibiotic prophylaxis preoperatively to reduce maternal infectious morbidities. Further research may be needed to elucidate short‐ and long‐term adverse effects for neonates.
PICO
Laički sažetak
Kada primijeniti antibiotike za sprječavanje infekcije nakon poroda carskim rezom?
Kirurški zahvat nosi rizik od infekcije, što produžava oporavak nakon zahvata. Antibiotici se primjenjuju preventivno (ili profilaktički) sa svrhom sprječavanja infekcije i smanjenja komplikacija. U pravilu se antibiotici primjenjuju 60 minuta prije operativnog zahvata jer se tada postignu odgovarajuće koncentracije u tkivu već prije prvog reza. Kod carskog reza se mora uzeti u obzir učinak antibiotika na dijete te se antibiotici daju nakon postavljanja stezaljke na pupčanu vrpcu. U tom slučaju u tkivu majke nije postignuta odgovarajuća koncentracija antibiotika za sprječavanje infekcije uslijed kirurškog zahvata, a odgađanje primjene antibiotika ne donosi korist djetetu.
U ovom Cochrane sustavnom pregledu randomiziranih kliničkih studija istražen je učinak različitog vremena preventivnog davanja antibiotika sa svrhom sprječavanja infekcije uslijed carskog reza. Učinjena je usporedba davanja antibiotika prije zahvata u odnosu na primjenu nakon stezanja pupčane vrpce.
Ovaj sustavni pregled uključuje 10 studija (podatci sakupljeni od 5041 žene). Studije su imali mali rizik pristranosti. Primjena antibiotika prije carskog reza je smanjila na polovicu rizik infekcije (42%), endometritisa (46%; upala maternice) i infekcije rane (41%) u usporedbi s primjenom antibiotika nakon stezanja pupčane vrpce. Ostale infekcije u majke, primjerice mokraćna infekcija ili dišna (respiratorna) se nisu razlikovale između skupina, a nisu se razlikovale niti nuspojave u novorođenčadi. Dokaz visoke kakvoće pokazuju da prijeoperativna intravenska primjena antibiotika smanjuje infekcije nakon porođaja te je važna za zdravlje majke. Nuspojave u majke nisu sustavno ispitivane. Podaci o novorođenčadi i nuspojave u njih su bili oskudni. Potrebna su dodatna istraživanja za ustanovljavanje mogućih nuspojava u djece.
Authors' conclusions
Summary of findings
| Prophylactic antibiotics for preventing postpartum infectious morbidity in women and infants after cesarean delivery | ||||||
| Population: women undergoing cesarean delivery. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Maternal and neonatal postpartum infectious morbidity | |||||
| Maternal composite morbidity | Study population | RR 0.67 | 5041 | ⊕⊕⊕⊕ | ||
| 85 per 1000 | 57 per 1000 | |||||
| Moderate | ||||||
| 97 per 1000 | 65 per 1000 | |||||
| Maternal endomyometritis | Study population | RR 0.54 | 5041 | ⊕⊕⊕⊕ | ||
| 28 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 26 per 1000 | 14 per 1000 | |||||
| Maternal wound Infection | Study population | RR 0.59 | 5041 | ⊕⊕⊕⊕ | ||
| 41 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 51 per 1000 | 30 per 1000 | |||||
| Maternal UTI/cystitis/pyelonephritis | Study population | RR 1.02 | 4001 | ⊕⊕⊕⊝ | ||
| 18 per 1000 | 18 per 1000 | |||||
| Moderate | ||||||
| 13 per 1000 | 13 per 1000 | |||||
| Maternal respiratory infection (pneumonia) | Study population | RR 2.3 | 1158 | ⊕⊕⊝⊝ | ||
| 2 per 1000 | 4 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Neonatal sepsis | Study population | RR 0.76 | 2907 | ⊕⊕⊕⊝ | ||
| 37 per 1000 | 28 per 1000 | |||||
| Moderate | ||||||
| 40 per 1000 | 30 per 1000 | |||||
| Neonatal ICU admission | Study population | RR 0.91 | 3708 | ⊕⊕⊕⊕ | ||
| 86 per 1000 | 78 per 1000 | |||||
| Moderate | ||||||
| 71 per 1000 | 65 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide confidence interval crossing the line of no effect. | ||||||
Background
Description of the condition
Cesarean birth is one of the most common surgical procedures performed worldwide. Internationally, 46 countries are noted to have cesarean delivery rates greater than 20%. Of these 46, China and Brazil account for almost 50% of total cesarean births (Gibbons 2012). The World Health Organization (WHO) Multicountry Survey on Maternal and Newborn Health survey conducted in 29 countries showed the overall rate of cesarean delivery was 28.6%, and the coverage of prophylactic antibiotics for cesarean birth was 87.3%, globally (Souza 2013). Worldwide, infectious morbidity consisting primarily of endomyometritis and wound infection occurs in approximately 5% to 10% of cesarean births (Henderson 1995; Olsen 2008; Opoien 2007; Yokoe 2001). As the cesarean birth rate continues to rise in most developed countries, postpartum infectious morbidity will become an even more significant problem. Therefore, measures aimed at decreasing postpartum infectious morbidity are an important area of focus.
Surgical site infections are the most common nosocomial infections among surgical patients, accounting for about 23% of all such infections from the data reported to the National Healthcare Safety Network (NHSN) in the United States during 2009 to 2010 (Sievert 2013). A prospective multicenter cohort study conducted in England reported that 9.6% of women in the study developed a postsurgical infection after cesarean delivery (Wloch 2012). Infectious complications after cesarean birth include, but are not limited to, wound infection, endomyometritis, urinary tract infection, pelvic abscess, septic shock, septic pelvic thrombophlebitis, necrotizing fasciitis, and pneumonia.
The risk of postpartum infection after cesarean birth is nearly five‐fold that of vaginal birth (Leth 2009). Many interventions have been studied in an attempt to decrease the incidence of surgical site infections after cesarean birth including prophylactic antibiotics, surgical hand antisepsis, skin preparation methods, surgical techniques, closure of subcutaneous fat, subcutaneous drain placement, and postoperative surveillance (Barwolff 2006; Dahlke 2013; Edi‐Osagie 1998; Hellums 2007; Lorenz 1988; Magann 1993; Starr 2005; Ventolini 2004).
Description of the intervention
In 2008, the WHO established a 'Safe Surgery Saves Lives' campaign. An intraoperative surgical checklist was developed to reduce post‐surgical infection as well as other associated morbidities. This checklist acknowledged the evidence‐based value of prophylactic antibiotic administration 60 minutes prior to skin incision (Soar 2009).
The obstetric population poses a unique challenge for antibiotic prophylaxis as there is known transplacental delivery of antibiotics to the fetus. Traditionally, the administration of prophylactic antibiotics has been withheld until after neonatal umbilical cord clamping during cesarean birth in order to avoid transfer of antibiotics to the fetus. The theoretical concerns of neonatal antibiotic exposure include the masking of neonatal infection, interference with sepsis workup and the selection of antibiotic‐resistant bacterial strains in both neonatal colonization and infection (Cunningham 1983). Due to these theoretical risks, the Centers for Disease Control and Prevention's 'Guideline for Prevention of Surgical Site Infection, 1999' stated with high level evidence, that for high‐risk cesarean birth, prophylactic antimicrobial agents should be given immediately after umbilical cord clamping, rather than preoperatively (Mangram 1999).
How the intervention might work
The general surgery tenet of antibiotic prophylaxis was born out of animal studies that demonstrated maximum suppression of infection when adequate tissue antibiotic levels were present at the time of microbial contamination (Burke 1961). These findings were confirmed in clinical practice: less surgical‐wound infections were noted when antibiotics were administered within two hours before skin incision as compared to when antibiotics were administered postoperatively (Classen 1992). One of the most commonly used antibiotics for cesarean birth is cefazolin. Pharmacokinetic studies of cefazolin demonstrate that mean inhibiting concentration levels for group B streptococcus are attained in maternal, fetal and amniotic fluid samples within 30 minutes of administration (Fiore 2001). It has also been demonstrated that bactericidal levels against group B streptococcus are achieved in maternal, fetal and amniotic fluid samples within five minutes of ampicillin administration (Bloom 1996).
Why it is important to do this review
The benefit of using prophylactic antibiotics in order to prevent surgical site and other infections has been demonstrated in the obstetric literature. A 2010 Cochrane review concluded that antibiotic prophylaxis, as compared to no prophylaxis, was associated with a reduction in the incidence of febrile morbidity, wound infection, endomyometritis and other serious maternal infectious complications (Smaill 2010). This prior review stated that data were insufficient to compare timing of antibiotic administration.
Several recent studies have evaluated timing of the administration of prophylactic antibiotics, specifically administration prior to skin incision versus after neonatal umbilical cord clamping. A meta‐analysis comparing the administration of prophylactic antibiotics prior to skin incision versus after clamping of the umbilical cord concluded that pre‐incision antibiotic prophylaxis for cesarean birth not only decreased the incidence of postpartum endomyometritis and total infectious morbidity, but also did not adversely affect neonatal outcomes (Costantine 2008). Several institutions have evaluated the impact of protocol changes in the timing of perioperative antibiotic administration on postoperative infectious complications, and have found decreased infectious complications with antibiotics given prior to skin incision compared to after cord clamping (Kaimal 2008; Owens 2009).
In response to the recent attention on the timing of prophylactic antibiotics in cesarean birth, the American College of Obstetricians and Gynecologists (ACOG) recommended antimicrobial prophylaxis for all cesarean births, and further stated that prophylaxis should be administered within 60 minutes of the start of the cesarean delivery (ACOG 2010). These recommendations are consistent with recommendations from the National Surgical Infection Prevention Project (Bratzler 2005).
Given the continued rise in cesarean birth rate and the relatively high risk of surgical site infections after cesarean birth as compared to other surgical procedures (NNIS System 2004), measures must be taken to prevent surgical site infections. Prior Cochrane reviews have looked at the maternal morbidity associated in relation to administration of prophylactic antibiotics at time of cesarean delivery (Smaill 2010) and various antibiotic regimens for cesarean delivery (Hopkins 1999). This review will focus on studies comparing antibiotic prophylaxis administered prior to skin incision compared with administration after umbilical cord clamping for cesarean birth. We will compare infectious morbidity and address the concern for neonatal harm.
Objectives
To assess the differences in infectious morbidity for mother and neonate when prophylactic cesarean antibiotics are administered preoperatively versus after neonatal cord clamping.
Methods
Criteria for considering studies for this review
Types of studies
Only randomized controlled trials were included. We excluded quasi‐randomized studies.
Abstracts were included if they provided sufficient information to make an assessment of methodologic quality, i.e., information allows for completion of at least some of the domains within the 'Risk of bias' tables. We attempted to contact authors of such manuscripts before deciding to exclude a study on these grounds.
Types of participants
Pregnant women who have undergone cesarean delivery and received prophylactic antibiotics.
Types of interventions
Prophylactic intravenous (IV) antibiotic administration for cesarean birth 0 to 30 and 30 to 60 minutes prior to skin incision versus prophylactic antibiotic administration for cesarean birth after neonatal umbilical cord clamping. We planned to exclude studies of women who received antibiotics after skin incision but before cord clamping.
Types of outcome measures
Primary outcomes
Composite maternal postpartum infectious morbidity (including serious infectious complications such as sepsis (including septic shock), endomyometritis, wound infection, or death attributed to infection). Though not pre‐specified in the protocol, we stratified composite morbidity by whether 1 g or 2 g of cephalosporin were administered.
Secondary outcomes
Maternal
1. Maternal mortality.
2. Maternal postpartum infection (the following are as defined by the individual trials):
-
endomyometritis (though not pre‐specified in the protocol, we stratified endomyometritis by whether 1 g or 2 g of cephalosporin were administered);
-
wound infection (though not pre‐specified in the protocol, we stratified wound infection by whether 1 g or 2 g of cephalosporin were administered);
-
urinary tract infection (UTI), cystitis and pyelonephritis;
-
sepsis, including septic shock;
-
pelvic abscess;
-
septic pelvic thrombophlebitis;
-
respiratory infection (e.g. pneumonia);
-
length of hospital stay (days);
-
Intensive care unit (ICU) admission;
-
cost of antibiotics;
-
antibiotic‐related adverse events (e.g. anaphylaxis);
-
febrile illness.
3. Placental transfer of antibiotics
4. Breastfeeding
Neonatal outcomes
1. Neonatal mortality.
2. Neonatal morbidity (the following are as defined by the individual trials):
-
sepsis;
-
neonatal sepsis workup;
-
infection with resistant organism;
-
infection (other);
-
admission to ICU;
-
length of ICU stay (days);
-
neonatal antibiotic treatment;
-
febrile illness.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (1 March 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
weekly searches of Embase;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
At least two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion with the whole group.
Data extraction and management
We designed a form to abstract data. For eligible studies, two review authors independently abstracted the data using the agreed form. We resolved discrepancies through group discussion. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for the studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We have assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. However, as overall methodologic quality was considered low risk, sensitivity analyses were not undertaken. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
The quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following key outcomes for the main comparison.
-
Composite morbidity
-
Endomyometritis
-
Wound infection
-
Urinary tract infection (UTI)/cystitis/pyelonephritis
-
Respiratory infections (e.g. pneumonia)
-
Neonatal sepsis
-
Neonatal ICU admission
GRADEprofiler (GRADE 2008) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes were produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. If necessary, we planned to use the standardized mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
We did not identify any cluster‐randomized trials, but we would include cluster‐randomized trials along with individually‐randomized trials in the analysis in future updates, if identified. We will adjust their sample size using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify cluster‐randomized trials, we plan to synthesize the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomization unit and perform a sensitivity analysis to investigate the effects of the randomization unit.
We did not include cross‐over trials.
Dealing with missing data
For included studies, we noted levels of attrition. In a subsequent update of this review, we will include a sensitivity analysis excluding poor quality studies with high levels of attrition.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
As there were 10 studies in the meta‐analysis for the primary outcome, we investigated reporting biases (such as publication bias) using a funnel plot. We assessed funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment in the future update, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and the clinical implications of treatment effects differing between trials were discussed. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Substantial heterogeneity was not present, therefore we did not perform subgroup analyses in relation to heterogeneity. In future updates, if we identify substantial heterogeneity, we will perform subgroup analyses for composite maternal infectious morbidity based on:
-
cesarean as compared to labor: laboring versus non‐laboring (i.e. prelabor);
-
cesarean performed in presence of chorioamnionitis with treatment versus without treatment;
-
cesarean performed on women who have received antibiotic prophylaxis for other indications (e.g. GroupBeta Streptococcus) versus those without antibiotic treatment for any other indication;
-
timing of antibiotics prior to skin incision (e.g. less than 30 minutes to skin incision versus greater than 30 minutes prior to skin incision).
We considered whether an overall summary was meaningful and used random‐effects analysis to produce it. We assessed subgroup differences based on dose of cephalosporin (1 g versus 2 g) by interaction tests available within RevMan (RevMan 2014). Interaction test results are reported, along with the Chi² statistic, P value, and I² value.
Sensitivity analysis
We planned to carry out sensitivity analysis for the primary outcomes by restricting our analysis to trials assessed as having a low risk of bias for the domain attrition bias; if > 30% of participants were lost to follow‐up, these studies would have been excluded from sensitivity analyses. This was not necessary because none of the studies were assessed as having a high risk of bias. Planned sensitivity analysis will be conducted in future updates of this review, if appropriate.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group's Register retrieved 25 reports (see: Figure 1). Ten studies (12 trial reports) were included with 5041 women contributing data to the primary outcome. Eleven trials were excluded. One study is awaiting classification (Pevzner 2009). One study is ongoing (Zhang 2012) (see Characteristics of ongoing studies).
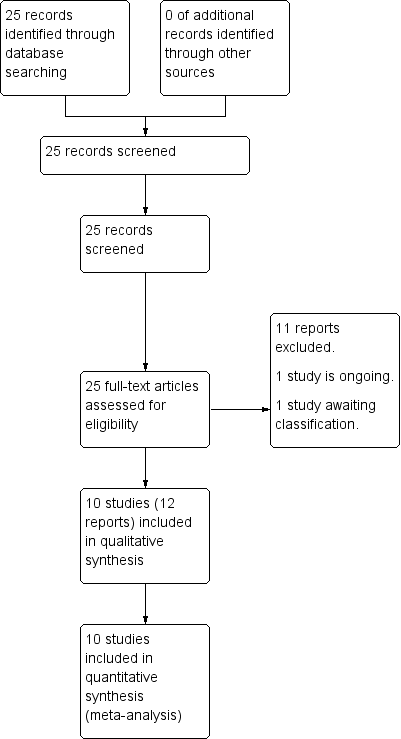
Study flow diagram.
Included studies
Ten trials met our inclusion criteria and contributed data on the 5041 women they enrolled for the primary outcome of composite infectious morbidity: 2531 received antibiotics preoperatively and 2510 received antibiotics after cord clamp.
For detailed information on all studies, see Characteristics of included studies tables.
Participants
Most studies described characteristics of the women who were included and excluded in detail. Most studies included women with non‐emergent or elective cesarean delivery at term (some studies included from 24 weeks estimated gestational age, others included from 34 or 36 weeks of gestation). Several studies excluded women with obstetric complications, while other studies only excluded women with infection at enrolment or allergy to antibiotics.
Settings
Eight trials were conducted in developed countries, seven from the United States of America and one from Austria. Five trials were performed in developing countries, with two trials from India and one trial each from Egypt, South Africa and Turkey.
Interventions
Antibiotics for prophylaxis were administered intravenously either before the incision versus after clamping of the neonatal umbilical cord. Studies administered antibiotics before incision at various time frames with the vast majority ranging from 15 to 60 minutes.
The antimicrobial agents used included a first generation cephalosporin (cefazolin 1 g or 2 g) in seven trials (Kandil 2014 (2 g); Macones 2012 (1 g); Sullivan 2007 (1 g); Thigpen 2005 (2 g); Wax 1997 (1 g); Witt 2011 (2 g); Yildirim 2009 (1 g)). The remaining three trials used a third generation cephalosporin (ceftriaxone 1 g or 2 g) (Bhattacharjee 2013 (2 g); Francis 2013 (2 g); Kalaranjini 2013 (1 g)). Clindamycin was typically the agent of choice for women who had known allergy to cephalosporins.
Outcomes
The primary outcome was a composite of maternal postpartum infectious morbidities including septic shock, endometritis, wound infection, or death attributed to infection. However, these were defined individually by the trialists.
The clinical criteria listed to define endometritis and urinary tract infection were remarkably consistent across trials. Wound infection was usually a clinical diagnosis and generally included induration, erythema, cellulitis or various degrees of drainage. Duration of maternal hospital stay and neonatal ICU length of stay were included when reported.
Two studies reported on septic pelvic thrombophlebitis, though there were no occurrences (Kalaranjini 2013; Yildirim 2009). One study (Kalaranjini 2013) reported on septic shock and maternal death, though there were no occurrences. None of the included studies reported on ICU admission, cost of antibiotics, antibiotic‐related adverse events, placental transfer of antibiotics, breastfeeding or neonatal mortality.
Excluded studies
Seven trials were excluded from analysis as antibiotic administrations were not limited to preoperative versus post‐cord clamp: women continued to receive antibiotics postoperatively in five (De Palma 1980; Gordon 1979; Gul 1999; Nokiani 2009; Tassi 1987) and single dose was compared to multiple day regimens in two (van Beekhuizen 2008; Van Velzen 2009). One study was excluded as antibiotics were given preoperatively and postoperatively, but were not given at neonatal cord clamp (Xu 1997). One study was excluded because it was not a randomized controlled trial (Cunningham 1983). Three studies published in abstract form did not provide sufficient information: two were excluded (Rodriguez 1990; Seton 1996) and one (Pevzner 2009) is awaiting classification pending publication of the final manuscript.
(Please refer to Characteristics of excluded studies for further details).
Risk of bias in included studies
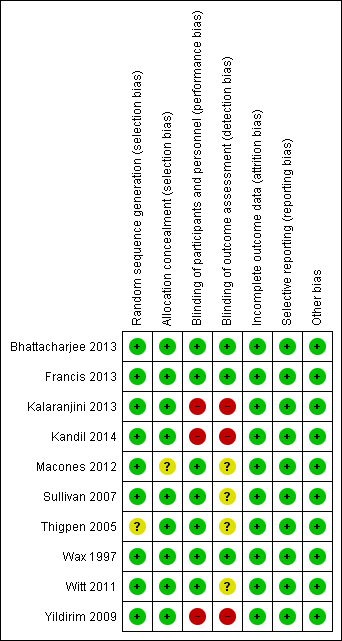
The overall methodological quality of the trials 10 trials that contributed data towards the analysis was considered low risk of bias (see Figure 2; Figure 3).
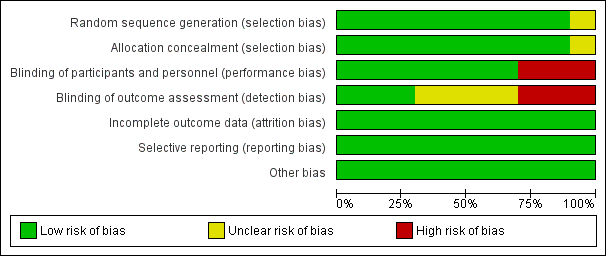
'Risk of bias. graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
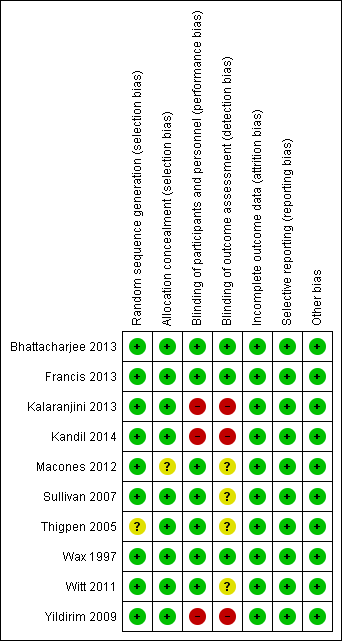
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
There was no serious publication bias in the primary outcome due to the large number of women in the symmetrical part of the funnel plot (Figure 4).
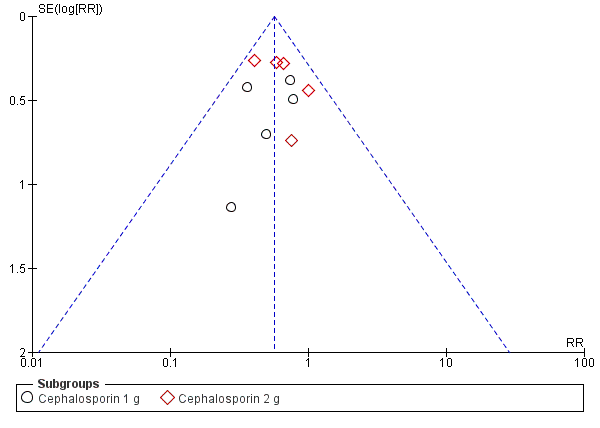
Funnel plot of comparison: 1 Maternal postpartum infectious morbidity, outcome: 1.1 Composite morbidity (as defined by trials).
Allocation
The studies used various methods of randomization, e.g. computer‐generated (Bhattacharjee 2013; Wax 1997), cards shuffled (Kandil 2014), and random number tables (Sullivan 2007), all of which were judged as low risk, except for Thigpen 2005 which was judged as unclear risk as it was only mentioned that randomization was performed by pharmacy personnel.
Four studies used sealed envelopes for allocation concealment (Bhattacharjee 2013; Kalaranjini 2013; Kandil 2014; Yildirim 2009) and five had pharmacy or nursing staff provide the antibiotics (Francis 2013; Sullivan 2007; Thigpen 2005; Wax 1997; Witt 2011). Macones 2012 does not mention method of allocation concealment.
Blinding
Seven trials were adequately blinded in regards to participants as well as providers, and therefore, deemed to have a low risk of performance bias. Three studies did not report performance bias but we suspect (from the study methods) that no blinding occurred and therefore categorized them as high risk of bias (Kalaranjini 2013; Kandil 2014; Yildirim 2009) for both blinding domains.
Four trials did not clearly report detection bias and we were unable to infer the potential for bias from the methods, so it was judged to be unclear risk (Macones 2012; Sullivan 2007; Thigpen 2005; Witt 2011). Three trials that described blinding of outcome assessment and were categorized as low risk of bias (Bhattacharjee 2013; Francis 2013; Wax 1997).Kalaranjini 2013; Yildirim 2009; and Kandil 2014 were again deemed high risk of bias as no mention was made in the associated text about detection bias and we suspect that no blinding occurred.
Incomplete outcome data
All 10 studies reported that all women who were initially randomized were included in the analysis; loss to follow‐up was low and typically reasons were provided.
Selective reporting
We found the reporting bias to be low risk as the primary outcomes were stated in the study methods of all 10 studies.
Other potential sources of bias
No other potential sources of bias were identified in the 10 trials.
Effects of interventions
The overall risk of bias was low. The quality of the evidence using GRADE was high for composite morbidity, endomyometritis, wound infection and neonatal intensive care unit admission, moderate for UTI/cystitis/pyelonephritis and neonatal sepsis, and low for maternal respiratory infection.
1. Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes) ‐ Analyses 1.1 to 1.10
Primary outcomes
Composite morbidity (as defined by trials)
There were significant reductions in composite morbidity (as defined by trials) for women who received antibiotics preoperatively as compared to those who received antibiotics after cord clamp (risk ratio (RR) 0.57; 95% confidence interval (CI) 0.45 to 0.72, 10 trials, 5041 women, high quality evidence) (Analysis 1.1). An interaction test for subgroup differences (1 g versus 2 g of cephalosporin) found no clear difference between studies using 1g of cephalosporin and those using 2 g of cephalosporin (Chi² = 0.02, df = 1 (P = 0.88); I² = 0%).
Secondary outcomes (maternal)
There were significant reductions in endomyometritis (RR 0.54; 95% CI 0.36 to 0.79, 10 trials, 5041 women, high quality evidence (Analysis 1.2); wound infection (RR 0.59; 95% CI 0.44 to 0.81, 10 trials, 5041 women, high quality evidence (Analysis 1.3)) and length of hospital stay (mean difference (MD) ‐0.17; 95% CI ‐0.30 to ‐0.04, two trials, 1342 women (Analysis 1.7)) in women who received antibiotics preoperatively as compared to those who received antibiotics after cord clamp.
An interaction test for subgroup differences (1 g versus 2 g of cephalosporin) found no clear difference between studies for endometritis (Chi² = 0.10, (P = 0.75); I² = 0%) or wound infections (Chi² = 0.08, (P = 0.78); I² = 0%).
There were no clear differences in occurrence of UTI/cystitis/pyelonephritis (RR 1.02; 95% CI 0.65 to 1.59, eight trials, 4001 women, moderate quality evidence (Analysis 1.4)); pelvic abscesses (RR 1.00; 95% CI 0.06 to 15.97, one trial, 741 women (Analysis 1.5)); respiratory infections (e.g. pneumonia) (RR 2.30; 95% CI 0.34 to 15.45, four trials, 1849 women, low quality evidence (Analysis 1.6)); or febrile illness (RR 0.93; 95% CI 0.63 to 1.35, four trials, 2650 women (Analysis 1.8)) between the groups.
Two studies reported on septic pelvic thrombophlebitis, though there were no occurrences (Kalaranjini 2013; Yildirim 2009); so RR could not be calculated. One study (Kalaranjini 2013) reported on septic shock and maternal death, though there were no occurrences; so RR could not be calculated. None of the included studies reported on ICU admission, cost of antibiotics, antibiotic‐related adverse events, placental transfer of antibiotics, breastfeeding or neonatal mortality.
2. Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes) ‐ Analyses 2.1 to 2.8
Secondary outcomes
There were no clear differences for any of the neonatal outcomes when comparing preoperative administration of antibiotics to administration after cord clamp: neonatal sepsis (RR 0.76; 95% CI 0.51 to 1.13, five trials, 2907 neonates, moderate quality evidence (Analysis 2.1)); neonatal sepsis work up (RR 0.92; 95% CI 0.69 to 1.23, four trials, 1170 neonates) (Analysis 2.2)); infection with a resistant organism (RR 0.70; 95% CI 0.12 to 4.14, one trial, 379 neonates (Analysis 2.3)); infection (other) (RR 0.93; 95% CI 0.52 to 1.64, one trial, 302 neonates (Analysis 2.4)); ICU admission (RR 0.91; 95% CI 0.74 to 1.13, six trials, 3708 neonates (Analysis 2.5));ICU length of stay (days) (MD ‐0.07; 95% CI ‐2.60 to 2.46, three trials, 1731 neonates, random‐effects, Tau² = 4.07; I² = 97% (Analysis 2.6)); neonatal antibiotic treatment (RR 0.84; 95% CI 0.12 to 5.68, one trial, 90 neonates (Analysis 2.7)); and febrile illness (RR 0.67; 95% CI 0.28 to 1.62, one trial, 953 neonates (Analysis 2.8)).
Among the included studies, no data were available for the neonatal outcome of mortality.
Discussion
Summary of main results
This review included 10 trials of 5041 women who received prophylactic antibiotic administration for cesarean birth prior to skin incision versus after neonatal umbilical cord clamping to assess which is superior in preventing postpartum infectious morbidity.
Those who received antibiotics preoperatively were 43% less likely to have infectious morbidity as compared to those who received antibiotics after neonatal cord clamping. Those who received antibiotics preoperatively were 46% and 41% less likely to have endomyometritis and wound infection, respectively, as compared to those who received antibiotics after neonatal cord clamping.
Although a significant difference was noted in the hospital length of stay favoring preoperative antibiotics, the difference is unlikely to be of clinical significance. For other maternal infections such as UTI, pelvic abscess, respiratory infection (pneumonia), febrile illness, and fever showed no differences. There were no clear differences in neonatal outcomes regardless of when antibiotics were administered (summary of findings Table for the main comparison).
Overall completeness and applicability of evidence
The included trials enrolled 5041 women from six (developing and developed) countries from 1990 to 2013. Overall, the trials in this review were of reasonably sound methodology and the results were generally consistent across the trials.
Prophylactic administration of antibiotics for cesarean delivery given prior to skin incision was more effective in preventing infectious morbidity (specifically, endomyometritis and wound infection) than when antibiotics were given after neonatal cord clamp.
Some secondary outcomes were not (or rarely) reported: septic pelvic thrombophlebitis, septic shock, maternal death, pelvic abscess, hospital length of stay, ICU admission, cost of antibiotics, antibiotic‐related adverse events, placental transfer of antibiotics, breastfeeding. Maternal side effects were not reported (or might not have been collected consistently across trials) in the included studies; however, all the studies consistently reported on wound infection and endomyometritis,which are included in the composite primary outcome. Neonatal outcomes were not consistently reported from all trials, although no evidence of adverse effects were found regardless of timing of antibiotic administration. No study reported on neonatal mortality.
The lack of consistent reporting of neonatal outcomes is certainly a limitation of this review. Although the primary outcome of composite maternal morbidity is well represented, the lack of neonatal data within several of the included studies creates an artificial separation of the effects of antibiotics on the maternal/fetal diad. Additionally, no maternal deaths or ICU admissions were noted in any of the studies.
Quality of the evidence
The quality of the evidence using GRADE was high for composite morbidity, endomyometritis, wound infection and neonatal ICU admission. UTI/cystitis/pyelonephritis and neonatal sepsis were moderate for quality of the evidence, downgraded one due to wide confidence interval crossing the line of no effect. Maternal respiratory infection (pneumonia) was considered to be of low quality of evidence due to wide confidence intervals crossing the line of no effect, few events and small sample size (summary of findings Table for the main comparison).
Potential biases in the review process
We followed to the Cochrane Pregnancy and Childbirth Group search strategies and review process. Two review authors conducted the study selection, data collection independently to avoid potential biases, and we are not aware of any potential bias in the review process.
Agreements and disagreements with other studies or reviews
There are two reviews that examined maternal and neonatal infectious morbidity in women undergoing cesarean delivery receiving preoperative prophylaxis compared with those receiving intraoperative administration (Baaqeel 2013; Costantine 2008). Costantine 2008 included three trials. Baaqeel 2013 included six trials. Our review includes 10 trials. Overall, the results of our review are in agreement with the previous reviews. All three reviews found that preoperative administration of antibiotics lead to a significant decrease in endomyometritis and total maternal infectious morbidity. Additionally, all three reviews found no significant differences in neonatal sepsis or neonatal ICU admission. In contrast, this review was able to demonstrate a significant decrease in wound infection when women received preoperative antibiotics, whereas the previous two reviews were only able to demonstrate a non‐significant trend towards decrease in wound infection. Other outcomes of the two previous reviews (including maternal febrile morbidity and neonatal morbidity) were found to have non‐significant reductions which was consistent with this review.

Study flow diagram.

'Risk of bias. graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Maternal postpartum infectious morbidity, outcome: 1.1 Composite morbidity (as defined by trials).
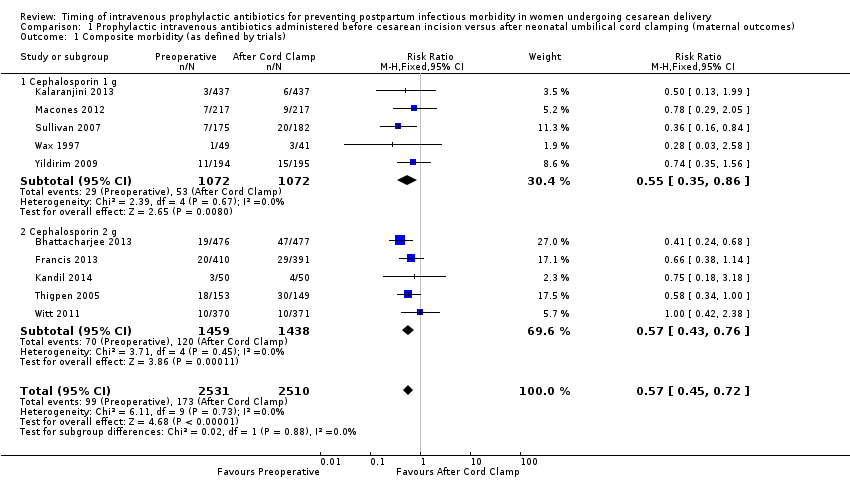
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 1 Composite morbidity (as defined by trials).
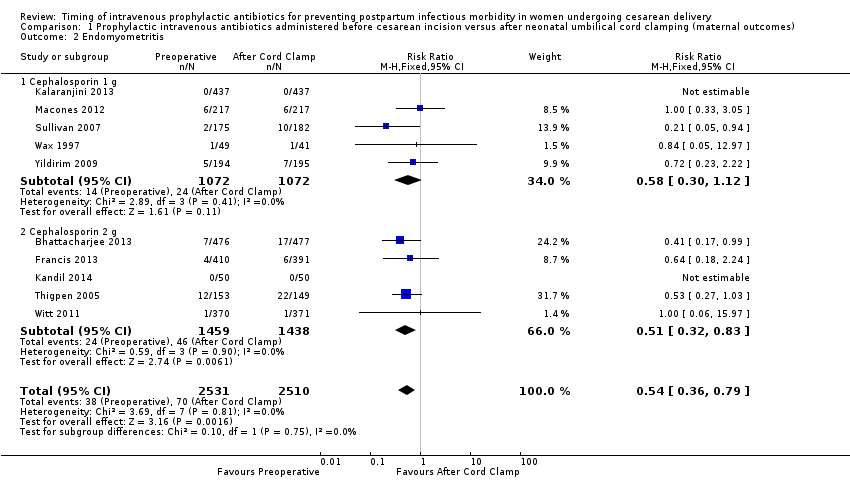
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 2 Endomyometritis.
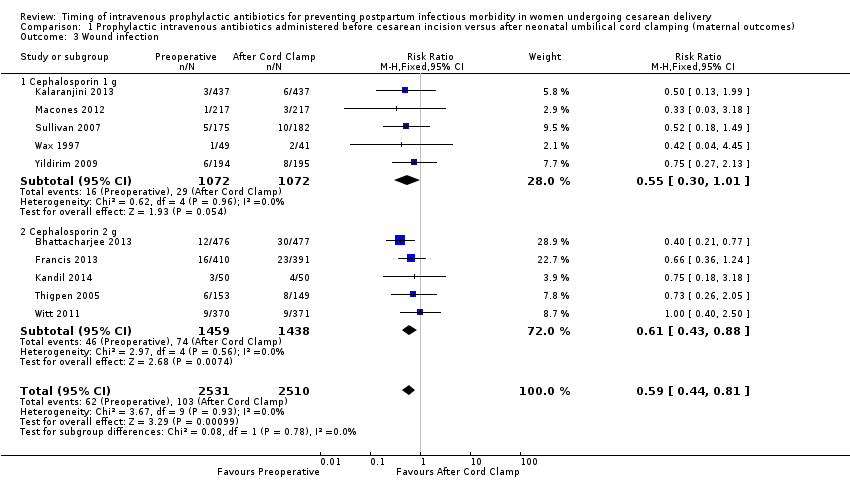
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 3 Wound infection.

Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 4 UTI/cystitis/pyelonephritis.

Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 5 Pelvic abscess.

Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 6 Respiratory infection (pneumonia).
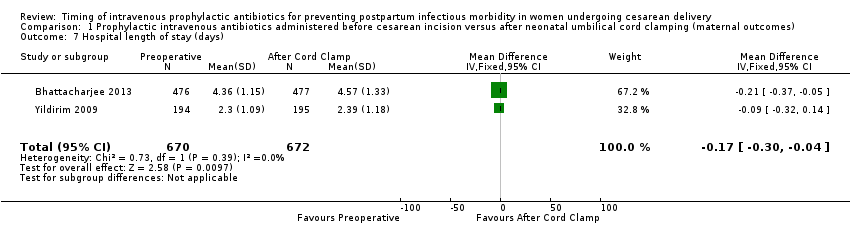
Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 7 Hospital length of stay (days).

Comparison 1 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (maternal outcomes), Outcome 8 Febrile Illness.
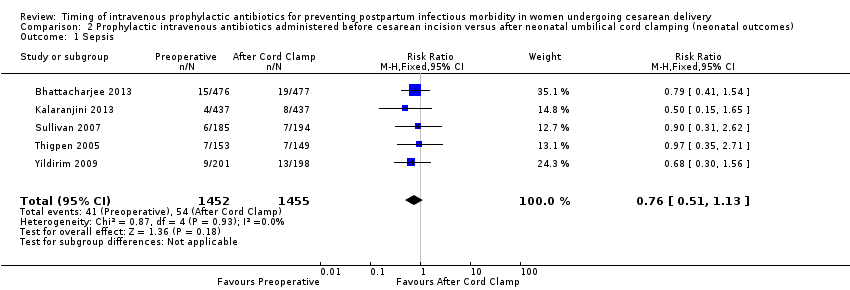
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 1 Sepsis.

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 2 Neonatal sepsis work up.
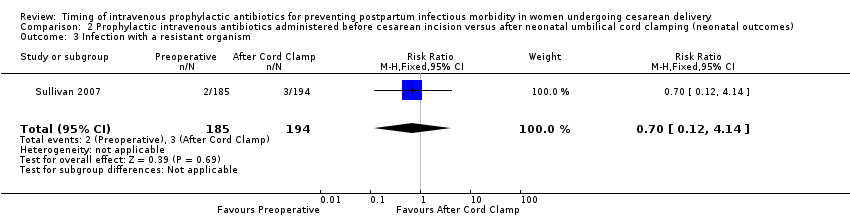
Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 3 Infection with a resistant organism.

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 4 Infection (other).

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 5 ICU admission.

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 6 ICU length of stay (days).

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 7 Neonatal antibiotic treatment.

Comparison 2 Prophylactic intravenous antibiotics administered before cesarean incision versus after neonatal umbilical cord clamping (neonatal outcomes), Outcome 8 Fever.
| Prophylactic antibiotics for preventing postpartum infectious morbidity in women and infants after cesarean delivery | ||||||
| Population: women undergoing cesarean delivery. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Maternal and neonatal postpartum infectious morbidity | |||||
| Maternal composite morbidity | Study population | RR 0.67 | 5041 | ⊕⊕⊕⊕ | ||
| 85 per 1000 | 57 per 1000 | |||||
| Moderate | ||||||
| 97 per 1000 | 65 per 1000 | |||||
| Maternal endomyometritis | Study population | RR 0.54 | 5041 | ⊕⊕⊕⊕ | ||
| 28 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 26 per 1000 | 14 per 1000 | |||||
| Maternal wound Infection | Study population | RR 0.59 | 5041 | ⊕⊕⊕⊕ | ||
| 41 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 51 per 1000 | 30 per 1000 | |||||
| Maternal UTI/cystitis/pyelonephritis | Study population | RR 1.02 | 4001 | ⊕⊕⊕⊝ | ||
| 18 per 1000 | 18 per 1000 | |||||
| Moderate | ||||||
| 13 per 1000 | 13 per 1000 | |||||
| Maternal respiratory infection (pneumonia) | Study population | RR 2.3 | 1158 | ⊕⊕⊝⊝ | ||
| 2 per 1000 | 4 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Neonatal sepsis | Study population | RR 0.76 | 2907 | ⊕⊕⊕⊝ | ||
| 37 per 1000 | 28 per 1000 | |||||
| Moderate | ||||||
| 40 per 1000 | 30 per 1000 | |||||
| Neonatal ICU admission | Study population | RR 0.91 | 3708 | ⊕⊕⊕⊕ | ||
| 86 per 1000 | 78 per 1000 | |||||
| Moderate | ||||||
| 71 per 1000 | 65 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide confidence interval crossing the line of no effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Composite morbidity (as defined by trials) Show forest plot | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.45, 0.72] |
| 1.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.35, 0.86] |
| 1.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.43, 0.76] |
| 2 Endomyometritis Show forest plot | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.36, 0.79] |
| 2.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.12] |
| 2.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.32, 0.83] |
| 3 Wound infection Show forest plot | 10 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.44, 0.81] |
| 3.1 Cephalosporin 1 g | 5 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| 3.2 Cephalosporin 2 g | 5 | 2897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.43, 0.88] |
| 4 UTI/cystitis/pyelonephritis Show forest plot | 8 | 4001 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.65, 1.59] |
| 5 Pelvic abscess Show forest plot | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.97] |
| 6 Respiratory infection (pneumonia) Show forest plot | 4 | 1849 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.34, 15.45] |
| 7 Hospital length of stay (days) Show forest plot | 2 | 1342 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.30, ‐0.04] |
| 8 Febrile Illness Show forest plot | 4 | 2650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.63, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sepsis Show forest plot | 5 | 2907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.51, 1.13] |
| 2 Neonatal sepsis work up Show forest plot | 4 | 1170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.23] |
| 3 Infection with a resistant organism Show forest plot | 1 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.12, 4.14] |
| 4 Infection (other) Show forest plot | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.52, 1.64] |
| 5 ICU admission Show forest plot | 6 | 3708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| 6 ICU length of stay (days) Show forest plot | 3 | 1731 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐2.60, 2.46] |
| 7 Neonatal antibiotic treatment Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.12, 5.68] |
| 8 Fever Show forest plot | 1 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.28, 1.62] |

