Perletakan stoma pararektal lateral berbanding transrektal untuk pencegahan hernia parastoma
Abstract
Background
A parastomal hernia is defined as an incisional hernia related to a stoma, and belongs to the most common stoma‐related complications. Many factors, which are considered to influence the incidence of parastomal herniation, have been investigated. However, it remains unclear whether the enterostomy should be placed through, or lateral to the rectus abdominis muscle, in order to prevent parastomal herniation and other important stoma complications.
Objectives
To assess if there is a difference regarding the incidence of parastomal herniation and other stoma complications, such as ileus and stenosis, in lateral pararectal versus transrectal stoma placement in people undergoing elective or emergency abdominal wall enterostomy.
Search methods
For this update, we searched for all types of published and unpublished randomized and non‐randomized studies in four medical databases: CENTRAL, PubMed, LILACS, Science Ciation Index, and two trials registers: ICTRP Search Portal and ClinicalTrials.gov to 9 November 2018. We applied no language restrictions.
Selection criteria
Randomized and non‐randomized studies comparing lateral pararectal versus transrectal stoma placement with regard to parastomal herniation and other stoma‐related complications.
Data collection and analysis
Two authors independently assessed study quality and extracted data. We conducted data analyses according to the recommendations of Cochrane and the Cochrane Colorectal Cancer Group (CCCG). We rated quality of evidence according to the GRADE approach.
Main results
Randomized controlled trials (RCT)
Only one RCT met the inclusion criteria. The participants underwent enterostomy placement in the frame of an operation for: rectal cancer (37/60), ulcerative colitis (14/60), familial adenomatous polyposis (7/60), and other (2/60).
The results between the lateral pararectal and the transrectal approach groups were inconclusive for the incidence of parastomal herniation (risk ratio (RR) 1.34, 95% confidence interval (CI) 0.40 to 4.48; low‐quality evidence); development of ileus or stenosis (RR 2.0, 95% CI 0.19 to 20.9; low‐quality evidence); or skin irritation (RR 0.67, 95% CI 0.21 to 2.13; moderate‐quality evidence). The results were also inconclusive for the subgroup analysis in which we compared the effect of ileostomy versus colostomy on parastomal herniation. The study did not measured other stoma‐related morbidities, or stoma‐related mortality, but did measure quality of life, which was not one of our outcomes of interest.
Non‐randomized studies (NRS)
Ten retrospective cohort studies, with a total of 864 participants, met the inclusion criteria. The indications for enterostomy placement and the baseline characteristics of the participants (age, co‐morbidities, disease‐severity) varied between studies. All included studies reported results for the primary outcome (parastomal herniation) and one study also reported data on one of the secondary outcomes (stomal prolapse).
The effects of different surgical approaches on parastomal herniation (RR 1.22, 95% CI 0.84 to 1.75; 10 studies, 864 participants; very low‐quality evidence) and the occurrence of stomal prolapse (RR 1.23, 95% CI 0.39 to 3.85; 1 study, 145 participants; very low‐quality evidence) are uncertain.
None of the included studies measured other stoma‐related morbidity or stoma‐related mortality.
Authors' conclusions
The present systematic review of randomized and non‐randomized studies found inconsistent results between the two compared interventions regarding their potential to prevent parastomal herniation.
In conclusion, there is still a lack of high‐quality evidence to support the ideal surgical technique of stoma formation. The available moderate‐, low‐, and very low‐quality evidence, does not support or refute the superiority of one of the studied stoma formation techniques over the other.
PICOs
Ringkasan bahasa mudah
Perletakan stoma pararektal lateral berbanding transrektal untuk pencegahan hernia parastoma
Kami bertanya
Adakah perbezaan kadar hernia parastoma dan komplikasi lain yang berkaitan stoma dalam kalangan orang yang menjalani enterostomi dinding abdomen, apabila membandingkan dua teknik pembentukan stoma: pararektal lateral, di mana stoma diletak di sebelah otot rektus abdominis, salah satu otot dinding abdomen, berbanding transrektal, di mana stoma ditarik melalui otot rektus abdominis?
Latar belakang
Stoma adalah pembukaan di abdomen, yang dibuat melalui pembedahan untuk mengalihkan aliran air kencing atau najis; enterostomi adalah stoma yang bermula dari usus. Hernia parastoma adalah hernia insisi, di mana kandungan abdomen menonjol melalui kecacatan pada dinding abdomen di tapak pembedahan terdahulu. Ia berkait dengan stoma, dan merupakan salah satu komplikasi berkaitan stoma yang paling lazim. Banyak faktor yang berpotensi mempengaruhi kejadian hernia parastoma telah disiasat. Walau bagaimanapun, masih tidak jelas sama ada enterostomi perlu diletakkan melalui atau di sebelah otot rektus abdominis untuk mencegah hernia parastoma.
Ciri‐ciri kajian
Bukti adalah terkini sehingga 9 November 2018. Dalam kemaskini ini, kami menyertakan 10 kajian kohort retrospektif dengan sejumlah 864 peserta, dan satu kajian rawak terkawal (RCT: kajian di mana para peserta secara rawak diperuntukkan kepada kumpulan rawatan), yang melibatkan 60 peserta. Kumpulan sasar adalah individu tanpa mengira umur, yang menerima enterostomi sementara atau kekal untuk sebarang sebab sama ada elektif (dirancang) mahupun kecemasan.
Keputusan utama
Hasil kajian mendapati keputusan yang tidak konklusif di antara kedua‐dua teknik untuk risiko hernia parastoma (11 kajian, 924 peserta), prolaps stoma (1 kajian, 145 peserta), ileus atau stenosis (1 kajian, 60 peserta), dan kerengsaan kulit (1 kajian , 60 peserta).
Tiada teknik yang lebih baik daripada yang lain untuk sebarang hasil yang berkaitan dengan stoma.
Tiada kajian yang mengukur masalah lain yang berkaitan stoma, atau kematian.
Kualiti bukti
Kami menurunkan kualiti bukti kepada sederhana, rendah atau sangat rendah, kerana risiko bias, saiz sampel yang kecil, kejadian yang sedikit, dan kepelbagaian dalam kajian.
Kesimpulan
Berdasarkan pengetahuan semasa yang dibentangkan dalam ulasan ini, tiada bukti untuk menyokong penggunaan satu teknik pembentukan stoma berbanding yang lain. Penyelidikan lanjut mungkin memberi impak penting dalam keyakinan kami terhadap anggaran kesan.
Authors' conclusions
Summary of findings
| Lateral pararectal versus transrectal enterostomy placement for prevention of parastomal herniation | ||||||
| Patient or population: people undergoing enterostomy placement for any reason | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Transrectal enterostomy placement | Lateral pararectal enterostomy placement | |||||
| parastomal hernia (NRS) clinical examination, CT scan, or botha Follow‐up: 2 to 240 months | Study population | RR 1.22 | 864 | ⊕⊝⊝⊝ | ||
| 295 per 1000 | 360 per 1000 | |||||
| Moderate | ||||||
| 348 per 1000 | 425 per 1000 | |||||
| parastomal hernia (RCT) clinical exam, sonography, intraoperatively, or combination, during stoma reversal Follow‐up: 0.5 to 21.4 months (15 to 642 days) | Study population | RR 1.34 | 56 | ⊕⊕⊝⊝ | ||
| 138 per 1000 | 185 per 1000 | |||||
| Moderate | ||||||
| 138 per 1000 | 185 per 1000 | |||||
| stomal prolapse clinical exam, documented on a standard pro forma Follow‐up: mean 110.4 months | Study population | RR 1.23 | 145 | ⊕⊝⊝⊝ | ||
| 78 per 1000 | 96 per 1000 | |||||
| Moderate | ||||||
| 78 per 1000 | 96 per 1000 | |||||
| ileus or stenosis clinical exam, sonography Follow‐up: 0.5 to 21.4 months (15 to 642 days) | Study population | RR 2 | 60 | ⊕⊕⊝⊝ | ||
| 33 per 1000 | 67 per 1000 | |||||
| Moderate | ||||||
| 33 per 1000 | 66 per 1000 | |||||
| skin irritation clinical exam, intraoperative exam, or both, during stoma reversal Follow‐up: 0.5 to 21.4 months (15 to 642 days) | Study population | RR 0.67 | 60 | ⊕⊕⊕⊝ | ||
| 200 per 1000 | 134 per 1000 | |||||
| Moderate | ||||||
| 200 per 1000 | 134 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aIn four studies the presence of parastomal hernia was assessed by clinical examination and CT scan, or CT scan alone. In the remaining studies, participants were clinically examined, e.g. in stoma therapy or outpatient clinic at follow‐up visits, and the findings were documented in the patient chart. | ||||||
Background
Description of the condition
A parastomal hernia is an incisional hernia related to a stoma (Pearl 1989). Parastomal herniation is one of the most common stoma‐related complications (Carne 2003). On physical examination, a parastomal hernia is diagnosed if bulging restricted to the peristomal area occurs during the Valsalva maneuver, and a fascial defect can be palpated (Cingi 2006; Sjodahl 1988). On a computed tomography (CT) scan, a parastomal hernia can be described as 'a loop of intestine or any abdominal organ, as well as preperitoneal fat, protruding through the defect alongside the ostomy' (Cingi 2006).
The incidence of parastomal hernias varies greatly between studies, but most likely ranges from 30% to 50%, depending on the length of follow‐up and on the type of enterostomy (Israelsson 2008). The consequences and implications of parastomal hernias are highly relevant for people. Many people complain of pain, suffer from an impaired body image, and have increasing difficulties in applying the stoma bag properly, leading to leakage and skin irritation (Martin 1996; Ripoche 2011). In rare cases, bowel incarceration occurs, requiring emergency surgery (Cuthbertson 1977). In addition, stomal herniation necessitating reoperation and hospitalization has a negative socioeconomic impact, especially since recurrence rates after surgical correction are high (Israelsson 2008; Tekkis 1999), ranging up to 50% within an average of six months after operative repair (Ripoche 2011). With regard to the etiology of parastomal herniation, one has to differentiate between patient‐related and surgical or technical risk factors. Ripoche 2011 studied several patient‐related potential risk factors, such as age, gender, past medical history (ventral hernia, diabetes, corticosteroid therapy), and diagnosed condition requiring stoma formation (for example malignancy, inflammatory bowel disease, diverticulosis). The only patient factor that was significantly associated with parastomal herniation in a multivariate analysis was age > 60 years at the time of stoma placement (Ripoche 2011), which is supported by data from other studies investigating long‐term hernia rate and risk factors (Hong 2013; Mylonakis 2001; Pilgrim 2010). The influence of operative factors is also much debated. Trephine size, closure of the lateral space, stomal fixation to the fascia, intra‐ versus extraperitoneal route, and location in relation to the rectus abdominis muscle may have an influence on the occurrence of parastomal herniation (Carne 2003; Hotouras 2013). Furthermore, type of enterostomy (ileostomy versus colostomy, end versus loop ostomy), operative setting (emergency versus elective surgery), and whether a stoma therapist marked the site of the stoma preoperatively, seem to have an impact on the risk of parastomal herniation.
Description of the intervention
Two interventions were compared; placement of the stoma lateral to versus through the rectus abdominis muscle (lateral pararectal versus transrectal).
How the intervention might work
Lateral pararectal stoma placement may have a different incidence of parastomal herniation in comparison to transrectal stoma formation. Since the transrectal stoma is surrounded by the thick muscle layers of the rectus abdominis muscle, one could expect a tighter fit and a reduced incidence of parastomal herniation due to the muscle contractions. On the other hand, one could argue that preserving the integrity of the rectus abdominis muscle and its sheath, as is the case in lateral pararectal stomas, minimizes anterior abdominal wall disruption, and consequently reduces the risk of parastomal hernia (Evans 2011; Stephenson 2010).
Why it is important to do this review
Parastomal herniation is one of the most common complications after stoma placement. Since parastomal hernias often have a negative impact on health and quality of life, the operative techniques should be optimized in order to prevent this postoperative complication. The need for prevention becomes even more relevant in the light of the high recurrence rates after operative correction of parastomal hernia, ranging from 50% to 76% after aponeurotic repair and from 24% to 86% after relocation (Israelsson 2008). People with a permanent stoma, for example after abdominoperineal resection for low rectal cancer, especially suffer from the morbidity caused by parastomal herniation. Many surgical approaches have been attempted and propagated.
Two studies showed a significant reduction in the incidence of parastomal herniation if the stoma was situated through the rectus abdominis muscle versus a location lateral to the muscle (Eldrup 1982; Sjodahl 1988). One prospective uncontrolled cohort study, which included 72 consecutive, unselected patients, described a novel anatomical approach to stoma formation, the lateral rectus abdominis positioned stoma (LRAPS), which involves minimal anterior abdominal wall disruption. The authors presented parastomal herniation rates of 5% (3/62) at one‐year follow‐up and 10% (4/41) after two years (Evans 2011; Stephenson 2010), which is consistent with a relevant reduction in parastomal hernia rate when compared to data from the literature (Robertson 2005).
When we conducted the first systematic review, there was no clinical evidence from randomized controlled trials (RCT) on the best stoma location in relation to the rectus abdominis muscle (Hardt 2013). At that time, we assumed clinical equipoise in regard to the review question, and designed the PATRASTOM trial, a phase 1 single center RCT, which assessed the safety and efficacy of the LRAPS in reducing the incidence of parastomal herniation in patients with temporary loop ileostomies, as a pilot trial, in preparation for a prospective multicenter RCT, comparing lateral pararectal to transrectal positioning of a permanent stoma, in regard to parastomal herniation (Hardt 2016).
The current update of our systematic review was considered justified, since the first RCT addressing the review's research question was recently published. The aim of the update was to integrate the newly identified randomized and non‐randomized evidence into the existing body of evidence, in order to synthesize and summarize the current state of knowledge in regard to the review objective.
Objectives
To assess if there is a difference regarding the incidence of parastomal herniation and other stoma complications, such as ileus and stenosis, in lateral pararectal versus transrectal stoma placement in people undergoing elective or emergency abdominal wall enterostomy.
Methods
Criteria for considering studies for this review
Types of studies
Randomized (RCT) and non‐randomized studies (NRS) were eligible. The initial rationale for including non‐randomized studies in the first version of this Cochrane Review was the fact that our preliminary search did not identify any randomized trials that compared the two interventions of interest. Therefore, we conducted a systematic review of non‐randomized studies. Since the only randomized trial, which was eligible for inclusion in the current, updated review version, has a relatively small sample size and short follow‐up, we decided to present results from both randomized and non‐randomized studies regarding the review objective, in order to depict the complete body of evidence. We included non‐randomized studies if they met the inclusion criteria, and compared a lateral pararectal versus a transrectal enterostomy placement. We excluded studies that only reported data on transrectal (for example Carlstedt 1987; Leenen 1989) or lateral pararectal enterostomies (for example Stephenson 2010).
Types of participants
We included trials with individuals who received a temporary or permanent abdominal wall enterostomy, for any reason, in either the elective or the emergency setting, with no regard for the underlying disease.
Types of interventions
Two interventions were compared: lateral pararectal versus transrectal stoma placement.
Types of outcome measures
Primary outcomes
-
Incidence of parastomal herniation.
Secondary outcomes
-
Stoma‐related morbidity, especially stenosis, obstruction, prolapse, necrosis, retraction, fistulization, and skin irritation;
-
Stoma‐related mortality.
Search methods for identification of studies
In November 2018, we searched for all types of published and unpublished RCTs and NRS with no restriction on language or country. We undertook a broad search approach, due to the scarcity of RCTs regarding the review objective. Therefore, we did not apply search filters for study designs on any of our searches.
Electronic searches
For this update, we identified studies by searching the following databases and trials registers:
-
Cochrane Central Register of Controlled Trials (CENTRAL; Cochrane Register of Studies Online; searched 9 November 2018), Appendix 1.
-
PubMed (searched 9 November 2018), Appendix 2.
-
Science Citation Index Expanded Web of Science (searched 9 November 2018), Appendix 3.
-
LILACS (Latin American and Caribbean Health Science Information database; searched 9 November 2018), Appendix 4.
-
ClinicalTrials.gov (searched 9 November 2018), Appendix 5.
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/; (searched 9 November 2018), Appendix 6.
For this search update, we did not include Embase, as it was no longer available at our institutions. However, RCTs from Embase are now prospectively added to CENTRAL via a highly sensitive screening process (CENTRAL creation details). In addition, all of the included retrospective cohort studies in the previous review version were indexed in MEDLINE, and the new included NRS for this update was also indexed in MEDLINE.
Searching other resources
We endeavored to identify other potentially eligible studies or ancillary publications by screening the reference lists of included studies, systematic reviews, and meta‐analyses. In addition, we contacted the authors of the included studies if we required additional information on their studies.
Data collection and analysis
Selection of studies
Two authors (JH and FH) independently screened all titles and abstracts of papers identified by the search strategies for relevance. They only excluded clearly irrelevant reports at this stage. We obtained full copies of all potentially relevant papers. Afterwards, the two review authors (JH and FH) independently screened the full texts, identified relevant studies, and assessed eligibility of studies for inclusion. They resolved disagreements on the eligibility of studies by discussion and consensus, or if necessary, by consulting a third review author (JM or PK). We excluded all irrelevant records, and recorded the reasons for exclusion.
Data extraction and management
JH and FH independently extracted the data for the review. In addition to the outcomes, they extracted data on population characteristics (such as sex, age, underlying disease, obesity (BMI > 30 kg/m²)), and type of stoma (ileostomy versus colostomy, end ostomy versus loop ostomy), if available. We carefully customized data collection forms to the review question being investigated. During the review process, we repeatedly revised the data collection forms to enable us to handle the different kinds of information about study findings.
Assessment of risk of bias in included studies
Assessment of risk of bias in RCT
We applied the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We assessed risk of bias in the included RCT in the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, reporting bias, and other bias.
Assessment of risk of bias in NRS
As recommended by Reeves and colleagues in Chapter 13 in the Cochrane Handbook for Systematic Reviews of Interventions (Reeves 2011), we applied the Newcastle‐Ottawa Scale (NOS) to assess the methodological quality of the included cohort studies in the following domains: selection of cohorts, comparability of cohorts, and assessment of outcome (Wells 2011). We also assessed reporting bias and other bias which are not included in the NOS. In addition, we attempted the use of the Downs and Black Checklist for 'Risk of bias' assessment, which is applicable to both RCTs and NRS, and is also recommended by Reeves and colleagues, but we had to conclude that the NOS was easier to use with the included cohort studies (Downs 1998).
For all included studies, JH and FH independently assessed the risk of bias.
Measures of treatment effect
We treated the presence of parastomal herniation, as well as the presence of other stoma complications, such as stomal prolapse, ileus or stenosis, and skin irritation, as dichotomous variables. For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval (CI).
Unit of analysis issues
The unit of analysis was each participant recruited into the trials.
Dealing with missing data
We carefully assessed the risk of incomplete outcome data using the NOS and the Cochrane tool for assessing risk of bias. Since all included NRS were retrospective cohort studies, with the majority published more than 20 years ago, we refrained from contacting the authors for missing data on losses to follow‐up and their reasons. However, we successfully contacted the authors of Ho 2018 for missing data on parastomal hernia rates, and discussed the potential impact of missing outcome data (for example due to losses of follow‐up or exclusions from analysis) in the discussion section (Appendix 7).
Assessment of heterogeneity
We used the Cochrane Q test to assess for statistical heterogeneity. We considered a P value of 0.10 to be statistically significant. Even more relevant than the assessment of heterogeneity, is the quantification of its impact on the meta‐analysis. Thus, we applied the I² value to quantify the inconsistency of the included NRS (Deeks 2011). An I² value less than 40% might not be important; greater values may represent moderate (< 60%), substantial, or even considerable (> 75%) heterogeneity. Nonetheless, we were aware of the fact that there is much uncertainty in measures, such as the I² statistic, when there are few studies.
Assessment of reporting biases
Reporting biases occur if the dissemination of research findings is influenced by the nature and direction of trial results. There are several types of reporting bias: publication bias, multiple publication bias, time lag bias, location bias, citation bias, language bias, and outcome reporting bias (Sterne 2011).
We used a comprehensive search strategy, including the search of two different trials registers, in order to minimize the likelihood of publication bias (see Electronic searches), but we were aware of the problem of inconsistent indexing in trials registers, especially with NRS. Furthermore, we searched LILACS, the most important and comprehensive index of scientific and technical Latin‐American and Caribbean literature, to diminish the risk of language bias.
To assess publication bias in our review, we used a funnel plot (see Figure 1). Publication bias is considered minimum if the plot resembles a funnel with the base down. The funnel plot test by Harbord did not suggest asymmetry in the funnel plot (P = 0.6667 (Harbord 2006)). However, we were aware of the fact that asymmetry is difficult to detect with such a small number of included studies (Sterne 2011).

Funnel plot of comparison 1. Lateral pararectal versus transrectal enterostomy placement, outcome 1.1. parastomal hernia. Only non‐randomized studies
Data synthesis
From the analysis options in Review Manager 5.3, we applied the DerSimonian and Laird random‐effects model, assuming that the effects being estimated in the different studies were not identical but followed some distribution (DerSimonian 1986). The random‐effects model estimates the extent of variation among the intervention effects of the included studies (Deeks 2011).
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analysis:
-
ileostomy versus colostomy.
For the following intended subgroup analyses, the included studies reported the required data insufficiently or not at all:
-
obesity (BMI > 30 kg/m²), overweight (BMI > 25 < 30 kg/m²) versus normal weight (BMI > 20 < 25 kg/m²);
-
end versus loop ostomy.
Sensitivity analysis
Sensitivity analysis investigates how the variation in the output of a statistical model depends on the different variations in the inputs of the model. We could not perform either of the two planned sensitivity analyses because of the following reasons:
-
Sensitivity analysis based on assessment of standardization of stoma surgery was not performed, since there was insufficient evidence of standardization of the surgical interventions in the included studies.
-
Sensitivity analysis based on assessment of risk of bias was not performed, since the majority of included studies were at high risk of bias in at least one of the assessed domains, and showed very similar risk of bias profiles (see Figure 2; Figure 3; Table 1).
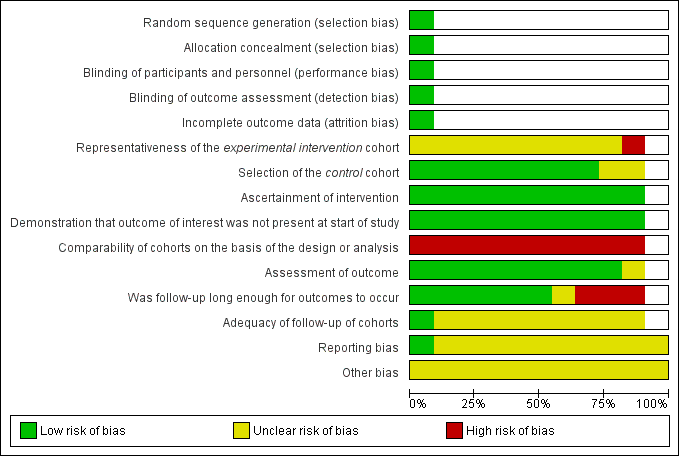
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
| Representativeness of the experimental intervention cohort | Selection of the control cohort | Ascertainment of intervention | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow‐up long enough for outcomes to occur | Adequacy of follow‐up of cohorts | |
| ★ | ★ | ★ | ||||||
| ★ | ★ | ★ | ★ | |||||
| ★ | ★ | ★ | ★ | ★ | ★ | |||
| ★ | ★ | ★ | ★ | ★ | ||||
| ★ | ★ | ★ | ★ | ★ | ||||
| ★ | ★ | ★ | ★ | ★ | ||||
| ★ | ★ | ★ | ||||||
| ★ | ★ | ★ | ★ | ★ | ||||
| ★ | ★ | ★ | ★ | |||||
| ★ | ★ | ★ | ★ | ★ |
A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability. A maximum of nine stars can be awarded overall.
Results
Description of studies
See: Characteristics of included studies
Results of the search
As recommended by the PRISMA statement, a study flow diagram summarizes the study selection process for the review update (Liberati 2009; Figure 4). The flow diagram presenting the study selection process of the original version of the review is available in Hardt 2013.
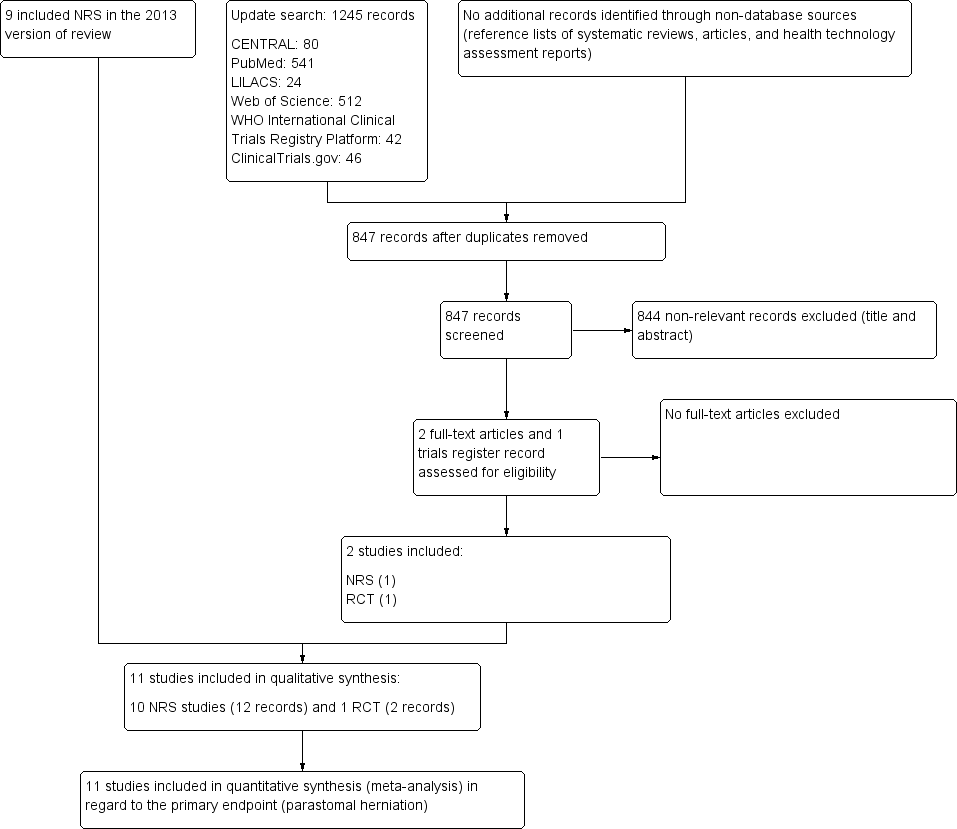
Trial flow diagram
RCT: randomized controlled trial; NRS: non‐randomized study
The electronic searches, run on 9 November 2018, retrieved a total of 1245 references; The Cochrane Library (CENTRAL 80), PubMed (541), Web of Science (512), LILACS (24), ClinicalTrials.gov (46) and ICTRP (42). We excluded 398 duplicates and 844 clearly irrelevant references, identified by reading titles and abstracts. We retrieved the three remaining references for further assessment. All three references, relating to one RCT and one NRS, fulfilled our inclusion criteria (see: Included studies).
Included studies
One randomized controlled trial (RCT) and ten retrospective non‐randomized studies (NRS) met the inclusion criteria. In order to determine the type of NRS and distinguish between case series and cohort studies, we applied the criteria outlined by Dekkers and colleagues (Dekkers 2012). According to their proposal, a cohort study is defined by the following main features:
-
sampling is based on exposure;
-
the occurrence of outcomes is assessed during a specified follow‐up period;
-
exposure can be a risk factor, a disease, or an intervention;
-
absolute risk (or rate) calculation is possible;
-
if a comparison group is included, relative risk can be calculated.
All included NRS showed these features, and we consequently classified them as cohort studies.
For more details on the included studies, see the 'Characteristics of included studies' tables.
Excluded studies
We excluded 11 studies due to various reasons, as presented in the 'Characteristics of excluded studies' tables.
Risk of bias in included studies
Assessment of risk of bias in RCT
Figure 2 and Figure 3 contain the risk of bias assessments for both the RCT and the NRS. Since not all risk of bias domains apply to both study types, some lines and cells of the two figures remain blank.
Assessment of risk of bias in NRS
Reeves and colleagues hypothesized that the risk of bias in NRS depends on the specific study features rather than on the design labels (such as cohort or case‐control study (Reeves 2011)). Thus, we attempted to assess the study features that had a major impact on the risk of bias using the Newcastle‐Ottawa Scale (NOS) for cohort studies (Wells 2011). See Figure 2; Figure 3; Table 1.
Selection bias
RCT
In the PATRASTOM trial (Hardt 2016), the only included RCT, successful randomization, including adequate sequence generation and allocation sequence concealment prevented selection bias. Thus, we considered the PATRASTOM trial at low risk of selection bias.
NRS
In NRS, groups are unlikely to be comparable due to the lack of concealed randomization within the allocation process. Consequently, NRS are more susceptible to selection bias compared to RCTs, which is regarded as the main difference between RCTs and NRS (Reeves 2011). We checked the representativeness of the intervention cohort (lateral pararectal stoma placement) and the comparability of the intervention (lateral pararectal) and control (transrectal) cohorts. The majority of the included studies were at unclear risk of selection bias since the authors did not sufficiently describe the derivation of the intervention cohort, and whether the intervention and control cohorts were drawn from the same community.
Performance bias
RCT
We judged the risk of performance bias as low in the PATRASTOM trial because participants were blinded to the intervention they received. As stated in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions, blinding is not always possible (Higgins 2011). This was the case with the operating surgeon who could not be blinded to the intervention he performed. Nonetheless, it is unlikely that the lack of blinding of the surgeon influenced the outcome.
NRS
We considered the risk of performance bias to be relatively low in the included studies because the surgical procedure (lateral pararectal versus transrectal stoma placement) was ascertained reliably either by reviewing secure records, such as operative reports and patient charts, or by computerized tomography (CT), or clinical examination, or both. In two radiological studies, the position of the stoma in relation to the rectus abdominis muscle was assessed by CT scanning (Cingi 2006; Williams 1990). In the most recently included study, the type of intervention was ascertained both by database review and by CT scans (Ho 2018). In another study, the intervention was reassessed by CT examination if it remained unclear after clinical evaluation (Ortiz 1994). In one study, the stomal site in relation to the rectus muscle was determined by clinical examination, by a surgeon and a staff nurse at the stoma therapy service (Sjodahl 1988). In the remaining studies, the intervention must have been ascertained by chart review (though this was not always explicitly stated (Eldrup 1982; Pilgrim 2010; von Smitten 1986)), or review of a standard operative 'pro forma' (Leong 1994; Londono‐Schimmer 1994).
Detection bias
RCT
There were no systematic differences between groups in how outcomes were determined in the PATRASTOM trial. Although the outcome assessor (i.e. the surgeon who performed the stoma reversal operation) was not blinded to the previously performed study intervention, it is unlikely that this influenced the outcome assessment results.
NRS
We also estimated the risk of detection bias to be low in most of the included studies, as the outcome (parastomal herniation) was assessed by either clinical examination, or CT scanning, or both, or by reviewing reliable patient records. In four studies, the outcome was assessed by physical examination and CT scanning (Cingi 2006; Ortiz 1994; Williams 1990) or by CT scanning alone (Ho 2018). In two Scandinavian studies from the 1980s, parastomal herniation was assessed only by clinical examination (Sjodahl 1988; von Smitten 1986). In another study, the outcome was ascertained by physical examination including 'digital examination through the stomal opening' by enterostomal nurse specialists (Pilgrim 2010). In both studies from St. Mark's Hospital in London, UK, the outcomes (parastomal herniation and stomal prolapse) were assessed by reviewing standard operative pro formas (Leong 1994; Londono‐Schimmer 1994). In the oldest of the included studies, a Danish study including patients from two Copenhagen county hospitals, all eligible living participants were screened for parastomal hernia by physical examination, whereas all eligible dead patients were assessed for the outcome by chart review (Eldrup 1982).
Incomplete outcome data
RCT
Outcome data were not available in 4 of the 60 randomized patients (Hardt 2016), but the reasons for missing outcome data were similar across the intervention groups and unlikely to be related to the true outcome. Therefore, the risk of attrition bias is judged as low in this study.
NRS
We found the majority of included NRS at high risk of presenting incomplete outcome data (attrition bias). Applying the NOS, we identified that five of the ten included studies showed follow‐up rates < 80% (Cingi 2006; Eldrup 1982; Leong 1994; Londono‐Schimmer 1994; Williams 1990). Four studies either did not present the number of people examined for eligibility or lost to follow‐up (Ortiz 1994; Pilgrim 2010; Sjodahl 1988), or the numbers given seemed unreliable from a clinician's standpoint (von Smitten 1986). Only the most current included NRS described the exact number of people examined for eligibility and the reasons for exclusion (Ho 2018). Since 103 of 112 patients were confirmed eligible, the follow‐up rate was about 92%. Accordingly, we considered the risk for attrition bias in this study as low.
Selective outcome reporting bias
RCT
Reporting bias due to selective outcome reporting seemed unlikely because the PATRASTOM trial was registered prospectively in the German Clinical Trials Register, and all of the study’s prespecified outcomes were reported in the prespecified way (see Appendix 8).
NRS
The common lack of a study protocol for NRS leads to a higher risk of potential selective outcome reporting bias for NRS compared to RCTs (Reeves 2011). Features of RCTs that prevent reporting bias (for example a prespecified protocol, ethical approval, progress and final reports, and the CONSORT statement) were missing in all included NRS. Comparing the information given in the methods and results sections regarding outcomes to be assessed and the outcomes assessed, we did not discover any discrepancies. Hence, we concluded that the likelihood of selective outcome reporting remained unclear. We considered the risk of reporting bias in the most recently included NRS was lower than in the remaining nine NRS because this study was reported in accordance with the STROBE guidelines (Elm 2014) and the European Hernia Society (EHS) recommendations on the reporting of hernia surgery (Muysoms 2013). Nonetheless, in the absence of a study protocol, it remains unclear whether selective (or incomplete) outcome reporting was present (Ho 2018).
Other potential sources of bias
One study was supported by a grant, but no further information was provided (Ortiz 1994). This could potentially introduce additional bias.
Effects of interventions
The only identified RCT reported data on the primary endpoint (parastomal herniation), as well as on the secondary outcomes ileus or stenosis and stoma‐related skin irritation (Hardt 2016). They also assessed quality of life before and after ileostomy placement, using the European Organisation for Research and Treatment of Cancer (EORTC) questionnaires QLQ‐CR29 and QLQ‐C30.
All NRS included in the meta‐analysis reported results for the primary outcome of parastomal herniation, and one study also reported data on one of the secondary outcomes, stomal prolapse (Leong 1994). None of the included studies compared the two interventions with regard to other secondary outcomes.
The results from one RCT (N = 56) were inconclusive between the lateral pararectal (5 of 27) and the transrectal groups (4 of 29) for the incidence of parastomal herniation (risk ratio (RR) 1.34, 95% confidence interval (CI) 0.40 to 4.48; P = 0.63; Analysis 1.1); development of ileus or stenosis (RR 2.0, 95% CI 0.19 to 20.90; P = 0.56; Analysis 1.4); stoma‐related skin irritation (RR 0.67, 95% CI 0.21 to 2.13; P = 0.49; Analysis 1.5), and quality of life.
The results from the NRS were also inconclusive for parastomal herniation rates (80/258 in the lateral pararectal, 179/606 in the transrectal group; RR 1.22, 95% CI 0.84 to 1.75; P = 0.29; 10 NRS, 864 participants; Analysis 1.2), and the development of stomal prolapse (RR 1.23, 95% CI 0.39 to 3.85; P = 0.73; 1 NRS, 145 participants; Analysis 1.3).
The subgroup analysis found inconclusive results for the incidence of parastomal herniation between participants who received an ileostomy and those who received a colostomy (RR 1.16, 95% CI 0.81 to 1.65; 7 NRS, 624 participants; Analysis 2.1). The results were also inconclusive in both the ileostomy subgroup (RR 0.70, 95% CI 0.21 to 2.29; 2 NRS, 173 participants; Analysis 2.1) and the colostomy subgroup (RR 1.30, 95% CI 0.80 to 2.11; 5 NRS, 451 participants; Analysis 2.1).
Discussion
Parastomal herniation is one of the most common stoma complications and causes significant stoma‐related morbidity, especially in people with permanent enterostomies, for example people with low rectal cancer who had to undergo abdominoperineal resection. The patient relevance of this complication is high because parastomal herniation often results in further morbidity, such as skin irritation and maceration due to leakage. Moreover, it can necessitate emergency surgery for strangulated bowel. A recent retrospective study from France, which included 782 people with a stoma, showed that after a mean follow‐up of 10.5 years, the prevalence of parastomal herniation was 25.6% (n = 202). Of the 202 participants with parastomal hernia, more than one third (35%) complained of pain and almost one third (28%) suffered from leakage due to difficulties in fitting the stomal appliance. More than half of the people (n = 114) underwent operative repair, but again, in half of these (n = 57) parastomal herniation recurred within six months (Ripoche 2011). The high recurrence and morbidity rates after hernia repair make the situation of the affected people even more precarious and underline the need for effective preventive measures.
Therefore, and on the premise that surgical prevention of parastomal herniation without the use of mesh is worthy of consideration, we aimed to collect all existing evidence regarding the question of whether a stoma should be placed through or lateral to the rectus abdominis muscle. The main results of our reviewing process are summarized in the Summary of main results.
Summary of main results
Currently, the evidence for our review objectives consists of a small single center pilot randomized controlled trial (RCT) and ten retrospective controlled cohort studies (NRS). Due to the lack of further RCT evidence, we still included NRS in this updated version of our Cochrane Review. All included NRS reported the primary outcome (parastomal hernia), and one study (145 participants), also reported one of the secondary outcomes (stomal prolapse (Leong 1994)). Eight of the ten included cohort studies (594 participants), did not find a statistically significant difference in parastomal herniation rates after lateral pararectal compared to transrectal stoma formation. In contrast, the remaining two studies found a significant reduction of parastomal herniation if the stoma was pulled out through the belly of the rectus abdominis muscle (Eldrup 1982; Sjodahl 1988).
Pooling the data of all included NRS did not provide conclusive results. Reasons for the widely differing estimates of the intervention effect (heterogeneity) across studies could be due to the lack of standardization of the surgical procedure, as well as the absence of a uniform definition and detection method for parastomal hernia. An I² value of 64% indicated substantial statistical heterogeneity in the meta‐analysis (Analysis 1.2). The I² statistic describes the percentage of total variation across studies that is caused by heterogeneity rather than by chance (Deeks 2011). In Analysis 1.2, the calculated P value was interpreted as a finding of uncertainty. The wide confidence interval could be the result of the small number of studies combined in the meta‐analysis, as well as the substantial heterogeneity.
The wide confidence intervals in the other analyses were likely caused by the small sample size of the only included RCT, the PATRASTOM trial, which found inconclusive results between lateral pararectal and transrectal stoma placement for the incidence of parastomal herniation, other stoma‐related complications, and quality of life (Hardt 2016). However, due to the limited sample size and the wide confidence intervals, a small and even a moderately sized difference in favor of one of the two stoma placement techniques cannot be entirely ruled out.
In conclusion, there is no evidence supporting the surgical dogma that the transrectal route of stoma formation should be preferred in order to prevent parastomal herniation.
Overall completeness and applicability of evidence
The present review aimed to compare lateral pararectal to transrectal stoma placement for prevention of parastomal herniation and other patient‐relevant stoma‐related complications. This was only partly accomplished. All included studies reported parastomal herniation, the primary outcome, but only one of the NRS and the RCT presented data on stomal prolapse. Only the RCT, but none of the NRS, reported any other secondary outcomes. Furthermore, we could only conduct one of the planned subgroup analyses and none of the sensitivity analyses because the required data were not reported in the included studies. Thus, the evidence with regard to the review objective is very limited, and the identified studies could not address all of the objectives of the review. The NRS identified are all retrospective cohort studies and at high risk of bias, especially with regard to selection bias and incomplete outcome data (attrition bias). Since the participants were not randomly assigned to the two intervention groups, the risk of selection bias is high. Moreover, it mostly remained unclear whether the individual participants were representative of the population. Hence, external validity and generalizability of the results from the meta‐analysis of NRS remain unclear. Although overall risk of bias is considered moderate to low in the included RCT, there are some obvious limitations (small sample size, short follow‐up, non‐blinding of the surgeons assessing the primary endpoint), which restrict the applicability of the study results.
Quality of the evidence
We included one RCT and ten retrospective cohort studies in this systematic review. For key methodological features and limitations of the studies, please see Characteristics of included studies. Confidence in the estimates of the treatment effect is limited because of risk of bias, publication bias, imprecision, inconsistency, and indirectness. All these issues are included in the GRADE system and are summarized in the summary of findings Table for the main comparison. Overall risk of bias in the included observational studies is high, especially due to possible selection bias (no randomization, unclear representativeness and comparability of the two cohorts), and high risk of attrition bias. It remains unclear whether publication bias affected the estimates of treatment effect, but it cannot be excluded, because observational studies reporting higher rates of parastomal herniation and other stoma‐related complications might be less likely to be published than studies presenting lower complication rates. This may be a particular concern in surgery. Since the 95% confidence interval around the estimate of effect includes both no effect and appreciable benefit or appreciable harm, and because the total number of events is less than 300, we assume that there is considerable imprecision. Moreover, the quality of evidence is diminished due to inconsistency, because of the widely differing estimates of the intervention effect (heterogeneity) across studies, for which we failed to identify a plausible explanation. However, we did not find any evidence of indirectness with respect to the target population, intervention, or outcome of interest.
Hence, we downgraded the quality of the evidence from NRS for the primary endpoint (parastomal herniation) by one level, from low to very low, due to high risk of bias, imprecision, and inconsistency.
In summary, the body of evidence identified does not allow a robust conclusion regarding the objectives of the review.
Potential biases in the review process
Although we followed a strict protocol consistent with the new version of the MECIR, published in 2016 (Higgins 2016), and the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) statements (Stroup 2000), and applied a comprehensive search strategy, this review is still prone to bias. For example, publication bias cannot be excluded in our review because NRS are not consistently indexed in trials registers (Reeves 2011). Consequently, it must be taken into consideration that we may have missed completed or ongoing NRS. Moreover, we did not screen conference proceedings, which could potentially lead to further publication bias.
As also stated in the "Declarations of interest" paragraph, four of the review authors (JH, FH, PK, SP) conducted one of the included studies, the PATRASTOM trial. In order to prevent confounding and to guarantee transparency and objectivity, an external clinician not involved in the trial checked data extraction, risk of bias assessment and interpretation of the PATRASTOM trial.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the only systematic review addressing this particular review question. However, several narrative reviews have discussed the objective of our review (Carne 2003; Hotouras 2013; Israelsson 2008; Martin 1996; Pearl 1989).
Regardless of the fact that until recently, when the results of the PATRASTOM trial were published, there was no evidence from randomized trials, surgical dogma has emerged that a stoma should be placed through the rectus abdominis rather than lateral to the muscle, in order to prevent parastomal herniation. This credo has been persistently repeated in the surgical literature, usually without any scientific evidence (Park 1999; Pearl 1985). But how could this surgical dictum arise? And why does it still persist, despite the lack of evidence? In the mid 1930s, Gabriel and Lloyd‐Davies recommended transrectal colostomy formation to prevent parastomal herniation, which was approved by Turnbull 20 years later (Gabriel 1935; Turnbull 1958). In the mid‐1970s, a study from St. Mark's Hospital in London, UK, was published, reporting data on 227 people undergoing surgery for rectal adenocarcinoma. In 35 of the included participants, the colostomy was created transrectally, and after a follow‐up of one to six years, six of the 35 participants were diagnosed with a parastomal hernia (Marks 1975). Thus, on the basis of their data, the authors concluded that the transrectal route could not generally be recommended. One explanation of why surgeons continued to adhere to the thesis that the stomata should be placed transrectally could be the assumption that pulling the stoma through the rectus abdominis muscle provided a more snug fit, since the muscle contracted around the stomal aperture. On the other hand, the same thinking could lead to the concern that there was a higher risk of stoma‐related stenosis and ileus, due to tight muscle contractions around the enterostomy.
In 2010, Stephenson described a novel approach to stoma formation, the lateral rectus abdominis positioned stoma (LRAPS), being convinced that preservation of the rectus abdominis muscle and its sheath could prevent parastomal herniation (Stephenson 2010). The new technique implied that the anterior and posterior rectus sheath were only divided horizontally (not vertically) and that the rectus muscle was not incised, but separated from its sheath, by sharp and blunt dissection and was then retracted medially. After the bowel was delivered, any defect of the medial margins of the incisions in the anterior and posterior sheath was closed with interrupted absorbable sutures. Stephenson presented data from 29 consecutive, unselected people who received a LRAPS. After a mean follow‐up of 13 months (range 7 to 18), no parastomal hernia had been detected. In 2011, Evans published a further follow‐up of the LRAPS; at one year of follow‐up, the authors observed parastomal hernias in 5% of the participants with a LRAPS (3/62), and at two years, the parastomal hernia rate had increased to 10% (4/41 (Evans 2011)). Although the follow‐up duration was still rather short, the reported parastomal herniation rates were lower than what was usually reported in the literature (Israelsson 2008; Robertson 2005).
Another emerging surgical trend is the use of prophylactic mesh for prevention of parastomal herniation. In the last decade, multiple systematic reviews of randomized trials addressed the question of whether prophylactic implantation of prosthetic mesh could reduce the rate of parastomal herniation. The most recently published systematic reviews pooled the data of six to ten RCTs; meta‐analyses showed that mesh reinforcement at the time of stoma formation significantly reduced the incidence of clinically‐ and radiologically‐detected parastomal herniation without increasing stoma‐ or mesh‐related morbidity and other surgical complications (Chapman 2017; Cornille 2017; Cross 2017; López‐Cano 2017; Patel 2017; Pianka 2017; Wang 2016). However, these results should be interpreted with caution, due to the heterogeneity among the included studies, the high risk of bias, and the small sample sizes of the individual studies (the median sample size of the RCTs included in the systematic review by Chapman was 54 (range 34 to 113)). Because of these severe limitations, some of the authors of the most recent systematic reviews call for larger, higher‐quality RCTs, which also assessed different mesh types, locations, and techniques, as well as long‐term cost‐effectiveness (Chapman 2017; Cornille 2017; Patel 2017; Wang 2016). Interestingly, the very recently published high‐quality multicenter double‐blinded RCT from Sweden, STOMAMESH, with a remarkably larger sample size than all previously conducted RCTs (211 analyzed participants), could not confirm the findings of the above‐cited meta‐analyses: Odensten and colleagues described that mesh reinforcement around the stoma did not alter the rate of parastomal herniation at one‐year follow‐up (Odensten 2019). In summary, there is still contradictory evidence for the effect of prophylactic mesh placement at the time of stoma formation, and at least on the basis of the results of the current large RCT, the standard use of prophylactic mesh reinforcement cannot be recommended.

Funnel plot of comparison 1. Lateral pararectal versus transrectal enterostomy placement, outcome 1.1. parastomal hernia. Only non‐randomized studies

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Trial flow diagram
RCT: randomized controlled trial; NRS: non‐randomized study

Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 1 parastomal hernia (RCT).

Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 2 parastomal hernia (NRS).

Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 3 stomal prolapse.

Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 4 ileus or stenosis.

Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 5 skin irritation.

Comparison 2 Subgroup analyses ‐ ileostomy versus colostomy, Outcome 1 parastomal hernia.
| Lateral pararectal versus transrectal enterostomy placement for prevention of parastomal herniation | ||||||
| Patient or population: people undergoing enterostomy placement for any reason | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Transrectal enterostomy placement | Lateral pararectal enterostomy placement | |||||
| parastomal hernia (NRS) clinical examination, CT scan, or botha Follow‐up: 2 to 240 months | Study population | RR 1.22 | 864 | ⊕⊝⊝⊝ | ||
| 295 per 1000 | 360 per 1000 | |||||
| Moderate | ||||||
| 348 per 1000 | 425 per 1000 | |||||
| parastomal hernia (RCT) clinical exam, sonography, intraoperatively, or combination, during stoma reversal Follow‐up: 0.5 to 21.4 months (15 to 642 days) | Study population | RR 1.34 | 56 | ⊕⊕⊝⊝ | ||
| 138 per 1000 | 185 per 1000 | |||||
| Moderate | ||||||
| 138 per 1000 | 185 per 1000 | |||||
| stomal prolapse clinical exam, documented on a standard pro forma Follow‐up: mean 110.4 months | Study population | RR 1.23 | 145 | ⊕⊝⊝⊝ | ||
| 78 per 1000 | 96 per 1000 | |||||
| Moderate | ||||||
| 78 per 1000 | 96 per 1000 | |||||
| ileus or stenosis clinical exam, sonography Follow‐up: 0.5 to 21.4 months (15 to 642 days) | Study population | RR 2 | 60 | ⊕⊕⊝⊝ | ||
| 33 per 1000 | 67 per 1000 | |||||
| Moderate | ||||||
| 33 per 1000 | 66 per 1000 | |||||
| skin irritation clinical exam, intraoperative exam, or both, during stoma reversal Follow‐up: 0.5 to 21.4 months (15 to 642 days) | Study population | RR 0.67 | 60 | ⊕⊕⊕⊝ | ||
| 200 per 1000 | 134 per 1000 | |||||
| Moderate | ||||||
| 200 per 1000 | 134 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aIn four studies the presence of parastomal hernia was assessed by clinical examination and CT scan, or CT scan alone. In the remaining studies, participants were clinically examined, e.g. in stoma therapy or outpatient clinic at follow‐up visits, and the findings were documented in the patient chart. | ||||||
| Representativeness of the experimental intervention cohort | Selection of the control cohort | Ascertainment of intervention | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow‐up long enough for outcomes to occur | Adequacy of follow‐up of cohorts | |
| ★ | ★ | ★ | ||||||
| ★ | ★ | ★ | ★ | |||||
| ★ | ★ | ★ | ★ | ★ | ★ | |||
| ★ | ★ | ★ | ★ | ★ | ||||
| ★ | ★ | ★ | ★ | ★ | ||||
| ★ | ★ | ★ | ★ | ★ | ||||
| ★ | ★ | ★ | ||||||
| ★ | ★ | ★ | ★ | ★ | ||||
| ★ | ★ | ★ | ★ | |||||
| ★ | ★ | ★ | ★ | ★ | ||||
| A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability. A maximum of nine stars can be awarded overall. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 parastomal hernia (RCT) Show forest plot | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.40, 4.48] |
| 2 parastomal hernia (NRS) Show forest plot | 10 | 864 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.75] |
| 3 stomal prolapse Show forest plot | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.39, 3.85] |
| 4 ileus or stenosis Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 20.90] |
| 5 skin irritation Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 parastomal hernia Show forest plot | 7 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.81, 1.65] |
| 1.1 ileostomy | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.21, 2.29] |
| 1.2 colostomy | 5 | 451 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.80, 2.11] |

