Primjena izotonične u odnosu na hipotonične otopine za intravensku hidraciju djece
Abstract
Background
Maintenance intravenous fluids are frequently used in hospitalised children who cannot maintain adequate hydration through enteral intake. Traditionally used hypotonic fluids have been associated with hyponatraemia and subsequent morbidity and mortality. Use of isotonic fluid has been proposed to reduce complications.
Objectives
To establish and compare the risk of hyponatraemia by systematically reviewing studies where isotonic is compared with hypotonic intravenous fluid for maintenance purposes in children.
Secondly, to compare the risk of hypernatraemia, the effect on mean serum sodium concentration and the rate of attributable adverse effects of both fluid types in children.
Search methods
We ran the search on 17 June 2013. We searched the Cochrane Injuries Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library), MEDLINE (OvidSP), Embase (OvidSP), and ISI Web of Science. We also searched clinical trials registers and screened reference lists. We updated this search in October 2014 but these results have not yet been incorporated.
Selection criteria
We included randomised controlled trials that compared isotonic versus hypotonic intravenous fluids for maintenance hydration in children.
Data collection and analysis
At least two authors assessed and extracted data for each trial. We presented dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (CIs) and continuous outcomes as mean differences with 95% CIs.
Main results
Ten studies met the inclusion criteria, with a total of 1106 patients. The majority of the studies were performed in surgical or intensive care populations (or both). There was considerable variation in the composition of intravenous fluid, particularly hypotonic fluid, used in the studies. There was a low risk of bias for most of the included studies. Ten studies provided data for our primary outcome, a total of 449 patients in the analysis received isotonic fluid, while 521 received hypotonic fluid. Those who received isotonic fluid had a substantially lower risk of hyponatraemia (17% versus 34%; RR 0.48; 95% CI 0.38 to 0.60, high quality evidence). It is unclear whether there is an increased risk of hypernatraemia when isotonic fluids are used (4% versus 3%; RR 1.24; 95% CI 0.65 to 2.38, nine studies, 937 participants, low quality evidence), although the absolute number of patients developing hypernatraemia was low. Most studies had safety restrictions included in their methodology, preventing detailed investigation of serious adverse events.
Authors' conclusions
Isotonic intravenous maintenance fluids with sodium concentrations similar to that of plasma reduce the risk of hyponatraemia when compared with hypotonic intravenous fluids. These results apply for the first 24 hours of administration in a wide group of primarily surgical paediatric patients with varying severities of illness.
PICOs
Laički sažetak
Jesu li bolje tekućine s manje ili više soli za intravensko davanje tekućine djeci
Dosadašnje spoznaje
Velikom broju djece u bolnici potrebno je davati tekućinu kroz intravenozne linije (ili "drip"), jer ne mogu ni jesti ni piti dovoljno, a što im je potrebno da ostanu hidrirani.Tekućina koja se djeci daje intravenski može izazvati rijetke, ali ozbiljne nuspojave uslijed smanjenja razine soli u tijelu. Kad se razina soli u tijelu naglo smanji, može se pojaviti oticanje mozga, što može dovesti do smrti.
Nije jasno koliko soli intravenozna tekućina mora sadržavati.
Istraživačko pitanje
Tradicionalno se daju tekućine koje sadrže niže razine soli od krvi (hipotonične). Ovaj Cochrane sustavni pregled uspoređuje te tekućine s tekućinom koja sadrži sličnu razinu soli kao i krv (izotonične tekućine). Autori su htjeli utvrditi koliko pacijenata je imalo nisku razinu soli u krvi kada se koriste izotonične tekućine u usporedbi s hipotoničnim tekućinama.
Ključni rezultati
Analizirana su istraživanja objavljena do 17. lipnja 2013. Uključeno je 10 studija u analizu, s ukupno 1.106 djece. Kad se koriste izotonične tekućine, manje je vjerojatno da će razina natrija u tijelu biti niska. Sto šezdeset i devet djece na 1000 su imali nisku razinu natrija u krvi kada su dobili izotoničnu tekućinu, u usporedbi s 338 djece na 1000, kada je korištena hipotonična tekućina. Rezultati za ozbiljne nuspojave povezane s izotoničnim ili hipotoničnim tekućinama su bili nejasni.
Ovaj pregled je uglavnom analizirao pacijenate koji su imali operaciju i/ili su bili na intenzivnoj njezi, a većina je trebala intravensku tekućinu tijekom manje od jednog dana.
Kvaliteta dokaza
Uključene studije su u pravilu dobro provedene i bile su visoke kvalitete.
Authors' conclusions
Summary of findings
| Isotonic intravenous fluid compared with hypotonic intravenous fluid to maintain hydration | ||||||
| Patient or population: children requiring intravenous fluid to maintain hydration Settings: inpatient hospital setting Intervention: isotonic intravenous fluid Comparison: hypotonic intravenous fluid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hypotonic intravenous fluid | Isotonic intravenous fluid | |||||
| Hyponatraemia (serum sodium < 135 mmol/L) | Study population | RR 0.48 (0.38 to 0.60) | 970 | ⊕⊕⊕⊕ | ||
| 338 per 1000 | 169 per 1000 | |||||
| Surgical patients | RR 0.48 (0.36 to 0.64) | 529 (7) | ⊕⊕⊕⊕ | |||
| 379 per 1000 | 185 per 1000 | |||||
| Medical patients | RR 0.29 (0.16 to 0.55) | 279 (4) | ⊕⊕⊕⊝ | |||
| 276 per 1000 | 83 per 1000 | |||||
| Intensive care patients | RR 0.48 (0.37 to 0.64) | 443 (5) | ⊕⊕⊕⊕ | |||
| 446 per 1000 | 217 per 1000 | |||||
| Non‐intensive care patients | RR 0.45 (0.29 to 0.68) | 359 (5) | ⊕⊕⊕⊝ | |||
| 312 per 1000 | 135 per 1000 | |||||
| Hypernatraemia | Study population | RR 1.24 (0.65 to 2.38) | 937 | ⊕⊕⊝⊝ | Quality of evidence downgraded due to imprecision ‐ small number of events, wide confidence interval | |
| 34 per 1000 | 37 per 1000 | |||||
| Death | Study population | 5.59 (0.23 to 135.17) | 996 | ⊕⊕⊝⊝ | Quality of evidence downgraded due to imprecision ‐ small number of events, wide confidence interval Study design reduced the likelihood of this outcome | |
| 0 per 1000 | 2 per 1000 | |||||
| Seizures | Study population | RR 0.62 (0.03 to 15.02) | 996 | ⊕⊕⊝⊝ | Quality of evidence downgraded due to imprecision ‐ small number of events, wide confidence interval Study design reduced the likelihood of this outcome | |
| 2 per 1000 | 0 per 1000 | |||||
| Cerebral oedema | Study population | RR incalculable | 9 studies | ⊕⊝⊝⊝ | Quality of evidence downgraded due to imprecision ‐ no events, incalculable confidence interval Study design reduced the likelihood of this outcome | |
| 0 per 1000 | 0 per 1000 | |||||
| Overhydration | Study population | RR 1.14 (0.46 to 2.87) | 615 | ⊕⊕⊝⊝ | Quality of evidence downgraded due to imprecision ‐ small number of events, wide confidence interval Heterogeneity in the criteria for assessing this outcome | |
| 26 per 1000 | 30 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Maintenance intravenous fluids are frequently used in hospitalised children who cannot maintain adequate hydration through enteral intake.
Traditionally, hypotonic fluids, containing approximately 30 to 50 mmol/L of sodium, have been prescribed for maintenance hydration. Fluid of this composition, prescribed at standard maintenance rates, provides approximately 2 to 4 mmol/kg of sodium each day. This is consistent with requirements described in a landmark paper published in 1957 examining maintenance fluid requirements in children (Holliday 1957). However, this fluid is markedly hypotonic when compared with plasma, which contains approximately 140 mmol/L of sodium. It has been postulated that this may lead to hyponatraemia and cerebral oedema, which has significant neurological morbidity.
There are a number of case series reporting deaths secondary to hyponatraemia in association with maintenance intravenous fluid (Arieff 1992; Halberthal 2001; Hoorn 2004; Hughes 1998; Koczmara 2010; Moritz 2005). It has been proposed that using an isotonic maintenance intravenous fluid may reduce complications secondary to hyponatraemia.
Description of the condition
Maintenance volumes of hypotonic fluid have previously been considered safe in most children due to the adaptive mechanisms of the kidney, which enable the excretion of excess free water and thus the maintenance of sodium balance. However, increased levels of circulating antidiuretic hormone are more common in hospitalised children than previously appreciated (Moritz 2003), decreasing their ability to excrete excess water and placing them at risk of hyponatraemia. Osmotic fluid shifts from the extracellular to intracellular space secondary to hyponatraemia can cause cerebral oedema, which can result in significant irreversible neurological morbidity and death.
Description of the intervention
When describing a fluid as hypotonic, isotonic or hypertonic, we are referring to the in vivo tonicity. Given that dextrose metabolises rapidly to free water, the in vivo tonicity of fluids containing dextrose differs from the in vitro tonicity or osmolarity. The in vitro osmolarity refers to the number of osmoles of solute per litre of solution, while the in vivo tonicity is the total concentration of solutes available to exert an osmotic force across the cell membrane.
In practice, an isotonic fluid is one containing a similar concentration of sodium to plasma, while a hypotonic fluid contains less sodium than plasma.
Maintenance volume refers to the fluid required to maintain adequate hydration in a child who is not eating and drinking but who is otherwise euvolaemic. It is the volume required for the kidneys to excrete excess solute load in an isotonic urine and replace insensible losses.
How the intervention might work
An isotonic fluid is considered physiologic as it has a similar sodium concentration to the extracellular space into which it is being administered. By using an isotonic rather than a hypotonic fluid, it is anticipated that there will be less likelihood of hyponatraemia and, therefore, the osmotic difference between the extracellular and intracellular spaces will be minimised. This should lessen the fluid shifts between compartments and reduce the risk of cerebral oedema.
While an isotonic fluid could still potentially result in hyponatraemia in the context of impaired urinary dilution, it is anticipated that the likelihood of this will be markedly diminished.
Why it is important to do this review
Intravenous fluid therapy is one of the most common interventions for hospitalised children. There is currently no clear consensus on the optimal composition of maintenance intravenous therapy, leading to wide practice variation (Davies 2008; Freeman 2012; Way 2006).
Children are still dying or suffering significant morbidity due to hyponatraemia associated with intravenous fluid administration. If an isotonic fluid is found to be superior in terms of clinically significant hyponatraemia, there will be a strong argument to shift routine maintenance fluid to the higher sodium‐containing solutions. This shift in the default for fluid therapy will alter therapy for millions of children worldwide, potentially saving lives and reducing morbidity.
Objectives
To establish and compare the risk of hyponatraemia by systematically reviewing studies where isotonic is compared with hypotonic intravenous fluid for maintenance purposes in children.
Secondly, to compare the risk of hypernatraemia, the effect on mean serum sodium concentration and the rate of attributable adverse effects of both fluid types in children.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that compared isotonic or near isotonic (sodium ≥ 125 to 160 mmol/L) versus hypotonic (sodium < 125 mmol/L) intravenous fluids for maintenance hydration in children.
Blinding was not a requisite for inclusion.
Types of participants
We included trials where the majority of participants were children (aged three months to 18 years) who required intravenous fluids for maintenance hydration.
We did not include or exclude studies on the basis of any specific medical diagnoses examined.
Types of interventions
The intervention group were patients who received isotonic or near isotonic fluid (a fluid with a sodium concentration approximately equal to that of human plasma). The comparison group were patients who received hypotonic fluid (a fluid with a sodium concentration less than that of human plasma).
For the purposes of the review, we considered fluids with a sodium concentration ≥ 125 to 160 mmol/L isotonic or near isotonic, while we considered those with a sodium concentration < 125 mmol/L hypotonic. When determining these ranges, we took into account the normal serum sodium range (135 to 145 mmol/L) and the sodium concentration of commercially available, commonly used fluids (see Table 1 ‐ 'Common commercially available intravenous fluids').
|
| Na+ (mmol/L) | Cl‐ (mmol/L) | K+ (mmol/L) | Mg++ (mmol/L) | Calcium (mmol/L) | Lactate (mmol/L) | Acetate (mmol/L) | Gluconate (mmol/L) | Glucose (gram/L) |
| Physiologically isotonic/near isotonic | |||||||||
| 0.9% sodium chloride | 154 | 154 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| 0.9% sodium chloride with 2.5/5% dextrose | 154 | 154 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 25 / 50 |
| Hartmann's solution (similar in ionic composition to Ringer's lactate) | 131 | 111 | 5 | ‐ | 2 | 29 | ‐ | ‐ | ‐ |
| Plasmalyte 148 solution | 140 | 98 | 5 | 1.5 | ‐ | ‐ | 27 | 23 | ‐ |
| Plasmalyte 148 solution with 5% dextrose | 140 | 98 | 5 | 1.5 | ‐ | ‐ | 27 | 23 | 50 |
| Physiologically moderately hypotonic | |||||||||
| 0.45% sodium chloride (N/2) with 5% dextrose | 77 | 77 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 50 |
| Physiologically very hypotonic | |||||||||
| 0.3% sodium chloride (N/3) with 3.3% dextrose | 51 | 51 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 33 |
| 0.18% sodium chloride (N/5) with 4% dextrose | 30 | 30 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 40 |
Note: Minor variations in composition occur at the point of manufacture.
We only included studies where the fluid was primarily administered for maintenance hydration (that is, not for resuscitation purposes or to replace a pre‐existing deficit).
Types of outcome measures
Primary outcomes
The primary outcome was the proportion of participants in each treatment group with hyponatraemia (serum sodium < 135 mmol/L) at any time point while receiving intravenous fluids.
Each participant was counted only once, despite the number of hyponatraemic events he or she had.
Secondary outcomes
Other outcomes of interest were:
-
the proportion of participants in each treatment group who developed hypernatraemia (serum sodium > 145 mmol/L) while receiving intravenous fluids;
-
mean serum sodium;
-
adverse clinical effects including:
-
death;
-
seizures;
-
cerebral oedema;
-
overhydration (author defined clinical assessment);
-
-
antidiuretic hormone levels.
-
urinary osmolarity and electrolytes.
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by language, date or publication status.
Electronic searches
We searched the following:
-
Cochrane Injuries Group Specialised Register (9 May 2013);
-
Cochrane Central Register of Controlled Trials (CENTRAL,The Cochrane Library, issue 4 of 12 2013);
-
MEDLINE (OvidSP) (1946 to June, week 4 2013);
-
Embase (OvidSP) (1974 to 2013 week 27);
-
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to 17 June 2013);
-
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to 17 June 2013).
Search strategies are reported in (Appendix 1). We adapted the MEDLINE search strategy as necessary for each of the other databases: the added study filter is the Ovid MEDLINE Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011); we added some of the search strategy study design terms as used by the UK Cochrane Centre (Lefebvre 2011) to the Embase Strategy.
We performed a further search in October 2014. We added three studies to Characteristics of studies awaiting classification and we will incorporate them into the review at the next update.
Searching other resources
To identify unpublished studies and those in progress, we searched the following trials registers:
-
ClinicalTrials.gov (www.clinicaltrials.gov);
-
Current Controlled Trials (www.controlled‐trials.com);
-
Australia New Zealand Clinical Trials Registry (http://www.anzctr.org.au/);
-
Clinical Trial Results (www.clinicaltrialresults.org);
-
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en).
We examined the reference lists of all eligible trials and relevant systematic reviews to identify any further trials that may have been missed by the electronic searches.
Data collection and analysis
Assistance with running the search and collating results was provided by the Cochrane Injuries Group's Trials Search Co‐ordinator.
Selection of studies
Two review authors (McNab and Dorofaeff) screened the titles and abstracts of all trials identified through the search for potential inclusion on the basis of study design, intervention and participants. Following this, two authors examined in further detail the full text of potentially eligible studies (McNab reviewed each, with each additional author reviewing one to two studies) to determine which trials met the full inclusion criteria. We resolved any uncertainty or discrepancy through discussion.
Data extraction and management
Two authors independently performed data extraction for each study and recorded this information on a data extraction form. We resolved any difference of opinion by discussion. We contacted the original study authors regarding missing data or data queries.
Assessment of risk of bias in included studies
We recorded the following information for all included studies:
-
the method of randomisation sequence generation;
-
the method of allocation concealment;
-
whether the treatment allocation was blinded/not blinded and to whom (participants, clinicians, outcome assessors);
-
whether there was incomplete outcome data and whether withdrawals and drop‐outs were described;
-
whether all participants were analysed using the intention‐to‐treat principle.
We applied a judgement to each of these domains as to whether there was a low, high or unclear risk of bias. This was based on guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We presented dichotomous outcomes as summary risk ratios with 95% confidence intervals. Whenever there were no events for one treatment group, we added 0.5 to each cell to allow the calculation of effect estimates, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions. For continuous outcomes, we calculated mean differences for individual studies and also for the pooled estimates. We presented these with 95% confidence intervals.
Unit of analysis issues
In many circumstances, repeated observations were performed on each participant (e.g. serum sodium could be tested at multiple time points). In addition, differently designed studies measured observations at different time points to each other: for example, one study measured serum electrolytes after 12 hours of fluid therapy (Saba 2011), while another measured after six hours and then 24 hours of fluid therapy (Montañana 2008).
The primary outcome for the systematic review was hyponatraemia at any time point while maintenance intravenous fluids were being administered. This allows for repeated observations. Where hyponatraemia was noted in the same participant on multiple occasions, we analysed the observation only once. In some studies it was unclear whether the same patient was hyponatraemic at multiple time points or whether a new participant had become hyponatraemic. In this situation, we contacted the authors to ascertain this information. If additional information was not available, we only included the outcome data collected at the first time point in the primary analysis.
For continuous outcomes (e.g. mean serum sodium), there may be multiple observations for the same outcome, or the same outcome may be recorded at different time points in different studies, or both. To account for this, we studied arbitrary time 'blocks'. The time blocks for continuous outcomes were:
-
6 to 12 hours; and
-
> 12 to 24 hours.
Where fluid composition was investigated as part of a larger factorial trial, we combined the summary estimates of the main effect for fluid type whenever there was no interaction between the fluid type and the other intervention being investigated. For three‐armed trials, we combined arms with the same fluid composition, regardless of the fluid rate being administered. We performed a sensitivity analysis, including only arms where the administered rate was balanced.
Dealing with missing data
Where data were missing for trials which met the inclusion criteria, we contacted the trial authors. Where data had not been collected for a study or were not available, we only analysed the available data. We did not impute missing data for drop‐outs.
Assessment of heterogeneity
We performed a meta‐analysis of all trials meeting the inclusion criteria using a fixed‐effect model. We used the I2 statistic to detect significant levels of heterogeneity.
Assessment of reporting biases
We searched trial registries and contacted the investigators of unpublished, registered trials.
Data synthesis
We used Review Manager software to carry out the statistical analysis (RevMan 2011). We used a fixed‐effect model of meta‐analysis to estimate the combination of intervention effects across the studies.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses on the primary outcome to investigate the effects of the following:
-
The sodium concentration of the hypotonic fluid. The sodium concentration can differ markedly in fluids considered to be hypotonic. We performed a subgroup analysis, grouping the hypotonic fluids according to sodium concentration:
-
< 70 mmol/L sodium;
-
≥ 70 mmol/L and < 125 mmol/L sodium.
-
-
Surgical patients versus medical patients.
-
Fluid rate:
-
maintenance rate (80% to 120% of standard maintenance rate as defined by Holliday 1957;
-
restricted rate ≤ 70% of standard maintenance rate.
-
-
Age:
-
less than one year old;
-
one to five years old;
-
five to 18 years old.
-
-
Severity of illness:
-
intensive/critical care unit patients;
-
non‐intensive/critical care unit patients.
-
Sensitivity analysis
We performed a sensitivity analysis excluding studies where the intervention and control groups were not balanced for all factors other than fluid composition. We performed a further sensitivity analysis excluding studies that randomised patients who were hyponatraemic at baseline. We planned neither of these sensitivity analyses a priori.
Results
Description of studies
Results of the search
The initial search of titles and abstracts revealed 14 potentially eligible studies, for which we obtained the full text (Figure 1). One study, written in Turkish, required full translation (Dağli 1997); after this was obtained it became clear that the study predominantly examined intraoperative fluid replacement rather than maintenance hydration. We therefore excluded this study (see Excluded studies).

Study flow diagram.
We also excluded a further study examining intravenous fluid composition in gastroenteritis after it was established that the fluid was given predominantly to replace a pre‐existing deficit at rates that far exceeded standard maintenance hydration (Neville 2006).
One title also not included was published only in protocol form (Flaring 2011). This three‐armed design aimed to compare 40, 70 and 140 mmol/L of sodium in 51 children with complicated appendicitis. Contact with the author revealed that the study had been prematurely ceased due to low recruitment, with outcome data on only one participant.
One study was available only in abstract form (conference presentation Ang 2010). This study of 19 children admitted to an Emergency Department in the Philippines compared 140 mmol/L of sodium with 40 mmol/L of sodium. Without the full details of the study available, insufficient information was available for inclusion in the meta‐analysis. A further study was also published in abstract form (conference presentation Cuello 2012). However, contact with the author provided additional information to allow inclusion in this review.
We searched databases of registered protocols for potentially eligible studies in progress. This revealed a further five studies. Three studies (McNab 2014; Baron 2013; Pemde 2014) have since been published and will be incorporated into the review at the next update. Additional information is not available regarding the remaining two studies (CTRI/2010/091/000398; NCT00632775).
Following the search process, we deemed 10 studies eligible for inclusion.
We have added three study reports from an updated search in October 2014 to Studies awaiting classification and will incorporate these in the next update.
Included studies
Ten studies were eligible for inclusion (Characteristics of included studies; Table 2). We made contact with all authors to collect additional information. All authors assisted with this request.
| Study | Number of participants | Population | Study arms | Primary outcome |
| 12 | Patients undergoing primary corrective surgery for idiopathic scoliosis | Isotonic (Hartmann's) Hypotonic (either 0.3% saline + 3% dextrose or 0.18% saline + 4% dextrose) Rate: 1.5 ml/kg/hr | Development of SIADH | |
| 122 | PICU patients | Isotonic fluid (sodium 140 mEq/L) Hypotonic fluid (sodium between 20 and 100 mEq/L corresponding to 2 to 4 mEq/kg/24 hr) Exact composition of fluids not stated Rate: standard maintenance | Hyponatraemia < 135 mEq/L | |
| 50 | PICU patients | 0.9% saline at standard maintenance rate 0.9% saline at 2/3 restricted rate 0.18% saline + 4% dextrose at standard maintenance rate 0.18% saline + 4% dextrose at 2/3 restricted rate | Change in plasma sodium from admission to 12 to 24 hrs later | |
| 167 | Broad paediatric population (university hospital in India) | 0.9% saline + 5% dextrose at standard maintenance rate 0.18% saline + 5% dextrose at standard maintenance rate 0.18% saline + 5% dextrose at 2/3 restricted rate | Hyponatraemia < 130mEq/L | |
| 124 | Elective or emergency surgery | 0.9% saline + 2.5% dextrose at standard maintenance rate 0.9% saline + 5% dextrose at 50% restricted rate 0.45% saline + 2.5% dextrose at standard maintenance rate 0.45% saline + 5% dextrose at 50% restricted rate | Change in plasma sodium from induction of anaesthesia to T8 | |
| 125 | PICU patients | Isotonic (sodium 136 mmol/L) Hypotonic (sodium 30 to 50 mmol/L) Exact composition not stated Rate: standard maintenance | Change in plasma sodium from admission to 12 and 24 hrs later | |
| 258 | Surgical patients | 0.9% saline + 5% dextrose 0.45% saline + 5% dextrose Rate: Physician's discretion | Hyponatraemia < 135 mmol/L | |
| 37 | Medical patients admitted via ED Elective surgical patients | 0.9% saline + 5% dextrose 0.45% saline + 5% dextrose Rate: physician's discretion | Rate of change of sodium | |
| 82 | Patients undergoing spinal instrumentation, craniotomy for brain tumour resection or cranial vault remodelling | Hartmann's + 5% dextrose at full maintenance rate 0.45% saline + 5% dextrose at 2/3 restricted rate | Mean plasma sodium 16 to 18 hrs postoperatively | |
| (abstract only) | 72 | Participants with either of 2 conditions: a) gastroenteritis with moderate dehydration and unable to tolerate fluids b) children requiring non‐urgent surgery and requiring maintenance intravenous hydration during their admission | 0.9% saline + 5% dextrose +/‐ 20 mmol/L KCl 0.2% saline + 5% dextrose +/‐ 20 mmol/L KCl Rate: standard maintenance | Mean plasma sodium at 4 (T4) and 8 (T8) hours, and the percentage of patients who developed hyponatraemia (> 125 mEq/L and < 135 mEq/L) |
ED: emergency department
hr: hour
KCl: potassium choride
PICU: paediatric intensive care unit
SIADH: syndrome of inappropriate antidiuretic hormone secretion
Trial design characteristics
Composition and rate of intravenous fluid administered
There were significant clinical differences between studies regarding the composition of intravenous fluid administered (Table 2). As stipulated in our inclusion criteria, all used an isotonic fluid as a comparator, although this varied between Hartmann's solution (Brazel 1996), Hartmann's solution with 5% dextrose (Coulthard 2012), 0.9% sodium chloride (Yung 2009), and 0.9% sodium chloride with 5% dextrose (Choong 2011; Cuello 2012; Kannan 2010; Saba 2011). One study used both 0.9% sodium chloride with 2.5% dextrose and 0.9% sodium chloride with 5% dextrose (Neville 2010). Two studies, Montañana 2008 and Rey 2011, used unnamed solutions containing 140 mEq/L of sodium and 136 mmol/L of sodium, respectively.
There was further, and arguably more significant, heterogeneity regarding the hypotonic intervention fluid used by each study. Yung 2009 used 0.18% sodium chloride with 4% dextrose, while Kannan 2010 added slightly more dextrose (0.18% sodium chloride with 5% dextrose). Cuello 2012 used a similar fluid (0.2% sodium chloride with 5% dextrose). Brazel 1996 used both 0.3% sodium chloride with 3% dextrose and 0.18% sodium chloride with 4% dextrose for its hypotonic intervention fluid. Three studies used 0.45% sodium chloride with 5% dextrose (Choong 2011; Coulthard 2012; Saba 2011). Neville 2010 again used two fluids with differing dextrose concentrations (0.45% sodium chloride with 2.5% dextrose and 0.45% sodium chloride with 5% dextrose). Montañana 2008 and Rey 2011 again used unnamed solutions. Montañana's composition differed depending on the participant's weight, but contained a sodium concentration of between 20 and 100 mEq/L corresponding to 2 to 4 mEq/kg/24 hours. Rey's hypotonic fluid contained a sodium between 30 mmol/L and 50 mmol/L.
The rates at which fluid was administered differed between studies (Table 2). Choong 2011 and Saba 2011 both left the rate to the treating physician's discretion, while Cuello 2012, Montañana 2008 and Rey 2011 stipulated that the rate should be prescribed according to Holliday and Segar's formula (Holliday 1957). The patients in the Brazel 1996 study all received fluid at 1.5 ml/kg/hour. Neville 2010 and Yung 2009 both conducted four‐armed studies: Neville randomised the isotonic and hypotonic groups to 100% or 50% maintenance rates, while Yung randomised them to full or 2/3 maintenance rates. The three‐armed study, Kannan 2010, gave isotonic fluid at full maintenance rates and hypotonic fluid at either maintenance or 2/3 maintenance rates. In the study conducted by Coulthard 2012, patients in the isotonic arm received full maintenance rates, while those in the hypotonic arm received 2/3 maintenance rates.
Duration of fluid therapy and timing of outcome measurements
The duration of fluid therapy and timing of outcome measurements differed between studies (Table 3). As previously discussed, our primary outcome was hyponatraemia at any time point, which allowed for clinical heterogeneity in this area.
| First author | Duration of fluid therapy | Timing of outcome measurements |
| Brazel | Max 72 hrs | End of procedure, T6, T24, T48, T72
|
| Montañana | Max 24 hrs | T6, T24 (only T6 primary outcome data included in analysis) |
| Yung | 12 to 24 hrs | T12 to 24 |
| Kannan | Max 72 hrs | T12, T24, T36, T48, T60, T72
|
| Neville | Max 24 hrs | At intubation, T8, T24 |
| Rey | Max 24 hrs | T12, T24 |
| Choong | Max 48 hrs | T12, T24, T36, T48 |
| Saba | 8 to 12 hrs | T12 |
| Coulthard | 16 to 18 hrs | T16 to 18 |
| Cuello | 8 hrs | T4, T8 |
hr: hour
Baseline characteristics of participants
Setting
All studies were performed in tertiary paediatric hospitals, with the exception of Kannan 2010, which was performed in a general (adult and paediatric) university hospital and Rey 2011, which was conducted across three university hospitals. Four studies were conducted in Australia (Brazel 1996; Coulthard 2012; Neville 2010; Yung 2009), two studies occurred in both Spain (Montañana 2008; Rey 2011) and Canada (Choong 2011; Saba 2011), while the remaining studies took place in India (Kannan 2010) and Mexico (Cuello 2012).
Inclusion and exclusion criteria
The inclusion and exclusion criteria for the included studies are outlined in Table 4.
| Primary author | Inclusion criteria | Exclusion criteria |
| Brazel | Adolescent patients undergoing primary corrective surgery for adolescent idiopathic scoliosis
| — |
| Montañana | PICU patients requiring maintenance intravenous fluid
| Chronic or acute kidney failure Patients at risk of cerebral oedema (diabetic ketoacidosis or craneoencephalic trauma) Patients with plasma sodium level at admission < 130 mEq/L or > 150 mEq/L, and/or dehydration > 5% of the patient's body weight
|
| Yung | Patients admitted to PICU who would normally require IV fluids at standard maintenance rates for 12 hrs, with normal sodium levels and not hypoglycaemic
| Neonates, diabetes, renal failure, shock Cardiac and neurosurgical patients were eligible for the restricted rate arm only |
| Kannan | Patients aged between 3 months and 12 years requiring IV maintenance fluid administration for at least 24 hrs
| Na < 130mEq/L, Na > 150mEq/L, blood glucose > 180 mg/dL, dehydration, shock, severe malnutrition, cirrhosis of liver, congestive heart failure, acute or chronic renal failure and nephrotic syndrome Patients receiving drugs that may alter plasma sodium levels Patients requiring fluid boluses for volume depletion and/or shock |
| Neville | Patients undergoing elective or emergency surgery, expected to take nothing by mouth after surgery for at least 8 hrs. Weight > 8kg | Significant blood loss expected during surgery Surgery types known to be associated with excess ADH secretion (cranial and thoracic surgery) Known abnormality of ADH secretion, nephrogenic diabetes insipidus, pituitary or hypothalamic disease, kidney disease, acute or chronic lung disease Patients receiving drugs known to stimulate ADH secretion |
| Rey | PICU patients requiring maintenance IV fluids
| Impairment in body water homeostasis (e.g. congestive heart failure) Electrolytic alterations requiring a different IV fluid than that in the study Renal function abnormalities Patients requiring fluid restriction |
| Choong | Euvolaemic patients within 6 hours after elective surgery if anticipated need for IV maintenance was > 24 hours
| Uncorrected plasma sodium level abnormalities before the end of surgery Patients with known abnormalities of ADH secretion Patients requiring volume resuscitation and/or vasoactive infusions Recent loop diuretic use Total parenteral nutrition required with 24 hours following surgery Patients for whom either a hypotonic or isotonic Isotonic fluid was considered necessary or contraindicated (e.g. because of a risk of cerebral oedema, acute burns or the risk of third space and/or sodium overload in patients with pre‐existing congestive cardiac failure, renal failure, liver failure or cirrhosis) |
| Saba | Patients requiring at least 8 hours of IV fluids
| Baseline Na of < 133 or > 145 mmol/L Patients with any of renal disease, cardiac dysfunction, pre‐existing hypertension, diuretic use, oedema, known adrenal dysfunction, acute or severe chronic neurological illness |
| Coulthard | Patients admitted to PICU following spinal instrumentation for correction of scoliosis, craniotomy for excision of brain tumours and cranial vault remodelling | Lengthening only of spinal instrumentation rods, insertion or revision of ventriculoperitoneal shunts, intracerebral cyst fenestration or previously enrolled |
| Cuello | Patients 6 months to 14 years old; with a serum sodium level between 125 mmol/L to 150 mmol/L; previously healthy, admitted to the emergency room or hospitalisation ward with any of 2 conditions: a) gastroenteritis with moderate dehydration and unable to drink fluids; b) children undergoing non‐urgent surgery and requiring maintenance IV hydration during their hospitalisation (i.e. non‐incarcerated hernia, adenotonsillectomies, tympanostomy, fracture reductions of the extremities, etc.) | Chronic illnesses (e.g. cystic fibrosis, cerebral palsy, etc.); taking antidiuretics; major trauma that required intensive care; hyper or hyponatraemia at admission; severe dehydration |
ADH: antidiuretic hormone secretion
hr: hour
IV: intravenous
PICU: paediatric intensive care unit
Four studies were performed only in surgical populations. Of these, Choong 2011 recruited patients undergoing elective surgery, while Neville 2010 recruited patients requiring either elective or emergency surgery. Patients in Coulthard 2012 were those admitted to the paediatric intensive care unit (PICU) following spinal instrumentation or craniotomies, while Brazel 1996 included only those who underwent primary corrective surgery for adolescent idiopathic scoliosis. Four studies were performed exclusively in PICU populations (Coulthard 2012; Montañana 2008; Rey 2011; Yung 2009). Cuello 2012 included two patient groups: those admitted with gastroenteritis or those undergoing non‐urgent surgery. Kannan 2010 and Saba 2011 did not restrict recruitment on the basis of surgery or PICU requirements.
The exclusion criteria varied between studies; in general, patients were excluded if isotonic or hypotonic fluids were considered to be contraindicated. This was usually where an underlying diagnosis or medication affected water and electrolyte homeostasis. Cuello 2012 also excluded patients with chronic illnesses.
Studied outcomes
The primary outcomes for all included studies are listed in Table 2. There was significant variation between studies regarding the chosen primary outcome.
Three studies elected to use hyponatraemia as their primary outcome (Choong 2011; Kannan 2010; Montañana 2008), although the definitions of hyponatraemia differed (< 135 mEq/L for the former two and < 130 mEq/L for Kannan's study).
Three studies reported change in plasma sodium as their primary outcome (Neville 2010; Rey 2011; Yung 2009). Two of these elected the time frame for change to be from admission to 12 to 24 hours later, while one examined change in sodium from induction of anaesthesia to eight hours postoperatively.
The remaining studies had unique primary outcomes; Brazel 1996 reported the development of syndrome of inappropriate antidiuretic hormone secretion (SIADH), defining this on the basis of serum sodium, serum osmolarity and urine osmolarity; Saba 2011 reported rate of change of sodium; and Coulthard 2012 reported mean plasma sodium 16 to 18 hours postoperatively, while Cuello 2012 described mean plasma sodium after four and eight hours of treatment, as well as the percentage of patients who developed hyponatraemia.
All 10 studies could provide data for the primary outcome for this systematic review (hyponatraemia of < 135 mmol/L at any time point during the study).
Excluded studies
See Characteristics of excluded studies.
We examined the full text of two studies in detail prior to exclusion. Neville 2006 performed a study comparing 0.9% sodium chloride + 2.5% dextrose with 0.45% sodium chloride + 2.5% dextrose in children with a presumed diagnosis of gastroenteritis. The rate of fluid replacement was determined by the treating clinician and followed one of two clinical protocols: 10 ml/kg/hour for four hours or maintenance rate plus estimated dehydration replaced over 24 hours. Of note, 76% of patients were administered fluid using the former protocol, which is at least 250% of standard maintenance rates. As such, the patients were not given intravenous fluid for the primary purpose of maintenance hydration but, rather, to replace a pre‐existing deficit.
The study by Dağli 1997 was similarly examined in detail, including obtaining a Turkish translation. Children undergoing surgery were randomised to receive one of three different fluid compositions intraoperatively: Ringer's lactate, Ringer's lactate + 1% dextrose or 0.3% sodium chloride + 3.3% dextrose. However, the rate administered once again indicated that fluids were given for purposes other than maintenance hydration. Based on the patient's age, fluids were given at either 25 ml/kg/hour or 15 ml/kg/hour for the first hour, followed by 6 ml/kg/hour. Almost all the treatment periods were less than an hour, meaning the rates received were well in excess of accepted maintenance.
One excluded study was published only in protocol form (Flaring 2011). This three‐armed design aimed to compare 40, 70 and 140 mmol/L of sodium in 51 children with complicated appendicitis. Contact with the author revealed that the study had been prematurely ceased due to low recruitment, with outcome data on only one participant.
A conference presentation (Ang 2010) was published only in abstract form and was also excluded. This study of 19 children admitted to an Emergency Department in the Philippines compared 140 mmol/L of sodium with 40 mmol/L of sodium. Without the full details of the study available, insufficient information was available for inclusion in the review.
Risk of bias in included studies
Allocation
Brazel 1996, the first quasi‐randomised controlled trial to compare hypotonic and isotonic fluid, had a potential selection bias as there was no random sequence generation or allocation concealment. Consecutive patients were given alternating solutions. The rest of the studies all had a low risk of selection bias.
Blinding
Choong 2011, Saba 2011 and Yung 2009 conducted studies with the participants, treating team and research team all blinded to the fluid type. Choong also blinded the treating team to the primary outcome measure. While the other studies did not blind their intervention fluids to the treating team, this still presents a relatively low risk of bias. Our primary outcome measure is objective (serum sodium) and all studies stipulated times for bloods to be taken. However, if additional blood tests were taken at non‐specified time points, these results were also included in our meta‐analysis, which could introduce a performance bias. That is, if a clinician is concerned because a patient is receiving a certain composition of fluid, they may request additional testing, increasing the likelihood of reaching our primary outcome.
Incomplete outcome data
With the exception of Saba's small study (Saba 2011), which had a moderate number of drop‐outs, there was reasonable retention of patients (between 83% and 100%). Saba's study enrolled 25 patients in each intervention arm. However, only 16 (64%) and 21 (84%) patients in each arm completed the study.
Selective reporting
While not all studies had registered protocols, all eligible studies were able to provide data for the primary outcome of the meta‐analysis, making reporting bias unlikely.
Other potential sources of bias
A systematic bias may have been introduced in the measurement of the serum sodium levels. While Choong 2011 described an additional intravenous line being placed in the majority of her patients specifically for blood sampling and Kannan 2010, Coulthard 2012 and Cuello 2012 also placed a separate line for blood sampling (personal communication), the remaining studies did not describe how the blood samples were obtained. If the samples were removed from the same intravenous line into which study fluid was being administered, there is potential for contamination. Even a small volume of contamination from a hypotonic fluid could result in a false positive primary outcome result. This could markedly influence the results of the meta‐analysis.
Effects of interventions
See: Summary of findings for the main comparison
Hyponatraemia
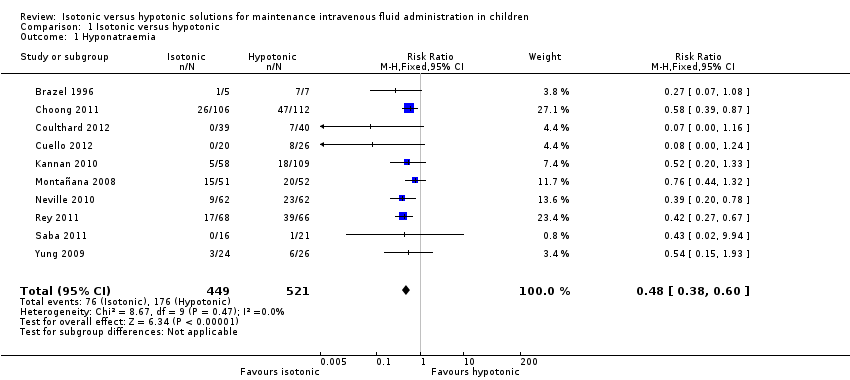
The primary outcome for the systematic review was hyponatraemia (< 135 mmol/L) occurring at any time point during the study. This information was available for all eligible studies and is presented in Analysis 1.1. When examining the data from eligible studies, isotonic fluid appears to halve the risk of hyponatraemia (risk ratio (RR) 0.48; 95% confidence interval (CI) 0.38 to 0.60).
We only used limited data for Montañana 2008. This study presented the incidence of hyponatraemia at specific time points (six hours and 24 hours after commencing fluid). However, given that the same patient could be counted on more than one occasion, the overall rate of hyponatraemia during the study was indeterminable. Given this, we only included data obtained up until the first time point (T6) in our primary analysis to avoid possible duplication. We chose the first time point as, by the second time point, there was relatively large attrition and patients could potentially have been withdrawn unequally due to earlier hyponatraemia.
Cuello 2012 provided hyponatraemic data only for patients who were normonatraemic at baseline; patients who remained hyponatraemic were not included in this analysis.
All data were available and used for the remaining studies. There was little statistical heterogeneity between the groups: I2 = 0%. A total of 449 patients received isotonic fluid, while 521 received hypotonic fluid, with 76 and 176 primary outcome events occurring in each group respectively.
Hypernatraemia
Whether isotonic fluid increases the risk of hypernatraemia (> 145 mmol/L) remains unclear (see Analysis 1.2), with hypernatraemia being an uncommon event in the meta‐analysis.
Data from nine studies were available for inclusion with Montañana's data again only included until the patient had received six hours of intravenous fluid. In total, 16 of the 437 patients receiving isotonic fluid and 17 of the 500 patients receiving hypotonic fluid developed hypernatraemia. A broad confidence interval of 0.65 to 2.38 makes it difficult to determine whether there is an increased risk of hypernatraemia with isotonic fluid. The confidence intervals of three individual studies were exceptionally large (Neville 2010; Rey 2011; Saba 2011), while risk ratios were not calculable for a further three studies as they had no episodes of hypernatraemia (Brazel 1996; Coulthard 2012; Cuello 2012).
The large study performed by Choong 2011 in a post‐surgical population, as well as the three‐armed study by Kannan 2010, suggest that there is unlikely to be a clinically significant difference in rates of hypernatraemia between groups, however further large studies, currently underway (Table 2), will provide more information regarding this.
Mean serum sodium
Mean serum sodium was examined following six to 12 hours and > 12 to 24 hours of intravenous fluid therapy (Analysis 1.3; Analysis 1.4). No study examined serum sodium on more than one occasion within these time blocks. The mean serum sodium was lower in the hypotonic group at both time points.
Seven studies had data included in the six to 12‐hour analysis; for one study (Montañana 2008), the blood samples were taken six hours after starting intravenous fluid, Neville 2010 and Cuello 2012 collected samples at after eight hours, and four studies had blood samples collected 12 hours after starting intravenous fluid (Choong 2011; Kannan 2010; Rey 2011; Saba 2011). The mean serum sodium was lower for those receiving hypotonic fluid, with a mean difference between treatment groups of 1.99 mmol/L (95% CI 1.55 to 2.42). Only one study reported a low overall mean serum sodium for patients receiving hypotonic fluid (Rey 2011: 133.7 mmol/L). No study reported an overall mean serum sodium in the hypernatraemic range for children receiving hypotonic or isotonic fluid.
Six studies had data available for inclusion in the > 12 to 24‐hour analysis (Choong 2011; Coulthard 2012; Kannan 2010; Montañana 2008; Neville 2010; Rey 2011). For Coulthard's study, the bloods were collected between 16 and 18 hours after commencing intravenous fluid, while Choong's were collected the morning of postoperative day two. The remaining studies collected samples after 24 hours of treatment. The mean difference between treatment groups was less than the earlier time point, being 1.33 mmol/L (95% CI 0.81 to 1.85). Once again, after 24 hours of intravenous fluid, the overall mean serum sodium was only in the low range (134.2 mmol/L) for those receiving hypotonic fluids in Rey 2011.
Adverse events
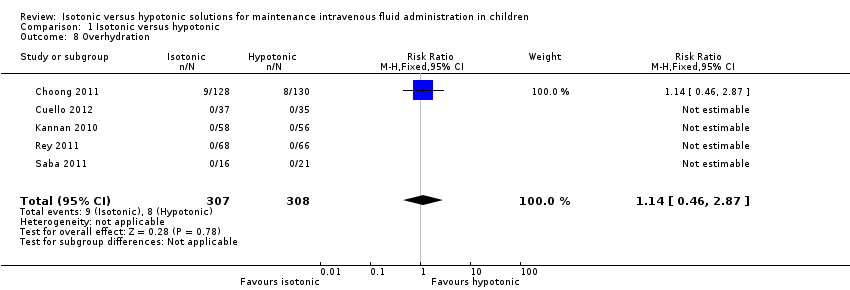
We examined death, seizures, cerebral oedema and overhydration as adverse events (Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8). Out of 996 participants there was one death, one seizure and there were no episodes of cerebral oedema, making these very rare events within the studies. The post‐surgical study by Choong 2011 was the only study to record any episodes of overhydration.
We did not find a statistically significant difference for any adverse event. However, particularly for seizures (RR 0.62; 95% CI 0.03 to 15.02) and death (RR 5.59; 95% CI 0.23 to 135.17), broad confidence intervals indicate that, despite the meta‐analysis, the power was insufficient to detect potential differences. We found no difference in clinical episodes of overhydration (RR 1.14; 95% CI 0.46 to 2.87). As no episodes of cerebral oedema were reported in any study, risk ratios were incalculable for this rare outcome.
It is notable that most studies had safety restrictions built into their methodology to ensure that high‐risk patients were either excluded or withdrawn from the study prior to serious adverse events occurring.
Antidiuretic hormone, urine osmolality and urine electrolytes
Choong 2011, Kannan 2010 and Neville 2010 all reported serum antidiuretic hormone (ADH) levels. The data were not normally distributed, so we did not carry out meta‐analysis. However, in each study there was no statistically significant difference between the hypotonic and isotonic groups. ADH was frequently elevated to a level above that normally associated with maximal antidiuresis (3 to 5 pg/ml). This may explain why some patients are unable to excrete effectively free water and develop hyponatraemia in the presence of free water administration.
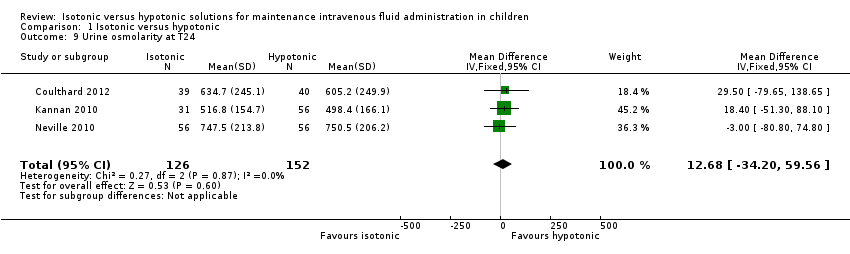
Three studies performed urine osmolality testing after 24 hours of intravenous fluid, with no difference in urine osmolality between the treatment groups (Coulthard 2012; Kannan 2010; Neville 2010) (Analysis 1.9). Four additional studies also provided information on urine osmolality: Cuello 2012 performed urine osmolality after eight hours of treatment and found no significant difference between treatment groups; Brazel 1996 presented data only in a box plot format, but again found no significant difference; Yung 2009 provided only change in osmolality data and Montañana 2008 presented non‐normally distributed data after six hours of intravenous fluid.
There were unsurprising differences in the urinary sodium concentration between treatment groups after 24 hours of treatment (Analysis 1.10) (Choong 2011; Coulthard 2012; Kannan 2010; Neville 2010). Again, further studies provided data that were not included in the meta‐analysis: Cuello 2012 provided data after eight hours of intravenous fluid and found no significant difference between treatment groups; Yung 2009 described change in urinary sodium, while Montañana 2008 again presented data at six hours of treatment, which were not normally distributed. Urine biochemistry is difficult to interpret in the context of a meta‐analysis, as it is more relevant for individual patients and dependent on the hydration status, serum biochemistry and fluid received. It would be interesting, although not possible within the meta‐analysis, to evaluate the urine biochemistry for specific hyponatraemic patients.
Subgroup analyses
Sodium concentration of hypotonic fluid
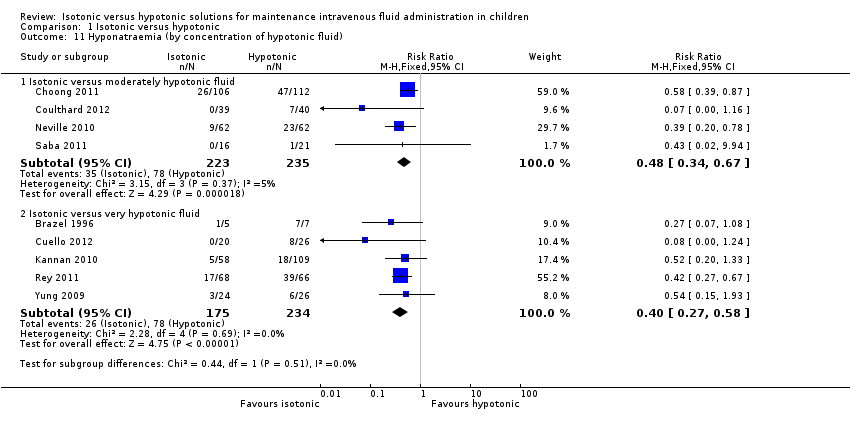
The range of sodium concentrations in hypotonic fluids is broad (e.g. some studies may use a hypotonic fluid containing 30 mmol/L of sodium, while another may use a hypotonic fluid containing 77 mmol/L of sodium). It could be reasoned that, if hypotonic fluid increases the risk of hyponatraemia, a more profoundly hypotonic fluid would exacerbate this. We performed a subgroup analysis, grouping the hypotonic fluids according to sodium concentration:
-
sodium ≥ 70 mmol/L and ≤ 125 mmol/L (moderately hypotonic fluid);
-
sodium < 70 mmol/L (very hypotonic fluid).
Four studies used a moderately hypotonic fluid (Choong 2011; Coulthard 2012; Neville 2010; Saba 2011), while five used a very hypotonic fluid (Brazel 1996; Cuello 2012; Kannan 2010; Rey 2011; Yung 2009). The composition of hypotonic fluid used in Montañana 2008 differed according to weight and was not included in this analysis.
Somewhat surprisingly, this subgroup analysis suggests that, when compared with isotonic fluid, there is a similar risk of hyponatraemia whether using a profoundly or moderately hypotonic fluid (Analysis 1.11).
Surgical patients versus medical patients
We performed a subgroup analysis to explore whether being a surgical or medical patient influences the risk of developing hyponatraemia (Analysis 1.12). Four studies with data available for our primary outcome were performed exclusively in postoperative patients (Brazel 1996; Choong 2011; Coulthard 2012; Neville 2010), while Kannan 2010 examined medical patients. Cuello 2012, Rey 2011 and Saba 2011 performed studies examining both medical and surgical patients; data on each subgroup could be analysed. The remaining studies did not provide the case mix data to allow analysis according to surgical and medical subgroups.
In the 529 surgical patients with available data, the meta‐analysis shows that isotonic fluid is protective against hyponatraemia when compared with hypotonic fluid (RR 0.48; 95% CI 0.36 to 0.66). When examining the medical cohort, Saba 2011 reported no cases of hyponatraemia in the six patients each receiving isotonic and hypotonic fluid. A protective difference was shown in the gastroenteritis population in Cuello 2012, the medical intensive care population in Rey 2011 and the general medical population in Kannan 2010, when using isotonic compared with hypotonic fluid (RR 0.29; 95% CI 0.16 to 0.55).
Fluid rate
We compared the results of those randomised to receive fluid at maintenance rates (as per Holliday 1957) with the results of those randomised to receive fluid at ≤ 70% maintenance (Analysis 1.13). Studies where rates were prescribed at the treating physician's discretion were not included in this analysis. Five studies had data available for the maintenance rate analysis (Cuello 2012; Kannan 2010; Montañana 2008; Neville 2010; Rey 2011). They showed strong evidence of a decreased risk of hyponatraemia when isotonic fluid compared with hypotonic fluid was used at maintenance rates (RR 0.45; 95% CI 0.33 to 0.61). When rates were restricted to ≤ 70% maintenance, only one study had data available for analysis (Neville 2010). There was a similar risk ratio for hyponatraemia to the overall comparison (RR 0.45; 95% CI 0.18 to 1.16). This suggests that fluid restriction does not protect against hyponatraemia, although this cannot be concluded with certainty, likely due to insufficient power.
Three further studies examined the issue of fluid restriction, but were not included in the meta‐analysis. Yung 2009 conducted a four‐armed factorial study comparing 0.9% sodium chloride with 0.18% sodium chloride + 4% dextrose at full and 2/3 of maintenance rates. These data were not included in the subgroup analysis as data on episodes of hyponatraemia were not available for these subgroups. Kannan 2010 conducted a three‐armed study comparing isotonic fluid at full maintenance rates with hypotonic fluid at full maintenance and 2/3 of maintenance rates. While the full maintenance data could be analysed as there was both an isotonic and hypotonic arm, only hypotonic fluid was given at a restricted rate, and therefore could not be analysed. Similarly, Coulthard 2012 gave isotonic fluid at a maintenance rate and hypotonic fluid at a restricted rate. Without isotonic and hypotonic arms for both subgroups, we could draw no conclusions regarding the impact of rate.
Age
Seven studies were able to provide information on rates of hyponatraemia according to age (Choong 2011; Coulthard 2012; Cuello 2012; Kannan 2010; Neville 2010; Rey 2011; Saba 2011). There appears to be a similar association between fluid type and hyponatraemia in each age stratum (Analysis 1.14).
Severity of illness
Isotonic fluid decreases the risk of hyponatraemia when compared with hypotonic fluid in both intensive care (RR 0.48; 95% CI 0.37 to 0.64) and non‐intensive care patients (RR 0.45; 95% CI 0.29 to 0.68) (Analysis 1.15).
Sensitivity analyses
Unbalanced interventions
Two studies included in the primary analysis had data which could be considered unbalanced as the isotonic and hypotonic arms were randomised to receive different volumes of fluid. Kannan 2010 performed a three‐armed study comparing isotonic fluid at maintenance rates with hypotonic fluid at maintenance rates as well as hypotonic fluid at restricted rates. For the primary meta‐analysis, we amalgamated the data from the hypotonic arms together. However, a potential bias could be introduced by this approach as the isotonic and hypotonic groups are no longer equivalent for all factors other than fluid composition. Similarly, Coulthard 2012 compared isotonic fluid at maintenance rates with hypotonic fluid at restricted rates. Again, these groups are not balanced, potentially introducing a bias. For the sensitivity analysis, we removed Coulthard's data and only included the maintenance rate arms of Kannan's study (Analysis 1.16).
The findings of the sensitivity analysis are almost identical to that of the primary analysis; using an isotonic fluid for maintenance hydration appears to be protective against the development of hyponatraemia (RR 0.45; 95% CI 0.35 to 0.58).
Hyponatraemia at baseline
Some studies included in the primary analysis recruited patients with abnormal serum sodium levels (< 135 mmol/L) at enrolment. Saba 2011 excluded patients only if their serum sodium was < 133 mmol/L, while Kannan 2010, Montañana 2008 and Rey 2011 excluded patients with lower serum sodiums (< 130 mmol/L). Neither Neville 2010 nor Coulthard 2012 restricted enrolment on the basis of initial sodium. The primary outcome for the meta‐analysis was hyponatraemia (an undesirable outcome) at any time point after commencing intravenous fluid; however, it is likely that some patients in these studies were recorded as having met the primary outcome despite a clinical improvement in serum sodium. That is, an initially hyponatraemic patient whose sodium improved towards the normal range could be judged as having met the primary outcome for the meta‐analysis.
To account for this, we undertook a sensitivity analysis, excluding studies where any patients were hyponatraemic at baseline (Analysis 1.17). Of note, we included data from Cuello 2012 in this analysis; while this study recruited patients with sodium as low as 125 mmol/L, only those who were normonatraemic at baseline were included in its hyponatraemia analysis.
Again, this sensitivity analysis did not substantially alter the finding that isotonic fluid appears to be protective against hyponatraemia when compared with hypotonic fluid, when given for maintenance hydration (RR 0.49; 95% CI 0.34 to 0.71).
Discussion
Summary of main results
In the populations studied, those who received isotonic fluid for intravenous maintenance hydration, when compared with those who received hypotonic fluid, had a substantially lower risk of hyponatraemia.
The protective effect of isotonic fluid in reducing hyponatraemia was maintained when we examined most subgroups.
Overall completeness and applicability of evidence
Populations studied
Some gaps in the evidence remain; in particular, the majority of studies were conducted in surgical and/or intensive care populations. There was a paucity of evidence for those receiving fluids beyond 24 hours.
Timing of outcomes and follow‐up period
Continuous outcomes were examined in time blocks of six to 12 hours and ≥ 12 to 24 hours. There were few meaningful 48‐hour data, as studies either did not examine this later time point or most patients had ceased intravenous fluids by this time point. It is, therefore, difficult to extrapolate our data for patients requiring more than a day of intravenous fluid. The short follow‐up period for some of the studies did introduce a potentially significant problem: if a patient became hyponatraemic for the first time after the study ended, the true effect of the fluid type would not have been captured. Similarly, most studies did not examine the primary outcome prior to 12 hours of therapy. This may miss patients who rapidly became hyponatraemic, then had an improving sodium level.
Quality of the evidence
Study limitations
In general, all the studies appeared to be conducted in a robust and systematic manner. We excluded no studies due to concerns with poor quality or potential bias. However, a potential factor negatively affecting the quality of the primary outcome exists, as only one study described the method of blood sampling (Choong 2011), with a further three studies providing unpublished information (Coulthard 2012; Cuello 2012; Kannan 2010). If the remaining studies sampled blood from an intravenous line running study fluid, false positive results arising from contamination could have markedly influenced the results of this meta‐analysis. However, those studies that described a separate intravenous line being used for blood sampling found comparable treatment effects to the overall meta‐analysis, reducing the likelihood that this has had a significant impact on the primary result.
Indirectness
No indirect outcomes were assessed.
Heterogeneity
This meta‐analysis was limited by the clinical heterogeneity of the studies, which compromises the ability to compare studies fairly. In particular, the difference in sodium concentrations of the hypotonic fluid studied was vast, reflecting the broad range of practice occurring internationally (Davies 2008; Freeman 2012; Way 2006). Despite this, the results were consistent between studies and maintained in most of the subgroup analyses, implying that the results may be generalised to a wide range of settings.
There was no apparent heterogeneity of results between studies or subgroup analyses.
Imprecision of results
There was imprecision in a number of secondary outcomes due to the low event numbers. This is reflected in the downgrading of the GRADE domain (see summary of findings Table for the main comparison).
Publication bias
We searched databases of registered protocols to identify unpublished studies and all authors of included studies replied to requests for additional, unpublished data.
Potential biases in the review process
Primary outcome limitations
We selected hyponatraemia (< 135 mmol/L) as the most appropriate, pragmatic and ethical primary outcome for the meta‐analysis, acknowledging that there are some limitations with this.
Sodium stability or a serum sodium level that slowly moves towards the normal range, rather than normonatraemia, may be a more clinically appropriate outcome for a patient with a low serum sodium at baseline. However, these data are dependent on the baseline sodium level for an individual patient: these data are not available in the context of a meta‐analysis.
We chose mild to moderate hyponatraemia as the primary outcome despite it being unlikely to be associated with any adverse neurological consequences. We used it in this context as a surrogate marker for risk of more severe hyponatraemia, which has been associated with cerebral oedema. While severe hyponatraemia (< 130 mmol/L) was an outcome for some of the included studies, allowing at‐risk patients to become severely hyponatraemic raises significant safety concerns and it was, therefore, not considered appropriate for use in this meta‐analysis. In addition, some studies included safety measures that prevented the development of severe hyponatraemia. If sodium decreased, the fluid prescription was changed and the patient was removed from subsequent data analysis. This made it unlikely for any patients to develop severe hyponatraemia, but also limits the interpretation of data beyond the first measurement of hyponatraemia.
Secondary outcome limitations
Mean serum sodium
When examining mean sodium as a secondary outcome, choosing the ideal time point to study was difficult. It would seem plausible that, if hypotonic fluids decrease mean serum sodium, a protracted length of treatment would decrease the serum sodium further. That is, the longer the treatment length, the lower the serum sodium. However, it is also logical that, for the majority of patients, the severity of their illness will improve with length of treatment. This, in turn, should decrease the likelihood of nonosmotic antidiuretic hormone (ADH) secretion with time, allowing the body to excrete excess water and minimising any reduction in serum sodium. It could, therefore, be expected that the nadir in serum sodium will be affected by an interplay between severity of illness and length of treatment. There is a lack of evidence to suggest when the nadir would be expected in any given patient population, and whether it was captured by our time brackets. Regardless, in the two time brackets studied, mean serum sodium was consistently lower in the treatment group receiving hypotonic fluid.
Adverse events
Little can be said regarding serious adverse events as most studies had safety restrictions built into their methodology to ensure adverse events were avoided, or high‐risk patients were withdrawn from the study prior to the occurrence of serious adverse events, or both. There is inadequate evidence to suggest isotonic fluids increase the risk of hypernatraemia and overhydration, which have been suggested as potential adverse outcomes (Coulthard 2008; Hatherill 2004; Holliday 2004; Holliday 2005).
While it is unclear whether there is an increased risk of hypernatraemia when isotonic fluids are used, it is worth noting that the total number of episodes of hypernatraemia in the meta‐analysis (33/937) was significantly lower than the number of episodes of hyponatraemia (252/970). In addition, in studies where hypernatraemia was examined as an outcome, the data do not address whether this is from hypernatraemic dehydration or excess sodium infusion. However, further large clinical trials, currently underway, examining hypernatraemia as an outcome of isotonic fluid administration, may be beneficial.
Overhydration has been suggested as a possible consequence of the prescription of isotonic fluids, as an increased sodium load could potentially lead to interstitial fluid overload (Holliday 2005). This situation could be particularly problematic in developing countries where 'rescue' medications or access to ventilatory support may not be available. Again, further large trials examining this outcome may assist.
Agreements and disagreements with other studies or reviews
The result of the primary meta‐analysis is consistent with all randomised controlled trials comparing isotonic with hypotonic fluid for maintenance hydration in children. Each study conducted has concluded that isotonic fluid is protective against hyponatraemia.
Comparison 1 Isotonic versus hypotonic, Outcome 1 Hyponatraemia.

Comparison 1 Isotonic versus hypotonic, Outcome 2 Hypernatraemia.

Comparison 1 Isotonic versus hypotonic, Outcome 3 Mean serum sodium T6‐T12.

Comparison 1 Isotonic versus hypotonic, Outcome 4 Mean serum sodium at T > T12 to T24.

Comparison 1 Isotonic versus hypotonic, Outcome 5 Death.

Comparison 1 Isotonic versus hypotonic, Outcome 6 Seizures.

Comparison 1 Isotonic versus hypotonic, Outcome 7 Cerebral oedema.
Comparison 1 Isotonic versus hypotonic, Outcome 8 Overhydration.
Comparison 1 Isotonic versus hypotonic, Outcome 9 Urine osmolarity at T24.

Comparison 1 Isotonic versus hypotonic, Outcome 10 Urinary sodium concentration at T24.
Comparison 1 Isotonic versus hypotonic, Outcome 11 Hyponatraemia (by concentration of hypotonic fluid).
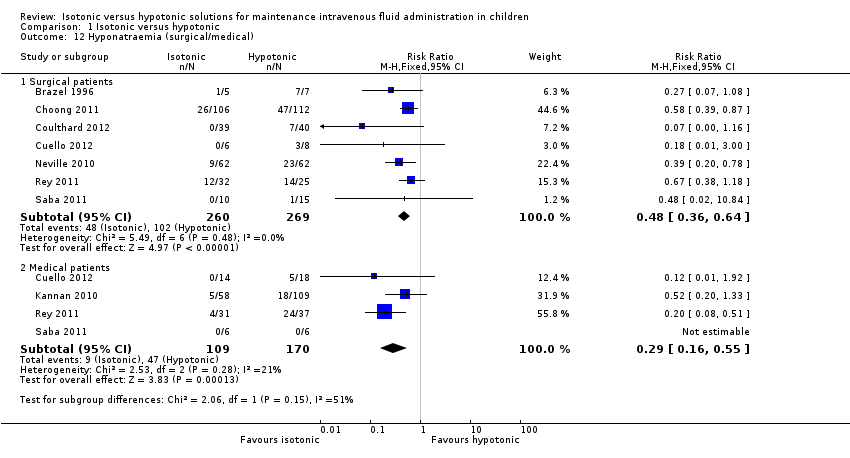
Comparison 1 Isotonic versus hypotonic, Outcome 12 Hyponatraemia (surgical/medical).

Comparison 1 Isotonic versus hypotonic, Outcome 13 Hyponatraemia (by fluid rate).

Comparison 1 Isotonic versus hypotonic, Outcome 14 Hyponatraemia (by age).
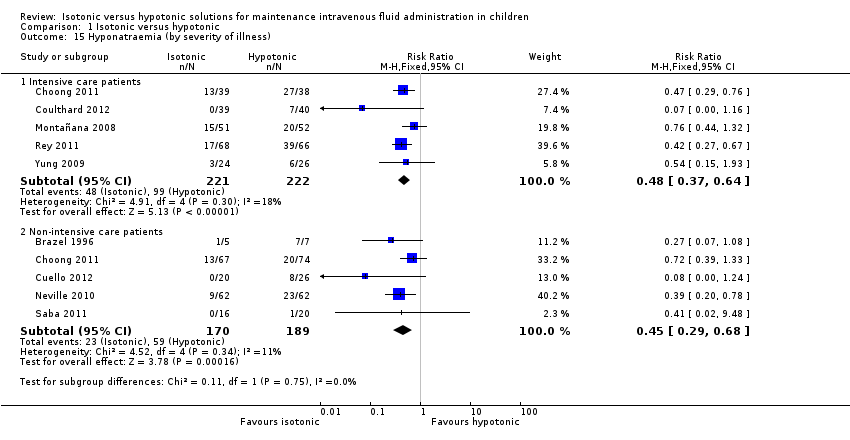
Comparison 1 Isotonic versus hypotonic, Outcome 15 Hyponatraemia (by severity of illness).

Comparison 1 Isotonic versus hypotonic, Outcome 16 Sensitivity analysis ‐ balanced fluid rates.
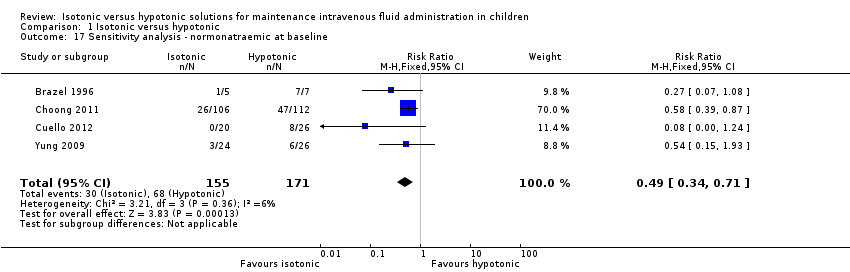
Comparison 1 Isotonic versus hypotonic, Outcome 17 Sensitivity analysis ‐ normonatraemic at baseline.
| Isotonic intravenous fluid compared with hypotonic intravenous fluid to maintain hydration | ||||||
| Patient or population: children requiring intravenous fluid to maintain hydration Settings: inpatient hospital setting Intervention: isotonic intravenous fluid Comparison: hypotonic intravenous fluid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hypotonic intravenous fluid | Isotonic intravenous fluid | |||||
| Hyponatraemia (serum sodium < 135 mmol/L) | Study population | RR 0.48 (0.38 to 0.60) | 970 | ⊕⊕⊕⊕ | ||
| 338 per 1000 | 169 per 1000 | |||||
| Surgical patients | RR 0.48 (0.36 to 0.64) | 529 (7) | ⊕⊕⊕⊕ | |||
| 379 per 1000 | 185 per 1000 | |||||
| Medical patients | RR 0.29 (0.16 to 0.55) | 279 (4) | ⊕⊕⊕⊝ | |||
| 276 per 1000 | 83 per 1000 | |||||
| Intensive care patients | RR 0.48 (0.37 to 0.64) | 443 (5) | ⊕⊕⊕⊕ | |||
| 446 per 1000 | 217 per 1000 | |||||
| Non‐intensive care patients | RR 0.45 (0.29 to 0.68) | 359 (5) | ⊕⊕⊕⊝ | |||
| 312 per 1000 | 135 per 1000 | |||||
| Hypernatraemia | Study population | RR 1.24 (0.65 to 2.38) | 937 | ⊕⊕⊝⊝ | Quality of evidence downgraded due to imprecision ‐ small number of events, wide confidence interval | |
| 34 per 1000 | 37 per 1000 | |||||
| Death | Study population | 5.59 (0.23 to 135.17) | 996 | ⊕⊕⊝⊝ | Quality of evidence downgraded due to imprecision ‐ small number of events, wide confidence interval Study design reduced the likelihood of this outcome | |
| 0 per 1000 | 2 per 1000 | |||||
| Seizures | Study population | RR 0.62 (0.03 to 15.02) | 996 | ⊕⊕⊝⊝ | Quality of evidence downgraded due to imprecision ‐ small number of events, wide confidence interval Study design reduced the likelihood of this outcome | |
| 2 per 1000 | 0 per 1000 | |||||
| Cerebral oedema | Study population | RR incalculable | 9 studies | ⊕⊝⊝⊝ | Quality of evidence downgraded due to imprecision ‐ no events, incalculable confidence interval Study design reduced the likelihood of this outcome | |
| 0 per 1000 | 0 per 1000 | |||||
| Overhydration | Study population | RR 1.14 (0.46 to 2.87) | 615 | ⊕⊕⊝⊝ | Quality of evidence downgraded due to imprecision ‐ small number of events, wide confidence interval Heterogeneity in the criteria for assessing this outcome | |
| 26 per 1000 | 30 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
|
| Na+ (mmol/L) | Cl‐ (mmol/L) | K+ (mmol/L) | Mg++ (mmol/L) | Calcium (mmol/L) | Lactate (mmol/L) | Acetate (mmol/L) | Gluconate (mmol/L) | Glucose (gram/L) |
| Physiologically isotonic/near isotonic | |||||||||
| 0.9% sodium chloride | 154 | 154 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| 0.9% sodium chloride with 2.5/5% dextrose | 154 | 154 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 25 / 50 |
| Hartmann's solution (similar in ionic composition to Ringer's lactate) | 131 | 111 | 5 | ‐ | 2 | 29 | ‐ | ‐ | ‐ |
| Plasmalyte 148 solution | 140 | 98 | 5 | 1.5 | ‐ | ‐ | 27 | 23 | ‐ |
| Plasmalyte 148 solution with 5% dextrose | 140 | 98 | 5 | 1.5 | ‐ | ‐ | 27 | 23 | 50 |
| Physiologically moderately hypotonic | |||||||||
| 0.45% sodium chloride (N/2) with 5% dextrose | 77 | 77 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 50 |
| Physiologically very hypotonic | |||||||||
| 0.3% sodium chloride (N/3) with 3.3% dextrose | 51 | 51 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 33 |
| 0.18% sodium chloride (N/5) with 4% dextrose | 30 | 30 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 40 |
| Note: Minor variations in composition occur at the point of manufacture. | |||||||||
| Study | Number of participants | Population | Study arms | Primary outcome |
| 12 | Patients undergoing primary corrective surgery for idiopathic scoliosis | Isotonic (Hartmann's) Hypotonic (either 0.3% saline + 3% dextrose or 0.18% saline + 4% dextrose) Rate: 1.5 ml/kg/hr | Development of SIADH | |
| 122 | PICU patients | Isotonic fluid (sodium 140 mEq/L) Hypotonic fluid (sodium between 20 and 100 mEq/L corresponding to 2 to 4 mEq/kg/24 hr) Exact composition of fluids not stated Rate: standard maintenance | Hyponatraemia < 135 mEq/L | |
| 50 | PICU patients | 0.9% saline at standard maintenance rate 0.9% saline at 2/3 restricted rate 0.18% saline + 4% dextrose at standard maintenance rate 0.18% saline + 4% dextrose at 2/3 restricted rate | Change in plasma sodium from admission to 12 to 24 hrs later | |
| 167 | Broad paediatric population (university hospital in India) | 0.9% saline + 5% dextrose at standard maintenance rate 0.18% saline + 5% dextrose at standard maintenance rate 0.18% saline + 5% dextrose at 2/3 restricted rate | Hyponatraemia < 130mEq/L | |
| 124 | Elective or emergency surgery | 0.9% saline + 2.5% dextrose at standard maintenance rate 0.9% saline + 5% dextrose at 50% restricted rate 0.45% saline + 2.5% dextrose at standard maintenance rate 0.45% saline + 5% dextrose at 50% restricted rate | Change in plasma sodium from induction of anaesthesia to T8 | |
| 125 | PICU patients | Isotonic (sodium 136 mmol/L) Hypotonic (sodium 30 to 50 mmol/L) Exact composition not stated Rate: standard maintenance | Change in plasma sodium from admission to 12 and 24 hrs later | |
| 258 | Surgical patients | 0.9% saline + 5% dextrose 0.45% saline + 5% dextrose Rate: Physician's discretion | Hyponatraemia < 135 mmol/L | |
| 37 | Medical patients admitted via ED Elective surgical patients | 0.9% saline + 5% dextrose 0.45% saline + 5% dextrose Rate: physician's discretion | Rate of change of sodium | |
| 82 | Patients undergoing spinal instrumentation, craniotomy for brain tumour resection or cranial vault remodelling | Hartmann's + 5% dextrose at full maintenance rate 0.45% saline + 5% dextrose at 2/3 restricted rate | Mean plasma sodium 16 to 18 hrs postoperatively | |
| (abstract only) | 72 | Participants with either of 2 conditions: a) gastroenteritis with moderate dehydration and unable to tolerate fluids b) children requiring non‐urgent surgery and requiring maintenance intravenous hydration during their admission | 0.9% saline + 5% dextrose +/‐ 20 mmol/L KCl 0.2% saline + 5% dextrose +/‐ 20 mmol/L KCl Rate: standard maintenance | Mean plasma sodium at 4 (T4) and 8 (T8) hours, and the percentage of patients who developed hyponatraemia (> 125 mEq/L and < 135 mEq/L) |
| ED: emergency department KCl: potassium choride | ||||
| First author | Duration of fluid therapy | Timing of outcome measurements |
| Brazel | Max 72 hrs | End of procedure, T6, T24, T48, T72
|
| Montañana | Max 24 hrs | T6, T24 (only T6 primary outcome data included in analysis) |
| Yung | 12 to 24 hrs | T12 to 24 |
| Kannan | Max 72 hrs | T12, T24, T36, T48, T60, T72
|
| Neville | Max 24 hrs | At intubation, T8, T24 |
| Rey | Max 24 hrs | T12, T24 |
| Choong | Max 48 hrs | T12, T24, T36, T48 |
| Saba | 8 to 12 hrs | T12 |
| Coulthard | 16 to 18 hrs | T16 to 18 |
| Cuello | 8 hrs | T4, T8 |
| hr: hour | ||
| Primary author | Inclusion criteria | Exclusion criteria |
| Brazel | Adolescent patients undergoing primary corrective surgery for adolescent idiopathic scoliosis
| — |
| Montañana | PICU patients requiring maintenance intravenous fluid
| Chronic or acute kidney failure Patients at risk of cerebral oedema (diabetic ketoacidosis or craneoencephalic trauma) Patients with plasma sodium level at admission < 130 mEq/L or > 150 mEq/L, and/or dehydration > 5% of the patient's body weight
|
| Yung | Patients admitted to PICU who would normally require IV fluids at standard maintenance rates for 12 hrs, with normal sodium levels and not hypoglycaemic
| Neonates, diabetes, renal failure, shock Cardiac and neurosurgical patients were eligible for the restricted rate arm only |
| Kannan | Patients aged between 3 months and 12 years requiring IV maintenance fluid administration for at least 24 hrs
| Na < 130mEq/L, Na > 150mEq/L, blood glucose > 180 mg/dL, dehydration, shock, severe malnutrition, cirrhosis of liver, congestive heart failure, acute or chronic renal failure and nephrotic syndrome Patients receiving drugs that may alter plasma sodium levels Patients requiring fluid boluses for volume depletion and/or shock |
| Neville | Patients undergoing elective or emergency surgery, expected to take nothing by mouth after surgery for at least 8 hrs. Weight > 8kg | Significant blood loss expected during surgery Surgery types known to be associated with excess ADH secretion (cranial and thoracic surgery) Known abnormality of ADH secretion, nephrogenic diabetes insipidus, pituitary or hypothalamic disease, kidney disease, acute or chronic lung disease Patients receiving drugs known to stimulate ADH secretion |
| Rey | PICU patients requiring maintenance IV fluids
| Impairment in body water homeostasis (e.g. congestive heart failure) Electrolytic alterations requiring a different IV fluid than that in the study Renal function abnormalities Patients requiring fluid restriction |
| Choong | Euvolaemic patients within 6 hours after elective surgery if anticipated need for IV maintenance was > 24 hours
| Uncorrected plasma sodium level abnormalities before the end of surgery Patients with known abnormalities of ADH secretion Patients requiring volume resuscitation and/or vasoactive infusions Recent loop diuretic use Total parenteral nutrition required with 24 hours following surgery Patients for whom either a hypotonic or isotonic Isotonic fluid was considered necessary or contraindicated (e.g. because of a risk of cerebral oedema, acute burns or the risk of third space and/or sodium overload in patients with pre‐existing congestive cardiac failure, renal failure, liver failure or cirrhosis) |
| Saba | Patients requiring at least 8 hours of IV fluids
| Baseline Na of < 133 or > 145 mmol/L Patients with any of renal disease, cardiac dysfunction, pre‐existing hypertension, diuretic use, oedema, known adrenal dysfunction, acute or severe chronic neurological illness |
| Coulthard | Patients admitted to PICU following spinal instrumentation for correction of scoliosis, craniotomy for excision of brain tumours and cranial vault remodelling | Lengthening only of spinal instrumentation rods, insertion or revision of ventriculoperitoneal shunts, intracerebral cyst fenestration or previously enrolled |
| Cuello | Patients 6 months to 14 years old; with a serum sodium level between 125 mmol/L to 150 mmol/L; previously healthy, admitted to the emergency room or hospitalisation ward with any of 2 conditions: a) gastroenteritis with moderate dehydration and unable to drink fluids; b) children undergoing non‐urgent surgery and requiring maintenance IV hydration during their hospitalisation (i.e. non‐incarcerated hernia, adenotonsillectomies, tympanostomy, fracture reductions of the extremities, etc.) | Chronic illnesses (e.g. cystic fibrosis, cerebral palsy, etc.); taking antidiuretics; major trauma that required intensive care; hyper or hyponatraemia at admission; severe dehydration |
| ADH: antidiuretic hormone secretion | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hyponatraemia Show forest plot | 10 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.38, 0.60] |
| 2 Hypernatraemia Show forest plot | 9 | 937 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.38] |
| 3 Mean serum sodium T6‐T12 Show forest plot | 7 | 851 | Mean Difference (IV, Fixed, 95% CI) | 1.99 [1.55, 2.42] |
| 4 Mean serum sodium at T > T12 to T24 Show forest plot | 6 | 579 | Mean Difference (IV, Fixed, 95% CI) | 1.33 [0.81, 1.85] |
| 5 Death Show forest plot | 10 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.59 [0.23, 135.17] |
| 6 Seizures Show forest plot | 10 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.03, 15.02] |
| 7 Cerebral oedema Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8 Overhydration Show forest plot | 5 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.46, 2.87] |
| 9 Urine osmolarity at T24 Show forest plot | 3 | 278 | Mean Difference (IV, Fixed, 95% CI) | 12.68 [‐34.20, 59.56] |
| 10 Urinary sodium concentration at T24 Show forest plot | 4 | 516 | Mean Difference (IV, Fixed, 95% CI) | 14.72 [9.02, 20.42] |
| 11 Hyponatraemia (by concentration of hypotonic fluid) Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Isotonic versus moderately hypotonic fluid | 4 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.67] |
| 11.2 Isotonic versus very hypotonic fluid | 5 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.27, 0.58] |
| 12 Hyponatraemia (surgical/medical) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Surgical patients | 7 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.36, 0.64] |
| 12.2 Medical patients | 4 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.16, 0.55] |
| 13 Hyponatraemia (by fluid rate) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Full maintenance | 5 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.33, 0.61] |
| 13.2 Restricted rate | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.18, 1.16] |
| 14 Hyponatraemia (by age) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Age < 1 year | 7 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.12, 0.88] |
| 14.2 Age 1 to 5 years | 7 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.19, 0.57] |
| 14.3 Age > 5 years | 8 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.38, 0.69] |
| 15 Hyponatraemia (by severity of illness) Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Intensive care patients | 5 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.37, 0.64] |
| 15.2 Non‐intensive care patients | 5 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.29, 0.68] |
| 16 Sensitivity analysis ‐ balanced fluid rates Show forest plot | 8 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.35, 0.58] |
| 17 Sensitivity analysis ‐ normonatraemic at baseline Show forest plot | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.34, 0.71] |

