Anticoagulación para la tromboprofilaxis perioperatoria en pacientes con cáncer
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized double‐blind double‐dummy study | |
| Participants | 1941 (67.9%) participants undergoing abdominal surgery for cancer, expected to last > 45 minutes under general anesthesia and aged > 60 years, or aged > 40 years with ≥ 1 additional risk factors for thromboembolic complications in 131 hospitals in 22 countries | |
| Interventions | Intervention: fondaparinux 2.5 mg subcutaneously once daily started 6 h after surgical closure plus placebo Control: dalteparin 2500 U subcutaneously once daily started 2 h before induction of anesthesia and 12 h later than 5000 U once‐daily plus placebo | |
| Outcomes | Duration of follow‐up: 10 days (for screening) and 30 days (for symptomatic) days postoperation
Screening test for DVT: bilateral ascending contrast venography of the legs Diagnostic test for DVT: ultrasonography of the leg veins followed by bilateral venography Diagnostic test for PE: high‐probability lung scan, pulmonary angiography, helical computed tomography, or at autopsy | |
| Notes | Funding: Sanofi‐Synthélabo and NV Organon Ethical approval: "the study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki and local regulations." Conflict of interest: "none of the authors had financial conflicts of interest in relation to the study." Intention‐to‐treat analysis: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "double‐blind double‐dummy randomized study" Comment: probably yes |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind double‐dummy randomized study. Patients given fondaparinux received a placebo injection 2 h before surgery and again 12 h later to correspond with the dalteparin dosing schedule. Patients given dalteparin received a placebo injection 6 h after surgery." Comment: definitely yes |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "study outcome measures, including venography results, clinically suspected thromboembolic and bleeding events, and deaths, were adjudicated by a central independent committee that was unaware of the patients’ treatment assignment and the local assessment." Comment: definitely yes |
| Incomplete outcome data (attrition bias) | High risk | Comment: judgment based on comparison between MPD rate (VTE: 536/1941 = 27.61%) and event rate (VTE: 88/1408 = 6.25%) |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on. Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized double‐blind trial | |
| Participants | 102 participants aged 40‐70 years, non‐smokers and no history of peripheral arterial disease or thrombosis, undergoing surgery for gynecologic malignancy. Mean age: 57 years, previous VTE: not reported | |
| Interventions | Intervention: enoxaparin 2500 U subcutaneously 2 h preoperatively then once daily (LMWH) Control: UFH 5000 U subcutaneously every 8 h Discontinued treatment: not clear | |
| Outcomes | Duration of follow‐up: not clear
Screening testing for DVT/PE: none Diagnostic testing for DVT: duplex ultrasonography and if required venography Diagnostic test for PE: ventilation‐perfusion scan and pulmonary arteriography | |
| Notes | Funding: Eczacibasi‐Rhône Poulenc, Turkey Ethical approval: not reported Conflict of interest: not reported Intention‐to‐treat analysis: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to author contact: random number table |
| Allocation concealment (selection bias) | Low risk | According to author contact: "sequentially numbered sealed envelopes" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "randomised double blind trial" Comment: according to author contact: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "the surgical team and those collecting laboratory and clinical data were not informed about the prophylactic anticoagulation being used." Comment: according to author contact: yes |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up 100% |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on. Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized double‐blind multicenter trial | |
| Participants | 637 (64%) participants aged ≥ 40 years, with cancer undergoing major elective general abdominal surgery (study subgroup) from 7 centers Mean age: 71 years, men 52% (329/637), previous VTE 6% (40/637) | |
| Interventions | Intervention: dalteparin 5000 U subcutaneously at 22.00 h on the evening preoperatively then twice daily for 5‐8 days (an LMWH) Control: UFH 5000 U/0.2 ml subcutaneously 2 h preoperatively then twice daily from 5 to 8 days Discontinued treatment: not clear | |
| Outcomes | Duration of follow‐up: 30 days
Screening testing for DVT: iodine‐radiolabeled fibrinogen uptake test for 7 days Diagnostic test for PE: scintigraphy | |
| Notes | Funding: Swedish Medical Research Council (No. 00759) Ethical approval: study approved by Ethics Committee, University of Lund, and local ethics committees Conflict of interest: not reported Intention‐to‐treat analysis: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a total of 1040 patients were randomised" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "randomised double blind multicenter trial" Comment: definitely yes |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo used Comment: not reported, probably not; however, the knowledge of the assigned intervention may not have impacted the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up 100% |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized double‐blind multicenter trial | |
| Participants | 1116 participants undergoing planned curative abdominal or pelvic surgery for cancer (study subgroup) were randomized in the study. Venograms were inadequate in 460 (41.3%) leaving "631 evaluable patients." Minimum age: 40 years. Mean age: 68.5 years, 53% men, previous DVT 3% (20/631) | |
| Interventions | Intervention: enoxaparin 40 mg (0.2 ml) subcutaneously started 2 h before surgery (an LMWH) and then once daily in addition to placebo twice daily Control: low‐dose UFH 5000 U (0.2 ml) subcutaneously started 2 h before surgery and then 3 times daily Discontinuation treatment: 243/556 participants randomized to LMWH and 241/560 participants randomized to UFH | |
| Outcomes | Duration of follow‐up: 3 months
Screening testing for DVT/PE: venography "Scheduled bilateral ascending venography was performed 24 hours after the last injection of the trial substance." Diagnostic test for DVT: venography; "If the patient developed clinical symptoms or signs of DVT, unilateral venography was performed within 24h." Diagnostic test for PE: ventilation‐perfusion lung scan or pulmonary angiography, or both | |
| Notes | Funding: Swedish Medical Research Council grant No. 00759 Ethical approval: "the study was performed according to the Declaration of Helsinki and good clinical practice. Regional ethics committees in the various countries approved the trial." Conflict of interest: not reported Intention‐to‐treat analysis: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "separate randomisation were made per country and per hospital to one of two groups." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind randomised trial" Comment: definitely yes |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "the venographic results were evaluated and agreed on by an independent panel before the code was broken." Comment: definitely yes |
| Incomplete outcome data (attrition bias) | High risk | Comment: judgment based on comparison between MPD rate (symptomatic DVT: 485/1116 = 43.45%; asymptomatic DVT: 485/1116 = 43.45%); and event rate (symptomatic DVT: 10/631 = 1.58%; asymptomatic DVT: 91/631 = 14.42%) |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on. Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized trial | |
| Participants | 50 participants aged 45 to 75 years with localized prostate cancer (stage T1c‐T2) undergoing radical retropubic prostatectomy for prostate cancer Mean age: 60 years, previous VTE: not reported | |
| Interventions | Intervention: calcium nadroparin 2850 IU (0.3 mL) given as single daily subcutaneously (an LMWH) started 12 h before surgery Control: UFH 5000 U subcutaneously 3 times daily started 2 h before surgery In both groups, prophylaxis began preoperatively and maintained throughout the hospital stay (mean 15 days) Discontinued treatment: none | |
| Outcomes | Duration of follow‐up: 15 days
Screening testing for DVT/PE: none Diagnostic testing for DVT/PE: ultrasound‐Doppler | |
| Notes | Funding: not reported Ethical approval: not reported Conflict of interest: not reported Intention‐to‐treat analysis: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned two groups." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) | High risk | No placebo used Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behavior across intervention group (e.g. differential drop‐out, differential cross‐over to an alternative intervention, or differential administration of co interventions. |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo used Comment: not reported, probably not, however the knowledge of the assigned intervention may not have impacted the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up 100% |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized trial | |
| Participants | 100 participants undergoing thoracic surgery for cancer; aged > 18 years Mean age: 59 years, 92% men, previous VTE: not reported | |
| Interventions | Intervention: nadroparin 7500 U subcutaneously 12 h preoperatively and 12 h postoperatively until the second postoperative day then 10,000 U once daily on postoperative days 3‐7 Control: UFH 5000 U subcutaneously 2 h preoperatively and 12 h postoperatively then 3 times daily until the second postoperative day then a dose adjusted to activated partial thromboplastin time on postoperative days 3‐7 twice daily Discontinued treatment: none | |
| Outcomes | Duration of follow‐up: not clear
Screening testing for DVT/PE: participants were screened with 125I‐fibrinogen uptake test Diagnostic testing for DVT/PE: none | |
| Notes | Funding: not reported Ethical approval: not reported Conflict of interest: not reported Intention‐to‐treat analysis: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised study" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) | High risk | No placebo used Quote: "This trial was a prospective multicentre, partially double‐blind;" "a first phase conducted double blindly from day ‐ I (D ‐ I) to D + 2"; "a second, open phase from D3 to D7" Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behavior across intervention group (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions. |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo used Quote: "partially double blind;" "first phase conducted double blind"; "a second, open phase from D3 to D7" Comment: probably not blinded; however, the knowledge of the assigned intervention was not likely to impact the analysis of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up 100% |
| Selective reporting (reporting bias) | Unclear risk | Study not registered. No published protocol. No outcomes listed in methods section Comment: unclear |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized multicenter trial | |
| Participants | 704 participants aged > 40 years with cancer (37% study subgroup) and scheduled for elective abdominal surgery Mean age: 61 years, 52% men, previous VTE: not reported | |
| Interventions | Intervention: fraxiparin 7500 anti‐Xa U subcutaneously (an LMWH) Control: calcium heparin 5000 U subcutaneously 3 times daily Treatment initiated 2 h before surgery, second injection given 8 h after surgery. Subsequent injections given every 24 h between 07.00 and 10.00 h from the 1st to the 7th postoperative day Discontinuation treatment: not clear | |
| Outcomes | Duration of follow‐up: 7 days
Screening testing for DVT/PE: radiolabeled iodine fibrinogen leg scanning on the day of the surgery and then daily for 7 consecutive days Diagnostic testing for DVT/: phlebography Diagnostic testing for PE: ventilation‐perfusion scanning or angiography | |
| Notes | Funding: Sanofi Labaz, GmbH, Pharmzeutische Praparate Ethical approval: trial protocol approved by Ethical Committee of the University of Frankfurt Conflict of interest: not reported Intention‐to‐treat analysis: no. Quote: "In all, 1909 patients qualified for the trial and were randomized. However, 13 of these patients received neither Fraxiparin nor Calciparin and therefore were excluded from analysis of efficacy and tolerance." "A total of 35 patients (21 in the Fraxiparin group and 14 in the calcium heparin group) did not receive the full study medication for various reasons; they were, however, included in the final analysis." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients were assigned to treatment with either Fraxiparin or calcium heparin following randomised schedule." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) | High risk | No placebo used Quote: "the trial was not performed in double blind manner." Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo used Quote: "the trial was not performed in double blind manner." Comment: probably not; however, the knowledge of the assigned intervention may not have impacted the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | Unclear risk | Follow‐up 100% |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on. Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized trial | |
| Participants | 80 participants aged ≥ 40 years undergoing surgery for abdominal and pelvic malignancy Mean age: 57.6 years, 93% women, previous VTE 13.7% | |
| Interventions | Intervention: 2500 anti‐Xa U subcutaneously 2 h before surgery and 12 h after the first injection and then dalteparin sodium (Fragmin) 5000 anti‐Xa U injection every morning for 10 days Control: calcium heparin 5000 IU subcutaneously injection 2 h before the surgery and then at 8‐h intervals for the next 10 days Discontinuation of treatment: 0 | |
| Outcomes | Follow‐up: 10 days
Screening testing for DVT/PE: radiolabeled fibrinogen tests used for postoperative screening of DVT Diagnostic testing for DVT: venography Diagnostic testing for PE: lung scintigraphy with 99mTc‐aggregated albumin perfusion study | |
| Notes | Funding: not reported Ethical approval: approved by local Ethics Committee (Groupe de Recherche sur le Medicament, Université Louis Pasteur, Strasbourg, France) Conflict of interest: not reported Intention‐to‐treat analysis: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eighty patients undergoing pelvic or abdominal surgery for cancer were randomised in two groups." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) | High risk | No placebo used Quote: "we have undertaken a prospective open randomised trial." Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo used Quote: "the trial was not performed in double blind manner." Comment: probably not; however, the knowledge of the assigned intervention may not have impacted the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up 100% |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on. Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized double‐blind trial | |
| Participants | 514 participants aged > 40 years undergoing major abdominal or thoracic surgery for cancer at the Royal Melbourne and Austin Hospitals (Melbourne, Australia), the Middlemore Hospital (Auckland, New Zealand), and the Flinders Medical Centre (Adelaide, Australia) Mean age: 65 years, 62% men, previous VTE 2.5% | |
| Interventions | Intervention: orgaran 750 U subcutaneously 1‐2 h preoperatively then at 12‐h intervals × 6 days (an LMWH) Control: UFH 5000 U subcutaneously 1‐2 h preoperatively then at 12‐h intervals × 6 days Discontinued treatment: 16/241 randomized to LMWH and 7/249 randomized to UFH | |
| Outcomes | Duration of follow‐up: 4‐6 weeks after discharge from hospital. Follow‐up period defined as starting 2 days after end of trial therapy
Screening test for DVT: radiolabeled fibrinogen tests used for screening of postoperative DVT every 2nd day on the week days Diagnostic test for DVT: ascending contrast medium venography Diagnostic test for PE: ventilation‐perfusion lung scanning | |
| Notes | Funding: Organon International, Oss, The Netherlands Ethical approval: trial protocol approved by relevant institutional clinical investigations committees Conflict of interest: not reported Intention‐to‐treat analysis: yes, quote: "intent to treat analysis showed statistically non‐significant toward trend towards less VT [venous thrombosis] during Orgaran prophylaxis." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using predetermined randomisation sequences for each trial center." |
| Allocation concealment (selection bias) | Low risk | Quote: "coded ampoules of Orgaran and Na [sodium] heparin were supplied by Organon International B.V. and dispensed in numbered boxes by hospital pharmacies using predetermined randomisation sequences for each trial center." Comment: yes |
| Blinding of participants and personnel (performance bias) | High risk | No placebo used Quote: "double blind multicenter trial" Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo used Comment: probably not; however, knowledge of assigned intervention may not have impacted the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: judgment based on comparison between MPD rate (asymptomatic DVT: 23/513 = 4.48%, PE: 23/513 = 4.48%) and event rate (asymptomatic DVT 47/490 = 9.59%, PE 4/513 = 0.77%) |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on. Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized double‐blind trial | |
| Participants | 904 participants undergoing abdominal or pelvic surgery for cancer Mean age: not reported, % males: not reported, previous VTE % not reported | |
| Interventions | Intervention: RDH (Normiflo) 50 U/kg subcutaneously 2 h preoperatively and then 50 U/kg twice daily or 90 U/Kg once daily (an LMWH) Control: UFH 5000 U subcutaneously 2 h preoperatively and then 5000 U twice daily Discontinued treatment: 0 | |
| Outcomes | Duration of follow‐up: not clear
Screening testing for DVT: preoperatively by non‐invasive venous tests, either impedance plethysmography or duplex ultrasound scan Diagnostic testing for DVT: venography Diagnostic testing for PE: ventilation‐perfusion lung scan or pulmonary angiography | |
| Notes | Funding: KabiVitrum Ethical approval: not reported Conflict of interest: not reported Intention‐to‐treat analysis: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a total of 904 patients were randomised into three groups." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) | High risk | No placebo used Quote: "double blind randomised trial" Comment: definitely not blinded; knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo used Comment: probably not; however, the knowledge of the assigned intervention may not have impacted the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up 100% |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on. Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized, double‐blind clinical trial | |
| Participants | 150 participants undergoing craniotomy for primary or metastatic brain tumor | |
| Interventions | Intervention: enoxaparin 40 mg subcutaneously in the morning and a placebo injection in the evening Control: UFH 5000 U subcutaneously twice daily Cointervention: perioperative prophylaxis with graduated compression stockings and sequential intermittent pneumatic compression devices | |
| Outcomes | Duration of follow‐up: 30 days
Screening testing for DVT/PE: duplex venous ultrasonography examination Diagnostic testing for DVT/PE: criterion for diagnosing DVT was loss of venous compressibility | |
| Notes | Funding: clinical research grant from Aventis Ethical approval: not reported Conflict of interest: not reported Intention‐to‐treat analysis: yes, quote: "Patients were analyzed according to the intention‐to‐treat principle." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "one hundred fifty patients were randomized from June 1999 through September 2001, 75 patients to each of the two prophylaxis strategies." Comment: probably yes |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Randomized, prospective, double‐blind clinical trial Quote: "either enoxaparin, 40 mg, in the morning and a placebo injection in the evening vs 5,000 U of subcutaneous unfractionated heparin bid [twice daily]. Drug assignment was double blinded." Comment: definitely yes |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported Comment: probably not; however, the knowledge of the assigned intervention may not have impacted the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | High risk | Comment: varied by outcome; high for mortality with judgment based on comparison between MPD rate (mortality: 5/150 = 3.33%) and event rate (mortality: 0/150); high risk for symptomatic DVT (MPD rate: 10/150 = 6.66% and event rate 0/150) |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Prospective, single‐blind, non‐inferiority randomized trial | |
| Participants | 298 participants aged ≥ 40 year undergoing surgery for urologic malignancies Mean age: 64 years, 94% men | |
| Interventions | Intervention: enoxaparin (a LMWH) 2000 U subcutaneously twice daily Control: fondaparinux 2.5 mg subcutaneously once daily Cointervention: mechanical thromboprophylaxis | |
| Outcomes | Duration of follow‐up: up to 3 months
Screening testing for DVT/PE: none Diagnostic testing for DVT/PE: multi detector‐row computed tomography. | |
| Notes | Funding: Glaxo Smith Kline KK and Kaken Pharmaceutical Co. Ltd Ethical approval: carried out under the Declaration of Helsinki and applicable clinical practice. Institutional ethics committees approved the study protocol, and informed consent was obtained from all participants. Conflict of interest: none declared Intention‐to‐treat analysis: yes, quote: "All the analyses were carried out in the intention‐to‐treat." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "prospective, single blind, non‐inferiority randomized trial" Personal communication with the author, "It was done by a software as a randomization." Comment: probably yes |
| Allocation concealment (selection bias) | Low risk | Personal communication with the author: "Allocation was concealed" Comment: definitely yes |
| Blinding of participants and personnel (performance bias) | High risk | No placebo was used. Quote: "prospective, single blind, non‐inferiority randomized trial...all (surgeons) were blinded to drug allocation until the end of the surgical procedure." Personal communication with the author, "All surgeons and medical staff were blinded to drug allocation until the end of the surgical procedure. All patients were blinded to it before they were given the allocation drug." Comment: probably not, knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions). |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo used Quote: "prospective, single blind, non‐inferiority randomized trial...all (surgeons) were blinded to drug allocation until the end of the surgical procedure." Personal communication with the author, "All surgeons and medical staff were blinded to drug allocation until the end of the surgical procedure. All patients were blinded to it before they were given the allocation drug." Comment: probably not; however, the knowledge of the assigned intervention may not have impacted the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | High risk | Rate of participants who did not receive allocated treatment 5% in intervention and control arms Comment: judgment based on comparison between MPD rate (VTE: 16/298 = 5.36%) and event rate (VTE: 2/282 = 0.7%) |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on. Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized double‐blind trial | |
| Participants | 706 participants aged ≥ 40 years with an underlying malignancy (of 1351 participants (52%)) undergoing general or gynecologic surgery Mean age: 59.6 years, % men not reported, previous VTE % not reported | |
| Interventions | Intervention: reviparin sodium (a LMWH) 1750 anti‐Xa IU subcutaneously once daily with 2nd injection of saline (placebo) 12 h later Control: UFH 5000 IU subcutaneously every 12 h Treatment commenced 2 h prior to surgery followed by 2nd injection 8 h postoperatively and continued for at least 5 days (longer if the participant was still confined to bed) Discontinued treatment: 0 | |
| Outcomes | Duration of follow‐up: not clear
Screening testing for DVT/PE: scheduled radioactive fibrinogen uptake test was done daily for DVT screening Diagnostic testing for DVT: phlebography Diagnostic testing for PE: ventilation‐perfusion lung scanning, pulmonary angiography, or both | |
| Notes | Funding: Knoll AG, Germany Ethical approval: "the study was conducted in compliance with the revised Declaration of Helsinki, Good Clinical Practice (GCP), and the regulations of the national health authorities of each country." Conflict of interest: not reported Intention‐to‐treat analysis: yes, quote: "the study was analysed in accordance with the intention‐to‐treat principle." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind multicenter trial;" "patients were randomly allocated to receive either LMWH 1750 anti‐Xa IU administered subcutaneously (SC) once daily with a second injection of saline (placebo) 12 hours later, or UFH 5000 IU SC every 12 hours." Comment: probably yes |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "the final diagnosis of DVT or PE was based on the assessment of a blinded expert committee." Comment: probably yes; however, the knowledge of the assigned intervention may not have impacted the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | High risk | Comment: varies: low for asymptomatic DVT with judgment based on comparison between MPD rate (asymptomatic DVT 9/1351 = 0.66%) and event rate (asymptomatic DVT 58/1342 = 4.32%). Might be considered as high risk for mortality with MPD rate 9/1351 = 0.66% and event rate 8/1342 = 0.59%. |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on. Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized double‐blind controlled trial | |
| Participants | 6124 (27%) participants aged > 40 years undergoing surgery of ≥ 30 minutes' duration at 67 centers in Germany, Austria, and the Czech Republic Minimum age 40 years, mean age 62 years, % men not reported, previous VTE % not reported | |
| Interventions | Intervention: LMWH certoparin 3000 anti‐Xa IU subcutaneously once daily Control: UFH 5000 IU, administered subcutaneously 3 times daily Discontinued treatment: not applicable | |
| Outcomes | Duration of follow‐up: 14 days
Screening testing for DVT/PE: none Diagnostic testing for DVT/PE: none (fatal PE determined by autopsy) | |
| Notes | Funding: Novartis Pharma GmbH, Nürnberg, Germany Ethical approval: conducted in accordance with the Declaration of Helsinki. The ethics review board at each local center approved the study, under the supervision and guidance of a central ethics committee (The Ethics Committee, Regensburg). Conflict of interest: not reported Intention‐to‐treat analysis: yes, quote: "the analyses included all randomised patients (intention‐to‐treat)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised to one of two treatment groups using a centralised computer generated randomizations list." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind clinical trial;" "placebo injections were given to Certoparin patients to conform to the double blind trial design." Comment: definitely yes |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The statistical analysis was performed by an independent statistician and under the guidance of the Steering Committee." Comment: unclear; however, the knowledge of the assigned intervention may not have impacted the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | Unclear risk | Follow‐up 100% for mortality; 70% for fatal PE |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on Comment: probably no |
| Other bias | High risk | Quote: "the decision was taken to end the study prematurely as the study would not be sufficiently powered to show superiority of Certoparin over UFH." Comment: probably yes |
| Methods | Prospective, randomized, double‐blind multicentric study | |
| Participants | 673 participants aged ≥ 40 years undergoing major elective abdominal surgery 54.5% receiving LMWH and 58.5% receiving low‐dose heparin had malignant diseases | |
| Interventions | Intervention: 1 injection of LMWH sodium heparin 3,000 anti‐Xa U subcutaneously and 2 placebo injections per day started 2 h prior to surgery Control: 3 applications of UFH 5000 U subcutaneously per day started 2 h prior to surgery Discontinued treatment: 13 participants in LMWH group and 7 in heparin group | |
| Outcomes | Duration of follow‐up: not clear
Screening test for DVT: radiofibrinogen uptake test Diagnostic test for DVT: phlebography | |
| Notes | Funding: not reported Ethical approval: not reported Conflict of interest: not reported Intention‐to‐treat analysis: not reported Quote: "39 patients (78.0%) who developed DVT suffered from malignant diseases, compared to 330 (50.5%) without DVT." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "prospective, randomized, double‐blind multicentric study" Comment: probably yes |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "prospective, randomized, double‐blind multicentric study;" "the patients received either one injection of LMWH and two placebo injections or three applications of 5,000 U of unfractionated heparin per day." Comment: probably yes |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported Comment: probably not; however, the knowledge of the assigned intervention may not have impacted the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | High risk | Comment: judgment based on comparison between MPD rate (mortality: 20/673 = 2.9% symptomatic DVT: 20/673 = 2.9%) and event rate (mortality 5/653 = 0.76% symptomatic DVT 7/653 = 1.07%) |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized double‐blind trial | |
| Participants | 52 participants aged ≥ 40 years; undergoing surgery for gastric, colonic, and rectal malignancy Mean age: 70.35 years, % men not reported, previous VTE 5.8% | |
| Interventions | Intervention: dalteparin 5000 IU subcutaneously 2 h preoperatively then once daily for 6 days and placebo injection given each evening Control: heparin Kabi 2165 5000 U subcutaneously 2 h preoperatively then twice daily for 6 days Discontinued treatment: not clear | |
| Outcomes | Duration of follow‐up: 30 days
Screening testing for DVT/PE: radioactive fibrinogen uptake test used for DVT screening and performed preoperatively and then daily or every 2nd day for at least 7 postoperative days Diagnostic testing for DVT/PE: phlebography | |
| Notes | Funding: Kabivitrum Ethical approval: study approved by hospital's ethical committee. Conflict of interest: not reported Intention‐to‐treat analysis: yes, quote: "the data collected from 52 patients were therefore uniformly analysed on an "intention to treat" basis." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated to receive conventional heparin (heparin group) or LMWH KABI 2165 (LMWH group)." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind trial;" "a placebo injection was given each evening, in order in order to keep the study completely blind." Comment: probably yes |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported Quote: "double blind trial;" "a placebo injection was given each evening, in order in order to keep the study completely blind." Comment: probably not; however, the knowledge of the assigned intervention may not have impacted the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up: 100% |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized double‐blind parallel‐group trial | |
| Participants | A total of 129 patients aged between 18 to 75, with esophageal cancer patients and undergoing minimally invasive esophagectomy were enrolled from January 2011 to July 2012. | |
| Interventions | Intervention: fondaparinux sodium 2.5 mg subcutaneously once daily starting 6h after procedures. Control: nadroparin calcium 2850 anti‐Xa IU subcutaneously once daily starting 6h after procedures. Discontinued treatment: 3 participant in LMWH group and 1 in fondaparinux group | |
| Outcomes | Duration of follow‐up: 7 days
Screening testing for DVT/PE: ultrasound machine was used for DVT screening and was performed immediately after Diagnostic testing for DVT/PE: computed tomography pulmonary | |
| Notes | Funding: No Ethical approval: The Ethics Committee of Zhongshan Hospital approved the protocol (No. 2010‐186) Conflict of interest: No Intention‐to‐treat analysis: yes, Quote: "All primary analyses were performed on an intention‐to‐treat basis." Registered in ClinicalTrials.gov (NCT01267305) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Immediately after admission in SICU, the participants randomly received either subcutaneous nadroparin calcium 2850 IU (Fraxiparine ®, Glaxo Smith Kline, UK, Group H) or fondaparinux sodium 2.5 mg (Arixtra ®, Glaxo Smith Kline, UK, Group F) once daily in a 1:1 ratio based on a computer‐generated randomization list." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "In order to achieve a double‐blind study, the two kinds of anticoagulants were loaded into the similar syringes before use." |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported Comment: probably not; however, the knowledge of the assigned intervention may not have impacted the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up: 100% |
| Selective reporting (reporting bias) | Low risk | Study registered in ClinicalTrials.gov (NCT01267305). The protocol was published (No. 2010‐186), All relevant outcomes listed in the methods section were reported on. Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized double‐blind trial | |
| Participants | 60 participants with ovarian cancer undergoing surgery and chemotherapy Mean age 56.7 years, previous VTE 3.3% | |
| Interventions | Intervention: LMWH, certoparin sodium 3000 anti‐Xa U/day subcutaneously plus 2 placebo injections Control: UFH 5000 IU/day subcutaneously 3 times a day Prophylaxis was begun 2 h before operation and continued until the 7th postoperative day Discontinuation treatment: 0 | |
| Outcomes | Duration of follow‐up: 7 days
Screening testing for DVT/PE: impedance plethysmography was used for DVT screening on days 1, 3, 5, 7, and 10 Diagnostic testing for DVT/PE: ascending phlebography | |
| Notes | Funding: not reported Ethical approval: not reported Conflict of interest: not reported Intention‐to‐treat analysis: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "all patients were eligible for surgery and randomised to receive either daily LMWH or UFH." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "all 60 patients were randomised in double blind manner to receive either LMWH or UFH." "The dose of the LMWH was once 3000 anti Xa units/day s.c. [subcutaneously] plus 2 placebo injections and of UFH three times 5000 IU/day s.c." Comment: probably yes |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported Comment: probably not; however, the knowledge of the assigned intervention may not have impacted the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up 100% |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized double‐blind trial | |
| Participants | 350 participants with either histologically confirmed carcinoma of the breast, endometrium, vulva, or vagina, or with suspected ovarian malignancy; minimum age 40 years; mean age 61 years | |
| Interventions | Intervention: certoparin 3000 anti‐Xa U subcutaneously once daily plus 2 placebo injections (0.9% saline) Control: UFH 5000 IU subcutaneously 3 times daily Initial injection 2 h before the surgery always contained active drug. In both treatment arms, study medication was given at 8‐h intervals until 7th postoperative day Discontinuation treatment: not clear | |
| Outcomes | Duration of follow‐up: median of 1849 days in LMWH group and 1954 days in UFH group
Screening testing for DVT/PE: none Diagnostic testing for DVT/PE: none | |
| Notes | Funding: Novartis, Germany Ethical approval: study protocol reviewed and approved by an independent Ethics Committee Conflict of interest: not reported Intention‐to‐treat analysis: no, quote: "patients were not randomised according to intention to treat principle." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patient who randomly received LMW [low‐molecular weight] heparin (certoparin) compared to patients given UF [unfractionated] heparin for thrombosis prophylaxis during primary surgery." Comment: probably yes, particularly given the method of allocation concealment used |
| Allocation concealment (selection bias) | Low risk | Quote: "the boxes and ampoules of both heparins were labelled with a trial code number but were identical in appearance so neither the patient nor the staff were aware of the kind of heparin administered." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "randomised double blind trial" "LMW [low‐molecular heparin] heparin was given at a dose of 3,000 anti‐Xa units subcutaneously once daily in combination with 2 placebo injections (0.9% NaCl) [sodium chloride]." "The boxes and ampoules of both heparins were labeled with a trial code number but were identical in appearance so neither the patient nor the staff were aware of the kind of heparin administered." "The list with the trial code numbers remained at the manufacturer and the double‐blind conditions (medical staff, patients, and investigators) were maintained until the database was closed, and protocols were inspected by a study monitor." Comment: definitely yes |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported Comment: probably not, however the knowledge of the assigned intervention may not have impacted the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up 100% |
| Selective reporting (reporting bias) | High risk | Study appeared to have collected data on VTE outcomes but did not report them Comment: probably yes |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
| Methods | Randomized controlled trial | |
| Participants | 566 consecutive women attending the Gynaecological Oncology Unit at the Royal Women’s Hospital, Brisbane, for planned surgery Mean age; 55 years, 461 (81%) participants had malignant disease | |
| Interventions | Intervention: dalteparin sodium (Fragmin; an LMWH) 5000 U subcutaneously once daily Control: sodium heparin 5000 U subcutaneously twice daily Both interventions were given as subcutaneous injection at a site distant from the surgical site, most commonly into the thigh 12 h prior to surgery and continued for 5 days or until full activity was resumed, whichever was the longer. Cointervention: compression stockings and intermittent calf‐compression devices were also used by a small number of women with a previous history of DVT or PE. | |
| Outcomes | Duration of follow‐up: 6 weeks postoperative
Screening testing for DVT/PE: none Diagnostic testing for DVT/PE: ventilation‐perfusion lung scans, Doppler ultrasound, or venography, depending on the clinical situation | |
| Notes | Funding: not reported Ethical approval: "approval had previously been granted to the study by the ethics committee of the hospital" Conflict of interest: not reported Intention‐to‐treat analysis: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was by computer‐generated random numbers and was concealed from the treating surgeon until after the operation." |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was by computer‐generated random numbers and was concealed from the treating surgeon until after the operation." |
| Blinding of participants and personnel (performance bias) | High risk | No placebo used Comment: probably not, knowledge of the assigned intervention may have led to differential behaviors across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported Comment: probably not; however, the knowledge of the assigned intervention may not have impacted the assessment of the physiologic outcomes (mortality, DVT, PE, bleeding, etc.). |
| Incomplete outcome data (attrition bias) | High risk | Comment: judgment based on comparison between MPD rate (symptomatic DVT: 14/566 = 2.47%; PE: 14/566 = 2.47%) and event rate (symptomatic DVT: 1/552 = 0.18%; PE 6/552: = 1.08%) |
| Selective reporting (reporting bias) | Low risk | Study not registered. No published protocol. All relevant outcomes listed in the methods section were reported on Comment: probably no |
| Other bias | Low risk | Study not reported as stopped early for benefit Comment: probably no |
DVT: deep venous thrombosis; h: hour; IU: international units; LMWH: low‐molecular weight heparin; MPD: missing participant data ; PE: pulmonary embolism; U: unit; UFH: unfractionated heparin; V/Q: ventilation‐perfusion; VTE: venous thromboembolism.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not population of interest (people with cancer without VTE undergoing a surgical procedure) OR not comparison of interest (pharmacologic and mechanical thromboprophylaxis vs mechanical thromboprophylaxis only) | |
| Not population of interest (people with cancer with VTE); included 2 reports | |
| Not population of interest (hospitalized people with cancer) OR not comparison of interest (anticoagulation vs no anticoagulation); included 2 reports | |
| Comparison not of interest: UFH vs no anticoagulant | |
| Comparison not of interest: different doses of LMWH | |
| Comparison not of interest: extended vs standard perioperative thromboprophylaxis | |
| Not population of interest (people with cancer without VTE who had a surgical procedure) OR not comparison of interest (continue or discontinue thromboprophylaxis) | |
| Study included people with cancer as a subgroup for which outcome data were not available. | |
| Study included people with cancer as a subgroup for which outcome data were not available. | |
| Comparison not of interest: extended vs standard duration of thromboprophylaxis | |
| Not population of interest (surgical setting) | |
| Study included people with cancer as a subgroup for which outcome data were not available. | |
| Study included people with cancer as a subgroup for which outcome data were not available. | |
| Study included people with cancer as a subgroup for which outcome data were not available. | |
| Comparison not of interest: UFH vs no anticoagulant | |
| Comparison not of interest: study compared efficacy of a higher dose of heparin (7500 U twice daily) with the commonly used dose of 5000 U | |
| Outcome not of interest: study reported tPA and PAI‐1 activity | |
| Outcome not of interest: study compared the relative risk of bleeding when starting enoxaparin 2 h vs 12 h before surgery for colorectal cancer. | |
| Comparison not of interest: extended vs standard duration of thromboprophylaxis | |
| Not population of interest (ambulatory people with cancer without VTE) OR not intervention of interest (oral anticoagulant) | |
| Comparison not of interest: UFH vs no anticoagulant | |
| Comparison not of interest: comparison between 2 doses of UFH | |
| Comparison not of interest: UFH vs no anticoagulant | |
| Comparison not of interest: LMWH vs no anticoagulant | |
| Not population of interest (people with cancer without VTE undergoing a surgical procedure) OR not comparison of interest (pharmacologic thromboprophylaxis vs mechanical thromboprophylaxis) | |
| Not population of interest (hospitalitzed) | |
| Not population of interest (hospitalized people with cancer) OR not comparison of interest (anticoagulation vs no anticoagulation); included 3 reports | |
| Not population of interest (people with cancer with CVC without VTE); included 3 reports | |
| Comparison not of interest: defibrotide vs heparin | |
| Not population of interest (people with cancer without VTE undergoing a surgical procedure) OR not comparison of interest (pharmacologic thromboprophylaxis vs mechanical thromboprophylaxis vs both) | |
| Comparison not of interest: defibrotide | |
| Comparison not of interest: LMWH vs no anticoagulant | |
| Not population of interest (hospitalized people with cancer) OR not comparison of interest (LMWH vs UFH); included 3 reports | |
| Not population of interest (hospitalized people with cancer) OR not comparison of interest (LMWH vs UFH); included 2 reports | |
| Comparison not of interest: LMWH vs no anticoagulant | |
| Meta‐analysis | |
| Study included people with cancer as a subgroup for which outcome data were not available. | |
| Study included people with cancer as a subgroup for which outcome data were not available. | |
| Comparison not of interest: Extended vs time‐limited thromboprophylaxis | |
| Not population of interest (people with cancer who had a surgical procedure) OR not comparison of interest (continue vs discontinue thromboprophylaxis); included 2 reports | |
| Comparison not of interest: Extended vs time‐limited thromboprophylaxis | |
| Comparison not of interest: different types of LMWH | |
| Not population of interest (people with cancer without VTE undergoing a surgical procedure) OR not comparison of interest (initiate prophylaxis before vs after surgery); included 2 reports | |
| Not population of interest (ambulatory people with cancer without VTE) OR not comparison of interest (parenteral anticoagulant); included 2 reports | |
| Not comparison of interest (LMWH vs aspirin) | |
| Comparison was not of interest: extended vs time‐limited thromboprophylaxis | |
| Not population of interest (people with cancer with VTE); included 9 reports | |
| Study included people with cancer as a subgroup for which outcome data were not available. | |
| Study included people with cancer as a subgroup for which outcome data were not available. | |
| Not population of interest (ambulatory people with cancer without VTE) OR not comparison of interest (parenteral anticoagulant); included 4 reports | |
| Study included people with cancer as a subgroup for which outcome data were not available. | |
| Comparison not of interest: LMWH vs no anticoagulant | |
| Not population of interest (people with cancer without VTE undergoing a surgical procedure) OR not comparison of interest (pharmacologic thromboprophylaxis vs mechanical thromboprophylaxis) | |
| Not population of interest (people with cancer with VTE) | |
| Not population of interest (surgical setting) | |
| Not population of interest (people with cancer without VTE undergoing a surgical procedure) OR not comparison of interest (pharmacologic thromboprophylaxis vs mechanical thromboprophylaxis) | |
| Data for outcome of interest not available from report or author | |
| Not population of interest (people with cancer without VTE undergoing a surgical procedure) OR not comparison of interest (pharmacologic and mechanical thromboprophylaxis vs mechanical thromboprophylaxis only) | |
| Not population of interest (ambulatory people with low‐risk multiple myeloma without VTE) OR not comparison of interest (aspirin vs warfarin); included 6 reports | |
| Not population of interest (ambulatory people with cancer without VTE) OR not comparison of interest (parenteral anticoagulant); included 10 reports | |
| Not population of interest (people with cancer with VTE); included 2 reports | |
| Not population of interest (people with cancer with VTE); included 3 reports | |
| Not population of interest (people with cancer with VTE); included 1 report | |
| Comparison not of interest: LMWH (4 weeks) vs LMWH (1 week) | |
| Not population of interest (people with cancer without VTE undergoing a surgical procedure) OR not comparison of interest (pharmacologic thromboprophylaxis vs mechanical thromboprophylaxis) | |
| Study included people with cancer as a subgroup for which outcome data were not available. | |
| Not population of interest (people with VTE) | |
| Not population of interest (people with cancer with VTE) | |
| Not population of interest (people with cancer with VTE) | |
| Comparison not of interest: LMWH vs no anticoagulant | |
| Comparison not of interest: different types of LMWH | |
| Not population of interest (people with cancer without VTE undergoing a surgical procedure) OR not comparison of interest (pharmacologic and mechanical thromboprophylaxis vs mechanical thromboprophylaxis only) | |
| Comparison not of interest: LMWH vs no anticoagulant | |
| Not population of interest (ambulatory people with cancer without VTE) OR not comparison of interest (parenteral anticoagulant) | |
| Comparison was not of interest: extended vs standard duration of thromboprophylaxis | |
| Not population of interest (people with cancer who had a surgical procedure) OR not comparison of interest (continue vs discontinue thromboprophylaxis); included 5 reports | |
| Not population of interest (people with cancer with CVC without VTE); included 4 reports | |
| Not population of interest (people with cancer with VTE); included 1 report | |
| Not population of interest (people with cancer without VTE undergoing a surgical procedure) OR not comparison of interest (pharmacologic and mechanical thromboprophylaxis vs mechanical thromboprophylaxis only) | |
| Not population of interest (ambulatory people with cancer without VTE) OR not comparison of interest (parenteral anticoagulant); included 2 reports |
CVC: central venous catheter; h: hour; LMWH: low‐molecular weight heparin; PAI‐1: plasminogen activator inhibitor‐1; tPA: tissue plasminogen activator; UFH: unfractionated heparin; VTE: venous thromboembolism.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A Randomized, Controlled, Open Label Study of the Efficacy and Safety of the Low Molecular Weight Heparin (LMWH), LovenoxTM (Enoxaparin) versus HeparinTM (Unfractionated Heparin) for Prevention of Venous Thromboembolism (VTE) in Gynecologic Oncology Patients |
| Methods | Phase IIIB, randomized, open‐label, non‐comparative controlled trial |
| Participants | 150 gynecologic oncology participants with diagnosis of malignancy or suspension of malignancy in the Kingdom of Saudi Arabia who required major surgery or admission for the prevention of VTE, aged > 18 years |
| Interventions | Intervention: enoxaparin (LMWH) subcutaneously Control: UFH subcutaneously |
| Outcomes |
Diagnostic test for thromboembolic events: spiral computed tomography or ventilation‐perfusion scan, Doppler ultrasound, and coagulation profile parameter |
| Starting date | October 2009 |
| Contact information | Faisal Safi, MD Gynecology |
| Notes | NCT01356329 Status as of June 2018: suspended (difficulty enrolling participants) Sponsor: National Guard Health Affairs |
LMWH: low‐molecular weight heparin; UFH: unfractionated heparin; VTE: venous thromboembolism.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 8 | 4260 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.07] |
| Analysis 1.1 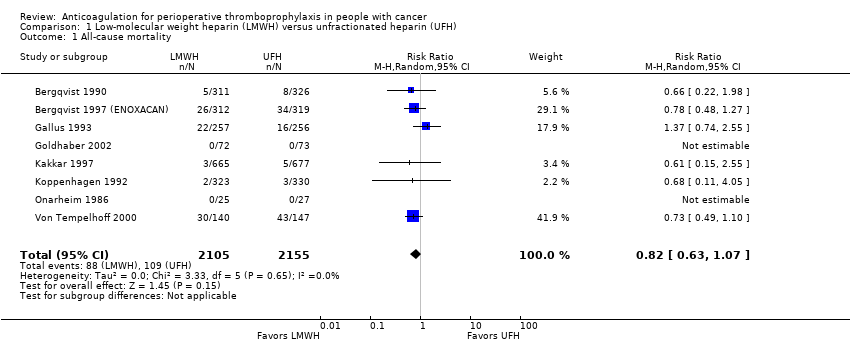 Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 1 All‐cause mortality. | ||||
| 2 Pulmonary embolism (PE) Show forest plot | 14 | 5588 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.17, 1.47] |
| Analysis 1.2 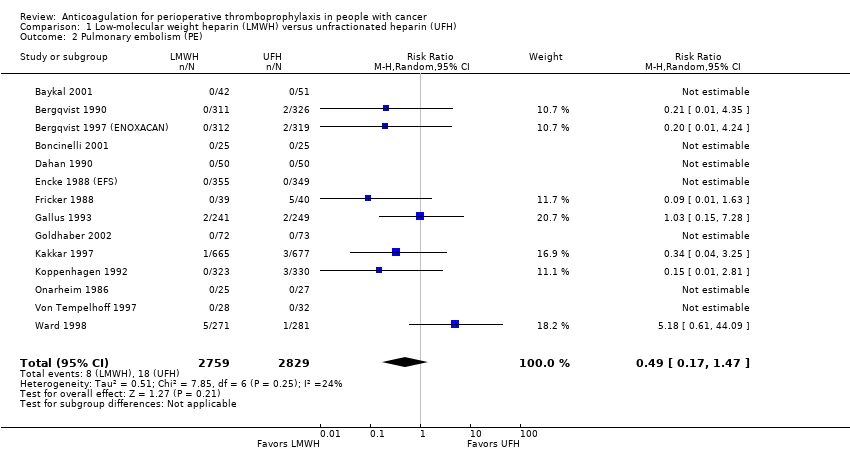 Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 2 Pulmonary embolism (PE). | ||||
| 3 Symptomatic deep venous thrombosis (DVT) Show forest plot | 8 | 2250 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.27, 1.69] |
| Analysis 1.3 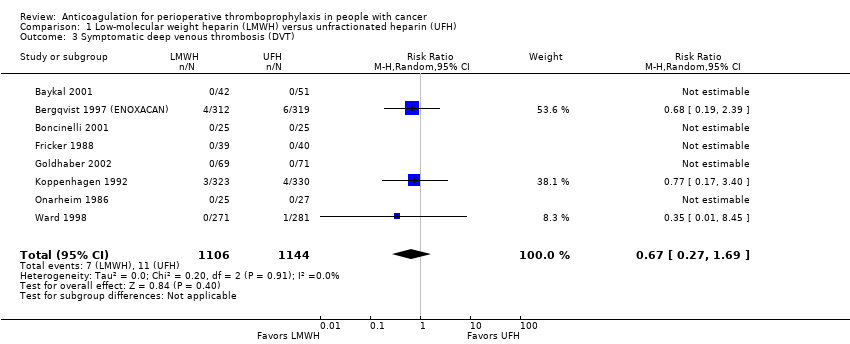 Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 3 Symptomatic deep venous thrombosis (DVT). | ||||
| 4 Asymptomatic DVT Show forest plot | 12 | 4938 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.71, 1.05] |
| Analysis 1.4 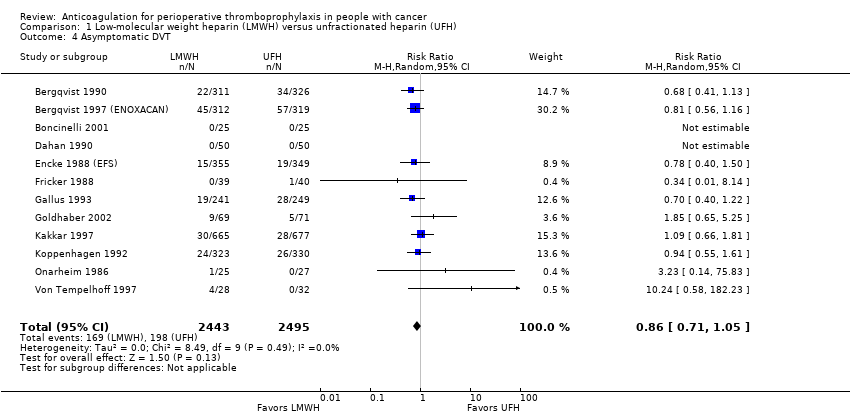 Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 4 Asymptomatic DVT. | ||||
| 5 Major bleeding Show forest plot | 9 | 3473 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.69, 1.48] |
| Analysis 1.5  Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 5 Major bleeding. | ||||
| 6 Minor bleeding Show forest plot | 2 | 1194 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.76, 1.33] |
| Analysis 1.6  Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 6 Minor bleeding. | ||||
| 7 Wound hematoma Show forest plot | 6 | 2827 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.54, 0.92] |
| Analysis 1.7  Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 7 Wound hematoma. | ||||
| 8 Reoperation for bleeding Show forest plot | 4 | 1246 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.57, 1.50] |
| Analysis 1.8  Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 8 Reoperation for bleeding. | ||||
| 9 Intraoperative transfusion Show forest plot | 2 | 737 | Mean Difference (IV, Random, 95% CI) | ‐35.36 [‐253.19, 182.47] |
| Analysis 1.9 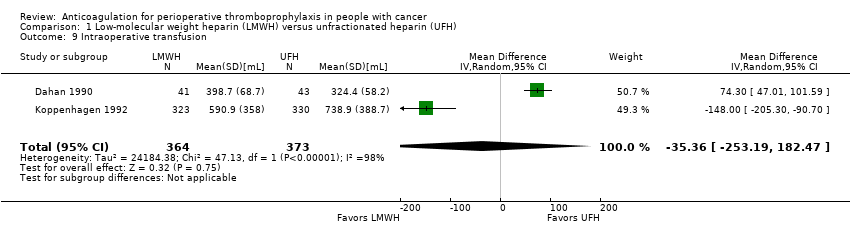 Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 9 Intraoperative transfusion. | ||||
| 10 Postoperative transfusion Show forest plot | 2 | 734 | Mean Difference (IV, Random, 95% CI) | 190.03 [‐23.65, 403.72] |
| Analysis 1.10  Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 10 Postoperative transfusion. | ||||
| 11 Intraoperative blood loss Show forest plot | 4 | 761 | Mean Difference (IV, Random, 95% CI) | ‐6.75 [‐85.49, 71.99] |
| Analysis 1.11  Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 11 Intraoperative blood loss. | ||||
| 12 Postoperative drain volume Show forest plot | 3 | 1459 | Mean Difference (IV, Random, 95% CI) | 30.18 [‐36.26, 96.62] |
| Analysis 1.12 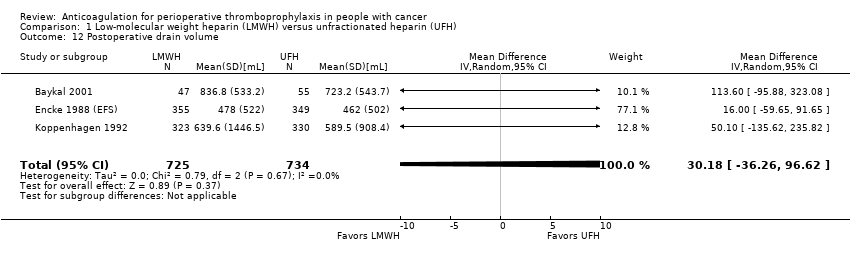 Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 12 Postoperative drain volume. | ||||
| 13 Thrombocytopenia Show forest plot | 2 | 683 | Risk Ratio (M‐H, Random, 95% CI) | 3.07 [0.32, 29.33] |
| Analysis 1.13  Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 13 Thrombocytopenia. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
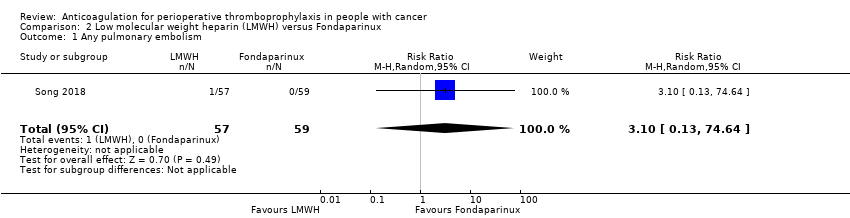
| 1 Any pulmonary embolism Show forest plot | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [0.13, 74.64] |
| Analysis 2.1 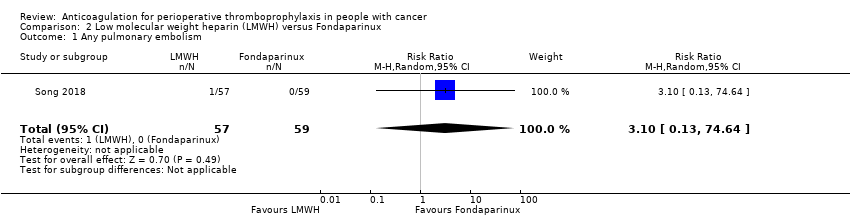 Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 1 Any pulmonary embolism. | ||||
| 2 Any venous thromboembolism (VTE) Show forest plot | 3 | 1806 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [0.89, 7.03] |
| Analysis 2.2 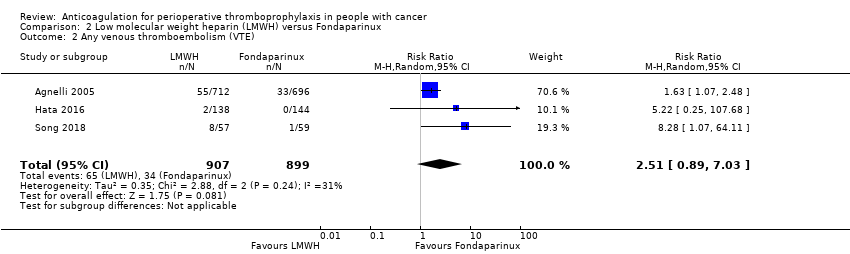 Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 2 Any venous thromboembolism (VTE). | ||||
| 3 Major Bleeding Show forest plot | 3 | 2339 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.45, 1.23] |
| Analysis 2.3 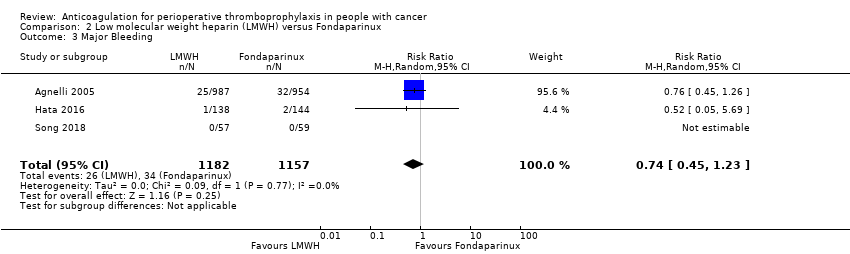 Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 3 Major Bleeding. | ||||
| 4 Minor Bleeding Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.34, 2.05] |
| Analysis 2.4  Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 4 Minor Bleeding. | ||||
| 5 Postoperative drain volume Show forest plot | 1 | 116 | Mean Difference (IV, Random, 95% CI) | ‐20.0 [‐114.34, 74.34] |
| Analysis 2.5  Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 5 Postoperative drain volume. | ||||
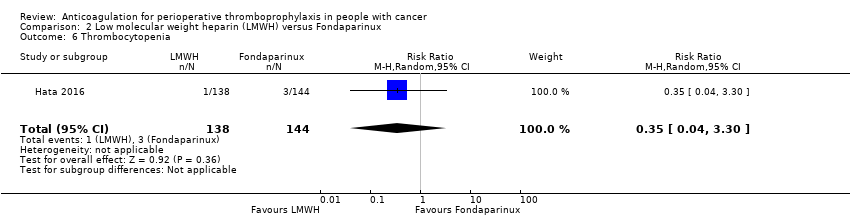
| 6 Thrombocytopenia Show forest plot | 1 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.30] |
| Analysis 2.6  Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 6 Thrombocytopenia. | ||||

Study flow diagram. CVC: central venous catheter; LMWH: low‐molecular weight heparin; RCT: randomized controlled trial; UFH: unfractionated heparin; VTE: venous thromboembolism.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 1 All‐cause mortality.

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 2 Pulmonary embolism (PE).

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 3 Symptomatic deep venous thrombosis (DVT).

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 4 Asymptomatic DVT.
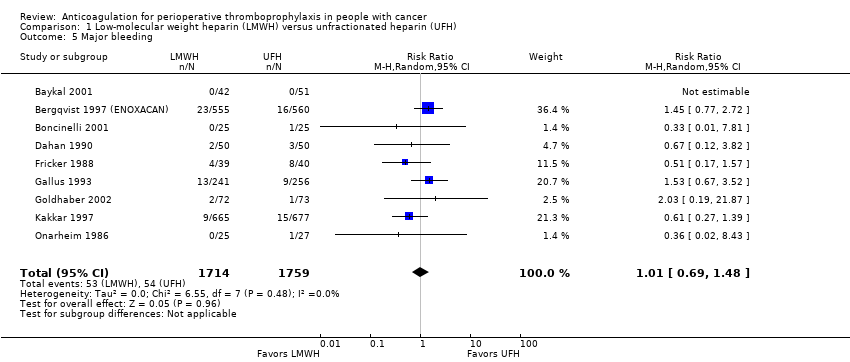
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 5 Major bleeding.
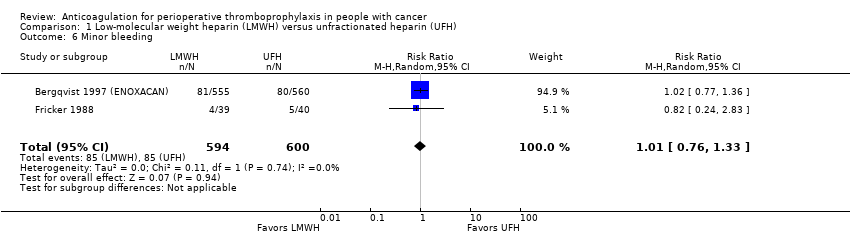
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 6 Minor bleeding.
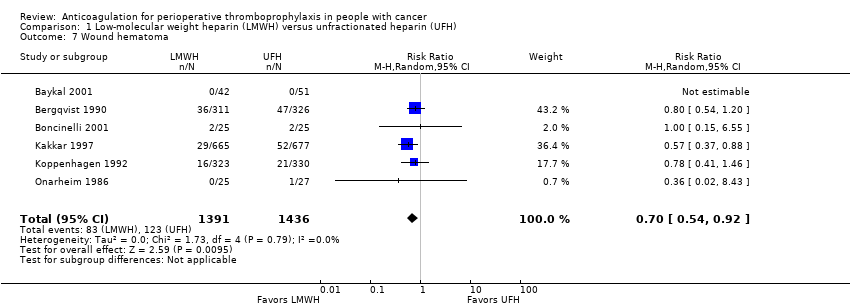
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 7 Wound hematoma.
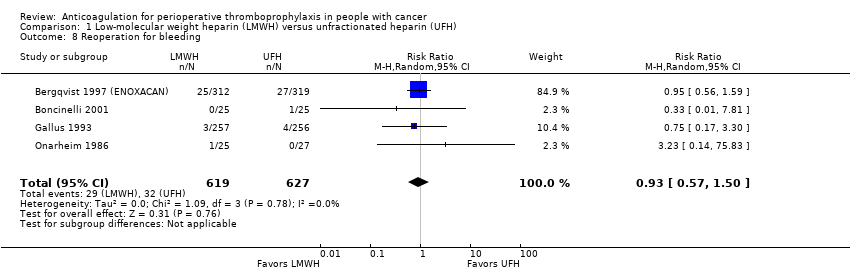
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 8 Reoperation for bleeding.

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 9 Intraoperative transfusion.
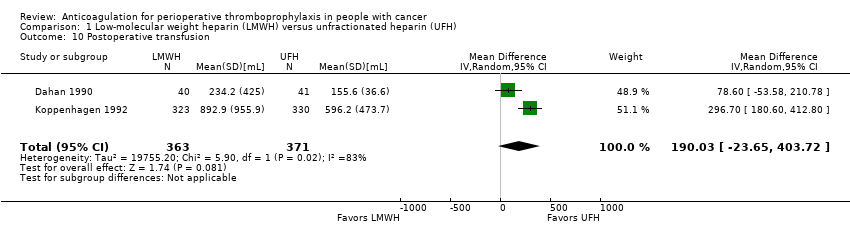
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 10 Postoperative transfusion.
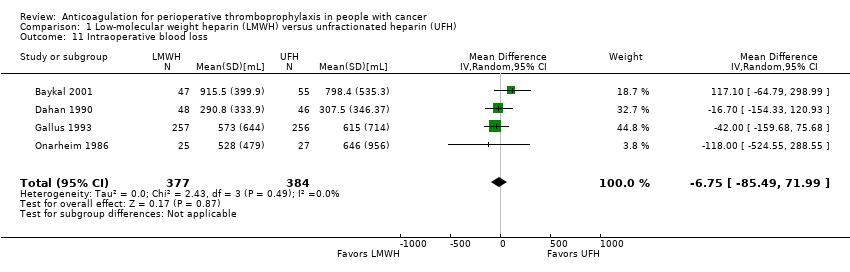
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 11 Intraoperative blood loss.

Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 12 Postoperative drain volume.
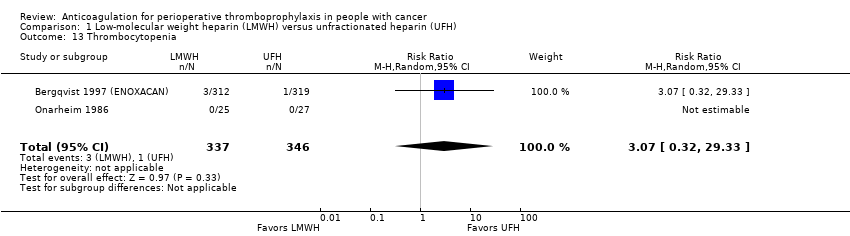
Comparison 1 Low‐molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 13 Thrombocytopenia.
Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 1 Any pulmonary embolism.

Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 2 Any venous thromboembolism (VTE).

Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 3 Major Bleeding.

Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 4 Minor Bleeding.

Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 5 Postoperative drain volume.
Comparison 2 Low molecular weight heparin (LMWH) versus Fondaparinux, Outcome 6 Thrombocytopenia.
| LMWH prophylaxis compared to UFH prophylaxis in people with cancer without VTE undergoing a surgery | |||||
| Patient or population: People with cancer with perioperative thromboprophylaxis | |||||
| Outcomes | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with UFH prophylaxis | Risk difference with LMWH prophylaxis | ||||
| Mortality | 4260 | ⊕⊕⊕⊝ | RR 0.82 | Study population | |
| 51 per 1000 | 9 fewer per 1000 | ||||
| Any PE | 5588 | ⊕⊕⊕⊝ | RR 0.49 | Study population | |
| 6 per 1000 | 3 fewer per 1000 | ||||
| Symptomatic DVT | 2250 | ⊕⊕⊕⊝ | RR 0.67 | Study population | |
| 10 per 1000 | 3 fewer per 1000 | ||||
| Symptomatic DVT measured as asymptomatic DVT | 4938 | ⊕⊕⊝⊝ | RR 0.86 | Study population | |
| 79 per 1000 | 11 fewer per 1000 | ||||
| Major bleeding | 3473 | ⊕⊕⊕⊝ | RR 1.01 | Study population | |
| 31 per 1000 | 0 fewer per 1000 | ||||
| Minor bleeding | 1194 | ⊕⊕⊕⊝ | RR 1.01 | Study population | |
| 142 per 1000 | 1 more per 1000 | ||||
| Wound hematoma | 2827 | ⊕⊕⊕⊝ | RR 0.70 | Study population | |
| 86 per 1000 | 26 fewer per 1000 | ||||
| Reoperation for bleeding | 1246 | ⊕⊕⊕⊝ | RR 0.93 | Study population | |
| 51 per 1000 | 4 fewer per 1000 | ||||
| Intraoperative transfusion | 737 | ⊕⊕⊝⊝ | ‐ | MD 35.36 lower | |
| Postoperative transfusion | 734 | ⊕⊕⊝⊝ | ‐ | MD 190.03 higher | |
| Intraoperative blood loss | 761 | ⊕⊕⊕⊝ | ‐ | MD 6.75 lower | |
| Postoperative drain volume | 1459 | ⊕⊕⊕⊝ | ‐ | MD 30.18 higher | |
| Thrombocytopenia | 683 | ⊕⊕⊕⊝ | RR 3.07 | Study population | |
| 3 per 1000 | 6 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (19 fewer per 1000 absolute reduction) and possibility of no effect (4 more per 1000 absolute increase), including 197 events in total. 2 Downgraded due to serious imprecision. Low event rate, 26 events in total 3 Downgraded due to serious imprecision. Low event rate, 18 events in total 4 Downgraded by one level due to serious inconsistency, outcome measured as surrogate outcome 5 Downgraded due to serious imprecision. 95% CI is consistent with the possibility of important benefit (23 fewer more per 1000 absolute reduction) and possibility of harm (4 more per 1000 increase), including 367 events in total. 6 Downgraded due to serious imprecision. 95% CI is consistent with the possibility of important benefit (10 fewer more per 1000 absolute reduction) and possibility of harm (15 more per 1000 increase), including 107 events in total. 7 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (34 fewer per 1000 absolute reduction) and possibility of no effect (47 more per 1000 absolute increase), including 170 events in total. 8 Downgraded due to serious risk of bias; allocation concealment was not clear in 5 out of 6 studies. 9 Downgraded due to serious imprecision. Low event rate, 206 events in total 10 Downgraded due to serious imprecision. 95% CI is consistent with the possibility of benefit (22 fewer per 1000 absolute reduction) and possibility of important harm (26 more per 1000 absolute increase), including 61 events in total. 11 Downgraded due to serious inconsistency. I2= 98%; Dahan 1990 included patients undergoing thoracic surgery for cancer whereas Koppenhagen 1992 included patients undergoing major elective abdominal surgery 12 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (253.19 mL less) and possibility of harm (182.47 mL more) 13 Downgraded due to serious inconsistency. I2 = 83%; Dahan 1990 included patients undergoing thoracic surgery for cancer whereas Koppenhagen 1992 included patients undergoing major elective abdominal surgery 14 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (23.65mL less) and possibility of harm (40.3.72mL more) 15 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (85.49 mL less) and possibility of harm (71.99 mL more) 16 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (36.26 mL less) and possibility of harm (96.62 mL more) 17 Downgraded due to serious imprecision. Low event rate, 4 events in total | |||||
| LMWH prophylaxis compared to fondaparinux prophylaxis in people with cancer without VTE undergoing a surgical procedure | |||||
| Patient or population: People with perioperative thromboprophylaxis in people with cancer | |||||
| Outcomes | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with Fondaparinux prophylaxis | Risk difference with LMWH prophylaxis | ||||
| Mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Any VTE | 1806 | ⊕⊕⊝⊝ | RR 2.51 | Study population | |
| 38 per 1000 | 57 more per 1000 | ||||
| Major Bleeding | 2339 | ⊕⊕⊝⊝ | RR 0.74 | Study population | |
| 29 per 1000 | 8 fewer per 1000 | ||||
| Minor Bleeding | 398 | ⊕⊕⊝⊝ | RR 0.83 | Study population | |
| 49 per 1000 | 8 fewer per 1000 | ||||
| Thrombocytopenia | 282 | ⊕⊕⊝⊝ | RR 0.35 | Study population | |
| 21 per 1000 | 14 fewer per 1000 | ||||
| Any Pulmonary embolism | 116 | ⊕⊕⊝⊝ | RR 3.10 | Study population | |
| 0 per 1000 | 0 fewer per 1000 | ||||
| Low | |||||
| 1 per 1000 | 2 more per 1000 | ||||
| Postoperative drain volume | 116 | ⊕⊕⊝⊝ | ‐ | The mean postoperative drain volume was 0 ml | MD 20 ml lower |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded by one level for concerns about both imprecision and indirectness. 95% CI is consistent with the possibility for benefit (4 per 1000 absolute reduction) and possibility of important harm (22 per 1000 absolute increase), including 99 events in total. VTE events included both symptomatic and asymptomatic events for patients with cancer which introduces some level of indirectness. 2 Downgraded by one level due to high risk of bias (lack of allocation concealment and incomplete outcome data in Agnelli 2005; lack of blinding of patients and personnel and incomplete outcome data in Hata 2016 unclear allocation concealment in song 2018) 3 Although the event rate used from the fondaprinux arm includes asymptomatic events, it is very close to rate of symptomatic VTE (3.1%) found in a retrospective cohort Changolkar 2014 4 Downgraded for serious imprecision. 95% CI is consistent with the possibility for important benefit (16 per 1000 absolute reduction) and possibility of important harm (7 per 1000 absolute increase), including 60 events in total. 5 Downgraded for high risk of bias (lack of blinding of patients and personnel and incomplete outcome data in Hata 2016 and unclear allocation concealment in Song 2018) 6 Downgraded for serious imprecision. 95% CI is consistent with the possibility for important benefit (33 per 1000 absolute reduction) and possibility of important harm (52 per 1000 absolute increase), including 18 events in total. 7 Downgraded for high risk of bias (lack of blinding of patients and personnel and incomplete outcome data in Hata 2016) 8 Downgraded for serious imprecision. 95% CI is consistent with the possibility for important benefit (20 per 1000 absolute reduction) and possibility of important harm (48 per 1000 absolute increase), including 4 events in total. 9 Downgraded by two levels for very serious imprecision. 95% CI is consistent with the possibility for important benefit (1 per 1000 absolute reduction) and possibility of important harm (78 per 1000 absolute increase), including 1 event in total. 10 Downgraded due to serious imprecision. 95% CI is consistent with the possibility for important benefit (114.34 mL less) and possibility of harm (74.34 mL more) | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 8 | 4260 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.07] |
| 2 Pulmonary embolism (PE) Show forest plot | 14 | 5588 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.17, 1.47] |
| 3 Symptomatic deep venous thrombosis (DVT) Show forest plot | 8 | 2250 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.27, 1.69] |
| 4 Asymptomatic DVT Show forest plot | 12 | 4938 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.71, 1.05] |
| 5 Major bleeding Show forest plot | 9 | 3473 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.69, 1.48] |
| 6 Minor bleeding Show forest plot | 2 | 1194 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.76, 1.33] |
| 7 Wound hematoma Show forest plot | 6 | 2827 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.54, 0.92] |
| 8 Reoperation for bleeding Show forest plot | 4 | 1246 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.57, 1.50] |
| 9 Intraoperative transfusion Show forest plot | 2 | 737 | Mean Difference (IV, Random, 95% CI) | ‐35.36 [‐253.19, 182.47] |
| 10 Postoperative transfusion Show forest plot | 2 | 734 | Mean Difference (IV, Random, 95% CI) | 190.03 [‐23.65, 403.72] |
| 11 Intraoperative blood loss Show forest plot | 4 | 761 | Mean Difference (IV, Random, 95% CI) | ‐6.75 [‐85.49, 71.99] |
| 12 Postoperative drain volume Show forest plot | 3 | 1459 | Mean Difference (IV, Random, 95% CI) | 30.18 [‐36.26, 96.62] |
| 13 Thrombocytopenia Show forest plot | 2 | 683 | Risk Ratio (M‐H, Random, 95% CI) | 3.07 [0.32, 29.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any pulmonary embolism Show forest plot | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [0.13, 74.64] |
| 2 Any venous thromboembolism (VTE) Show forest plot | 3 | 1806 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [0.89, 7.03] |
| 3 Major Bleeding Show forest plot | 3 | 2339 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.45, 1.23] |
| 4 Minor Bleeding Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.34, 2.05] |
| 5 Postoperative drain volume Show forest plot | 1 | 116 | Mean Difference (IV, Random, 95% CI) | ‐20.0 [‐114.34, 74.34] |
| 6 Thrombocytopenia Show forest plot | 1 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.30] |

