Administración de suplementos de vitamina E en pacientes con fibrosis quística
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009422.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 09 diciembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Peter Okebukola conceived of and wrote the text of the protocol and review alongside Sonal Kansra with comments from Joanne Barratt.
Sources of support
Internal sources
-
None, Other.
External sources
-
No sources of support supplied
Declarations of interest
None known.
Acknowledgements
We would like to thank Larissa Shamseer for her input into an early draft of the protocol and also Helen McCabe who was an author on the published protocol. Thanks also to Nikki Jahnke for all her help with editing the review and other administrative matters. Finally we would like to thank the authors of the included Keljo study for providing additional data.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Sep 06 | Vitamin E supplementation in people with cystic fibrosis | Review | Peter O Okebukola, Sonal Kansra, Joanne Barrett | |
| 2017 Mar 06 | Vitamin E supplementation in people with cystic fibrosis | Review | Peter O Okebukola, Sonal Kansra, Joanne Barrett | |
| 2014 Dec 09 | Vitamin E supplementation in people with cystic fibrosis | Review | Peter O Okebukola, Sonal Kansra, Joanne Barrett | |
| 2011 Nov 09 | Vitamin E supplementation in people with cystic fibrosis | Protocol | Peter O Okebukola, Sonal Kansra, Helen McCabe | |
Differences between protocol and review
We originally planned to present all formulations of vitamin E supplements as a single intervention, but in the full review we have presented the comparisons of water‐soluble vitamin E supplements versus control and fat‐soluble vitamin E supplements versus control separately.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Child; Child, Preschool; Humans; Infant;
PICO

Study flow diagram

Comparison 1 Water‐miscible vitamin E supplementation versus control, Outcome 1 Serum vitamin E levels.
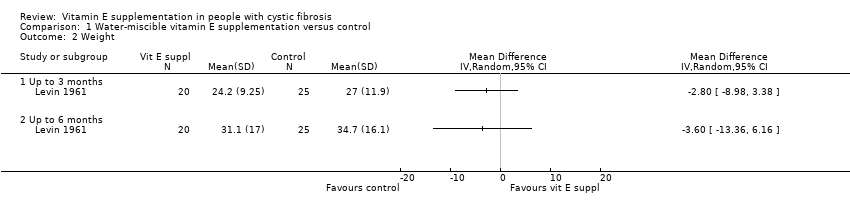
Comparison 1 Water‐miscible vitamin E supplementation versus control, Outcome 2 Weight.
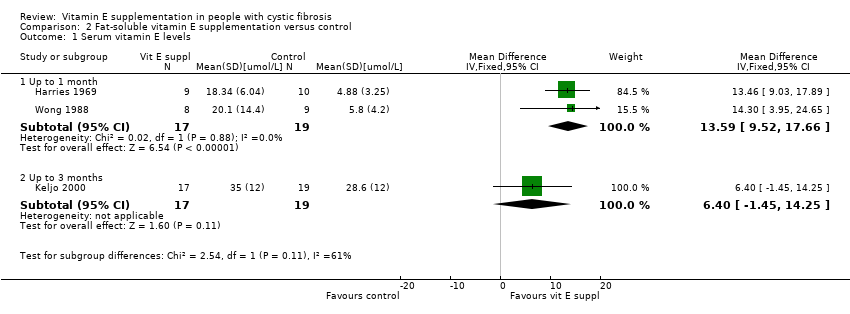
Comparison 2 Fat‐soluble vitamin E supplementation versus control, Outcome 1 Serum vitamin E levels.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serum vitamin E levels Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to 1 month | 2 | 32 | Mean Difference (IV, Fixed, 95% CI) | 17.66 [10.59, 24.74] |
| 1.2 Up to 3 months | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 11.61 [4.77, 18.45] |
| 1.3 Up to 6 months | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 19.74 [13.48, 26.00] |
| 2 Weight Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Up to 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Up to 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serum vitamin E levels Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to 1 month | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | 13.59 [9.52, 17.66] |
| 1.2 Up to 3 months | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 6.40 [‐1.45, 14.25] |

