Neuromuskuläre Elektrische Stimulation zur Behandlung von Muskelschwäche für Erwachsene mit Krankheiten im fortgeschrittenen Stadium
Appendices
Appendix 1. Search strategies
2016 search strategies
CENTRAL, DARE & CDSR (the Cochrane Library)
#1 MeSH descriptor: [Electric Stimulation Therapy] explode all trees
#2 ((muscle* or muscular or neuromuscular or neuro‐muscular) and electric* and stimulat*):ti,ab,kw (Word variations have been searched)
#3 NMES:ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
#5 MeSH descriptor: [Muscle Weakness] this term only
#6 ((muscle* or muscular) and (weak* or fatigue or strength)):ti,ab,kw (Word variations have been searched)
#7 #5 or #6
#8 (advance* near/6 (disease* or illness*)):ti,ab,kw (Word variations have been searched)
#9 MeSH descriptor: [Neoplasms] explode all trees
#10 (cancer* or neoplas* or malignan* or carcinoma* or tumor* or tumour* or metasta* or adenocarcinoma* or lymphoma* or leukemia* or leukaemia*):ti,ab,kw (Word variations have been searched)
#11 MeSH descriptor: [Pulmonary Disease, Chronic Obstructive] explode all trees
#12 (chronic and obstruct* and (pulmonary or airway* or airflow or lung*)):ti,ab,kw (Word variations have been searched)
#13 COPD:ti,ab,kw (Word variations have been searched)
#14 ((pulmonary or respiratory) near/6 disease*):ti,ab,kw (Word variations have been searched)
#15 MeSH descriptor: [Heart Diseases] explode all trees
#16 (((cardi* or heart) near/6 (disease* or failure)) or CHF):ti,ab,kw (Word variations have been searched)
#17 MeSH descriptor: [HIV] explode all trees
#18 human immunodeficiency virus*:ti,ab,kw (Word variations have been searched)
#19 human immuno‐deficiency virus*:ti,ab,kw (Word variations have been searched)
#20 acquired immunodeficiency syndrome*:ti,ab,kw (Word variations have been searched)
#21 acquired immuno‐deficiency syndrome*:ti,ab,kw (Word variations have been searched)
#22 (HIV or AIDS):ti,ab,kw (Word variations have been searched)
#23 (#8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22)
#24 (#4 and #7 and #23)
MEDLINE (OVID)
1 exp Electric Stimulation Therapy/
2 ((muscle* or muscular or neuromuscular or neuro‐muscular) and electric* and stimulat*).mp.
3 NMES.mp.
4 or/1‐3
5 Muscle Weakness/
6 ((muscle* or muscular) and (weak* or fatigue or strength)).mp.
7 5 or 6
8 (advance* adj6 (disease* or illness*)).mp.
9 exp neoplasms/
10 (cancer* or neoplas* or malignan* or carcinoma* or tumor* or tumour* or metasta* or adenocarcinoma* or lymphoma* or leukemia* or leukaemia*).mp.
11 exp Pulmonary Disease, Chronic Obstructive/
12 (chronic and obstruct* and (pulmonary or airway* or airflow or lung*)).mp.
13 COPD.mp.
14 ((pulmonary or respiratory) adj6 disease*).mp.
15 exp heart diseases/
16 (((cardi* or heart) adj6 (disease* or failure)) or CHF).mp.
17 exp HIV/
18 human immunodeficiency virus*.mp.
19 human immuno‐deficiency virus*.mp.
20 acquired immunodeficiency syndrome*.mp.
21 acquired immuno‐deficiency syndrome*.mp.
22 (HIV or AIDS).mp.
23 or/8‐22
24 4 and 7 and 23
25 (201207* or 201208* or 201209* or 201210* or 201211* or 201212* or 2013* or 2014* or 2015* or 2016*).ed.
26 24 and 25
Embase (OVID)
1 neuromuscular electrical stimulation/
2 ((muscle* or muscular or neuromuscular or neuro‐muscular) and electric* and stimulat*).mp.
3 NMES.mp.
4 or/1‐3
5 exp Muscle Weakness/
6 ((muscle* or muscular) and (weak* or fatigue or strength)).mp.
7 5 or 6
8 (advance* adj6 (disease* or illness*)).mp.
9 exp neoplasm/
10 (cancer* or neoplas* or malignan* or carcinoma* or tumor* or tumour* or metasta* or adenocarcinoma* or lymphoma* or leukemia* or leukaemia*).mp.
11 chronic obstructive lung disease/
12 (chronic and obstruct* and (pulmonary or airway* or airflow or lung*)).mp.
13 COPD.mp.
14 ((pulmonary or respiratory) adj6 disease*).mp.
15 exp heart disease/
16 (((cardi* or heart) adj6 (disease* or failure)) or CHF).mp.
17 exp Human immunodeficiency virus/
18 human immunodeficiency virus*.mp.
19 human immuno‐deficiency virus*.mp.
20 acquired immunodeficiency syndrome*.mp.
21 acquired immuno‐deficiency syndrome*.mp.
22 (HIV or AIDS).mp.
23 or/8‐22
24 4 and 7 and 23
25 (201207* or 201208* or 201209* or 201210* or 201211* or 201212* or 2013* or 2014* or 2015* or 2016*).dd.
26 24 and 25
27 random$.tw.
28 factorial$.tw.
29 crossover$.tw.
30 cross over$.tw.
31 cross‐over$.tw.
32 placebo$.tw.
33 (doubl$ adj blind$).tw.
34 (singl$ adj blind$).tw.
35 assign$.tw.
36 allocat$.tw.
37 volunteer$.tw.
38 Crossover Procedure/
39 double‐blind procedure.tw.
40 Randomized Controlled Trial/
41 Single Blind Procedure/
42 or/27‐41
43 (animal/ or nonhuman/) not human/
44 42 not 43
45 26 and 44
PsycINFO (OVID)
1 ((muscle* or muscular or neuromuscular or neuro‐muscular) and electric* and stimulat*).mp.
2 NMES.mp.
3 ((muscle* or muscular) and (weak* or fatigue or strength)).mp.
4 (advance* adj6 (disease* or illness*)).mp.
5 exp neoplasm/
6 (cancer* or neoplas* or malignan* or carcinoma* or tumor* or tumour* or metasta* or adenocarcinoma* or lymphoma* or leukemia* or leukaemia*).mp.
7 chronic obstructive pulmonary disease/
8 (chronic and obstruct* and (pulmonary or airway* or airflow or lung*)).mp.
9 COPD.mp.
10 ((pulmonary or respiratory) adj6 disease*).mp.
11 exp heart disorders/
12 (((cardi* or heart) adj6 (disease* or failure)) or CHF).mp.
13 exp Human immunodeficiency virus/
14 human immunodeficiency virus*.mp.
15 human immuno‐deficiency virus*.mp.
16 acquired immunodeficiency syndrome*.mp.
17 acquired immuno‐deficiency syndrome*.mp.
18 (HIV or AIDS).mp.
19 or/4‐18
20 1 or 2
21 3 and 19 and 20
22 limit 21 to yr="2012 ‐Current"
CINAHL (EBSCO)
S26 S24 AND S25
S25 EM 20120701‐20150131
S24 S4 AND S7 AND S23
S23 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22
S22 (HIV or AIDS)
S21 acquired immuno‐deficiency syndrome*
S20 acquired immunodeficiency syndrome*
S19 human immuno‐deficiency virus*
S18 human immunodeficiency virus*
S17 (MH "Human Immunodeficiency Virus+")
S16 (((cardi* or heart) N6 (disease* or failure)) or CHF)
S15 (MH "Heart Diseases+")
S14 ((pulmonary or respiratory) N6 disease*)
S13 COPD
S12 (chronic and obstruct* and (pulmonary or airway* or airflow or lung*))
S11 (MH "Pulmonary Disease, Chronic Obstructive+")
S10 (cancer* or neoplas* or malignan* or carcinoma* or tumor* or tumour* or metasta* or adenocarcinoma* or lymphoma* or
leukemia* or leukaemia*)
S9 (MH "Neoplasms+")
S8 (advance* N6 (disease* or illness*))
S7 S5 OR S6
S6 ((muscle* or muscular) and (weak* or fatigue or strength))
S5 (MH "Muscle Weakness")
S4 S1 OR S2 OR S3
S3 NMES
S2 ((muscle* or muscular or neuromuscular or neuro‐muscular) and electric* and stimulat*)
S1 (MH "Electric Stimulation+")
2012 search strategies
MEDLINE, CINAHL, EMBASE, and PsycINFO (Ovid Web)
1. exp Electric Stimulation Therapy/
2. ((muscle* or muscular or neuromuscular or neuro‐muscular) and electric* and stimulat*).mp.
3. NMES.mp.
4. 1 or 2 or 3
5. Muscle Weakness/
6. ((muscle* or muscular) and (weak* or fatigue or strength)).mp.
7. 5 or 6
8. (advance* adj6 (disease* or illness*)).mp.
9. exp neoplasms/
10. (cancer* or neoplas* or malignan* or carcinoma* or tumor* or tumour* or metasta* or adenocarcinoma* or lymphoma* or leukemia* or leukaemia*).mp.
11. exp Pulmonary Disease, Chronic Obstructive/
12. (chronic and obstruct* and (pulmonary or airway* or airflow or lung*)).mp.
13. COPD.mp.
14. ((pulmonary or respiratory) adj6 disease*).mp.
15. exp heart diseases/
16. (((cardi* or heart) adj6 (disease* or failure)) or CHF).mp.
17. exp HIV/
18. human immunodeficiency virus*.mp.
19. human immuno‐deficiency virus*.mp.
20. acquired immunodeficiency syndrome*.mp.
21. acquired immuno‐deficiency syndrome*.mp.
22. (HIV or AIDS).mp.
23. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24. 4 and 7 and 23
British Nursing Index (ProQuest)
1. NMES
2. muscle stimulation
3. neuromuscular electrical stimulation
4. (1 or 2 or 3)
Science Citation Index Expanded (Web of Science)
1. ((muscle* or muscular or neuromuscular or neuro‐muscular) and electric* and stimulat*). ti.
2. NMES. ti.
3. 1 or 2
4. (cancer* or neoplas* or malignan* or carcinoma* or tumor* or tumour* or metasta* or adenocarcinoma* or lymphoma* or leukemia* or leukaemia*). ti.
5. ((chronic and obstruct* and (pulmonary or airway* or airflow or lung*)) or COPD). ti.
6. (((cardi* or heart) adj6 (disease* or failure)) or CHF). ti.
7. (human immunodeficiency virus* or HIV or AIDS). ti.
8. 4 or 5 or 6 or 7
9. 3 and 8
The Cochrane Library (Wiley Online Library)
1. ELECTRIC STIMULATION THERAPY single term (MeSH)
2. RESISTANCE TRAINING single term (MeSH)
3. ((muscle* or muscular or neuromuscular or neuro‐muscular) and electric* and stimulat*).ti.
4. (cancer* or neoplas* or malignan* or carcinoma* or tumor* or tumour* or metasta* or adenocarcinoma* or lymphoma* or leukemia* or leukaemia*)
5. (COPD or chronic and obstruct* and (pulmonary or airway* or airflow or lung*))
6. (((cardi* or heart) adj6 (disease* or failure)) or CHF)
7. HIV or human immunodeficiency virus*
8. 3 and (4 or 5 or 6 or 7)
key: [mp = protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] [ti = title].

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
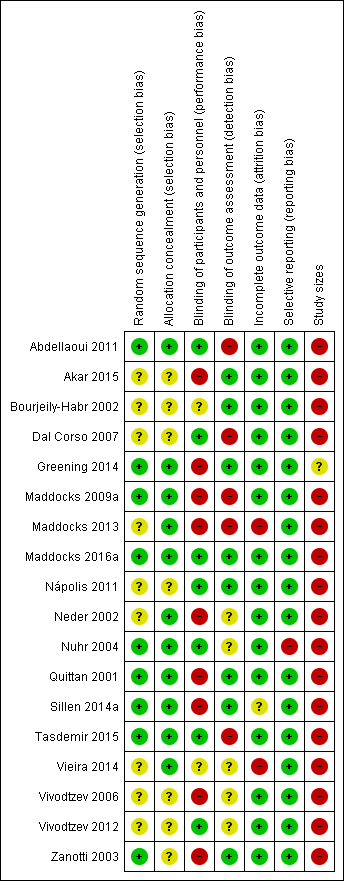
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of quadriceps muscle strength for NMES versus control.
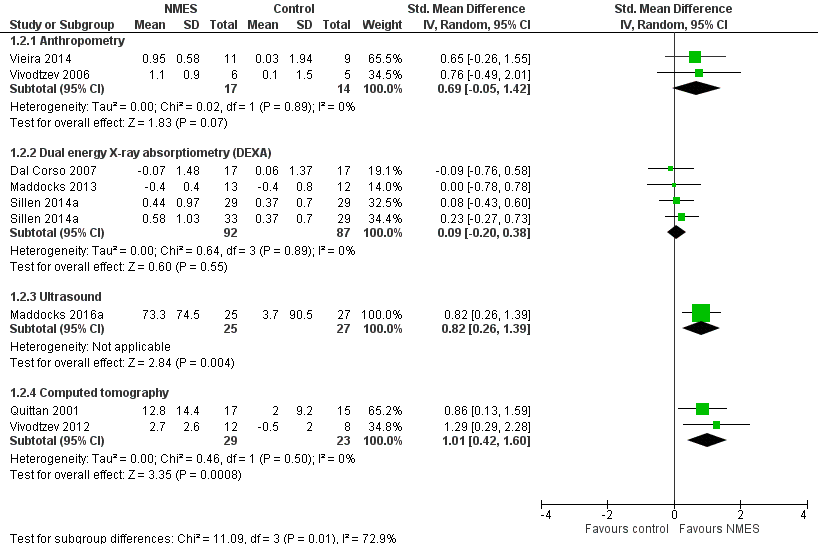
Forest plot of muscle mass for NMES versus control.

Forest plot of exercise performance for NMES versus control.

Comparison 1 Neuromuscular electrical stimulation versus control, Outcome 1 Quadriceps muscle strength.

Comparison 1 Neuromuscular electrical stimulation versus control, Outcome 2 Muscle mass.

Comparison 1 Neuromuscular electrical stimulation versus control, Outcome 3 Exercise performance.
| NMES for adults with advanced disease for muscle weakness | ||||
| Patient or population: adults with advanced disease for muscle weakness Control: no intervention (7 studies), placebo NMES (8 studies), or resistance training (1 study) | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | |||
| Control | NMES | |||
| Quadriceps muscle strength | The mean change was 0.43 standard deviations from baseline. | The mean change in the intervention groups was 0.53 standard deviations higher (ranging from 0.19 to 0.87 standard deviations higher). | 781 | ⊕⊕⊝⊝ |
| Safety | No serious adverse events related to control interventions reported. | No serious adverse events related to NMES reported. | 933 | ⊕⊕⊕⊝ |
| Safety Adverse events: Muscle discomfort | 0/415 (0%) participants reported muscle discomfort following control interventions. | 19/518 (3.7%) participants reported muscle discomfort following NMES. | 933 (18 studies) | ⊕⊕⊕⊝ |
| Muscle mass | The mean change in muscle mass ranged from 0.04 to 0.49 standard deviations from baseline across the different assessment modalities used. | The mean change in muscle mass ranged from 0.09 to 1.01 standard deviations higher across the different assessment modalities used. | 314 | ⊕⊝⊝⊝ |
| Exercise performance ‐ walking distance | The mean change in distance walked was 21, 36, and 37 metres from baseline across the different walking tests used. | The mean change in distance walked was 35, 9, and 64 metres further across the different walking tests used. | 788 | ⊕⊝⊝⊝ |
| Exercise performance ‐ peak oxygen uptake | The mean change in peak oxygen uptake was ‐0.4 mL/min from baseline. | The mean exercise performance ‐ peak oxygen uptake in the intervention groups was 44.8 mL/min higher (95% CI 7.3 lower to 97.0 higher) | 109 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk is the mean change from baseline in the control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1Downgraded once: the lower 95% CI for the estimate of effect was below what would be considered a small effect (standardised mean difference 0.2). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quadriceps muscle strength Show forest plot | 12 | 781 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [0.19, 0.87] |
| 2 Muscle mass Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Anthropometry | 2 | 31 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [‐0.05, 1.42] |
| 2.2 Dual energy X‐ray absorptiometry (DEXA) | 3 | 179 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.20, 0.38] |
| 2.3 Ultrasound | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.82 [0.26, 1.39] |
| 2.4 Computed tomography | 2 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 1.01 [0.42, 1.60] |
| 3 Exercise performance Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 6‐minute walk test (m) (6MWT) | 7 | 317 | Mean Difference (IV, Random, 95% CI) | 34.78 [13.52, 56.05] |
| 3.2 Incremental shuttle walk test (m) (ISWT) | 3 | 434 | Mean Difference (IV, Random, 95% CI) | 8.72 [‐34.87, 52.31] |
| 3.3 Endurance shuttle walk test (m) (ESWT) | 4 | 452 | Mean Difference (IV, Random, 95% CI) | 64.13 [‐17.79, 146.05] |
| 3.4 Cardiopulmonary exercise testing (mL/min) (CPET) | 4 | 109 | Mean Difference (IV, Random, 95% CI) | 44.82 [‐7.34, 96.99] |

