Haloperidol untuk psikosis berpunca dari keagresifan atau gelisah (tranquilisisasi pantas)
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised. Blindness: blind for some outcomes. Duration: 72 hours. | |
| Participants | Diagnosis: Research Diagnostic Criteria (11) diagnosis of schizophrenia, any type, or manic depressive illness, manic type and grossly abnormal behavior. N = 34. Age: mean 25.2 years. Sex: 26 males, 8 females. History: "violent or assaultive or too psychologically disorganized to be maintained on a unit without significant extra supervision." (p.128). Excluded: not reported. Setting: inpatient, USA. | |
| Interventions | 1. Haloperidol IM: dose 5 mg or 10 mg/IM, maximum cumulative dose of 100 mg during 24 hours (mean number of IMs not reported). N = 20. 2. Chlorpromazine IM: dose 50 mg or 100 mg/IM, maximum cumulative dose of 500 mg during 24 hours (mean number of IMs not reported). N = 14. | |
| Outcomes | Adverse events: leaving the study early. Unable to use: Mental state: BPRS (mean and overall P value only for significant findings reported, SD/SE/CI not reported). Global state: GAS (not published, mean and overall P value only for significant findings reported, SD/SE/CI not reported). Global state: NOSIE (overall P value only for significantly findings reported, mean/SD/SE/CI not reported). Adverse events: SAS (overall P value only for significantly findings reported, mean/SD/SE/CI not reported). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number sequence". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | BPRS was rater‐blinded. However, it is not clear whether other outcomes were blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is reported that no one discontinued due to adverse effects, however it does not state if there were discontinuations due to any other reason. |
| Selective reporting (reporting bias) | High risk | Only the significant findings/factors are reported for a number of outcomes. |
| Other bias | High risk | Small short study. |
| Methods | Allocation: randomised. Blindness: single (rater‐blinded)*. Duration: 12 hours. | |
| Participants | Diagnosis: DSM‐IV‐TR criteria for bipolar (maniac or mixed episode) or psychotic disorder. N = 150. Age: 18‐50 years. Sex: 91 males, 59 females. History: "admitted to the Psychiatric Emergency Room" (p.31). Excluded: "disorders due to drug abuse, organic disorder, anxiety or personality disorder (DSM‐IV‐TR criteria), failure to agree to participate in the study, incapability of completing all steps and unstable clinical disease" (p.31). Setting: emergency room, São Paulo. | |
| Interventions | 1. Haloperidol IM: fixed dose 5 mg/IM. N = 30. 2. Olanzapine IM: fixed dose 10 mg/IM. N = 30. 3. Ziprasidone IM: fixed dose 20 mg/IM. N = 30. 4. Haloperidol IM: fixed dose 5 mg/IM + promethazine IM: fixed dose 50 mg/IM. N = 30. 5. Haloperidol IM: fixed dose 5 mg/IM + midazolam IM: fixed dose 15 mg/IM. N = 30. "Only additional doses of haloperidol + promethazine could be used" (p.31). | |
| Outcomes | Need for additional drugs for tranquillisation. Agitation: OASS**. Aggression: OAS**. Global state: restriction. Adverse events: death, RSS, EPS, excessive sedation, hypotension. | |
| Notes | *"patients were instructed not to reveal their current treatment to the investigators" (p.31). **endpoint scores at 1 hour are discordant between text (p.32‐33) and Table 2 (p.35); scores from Table 2 were used, due to compatibility with Figure 2 (p.36). Authors were contacted (3rd July 2016, 7th July 2016, 9th July 2016). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomization employed in this clinical trial was allocation by permuted blocks" (p.31); no further informations on the randomisation process. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | "study medications were packaged in identical color‐coded boxes" (p.31); stated as double blind, however "patients were instructed not to reveal their current treatment to the investigators" (p.31); no information whether the blindness was effective. |
| Blinding of outcome assessment (detection bias) | High risk | "Patients were assessed by two psychiatrists. The psychiatrists were all masked with regard to the patient’s treatment assignment" (p.31); no information whether the blindness was effective. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | Endpoint scores at 1 hour are discordant between text (p.32‐33) and Table 2 (p.35). |
| Other bias | Low risk | None obvious. |
| Methods | Allocation: randomised. Blindness: double. Duration: 24 hours. | |
| Participants | Diagnosis: mania (N = 13), psychoactive substance use (N = 16), psychosis not otherwise specified (N = 27), schizophrenia (N = 47), schizophreniform disorder (N = 1).* N = 100.** Age: range 18‐57 years. Sex: 73 males, 25 females. History: "psychosis and behavioral dyscontrol (agitated, aggressive, destructive, assaultive, or restless behavior." Excluded: "clinically obvious alcohol intoxication, central nervous system (CNS) depression, pregnancy, allergic hypersensitivity, delirium, neuroleptic malignant syndrome, airway obstruction, severe hypotension or hypertension, acute narrow angle glaucoma and treatment with a benzodiazepine or neuroleptic within the previous 24 hours (previous two weeks for fluphenazine decanoate and previous 4 weeks for haloperidol decanoate)." Setting: Emergency department, USA hospitals. | |
| Interventions | 1. Haloperidol IM: dose up to 6 injections of 5 mg (mean number of IMs 2.86). N = 35. 2. Lorazepam IM: dose up to 6 injections of 2 mg (mean number of IMs 2.87). N = 31. 3. Lorazepam IM: dose up to 6 injections of 2 mg + haloperidol 5 mg (mean number of IMs 2.41). N = 32. First 3 injections ‐ at least 1 hour apart, remainder 2 hours apart. Need for subsequent doses made by blinded evaluator. | |
| Outcomes | Tranquillisation or asleep. Global state: number of additional injections.** Adverse events: EPS, hypertension, others in over ≥ 9% of people. Unable to use ‐ Global state: CGI (reported, out of necessity, only for those awake), Efficacy Index (no data reported). Agitation: ABS (mean, SE/SD not reported, P values of significant findings reported, overall F value). Mental state: MBPRS (modified, unpublished; mean, SE/SD not reported, P values of significant findings reported, overall F value), Alertness Scale (no mean, SE/SD not reported, P values of significant findings reported). Physiological: vital signs (not reported). | |
| Notes | * 6 people had more than one diagnosis. ** 2 people excluded from efficacy analysis ‐ after enrolment they had received proscribed antipsychotic medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | "The ED [Emergency Department] psychiatrist who treated and rated the patient remained blinded to the identity of the patient's medication throughout treatment" (p.336). [italic added by reviewers] "Double blind" ‐ participant blinding not specifically mentioned. |
| Blinding of outcome assessment (detection bias) | Low risk | "The ED [Emergency Department] psychiatrist who treated and rated the patient remained blinded to the identity of the patient's medication throughout treatment" (p.336).[italic added by reviewers]. Not tested. |
| Incomplete outcome data (attrition bias) | High risk | "two patients were excluded from the efficacy analysis. It was determined after enrolment that both received proscribed antipsychotic medication before entering the study." |
| Selective reporting (reporting bias) | High risk | More details on level of significance for outcomes with P < 0.05. Adverse effects for central nervous system reported only if present in ≥ 9%. |
| Other bias | High risk | Sponsored by drug company. |
| Methods | Allocation: randomised. Blindness: double. Duration: 24 hours, preceded by a 2 hour screening period. | |
| Participants | Diagnosis: DSM‐IV schizophrenia, schizophreniform disorder, or schizoaffective disorder. N = 311 Age: mean 38.2 years. Sex: 204 males, 107 females. History: mean age of illness onset, years (SD); haloperidol 25.1 (8.3), olanzapine 23.5 (8.9), placebo 24.9 (8.0). Excluded: Pregnant or lactating women, people with serious medical illnesses or people currently prescribed antipsychotic medication via depot injection. Setting: Multi‐centre (Australia, Austria, Belgium, Canada, the Czech Republic, France, Greece, Hungary, Israel, the Republic of South Africa, Spain, the UK, and the USA). | |
| Interventions | 1. Haloperidol IM: dose up to 3 injections of 7.5 mg (mean number of IMs 1.27). N = 126. 2. Olanzapine IM: dose up to 3 injections of 10 mg (mean number of IMs 1.3). N = 131. 3. Placebo IM: up to 3 injections (mean number of IMs ˜ 1.5). N = 54. Need for subsequent doses at the discretion of the investigator. | |
| Outcomes | Mental state: BPRS. Global state: CGI, number of additional injections, use of benzodiazepine. Adverse effects: SAS, BAS, COSTART, QT changes, use of anticholinergic drugs. Agitation: ACES, PANSS‐EC. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | "Both drugs and placebo were administered in identical, color‐blinded, translucent standard syringes, and raters and study personnel were blind to treatment assignment" (Wright 2001, p.1149). [italic added by reviewers] |
| Blinding of outcome assessment (detection bias) | Low risk | "Both drugs and placebo were administered in identical, color‐blinded, translucent standard syringes, and raters and study personnel were blind to treatment assignment" (Wright 2001, p.1149). [italic added by reviewers] |
| Incomplete outcome data (attrition bias) | High risk | The authors only account for 5 of the 26 people who did not complete the 24 hours study. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. |
| Other bias | High risk | Sponsored by the manufacturers of olanzapine. |
| Methods | Allocation: randomised. Blindness: double. Duration: 24 hours. | |
| Participants | Diagnosis: schizophrenia, schizophreniform or schizoaffective disorder. N = 270. Age: range 18‐73 years. Sex: 155 males, 115 females. History: "recently hospitalised with acute agitation." Mean age at onset of illness 25.1 (SD 7.3) years. Excluded: people with "significant medical disorders, including alcohol and/or drug dependency, were excluded from this trial." Setting: Multi‐centre (Croatia, Italy, Romania and South Africa). | |
| Interventions | 1. Haloperidol IM: dose up to 3 injections of 7.5 mg/IM (mean number of IMs not reported, mean dose 9.9 mg (SD 4.6). N = 40. 2. Olanzapine IM: dose up to 3 injections of 2.5 mg/IM (mean number of IMs not reported, mean dose 4 mg (SD 1.5). N = 48. 3. Olanzapine IM: dose up to 3 injections of 5 mg/IM (mean number of IMs not reported, mean dose 6.9 mg (SD 2.7). N = 45. 4. Olanzapine IM: dose up to 3 injections of 7.5 mg/IM (mean number of IMs not reported, mean dose 9.8 mg (SD 3.8). N = 46. 5. Olanzapine IM: dose up to 3 injections of 10 mg/IM (mean number of IMs not reported, mean dose 12.6 mg (SD 4.9). N = 46*. 6. Placebo IM. N = 50. 2nd injection allowed 2 hours after 1st, 3rd injection allowed 4 hours after 2nd injection. Both to be administered within 20 hours of 1st injection. Need for subsequent doses at the discretion of the investigator. | |
| Outcomes | Mental state: BPRS Total, BPRS Positive. Global state: CGI‐S, additional injections, need for benzodiazepine. Agitation: ABS, PANSS‐EC. Adverse events: QT interval at 24 hours, hypotension**, acute dystonia**, EPS**. Unable to use*** ‐ Leaving the study early: reasons are not given for why participants did not complete the study. Adverse effects: SAS (mean, SE/SD not reported). Adverse effects: BAS (mean, SE/SD not reported). Adverse effects: need for anticholinergic therapy (olanzapine 10 mg/IM not reported). Adverse effects: QT interval at 2 hours (mean, SE/SD not reported). | |
| Notes | * For data analysis, where continuous outcomes were used, haloperidol was compared with Olanzapine 10 mg/IM as this is the dose referred to as usual by the BNF (BNF 2011). ** It is possible that this binary data was derived from the BAS scale. *** Additional data are reported by dose but not used in this review (Table 4 4; Table 5 5; Table 6 6; Table 7 7; Table 8 8) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Low risk | "Allocation concealed placebo‐controlled trial." Method of randomisation not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "unblinded third‐party personnel, who played no role in evaluating patients, were trained to handle and administer injections in identical, unmarked syringes." "Investigators, patients, clinical carers and raters were blinded." |
| Blinding of outcome assessment (detection bias) | Low risk | "Investigators, patients, clinical carers and raters were blinded." |
| Incomplete outcome data (attrition bias) | High risk | Reasons why all participants that left the study early were not reported. |
| Selective reporting (reporting bias) | Unclear risk | It is not clear whether all side effects are reported. |
| Other bias | High risk | Trial sponsored by manufacturers of olanzapine IM. |
| AE | Dose | 1 mg (N = 57) | 5 mg (N = 63) | 15 mg (N = 58) |
| At least 1 AE | 28 | 30 | 27 | |
| AE rated as serious | 1 | 2 | 0 | |
| Tachycardia | 3 | 2 | 0 | |
| Sinus tachycardia | 1 | 0 | 0 | |
| Vomiting | 1 | 0 | 3 | |
| Nausea | 0 | 6 | 2 | |
| Dizziness | 4 | 7 | 7 | |
| Headache | 4 | 11 | 8 | |
| Somnolence | 3 | 5 | 6 | |
| Akathisia | 0 | 2 | 0 | |
| Dystonia | 0 | 0 | 1 | |
| Agitation | 0 | 0 | 3 | |
| Pain at injection site | Not reported | 1 | 1 | |
| QTC abnormality | 4 | 4 | 3 | |
AE: adverse event
| Outcome | Dose | Mean | SD | N |
| Mean dose of study drug | 2.5 mg | 4 | 1.5 | 48 |
| 5 mg | 6.9 | 2.7 | 45 | |
| 7.5 mg | 9.8 | 3.8 | 46 | |
| Mean dose of benzodiazepines | 2.5 mg | 3.2 | 1.1 | 48 |
| 5 mg | 2.0 | 0 | 45 | |
| 7.5 mg | 3.0 | 1.4 | 46 |
| Rating scale (Mean change at 2 hr) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 2.5 mg | 1.3 | 1.5 | 48 |
| 5 mg | 2.3 | 1.9 | 45 | |
| 7.5 mg | 2.4 | 1.7 | 46 | |
| Specific behaviour: ABS | 2.5 mg | ‐5.8 | 5.5 | 48 |
| 5 mg | ‐9.0 | 5.5 | 45 | |
| 7.5 mg | ‐10.5 | 5.6 | 46 | |
| Specific behaviour: PANSS‐EC | 2.5 mg | ‐5.5 | 4.6 | 48 |
| 5 mg | ‐8.1 | 5.3 | 45 | |
| 7.5 mg | ‐8.7 | 5 | 46 | |
| Mental state: BPRS Total | 2.5 mg | ‐8.2 | 9.1 | 48 |
| 5 mg | ‐10.4 | 7.5 | 45 | |
| 7.5 mg | ‐12.0 | 7 | 46 | |
| Mental state: BPRS Positive | 2.5 mg | ‐1.5 | 3.1 | 48 |
| 5 mg | ‐1.7 | 2.8 | 45 | |
| 7.5 mg | ‐2.1 | 2.9 | 46 |
ABS: Agitated Behavior Scale
ACES: Agitation‐Calmness Evaluation Scale
BPRS: Brief Psychiatric Rating Scale
PANSS‐EC: Positive and Negative Syndrome Scale Excited Component
| Rating scale (mean change at 24 hr) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 2.5 mg | 0.9 | 0.8 | 48 |
| 5 mg | 1.1 | 1.1 | 45 | |
| 7.5 mg | 1 | 1 | 46 | |
| Specific behaviour: ABS | 2.5 mg | ‐5.7 | 4.2 | 48 |
| 5 mg | ‐6.7 | 5.9 | 45 | |
| 7.5 mg | ‐7.7 | 5.8 | 46 | |
| Specific behaviour: PANSS‐EC | 2.5 mg | ‐4.9 | 4.3 | 48 |
| 5 mg | ‐5.5 | 4.9 | 45 | |
| 7.5 mg | ‐5.5 | 4.1 | 46 | |
| Global outcomes: CGI‐S (24 hr) | 2.5 mg | ‐0.3 | 0.5 | 48 |
| 5 mg | ‐0.5 | 0.8 | 45 | |
| 7.5 mg | ‐0.6 | 0.7 | 46 | |
| Mental state: BPRS Total | 2.5 mg | ‐8.4 | 7.4 | 48 |
| 5 mg | ‐9.2 | 7.8 | 45 | |
| 7.5 mg | ‐9.6 | 7.5 | 46 | |
| Mental state: BPRS Positive | 2.5 mg | ‐1.5 | 2.3 | 48 |
| 5 mg | ‐2.0 | 2.6 | 45 | |
| 7.5 mg | ‐1.9 | 2.7 | 46 |
ABS: Agitated Behavior Scale
ACES: Agitation‐Calmness Evaluation Scale
BPRS: Brief Psychiatric Rating Scale
CGI‐S: Clinical Global Impression – Severity
PANSS‐EC: Positive and Negative Syndrome Scale Excited Component
|
| Dose | 2.5 mg | 5 mg | 7.5 mg |
| Outcome |
| N = 48 | N = 45 | N = 46 |
| Global outcome: > 1 injection | 25 | 17 | 14 | |
| Leaving the study early: lack of efficacy | 0 | 2 | 0 | |
| Adverse effects: EPS | 0 | 0 | 0 | |
| Adverse effects: akathisia | 0 | 2 | 0 | |
| Adverse effects: QT abnormality | 0 | 4 | 2 | |
EPS: Extrapyramidal Side Effects
| Methods | Allocation: randomised, 2:2:1 ratio. Blindness: double. Duration: 2‐hour screening period, 24 hours initial phase, 4‐day second phase, 35 days follow‐up. | |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia (N = 324) or schizoaffective disorder (N = 124). N = 448. Age: range 18‐69 years. Sex: males and females. History: voluntarily hospitalised, experiencing acute agitation, mean age of diagnosis ˜ 26.3 years. Excluded: a diagnosis of schizophreniform disorder; a psychiatric disorder other than schizophrenia requiring pharmacotherapy, those at significant risk of suicide, neurological diagnoses (including migraine, Parkinson's disease, epilepsy, Alzheimer's disease, residual of stroke, multiple sclerosis, transient cerebral ischaemic attacks, learning disability, cerebral palsy); a history of seizures, severe head trauma, abnormal ECG, stroke or evidence of other unstable medical conditions or adverse event, substance or alcohol dependence within 2 months of the study, history of neuroleptic malignant syndrome from antipsychotic agents, suspected substance‐induced psychiatric disorder or behavioural disturbance, benzodiazepine or anticholinergics within 4 hours before the first injection of study medication; lack of response to previous antipsychotic medication. Setting: Multi‐centre (USA, Czech Republic, France, Estonia, Latvia, Poland, Croatia, Italy, Puerto Rico, South Africa, and Spain). | |
| Interventions | 1. Haloperidol: dose 6.5 mg/IM, maximum 3 doses during first 24 hours (mean number of IMs 1.43). N = 185. Followed by haloperidol: max dose 7 mg/oral to 10 mg/oral in 24 hours for 4 days. N = 151. 2. Aripiprazole: dose 10 mg/IM, maximum 3 doses during first 24 hours (mean number of IMs 1.54). N = 175. Followed by aripiprazole: max dose 10 mg/oral to 15 mg/oral in 24 hours for 4 days. N = 153. 3. Placebo IM, maximum 2 doses during first 24 hours. If 3rd dose needed aripiprazole: dose 10 mg/IM administered (mean number of IMs 1.92). N = 88. Followed by aripiprazole: max dose 10 mg/oral to 15 mg/oral in 24 hours for 4 days. N = 76. | |
| Outcomes | Global state: CGI‐S, CGI‐I, additional medication, need for benzodiazepine. Leaving the study early. Adverse events: concomitant medication, death, ECG, physical examination, vital signs. Leaving the study early. Unable to use: Adverse effects: SAS (SE/SD/CI not reported). Adverse effects: BAS (SE/SD/CI not reported). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Low risk | "Randomization was conducted via a centralized call‐in system and was organized by study center using permuted block randomization". |
| Blinding of participants and personnel (performance bias) | Unclear risk | During the IM phase, haloperidol, aripiprazole and placebo were delivered in 1.3 mL injections. However, in the oral phase a tablet was used for aripiprazole and a capsule was used for haloperidol and so it is possible that this presented a risk to blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as "double blind", however no further details are given. |
| Incomplete outcome data (attrition bias) | High risk | Reasons why all participants that left the study early were not reported. |
| Selective reporting (reporting bias) | High risk | The author mentions that there is increased glucose levels in both aripiprazole and haloperidol group but does not provide data. The authors only report the side effects that occurred in ≥ 2% of people. |
| Other bias | High risk | Sponsored by the manufacturers of aripiprazole. |
| Methods | Allocation: randomised. Blindness: double. Duration: 24 hours (preceded by 2‐hour screening period). | |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia (N = 237), schizoaffective disorder (N = 113) or schizophreniform disorder (N = 7). N = 357*. Age: 18‐66 years. Sex: 214 males, 143 females. History: acute agitation. Excluded: psychoactive substance dependence 2 months prior to the start of the study, required involuntary restraint, were suicidal, had a neurological or psychiatric condition other than schizophrenia, schizoaffective disorder or schizophreniform disorder, had a significant medical condition or were known non responders to antipsychotic medication. Setting: Multi‐centre (USA, Canada, Czech Republic, France, Estonia, Latvia, Lithuania, Spain). | |
| Interventions | 1. Haloperidol IM: dose up to 3 injections of 7.5 mg/IM (mean number of IMs 1.33). N = 60. 2. Aripiprazole IM: dose up to 3 injections of 1 mg/IM (mean number of IMs not reported). N = 57. 3. Aripiprazole IM: dose up to 3 injections of 5 mg/IM (mean number of IMs not reported). N = 63. 4. Aripiprazole IM: dose up to 3 injections of 10 mg/IM (mean number of IMs not reported). N = 57*. 5. Aripiprazole IM: dose up to 3 injections of 15 mg/IM (mean number of IMs not reported). N = 58. 6. Placebo IM (mean number of IMs not reported). N = 50. | |
| Outcomes | Mental state: BPRS Total and Positive Scores. Global state: CGI‐I, CGI‐S, number of re‐injections*. Agitation: ACES, CABS, PANSS‐EC. Leaving the study early. Adverse events: adverse event reports.Table 9 9 Unable to use***: Adverse effects: SAS (SE/SD/CI not reported). Adverse effects: BAS (SE/SD/CI not reported). Physiological: QTc is not reported for 9.75 mg. Results of clinical laboratory tests, physical examination, vital sign measurement are not reported. | |
| Notes | * For data analysis, where continuous outcomes were used, haloperidol was compared with aripiprazole 10 mg/IM as this is the dose referred to as usual by the BNF (BNF 2011). **The number of additional injections needed was not reported individually for each of the aripiprazole doses but given as a range (i.e. 56% to 60% received 1 injection), therefore the author chose the mean value (i.e. 58%). *** Additional data are reported by dose but not used in this review (Table 9 9;Table 10 10) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding was used during the preparation of injections and in most cases the person preparing the drug administered the injection. |
| Blinding of outcome assessment (detection bias) | Low risk | Study investigators conducting the assessments were blinded to treatment. |
| Incomplete outcome data (attrition bias) | Unclear risk | The reasons for leaving the study early were reported for those participants who did not complete the study. However, the number of participants completing different assessments at the same time point vary and reasons are not given. |
| Selective reporting (reporting bias) | High risk | The authors only report the adverse effects that occurred in ≥ 5% of people. The QTc abnormality for 9.75 mg is not reported. |
| Other bias | High risk | Sponsored by the manufacturers of aripiprazole. |
| Rating scale (Mean change 2 hr after 1st injection) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 1 mg | 0.65 | 1.64 | 56 |
| 5 mg | 1.01 | 1.65 | 62 | |
| 15 mg | 0.99 | 1.73 | 58 | |
| Specific behaviour: CABS | 1 mg | ‐5.15 | 6.8 | 56 |
| 5 mg | ‐5.97 | 6.81 | 62 | |
| 15 mg | ‐7.04 | 7.01 | 58 | |
| Global outcomes: CGI‐I | 1 mg | 3.07 | 0.98 | 56 |
| 5 mg | 2.82 | 1.10 | 62 | |
| 15 mg | 2.66 | 1.07 | 58 | |
| Global outcomes: CGI‐S | 1 mg | ‐0.63 | 1.15 | 56 |
| 5 mg | ‐0.82 | 1.12 | 62 | |
| 15 mg | ‐0.99 | 1.17 | 58 | |
| Mental state: BPRS Total | 1 mg | ‐6.53 | 9.61 | 55 |
| 5 mg | ‐8.16 | 9.66 | 61 | |
| 15 mg | ‐8.88 | 9.89 | 56 | |
| Mental state: BPRS Positive | 1 mg | ‐1.20 | 2.72 | 55 |
| 5 mg | ‐1.47 | 2.71 | 61 | |
| 15 mg | ‐1.86 | 2.82 | 57 |
ACES: Agitation‐Calmness Evaluation Scale
BPRS: Brief Psychiatric Rating Scale
CABS: CABS ‐ Corrigan Agitated Behavior Scale
CGI‐I: Clinical Global Impression – Improvement
CGI‐S: Clinical Global Impression – Severity
|
| Dose | 1 mg | 5 mg | 15 mg |
| Outcome | N | N = 57 | N = 63 | N = 58 |
| PEC response ≥ 40% | 21 | 31 | 32 | |
| Need for rescue medication | 11 | 5 | 12 | |
| Discontinued for any reason | 2 | 3 | 1 | |
| Discontinued due to adverse event | 0 | 0 | 1 | |
| Withdrew consent | 2 | 2 | 0 | |
| Discontinued due to other known cause | 0 | 1 | 0 | |
PEC: Psychiatric Emergency Centre
| Methods | Allocation: randomised. Blindness: open. Duration: 7 days. | |
| Participants | Diagnosis: diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, brief psychotic disorder, schizophreniform disorder, delusional disorder, or psychotic disorders not otherwise specified as defined by DSM‐III‐R. N = 132. Age: range 19‐66 years. Sex: 123 males, 9 females. History: recently hospitalised with acute psychosis. Excluded: substance‐induced psychosis (confirmed by urinalysis), psychosis related to organic origin, clinically relevant medical illness, abnormal ECG, imminent risk of suicide or homicide, history of substance abuse or dependence in the previous 2 months. Setting: Multi‐centre (7 countries). | |
| Interventions | 1. Haloperidol: flexible dose 2.5 mg/IM to 10 mg/IM (mean number of IMs not reported), maximum 4 doses in 24 hours for 3 days. Followed by haloperidol: flexible dose 10 mg/oral to 80 mg/oral during 24 hours for 4 days. N = 42. 2. Ziprasidone: dose 10 mg/IM, maximum 4 doses in 24 hours for 3 days (mean number of IMs not reported). Followed by ziprasidone: flexible dose 80 mg/oral to 200 mg/oral in 24 hours for 4 days. N = 90. | |
| Outcomes | Mental state: BPRS. Global state: CGI‐S. Leaving the study early. Adverse events: COSTART, BAS, modified SAS, investigators assessment of severity, blood pressure, laboratory tests, concomitant medication, ECG, urinalysis, others in ≥ 10 of participants. Unable to use Global state: CGI‐I (SE/SD not reported). Sedation score: 5‐point categorical scale (not published, present mean/SD rather than binary data). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computerized randomization assigned". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | Open trial. |
| Blinding of outcome assessment (detection bias) | High risk | Open trial. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. A table is provided which presents a summary of discontinuations. |
| Selective reporting (reporting bias) | High risk | The number of participants reported as discontinuing due to adverse effects is reported inconsistently (see Brook 1998c table 2 and Brook 2000 table 4). It is not clear whether all adverse events are reported. Brook 2000 table 4 only reports adverse events occurring in ≥10 of people, however there are more adverse events mentioned throughout the text. |
| Other bias | High risk | Sponsored by drug company. |
| Methods | Allocation: randomised. Blindness: double. Duration: 2 hours. | |
| Participants | Diagnosis: mental illness (N = 114), drug‐induced psychosis (N = 70), intoxication (N = 17), threatened self‐harm (N = 6), other/unknown (N = 19) (p.225)*. N = 228. Age: major than 18 years. Sex: 144 males, 84 females. History: admitted involuntarily to the psychiatric intensive care unit from the psychiatric emergency care centre (p.223). Excluded: patients willing to take oral medication for sedation without physical restraint or seclusion. Setting: psychiatry intensive care unit. | |
| Interventions | 1. Haloperidol: fixed dose 10 mg/IM. N = 110. 2. Droperidol: fixed dose 10 mg/IM. N = 118. | |
| Outcomes | Tranquillisation or asleep, time to sedation. Need for additional benzodiazepines. Adverse events: respiratory rate less than 12 breaths/min, systolic blood pressure less than 90 mmHg, heart rate less than 60 bpm, oxygen saturation less than 90% or the presence of extrapyramidal side effects. | |
| Notes | *when available, outcomes are reported for the mental illness sub‐group; gently provided by the authors (contacted 5th July 2016). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomisation was used. Microsoft Excel was used to randomly create blocks of four (ABAB, AABB, etc.) or six (ABABAB, AAABBB, etc.)" (p.224). |
| Allocation concealment (selection bias) | Low risk | "Pre‐packed treatment kits [...] contained either droperidol (10mg in 2ml) or haloperidol (10mg in 2ml) [...] transferred into vials identical" (p.224). |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study. |
| Blinding of outcome assessment (detection bias) | Low risk | "labels A or B and not the drug names were known to the investigator. This investigator analysed the data independently and presented this to the other investigators" (p.224). |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | "In breach of the study protocol midazolam was given nine times simultaneously with the study drug" (p.226); no further evidence of selective reporting. |
| Other bias | High risk | Short study. |
| Methods | Allocation: randomised. Blindness: single‐blind (rater‐blinded). Duration: 24 hours. | |
| Participants | Diagnosis: DSM‐IV diagnosis of paranoid schizophrenia (N = 54), schizoaffective disorder (N = 36), bipolar disorder (N = 13), psychotic disorder not otherwise specified (N = 31), other (N = 28). N = 162. Age: range 18‐65 years. Sex: 105 males, 57 females. History: acute agitation. Excluded: pregnancy, delirium, epilepsy, learning disability, intoxication, symptoms of withdrawal from alcohol or other psychoactive substances, medical illness, treatment with any anti‐psychotic or benzodiazepine within 6 hours of screening, history of neuroleptic malignant syndrome, known hypersensitivity to any of the trial medications, treatment with depot injection, use of disallowed medication. Setting: multi‐centre, USA (24 sites) | |
| Interventions | 1. Haloperidol: dose 5 mg/IM + lorazepam 2 mg/oral (mean number of IMs not reported). N = 79. 2. Risperidone: dose 2 mg/oral + lorazepam 2 mg/oral (mean number of doses not reported). N = 83. | |
| Outcomes | Tranquillisation or asleep. Leaving the study early. Global state: CGI‐S, additional lorazepam. Agitation: PANSS‐EC. Aggression: OAS. Adverse effects: BAS, SAS, EPS, heart monitoring, sedation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Low risk | "A telephone‐based central service was used to randomly assign eligible patients to receive a single dose of oral treatment or IM injection." (p.387) |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blind study. |
| Blinding of outcome assessment (detection bias) | Low risk | "independent raters blinded to the treatment arm conducted efficacy assessments". (p.387) |
| Incomplete outcome data (attrition bias) | Unclear risk | A flow diagram is provided with the reasons for leaving the study early reported. However, the number of participants completing different assessments at the same time point vary and reasons are not given. |
| Selective reporting (reporting bias) | High risk | The authors only report the side effects that occurred in ≥ 5% of people. |
| Other bias | High risk | Sponsored by drug company (Janssen Phamaceutica). |
| Methods | Allocation: randomised. Blindness: double. Duration: 2 hours. | |
| Participants | Diagnosis: DSM‐IV diagnosis of paranoid schizophrenia (N = 19), schizoaffective disorder (N = 7), bipolar disorder (N = 2). N = 28. Age: range 20‐60 years. Sex: 13 males, 15 females. History: "actively psychotic inpatients". (p.142). Excluded: Not reported. Setting: Psychiatric hospital, Tel Aviv. | |
| Interventions | 1. Haloperidol: dose 5 mg/IM (mean number of IMs not reported). N = 13. 2. Flunitrazepam: dose 1 mg/IM (mean number of IMs not reported). N = 15. | |
| Outcomes | Global state: need for seclusion or restraint. Aggression: OAS. Adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "assigned by a table of random numbers." (p.142). |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind, however further details regarding the blinding of participants and personnel are not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | "All ratings were completed by the same rater (N.K.), who was blind to the study medications." (p.143). |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | It is not clear whether all side effects are reported. |
| Other bias | Unclear risk | Small short study. |
| Methods | Allocation: randomised. Blindness: double. Duration: 24‐72 hours. | |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia. N = 49. Age: range 18‐65* years. Sex: 35 males, 14 females. History: "The mean length of current psychotic episode was 13.8 and 17.9 days in the olanzapine and haloperidol groups respectively." (p.6). Excluded: dementia, documented allergy to study drugs, treatment with injectable depot within 1 injection interval, unstable illnesses where death is anticipated within 1 year, or treatment in intensive care expected within 6 months, previous participation in a Lilly sponsored intra‐muscular olanzapine clinical trial. Setting: multi‐centre, Taiwan. | |
| Interventions | 1. Haloperidol: dose 7.5 mg/IM (mean number of IMs 1.13). N = 24. 2. Olanzapine: dose 10 mg/IM (mean number of IMs 1.32). N = 25. | |
| Outcomes | Mental state: BPRS total score, BPRS positive sub‐scale. Global state: CGI‐I, additional medication. Agitation: ACES, PANSS‐EC. Leaving the study early. Adverse effects: SAS, BAS, COSTART, death, laboratory analytes, ECG. Unable to use: Global state: CGI‐S. Adverse effects: change in vital signs (mean, SD CI not reported). | |
| Notes | *The age difference between the two groups was statistically significant (P = 0.011). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind, however no further details reported regarding the blinding of participants and personnel are not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind, however no further details reported regarding rater‐blinding. |
| Incomplete outcome data (attrition bias) | High risk | The number of patients that completed the study is unclear: authors assert 45 in a study, 46 participants in the other one out of a possible 49 participants. However, only 42 participants are included in the analysis for the CGI‐I (at the end of the study), SAS, BAS, clinical laboratory evaluations, vital signs and ECG, without explanation for the missing participants. |
| Selective reporting (reporting bias) | High risk | Only the CGI‐S baseline score is reported. |
| Other bias | High risk | Sponsored by the manufacturers of olanzapine. |
| Methods | Allocation: randomised. Blindness: open. Duration: 120 hours (as for session I). | |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia (N = 192), schizophreniform (N = 11) or schizoaffective (N = 0) disorders, no information* (N = 2); PANSS‐EC ≥14; PANSS ≥60. N = 208 screened, 205 randomised. Age: range 18‐45 years. Sex: 99 males, 106 females. History: acute agitation. Excluded: "women pregnant, breastfeeding or planning to become pregnant during the study", "psychotic agitation caused by delirium, epilepsy, mental retardation, or affective disorder", "intoxication or symptoms of withdrawal from alcohol or other psychoactive substances", "serious medical illness", "known hypersensitivity to any of the study medications or no response to risperidone or haloperidol during previous treatment", "treatment with a depot antipsychotic with one cycle of screening", "use of disallowed medication". Setting: multi‐centre (6), China. | |
| Interventions | 1. Haloperidol: flexible dose IM 10 mg/day to 20 mg/day (mean prescribed daily dose 12.2 ± 3.7 mg), N = 101. 2. Risperidone: flexible dose oral 2 mL/day to 6 mL/day (mean prescribed daily dose 3.4 ± 0.7 mg), N = 104 + clonazepam: flexible dose oral 0 mg/day to 8 mg/day (mean prescribed daily dose 2.9 ± 1.5 mg), N = 99. | |
| Outcomes | Not asleep. Mental State: PANSS total. Agitation: PANSS‐EC. Adverse events: insomnia, tachycardia, EPS, akathisia. Unable to use: Adverse effects: SAS (mean, SE/SD not reported). Adverse effects: BAS (mean, SE/SD not reported). | |
| Notes | *Author assume a typing error in the demographic‐clinical "table 1". An attempt to contact the authors was made (2nd July 2016). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A telephone‐based central service was used to randomly assign eligible patients" (p.108). |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | Open trial. |
| Blinding of outcome assessment (detection bias) | High risk | Open trial. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | SAS and BAS scores at 2, 4, 24, 72, and 120 hours are not reported. Only side effects occurred in ≥ 5% of people are reported. |
| Other bias | High risk | Sponsored by drug company (Xian‐Janssen Pharmaceutical Ltd). |
| Methods | Allocation: randomised. Blindness: double. Duration: 48 hours. | |
| Participants | Diagnosis: "acute psychotic behavior". "Eleven of the haloperidol recipients and 12 of the perphenazine recipients exhibited paranoid reactions". (p.515). N = 44. Age: mean 41 years (haloperidol group), 36 years (perphenazine group). Sex: 17 males, 27 females. History: "acutely disturbed. Newly admitted patients exhibiting severe flat affect, inappropriateness, agitation, hostility, overactivity or various combinations". (p.515). Excluded: Not reported. Setting: inpatient, USA. | |
| Interventions | 1. Haloperidol: dose 5 mg/IM (mean number of IMs not reported, mean dose 25.7 mg). N = 23. 2. Perphenazine: dose 5 mg/IM (mean number of IMs not reported, mean dose 27.6 mg). N = 21. | |
| Outcomes | Global state: clinical observation of global improvement. Leaving the study early. Adverse effects: vital signs, need for anti‐parkinsonian medication. Not usable: Mental state: Target symptoms (unpublished). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | "haloperidol and perphenazine were supplied in identically appearing 1‐mL ampoules each containing 5 mg of drug." (p.515). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind ‐ no further details given regarding rater‐blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is reported that 3 participants were discontinued on the medication. According to Table 9 9, not all participants completed all outcome measures, it is not clear if this is due to attrition or because people were not available for assessment. |
| Selective reporting (reporting bias) | Unclear risk | "Most adverse reactions disappeared with the use of control measures" ‐ author suspects that only the adverse effects severe enough for discontinuation are reported. |
| Other bias | Unclear risk | No clear interested funding. |
| Methods | Allocation: randomised. Blindness: double. Duration: 4 hours. | |
| Participants | Diagnosis: DSM‐III diagnosis of schizophrenia (N = 13), schizoaffective disorder (N = 4), bipolar disorder (N = 13), psychotic disorder not otherwise specified (N = 7). N = 37. Age: range 18‐61 years. Sex: 26 males, 11 females. History: "patients presenting at a psychiatric emergency room service." (p.177). Excluded: Not reported. Setting: psychiatric emergency room, USA. | |
| Interventions | 1. Haloperidol: dose 5 mg/oral/IM, repeated at 30 minute intervals if necessary for up to 4 hours (mean number of IMs 2.25). N = 17. 2. Lorazepam: dose 2 mg/oral/IM, repeated at 30 minute intervals if necessary for up to 4 hours (mean number of IMs 1.82). N = 20. | |
| Outcomes | Mental state: BPRS. Unable to use: Global State: need for additional oral/IM medication of study drug (N/SD not reported, mean doses reported). Behaviour: use of additional benzodiazepine (not reported). Physiological: vital signs (not reported). | |
| Notes | *Author assumes GCI is the same as CGI. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind, however it is not clear whether the study drugs appeared identical. |
| Blinding of outcome assessment (detection bias) | Low risk | "All raters were unaware of the specific medication received by the patient". (p.176). |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | "patients continued to receive treatment with a benzodiazepine, if necessary, for the duration of their stay in the emergency department." (p.176). The amount was not reported. |
| Other bias | High risk | Small short study. |
| Methods | Allocation: randomised. Blindness: double. Duration: 72 hours. | |
| Participants | Diagnosis: acute schizophrenia (N = 12), psychogenic psychosis (N = 18). N = 30. Age: range 19‐65 years. Sex: 7 males, 23 females. History: "newly admitted patients with a diagnosis of acute psychosis and characterized by symptoms such as agitation, excitement, aggressiveness, delusions and hallucinations." (p.257). Onset < 7 days (N = 14), 1 week ‐ 1 month (N = 13), 1‐6 months (N = 3). Excluded: "pregnancy, manic depressive illness, electroconvulsive therapy within the preceding 8 weeks, organic brain syndrome with marked dementia, convulsive disorders, alcoholism or drug dependence, serious impairment of renal, hepatic, cardiovascular or metabolic functions, and present or former increased intra‐ocular pressure."(p.257). Setting: psychiatric hospital, Denmark. | |
| Interventions | 1. Haloperidol: flexible dose 2.5 mg/IM to 5 mg/IM + biperiden: dose 2.5 mg/IM to 5 mg/IM (mean number of IMs not reported). N = 15. 2. Loxapine: flexible dose 25 mg/IM to 50 mg/IM + biperiden: dose 2.5 mg/IM (mean number of IMs not reported). N = 15. | |
| Outcomes | Adverse effects: recording of side effects, laboratory tests.. Agitation: observation*. Not able to use: Mental state: BPRS (mean reported, SD/CI not reported). Global state: CGI (mean reported, SD/CI not reported). Adverse effects: blood pressure, pulse rate (reports a decrease in blood pressure/pulse rate in both groups but does not give N/mean/SD/CI). | |
| Notes | * Scale derived data for agitation/excitation scores was not included as the measure was not validated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | "Both drugs were supplied in ampules of identical appearance." (p.257). |
| Blinding of outcome assessment (detection bias) | Low risk | Described as double‐blind, however no further details reported regarding rater‐blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | Small study. |
| Methods | Allocation: randomised. Blindness: open trial. Duration: not reported. | |
| Participants | Diagnosis: Schizophrenia (N = 16), mania (N = 22), "atypical psychotic patients" (N = 6), "miscellaneous" (N = 14). N = 68. Age: Not reported. Sex: 41 males, 27 females. History: "judged to require immediate treatment for agitated or assaultive behavior, with either drugs, seclusion or both." (p.1599). Excluded: Not reported. Setting: psychiatric hospital, USA. | |
| Interventions | 1. Haloperidol: dose 5 mg/IM (mean number of IMs not reported). N = 21. 2. Haloperidol: dose 5 mg/IM + lorazepam 4 mg/IM (mean number of IMs not reported). N = 24. 3. Lorazepam: dose 4 mg/IM (mean number of IMs not reported). N = 23. | |
| Outcomes | Agitation: VAS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | Open trial. |
| Blinding of outcome assessment (detection bias) | High risk | Open trial. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete data. |
| Selective reporting (reporting bias) | High risk | There is no reporting regarding the incidence of adverse effects. |
| Other bias | Unclear risk | The duration of the study is not reported. |
| Methods | Allocation: randomised. Blindness: open trial. Duration: 14 days. | |
| Participants | Diagnosis: CCMD‐3 diagnosis of schizophrenia with agitation/aggression. N = 60. Age: range 18‐49 years. Sex: males and females. History: mean length of illness 56.7 (SD 7.8) months. Excluded: severe physical illness, drug/alcohol dependence. Setting: inpatient, China. | |
| Interventions | Haloperidol: dose 5 mg/day to 10 mg/day for 3 days, 10 mg/IM/day to 30 mg/IM/day afterwards (mean number of IMs not reported. N = 30. Quetiapine: dose 100 mg/day to 800 mg/day + magnesium valproate dose: 0.5 g/p.o./day to 1 g/p.o./day (mean number of IMs not reported) N = 30. | |
| Outcomes | Agitation: PANSS‐EC. Not able to use: Global outcome: CGI (not outcome data reported). Adverse effects: TESS (no outcome data reported). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | High risk | CGI and TESS were measured, but not reported. |
| Other bias | Unclear risk | None obvious. |
| Methods | Allocation: randomised. Blindness: open trial. Duration: 8 weeks. | |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia. N = 19. Age: mean 29.5 years (monotherapy), mean 26.6 years (combined therapy). Sex: 12 males, 7 females. History: "acute exacerbation of psychotic symptoms." (p.380). Excluded: Not reported. Setting: Psychiatric hospital, Japan. | |
| Interventions | Haloperidol: average endpoint dose 5.4 (2.7) mg/p.o./day. N = 10. Haloperidol + levomepromazine: average endpoint dose ratio 1:10, 5.4 (2.4) mg/p.o./day. N = 9. | |
| Outcomes | Global state: no overall improvement. Unable to use: Mental state: BPRS. Adverse effects: not reported. Adverse effects: the quantity of anticholinergic medication prescribed was not reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | Open trial. |
| Blinding of outcome assessment (detection bias) | High risk | Open trial. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete data. |
| Selective reporting (reporting bias) | High risk | The discussion describes how the combination drug "produced moderate sedation without any cardiovascular adverse effect". However, there is no other reporting regarding the incidence adverse effects. |
| Other bias | High risk | Small N study. |
| Methods | Allocation: randomised. Blindness: open trial. Duration: 14 days. | |
| Participants | Diagnosis: psychosis (N = 244), substance misuse (N = 58), others (N = 11), no information (N = 3). N = 316. Age: mean 40.1 years. Sex: males. History: people presenting at a psychiatric emergency room service. 1st attendance (N = 59), previous attendance (N = 224), unknown history (N = 33). Excluded: where the "clinician believed that one of the treatment options represented an additional risk for the patient." (p.3). Setting: psychiatric emergency room, Brazil. | |
| Interventions | 1. Haloperidol: dose 5 mg/IM to 10 mg/IM. N = 156. 2. Haloperidol: dose 5 mg/IM to 10 mg/IM + promethazine 25 mg/IM to 50 mg/IM. N = 160. | |
| Outcomes | Tranquillisation or asleep: tranquil/asleep, time to tranquillisation/sleep, need for additional tranquillising drug. Specific behaviours: another episode of aggression by 24 hours. Global state: use of restraints, needing extra visits from the doctor, refusing oral medication. Leaving the study early. Service outcomes: duration of hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomised sequence was applied to a table of numbers. |
| Allocation concealment (selection bias) | Low risk | "Boxes were consecutively numbered, sealed, opaque, and identical appearance and weight; on the outside was a form with questions to be completed by the attending doctor while "blind" to the contents of the box." (p.2). |
| Blinding of participants and personnel (performance bias) | High risk | Open trial. |
| Blinding of outcome assessment (detection bias) | High risk | Open trial. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | The study was stopped early due to adverse effects, therefore it is possible that the available data may be biased. |
| Methods | Allocation: randomised. Blindness: double. Duration: 5 hours. | |
| Participants | Diagnosis: DSM‐III diagnosis of schizophrenia (N = 13), schizoaffective disorder (N = 4), bipolar disorder (N = 13), psychotic disorder not otherwise specified (N = 7). N = 44. Age: range 19‐56 years. Sex: 21 males, 23 females. History: "newly admitted patients...mean duration of illness 7.75 years." (p.77). Excluded: Not reported. Setting: psychiatric hospital, USA. | |
| Interventions | 1. Haloperidol: flexible dose 2.5 mg/IM to 10 mg/IM, maximum 4 injections/cumulative dosage of 27.5 mg during 24 hours (mean number of IMs not reported, mean cumulative dose ˜ 12.1 mg). N = 24. 2. Thiothixene: flexible dose 2.5 mg/IM to 10 mg/IM, maximum 4 injections/cumulative dosage of 27.5 mg during 24 hours (mean number of IMs not reported, mean cumulative dose ˜ 16.1 mg). N = 20. | |
| Outcomes | Global state: global evaluation. Adverse effects. Unable to use: Mental state: BPRS (SE/SD/CI not reported, mean reported). Mental state: Study Symptom Profile (mean, SE/SD not reported, P values of significant findings reported). Physiological: the authors report that there were no abnormal laboratory results, although no data was given. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind, however no further details reported regarding the blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind, however no further details reported regarding rater‐blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting. |
| Other bias | High risk | Recieved grant from Roerig, a division of Phizer Pharmaceuticals (thiothixene manufacturers), small short study. |
| Methods | Allocation: randomised. Blindness: double. Duration: 21 days. | |
| Participants | Diagnosis: schizophrenia (N = 56), schizophreniform disorder (N = 42), schizoaffective disorder (N = 2). N = 100. Age: range 18‐50 years. Sex: 71 males, 29 females. History: "newly admitted patients who were acutely agitated." (p.181). Excluded: pregnant or lactating women, people with "serious unstable illnesses, including hepatic, renal, gastroenterologic, respiratory, cardiovascular, endocrinologic, neurologic, immunologic, or hematologic disease, in which pharmacotherapy posed a substantial clinical risk or confounded diagnosis were excluded from this trial." (p.181). Setting: not reported. | |
| Interventions | 1. Haloperidol: accelerated dose titration 10 mg/p.o. to 20 mg/p.o. during 24 hours. N = 48. 2. Olanzapine: accelerated dose titration 10 mg/p.o. to 20 mg/p.o. during 24 hours. N = 52. | |
| Outcomes | Mental state: PANSS total, need for increased dose of study drug. Global state: CGI‐I, time in restraints, seclusion and special observation, need for increased dose of study drug, use of benzodiazepine. Agitation: Seclusion and special observation. Leaving the study early. Adverse effects: Tranquillisation Scale, recording rates of discontinuation, recording treatment emergent adverse events in ≥10% of participants, need for parkinsonian drugs. Unable to use: Global state: CGI (between‐group data was described as not significant but figures not reported ‐ only within‐group data reported), CGI‐S (not reported). Agitation: OASS (only present the data from 2 items of the scale), NOSIE (between‐group data were described as not significant but figures not reported ‐ only within‐group data reported), PANSS Agitation sub‐scale (only present data from certain items of the PANSS), SAS (between‐group data were described as not significant but figures not reported ‐ only within‐group data reported). Adverse effects: BAS (between‐group data was described as not significant but figures not reported ‐ only within‐group data reported), vital signs (not reported), laboratory analytes (described as no clinically relevant changes, although no data given), weight gain (mean, P values of significant findings, overall F value reported, SD/SE/CI not reported). Satisfaction: DAI‐10 (significant within‐group data reported, for between‐group P values of non significant findings reported, overall F value). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind, however no further details reported regarding the blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind, however no further details reported regarding rater‐blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | Significant findings from the within‐group data are presented on a number of occasions, whilst acknowledging but omitting the non‐significant between‐group findings. The OASS is a 16 item rating scale, yet only the results for two items where the olanzapine group had significantly greater improvement than the haloperidol group were reported. Treatment‐emergent adverse effects were only reported if they occurred with a frequency ≥10% or P< .05 through the end of the study. |
| Other bias | High risk | Sponsored by the manufacturers of olanzapine. |
| Methods | Allocation: randomised. Blindness: single. Duration: 72 hours. | |
| Participants | Diagnosis: CCMD‐3 diagnosis of schizophrenia with agitation/aggression. N = 231. Age: mean 34 (SD 12) years. Sex: males and females. History: mean length of illness, haloperidol group 30 (± 68) months, ziprasidone 18 (± 35) months. Excluded: severe physical or neurological impairment/diseases; drug or alcohol abuse, dependence, pregnant women. Setting: not reported. | |
| Interventions | 1. Haloperidol: flexible dose 5 mg/IM to 10 mg/IM, repeated every 4‐6 hours if necessary, maximum dose 30 mg during 24 hours. N = 116. 2. Ziprasidone: flexible dose 10 mg/IM to 20 mg/IM, repeated every 4‐6 hours if necessary, maximum dose 40 mg during 24 hours. N = 115. | |
| Outcomes | Mental state: PANSS endpoint. Agitation: PANSS‐EC. Adverse effects: TESS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Single‐blind – however the author did not state who had been blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Single‐blind – however the author did not state who had been blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Unclear risk | TESS and SAS were used for assessment of adverse events. No continuous data were reported from either scales, but binary outcomes for adverse events were reported. |
| Other bias | Unclear risk | None obvious. |
| Methods | Allocation: randomised. Blinding: not stated. Duration: 28 days. | |
| Participants | Diagnosis: ICD‐10 diagnosis of schizophrenia. N = 80. Age: range 18‐60 years. Sex: 42 males, 38 females. History: acute agitation and excitement; length of Illness 7.1 ± 7.7 (quetiapine group), 7.0 ± 6.3 (haloperidol group). Excluded: "pregnancy, serious physical sickness, addiction to alcohol or drugs, intolerant to quetiapine, haloperidol or orally taking medicines, taking quetiapine or haloperidol or other long‐term schizophrenia medicines one month before recruitment, neutrophil cells ≤ 1.5× 109/L". Setting: inpatients, China. | |
| Interventions | 1. Haloperidol: dose 2 mg/oral/day to 4 mg/oral/day, by 3rd day 6 mg/day, by 7th day 10 mg/day to 12 mg/day. N = 40. 2. Quetiapine: dose 50 100 mg/oral/day to 100 mg/oral/day, by 3rd day 300 mg/day, by 7th day 600 mg/day to 750 mg/day. N = 40. | |
| Outcomes | Mental state: PANSS total and sub‐scale. Agitation: PANSS‐EC. Adverse effects: general. Adverse effects: movement ‐ SAS. Dropout from the study. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised by using random number table". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | A total of 5 dropped out in haloperidol group (adverse events = 4, lost to contact = 1) and 4 dropped out in quetiapine group (adverse events = 1, lost to contact = 3); missing data have not been imputed using appropriate methods such as LOCF. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | None obvious. |
| Methods | Allocation: randomised. Blindness: open‐label, rater‐blinded. Duration: 24 hours. | |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia (N = 78), bipolar disorder (N = 43), others (N = 3). N = 124. Age: range 18‐65 years. Sex: 66 males, 58 females. History: "acute psychotic agitation in the emergency room." (p.82). Excluded: neurological disorder, severe medical disease, alcohol or other psychoactive substance misusers, history of neuromalignant syndrome or hypersensitivity to trial medications, pregnant or lactating women, people treated with antipsychotics or benzodiazepines within 6 hours to the start of the trial or with depot antipsychotic within one treatment cycle of enrolment. Setting: psychiatric emergency room, Korea. | |
| Interventions | 1. Haloperidol: dose 5 mg/IM, maximum 15 mg during 24 hours (mean number of IMs not reported). N = 62. 2. Risperidone: dose 2 mg/p.o, maximum 6 mg during 24 hours (mean dose not reported). N = 62. | |
| Outcomes | Global state: need for benzodiazepine. Agitation: PANSS‐EC. Leaving the study early. Adverse effects. Unable to use: Mental state: PANSS Total (mean, SE/SD not reported, P values of significant findings reported, overall F value). Mental state: YMRS Total (mean, SE/SD/CI not reported). Global state: CGI‐S (mean, SE/SD not reported, P values of significant findings reported, overall F value). Adverse effects: use of antiparkinsonian drugs not reported. Adverse effects: AIMS (mean, SE/SD/CI not reported). Adverse effects: SAS (mean, SE/SD/CI not reported). Behaviour: Behaviorally Anchored Rating Scale (mean, SE/SD/CI not reported). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned "according to a predefined randomization code that was balanced to ensure even distribution of patients in each treatment group." (p.82). |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | This was an open‐label study where one group received IM and the other group received an orodispersible tablet. |
| Blinding of outcome assessment (detection bias) | Low risk | The study was rater‐blinded. |
| Incomplete outcome data (attrition bias) | Low risk | There is no evidence of incomplete outcome data. The authors give reasons for the participants who discontinued the study. |
| Selective reporting (reporting bias) | High risk | The authors report that antiparkinsonian drugs could be given at the lowest effective dose, however the amount administered is not described in the results. |
| Other bias | High risk | Sponsored by drug company (Janssen Phamaceutica Korea). |
| Methods | Allocation: randomised. Blinding: not stated. Duration: 2 weeks. | |
| Participants | Diagnosis: CCMD‐3 diagnosis of schizophrenia. N = 150. Age: range 18‐59 years. Sex: 69 males, 81 females. History: agitated behaviour; length of Illness 0‐ 30 months. Excluded: "serious physical sickness, additive to alcohol and drugs, intolerant to schizophrenia treatment, pregnancy". Setting: inpatients, China. | |
| Interventions | 1. Haloperidol: dose 10 mg/IM/day to 20 mg/IM/day (mean dose 16.15 ± 4.67 mg/day). N = 50. 2. Risperidone: dose 3 mg/oral/day to 6 mg/oral/day (mean dose 4.57 ± 0.65 mg/day) + haloperidol dose 10 mg/IM/day to 20 mg/IM/day (mean dose 15.73 ± 4.26 mg/day). N = 50. 3. Risperidone: dose 3 mg/oral/day to 6 mg/oral/day + clonazepam dose 3 mg/IM/day to 6 mg/IM/day (mean dose 4.05 ± 0.81 mg/day). N = 50. All IM injections changed to oral administration by 10 days. | |
| Outcomes | Global state: clinical observation of global improvement. Agitation: PANSS‐EC. Adverse effects: TESS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised by using random number table". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | None obvious. |
| Methods | Allocation: not reported. Blindness: double. Duration: 72 hours. | |
| Participants | Diagnosis: acute exacerbation of chronic psychosis ‐ “severe agitation with marked psychomotor hyperactivity, extreme assaultiveness, hostility and mania. The patients presented a clear danger to themselves, to other patients and to staff personnel”. N = 30. Age: mean 33 years. Sex: 15 males and 15 females. History: acute agitation, 90% had previous admission to a psychiatric hospital. Excluded: anyone who used anti‐psychotic or anti‐convulsant within one week prior to the trial or anyone who used long lasting drug within one month prior to the trial. Setting: inpatients, China. | |
| Interventions | 1. Haloperidol: dose 5 mg/IM, repeated every 30 minutes if necessary, maximum 15 mg during 24 hours (mean number of IMs 3.33). N = 15. 2. Chlorpromazine: dose 50 mg/IM, repeated every 30 minutes if necessary, maximum 600 mg during 24 hours (mean number of IMs 2.93). N = 15. | |
| Outcomes | Global state: need for additional injections. Adverse effects: reporting of EPS. Leaving the study early. Unable to use: Mental state: BPRS (SD/SE/CI not reported, mean not reported). Global state: TSRS (unpublished, mean reported, SD/SE/CI not reported). Adverse effects: it is reported that there were no significant changes in blood pressure, pulse rate and respirations, however no data given. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not explicitly described as randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind, however it is unclear whether the syringes appeared identical given the varied doses. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind, however no further details reported regarding rater blinding. |
| Incomplete outcome data (attrition bias) | High risk | The authors give reasons for the participants who discontinued the study. However, the results are only presented for the 27 participants who completed the study. Missing data have not been imputed using appropriate methods such as LOCF. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | Small study. |
| Methods | Allocation: randomised. Blindness: double. Duration: not reported. | |
| Participants | Diagnosis: violent behaviour and severe agitation. N = 111. Age: mean 40.7 years. Sex: not reported. History: prior psychiatric history (N = 54), no psychiatric history (N = 8), unknown psychiatric history (N = 49). Excluded: known allergy to study drugs, hypotensive, unable to maintain own airway, tachycardia, bradycardia, respiratory distress, <18 or pregnant. Setting: emergency psychiatric department, USA. | |
| Interventions | 1. Haloperidol: fixed dose 5 mg/IM (mean number of IMs not reported). N = 42. 2. Midazolam: fixed dose 5 mg/IM (mean number of IMs not reported). N = 42.* | |
| Outcomes | Tranquil or asleep: modified TCS. Global state: need for additional medication. Adverse effects. Unable to use: Adverse effects: it is reported that there were no significant differences between groups for changes to blood pressure, heart rate and oxygen. However, data not given. | |
| Notes | *There was also a lorazepam group, however we cannot include the data because randomisation was broken. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The administered drugs were prepared in the pharmacy using a computer generated randomisation code." (p.745). |
| Allocation concealment (selection bias) | Low risk | "The administered drugs were prepared in the pharmacy using a computer generated randomisation code." (p.745). |
| Blinding of participants and personnel (performance bias) | Low risk | "The research assistant [rater], the administering physician, and the patient were all blinded to the drug delivered." (p.745). |
| Blinding of outcome assessment (detection bias) | Low risk | "The research assistant [rater], the administering physician, and the patient were all blinded to the drug delivered." (p.745). |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | The duration of the study was not reported, therefore it is difficult to establish how long participants were monitored for adverse effects. |
| Methods | Allocation: randomised. Blindness: double. Duration: 4‐day IM phase followed by up to 4 week oral phase. | |
| Participants | Diagnosis: "acute psychosis with manifest disturbed psychotic symptoms characterised by agitation, excitement, aggressiveness, hostility, delusions and hallucinations." (p.81). N = 35. Age: range 18‐49 years. Sex: females. History: onset of present episode: < 1 week (N = 10), > 1 week < 1 month (N = 26)*. Excluded: psychotropic drugs prior to 24 hours of the start of the study, "known hypersensitivity to dibenzazepine compounds, electroconvulsive therapy or insulin coma or subcoma therapy within the preceding eight weeks, presence of organic brain syndrome with marked dementia and inability to communicate during interviews, history of convulsive disorders, alcoholism or drug dependence as a significant feature of clinical history, presence of serious impairment of renal or hepatic function, increased intra ocular pressure or history of narrow angle glaucoma or urinary retention, cardiovascular or metabolic disease, pregnancy suspected or confirmed." (p.82). Setting: emergency psychiatric department, USA. | |
| Interventions | 1. Haloperidol: dose 2.5 mg/IM to 5 mg/IM, repeated every 6 to 12 hours if necessary (IM phase), dose 5 mg to 15 mg (oral phase) (mean number of IMs not reported, mean daily dose 11.5 mg). N = 18. 2. Loxapine: dose 25 mg/IM to 50 mg/IM, repeated every 6 to 12 hours if necessary (IM phase), dose 50 mg to 150 mg (oral phase) (mean number of IMs not reported, mean daily dose 115.4 mg). N = 17. | |
| Outcomes | Global state: CGI. Leaving the study early Adverse effects: observations, laboratory tests. Unable to use: Mental state: BPRS (overall P value and level of significance reported, SD/SE/CI not reported). Global state: NOSIE (overall P value and level of significance reported, SD/SE/CI not reported). Adverse effects: sedation score (mean reported, SD/SE/CI not reported). | |
| Notes | *This would suggest there were 36 participants. It is not clear if this is a typing error because it is reported in the rest of the paper that there were 35 participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind ‐ "supplied in 1ml ampules...of identical appearance, individually packaged and labelled with the code number assigned to each patient". Capsules used in the oral phase were also of identical appearance. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind ‐ no further details given regarding rater blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | The number of participants reported is inconsistent (reported at 36 in one instance and 35 at other times) and it is not clear if this is a typing error. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | Small study. |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia (no specified criteria). History: severe agitation ‐ "Psychiatric emergencies with symptoms such as agitation, excitement, and assaultiveness of such severity that rapid tranquillisation was necessary". Excluded: not reported. | |
| Interventions | 1. Haloperidol IM: dose up to 4 injections of 1 mg (mean number of IMs not reported). N = 10. 2. Haloperidol IM: dose up to 4 injections of 2 mg (mean number of IMs 3.7). N = 11. 3. Haloperidol IM: dose up to 4 injections of 5 mg (mean number of IMs 2.8). N = 8. 4. Chlorpromazine IM: dose up to 4 injections of 25 mg (mean number of IMs not reported). N = 10. 5. Placebo IM: dose up to 4 injections of 1 mg (mean number of IMs not reported). N = 11. | |
| Outcomes | Not tranquil or asleep: not asleep at 2 hours. Global state: own 5‐point rating scale. Unable to use: | |
| Notes | Data for the haloperidol interventions were combined within the paper for some outcomes, for the binary outcomes that were not already presented in this way we added events for the different doses together following the protocol (see Unit of analysis issues). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind, however no further details are given. It is not clear whether the study drugs appeared identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind, however no further details reported regarding rater blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | Small study. |
| Methods | Allocation: randomised. Blindness: double. Duration: 24 hours. | |
| Participants | Diagnosis: acute agitation. N = 27. Age: range 18‐65 years. Sex: not reported. History: involuntary hospitalised. Excluded: intoxicated, known sensitivity to droperidol or haloperidol, evidence of active renal, hepatic, or cardiac disease. Setting: emergency and psychiatric crisis unit, USA. | |
| Interventions | 1. Haloperidol: dose up to 4 injections of 5 mg (mean number of IMs not reported). N = 16. 2. Droperidol: dose up to 4 injections of 5 mg (mean number of IMs 1.36). N = 11. | |
| Outcomes | Global state: need for additional injection. Adverse effects: reporting of EPS. Unable to use: Mental state: BPRS (mean/SD/SE/CI not reported). Adverse effects: it is reported that there were no significant changes in blood pressure, pulse rate and respirations, however no data given. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Low risk | "packages of medication were identified only by a code which was kept in the pharmacy until the conclusion of the study" (p.298). |
| Blinding of participants and personnel (performance bias) | Low risk | "packages of medication were identified only by a code which was kept in the pharmacy until the conclusion of the study" (p.298). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind, however no further details reported regarding rater blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | Small short study. |
| Methods | Allocation: randomised, no further details. | |
| Participants | Diagnosis: paranoid schizophrenia (N = 48), involutional melancholia (N = 1), manic‐depression (N = 1). History: all exhibited symptoms such as aggressiveness, assaultiveness or uncooperativeness that disrupted normal hospital operation and interfered with attempts at therapy. Excluded: not reported. | |
| Interventions | 1. Haloperidol: fixed dose 5 mg/IM, repeated every 6‐8 hours as necessary (mean number of IMs not reported). N = 25. | |
| Outcomes | Global state: own 5‐point scale. Adverse events: Leucopenia, SGPT increase. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind ‐ the test drugs were supplied in identically appearing ampoules. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind, however no further details reported regarding rater blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | Do not report all adverse effects. The authors do not report the mean dosages/number of injections needed. |
| Other bias | Unclear risk | None obvious. |
| Methods | Allocation: not reported. Blindness: double. Duration: 48 hours. | |
| Participants | Diagnosis: schizophrenia (N = 26), schizoaffective disorder (N = 4), bipolar disorder (N = 11), organic mental disorder (N = 6), other (N = 13). N = 60. Age: mean 30.5 years (haloperidol group), mean 37.9 years (lorazepam group). Sex: not reported. History: involuntary hospitalised ‐ "Aggressive, assaultive or disruptive behaviour, thought to be a danger to the patient or other people". Excluded: intoxicated, known sensitivity to droperidol or haloperidol, evidence of active renal, hepatic, or cardiac disease. Setting: emergency and psychiatric crisis unit, USA. | |
| Interventions | 1. Haloperidol: fixed dose 5 mg/IM (mean number of IMs 1.1). N = 30. 2. Lorazepam: fixed dose 2 mg/IM (mean number of IMs 1.13). N = 30. | |
| Outcomes | Adverse effects. Unable to use: Mental state: BPRS (mean change given, SD/SE/CI not reported). Global state: CGI‐S (mean change given, SD/SE/CI not reported). Aggression: OAS (mean change given, SD/SE/CI not reported). Global state: additional injection (mean dose given, SD/SE/CI not reported). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not explicitly described as randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | "Parenteral medication was prepared in advance by a member of staff who had no direct clinical responsibility for the patient." (p.177). |
| Blinding of outcome assessment (detection bias) | Low risk | "Raters were blind to the treatment condition received by the patient." (p.178). |
| Incomplete outcome data (attrition bias) | High risk | It is reported that the number of participants declined after the baseline assessment because they were discharged or transferred. However, exact numbers are not given and it is not clear if the participants were transferred because they had a positive or negative outcome. Attrition approaching 50% for some outcomes at 24 hours. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | Sponsored by drug company. |
| Methods | Allocation: randomised. Blindness: not stated. Duration: 2 weeks. | |
| Participants | Diagnosis: CCMD‐3 diagnosis of schizophrenia. N = 64. Age: range 18‐50 years. Sex: 37 males, 27 females. History: agitation and aggression. Excluded: serious physical illness; addiction to alcohol or drugs; pregnancy. Setting: inpatients, China. | |
| Interventions | 1. Haloperidol IM (mean dose 15 ± 2.5 mg/day). N = 31. 2. Olanzapine oral (mean dose 10 ± 2.5 mg/day) + magnesium valproate oral (mean 1.25 ± 0.25 g/day). N = 33. | |
| Outcomes | Global state: clinical observation of global improvement. Mental state: PANSS total score. Agitation: PANSS‐EC. Adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised by using random number table". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | None obvious. |
| Methods | Allocation: randomised. Blindness: open‐label, rater‐blinded. Duration: 72 hours. | |
| Participants | Diagnosis: ICD‐10 diagnosis of schizophrenia. N = 376. Age: range 18‐65 years. Sex: males and females. History: mean duration of acute agitation 3.4 days (haloperidol group), 3.2 days (ziprasidone group); mean duration of schizophrenia 4 years (haloperidol group), 5.6 years (ziprasidone group). Excluded: history of clinically significant physical illness especially myocardial infarction, non compensatory heart failure, people receiving an investigational agent in the previous 3 months to screening, us of antipsychotic agents within 12 hours or parenteral benzodiazepines within 4 hours prior to randomisation and during the study. Setting: multi‐centre, China. | |
| Interventions | 1. Haloperidol: dose 5 mg/IM, maximum 20 mg during 24 hours (mean number of IMs not reported). N = 187. 2. Ziprasidone: flexible dose 10 mg/IM to 20 mg/IM, maximum 40 mg in 24 hours (mean number of IMs not reported). N = 189. | |
| Outcomes | Mental state: BPRS. Adverse effects: laboratory tests, ECG's, physical examination. Leaving the study early. Unable to use: Specific behaviour: agitation ‐ BPRS agitation items (modified from BPRS, only report LS means; means/SD not reported). Specific behaviour: agitation ‐ BARS (only give upper quartile value and LS means; means/SD not reported). Global state: CGI‐I (only report CI and LS means, means/SD not reported). Global state: CGI‐S (only give upper quartile value and LS means, means/SD not reported). Adverse effects: BAS and SAS (only report LS means). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized in a 1:1 ratio (in blocks of 4)" (p.179); no further informations on the randomization procedure is stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) | Low risk | Rater‐blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | The reasons for leaving the study early were reported for those participants who did not complete the study. However, the number of participants completing different assessments at the same time point vary and reasons are not given. |
| Selective reporting (reporting bias) | Unclear risk | Only report side effects experienced by ≥2% of people. |
| Other bias | High risk | Sponsored by drug company. |
| Methods | Allocation: randomised. Blindness: double. Duration: 6 hours. | |
| Participants | Diagnosis: schizophrenia (N = 24), bipolar disorder (N = 2), psychotic depressive reaction (N = 4). N = 30. Age: range 18‐66 years. Sex: 13 males, 17 females. History: disturbed, acutely disruptive symptomology outpatients, 16 of whom had prior inpatient treatment, and 21 of whom had a history of outpatient treatment. Excluded: known hypersensitivity to haloperidol or thiothixene, active hepatic, renal or cardiac disease or organic brain disease. Setting: emergency and psychiatric crisis unit, USA. | |
| Interventions | 1. Haloperidol: flexible dose 4 mg/IM or 8 mg/IM, maximum dose 32 mg during 6 hours (mean number of IMs 3.07, mean dose 15 mg). N = 15. 2. Thiothixene: flexible dose 4 mg/IM or 8 mg/IM, maximum dose 32 mg during 6 hours (mean number of IMs 2.6, mean dose 10.1 mg). N = 15. | |
| Outcomes | Global state: need for additional injection. Adverse effects. Unable to use: Mental state: BPRS (mean scores given, SD/SE/CI not reported). Mental state: symptom profile (not a validated scale). Adverse effects: vital signs (reports significant between‐group changes but does not give data). Global state: mean dose given (SD/SE/CI not reported). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised in blocks of 10. Method of sequence generation not clear. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | "Medication was provided in identically appearing 2cc. ampules each containing 4 mg's. Prior to the administration of the drug labels were removed by a person not involved either in the prescription or administration of the drug." (p.968). |
| Blinding of outcome assessment (detection bias) | Low risk | "Ratings were performed by the psychiatrist ordering the medication who was not aware which drug was administered to the patient. When asked to guess which drug was given, the psychiatrists administering the drug were unable to guess significantly better than chance." (p.968). |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | Small short study, sponsored by drug company. |
| Methods | Allocation: randomised. Blindness: present for some outcomes. Duration: 7 days. | |
| Participants | Diagnoses: ICD‐10 diagnosis of schizophrenia with acute exacerbation of symptoms (N = 39), mania (N = 1), other (N = 6). N = 70. Age: range 18‐65 years. Sex: males and females. History: disturbed and aggressive behaviour, unresponsive to verbal intervention. Mean onset of acute episode, 20.09 days (haloperidol group), 13.63 days (zuclopenthixol group). Excluded: psychosis caused by medication, medical condition, learning disability, people who had anti‐psychotic in the previous 2 weeks. Setting: psychiatric hospital, Thailand. | |
| Interventions | 1. Haloperidol IM: flexible dose 5 mg to 10 mg repeated every 6 hours as required (mean number of IMs 5). N = 32. 2. Zuclopenthixol acetate IM: flexible dose 50 mg to 100 mg repeated every 12 hours as required (mean number of IMs 2). N = 38. Oral antipsychotics and mood stabilisers allowed. | |
| Outcomes | Global state: additional injections. Adverse effects: presence of tremor, reaction at injection site. Unable to use ‐ Mental state: BPRS, BRMS (means, inadequate data on SD). Global state: CGI (means, no SD). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The BPRS was completed by a psychiatric nurse who was blind to the intervention. However, it is not reported whether the rater for CGI and adverse effects was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | None obvious. |
| Methods | Allocation: randomised. Blinding: “modified double” ‐ staff administering drugs not blinded, assessments blinded. Duration: 24‐72 hours (IM phase) ‐ oral phase data not included. | |
| Participants | Diagnosis: schizophrenia, acutely psychotic (DSM‐III used beyond 3 days). N = 54*. Age: range 18‐65, mean 35 (SD 10) years. Sex: 33 males, 19 females, 2 not reported. History: newly admitted. Excluded: ill health, co‐existing mental illness condition. Setting: psychiatric hospital, USA. | |
| Interventions | 1. Haloperidol: dose 5 mg/IM, then flexible dose 2.5 mg/hour/IM to 5 mg/hour/IM, maximum 100 mg/day (mean daily dose reported only for day one). N = 29. 2. Loxapine: dose 25 mg/IM, then flexible dose 12.5 mg/hour/IM to 25 mg/hour/IM, maximum 250 mg/day (mean daily dose reported only for day one). N = 25. Antiparkinsonian drugs and chloral hydrate as required. | |
| Outcomes | Mental state: BPRS.* Global state: CGI‐I, needing an extended period of medication > 24 hours. Leaving the study early. Adverse effects: sedation ‐ ESBE. Unable to use: Global state: CGI‐S (no usable data). Adverse effects: amount of antiparkinsonian drugs/sleeping tablets administered not reported. | |
| Notes | *BPRS scores reported with SD. Data thought not to be SD but SE and review authors have converted to SD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | Staff administering the medication were not blind to the intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Rater‐blinded. |
| Incomplete outcome data (attrition bias) | High risk | Participants who were discharged/transferred were not followed up. The number of participants reported as leaving the study is contradictory as follows; "On Day 3, 19% of the haloperidol patients were withdrawn from the study while all loxapine patients remained" "the others (6 loxapine and 11 haloperidol) were dropped on Day 2, when all were still rated severely or very severely ill." (p.127). By day 10, over 50% attrition in both groups. |
| Selective reporting (reporting bias) | High risk | The amount of anticholinergic and sleeping medication administered was not reported. Table 9 9 and 3 present data from 48 hours after the 1st injection, rather than from 24 hours, despite referring to two of the outcomes in the text; "Twenty‐four hours after the first I.M. dose, 72% (18 of 25) of the loxapine patients and 59% (12 of 27) of the haloperidol patients showed moderate or marked improvement" (p.128). |
| Other bias | Unclear risk | None obvious. |
| Methods | Allocation: randomised. Blinding: not stated. Duration: 96 hours. | |
| Participants | Diagnosis: DSM‐IV criteria for schizophrenia (N = 27), schizoaffective (N = 6), or schizophreniform disorder (N = 10); PANSS‐PES ≥20. N = 43*. Age: range 18‐55. Sex: 31 males, 12 females. History: admitted to acute care inpatient unit. Excluded: relevant medical or neurological disorders, pregnancy, ongoing intake of illicit drugs and alcohol. Setting: psychiatric hospital of Bern, Switzerland. | |
| Interventions | 1. Haloperidol: fixed dose 10 mg/oral for the first day**, then fixed dose 7.5 mg/twice daily/oral***. N = 14. 2. Risperidone: fixed dose 2 mg/oral for the first day**, fixed dose 2 mg/twice daily/oral***, then fixed dose 3 mg/twice daily/oral***. N = 14. 3. Olanzapine: fixed dose 15 mg/oral for the first day**, then fixed dose 20 mg/oral***. N = 15. | |
| Outcomes | Specific behaviour: agitation ‐ PANSS‐PAS. Adverse effects: general ‐ death. Adverse effects: movement ‐ AIMS. Adverse effects: movement ‐ BARS. Adverse effects: movement ‐ SAS. Leaving the study early. Unable to use: Mental state: PANSS (mean scores and SD/SE/CI not provided). Adverse effects: amount of patients that needed antiparkinsonian drugs/benzodiazepine not reported. | |
| Notes | *Eligible patients were 52 participants but 9 refused to provide post‐hoc consent. **Additional use of diazepam up to 30 mg was permitted in day 1. ***Additional use of diazepam up to 60 mg was permitted from day 2 to day 5. Authors were contacted (2nd July 2016, 9th July 2016). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed by creating 3 sets of random numbers between 1 and 60 using a computer‐based research randomizer" (p.125). |
| Allocation concealment (selection bias) | Low risk | "The randomization list was locked in the office of the principal investigator (T.J.M.), who was engaged neither in treatment nor in study assessments" (p.125). |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | 9 participants were excluded post‐randomisation due to refusal to provide post‐hoc consent; their treatment assignment was not provided. |
| Selective reporting (reporting bias) | Unclear risk | PANSS‐PAS scores at 24, 48, 72, and 96 hours not provided. PANSS scores at 96 hours not provided. Number of patients that needed additional BDZ not provided. Number of patients that needed additional biperiden not provided. |
| Other bias | Unclear risk | Small study. |
| Methods | Allocation: randomised. Blinding: researcher‐blinded. Duration: 72 hours. | |
| Participants | Diagnosis: ICD‐10 diagnosis of schizophrenia. N = 60 Age: range 20‐54 years. Sex: 33 males, 27 females. History: acute agitation; length of Illness: 1‐ 23 years. Excluded: "taking ziprasidone 3 months before recruitment; taking other schizophrenia medications 12 hours before recruitment; taking long‐term schizophrenia medication 2 weeks before recruitment; additive to drugs 3 months before recruitment; ECG irregular (QTc > 500msec)". Setting: inpatients, China. | |
| Interventions | 1. Haloperidol: starting dose 5 mg/IM/day; maximum dose 20 mg. N = 30. 2. Ziprasidone: starting dose 10 mg/IM/day; maximum dose 40 mg. N = 30. | |
| Outcomes | Mental state: BPRS total. Adverse effects. Adverse effects: movement ‐ SAS. | |
| Notes | *It is not clear if this paper presents the results from one centre of the Fang 2012 multi‐centre trial. Attempted to contact author to clarify. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised ‐ method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Researcher blinded to assignment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | None obvious. |
| Methods | Allocation: randomised. Blinding: not stated. Duration: 4 weeks*. | |
| Participants | Diagnosis: CCMD‐3 diagnosis of schizophrenia. N = 76. Age: range 16‐60 years. Sex: 39 males, 37 females. History: "excited/agitated symptoms of acute‐stage schizophrenia"; "length of Illness 1‐32 months". Excluded: "serious physical sickness, additive to alcohol and drugs, pregnant, history of taking long‐term schizophrenia medicine, suicide tendency and allergic to treatment". Setting: inpatients, China. | |
| Interventions | 1. Haloperidol: flexible dose 5 mg/IM/day to 30 mg/IM/day (mean dose 16.4 ± 6.1 mg/day). N = 38. 2. Ziprasidone: flexible dose 40 mg/oral/day to 160 mg/oral/day (mean dose 90.6 ± 20.8 mg/day) + clonazepam flexible dose 2 mg/oral/day to 6 mg/oral/day. N = 38. | |
| Outcomes | Mental state: PANSS total score, positive sub‐scale, negative sub‐scale, general psychopathology sub‐scale. Agitation: PANSS‐EC. Global outcome: CGI‐S. Leaving the study early. | |
| Notes | *Data were extracted only for the first 7 days because at the 8th day research group stopped clonazepam and switched from haloperidol to ziprasidone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised by using random number table". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | A total of 3 dropped out in haloperidol group (serious adverse events = 2, not following protocol = 1) and 2 dropped out in ziprasidone + clonazepam (lost to contact = 1, not following protocol = 1); missing data have not been imputed using appropriate methods such as LOCF. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | None obvious. |
ABS: Agitated Behaviour Scale
ACES: Agitation‐Calmness Evaluation Scale
AIMS: Abnormal Involuntary Movement Scale
BARS: Behavioural Activity Rating Scale
BAS: Barnes Akathisia Scale
BDZ: benzodiazepine
BPRS: Brief Psychiatric Rating Scale
BRMS:
CABS: Corrigan Agitated Behavior Scale
CCMD: Chinese Classification of Mental Disorders
CGI: Clinical Global Impression
CGI‐I: Clinical Global Impression ‐ Improvement
CGI‐S: Clinical Global Impression ‐ Severity
CI: confidence interval
COSTART: Coding Symbols and Thesaurus for Adverse Reaction Terms
DAI‐10: Drug Attitude Inventory
DSM: Diagnostic and Statistical Manual
ECG: electrocardiogram
EPS: Extrapyramidal Side Effects
ESBE:
GAS: Global Adjustment Scale
ICD: International Classification of Diseases
IM: intramuscular
LOCF: last observation carried forward
LS:
MBPRS: Modified Brief Psychiatric Rating Scale
NOSIE: Nurses Observation Scale for Inpatient Evaluation
OAS: Overt Aggression Scale
OASS: Overt Agitation Severity Scale
PANSS: Positive and Negative Syndrome Scale
PANSS‐EC: Positive and Negative Syndrome Scale Excited Component
PANSS‐PAS: Positive and Negative Syndrome Scale Psychotic Agitation Component
PANSS‐PES:
p.o.: orally
RSS: Ramsay Sedation Scale
SAS: Simpson‐Angus Scale
SD: standard deviation
SE: standard error
SGPT: serum glutamic pyruvic transaminase
TCS:
TESS: Treatment Emergent Symptom Scale
TSRS: Target Rating Symptom Scale
VAS: Visual analogue scale
YMRS: Young Mania Rating Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: randomised. | |
| Allocation: randomised. Participants: acute agitation and dangerous behaviour. Intervention: haloperidol + promethazine vs olanzapine, no haloperidol alone group. | |
| Allocation: not randomised, review. | |
| Allocation: randomised. Participants: psychotic disorders (N = 52), mood disorders (N = 23), cognitive impairment (N = 23), adjustment disorders (N = 17), others (infection, substance intoxication or withdrawal) (N = 35), and unknown aetiology (N = 10). Therefore, eligible patients are < 50% of the sample. An attempt to contact the authors for partial outcomes was made (3rd July 2016). | |
| Allocation: randomised. Participants: people with first episode psychosis, but not specifically aggressive. | |
| Allocation: randomised. Participants: newly admitted patients to a psychiatric emergency service who were uncooperative, threatening, and unresponsive to verbal interventions. Intervention: haloperidol + lorazepam vs lorazepam, no haloperidol alone group. | |
| Allocation: randomised. | |
| Allocation: randomised. Participants: children with aggressive behaviour, but the number of those with suspected psychosis < 50% of the sample. | |
| Allocation: quasi‐randomised. | |
| Allocation: quasi‐randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. Participants: admitted patients to an emergency setting experiencing agitation associated with active psychosis; inpatients experiencing exacerbation or agitation with active psychosis. Intervention: haloperidol + clonazepam vs risperidone + clonazepam, no haloperidol alone group. | |
| Allocation: randomised. Participants: people with chronic schizophrenia, acute exacerbation of symptoms, not specifically aggressive. | |
| Allocation: not randomised. | |
| Allocation: not randomised, review. | |
| Allocation: randomised. Participants: people with acute schizophrenia, not acutely agitated or aggressive. | |
| Allocation: randomised. Participants: people with psychosis, not acutely agitated or aggressive. | |
| Quasi‐randomised controlled trial: "the participants were assigned by the order of admission". | |
| Allocation: randomised. Participants: children with suspected schizophrenia, not specifically aggressive. | |
| Allocation: not randomised, retrospective case note study. | |
| Allocation: randomised. Participants: people with acute psychosis, not aggressive. | |
| Allocation: randomised. Participants: people with mild symptoms of schizophrenia, not aggressive. | |
| Allocation: quasi‐randomised. | |
| Quasi‐randomised controlled trial: "the participants were assigned by the order of admission". | |
| Allocation: not randomised, review. | |
| Allocation: randomised. Participants: people with acute schizophrenia, not specifically aggressive. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. Participants: people with schizophrenia and persistent aggressive behaviour, not an emergency situation. | |
| Allocation: randomised. Participants: people with schizophrenia and persistent aggressive behaviour, not an emergency situation. | |
| Allocation: randomised. Participants: people with schizophrenia and persistent aggressive behaviour, not an emergency situation. | |
| Allocation: randomised. Participants: people with acute excitement in schizophrenia. Intervention: haloperidol vs tiapride. Outcome: there was no specific outcome for acute agitation (T1 at 7 days). | |
| Allocation: quasi‐randomised. | |
| Quasi‐randomised controlled trials: "the participants were randomly assigned by the order of admission". | |
| Allocation: randomised. Participants: people with schizophrenia and acute agitation. Intervention: haloperidol vs modified electroconvulsive therapy (MECT), no comparison drug. | |
| Allocation: randomised. Participants: people with schizophrenia, not specifically aggressive. | |
| Allocation: not randomised, naturalistic observational study. | |
| Allocation: randomised. Participants: people with schizophrenia, not specifically aggressive. | |
| Allocation: not randomised, review. | |
| Allocation: was to be randomised, but trial not conducted because ethics approval not granted. | |
| Allocation: randomised. Participants: people with acute schizophrenia, not specifically aggressive. | |
| Allocation: randomised. Participants: people with schizophrenia and agitation. Intervention: haloperidol plus lorazepam vs olanzapine ‐ no haloperidol alone group. | |
| Allocation: quasi‐randomised. | |
| Allocation: randomised. Participants: people with schizophrenia, not specifically aggressive. | |
| Allocation: not randomised ‐ cohort study. | |
| Quasi‐randomised controlled trial: "the participants were assigned by the order of admission". | |
| Allocation: randomised. Participants: people with acute schizophrenia, not specifically aggressive. | |
| Allocation: randomised. Participants: people with intellectual disability and challenging behaviour, not psychosis‐induced aggression. | |
| Allocation: randomised. Participants: people with agitation in the emergency setting. Intervention: haloperidol plus benztropine mesylate vs lorazepam vs quetiapine ‐ no haloperidol alone group. | |
| Allocation: not reported, described as double‐blind. Participants: people with schizophrenia, not acute exacerbation of symptoms. | |
| Allocation: randomised. Participants: people with senile psychosis, not specifically aggressive. | |
| Allocation: randomised. Participants: "patients who presented with acute psychotic agitation". Intervention: haloperidol + promethazine vs lorazepam, no haloperidol alone group. | |
| Allocation: randomised. Participants: people with chronic schizophrenia, not specifically aggressive. | |
| Allocation: randomised. Participants: violent and agitated people, mostly intoxicated, not necessarily psychosis‐induced aggression. | |
| Allocation: randomised. Participants: acute agitation and dangerous behaviour. Intervention: haloperidol + promethazine vs midazolam (no haloperidol alone group). | |
| Allocation: randomised. Participants: people with acute schizophrenia, not specifically aggressive. | |
| Allocation: randomised. Participants: people with intellectual disability and agitation, not psychosis‐induced aggression. | |
| Allocation: randomised. Participants: people with psychosis‐induced agitation, not specifically aggressive (exclusion criteria included an inability to give informed consent). | |
| Allocation: randomised. Participants: agitated schizophrenic patients in the emergency setting. Intervention: haloperidol intranasal vs haloperidol intramuscular, no other drugs except haloperidol. | |
| Allocation: randomised. Participants: people with schizophrenia and agitation/aggression. Intervention: haloperidol vs chlorpromazine vs risperidone + clonazepam. Outcome: excitement and agitation scale (not validated), also the data for the haloperidol and chlorpromazine groups are combined. | |
| Allocation: quasi‐randomised. | |
| Allocation: quasi‐randomised. | |
| Allocation: randomised. | |
| Allocation: not reported, described as double‐blind. Participants: people with schizophrenia, not specifically aggressive. |
CGI: Clinical Global Impression
vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Allocation: randomised. Blindness: not stated. Duration: 24‐hour IM phase followed by 4‐day oral phase. |
| Participants | Diagnosis: schizophrenia with agitation. N = 360. Age: range 18‐60 years. Sex: not stated. History: not stated. Exclusion: not stated. Setting: not stated. |
| Interventions | 1. Haloperidol: dose 6.5 mg/IM, maximum 3 injections during 24 hours, followed by haloperidol 7 mg/p.o./day to 10 mg/p.o./day for 4 days. 2. Aripiprazole: dose 10 mg/IM, maximum 3 injections during 24 hours, followed by haloperidol 10 mg/p.o./day to 15 mg/p.o./day for 4 days. |
| Outcomes | Global state: CGI‐I, CGI‐S. Agitation: PANSS‐EC, ACES, CABS. |
| Notes | Conference proceeding, full characteristics and outcome data not reported. Additionally, unclear whether this is the same study as Bristol‐Myers 2005b ‐ attempted to contact Bristol Myers. |
| Methods | Allocation: randomised. Blindness: not stated. Duration: 24 hours. |
| Participants | Diagnosis: schizophrenia. N = 62. Age: not stated. Sex: not stated. History: "agitated schizophrenic patient admitted to hospital." Exclusion: not stated. Setting: not stated. |
| Interventions | 1. Haloperidol: dose 5 mg/IM/day to 15 mg/IM/day. N = 21. 2. Haloperidol: dose 5 mg/IM/day to 15 mg/IM/day + clonazepam: dose 2 mg/IM/day to 6 mg/IM/day. N = 21. 3. Clonazepam: dose 2 mg/IM/day to 6 mg/IM/day. N = 20. |
| Outcomes | Mental state: BPRS Total, BPRS Psychosis sub‐scale. Global state: CGI. Agitation: BPRS agitation sub‐scale. Adverse effects. |
| Notes | Conference proceeding, full characteristics and outcome data not reported. Attempted to contact author. |
| Methods | Allocation: randomised. Blindness: single. Duration: not stated. |
| Participants | Diagnosis: schizophrenia (N = 77), mania (N = 37), psychosis aetiology unknown (N = 25), amphetamine intoxication (N = 11), alcohol intoxication (N = 5), brief reactive psychosis (N = 3), brain organic syndrome (N = 1). N = 159. Age: range 18‐65 years. Sex: 77 males and 82 females. History: newly admitted ‐ previous history not stated. Exclusion: pregnancy, central nervous system depression, history of urinary retention or glaucoma, known sensitivity to phenothiazines, conditions such as epilepsy, severe hypotension or hypertension, head injuries, and haematologic, cardiovascular, renal or hepatic impairments, and ingestion of antipsychotic, sedative‐hypnotic, or antianxiety drugs within 24 hours of admission. Setting: psychiatric emergency hospital, USA. |
| Interventions | 1. Haloperidol: dose 5 mg/oral concentrate (mean number of doses 3.8). N = 15. 2. Haloperidol: dose 5 mg/IM (mean number of IMs not reported 3.2). N = 14. 3. Haloperidol: dose 10 mg/oral concentrate (mean number of doses 3.5). N = 14. 4. Haloperidol: dose 10 mg/IM (mean number of IMs 2.2). N = 17. 5. Thiothixene: dose 10 mg/IM (mean number of IMs 3.1). N = 24. 6. Thiothixene: dose 10 mg/oral concentrate (mean number of doses 4.7). N = 14. 7. Thiothixene: dose 20 mg/oral concentrate (mean number of doses 3.5). N = 14. 8. Thioridazine: dose 25 mg/oral suspension (mean number of doses 3.8). N = 16. 9. Thioridazine: dose 50 mg/oral suspension (mean number of doses 3.8). N = 13. 10. Thioridazine: dose 100 mg/oral suspension (mean number of doses 3.2). N = 18. |
| Outcomes | Global state: additional injections. Mental state: BPRS (mean/SD/SE/CI not reported). Adverse effects: % of whole sample experiencing side effects reported.* |
| Notes | *Attempted to contact author to see if there are any usable data available. |
| Methods | Allocation: randomised. Blindness: double. Duration: not stated. |
| Participants | Diagnosis: patients who referred to the emergency department because of medical diseases, drug poisoning, or trauma, and need the sedative agent to sedation. N = 48. Age: 18‐65 years. Sex: 36 males and 12 females. History: internal (N = 36)*, drug poisoning (N = 2), trauma (N = 10). Exclusion: sensitivity to haloperidol or midazolam, contraindications to these drugs, sympathomimetic agents, agitation because of reversible factors (such as hypotension, hypoxia, and hypoglycaemia), tachycardia or bradycardia, respiratory distress, pregnancy, symptoms of withdrawal syndrome, and receiving sedative agents within the past 12 hours. Setting: emergency department, Iran. |
| Interventions | 1. Haloperidol: fixed dose 5 mg/IM. 2. Midazolam: flexible doses 2.5 mg/IM to 5 mg/IM according to weight. |
| Outcomes | General: time to sedation, time to full consciousness, need for additional doses, need for re‐sedation within 60 minutes. Adverse effects. |
| Notes | *not clear which diagnosis includes; an attempt to contact the authors was made (3rd July 2016). |
| Methods | Allocation: randomised. Blindness: double. Duration: not stated. |
| Participants | Diagnosis: psychosis with agitation and/or violence. N = 20. Age: not stated. Sex: not stated. History: not stated. Exclusion: not stated. Setting: psychiatric emergency department. |
| Interventions | 1. Haloperidol: dose 10 mg/IM. 2. Risperidone: dose 2 mg/liquid. |
| Outcomes | Agitation: PANSS‐EC. |
| Notes | Conference proceeding, full characteristics and outcome data not reported. Attempted to contact author. |
| Methods | Allocation: randomised. Blindness: single. Duration: 24 hours. |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia (N = 20), bipolar disorder (N = 18), schizoaffective disorder (N = 1), delusional disorder or others (N = 3). N = 42. Age: range 18‐65 years. Sex: 20 males and 22 females. History: within 24 hours of admission ‐ previous psychiatric history not stated. Exclusion: "pregnant or lactating women; patients with serious medical illnesses; patients with closed‐angle glaucoma; patients with an allergic reaction to olanzapine, risperidone, or haloperidol; or patients who had received a long‐acting antipsychotic agent injection within 30 days were excluded". Setting: acute medical centre, Taiwan. |
| Interventions | 1. Haloperidol: dose 7.5 mg/IM. N = 11. 2. Risperidone: dose 3 mg/liquid. N = 10. 3. Olanzapine: dose 10 mg/IM. N = 11. 4. Olanzapine: dose 10 mg/velotab. N = 10. |
| Outcomes | Agitation: PANSS‐EC.* |
| Notes | *Attempted to contact author to see if there is any usable data available. |
| Methods | Allocation: randomised. Blindness: rater‐blinded. Duration: not stated. |
| Participants | Diagnosis: psychosis with agitation. N = 120. Age: not stated. Sex: males and females. History: not stated. Exclusion: not stated. Setting: psychiatric emergency room, Greece. |
| Interventions | 1. Haloperidol IM (dose not reported). 2. Aripiprazole IM (dose not reported). |
| Outcomes | Mental state: PANSS, YMRS. Global state: CGI‐I. Agitation: AIMS. Adverse effects: SAS. Quality of life: QoL |
| Notes | Conference proceeding, full characteristics and outcome data not reported. Unable to establish contact details at this time. |
| Methods | Allocation: randomised. Blindness: open‐label. Duration: 2 hours. |
| Participants | Diagnosis: acutely agitated patients. N = 37. Age: 18 years or older. Sex: not stated. History: "DSM‐IV diagnosis of schizophrenia, schizophreniform disorder, brief psychotic disorder, or schizoaffective disorder, bipolar dosprder" (p.S525). Exclusion: not stated. Setting: psychiatry ward, Inje University, China. |
| Interventions | 1. Haloperidol: fixed dose 5 mg/IM. N = 10. 2. Olanzapine: fixed dose 10 mg/IM. N = 12. 3. Haloperidol: fixed dose 5 mg/IM + lorazepam fixed dose 4 mg/IM. N = 15. |
| Outcomes | Global State: CGI‐S, CGI‐I. Agitation: PANSS‐EC. Adverse effects. Seclusions. |
| Notes | Abstract, full characteristics and outcome data not reported. Additionally, it is probably the same study as Shim 2009 (discordant doses and reported adverse effects). An attempt to contact the authors was made (3rd July 2016). |
| Methods | Allocation: randomised. Blindness: not stated. Duration: up to 3 months. |
| Participants | Diagnosis: ICD X diagnosis of acute exacerbation of schizophrenia or schizoaffective disorder, mania with psychotic features, acute paranoid reaction, or delusional disorders. N = 60. Age: > 18. Sex: males and females. History: not stated. Exclusion: "delirium, epilepsy, or mental retardation; intoxication or symptoms of withdrawal from alcohol or other psychoactive substances; clinical laboratory values indicating serious medical illness; treatment with any antipsychotic or benzodiazepine within 6 hours of screening; a history of neuroleptic malignant syndrome or known hypersensitivity to any of the trial medications; treatment with a depot antipsychotic within 1 treatment cycle of screening and use of disallowed medications". Setting: acute psychiatric inpatient ward, Croatia. |
| Interventions | 1. Haloperidol IM (dose not reported). 2. Risperidol liquid (dose not reported). |
| Outcomes | Mental state: PANSS. Global state: CGI‐I. Agitation: BARS, PANSS agitation cluster. Adverse effects |
| Notes | Conference proceeding, full characteristics and outcome data not reported. Attempted to contact author. |
| Methods | Allocation: not clearly stated; "each unit of patients were randomly assigned to one of three treatment groups by random number and hospital serial number". Blindness: double‐blind. Duration: 24 hours. |
| Participants | Diagnosis: CCMD‐3 diagnosis of schizophrenia. N = 45. Age: range 18‐45 years. Sex: males (20) and females (25). History: acute agitation. Exclusion: serious physical illness; additive to alcohol, drugs; taking schizophrenia medication one month before recruitment. Setting: psychiatric inpatient ward, China. |
| Interventions | 1. Haloperidol: dose 5 mg/IM/day to 15 mg/IM/day. N = 15. 2. Haloperidol: dose 5 mg/IM/day to 15 mg/IM/day + clonazepam dose 2 mg/IM/day to 6 mg/IM/day. N = 15. 3. Clonazepam: dose 2 mg/IM/day to 6 mg/IM/day. N = 15. |
| Outcomes | Mental state: BPRS. Adverse effects. Unable to use: CGI: data not available. |
| Notes | Allocation methodology not clearly stated. |
| Methods | Allocation: randomised. Blindness: rater‐blinded. Duration: 72 hours. |
| Participants | Diagnosis: CCMD‐3 diagnosis of schizophrenia. N = 60. Age: mean 33.5 (SD 12.3) years. Sex: male and female. History: acute agitation. Excluded: anyone who used anti‐psychotic or anti‐convulsant within one week prior to the trial or anyone who used long lasting drug within one month prior to the trial. Setting: inpatients, China*. |
| Interventions | 1. Haloperidol: dose 5 mg/IM, to 10 mg/IM, maximum dose 30 mg during 24 hours. N = 30. 2. Ziprasidone: dose 10 mg/IM, to 20 mg/IM, maximum dose 40 mg during 24 hours. N = 30. |
| Outcomes | Global state: CGI‐IS. Adverse effects: TESS. Agitation: PANSS‐EC. Unable to use: Adverse effects: the amount of anticholinergic drugs used was not reported. |
| Notes | *It is not clear if this paper presents the results from one centre of the Li 2006 multi‐centre trial. Attempted to contact author to clarify. |
| Methods | Allocation: randomised. Blindness: single. Duration: up to 47 days. |
| Participants | Diagnosis: DSM‐IV diagnosis of acute exacerbation of schizophrenia or schizoaffective disorder with agitation. N = not stated. Age: range > 18 < 45 years. Sex: males and females. History: not stated. Exclusion: pregnant or lactating women, serious medical illness, known sensitivity to study medication, treatment with a depot antipsychotic with 1 cycle of screening, use of disallowed medication, psychosis caused by "delirium, epilepsy, mental retardation and affective disorder; intoxication or symptoms of withdrawal from alcohol of other psychoactive substances." Setting: psychiatric inpatient ward, China. |
| Interventions | 1. Haloperidol: dose 5 mg/IM/day to 20 mg/IM/day. 2. Risperidone: dose 2 mg/oral/day to 6 mg/oral/day + clonazepam: dose 4 mg/oral/day to 8 mg/oral/day. |
| Outcomes | Mental state: PANSS. Agitation: PANSS‐EC. |
| Notes | *Protocol, full characteristics and outcome data not reported. Unable to establish contact details at this time. |
| Methods | Allocation: randomised. Blindness: single. Duration: 72 hours. |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia or schizophreniform psychosis. N = not stated. Age: range > 18 < 65 years. Sex: males and females. History: not stated. Exclusion: investigator and his/her relatives, participation in another drug trial within 3 months prior to enrolment into this study, pregnant or lactating women, serious medical illness, a significantly abnormal value in ECG or lab results, family history of sudden death, meet the DSM‐IV criteria for substance abuse within 1 year prior to enrolment, regular use of antipsychotics (clozapine within 90 days), antidepressants, mood stabilisers, anti‐epileptics or prolonged‐action preparations within 2 weeks prior to enrolment, use of ECT within 30 days prior to enrolment, systematic use of sulpiride, levosulpiride, or haloperidol therapy within 30 days prior enrolment, history of neuroleptic malignant syndrome, severe EPS or significant tardive dyskinesia, severe suicide attempt, know hypersensitivity to sulpiride, levosulpiride or haloperidol, history of hypersensitivity to more than 2 drugs, use of psychotropics (except for permitted drugs) within 12 hours prior to enrolment, known lack of efficacy to levosulpiride or haloperidol by formal treatment before, organic mental disorders including learning disability, history of psychosurgery treatment, people who could not comply with the study protocol. Setting: psychiatric inpatient ward, China. |
| Interventions | 1. Haloperidol: dose 5 mg/IM, ≥ 4 hours apart, maximum 10 mg during 24 hours. 2. Levosulpiride: dose 50 mg/IM, ≥ 4 hours apart, maximum 100 mg during 24 hours. |
| Outcomes | Mental state: BPRS, PANSS Total. Global State: CGI, CGI‐I, CGI‐S. Agitation: ACES, PANSS‐EC. Adverse effects: BAS, ECG, laboratory tests, physical examination, RSESE. |
| Notes | Protocol, full characteristics and outcome data not reported. Attempted to contact author to enquire whether there is any available data. |
| Methods | Allocation: randomised. Blindness: open‐label. Duration: 2 hours. |
| Participants | Diagnosis: acutely agitated patients. N = 37. Age: not stated. Sex: not stated. History: "hospitalized patients with acute psychotic symptom in association with schizophrenia, bipolar I disorder, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, delusional disorder" (p.S512). Exclusion: not stated. Setting: psychiatry ward, Inje University, China. |
| Interventions | 1. Haloperidol: fixed dose 4 mg/IM. N = 10. 2. Olanzapine: fixed dose 10mg/IM. N = 12. 3. Haloperidol: fixed dose 4 mg/IM + lorazepam fixed dose 5 mg/IM. N = 15. |
| Outcomes | Global State: CGI‐S. Agitation: PANSS‐EC, BARS. Adverse effects. |
| Notes | Abstract, full characteristics and outcome data not reported. Additionally, it is probably the same study as Kong 2009 (discordant doses and reported adverse effects). An attempt to contact the authors was made (3rd July 2016). |
| Methods | Allocation: randomised. Blindness: open‐label. Duration: 28 days. |
| Participants | Diagnosis: acute exacerbation of schizophrenia with agitation and hostility. N = 80. Age: not stated. Sex: not stated. History: not stated. Exclusion: not stated. Setting: psychiatric inpatient ward, China. |
| Interventions | 1. Haloperidol: dose 10 mg/oral/day to 12 mg/oral/day. N = 40. 2. Quetiapine: dose 600 mg/oral/day to 750 mg/oral/day. N = 40. |
| Outcomes | Mental state: BPRS, PANSS Total. Global State: CGI, CGI‐I, CGI‐S. Agitation: ACES, PANSS‐EC. Adverse effects: BAS, ECG, laboratory tests, physical examination, RSESE. |
| Notes | Conference proceeding, full characteristics and outcome data not reported. Attempted to contact author. |
| Methods | Allocation: randomised. Blindness: double‐blind. Duration: 48 hours. |
| Participants | Diagnosis: "acutely agitated psychiatric patients". N = 150 (three trials combined). Age: not stated. Sex: not stated. History: not stated. Exclusion: not stated. Setting: not stated. |
| Interventions | 1. Haloperidol IM (dose not reported). N = 75 (three trials combined). 2. Chorpromazine IM (dose not reported). N = 75 (three trials combined). |
| Outcomes | Mental state: BPRS. |
| Notes | Conference proceeding, full characteristics and outcome data for the three trials not reported. Unable to establish contact details at this time. |
| Methods | Allocation: not reported. Blindness: double. Duration: 10 days. |
| Participants | Diagnosis: DSM‐III diagnosis of schizophrenia with acute agitation. N = 16. Age: not stated. Sex: not stated. History: not stated. Exclusion: at risk of pregnancy, significant medical disorders, history of EPS or hypotensive crisis upon administration of other injectable antipsychotic agents, significantly abnormal laboratory test results, recent alcoholism or drug dependency. Setting: inpatient, USA. |
| Interventions | 1. Haloperidol: dose 2.5 mg/IM to 80 mg/IM, for 2 to 10 days. 2. Molindone: dose 12.5 mg/IM to 150 mg/IM, for 4‐7 days. |
| Outcomes | Mental state: BPRS. Global state: CGI. Agitation: TSRS*. |
| Notes | *Attempted to contact author to see if there is any available usable data available. |
| Methods | Allocation: randomised. Blindness: simple. Duration: not stated. |
| Participants | Diagnosis: DSM‐IV diagnosis of acute psychosis ‐ "schizophrenic or affective". N = 30. Age: range 18‐83 years. Sex: not stated. History: not stated. Exclusion: not stated. Setting: emergency room, Peru. |
| Interventions | 1. Haloperidol: dose 5 mg/IM. N = 15. 2. Midazolam: dose 15 mg/IM. N = 15. |
| Outcomes | Aggression: OAS. |
| Notes | Conference proceeding, full characteristics and outcome data for the three trials not reported. Unable to establish contact details at this time. |
| Methods | Allocation: randomised. Blinding: single‐blind. Duration: 72 hours. |
| Participants | Diagnosis: CCMD‐3 diagnosis of schizophrenia. N = 60. Age: mean 27.40 ± 9.64 years (ziprasidone group), mean 27.40 ± 9.64 years (haloperidol group). Sex: 39 males and 21 females. History: total score of PANSS ≥ 60; total score of excitement component of PANSS ≥14 and at least one section ≥ 4. Exclusion: physical illness; additive to alcohol, drugs; pregnant or plan to be pregnant. Setting: inpatients*, China. |
| Interventions | 1. Haloperidol: dose 5 mg/IM to 10 mg/IM, maximum 30 mg/day. N = 30. 2. Ziprasidone: dose 10 mg/IM to 20 mg/IM, maximum 40 mg/day. N = 30. |
| Outcomes | Mental state: PANSS‐EC. Adverse effects: TESS. Adverse effects: movement ‐ SAS. |
| Notes | *It is not clear if this paper presents the results from one centre of the Li 2006 multi‐centre trial. Attempted to contact author to clarify. |
| Methods | Allocation: randomised. Blindness: single. Duration: 72 hours. |
| Participants | Diagnosis: CCND‐3 diagnosis of schizophrenia with agitation/aggression. N = 32. Age: mean 25.8 (SD 10.4) years (haloperidol group), 28.6 (SD 9.4) years (ziprasidone group). Sex: 16 males and 16 females. History: first episode (N = 27), relapse (N = 5). Excluded: direct blood relatives of staff in current trial, participation in other drug trials 30 days prior to enrolment, pregnant or lactating women, severe disease/unstable medical condition, clinically significant abnormal EEG or laboratory results, QTC interval ≥ 450, family history of sudden death, drug abuse in past year, regular user of antipsychotic, antidepressant, depot medication, ziprasidone or haloperidol in past 30 days, people with drug‐induced malignant syndrome, suicidal ideation, or known allergy to ziprasidone or haloperidol. Setting: inpatient, China*. |
| Interventions | 1. Haloperidol: flexible dose 5 mg/IM, to 10 mg/IM, repeated every 4‐6 hours if necessary, maximum dose 30 mg during 24 hours. N = 16. 2. Ziprasidone: flexible dose 10 mg/IM, to 20 mg/IM, repeated every 4‐6 hours if necessary, maximum dose 40 mg during 24 hours. N = 16. |
| Outcomes | Mental state: PANSS. Global State: CGI. Agitation: PANNS‐EC, ACES. Adverse effects: BAS. |
| Notes | *It is not clear if this paper presents the results from one centre of the Li 2006 multi‐centre trial. Attempted to contact author to clarify. |
| Methods | Allocation: not stated. Blindness: not stated. Duration: 2 hours. |
| Participants | Diagnosis: "acutely agitated psychiatric patients". N = not stated. Age: not stated. Sex: not stated. History: not stated. Excluded: not stated. Setting: acute psychiatry service. |
| Interventions | 1. Haloperidol: flexible dose 2 mg/IM to 5 mg/IM. N = not stated. 2. Risperidone: flexible dose 1 mg/oral to 2 mg/oral. N = not stated. Concomitant use of lorazepam was allowed. |
| Outcomes | Unable to use: Agitation: "four different agitation rating scales", not reported. |
| Notes | Abstract of poser, full characteristics and outcome data not reported. Unable to establish contact details at this time. |
| Methods | Allocation: randomised. Blinding: not stated. Duration: 7 days. |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia. N = 60. Age: range 18‐65 years. Sex: 36 males, 24 females. History: acute agitation; length of Illness 124.7 ± 115.3 months (haloperidol group), 157.5 ± 113.0 months (risperidone+clonazepam group). Excluded: "allergy to compound utilised in the treatment, serious physical sickness, addiction to alcohol or drugs, history of taking long‐term schizophrenia medicine, suicide tendency, pregnancy". Setting: inpatients, China. |
| Interventions | 1. Haloperidol: dose 10 mg/IM/day to 20 mg/IM/day. N = 30. 2. Risperidone: dose 2 mg/oral/day to 6 mg/oral/day + clonazepam dose 2 mg/oral/day to 8 mg/oral/day. N = 30. |
| Outcomes | Mental state: PANSS total and positive sub‐scale. Agitation: PANSS‐EC. Adverse effects: TESS. |
| Notes |
| Methods | Allocation: randomised. Blindness: single. Duration: 72 hours. |
| Participants | Diagnosis: DSM‐IV diagnosis of schizophrenia. N = 60. Age: mean 28.37 ±1 0.20 years (haloperidol group), mean 27.40 ± 9.63 years (ziprasidone group). Sex: 39 males, 21 females. History: acute agitation. Excluded: "physical illness; addiction to alcohol or drugs; pregnancy or plan to be pregnant". Setting: inpatients, China*. |
| Interventions | 1. Haloperidol: dose 5 mg/IM to 10 mg/IM, maximum 30 mg/day. N = 30. 2. Ziprasidone: dose 10 mg/IM to 20 mg/IM, maximum 40 mg/day. N = 30. |
| Outcomes | Mental state: PANSS total. Agitation: PANSS‐EC. Adverse events. |
| Notes | *It is not clear if this paper presents the results from one centre of the Li 2006 multi‐centre trial. Authors contact unavailable. |
| Methods | Allocation: randomised. Blindness: not stated. Duration: 7 days. |
| Participants | Diagnosis: CCMD‐3 diagnosis of schizophrenia. N = 65. Age: range 18‐60 years. Sex: 36 males, 29 females. History: acute psychotic agitation; length of Illness of 1.1 to 22.3 months. Excluded: pregnancy, serious physical illness, addiction to alcohol or drugs. Setting: inpatients, China*. |
| Interventions | 1. Haloperidol: dose 10 mg/IM/day to 20 mg/IM/day. N = 32. 2. Risperidone: dose 4 mg/oral/day to 6 mg/oral/day + clonazepam dose 2 mg/oral/day to 4 mg/oral/day. N = 33. |
| Outcomes | Unable to use: Mental state: PANSS total (data not reported). Agitation: PANSS‐EC (data not reported). Adverse events (data not reported). |
| Notes | *It is not clear if this paper presents the results from one centre of the Fang 2012 multi‐centre trial. Authors contact unavailable. |
ACES: Agitation‐Calmness Evaluation Scale
AIMS: Abnormal Involuntary Movement Scale
BARS: Behavioural Activity Rating ScaleBAS: Barnes Akathisia Scale
BPRS: Brief Psychiatric Rating Scale
CABS: Corrigan Agitated Behavior Scale
CCMD: Chinese Classification of Mental Disorders
CGI: Clinical Global Impression
CGI‐I: Clinical Global Impression – Improvement
CGI‐S: Clinical Global Impression – Severity
CI: confidence interval
DSM: Diagnostic and Statistical Manual
ECG: electrocardiogram
ECT: electroconvulsive therapy
EPS: Extrapyramidal Side Effects
ICD: International Classification of Diseases
IM: intramuscular
OAS: Overt Aggression Scale
PANSS: Positive and Negative Syndrome Scale
PANSS‐EC: Positive and Negative Syndrome Scale Excited Component
p.o.: by mouth
RSESE: Rating Scale for Extrapyramidal Side Effects.
SAS: Simpson‐Angus Scale
SD: standard deviation
SE: standard error
TESS: Treatment Emergent Symptom Scale
TSRS: TSRS ‐ Target Rating Symptom Scale
YMRS ‐ Young Mania Rating Scale
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Pilot study to evaluate the efficacy and safety of quetiapine fumarate instant‐release (Seroquel IR) in controlling agitation and aggressive symptoms in the acute treatment of patients with schizophrenia |
| Methods | Allocation: randomised. Blindness: single. Duration: 14 days |
| Participants | Diagnosis: CCMD‐3 diagnosis of schizophrenia with agitation. N = not stated. Age: > 18 < 65. Sex: males and females. History: not stated. Exclusion: pregnancy or lactation, risk of suicide, self‐harm or harm to others, known intolerance or lack of response to quetiapine fumarate or haloperidol, use of ketonazole, itraconazole, fluconazole, erythromycin, clarithromycin, troleandomycin, indinavir, nelfinavir, ritonavir, fluvoxamine, saquinavir, phenytoin, carbamazepine, rifampin, St. John's Wort and glucocorticoids two weeks prior to the trial, depot anti‐psychotic within one dosing interval (for the depot) before randomisation, CCMD diagnosis of substance or alcohol dependence at enrolment (exception of caffeine or nicotine), use of opiates, amphetamine, cocaine, cannabis, barbiturates or hallucinogens within four weeks prior, medical conditions that would affect the absorption, distribution, metabolism, or excretion of study treatment, unstable or inadequately treated medical illnesses, involvement in the planning and conduct of the study, previous enrolment or randomisation in the present study, participation in another drug trial within four weeks prior to enrolment or in accordance with local requirements, a person with diabetes mellitus, an absolute neutrophil count of 1.5 x 109 per litre, 2 x higher than normal upper limit of ALT or AST, use of clozapine within 28 days prior to randomisation. Setting: psychiatric inpatient ward, China. |
| Interventions | 1. Haloperidol. 2. Quetiapine. |
| Outcomes | Not stated. |
| Starting date | August 2008. |
| Contact information | Bo Zhang, PhD 13808203275 [email protected] |
| Notes | Protocol, full characteristics and outcome data not reported. Attempted to contact author to enquire whether there are any available data. |
ALT: alanine aminotransferase
AST: aspartate transaminase
CCMD: Chinese Classification of Mental Disorders
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ asleep Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 1 Tranquillisation or asleep ‐ asleep. | ||||
| 1.1 not asleep up to 2 hours | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.82, 0.95] |
| 2 Repeated need for tranquillisation Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 2 Repeated need for tranquillisation. | ||||
| 2.1 needing additional injection during 24 hours (agitation only) | 4 | 660 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.42, 0.62] |
| 3 Specific behaviour: 1a. Agitation ‐ various measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 3 Specific behaviour: 1a. Agitation ‐ various measures. | ||||
| 3.1 PANSS‐EC response at 2 hours (at least 40% change on PANSS‐EC from baseline) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.28, 2.07] |
| 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours. | ||||
| 4.1 change score (ABS, high=worse) | 3 | 474 | Mean Difference (IV, Fixed, 95% CI) | ‐4.48 [‐5.78, ‐3.18] |
| 4.2 change score (ACES, low=agitated, high=sedated) | 2 | 390 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.39, 1.26] |
| 4.3 score (PANSS‐EC, high=worse) | 3 | 514 | Mean Difference (IV, Fixed, 95% CI) | ‐3.67 [‐4.75, ‐2.59] |
| 4.4 change score (PANSS‐EC, high=worse, schizophrenia subgroup) | 1 | 199 | Mean Difference (IV, Fixed, 95% CI) | ‐2.57 [‐4.05, ‐1.09] |
| 4.5 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on ACES) | 1 | 240 | Mean Difference (IV, Fixed, 95% CI) | ‐2.64 [‐3.95, ‐1.33] |
| 4.6 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on ACES) | 1 | 263 | Mean Difference (IV, Fixed, 95% CI) | ‐2.97 [‐4.27, ‐1.67] |
| 5 Specific behaviour: 1c. Agitation ‐ average scores ‐ ii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 5 Specific behaviour: 1c. Agitation ‐ average scores ‐ ii. up to 24 hours. | ||||
| 5.1 change score (ABS, high=worse) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐2.4 [‐4.13, ‐0.67] |
| 5.2 change score (PANSS‐EC, high=worse) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐1.4 [‐2.97, 0.17] |
| 6 Global outcome: 1. Not improved Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 6 Global outcome: 1. Not improved. | ||||
| 6.1 not marked improvement | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.44, 0.84] |
| 6.2 not any improvement | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 1.07] |
| 7 Global outcome: 2. Need for benzodiazepine up to 24 hours Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7 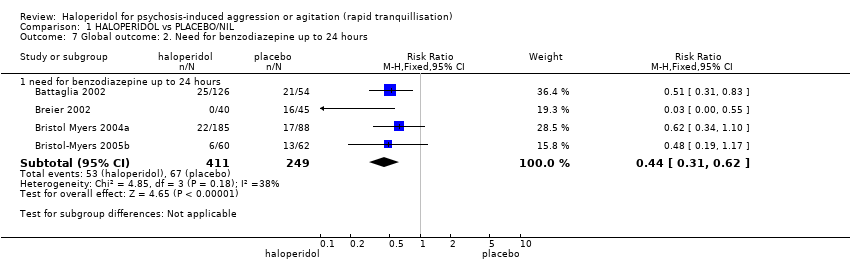 Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 7 Global outcome: 2. Need for benzodiazepine up to 24 hours. | ||||
| 7.1 need for benzodiazepine up to 24 hours | 4 | 660 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.31, 0.62] |
| 8 Global outcome: 3a. Average scores ‐ i. up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8 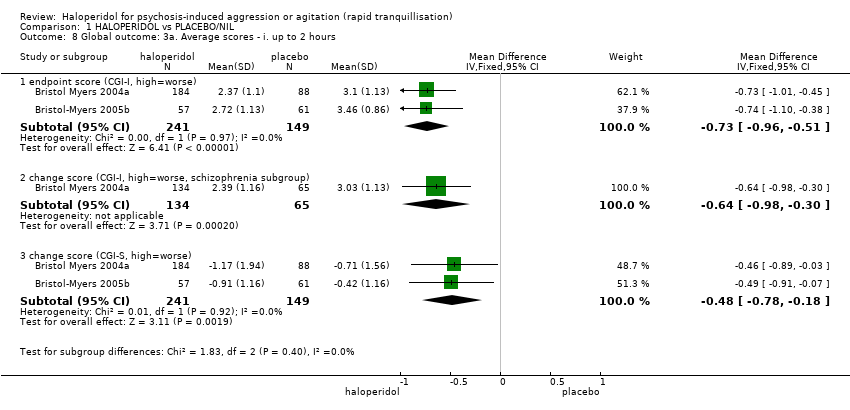 Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 8 Global outcome: 3a. Average scores ‐ i. up to 2 hours. | ||||
| 8.1 endpoint score (CGI‐I, high=worse) | 2 | 390 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐0.96, ‐0.51] |
| 8.2 change score (CGI‐I, high=worse, schizophrenia subgroup) | 1 | 199 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐0.98, ‐0.30] |
| 8.3 change score (CGI‐S, high=worse) | 2 | 390 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.78, ‐0.18] |
| 9 Global outcome: 3b. Average scores ‐ ii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 9 Global outcome: 3b. Average scores ‐ ii. up to 24 hours. | ||||
| 9.1 change score (CGI‐I, high=worse) | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐0.62, ‐0.18] |
| 9.2 change score (CGI‐S, high=worse) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐0.2 [‐0.46, 0.06] |
| 10 Mental state: 1a. Average scores ‐ i. up to 2 hours Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 10 Mental state: 1a. Average scores ‐ i. up to 2 hours. | ||||
| 10.1 change score up to 2 hours (BPRS positive sub‐scale, high=worse) | 3 | 372 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.54, ‐0.54] |
| 10.2 change score up to 2 hours (BPRS total, high=worse) | 3 | 371 | Mean Difference (IV, Fixed, 95% CI) | ‐5.69 [‐7.38, ‐4.00] |
| 11 Mental state: 1b. Average scores ‐ ii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 11 Mental state: 1b. Average scores ‐ ii. up to 24 hours. | ||||
| 11.1 change score up to 24 hours (BPRS positive sub‐scale, high=worse) | 2 | 264 | Mean Difference (IV, Fixed, 95% CI) | ‐1.61 [‐2.34, ‐0.89] |
| 11.2 change score up to 24 hours (BPRS total, high=worse) | 2 | 264 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐6.82, ‐2.81] |
| 12 Adverse effects: 1a. General Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 12 Adverse effects: 1a. General. | ||||
| 12.1 one or more drug related adverse events during 24 hours | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.22, 2.20] |
| 12.2 increased severity of adverse effects after 2nd injection | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.88, 5.63] |
| 12.3 overall adverse events during 72 hours | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.23, 2.59] |
| 13 Adverse effects: 1b. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13 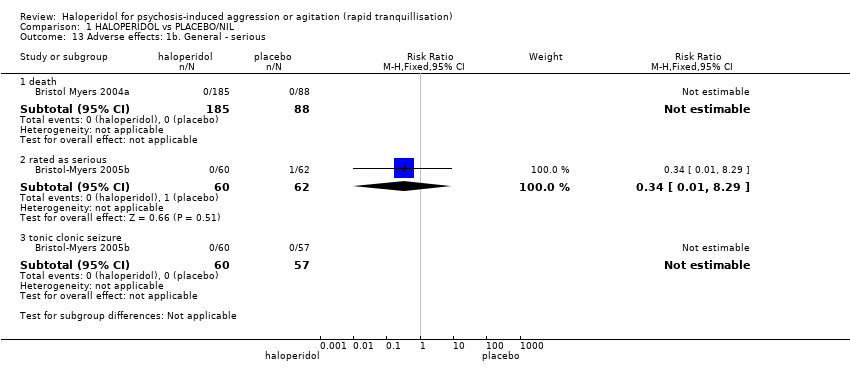 Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 13 Adverse effects: 1b. General ‐ serious. | ||||
| 13.1 death | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 rated as serious | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.29] |
| 13.3 tonic clonic seizure | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Adverse effects: 2a. Specific ‐ arousal level Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.14 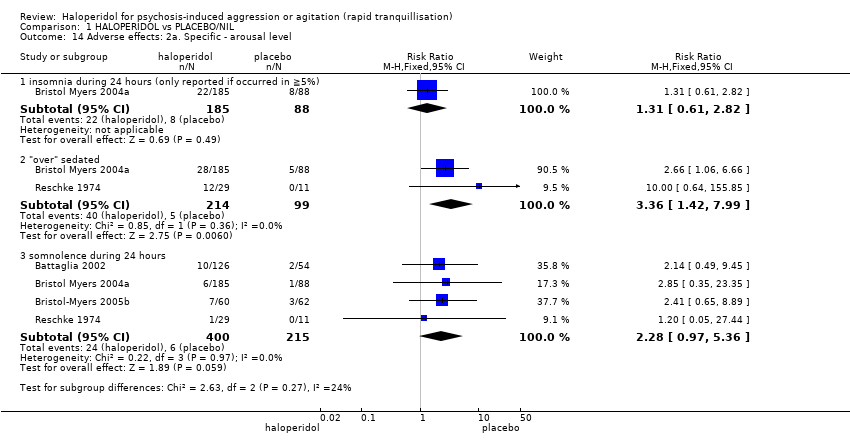 Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 14 Adverse effects: 2a. Specific ‐ arousal level. | ||||
| 14.1 insomnia during 24 hours (only reported if occurred in ≧5%) | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.61, 2.82] |
| 14.2 "over" sedated | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.36 [1.42, 7.99] |
| 14.3 somnolence during 24 hours | 4 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [0.97, 5.36] |
| 15 Adverse effects: 2b. Specific ‐ cardiovascular ‐ miscellaneous outcomes Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 15 Adverse effects: 2b. Specific ‐ cardiovascular ‐ miscellaneous outcomes. | ||||
| 15.1 dizziness during 24 hours (only reported if occurred in ≧5%) | 2 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.48, 3.65] |
| 15.2 hypotension during 24 hours | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.05, 27.44] |
| 15.3 QTc abnormality | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.1 [0.33, 28.98] |
| 15.4 sinus tachycardia during 24 hours (only reported if occurred in ≥5%) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.1 [0.33, 28.98] |
| 15.5 tachycardia during 24 hours (only reported if occurred in ≥5%) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 16.15] |
| 16 Adverse effects: 2c. Specific ‐ cardiovascular ‐ QTc interval (average change at 24 hours) Show forest plot | 2 | 265 | Mean Difference (IV, Fixed, 95% CI) | 3.63 [‐2.67, 9.93] |
| Analysis 1.16 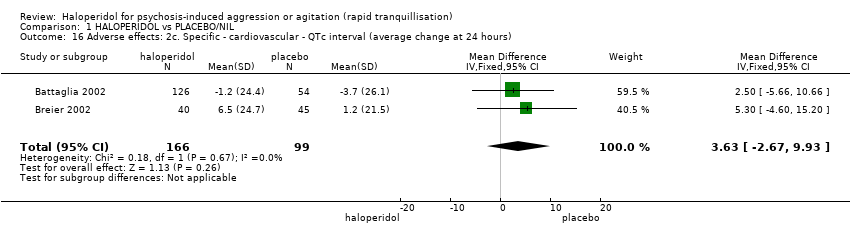 Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 16 Adverse effects: 2c. Specific ‐ cardiovascular ‐ QTc interval (average change at 24 hours). | ||||
| 17 Adverse effects: 2d. Specific ‐ movement disorders Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 17 Adverse effects: 2d. Specific ‐ movement disorders. | ||||
| 17.1 akathisia during 24 hours (only reported if occurred in ≥5%) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.43 [0.77, 233.23] |
| 17.2 dystonia during 24 hours | 2 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.49 [0.93, 60.21] |
| 17.3 extrapyramidal effects ‐ use of antiparkinson drugs during 24 hours | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.57 [1.37, 22.65] |
| 17.4 EPS during 24 hours | 3 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.79 [2.19, 21.07] |
| 18 Adverse effects: 2e. Specific ‐ movement disorders: i. average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.18 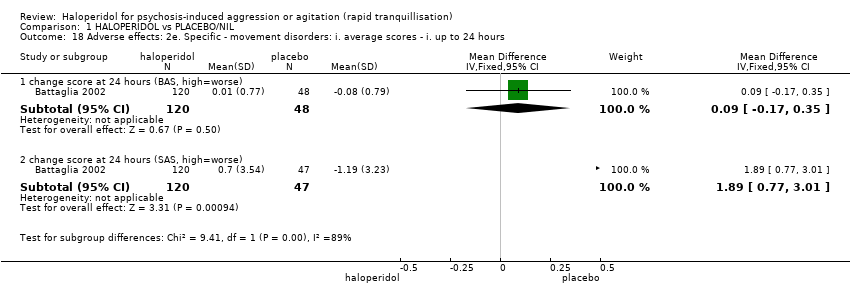 Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 18 Adverse effects: 2e. Specific ‐ movement disorders: i. average scores ‐ i. up to 24 hours. | ||||
| 18.1 change score at 24 hours (BAS, high=worse) | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.17, 0.35] |
| 18.2 change score at 24 hours (SAS, high=worse) | 1 | 167 | Mean Difference (IV, Fixed, 95% CI) | 1.89 [0.77, 3.01] |
| 19 Adverse effects: 2f. Specific ‐ miscellaneous Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.19  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 19 Adverse effects: 2f. Specific ‐ miscellaneous. | ||||
| 19.1 agitation during 24 hours (only reported if occurred in ≧5%) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.29, 2.19] |
| 19.2 dry mouth | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.6 [0.21, 61.86] |
| 19.3 headache during 24 hours (only reported if occurred in ≧5%) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.55, 3.00] |
| 19.4 pain at injection site | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 19.5 pain (1st injection) | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.00, 1.97] |
| 19.6 pain (2nd injection) | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.88, 5.63] |
| 19.7 nausea during 24 hours (only reported if occurred in ≧5%) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.13, 3.67] |
| 19.8 vomiting during 24 hours (only reported if occurred in ≧5%) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 16.15] |
| 20 Leaving the study early Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.20  Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 20 Leaving the study early. | ||||
| 20.1 any reason | 3 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.53, 2.11] |
| 20.2 lack of efficacy | 3 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.49] |
| 20.3 withdrew | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [0.17, 64.15] |
| 20.4 consent | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.2 [0.77, 49.98] |
| 20.5 adverse effects | 3 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.21, 15.39] |
| 20.6 could not be evaluated due to being asleep | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.05, 27.44] |
| 20.7 other | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.27, 12.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for rapid tranquillisation: needing additional injection Show forest plot | 2 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| Analysis 2.1  Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 1 Repeated need for rapid tranquillisation: needing additional injection. | ||||
| 2 Specific behaviour: 1a. Agitation ‐ PANSS‐EC response up to 2 hours (at least 40% change on PANSS‐EC from baseline) Show forest plot | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.26] |
| Analysis 2.2 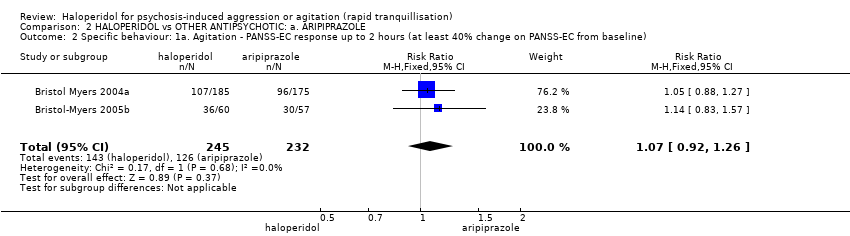 Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 2 Specific behaviour: 1a. Agitation ‐ PANSS‐EC response up to 2 hours (at least 40% change on PANSS‐EC from baseline). | ||||
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ up to 2 hours. | ||||
| 3.1 change score (ACES, low=agitated, high=sedated) | 2 | 470 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.30, 0.55] |
| 3.2 change score (CABS, high=worse) | 2 | 470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐2.10, 1.01] |
| 3.3 change score (PANSS‐EC, high=worse) | 1 | 357 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐2.12, 1.16] |
| 3.4 change score (PANSS‐EC, high=worse, schizophrenia subgroup) | 1 | 257 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐1.48, 0.96] |
| 3.5 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on ACES) | 1 | 316 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐1.42, 0.76] |
| 3.6 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on AEs) | 1 | 346 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.40, 0.72] |
| 4 Global outcome: 1. Need for benzodiazepine Show forest plot | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.74, 2.16] |
| Analysis 2.4  Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 4 Global outcome: 1. Need for benzodiazepine. | ||||
| 5 Global outcome: 2. Average scores ‐ up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 5 Global outcome: 2. Average scores ‐ up to 2 hours. | ||||
| 5.1 change score (CGI‐S, high=worse) | 2 | 470 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.22, 0.36] |
| 5.2 endpoint score (CGI‐I, high=worse) | 2 | 470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.23, 0.19] |
| 5.3 change score (CGI‐I, high=worse, schizophrenia subgroup) | 1 | 257 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.30, 0.26] |
| 6 Mental state: 1. Average scores ‐ up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6 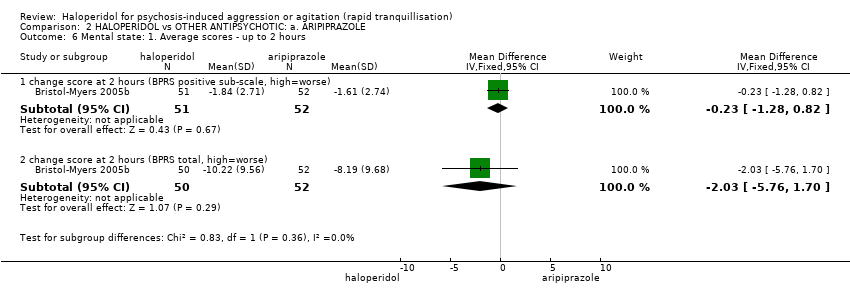 Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 6 Mental state: 1. Average scores ‐ up to 2 hours. | ||||
| 6.1 change score at 2 hours (BPRS positive sub‐scale, high=worse) | 1 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐1.28, 0.82] |
| 6.2 change score at 2 hours (BPRS total, high=worse) | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐2.03 [‐5.76, 1.70] |
| 7 Adverse effects: 1a. General Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 7 Adverse effects: 1a. General. | ||||
| 7.1 one or more drug related adverse effects during 24 hours | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.95, 1.46] |
| 7.2 increased severity of adverse effects after 2nd injection | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.03, 1.74] |
| 7.3 overall adverse events during 72 hours | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.04, 1.70] |
| 8 Adverse effects: 1b. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 8 Adverse effects: 1b. General ‐ serious. | ||||
| 8.1 any | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.18, 1.81] |
| 8.2 tonic clonic seizure | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.62] |
| 8.3 death | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse effects: 2a. Specific ‐ arousal level Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.9  Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 9 Adverse effects: 2a. Specific ‐ arousal level. | ||||
| 9.1 insomnia ‐ between 0‐1 days (IM phase) (only reported if occurred in ≥5%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.01, 4.27] |
| 9.2 insomnia ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.47, 1.44] |
| 9.3 "over" sedated | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.93, 2.95] |
| 9.4 somnolence ‐ between 0‐1 days | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.25, 2.00] |
| 9.5 somnolence ‐ between 0‐1 days (IM phase) (only reported if occurred in ≥5%) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.60, 8.16] |
| 9.6 somnolence ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.30, 27.02] |
| 10 Adverse effects: 2b. Specific ‐ cardiac Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.10  Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 10 Adverse effects: 2b. Specific ‐ cardiac. | ||||
| 10.1 dizziness ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.33, 1.48] |
| 10.2 sinus tachycardia ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.66 [0.35, 126.06] |
| 10.3 tachycardia ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.06] |
| 11 Adverse effects: 2c. Specific ‐ gastrointestinal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.11  Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 11 Adverse effects: 2c. Specific ‐ gastrointestinal. | ||||
| 11.1 dyspepsia ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.41 [1.36, 79.76] |
| 11.2 diarrhoea ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.28, 3.21] |
| 11.3 nausea ‐ between 0‐1 days (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.60] |
| 11.4 nausea ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.09] |
| 11.5 vomiting ‐ between 0‐1 days (IM phase) (only reported if occurred in ≥5%) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.10] |
| 11.6 vomiting ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.07, 1.92] |
| 12 Adverse effects: 2d. Specific ‐ movement disorder Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.12  Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 12 Adverse effects: 2d. Specific ‐ movement disorder. | ||||
| 12.1 akathisia ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.94, 8.61] |
| 12.2 akathisia ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.58, 8.40] |
| 12.3 dystonia ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.63 [1.52, 28.86] |
| 12.4 EPS ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.46 [1.22, 73.13] |
| 12.5 EPS ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.09 [1.65, 30.58] |
| 12.6 Use of antiparkinson drugs ‐ between 3 ‐ 7 days (oral phase) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.94 [2.50, 9.78] |
| 12.7 dystonia | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 69.22] |
| 13 Adverse effects: 2e. Specific ‐ miscellaneous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.13  Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 13 Adverse effects: 2e. Specific ‐ miscellaneous. | ||||
| 13.1 agitation ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.46, 3.22] |
| 13.2 agitation ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.68, 1.74] |
| 13.3 anxiety ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.54, 3.16] |
| 13.4 headache ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.45, 1.59] |
| 13.5 headache ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.57, 1.97] |
| 13.6 pain at injection site | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.62] |
| 13.7 pain (1st injection) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.06, 1.54] |
| 13.8 pain (2nd injection) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.03, 1.74] |
| 14 Leaving the study early: 1. For specific reasons Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.14 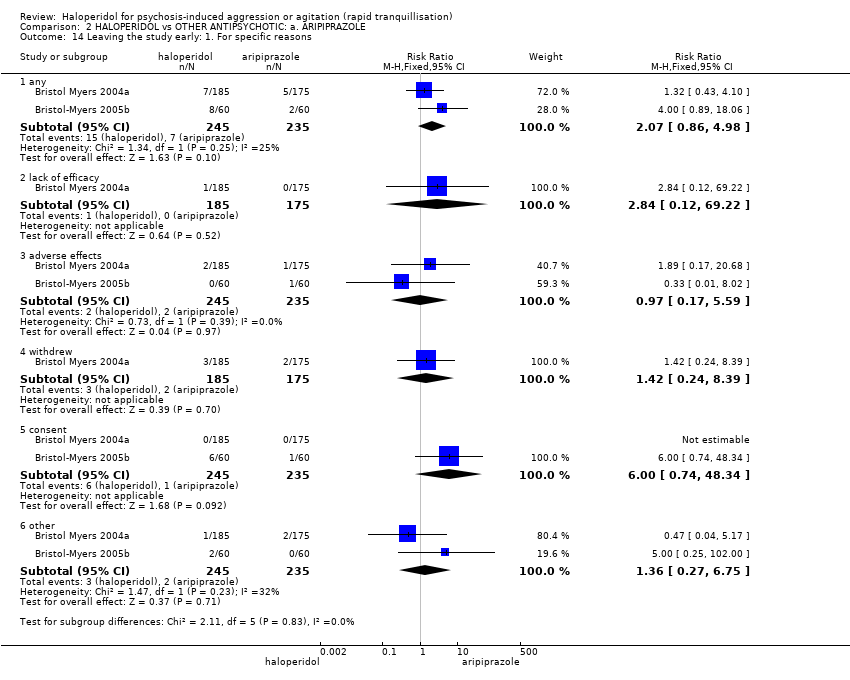 Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 14 Leaving the study early: 1. For specific reasons. | ||||
| 14.1 any | 2 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.86, 4.98] |
| 14.2 lack of efficacy | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 69.22] |
| 14.3 adverse effects | 2 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.17, 5.59] |
| 14.4 withdrew | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.24, 8.39] |
| 14.5 consent | 2 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.74, 48.34] |
| 14.6 other | 2 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.27, 6.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ asleep Show forest plot | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.04, 3.60] |
| Analysis 3.1  Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 1 Tranquillisation or asleep ‐ asleep. | ||||
| 1.1 not asleep up to 2 hours | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.04, 3.60] |
| 2 Repeated need for rapid tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2 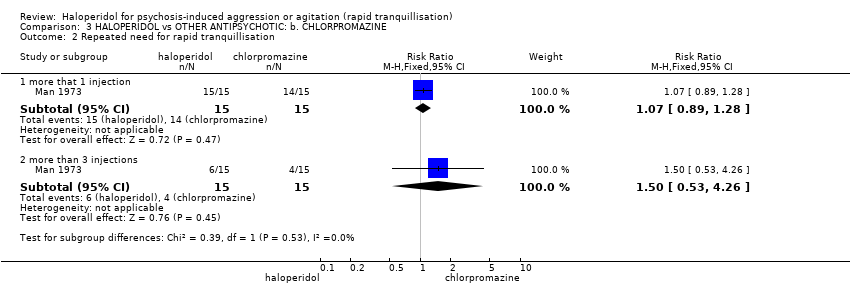 Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 2 Repeated need for rapid tranquillisation. | ||||
| 2.1 more that 1 injection | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| 2.2 more than 3 injections | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.53, 4.26] |
| 3 Global outcome: 1. Not improved Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 3 Global outcome: 1. Not improved. | ||||
| 3.1 not marked improvement | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.61, 1.02] |
| 3.2 not any improvement | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.05, 0.49] |
| 4 Adverse effects: any serious or specific adverse effects Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4 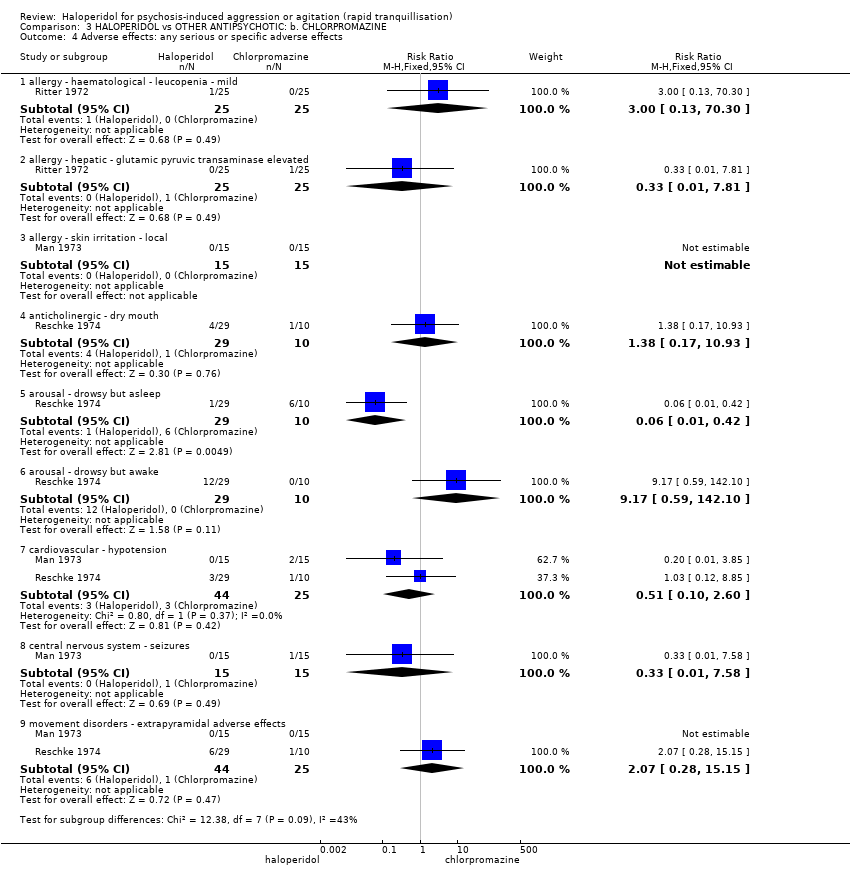 Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 4 Adverse effects: any serious or specific adverse effects. | ||||
| 4.1 allergy ‐ haematological ‐ leucopenia ‐ mild | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 4.2 allergy ‐ hepatic ‐ glutamic pyruvic transaminase elevated | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
| 4.3 allergy ‐ skin irritation ‐ local | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 anticholinergic ‐ dry mouth | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.17, 10.93] |
| 4.5 arousal ‐ drowsy but asleep | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.42] |
| 4.6 arousal ‐ drowsy but awake | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.17 [0.59, 142.10] |
| 4.7 cardiovascular ‐ hypotension | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.60] |
| 4.8 central nervous system ‐ seizures | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 4.9 movement disorders ‐ extrapyramidal adverse effects | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.28, 15.15] |
| 5 Leaving the study early Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 5 Leaving the study early. | ||||
| 5.1 any reason/general reasons | 4 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.71] |
| 5.2 could not be evaluated due to being asleep | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.52] |
| 5.3 severe hypotensive reaction | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.85] |
| 5.4 deviation from protocol | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep: 1. Not asleep Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1 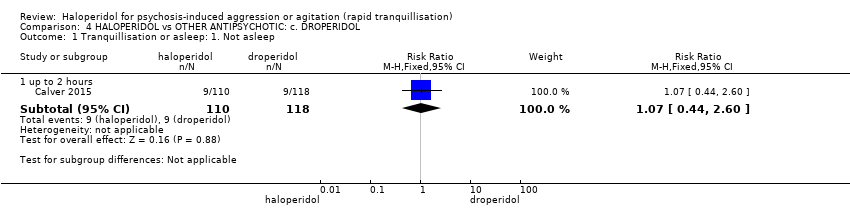 Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 1 Tranquillisation or asleep: 1. Not asleep. | ||||
| 1.1 up to 2 hours | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.44, 2.60] |
| 2 Tranquillisation or asleep: 2. Time to sleep Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 2 Tranquillisation or asleep: 2. Time to sleep. | ||||
| 2.1 up to 2 hours | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 5.20 [‐6.05, 16.45] |
| 3 Repeated need for rapid tranquillisation Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 3 Repeated need for rapid tranquillisation. | ||||
| 3.1 more than 1 injection | 2 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [1.27, 4.47] |
| 3.2 more than 3 injections | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.09, 47.68] |
| 4 Global outcome: 1. Need for benzodiazepine Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.4 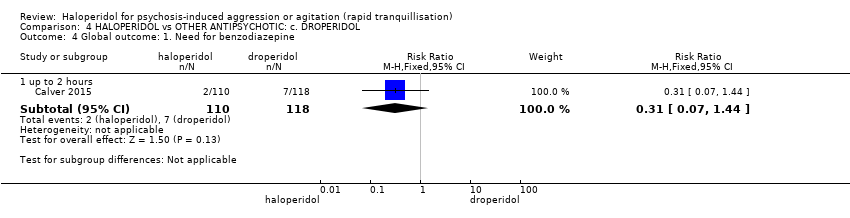 Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 4 Global outcome: 1. Need for benzodiazepine. | ||||
| 4.1 up to 2 hours | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.07, 1.44] |
| 5 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.5  Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 5 Adverse effects: 1. General. | ||||
| 5.1 overall adverse events up to 2 hours | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.23] |
| 5.2 patients with one or more AEs | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.46] |
| 6 Adverse effects: 2a. Specific ‐ arousal level Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.6 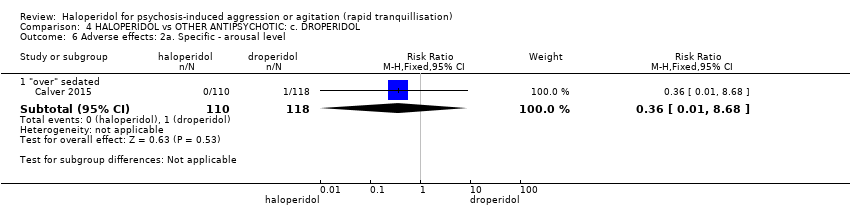 Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 6 Adverse effects: 2a. Specific ‐ arousal level. | ||||
| 6.1 "over" sedated | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.68] |
| 7 Adverse effects: 2b. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.7  Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 7 Adverse effects: 2b. Specific ‐ cardiovascular. | ||||
| 7.1 hypotension up to 2 hours | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.36] |
| 8 Adverse effects: 2c. Specific ‐ movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.8 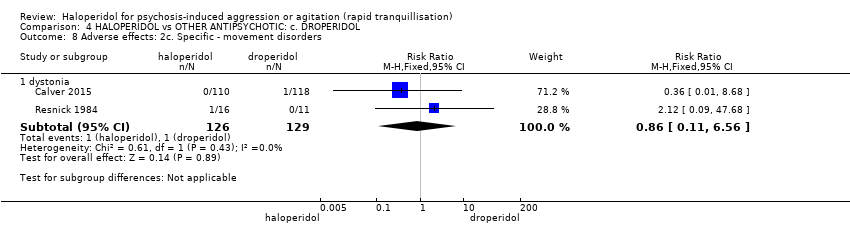 Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 8 Adverse effects: 2c. Specific ‐ movement disorders. | ||||
| 8.1 dystonia | 2 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.11, 6.56] |
| 9 Adverse effects: 2d. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.9 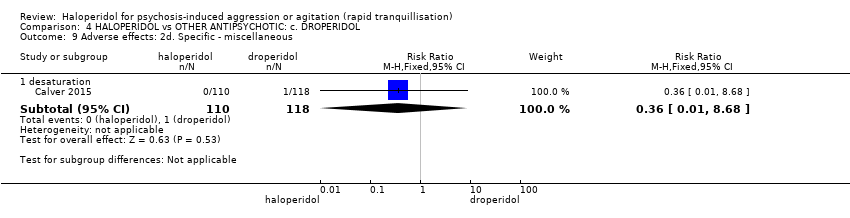 Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 9 Adverse effects: 2d. Specific ‐ miscellaneous. | ||||
| 9.1 desaturation | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ not asleep up to 24 hours Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.31 [0.54, 34.48] |
| Analysis 5.1  Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 1 Tranquillisation or asleep ‐ not asleep up to 24 hours. | ||||
| 2 Specific behaviour: 2. Aggression ‐ various measures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.2 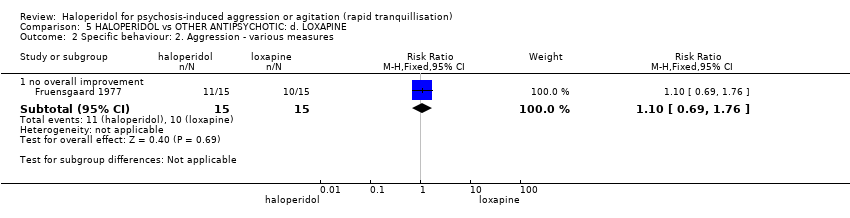 Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 2 Specific behaviour: 2. Aggression ‐ various measures. | ||||
| 2.1 no overall improvement | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.69, 1.76] |
| 3 Global outcome: 1. General ‐ no change ‐ i. over 24 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 3 Global outcome: 1. General ‐ no change ‐ i. over 24 hours. | ||||
| 3.1 up to 48 hours (CGI‐I, high=worse) | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.14, 6.15] |
| 3.2 up to 72 hours (CGI‐I, high=worse) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.04, 3.49] |
| 3.3 at endpoint (CGI‐I, high=worse) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.12, 1.32] |
| 4 Global outcome: 2a. Specific ‐ not sedated ‐ i. by 30 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 4 Global outcome: 2a. Specific ‐ not sedated ‐ i. by 30 minutes. | ||||
| 4.1 at 30 minutes | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.65, 2.54] |
| 5 Global outcome: 2b. Specific ‐ not sedated ‐ ii. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.5 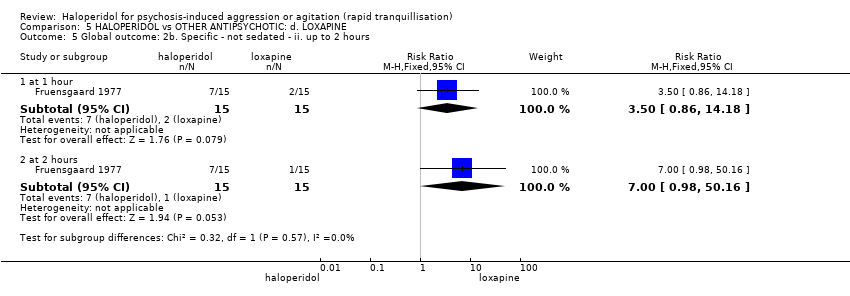 Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 5 Global outcome: 2b. Specific ‐ not sedated ‐ ii. up to 2 hours. | ||||
| 5.1 at 1 hour | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [0.86, 14.18] |
| 5.2 at 2 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.98, 50.16] |
| 6 Mental state: 1. Average scores Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [4.48, 7.72] |
| Analysis 5.6  Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 6 Mental state: 1. Average scores. | ||||
| 6.1 endpoint score (BPRS, high=worse) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [4.48, 7.72] |
| 7 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.7 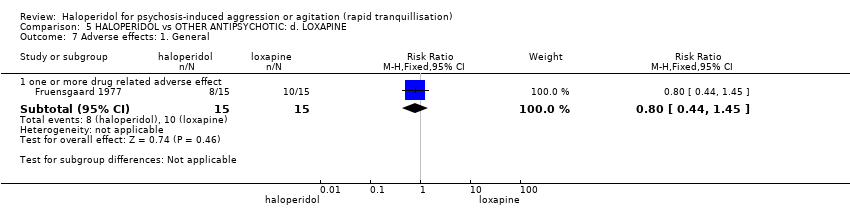 Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 7 Adverse effects: 1. General. | ||||
| 7.1 one or more drug related adverse effect | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.44, 1.45] |
| 8 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.8 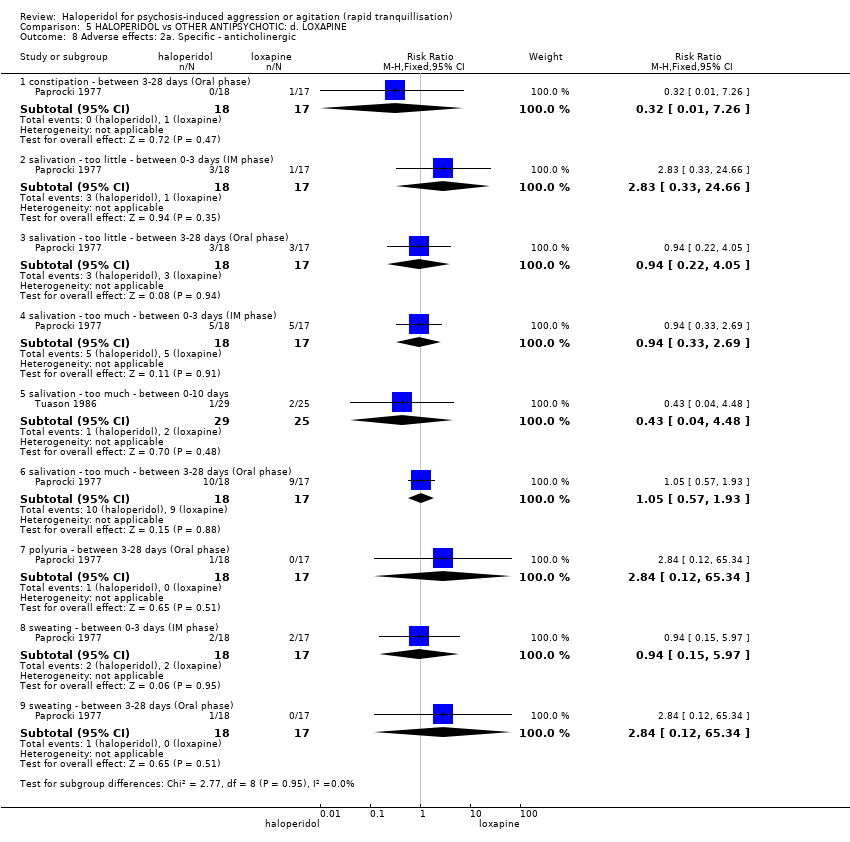 Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 8 Adverse effects: 2a. Specific ‐ anticholinergic. | ||||
| 8.1 constipation ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 8.2 salivation ‐ too little ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.33, 24.66] |
| 8.3 salivation ‐ too little ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.22, 4.05] |
| 8.4 salivation ‐ too much ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.33, 2.69] |
| 8.5 salivation ‐ too much ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.04, 4.48] |
| 8.6 salivation ‐ too much ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.57, 1.93] |
| 8.7 polyuria ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 8.8 sweating ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.15, 5.97] |
| 8.9 sweating ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 9 Adverse effects: 2b. Specific ‐ arousal level Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.9  Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 9 Adverse effects: 2b. Specific ‐ arousal level. | ||||
| 9.1 asleep ‐ by 12 hours | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.04] |
| 9.2 drowsiness ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| 9.3 drowsiness ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 33.16 [2.15, 511.57] |
| 9.4 drowsiness during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.20, 2.79] |
| 9.5 insomnia ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 9.6 insomnia ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.63 [0.37, 119.59] |
| 10 Adverse effects: 2c. Specific ‐ cardiovascular Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.10  Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 10 Adverse effects: 2c. Specific ‐ cardiovascular. | ||||
| 10.1 blood pressure ‐ increased ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.45] |
| 10.2 dizziness ‐ between 3‐28 days | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.09, 1.52] |
| 10.3 dyspnoea ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 10.4 palpitations during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 10.5 pulse rate ‐ increased ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.79] |
| 11 Adverse effects: 2d. Specific ‐ movement disorders Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.11  Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 11 Adverse effects: 2d. Specific ‐ movement disorders. | ||||
| 11.1 akathisia between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.16, 2.02] |
| 11.2 akathisia between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.26, 1.61] |
| 11.3 akathisia between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.62, 2.27] |
| 11.4 dysarthria between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.44] |
| 11.5 dyskinesia between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.79] |
| 11.6 dystonia between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.06, 13.93] |
| 11.7 dystonia between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.26, 1.61] |
| 11.8 involuntary movement between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 11.9 motor retardation between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.06, 13.93] |
| 11.10 muscle spasms between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.33, 4.82] |
| 11.11 oculogyric crisis between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.11, 61.11] |
| 11.12 rigidity between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.19] |
| 11.13 rigidity between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.13, 5.68] |
| 11.14 rigidity during between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.93, 1.38] |
| 11.15 tremor between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.42, 2.13] |
| 11.16 tremor during between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.71, 1.76] |
| 11.17 tremor between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.11, 61.11] |
| 11.18 use of antiparkinson medication | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.66, 12.16] |
| 11.19 dystonia during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 96.13] |
| 11.20 extrapyramidal effects ‐ use of antiparkinson drugs during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.17, 2.07] |
| 11.21 EPS during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.98, 50.16] |
| 11.22 thick tongue ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.79] |
| 12 Adverse effects: 2e. Specific ‐ miscellaneous Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.12  Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 12 Adverse effects: 2e. Specific ‐ miscellaneous. | ||||
| 12.1 allergy ‐ itch ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 12.2 allergy ‐ tissue reaction at injection site ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 nervousness ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.79] |
| 12.4 pain ‐ during 24 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.44] |
| 12.5 pain ‐ headache ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 12.6 pain ‐ myalgia ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 13 Leaving the study early: 1. For general reasons Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 90% CI) | Subtotals only | |
| Analysis 5.13  Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 13 Leaving the study early: 1. For general reasons. | ||||
| 13.1 by 72 hours | 1 | 35 | Risk Ratio (M‐H, Fixed, 90% CI) | 0.63 [0.21, 1.85] |
| 13.2 by 10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 90% CI) | 1.18 [0.67, 2.07] |
| 13.3 by endpoint | 1 | 35 | Risk Ratio (M‐H, Fixed, 90% CI) | 0.94 [0.42, 2.13] |
| 14 Leaving the study early: 2. Specific reasons Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.14  Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 14 Leaving the study early: 2. Specific reasons. | ||||
| 14.1 adverse effects by 72 hours | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Tranquillisation or asleep ‐ asleep Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.1  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 1 Tranquillisation or asleep ‐ asleep. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 not asleep up to 2 hours | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.02, 1.32] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Repeated need for tranquillisation ‐ needing additional injection Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.2  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 2 Repeated need for tranquillisation ‐ needing additional injection. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.1 by 2 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.47, 33.73] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.2 by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.67, 6.47] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.3 by 24 hours | 3 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.51] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Specific behaviour: 1a. Agitation ‐ various measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.3  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 3 Specific behaviour: 1a. Agitation ‐ various measures. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.1 response ‐ up to 2 hours (≥40% reduction in PANSS‐EC) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.58, 1.58] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2 agitation ‐ reported as 'adverse effect' | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.73] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.3 agitation during 21 days (if occurred in ≧10% at P < 0.05) ‐ reported as 'adverse effect' | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.33, 3.51] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. by 30 minutes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.4 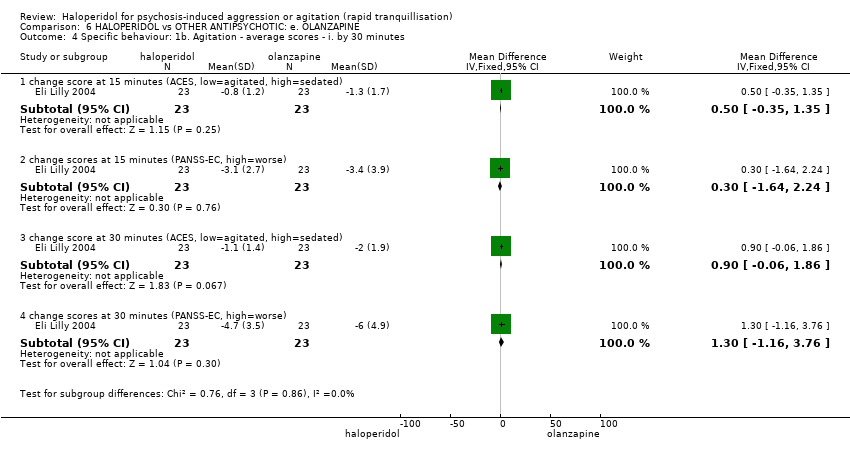 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. by 30 minutes. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 change score at 15 minutes (ACES, low=agitated, high=sedated) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.35, 1.35] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 change scores at 15 minutes (PANSS‐EC, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.64, 2.24] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.3 change score at 30 minutes (ACES, low=agitated, high=sedated) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.06, 1.86] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.4 change scores at 30 minutes (PANSS‐EC, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐1.16, 3.76] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 Specific behaviour: 1c. Agitation ‐ average scores ‐ ii. up to 2 hours Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.5 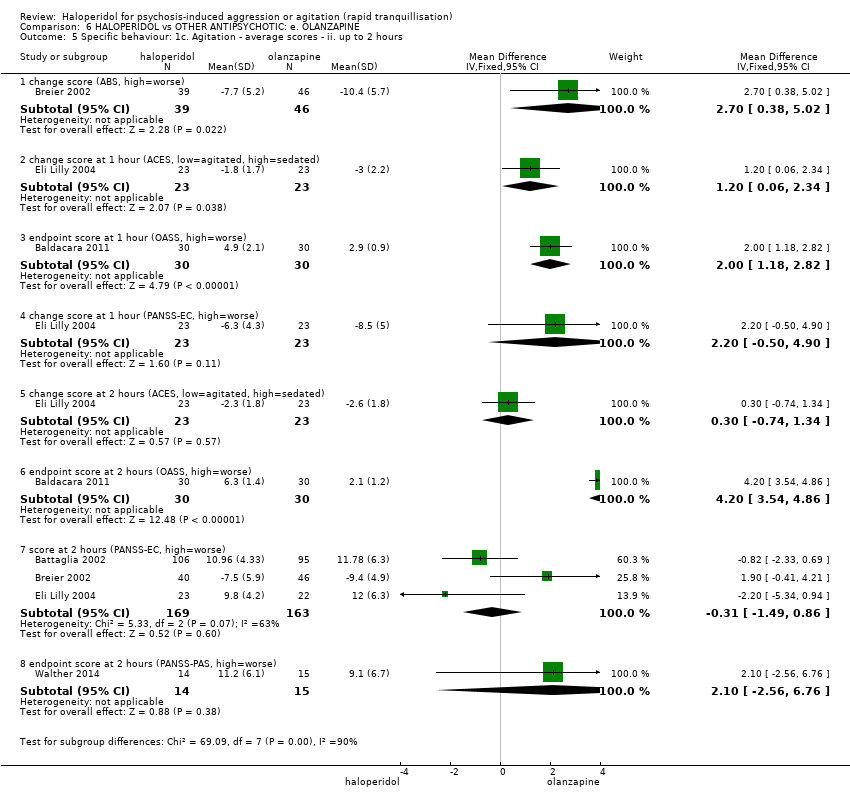 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 5 Specific behaviour: 1c. Agitation ‐ average scores ‐ ii. up to 2 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.1 change score (ABS, high=worse) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | 2.7 [0.38, 5.02] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.2 change score at 1 hour (ACES, low=agitated, high=sedated) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.06, 2.34] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.3 endpoint score at 1 hour (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [1.18, 2.82] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.4 change score at 1 hour (PANSS‐EC, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 2.2 [‐0.50, 4.90] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.5 change score at 2 hours (ACES, low=agitated, high=sedated) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.74, 1.34] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.6 endpoint score at 2 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [3.54, 4.86] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.7 score at 2 hours (PANSS‐EC, high=worse) | 3 | 332 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.49, 0.86] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.8 endpoint score at 2 hours (PANSS‐PAS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐2.56, 6.76] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 Specific behaviour: 1d. Agitation ‐ average scores ‐ iii. up to 4 hours Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.45] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.6 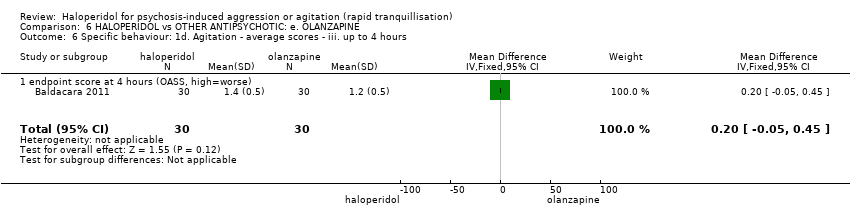 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 6 Specific behaviour: 1d. Agitation ‐ average scores ‐ iii. up to 4 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.1 endpoint score at 4 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.45] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 Specific behaviour: 1e. Agitation ‐ average scores ‐ iv. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.7 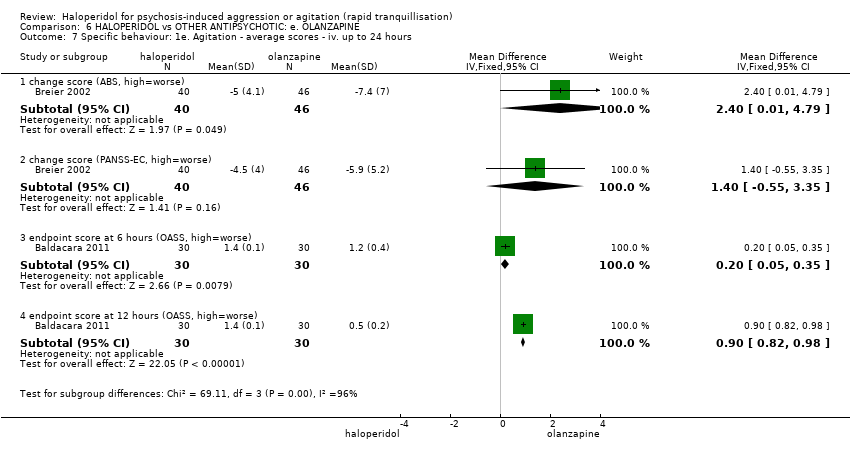 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 7 Specific behaviour: 1e. Agitation ‐ average scores ‐ iv. up to 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.1 change score (ABS, high=worse) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [0.01, 4.79] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.2 change score (PANSS‐EC, high=worse) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐0.55, 3.35] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.3 endpoint score at 6 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.05, 0.35] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.4 endpoint score at 12 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.9 [0.82, 0.98] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.8  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 8 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.1 endpoint score at 1 hour (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.13, 1.67] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.2 endpoint score at 2 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.41, 1.41] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.9 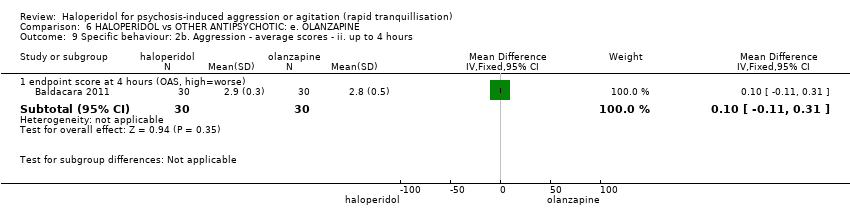 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 9 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.1 endpoint score at 4 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.11, 0.31] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.10 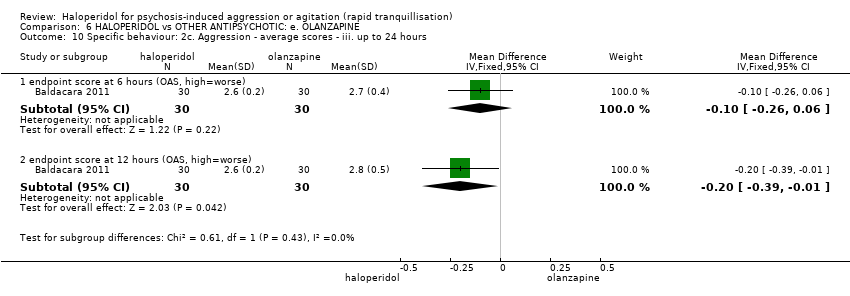 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 10 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.1 endpoint score at 6 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.26, 0.06] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.2 endpoint score at 12 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.39, ‐0.01] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 Specific behaviour: 3. Hostility Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.12] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.11  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 11 Specific behaviour: 3. Hostility. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 Global outcome: 1a. General ‐ need for additional measures Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.12  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 12 Global outcome: 1a. General ‐ need for additional measures. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12.1 need for benzodiazepine during 24 hours | 2 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.74] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12.2 need for benzodiazepine during 7 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.27] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12.3 additional restraint, seclusion or special observation | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.70, 3.17] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 Global outcome: 1b. General ‐ time and doses Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.13 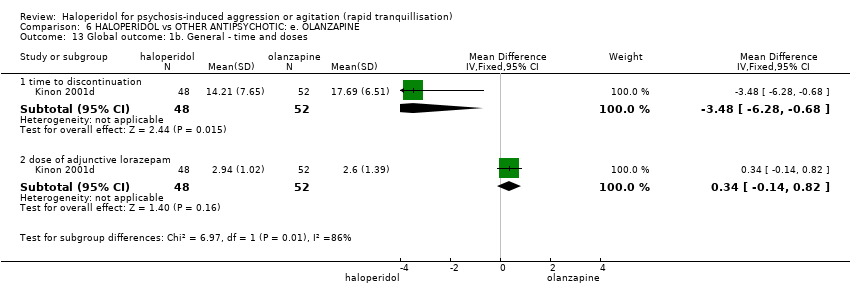 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 13 Global outcome: 1b. General ‐ time and doses. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13.1 time to discontinuation | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐3.48 [‐6.28, ‐0.68] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13.2 dose of adjunctive lorazepam | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐0.14, 0.82] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 Global outcome: 1c. General ‐ average scores ‐ up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.14  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 14 Global outcome: 1c. General ‐ average scores ‐ up to 2 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14.1 endpoint score (CGI‐I, high=worse) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.65, 0.45] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 Global outcome: 1d. General ‐ average scores ‐ over 24 hours Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.15 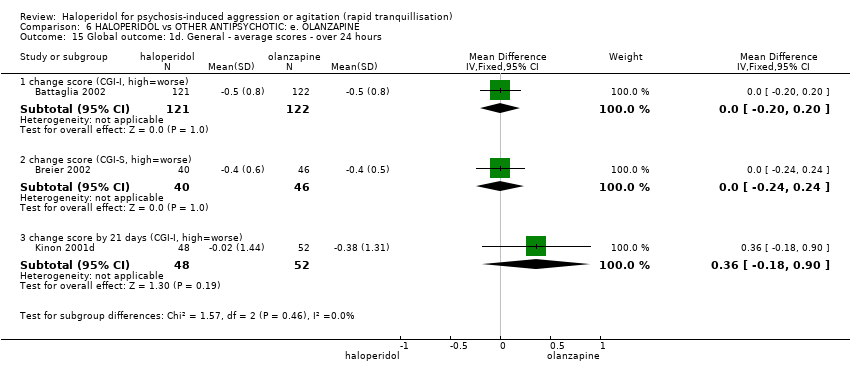 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 15 Global outcome: 1d. General ‐ average scores ‐ over 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15.1 change score (CGI‐I, high=worse) | 1 | 243 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.20, 0.20] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15.2 change score (CGI‐S, high=worse) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.24, 0.24] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15.3 change score by 21 days (CGI‐I, high=worse) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.18, 0.90] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 Global outcome: 2a. Specific ‐ alert ‐ i. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.16  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 16 Global outcome: 2a. Specific ‐ alert ‐ i. up to 2 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16.1 alert at 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.99, 1.55] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 Global outcome: 2b. Specific ‐ alert ‐ ii. up to 4 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.17 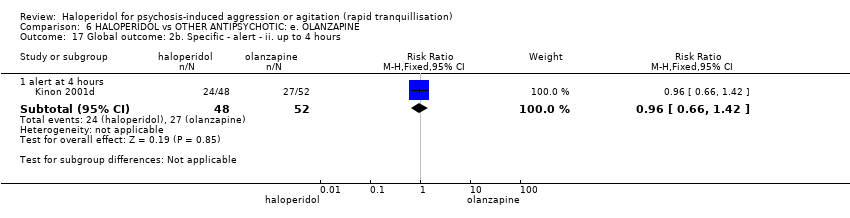 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 17 Global outcome: 2b. Specific ‐ alert ‐ ii. up to 4 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.1 alert at 4 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.42] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 Global outcome: 2c. Specific ‐ alert ‐ iii. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.18  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 18 Global outcome: 2c. Specific ‐ alert ‐ iii. up to 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18.1 alert at 8 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.67, 1.60] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18.2 alert at 16 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.58, 1.78] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18.3 alert at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.20] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 Global outcome: 2d. Specific ‐ tranquil ‐ i. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.19 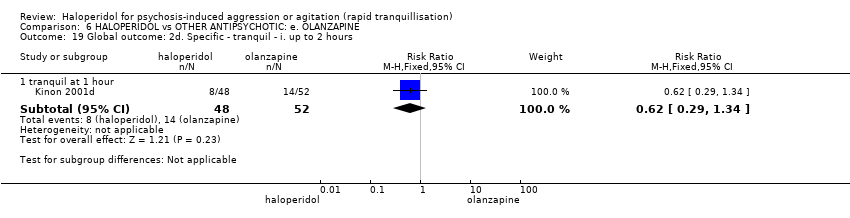 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 19 Global outcome: 2d. Specific ‐ tranquil ‐ i. up to 2 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19.1 tranquil at 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.29, 1.34] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20 Global outcome: 2e. Specific ‐ tranquil ‐ ii. up to 4 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.20  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 20 Global outcome: 2e. Specific ‐ tranquil ‐ ii. up to 4 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20.1 tranquil at 4 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.94, 3.62] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 21 Global outcome: 2f. Specific ‐ tranquil ‐ iii. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.21 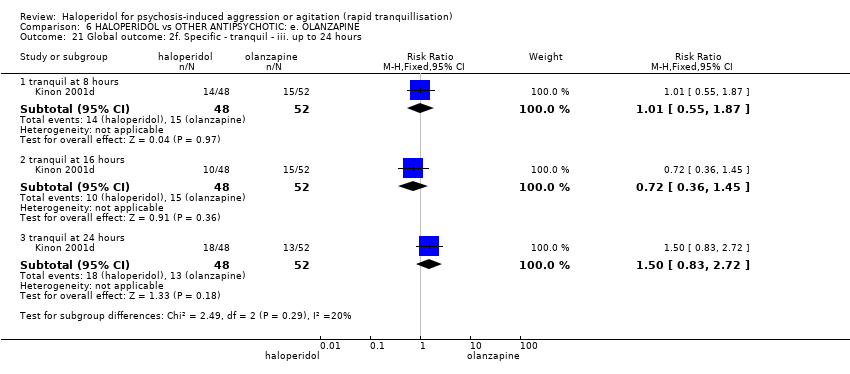 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 21 Global outcome: 2f. Specific ‐ tranquil ‐ iii. up to 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 21.1 tranquil at 8 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.55, 1.87] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 21.2 tranquil at 16 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.36, 1.45] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 21.3 tranquil at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.83, 2.72] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 22 Global outcome: 2g. Specific ‐ sedated ‐ i. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.22 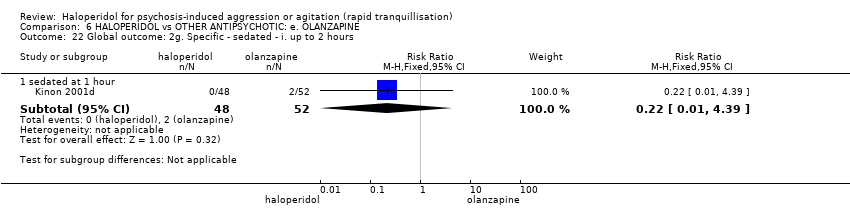 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 22 Global outcome: 2g. Specific ‐ sedated ‐ i. up to 2 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 22.1 sedated at 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.39] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 23 Global outcome: 2h. Specific ‐ sedated ‐ ii. up to 4 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.23  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 23 Global outcome: 2h. Specific ‐ sedated ‐ ii. up to 4 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 23.1 sedated at 4 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.18, 1.03] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24 Global outcome: 2i. Specific ‐ sedated ‐ iii. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.24 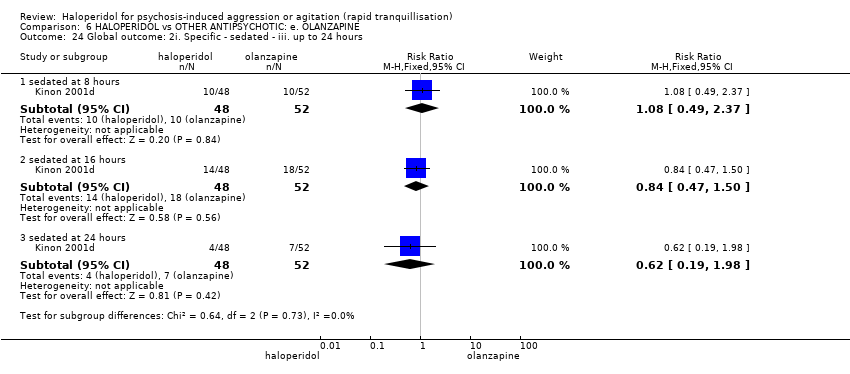 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 24 Global outcome: 2i. Specific ‐ sedated ‐ iii. up to 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24.1 sedated at 8 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.49, 2.37] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24.2 sedated at 16 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.50] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24.3 sedated at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.19, 1.98] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25 Global outcome: 2j. Specific ‐ asleep ‐ i. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.25 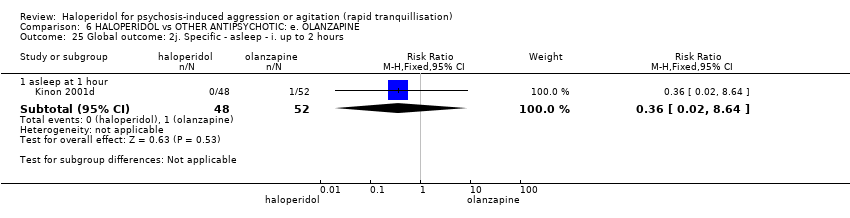 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 25 Global outcome: 2j. Specific ‐ asleep ‐ i. up to 2 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25.1 asleep at 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.64] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 26 Global outcome: 2k. Specific ‐ asleep ‐ ii. up to 4 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.26 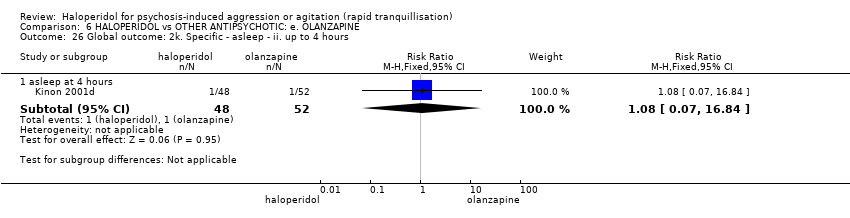 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 26 Global outcome: 2k. Specific ‐ asleep ‐ ii. up to 4 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 26.1 asleep at 4 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.07, 16.84] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 27 Global outcome: 2l. Specific ‐ asleep ‐ iii. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.27 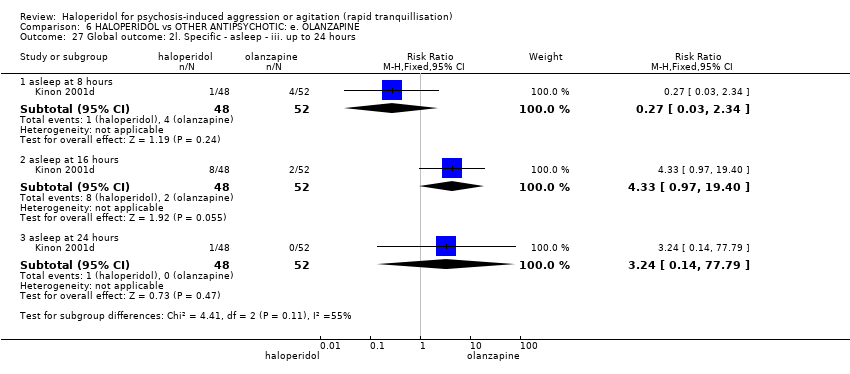 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 27 Global outcome: 2l. Specific ‐ asleep ‐ iii. up to 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 27.1 asleep at 8 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.34] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 27.2 asleep at 16 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [0.97, 19.40] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 27.3 asleep at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.14, 77.79] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 28 Service use: 1. Average days to discharge Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.85, 0.65] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.28 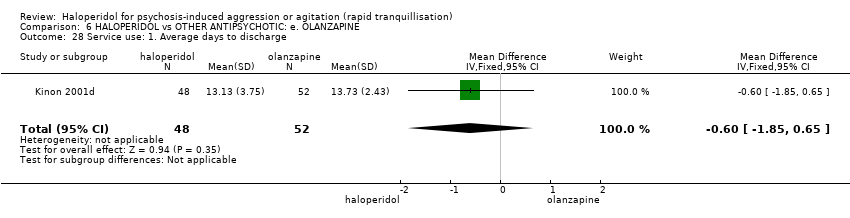 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 28 Service use: 1. Average days to discharge. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 29 Service use: 2. Average patient‐hours used during 24 hours (skewed data) Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.29
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 29 Service use: 2. Average patient‐hours used during 24 hours (skewed data). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 29.1 days 1‐7 | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 29.2 days 8‐14 | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 29.3 days 15‐21 | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30 Mental state: 1. Various outcomes reported as 'adverse events' Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.30 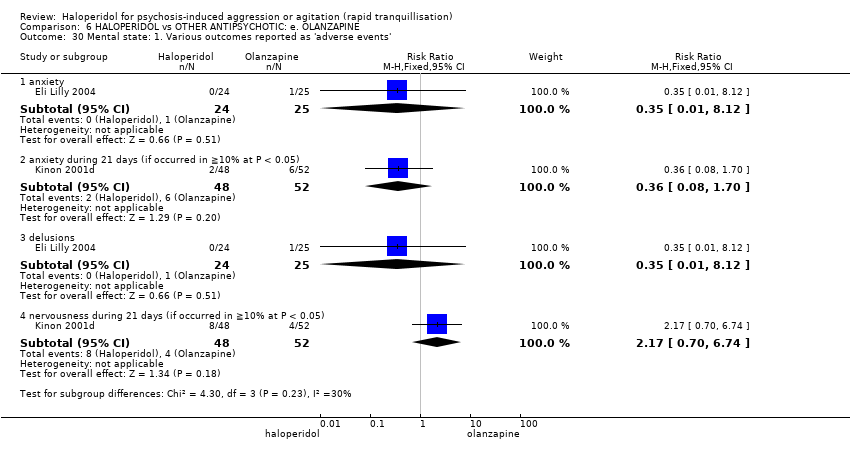 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 30 Mental state: 1. Various outcomes reported as 'adverse events'. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30.1 anxiety | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.12] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30.2 anxiety during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.08, 1.70] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30.3 delusions | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.12] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30.4 nervousness during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.70, 6.74] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 31 Mental state: 2a. Average scores ‐ i. by 30 minutes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.31  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 31 Mental state: 2a. Average scores ‐ i. by 30 minutes. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 31.1 change score at 15 minutes (BPRS positive sub‐scale, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.11, 1.51] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 31.2 change score at 30 minutes (BPRS positive sub‐scale, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.70, 1.90] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 31.3 change score at 15 minutes (BPRS total, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐5.42, 4.02] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 31.4 change score at 30 minutes (BPRS total, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐7.35, 6.75] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32 Mental state: 2b. Average scores ‐ ii. up to 2 hours Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.32 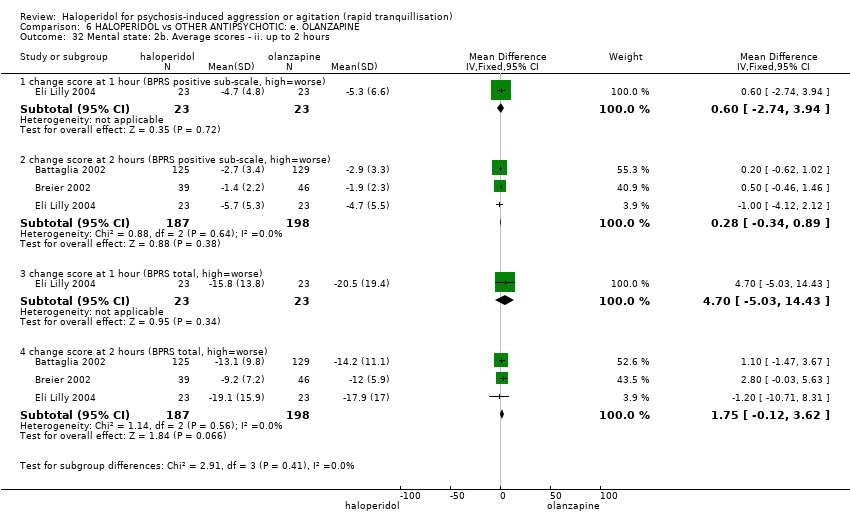 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 32 Mental state: 2b. Average scores ‐ ii. up to 2 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32.1 change score at 1 hour (BPRS positive sub‐scale, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.74, 3.94] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32.2 change score at 2 hours (BPRS positive sub‐scale, high=worse) | 3 | 385 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.34, 0.89] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32.3 change score at 1 hour (BPRS total, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [‐5.03, 14.43] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32.4 change score at 2 hours (BPRS total, high=worse) | 3 | 385 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [‐0.12, 3.62] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 33 Mental state: 2c. Average scores ‐ iii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.33  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 33 Mental state: 2c. Average scores ‐ iii. up to 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 33.1 change score at 24 hours (BPRS positive sub‐scale, high=worse) | 2 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.97, 0.37] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 33.2 change score at 24 hours (BPRS total, high=worse) | 2 | 340 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐1.34, 2.29] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 34 Mental state: 2d. Average scores ‐ iv. over 24 hours Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [‐0.18, 9.58] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.34 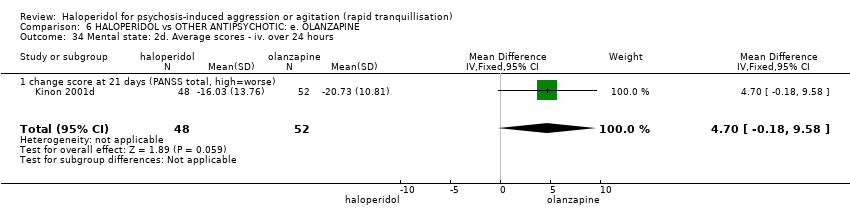 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 34 Mental state: 2d. Average scores ‐ iv. over 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 34.1 change score at 21 days (PANSS total, high=worse) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [‐0.18, 9.58] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35 Adverse effects: 1a. General Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.35  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 35 Adverse effects: 1a. General. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35.1 one or more drug related adverse events | 3 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.09, 1.76] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36 Adverse effects: 1b. General ‐ serious Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.36  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 36 Adverse effects: 1b. General ‐ serious. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36.1 one or more treatment emergent adverse events | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.36, 1.83] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36.2 treatment emergent adverse events ‐ all | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.69, 2.19] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36.3 overall serious adverse effects | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36.4 death | 3 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 37 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.37 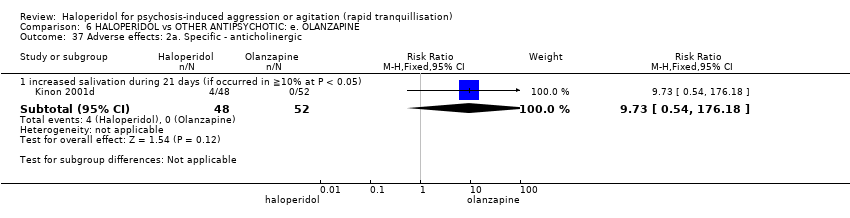 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 37 Adverse effects: 2a. Specific ‐ anticholinergic. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 37.1 increased salivation during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.54, 176.18] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 38 Adverse effects: 2b. Specific ‐ arousal level ‐ i. various Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.38 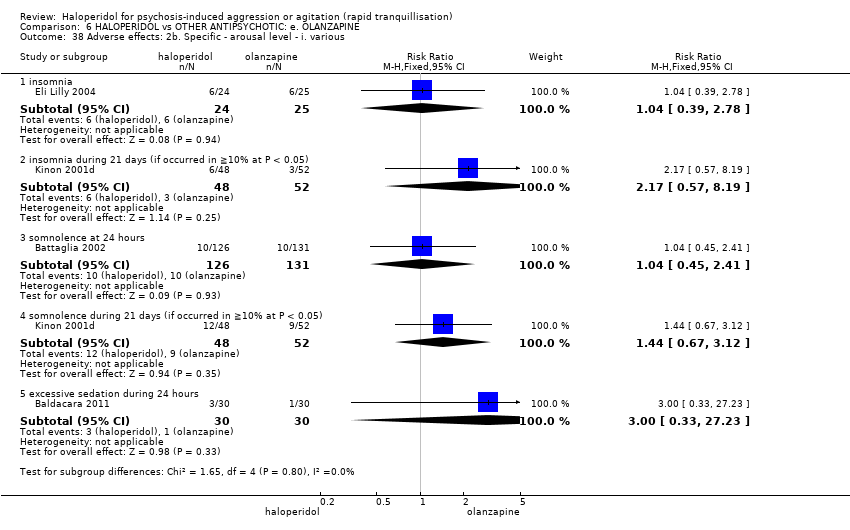 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 38 Adverse effects: 2b. Specific ‐ arousal level ‐ i. various. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 38.1 insomnia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.39, 2.78] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 38.2 insomnia during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.57, 8.19] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 38.3 somnolence at 24 hours | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.45, 2.41] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 38.4 somnolence during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.67, 3.12] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 38.5 excessive sedation during 24 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 39 Adverse effects: 2c. Specific ‐ arousal level ‐ ii. average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.39  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 39 Adverse effects: 2c. Specific ‐ arousal level ‐ ii. average scores ‐ i. up to 2 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 39.1 endpoint score at 1 hour (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.13, 0.73] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 39.2 endpoint score at 2 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.36, 0.36] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 40 Adverse effects: 2d. Specific ‐ arousal level ‐ ii. average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.40 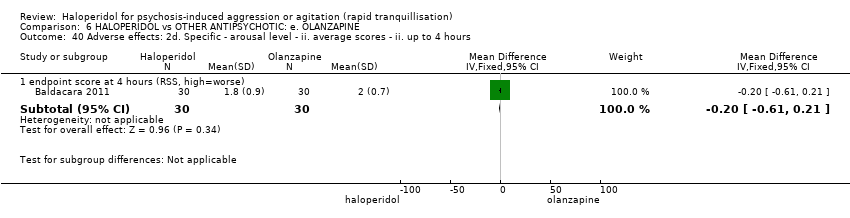 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 40 Adverse effects: 2d. Specific ‐ arousal level ‐ ii. average scores ‐ ii. up to 4 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 40.1 endpoint score at 4 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.61, 0.21] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 41 Adverse effects: 2e. Specific ‐ arousal level ‐ ii. average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.41  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 41 Adverse effects: 2e. Specific ‐ arousal level ‐ ii. average scores ‐ iii. up to 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 41.1 endpoint score at 6 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.38, 0.18] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 41.2 endpoint score at 12 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.26, 0.26] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 42 Adverse effects: 2f. Specific ‐ cardiovascular i. binary Show forest plot | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.38] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.42  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 42 Adverse effects: 2f. Specific ‐ cardiovascular i. binary. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 42.1 clinically significant ECG change | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 42.2 hypotension during 24 hours | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.38] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 43 Adverse effects: 2g. Specific ‐ cardiovascular ii. continuous Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.43 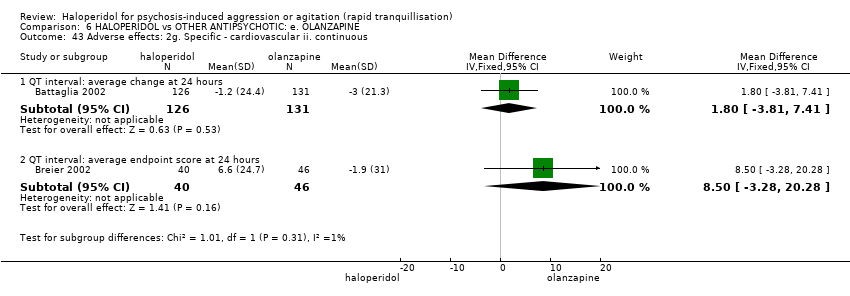 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 43 Adverse effects: 2g. Specific ‐ cardiovascular ii. continuous. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 43.1 QT interval: average change at 24 hours | 1 | 257 | Mean Difference (IV, Fixed, 95% CI) | 1.8 [‐3.81, 7.41] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 43.2 QT interval: average endpoint score at 24 hours | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 8.5 [‐3.28, 20.28] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 44 Adverse effects: 2h. Specific ‐ gastrointestinal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.44  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 44 Adverse effects: 2h. Specific ‐ gastrointestinal. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 44.1 abdominal pain | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 73.04] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 44.2 vomiting | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.2 [0.26, 103.03] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45 Adverse effects: 2i. Specific ‐ movement disorder ‐ i. various Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.45  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 45 Adverse effects: 2i. Specific ‐ movement disorder ‐ i. various. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45.1 dystonia during 24 hours | 2 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.92 [1.67, 99.78] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45.2 dystonia during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.54, 176.18] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45.3 hypertonia during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.54, 176.18] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45.4 EPS during 24 hours | 3 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.35 [2.27, 30.63] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45.5 EPS during study | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.73] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45.6 extrapyramidal effects ‐ use of antiparkinson drugs during 24 hours | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.51 [1.92, 10.58] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45.7 extrapyramidal effects ‐ use of antiparkinson drugs during 21 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.54, 176.18] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45.8 extrapyramidal effects ‐ use of antiparkinson drugs during study | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.73] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 46 Adverse effects: 2j. Specific ‐ movement disorder ‐ ii. average scores ‐ i. up to 24 hours Show forest plot | 1 | 483 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.16, 0.53] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.46 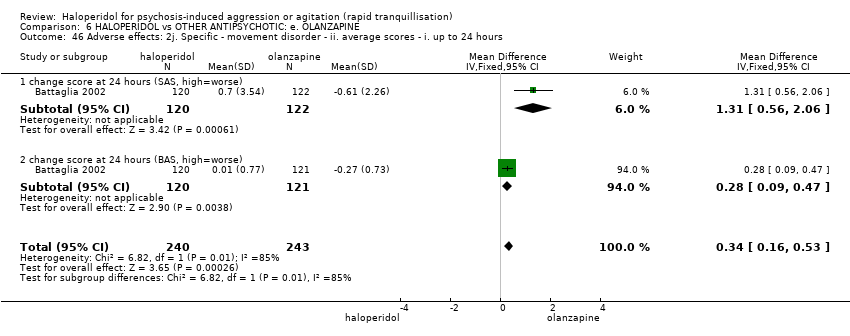 Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 46 Adverse effects: 2j. Specific ‐ movement disorder ‐ ii. average scores ‐ i. up to 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 46.1 change score at 24 hours (SAS, high=worse) | 1 | 242 | Mean Difference (IV, Fixed, 95% CI) | 1.31 [0.56, 2.06] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 46.2 change score at 24 hours (BAS, high=worse) | 1 | 241 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.09, 0.47] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 47 Adverse effects: 2k. Specific ‐ movement disorder ‐ iii. average scores ‐ i. up to 24 hours Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.47
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 47 Adverse effects: 2k. Specific ‐ movement disorder ‐ iii. average scores ‐ i. up to 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 47.1 endpoint score at 24 hours (SAS, high=worse) ‐ (skewed data) | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 47.2 endpoint score at 24 hours (BAS, high=worse) ‐ (skewed data) | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48 Adverse effects: 2l. Specific ‐ movement disorder ‐ vi. average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.48  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 48 Adverse effects: 2l. Specific ‐ movement disorder ‐ vi. average scores ‐ i. over 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48.1 endpoint score at 96 hours (AIMS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.87, 1.27] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48.2 endpoint score at 96 hours (BARS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.8 [‐0.01, 1.61] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48.3 endpoint score at 96 hours (SAS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.2 [‐0.07, 4.47] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 49 Adverse effects: 2m. Specific ‐ miscellaneous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.49  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 49 Adverse effects: 2m. Specific ‐ miscellaneous. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 49.1 pain | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 73.04] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 49.2 pain during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.33, 3.51] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 49.3 pain ‐ headache during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.88, 5.32] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 49.4 other ‐ nose bleed | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 73.04] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 49.5 other ‐ clinically relevant laboratory changes | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50 Leaving the study early Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 6.50  Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 50 Leaving the study early. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50.1 any reason | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.04, 2.65] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50.2 adverse effects | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.67 [1.13, 66.75] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50.3 lack of efficacy | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.09, 1.44] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50.4 lost at follow‐up | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.10, 2.82] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50.5 participants decision | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.57, 8.19] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50.6 non compliance | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.42, 11.30] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50.7 physician decision | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [0.50, 37.42] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50.8 sponsor decision | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.64] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global outcome: No improvement Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 4.68] |
| Analysis 7.1 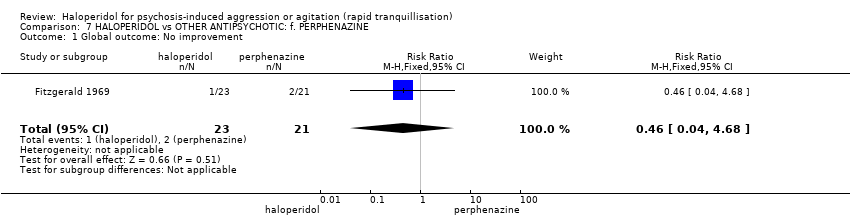 Comparison 7 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: f. PERPHENAZINE, Outcome 1 Global outcome: No improvement. | ||||
| 2 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.2  Comparison 7 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: f. PERPHENAZINE, Outcome 2 Adverse effects: 1. General. | ||||
| 2.1 one or more adverse effect | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.61, 2.80] |
| 2.2 clinically significant laboratory changes | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Adverse effects: 2. Specific Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.3 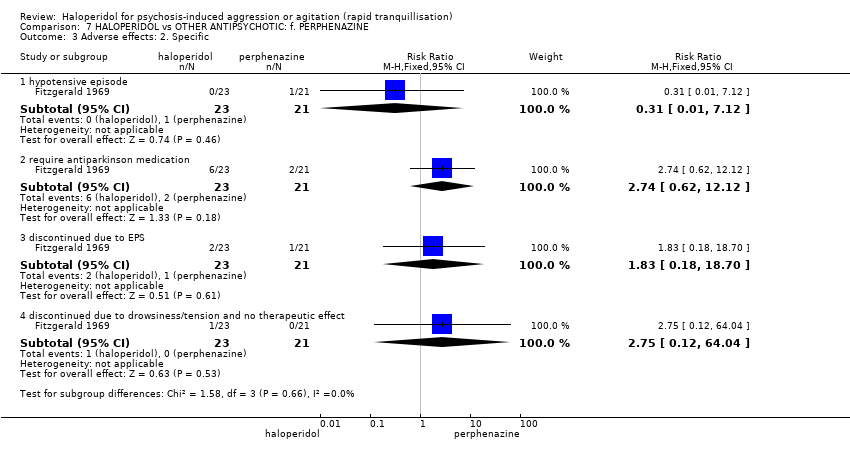 Comparison 7 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: f. PERPHENAZINE, Outcome 3 Adverse effects: 2. Specific. | ||||
| 3.1 hypotensive episode | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.12] |
| 3.2 require antiparkinson medication | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.62, 12.12] |
| 3.3 discontinued due to EPS | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.18, 18.70] |
| 3.4 discontinued due to drowsiness/tension and no therapeutic effect | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.12, 64.04] |
| 4 Leaving the study early: 1. Specific reasons Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.4  Comparison 7 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: f. PERPHENAZINE, Outcome 4 Leaving the study early: 1. Specific reasons. | ||||
| 4.1 discontinued due to EPS | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.18, 18.70] |
| 4.2 discontinued due to drowsiness/tension and no therapeutic effect | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.12, 64.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 8.1 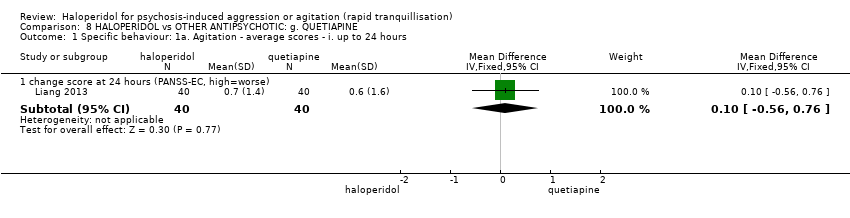 Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours. | ||||
| 1.1 change score at 24 hours (PANSS‐EC, high=worse) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.56, 0.76] |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.2  Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours. | ||||
| 2.1 change score at 72 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 change score at 7 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 change score at 10 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 change score at 14 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 change score at 28 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mental state: Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.3  Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 3 Mental state: Average scores ‐ i. over 24 hours. | ||||
| 3.1 change score at 28 days (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 change score at 28 days (PANSS positive sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 change score at 28 days (PANSS negative sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.4 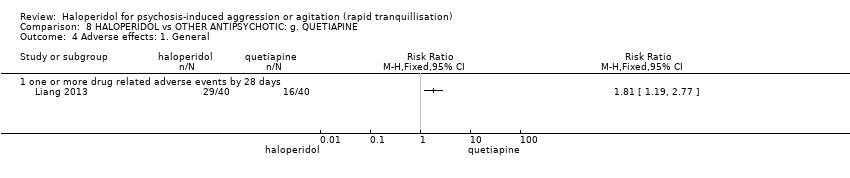 Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 4 Adverse effects: 1. General. | ||||
| 4.1 one or more drug related adverse events by 28 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects: 2. Specific ‐ movement disorder ‐ i. average scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.5 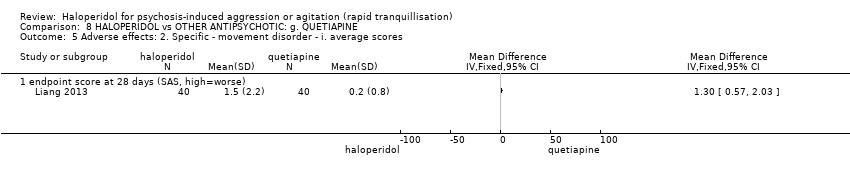 Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 5 Adverse effects: 2. Specific ‐ movement disorder ‐ i. average scores. | ||||
| 5.1 endpoint score at 28 days (SAS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.6  Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 6 Leaving the study early. | ||||
| 6.1 any reason | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 lost at follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ i. by 30 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.1 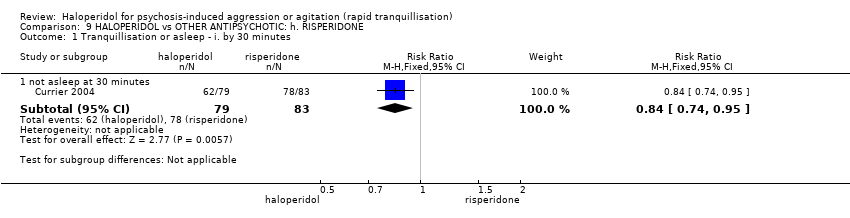 Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 1 Tranquillisation or asleep ‐ i. by 30 minutes. | ||||
| 1.1 not asleep at 30 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.95] |
| 2 Tranquillisation or asleep ‐ ii. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.2  Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 2 Tranquillisation or asleep ‐ ii. up to 2 hours. | ||||
| 2.1 not asleep at 1 hour | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.92] |
| 2.2 not asleep at 2 hours | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.51, 0.99] |
| 3 Specific behaviour: 1a. Agitation ‐ various measures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.3 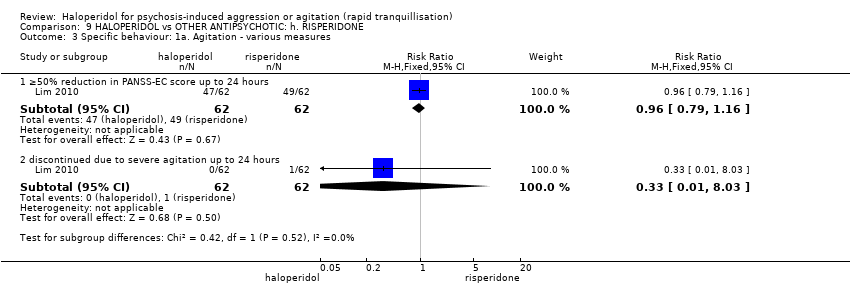 Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 3 Specific behaviour: 1a. Agitation ‐ various measures. | ||||
| 3.1 ≥50% reduction in PANSS‐EC score up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.79, 1.16] |
| 3.2 discontinued due to severe agitation up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.03] |
| 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.4  Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours. | ||||
| 4.1 endpoint score at 2 hours (PANSS‐PAS, high=worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐5.22, 4.42] |
| 5 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. by 30 minutes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.5 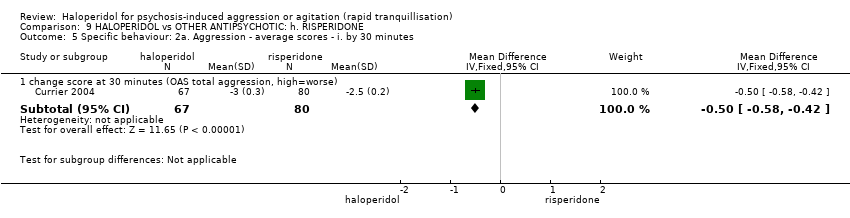 Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 5 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. by 30 minutes. | ||||
| 5.1 change score at 30 minutes (OAS total aggression, high=worse) | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.58, ‐0.42] |
| 6 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.6 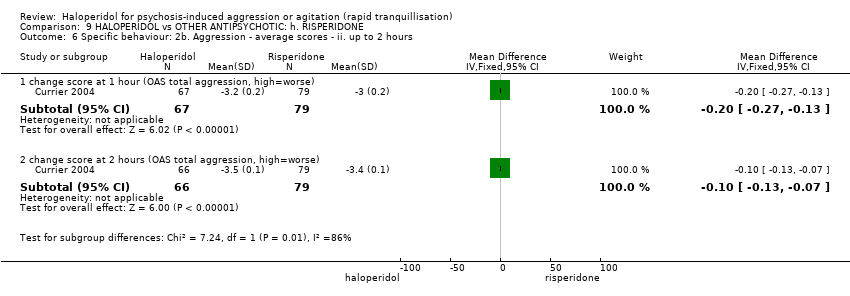 Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 6 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 2 hours. | ||||
| 6.1 change score at 1 hour (OAS total aggression, high=worse) | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.27, ‐0.13] |
| 6.2 change score at 2 hours (OAS total aggression, high=worse) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.13, ‐0.07] |
| 7 Global outcome: 1. Binary measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.7 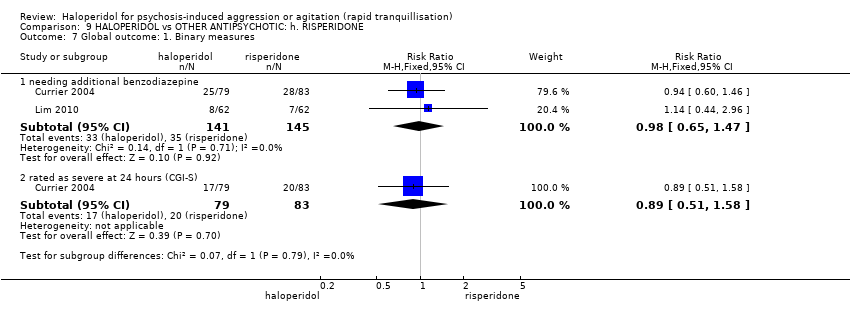 Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 7 Global outcome: 1. Binary measures. | ||||
| 7.1 needing additional benzodiazepine | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.47] |
| 7.2 rated as severe at 24 hours (CGI‐S) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.51, 1.58] |
| 8 Global outcome: 2. Continuous measures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.8  Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 8 Global outcome: 2. Continuous measures. | ||||
| 8.1 dose of lorazapam | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.49, 0.29] |
| 8.2 time to additional dose | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐2.17, 2.57] |
| 9 Adverse effects: 1a. General ‐ one or more adverse effects Show forest plot | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.23] |
| Analysis 9.9  Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 9 Adverse effects: 1a. General ‐ one or more adverse effects. | ||||
| 9.1 up to 24 hours | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.23] |
| 10 Adverse effects: 2a. Specific ‐ arousal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.10  Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 10 Adverse effects: 2a. Specific ‐ arousal. | ||||
| 10.1 sedated at 30 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.39, 2.59] |
| 10.2 sedated at 60 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.15, 1.72] |
| 10.3 sedated at 120 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.98, 1.23] |
| 10.4 somnolence during 24 hours (only reported if occurred in ≥5%) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.43, 2.12] |
| 10.5 somnolence during 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.96] |
| 10.6 insomnia ‐ severe | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.47] |
| 10.7 somnolence ‐ severe | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.13, 76.20] |
| 11 Adverse effects: 2b. Specific ‐ cardiovascular ‐ i. binary outcomes Show forest plot | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.45, 4.70] |
| Analysis 9.11  Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 11 Adverse effects: 2b. Specific ‐ cardiovascular ‐ i. binary outcomes. | ||||
| 11.1 dizziness during 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.82] |
| 11.2 orthostatic hypertension | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.25 [0.26, 107.67] |
| 12 Adverse effects: 2c. Specific ‐ cardiovascular ‐ ii. pulse rate ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.12  Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 12 Adverse effects: 2c. Specific ‐ cardiovascular ‐ ii. pulse rate ‐ i. up to 2 hours. | ||||
| 12.1 heartbeat change at 60 minutes | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐9.4 [‐9.99, ‐8.81] |
| 13 Adverse effects: 2d. Specific ‐ cardiovascular ‐ ii. pulse rate ‐ ii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.13 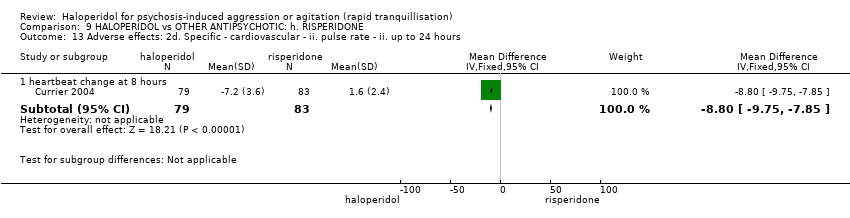 Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 13 Adverse effects: 2d. Specific ‐ cardiovascular ‐ ii. pulse rate ‐ ii. up to 24 hours. | ||||
| 13.1 heartbeat change at 8 hours | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐8.8 [‐9.75, ‐7.85] |
| 14 Adverse effects: 2e. Specific ‐ movement disorder ‐ i. binary outcomes Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.14  Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 14 Adverse effects: 2e. Specific ‐ movement disorder ‐ i. binary outcomes. | ||||
| 14.1 dystonia during 24 hours | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.28, 7.28] |
| 14.2 dyskinesia during 24 hours | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.13, 76.20] |
| 14.3 hyperkinesia during 24 hours (only reported if occurred in ≥5%) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.20 [0.48, 36.79] |
| 14.4 hypertonia/rigidity during 24 hours | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.47] |
| 14.5 tremor during 24 hours | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.25 [0.26, 107.67] |
| 14.6 movement disorder | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.55, 4.62] |
| 15 Adverse effects: 2f. Specific ‐ movement disorders ‐ ii. average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.15  Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 15 Adverse effects: 2f. Specific ‐ movement disorders ‐ ii. average scores ‐ i. up to 24 hours. | ||||
| 15.1 change score at 24 hours (BAS akathisia, high=worse) | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.3 [0.24, 0.36] |
| 15.2 change score at 24 hours (SAS movement disorders, high=worse) | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [0.34, 0.46] |
| 16 Adverse effects: 2g. Specific ‐ movement disorder ‐ ii. average scores ‐ ii. over 24 hours Show forest plot | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [‐0.32, 1.47] |
| Analysis 9.16  Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 16 Adverse effects: 2g. Specific ‐ movement disorder ‐ ii. average scores ‐ ii. over 24 hours. | ||||
| 16.1 endpoint score at 96 hours (AIMS, high=worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 endpoint score at 96 hours (BARS, high=worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.36, 1.56] |
| 16.3 endpoint score at 96 hours (SAS, high=worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.20, 3.00] |
| 17 Adverse effects: 2h. Specific ‐ miscellaneous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.17  Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 17 Adverse effects: 2h. Specific ‐ miscellaneous. | ||||
| 17.1 agitation during 24 hours (only reported if occurred in ≥5%) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.27, 4.06] |
| 17.2 agitation ‐ severe | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.19, 22.72] |
| 17.3 anxiety ‐ severe | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.13, 76.20] |
| 17.4 headache during 24 hours (only reported if occurred in ≥5%) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.37, 4.71] |
| 17.5 headache during 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.71] |
| 18 Leaving the study early ‐ i. up to 24 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.18 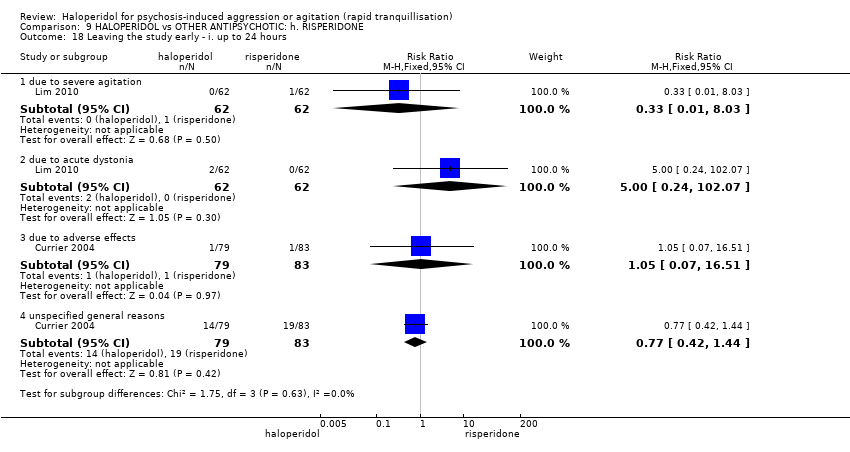 Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 18 Leaving the study early ‐ i. up to 24 hours. | ||||
| 18.1 due to severe agitation | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.03] |
| 18.2 due to acute dystonia | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.07] |
| 18.3 due to adverse effects | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.07, 16.51] |
| 18.4 unspecified general reasons | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.42, 1.44] |
| 19 Leaving the study early ‐ ii. over 24 hours Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.89] |
| Analysis 9.19 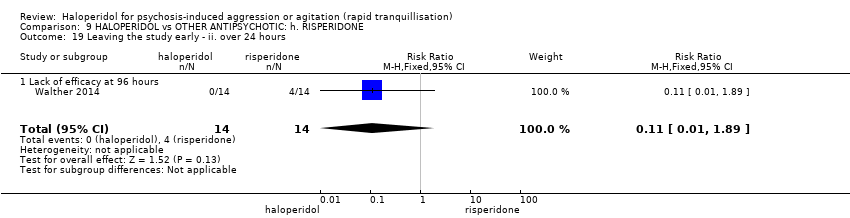 Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 19 Leaving the study early ‐ ii. over 24 hours. | ||||
| 19.1 Lack of efficacy at 96 hours | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for rapid tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 10.1  Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 1 Repeated need for rapid tranquillisation. | ||||
| 1.1 more than 1 injection | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| 1.2 more than 3 injections | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.57, 10.93] |
| 2 Specific behaviour: 1. Agitation ‐ rated as 'adverse effect' Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.52] |
| Analysis 10.2  Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 2 Specific behaviour: 1. Agitation ‐ rated as 'adverse effect'. | ||||
| 3 Global outcome Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 10.3  Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 3 Global outcome. | ||||
| 3.1 no response to 1st injection | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.57, 11.05] |
| 3.2 no improvement at 1 hour | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.14, 1.84] |
| 3.3 no improvement at 2 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.31] |
| 3.4 no improvement at 3 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.49] |
| 3.5 no improvement at 4 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.49] |
| 3.6 no improvement at 5 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.2 [0.21, 82.72] |
| 4 Adverse effects: 1. General ‐ one or more adverse effects Show forest plot | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.97, 2.22] |
| Analysis 10.4 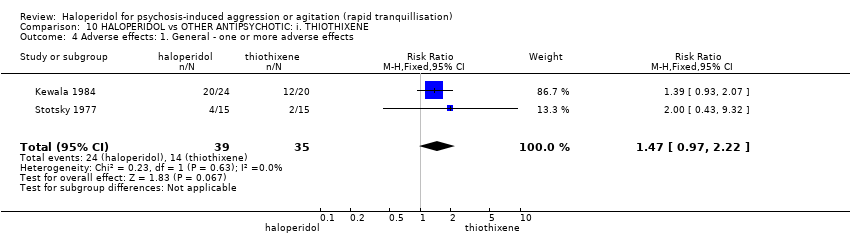 Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 4 Adverse effects: 1. General ‐ one or more adverse effects. | ||||
| 5 Adverse effects: 2a. Specific ‐ movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 10.5  Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 5 Adverse effects: 2a. Specific ‐ movement disorders. | ||||
| 5.1 ataxia | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.49] |
| 5.2 thick tongue | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.11, 58.67] |
| 5.3 use of antiparkinson drugs | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse effects: 2b. Specific ‐ others Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 10.6  Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 6 Adverse effects: 2b. Specific ‐ others. | ||||
| 6.1 anticholinergic ‐ blurred vision | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.2 [0.21, 82.72] |
| 6.2 anticholinergic ‐ dry mouth | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.31] |
| 6.3 arousal ‐ drowsiness | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.02, 2.90] |
| 6.4 arousal ‐ lightheadedness | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.2 [0.21, 82.72] |
| 6.5 cardiac ‐ chest pain | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.52] |
| 6.6 cardiac ‐ dizziness | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.28, 22.20] |
| 6.7 cardiac ‐ hypotensive crisis | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 cardiac ‐ orthostatic hypotension | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.49] |
| 6.9 cardiac ‐ tachycardia | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.52] |
| 6.10 other ‐ depressed mood | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.11, 58.67] |
| 6.11 other ‐ diaphoresis | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.52] |
| 6.12 other ‐ weakness | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.30, 25.21] |
| 6.13 other ‐ unsteadiness | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for tranquillisation ‐ need for additional drugs for tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.1 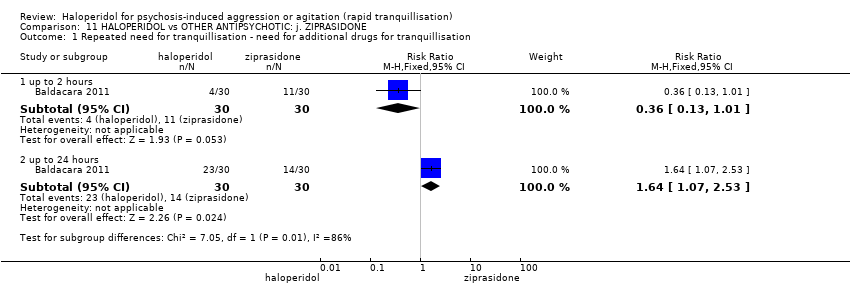 Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 1 Repeated need for tranquillisation ‐ need for additional drugs for tranquillisation. | ||||
| 1.1 up to 2 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.13, 1.01] |
| 1.2 up to 24 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.07, 2.53] |
| 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.2  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours. | ||||
| 2.1 endpoint score at 1 hour (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐7.70 [‐9.41, ‐5.99] |
| 2.2 endpoint score at 2 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐6.38, ‐1.42] |
| 2.3 endpoint score at 2 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐1.13, 1.25] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.3 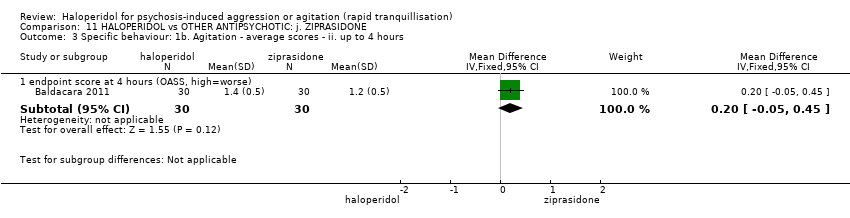 Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours. | ||||
| 3.1 endpoint score at 4 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.45] |
| 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.4 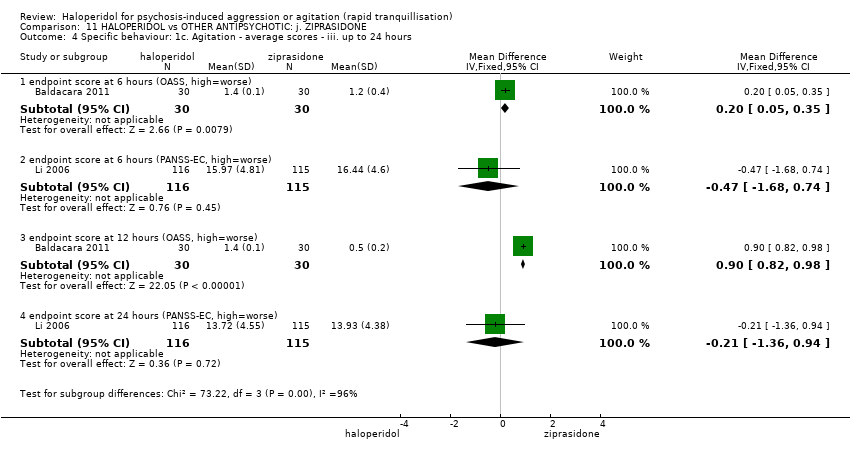 Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours. | ||||
| 4.1 endpoint score at 6 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.05, 0.35] |
| 4.2 endpoint score at 6 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.68, 0.74] |
| 4.3 endpoint score at 12 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.9 [0.82, 0.98] |
| 4.4 endpoint score at 24 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐1.36, 0.94] |
| 5 Specific behaviour: 1d. Agitation ‐ average scores ‐ iv. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.5  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 5 Specific behaviour: 1d. Agitation ‐ average scores ‐ iv. over 24 hours. | ||||
| 5.1 endpoint scores at 48 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐1.03, 1.15] |
| 5.2 endpoint scores at 72 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [‐0.45, 1.69] |
| 6 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.6  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 6 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours. | ||||
| 6.1 endpoint score at 1 hour (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.77, 0.77] |
| 6.2 endpoint score at 2 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.25, 1.65] |
| 7 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.7  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 7 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours. | ||||
| 7.1 endpoint score at 4 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.04, 0.64] |
| 8 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.8 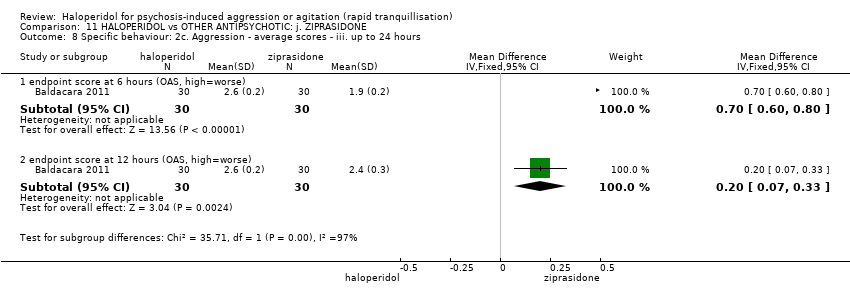 Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 8 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours. | ||||
| 8.1 endpoint score at 6 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.60, 0.80] |
| 8.2 endpoint score at 12 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.07, 0.33] |
| 9 Global outcome: 1. General ‐ need for additional measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.9 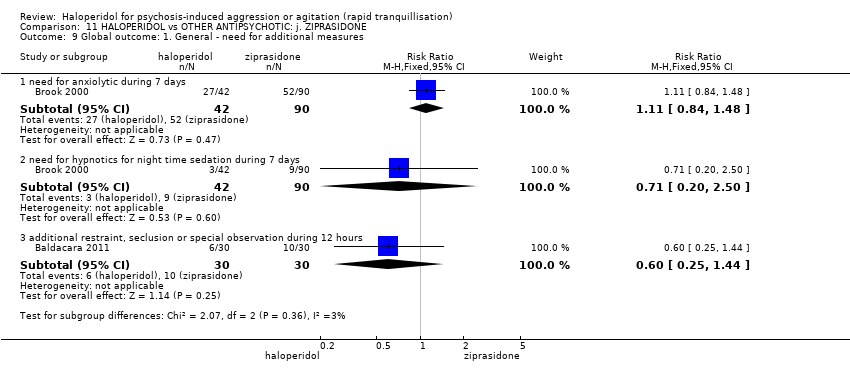 Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 9 Global outcome: 1. General ‐ need for additional measures. | ||||
| 9.1 need for anxiolytic during 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.84, 1.48] |
| 9.2 need for hypnotics for night time sedation during 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.20, 2.50] |
| 9.3 additional restraint, seclusion or special observation during 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.25, 1.44] |
| 10 Global outcome: 2. Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.10  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 10 Global outcome: 2. Average scores ‐ i. over 24 hours. | ||||
| 10.1 change score at 72 hours (CGI‐S, high=worse) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.13, 0.55] |
| 10.2 change score at 7 days (CGI‐S, high=worse) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [0.07, 0.95] |
| 11 Mental state: 1a. Average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.11  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 11 Mental state: 1a. Average scores ‐ i. up to 2 hours. | ||||
| 11.1 endpoint score at 2 hours (BPRS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Mental state: 1b. Average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.12  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 12 Mental state: 1b. Average scores ‐ ii. up to 4 hours. | ||||
| 12.1 endpoint score at 4 hours (BPRS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Mental state: 1c. Average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.13  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 13 Mental state: 1c. Average scores ‐ iii. up to 24 hours. | ||||
| 13.1 endpoint score at 24 hours (BPRS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Mental state: 1d. Average scores ‐ iv. over 24 hours Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.14  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 14 Mental state: 1d. Average scores ‐ iv. over 24 hours. | ||||
| 14.1 endpoint score at 48 hours (BPRS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.05 [‐6.45, 2.35] |
| 14.2 endpoint score at 72 hours (PANSS total, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 2.45 [‐2.19, 7.09] |
| 14.3 endpoint score at 72 hours (PANSS positive sub‐scale, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.45, 2.67] |
| 14.4 endpoint score at 72 hours (PANSS negative sub‐scale, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐4.19 [‐5.71, ‐2.67] |
| 14.5 endpoint score at 72 hours (PANSS general pathology score, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.27, 2.27] |
| 14.6 score at 72 hours (BPRS, high=worse) | 3 | 511 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.93, 1.07] |
| 14.7 change score at 7 days (BPRS, high=worse) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | 2.93 [‐0.81, 6.67] |
| 15 Adverse effects: 1a. General ‐ i. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.15 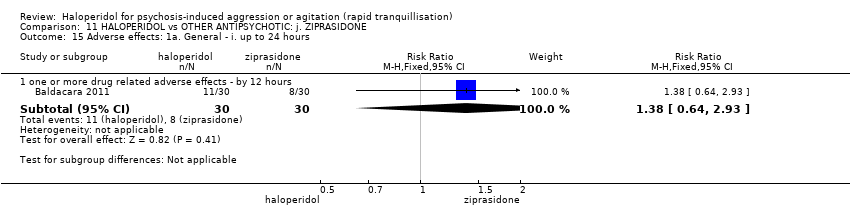 Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 15 Adverse effects: 1a. General ‐ i. up to 24 hours. | ||||
| 15.1 one or more drug related adverse effects ‐ by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.64, 2.93] |
| 16 Adverse effects: 1b. General ‐ ii. over 24 hours Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.16  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 16 Adverse effects: 1b. General ‐ ii. over 24 hours. | ||||
| 16.1 one or more drug related adverse effects ‐ by 72 hours | 4 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.47, 2.06] |
| 16.2 one or more drug related adverse effects ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.93, 1.83] |
| 17 Adverse effects: 1c. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.17 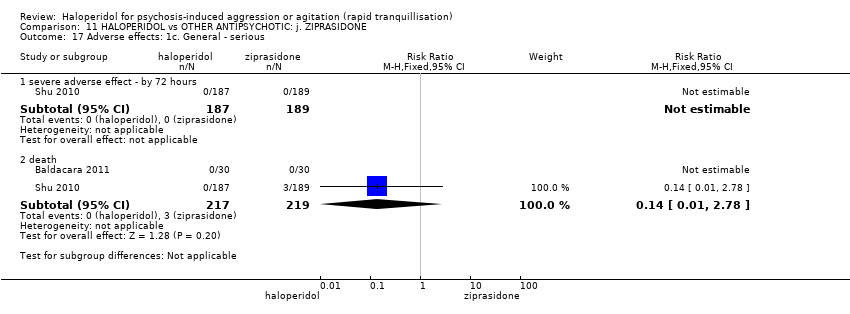 Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 17 Adverse effects: 1c. General ‐ serious. | ||||
| 17.1 severe adverse effect ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 death | 2 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.78] |
| 18 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.18  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 18 Adverse effects: 2a. Specific ‐ anticholinergic. | ||||
| 18.1 blurred vision by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.97 [1.15, 13.68] |
| 18.2 constipation by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
| 18.3 dry mouth by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [1.22, 7.22] |
| 18.4 hypersalivation by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.11, 5.87] |
| 19 Adverse effects: 2b. Specific ‐ arousal level ‐ i. various Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.19  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 19 Adverse effects: 2b. Specific ‐ arousal level ‐ i. various. | ||||
| 19.1 excessive sedation at 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.22, 4.56] |
| 19.2 insomnia ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.53] |
| 19.3 lethargy ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.67, 2.35] |
| 19.4 somnolence ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.43, 3.12] |
| 20 Adverse effects: 2c. Specific ‐ arousal level ‐ ii. average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.20  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 20 Adverse effects: 2c. Specific ‐ arousal level ‐ ii. average scores ‐ i. up to 2 hours. | ||||
| 20.1 endpoint score at 1 hour (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.46, 0.46] |
| 20.2 endpoint score at 2 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.46, 0.46] |
| 21 Adverse effects: 2d. Specific ‐ arousal level ‐ ii. average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.21 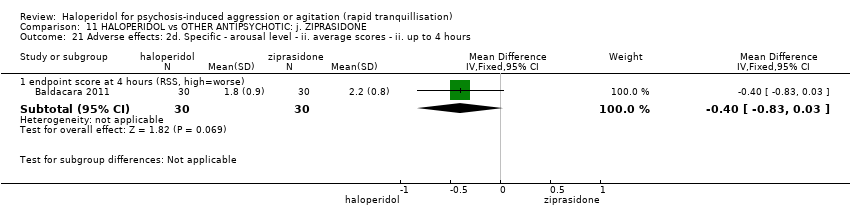 Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 21 Adverse effects: 2d. Specific ‐ arousal level ‐ ii. average scores ‐ ii. up to 4 hours. | ||||
| 21.1 endpoint score at 4 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.83, 0.03] |
| 22 Adverse effects: 2e. Specific ‐ arousal level ‐ ii. average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.22  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 22 Adverse effects: 2e. Specific ‐ arousal level ‐ ii. average scores ‐ iii. up to 24 hours. | ||||
| 22.1 endpoint score at 6 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.40, 0.20] |
| 22.2 endpoint score at 12 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.40, 0.20] |
| 23 Adverse effects: 2f. Specific ‐ cardiovascular Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.23  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 23 Adverse effects: 2f. Specific ‐ cardiovascular. | ||||
| 23.1 hypotension at 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.31] |
| 23.2 dizziness ‐ by 72 hours | 2 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.28, 1.19] |
| 23.3 ECG ‐ abnormal ‐ by 72 hours | 2 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.17, 0.84] |
| 23.4 ECG change ‐ clinically significant ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.60, 1.71] |
| 23.5 tachycardia ‐ by 72 hours | 3 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.23, 1.04] |
| 24 Adverse effects: 2g. Specific ‐ gastrointestinal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.24  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 24 Adverse effects: 2g. Specific ‐ gastrointestinal. | ||||
| 24.1 nausea by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
| 24.2 vomiting by 72 hours | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.02, 5.72] |
| 24.3 vomiting by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.82] |
| 25 Adverse effects: 2h. Specific ‐ hematological tests Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.25  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 25 Adverse effects: 2h. Specific ‐ hematological tests. | ||||
| 25.1 abnormal lab results ‐ by 72 hours | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.69, 1.17] |
| 25.2 abnormal lab results ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.47, 2.15] |
| 25.3 aspartate aminotransference ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.12 [0.62, 199.64] |
| 25.4 glucose increased ‐ by 24 hours | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.31, 2.92] |
| 25.5 haemogram abnormal ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.35] |
| 25.6 lactate dehydrogenase increase ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.39] |
| 25.7 liver function abnormal ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.39] |
| 25.8 triglyceride increase ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.96 [0.24, 102.14] |
| 26 Adverse effects: 2i. Specific ‐ movement disorders ‐ i. binary measures Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.26  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 26 Adverse effects: 2i. Specific ‐ movement disorders ‐ i. binary measures. | ||||
| 26.1 akathisia ‐ by 72 hours | 3 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.34, 4.01] |
| 26.2 akathisia ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [1.13, 16.31] |
| 26.3 dystonia ‐ by 72 hours | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.26 [1.67, 63.17] |
| 26.4 dystonia ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [0.76, 9.47] |
| 26.5 EPS ‐ by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.64, 190.53] |
| 26.6 EPS ‐ by 72 hours | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.13 [7.59, 48.21] |
| 26.7 EPS ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 34.29 [4.70, 250.02] |
| 26.8 extrapyramidal effects ‐ use of antiparkinson drugs ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.30 [1.82, 5.97] |
| 26.9 hypertonia/rigidity ‐ by 72 hours | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.81 [0.78, 280.47] |
| 26.10 hypertonia ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [0.90, 14.25] |
| 26.11 myotonia ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.84 [1.85, 8.00] |
| 26.12 pyramidal tract syndrome ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.18 [1.00, 295.55] |
| 26.13 reduced movement ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.17, 3.91] |
| 26.14 torsional spasm ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.30 [0.93, 11.70] |
| 26.15 tremor ‐ by 72 hours | 2 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.37, 5.11] |
| 26.16 tremor ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [0.82, 22.48] |
| 27 Adverse effects: 2j. Specific ‐ movement disorders ‐ ii. average scores ‐ i. over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.27  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 27 Adverse effects: 2j. Specific ‐ movement disorders ‐ ii. average scores ‐ i. over 24 hours. | ||||
| 27.1 change score at 72 hours (BAS, high=worse) | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [0.18, 0.76] |
| 27.2 score at 72 hours (SAS, high=worse) | 2 | 191 | Mean Difference (IV, Fixed, 95% CI) | 3.59 [2.15, 5.03] |
| 27.3 change score at 7 days (BAS, high=worse) | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.9 [0.51, 1.29] |
| 27.4 change score at 7 days (SAS, high=worse) | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 6.1 [3.91, 8.29] |
| 28 Adverse effects: 2k. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.28 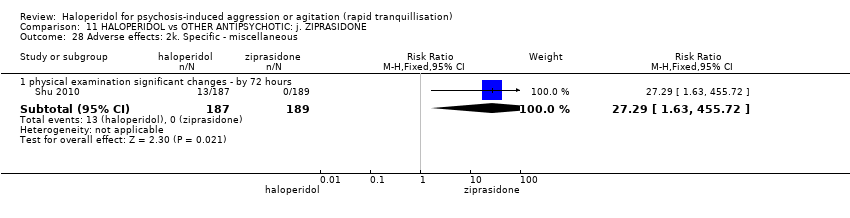 Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 28 Adverse effects: 2k. Specific ‐ miscellaneous. | ||||
| 28.1 physical examination significant changes ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 27.29 [1.63, 455.72] |
| 29 Leaving the study early: 1. For any reason ‐ i. over 24 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.29 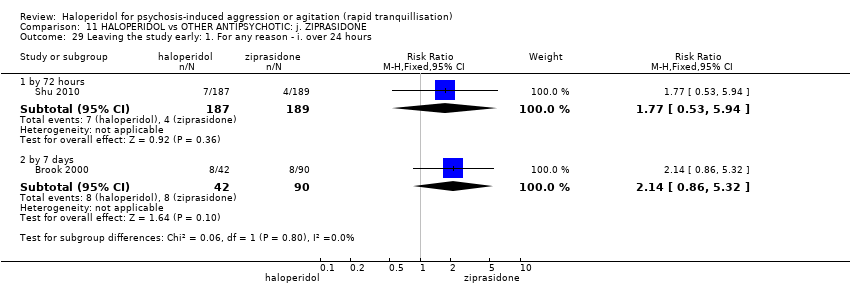 Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 29 Leaving the study early: 1. For any reason ‐ i. over 24 hours. | ||||
| 29.1 by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.53, 5.94] |
| 29.2 by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.86, 5.32] |
| 30 Leaving the study early: 2. For specific reasons ‐ i. over 24 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 11.30  Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 30 Leaving the study early: 2. For specific reasons ‐ i. over 24 hours. | ||||
| 30.1 adverse events ‐ by 72 hours | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.45, 12.75] |
| 30.2 adverse events ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.06, 4.65] |
| 30.3 withdrew consent ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.24, 104.55] |
| 30.4 other ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for tranquillisation: more than 3 injections Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [1.19, 5.46] |
| Analysis 12.1  Comparison 12 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE, Outcome 1 Repeated need for tranquillisation: more than 3 injections. | ||||
| 2 Adverse effects: 1a. Specific ‐ movement disorders Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 12.2  Comparison 12 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE, Outcome 2 Adverse effects: 1a. Specific ‐ movement disorders. | ||||
| 2.1 movement disorder ‐ tremor by 7 days | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.16 [0.93, 18.62] |
| 3 Adverse effects: 1b. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 12.3  Comparison 12 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE, Outcome 3 Adverse effects: 1b. Specific ‐ miscellaneous. | ||||
| 3.1 pain/allergy ‐ reaction at injection site | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.55 [0.15, 84.14] |
| 4 Leaving the study early Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 12.4  Comparison 12 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE, Outcome 4 Leaving the study early. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1. Aggression ‐ binary measures ‐ i. up to 2 hours Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.86, 1.55] |
| Analysis 13.1  Comparison 13 HALOPERIDOL vs BENZODIAZEPINE: a. FLUNITRAZEPAM, Outcome 1 Specific behaviour: 1. Aggression ‐ binary measures ‐ i. up to 2 hours. | ||||
| 1.1 ≥50% reduction at 90 minutes (OAS) | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.86, 1.55] |
| 2 Global outcome: 1. Need for seclusion or restraint Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 13.2 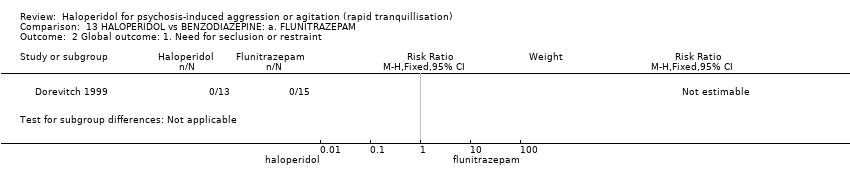 Comparison 13 HALOPERIDOL vs BENZODIAZEPINE: a. FLUNITRAZEPAM, Outcome 2 Global outcome: 1. Need for seclusion or restraint. | ||||
| 3 Adverse effects: 1. Specific ‐ movement disorders ‐ i. binary measures ‐ i. by 30 minutes Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 13.3  Comparison 13 HALOPERIDOL vs BENZODIAZEPINE: a. FLUNITRAZEPAM, Outcome 3 Adverse effects: 1. Specific ‐ movement disorders ‐ i. binary measures ‐ i. by 30 minutes. | ||||
| 3.1 EPS by 30 minutes | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ not asleep Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 14.1  Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 1 Tranquillisation or asleep ‐ not asleep. | ||||
| 1.1 at 1 hour | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.76, 1.44] |
| 1.2 by 3 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.14, 3.27] |
| 2 Repeated need for rapid tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 14.2  Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 2 Repeated need for rapid tranquillisation. | ||||
| 2.1 more than 1 injection | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.91, 1.43] |
| 2.2 more than 3 injections | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.50, 2.45] |
| 3 Global outcome: 1. No overall improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 14.3 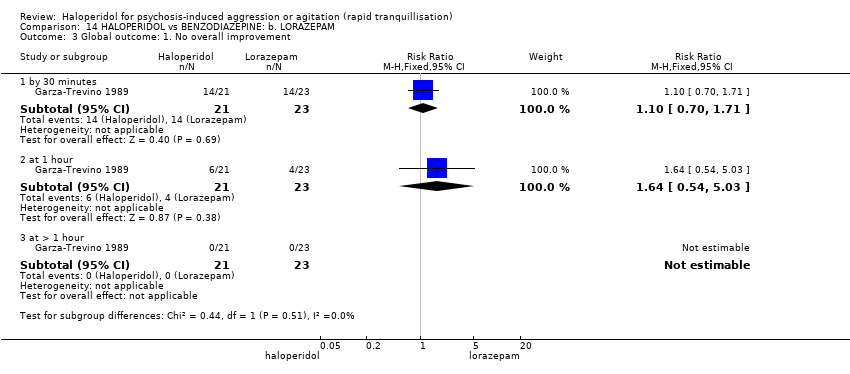 Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 3 Global outcome: 1. No overall improvement. | ||||
| 3.1 by 30 minutes | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.70, 1.71] |
| 3.2 at 1 hour | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.54, 5.03] |
| 3.3 at > 1 hour | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mental state: 1a. Average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 14.4  Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 4 Mental state: 1a. Average scores ‐ i. up to 2 hours. | ||||
| 4.1 endpoint score at 1 hour (BPRS, high=worse) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 3.26 [‐4.13, 10.65] |
| 4.2 endpoint score at 2 hours (BPRS, high=worse) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 4.07 [‐2.62, 10.76] |
| 5 Mental state: 1b. Average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 14.5 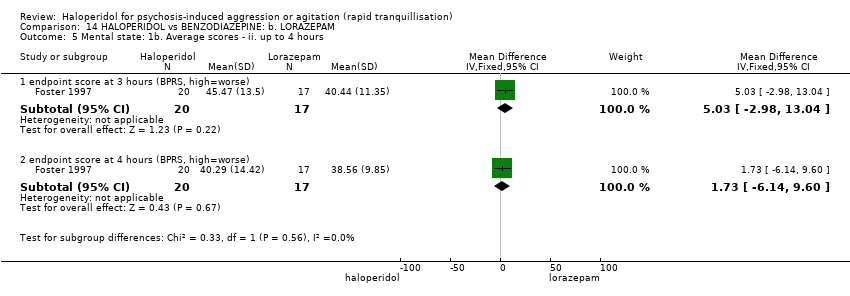 Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 5 Mental state: 1b. Average scores ‐ ii. up to 4 hours. | ||||
| 5.1 endpoint score at 3 hours (BPRS, high=worse) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 5.03 [‐2.98, 13.04] |
| 5.2 endpoint score at 4 hours (BPRS, high=worse) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 1.73 [‐6.14, 9.60] |
| 6 Adverse effects: 1a. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 14.6  Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 6 Adverse effects: 1a. General. | ||||
| 6.1 one or more drug‐related adverse effect up to 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.60, 2.10] |
| 7 Adverse effects: 1b. General ‐ serious Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 14.7 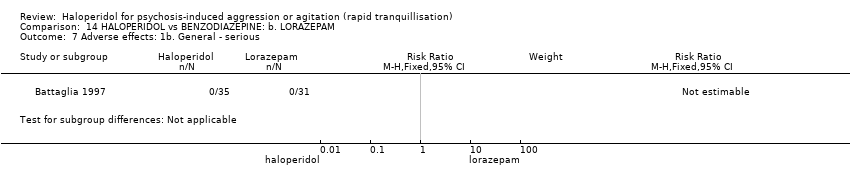 Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 7 Adverse effects: 1b. General ‐ serious. | ||||
| 8 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.14, 2.04] |
| Analysis 14.8  Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 8 Adverse effects: 2a. Specific ‐ anticholinergic. | ||||
| 8.1 dry mouth up to 24 hours (only reported if occurred ≥9%) | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.14, 2.04] |
| 9 Adverse effects: 2b. Specific ‐ arousal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 14.9  Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 9 Adverse effects: 2b. Specific ‐ arousal. | ||||
| 9.1 asleep ‐ at 1 hour | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.40, 1.99] |
| 10 Adverse effects: 2c. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 14.10 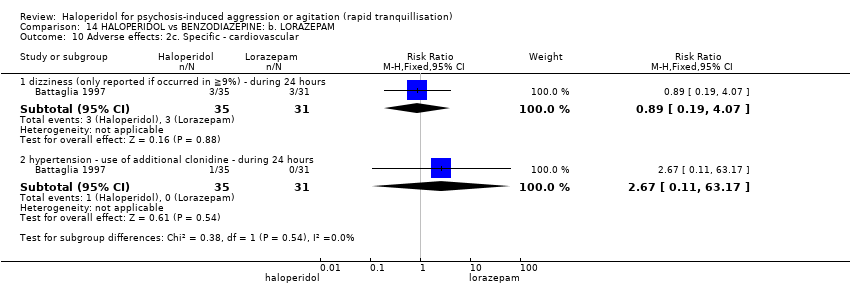 Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 10 Adverse effects: 2c. Specific ‐ cardiovascular. | ||||
| 10.1 dizziness (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.19, 4.07] |
| 10.2 hypertension ‐ use of additional clonidine ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.11, 63.17] |
| 11 Adverse effects: 2d. Specific ‐ movement disorder Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 14.11  Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 11 Adverse effects: 2d. Specific ‐ movement disorder. | ||||
| 11.1 ataxia (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.04, 4.65] |
| 11.2 dystonia (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.54 [0.42, 30.03] |
| 11.3 EPS | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.0 [2.11, 106.49] |
| 11.4 hypertonia/rigidity (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.22 [0.33, 115.91] |
| 11.5 speech disorder (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.35, 9.01] |
| 11.6 tremor (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.17, 18.60] |
| 11.7 use of additional benztropine ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.68, 5.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Tranquillisation or asleep: average times in minutes ‐ (skewed data) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 15.1
Comparison 15 HALOPERIDOL vs BENZODIAZEPINE: c. MIDAZOLAM, Outcome 1 Tranquillisation or asleep: average times in minutes ‐ (skewed data). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 average time to sedation | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.2 average time to arousal | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Global outcome: 1. Need for rescue drug Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.46, 2.87] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 15.2  Comparison 15 HALOPERIDOL vs BENZODIAZEPINE: c. MIDAZOLAM, Outcome 2 Global outcome: 1. Need for rescue drug. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Adverse effects: 1. General ‐ one or more drug related adverse effect Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.11] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 15.3  Comparison 15 HALOPERIDOL vs BENZODIAZEPINE: c. MIDAZOLAM, Outcome 3 Adverse effects: 1. General ‐ one or more drug related adverse effect. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Adverse effects: 2. Specific Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 15.4  Comparison 15 HALOPERIDOL vs BENZODIAZEPINE: c. MIDAZOLAM, Outcome 4 Adverse effects: 2. Specific. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 hypotensive | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.61] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 apnoea | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.61] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global outcome: 1. No overall improvement Show forest plot | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.18 [0.50, 133.66] |
| Analysis 16.1  Comparison 16 HALOPERIDOL vs COMBINATIONS: a. HALOPERIDOL + LEVOMEPROMAZINE, Outcome 1 Global outcome: 1. No overall improvement. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ asleep Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.11, 3.02] |
| Analysis 17.1  Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 1 Tranquillisation or asleep ‐ asleep. | ||||
| 1.1 not asleep by 3 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.11, 3.02] |
| 2 Repeated need for rapid tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 17.2 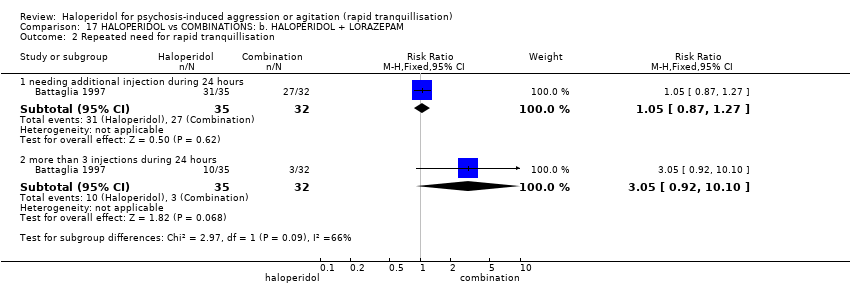 Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 2 Repeated need for rapid tranquillisation. | ||||
| 2.1 needing additional injection during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.87, 1.27] |
| 2.2 more than 3 injections during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.92, 10.10] |
| 3 Global outcome: 1. No overall improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 17.3  Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 3 Global outcome: 1. No overall improvement. | ||||
| 3.1 by 30 minutes | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.25, 5.68] |
| 3.2 at 1 hour | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.77 [0.88, 247.54] |
| 3.3 at > 1 hour | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Adverse effects: 1. General Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.62, 2.18] |
| Analysis 17.4  Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 4 Adverse effects: 1. General. | ||||
| 4.1 one or more drug‐related adverse effect during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.62, 2.18] |
| 5 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 17.5  Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 5 Adverse effects: 2a. Specific ‐ anticholinergic. | ||||
| 5.1 dry mouth (only reported if occurred in ≧9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.20, 4.21] |
| 6 Adverse effects: 2b. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 17.6 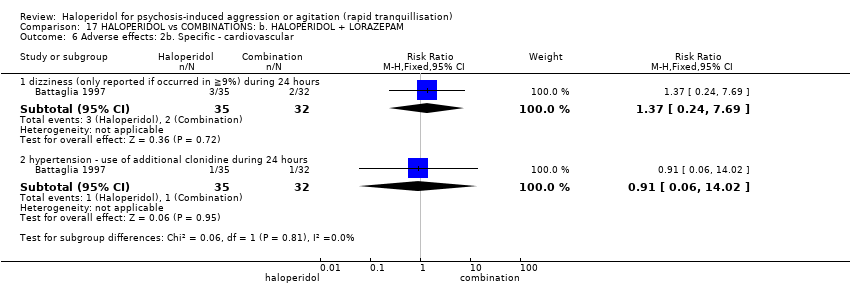 Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 6 Adverse effects: 2b. Specific ‐ cardiovascular. | ||||
| 6.1 dizziness (only reported if occurred in ≧9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.24, 7.69] |
| 6.2 hypertension ‐ use of additional clonidine during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.06, 14.02] |
| 7 Adverse effects: 2c. Specific ‐ movement disorders Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 17.7  Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 7 Adverse effects: 2c. Specific ‐ movement disorders. | ||||
| 7.1 ataxia (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 2.78] |
| 7.2 dystonia (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.25 [0.46, 147.45] |
| 7.3 hypertonia/rigidity (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.30, 25.05] |
| 7.4 tremor (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.17, 19.21] |
| 7.5 speech disorder (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.30, 5.03] |
| 7.6 use of additional benztropine during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.81, 9.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.1 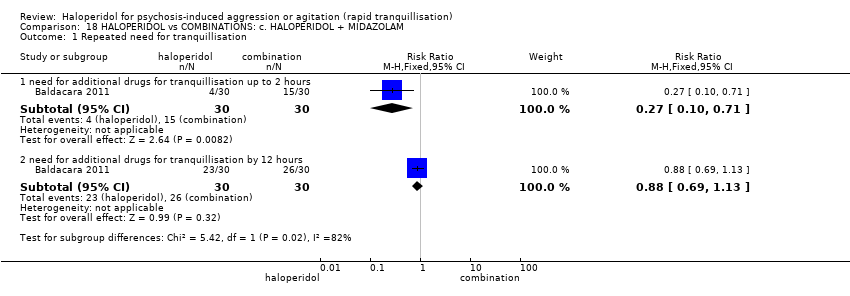 Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 1 Repeated need for tranquillisation. | ||||
| 1.1 need for additional drugs for tranquillisation up to 2 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.10, 0.71] |
| 1.2 need for additional drugs for tranquillisation by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
| 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.2  Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours. | ||||
| 2.1 endpoint score at 1 hour (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐8.5 [‐9.93, ‐7.07] |
| 2.2 endpoint score at 2 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐6.7 [‐7.46, ‐5.94] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.3 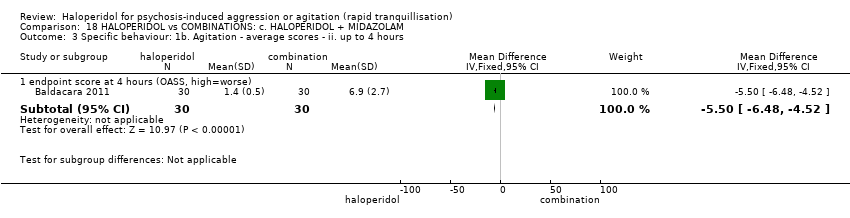 Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours. | ||||
| 3.1 endpoint score at 4 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐5.5 [‐6.48, ‐4.52] |
| 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.4  Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours. | ||||
| 4.1 endpoint score at 6 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐4.56, ‐2.84] |
| 4.2 endpoint score at 12 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐11.2 [‐12.24, ‐10.16] |
| 5 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.5  Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 5 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours. | ||||
| 5.1 endpoint score at 1 hour (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.44, 0.04] |
| 5.2 endpoint score at 2 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐4.21, ‐0.59] |
| 6 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.6  Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 6 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours. | ||||
| 6.1 endpoint score at 4 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.68, 0.48] |
| 7 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.7  Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 7 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours. | ||||
| 7.1 endpoint score at 6 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.30, 0.50] |
| 7.2 endpoint score at 12 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.9 [‐2.58, ‐1.22] |
| 8 Global outcomes: 1. General ‐ need for additional measures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.8 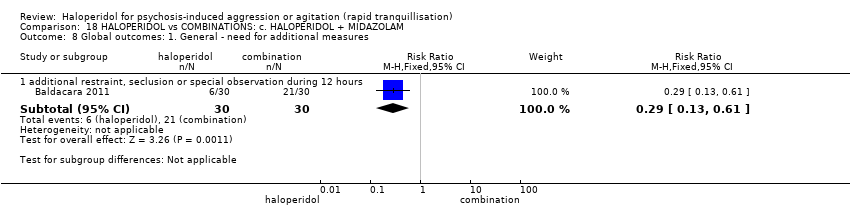 Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 8 Global outcomes: 1. General ‐ need for additional measures. | ||||
| 8.1 additional restraint, seclusion or special observation during 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.13, 0.61] |
| 9 Adverse effects: 1b. General ‐ one or more adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.9  Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 9 Adverse effects: 1b. General ‐ one or more adverse events. | ||||
| 9.1 during 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.41, 1.32] |
| 10 Adverse effects: 1a. General ‐ serious Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.10  Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 10 Adverse effects: 1a. General ‐ serious. | ||||
| 10.1 death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Adverse effects: 2a. Specific ‐ arousal ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.11  Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 11 Adverse effects: 2a. Specific ‐ arousal ‐ i. various. | ||||
| 11.1 excessive sedation by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.80] |
| 12 Adverse effects: 2b. Specific ‐ arousal ‐ ii. average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.12 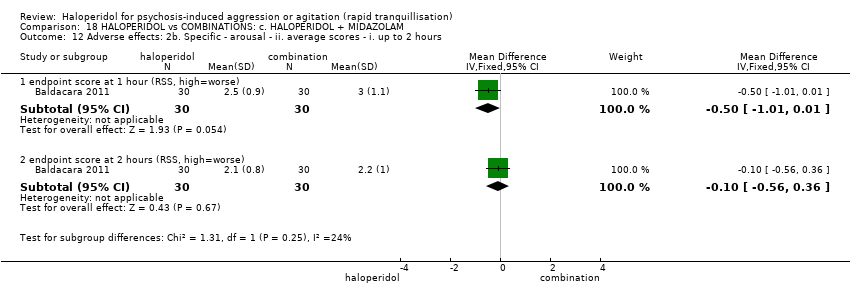 Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 12 Adverse effects: 2b. Specific ‐ arousal ‐ ii. average scores ‐ i. up to 2 hours. | ||||
| 12.1 endpoint score at 1 hour (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.01, 0.01] |
| 12.2 endpoint score at 2 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.56, 0.36] |
| 13 Adverse effects: 2c. Specific ‐ arousal ‐ ii. average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.13  Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 13 Adverse effects: 2c. Specific ‐ arousal ‐ ii. average scores ‐ ii. up to 4 hours. | ||||
| 13.1 endpoint score at 4 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.78, 0.18] |
| 14 Adverse effects: 2d. Specific ‐ arousal ‐ iii. average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.14 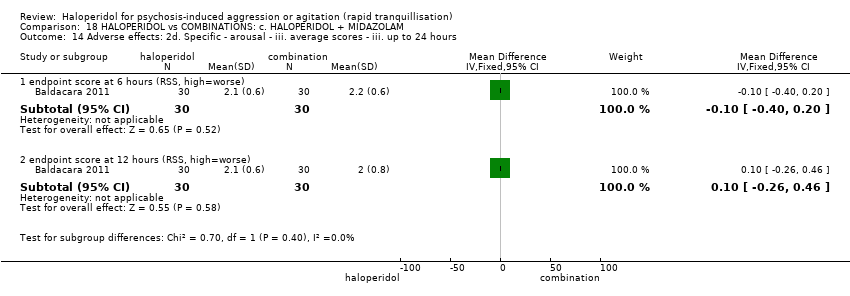 Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 14 Adverse effects: 2d. Specific ‐ arousal ‐ iii. average scores ‐ iii. up to 24 hours. | ||||
| 14.1 endpoint score at 6 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.40, 0.20] |
| 14.2 endpoint score at 12 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.26, 0.46] |
| 15 Adverse effects: 2e. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.15 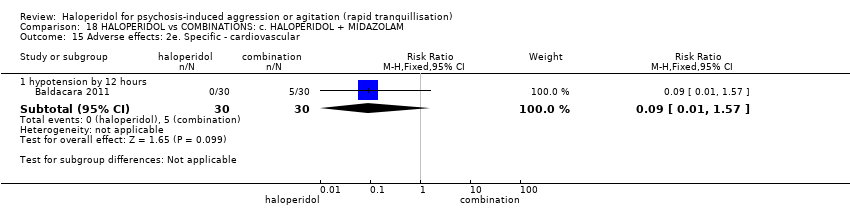 Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 15 Adverse effects: 2e. Specific ‐ cardiovascular. | ||||
| 15.1 hypotension by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.57] |
| 16 Adverse effects: 2f. Specific ‐ movement disorders Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 18.16  Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 16 Adverse effects: 2f. Specific ‐ movement disorders. | ||||
| 16.1 EPS by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.44, 6.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ not tranquil or asleep ‐ i. by 30 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.1  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 1 Tranquillisation or asleep ‐ not tranquil or asleep ‐ i. by 30 minutes. | ||||
| 1.1 at 20 minutes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.18, 2.16] |
| 2 Tranquillisation or asleep ‐ not tranquil or asleep ‐ ii. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.2  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 2 Tranquillisation or asleep ‐ not tranquil or asleep ‐ ii. up to 2 hours. | ||||
| 2.1 at 40 minutes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.83, 1.91] |
| 2.2 at 1 hour | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.85, 2.37] |
| 2.3 at 2 hours | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.68, 2.56] |
| 3 Repeated need for tranquillisation ‐ need for additional drugs for tranquillisation Show forest plot | 2 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.29] |
| Analysis 19.3  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 3 Repeated need for tranquillisation ‐ need for additional drugs for tranquillisation. | ||||
| 3.1 by 2 hours | 2 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.41] |
| 3.2 by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.81, 1.49] |
| 4 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.4  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 4 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours. | ||||
| 4.1 endpoint score at 1 hour (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐24.5 [‐27.32, ‐21.68] |
| 4.2 endpoint score at 2 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐9.40 [‐10.39, ‐8.41] |
| 5 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.5  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 5 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours. | ||||
| 5.1 endpoint score at 4 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [3.27, 4.33] |
| 6 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.6  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 6 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours. | ||||
| 6.1 endpoint score at 6 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.6 [2.13, 3.07] |
| 6.2 endpoint score at 12 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.55, 1.05] |
| 7 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.7 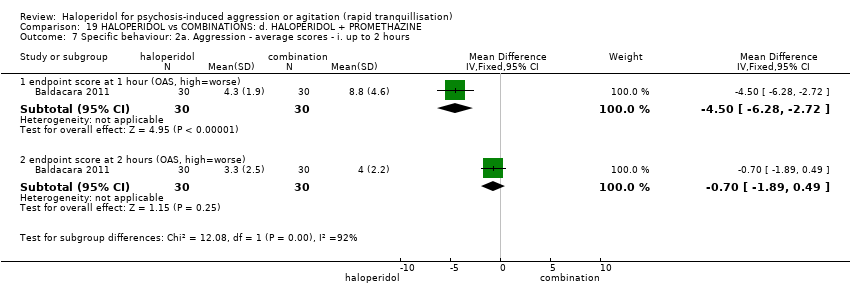 Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 7 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours. | ||||
| 7.1 endpoint score at 1 hour (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.50 [‐6.28, ‐2.72] |
| 7.2 endpoint score at 2 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.89, 0.49] |
| 8 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.8  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 8 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours. | ||||
| 8.1 endpoint score at 4 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.49, 0.71] |
| 9 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.9  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 9 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours. | ||||
| 9.1 endpoint score at 6 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [0.91, 1.29] |
| 9.2 endpoint score at 12 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.8 [1.67, 1.93] |
| 10 Specific behaviour: 2d. Aggression ‐ further aggressive episode ‐ i. up to 24 hours Show forest plot | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.65] |
| Analysis 19.10 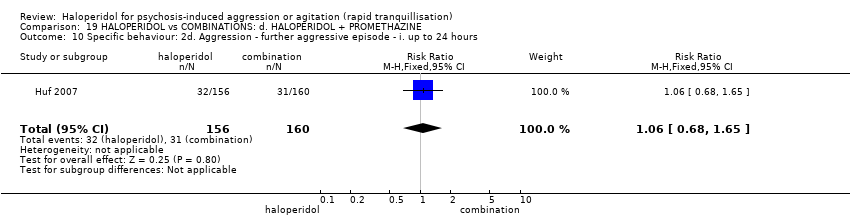 Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 10 Specific behaviour: 2d. Aggression ‐ further aggressive episode ‐ i. up to 24 hours. | ||||
| 11 Global outcomes: 1. General ‐ need for additional measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.11 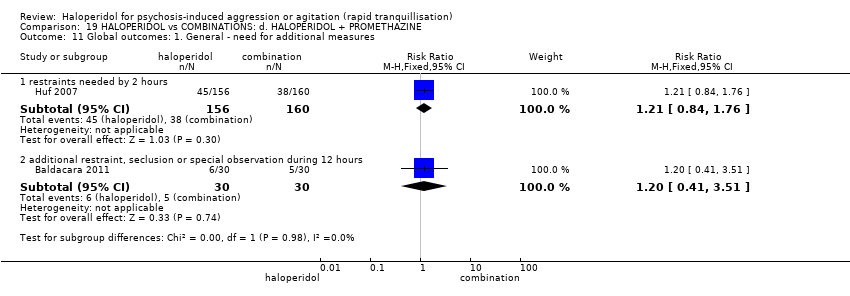 Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 11 Global outcomes: 1. General ‐ need for additional measures. | ||||
| 11.1 restraints needed by 2 hours | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.84, 1.76] |
| 11.2 additional restraint, seclusion or special observation during 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.41, 3.51] |
| 12 Global outcomes: 2. Specific Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.12  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 12 Global outcomes: 2. Specific. | ||||
| 12.1 doctor called to see patient | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.05, 2.14] |
| 12.2 refuse oral drugs | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.61, 1.60] |
| 13 Adverse effects: 1a. General Show forest plot | 2 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.07, 3.80] |
| Analysis 19.13  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 13 Adverse effects: 1a. General. | ||||
| 13.1 one or more adverse effects up to 24 hours | 2 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.07, 3.80] |
| 14 Adverse effects: 1b. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.14 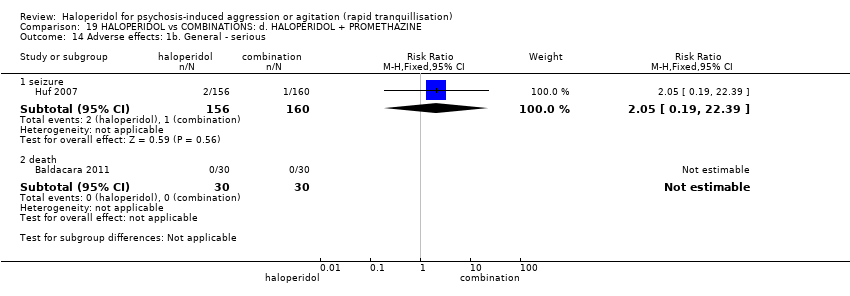 Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 14 Adverse effects: 1b. General ‐ serious. | ||||
| 14.1 seizure | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.19, 22.39] |
| 14.2 death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Adverse effects: 2a. Specific ‐ arousal ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.15 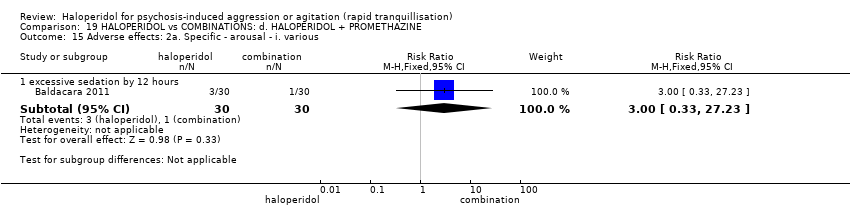 Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 15 Adverse effects: 2a. Specific ‐ arousal ‐ i. various. | ||||
| 15.1 excessive sedation by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 16 Adverse effects: 2b. Specific ‐ arousal ‐ ii. average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.16  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 16 Adverse effects: 2b. Specific ‐ arousal ‐ ii. average scores ‐ i. up to 2 hours. | ||||
| 16.1 endpoint score at 1 hour (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.38, 0.58] |
| 16.2 endpoint score at 2 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.50, 0.30] |
| 17 Adverse effects: 2c. Specific ‐ arousal ‐ ii. average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.17  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 17 Adverse effects: 2c. Specific ‐ arousal ‐ ii. average scores ‐ ii. up to 4 hours. | ||||
| 17.1 endpoint score at 4 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.78, 0.18] |
| 18 Adverse effects: 2d. Specific ‐ arousal ‐ ii. average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.18 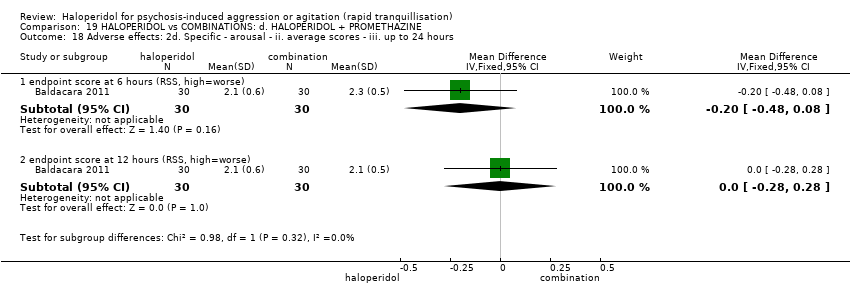 Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 18 Adverse effects: 2d. Specific ‐ arousal ‐ ii. average scores ‐ iii. up to 24 hours. | ||||
| 18.1 endpoint score at 6 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.48, 0.08] |
| 18.2 endpoint score at 12 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 19 Adverse effects: 2e. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.19 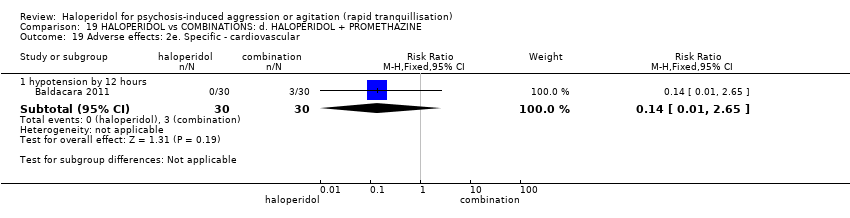 Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 19 Adverse effects: 2e. Specific ‐ cardiovascular. | ||||
| 19.1 hypotension by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.65] |
| 20 Adverse effects: 2f. Specific ‐ movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.20  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 20 Adverse effects: 2f. Specific ‐ movement disorders. | ||||
| 20.1 acute dystonia | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.48 [1.14, 331.92] |
| 20.2 EPS by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.32, 3.10] |
| 21 Service outcomes: 1. Not discharged by 14 days Show forest plot | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.05] |
| Analysis 19.21  Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 21 Service outcomes: 1. Not discharged by 14 days. | ||||
| 22 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 19.22 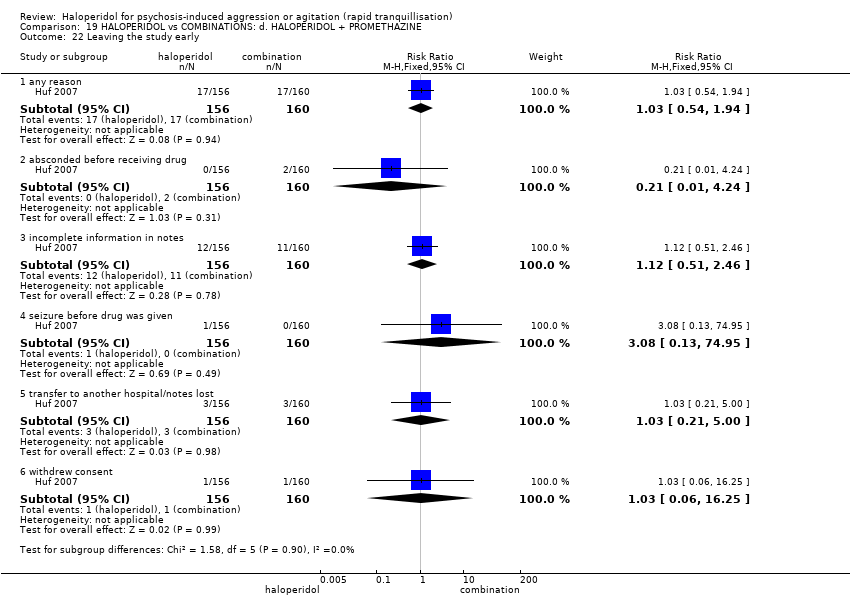 Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 22 Leaving the study early. | ||||
| 22.1 any reason | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.54, 1.94] |
| 22.2 absconded before receiving drug | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.24] |
| 22.3 incomplete information in notes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.51, 2.46] |
| 22.4 seizure before drug was given | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.95] |
| 22.5 transfer to another hospital/notes lost | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.21, 5.00] |
| 22.6 withdrew consent | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.06, 16.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 20.1 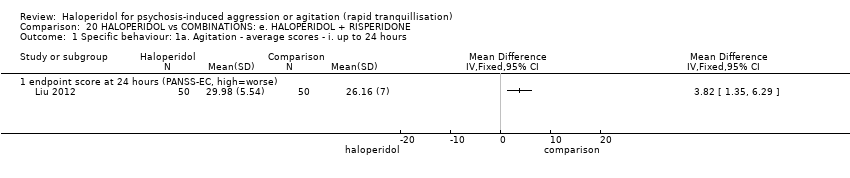 Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours. | ||||
| 1.1 endpoint score at 24 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 20.2  Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours. | ||||
| 2.1 endpoint score at 3 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 endpoint score at 5 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 endpoint score at 1 week (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 endpoint score at 2 weeks (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Global outcome: No improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 20.3  Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 3 Global outcome: No improvement. | ||||
| 3.1 at 72 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 at 5 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 at 1 week | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 at 2 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: 1a. Specific ‐ arousal level ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 20.4 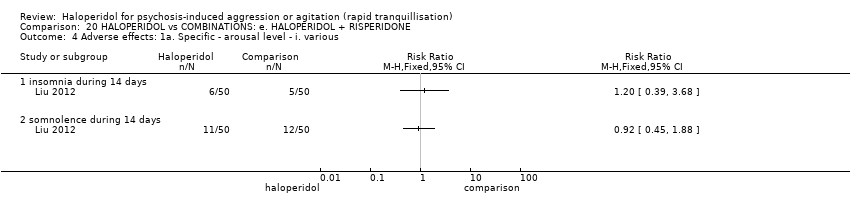 Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 4 Adverse effects: 1a. Specific ‐ arousal level ‐ i. various. | ||||
| 4.1 insomnia during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 somnolence during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects: 1b. Specific ‐ movement disorders ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 20.5  Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 5 Adverse effects: 1b. Specific ‐ movement disorders ‐ i. various. | ||||
| 5.1 tremor during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 akathisia during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Adverse effects: 1c. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 20.6  Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 6 Adverse effects: 1c. Specific ‐ miscellaneous. | ||||
| 6.1 Dry mouth during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Constipation during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Increased salivation during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1. Agitation ‐ average scores ‐ over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 21.1  Comparison 21 HALOPERIDOL vs COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE, Outcome 1 Specific behaviour: 1. Agitation ‐ average scores ‐ over 24 hours. | ||||
| 1.1 endpoint score at 72 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 endpoint score at 7 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 endpoint score at 14 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Global outcome: No improvement Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.38, 2.95] |
| Analysis 21.2  Comparison 21 HALOPERIDOL vs COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE, Outcome 2 Global outcome: No improvement. | ||||
| 3 Mental state: Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 21.3  Comparison 21 HALOPERIDOL vs COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE, Outcome 3 Mental state: Average scores ‐ i. over 24 hours. | ||||
| 3.1 endpoint score at 72 hours (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 endpoint score at 7 days (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 endpoint score at 14 days (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: General ‐ over 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 21.4 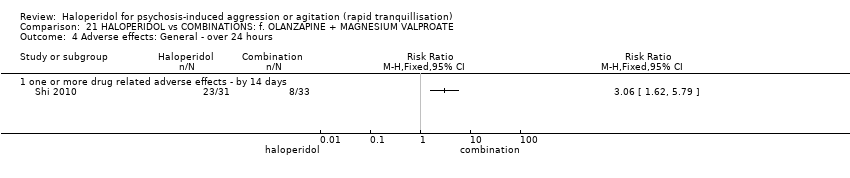 Comparison 21 HALOPERIDOL vs COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE, Outcome 4 Adverse effects: General ‐ over 24 hours. | ||||
| 4.1 one or more drug related adverse effects ‐ by 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ binary measures Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.44, 3.06] |
| Analysis 22.1  Comparison 22 HALOPERIDOL vs COMBINATIONS: g. QUETIAPINE + MAGNESIUM VALPROATE, Outcome 1 Specific behaviour: 1a. Agitation ‐ binary measures. | ||||
| 1.1 <25% reduction, no effect (PANSS‐EC) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.44, 3.06] |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 22.2  Comparison 22 HALOPERIDOL vs COMBINATIONS: g. QUETIAPINE + MAGNESIUM VALPROATE, Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. over 24 hours. | ||||
| 2.1 endpoint score at 3 days (PANSS‐EC, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐2.31, 2.35] |
| 2.2 endpoint score at 7 days (PANSS‐EC, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.16 [‐0.98, 3.30] |
| 2.3 endpoint score at 14 days (PANSS‐EC, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.24 [‐0.49, 2.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 23.1 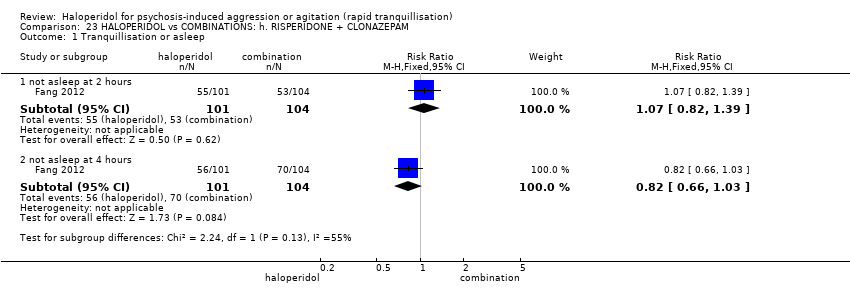 Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 1 Tranquillisation or asleep. | ||||
| 1.1 not asleep at 2 hours | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.39] |
| 1.2 not asleep at 4 hours | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.66, 1.03] |
| 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 23.2  Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours. | ||||
| 2.1 change score at 2 hours (PANSS‐EC, high=worse) | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.80, 2.80] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 23.3  Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 4 hours. | ||||
| 3.1 change score at 4 hours (PANSS‐EC, high=worse) | 1 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐5.20, 0.40] |
| 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 23.4 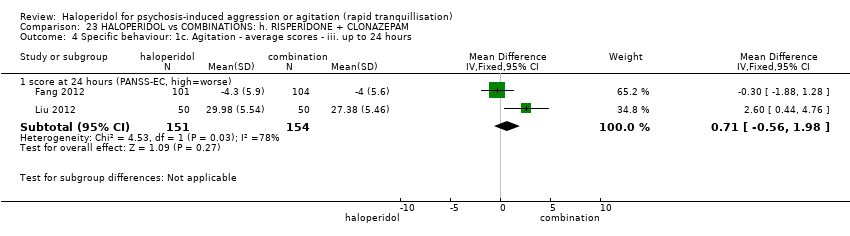 Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours. | ||||
| 4.1 score at 24 hours (PANSS‐EC, high=worse) | 2 | 305 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [‐0.56, 1.98] |
| 5 Specific behaviour: 1d. Agitation ‐ average scores ‐ iv. over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 23.5 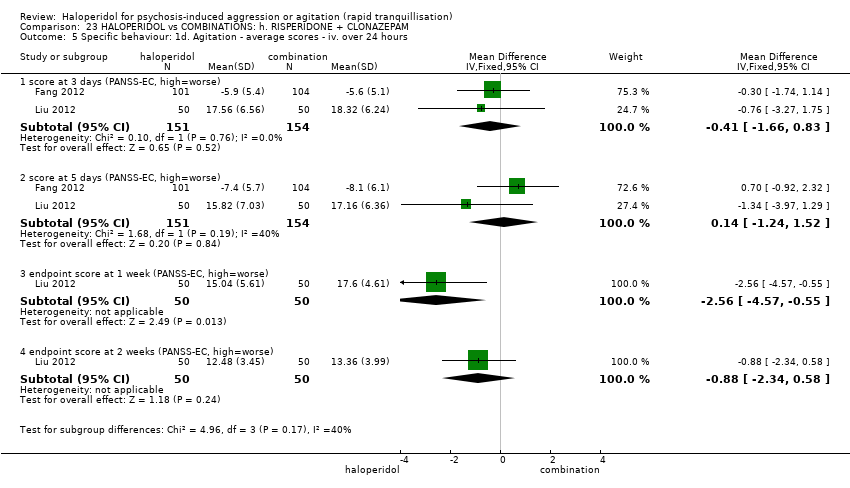 Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 5 Specific behaviour: 1d. Agitation ‐ average scores ‐ iv. over 24 hours. | ||||
| 5.1 score at 3 days (PANSS‐EC, high=worse) | 2 | 305 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.66, 0.83] |
| 5.2 score at 5 days (PANSS‐EC, high=worse) | 2 | 305 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐1.24, 1.52] |
| 5.3 endpoint score at 1 week (PANSS‐EC, high=worse) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.56 [‐4.57, ‐0.55] |
| 5.4 endpoint score at 2 weeks (PANSS‐EC, high=worse) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐2.34, 0.58] |
| 6 Global outcome: No improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 23.6  Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 6 Global outcome: No improvement. | ||||
| 6.1 at 72 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 at 5 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 at 1 week | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 at 2 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Mental state: 1. Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 23.7 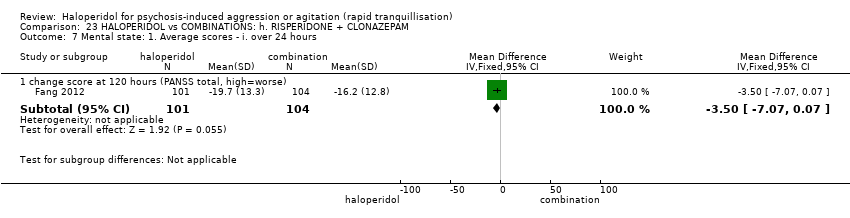 Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 7 Mental state: 1. Average scores ‐ i. over 24 hours. | ||||
| 7.1 change score at 120 hours (PANSS total, high=worse) | 1 | 205 | Mean Difference (IV, Fixed, 95% CI) | ‐3.5 [‐7.07, 0.07] |
| 8 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 23.8 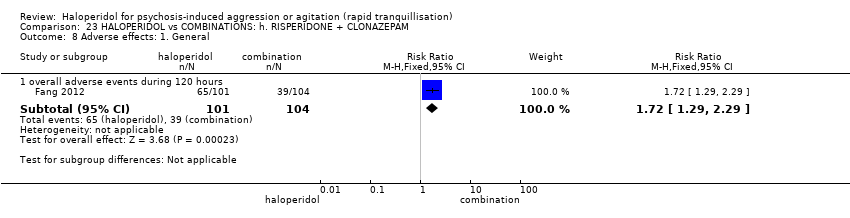 Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 8 Adverse effects: 1. General. | ||||
| 8.1 overall adverse events during 120 hours | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.29, 2.29] |
| 9 Adverse effects: 2a. Specific ‐ arousal ‐ i. various Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 23.9  Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 9 Adverse effects: 2a. Specific ‐ arousal ‐ i. various. | ||||
| 9.1 insomnia during 5 days (only reported if occurred in ≥5%) | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.45, 5.31] |
| 9.2 insomnia during 14 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.20, 1.23] |
| 9.3 somnolence during 14 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.94, 8.06] |
| 10 Adverse effects: 2b. Specific ‐ cardiovascular ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 23.10  Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 10 Adverse effects: 2b. Specific ‐ cardiovascular ‐ i. various. | ||||
| 10.1 tachycardia during 120 hours (only reported if occurred in ≥5%) | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.36, 4.66] |
| 11 Adverse effects: 2c. Specific ‐ movement disorders ‐ i. various Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 23.11  Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 11 Adverse effects: 2c. Specific ‐ movement disorders ‐ i. various. | ||||
| 11.1 akathisia during 5 days (only reported if occurred in ≥5%) | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.12, 2.38] |
| 11.2 akathisia during 14 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.98, 5.58] |
| 11.3 EPS during 5 days (only reported if occurred in ≥5%) | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.52, 3.23] |
| 11.4 tremor during 14 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.2 [1.27, 8.07] |
| 12 Adverse effects: 2d. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 23.12 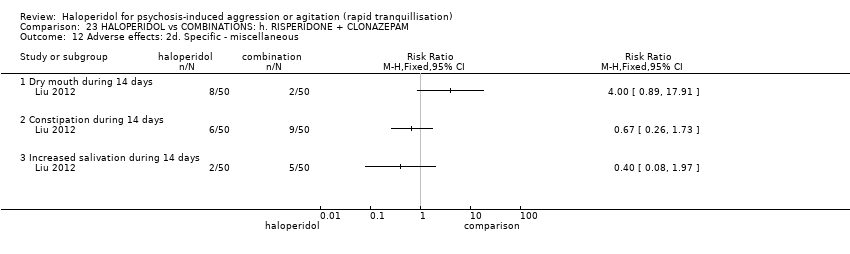 Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 12 Adverse effects: 2d. Specific ‐ miscellaneous. | ||||
| 12.1 Dry mouth during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Constipation during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Increased salivation during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 24.1  Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours. | ||||
| 1.1 endpoint score at 12 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 24.2  Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours. | ||||
| 2.1 endpoint score at 48 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 endpoint score at 3 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 endpoint score at 5 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 endpoint score at 7 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Global outcome: 1. Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 24.3  Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 3 Global outcome: 1. Average scores ‐ i. over 24 hours. | ||||
| 3.1 endpoint score at 7 days (CGI‐S, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mental state: 1. Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 24.4  Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 4 Mental state: 1. Average scores ‐ i. over 24 hours. | ||||
| 4.1 endpoint score at 7 days (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 endpoint score at 7 days (PANSS positive sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 endpoint score at 7 days (PANSS negative sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 endpoint score at 7 days (PANSS general psychopathology sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 24.5  Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 5 Leaving the study early. | ||||
| 5.1 any reason | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 lost at follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 non compliance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Study flow diagram.
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
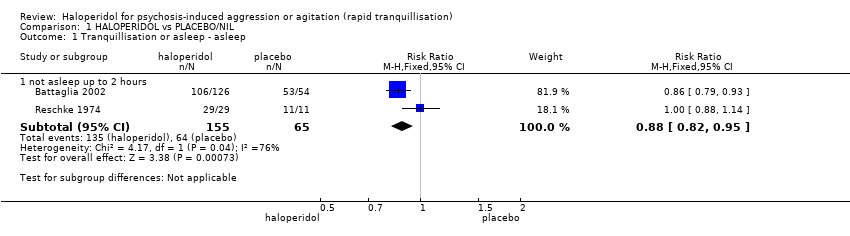
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 1 Tranquillisation or asleep ‐ asleep.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 2 Repeated need for tranquillisation.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 3 Specific behaviour: 1a. Agitation ‐ various measures.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours.
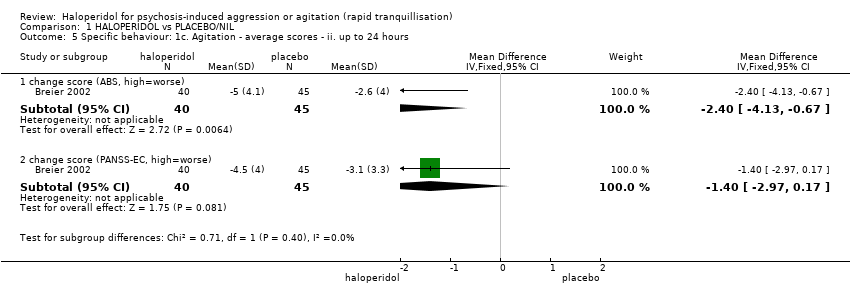
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 5 Specific behaviour: 1c. Agitation ‐ average scores ‐ ii. up to 24 hours.
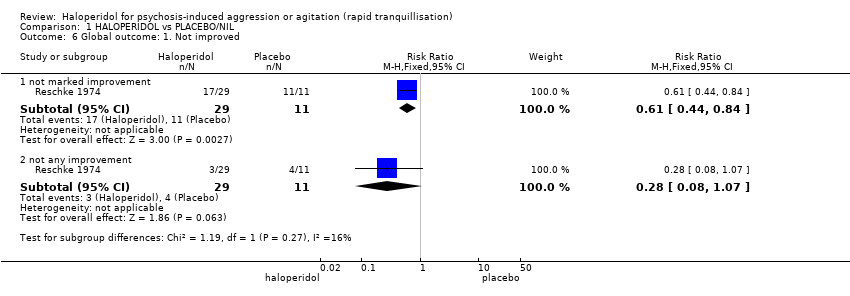
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 6 Global outcome: 1. Not improved.
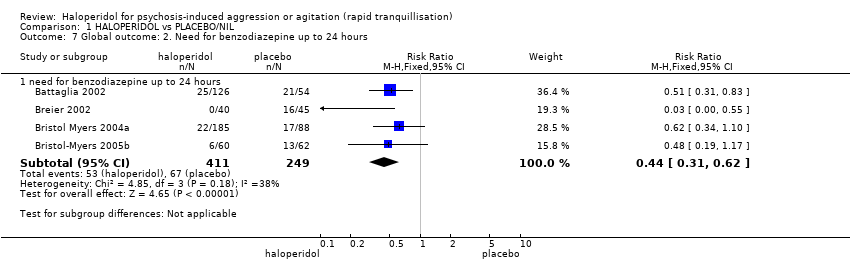
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 7 Global outcome: 2. Need for benzodiazepine up to 24 hours.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 8 Global outcome: 3a. Average scores ‐ i. up to 2 hours.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 9 Global outcome: 3b. Average scores ‐ ii. up to 24 hours.
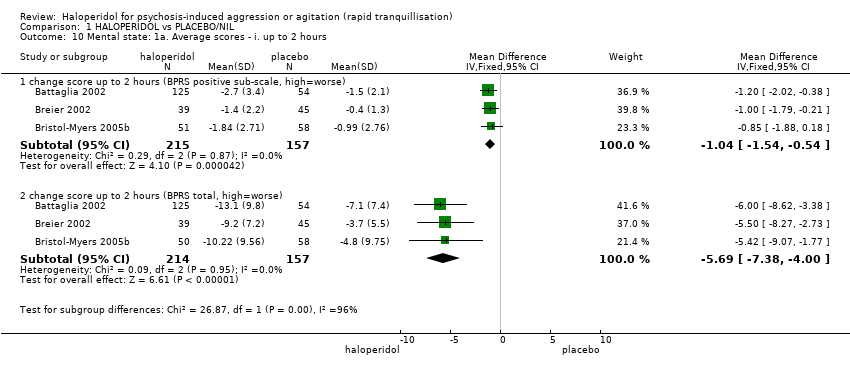
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 10 Mental state: 1a. Average scores ‐ i. up to 2 hours.
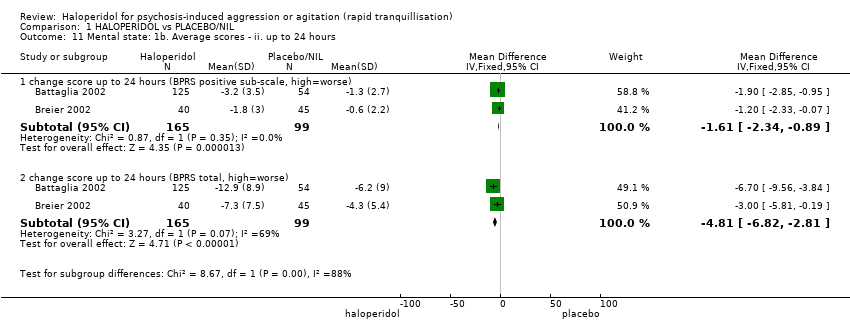
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 11 Mental state: 1b. Average scores ‐ ii. up to 24 hours.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 12 Adverse effects: 1a. General.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 13 Adverse effects: 1b. General ‐ serious.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 14 Adverse effects: 2a. Specific ‐ arousal level.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 15 Adverse effects: 2b. Specific ‐ cardiovascular ‐ miscellaneous outcomes.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 16 Adverse effects: 2c. Specific ‐ cardiovascular ‐ QTc interval (average change at 24 hours).
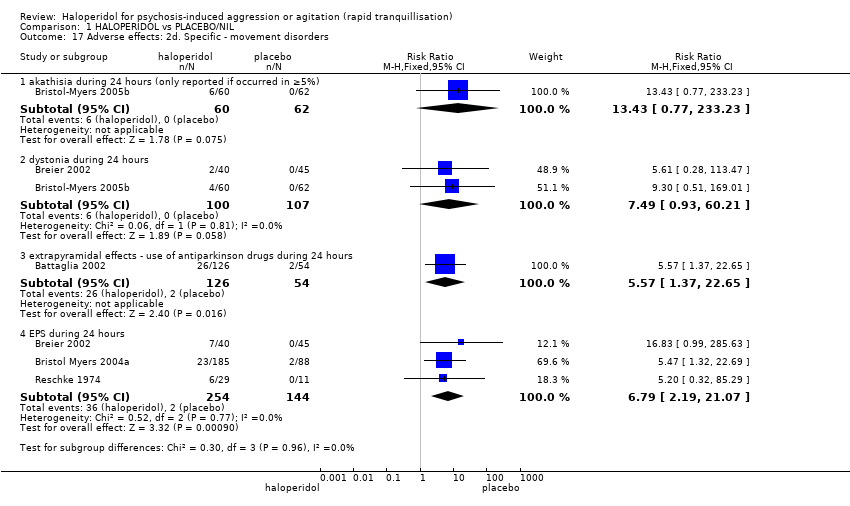
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 17 Adverse effects: 2d. Specific ‐ movement disorders.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 18 Adverse effects: 2e. Specific ‐ movement disorders: i. average scores ‐ i. up to 24 hours.
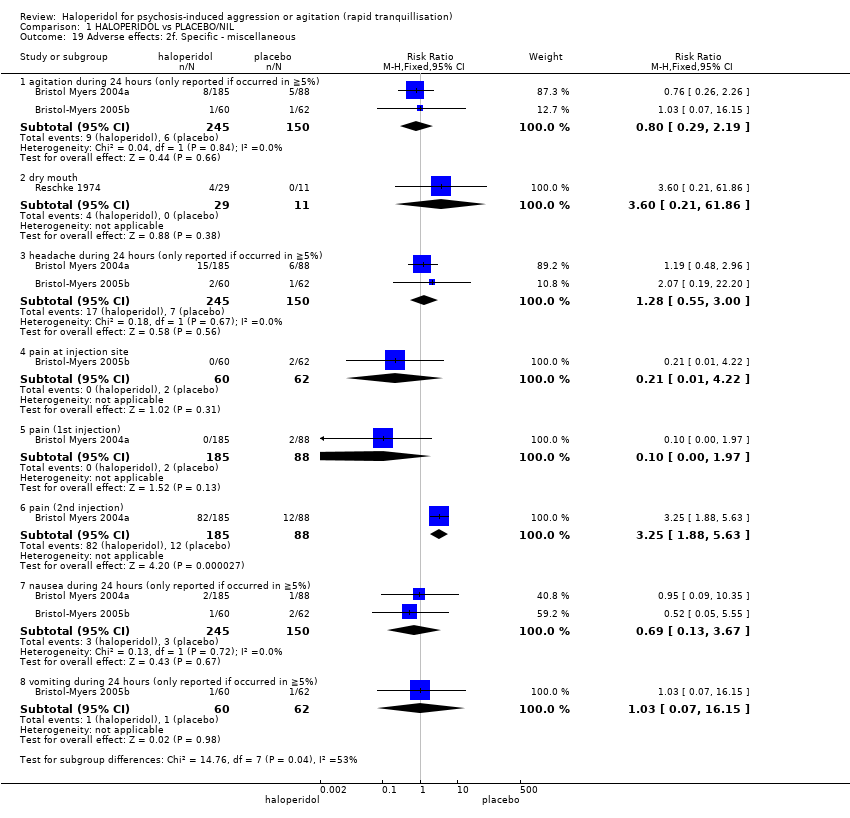
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 19 Adverse effects: 2f. Specific ‐ miscellaneous.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 20 Leaving the study early.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 1 Repeated need for rapid tranquillisation: needing additional injection.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 2 Specific behaviour: 1a. Agitation ‐ PANSS‐EC response up to 2 hours (at least 40% change on PANSS‐EC from baseline).

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ up to 2 hours.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 4 Global outcome: 1. Need for benzodiazepine.
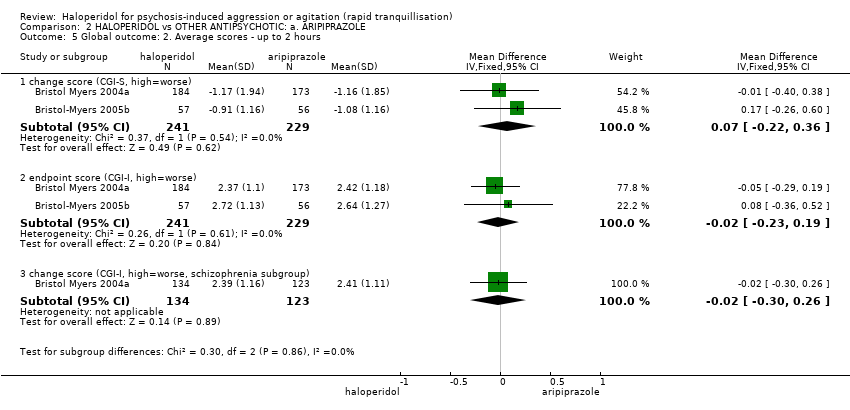
Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 5 Global outcome: 2. Average scores ‐ up to 2 hours.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 6 Mental state: 1. Average scores ‐ up to 2 hours.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 7 Adverse effects: 1a. General.
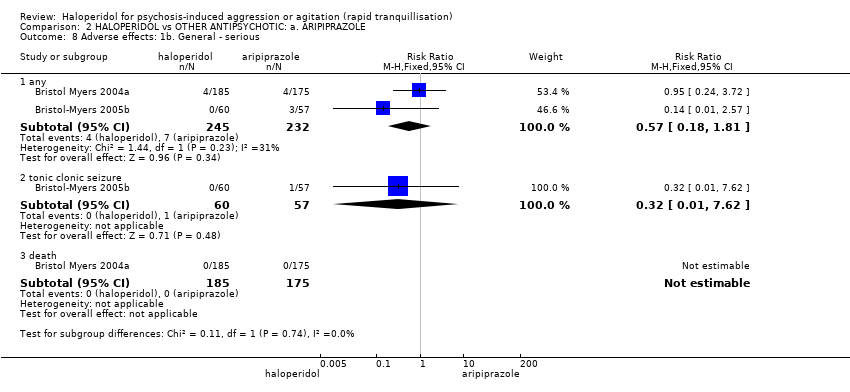
Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 8 Adverse effects: 1b. General ‐ serious.
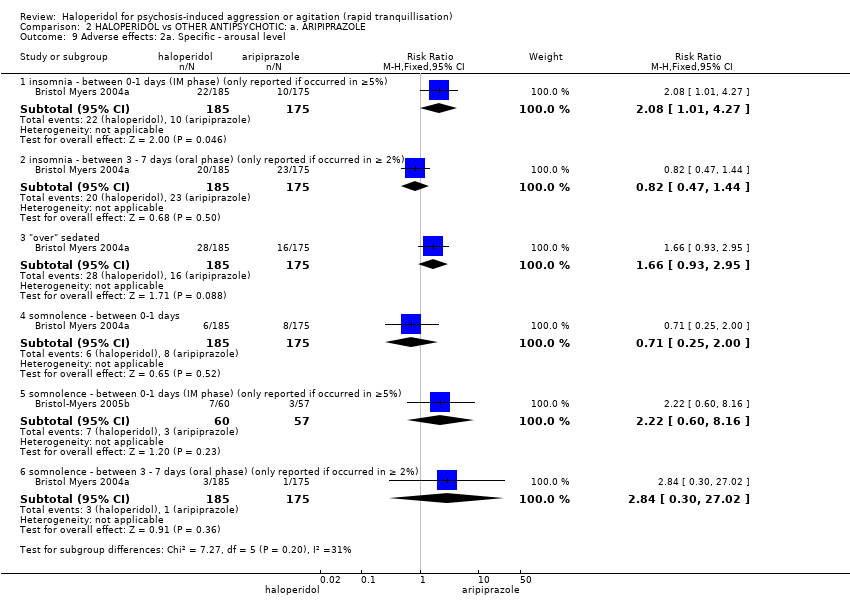
Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 9 Adverse effects: 2a. Specific ‐ arousal level.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 10 Adverse effects: 2b. Specific ‐ cardiac.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 11 Adverse effects: 2c. Specific ‐ gastrointestinal.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 12 Adverse effects: 2d. Specific ‐ movement disorder.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 13 Adverse effects: 2e. Specific ‐ miscellaneous.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 14 Leaving the study early: 1. For specific reasons.

Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 1 Tranquillisation or asleep ‐ asleep.
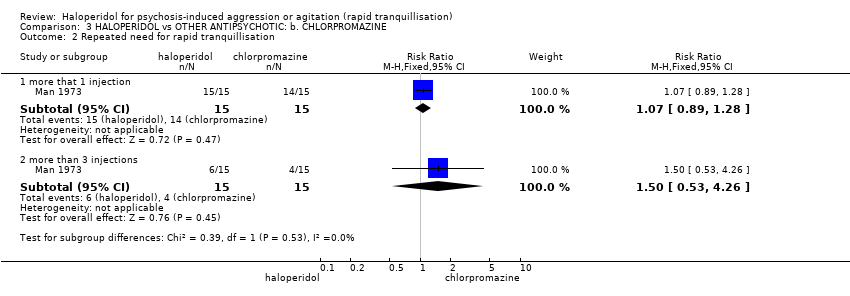
Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 2 Repeated need for rapid tranquillisation.

Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 3 Global outcome: 1. Not improved.

Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 4 Adverse effects: any serious or specific adverse effects.

Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 5 Leaving the study early.
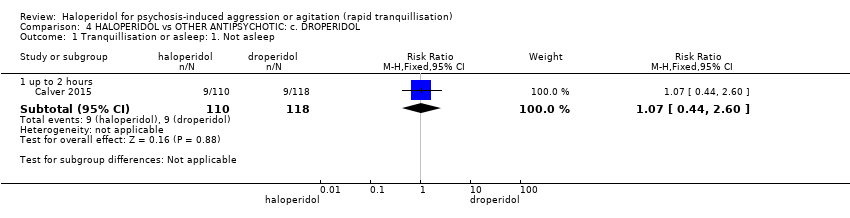
Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 1 Tranquillisation or asleep: 1. Not asleep.

Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 2 Tranquillisation or asleep: 2. Time to sleep.

Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 3 Repeated need for rapid tranquillisation.

Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 4 Global outcome: 1. Need for benzodiazepine.

Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 5 Adverse effects: 1. General.

Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 6 Adverse effects: 2a. Specific ‐ arousal level.

Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 7 Adverse effects: 2b. Specific ‐ cardiovascular.
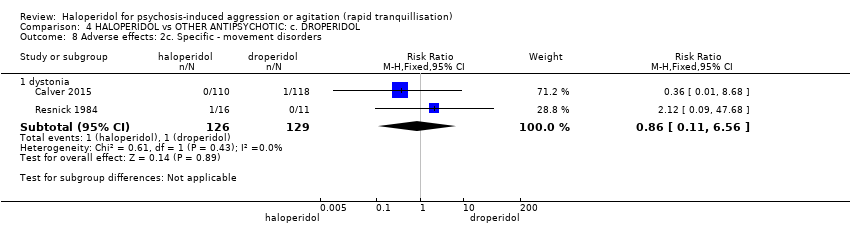
Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 8 Adverse effects: 2c. Specific ‐ movement disorders.

Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 9 Adverse effects: 2d. Specific ‐ miscellaneous.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 1 Tranquillisation or asleep ‐ not asleep up to 24 hours.
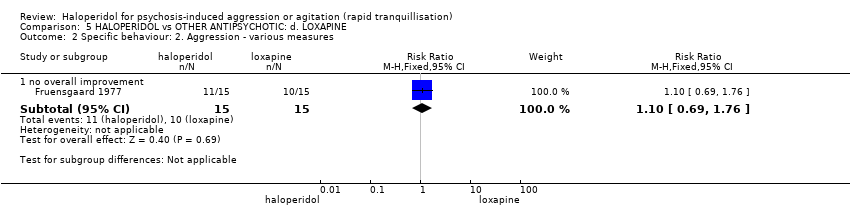
Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 2 Specific behaviour: 2. Aggression ‐ various measures.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 3 Global outcome: 1. General ‐ no change ‐ i. over 24 hours.
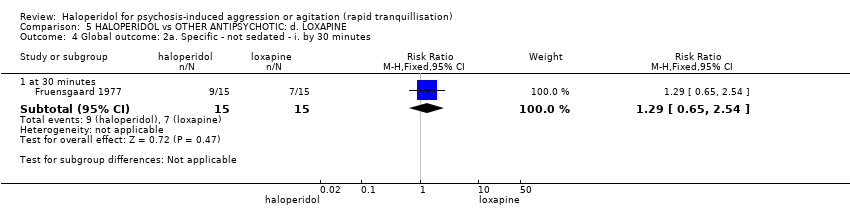
Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 4 Global outcome: 2a. Specific ‐ not sedated ‐ i. by 30 minutes.
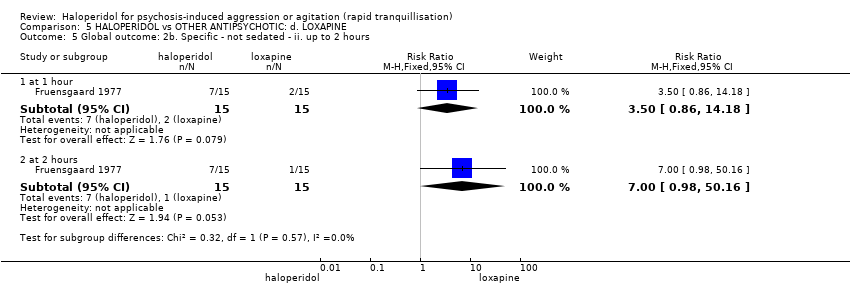
Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 5 Global outcome: 2b. Specific ‐ not sedated ‐ ii. up to 2 hours.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 6 Mental state: 1. Average scores.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 7 Adverse effects: 1. General.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 8 Adverse effects: 2a. Specific ‐ anticholinergic.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 9 Adverse effects: 2b. Specific ‐ arousal level.
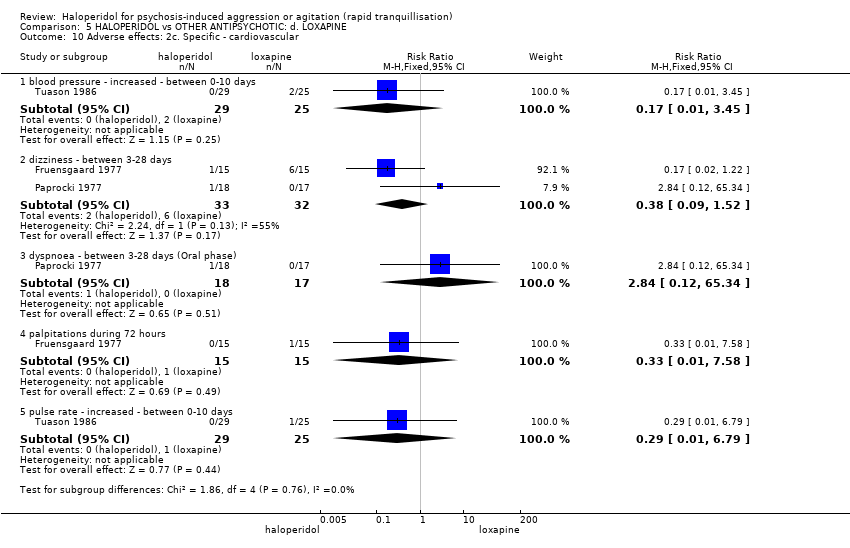
Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 10 Adverse effects: 2c. Specific ‐ cardiovascular.
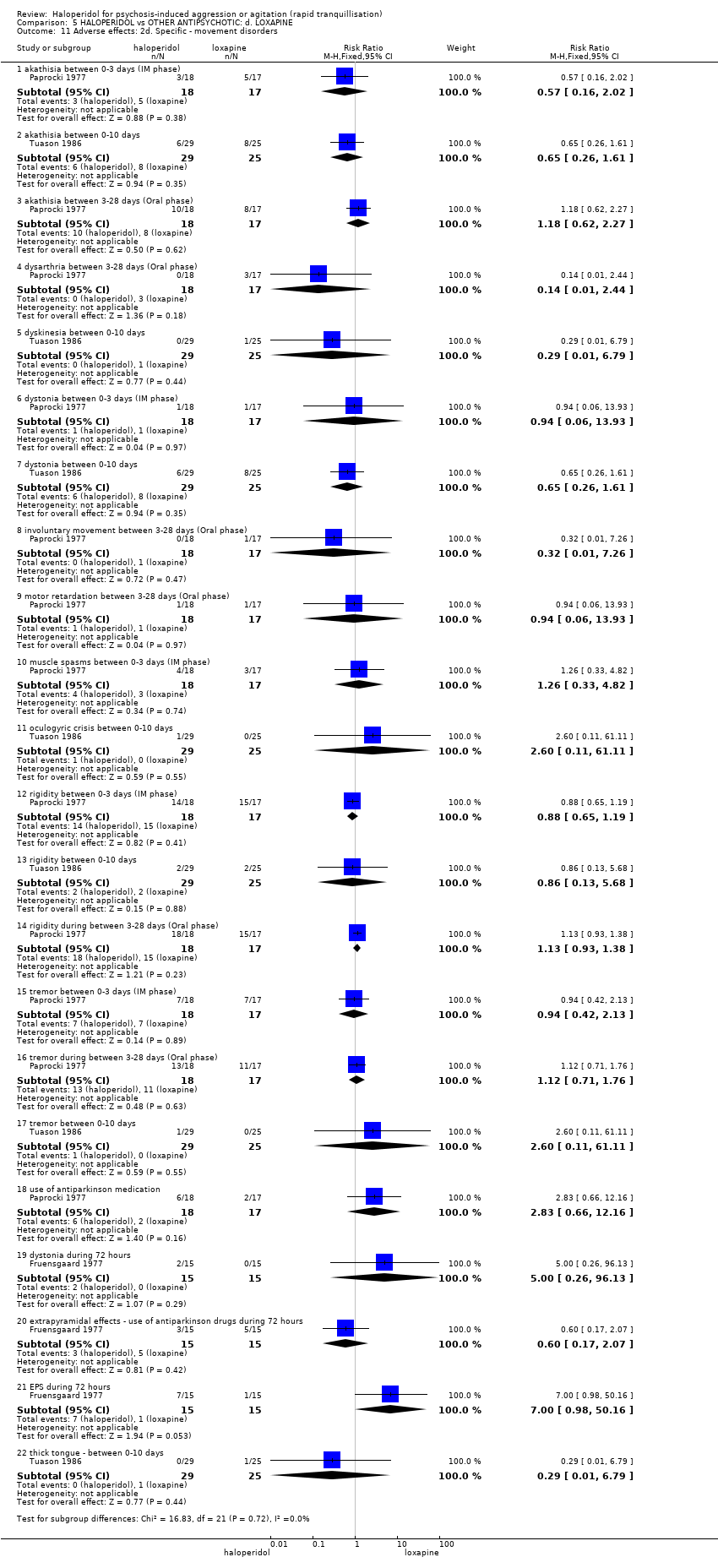
Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 11 Adverse effects: 2d. Specific ‐ movement disorders.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 12 Adverse effects: 2e. Specific ‐ miscellaneous.
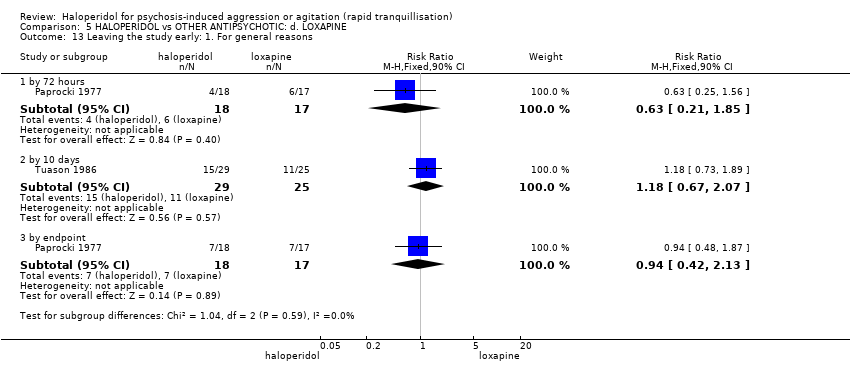
Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 13 Leaving the study early: 1. For general reasons.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 14 Leaving the study early: 2. Specific reasons.
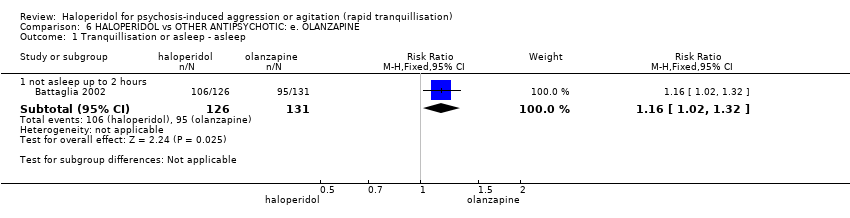
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 1 Tranquillisation or asleep ‐ asleep.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 2 Repeated need for tranquillisation ‐ needing additional injection.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 3 Specific behaviour: 1a. Agitation ‐ various measures.
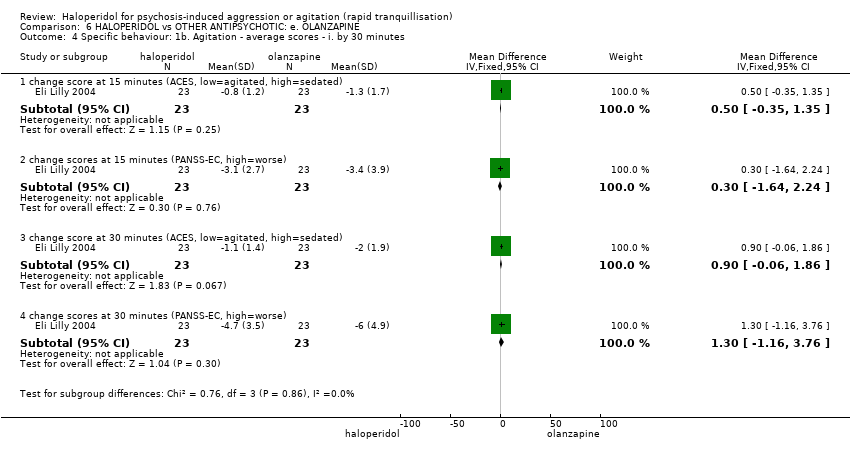
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. by 30 minutes.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 5 Specific behaviour: 1c. Agitation ‐ average scores ‐ ii. up to 2 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 6 Specific behaviour: 1d. Agitation ‐ average scores ‐ iii. up to 4 hours.
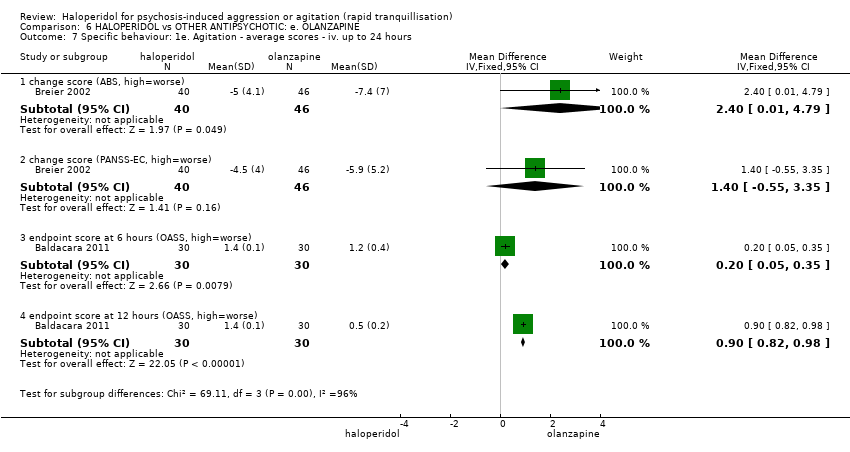
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 7 Specific behaviour: 1e. Agitation ‐ average scores ‐ iv. up to 24 hours.
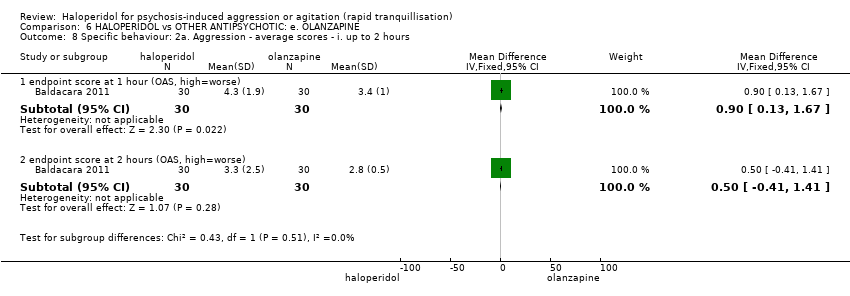
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 8 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 9 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 10 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours.
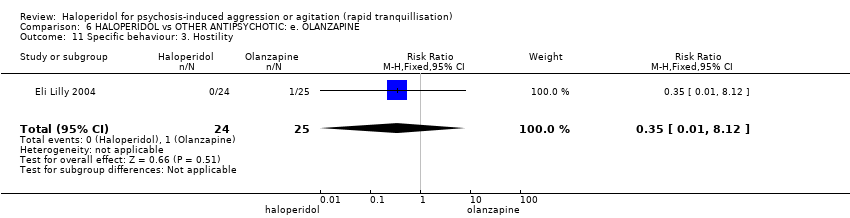
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 11 Specific behaviour: 3. Hostility.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 12 Global outcome: 1a. General ‐ need for additional measures.
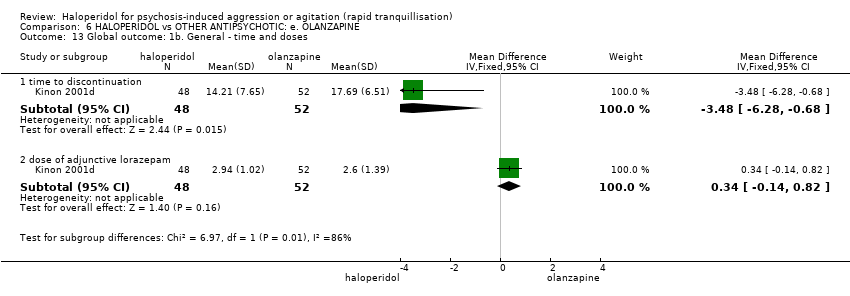
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 13 Global outcome: 1b. General ‐ time and doses.
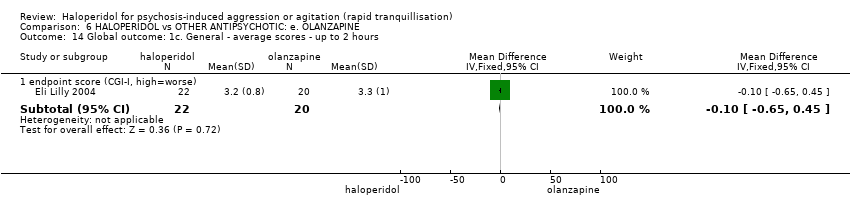
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 14 Global outcome: 1c. General ‐ average scores ‐ up to 2 hours.
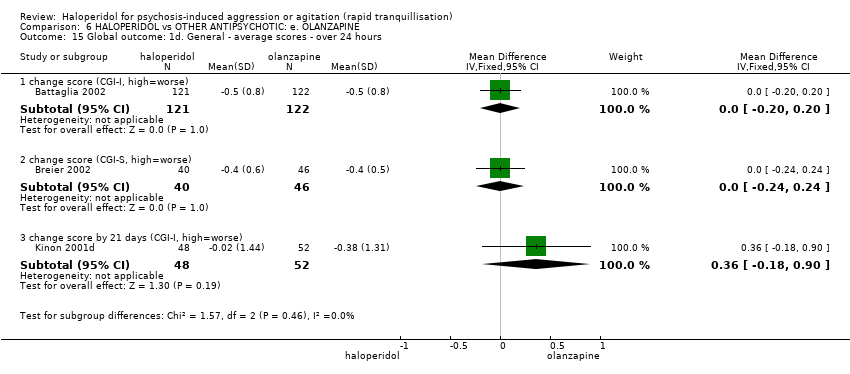
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 15 Global outcome: 1d. General ‐ average scores ‐ over 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 16 Global outcome: 2a. Specific ‐ alert ‐ i. up to 2 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 17 Global outcome: 2b. Specific ‐ alert ‐ ii. up to 4 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 18 Global outcome: 2c. Specific ‐ alert ‐ iii. up to 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 19 Global outcome: 2d. Specific ‐ tranquil ‐ i. up to 2 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 20 Global outcome: 2e. Specific ‐ tranquil ‐ ii. up to 4 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 21 Global outcome: 2f. Specific ‐ tranquil ‐ iii. up to 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 22 Global outcome: 2g. Specific ‐ sedated ‐ i. up to 2 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 23 Global outcome: 2h. Specific ‐ sedated ‐ ii. up to 4 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 24 Global outcome: 2i. Specific ‐ sedated ‐ iii. up to 24 hours.
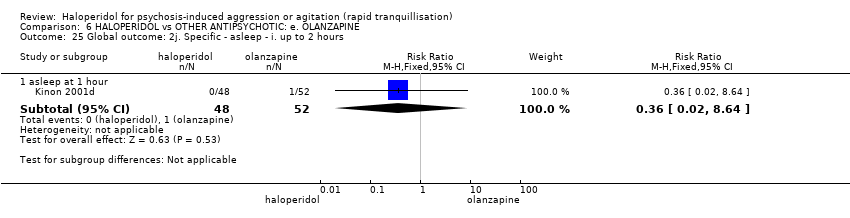
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 25 Global outcome: 2j. Specific ‐ asleep ‐ i. up to 2 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 26 Global outcome: 2k. Specific ‐ asleep ‐ ii. up to 4 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 27 Global outcome: 2l. Specific ‐ asleep ‐ iii. up to 24 hours.
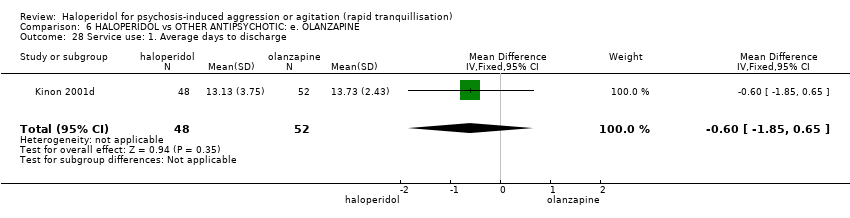
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 28 Service use: 1. Average days to discharge.
| Study | Intervention | Mean | SD | N |
| days 1‐7 | ||||
| Kinon 2001d | Haloperidol | 2.59 | 6.79 | 48 |
| Kinon 2001d | Olanzapine | 1.57 | 5.52 | 52 |
| days 8‐14 | ||||
| Kinon 2001d | Haloperidol | 0.92 | 4.05 | 48 |
| Kinon 2001d | Olanzapine | 0.33 | 2.23 | 52 |
| days 15‐21 | ||||
| Kinon 2001d | Haloperidol | 0.55 | 2.74 | 48 |
| Kinon 2001d | Olanzapine | 0 | 0 | 52 |
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 29 Service use: 2. Average patient‐hours used during 24 hours (skewed data).

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 30 Mental state: 1. Various outcomes reported as 'adverse events'.
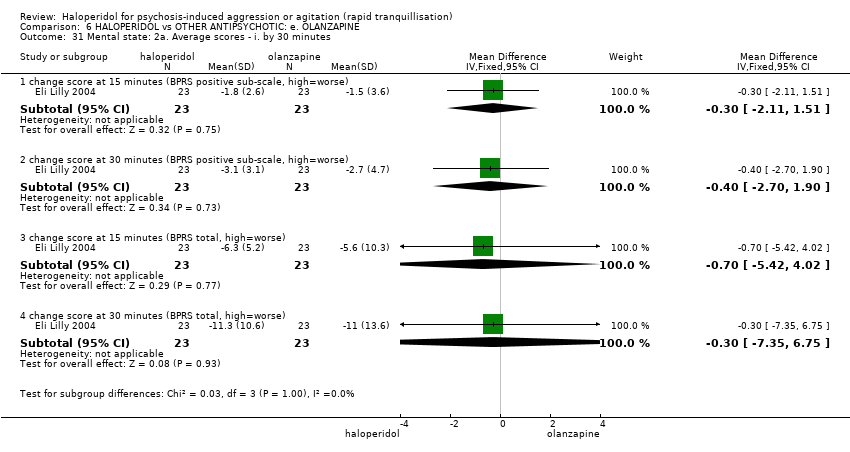
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 31 Mental state: 2a. Average scores ‐ i. by 30 minutes.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 32 Mental state: 2b. Average scores ‐ ii. up to 2 hours.
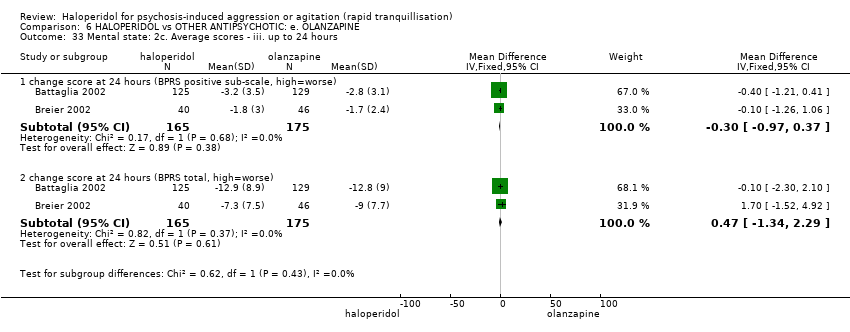
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 33 Mental state: 2c. Average scores ‐ iii. up to 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 34 Mental state: 2d. Average scores ‐ iv. over 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 35 Adverse effects: 1a. General.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 36 Adverse effects: 1b. General ‐ serious.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 37 Adverse effects: 2a. Specific ‐ anticholinergic.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 38 Adverse effects: 2b. Specific ‐ arousal level ‐ i. various.
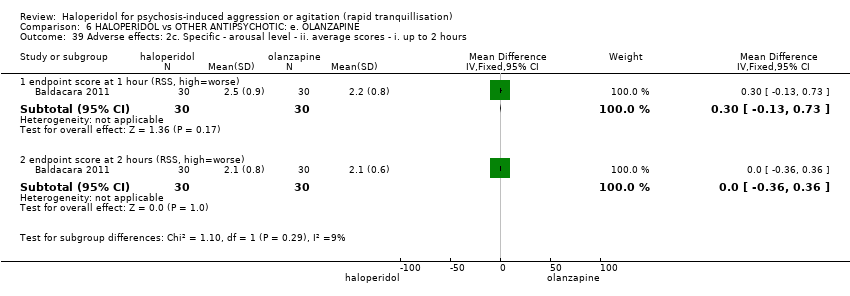
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 39 Adverse effects: 2c. Specific ‐ arousal level ‐ ii. average scores ‐ i. up to 2 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 40 Adverse effects: 2d. Specific ‐ arousal level ‐ ii. average scores ‐ ii. up to 4 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 41 Adverse effects: 2e. Specific ‐ arousal level ‐ ii. average scores ‐ iii. up to 24 hours.
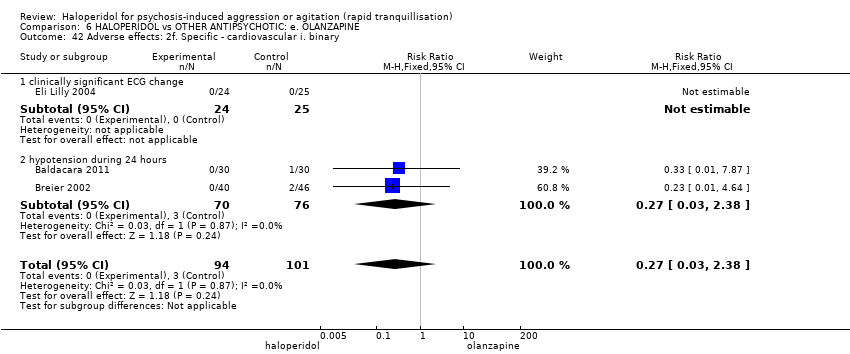
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 42 Adverse effects: 2f. Specific ‐ cardiovascular i. binary.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 43 Adverse effects: 2g. Specific ‐ cardiovascular ii. continuous.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 44 Adverse effects: 2h. Specific ‐ gastrointestinal.
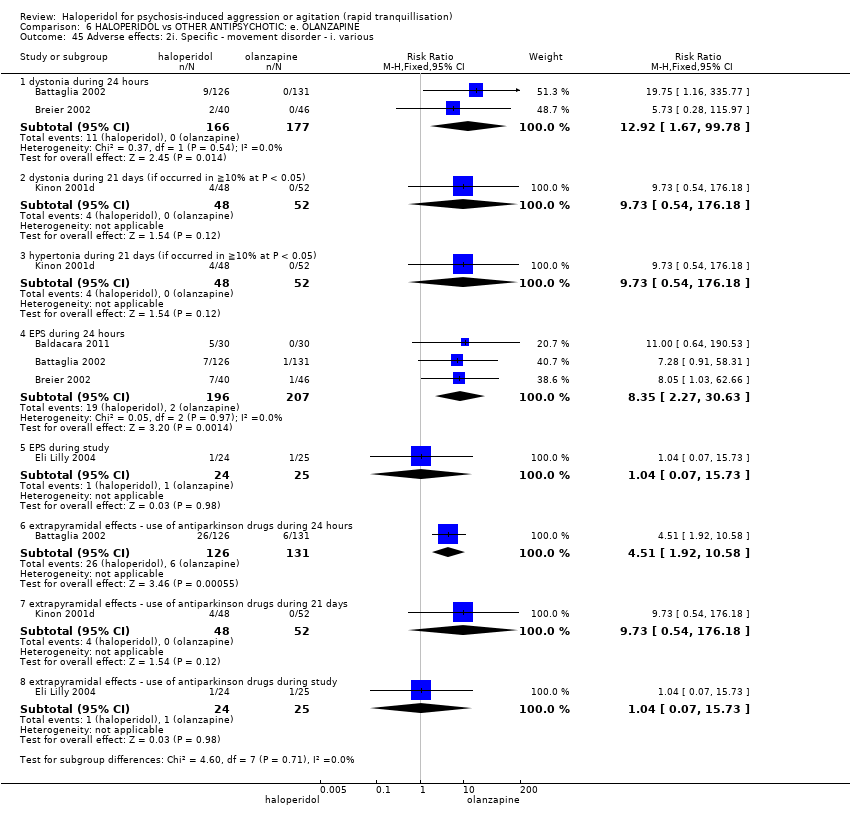
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 45 Adverse effects: 2i. Specific ‐ movement disorder ‐ i. various.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 46 Adverse effects: 2j. Specific ‐ movement disorder ‐ ii. average scores ‐ i. up to 24 hours.
| Study | Intervention | Mean | SD | N | Notes |
| endpoint score at 24 hours (SAS, high=worse) ‐ (skewed data) | |||||
| Eli Lilly 2004 | Haloperidol | 1.4 | 2.8 | 22 | |
| Eli Lilly 2004 | Olanzapine | 2.2 | 3.5 | 20 | |
| endpoint score at 24 hours (BAS, high=worse) ‐ (skewed data) | |||||
| Eli Lilly 2004 | Haloperidol | 1.5 | 1.7 | 22 | |
| Eli Lilly 2004 | Olanzapine | 1.7 | 2.6 | 20 | |
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 47 Adverse effects: 2k. Specific ‐ movement disorder ‐ iii. average scores ‐ i. up to 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 48 Adverse effects: 2l. Specific ‐ movement disorder ‐ vi. average scores ‐ i. over 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 49 Adverse effects: 2m. Specific ‐ miscellaneous.
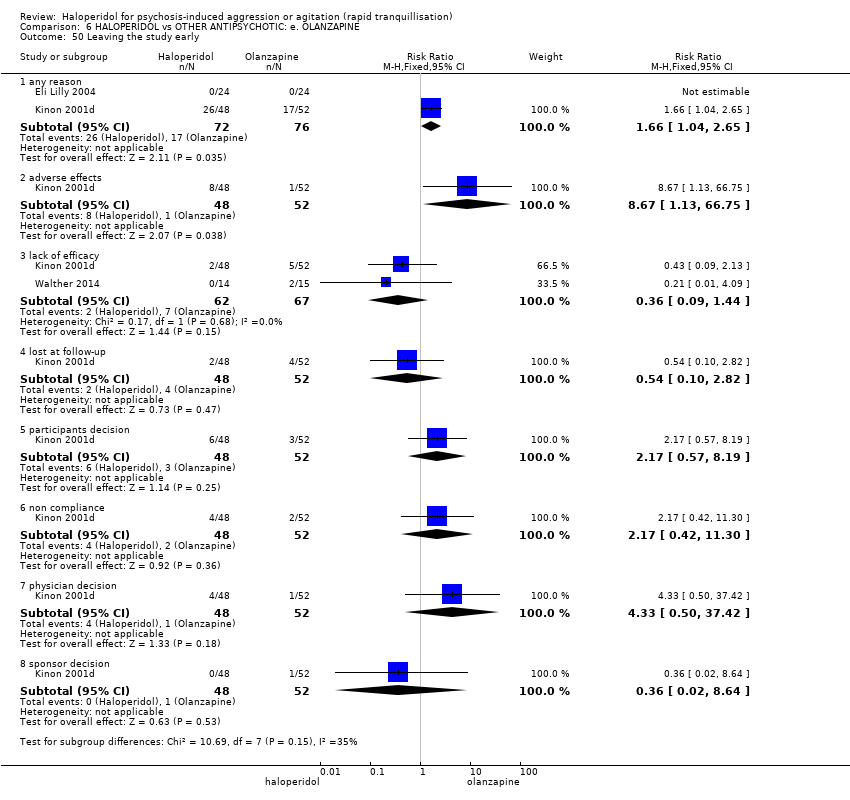
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 50 Leaving the study early.

Comparison 7 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: f. PERPHENAZINE, Outcome 1 Global outcome: No improvement.
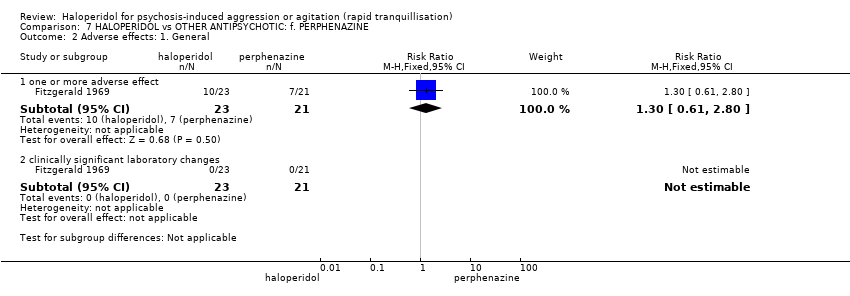
Comparison 7 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: f. PERPHENAZINE, Outcome 2 Adverse effects: 1. General.
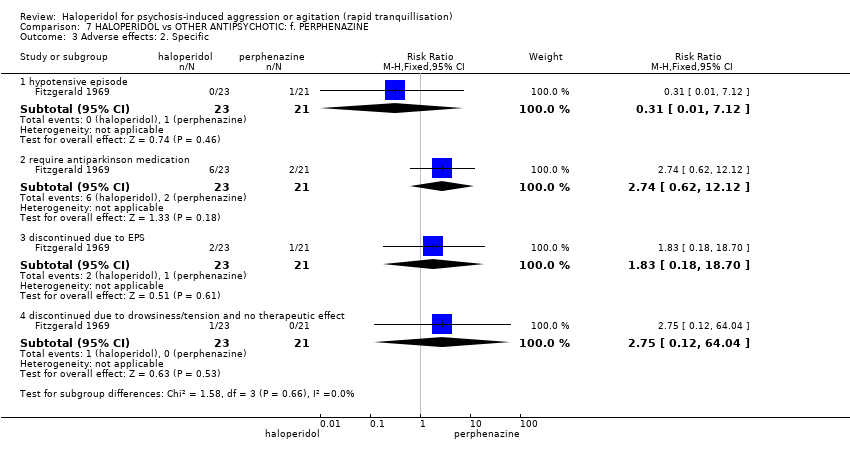
Comparison 7 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: f. PERPHENAZINE, Outcome 3 Adverse effects: 2. Specific.

Comparison 7 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: f. PERPHENAZINE, Outcome 4 Leaving the study early: 1. Specific reasons.

Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours.
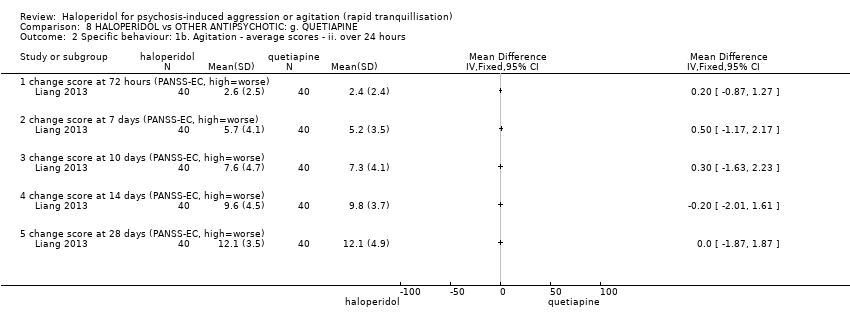
Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours.

Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 3 Mental state: Average scores ‐ i. over 24 hours.

Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 4 Adverse effects: 1. General.

Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 5 Adverse effects: 2. Specific ‐ movement disorder ‐ i. average scores.
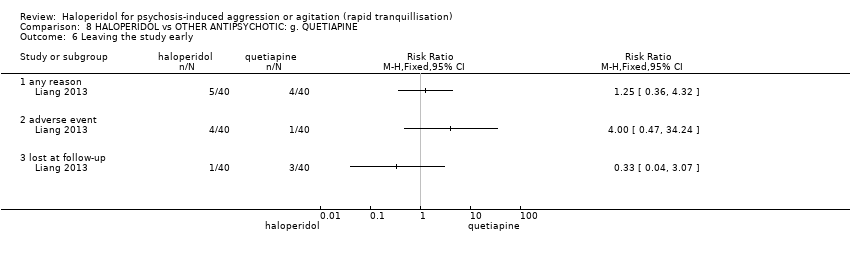
Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 6 Leaving the study early.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 1 Tranquillisation or asleep ‐ i. by 30 minutes.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 2 Tranquillisation or asleep ‐ ii. up to 2 hours.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 3 Specific behaviour: 1a. Agitation ‐ various measures.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 5 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. by 30 minutes.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 6 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 2 hours.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 7 Global outcome: 1. Binary measures.
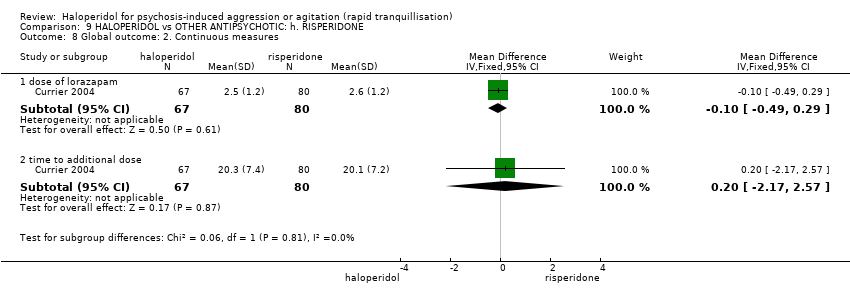
Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 8 Global outcome: 2. Continuous measures.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 9 Adverse effects: 1a. General ‐ one or more adverse effects.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 10 Adverse effects: 2a. Specific ‐ arousal.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 11 Adverse effects: 2b. Specific ‐ cardiovascular ‐ i. binary outcomes.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 12 Adverse effects: 2c. Specific ‐ cardiovascular ‐ ii. pulse rate ‐ i. up to 2 hours.
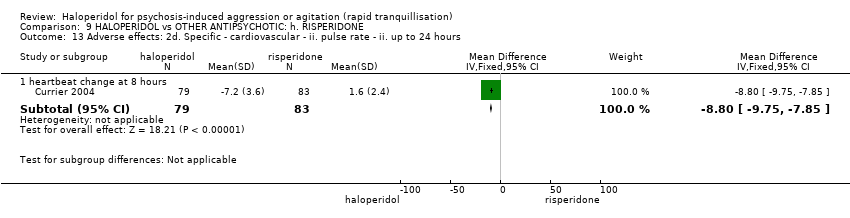
Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 13 Adverse effects: 2d. Specific ‐ cardiovascular ‐ ii. pulse rate ‐ ii. up to 24 hours.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 14 Adverse effects: 2e. Specific ‐ movement disorder ‐ i. binary outcomes.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 15 Adverse effects: 2f. Specific ‐ movement disorders ‐ ii. average scores ‐ i. up to 24 hours.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 16 Adverse effects: 2g. Specific ‐ movement disorder ‐ ii. average scores ‐ ii. over 24 hours.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 17 Adverse effects: 2h. Specific ‐ miscellaneous.
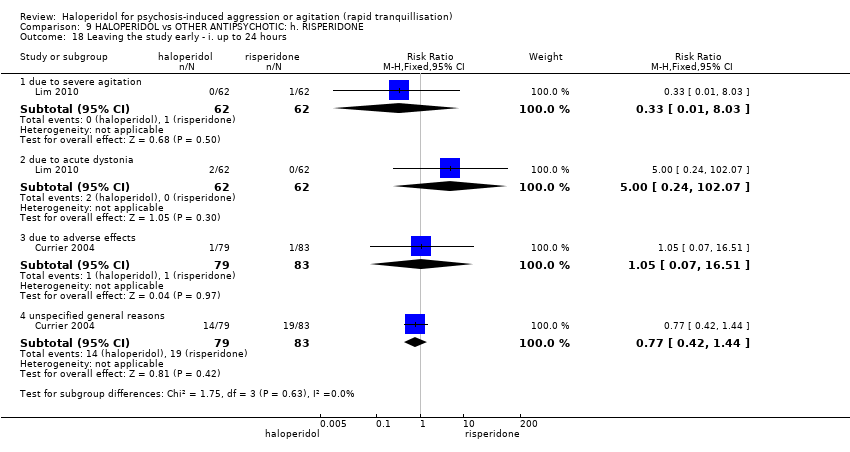
Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 18 Leaving the study early ‐ i. up to 24 hours.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 19 Leaving the study early ‐ ii. over 24 hours.

Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 1 Repeated need for rapid tranquillisation.

Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 2 Specific behaviour: 1. Agitation ‐ rated as 'adverse effect'.
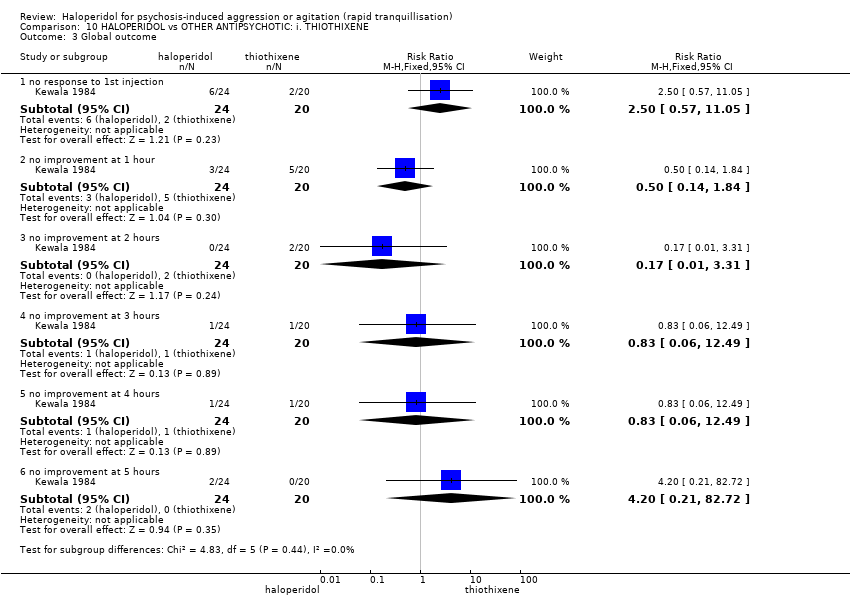
Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 3 Global outcome.

Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 4 Adverse effects: 1. General ‐ one or more adverse effects.
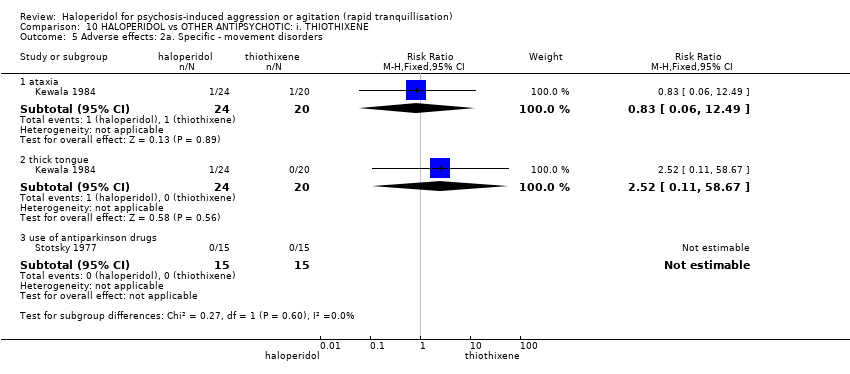
Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 5 Adverse effects: 2a. Specific ‐ movement disorders.
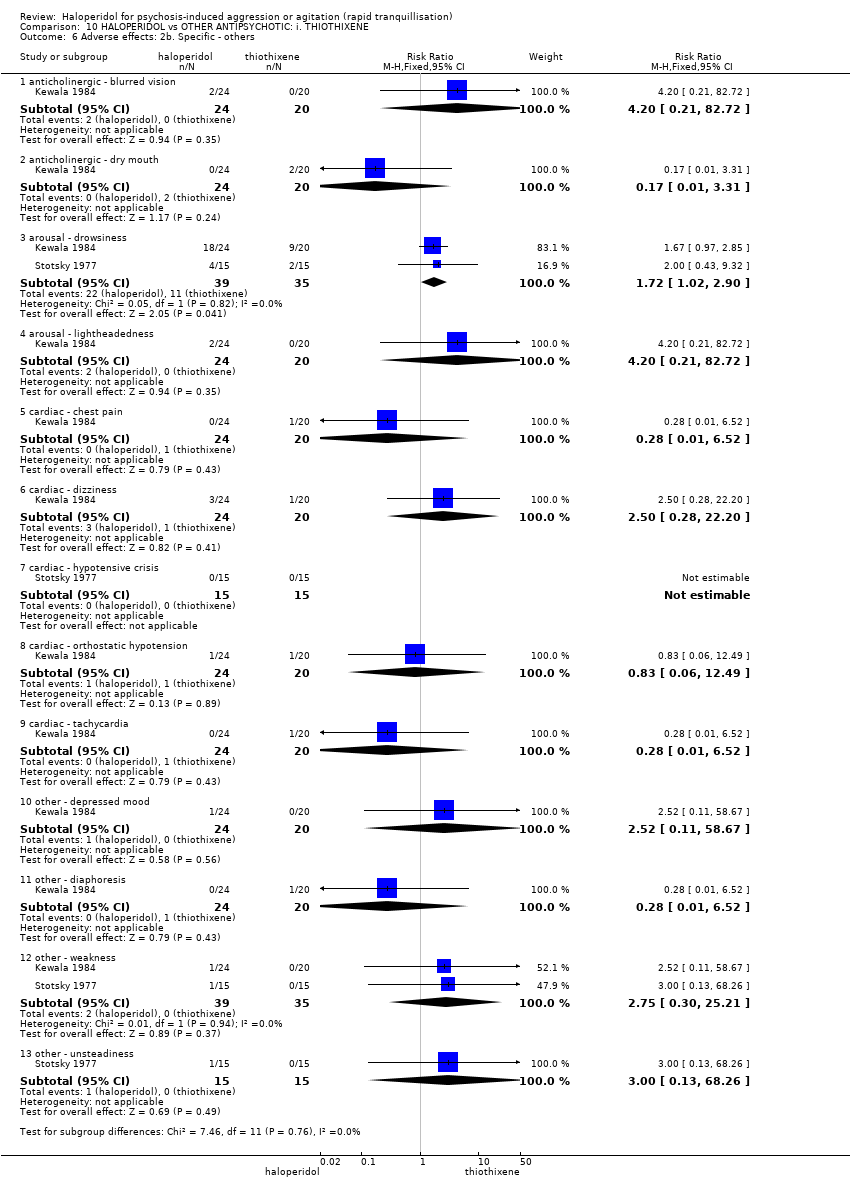
Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 6 Adverse effects: 2b. Specific ‐ others.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 1 Repeated need for tranquillisation ‐ need for additional drugs for tranquillisation.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours.
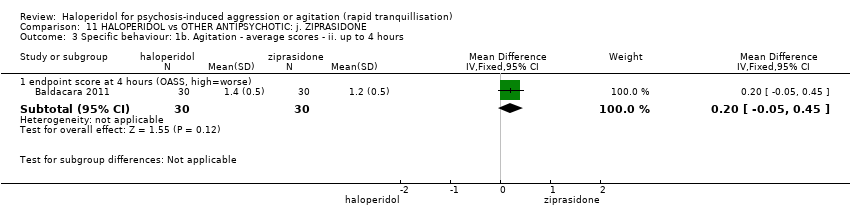
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 5 Specific behaviour: 1d. Agitation ‐ average scores ‐ iv. over 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 6 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours.
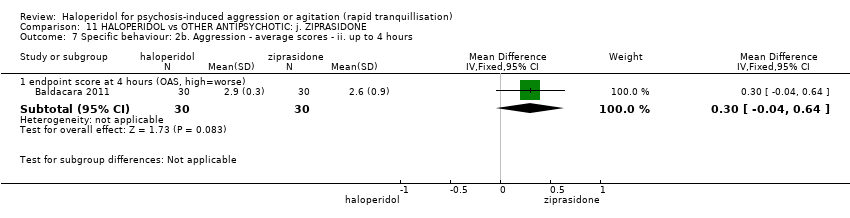
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 7 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours.
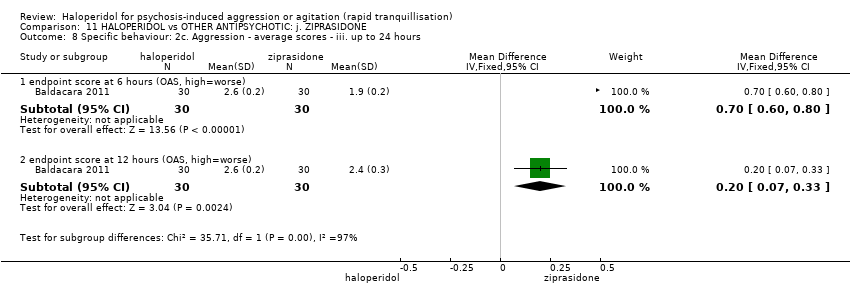
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 8 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 9 Global outcome: 1. General ‐ need for additional measures.
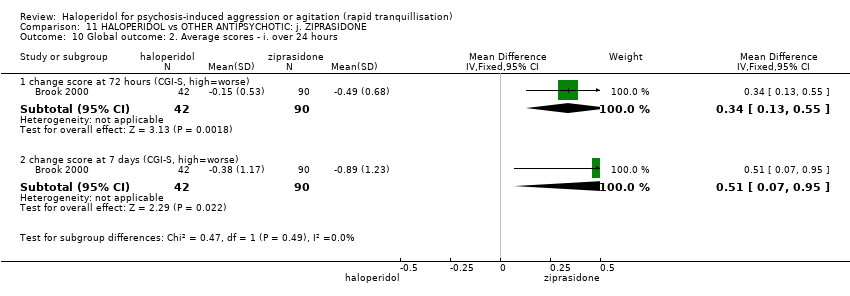
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 10 Global outcome: 2. Average scores ‐ i. over 24 hours.
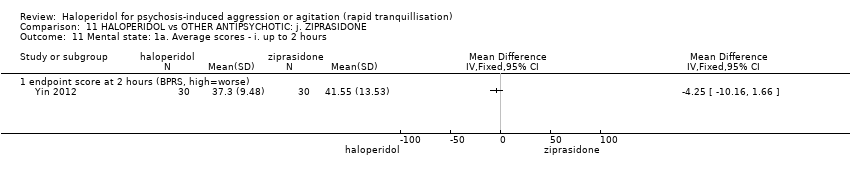
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 11 Mental state: 1a. Average scores ‐ i. up to 2 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 12 Mental state: 1b. Average scores ‐ ii. up to 4 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 13 Mental state: 1c. Average scores ‐ iii. up to 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 14 Mental state: 1d. Average scores ‐ iv. over 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 15 Adverse effects: 1a. General ‐ i. up to 24 hours.
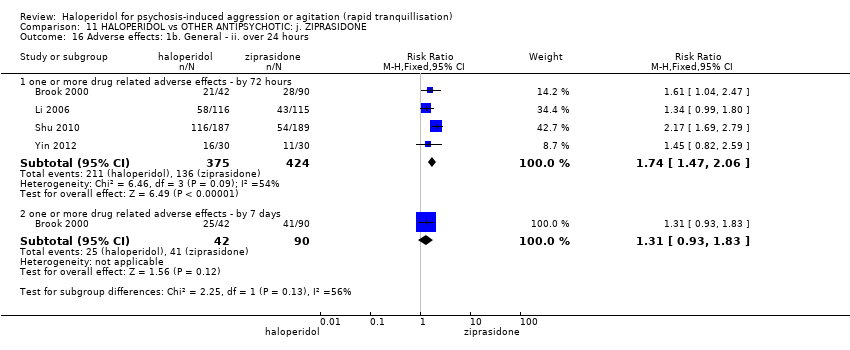
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 16 Adverse effects: 1b. General ‐ ii. over 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 17 Adverse effects: 1c. General ‐ serious.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 18 Adverse effects: 2a. Specific ‐ anticholinergic.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 19 Adverse effects: 2b. Specific ‐ arousal level ‐ i. various.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 20 Adverse effects: 2c. Specific ‐ arousal level ‐ ii. average scores ‐ i. up to 2 hours.
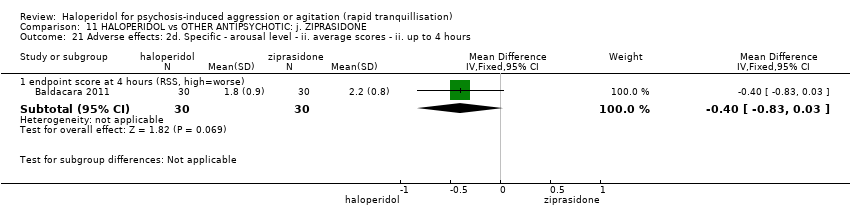
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 21 Adverse effects: 2d. Specific ‐ arousal level ‐ ii. average scores ‐ ii. up to 4 hours.
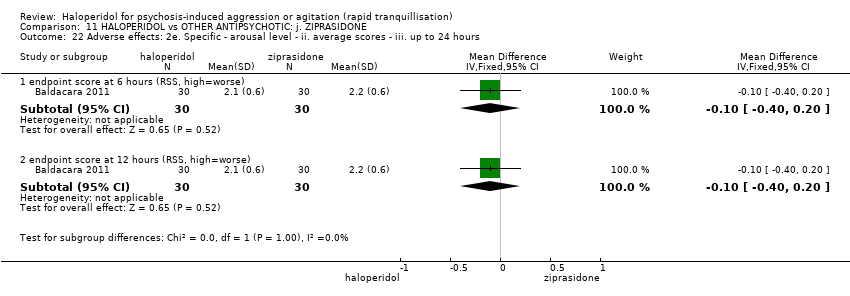
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 22 Adverse effects: 2e. Specific ‐ arousal level ‐ ii. average scores ‐ iii. up to 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 23 Adverse effects: 2f. Specific ‐ cardiovascular.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 24 Adverse effects: 2g. Specific ‐ gastrointestinal.
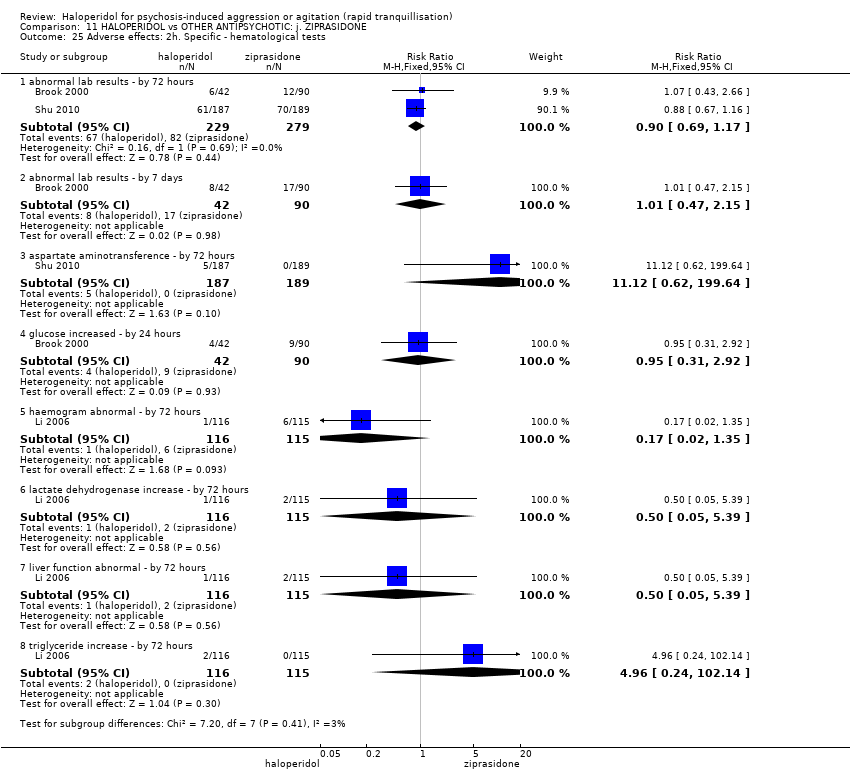
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 25 Adverse effects: 2h. Specific ‐ hematological tests.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 26 Adverse effects: 2i. Specific ‐ movement disorders ‐ i. binary measures.
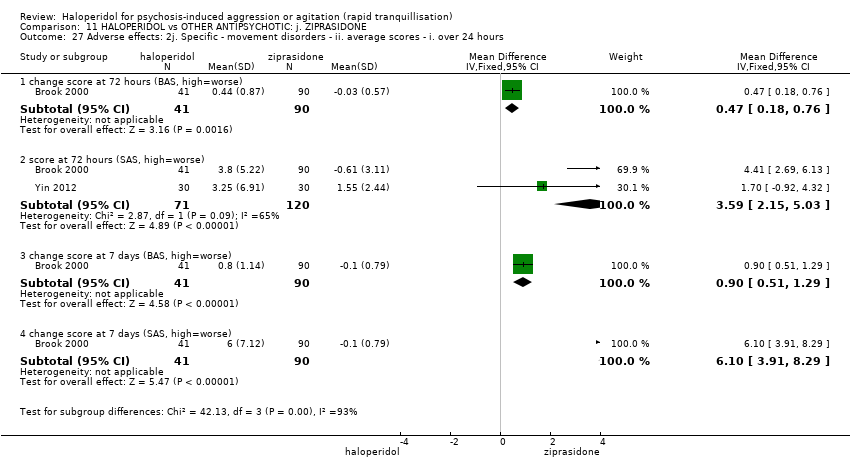
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 27 Adverse effects: 2j. Specific ‐ movement disorders ‐ ii. average scores ‐ i. over 24 hours.
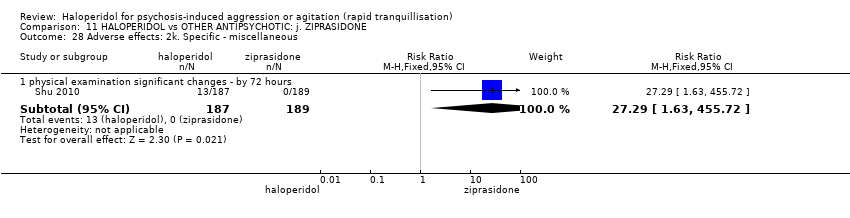
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 28 Adverse effects: 2k. Specific ‐ miscellaneous.
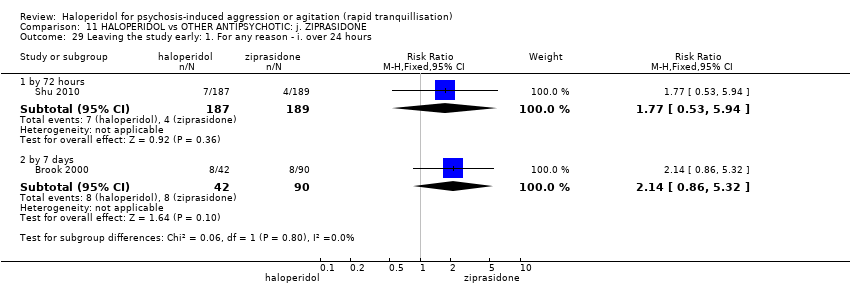
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 29 Leaving the study early: 1. For any reason ‐ i. over 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 30 Leaving the study early: 2. For specific reasons ‐ i. over 24 hours.

Comparison 12 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE, Outcome 1 Repeated need for tranquillisation: more than 3 injections.

Comparison 12 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE, Outcome 2 Adverse effects: 1a. Specific ‐ movement disorders.
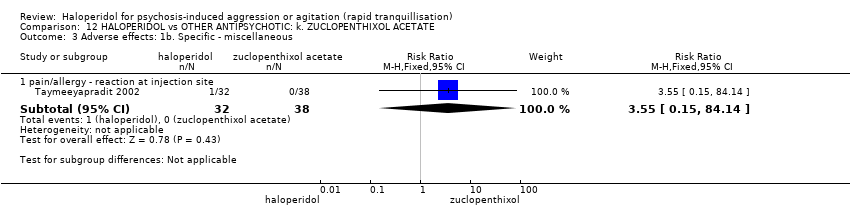
Comparison 12 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE, Outcome 3 Adverse effects: 1b. Specific ‐ miscellaneous.

Comparison 12 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE, Outcome 4 Leaving the study early.

Comparison 13 HALOPERIDOL vs BENZODIAZEPINE: a. FLUNITRAZEPAM, Outcome 1 Specific behaviour: 1. Aggression ‐ binary measures ‐ i. up to 2 hours.
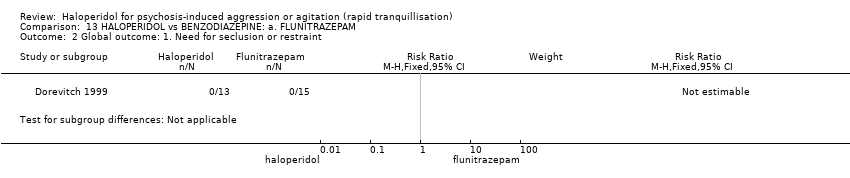
Comparison 13 HALOPERIDOL vs BENZODIAZEPINE: a. FLUNITRAZEPAM, Outcome 2 Global outcome: 1. Need for seclusion or restraint.

Comparison 13 HALOPERIDOL vs BENZODIAZEPINE: a. FLUNITRAZEPAM, Outcome 3 Adverse effects: 1. Specific ‐ movement disorders ‐ i. binary measures ‐ i. by 30 minutes.
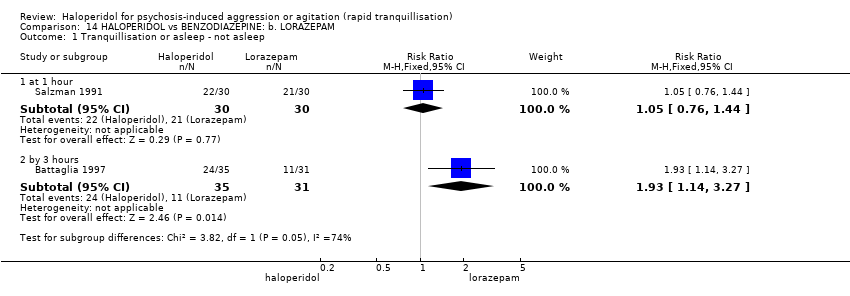
Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 1 Tranquillisation or asleep ‐ not asleep.
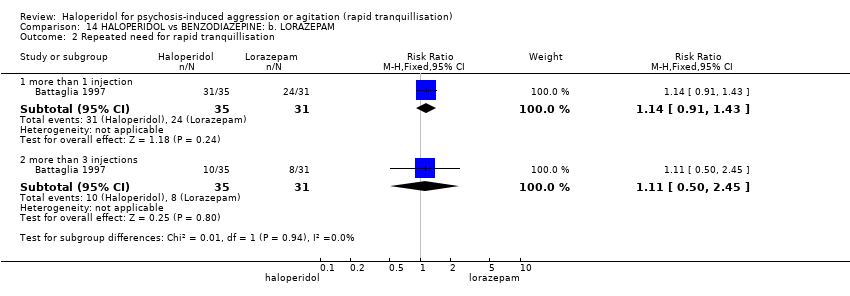
Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 2 Repeated need for rapid tranquillisation.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 3 Global outcome: 1. No overall improvement.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 4 Mental state: 1a. Average scores ‐ i. up to 2 hours.
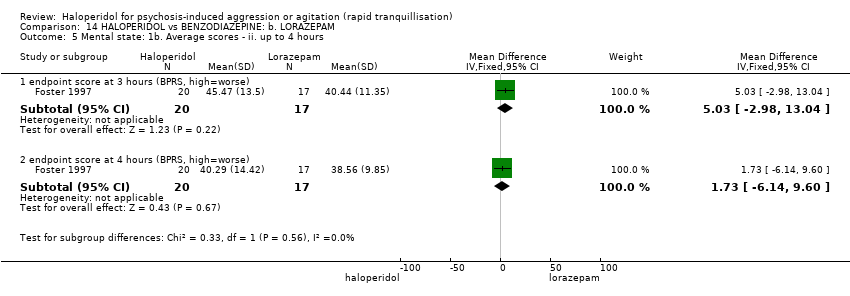
Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 5 Mental state: 1b. Average scores ‐ ii. up to 4 hours.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 6 Adverse effects: 1a. General.
Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 7 Adverse effects: 1b. General ‐ serious.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 8 Adverse effects: 2a. Specific ‐ anticholinergic.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 9 Adverse effects: 2b. Specific ‐ arousal.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 10 Adverse effects: 2c. Specific ‐ cardiovascular.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 11 Adverse effects: 2d. Specific ‐ movement disorder.
| Study | Intervention | Average | SD | N | Notes |
| average time to sedation | |||||
| Nobay 2004 | Haloperidol | 28.3 | 25 | 42 | |
| Nobay 2004 | Midazolam | 18.3 | 14 | 42 | |
| average time to arousal | |||||
| Nobay 2004 | Haloperidol | 126.6 | 85 | 42 | |
| Nobay 2004 | Midazolam | 81.9 | 66 | 42 | |
Comparison 15 HALOPERIDOL vs BENZODIAZEPINE: c. MIDAZOLAM, Outcome 1 Tranquillisation or asleep: average times in minutes ‐ (skewed data).

Comparison 15 HALOPERIDOL vs BENZODIAZEPINE: c. MIDAZOLAM, Outcome 2 Global outcome: 1. Need for rescue drug.

Comparison 15 HALOPERIDOL vs BENZODIAZEPINE: c. MIDAZOLAM, Outcome 3 Adverse effects: 1. General ‐ one or more drug related adverse effect.

Comparison 15 HALOPERIDOL vs BENZODIAZEPINE: c. MIDAZOLAM, Outcome 4 Adverse effects: 2. Specific.

Comparison 16 HALOPERIDOL vs COMBINATIONS: a. HALOPERIDOL + LEVOMEPROMAZINE, Outcome 1 Global outcome: 1. No overall improvement.
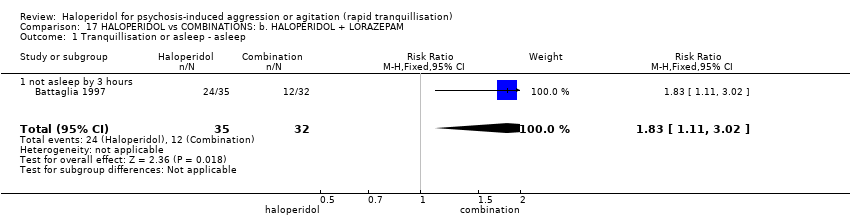
Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 1 Tranquillisation or asleep ‐ asleep.

Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 2 Repeated need for rapid tranquillisation.
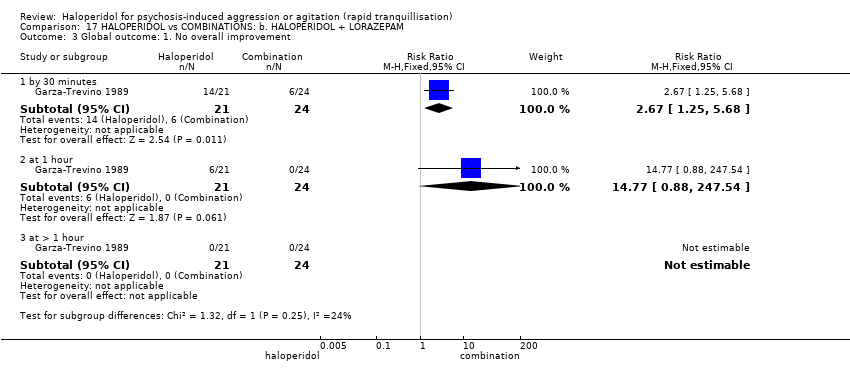
Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 3 Global outcome: 1. No overall improvement.
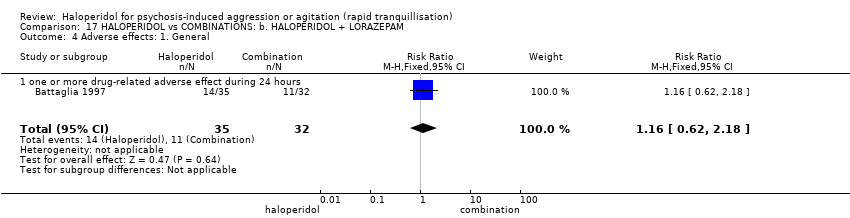
Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 4 Adverse effects: 1. General.
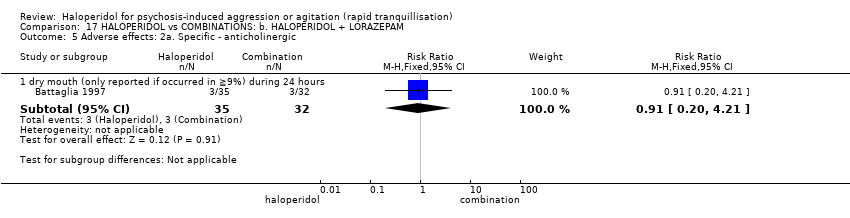
Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 5 Adverse effects: 2a. Specific ‐ anticholinergic.

Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 6 Adverse effects: 2b. Specific ‐ cardiovascular.

Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 7 Adverse effects: 2c. Specific ‐ movement disorders.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 1 Repeated need for tranquillisation.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours.
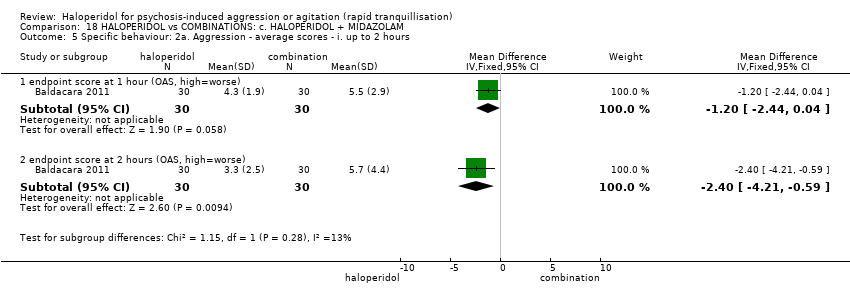
Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 5 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 6 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours.
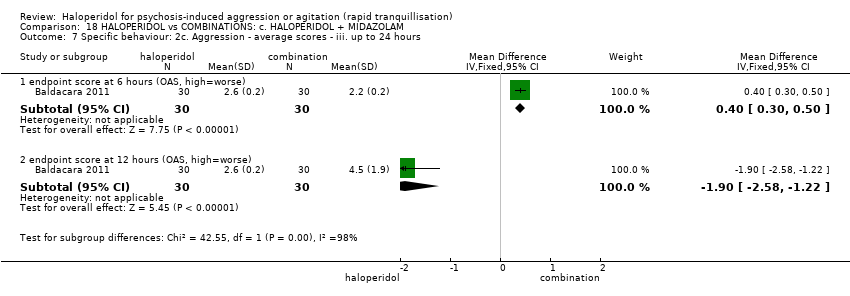
Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 7 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours.
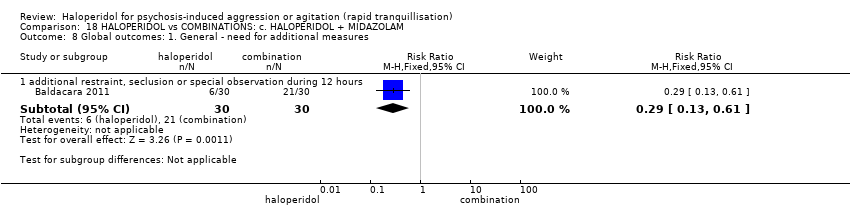
Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 8 Global outcomes: 1. General ‐ need for additional measures.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 9 Adverse effects: 1b. General ‐ one or more adverse events.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 10 Adverse effects: 1a. General ‐ serious.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 11 Adverse effects: 2a. Specific ‐ arousal ‐ i. various.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 12 Adverse effects: 2b. Specific ‐ arousal ‐ ii. average scores ‐ i. up to 2 hours.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 13 Adverse effects: 2c. Specific ‐ arousal ‐ ii. average scores ‐ ii. up to 4 hours.
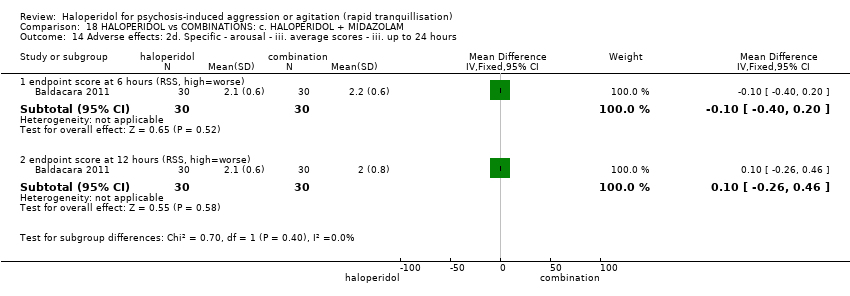
Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 14 Adverse effects: 2d. Specific ‐ arousal ‐ iii. average scores ‐ iii. up to 24 hours.
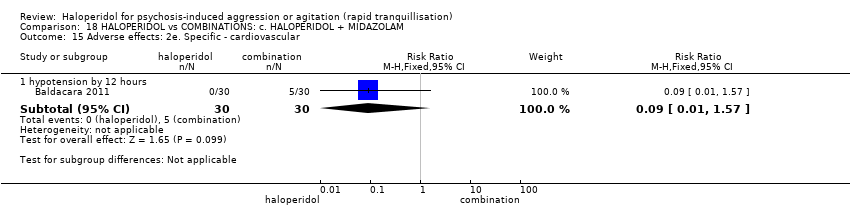
Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 15 Adverse effects: 2e. Specific ‐ cardiovascular.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 16 Adverse effects: 2f. Specific ‐ movement disorders.
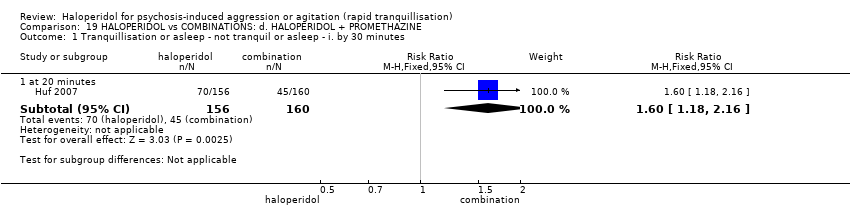
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 1 Tranquillisation or asleep ‐ not tranquil or asleep ‐ i. by 30 minutes.
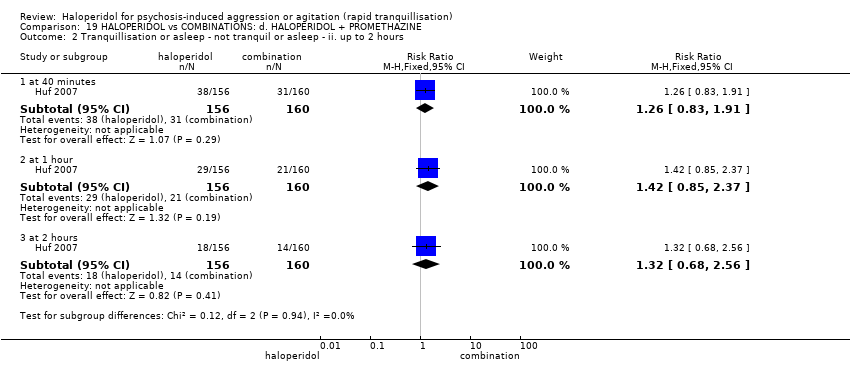
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 2 Tranquillisation or asleep ‐ not tranquil or asleep ‐ ii. up to 2 hours.
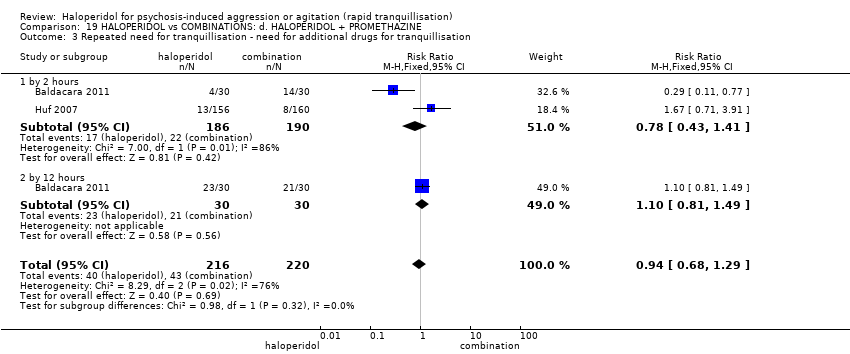
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 3 Repeated need for tranquillisation ‐ need for additional drugs for tranquillisation.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 4 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 5 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours.
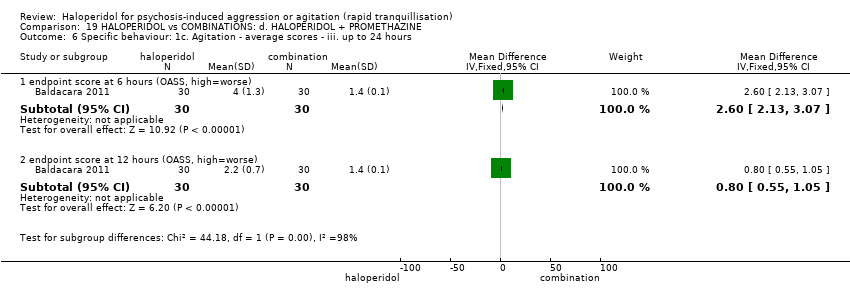
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 6 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 7 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours.
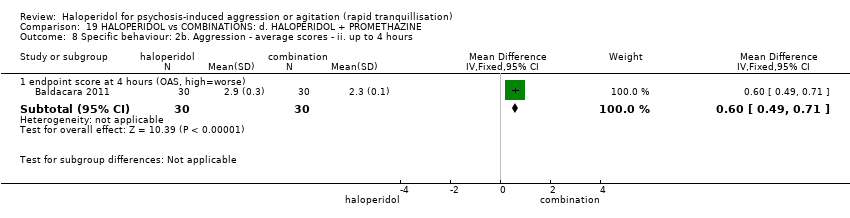
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 8 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 9 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 10 Specific behaviour: 2d. Aggression ‐ further aggressive episode ‐ i. up to 24 hours.
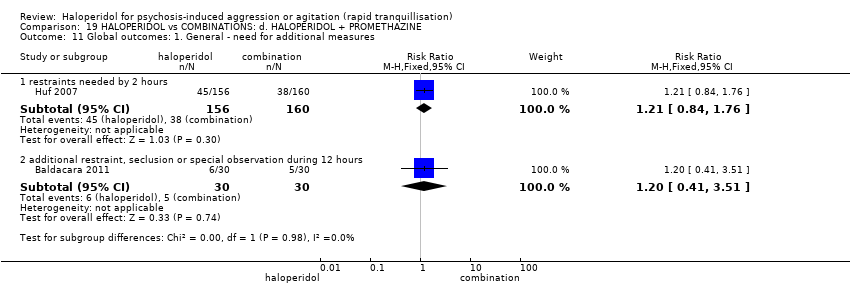
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 11 Global outcomes: 1. General ‐ need for additional measures.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 12 Global outcomes: 2. Specific.
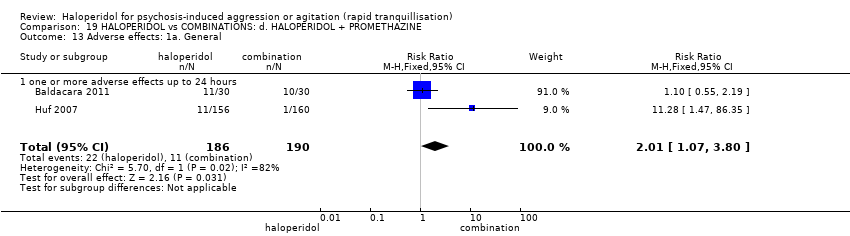
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 13 Adverse effects: 1a. General.
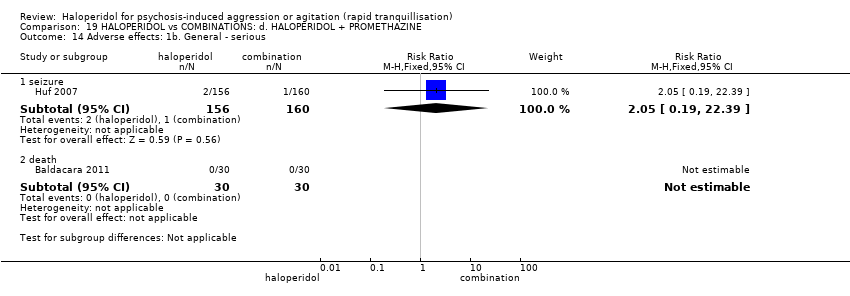
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 14 Adverse effects: 1b. General ‐ serious.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 15 Adverse effects: 2a. Specific ‐ arousal ‐ i. various.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 16 Adverse effects: 2b. Specific ‐ arousal ‐ ii. average scores ‐ i. up to 2 hours.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 17 Adverse effects: 2c. Specific ‐ arousal ‐ ii. average scores ‐ ii. up to 4 hours.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 18 Adverse effects: 2d. Specific ‐ arousal ‐ ii. average scores ‐ iii. up to 24 hours.
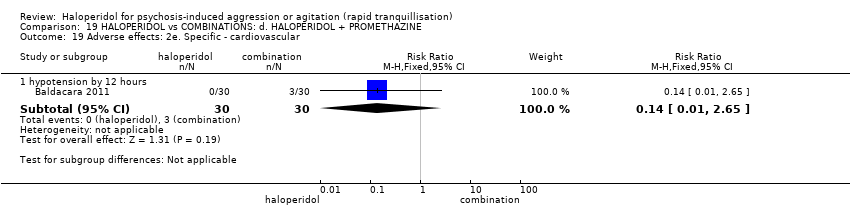
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 19 Adverse effects: 2e. Specific ‐ cardiovascular.
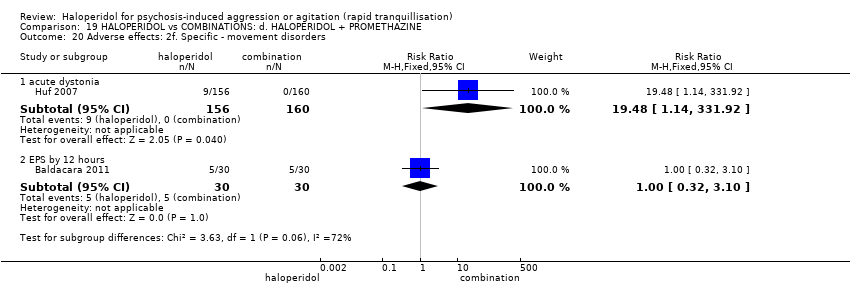
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 20 Adverse effects: 2f. Specific ‐ movement disorders.
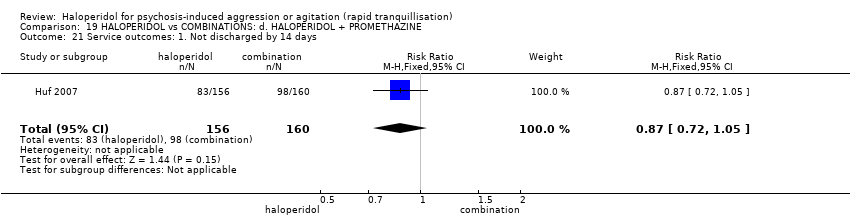
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 21 Service outcomes: 1. Not discharged by 14 days.
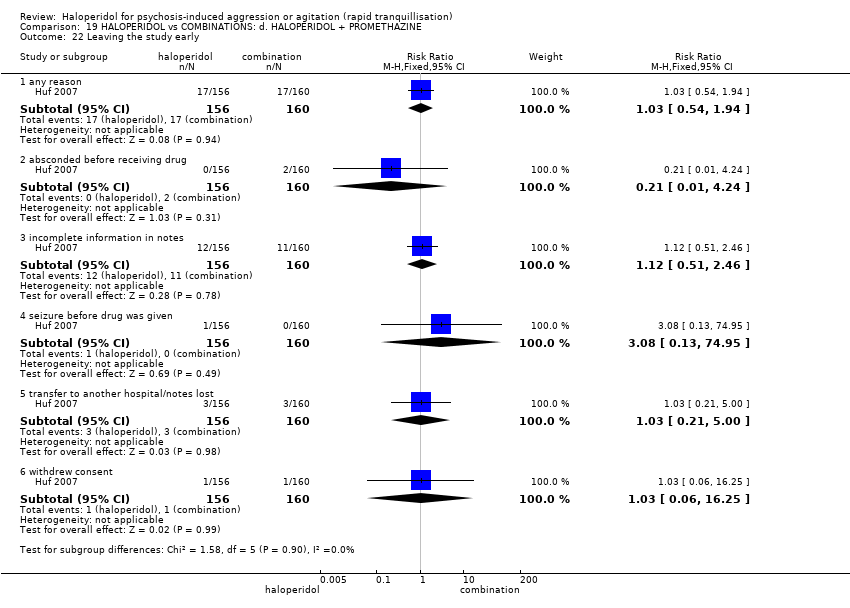
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 22 Leaving the study early.

Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours.
Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours.

Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 3 Global outcome: No improvement.

Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 4 Adverse effects: 1a. Specific ‐ arousal level ‐ i. various.

Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 5 Adverse effects: 1b. Specific ‐ movement disorders ‐ i. various.

Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 6 Adverse effects: 1c. Specific ‐ miscellaneous.

Comparison 21 HALOPERIDOL vs COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE, Outcome 1 Specific behaviour: 1. Agitation ‐ average scores ‐ over 24 hours.

Comparison 21 HALOPERIDOL vs COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE, Outcome 2 Global outcome: No improvement.
Comparison 21 HALOPERIDOL vs COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE, Outcome 3 Mental state: Average scores ‐ i. over 24 hours.
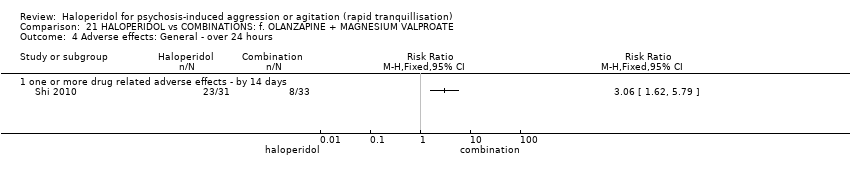
Comparison 21 HALOPERIDOL vs COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE, Outcome 4 Adverse effects: General ‐ over 24 hours.

Comparison 22 HALOPERIDOL vs COMBINATIONS: g. QUETIAPINE + MAGNESIUM VALPROATE, Outcome 1 Specific behaviour: 1a. Agitation ‐ binary measures.

Comparison 22 HALOPERIDOL vs COMBINATIONS: g. QUETIAPINE + MAGNESIUM VALPROATE, Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. over 24 hours.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 1 Tranquillisation or asleep.
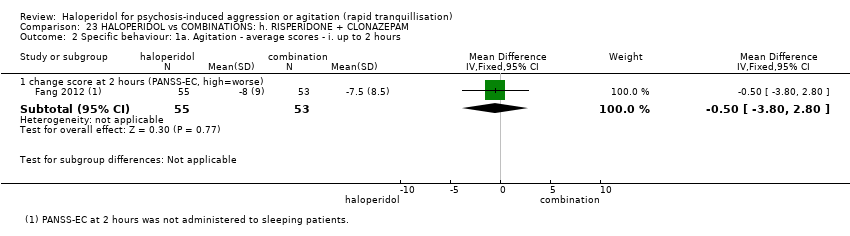
Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours.
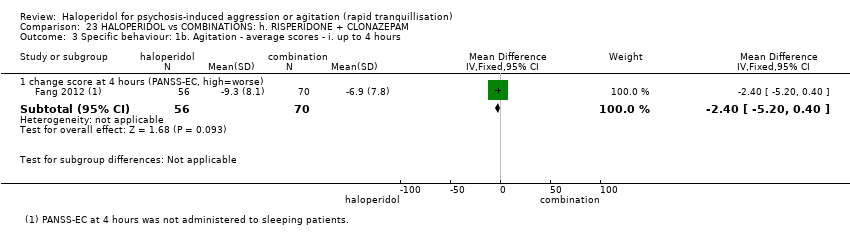
Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 4 hours.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours.
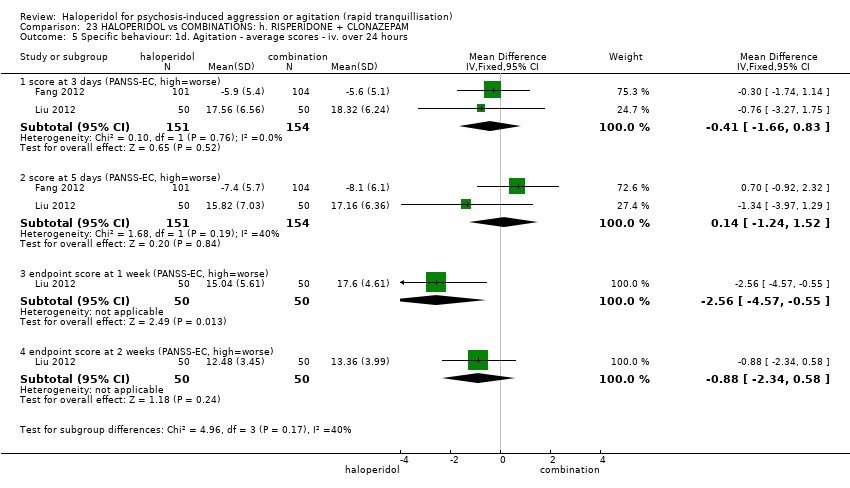
Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 5 Specific behaviour: 1d. Agitation ‐ average scores ‐ iv. over 24 hours.
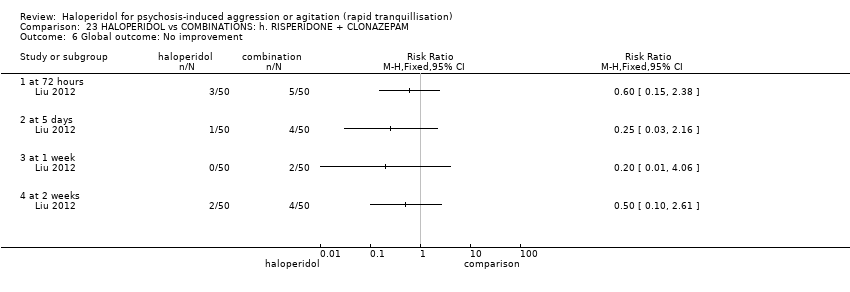
Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 6 Global outcome: No improvement.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 7 Mental state: 1. Average scores ‐ i. over 24 hours.
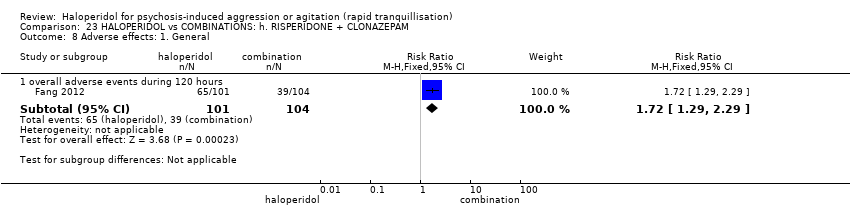
Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 8 Adverse effects: 1. General.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 9 Adverse effects: 2a. Specific ‐ arousal ‐ i. various.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 10 Adverse effects: 2b. Specific ‐ cardiovascular ‐ i. various.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 11 Adverse effects: 2c. Specific ‐ movement disorders ‐ i. various.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 12 Adverse effects: 2d. Specific ‐ miscellaneous.
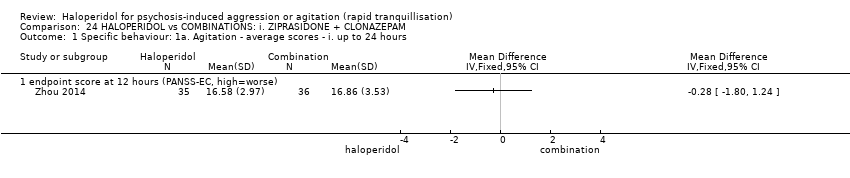
Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours.
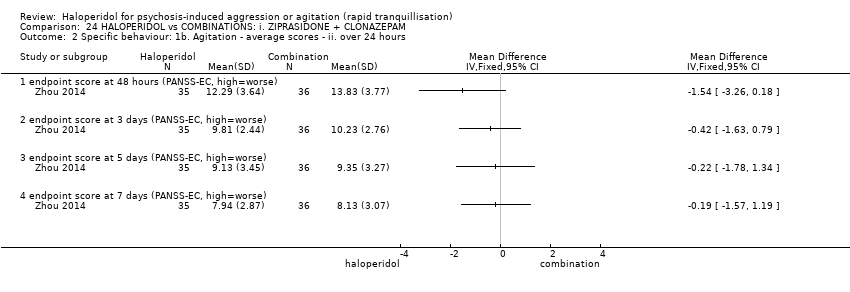
Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours.

Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 3 Global outcome: 1. Average scores ‐ i. over 24 hours.

Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 4 Mental state: 1. Average scores ‐ i. over 24 hours.
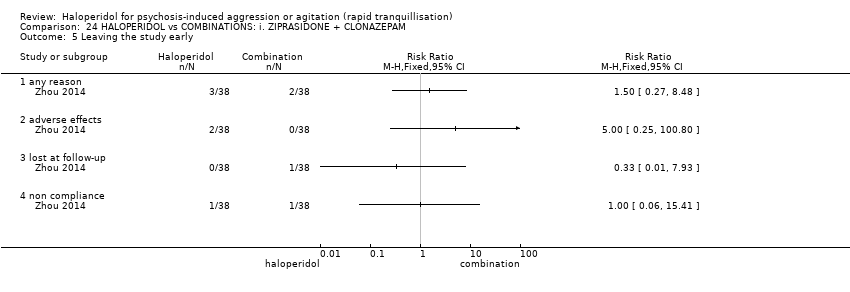
Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 5 Leaving the study early.
| Methods | Allocation: randomised, clearly described, concealed. |
| Participants | Diagnosis: thought to have psychoses. |
| Interventions | 1. Haloperidol IM: dose flexible within recommended limits. N = 150. |
| Outcomes | All outcomes are grouped by time: by 30 minutes, up to two hours, up to four hours, up to 24 hours and finally over 24 hours. Tranquillisation or asleep (measured at 30 minutes, 2 hours, 4 hours and 24 hours). |
| Notes | * Powered to be able to identify a difference of ˜20% between groups for primary outcome with adequate degree of certainty. |
| IM: intramuscular | |
| HALOPERIDOL compared with PLACEBO/NIL for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO/NIL | HALOPERIDOL | |||||
| Tranquillisation or asleep | Low1 | RR 0.88 | 220 | ⊕⊝⊝⊝ | ||
| 965 per 1000 | 849 per 1000 | |||||
| Moderate1 | ||||||
| 980 per 1000 | 862 per 1000 | |||||
| High1 | ||||||
| 995 per 1000 | 876 per 1000 | |||||
| Repeated need for tranquillisation | Low1 | RR 0.51 | 660 | ⊕⊕⊝⊝ | ||
| 400 per 1000 | 204 per 1000 | |||||
| Moderate1 | ||||||
| 600 per 1000 | 306 per 1000 | |||||
| High1 | ||||||
| 800 per 1000 | 408 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others | The mean specific behaviour ‐ threat or injury to self or others in the intervention groups was | 474 | ⊕⊝⊝⊝ | |||
| Global outcome ‐ overall improvement (not any improvement) | Low1 | RR 0.28 | 40 | ⊕⊕⊝⊝ | ||
| 200 per 1000 | 56 per 1000 | |||||
| Moderate1 | ||||||
| 350 per 1000 | 98 per 1000 | |||||
| High1 | ||||||
| 500 per 1000 | 140 per 1000 | |||||
| Adverse effects: Specific ‐ dystonia within 24 hours | Moderate1 | RR 7.49 | 207 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| High1 | ||||||
| 10 per 1000 | 75 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control risk ‐ moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTICS: a. ARIPIPRAZOLE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTICS: a. ARIPIPRAZOLE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation ‐ needing additional injection | Low1 | RR 0.78 | 473 | ⊕⊕⊝⊝ | ||
| 200 per 1000 | 156 per 1000 | |||||
| Moderate1 | ||||||
| 400 per 1000 | 312 per 1000 | |||||
| High1 | ||||||
| 650 per 1000 | 507 per 1000 | |||||
| Specific behaviours ‐ agitation: CABS average change score at 2 hours | The mean specific behaviours ‐ agitation: average change score at 2 hours in the intervention groups was | 470 | ⊕⊝⊝⊝ | |||
| Global outcomes: need for benzodiazepine 2 | Low1 | RR 1.26 | 477 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 63 per 1000 | |||||
| Moderate1 | ||||||
| 100 per 1000 | 126 per 1000 | |||||
| High1 | ||||||
| 200 per 1000 | 252 per 1000 | |||||
| Adverse effects: any serious, specific ‐ dystonia during 24 hours | Low6 | RR 6.63 | 477 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate6 | ||||||
| 50 per 1000 | 332 per 1000 | |||||
| High6 | ||||||
| 100 per 1000 | 663 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk roughly equates with that of the trial control group. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE | HALOPERIDOL | |||||
| Tranquillisation or asleep | Low1 | RR 1.93 | 39 | ⊕⊝⊝⊝ | ||
| 200 per 1000 | 386 per 1000 | |||||
| Moderate1 | ||||||
| 500 per 1000 | 965 per 1000 | |||||
| High1 | ||||||
| 700 per 1000 | 1000 per 1000 | |||||
| Repeated need for rapid tranquillisation ‐ more that 1 injection | Low1 | RR 1.07 | 30 | ⊕⊝⊝⊝ | ||
| 800 per 1000 | 856 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 963 per 1000 | |||||
| High1 | ||||||
| 990 per 1000 | 1000 per 1000 | |||||
| Specific behaviour ‐ threat or injury of harm to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome ‐ not any improvement | Low1 | RR 0.15 | 89 | ⊕⊝⊝⊝ | ||
| 1000 per 1000 | 150 per 1000 | |||||
| Moderate1 | ||||||
| 300 per 1000 | 45 per 1000 | |||||
| High1 | ||||||
| 500 per 1000 | 75 per 1000 | |||||
| Adverse effects: specific ‐ cardiovascular ‐ hypotension | Low1 | RR 0.51 | 30 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 10 per 1000 | |||||
| Moderate1 | ||||||
| 150 per 1000 | 30 per 1000 | |||||
| High1 | ||||||
| 250 per 1000 | 50 per 1000 | |||||
| Adverse effects: specific ‐ central nervous system ‐ seizures | Low1 | RR 0.33 | 30 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 3 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 17 per 1000 | |||||
| High1 | ||||||
| 150 per 1000 | 50 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control risk ‐ moderate risk roughly equates to that of the control group | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: c. DROPERIDOL for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: c. DROPERIDOL | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not asleep up to 2 hours | Study population | RR 1.07 | 228 | ⊕⊕⊕⊝ | ||
| 82 per 1.000 | 88 per 1.000 | |||||
| Repeated need for rapid tranquillisation ‐ more than 1 injection | Low | RR 2.38 | 255 | ⊕⊕⊕⊝ | ||
| 50 per 1.000 | 119 per 1.000 | |||||
| Moderate | ||||||
| 70 per 1.000 | 167 per 1.000 | |||||
| High | ||||||
| 360 per 1.000 | 857 per 1.000 | |||||
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Global outcome ‐ need for benzodiazepine | Study population | RR 0.31 | 228 | ⊕⊕⊕⊝ | ||
| 59 per 1.000 | 18 per 1.000 | |||||
| Adverse effects: specific ‐ dystonia | Study population | RR 0.86 | 255 | ⊕⊕⊕⊝ | ||
| 8 per 1.000 | 7 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 CI include no effect 2 Risk of bias: rated 'serious' ‐ method of randomisation not reported, described as double blind but no further information given regarding rater blinding, small short study. 3 Publication bias: rated 'strongly suspected' ‐ small study. 4 Moderate risk roughly equates with that of the trial control group, 5 Adverse effects ‐ imprecision ‐ 95% confidence interval. 6 Adverse effects ‐ publication bias ‐ small study. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTICS: d. LOXAPINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients to psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTICS: d. LOXAPINE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not asleep by 12 hours | Low1 | RR 4.31 | 54 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 43 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 215 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 431 per 1000 | |||||
| Global outcome: specific | Low1 | RR 3.50 | 30 | ⊕⊝⊝⊝ | * data for prespecified outcome Repeated need for rapid tranquillisation were not reported | |
| 10 per 1000 | 57 per 1000 | |||||
| Moderate1 | ||||||
| 100 per 1000 | 569 per 1000 | |||||
| High1 | ||||||
| 200 per 1000 | 1000 per 1000 | |||||
| Specific behaviour ‐ aggression ‐ no overall improvement | Low1 | RR 1.10 | 30 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 550 per 1000 | |||||
| Moderate1 | ||||||
| 650 per 1000 | 715 per 1000 | |||||
| High1 | ||||||
| 800 per 1000 | 880 per 1000 | |||||
| Global outcome: no change at 48 hours | Low1 | RR 0.93 | 56 | ⊕⊕⊝⊝ | ||
| 10 per 1000 | 9 per 1000 | |||||
| Moderate1 | ||||||
| 70 per 1000 | 65 per 1000 | |||||
| High1 | ||||||
| 140 per 1000 | 130 per 1000 | |||||
| Adverse effects: Specific ‐ dystonia between 0‐3 days (IM phase) | Low1 | RR 0.94 | 35 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 9 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 47 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 94 per 1000 | |||||
| Adverse effects: Specific ‐ rigidity between 0‐3 days (IM phase) | Low1 | RR 0.88 | 35 | ⊕⊝⊝⊝ | ||
| 750 per 1000 | 660 per 1000 | |||||
| Moderate1 | ||||||
| 850 per 1000 | 748 per 1000 | |||||
| High1 | ||||||
| 950 per 1000 | 836 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: e. OLANZAPINE for psychosis induced aggression or agitation | ||||||
| Patient or population: psychosis induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: e. OLANZAPINE | Risk with HALOPERIDOL | |||||
| Tranquilisation or asleep | Low | RR 1.16 | 257 | ⊕⊝⊝⊝ | ||
| 200 per 1.000 | 232 per 1.000 | |||||
| Moderate | ||||||
| 700 per 1.000 | 812 per 1.000 | |||||
| High | ||||||
| 900 per 1.000 | 1000 per 1.000 | |||||
| Repeated need for tranquillisation | Low | RR 1.06 | 392 | ⊕⊝⊝⊝ | ||
| 50 per 1.000 | 53 per 1.000 | |||||
| Moderate | ||||||
| 200 per 1.000 | 212 per 1.000 | |||||
| High | ||||||
| 350 per 1.000 | 371 per 1.000 | |||||
| Specific behaviour ‐ threat or injury to self or others | Low | RR 0.96 | 45 | ⊕⊝⊝⊝ | ||
| 200 per 1.000 | 192 per 1.000 | |||||
| Moderate | ||||||
| 400 per 1.000 | 384 per 1.000 | |||||
| High | ||||||
| 600 per 1.000 | 576 per 1.000 | |||||
| Global outcome ‐ need for benzodiazepine during 24 hours | Low | RR 1.05 | 343 | ⊕⊝⊝⊝ | ||
| 50 per 1.000 | 53 per 1.000 | |||||
| Moderate | ||||||
| 150 per 1.000 | 158 per 1.000 | |||||
| High | ||||||
| 250 per 1.000 | 263 per 1.000 | |||||
| Adverse effects: Specific ‐ dystonia during 24 hours | Low | RR 12.92 | 343 | ⊕⊝⊝⊝ | ||
| 0 per 1.000 | 0 per 1.000 | |||||
| Moderate | ||||||
| 0 per 1.000 | 0 per 1.000 | |||||
| High | ||||||
| 5 per 1.000 | 65 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. 2 Risk of bias: rated 'very serious' ‐ method of randomisation not reported, allocation concealment not stated, described as double blind but no further details are given regarding blinding, selective reporting, sponsored by drug company. 3 Indirectness: rated 'serious' ‐ tranquil at 30 minutes is not measured, therefore used not asleep at 2 hour. 4 Publication bias: rated 'strongly suspected' ‐ sponsored by drug company. 5 Indirectness: rated 'serious' ‐ not threat or injury to self or others as stated in protocol, therefore inferred further aggressive behaviour by less than a 40% reduction in PANSS‐EC score. 6 Risk of bias: rated 'serious' ‐ method of randomisation is not reported, allocation concealment not stated, described as double blind but no further information given, incomplete outcome data, selective reporting, sponsored by drug company. 7 Imprecision: rated 'serious' ‐ 95% confidence interval is wide. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTIC: f. PERPHENAZINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTIC: f. PERPHENAZINE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviour ‐ injury or threat of self to others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome: general ‐ no improvement | Low1 | RR 0.46 | 44 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 5 per 1000 | |||||
| Moderate1 | ||||||
| 100 per 1000 | 46 per 1000 | |||||
| High1 | ||||||
| 200 per 1000 | 92 per 1000 | |||||
| Adverse effect: Specific ‐ hypotensive episode | Low1 | RR 0.31 | 44 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 3 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 15 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 31 per 1000 | |||||
| Adverse effect ‐ discontinued due to EPS | Low1 | RR 1.83 | 44 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 18 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 92 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 183 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTICS: g. QUETIAPINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTICS: g. QUETIAPINE | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 0.10 higher | ‐ | 80 | ⊕⊕⊝⊝ | ||
| Global outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Adverse effects | Low | RR 1.81 | 80 | ⊕⊕⊝⊝ | ||
| 200 per 1.000 | 362 per 1.000 | |||||
| Moderate | ||||||
| 400 per 1.000 | 724 per 1.000 | |||||
| High | ||||||
| 600 per 1.000 | 1000 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Imprecision: rated 'very serious' ‐ small study, 95% CI are wide. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTIC: g. RISPERIDONE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTIC: g. RISPERIDONE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not asleep | Low1 | RR 0.84 | 162 | ⊕⊕⊕⊝ | ||
| 700 per 1000 | 588 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 756 per 1000 | |||||
| High1 | ||||||
| 1000 per 1000 | 840 per 1000 | |||||
| Repeated need for rapid tranquillisation ‐ needing additional benzodiazepine | Low | RR 0.98 | 286 | ⊕⊕⊝⊝ | ||
| 100 per 1000 | 98 per 1000 | |||||
| Moderate | ||||||
| 200 per 1000 | 196 per 1000 | |||||
| High | ||||||
| 300 per 1000 | 294 per 1000 | |||||
| Specific behaviours ‐ agitation | Low1 | RR 1.15 | 124 | ⊕⊕⊝⊝ | ||
| 50 per 1000 | 58 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 230 per 1000 | |||||
| High1 | ||||||
| 350 per 1000 | 402 per 1000 | |||||
| Global outcome ‐ rated as severe ‐ CGI‐S Follow‐up: 1 days | Low1 | RR 0.89 | 162 | ⊕⊝⊝⊝ | ||
| 100 per 1000 | 89 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 178 per 1000 | |||||
| High1 | ||||||
| 300 per 1000 | 267 per 1000 | |||||
| Adverse effects: Specific ‐ EPS during 24 hours | Low1 | RR 1.6 | 124 | ⊕⊕⊝⊝ | ||
| 10 per 1000 | 16 per 1000 | |||||
| Moderate1 | ||||||
| 100 per 1000 | 160 per 1000 | |||||
| High1 | ||||||
| 200 per 1000 | 320 per 1000 | |||||
| Adverse effects: Specific ‐ acute dystonia during 24 hours | Low1 | RR 5 | 286 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 50 per 1000 | |||||
| Moderate1 | ||||||
| 20 per 1000 | 100 per 1000 | |||||
| High1 | ||||||
| 30 per 1000 | 150 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: h. THIOTHIXENE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTIC: h. THIOTHIXENE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation | Low1 | RR 1.07 | 30 | ⊕⊝⊝⊝ | ||
| 850 per 1000 | 910 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 963 per 1000 | |||||
| High1 | ||||||
| 950 per 1000 | 1000 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome | Low1 | RR 0.50 | 44 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 25 per 1000 | |||||
| Moderate1 | ||||||
| 250 per 1000 | 125 per 1000 | |||||
| High1 | ||||||
| 450 per 1000 | 225 per 1000 | |||||
| Adverse effects: specific ‐ hypotensive episode | Low | Not estimable | 30 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Adverse effects: specific ‐ tachycardia | Low1 | RR 0.28 | 44 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 3 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 14 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 28 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control risk ‐ moderate risk roughly equates to that of the trial control group. | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: i. ZIPRASIDONE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: i. ZIPRASIDONE | Risk with HALOPERIDOL | |||||
| Tranquilisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No trial reported this outcome. |
| Repeated need for tranquilisation | Study population | RR 1.64 | 60 | ⊕⊝⊝⊝ | ||
| 467 per 1.000 | 765 per 1.000 | |||||
| Specific behaviour ‐ agitation | MD 0.06 higher | ‐ | 231 | ⊕⊕⊝⊝ | ||
| Global outcome | MD 0.34 higher | ‐ | 132 | ⊕⊝⊝⊝ | ||
| Adverse effects: Specific ‐ dystonia during 72 hours | Low | RR 10.26 | 508 | ⊕⊝⊝⊝ | ||
| 2 per 1.000 | 21 per 1.000 | |||||
| Moderate | ||||||
| 4 per 1.000 | 41 per 1.000 | |||||
| High | ||||||
| 10 per 1.000 | 103 per 1.000 | |||||
| Adverse effects: Specific ‐ clinically significant abnormal ECG during 72 hours | Study population | RR 1.01 | 376 | ⊕⊝⊝⊝ | ||
| 127 per 1.000 | 128 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No trial reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ method of allocation concealment not stated, blinding of participants uneffective 2 Imprecision: rated 'very serious' ‐ less than 100 patients, 95% CI extends beyond the no effect point 3 Risk of bias: rated 'serious' ‐ method of randomisation is not described, allocation concealment is not stated, single‐blind. 4 Indirectness: rated 'serious' ‐ not threat or injury to self or others, therefore had to use scale derived data for agitation. 5 Risk of bias: rated 'very serious' ‐ allocation of concealment not stated, open trial, adverse effects only reported where they occurred in ≥10% of people, sponsored by drug company. 6 Publication bias: rated 'strongly suspected' ‐ sponsored by drug company. 7 Imprecision: rated 'serious' ‐ 95% confidence intervals are wide. 8 Moderate risk roughly is roughly equal to that of the control group. 9 Indirectness: rated 'serious' ‐ abnormal ECG ‐ not necessarily a serious adverse effect. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTICS: j. ZUCLOPENTHIXOL ACETATE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTICS: j. ZUCLOPENTHIXOL ACETATE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation ‐ more than 3 injections | Low1 | RR 2.54 | 70 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 127 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 508 per 1000 | |||||
| High1 | ||||||
| 350 per 1000 | 889 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Adverse effects: specific ‐ tremor by 7 days | Low1 | RR 4.16 | 70 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 42 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 208 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 416 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared with BENZODIAZEPINE: a. FLUNITRAZEPAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| BENZODIAZEPINE: a. FLUNITRAZEPAM | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for tranquillisation within 24 hours ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviours ‐ aggression | Low1 | RR 1.15 | 28 | ⊕⊝⊝⊝ | ||
| 650 per 1000 | 747 per 1000 | |||||
| Moderate1 | ||||||
| 800 per 1000 | 920 per 1000 | |||||
| High1 | ||||||
| 950 per 1000 | 1000 per 1000 | |||||
| Global outcome ‐ need for restraint or seclusion | See comment | See comment | Not estimable | 28 | ⊕⊝⊝⊝ | No events in either group. |
| Adverse effects: specific ‐ EPS | See comment | See comment | Not estimable | 28 | ⊕⊝⊝⊝ | No events in either group. |
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared with BENZODIAZEPINE: b. LORAZEPAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| BENZODIAZEPINE: b. LORAZEPAM | HALOPERIDOL | |||||
| Tranquillisation or asleep | Low1 | RR 1.05 | 60 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 525 per 1000 | |||||
| Moderate1 | ||||||
| 700 per 1000 | 735 per 1000 | |||||
| High1 | ||||||
| 900 per 1000 | 945 per 1000 | |||||
| Repeated need for rapid tranquillisation | Low1 | RR 1.14 | 66 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 570 per 1000 | |||||
| Moderate1 | ||||||
| 750 per 1000 | 855 per 1000 | |||||
| High1 | ||||||
| 900 per 1000 | 1000 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others within 24 hours ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome: no overall improvement ‐ at 60 minutes | Low1 | RR 1.64 | 44 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 82 per 1000 | |||||
| Moderate1 | ||||||
| 150 per 1000 | 246 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 164 per 1000 | |||||
| Adverse effects: specific ‐ dystonia (only reported if occurred in ≥9% during 24 hours) | Low1 | RR 3.54 | 66 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 35 per 1000 | |||||
| Moderate1 | ||||||
| 30 per 1000 | 106 per 1000 | |||||
| High1 | ||||||
| 50 per 1000 | 177 per 1000 | |||||
| Adverse effects: specific ‐ hypertonia/rigidity (only reported if occurred in ≥9%) during 24 hours | Low7 | RR 6.22 | 66 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate7 | ||||||
| 50 per 1000 | 311 per 1000 | |||||
| High7 | ||||||
| 100 per 1000 | 622 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared with BENZODIAZEPINE: c. MIDAZOLAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| BENZODIAZEPINE: c. MIDAZOLAM | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome | Low1 | RR 1.14 | 84 | ⊕⊕⊝⊝ | ||
| 50 per 1000 | 57 per 1000 | |||||
| Moderate1 | ||||||
| 150 per 1000 | 171 per 1000 | |||||
| High1 | ||||||
| 250 per 1000 | 285 per 1000 | |||||
| Adverse effects ‐ general ‐ one or more drug‐related adverse effect | Low4 | RR 5.00 | 84 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate4 | ||||||
| 50 per 1000 | 250 per 1000 | |||||
| High4 | ||||||
| 100 per 1000 | 500 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Medium risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared with 1. COMBINATIONS ‐ HALOPERIDOL + LEVOMEPROMAZINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| COMBINATIONS: a. HALOPERIDOL + LEVOMEPROMAZINE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviours ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome: general ‐ no overall improvement | Low1 | RR 8.18 | 19 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 1000 per 1000 | |||||
| High1 | ||||||
| 400 per 1000 | 1000 per 1000 | |||||
| Adverse effect: any serious, specific ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Economic outcome ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Low risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared with COMBINATIONS: b. HALOPERIDOL + LORAZEPAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| COMBINATIONS: b. HALOPERIDOL + LORAZEPAM | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not asleep by 3 hours | Low1 | RR 1.83 | 67 | ⊕⊝⊝⊝ | ||
| 200 per 1000 | 366 per 1000 | |||||
| Moderate1 | ||||||
| 400 per 1000 | 732 per 1000 | |||||
| High1 | ||||||
| 600 per 1000 | 1000 per 1000 | |||||
| Repeated need for rapid tranquillisation ‐ needing additional injection during 24 hours | Low1 | RR 1.05 | 67 | ⊕⊝⊝⊝ | ||
| 750 per 1000 | 788 per 1000 | |||||
| Moderate1 | ||||||
| 850 per 1000 | 892 per 1000 | |||||
| High1 | ||||||
| 950 per 1000 | 997 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Global outcome: no overall improvement ‐ at 30 minutes | Low1 | RR 2.67 | 45 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 134 per 1000 | |||||
| Moderate1 | ||||||
| 250 per 1000 | 668 per 1000 | |||||
| High1 | ||||||
| 450 per 1000 | 1000 per 1000 | |||||
| Adverse effects: Specific ‐ dystonia (only reported if occurred in ≥9%) during 24 hours | Low7 | RR 8.25 | 67 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate7 | ||||||
| 50 per 1000 | 412 per 1000 | |||||
| High7 | ||||||
| 100 per 1000 | 825 per 1000 | |||||
| Adverse effects: Specific ‐ hypertonia/rigidity (only reported if occurred in ≥9%) during 24 hours | Low1 | RR 2.74 | 67 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 27 per 1000 | |||||
| Moderate1 | ||||||
| 30 per 1000 | 82 per 1000 | |||||
| High1 | ||||||
| 50 per 1000 | 137 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared to COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation | Study population | RR 0.88 | 60 | ⊕⊝⊝⊝ | ||
| 867 per 1.000 | 763 per 1.000 | |||||
| Specific behaviour ‐ agitation | MD 11.20 lower | ‐ | 60 | ⊕⊝⊝⊝ | ||
| Global outcome | Low | RR 0.29 | 60 | ⊕⊝⊝⊝ | ||
| 350 per 1.000 | 102 per 1.000 | |||||
| Moderate | ||||||
| 700 per 1.000 | 203 per 1.000 | |||||
| High | ||||||
| 950 per 1.000 | 275 per 1.000 | |||||
| Adverse effects | Study population | RR 1.67 | 60 | ⊕⊝⊝⊝ | ||
| 100 per 1.000 | 167 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ method of allocation not stated, patients instructed to not reveal their allocation although the trial is described as double blind, selective reporting, small study. 2 Imprecision: rated 'very serious' ‐ small study, 95% CI cross the no effect line. | ||||||
| HALOPERIDOL compared to COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep | Study population | RR 1.60 | 316 | ⊕⊕⊕⊝ | ||
| 281 per 1.000 | 450 per 1.000 | |||||
| Repeated need for tranquillisation | Low | RR 0.78 | 376 | ⊕⊝⊝⊝ | ||
| 50 per 1.000 | 39 per 1.000 | |||||
| Moderate | ||||||
| 120 per 1.000 | 94 per 1.000 | |||||
| High | ||||||
| 450 per 1.000 | 351 per 1.000 | |||||
| Specific behaviours ‐ aggression | Study population | RR 1.06 | 316 | ⊕⊕⊕⊝ | ||
| 194 per 1.000 | 205 per 1.000 | |||||
| Global outcomes | Study population | RR 1.21 | 316 | ⊕⊕⊕⊝ | ||
| 238 per 1.000 | 287 per 1.000 | |||||
| Adverse effects: specific ‐ acute dystonia | Low | RR 19.48 | 316 | ⊕⊕⊝⊝ | ||
| 0 per 1.000 | 0 per 1.000 | |||||
| Moderate | ||||||
| 50 per 1.000 | 974 per 1.000 | |||||
| High | ||||||
| 100 per 1.000 | 1000 per 1.000 | |||||
| Adverse effects: specific ‐ seizure | Study population | RR 2.05 | 316 | ⊕⊕⊝⊝ | ||
| 6 per 1.000 | 13 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No trial reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ open trial. 2 Moderate risk is roughly equal to that of the trial control group. 3 Inconsistency: rated 'very serious' ‐ I2 86%. 4 Publication bias: rated 'strongly suspected' ‐ visual inspection of the funnel plot. 5 Indirectness: overall improvement not reported, therefore global improvement inferred from need for restraints at by 120 minutes. 6 Imprecision: rated 'serious' ‐ 95% confidence intervals are wide. 7 Low risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared to COMBINATIONS: e. HALOPERIDOL + RISPERIDONE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: e. HALOPERIDOL + RISPERIDONE | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 3.82 higher | ‐ | 100 | ⊕⊕⊝⊝ | ||
| Global outcome assessed with: no improvement at 72 hours | Study population | RR 0.38 | 100 | ⊕⊕⊝⊝ | ||
| 160 per 1.000 | 61 per 1.000 | |||||
| Adverse effects: specific assessed with akathisia during 14 days | Study population | RR 0.88 | 100 | ⊕⊕⊝⊝ | ||
| 320 per 1.000 | 282 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ allocation concealment procedures not stated, blinding procedures not stated. 2 Imprecision: rated 'serious' ‐ 95% CIs are wide. | ||||||
| HALOPERIDOL compared to COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 0.04 lower | ‐ | 64 | ⊕⊝⊝⊝ | ||
| Global outcome | Study population | RR 1.06 | 64 | ⊕⊝⊝⊝ | ||
| 182 per 1.000 | 193 per 1.000 | |||||
| Adverse effects | Low | RR 3.06 | 64 | ⊕⊝⊝⊝ | ||
| 100 per 1.000 | 306 per 1.000 | |||||
| Moderate | ||||||
| 240 per 1.000 | 734 per 1.000 | |||||
| High | ||||||
| 500 per 1.000 | 1000 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ allocation concealment procedures not stated, blinding procedures not stated, small study. 2 Imprecision: rated 'very serious' ‐ small study, 95% CI cross the no effect area. | ||||||
| HALOPERIDOL compared with COMBINATIONS: d. QUETIAPINE + MAGNESIUM VALPROATE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| COMBINATIONS: d. QUETIAPINE + MAGNESIUM VALPROATE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviours ‐ agitation at 3 days | The mean specific behaviours ‐ agitation at 3 days in the intervention groups was | 60 | ⊕⊝⊝⊝ | |||
| Global effect | Low3 | RR 1.17 | 60 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 58 per 1000 | |||||
| Moderate3 | ||||||
| 200 per 1000 | 234 per 1000 | |||||
| High3 | ||||||
| 350 per 1000 | 409 per 1000 | |||||
| Adverse effects ‐ any specific, serious ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ method of randomisation is not reported, allocation concealment not stated, open‐label, selective reporting. | ||||||
| HALOPERIDOL compared to COMBINATIONS: h. RISPERIDONE + CLONAZEPAM for psychosis induced aggression or agitation | ||||||
| Patient or population: psychosis induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: h. RISPERIDONE + CLONAZEPAM | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep | Study population | RR 0.82 | 205 | ⊕⊝⊝⊝ | ||
| 673 per 1.000 | 552 per 1.000 | |||||
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 0.50 lower | ‐ | 108 | ⊕⊝⊝⊝ | ||
| Global outcome | Study population | RR 0.60 | 100 | ⊕⊕⊝⊝ | ||
| 100 per 1.000 | 60 per 1.000 | |||||
| Adverse effects | Low | RR 1.63 | 205 | ⊕⊝⊝⊝ | ||
| 150 per 1.000 | 244 per 1.000 | |||||
| Moderate | ||||||
| 300 per 1.000 | 489 per 1.000 | |||||
| High | ||||||
| 500 per 1.000 | 815 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ open study, evidence of reporting bias, sponsored trial. 2 Imprecision: rated 'serious' ‐ 95% CI cross the no effect area. 3 Risk of bias: rated 'serious' ‐ allocation concealment and blinding procedures not stated. 4 Imprecision: rated 'serious' ‐ 95% CI are wide. 5 Imprecision: rated 'serious' ‐ 95% CI are wide. | ||||||
| HALOPERIDOL compared to COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 0.28 lower | ‐ | 71 | ⊕⊝⊝⊝ | ||
| Global outcome ‐ no improvement ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Adverse effects ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ randomisation, allocation and blinding procedures not stated, evidence of attrition bias, small study. 2 Imprecision: rated 'very serious' ‐ small study, 95% CI are wide. | ||||||
| Focus of review | Reference |
| Completed and maintained reviews | |
| 'As required' medication regimens for seriously mentally ill people in hospital | |
| Benzodiazepines for psychosis‐induced aggression or agitation | |
| Chlorpromazine for psychosis induced aggression or agitation | |
| Clotiapine for acute psychotic illnesses | |
| Containment strategies for people with serious mental illness | |
| Droperidol for acute psychosis | |
| Haloperidol plus promethazine for psychosis‐induced aggression | |
| Olanzapine IM or velotab for acutely disturbed/agitated people with suspected serious mental illnesses | |
| Seclusion and restraint for serious mental illnesses | |
| Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses | |
| Reviews in the process of being completed | |
| Risperidone for psychosis induced aggression or agitation | |
| Haloperidol for long term aggression in psychosis | |
| Loxapine inhaler for psychosis‐induced aggression | |
| Quetiapine for psychosis‐induced aggression | |
| De‐escalation techniques for psychosis‐induced aggression | |
| Description | Study |
| Explicitly referred to how participants, study personnel and raters were blinded + tested blinding. | |
| Explicitly referred to how participants, study personnel and raters were blinded. | |
| Report that drugs had identical appearance ‐ no further details regarding rater blinding. | Fitzgerald 1969, Fruensgaard 1977, Paprocki 1977, Resnick 1984, Ritter 1972 |
| Explicitly state that rater was blinded ‐ no reference to participant blinding. Not all study personnel could have been blinded because person preparing drug (not blinded), often administered drug. | |
| Study personnel and raters blinded, not clear if attempts made to blind participants. | |
| Raters blinded ‐ not clear if study personnel and participants were blinded. | |
| "Double blind" ‐ no further information regarding blinding of participants, study personnel and raters. | |
| Described as double‐blind, but during oral phase haloperidol described as being in capsule form, and aripiprazole in tablet form, therefore unclear whether drugs had identical appearance. Not explicit if raters were blinded. | |
| Described as double‐blind during IM phase ‐ open during oral phase ‐ no further information given regarding blinding. | |
| Described as "modified double", whereby staff administering drugs were not blinded, but raters were ‐ not clear if participants were blinded. | |
| Single‐blind studies ‐ "open label, rater blind". | |
| Reported to be single‐blind, but unclear who was blind. | |
| Open‐label and rater blinding for some outcomes (BPRS). | |
| Rater blinding for assessment of BPRS, but unclear regarding rater blinding for other outcomes and whether participants and study personnel were blind. | |
| Open studies | Brook 2000, Fang 2012, Garza‐Trevino 1989, Guo 2007, Higashima 2004, Huf 2007 |
| Not stated | |
| BPRS ‐ Brief Psychiatric Rating Scale | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ asleep Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 not asleep up to 2 hours | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.82, 0.95] |
| 2 Repeated need for tranquillisation Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 needing additional injection during 24 hours (agitation only) | 4 | 660 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.42, 0.62] |
| 3 Specific behaviour: 1a. Agitation ‐ various measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 PANSS‐EC response at 2 hours (at least 40% change on PANSS‐EC from baseline) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.28, 2.07] |
| 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 change score (ABS, high=worse) | 3 | 474 | Mean Difference (IV, Fixed, 95% CI) | ‐4.48 [‐5.78, ‐3.18] |
| 4.2 change score (ACES, low=agitated, high=sedated) | 2 | 390 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.39, 1.26] |
| 4.3 score (PANSS‐EC, high=worse) | 3 | 514 | Mean Difference (IV, Fixed, 95% CI) | ‐3.67 [‐4.75, ‐2.59] |
| 4.4 change score (PANSS‐EC, high=worse, schizophrenia subgroup) | 1 | 199 | Mean Difference (IV, Fixed, 95% CI) | ‐2.57 [‐4.05, ‐1.09] |
| 4.5 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on ACES) | 1 | 240 | Mean Difference (IV, Fixed, 95% CI) | ‐2.64 [‐3.95, ‐1.33] |
| 4.6 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on ACES) | 1 | 263 | Mean Difference (IV, Fixed, 95% CI) | ‐2.97 [‐4.27, ‐1.67] |
| 5 Specific behaviour: 1c. Agitation ‐ average scores ‐ ii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 change score (ABS, high=worse) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐2.4 [‐4.13, ‐0.67] |
| 5.2 change score (PANSS‐EC, high=worse) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐1.4 [‐2.97, 0.17] |
| 6 Global outcome: 1. Not improved Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 not marked improvement | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.44, 0.84] |
| 6.2 not any improvement | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 1.07] |
| 7 Global outcome: 2. Need for benzodiazepine up to 24 hours Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 need for benzodiazepine up to 24 hours | 4 | 660 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.31, 0.62] |
| 8 Global outcome: 3a. Average scores ‐ i. up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 endpoint score (CGI‐I, high=worse) | 2 | 390 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐0.96, ‐0.51] |
| 8.2 change score (CGI‐I, high=worse, schizophrenia subgroup) | 1 | 199 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐0.98, ‐0.30] |
| 8.3 change score (CGI‐S, high=worse) | 2 | 390 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.78, ‐0.18] |
| 9 Global outcome: 3b. Average scores ‐ ii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 change score (CGI‐I, high=worse) | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐0.62, ‐0.18] |
| 9.2 change score (CGI‐S, high=worse) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐0.2 [‐0.46, 0.06] |
| 10 Mental state: 1a. Average scores ‐ i. up to 2 hours Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 change score up to 2 hours (BPRS positive sub‐scale, high=worse) | 3 | 372 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.54, ‐0.54] |
| 10.2 change score up to 2 hours (BPRS total, high=worse) | 3 | 371 | Mean Difference (IV, Fixed, 95% CI) | ‐5.69 [‐7.38, ‐4.00] |
| 11 Mental state: 1b. Average scores ‐ ii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 change score up to 24 hours (BPRS positive sub‐scale, high=worse) | 2 | 264 | Mean Difference (IV, Fixed, 95% CI) | ‐1.61 [‐2.34, ‐0.89] |
| 11.2 change score up to 24 hours (BPRS total, high=worse) | 2 | 264 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐6.82, ‐2.81] |
| 12 Adverse effects: 1a. General Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 one or more drug related adverse events during 24 hours | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.22, 2.20] |
| 12.2 increased severity of adverse effects after 2nd injection | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.88, 5.63] |
| 12.3 overall adverse events during 72 hours | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.23, 2.59] |
| 13 Adverse effects: 1b. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 death | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 rated as serious | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.29] |
| 13.3 tonic clonic seizure | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Adverse effects: 2a. Specific ‐ arousal level Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 insomnia during 24 hours (only reported if occurred in ≧5%) | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.61, 2.82] |
| 14.2 "over" sedated | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.36 [1.42, 7.99] |
| 14.3 somnolence during 24 hours | 4 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [0.97, 5.36] |
| 15 Adverse effects: 2b. Specific ‐ cardiovascular ‐ miscellaneous outcomes Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 dizziness during 24 hours (only reported if occurred in ≧5%) | 2 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.48, 3.65] |
| 15.2 hypotension during 24 hours | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.05, 27.44] |
| 15.3 QTc abnormality | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.1 [0.33, 28.98] |
| 15.4 sinus tachycardia during 24 hours (only reported if occurred in ≥5%) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.1 [0.33, 28.98] |
| 15.5 tachycardia during 24 hours (only reported if occurred in ≥5%) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 16.15] |
| 16 Adverse effects: 2c. Specific ‐ cardiovascular ‐ QTc interval (average change at 24 hours) Show forest plot | 2 | 265 | Mean Difference (IV, Fixed, 95% CI) | 3.63 [‐2.67, 9.93] |
| 17 Adverse effects: 2d. Specific ‐ movement disorders Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 akathisia during 24 hours (only reported if occurred in ≥5%) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.43 [0.77, 233.23] |
| 17.2 dystonia during 24 hours | 2 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.49 [0.93, 60.21] |
| 17.3 extrapyramidal effects ‐ use of antiparkinson drugs during 24 hours | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.57 [1.37, 22.65] |
| 17.4 EPS during 24 hours | 3 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.79 [2.19, 21.07] |
| 18 Adverse effects: 2e. Specific ‐ movement disorders: i. average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 change score at 24 hours (BAS, high=worse) | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.17, 0.35] |
| 18.2 change score at 24 hours (SAS, high=worse) | 1 | 167 | Mean Difference (IV, Fixed, 95% CI) | 1.89 [0.77, 3.01] |
| 19 Adverse effects: 2f. Specific ‐ miscellaneous Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 agitation during 24 hours (only reported if occurred in ≧5%) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.29, 2.19] |
| 19.2 dry mouth | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.6 [0.21, 61.86] |
| 19.3 headache during 24 hours (only reported if occurred in ≧5%) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.55, 3.00] |
| 19.4 pain at injection site | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 19.5 pain (1st injection) | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.00, 1.97] |
| 19.6 pain (2nd injection) | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.88, 5.63] |
| 19.7 nausea during 24 hours (only reported if occurred in ≧5%) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.13, 3.67] |
| 19.8 vomiting during 24 hours (only reported if occurred in ≧5%) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 16.15] |
| 20 Leaving the study early Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 any reason | 3 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.53, 2.11] |
| 20.2 lack of efficacy | 3 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.49] |
| 20.3 withdrew | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [0.17, 64.15] |
| 20.4 consent | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.2 [0.77, 49.98] |
| 20.5 adverse effects | 3 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.21, 15.39] |
| 20.6 could not be evaluated due to being asleep | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.05, 27.44] |
| 20.7 other | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.27, 12.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for rapid tranquillisation: needing additional injection Show forest plot | 2 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| 2 Specific behaviour: 1a. Agitation ‐ PANSS‐EC response up to 2 hours (at least 40% change on PANSS‐EC from baseline) Show forest plot | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.26] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 change score (ACES, low=agitated, high=sedated) | 2 | 470 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.30, 0.55] |
| 3.2 change score (CABS, high=worse) | 2 | 470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐2.10, 1.01] |
| 3.3 change score (PANSS‐EC, high=worse) | 1 | 357 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐2.12, 1.16] |
| 3.4 change score (PANSS‐EC, high=worse, schizophrenia subgroup) | 1 | 257 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐1.48, 0.96] |
| 3.5 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on ACES) | 1 | 316 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐1.42, 0.76] |
| 3.6 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on AEs) | 1 | 346 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.40, 0.72] |
| 4 Global outcome: 1. Need for benzodiazepine Show forest plot | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.74, 2.16] |
| 5 Global outcome: 2. Average scores ‐ up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 change score (CGI‐S, high=worse) | 2 | 470 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.22, 0.36] |
| 5.2 endpoint score (CGI‐I, high=worse) | 2 | 470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.23, 0.19] |
| 5.3 change score (CGI‐I, high=worse, schizophrenia subgroup) | 1 | 257 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.30, 0.26] |
| 6 Mental state: 1. Average scores ‐ up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 change score at 2 hours (BPRS positive sub‐scale, high=worse) | 1 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐1.28, 0.82] |
| 6.2 change score at 2 hours (BPRS total, high=worse) | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐2.03 [‐5.76, 1.70] |
| 7 Adverse effects: 1a. General Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 one or more drug related adverse effects during 24 hours | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.95, 1.46] |
| 7.2 increased severity of adverse effects after 2nd injection | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.03, 1.74] |
| 7.3 overall adverse events during 72 hours | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.04, 1.70] |
| 8 Adverse effects: 1b. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 any | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.18, 1.81] |
| 8.2 tonic clonic seizure | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.62] |
| 8.3 death | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse effects: 2a. Specific ‐ arousal level Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 insomnia ‐ between 0‐1 days (IM phase) (only reported if occurred in ≥5%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.01, 4.27] |
| 9.2 insomnia ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.47, 1.44] |
| 9.3 "over" sedated | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.93, 2.95] |
| 9.4 somnolence ‐ between 0‐1 days | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.25, 2.00] |
| 9.5 somnolence ‐ between 0‐1 days (IM phase) (only reported if occurred in ≥5%) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.60, 8.16] |
| 9.6 somnolence ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.30, 27.02] |
| 10 Adverse effects: 2b. Specific ‐ cardiac Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 dizziness ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.33, 1.48] |
| 10.2 sinus tachycardia ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.66 [0.35, 126.06] |
| 10.3 tachycardia ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.06] |
| 11 Adverse effects: 2c. Specific ‐ gastrointestinal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 dyspepsia ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.41 [1.36, 79.76] |
| 11.2 diarrhoea ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.28, 3.21] |
| 11.3 nausea ‐ between 0‐1 days (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.60] |
| 11.4 nausea ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.09] |
| 11.5 vomiting ‐ between 0‐1 days (IM phase) (only reported if occurred in ≥5%) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.10] |
| 11.6 vomiting ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.07, 1.92] |
| 12 Adverse effects: 2d. Specific ‐ movement disorder Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 akathisia ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.94, 8.61] |
| 12.2 akathisia ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.58, 8.40] |
| 12.3 dystonia ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.63 [1.52, 28.86] |
| 12.4 EPS ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.46 [1.22, 73.13] |
| 12.5 EPS ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.09 [1.65, 30.58] |
| 12.6 Use of antiparkinson drugs ‐ between 3 ‐ 7 days (oral phase) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.94 [2.50, 9.78] |
| 12.7 dystonia | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 69.22] |
| 13 Adverse effects: 2e. Specific ‐ miscellaneous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 agitation ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.46, 3.22] |
| 13.2 agitation ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.68, 1.74] |
| 13.3 anxiety ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.54, 3.16] |
| 13.4 headache ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.45, 1.59] |
| 13.5 headache ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.57, 1.97] |
| 13.6 pain at injection site | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.62] |
| 13.7 pain (1st injection) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.06, 1.54] |
| 13.8 pain (2nd injection) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.03, 1.74] |
| 14 Leaving the study early: 1. For specific reasons Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 any | 2 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.86, 4.98] |
| 14.2 lack of efficacy | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 69.22] |
| 14.3 adverse effects | 2 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.17, 5.59] |
| 14.4 withdrew | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.24, 8.39] |
| 14.5 consent | 2 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.74, 48.34] |
| 14.6 other | 2 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.27, 6.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ asleep Show forest plot | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.04, 3.60] |
| 1.1 not asleep up to 2 hours | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.04, 3.60] |
| 2 Repeated need for rapid tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 more that 1 injection | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| 2.2 more than 3 injections | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.53, 4.26] |
| 3 Global outcome: 1. Not improved Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 not marked improvement | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.61, 1.02] |
| 3.2 not any improvement | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.05, 0.49] |
| 4 Adverse effects: any serious or specific adverse effects Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 allergy ‐ haematological ‐ leucopenia ‐ mild | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 4.2 allergy ‐ hepatic ‐ glutamic pyruvic transaminase elevated | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
| 4.3 allergy ‐ skin irritation ‐ local | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 anticholinergic ‐ dry mouth | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.17, 10.93] |
| 4.5 arousal ‐ drowsy but asleep | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.42] |
| 4.6 arousal ‐ drowsy but awake | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.17 [0.59, 142.10] |
| 4.7 cardiovascular ‐ hypotension | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.60] |
| 4.8 central nervous system ‐ seizures | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 4.9 movement disorders ‐ extrapyramidal adverse effects | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.28, 15.15] |
| 5 Leaving the study early Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 any reason/general reasons | 4 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.71] |
| 5.2 could not be evaluated due to being asleep | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.52] |
| 5.3 severe hypotensive reaction | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.85] |
| 5.4 deviation from protocol | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep: 1. Not asleep Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 up to 2 hours | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.44, 2.60] |
| 2 Tranquillisation or asleep: 2. Time to sleep Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 up to 2 hours | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 5.20 [‐6.05, 16.45] |
| 3 Repeated need for rapid tranquillisation Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 more than 1 injection | 2 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [1.27, 4.47] |
| 3.2 more than 3 injections | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.09, 47.68] |
| 4 Global outcome: 1. Need for benzodiazepine Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 up to 2 hours | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.07, 1.44] |
| 5 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 overall adverse events up to 2 hours | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.23] |
| 5.2 patients with one or more AEs | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.46] |
| 6 Adverse effects: 2a. Specific ‐ arousal level Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 "over" sedated | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.68] |
| 7 Adverse effects: 2b. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 hypotension up to 2 hours | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.36] |
| 8 Adverse effects: 2c. Specific ‐ movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 dystonia | 2 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.11, 6.56] |
| 9 Adverse effects: 2d. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 desaturation | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ not asleep up to 24 hours Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.31 [0.54, 34.48] |
| 2 Specific behaviour: 2. Aggression ‐ various measures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 no overall improvement | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.69, 1.76] |
| 3 Global outcome: 1. General ‐ no change ‐ i. over 24 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 up to 48 hours (CGI‐I, high=worse) | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.14, 6.15] |
| 3.2 up to 72 hours (CGI‐I, high=worse) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.04, 3.49] |
| 3.3 at endpoint (CGI‐I, high=worse) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.12, 1.32] |
| 4 Global outcome: 2a. Specific ‐ not sedated ‐ i. by 30 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 at 30 minutes | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.65, 2.54] |
| 5 Global outcome: 2b. Specific ‐ not sedated ‐ ii. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 at 1 hour | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [0.86, 14.18] |
| 5.2 at 2 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.98, 50.16] |
| 6 Mental state: 1. Average scores Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [4.48, 7.72] |
| 6.1 endpoint score (BPRS, high=worse) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [4.48, 7.72] |
| 7 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 one or more drug related adverse effect | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.44, 1.45] |
| 8 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 constipation ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 8.2 salivation ‐ too little ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.33, 24.66] |
| 8.3 salivation ‐ too little ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.22, 4.05] |
| 8.4 salivation ‐ too much ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.33, 2.69] |
| 8.5 salivation ‐ too much ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.04, 4.48] |
| 8.6 salivation ‐ too much ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.57, 1.93] |
| 8.7 polyuria ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 8.8 sweating ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.15, 5.97] |
| 8.9 sweating ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 9 Adverse effects: 2b. Specific ‐ arousal level Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 asleep ‐ by 12 hours | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.04] |
| 9.2 drowsiness ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| 9.3 drowsiness ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 33.16 [2.15, 511.57] |
| 9.4 drowsiness during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.20, 2.79] |
| 9.5 insomnia ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 9.6 insomnia ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.63 [0.37, 119.59] |
| 10 Adverse effects: 2c. Specific ‐ cardiovascular Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 blood pressure ‐ increased ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.45] |
| 10.2 dizziness ‐ between 3‐28 days | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.09, 1.52] |
| 10.3 dyspnoea ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 10.4 palpitations during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 10.5 pulse rate ‐ increased ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.79] |
| 11 Adverse effects: 2d. Specific ‐ movement disorders Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 akathisia between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.16, 2.02] |
| 11.2 akathisia between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.26, 1.61] |
| 11.3 akathisia between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.62, 2.27] |
| 11.4 dysarthria between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.44] |
| 11.5 dyskinesia between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.79] |
| 11.6 dystonia between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.06, 13.93] |
| 11.7 dystonia between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.26, 1.61] |
| 11.8 involuntary movement between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 11.9 motor retardation between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.06, 13.93] |
| 11.10 muscle spasms between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.33, 4.82] |
| 11.11 oculogyric crisis between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.11, 61.11] |
| 11.12 rigidity between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.19] |
| 11.13 rigidity between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.13, 5.68] |
| 11.14 rigidity during between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.93, 1.38] |
| 11.15 tremor between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.42, 2.13] |
| 11.16 tremor during between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.71, 1.76] |
| 11.17 tremor between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.11, 61.11] |
| 11.18 use of antiparkinson medication | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.66, 12.16] |
| 11.19 dystonia during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 96.13] |
| 11.20 extrapyramidal effects ‐ use of antiparkinson drugs during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.17, 2.07] |
| 11.21 EPS during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.98, 50.16] |
| 11.22 thick tongue ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.79] |
| 12 Adverse effects: 2e. Specific ‐ miscellaneous Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 allergy ‐ itch ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 12.2 allergy ‐ tissue reaction at injection site ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 nervousness ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.79] |
| 12.4 pain ‐ during 24 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.44] |
| 12.5 pain ‐ headache ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 12.6 pain ‐ myalgia ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 13 Leaving the study early: 1. For general reasons Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 90% CI) | Subtotals only | |
| 13.1 by 72 hours | 1 | 35 | Risk Ratio (M‐H, Fixed, 90% CI) | 0.63 [0.21, 1.85] |
| 13.2 by 10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 90% CI) | 1.18 [0.67, 2.07] |
| 13.3 by endpoint | 1 | 35 | Risk Ratio (M‐H, Fixed, 90% CI) | 0.94 [0.42, 2.13] |
| 14 Leaving the study early: 2. Specific reasons Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 adverse effects by 72 hours | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ asleep Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 not asleep up to 2 hours | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.02, 1.32] |
| 2 Repeated need for tranquillisation ‐ needing additional injection Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 by 2 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.47, 33.73] |
| 2.2 by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.67, 6.47] |
| 2.3 by 24 hours | 3 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.51] |
| 3 Specific behaviour: 1a. Agitation ‐ various measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 response ‐ up to 2 hours (≥40% reduction in PANSS‐EC) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.58, 1.58] |
| 3.2 agitation ‐ reported as 'adverse effect' | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.73] |
| 3.3 agitation during 21 days (if occurred in ≧10% at P < 0.05) ‐ reported as 'adverse effect' | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.33, 3.51] |
| 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. by 30 minutes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 change score at 15 minutes (ACES, low=agitated, high=sedated) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.35, 1.35] |
| 4.2 change scores at 15 minutes (PANSS‐EC, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.64, 2.24] |
| 4.3 change score at 30 minutes (ACES, low=agitated, high=sedated) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.06, 1.86] |
| 4.4 change scores at 30 minutes (PANSS‐EC, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐1.16, 3.76] |
| 5 Specific behaviour: 1c. Agitation ‐ average scores ‐ ii. up to 2 hours Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 change score (ABS, high=worse) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | 2.7 [0.38, 5.02] |
| 5.2 change score at 1 hour (ACES, low=agitated, high=sedated) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.06, 2.34] |
| 5.3 endpoint score at 1 hour (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [1.18, 2.82] |
| 5.4 change score at 1 hour (PANSS‐EC, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 2.2 [‐0.50, 4.90] |
| 5.5 change score at 2 hours (ACES, low=agitated, high=sedated) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.74, 1.34] |
| 5.6 endpoint score at 2 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [3.54, 4.86] |
| 5.7 score at 2 hours (PANSS‐EC, high=worse) | 3 | 332 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.49, 0.86] |
| 5.8 endpoint score at 2 hours (PANSS‐PAS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐2.56, 6.76] |
| 6 Specific behaviour: 1d. Agitation ‐ average scores ‐ iii. up to 4 hours Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.45] |
| 6.1 endpoint score at 4 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.45] |
| 7 Specific behaviour: 1e. Agitation ‐ average scores ‐ iv. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 change score (ABS, high=worse) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [0.01, 4.79] |
| 7.2 change score (PANSS‐EC, high=worse) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐0.55, 3.35] |
| 7.3 endpoint score at 6 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.05, 0.35] |
| 7.4 endpoint score at 12 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.9 [0.82, 0.98] |
| 8 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 endpoint score at 1 hour (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.13, 1.67] |
| 8.2 endpoint score at 2 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.41, 1.41] |
| 9 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 endpoint score at 4 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.11, 0.31] |
| 10 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 endpoint score at 6 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.26, 0.06] |
| 10.2 endpoint score at 12 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.39, ‐0.01] |
| 11 Specific behaviour: 3. Hostility Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.12] |
| 12 Global outcome: 1a. General ‐ need for additional measures Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 need for benzodiazepine during 24 hours | 2 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.74] |
| 12.2 need for benzodiazepine during 7 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.27] |
| 12.3 additional restraint, seclusion or special observation | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.70, 3.17] |
| 13 Global outcome: 1b. General ‐ time and doses Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 time to discontinuation | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐3.48 [‐6.28, ‐0.68] |
| 13.2 dose of adjunctive lorazepam | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐0.14, 0.82] |
| 14 Global outcome: 1c. General ‐ average scores ‐ up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 endpoint score (CGI‐I, high=worse) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.65, 0.45] |
| 15 Global outcome: 1d. General ‐ average scores ‐ over 24 hours Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 change score (CGI‐I, high=worse) | 1 | 243 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.20, 0.20] |
| 15.2 change score (CGI‐S, high=worse) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.24, 0.24] |
| 15.3 change score by 21 days (CGI‐I, high=worse) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.18, 0.90] |
| 16 Global outcome: 2a. Specific ‐ alert ‐ i. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 alert at 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.99, 1.55] |
| 17 Global outcome: 2b. Specific ‐ alert ‐ ii. up to 4 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 alert at 4 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.42] |
| 18 Global outcome: 2c. Specific ‐ alert ‐ iii. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 alert at 8 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.67, 1.60] |
| 18.2 alert at 16 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.58, 1.78] |
| 18.3 alert at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.20] |
| 19 Global outcome: 2d. Specific ‐ tranquil ‐ i. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 tranquil at 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.29, 1.34] |
| 20 Global outcome: 2e. Specific ‐ tranquil ‐ ii. up to 4 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 tranquil at 4 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.94, 3.62] |
| 21 Global outcome: 2f. Specific ‐ tranquil ‐ iii. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 tranquil at 8 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.55, 1.87] |
| 21.2 tranquil at 16 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.36, 1.45] |
| 21.3 tranquil at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.83, 2.72] |
| 22 Global outcome: 2g. Specific ‐ sedated ‐ i. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 sedated at 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.39] |
| 23 Global outcome: 2h. Specific ‐ sedated ‐ ii. up to 4 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 sedated at 4 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.18, 1.03] |
| 24 Global outcome: 2i. Specific ‐ sedated ‐ iii. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 sedated at 8 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.49, 2.37] |
| 24.2 sedated at 16 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.50] |
| 24.3 sedated at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.19, 1.98] |
| 25 Global outcome: 2j. Specific ‐ asleep ‐ i. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 asleep at 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.64] |
| 26 Global outcome: 2k. Specific ‐ asleep ‐ ii. up to 4 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 asleep at 4 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.07, 16.84] |
| 27 Global outcome: 2l. Specific ‐ asleep ‐ iii. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 asleep at 8 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.34] |
| 27.2 asleep at 16 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [0.97, 19.40] |
| 27.3 asleep at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.14, 77.79] |
| 28 Service use: 1. Average days to discharge Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.85, 0.65] |
| 29 Service use: 2. Average patient‐hours used during 24 hours (skewed data) Show forest plot | Other data | No numeric data | ||
| 29.1 days 1‐7 | Other data | No numeric data | ||
| 29.2 days 8‐14 | Other data | No numeric data | ||
| 29.3 days 15‐21 | Other data | No numeric data | ||
| 30 Mental state: 1. Various outcomes reported as 'adverse events' Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 anxiety | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.12] |
| 30.2 anxiety during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.08, 1.70] |
| 30.3 delusions | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.12] |
| 30.4 nervousness during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.70, 6.74] |
| 31 Mental state: 2a. Average scores ‐ i. by 30 minutes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 31.1 change score at 15 minutes (BPRS positive sub‐scale, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.11, 1.51] |
| 31.2 change score at 30 minutes (BPRS positive sub‐scale, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.70, 1.90] |
| 31.3 change score at 15 minutes (BPRS total, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐5.42, 4.02] |
| 31.4 change score at 30 minutes (BPRS total, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐7.35, 6.75] |
| 32 Mental state: 2b. Average scores ‐ ii. up to 2 hours Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 32.1 change score at 1 hour (BPRS positive sub‐scale, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.74, 3.94] |
| 32.2 change score at 2 hours (BPRS positive sub‐scale, high=worse) | 3 | 385 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.34, 0.89] |
| 32.3 change score at 1 hour (BPRS total, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [‐5.03, 14.43] |
| 32.4 change score at 2 hours (BPRS total, high=worse) | 3 | 385 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [‐0.12, 3.62] |
| 33 Mental state: 2c. Average scores ‐ iii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 33.1 change score at 24 hours (BPRS positive sub‐scale, high=worse) | 2 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.97, 0.37] |
| 33.2 change score at 24 hours (BPRS total, high=worse) | 2 | 340 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐1.34, 2.29] |
| 34 Mental state: 2d. Average scores ‐ iv. over 24 hours Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [‐0.18, 9.58] |
| 34.1 change score at 21 days (PANSS total, high=worse) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [‐0.18, 9.58] |
| 35 Adverse effects: 1a. General Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 35.1 one or more drug related adverse events | 3 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.09, 1.76] |
| 36 Adverse effects: 1b. General ‐ serious Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 36.1 one or more treatment emergent adverse events | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.36, 1.83] |
| 36.2 treatment emergent adverse events ‐ all | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.69, 2.19] |
| 36.3 overall serious adverse effects | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 36.4 death | 3 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 37.1 increased salivation during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.54, 176.18] |
| 38 Adverse effects: 2b. Specific ‐ arousal level ‐ i. various Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 38.1 insomnia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.39, 2.78] |
| 38.2 insomnia during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.57, 8.19] |
| 38.3 somnolence at 24 hours | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.45, 2.41] |
| 38.4 somnolence during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.67, 3.12] |
| 38.5 excessive sedation during 24 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 39 Adverse effects: 2c. Specific ‐ arousal level ‐ ii. average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 39.1 endpoint score at 1 hour (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.13, 0.73] |
| 39.2 endpoint score at 2 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.36, 0.36] |
| 40 Adverse effects: 2d. Specific ‐ arousal level ‐ ii. average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 40.1 endpoint score at 4 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.61, 0.21] |
| 41 Adverse effects: 2e. Specific ‐ arousal level ‐ ii. average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 41.1 endpoint score at 6 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.38, 0.18] |
| 41.2 endpoint score at 12 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.26, 0.26] |
| 42 Adverse effects: 2f. Specific ‐ cardiovascular i. binary Show forest plot | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.38] |
| 42.1 clinically significant ECG change | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 42.2 hypotension during 24 hours | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.38] |
| 43 Adverse effects: 2g. Specific ‐ cardiovascular ii. continuous Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 43.1 QT interval: average change at 24 hours | 1 | 257 | Mean Difference (IV, Fixed, 95% CI) | 1.8 [‐3.81, 7.41] |
| 43.2 QT interval: average endpoint score at 24 hours | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 8.5 [‐3.28, 20.28] |
| 44 Adverse effects: 2h. Specific ‐ gastrointestinal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 44.1 abdominal pain | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 73.04] |
| 44.2 vomiting | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.2 [0.26, 103.03] |
| 45 Adverse effects: 2i. Specific ‐ movement disorder ‐ i. various Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 45.1 dystonia during 24 hours | 2 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.92 [1.67, 99.78] |
| 45.2 dystonia during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.54, 176.18] |
| 45.3 hypertonia during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.54, 176.18] |
| 45.4 EPS during 24 hours | 3 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.35 [2.27, 30.63] |
| 45.5 EPS during study | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.73] |
| 45.6 extrapyramidal effects ‐ use of antiparkinson drugs during 24 hours | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.51 [1.92, 10.58] |
| 45.7 extrapyramidal effects ‐ use of antiparkinson drugs during 21 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.54, 176.18] |
| 45.8 extrapyramidal effects ‐ use of antiparkinson drugs during study | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.73] |
| 46 Adverse effects: 2j. Specific ‐ movement disorder ‐ ii. average scores ‐ i. up to 24 hours Show forest plot | 1 | 483 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.16, 0.53] |
| 46.1 change score at 24 hours (SAS, high=worse) | 1 | 242 | Mean Difference (IV, Fixed, 95% CI) | 1.31 [0.56, 2.06] |
| 46.2 change score at 24 hours (BAS, high=worse) | 1 | 241 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.09, 0.47] |
| 47 Adverse effects: 2k. Specific ‐ movement disorder ‐ iii. average scores ‐ i. up to 24 hours Show forest plot | Other data | No numeric data | ||
| 47.1 endpoint score at 24 hours (SAS, high=worse) ‐ (skewed data) | Other data | No numeric data | ||
| 47.2 endpoint score at 24 hours (BAS, high=worse) ‐ (skewed data) | Other data | No numeric data | ||
| 48 Adverse effects: 2l. Specific ‐ movement disorder ‐ vi. average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 48.1 endpoint score at 96 hours (AIMS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.87, 1.27] |
| 48.2 endpoint score at 96 hours (BARS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.8 [‐0.01, 1.61] |
| 48.3 endpoint score at 96 hours (SAS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.2 [‐0.07, 4.47] |
| 49 Adverse effects: 2m. Specific ‐ miscellaneous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 49.1 pain | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 73.04] |
| 49.2 pain during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.33, 3.51] |
| 49.3 pain ‐ headache during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.88, 5.32] |
| 49.4 other ‐ nose bleed | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 73.04] |
| 49.5 other ‐ clinically relevant laboratory changes | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 50 Leaving the study early Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 50.1 any reason | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.04, 2.65] |
| 50.2 adverse effects | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.67 [1.13, 66.75] |
| 50.3 lack of efficacy | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.09, 1.44] |
| 50.4 lost at follow‐up | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.10, 2.82] |
| 50.5 participants decision | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.57, 8.19] |
| 50.6 non compliance | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.42, 11.30] |
| 50.7 physician decision | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [0.50, 37.42] |
| 50.8 sponsor decision | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global outcome: No improvement Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 4.68] |
| 2 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 one or more adverse effect | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.61, 2.80] |
| 2.2 clinically significant laboratory changes | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Adverse effects: 2. Specific Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 hypotensive episode | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.12] |
| 3.2 require antiparkinson medication | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.62, 12.12] |
| 3.3 discontinued due to EPS | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.18, 18.70] |
| 3.4 discontinued due to drowsiness/tension and no therapeutic effect | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.12, 64.04] |
| 4 Leaving the study early: 1. Specific reasons Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 discontinued due to EPS | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.18, 18.70] |
| 4.2 discontinued due to drowsiness/tension and no therapeutic effect | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.12, 64.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 change score at 24 hours (PANSS‐EC, high=worse) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.56, 0.76] |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 change score at 72 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 change score at 7 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 change score at 10 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 change score at 14 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 change score at 28 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mental state: Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 change score at 28 days (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 change score at 28 days (PANSS positive sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 change score at 28 days (PANSS negative sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 one or more drug related adverse events by 28 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects: 2. Specific ‐ movement disorder ‐ i. average scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 endpoint score at 28 days (SAS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 any reason | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 lost at follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ i. by 30 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 not asleep at 30 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.95] |
| 2 Tranquillisation or asleep ‐ ii. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 not asleep at 1 hour | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.92] |
| 2.2 not asleep at 2 hours | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.51, 0.99] |
| 3 Specific behaviour: 1a. Agitation ‐ various measures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 ≥50% reduction in PANSS‐EC score up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.79, 1.16] |
| 3.2 discontinued due to severe agitation up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.03] |
| 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 endpoint score at 2 hours (PANSS‐PAS, high=worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐5.22, 4.42] |
| 5 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. by 30 minutes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 change score at 30 minutes (OAS total aggression, high=worse) | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.58, ‐0.42] |
| 6 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 change score at 1 hour (OAS total aggression, high=worse) | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.27, ‐0.13] |
| 6.2 change score at 2 hours (OAS total aggression, high=worse) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.13, ‐0.07] |
| 7 Global outcome: 1. Binary measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 needing additional benzodiazepine | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.47] |
| 7.2 rated as severe at 24 hours (CGI‐S) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.51, 1.58] |
| 8 Global outcome: 2. Continuous measures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 dose of lorazapam | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.49, 0.29] |
| 8.2 time to additional dose | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐2.17, 2.57] |
| 9 Adverse effects: 1a. General ‐ one or more adverse effects Show forest plot | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.23] |
| 9.1 up to 24 hours | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.23] |
| 10 Adverse effects: 2a. Specific ‐ arousal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 sedated at 30 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.39, 2.59] |
| 10.2 sedated at 60 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.15, 1.72] |
| 10.3 sedated at 120 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.98, 1.23] |
| 10.4 somnolence during 24 hours (only reported if occurred in ≥5%) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.43, 2.12] |
| 10.5 somnolence during 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.96] |
| 10.6 insomnia ‐ severe | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.47] |
| 10.7 somnolence ‐ severe | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.13, 76.20] |
| 11 Adverse effects: 2b. Specific ‐ cardiovascular ‐ i. binary outcomes Show forest plot | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.45, 4.70] |
| 11.1 dizziness during 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.82] |
| 11.2 orthostatic hypertension | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.25 [0.26, 107.67] |
| 12 Adverse effects: 2c. Specific ‐ cardiovascular ‐ ii. pulse rate ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 heartbeat change at 60 minutes | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐9.4 [‐9.99, ‐8.81] |
| 13 Adverse effects: 2d. Specific ‐ cardiovascular ‐ ii. pulse rate ‐ ii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 heartbeat change at 8 hours | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐8.8 [‐9.75, ‐7.85] |
| 14 Adverse effects: 2e. Specific ‐ movement disorder ‐ i. binary outcomes Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 dystonia during 24 hours | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.28, 7.28] |
| 14.2 dyskinesia during 24 hours | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.13, 76.20] |
| 14.3 hyperkinesia during 24 hours (only reported if occurred in ≥5%) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.20 [0.48, 36.79] |
| 14.4 hypertonia/rigidity during 24 hours | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.47] |
| 14.5 tremor during 24 hours | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.25 [0.26, 107.67] |
| 14.6 movement disorder | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.55, 4.62] |
| 15 Adverse effects: 2f. Specific ‐ movement disorders ‐ ii. average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 change score at 24 hours (BAS akathisia, high=worse) | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.3 [0.24, 0.36] |
| 15.2 change score at 24 hours (SAS movement disorders, high=worse) | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [0.34, 0.46] |
| 16 Adverse effects: 2g. Specific ‐ movement disorder ‐ ii. average scores ‐ ii. over 24 hours Show forest plot | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [‐0.32, 1.47] |
| 16.1 endpoint score at 96 hours (AIMS, high=worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 endpoint score at 96 hours (BARS, high=worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.36, 1.56] |
| 16.3 endpoint score at 96 hours (SAS, high=worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.20, 3.00] |
| 17 Adverse effects: 2h. Specific ‐ miscellaneous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 agitation during 24 hours (only reported if occurred in ≥5%) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.27, 4.06] |
| 17.2 agitation ‐ severe | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.19, 22.72] |
| 17.3 anxiety ‐ severe | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.13, 76.20] |
| 17.4 headache during 24 hours (only reported if occurred in ≥5%) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.37, 4.71] |
| 17.5 headache during 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.71] |
| 18 Leaving the study early ‐ i. up to 24 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 due to severe agitation | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.03] |
| 18.2 due to acute dystonia | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.07] |
| 18.3 due to adverse effects | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.07, 16.51] |
| 18.4 unspecified general reasons | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.42, 1.44] |
| 19 Leaving the study early ‐ ii. over 24 hours Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.89] |
| 19.1 Lack of efficacy at 96 hours | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for rapid tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 more than 1 injection | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| 1.2 more than 3 injections | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.57, 10.93] |
| 2 Specific behaviour: 1. Agitation ‐ rated as 'adverse effect' Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.52] |
| 3 Global outcome Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 no response to 1st injection | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.57, 11.05] |
| 3.2 no improvement at 1 hour | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.14, 1.84] |
| 3.3 no improvement at 2 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.31] |
| 3.4 no improvement at 3 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.49] |
| 3.5 no improvement at 4 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.49] |
| 3.6 no improvement at 5 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.2 [0.21, 82.72] |
| 4 Adverse effects: 1. General ‐ one or more adverse effects Show forest plot | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.97, 2.22] |
| 5 Adverse effects: 2a. Specific ‐ movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 ataxia | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.49] |
| 5.2 thick tongue | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.11, 58.67] |
| 5.3 use of antiparkinson drugs | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse effects: 2b. Specific ‐ others Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 anticholinergic ‐ blurred vision | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.2 [0.21, 82.72] |
| 6.2 anticholinergic ‐ dry mouth | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.31] |
| 6.3 arousal ‐ drowsiness | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.02, 2.90] |
| 6.4 arousal ‐ lightheadedness | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.2 [0.21, 82.72] |
| 6.5 cardiac ‐ chest pain | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.52] |
| 6.6 cardiac ‐ dizziness | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.28, 22.20] |
| 6.7 cardiac ‐ hypotensive crisis | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 cardiac ‐ orthostatic hypotension | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.49] |
| 6.9 cardiac ‐ tachycardia | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.52] |
| 6.10 other ‐ depressed mood | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.11, 58.67] |
| 6.11 other ‐ diaphoresis | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.52] |
| 6.12 other ‐ weakness | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.30, 25.21] |
| 6.13 other ‐ unsteadiness | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for tranquillisation ‐ need for additional drugs for tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 up to 2 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.13, 1.01] |
| 1.2 up to 24 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.07, 2.53] |
| 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 endpoint score at 1 hour (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐7.70 [‐9.41, ‐5.99] |
| 2.2 endpoint score at 2 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐6.38, ‐1.42] |
| 2.3 endpoint score at 2 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐1.13, 1.25] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 endpoint score at 4 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.45] |
| 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 endpoint score at 6 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.05, 0.35] |
| 4.2 endpoint score at 6 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.68, 0.74] |
| 4.3 endpoint score at 12 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.9 [0.82, 0.98] |
| 4.4 endpoint score at 24 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐1.36, 0.94] |
| 5 Specific behaviour: 1d. Agitation ‐ average scores ‐ iv. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 endpoint scores at 48 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐1.03, 1.15] |
| 5.2 endpoint scores at 72 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [‐0.45, 1.69] |
| 6 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 endpoint score at 1 hour (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.77, 0.77] |
| 6.2 endpoint score at 2 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.25, 1.65] |
| 7 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 endpoint score at 4 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.04, 0.64] |
| 8 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 endpoint score at 6 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.60, 0.80] |
| 8.2 endpoint score at 12 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.07, 0.33] |
| 9 Global outcome: 1. General ‐ need for additional measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 need for anxiolytic during 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.84, 1.48] |
| 9.2 need for hypnotics for night time sedation during 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.20, 2.50] |
| 9.3 additional restraint, seclusion or special observation during 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.25, 1.44] |
| 10 Global outcome: 2. Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 change score at 72 hours (CGI‐S, high=worse) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.13, 0.55] |
| 10.2 change score at 7 days (CGI‐S, high=worse) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [0.07, 0.95] |
| 11 Mental state: 1a. Average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 endpoint score at 2 hours (BPRS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Mental state: 1b. Average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 endpoint score at 4 hours (BPRS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Mental state: 1c. Average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 endpoint score at 24 hours (BPRS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Mental state: 1d. Average scores ‐ iv. over 24 hours Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 endpoint score at 48 hours (BPRS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.05 [‐6.45, 2.35] |
| 14.2 endpoint score at 72 hours (PANSS total, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 2.45 [‐2.19, 7.09] |
| 14.3 endpoint score at 72 hours (PANSS positive sub‐scale, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.45, 2.67] |
| 14.4 endpoint score at 72 hours (PANSS negative sub‐scale, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐4.19 [‐5.71, ‐2.67] |
| 14.5 endpoint score at 72 hours (PANSS general pathology score, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.27, 2.27] |
| 14.6 score at 72 hours (BPRS, high=worse) | 3 | 511 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.93, 1.07] |
| 14.7 change score at 7 days (BPRS, high=worse) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | 2.93 [‐0.81, 6.67] |
| 15 Adverse effects: 1a. General ‐ i. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 one or more drug related adverse effects ‐ by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.64, 2.93] |
| 16 Adverse effects: 1b. General ‐ ii. over 24 hours Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 one or more drug related adverse effects ‐ by 72 hours | 4 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.47, 2.06] |
| 16.2 one or more drug related adverse effects ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.93, 1.83] |
| 17 Adverse effects: 1c. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 severe adverse effect ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 death | 2 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.78] |
| 18 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 blurred vision by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.97 [1.15, 13.68] |
| 18.2 constipation by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
| 18.3 dry mouth by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [1.22, 7.22] |
| 18.4 hypersalivation by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.11, 5.87] |
| 19 Adverse effects: 2b. Specific ‐ arousal level ‐ i. various Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 excessive sedation at 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.22, 4.56] |
| 19.2 insomnia ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.53] |
| 19.3 lethargy ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.67, 2.35] |
| 19.4 somnolence ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.43, 3.12] |
| 20 Adverse effects: 2c. Specific ‐ arousal level ‐ ii. average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 endpoint score at 1 hour (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.46, 0.46] |
| 20.2 endpoint score at 2 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.46, 0.46] |
| 21 Adverse effects: 2d. Specific ‐ arousal level ‐ ii. average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1 endpoint score at 4 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.83, 0.03] |
| 22 Adverse effects: 2e. Specific ‐ arousal level ‐ ii. average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.1 endpoint score at 6 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.40, 0.20] |
| 22.2 endpoint score at 12 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.40, 0.20] |
| 23 Adverse effects: 2f. Specific ‐ cardiovascular Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 hypotension at 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.31] |
| 23.2 dizziness ‐ by 72 hours | 2 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.28, 1.19] |
| 23.3 ECG ‐ abnormal ‐ by 72 hours | 2 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.17, 0.84] |
| 23.4 ECG change ‐ clinically significant ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.60, 1.71] |
| 23.5 tachycardia ‐ by 72 hours | 3 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.23, 1.04] |
| 24 Adverse effects: 2g. Specific ‐ gastrointestinal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 nausea by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
| 24.2 vomiting by 72 hours | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.02, 5.72] |
| 24.3 vomiting by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.82] |
| 25 Adverse effects: 2h. Specific ‐ hematological tests Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 abnormal lab results ‐ by 72 hours | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.69, 1.17] |
| 25.2 abnormal lab results ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.47, 2.15] |
| 25.3 aspartate aminotransference ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.12 [0.62, 199.64] |
| 25.4 glucose increased ‐ by 24 hours | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.31, 2.92] |
| 25.5 haemogram abnormal ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.35] |
| 25.6 lactate dehydrogenase increase ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.39] |
| 25.7 liver function abnormal ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.39] |
| 25.8 triglyceride increase ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.96 [0.24, 102.14] |
| 26 Adverse effects: 2i. Specific ‐ movement disorders ‐ i. binary measures Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 akathisia ‐ by 72 hours | 3 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.34, 4.01] |
| 26.2 akathisia ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [1.13, 16.31] |
| 26.3 dystonia ‐ by 72 hours | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.26 [1.67, 63.17] |
| 26.4 dystonia ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [0.76, 9.47] |
| 26.5 EPS ‐ by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.64, 190.53] |
| 26.6 EPS ‐ by 72 hours | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.13 [7.59, 48.21] |
| 26.7 EPS ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 34.29 [4.70, 250.02] |
| 26.8 extrapyramidal effects ‐ use of antiparkinson drugs ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.30 [1.82, 5.97] |
| 26.9 hypertonia/rigidity ‐ by 72 hours | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.81 [0.78, 280.47] |
| 26.10 hypertonia ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [0.90, 14.25] |
| 26.11 myotonia ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.84 [1.85, 8.00] |
| 26.12 pyramidal tract syndrome ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.18 [1.00, 295.55] |
| 26.13 reduced movement ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.17, 3.91] |
| 26.14 torsional spasm ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.30 [0.93, 11.70] |
| 26.15 tremor ‐ by 72 hours | 2 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.37, 5.11] |
| 26.16 tremor ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [0.82, 22.48] |
| 27 Adverse effects: 2j. Specific ‐ movement disorders ‐ ii. average scores ‐ i. over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 27.1 change score at 72 hours (BAS, high=worse) | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [0.18, 0.76] |
| 27.2 score at 72 hours (SAS, high=worse) | 2 | 191 | Mean Difference (IV, Fixed, 95% CI) | 3.59 [2.15, 5.03] |
| 27.3 change score at 7 days (BAS, high=worse) | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.9 [0.51, 1.29] |
| 27.4 change score at 7 days (SAS, high=worse) | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 6.1 [3.91, 8.29] |
| 28 Adverse effects: 2k. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 physical examination significant changes ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 27.29 [1.63, 455.72] |
| 29 Leaving the study early: 1. For any reason ‐ i. over 24 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 29.1 by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.53, 5.94] |
| 29.2 by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.86, 5.32] |
| 30 Leaving the study early: 2. For specific reasons ‐ i. over 24 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 adverse events ‐ by 72 hours | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.45, 12.75] |
| 30.2 adverse events ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.06, 4.65] |
| 30.3 withdrew consent ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.24, 104.55] |
| 30.4 other ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for tranquillisation: more than 3 injections Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [1.19, 5.46] |
| 2 Adverse effects: 1a. Specific ‐ movement disorders Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 movement disorder ‐ tremor by 7 days | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.16 [0.93, 18.62] |
| 3 Adverse effects: 1b. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 pain/allergy ‐ reaction at injection site | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.55 [0.15, 84.14] |
| 4 Leaving the study early Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1. Aggression ‐ binary measures ‐ i. up to 2 hours Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.86, 1.55] |
| 1.1 ≥50% reduction at 90 minutes (OAS) | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.86, 1.55] |
| 2 Global outcome: 1. Need for seclusion or restraint Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Adverse effects: 1. Specific ‐ movement disorders ‐ i. binary measures ‐ i. by 30 minutes Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 EPS by 30 minutes | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ not asleep Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 at 1 hour | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.76, 1.44] |
| 1.2 by 3 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.14, 3.27] |
| 2 Repeated need for rapid tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 more than 1 injection | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.91, 1.43] |
| 2.2 more than 3 injections | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.50, 2.45] |
| 3 Global outcome: 1. No overall improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 by 30 minutes | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.70, 1.71] |
| 3.2 at 1 hour | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.54, 5.03] |
| 3.3 at > 1 hour | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mental state: 1a. Average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 endpoint score at 1 hour (BPRS, high=worse) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 3.26 [‐4.13, 10.65] |
| 4.2 endpoint score at 2 hours (BPRS, high=worse) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 4.07 [‐2.62, 10.76] |
| 5 Mental state: 1b. Average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 endpoint score at 3 hours (BPRS, high=worse) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 5.03 [‐2.98, 13.04] |
| 5.2 endpoint score at 4 hours (BPRS, high=worse) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 1.73 [‐6.14, 9.60] |
| 6 Adverse effects: 1a. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 one or more drug‐related adverse effect up to 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.60, 2.10] |
| 7 Adverse effects: 1b. General ‐ serious Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.14, 2.04] |
| 8.1 dry mouth up to 24 hours (only reported if occurred ≥9%) | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.14, 2.04] |
| 9 Adverse effects: 2b. Specific ‐ arousal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 asleep ‐ at 1 hour | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.40, 1.99] |
| 10 Adverse effects: 2c. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 dizziness (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.19, 4.07] |
| 10.2 hypertension ‐ use of additional clonidine ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.11, 63.17] |
| 11 Adverse effects: 2d. Specific ‐ movement disorder Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 ataxia (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.04, 4.65] |
| 11.2 dystonia (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.54 [0.42, 30.03] |
| 11.3 EPS | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.0 [2.11, 106.49] |
| 11.4 hypertonia/rigidity (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.22 [0.33, 115.91] |
| 11.5 speech disorder (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.35, 9.01] |
| 11.6 tremor (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.17, 18.60] |
| 11.7 use of additional benztropine ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.68, 5.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep: average times in minutes ‐ (skewed data) Show forest plot | Other data | No numeric data | ||
| 1.1 average time to sedation | Other data | No numeric data | ||
| 1.2 average time to arousal | Other data | No numeric data | ||
| 2 Global outcome: 1. Need for rescue drug Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.46, 2.87] |
| 3 Adverse effects: 1. General ‐ one or more drug related adverse effect Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.11] |
| 4 Adverse effects: 2. Specific Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 hypotensive | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.61] |
| 4.2 apnoea | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global outcome: 1. No overall improvement Show forest plot | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.18 [0.50, 133.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ asleep Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.11, 3.02] |
| 1.1 not asleep by 3 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.11, 3.02] |
| 2 Repeated need for rapid tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 needing additional injection during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.87, 1.27] |
| 2.2 more than 3 injections during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.92, 10.10] |
| 3 Global outcome: 1. No overall improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 by 30 minutes | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.25, 5.68] |
| 3.2 at 1 hour | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.77 [0.88, 247.54] |
| 3.3 at > 1 hour | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Adverse effects: 1. General Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.62, 2.18] |
| 4.1 one or more drug‐related adverse effect during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.62, 2.18] |
| 5 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 dry mouth (only reported if occurred in ≧9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.20, 4.21] |
| 6 Adverse effects: 2b. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 dizziness (only reported if occurred in ≧9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.24, 7.69] |
| 6.2 hypertension ‐ use of additional clonidine during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.06, 14.02] |
| 7 Adverse effects: 2c. Specific ‐ movement disorders Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 ataxia (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 2.78] |
| 7.2 dystonia (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.25 [0.46, 147.45] |
| 7.3 hypertonia/rigidity (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.30, 25.05] |
| 7.4 tremor (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.17, 19.21] |
| 7.5 speech disorder (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.30, 5.03] |
| 7.6 use of additional benztropine during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.81, 9.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 need for additional drugs for tranquillisation up to 2 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.10, 0.71] |
| 1.2 need for additional drugs for tranquillisation by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
| 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 endpoint score at 1 hour (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐8.5 [‐9.93, ‐7.07] |
| 2.2 endpoint score at 2 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐6.7 [‐7.46, ‐5.94] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 endpoint score at 4 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐5.5 [‐6.48, ‐4.52] |
| 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 endpoint score at 6 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐4.56, ‐2.84] |
| 4.2 endpoint score at 12 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐11.2 [‐12.24, ‐10.16] |
| 5 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 endpoint score at 1 hour (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.44, 0.04] |
| 5.2 endpoint score at 2 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐4.21, ‐0.59] |
| 6 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 endpoint score at 4 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.68, 0.48] |
| 7 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 endpoint score at 6 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.30, 0.50] |
| 7.2 endpoint score at 12 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.9 [‐2.58, ‐1.22] |
| 8 Global outcomes: 1. General ‐ need for additional measures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 additional restraint, seclusion or special observation during 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.13, 0.61] |
| 9 Adverse effects: 1b. General ‐ one or more adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 during 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.41, 1.32] |
| 10 Adverse effects: 1a. General ‐ serious Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Adverse effects: 2a. Specific ‐ arousal ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 excessive sedation by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.80] |
| 12 Adverse effects: 2b. Specific ‐ arousal ‐ ii. average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 endpoint score at 1 hour (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.01, 0.01] |
| 12.2 endpoint score at 2 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.56, 0.36] |
| 13 Adverse effects: 2c. Specific ‐ arousal ‐ ii. average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 endpoint score at 4 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.78, 0.18] |
| 14 Adverse effects: 2d. Specific ‐ arousal ‐ iii. average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 endpoint score at 6 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.40, 0.20] |
| 14.2 endpoint score at 12 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.26, 0.46] |
| 15 Adverse effects: 2e. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 hypotension by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.57] |
| 16 Adverse effects: 2f. Specific ‐ movement disorders Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 EPS by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.44, 6.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ not tranquil or asleep ‐ i. by 30 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 at 20 minutes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.18, 2.16] |
| 2 Tranquillisation or asleep ‐ not tranquil or asleep ‐ ii. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 at 40 minutes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.83, 1.91] |
| 2.2 at 1 hour | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.85, 2.37] |
| 2.3 at 2 hours | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.68, 2.56] |
| 3 Repeated need for tranquillisation ‐ need for additional drugs for tranquillisation Show forest plot | 2 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.29] |
| 3.1 by 2 hours | 2 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.41] |
| 3.2 by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.81, 1.49] |
| 4 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 endpoint score at 1 hour (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐24.5 [‐27.32, ‐21.68] |
| 4.2 endpoint score at 2 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐9.40 [‐10.39, ‐8.41] |
| 5 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 endpoint score at 4 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [3.27, 4.33] |
| 6 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 endpoint score at 6 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.6 [2.13, 3.07] |
| 6.2 endpoint score at 12 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.55, 1.05] |
| 7 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 endpoint score at 1 hour (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.50 [‐6.28, ‐2.72] |
| 7.2 endpoint score at 2 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.89, 0.49] |
| 8 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 endpoint score at 4 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.49, 0.71] |
| 9 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 endpoint score at 6 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [0.91, 1.29] |
| 9.2 endpoint score at 12 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.8 [1.67, 1.93] |
| 10 Specific behaviour: 2d. Aggression ‐ further aggressive episode ‐ i. up to 24 hours Show forest plot | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.65] |
| 11 Global outcomes: 1. General ‐ need for additional measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 restraints needed by 2 hours | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.84, 1.76] |
| 11.2 additional restraint, seclusion or special observation during 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.41, 3.51] |
| 12 Global outcomes: 2. Specific Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 doctor called to see patient | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.05, 2.14] |
| 12.2 refuse oral drugs | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.61, 1.60] |
| 13 Adverse effects: 1a. General Show forest plot | 2 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.07, 3.80] |
| 13.1 one or more adverse effects up to 24 hours | 2 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.07, 3.80] |
| 14 Adverse effects: 1b. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 seizure | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.19, 22.39] |
| 14.2 death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Adverse effects: 2a. Specific ‐ arousal ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 excessive sedation by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 16 Adverse effects: 2b. Specific ‐ arousal ‐ ii. average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 endpoint score at 1 hour (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.38, 0.58] |
| 16.2 endpoint score at 2 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.50, 0.30] |
| 17 Adverse effects: 2c. Specific ‐ arousal ‐ ii. average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 endpoint score at 4 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.78, 0.18] |
| 18 Adverse effects: 2d. Specific ‐ arousal ‐ ii. average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 endpoint score at 6 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.48, 0.08] |
| 18.2 endpoint score at 12 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 19 Adverse effects: 2e. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 hypotension by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.65] |
| 20 Adverse effects: 2f. Specific ‐ movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 acute dystonia | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.48 [1.14, 331.92] |
| 20.2 EPS by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.32, 3.10] |
| 21 Service outcomes: 1. Not discharged by 14 days Show forest plot | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.05] |
| 22 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 any reason | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.54, 1.94] |
| 22.2 absconded before receiving drug | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.24] |
| 22.3 incomplete information in notes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.51, 2.46] |
| 22.4 seizure before drug was given | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.95] |
| 22.5 transfer to another hospital/notes lost | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.21, 5.00] |
| 22.6 withdrew consent | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.06, 16.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 endpoint score at 24 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 endpoint score at 3 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 endpoint score at 5 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 endpoint score at 1 week (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 endpoint score at 2 weeks (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Global outcome: No improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 at 72 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 at 5 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 at 1 week | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 at 2 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: 1a. Specific ‐ arousal level ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 insomnia during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 somnolence during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects: 1b. Specific ‐ movement disorders ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 tremor during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 akathisia during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Adverse effects: 1c. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Dry mouth during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Constipation during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Increased salivation during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1. Agitation ‐ average scores ‐ over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 endpoint score at 72 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 endpoint score at 7 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 endpoint score at 14 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Global outcome: No improvement Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.38, 2.95] |
| 3 Mental state: Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 endpoint score at 72 hours (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 endpoint score at 7 days (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 endpoint score at 14 days (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: General ‐ over 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 one or more drug related adverse effects ‐ by 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ binary measures Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.44, 3.06] |
| 1.1 <25% reduction, no effect (PANSS‐EC) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.44, 3.06] |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 endpoint score at 3 days (PANSS‐EC, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐2.31, 2.35] |
| 2.2 endpoint score at 7 days (PANSS‐EC, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.16 [‐0.98, 3.30] |
| 2.3 endpoint score at 14 days (PANSS‐EC, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.24 [‐0.49, 2.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 not asleep at 2 hours | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.39] |
| 1.2 not asleep at 4 hours | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.66, 1.03] |
| 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 change score at 2 hours (PANSS‐EC, high=worse) | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.80, 2.80] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 change score at 4 hours (PANSS‐EC, high=worse) | 1 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐5.20, 0.40] |
| 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 score at 24 hours (PANSS‐EC, high=worse) | 2 | 305 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [‐0.56, 1.98] |
| 5 Specific behaviour: 1d. Agitation ‐ average scores ‐ iv. over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 score at 3 days (PANSS‐EC, high=worse) | 2 | 305 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.66, 0.83] |
| 5.2 score at 5 days (PANSS‐EC, high=worse) | 2 | 305 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐1.24, 1.52] |
| 5.3 endpoint score at 1 week (PANSS‐EC, high=worse) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.56 [‐4.57, ‐0.55] |
| 5.4 endpoint score at 2 weeks (PANSS‐EC, high=worse) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐2.34, 0.58] |
| 6 Global outcome: No improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 at 72 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 at 5 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 at 1 week | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 at 2 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Mental state: 1. Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 change score at 120 hours (PANSS total, high=worse) | 1 | 205 | Mean Difference (IV, Fixed, 95% CI) | ‐3.5 [‐7.07, 0.07] |
| 8 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 overall adverse events during 120 hours | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.29, 2.29] |
| 9 Adverse effects: 2a. Specific ‐ arousal ‐ i. various Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 insomnia during 5 days (only reported if occurred in ≥5%) | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.45, 5.31] |
| 9.2 insomnia during 14 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.20, 1.23] |
| 9.3 somnolence during 14 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.94, 8.06] |
| 10 Adverse effects: 2b. Specific ‐ cardiovascular ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 tachycardia during 120 hours (only reported if occurred in ≥5%) | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.36, 4.66] |
| 11 Adverse effects: 2c. Specific ‐ movement disorders ‐ i. various Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 akathisia during 5 days (only reported if occurred in ≥5%) | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.12, 2.38] |
| 11.2 akathisia during 14 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.98, 5.58] |
| 11.3 EPS during 5 days (only reported if occurred in ≥5%) | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.52, 3.23] |
| 11.4 tremor during 14 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.2 [1.27, 8.07] |
| 12 Adverse effects: 2d. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.1 Dry mouth during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Constipation during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Increased salivation during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 endpoint score at 12 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 endpoint score at 48 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 endpoint score at 3 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 endpoint score at 5 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 endpoint score at 7 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Global outcome: 1. Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 endpoint score at 7 days (CGI‐S, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mental state: 1. Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 endpoint score at 7 days (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 endpoint score at 7 days (PANSS positive sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 endpoint score at 7 days (PANSS negative sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 endpoint score at 7 days (PANSS general psychopathology sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 any reason | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 lost at follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 non compliance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| AE | Dose | 1 mg (N = 57) | 5 mg (N = 63) | 15 mg (N = 58) |
| At least 1 AE | 28 | 30 | 27 | |
| AE rated as serious | 1 | 2 | 0 | |
| Tachycardia | 3 | 2 | 0 | |
| Sinus tachycardia | 1 | 0 | 0 | |
| Vomiting | 1 | 0 | 3 | |
| Nausea | 0 | 6 | 2 | |
| Dizziness | 4 | 7 | 7 | |
| Headache | 4 | 11 | 8 | |
| Somnolence | 3 | 5 | 6 | |
| Akathisia | 0 | 2 | 0 | |
| Dystonia | 0 | 0 | 1 | |
| Agitation | 0 | 0 | 3 | |
| Pain at injection site | Not reported | 1 | 1 | |
| QTC abnormality | 4 | 4 | 3 | |
| AE: adverse event | ||||
| Outcome | Dose | Mean | SD | N |
| Mean dose of study drug | 2.5 mg | 4 | 1.5 | 48 |
| 5 mg | 6.9 | 2.7 | 45 | |
| 7.5 mg | 9.8 | 3.8 | 46 | |
| Mean dose of benzodiazepines | 2.5 mg | 3.2 | 1.1 | 48 |
| 5 mg | 2.0 | 0 | 45 | |
| 7.5 mg | 3.0 | 1.4 | 46 |
| Rating scale (Mean change at 2 hr) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 2.5 mg | 1.3 | 1.5 | 48 |
| 5 mg | 2.3 | 1.9 | 45 | |
| 7.5 mg | 2.4 | 1.7 | 46 | |
| Specific behaviour: ABS | 2.5 mg | ‐5.8 | 5.5 | 48 |
| 5 mg | ‐9.0 | 5.5 | 45 | |
| 7.5 mg | ‐10.5 | 5.6 | 46 | |
| Specific behaviour: PANSS‐EC | 2.5 mg | ‐5.5 | 4.6 | 48 |
| 5 mg | ‐8.1 | 5.3 | 45 | |
| 7.5 mg | ‐8.7 | 5 | 46 | |
| Mental state: BPRS Total | 2.5 mg | ‐8.2 | 9.1 | 48 |
| 5 mg | ‐10.4 | 7.5 | 45 | |
| 7.5 mg | ‐12.0 | 7 | 46 | |
| Mental state: BPRS Positive | 2.5 mg | ‐1.5 | 3.1 | 48 |
| 5 mg | ‐1.7 | 2.8 | 45 | |
| 7.5 mg | ‐2.1 | 2.9 | 46 | |
| ABS: Agitated Behavior Scale | ||||
| Rating scale (mean change at 24 hr) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 2.5 mg | 0.9 | 0.8 | 48 |
| 5 mg | 1.1 | 1.1 | 45 | |
| 7.5 mg | 1 | 1 | 46 | |
| Specific behaviour: ABS | 2.5 mg | ‐5.7 | 4.2 | 48 |
| 5 mg | ‐6.7 | 5.9 | 45 | |
| 7.5 mg | ‐7.7 | 5.8 | 46 | |
| Specific behaviour: PANSS‐EC | 2.5 mg | ‐4.9 | 4.3 | 48 |
| 5 mg | ‐5.5 | 4.9 | 45 | |
| 7.5 mg | ‐5.5 | 4.1 | 46 | |
| Global outcomes: CGI‐S (24 hr) | 2.5 mg | ‐0.3 | 0.5 | 48 |
| 5 mg | ‐0.5 | 0.8 | 45 | |
| 7.5 mg | ‐0.6 | 0.7 | 46 | |
| Mental state: BPRS Total | 2.5 mg | ‐8.4 | 7.4 | 48 |
| 5 mg | ‐9.2 | 7.8 | 45 | |
| 7.5 mg | ‐9.6 | 7.5 | 46 | |
| Mental state: BPRS Positive | 2.5 mg | ‐1.5 | 2.3 | 48 |
| 5 mg | ‐2.0 | 2.6 | 45 | |
| 7.5 mg | ‐1.9 | 2.7 | 46 | |
| ABS: Agitated Behavior Scale | ||||
|
| Dose | 2.5 mg | 5 mg | 7.5 mg |
| Outcome |
| N = 48 | N = 45 | N = 46 |
| Global outcome: > 1 injection | 25 | 17 | 14 | |
| Leaving the study early: lack of efficacy | 0 | 2 | 0 | |
| Adverse effects: EPS | 0 | 0 | 0 | |
| Adverse effects: akathisia | 0 | 2 | 0 | |
| Adverse effects: QT abnormality | 0 | 4 | 2 | |
| EPS: Extrapyramidal Side Effects | ||||
| Rating scale (Mean change 2 hr after 1st injection) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 1 mg | 0.65 | 1.64 | 56 |
| 5 mg | 1.01 | 1.65 | 62 | |
| 15 mg | 0.99 | 1.73 | 58 | |
| Specific behaviour: CABS | 1 mg | ‐5.15 | 6.8 | 56 |
| 5 mg | ‐5.97 | 6.81 | 62 | |
| 15 mg | ‐7.04 | 7.01 | 58 | |
| Global outcomes: CGI‐I | 1 mg | 3.07 | 0.98 | 56 |
| 5 mg | 2.82 | 1.10 | 62 | |
| 15 mg | 2.66 | 1.07 | 58 | |
| Global outcomes: CGI‐S | 1 mg | ‐0.63 | 1.15 | 56 |
| 5 mg | ‐0.82 | 1.12 | 62 | |
| 15 mg | ‐0.99 | 1.17 | 58 | |
| Mental state: BPRS Total | 1 mg | ‐6.53 | 9.61 | 55 |
| 5 mg | ‐8.16 | 9.66 | 61 | |
| 15 mg | ‐8.88 | 9.89 | 56 | |
| Mental state: BPRS Positive | 1 mg | ‐1.20 | 2.72 | 55 |
| 5 mg | ‐1.47 | 2.71 | 61 | |
| 15 mg | ‐1.86 | 2.82 | 57 | |
| ACES: Agitation‐Calmness Evaluation Scale | ||||
|
| Dose | 1 mg | 5 mg | 15 mg |
| Outcome | N | N = 57 | N = 63 | N = 58 |
| PEC response ≥ 40% | 21 | 31 | 32 | |
| Need for rescue medication | 11 | 5 | 12 | |
| Discontinued for any reason | 2 | 3 | 1 | |
| Discontinued due to adverse event | 0 | 0 | 1 | |
| Withdrew consent | 2 | 2 | 0 | |
| Discontinued due to other known cause | 0 | 1 | 0 | |
| PEC: Psychiatric Emergency Centre | ||||
| AE | Dose | 1 mg (N = 57) | 5 mg (N = 63) | 15 mg (N = 58) |
| At least 1 AE | 28 | 30 | 27 | |
| AE rated as serious | 1 | 2 | 0 | |
| Tachycardia | 3 | 2 | 0 | |
| Sinus tachycardia | 1 | 0 | 0 | |
| Vomiting | 1 | 0 | 3 | |
| Nausea | 0 | 6 | 2 | |
| Dizziness | 4 | 7 | 7 | |
| Headache | 4 | 11 | 8 | |
| Somnolence | 3 | 5 | 6 | |
| Akathisia | 0 | 2 | 0 | |
| Dystonia | 0 | 0 | 1 | |
| Agitation | 0 | 0 | 3 | |
| Pain at injection site | Not reported | 1 | 1 | |
| QTC abnormality | 4 | 4 | 3 | |
| AE: adverse event | ||||
| Outcome | Dose | Mean | SD | N |
| Mean dose of study drug | 2.5 mg | 4 | 1.5 | 48 |
| 5 mg | 6.9 | 2.7 | 45 | |
| 7.5 mg | 9.8 | 3.8 | 46 | |
| Mean dose of benzodiazepines | 2.5 mg | 3.2 | 1.1 | 48 |
| 5 mg | 2.0 | 0 | 45 | |
| 7.5 mg | 3.0 | 1.4 | 46 |
| Rating scale (Mean change at 2 hr) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 2.5 mg | 1.3 | 1.5 | 48 |
| 5 mg | 2.3 | 1.9 | 45 | |
| 7.5 mg | 2.4 | 1.7 | 46 | |
| Specific behaviour: ABS | 2.5 mg | ‐5.8 | 5.5 | 48 |
| 5 mg | ‐9.0 | 5.5 | 45 | |
| 7.5 mg | ‐10.5 | 5.6 | 46 | |
| Specific behaviour: PANSS‐EC | 2.5 mg | ‐5.5 | 4.6 | 48 |
| 5 mg | ‐8.1 | 5.3 | 45 | |
| 7.5 mg | ‐8.7 | 5 | 46 | |
| Mental state: BPRS Total | 2.5 mg | ‐8.2 | 9.1 | 48 |
| 5 mg | ‐10.4 | 7.5 | 45 | |
| 7.5 mg | ‐12.0 | 7 | 46 | |
| Mental state: BPRS Positive | 2.5 mg | ‐1.5 | 3.1 | 48 |
| 5 mg | ‐1.7 | 2.8 | 45 | |
| 7.5 mg | ‐2.1 | 2.9 | 46 | |
| ABS: Agitated Behavior Scale | ||||
| Rating scale (mean change at 24 hr) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 2.5 mg | 0.9 | 0.8 | 48 |
| 5 mg | 1.1 | 1.1 | 45 | |
| 7.5 mg | 1 | 1 | 46 | |
| Specific behaviour: ABS | 2.5 mg | ‐5.7 | 4.2 | 48 |
| 5 mg | ‐6.7 | 5.9 | 45 | |
| 7.5 mg | ‐7.7 | 5.8 | 46 | |
| Specific behaviour: PANSS‐EC | 2.5 mg | ‐4.9 | 4.3 | 48 |
| 5 mg | ‐5.5 | 4.9 | 45 | |
| 7.5 mg | ‐5.5 | 4.1 | 46 | |
| Global outcomes: CGI‐S (24 hr) | 2.5 mg | ‐0.3 | 0.5 | 48 |
| 5 mg | ‐0.5 | 0.8 | 45 | |
| 7.5 mg | ‐0.6 | 0.7 | 46 | |
| Mental state: BPRS Total | 2.5 mg | ‐8.4 | 7.4 | 48 |
| 5 mg | ‐9.2 | 7.8 | 45 | |
| 7.5 mg | ‐9.6 | 7.5 | 46 | |
| Mental state: BPRS Positive | 2.5 mg | ‐1.5 | 2.3 | 48 |
| 5 mg | ‐2.0 | 2.6 | 45 | |
| 7.5 mg | ‐1.9 | 2.7 | 46 | |
| ABS: Agitated Behavior Scale | ||||
|
| Dose | 2.5 mg | 5 mg | 7.5 mg |
| Outcome |
| N = 48 | N = 45 | N = 46 |
| Global outcome: > 1 injection | 25 | 17 | 14 | |
| Leaving the study early: lack of efficacy | 0 | 2 | 0 | |
| Adverse effects: EPS | 0 | 0 | 0 | |
| Adverse effects: akathisia | 0 | 2 | 0 | |
| Adverse effects: QT abnormality | 0 | 4 | 2 | |
| EPS: Extrapyramidal Side Effects | ||||
| Rating scale (Mean change 2 hr after 1st injection) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 1 mg | 0.65 | 1.64 | 56 |
| 5 mg | 1.01 | 1.65 | 62 | |
| 15 mg | 0.99 | 1.73 | 58 | |
| Specific behaviour: CABS | 1 mg | ‐5.15 | 6.8 | 56 |
| 5 mg | ‐5.97 | 6.81 | 62 | |
| 15 mg | ‐7.04 | 7.01 | 58 | |
| Global outcomes: CGI‐I | 1 mg | 3.07 | 0.98 | 56 |
| 5 mg | 2.82 | 1.10 | 62 | |
| 15 mg | 2.66 | 1.07 | 58 | |
| Global outcomes: CGI‐S | 1 mg | ‐0.63 | 1.15 | 56 |
| 5 mg | ‐0.82 | 1.12 | 62 | |
| 15 mg | ‐0.99 | 1.17 | 58 | |
| Mental state: BPRS Total | 1 mg | ‐6.53 | 9.61 | 55 |
| 5 mg | ‐8.16 | 9.66 | 61 | |
| 15 mg | ‐8.88 | 9.89 | 56 | |
| Mental state: BPRS Positive | 1 mg | ‐1.20 | 2.72 | 55 |
| 5 mg | ‐1.47 | 2.71 | 61 | |
| 15 mg | ‐1.86 | 2.82 | 57 | |
| ACES: Agitation‐Calmness Evaluation Scale | ||||
|
| Dose | 1 mg | 5 mg | 15 mg |
| Outcome | N | N = 57 | N = 63 | N = 58 |
| PEC response ≥ 40% | 21 | 31 | 32 | |
| Need for rescue medication | 11 | 5 | 12 | |
| Discontinued for any reason | 2 | 3 | 1 | |
| Discontinued due to adverse event | 0 | 0 | 1 | |
| Withdrew consent | 2 | 2 | 0 | |
| Discontinued due to other known cause | 0 | 1 | 0 | |
| PEC: Psychiatric Emergency Centre | ||||

