Simpatectomía lumbar versus prostanoides para la isquemia crítica de miembros inferiores debida a enfermedades arteriales periféricas no reconstruibles
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009366.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 16 abril 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vascular
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
IS: wrote protocol; trial selection, assessed quality, extracted data, performed meta‐analyses, interpreted results, and wrote the review manuscript.
SA: helped in writing the protocol; extracted data, interpreted results, and wrote the review manuscript.
PT: helped in writing the protocol; assessed quality, extracted data, performed meta‐analysis, and wrote the review manuscript.
RF: assisted in writing the review manuscript and performing the meta‐analysis.
Sources of support
Internal sources
-
Christian Medical College, Vellore, India.
Salaries and infrastructure support for all authors
-
Prof. BV Moses & Indian Council of Medical Research (ICMR) Centre for Advanced Research in Evidence‐Informed Healthcare, India.
Technical support for protocol development and review completion
External sources
-
Indian Council of Medical Research (ICMR), India.
Funding for the Prof. BV Moses Centre for Evidence‐Informed Healthcare
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
IS: none known.
SA: none known.
PT: PT's institution has received funding from the Indian Council for Medical Research (ICMR): the Professor BV Moses Centre was funded by an educational grant from the Indian Council for Medical Research during the development of the protocol for this Cochrane Review.
RF: none known.
Acknowledgements
The protocol for this Cochrane Review is an output of a protocol development workshop conducted by the Prof. BV Moses and ICMR Centre for Advanced Research and Training in Evidence‐Informed Healthcare (Sen 2011).
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Apr 16 | Lumbar sympathectomy versus prostanoids for critical limb ischaemia due to non‐reconstructable peripheral arterial disease | Review | Indrani Sen, Sunil Agarwal, Prathap Tharyan, Rachel Forster | |
| 2011 Oct 05 | Lumbar sympathectomy versus prostanoids for critical limb ischaemia due to non‐reconstructable peripheral arterial disease | Protocol | Indrani Sen, Sunil Agarwal, Prathap Tharyan | |
Differences between protocol and review
The outcome referring to walking distances has been modified to 'intermittent and absolute claudication distance (pain‐free walking distance and maximum walking distance, respectively)'. The two separate walking distances were clarified and 'increase in' was removed as we want to report any change in walking distances and not only if there is an increase.
The outcome of ankle brachial pressure index (ABPI) was edited to only 'ABPI' and 'improvement of' was removed as we intend to report on any ABPI findings and not only those that show improvement.
The protocol stated that an intention‐to‐treat (ITT) analysis would be performed as the primary analysis, where possible. After inclusion of only a single study with a high rate of unexplained dropouts, we chose to report a per protocol analysis as the primary analysis and include the ITT population in a sensitivity analysis.
We combined three individual primary outcomes of 'relief of rest pain', 'ulcer healing' and 'avoidance of major amputation' into a single outcome to reflect the outcome reported in the only included study, 'complete healing of ulcer without rest pain or major amputation'. We chose to do this after careful consideration in order to reduce a possible bias when interpreting the individual outcomes.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Iloprost [*therapeutic use];
- Ischemia [*drug therapy, etiology, *surgery];
- Leg Ulcer [drug therapy, etiology, surgery];
- Pain Management [*methods];
- Peripheral Arterial Disease [*complications];
- Prostaglandins [therapeutic use];
- Sympathectomy [*methods];
- Thromboangiitis Obliterans [*complications];
- Vasodilator Agents [*therapeutic use];
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Prostanoids versus lumbar sympathectomy, Outcome 1 Complete ulcer healing without rest pain or major amputation at 24 weeks (per protocol analysis).
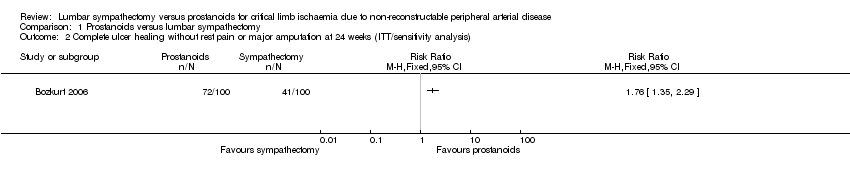
Comparison 1 Prostanoids versus lumbar sympathectomy, Outcome 2 Complete ulcer healing without rest pain or major amputation at 24 weeks (ITT/sensitivity analysis).

Comparison 1 Prostanoids versus lumbar sympathectomy, Outcome 3 Mortality (per protocol analysis).
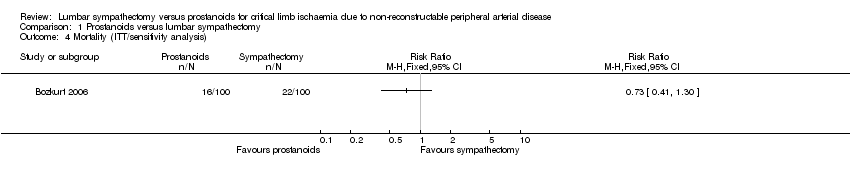
Comparison 1 Prostanoids versus lumbar sympathectomy, Outcome 4 Mortality (ITT/sensitivity analysis).
| Prostanoids versus lumbar sympathectomy for critical limb ischaemia due to non‐reconstructable peripheral arterial disease | ||||||
| Participants or population: people with critical limb ischaemia due to non‐reconstructable peripheral arterial disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Lumbar sympathectomy | Prostanoids | |||||
| Complete ulcer healing without rest pain or major amputation (per protocol) | 526 per 1000 | 331 per 1000 | RR 1.63 | 162 | ⊕⊕⊝⊝ | The outcomes 'relief of rest pain', 'complete ulcer healing' and 'avoidance of amputation' were all derived from a single outcome reported by Bozkurt 2006 as "complete healing without pain or major amputation". We chose to deviate from the review protocol and combine the outcomes, reflecting the single included study in order to limit potential bias. |
| Intermittent and absolute claudication distances | See comment | See comment | Not estimable | Not reported in included study. | ||
| Quality of life and functional status | See comment | See comment | Not estimable | Not reported in included study. | ||
| Adverse effects | See comment | See comment | Not estimable | 162 | ⊕⊕⊝⊝ | Adverse effects were not reported in a way that we could include in an analysis. Authors of the one included study reported more adverse effects in participants that received prostaglandin, but only one participant withdrew due to adverse effects. |
| Mortality | See comment | See comment | Not estimable | 162 | ⊕⊕⊝⊝ | No mortality reported in this trial. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is that the risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by two levels due to serious imprecision: study sample size was small with significant dropouts, and the data were only from a single trial. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete ulcer healing without rest pain or major amputation at 24 weeks (per protocol analysis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Complete ulcer healing without rest pain or major amputation at 24 weeks (ITT/sensitivity analysis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mortality (per protocol analysis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Mortality (ITT/sensitivity analysis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

