Losigamon jako leczenie uzupełniające padaczki ogniskowej
Abstract
Background
Epilepsy is a common neurologic disorder, affecting approximately 50 million people worldwide; nearly a third of these people have epilepsy that is not well controlled by a single antiepileptic drug (AED) and they usually require treatment with a combination of two or more AEDs. In recent years, many newer AEDs have been investigated as add‐on therapy for focal epilepsy; losigamone is one of these drugs and is the focus of this systematic review. This is an update of a Cochrane review first published in 2012 (Cochrane Database of Systematic Reviews 2012, Issue 6) and updated in 2015.
Objectives
To investigate the efficacy and safety of losigamone when used as an add‐on therapy for focal epilepsy.
Search methods
For the latest update on 9 February 2017, we searched the Cochrane Epilepsy Specialized Register, CENTRAL and MEDLINE . We searched trials registers and contacted the manufacturer of losigamone and authors of included studies for additional information. We did not impose any language restrictions.
Selection criteria
Randomized controlled, add‐on trials comparing losigamone with placebo for focal epilepsy.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. The primary outcomes were 50% or greater reduction in seizure frequency and seizure freedom; the secondary outcomes were treatment withdrawal and adverse events. Results are presented as risk ratios (RRs) with 95% confidence intervals (CIs) or 99% CIs (for the individual listed adverse events to make an allowance for multiple testing).
Main results
Two trials involving a total of 467 participants, aged over 18 years, were eligible for inclusion. Both trials assessed losigamone 1200 mg/day or 1500 mg/day as an add‐on therapy for focal epilepsy. We assessed one trial as being of good methodological quality while the other was of uncertain quality. For the efficacy outcomes, results did show that participants taking losigamone were significantly more likely to achieve a 50% or greater reduction in seizure frequency (RR 1.76, 95% CI 1.14 to 2.72), but associated with a significant increase of treatment withdrawal when compared with those taking placebo (RR 2.16, 95% CI 1.28 to 3.67). For the safety outcomes, results indicated that the proportion of participants who experienced adverse events in the losigamone group was higher than in the placebo group (RR 1.34, 95% CI 1.00 to 1.80), dizziness was the only adverse event significantly reported in relation to losigamone (RR 3.82, 99% CI 1.69 to 8.64). The proportion of participants achieving seizure freedom was not reported in either trial report. A subgroup analysis according to different doses of losigamone showed that a higher dose of losigamone (1500 mg/day) was associated with a greater reduction in seizure frequency than lower doses, but was also associated with more dropouts due to adverse events.
Authors' conclusions
The results of this review showed that losigamone did reduce seizure frequency but was associated with more treatment withdrawals when used as an add‐on therapy for people with focal epilepsy. However, the included trials were of short‐term duration and uncertain quality. Future well‐designed randomized, double‐blind, placebo‐controlled trials with a longer‐term duration are needed. No new studies have been found since the last version of this review. We judged the overall quality of the evidence for the outcomes assessed as moderate.
PICOs
Streszczenie prostym językiem
Losigamon jako leczenie uzupełniające padaczki ogniskowej
Pytanie badawcze
Niniejszy przegląd jest aktualizacją poprzednio opublikowanego przeglądu w Cochrane Database of Systematic Reviews (2015, Issue 12) pt. "Leczenie uzupełniające losigamonem w padaczce ogniskowej". Dokonaliśmy przeglądu danych dotyczących skuteczności i bezpieczeństwa losigamonu (lek niedostępny w Polsce; przyp.tłum.) stosowanego jako leczenie uzupełniające padaczki ogniskowej. Odnaleźliśmy dwa badania.
Wprowadzenie
Padaczka to częsta choroba neurologiczna, która dotyka około 50 milionów osób na całym świecie; blisko u jednej trzeciej z nich leczenie jednym lekiem przeciwdrgawkowym nie pozwala na wystarczającą kontrolę objawów i często wymagają oni stosowania dwóch lub większej liczby leków (leczenie uzupełniające). W ostatnich latach badano wiele nowych leków przeciwdrgawkowych w terapii uzupełniającej padaczki częściowej, a losigamon jest jednym z nich. Chcieliśmy się dowiedzieć, czy leczenie losigamonem jest skuteczne i bezpieczne u pacjentów z padaczką ogniskową.
Charakterystyka badania
Dane naukowe są aktualne do lutego 2017 r. Odnaleźliśmy dwa badania oceniające losigamon w leczeniu uzupełniającym padaczki ogniskowej, w których łącznie uczestniczyło 467 pacjentów w wieku powyżej 18 lat. W obydwu badaniach oceniano podawanie losigamonu w dawce 1200 lub 1500 mg/dobę jako leczenie uzupełniające padaczki ogniskowej.
Główne wyniki
Wyniki niniejszego przeglądu wykazały, że u pacjentów przyjmujących losigamon jako leczenie uzupełniające było większe prawdopodobieństwo redukcji napadów drgawkowych o 50% lub więcej w krótkiej obserwacji. Jednak w grupie leczonej więcej osób odstawiło lek z powodu działań niepożądanych niż w grupie placebo. Najczęściej występującymi działaniami niepożądanym związanymi z przyjmowaniem losigamonu były zawroty głowy.
Jakość danych naukowych
Pod względem metodologicznym jakość jednego badania została oceniona jako dobra, natomiast jakość drugiego była niejasna. Ogólna jakość danych naukowych ocnianych punktów końcowych została oceniona jako umiarkowana.
Authors' conclusions
Summary of findings
| Losigamone compared to placebo for focalepilepsy | |||||
| Patient or population: people with focal epilepsy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Losigamone | ||||
| 50% or greater reduction in seizure frequency | Study population | RR 1.76 | 467 | ⊕⊕⊕⊝ | |
| 132 per 1000 | 233 per 1000 | ||||
| Moderate | |||||
| 131 per 1000 | 231 per 1000 | ||||
| Treatment withdrawal | Study population | RR 2.16 | 467 | ⊕⊕⊕⊝ | |
| 90 per 1000 | 194 per 1000 | ||||
| Moderate | |||||
| 88 per 1000 | 190 per 1000 | ||||
| The proportion of participants experiencing any adverse events | Study population | RR 1.34 | 467 | ⊕⊕⊕⊝ | |
| 471 per 1000 | 631 per 1000 | ||||
| Moderate | |||||
| 482 per 1000 | 646 per 1000 | ||||
| Adverse event (dizziness) | Study population | RR 3.82 | 467 | ⊕⊕⊕⊝ | |
| 63 per 1000 | 243 per 1000 | ||||
| Moderate | |||||
| 60 per 1000 | 229 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1One of the two included trials did not describe the method used to generate the random list and did not mention allocation concealment. | |||||
Background
Description of the condition
Epilepsy is a common neurological disorder, affecting approximately 50 million people worldwide (Banerjee 2009; De Boer 2008). In low‐ and middle‐income countries, the median incidence of the disease is 68.7 per 100,000 population, which is higher than that in high‐income countries (43.4 per 100,000 population) (Kotsopoulos 2002). It is estimated that about 30% of patients have epilepsy that is refractory to one or more agents and they are considered to be drug‐resistant. These patients often need add‐on therapy with other antiepileptic drugs (AEDs) (Schuele 2008). People with epilepsy often have psychosocial, behavioral and cognitive problems, and may place a heavy burden on society (Devinsky 1999). As reported by the World Health Organization (WHO), the overall health burden due to epilepsy accounts for 0.5% of the global burden of disease; this can be considered as more than one‐third of the diabetes burden (1.3%) (WHO 2006). In Europe, the total annual costs of epilepsy are approximately EUR 15.5 billion (Pugliatti 2007). In China, about nine million people suffer from epilepsy; the overall annual costs are approximately USD 773 per patient, which represents half the average annual income (Hong 2009).
Description of the intervention
Losigamone is a newer AED that has been investigated as an add‐on therapy for focal seizures. When orally administered, losigamone is well absorbed through the gastrointestinal tract and reaches a peak plasma concentration within two to three hours of administration. Its plasma elimination half‐life is approximately four to seven hours (Luszczki 2009). In two double‐blind, placebo‐controlled, add‐on studies, losigamone has shown possible effects in people with focal epilepsy (Bauer 2001; Baulac 2003). One study tested losigamone at 1200 mg/day or 1500 mg/day, in addition to three standard anticonvulsants, in 264 people with focal epilepsy. They observed a dose‐dependent reduction of seizure frequency: 29.3% of participants receiving losigamone at 1500 mg/day had a 50% or greater reduction in seizure frequency, as compared to 17.2% in those receiving losigamone at 1200 mg/day and 11.8% in the placebo group (Baulac 2003). Generally, losigamone has been shown to be safe and well tolerated. The most commonly reported adverse events are dizziness, headache, somnolence, fatigue, ataxia, nausea, diplopia, abnormal vision and vertigo. No significant clinically pharmacokinetic interactions with other AEDs have been found, which allows the use of losigamone as an add‐on therapy (Bauer 2001; Baulac 2003).
How the intervention might work
The exact mechanism of action of losigamone remains unclear. Data from experimental studies have shown that losigamone stimulates the neuronal chloride channel without directly binding to γ‐aminobutyric acid (GABA), benzodiazepine or picrotoxin receptors, but it can enhance chloride influx in the absence of GABA and potentiate the effects of GABA (Dimpfel 1995; Willmore 2001). It has also been shown to reduce the frequency of spontaneous action potentials and depress repetitive firing of neurons through a decline in presynaptic activity (Draguhn 1997). Other proposed possible mechanisms by which losigamone may decrease neuronal excitability include a decrease in persistent sodium channel current and activation of potassium channel (Gebhardt 2001; Willmore 2001).
Why it is important to do this review
Randomized studies have been conducted to investigate the efficacy and safety of losigamone when used as an add‐on therapy for focal epilepsy, but they have not been systematically reviewed (Bauer 2001; Baulac 2003). This review therefore focused on the use of losigamone as an add‐on therapy for focal epilepsy, summarizing the evidence about efficacy and safety from randomized controlled trials (RCTs). This is an update of a Cochrane Review first published in 2012 (Xiao 2012) and previously updated in 2015 (Xiao 2015).
Objectives
To investigate the efficacy and safety of losigamone when used as an add‐on therapy for focal epilepsy.
Methods
Criteria for considering studies for this review
Types of studies
Published or unpublished randomized controlled trials (RCTs), with no language restrictions.
Types of participants
People of any age with focal epilepsy (defined as simple focal, complex focal or secondary generalized tonic‐clonic seizures) that has failed to respond to one or more antiepileptic drug(s) (AEDs), or refractory focal epilepsy (defined as focal seizures) according to the definition by the International League Against Epilepsy (ILAE) (Berg 2010; Kwan 2010) are eligible.
Types of interventions
-
The intervention (treatment) group used losigamone in addition to one or more other AEDs.
-
The control group used placebo in addition to the same co‐intervention as the treatment group.
Types of outcome measures
Primary outcomes
1. Fifty per cent or greater reduction in seizure frequency
The proportion of participants with a 50% or greater reduction in seizure frequency from pre‐randomization baseline to treatment period. We chose this outcome because it is often reported in this type of study and can be calculated for studies that did not report it, provided that baseline seizure data were recorded.
2. Seizure freedom
The proportion of participants with seizure freedom during the whole treatment period.
Secondary outcomes
1. Treatment withdrawal
We used the proportion of participants who withdrew during the course of the treatment period for any reason as a measure of global effectiveness. Adverse events were usually the main reason for treatment withdrawal.
2. Adverse events
-
The proportion of participants experiencing any adverse events.
-
The proportion of participants experiencing any of the following individual adverse events associated with AEDs, or clinically important adverse events reported in the original articles:
-
dizziness
-
headache
-
somnolence
-
fatigue
-
ataxia
-
nausea
-
diplopia
-
abnormal vision
-
vertigo
-
depression
-
Search methods for identification of studies
We conducted a systematic search without language restrictions to identify all relevant published and unpublished RCTs.
Electronic searches
This search was run for the original review on 1 May 2012. Subsequent searches were run on 30 January 2014, 16 February 2015, and 9 February 2017. For the latest update we searched the following databases.
-
The Cochrane Epilepsy Specialized Register (9 February 2017) using the strategy outlined in Appendix 1
-
The Cochrane Central Register of Controlled Trials (CENTRAL; 2017, issue 2) via the Cochrane Register of Studies Online (CRSO, 9 February 2017) using the strategy outlined in Appendix 2
-
MEDLINE (Ovid, 1946 to 9 February 2017) using the strategy outlined in Appendix 3
-
ClinicalTrials.gov (9 February 2017) using the search terms 'losigamone AND epilepsy'
-
WHO International Clinical Trials Registry Platform (ICTRP) (9 February 2017) using the search terms 'losigamone AND epilepsy'.
Searching other resources
We also searched Chinese Clinical Trial Register, reference lists of included studies and review articles, and relevant journals from recent years. We contacted the pharmaceutical company that produced losigamone (Dr. Willmar Schwabe GmbH & Co.) to obtain relevant data.
Data collection and analysis
Selection of studies
Two review authors (Xiao Y, Luo M) independently read the titles and abstracts of all studies identified by the search strategy. When we had retrieved all potentially relevant papers, each review author independently evaluated the full text of each paper for inclusion. We recorded the excluded studies and the reasons for exclusion. There was no disagreement between review authors about the selection of studies for inclusion.
Data extraction and management
Two review authors (Xiao Y, Luo M) independently extracted the following information, using a data extraction form.
-
Participants: seizure types, total and number in each group, age, gender, and seizure frequency at the time of randomization
-
Methods: study design, study duration, randomization method, allocation concealment method, and blinding methods
-
Interventions: details of losigamone treatment, such as administration method, dosage and duration, and number of background drugs
-
Outcomes: the proportion of participants with a 50% or greater reduction in seizure frequency, the proportion of participants with seizure freedom during the whole treatment period, the proportion of participants who withdrew during the course of the treatment period for any reason, the proportion of participants experiencing any adverse events, and individual listed adverse events
-
Other: country and setting, publication year, sources of funding, intention‐to‐treat (ITT) analysis, and factors for heterogeneity
There were some minor disagreements about the data extraction, however, any disagreements were resolved after discussion between review authors.
Assessment of risk of bias in included studies
Two review authors (Xiao Y, Luo M) independently assessed the risk of bias of the included studies using the 'Risk of bias' tool recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The 'Risk of bias' tool consists of six specific parameters: (1) sequence generation, (2) allocation concealment, (3) blinding, (4) incomplete outcome data, (5) selective outcome reporting and (6) other bias. For each entry, the judgment ('low risk' of bias, 'high risk' of bias, or 'unclear risk' of bias) was followed by a description of the design, and conduct or observations that explained the judgment (Higgins 2011). There was no disagreement in assessing the risk of bias between the review authors.
Measures of treatment effect
We managed data according to the ITT principle. For dichotomous outcomes (50% or greater reduction in seizure frequency, seizure freedom, treatment withdrawal, and adverse events), we used risk ratios (RRs) with 95% confidence intervals (CIs) to analyze the outcomes. For individual listed adverse effects, we used RR with 99% CIs to express the results to make allowance for multiple testing.
Unit of analysis issues
We did not identify any unit of analysis issues.
Dealing with missing data
We contacted study authors to obtain additional information that was not reported in the articles; unfortunately, we have not yet received a response from the authors. We could, therefore, analyze only the data that were available in the published reports.
Assessment of heterogeneity
We evaluated clinical and methodological heterogeneity of included trials by comparing the characteristics of participants (age, gender, seizure types, seizure frequency, epilepsy duration), interventions (administration method, dosage and duration, co‐interventions) and study designs (randomization, allocation concealment and blinding methods) between studies.
We evaluated statistical heterogeneity among the included studies using a Chi2 test with significance set at 0.1 and the I2 statistic (Higgins 2003). For the Chi2 test, we considered a P value of more than 0.1 indicative of no significant statistical heterogeneity. If a P value was less than 0.1, we interpreted heterogeneity according to the percentage ranges of the I2 statistic as follows (Deeks 2011):
-
0% to 40%: might not be important;
-
30% to 60%: may represent moderate heterogeneity*;
-
50% to 90%: may represent substantial heterogeneity*;
-
75% to 100%: considerable heterogeneity*.
*The importance of the observed value of the I2 statistic depends on (i) the magnitude and direction of effects and (ii) the strength of evidence for heterogeneity (e.g. P value from the Chi2 test or a CI for the I2 statistic).
Assessment of reporting biases
We could not investigate potential biases of publication using funnel plots and visual inspection for asymmetry to assess reporting bias according to the approach outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011) as we identified only two studies.
Data synthesis
We used Review Manager 5 (RevMan 5) (RevMan 2014) to synthesize the available data. Whether we applied a fixed‐effect or a random‐effects model mainly depended on the results of the Chi2 test and the I2 statistic for heterogeneity (Higgins 2011). If a P value was more than 0.1, indicating no significant statistical heterogeneity, we used a fixed‐effect model. If a P value was less than 0.1 and the I2 statistic indicated no important or moderate heterogeneity, we also used a fixed‐effect model. If a P value was less than 0.1 and the I2 statistic indicated substantial heterogeneity, we explored the factors contributing to heterogeneity first, to determine whether a subgroup analysis was needed. If the substantial heterogeneity could not be explained, we employed a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
-
Different age groups (e.g. children and adolescents (less than 17 years) and adults).
-
Different doses of losigamone.
However, because of the limited data from the included studies, we could only perform the subgroup analysis according to different doses of losigamone.
Sensitivity analysis
We planned to perform the following sensitivity analysis.
-
Re‐analysis excluding studies without adequate allocation concealment or blinding.
However, because we only identified two trials, we did not perform a sensitivity analysis.
Summarising and interpreting results
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013) to interpret findings, and GRADEpro GDT software (which imports data from Review Manager 5 software (Gradepro GDT 2015)), to create a 'Summary of findings' table for all the primary outcomes and the following secondary outcomes: treatment withdrawal; the proportion of participants experiencing any adverse events; adverse event (dizziness).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The original search on 1 May 2012 yielded a total of 29 potentially relevant articles (Cochrane Epilepsy Specialized Register: 5, CENTRAL: 8, MEDLINE: 16); we identified a further two relevant articles from reference lists of included studies. After reviewing the titles and abstracts we excluded 26 articles as they were not relevant or were obvious duplicate publications. Of the remaining five articles, we excluded three articles (Morris 1997; Runge 1993; Stefan 2001). Finally, two studies (Bauer 2001; Baulac 2003) were eligible to be included in the review (see Figure 1). We did not identify any ongoing trials or unpublished data. Subsequent updated searches have not identified any new studies.

Study flow diagram
Included studies
We included two studies that investigated the efficacy and safety of losigamone as an add‐on therapy for focal epilepsy (see Characteristics of included studies).
The first study conducted in Germany by Bauer and colleagues (Bauer 2001) was a randomized, double‐blind, placebo‐controlled, add‐on study. The study lasted for 16 weeks and consisted of an eight‐week baseline phase and an eight‐week double‐blind treatment phase. A total of 203 people with focal epilepsy taking no more than three AEDs prior to the study were randomized to treatment with either losigamone or placebo: 99 assigned to losigamone group and 104 to placebo; the mean age of participants in the losigamone group was 36.1 years (range 18 to 63 years) and 35.2 years (range 19 to 74 years) in the placebo group; the mean duration of epilepsy was 23.5 years in the losigamone group and 22.0 years in the placebo group; the mean frequency of focal seizures per 28 days in the losigamone group was 8.77 seizures, while in the placebo group it was 9.33 seizures. The dosage of losigamone administered was 750 mg/day in the first two days after randomization and then maintained at 1500 mg/day from the third day. The study authors reported change in seizure frequency and responder rate as primary outcome measures. At the end of the eight‐week follow‐up, 171 participants finished the study; 21 participants dropped out in the losigamone‐treated group, and 11 participants dropped out in the placebo group.
The second study conducted by Baulac and colleagues (Baulac 2003) was a multicenter, randomized, double‐blind, placebo‐controlled, add‐on study. The study lasted for 28 weeks and consisted of a 12‐week baseline period, a 12‐week double‐blind treatment phase and a four‐week post‐treatment period for safety observations. A total of 264 people with focal epilepsy, who had also had one to three AEDs prior to the study, were randomly assigned to the losigamone or placebo group: 87 participants were randomized to losigamone 1200 mg/day, 92 to losigamone 1500 mg/day and 85 to placebo. The mean age of the study population was 35.7 years, the mean duration of the disease was 21 years, and the mean seizure frequency per month before randomization was 9.2 seizures. The percentage reduction in seizure frequency and 50% or greater reduction in seizures were used as primary outcome measures. During the 12‐week double‐blind treatment period, 228 participants completed the study; 11 were withdrawn in the losigamone 1200 mg/day group, 19 in the losigamone 1500 mg/day group and six in the placebo group; 28 (77.8%) of the withdrawals were due to adverse events.
Excluded studies
We excluded both Morris 1997 and Runge 1993 because they had no control group; Stefan 2001 assessed losigamone monotherapy in patients with pharmacoresistant focal seizures undergoing presurgical evaluation (see Characteristics of excluded studies).
Risk of bias in included studies
We assessed one trial as being of good methodological quality (Baulac 2003) while the other was of uncertain quality (Bauer 2001). Overall results of all the risk of bias assessments are summarized in Figure 2 and Figure 3.
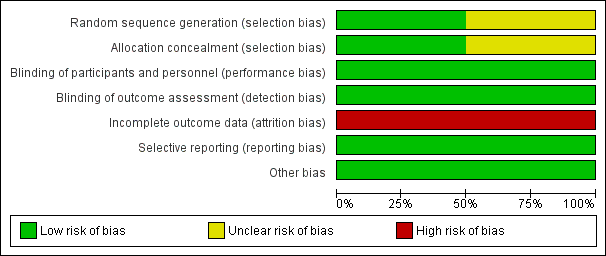
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies
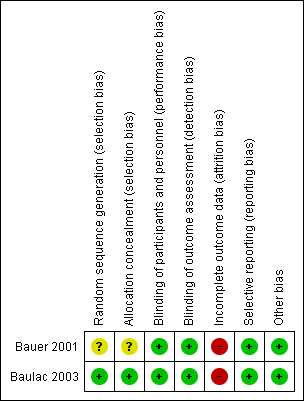
Risk of bias summary: review authors' judgments about each risk of bias item for each included study
Allocation
Both included studies were reported as randomized. However, only Baulac 2003 mentioned the method of generation of the random list describing the use of a random number generator and the use of permuted blocks. A third party, not involved in the assignment, was responsible for storing the allocation sequence to ensure adequate allocation concealment. The other study (Bauer 2001) did not describe the method used to generate the random list and did not mention allocation concealment. We contacted the study author asking for clarification of methods used, but so far we have not had a response. Accordingly, whether the randomization and allocation concealment was adequate is unclear.
Blinding
Although both included studies (Bauer 2001; Baulac 2003) were reported as double‐blind, no additional information has been provided regarding the details of blinding, especially whether outcome assessors were blinded or not. Despite the minor flaw, we would like to judge both studies at low risk of bias in this field at present.
Incomplete outcome data
Both Bauer 2001 and Baulac 2003 provided enough information about the number of, and the reasons for dropouts, but neither of them seems balanced across groups. In Bauer 2001, the percentage dropout in the losigamone group was 21.2% (21/99) and 10.6% (11/104) in the placebo group; in Baulac 2003, the percentage of dropout in losigamone 1200 mg/day was 12.6% (11/87), losigamone 1500 mg/day was 20.7% (19/92), and placebo was 7.1% (6/85). We judged both of the studies at high risk of attrition bias.
Selective reporting
Table 1 shows the outcome reporting matrix for the review's primary and secondary outcomes. It was difficult to confirm whether all prespecified outcomes were reported as we could not obtain protocols from either of the included studies. Given that both Bauer 2001 and Baulac 2003 reported favourable and unfavourable results, we judged both of them to have a low risk of selective reporting bias.
| Study ID | Review's primary outcomes | Review's secondary outcomes | Other study outcomes | |||
| 50% or greater reduction in seizure frequency | Seizure freedom | Treatment withdrawal | Adverse events | Change in seizure frequency | The percentage reduction in seizure frequency | |
| √ | × | √ | √ | √ | × | |
| √ | × | √ | √ | × | √ | |
√Full reporting of outcomes for treatment comparison
× No reporting of outcomes for treatment comparison
Other potential sources of bias
We did not identify other potential sources of bias for either included studies.
Effects of interventions
See: Summary of findings for the main comparison Losigamone compared to placebo for focal epilepsy
See: summary of findings Table for the main comparison
Primary outcomes
Fifty per cent or greater reduction in seizure frequency
Both trials (Bauer 2001; Baulac 2003) involving a total of 467 participants contributed to this outcome analysis; Bauer 2001 randomly allocated participants to losigamone 1500 mg/day or placebo, while Baulac 2003 allocated participants to losigamone 1200 mg/day, 1500 mg/day or placebo. When pooling these results, regardless of doses and follow‐up period, participants taking losigamone were significantly more likely to achieve a 50% or greater reduction in seizure frequency than those taking placebo (RR 1.76, 95% CI 1.14 to 2.72) (see Analysis 1.1), the test for heterogeneity indicated no significant heterogeneity (P = 0.56, I2 = 0%), so we applied the fixed‐effect model. When analyzing the results according to different doses, results did show that a 50% or greater reduction in seizure frequency favoured losigamone administered at 1500 mg/day compared to placebo (RR 1.94, 95% CI 1.25 to 3.01) (see Analysis 1.2), but no significant difference was detected between losigamone 1200 mg/day and placebo (RR 1.47, 95% CI 0.70 to 3.08) (see Analysis 1.3). It was not possible to do the adolescents/adults subgroup analyses because both trials only recruited participants aged over 18 years.
Seizure freedom
The proportion of participants with seizure freedom during the whole treatment period was not reported in either study.
Secondary outcomes
Treatment withdrawal
The number of treatment withdrawals and their reasons were carefully reported in both included trials (Bauer 2001; Baulac 2003). Adverse events were the main reason for treatment withdrawal; other reasons included lack of efficacy, increase of seizure frequency, status epilepticus and poor compliance. A pooled analysis of both studies, regardless of doses and follow‐up period, indicated that participants receiving losigamone experienced a significant increase in treatment withdrawal for any reason, compared to those receiving placebo (RR 2.16, 95% CI 1.28 to 3.67) (see Analysis 2.1), the test for heterogeneity indicated no significant heterogeneity (P = 0.76, I2 = 0%), so we applied the fixed‐effect model. When analyzing the results according to different doses, the dose of losigamone 1500 mg/day resulted in an increased risk of treatment withdrawal compared with placebo (RR 2.34, 95% CI 1.38 to 3.99) (see Analysis 2.2), while the dose of losigamone 1200 mg/day was not likely to lead to significant differences between both groups (RR 1.79, 95% CI 0.69 to 4.63) (see Analysis 2.3).
Adverse events
Both trials reported adverse events according to the WHO Adverse Reaction Terminology (WHO‐ART). Bauer 2001 performed the adverse events analysis according to the categorization of treatment‐emergent signs and symptoms (e.g. central and peripheral nervous system disorders, body as a whole ‐ general considerations, vision disorders, psychiatric disorders, gastrointestinal system disorders), and listed only the most frequent adverse events as individual symptoms; overall, they found no significant difference between treatment groups with regard to these adverse events during the short‐term follow‐up period. In contrast, in Baulac 2003, all listed adverse events were individual symptoms.
Therefore, we based our individually listed adverse event analysis mainly on the Baulac 2003 due to their method of reporting results. The proportion of participants receiving losigamone was higher than those receiving placebo in the occurrence of any adverse events during the short‐term follow‐up period, however, the difference was not significant (RR 1.34, 95% CI 1.00 to 1.80) (see Analysis 3.1). We applied a random‐effects model because of the possible substantial heterogeneity (P = 0.10, I2 = 64%), but when re‐analyzing it under a fixed‐effect model, we detected a significant difference (RR 1.33, 95% CI 1.12 to 1.57) (see Analysis 3.2). When analyzing the results according to different doses, the proportion of participants receiving a dose of losigamone 1500 mg/day was more likely to have an adverse event than those taking placebo (RR 1.40, 95% CI 1.14 to 1.71) (see Analysis 3.1), while we did not detect any significant difference between the dose of losigamone 1200 mg/day and placebo (RR 1.06, 95% CI 0.83 to 1.34) (see Analysis 3.1).
A pooling analysis of the individual listed adverse events indicated that dizziness was the only adverse event that was significantly more likely to occur in the losigamone group than in the placebo group (RR 3.82, 99% CI 1.69 to 8.64) (see Analysis 3.3), there was no significant heterogeneity (P = 0.21, I2 = 37%), so we applied the fixed‐effect model. The incidence of other adverse events was not significantly difference between treatment groups: headache (RR 1.19, 99% CI 0.43 to 3.30) (see Analysis 3.4); somnolence (RR 2.26, 99% CI 0.57 to 8.93) (see Analysis 3.5); fatigue (RR 2.26, 99% CI 0.57 to 8.93) (see Analysis 3.6); ataxia (RR 10.03, 99% CI 0.24 to 411.20) (see Analysis 3.7); nausea (RR 0.85, 99% CI 0.21 to 3.45) (see Analysis 3.8); diplopia (RR 2.85, 99% CI 0.59 to 13.70) (see Analysis 3.9); abnormal vision (RR 2.37, 99% CI 0.48 to 11.68) (see Analysis 3.10); vertigo (RR 6.17, 99% CI 0.44 to 87.50) (see Analysis 3.11); depression (RR 0.63, 99% CI 0.09 to 4.40) (see Analysis 3.12). When analyzing the results according to different doses, participants receiving losigamone 1500 mg/day were associated with significantly increased rates of dizziness than placebo (RR 3.96, 99% CI 1.79 to 8.76) (see Analysis 3.3) but there was no significant difference in other adverse events between both treatment groups: headache (RR 1.50, 99% CI 0.50 to 4.47); somnolence (RR 2.77, 99% CI 0.66 to 11.65); fatigue (RR 2.77, 99% CI 0.66 to 11.65); ataxia (RR 10.17, 99% CI 0.23 to 448.02); nausea (RR 1.29, 99% CI 0.30 to 5.56); diplopia (RR 3.39, 99% CI 0.66 to 17.33); abnormal vision (RR 3.08, 99% CI 0.59 to 16.05); vertigo (RR 6.47, 99% CI 0.42 to 98.79) and depression (RR 0.92, 99% CI 0.12 to 7.30). However, losigamone 1200 mg/day did not show any significant difference from placebo on all listed adverse events: dizziness (RR 5.37, 99% CI 0.77 to 37.42); headache (RR 0.85, 99% CI 0.24 to 3.06); somnolence (RR 1.71, 99% CI 0.36 to 8.19); fatigue (RR 1.71, 99% CI 0.36 to 8.19); ataxia (RR 10.75, 99% CI 0.24 to 473.22); nausea (RR 0.39, 99% CI 0.05 to 3.25); diplopia (RR 2.28, 99% CI 0.40 to 12.90); abnormal vision (RR 1.63, 99% CI 0.26 to 10.25); vertigo (RR 5.86, 99% CI 0.37 to 92.10) and depression (RR 0.98, 99% CI 0.12 to 7.71).
Discussion
Summary of main results
This systematic review assessed the short‐term efficacy and safety of losigamone administered as an add‐on therapy for focal epilepsy. Two RCTs involving a total of 467 participants contributed to the analysis; both trials reported a dose of losigamone 1200 mg/day or 1500 mg/day in addition to one to three AEDs for people with epilepsy older than 18 years. We assessed one trial (Baulac 2003) as good quality, and the other one (Bauer 2001) of uncertain quality (see: Risk of bias in included studies). For the efficacy outcomes, results did show that participants taking losigamone were significantly more likely to achieve a 50% or greater reduction in seizure frequency, but this was associated with a significant increase in treatment withdrawal when compared with those taking placebo. For the safety outcomes, results have indicated that the proportion of participants who experienced adverse events in the losigamone group was higher than the placebo group. Dizziness was the only adverse event significantly related to losigamone; other adverse events (headache, somnolence, fatigue, ataxia, nausea, diplopia, abnormal vision, vertigo, depression) did not differ significantly between both groups.
A subgroup analysis according to different doses of losigamone indicated that taking a higher dose of 1500 mg/day could increase the proportion of participants who achieved a 50% or greater reduction in seizure frequency, but it would also lead to an elevated risk of treatment withdrawal and adverse events (particularly dizziness). Taking a relatively low dose of losigamone 1200 mg/day did not show any significant difference in terms of all outcomes analysis.
Overall completeness and applicability of evidence
As a result of the insufficient number of included trials with placebo‐controlled design, short‐term duration and uncertain quality in this review, it is difficult to give any recommendations for current clinical practice of losigamone as an add‐on therapy for focal epilepsy. In this review, results suggest that the higher dose of losigamone 1500 mg/day seems a better starting dose according to its efficacy in reducing seizure frequency, but it is associated with more dropouts due to adverse events. Furthermore, because both of the included trials were focused on the adjunctive therapy of losigamone in focal epilepsy during a short‐term follow‐up (8 to 12 weeks), and recruited participants were exclusively aged over 18 years, there is still no evidence about the efficacy and safety of losigamone in children and adolescents, and no conclusion can be drawn about the long‐term efficacy of losigamone.
Quality of the evidence
We only assessed one trial (Baulac 2003) as being of good methodological quality; it used a double‐blind design, and had an adequate method of randomization and allocation concealment. We assessed the other trial (Bauer 2001) as being of uncertain methodological quality; it did not describe the method used to generate the random list and did not mention allocation concealment, although a double‐blind design was used. Unclear methods of randomization and allocation concealment contributed to high‐risk performance and detection bias and may therefore have led to an overestimation of intervention effects (Schulz 1995). We judged the overall quality of the evidence for the outcomes assessed as moderate.
It is noteworthy that both included trials reported a sample size calculation and performed an ITT analysis to provide adequate statistical power and an unbiased comparison among the treatment groups. In addition, we did not detect any significant clinical heterogeneity except for the different doses of losigamone used.
Potential biases in the review process
Potential biases from the trials
Although we undertook an extensive and comprehensive search to minimize bias in the review process, we could not confirm whether other studies with negative findings exist, because trials with negative findings are not published as often as trials with positive findings (Hopewell 2009). In addition, we did not receive further unreported data from correspondence with the study authors.
Potential biases from the review authors
There were no potential biases from the review authors in the review process. In the preparation of this review, two review authors independently read and screened trials retrieved for inclusion, independently completed data extraction and assessed the quality of included trials to minimize potential biases. No conflict of interests were found in relation to the review authors of this review.
Agreements and disagreements with other studies or reviews
The efficacy and safety of losigamone when used as an add‐on therapy for focal epilepsy has not been systematically reviewed previously.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgments about each risk of bias item for each included study
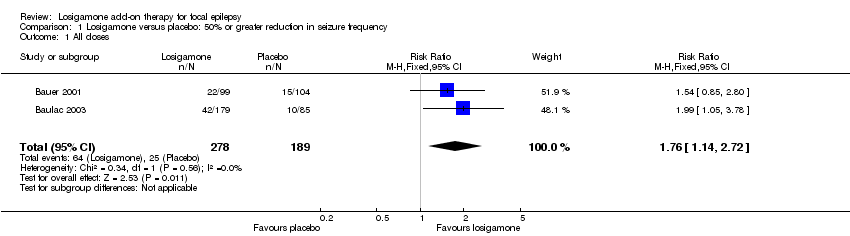
Comparison 1 Losigamone versus placebo: 50% or greater reduction in seizure frequency, Outcome 1 All doses.
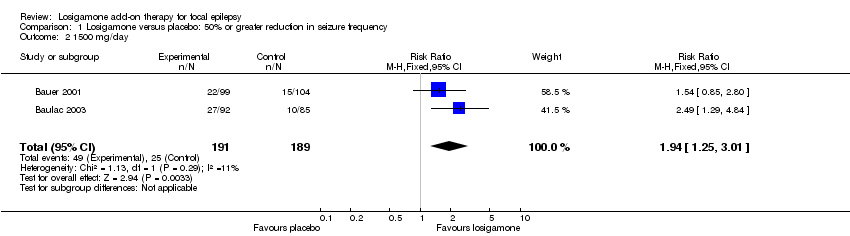
Comparison 1 Losigamone versus placebo: 50% or greater reduction in seizure frequency, Outcome 2 1500 mg/day.
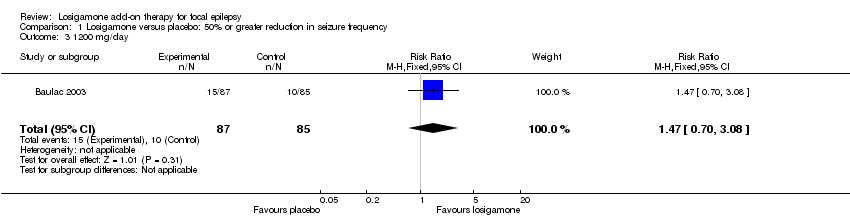
Comparison 1 Losigamone versus placebo: 50% or greater reduction in seizure frequency, Outcome 3 1200 mg/day.
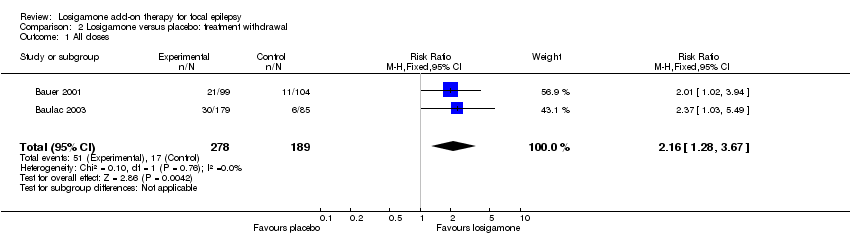
Comparison 2 Losigamone versus placebo: treatment withdrawal, Outcome 1 All doses.
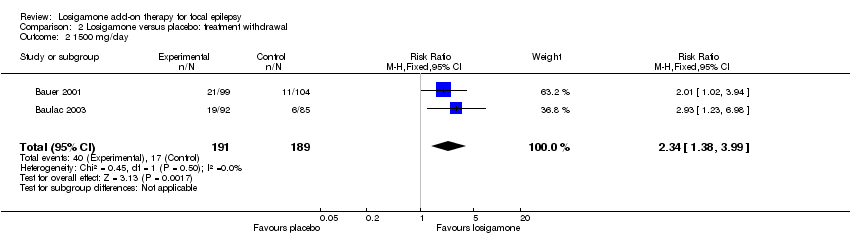
Comparison 2 Losigamone versus placebo: treatment withdrawal, Outcome 2 1500 mg/day.
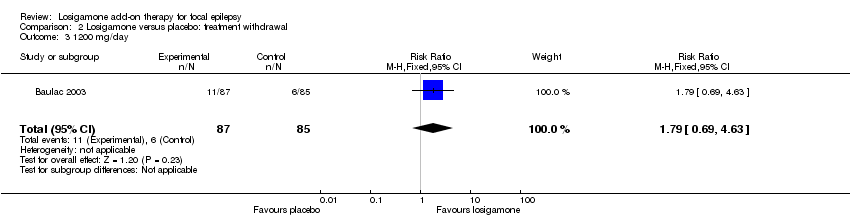
Comparison 2 Losigamone versus placebo: treatment withdrawal, Outcome 3 1200 mg/day.

Comparison 3 Losigamone versus placebo: adverse events, Outcome 1 The proportion of participants experiencing any adverse events (random model).

Comparison 3 Losigamone versus placebo: adverse events, Outcome 2 The proportion of participants experiencing any adverse events (fixed model).
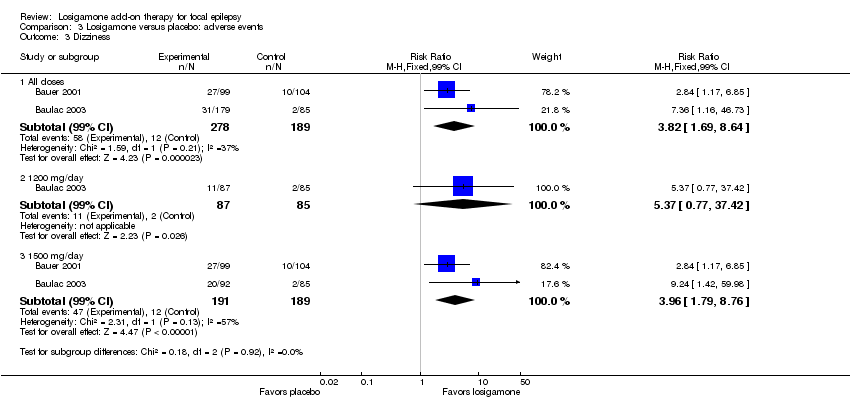
Comparison 3 Losigamone versus placebo: adverse events, Outcome 3 Dizziness.

Comparison 3 Losigamone versus placebo: adverse events, Outcome 4 Headache.

Comparison 3 Losigamone versus placebo: adverse events, Outcome 5 Somnolence.

Comparison 3 Losigamone versus placebo: adverse events, Outcome 6 Fatigue.

Comparison 3 Losigamone versus placebo: adverse events, Outcome 7 Ataxia.

Comparison 3 Losigamone versus placebo: adverse events, Outcome 8 Nausea.
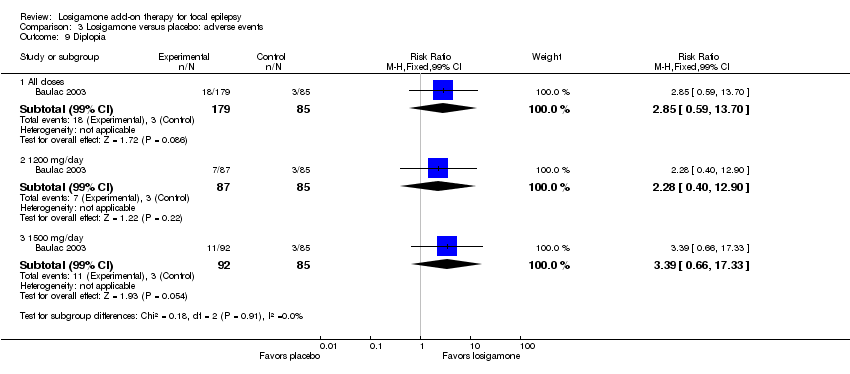
Comparison 3 Losigamone versus placebo: adverse events, Outcome 9 Diplopia.
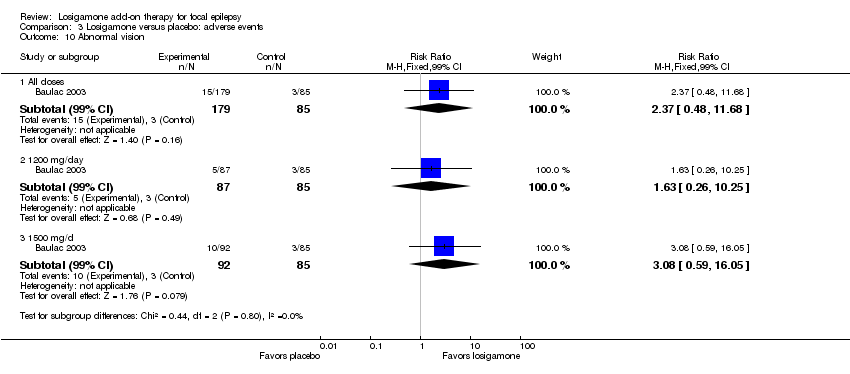
Comparison 3 Losigamone versus placebo: adverse events, Outcome 10 Abnormal vision.

Comparison 3 Losigamone versus placebo: adverse events, Outcome 11 Vertigo.
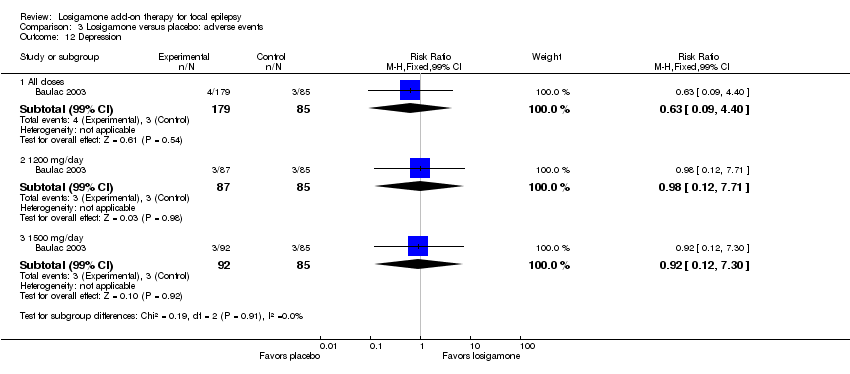
Comparison 3 Losigamone versus placebo: adverse events, Outcome 12 Depression.
| Losigamone compared to placebo for focalepilepsy | |||||
| Patient or population: people with focal epilepsy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Losigamone | ||||
| 50% or greater reduction in seizure frequency | Study population | RR 1.76 | 467 | ⊕⊕⊕⊝ | |
| 132 per 1000 | 233 per 1000 | ||||
| Moderate | |||||
| 131 per 1000 | 231 per 1000 | ||||
| Treatment withdrawal | Study population | RR 2.16 | 467 | ⊕⊕⊕⊝ | |
| 90 per 1000 | 194 per 1000 | ||||
| Moderate | |||||
| 88 per 1000 | 190 per 1000 | ||||
| The proportion of participants experiencing any adverse events | Study population | RR 1.34 | 467 | ⊕⊕⊕⊝ | |
| 471 per 1000 | 631 per 1000 | ||||
| Moderate | |||||
| 482 per 1000 | 646 per 1000 | ||||
| Adverse event (dizziness) | Study population | RR 3.82 | 467 | ⊕⊕⊕⊝ | |
| 63 per 1000 | 243 per 1000 | ||||
| Moderate | |||||
| 60 per 1000 | 229 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1One of the two included trials did not describe the method used to generate the random list and did not mention allocation concealment. | |||||
| Study ID | Review's primary outcomes | Review's secondary outcomes | Other study outcomes | |||
| 50% or greater reduction in seizure frequency | Seizure freedom | Treatment withdrawal | Adverse events | Change in seizure frequency | The percentage reduction in seizure frequency | |
| √ | × | √ | √ | √ | × | |
| √ | × | √ | √ | × | √ | |
| √Full reporting of outcomes for treatment comparison × No reporting of outcomes for treatment comparison | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All doses Show forest plot | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.14, 2.72] |
| 2 1500 mg/day Show forest plot | 2 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.25, 3.01] |
| 3 1200 mg/day Show forest plot | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.70, 3.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All doses Show forest plot | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.28, 3.67] |
| 2 1500 mg/day Show forest plot | 2 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [1.38, 3.99] |
| 3 1200 mg/day Show forest plot | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.69, 4.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 The proportion of participants experiencing any adverse events (random model) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 All doses | 2 | 467 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.00, 1.80] |
| 1.2 1200 mg/day | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.83, 1.34] |
| 1.3 1500 mg/day | 2 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.14, 1.71] |
| 2 The proportion of participants experiencing any adverse events (fixed model) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 All doses | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.12, 1.57] |
| 3 Dizziness Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 3.1 All doses | 2 | 467 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.82 [1.69, 8.64] |
| 3.2 1200 mg/day | 1 | 172 | Risk Ratio (M‐H, Fixed, 99% CI) | 5.37 [0.77, 37.42] |
| 3.3 1500 mg/day | 2 | 380 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.96 [1.79, 8.76] |
| 4 Headache Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 4.1 All doses | 1 | 264 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.19 [0.43, 3.30] |
| 4.2 1200 mg/day | 1 | 172 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.85 [0.24, 3.06] |
| 4.3 1500 mg/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.50 [0.50, 4.47] |
| 5 Somnolence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 5.1 All doses | 1 | 264 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.26 [0.57, 8.93] |
| 5.2 1200 mg/day | 1 | 172 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.71 [0.36, 8.19] |
| 5.3 1500 mg/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.77 [0.66, 11.65] |
| 6 Fatigue Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 6.1 All doses | 1 | 264 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.26 [0.57, 8.93] |
| 6.2 1200 mg/day | 1 | 172 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.71 [0.36, 8.19] |
| 6.3 1500 mg/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.77 [0.66, 11.65] |
| 7 Ataxia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 7.1 All doses | 1 | 264 | Risk Ratio (M‐H, Fixed, 99% CI) | 10.03 [0.24, 411.20] |
| 7.2 1200 mg/day | 1 | 172 | Risk Ratio (M‐H, Fixed, 99% CI) | 10.75 [0.24, 473.22] |
| 7.3 1500 mg/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 99% CI) | 10.17 [0.23, 448.02] |
| 8 Nausea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 8.1 All doses | 1 | 264 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.85 [0.21, 3.45] |
| 8.2 1200 mg/day | 1 | 172 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.39 [0.05, 3.25] |
| 8.3 1500 mg/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.29 [0.30, 5.56] |
| 9 Diplopia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 9.1 All doses | 1 | 264 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.85 [0.59, 13.70] |
| 9.2 1200 mg/day | 1 | 172 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.28 [0.40, 12.90] |
| 9.3 1500 mg/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.39 [0.66, 17.33] |
| 10 Abnormal vision Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 10.1 All doses | 1 | 264 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.37 [0.48, 11.68] |
| 10.2 1200 mg/day | 1 | 172 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.63 [0.26, 10.25] |
| 10.3 1500 mg/d | 1 | 177 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.08 [0.59, 16.05] |
| 11 Vertigo Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 11.1 All doses | 1 | 264 | Risk Ratio (M‐H, Fixed, 99% CI) | 6.17 [0.44, 87.50] |
| 11.2 1200 mg/day | 1 | 172 | Risk Ratio (M‐H, Fixed, 99% CI) | 5.86 [0.37, 92.10] |
| 11.3 1500 mg/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 99% CI) | 6.47 [0.42, 98.79] |
| 12 Depression Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 12.1 All doses | 1 | 264 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.63 [0.09, 4.40] |
| 12.2 1200 mg/day | 1 | 172 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.98 [0.12, 7.71] |
| 12.3 1500 mg/day | 1 | 177 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.92 [0.12, 7.30] |

