Cafeína como adyuvante analgésico para el dolor agudo en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009281.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 11 diciembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All authors contributed to writing the protocol.
For the original review CD and SD carried out searches and data extraction. RAM contacted pharmaceutical companies and conduct Internet searches for otherwise unpublished data. All authors were involved with data analysis and preparation of the manuscript. RAM will be responsible for any update, though the paucity of recent studies with caffeine make any update unlikely in the near future.
For this update SD and RAM carried out the searches. All authors contributed to updating the text.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
No sources of support supplied
Declarations of interest
CD has no conflicts relating to this review or any similar product.
SD has no conflicts relating to this review or any similar product.
RAM has no conflicts relating to this review or any similar product.
For transparency we have received research support from charities, government, and industry sources at various times, but none relate to this review. We are funded by the NIHR for work on a series of reviews informing the unmet need of chronic pain and providing the evidence for treatments of pain.
Acknowledgements
We wish to thank Prof Henry McQuay for his insight and useful discussions, and the peer reviewers and Cochrane PaPaS Group editorial team for their helpful comments on the original review.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is currently the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Dec 11 | Caffeine as an analgesic adjuvant for acute pain in adults | Review | Christopher J Derry, Sheena Derry, R Andrew Moore | |
| 2012 Mar 14 | Caffeine as an analgesic adjuvant for acute pain in adults | Review | Christopher J Derry, Sheena Derry, R Andrew Moore | |
| 2011 Aug 10 | Caffeine as an analgesic adjuvant for acute pain in adults | Protocol | Christopher J Derry, Sheena Derry, R Andrew Moore | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acetaminophen [therapeutic use];
- Acute Pain [*drug therapy];
- Analgesics [administration & dosage, *therapeutic use];
- Caffeine [administration & dosage, *therapeutic use];
- Chemotherapy, Adjuvant [methods];
- Diclofenac [therapeutic use];
- Drug Synergism;
- Dysmenorrhea [drug therapy];
- Headache [drug therapy];
- Ibuprofen [therapeutic use];
- Pain, Postoperative [drug therapy];
- Postpartum Period;
- Randomized Controlled Trials as Topic;
- ortho‐Aminobenzoates [therapeutic use];
Medical Subject Headings Check Words
Adolescent; Adult; Aged; Female; Humans; Male; Middle Aged; Pregnancy;
PICO

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
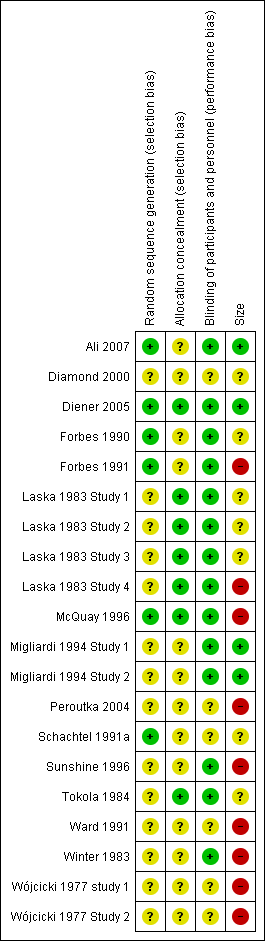
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Individual studies comparing the primary outcome for analgesic + caffeine versus analgesic alone ‐ any pain condition

Forest plot of comparison: 3 Analgesic plus caffeine versus analgesic alone by dose of caffeine, outcome: 3.1 Primary outcome.

Comparison 1 Analgesic plus caffeine versus analgesic alone by pain condition, Outcome 1 Primary outcome.
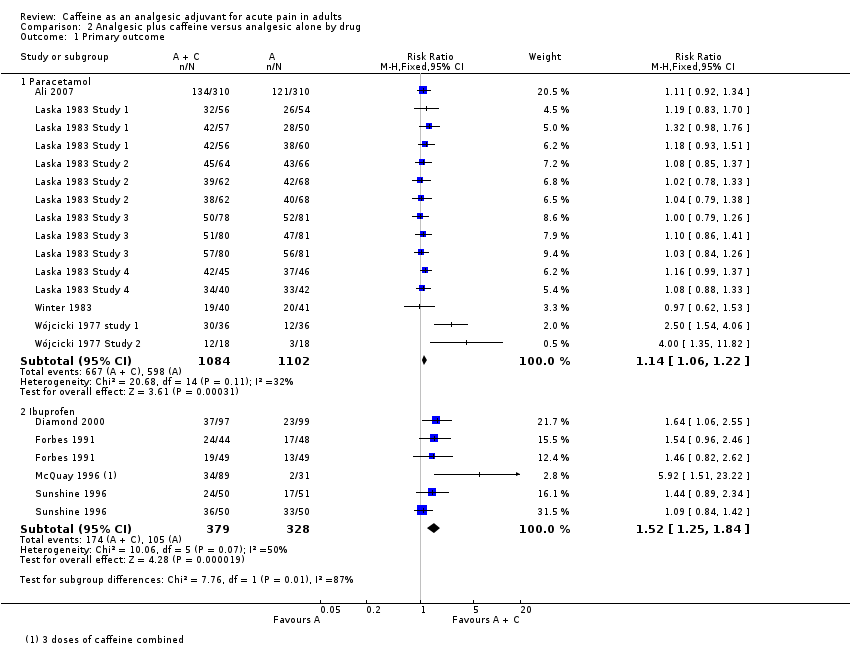
Comparison 2 Analgesic plus caffeine versus analgesic alone by drug, Outcome 1 Primary outcome.
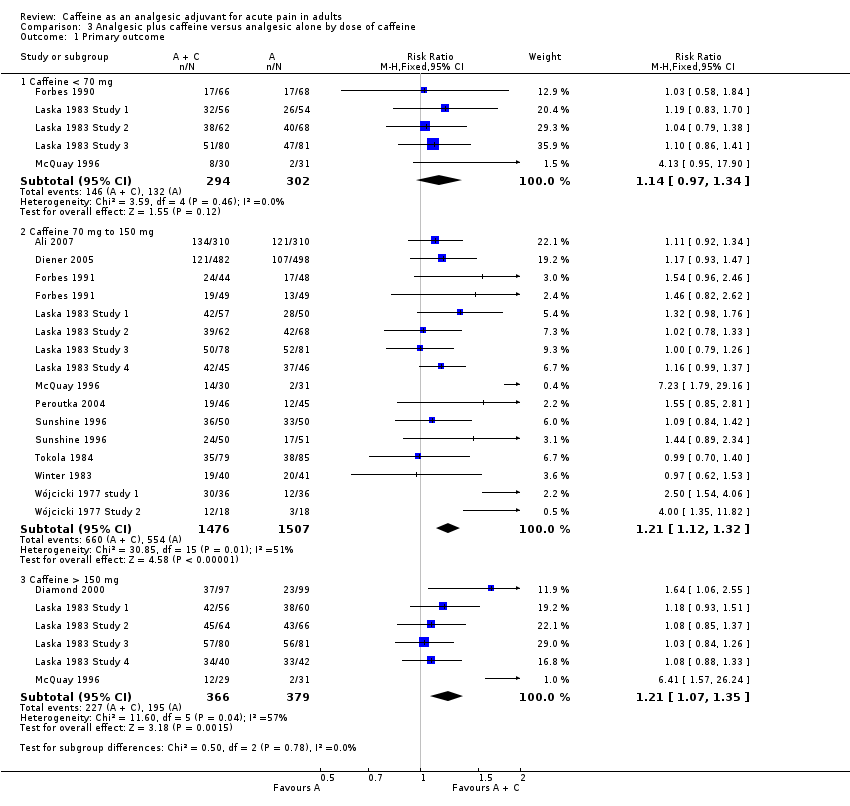
Comparison 3 Analgesic plus caffeine versus analgesic alone by dose of caffeine, Outcome 1 Primary outcome.
| Analgesic plus caffeine compared with analgesic alone for acute pain | ||||||
| Patient or population: adults with acute pain Settings: community Intervention: analgesic plus caffeine Comparison: same dose of analgesic alone | ||||||
| Outcomes | Outcome with analgesic alone | Outcome with analgesic plus caffeine | RR and NNT | No of participants | Quality of the evidence | Comments |
| Effective pain relief | 41% | 48% | RR 1.2 (1.1 to 1.3) NNT 14 (9.9 to 24) | 4262 (27 separate comparisons) | High | Small effect size but large numbers of participants contributing. There is a large amount of data that cannot be incorporated into this review, but this result is robust to analysis assuming all missing data show no effect. In fact, the results of this review are consistent with an almost completely different analysis in 10,000 participants demonstrating the effect of caffeine to have a similar effect size |
| Serious adverse events | 1 event | 1 event | Not calculated | Not calculated | Very low | Neither event judged related to study medication. Single dose studies are not powered to assess serious adverse events |
| CI: confidence interval; NNT: number needed to treat to benefit; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome Show forest plot | 16 | 4262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.11, 1.26] |
| 1.1 Postoperative/postpartum | 10 | 2139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.08, 1.25] |
| 1.2 Headache | 5 | 1503 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.11, 1.52] |
| 1.3 Dysmenorrhoea | 1 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.92, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Paracetamol | 8 | 2186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.06, 1.22] |
| 1.2 Ibuprofen | 4 | 707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.25, 1.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome Show forest plot | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Caffeine < 70 mg | 5 | 596 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.34] |
| 1.2 Caffeine 70 mg to 150 mg | 14 | 2983 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.12, 1.32] |
| 1.3 Caffeine > 150 mg | 6 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.07, 1.35] |

