Kafein sebagai adjuvan analgesik untuk sakit akut dalam kalangan dewasa
Abstract
Background
This is an updated version of the original Cochrane review published in Issue 3, 2012. Caffeine has been added to common analgesics such as paracetamol, ibuprofen, and aspirin, in the belief that it enhances analgesic efficacy. Evidence to support this belief is limited and often based on invalid comparisons.
Objectives
To assess the relative efficacy of a single dose of an analgesic plus caffeine against the same dose of the analgesic alone, without restriction on the analgesic used or the pain condition studied. We also assessed serious adverse events.
Search methods
We searched CENTRAL, MEDLINE, and EMBASE from inception to 28 August 2014, the Oxford Pain Relief Database, and also carried out Internet searches and contacted pharmaceutical companies known to have carried out trials that have not been published.
Selection criteria
We included randomised, double‐blind studies that compared a single dose of analgesic plus caffeine with the same dose of the analgesic alone in the treatment of acute pain.
Data collection and analysis
Two review authors independently assessed the eligibility and quality of studies, and extracted data. Any disagreements or uncertainties were settled by discussion with a third review author. We sought any validated measure of analgesic efficacy, but particularly the number of participants experiencing at least 50% of the maximum possible pain relief over four to six hours, participants reporting a global evaluation of treatment of very good or excellent, or headache relief after two hours. We pooled comparable data to look for a statistically significant difference, and calculated numbers needed to treat to benefit (NNT) with caffeine. We also looked for any numerical superiority associated with the addition of caffeine, and information about any serious adverse events.
Main results
We identified no new studies with available results for this update. The earlier review included 20 studies (7238 participants) in valid comparisons, but because we used different outcomes for some headache studies, the number of participants in the analyses of the effects of caffeine is now 4262 when previously it was 5243. The studies were generally of good methodological quality, using standard designs and mostly standard scales of pain measurement, although many of those treating postoperative pain were small.
Most studies used paracetamol or ibuprofen, with 100 mg to 130 mg caffeine, and the most common pain conditions studied were postoperative dental pain, postpartum pain, and headache. There was a small but statistically significant benefit with caffeine used at doses of 100 mg or more, which was not dependent on the pain condition or type of analgesic. About 5% to 10% more participants achieve a good level of pain relief (at least 50% of the maximum over four to six hours) with the addition of caffeine, giving a NNT of about 14 (high quality evidence).
Most comparisons individually demonstrated numerical superiority with caffeine, but not statistical superiority. One serious adverse event was reported with caffeine, but was considered unrelated to any study medication.
We know of the existence of around 25 additional studies with almost 12,500 participants for which data for analysis were not obtainable. The additional analgesic effect of caffeine remained statistically significant but clinically less important even if all the known missing data had no effect; the bulk of the unobtainable data are reported to have similar results as this review.
Authors' conclusions
The addition of caffeine (≥ 100 mg) to a standard dose of commonly used analgesics provides a small but important increase in the proportion of participants who experience a good level of pain relief.
PICO
Ringkasan bahasa mudah
Kafein sebagai adjuvan analgesik untuk sakit akut dalam kalangan dewasa
Kafein ditemui dalam pelbagai produk tumbuhan dan boleh diinges daripada teh, kopi dan sebahagian minuman berkarbonat serta minuman tenaga. Kafein adalah perangsang dan boleh memperbaiki kewaspadaandan mencegah keletihan untuk tempoh masa singkat. Ia mungkin mengganggu tidur sesetengah orang jika diambil sebelum tidur. Pengambilan biasa kafein (kurang dari 500 milligram sehari) tidak berbahaya kepada kesihatan. Kafein lazim diguna dalam ubat melegakan sakit yang boleh didapati dari farmasi tanpa preskripsi. Adjuvan adalah sesuatu yang ditambah dalam ubat‐ubatan untuk menjadikannya lebih berkesan.
Ulasan ini meneliti adakah kafein memperbaiki kesan melegakan sakit ubat‐ubatan tersebut. Kami mencari kajian‐kajian sehingga Ogos 2014 dan memasukkan dua puluh kajian (7238 peserta) yang mengkaji beberapa keadaan sakit seperti sakit kepala, sakit selepas rawatan gigi, sakit lepas bedah kerana bersalin dan sakit masa haid. Kajian‐kajian tersebut amnya berkualiti metodologi baik, menggunakan reka bentuk standard dan skala ukuran sakit yang standard. Kebanyakan kajian sakit selepas rawatan gigi dan sakit lepas bedah adalah kecil dan ini boleh menyebabkan lebih anggaran manfaat.
Dos kafein yang bersamaan dengan satu cawan kopi yang ditambah kepada satu dos standard analgesik lazim seperti parasetamol atau ibuprofen memberi kelegaan sakit yang lebih baik. Analgesik yang ditambah kafein meningkatkan bilangan orang yang mencapai tahap kelegaan sakit yang baik sebanyak 5% hingga 10% berbanding dengan penggunaan analgesik sahaja (bukti berkualiti tinggi).
Tiada peristiwa buruk yang serius berkaitan dengan analgesik atau kafein dilaporkan dalam kajian‐kajian tersebut (bukti berkualiti rendah). Adalah tidak mungkin penambahan kafein kepada analgesik akan memudaratkan jika tidak melampaui dos yang disyorkan.
Authors' conclusions
Summary of findings
| Analgesic plus caffeine compared with analgesic alone for acute pain | ||||||
| Patient or population: adults with acute pain Settings: community Intervention: analgesic plus caffeine Comparison: same dose of analgesic alone | ||||||
| Outcomes | Outcome with analgesic alone | Outcome with analgesic plus caffeine | RR and NNT | No of participants | Quality of the evidence | Comments |
| Effective pain relief | 41% | 48% | RR 1.2 (1.1 to 1.3) NNT 14 (9.9 to 24) | 4262 (27 separate comparisons) | High | Small effect size but large numbers of participants contributing. There is a large amount of data that cannot be incorporated into this review, but this result is robust to analysis assuming all missing data show no effect. In fact, the results of this review are consistent with an almost completely different analysis in 10,000 participants demonstrating the effect of caffeine to have a similar effect size |
| Serious adverse events | 1 event | 1 event | Not calculated | Not calculated | Very low | Neither event judged related to study medication. Single dose studies are not powered to assess serious adverse events |
| CI: confidence interval; NNT: number needed to treat to benefit; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
This is an updated version of the original Cochrane review, 'Caffeine as an analgesic adjuvant for acute pain in adults', published in Issue 3, 2012 (Derry 2012).
Description of the condition
Caffeine is added to a variety of basic analgesics that are used to treat a broad range of common painful conditions. We included information from any acute painful condition, with headache, postpartum pain, and postoperative pain the most commonly studied.
Description of the intervention
Caffeine is a naturally occurring compound found in the seeds, leaves, and fruit of many plants, where it is thought to function as a natural pesticide. It has a long (at least 5000 years) history of human consumption in the form of beverages such as tea and coffee, and foodstuffs such as chocolate. Caffeine intake varies widely among individuals and populations, but can be broadly divided into low (< 100 mg/day), moderate (100 mg to 400 mg/day), and high intake (> 400 mg/day), with the majority of people falling within the moderate intake range. Common sources of caffeine today include coffee (100 mg to 150 mg/mug), tea (75 mg/mug), cola drinks (up to 40 mg/drink), energy drinks (approximately 80 mg/drink), plain chocolate (up to 50 mg/bar), and caffeine tablets (100 mg/tablet). Some 'high‐energy' drinks have the caffeine content of five or six mugs of coffee.
Caffeine is a methylxanthine that is known to act as a central nervous system stimulant. It has a wide range of physiological effects in humans (Sawynok 1993), including increased wakefulness, alertness, endurance, heart rate, and blood pressure, and is regarded as a psychostimulant (enhances mood; Donovan 2001).
An adjuvant in this context is an agent that enhances the effects of a drug while having few if any direct effects when given by itself. There have been several reports of an intrinsic antinociceptive effect of caffeine from preclinical studies in rodents, but in general, only at very high doses of 50 mg/kg or more (Sawynok 2011a). A recent Cochrane review examined the use of high‐dose caffeine (300 mg) following post‐dural puncture headache; there were very few data (Basurto Ona 2013). Caffeine at dietary levels is not usually regarded as an analgesic in its own right in humans. Caffeine has been included as a constituent of both over‐the‐counter and prescription analgesic combinations for many years, based on the idea that it enhances analgesic efficacy.
The evidence supporting caffeine as a useful analgesic adjuvant has always been somewhat limited, with only a handful of often small studies providing any direct evidence of enhanced analgesia with a caffeine‐analgesic combination compared with the same analgesic alone. Randomised studies that have attempted to answer this question by comparing analgesic plus caffeine with the same dose of the analgesic alone have produced mixed results, with some showing a clear benefit for addition of caffeine (Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; Migliardi 1994 Study 1; Migliardi 1994 Study 2), and others showing no significant analgesic effect (Forbes 1990; McQuay 1996). An ongoing problem is that a large number of clinical trials have only been published in part, in reviews, without full publication or clinical trial reports made available; in one review only four of 30 studies had previously been published (Laska 1984). With only a relatively small benefit shown in some studies, this makes any estimate of adjuvant efficacy particularly susceptible to publication bias from unpublished studies with no effect.
It is possible that part of the reason for these mixed results is differing efficacy in different clinical pain models: for example it is suggested that caffeine may be a useful adjuvant in headache, but not postsurgical pain (Sawynok 2011a; Sawynok 2011b). Another complication is the fact that several studies, including some that are often cited as supporting the addition of caffeine to analgesics, attempt to draw their conclusions from comparisons of an analgesic plus caffeine with a different dose of the analgesic, or even a completely different analgesic (Jain 1978; Schachtel 1991 (in a trial of headache)).
A small number of reviews have attempted to investigate systematically the effect of adding caffeine to individual commonly used analgesics, including paracetamol (Palmer 2010; Zhang 1996), aspirin (Zhang 1997), and ibuprofen (Li Wan Po 1998). The three reviews published in the 1990s failed to provide any conclusive evidence for an analgesic adjuvant effect with any of these three drugs. The most recent review did demonstrate a marginal benefit of paracetamol and caffeine over paracetamol alone (Palmer 2010).
To add to the confusion, several preclinical studies have reported that very low doses of caffeine (lower than those that may exhibit adjuvant analgesic effects) actually inhibit antinociception by several agents (Sawynok 2011b), particularly paracetamol (Sawynok 2011c)), raising the possibility that dietary caffeine might interfere with the analgesic efficacy of some treatments. In the case of headache, another complication is that abrupt caffeine withdrawal is associated with the onset of headache, and this can be reversed by caffeine administration (Juliano 2004). Both of these issues have the potential to complicate any assessment of caffeine as an analgesic adjuvant.
How the intervention might work
The mechanisms by which caffeine may contribute to, or enhance the efficacy of other analgesics are not well understood. It is known to be a competitive antagonist of adenosine A1 and A2 receptors at plasma concentrations observed through normal dietary caffeine intake (in the 10 to 100 μM range). Many of the putative mechanisms of action are thought of in terms of this disruption of normal adenosine signalling. Proposed mechanisms of action include the following (Renner 2007; Sawynok 1993; Zhang 2001).
-
Improved drug absorption through lower gastric pH and increased gastric blood flow.
-
Reduced metabolic clearance of drugs through reduced hepatic blood flow.
-
Blockade of peripheral pro‐nociceptive adenosine signalling, and activation of the central noradenosine pathway (pain‐suppressing systems).
-
Transcriptional down‐regulation of cyclo‐oxygenase‐2 (COX‐2), via blockade of the adenosine A2a receptor.
-
Relief of inhibitor adenosine actions on central cholinergic nerve terminals.
-
Changes in mood and emotional state contributing to changes in the perception of pain.
Why it is important to do this review
Caffeine has been added to a large number of analgesics for years on the basis of a kind of inherited wisdom from a small number of trials showing an enhanced analgesic effect of combinations including caffeine. However, this is not the full story as some of these studies do not compare like with like, and there have been at least as many studies published suggesting no additional effect when caffeine is added. It is important to try to resolve this confusion to inform best clinical practice.
One of the major problems faced in trying to demonstrate an analgesic adjuvant effect of caffeine is the relatively small magnitude of this effect compared with normal variation in the course of an individual patient's pain and in the responses of different patients to the same analgesic, as noted 30 years ago (Beaver 1984). Many of the studies carried out have simply been underpowered to expose a statistically significant difference in treatment effects of analgesic plus caffeine versus the analgesic alone (Moore 1998). Meta‐analyses, in which data from individual comparisons are pooled, are an important tool for showing up these small effects that individual trials, on the whole, are unable to demonstrate. This methodology has been used successfully in the past to demonstrate a statistical superiority of higher dose aspirin, ibuprofen, or paracetamol over lower doses of the same drug (McQuay 2007).
This review provides an opportunity to apply systematically the same methodology, pooling individual comparison data wherever possible, to investigate the possible analgesic adjuvant effect of caffeine.
Objectives
To assess the relative efficacy of a single dose of an analgesic plus caffeine against the same dose of the analgesic alone, without restriction on the analgesic used or the pain condition studied. We also assessed serious adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included double‐blind studies comparing a single dose of oral analgesic plus caffeine with the same dose of the analgesic alone for the treatment of acute pain in adults; the caffeine had to be administered at the same time as the analgesic. Studies had to have a minimum of 10 participants randomly allocated to each treatment group and report some measure of patient‐reported pain relief.
We included studies using multiple doses to treat a single episode only if appropriate data from the first dose were available. We included cross‐over studies and studies reporting treatment of consecutive episodes (for example, consecutive migraine attacks) in pain conditions that result in comparable, recurrent, acute pain episodes, such as migraine. We used first dose only data where possible, but we accepted data from both phases of a cross‐over, or consecutive phases for recurrent conditions, if there was adequate washout (at least 48 hours pain‐ and medication‐free between phases).
Types of participants
Studies included adult participants (at least 16 years of age) with any acute painful condition. We did not include studies of experimental pain in healthy volunteers because these do not accurately correlate with clinical pain. Ideally participants had moderate to severe pain, to ensure sensitivity to detect a change in pain intensity, but mild to moderate pain was accepted.
Types of interventions
Included studies had to use a single dose of oral analgesic plus caffeine to treat an acute painful episode. The analgesics we were particularly interested in were paracetamol, ibuprofen, aspirin, diclofenac, naproxen, oxycodone, ergotamine, and the triptans, although studies using other analgesics were not excluded. There was no restriction on dose of analgesic or caffeine.
To investigate the effect of caffeine on the efficacy of the analgesic with which it is combined, it was essential that the comparator was the same drug and dose as the combination, minus caffeine.
Types of outcome measures
We collected data on the type of painful condition and baseline pain intensity.
Primary outcomes
We considered the following primary outcomes.
-
The number of participants with at least 50% of maximum pain relief at four to six hours.
-
The number of participants rating their treatment as "very good" or "excellent" on a five‐point categorical patient global evaluation of treatment (PGE) scale with the wording "poor, fair, good, very good, excellent" (or equivalent).
-
The number of participants achieving a self defined clinically meaningful level of pain relief.
-
The number of participants with headache relief at two hours.
In many postsurgical studies the outcome of "at least 50% of maximum pain relief at four to six hours" had to be transformed from group‐mean pain measures, as described in the 'Data synthesis' section.
We report the pain measures used in the 'Characteristics of included studies' table and have been as explicit as possible about how we transformed data from the various scales to the dichotomous outcomes specified above.
We considered only data obtained directly from the participant (pain reported by a physician, nurse, or carer was not included in the analysis).
Secondary outcomes
Although single dose studies in acute pain are generally underpowered to assess safety and tolerability and cannot provide information on repeat dosing strategies, we sought information on serious adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases:
-
the Cochrane Central Register of Controlled Trials (CENTRAL) via CRSO (to 28 August 2014);
-
MEDLINE via Ovid (1946 to 28 August 2014);
-
EMBASE via Ovid (1974 to 28 August 2014);
-
Oxford Pain Relief Database for the original review (Jadad 1996a). This database is no longer being updated.
See Appendix 1 for the search strategy for MEDLINE, Appendix 2 for EMBASE, and Appendix 3 for CENTRAL. We did not impose any language restrictions.
Searching other resources
We searched reference lists of retrieved studies and review articles for additional studies. We know of a number of unpublished trials using a caffeine analgesic combination, and we contacted relevant manufacturers to try to determine the extent of, and obtain, any unpublished data. We carried out Internet searches to identify any studies or study results that may have been reported to agencies such as the Food and Drug Administration (FDA).
For the update we searched two clinical trials databases (ClinicalTrials.gov (ClinicalTrials.gov)) and World Health Organization International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) to identify additional published or unpublished data.
We did not search grey literature and short abstracts (meeting reports).
Data collection and analysis
Selection of studies
Two review authors independently carried out the searches and selected studies for inclusion. We viewed the titles and abstracts of all studies identified by electronic searches on screen, and excluded any that clearly did not satisfy the inclusion criteria. We obtained full copies of the remaining studies to identify those suitable for inclusion, and settled any disagreements or uncertainty by discussion with the third review author.
Data extraction and management
Two review authors independently extracted data from included studies using a standard data extraction form. Disagreements and uncertainty were settled by discussion with the third review author. One review author entered data into RevMan 5.3 (RevMan 2014).
Assessment of risk of bias in included studies
We used the Oxford Quality Score as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum (Jadad 1996b).
Two authors independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study:
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, for example random number table; computer random number generator); or unclear risk of bias (when the method used to generate the sequence is not clearly stated). We excluded studies at a high risk of bias using a non‐random process (for example, odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (for example, telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); or unclear risk of bias (when the method is not clearly stated). We excluded studies that did not conceal allocation and are therefore at a high risk of bias (for example, open list).
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (for example, study stated that it was blinded and described the method used to achieve blinding, for example, identical tablets, matched in appearance and smell); or unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies at a high risk of bias that were not double‐blind.
-
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); or high risk of bias (fewer than 50 participants per treatment arm).
Measures of treatment effect
We used risk ratio (RR), calculated using a fixed‐effect model, to determine statistical difference between treatment groups, and number needed to treat to benefit (NNT) to provide an absolute measure of treatment effect. See 'Data synthesis' section for details.
Unit of analysis issues
We accepted randomisation of individual participants only. We did not, for example, accept studies where randomisation was by centre.
Dealing with missing data
The most likely source of missing data in single dose studies is cross‐over studies, in which multiple successive attacks are treated. In these studies, participants are excluded from the efficacy analyses after taking an initial dose simply because they do not have a sufficient number of qualifying pain episodes (for example, separate migraine attacks) to complete the cross‐over study. This is unlikely to introduce bias where it occurs equally in both treatment arms.
Assessment of heterogeneity
We assessed heterogeneity of studies visually using L'Abbé plots of the percentage of participants with the outcome with caffeine compared with those without caffeine (L'Abbé 1987), and with the use of the I2 statistic.
Assessment of reporting biases
Unpublished studies in this field are known to exist, as demonstrated in the review by Laska et al (Laska 1984), which analysed data from 30 clinical trials ‐ of which only four have been published in full. Obtaining unpublished studies, many of which were conducted 25 or more years ago, from the pharmaceutical companies sponsoring them was not possible. It is therefore difficult to make any meaningful assessment of reporting bias, and results must be interpreted with caution, with the knowledge that some degree of reporting bias is likely.
Approaches to pharmaceutical companies that may have had data were unsuccessful. In addition, although we know of data relating to three unpublished studies, we have been unable to obtain permission to use it. We identified further unpublished studies, with no available results, during this update.
We assessed the number of trials of average size amongst the included studies, with a RR of one (no effect), needed to reduce any statistically significant result to one that fails to meet statistical significance (following Moore 2008).
Data synthesis
Where possible, we used dichotomous data to calculate the RR with 95% confidence intervals (CIs) using a fixed‐effect model (Morris 1995). We calculated NNT with 95% CIs using the pooled number of events by the method of Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the RR did not include the number one.
Many studies provided data on pain measures using:
-
five‐point categorical pain relief (PR) scales with comparable wording to "none, slight, moderate, good or complete";
-
four‐point categorical pain intensity (PI) scales with comparable wording to "none, mild, moderate, severe";
-
visual analogue scale (VAS) for pain relief;
-
VAS for pain intensity.
We summed these pain measures over a four to six‐hour period to generate a measure of total pain relief (TOTPAR) or summed pain intensity difference (SPID) over this time period.
Where only non‐dichotomous, mean data are reported, we transformed them into dichotomous data using standardised methods. We converted any mean TOTPAR, SPID, VAS TOTPAR or VAS SPID values to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Cooper 1991). We calculated the proportion of participants in each treatment group achieving at least 50%maxTOTPAR using verified equations (Moore 1996; Moore 1997a; Moore 1997b), and converted these proportions into the number of participants achieving at least 50%maxTOTPAR by multiplying by the total number of participants in the treatment group. We then used information on the number of participants with at least 50%maxTOTPAR for the analgesic plus caffeine and the analgesic alone to calculate estimates of RR and NNT as before.
We tested for significant differences between groups in sensitivity analyses using the z test (Tramèr 1997).
Subgroup analysis and investigation of heterogeneity
We analysed data for different pain conditions and different analgesics separately where there were sufficient data (minimum of two studies and 200 participants).
Sensitivity analysis
We planned to carry out sensitivity analyses for dose of caffeine (< 70 mg, 70 mg to 150 mg, and > 150 mg), methodological quality (2 versus ≥ 3 on the Oxford Quality Scale), baseline pain intensity (mild versus ≥ moderate), and size (< 50 versus ≥ 50 participants in each treatment arm), where there were sufficient data (minimum of two studies and 200 participants). We also tried to ascertain whether any adjuvant effect of caffeine depended on the particular analgesic drug with which it was combined, and to investigate whether analgesic effect size with analgesic drug alone affects any adjuvant effects of caffeine through limiting the upside sensitivity of the analgesic assay (Cooper 1991).
Results
Description of studies
Results of the search
Searches of bibliographic databases for this update identified 279 potential studies in CENTRAL, 622 in MEDLINE, and 1216 in EMBASE; there were no new included or excluded studies. Searches of clinicaltrials.gov and the International Clinical Trials Registry Platform identified 42 and 21 potential studies, respectively. Of these, five appeared to satisfy the inclusion criteria, all of which have, or are likely to have, completed but none of which had any results available (IRCT201306121760N24; NCT00471952; NCT01172405; NCT01929031; NCT02183688) (Figure 1).

Study flow diagram.
The search for studies comparing a given dose of a given analgesic with the same dose of the same analgesic plus caffeine was complicated. The main reason was the large number of potentially relevant studies from which no data were available. The following narrative acts as a guide to the size the size of problem.
-
A 1994 review of caffeine as an analgesic adjuvant included 30 studies with over 10,600 participants (Laska 1984). Of these, it is likely that 12 studies with 4600 participants provided information regarding a suitable direct comparison. While the review provided no useful information for analysis, we believe that four studies (1206 participants) had been published previously and data from those were available (Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4). However, the likelihood is that there are at least eight studies with 3394 participants for which we know data exist but which were unavailable.
-
We obtained from the Internet an undated document in the form of a Citizen Petition Summary from Bristol‐Myers Squibb relating to use of caffeine as an analgesic adjuvant (BMS summary). The document had information about 17 studies involving 8772 participants. Eight of these studies (3231 participants) were submitted to the FDA in 1982, and we believe that they were probably included in the 1984 review (Laska 1984); nine studies (5541 participants) had submission dates of 1986 or later, and were probably not included in the 1984 review.
-
We are aware of three studies with up to 850 participants conducted by McNeil Consumer that are likely to have useful comparison data. We have been unable to obtain permission to use these data.
Although 20 studies were eventually included with data on 7238 participants, we know or suspect of the existence of 20 studies with 9785 participants for which data for analysis were not obtainable. There were also an additional 2689 participants in the five studies identified in clinical trial registries, for whom no results are yet available. About 12,500 participants have contributed to relevant adjuvant caffeine studies without available information.
The search for this update did not identify any new studies with data.
Included studies
Twenty studies (15 publications) fulfilled the inclusion criteria for this review; all 20 were published in full peer‐reviewed journals (Ali 2007; Diamond 2000; Diener 2005; Forbes 1990; Forbes 1991; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; McQuay 1996; Migliardi 1994 Study 1; Migliardi 1994 Study 2; Peroutka 2004; Schachtel 1991a; Sunshine 1996; Tokola 1984; Ward 1991; Winter 1983; Wójcicki 1977 study 1; Wójcicki 1977 Study 2). These studies provided data on 7238 participants.
We identified no additional studies by contacting manufacturers or searching the Internet, but searches of clinical trials registries for this update identified five additional studies (estimated 2689 participants) that may satisfy the inclusion criteria (IRCT201306121760N24; NCT00471952; NCT01172405; NCT01929031; NCT02183688). Although all of these studies have probably been completed, none have been published, and no results are posted in the trial registries. We have placed them in 'Studies awaiting classification', and have provided as many relevant details as possible. We sent emails to the principal investigators or sponsors of the studies where this information was provided, but at the time of publication of this update none had responded.
The majority of included studies recruited participants aged 18 years or over, with some placing an upper age limit of 60 to 85 years of age. One study recruited participants aged 16 to 75 years of age (Winter 1983), while another two recruited participants aged 15 years or older (Forbes 1990; Forbes 1991). Five studies did not report the age range for recruiting participants, and overall mean ages ranged from 21 to 46 years (Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; McQuay 1996). The majority of participants were female (58% to 100%) in 17 of the 19 included studies, and five of these had an exclusively female study population (Ali 2007; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; Sunshine 1996).
A number of different pain conditions were studied: postpartum pain (for example, episiotomy, uterine cramp), postoperative dental pain (third molar extraction), headache (tension, migraine, idiopathic), dysmenorrhoea, and sore throat. For analysis we combined postpartum pain and postoperative dental pain, since it was thought likely that most of the postpartum pain followed episiotomy.
The majority of studies prohibited participants from consuming any caffeine‐containing food, beverages, or medications within a defined period of treatment with study medication (ranging from three hours to midnight the night before), as well as during the study period. Three studies did not prohibit caffeine consumption before administration of study medication, but required that any caffeine consumed within four or 24 hours, respectively, of the study period was noted (Migliardi 1994 Study 1; Migliardi 1994 Study 2; Ward 1991). Seven studies did not comment on caffeine consumption before or during the study (Diener 2005; Forbes 1990; Peroutka 2004; Tokola 1984; Winter 1983; Wójcicki 1977 study 1; Wójcicki 1977 Study 2). Eight studies restricted participants from taking study medication within a defined time period of other analgesics and medications (Ali 2007; Diamond 2000; Diener 2005; Migliardi 1994 Study 1;Migliardi 1994 Study 2; Schachtel 1991a; Sunshine 1996; Winter 1983). The remaining 12 studies did not report on restricted use of other medications before administration of study medication.
Participants were generally excluded for: pregnancy or lactation, recent history of alcohol, analgesic or other substance abuse, allergy or intolerance to any of the study or rescue medications, or existing illness or medical condition that could compromise interpretation of the results. Reasons for exclusion specific to a particular study are described in the 'Characteristics of included studies' table.
The majority of studies required participants to have at least moderate pain (two or more on standard four‐point pain intensity scale, or equivalent) before treating with study medication. One study required only mild pain before treatment (Diener 2005), one study required a headache rated two or greater on the McGill Pain Questionnaire (Ward 1991), and two studies did not report baseline pain intensity (Diamond 2000; Tokola 1984).
Most of the included studies used a parallel‐group design (15/20), with the remaining five involving a treatment of multiple acute pain episodes (headache or migraine) in a cross‐over design (Ali 2007; Migliardi 1994 Study 1; Migliardi 1994 Study 2; Tokola 1984; Ward 1991).
The response to study treatment was measured using a standard four‐point pain intensity scale or five‐point pain relief scale, or both, in the majority of studies (15 studies and 13 studies respectively). The remaining studies used alternative pain intensity or pain relief scales (for example, 100 mm VAS) or patient global evaluations which are described in the 'Characteristics of included studies' table. Some headache studies also used a patient global outcome (Diamond 2000; Diener 2005).
The 20 studies reported on 17 different treatment comparisons:
-
Paracetamol 500 mg + caffeine 65 mg versus paracetamol 500 mg (Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3).
-
Paracetamol 648 mg + caffeine 65 mg versus paracetamol 648 mg (Ward 1991).
-
Paracetamol 648 mg + caffeine 130 mg versus paracetamol 648 mg (Ward 1991).
-
Paracetamol 1000 mg + caffeine 100 mg versus paracetamol 1000 mg (Wójcicki 1977 study 1; Wójcicki 1977 Study 2).
-
Paracetamol 1000 mg + caffeine 130 mg versus paracetamol 1000 mg (Ali 2007; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; Migliardi 1994 Study 1; Migliardi 1994 Study 2; Winter 1983).
-
Paracetamol 1500 mg + caffeine 195 mg versus paracetamol 1500 mg (Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3).
-
Paracetamol 2000 mg + caffeine 260 mg versus paracetamol 2000 mg (Laska 1983 Study 4).
-
Ibuprofen 100 mg + caffeine 100 mg versus ibuprofen 100 mg (Forbes 1991; Sunshine 1996).
-
Ibuprofen 200 mg + caffeine 50 mg versus ibuprofen 200 mg (McQuay 1996).
-
Ibuprofen 200 mg + caffeine 100 mg versus ibuprofen 200 mg (Forbes 1991; McQuay 1996; Sunshine 1996).
-
Ibuprofen 200 mg + caffeine 200 mg versus ibuprofen 200 mg (McQuay 1996).
-
Ibuprofen 400 mg + caffeine 200 mg versus ibuprofen 400 mg (Diamond 2000).
-
Aspirin 650 mg + caffeine 65 mg versus aspirin 650 mg (Forbes 1990).
-
Aspirin 800 mg + caffeine 64 mg versus aspirin 800 mg (Schachtel 1991a).
-
Aspirin 500 mg + paracetamol 400 mg + caffeine 100 mg versus aspirin 500 mg + paracetamol 400 mg (Diener 2005).
-
Diclofenac sodium softgel 100 mg + caffeine 100 mg versus diclofenac sodium softgel 100 mg (Peroutka 2004).
-
Tolfenamic acid 200 mg + caffeine 100 mg versus tolfenamic acid 200 mg (Tokola 1984).
Full details of included studies are provided in the Characteristics of included studies table.
Excluded studies
For the original review we excluded six reports after reading them in full (BMS summary (17 studies); Jain 1988; Laska 1984 (probably 12 relevant studies); Migliardi 1994a; Mitchell 2008; Schachtel 1991b). The BMS summary had information on 17 potentially relevant studies in tension‐type headache (HPD‐H203; 170‐01‐88; 170‐02‐88), postoperative dental pain (HPD‐D104; HPD‐D105; 171‐01‐88; 2569; 2711; 2570; 2571), and postpartum pain (2255; 2576; 2577, 2578; 2579; 2580; 2581). Eight of these (2569; 2711; 2255; 2576; 2577, 2578; 2579; 2580) were probably also included in Laska 1984. Laska 1984 reported on 30 studies, of which 12 were probably relevant to this review, and four are included studies (published separately as Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4).
We did not exclude any additional studies identified for this update. The reasons for exclusions are provided in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
Included studies were all randomised and double‐blind. Four studies scored five out of five on the Oxford Quality Scale (Ali 2007; Diener 2005; Forbes 1990; McQuay 1996), 11 studies scored four out of five (Forbes 1991; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; Migliardi 1994 Study 1; Migliardi 1994 Study 2; Schachtel 1991a; Sunshine 1996; Tokola 1984; Winter 1983), four studies scored three out of five (Diamond 2000; Peroutka 2004; Wójcicki 1977 study 1; Wójcicki 1977 Study 2), and one study scored two out of five (Ward 1991).
Comments on potential biases in individual studies related to random sequence generation, allocation concealment, blinding, and study size, are reported in the 'Risk of bias' section of the 'Characteristics of included studies' table. The findings are displayed in Figure 2 and Figure 3.
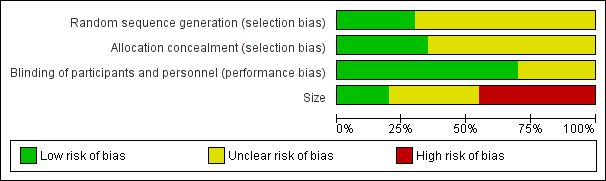
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
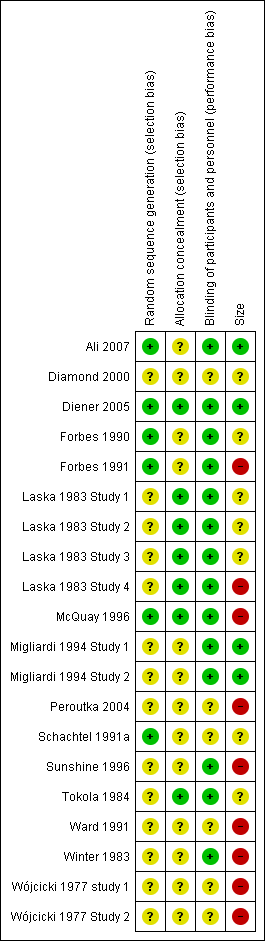
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
The large numbers of studies and participants excluded because of unavailability of data ‐ larger than the number with data for analysis ‐ provided a significant potential for publication bias.
Allocation
All studies stated that they were randomised but only six reported the method used to generate the random sequence, and only four described the method used to conceal the allocation of the sequence.
Blinding
All studies stated that they were double‐blind, but only 10 adequately described the method used to conceal the treatment allocation from the participants and study personnel.
Other potential sources of bias
We judged seven studies to be at high risk of bias because they randomised fewer than 50 participants to each treatment arm. We judged only three to be at low risk because they randomised at least 200 participants to each treatment arm.
Effects of interventions
See: Summary of findings for the main comparison
For all studies we report whether there was a simple numerical superiority for analgesic plus caffeine compared with analgesic alone using the primary outcomes (Appendix 4).
Most postoperative studies reported data that allowed calculation of the number of participants achieving at least 50% of the maximum possible pain relief over the duration of the study, and we used these studies for analyses in this review. One reported patients being pain‐free after four hours, and we used that (Wójcicki 1977 Study 2).
Headache studies reported various outcomes. One reported the number of participants rating their treatment as "very good" or "excellent" on a five‐point categorical patient global evaluation of treatment (Diamond 2000), while another reported a four‐point scale with the top value of "very good" (Diener 2005), which we used. Another reported the number of participants with headache relief at one hour: 41% (19/46) with diclofenac plus caffeine, and 27% (12/45) with diclofenac alone (Peroutka 2004). Tokola 1984 reported the number of participants with no pain or mild pain at 1.5 hours. Wójcicki 1977 study 1 reported the number of participants with headache relief at four hours with paracetamol + caffeine versus paracetamol alone. We did not convert average pain intensity and pain relief outcomes to dichotomous outcomes for two studies in tension headache (Migliardi 1994 Study 1; Migliardi 1994 Study 2), as the equations to do so were developed in postoperative pain and may not be appropriate. Ward 1991 also provided data on the mean pain intensity difference from baseline over two hours, but this could not be dichotomised as there is no valid method over this time period: the summed pain intensity difference (SPID) 2 for paracetamol 648 mg + caffeine 65 mg was 32.6, compared with 37.5 for paracetamol 648 mg + caffeine 130 mg, and 28.3 for paracetamol 648 mg alone.
Schachtel 1991a (sore throats) provided mean data for pain relief over two hours, but this could not be dichotomised as there is no valid method over this time period: the total pain relief (TOTPAR) at two hours for aspirin 800 mg + caffeine 64 mg was 6.3, compared with 4.7 for aspirin 800 mg alone.
Pain conditions
All pain conditions
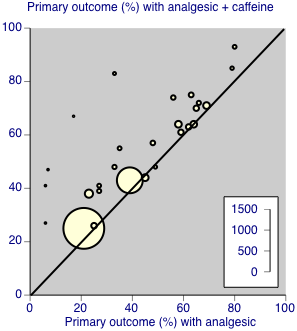
Twenty‐five comparisons (4262 participants) compared analgesic plus caffeine versus the same dose of analgesic alone (Figure 4). Caffeine provided additive analgesia irrespective of pain condition, dose of caffeine, analgesic, and proportion of responders with analgesic alone.
Individual studies comparing the primary outcome for analgesic + caffeine versus analgesic alone ‐ any pain condition
-
The proportion of participants with at least 50% of the maximum pain relief after analgesic plus caffeine was 48% (1033/2136; range 26% to 83%).
-
The proportion of participants with at least 50% of the maximum pain relief after analgesic alone was 41% (877/2126; range 6% to 66%).
-
The RR for the addition of caffeine was 1.2 (95% CI 1.1 to 1.3; Analysis 1.1), and the NNT was 14 (9.9 to 24).
Postoperative/postpartum pain
There were 19 comparisons (2239 participants) of analgesic plus caffeine versus the same dose of analgesic alone for postoperative or postpartum pain (Analysis 1.1).
-
The proportion of participants with at least 50% of the maximum pain relief after analgesic plus caffeine was 60% (657/1086; range 26% to 93%).
-
The proportion of participants with at least 50% of the maximum pain relief after analgesic alone was 51% (568/1115; range 6% to 80%).
-
The RR for the addition of caffeine was 1.2 (1.1 to 1.3; Analysis 1.1), and the NNT was 10 (7.3 to 18).
Headache pain
Five studies (1503 participants) provided data in migraine or tension‐type headache (Diamond 2000; Diener 2005; Tokola 1984; Wójcicki 1977 study 1).
-
The proportion of participants with at least 50% of the maximum pain relief after analgesic plus caffeine was 33% (242/740; range 25% to 83%).
-
The proportion of participants with at least 50% of the maximum pain relief after analgesic alone was 25% (172/763; range 21% to 43%).
-
The RR for the addition of caffeine was 1.3 (1.1 to 1.5; Analysis 1.1), and the NNT was 13 (8.3 to 34).
Dysmenorrhoea
Only one study (620 participants) provided data in dysmenorrhoea; 134/310 (43%) of participants had at least 50% of the maximum pain relief after analgesic plus caffeine, and 121/310 (39%) after analgesic alone (Ali 2007).
Choice of analgesic
At least 50% of maximum pain relief
Studies combined caffeine with paracetamol, ibuprofen, or aspirin alone, or with aspirin plus paracetamol.
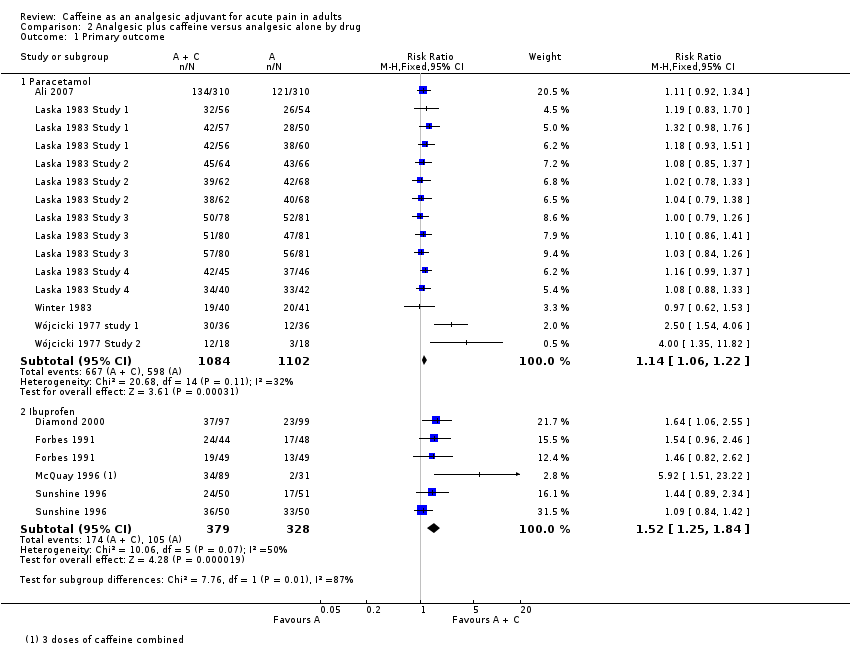
Paracetamol
Fifteen comparisons (2186 participants) compared paracetamol plus caffeine with paracetamol alone (Ali 2007; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; Winter 1983; Wójcicki 1977 study 1; Wójcicki 1977 Study 2). Doses of paracetamol ranged from 500 mg to 2000 mg.
-
The proportion of participants with at least 50% of the maximum pain relief after paracetamol plus caffeine was 62% (667/1084; range 43% to 93%).
-
The proportion of participants with at least 50% of the maximum pain relief after paracetamol alone was 54% (598/1102; range 39% to 80%).
-
The RR for the addition of caffeine was 1.1 (1.06 to 1.2; Analysis 2.1), and the NNT was 14 (8.8 to 32).
Ibuprofen
Six comparisons (707 participants) compared ibuprofen plus caffeine with ibuprofen alone (Diamond 2000; Forbes 1991; McQuay 1996; Sunshine 1996). Doses of ibuprofen ranged from 100 mg to 400 mg.
-
The proportion of participants with at least 50% of the maximum pain relief after ibuprofen plus caffeine was 46% (174/379; range 38% to 72%).
-
The proportion of participants with at least 50% of the maximum pain relief after ibuprofen alone was 32% (105/328; range 6% to 66%).
-
The RR for the addition of caffeine was 1.5 (1.3 to 1.8; Analysis 2.1), and the NNT was 7.2 (4.8 to 15).
Aspirin
One study (134 participants) used aspirin 650 mg (Forbes 1990), in a comparison with the aspirin plus caffeine; 17/66 (26%) participants experienced at least 50% of the maximum pain relief with aspirin plus caffeine, and 17/68 (25%) with aspirin alone.
One study (980 participants) used aspirin 500 mg plus paracetamol 400 mg in a comparison with the aspirin plus paracetamol plus caffeine; 429/482 (89%) participants experienced at least 50% of the maximum pain relief with aspirin plus paracetamol plus caffeine, and 435/566 (77%) with aspirin plus paracetamol only (Diener 2005).
Diclofenac
One study (91 participants) used diclofenac 100 mg in comparison with the diclofenac plus caffeine (Peroutka 2004); 19/46 (41%) had pain reduced to mild or none at one hour with diclofenac plus caffeine, and 12/45 with diclofenac alone.
Tolfenamic acid
One study (164 participants) used tolfenamic acid 200 mg in comparison with tolfenamic acid plus caffeine; 35/79 (44%) participants had no or mild pain at 1.5 hours with tolfenamic acid plus caffeine, and 38/85 (45%) with tolfenamic acid alone.
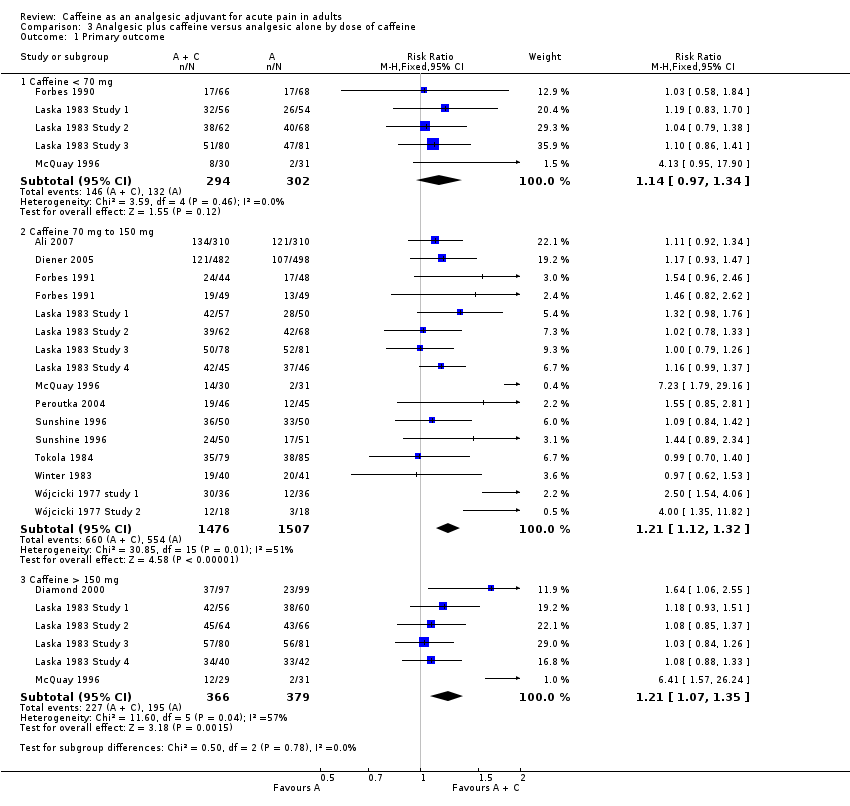
Dose of caffeine
Studies used doses of caffeine ranging from 50 mg to 260 mg, but typically they were 100 mg or 200 mg. We analysed all trials together to investigate whether there was a dose response for caffeine.
Caffeine < 70 mg
Five comparisons (596 participants) provided data (Forbes 1990; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; McQuay 1996). All were in postoperative pain.
-
The proportion of participants with at least 50% of the maximum pain relief after analgesic plus caffeine was 50% (146/294; range 26% to 64%).
-
The proportion of participants with at least 50% of the maximum pain relief after analgesic alone was 44% (132/302; range 6% to 59%).
-
The RR for the addition of caffeine was 1.1 (0.97 to 1.3; Analysis 3.1). There was no significant difference between treatment groups and the NNT was not calculated.
Caffeine 70 mg to 150 mg
Sixteen comparisons (2983 participants) provided data (Ali 2007; Diener 2005; Forbes 1991; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; McQuay 1996; Peroutka 2004; Sunshine 1996; Tokola 1984; Winter 1983; Wójcicki 1977 study 1; Wójcicki 1977 Study 2).
-
The proportion of participants with at least 50% of the maximum pain relief after analgesic plus caffeine was 45% (660/1476; range 25% to 83%).
-
The proportion of participants with at least 50% of the maximum pain relief after analgesic alone was 37% (554/1507; range 6% to 80%).
-
The RR for the addition of caffeine was 1.2 (1.1 to 1.3; Analysis 3.1), and the NNT was 13 (8.7 to 23).
Caffeine > 150 mg
Six comparisons (745 participants) provided data (Diamond 2000; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; McQuay 1996).
-
The proportion of participants with at least 50% of the maximum pain relief after analgesic plus caffeine was 62% (227/366; range 41% to 85%).
-
The proportion of participants with at least 50% of the maximum pain relief after analgesic alone was 51% (195/379; range 6% to 79%).
-
The RR for the addition of caffeine was 1.2 (1.1 to 1.4; Analysis 3.1), and the NNT was 9.5 (5.7 to 29).
There was no clear dose response (Figure 5).

Forest plot of comparison: 3 Analgesic plus caffeine versus analgesic alone by dose of caffeine, outcome: 3.1 Primary outcome.
Sensitivity analyses
Methodological quality
Only one study had a quality score of two out of five (Ward 1991), so no sensitivity could be carried out for this criterion.
Baseline pain intensity
One study did not state baseline pain (Diamond 2000), one administered the first dose when pain was mild (Tokola 1984), two treated when pain was "at least mild" (Diener 2005; Ward 1991), while the remainder treated when pain was moderate or severe. Tokola 1984 and Ward 1991 did not provide data suitable for analysis. Comparing Diamond 2000 and Diener 2005 with studies in moderate or severe pain gave no statistically significant difference (z = 0.1995, P value = 0.905).
Size
Using data for all pain conditions, comparing the 13 comparisons with treatments arms of 50 participants or fewer (964 participants; NNT for analgesic + caffeine versus analgesic alone 7.0 (4.9 to 13)) with the 14 comparisons with treatment arms of more than 50 participants (3298 participants; NNT 21 (12 to 70)) gave a statistically significant difference (z = 2.6032, P value = 0.009). Larger studies produced a smaller effect size.
Serious adverse events
One study did not report any information about adverse events during the study (Ward 1991). The remaining studies all provided some information, and only one reported any serious adverse events (Diener 2005). One participant experienced acute enteritis after treatment with the aspirin plus paracetamol plus caffeine combination, and one experienced an attack of ulcerative colitis following paracetamol alone: neither event was considered by the investigators to be drug‐related.
Discussion
Since publication of the previous version of this review, we found no new studies.
Summary of main results
This review update was able to analyse 25 comparisons of analgesic plus caffeine versus the same dose of analgesic alone in 4262 participants. Most studies used paracetamol (500 mg to 1500 mg) or ibuprofen (100 mg to 400 mg), with two using aspirin (650 mg and 800 mg), one aspirin (500 mg) plus paracetamol (400 mg), one diclofenac (100 mg), and one tolfenamic acid (200 mg). Caffeine was added at doses of 50 mg to 260 mg, with most studies using between 100 mg and 200 mg. A number of different pain conditions were studied: headache, postoperative pain, postpartum pain, dysmenorrhoea, and sore throat.
Numerical superiority (all primary outcomes) was demonstrated in all but three out of 28 comparisons (Appendix 4):
-
Paracetamol 1000 mg + caffeine 130 mg versus paracetamol 1000 mg alone (Laska 1983 Study 3; 159 participants).
-
Tolfenamic acid 200 mg + caffeine 100 mg versus tolfenamic acid 100 mg alone (Tokola 1984; 164 participants).
-
Paracetamol 1000 mg + caffeine 130 mg versus paracetamol 1000 mg alone (Winter 1983; 81 participants).
We carried out pooled analyses for studies in which one primary outcome was reported or could be calculated. For all studies combined there was a small significant benefit for adjuvant caffeine (RR 1.2 (1.1 to 1.3) and NNT 14 (9.9 to 24)).
Analysis according to pain condition demonstrated a similar benefit in postoperative pain (NNT 10 (7.3 to 18)) and headache (NNT 13 (8.3 to 34)). The benefit was the same for both paracetamol and ibuprofen. Analysis according to dose of caffeine demonstrated a statistically significant effect for doses of 100 mg and more with NNT of about 10, at 65 mg and below the result was not significant (RR 1.2 (0.94 to 1.4)). The absolute proportion of additional participants achieving at least 50% maximum pain relief was 6% at doses of 65 mg caffeine or less, 8% with doses between 70 and 150 mg, and 11% with doses of 150 mg or more. Failure to achieve a statistically significant improvement with lowest doses of caffeine (generally 65 mg) may have reflected the small number of studies and participants with the lowest dose.
A particular feature of the finding of an adjuvant effect of caffeine was its consistency in terms of the pain condition, analgesic used, and level of pain relief with analgesic alone. Only one study used an effective analgesic (ibuprofen 200 mg) with groups treated with several different doses of caffeine in addition (McQuay 1996); this study had the same result as the overall finding, namely that doses of caffeine of 65 mg or below are ineffective as an analgesic adjuvant.
Caffeine is commonly consumed worldwide at doses similar to those used in these studies, and its side effect profile is well known; nervousness and dizziness are common (Zhang 2001). No unexpected events occurred and the one serious event in a participant treated with adjuvant caffeine was not considered related to the study medication.
Overall completeness and applicability of evidence
Caffeine was added to only a limited number of analgesics in these studies, and it is unclear whether the small additional benefit demonstrated overall and with paracetamol and ibuprofen individually will apply to any other analgesic. Several different pain conditions were studied; while other acute conditions such as non‐surgical trauma were not included, it is unlikely that the result would be different, given that analgesics do not appear to be condition‐specific. It should be noted, though, that the review was limited to acute pain only. Whether caffeine has any effect in chronic painful conditions has yet to be elucidated.
Over the dose range of 65 mg to 200 mg, no increase in adjuvant effect was noted with increasing caffeine dose. This may be a function of limited data combined with small effect size, or the limited dose range studied, but it may also reflect the mechanism by which caffeine achieves an adjuvant response. The information in this review can pose rather than answer these questions.
Quality of the evidence
Most of the studies were relatively old, with only three (in headache) published since 2000, but they were generally of good methodological quality, using standard designs and mostly standard scales of pain measurement, though studies in tension‐type headache are problematical as to outcomes (Moore 2014). Individual studies, especially those in postpartum and dental pain, were small and individually they rarely demonstrated a significant benefit for caffeine. Meta‐analyses of small trials are susceptible to overestimation of effects (Dechartres 2013; Nüesch 2010). The larger studies were predominantly in headache pain, and individually demonstrated statistical significance.
Potential biases in the review process
The biggest threat to the validity of the results arises from the large amount of data that is known to exist in unpublished studies with data unavailable for this review. The small, significant, and arguably clinically relevant 5% to 10% increased number of responders was derived from the 4262 participants providing data for one primary outcome. We calculated that the result would remain statistically significant (though probably clinically much less relevant) if we added 10,000 additional participants in studies where there was no difference between analgesic and the same dose of analgesic plus caffeine (RR = 1.0).
This is the approximate size of the amount of unpublished data about which we know. However, there is considerable evidence that a positive effect of caffeine occurs in these studies (BMS summary; Laska 1984; Sawynok 1993). In this circumstance it is unlikely that publication bias would play any role in changing either the direction or magnitude of the result.
There were two other possible interferences in these assays. One was a possible interference of caffeine in analgesic effects of paracetamol (Sawynok 2011c). A very large part of the data came from studies comparing paracetamol with paracetamol plus caffeine, therefore reducing any adjuvant analgesic effects of caffeine so that any bias would be a negative bias. Moreover, there was no difference between studies in which paracetamol was the analgesic and those in which ibuprofen was the analgesic used. The other possible interference was from caffeine withdrawal headache (Juliano 2004), where use of caffeine might have a possible positive bias; the effects of adjuvant caffeine in headache were virtually identical to the effects in other painful conditions.
Agreements and disagreements with other studies or reviews
This review is in agreement with several reviews that have reported a small, but significant, analgesic adjuvant effect of caffeine at doses in excess of 65 mg when combined with common analgesics such as paracetamol and ibuprofen (Laska 1984; Palmer 2010; Sawynok 2011b). Other reviews in postsurgical pain only have reported an inconsistent effect for caffeine combined with ibuprofen (Li Wan Po 1998), no effect for caffeine combined with aspirin (Zhang 1997), and no effect for caffeine combined with paracetamol (Zhang 1996).
This update has taken a somewhat different approach to outcomes in some headache studies, which led to modest differences in the numbers available for analysis, but the results are similar to the original review.

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
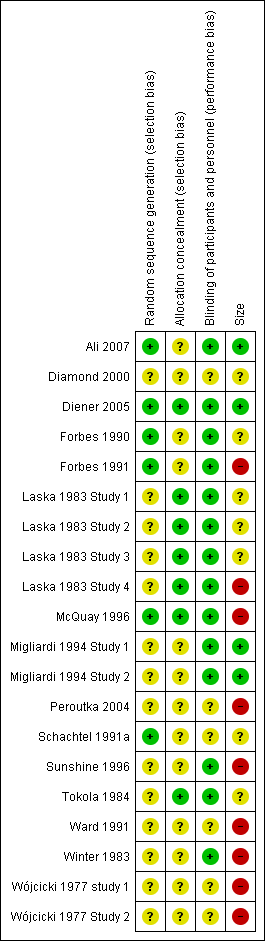
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Individual studies comparing the primary outcome for analgesic + caffeine versus analgesic alone ‐ any pain condition

Forest plot of comparison: 3 Analgesic plus caffeine versus analgesic alone by dose of caffeine, outcome: 3.1 Primary outcome.

Comparison 1 Analgesic plus caffeine versus analgesic alone by pain condition, Outcome 1 Primary outcome.
Comparison 2 Analgesic plus caffeine versus analgesic alone by drug, Outcome 1 Primary outcome.
Comparison 3 Analgesic plus caffeine versus analgesic alone by dose of caffeine, Outcome 1 Primary outcome.
| Analgesic plus caffeine compared with analgesic alone for acute pain | ||||||
| Patient or population: adults with acute pain Settings: community Intervention: analgesic plus caffeine Comparison: same dose of analgesic alone | ||||||
| Outcomes | Outcome with analgesic alone | Outcome with analgesic plus caffeine | RR and NNT | No of participants | Quality of the evidence | Comments |
| Effective pain relief | 41% | 48% | RR 1.2 (1.1 to 1.3) NNT 14 (9.9 to 24) | 4262 (27 separate comparisons) | High | Small effect size but large numbers of participants contributing. There is a large amount of data that cannot be incorporated into this review, but this result is robust to analysis assuming all missing data show no effect. In fact, the results of this review are consistent with an almost completely different analysis in 10,000 participants demonstrating the effect of caffeine to have a similar effect size |
| Serious adverse events | 1 event | 1 event | Not calculated | Not calculated | Very low | Neither event judged related to study medication. Single dose studies are not powered to assess serious adverse events |
| CI: confidence interval; NNT: number needed to treat to benefit; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome Show forest plot | 16 | 4262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.11, 1.26] |
| 1.1 Postoperative/postpartum | 10 | 2139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.08, 1.25] |
| 1.2 Headache | 5 | 1503 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.11, 1.52] |
| 1.3 Dysmenorrhoea | 1 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.92, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Paracetamol | 8 | 2186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.06, 1.22] |
| 1.2 Ibuprofen | 4 | 707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.25, 1.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome Show forest plot | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Caffeine < 70 mg | 5 | 596 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.34] |
| 1.2 Caffeine 70 mg to 150 mg | 14 | 2983 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.12, 1.32] |
| 1.3 Caffeine > 150 mg | 6 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.07, 1.35] |

