Psihosocijalne intervencije za smanjenje konzumacije alkohola u osoba koje su ovisne o alkoholu i drogama
Abstract
Background
Problem alcohol use is common among illicit drug users and is associated with adverse health outcomes. It is also an important factor contributing to a poor prognosis among drug users with hepatitis C virus (HCV) as it impacts on progression to hepatic cirrhosis or opiate overdose in opioid users.
Objectives
To assess the effects of psychosocial interventions for problem alcohol use in illicit drug users (principally problem drug users of opiates and stimulants).
Search methods
We searched the Cochrane Drugs and Alcohol Group trials register (June 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 11, June 2014), MEDLINE (1966 to June 2014); EMBASE (1974 to June 2014); CINAHL (1982 to June 2014); PsycINFO (1872 to June 2014) and the reference lists of eligible articles. We also searched: 1) conference proceedings (online archives only) of the Society for the Study of Addiction, International Harm Reduction Association, International Conference on Alcohol Harm Reduction and American Association for the Treatment of Opioid Dependence; 2) online registers of clinical trials: Current Controlled Trials, Clinical Trials.org, Center Watch and the World Health Organization International Clinical Trials Registry Platform.
Selection criteria
Randomised controlled trials comparing psychosocial interventions with another therapy (other psychosocial treatment, including non‐pharmacological therapies, or placebo) in adult (over the age of 18 years) illicit drug users with concurrent problem alcohol use.
Data collection and analysis
We used the standard methodological procedures expected by The Cochrane Collaboration.
Main results
Four studies, involving 594 participants, were included. Half of the trials were rated as having a high or unclear risk of bias. The studies considered six different psychosocial interventions grouped into four comparisons: (1) cognitive‐behavioural coping skills training versus 12‐step facilitation (one study; 41 participants), (2) brief intervention versus treatment as usual (one study; 110 participants), (3) group or individual motivational interviewing (MI) versus hepatitis health promotion (one study; 256 participants) and (4) brief motivational intervention (BMI) versus assessment‐only (one study; 187 participants). Differences between studies precluded any data pooling. Findings are described for each trial individually.
Comparison 1: low‐quality evidence; no significant difference for any of the outcomes considered
Alcohol abstinence as maximum number of weeks of consecutive alcohol abstinence during treatment: mean difference (MD) 0.40 (95% confidence interval (CI) ‐1.14 to 1.94); illicit drug abstinence as maximum number of weeks of consecutive abstinence from cocaine during treatment: MD 0.80 (95% CI ‐0.70 to 2.30); alcohol abstinence as number achieving three or more weeks of consecutive alcohol abstinence during treatment: risk ratio (RR) 1.96 (95% CI 0.43 to 8.94); illicit drug abstinence as number achieving three or more weeks of consecutive abstinence from cocaine during treatment: RR 1.10 (95% CI 0.42 to 2.88); alcohol abstinence during follow‐up year: RR 2.38 (95% CI 0.10 to 55.06); illicit drug abstinence as abstinence from cocaine during follow‐up year: RR 0.39 (95% CI 0.04 to 3.98), moderate‐quality evidence.
Comparison 2: low‐quality evidence, no significant difference for all the outcomes considered
Alcohol use as AUDIT scores at three months: MD 0.80 (95% ‐1.80 to 3.40); alcohol use as AUDIT scores at nine months: MD 2.30 (95% CI ‐0.58 to 5.18); alcohol use as number of drinks per week at three months: MD 0.70 (95% CI ‐3.85 to 5.25); alcohol use as number of drinks per week at nine months: MD ‐0.30 (95% CI ‐4.79 to 4.19); alcohol use as decreased alcohol use at three months: RR 1.13 (95% CI 0.67 to 1.93); alcohol use as decreased alcohol use at nine months: RR 1.34 (95% CI 0.69 to 2.58), moderate‐quality evidence.
Comparison 3 (group and individual MI), low‐quality evidence: no significant difference for all outcomes
Group MI: number of standard drinks consumed per day over the past month: MD ‐0.40 (95% CI ‐2.03 to 1.23); frequency of drug use: MD 0.00 (95% CI ‐0.03 to 0.03); composite drug score (frequency*severity for all drugs taken): MD 0.00 (95% CI ‐0.42 to 0.42); greater than 50% reduction in number of standard drinks consumed per day over the last 30 days: RR 1.10 (95% CI 0.82 to 1.48); abstinence from alcohol over the last 30 days: RR 0.88 (95% CI 0.49 to 1.58).
Individual MI: number of standard drinks consumed per day over the past month: MD ‐0.10 (95% CI ‐1.89 to 1.69); frequency of drug use (as measured using the Addiction Severity Index (ASI drug): MD 0.00 (95% CI ‐0.03 to 0.03); composite drug score (frequency*severity for all drugs taken): MD ‐0.10 (95% CI ‐0.46 to 0.26); greater than 50% reduction in number of standard drinks consumed per day over the last 30 days: RR 0.92 (95% CI 0.68 to 1.26); abstinence from alcohol over the last 30 days: RR 0.97 (95% CI 0.56 to 1.67).
Comparison 4: more people reduced alcohol use (by seven or more days in the past month at 6 months) in the BMI group than in the control group (RR 1.67; 95% CI 1.08 to 2.60), moderate‐quality evidence. No significant difference was reported for all other outcomes: number of days in the past 30 days with alcohol use at one month: MD ‐0.30 (95% CI ‐3.38 to 2.78); number of days in the past month with alcohol use at six months: MD ‐1.50 (95% CI ‐4.56 to 1.56); 25% reduction of drinking days in the past month: RR 1.23 (95% CI 0.96 to 1.57); 50% reduction of drinking days in the past month: RR 1.27 (95% CI 0.96 to 1.68); 75% reduction of drinking days in the past month: RR 1.21 (95% CI 0.84 to 1.75); one or more drinking days' reduction in the past month: RR 1.12 (95% CI 0.91 to 1.38).
Authors' conclusions
There is low‐quality evidence to suggest that there is no difference in effectiveness between different types of interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users and that brief interventions are not superior to assessment‐only or to treatment as usual. No firm conclusions can be made because of the paucity of the data and the low quality of the retrieved studies.
PICO
Laički sažetak
Koje vrste terapija razgovorom mogu pomoći ovisnicima koji imaju i probleme s alkoholom?
Istraživačko pitanje
Autori ovog Cochrane sustavnog pregleda su željeli utvrditi da li terapija savjetovanjem i motiviranjem pomaže ovisnicima o drogama koji imaju probleme sa alkoholom i ako da, je li neki od pristupa uspješniji od drugih.
Dosadašnje spoznaje
Zloupotreba alkohola (konzumacija iznad preporučenih dnevnih količina) može dovesti do teške psihičke i fizičke ovisnosti. Pretjerana konzumacija alkohola jako je česta kod intravenskih i drugih ovisnika o teškim drogama i često pogoršava njihovo kliničko stanje te može dovesti do teških posljedica za njihovo zdravlje.
Na grupnim terapijama nastoji se riješti problem ovisnosti međusobnim razgovorom i potporom te motivirati pojedinca da se uhvati u koštac sa svojom ovisnošću. Grupne terapije vodi profesionalno osoblje (npr. doktori, medicinske sestre, psiholozi i drugi stručni savjetnici). Ovakva vrsta terapije se pokazala dobra kod osoba koje imaju problema sa alkoholom, ali utjecaj na ovisnike koji uz alkohol zloupotrebljavaju i opojne droge je zasad nepoznat, odnosno nedovoljno istražen.
Datum pretraživanja literature:Uzete su u obzir sve studije koje se bave ovom problematikom i koje zadovoljavaju kriterije uključenja, a objavljene su do lipnja 2014. godine.
Značajke istraživanja
U literaturi su pronađene ukupno 4 studije koje uključuju ukupno 594 ovisnika o teškim drogama. U prvoj studiji je uspoređen pristup anonimnih alkoholičara (12 koraka) sa pristupom kojim se nastojalo motivirati pacijente da sami razviju želju da prestanu zloupotrebljavati alkohol ili droge. U drugoj studiji su autori su koristili pristup kojim se prepoznaje problem s alkoholom i zatim se te pacijente nastojalo motivirati da sami prestanu piti te se taj pristup usporedio sa uobičajenim liječenjem. Jedna od uključenih studija je usporedila savjetovanje u kojem se ljudima pokušalo pomoći na način da promijene svoje ponašanje (u grupnom i individualnom formatu) sa grupom pacijenata sa kojima se razgovaralo o hepatitisu u smislu promocije zdravlja. U posljednjoj studiji je uspoređen pristup kratke motivacijske intervencije sa samo procjenom pacijentova stanja.
Ključni rezultati
Ukupno gledano, pronađeni su dokazi loše kvalitete samo za usporedbe obuhvaćene ovim sustavnim pregledom.
‐ Studije koje su pronađene i uključene u ovaj sustavni pregled su jako različite i nije bilo moguće kombinirati njihove rezultate.
‐ Ostaje nejasno mogu li terapije razgovorom (savjetovanja) utjecati na smanjenje konzumacije alkohola kod intravenskih i drugih ovisnika o teškim drogama iz razloga sto dosadašnje studije sadrže dokaze loše kvalitete o tome pitanju.
‐ Isto tako ostaje nejasno da li savjetovanja o alkoholizmu utječu na korištenje teških droga kod pacijenata koji su ovisnici o drogama i alkoholu jer je također niska razina postojećih dokaza. Nije pronađeno dovoljno informacija da bi se usporedile različite vrste terapija razgovorom.
‐ Većina studija nije uzela u obzir eventualne izvore pristranosti.
‐ Potrebno je više studija sa jačom razinom dokaza (kao što su randomizirani klinički pokusi) da bismo odgovorili na postavljeno pitanje.
Authors' conclusions
Summary of findings
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | CBT versus TSF | |||||
| Maximum number of weeks of consecutive alcohol abstinence during treatment | The mean maximum number of weeks of consecutive alcohol abstinence during treatment in the control groups was | The mean maximum number of weeks of consecutive alcohol abstinence during treatment in the intervention group was | ‐ | 41 | ⊕⊕⊝⊝ | ‐ |
| Maximum number of weeks of consecutive abstinence from cocaine during treatment | The mean maximum number of weeks of consecutive abstinence from cocaine during treatment in the control groups was | The mean maximum number of weeks of consecutive abstinence from cocaine during treatment in the intervention group was | ‐ | 41 | ⊕⊕⊝⊝ | ‐ |
| Number of people achieving 3 or more weeks of consecutive alcohol abstinence during treatment | Study population | RR 1.96 | 41 | ⊕⊕⊝⊝ | ‐ | |
| 111 per 1000 | 218 per 1000 | |||||
| Moderate | ||||||
| 111 per 1000 | 218 per 1000 | |||||
| Alcohol abstinence | Study population | RR 2.38 | 41 | ⊕⊕⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Incomplete outcome data | ||||||
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | BI versus treatment as usual | |||||
| Number of standard drinks per week | The mean number of standard drinks per week in the control groups was | The mean number of standard drinks per week in the intervention groups was | ‐ | 110 | ⊕⊕⊝⊝ | ‐ |
| Number of standard drinks per week | The mean number of standard drinks per week in the control groups was | The mean number of standard drinks per week in the intervention groups was | ‐ | 110 | ⊕⊕⊝⊝ | ‐ |
| Decreased alcohol use | Study population | RR 1.13 | 110 | ⊕⊕⊝⊝ | ‐ | |
| 314 per 1000 | 355 per 1000 | |||||
| Moderate | ||||||
| 314 per 1000 | 355 per 1000 | |||||
| Decreased alcohol use | Study population | RR 1.34 | 110 | ⊕⊕⊝⊝ | ‐ | |
| 216 per 1000 | 289 per 1000 | |||||
| Moderate | ||||||
| 216 per 1000 | 289 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation and assessment of outcomes weren't blinded | ||||||
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | MI‐G versus HHP | |||||
| Number of standard drinks per day | The mean number of standard drinks per day in the control groups was | The mean number of standard drinks per day in the intervention groups was | ‐ | 147 | ⊕⊕⊝⊝ | ‐ |
| Over 50% less standard drinks per day | Study population | RR 1.1 | 166 | ⊕⊕⊝⊝ | ‐ | |
| 494 per 1000 | 544 per 1000 | |||||
| Moderate | ||||||
| 494 per 1000 | 543 per 1000 | |||||
| Alcohol abstinence | Study population | RR 0.88 | 166 | ⊕⊕⊝⊝ | ‐ | |
| 230 per 1000 | 202 per 1000 | |||||
| Moderate | ||||||
| 230 per 1000 | 202 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Masking: open label. Allocation and assessment of outcomes weren't blinded | ||||||
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | MI‐S versus hepatitis HHP | |||||
| Number of standard drinks consumed per day | The mean number of standard drinks consumed per day in the control groups was | The mean number of standard drinks consumed per day in the intervention groups was | ‐ | 155 | ⊕⊕⊝⊝ | ‐ |
| Over 50% less standard drinks per day | Study population | RR 0.92 | 177 | ⊕⊕⊝⊝ | ‐ | |
| 494 per 1000 | 455 per 1000 | |||||
| Moderate | ||||||
| 494 per 1000 | 454 per 1000 | |||||
| Alcohol abstinence | Study population | RR 0.97 | 177 | ⊕⊕⊝⊝ | ‐ | |
| 230 per 1000 | 223 per 1000 | |||||
| Moderate | ||||||
| 230 per 1000 | 223 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Masking: open label. Allocation and assessment of outcomes weren't blinded | ||||||
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | BMI) versus assessment‐only | |||||
| Number of days with alcohol use at 6 months | The mean number of days with alcohol use at 6 months in the control groups was | The mean number of days with alcohol use at 6 months in the intervention groups was | ‐ | 187 | ⊕⊕⊕⊝ | ‐ |
| 25% reduction of drinking days in the past 30 days | Study population | RR 1.23 | 187 | ⊕⊕⊕⊝ | ‐ | |
| 522 per 1000 | 642 per 1000 | |||||
| Moderate | ||||||
| 522 per 1000 | 642 per 1000 | |||||
| 50% reduction of drinking days in the past 30 days | Study population | RR 1.27 | 187 | ⊕⊕⊕⊝ | ‐ | |
| 457 per 1000 | 580 per 1000 | |||||
| Moderate | ||||||
| 457 per 1000 | 580 per 1000 | |||||
| Seven or more drinking days' reduction in the past 30 days | Study population | RR 1.67 | 187 | ⊕⊕⊕⊝ | ‐ | |
| 239 per 1000 | 399 per 1000 | |||||
| Moderate | ||||||
| 239 per 1000 | 399 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sparse data: only 1 study with relatively few participants included in comparison | ||||||
Background
Description of the condition
Problem alcohol use is common among illicit drug users and is associated with adverse health outcomes, which have physical, psychological and social implications (Staiger 2013). NIDA (National Institute on Drug Abuse) meta‐analyses of US clinical trial data found alcohol use disorders (AUDs) in 38% and 45% of opiate‐ and stimulant‐using treatment seekers, respectively (Hartzler 2010; Hartzler 2011). An earlier review of literature on the prevalence of 'heavy drinking' among drug users enrolled in a methadone maintenance treatment (MMT) found prevalence rates of 13% to 25% (Chen 2011), whereas more recent cross‐sectional studies report prevalence rates of 33% up to 50% in this setting (Islam 2013;.Wurst 2011).
Problem alcohol use is an expression that represents a spectrum of distinct drinking patterns (i.e. hazardous, harmful and dependent drinking). Hazardous drinking "is likely to result in harm should present habits persist", whereas harmful drinking, which is an International Classification of Diseases ‐ Tenth Revision (ICD‐10) diagnosis (WHO 1993), "causes harm to the health (physical or mental) of the individual" without the presence of dependence (Babor 2001). The term 'dependent drinkers' refers to individuals who meet criteria for the alcohol dependence syndrome under Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) or ICD‐10 criteria (DSM‐IV; WHO 1993).
Problem drug users are at high risk of liver disease resulting from hepatitis C virus (HCV) infection because of its high prevalence in this population (Smyth 1998). Problem alcohol use is an important factor contributing to a poor prognosis among people with HCV as it impacts on progression to hepatic cirrhosis, increased HCV‐RNA levels or fatal opiate overdose in opiate users (Du 2012; White 1999). Teplin 2007 noted that drug users have higher rates of mood, anxiety and personality disorders, all of which are exacerbated by alcohol use. In addition, there exists some evidence that alcohol may have a negative impact on outcomes of addiction treatment (Byrne 2011; Gossop 2000).
The emerging understanding of a high prevalence of problem alcohol use among current or former drug users, allied to the clear health implications of this problem for this population, necessitates a public health response to this issue.
Description of the intervention
Psychosocial interventions are best described as "psychologically‐based interventions aimed at reducing consumption behaviour or alcohol‐related problems" (Kaner 2007) that exclude any pharmacological treatments. The term refers to a heterogeneous collection of interventions, which vary depending on their: (a) theoretical underpinnings (e.g. psychodynamic, behavioural, motivational), (b) duration or intensity (e.g. brief, extended), (c) setting (e.g. primary‐care based, inpatient), (d) mode of delivery (e.g. group, individual, web‐based) or (e) treatment goals (e.g. abstinence oriented, harm reduction). To date, many psychosocial interventions specifically designed to address problem alcohol use have been described. The most frequently used interventions include: motivational interviewing (MI), cognitive‐behavioural therapy (CBT), psychodynamic approaches, screening and brief interventions (SBIs), family therapy, drug counselling, 12‐step programmes, therapeutic community (TC) and vocational rehabilitation (VR).
-
MI is a client‐centred approach, but in contrast to its non‐directive Rogerian origins, it is a directive therapy system. A central role is played by the client's motivation and readiness to change. Change within this approach is facilitated over a series of stages (Prochaska 1992). Relapse is not viewed as a failure to maintain healthy behaviour, but rather as a part of the process of change (Miller 2004).
-
CBT draws upon the principles of learning theory. Change in addictive behaviour is approached through altering irrational assumptions, coping skills training or other behavioural exercises. This therapy often deals with the identification and prevention of triggers contributing to drug use. Among the modern approaches utilising such behavioural techniques are relapse prevention (Marlatt 1996), contingency management (Budney 2001) and the community reinforcement approach, which combines both contingency management and positive reinforcement for non‐drinking behaviours (Hunt 1973).
-
Psychodynamic approaches are based on the assumptions of psychoanalytic theory, which focuses on addressing inner conflict, childhood trauma or problematic relationship themes. Such approaches include a range of different methods designed to deal with the underlying conflict (e.g. interpersonal therapy, supportive‐expressive techniques, etc.) (Crits‐Christoph 1999).
-
SBIs are time limited and therefore suitable for non‐specialist facilities. Usually, the length and intensity of the intervention is determined by the levels of risky alcohol consumption (i.e. screening results), and can range from a couple of minutes to several sessions (three to six). Each session includes the provision of information and advice (Babor 2001). Increasingly, brief interventions (BIs) are based on the principles and techniques of MI, so that the distinction between these two modalities is blurred in this regard.
-
Family therapy: the therapeutic change is achieved via intervening in the interaction between family members. Families are directly involved in a therapy session. The family therapist must be competent in eliciting the strengths and support of the wider family system. Frequently used family therapy models include multisystemic therapy and network therapy solution‐focused brief therapy (CSAT 2004).
-
Drug counselling: addiction is viewed as a chronic illness that has serious consequences to the individual's health and social functioning, in consonance with the 12‐step model (see below). Recovery includes spiritual components and attendance at fellowship meetings. The primary focus of this approach is to help the individual attain abstinence by promoting behavioural changes, including trigger avoidance, sport and other constructive activities. Both individual and group forms of drug counselling have been used in the largest collaborative cocaine treatment study (Crits‐Christoph 1999).
-
The 12‐step model emphasises the powerlessness of an individual over the addiction, which is seen as a disease, and the need for a spiritual recovery. The foundations of this approach lie in the 12 steps and an accompanying document ‐ 12 traditions (Alcoholics Anonymous 1939). The largest of all 12‐step programmes is that of Alcoholics Anonymous (AA), and all other programmes (e.g. those of Narcotics Anonymous, Al‐Anon etc.) have evolved from it. AA meetings, besides the 12 steps, utilise well‐established therapeutic factors of group psychotherapy, such as group cohesiveness, interpersonal learning (i.e. sponsorship), peer pressure, etc.
-
TC is a long‐term (18 to 24 months), drug‐free model of treatment, which usually runs in a residential form. This approach relies on the community itself, as the main therapeutic factor, and also on other factors, such as peer feedback, role‐modelling or recapitulation of the primary family experience. The community has a high degree of autonomy, is democratic and each member has a clearly defined role and responsibilities within the structure of TC. A structured regimen of daily activities in the TC often includes formal individual or group therapy sessions along with other educational and work activities (De Leon 2000; Staiger 2009).
-
VR employment is seen as an important element of successful rehabilitation from drug addiction and is often considered as one of its key indicators (Platt 1995). VR aims to increase the employability of drug users by developing their job interview skills or obtaining further qualifications. A necessary part of increasing ex‐users' access to the job market is linking with potential employers and addressing their concerns and prejudices related to drug users. An example of VR for unemployed individuals receiving MMT is the customised employment supports model (Blankertz 2004).
How the intervention might work
Substantial evidence has described the value of psychosocial interventions in treating problem alcohol use.
A review by Raistrick 2006 presented data on the effectiveness of many such interventions, including screening, further assessment, BIs, more intensive treatments that can still be considered 'brief' and alcohol‐focused specialist treatments. They reported mixed evidence on the longer‐term effects of BIs and whether extended BIs add anything to the effects of simple BIs.
The Mesa Grande project, which reviewed 361 controlled clinical trials (CCTs) (a three‐year update), found BIs to be the most strongly supported psychosocial treatment effective in treating AUDs (Miller 2002). These findings are supported by an Australian systematic review that found BIs to be effective in reducing alcohol consumption in drinkers without dependence or those with a low level of dependence (Shand 2003). Another meta‐analysis found the positive effect of BIs to be evident at the follow‐up points of 3, 6 and 12 months, and these results were more apparent when dependent drinkers were excluded (Moyer 2002). Indeed, dependent drinkers have been excluded from much of this research indicating that they are possibly unsuitable for BI and should be routinely referred to specialist treatment (Raistrick 2006).
While BIs are generally delivered across a range of settings, primary care has an important role in the delivery of BIs for problem alcohol use among problem drug users. BIs are well suited to primary care owing to their feasibility, and that they can be delivered in general settings by non‐specialist staff in a short period of time, and to individuals not actively seeking treatment (Kaner 2007; Raistrick 2006).
The benefits of primary care‐based interventions for people with problem alcohol use have been demonstrated in a Cochrane review (Kaner 2007), although the authors reported considerable variation in trials and that the effect of BIs appeared equivocal among women. Another systematic review of brief, multi‐contact behavioural counselling among adults attending primary care reported an average reduction of 13% to 34% in drinks per week (Whitlock 2004).
In conclusion, brief psychosocial interventions are feasible and potentially highly effective components of an overall public health approach to reducing problem alcohol use, although considerable variation in trials of effectiveness exists and problem drug users from primary care settings are under‐represented in these trials (Kaner 2007; Whitlock 2004).
Because BIs have been developed and evaluated mainly in conventional general practice settings, it is not clear whether they can be effectively applied to excessive drinking among illicit drug users, or whether new forms of intervention need to be developed and evaluated. Could the 'advice‐giving' form of BI be effective in illicit drug users or are motivational techniques, in which the impetus for change comes from the user, more likely to be effective in this population?
Why it is important to do this review
The high prevalence and serious consequences of problem alcohol use among illicit drug users highlights an opportunity for a Cochrane systematic review in this population. The question being asked in this review is also of importance because there are no other systematic reviews published that could help answer it.
Two narrative literature reviews have dealt with this question to date. The older of these reviews discussed six reports of four studies among methadone patients and saw some promise for contingency management procedures (Bickel 1987). A more recent review described the implications of combining behavioural and pharmacological treatments, which are effective in treating either alcohol‐ or drug‐use disorders alone, for the treatment of people who have both these disorders (Arias 2008). While pointing to the paucity of research specifically focused on the treatment of people with co‐occurring alcohol and other substance use disorders, the review concluded that successful treatment must take into account both alcohol‐ and drug‐use disorders. Additionally, one narrative review on treating people seeking therapy primarily for alcohol problems, but who also use other drugs, concurred with this idea (Miller 1996).
Cochrane reviews have so far examined the effectiveness of psychosocial interventions for stimulant, opiate and alcohol use disorders (Amato 2011a; Amato 2011b; Knapp 2007; Lui 2008; Mayet 2004; Minozzi 2011). Although other reviews and review protocols have targeted poly‐drug use, they concentrated either on specific populations, for example women and adolescents, or particular interventions, such as case management and MI, but not on 'alcohol‐specific' interventions (Dalsbø 2010; Hesse 2007; Smedslund 2011; Smith 2006; Terplan 2007; Thomas 2008). None of the published reviews on psychosocial interventions examined the effectiveness of alcohol‐specific interventions in problem drug users. The main problem driving the lack of good studies in this area seems to flow from the administrative separation of drug from alcohol problems. This separation has led researchers to focus on one or the other but not on both. In the USA, the National Institutes of Health plan to correct this separation by forming a new institute that covers both drugs and alcohol – the proposed National Institute of Substance Use and Addiction Disorders (NIH 2012).
The lack of systematic evaluation, together with the anticipated differences in the responsiveness of problem drug users to psychosocial interventions, provides additional reasons for conducting this review. In other words, the results of reviews on the effectiveness of this type of intervention among the general population might not be applicable to specific groups, such as drug users, because they may have a different responsiveness to psychosocial interventions (Nilsen 2010).
Several factors could possibly influence the responsiveness of drug users to treatment interventions (for example, stability of drug use, engagement with the service, concurrent personality disorders, etc). Evidence suggests that drug users with antisocial personality disorder are more likely to respond to rewarding than to punitive approaches (Messina 2003), and the use of more intensive psychosocial interventions is recommended in those who have achieved a sufficient degree of stability and compliance with a service regimen (Pilling 2010).
Objectives
To determine the effectiveness of psychosocial interventions targeting problem alcohol use versus other treatments in illicit drug users. Especially the effectiveness on reducing alcohol consumption.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and CCTs.
Types of participants
Adult (aged ≥ 18 years) problem drug users attending a range of services (i.e. community, inpatient or residential (including opiate substitution treatment)). Problem drug use was defined according to the definition of the European Monitoring Centre for Drugs and Drug Addiction, as "injecting drug use or long‐duration/regular use of opioids, cocaine and/or amphetamines" (EMCDDA 2008, p. 10). This definition also encompasses other, similar terms, for example substance use, misuse, abuse, dependence or addiction.
Only studies that defined participants as problem drug and alcohol users at randomisation were included. Studies including problem drug users without concurrent problem alcohol use were excluded. People whose primary drug of use was alcohol were excluded from this review.
Types of interventions
Experimental interventions: any psychosocial intervention that was described by the study's author as such.
Control interventions: other psychosocial interventions that will allow for comparisons between different types of interventions (e.g. CBT, contingency management, family therapy, etc.), standard care, no intervention, waiting list, placebo or any other non‐pharmacological therapy (including moderate drinking, assessment‐only).
We intended to exclude studies comparing psychosocial with pharmacological treatments. However, trials with two psychosocial arms in addition to pharmacological arms were exempted from this rule.
Types of outcome measures
Primary outcomes
-
Alcohol use (reduction or stabilisation), as measured by either biological markers or self‐report tests
Secondary outcomes
-
Illicit drug use (changes in illicit drug use), as measured by either biological markers or self‐report tests
-
Engagement in further treatment (i.e. drop‐out rates, utilisation of health services)
-
Alcohol‐related problems or harms, as represented by physical or mental health outcomes associated with problem alcohol use.
Search methods for identification of studies
Electronic searches
For the original review Klimas 2012a, we searched the following electronic databases (search date: 22 November 2011):
-
Cochrane Drugs and Alcohol Group (CDAG) Specialised Register* (1956 to November 2011; 230 hits);
-
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 11, November 2011);
-
MEDLINE (PubMed) (1966 to November 2011);
-
EMBASE (Elsevier, EMBASE.com) (1974 to November 2011);
-
CINAHL (EBSCO Host) (1982 to November 2011);
-
PsycINFO (ProQuest) (1872 to November 2011).
For this update, we searched the following electronic databases (search date: 23 June 2014):
-
CDAG Specialised Register* (November 2011 to June 2014; 67 hits)
-
CENTRAL (The Cochrane Library, Issue 6, Jun 2014);
-
MEDLINE (PubMed) (November 2011 to June 2014);
-
EMBASE (Elsevier, EMBASE.com) (November 2011 to June 2014);
-
CINAHL (EBSCO Host) (November 2011 to June 2014);
-
PsycINFO (ProQuest) (November 2011 to June 2014).
* All trials from the CDAG Specialised Register can be found in The Cochrane Library by searching on SR‐ADDICTN.
We searched the databases using a strategy developed incorporating the filter for the identification of RCTs (Higgins 2011), combined with selected medical subject heading (MeSH) terms and free‐text terms relating to alcohol use. The CDAG Group's Trials Search Co‐ordinator conducted the electronic searches of databases 1 to 5, listed above, and the first author of the review conducted the electronic search of database 6. We adapted the MEDLINE search strategy for use with the other databases using the appropriate controlled vocabulary, as applicable. Since the initial search yielded several RCTs, we continued to use the RCT filter for subsequent databases searches. We collated the results of the two sets of electronic searches into a single EndNote database.
The search strategies for all databases are shown in Appendix 1, Appendix 2, Appendix 3, Appendix 4 and Appendix 5.
In addition, we searched for ongoing clinical trials and unpublished studies via Internet searches on the following sites:
-
www.controlled‐trials.com (search date: 24 March 2014);
-
www.clinicaltrials.gov (search date: 25 March 2014);
-
www.centrewatch.com (search date: 26 March 2014);
-
www.who.int/ictrp/en/ World Health Organization International Clinical Trials Registry Platform (search date: 26 March 2014).
Searching other resources
We also searched:
-
reference lists of articles considered eligible based on full report screening and other relevant papers;
-
conference proceedings (online archives only) of the Society for the Study of Addiction, International Harm Reduction Association, International Conference on Alcohol Harm Reduction and American Association for the Treatment of Opioid Dependence.
In addition, we
contacted investigators and relevant trial authors seeking information about unpublished or incomplete trials.
All searches included non‐English language literature and we assessed any with English abstracts for inclusion. When considered likely to meet inclusion criteria, we obtained translations of any abstracts.
Data collection and analysis
Selection of studies
Two review authors (JK, HT) independently screened titles and abstracts and selected studies potentially relevant to the update. We resolved any differences between selection lists by discussion with a third and fourth review author with respective thematic and methodological expertise (WC, CSMOG). We obtained full‐text copies of each potentially relevant paper, as well as full reports of references with inadequate information in order to definitively determine relevance. Two review authors (JK, HT) independently re‐evaluated whether studies were eligible for the update or not, according to the inclusion criteria. A second opinion was not needed. We facilitated the processes of abstract screening, study selection and data extraction using Eppi Reviewer 4 software.
Data extraction and management
Two review authors (JK, HT) independently extracted data from the full‐text reports using an electronic version of an amended data extraction form of the Cochrane Drug and Alcohol review group (CDAG). We resolved disagreements by mutual discussion.
Assessment of risk of bias in included studies
We performed 'Risk of bias' assessments for RCTs and CCTs using the criteria recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The recommended approach for assessing risk of bias in studies included in a Cochrane review is a two‐part tool addressing five specific domains (namely sequence generation, allocation concealment, blinding, incomplete outcome data and other issues). The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry in terms of high, low or unclear risk. To make these judgements we used the criteria indicated in the Cochrane Handbook for Systematic Reviews of Interventions adapted to the addiction field. See the table in Appendix 6 for details.
We addressed the domains of sequence generation and allocation concealment (avoidance of selection bias) using a single entry for each study.
Blinding of participants and providers was not possible for this kind of intervention. We considered the blinding of outcome assessors (avoidance of detection bias) separately for objective outcomes (e.g. drop‐outs from therapy, substance use measured by urinalysis, participants relapsed at the end of follow up, participants engaged in further treatments) and subjective outcomes (e.g. duration and severity of signs and symptoms of withdrawal, individual self‐reported use of substance, side effects, social functioning as integration at school or at work, family relationship, etc.).
We considered incomplete outcome data (avoidance of attrition bias) for all outcomes with the exception of drop‐outs from therapy, which is usually the primary outcome measure in trials on addiction. We assessed this separately for results at the end of the study period, and for results at follow up.
Measures of treatment effect
For continuous data, we calculated mean differences (MD) between the intervention and comparator groups with 95% confidence intervals (CIs). We present dichotomous outcomes as risk ratios (RRs), with 95% CIs.
Unit of analysis issues
We did not perform a meta‐analysis, therefore unit‐of‐analysis error was not an issue. We identified only one multiarm trial in the review and it was not included more than once in any of the comparisons.
Dealing with missing data
We contacted the authors of the four original studies by email for missing data (April 2012) and sent reminders after two weeks. To date, two study authors have responded and provided additional information.
Assessment of heterogeneity
We did not pool results in a meta‐analysis owing to substantial clinical and statistical heterogeneity.
Assessment of reporting biases
We planned to further explore the potential for reporting bias using funnel plots if more than 10 RCTs were included; however, this was not possible because only four RCTs were identified.
Data synthesis
Formal meta‐analysis was not possible owing to substantial differences between studies; we considered that no two studies were sufficiently similar to allow pooling of data. We therefore report the results of included studies individually for each trial. We used a fixed‐effect model because there was only one study for each comparison.
Subgroup analysis and investigation of heterogeneity
We did not conduct investigations of heterogeneity. If sufficient information had been available, we had planned to conduct the following subgroup analyses:
-
types of psychosocial intervention (e.g. motivational versus behavioural or BIs);
-
length of the intervention (short, medium, extended).
We had also intended to conduct the following subgroup analyses, but did not due to insufficient data:
-
sustained benefit at 6 and 12 months after intervention;
-
gender differences;
-
single‐drug (alcohol) versus poly‐drug‐focused interventions;
-
single‐drug (alcohol) versus poly‐drug‐focused interventions that also address other health‐related behaviours.
Sensitivity analysis
We did not perform sensitivity analyses because we were unable to pool the study results. If sufficient information had been available, we intended to conduct the following sensitivity analyses according to the methodological quality criteria used for study inclusion:
-
excluding studies with a high risk of bias from the analysis; this decision was to be based on a predefined cut‐off score (i.e. studies judged to be at high risk of bias for three or more risk items, including selection bias, were to be excluded);
-
excluding CCTs.
Summary of findings tables
We used GRADE methodology to produce a 'Summary of findings' table for MI, as this is of more interest when considering the typical psychosocial interventions provided in opioid agonist treatments.
Consumer participation
We sought consumer participation in the preparation of the protocol and the original review: a) the first review author (JK) is a member of the Cochrane Consumers Network, b) the Cochrane Consumers Network was approached to assist with the plain language summary of the review, and c) one of the co‐authors of this review (EK) contributed to consumer consultation during the protocol and review development, as he was a practicing clinician in a healthcare facility with a high prevalence of this problem.
Results
Description of studies
See the 'Characteristics of included studies' and the 'Characteristics of excluded studies' tables.
Results of the search
This is an update of a Cochrane review first published in 2012 (Klimas 2012a). In the first version of our review, we retrieved a total of 7207 records from the initial search of the CDAG Register, CENTRAL, PubMed, EMBASE, CINAHL and PsycINFO. Removing duplicates left 5548 records. After screening titles and abstracts, we identified 25 potentially eligible studies; 18 full‐text reports were excluded and 7 reports were included (describing 4 RCTs). No additional studies were found through reference checking. A Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flowchart of study selection for the first version is shown in Figure 1 according to the PRISMA statement (Moher 2009).
For this 2014 update, we retrieved a total of 1836 records from a more up‐to‐date search of the CDAG Register, CENTRAL, PubMed, EMBASE, CINAHL and PsycINFO. Removing duplicates left 960. After screening titles and abstracts we identified 16 potentially eligible records and included one record (Feldman 2013). This record was a 2013 correction of a paper for one of the studies (Feldman 2013) included in our first Cochrane review (Klimas 2012a). A PRISMA flowchart of study selection for this review update is shown in Figure 2.

Study flow diagram from first publication of this review in 2012.
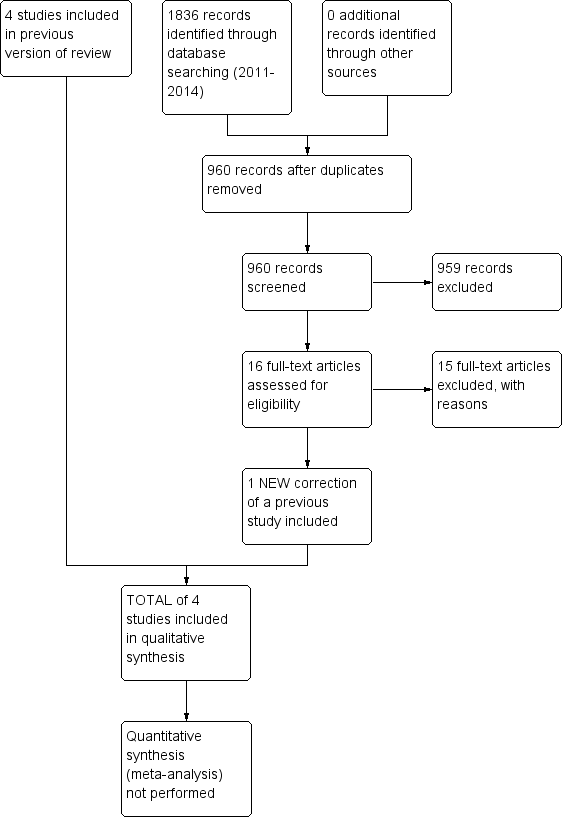
Study flow diagram for a review update: previous studies incorporated into results of new literature search
Included studies
We included four studies (594 participants)in this review. The studies assessed the effectiveness of six psychosocial interventions: CBT, 12‐step facilitation (TSF), BI, hepatitis health promotion (HHP), MI and brief motivational intervention (BMI).
Type of psychosocial intervention and setting
-
CBT versus TSF in an outpatient clinic (Carroll 1998)
-
BI versus treatment as usual in an outpatient clinic with/without opioid substitution treatment (Feldman 2013)
-
MI (group) versus HHP in an opioid substitution clinic (Nyamathi 2010)
-
MI (single) versus HHP in an opioid substitution clinic (Nyamathi 2010)
-
BMI versus assessment‐only in a needle exchange programme (Stein 2002a)
Three studies were conducted in the USA and one in Switzerland.
Duration of the trials ranged from 4 to 12 weeks (plus various follow ups) (mean 7.5 weeks). Between 1 and 16 sessions were offered to participants (mean 5.5,providing from 15 minutes to 16 hours of treatment time).
Participants included 594 problem drug users (one multiarm trial included 122 participants (Carroll 1998); however, only 41 participants from two psychosocial therapy arms were considered for this review); 33% were female; mean age was 38.3 years.
See the 'Characteristics of included studies' table for more detailed information.
Excluded studies
We excluded 33 studies (18 in 2012 and 15 in 2014) that did not meet the criteria for inclusion in this review; for more information see the 'Characteristics of excluded studies' table.
Grounds for exclusion were: type of intervention not in the inclusion criteria (no studies); type of participants not in the inclusion criteria (24 studies); types of outcomes not in the inclusion criteria (6 studies); study design not in the inclusion criteria (3 studies).
Risk of bias in included studies
For a summary of the our judgements regarding risk of bias for each domain in each included study and across studies, see Figure 3 and Figure 4. See the 'Characteristics of included studies' table for more detailed information.
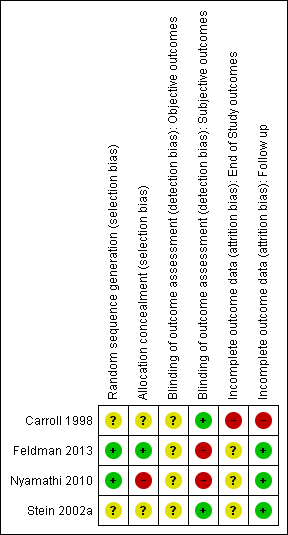
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
We judged random sequence generation to be adequate in two studies (for one this was based on unpublished information obtained via email communication with the study authors), and unclear in the remaining trials.
Allocation concealment
We judged only one study as being at low risk of bias, one at high risk of bias and the remaining studies at unclear risk of bias.
Blinding
Objective outcomes
-
Abstinence or use of substance, as measured by participants using urine tests, or breathalysers: participants and personnel were not blinded in all studies for these kind of interventions, and objective outcomes were not reported in the trials. They were used as an additional measure to confirm abstinence in two studies.
Subjective outcomes
-
Abstinence or use of substance, as measured by self‐reported or interviewer‐administered questionnaires: participants and personnel were not blinded in all studies for these kind of interventions; two studies (50%) specified that outcome assessors were blinded and were judged to be at low risk of bias. Two studies reported that the outcome assessor was not blinded and we judged these to be at high risk of bias; for one of them this was unpublished information obtained via email communication with the study authors.
Incomplete outcome data
End of study outcomes
-
With the exception of retention in treatment, only one study measured end‐of‐study outcomes and we judged it to be at high risk of bias because the drop‐out rates were not balanced across all groups (e.g. "the psychotherapy groups had significantly lower retention rates than the medication groups" (Carroll 1998)).
Follow‐up outcomes
-
With the exception of retention in treatment, we judged three studies to be at low risk of attrition bias because few participants (less than 10%) withdrew from the studies, there was a high rate of drop‐out but percentages were balanced across intervention groups and reasons for withdrawal were provided or authors performed an intention to treat (ITT) analysis. We judged one study to be at high risk of bias because of a high drop‐out rate that was unbalanced across groups.
Effects of interventions
See: Summary of findings for the main comparison Cognitive‐behavioural coping skills training (CBT) versus 12‐step facilitation (TSF) for alcohol use in concurrent problem alcohol and illicit drug users; Summary of findings 2 Brief intervention (BI) versus treatment as usual for alcohol use in concurrent problem alcohol and illicit drug users; Summary of findings 3 Motivational interviewing (group) (MI‐G) versus hepatitis health promotion (HHP) for alcohol use in concurrent problem alcohol and illicit drug users; Summary of findings 4 Motivational interviewing (single) (MI‐S) versus hepatitis health promotion (HHP) for alcohol use in concurrent problem alcohol and illicit drug users; Summary of findings 5 Brief motivational intervention (BMI) versus assessment‐only for alcohol use in concurrent problem alcohol and illicit drug users
We were unable to pool data from the included studies in order to conduct a meta‐analysis. We therefore summarise the results according to the type of psychosocial intervention, with comparisons of quantitative data where possible. The included studies used different questionnaires to measure their outcomes and for many the authors did not report post‐treatment/follow‐up scores or they did not state what was considered to represent mild, moderate and severe categories. This prevented comparison of results across studies. One study had three arms, which we entered into two separate comparisons (group and single format) so they were not counted twice. See the 'Characteristics of included studies' table for more detailed information.
We present the effects of the interventions by the comparisons examined in the primary studies. The primary outcome of this review was alcohol use or abstinence and the main secondary outcome was illicit drug use or abstinence. We were unable to report the other planned secondary outcomes (engagement in further treatment (i.e. drop‐out rates, utilisation of health services) and alcohol‐related problems or harms) because they were not measured in the identified trials. See: 'summary of findings Table for the main comparison'; 'summary of findings Table 2'; 'summary of findings Table 3'; 'summary of findings Table 4'; 'summary of findings Table 5' for all comparisons.
1. Cognitive‐behavioural coping skills training versus TSF
Continuous outcomes
1.1.1 Alcohol abstinence as maximum number of weeks of consecutive alcohol abstinence during treatment
One study, 41 participants (Carroll 1998), MD 0.40 (95% CI ‐1.14 to 1.94); the difference was not statistically significant, see Analysis 1.1.
1.1.2 Illicit drug abstinence as maximum number of weeks of consecutive abstinence from cocaine during treatment
One study, 41 participants (Carroll 1998), MD 0.80 (95% CI ‐0.70 to 2.30); the difference was not statistically significant, see Analysis 1.1.
1.2.1 Alcohol abstinence as number achieving three or more weeks of consecutive alcohol abstinence during treatment
One study, 41 participants (Carroll 1998), RR 1.96 (95% CI 0.43 to 8.94); the difference was not statistically significant, see Analysis 1.2.
1.2.2 Illicit drug abstinence as number achieving three or more weeks of consecutive abstinence from cocaine during treatment
One study, 41 participants (Carroll 1998), RR 1.10 (95% CI 0.42 to 2.88); the difference was not statistically significant, see Analysis 1.2.
1.2.3 Alcohol abstinence during follow‐up year
One study, 41 participants (Carroll 1998), RR 2.38 (95% CI 0.10 to 55.06); the difference was not statistically significant, see Analysis 1.2.
1.2.4 Illicit drug abstinence as abstinence from cocaine during follow‐up year
One study, 41 participants (Carroll 1998), RR 0.39 (95% CI 0.04 to 3.98); the difference was not statistically significant, see Analysis 1.2.
See: 'summary of findings Table for the main comparison' for this comparison.
2. BI versus treatment as usual
Continuous outcomes
2.1.1 Alcohol use as AUDIT scores at three months
One study, 110 participants (Feldman 2013), MD 0.80 (95% ‐1.80 to 3.40); the difference was not statistically significant, see Analysis 2.1.
2.1.2 Alcohol use as AUDIT scores at nine months
One study, 110 participants (Feldman 2013), MD 2.30 (95% CI ‐0.58 to 5.18); the difference was not statistically significant, see Analysis 2.1.
2.1.3 Alcohol use as number of drinks per week at three months
One study, 110 participants (Feldman 2013), MD 0.70 (95% CI ‐3.85 to 5.25); the difference was not statistically significant, see Analysis 2.1.
2.1.4 Alcohol use as number of drinks per week at nine months
One study, 110 participants (Feldman 2013), MD ‐0.30 (95% CI ‐4.79 to 4.19); the difference was not statistically significant, see Analysis 2.1.
Dichotomous outcomes
2.2.1 Alcohol use as decreased alcohol use at three months
One study, 110 participants (Feldman 2013), RR 1.13 (95% CI 0.67 to 1.93); the difference was not statistically significant, see Analysis 2.2.
2.2.2 Alcohol use as decreased alcohol use at nine months
One study, 110 participants (Feldman 2013), RR 1.34 (95% CI 0.69 to 2.58) the difference was not statistically significant, see Analysis 2.2.
See 'summary of findings Table 2' for this comparison.
3. MI (group) versus HHP
Continuous outcomes
3.1.1 Alcohol use (unpublished) as number of standard drinks consumed per day over the last 30 days
One study, 147 participants (Nyamathi 2010), MD ‐0.40 (95% CI ‐2.03 to 1.23); the difference was not statistically significant, see Analysis 3.1.
3.1.2 Illicit drug use (unpublished) as frequency of drug use (as measured by Addiction Severity Index (ASI drug))
One study, 147 participants (Nyamathi 2010), MD 0.00 (95% CI ‐0.03 to 0.03); the difference was not statistically significant, see Analysis 3.1.
3.1.3 Illicit drug use (unpublished) as a composite drug score (frequency*severity for all drugs taken)
One study, 151 participants (Nyamathi 2010), MD 0.00 (95% CI ‐0.42 to 0.42); the difference was not statistically significant, see Analysis 3.1.
This study reported an additional outcome as a change score for: daily drug use since baseline (past 30 days and six‐month recall). We do not report this calculated variable here because the authors provided us with unpublished results of two original variables that fed into this aggregate variable. Moreover, the published article reported scores for this variable as a mean change between assessment scores together with standard errors (SEs), which would have to be transformed into standard deviations (SDs).
Dichotomous outcomes
3.2.1 Alcohol use as greater than 50% reduction in number of standard drinks consumed per day over the last 30 days
One study, 166 participants (Nyamathi 2010), RR 1.10 (95% CI 0.82 to 1.48); the difference was not statistically significant, see Analysis 3.2.
3.2.2 Alcohol abstinence as abstinence from alcohol over the last 30 days
One study, 166 participants (Nyamathi 2010), RR 0.88 (95% CI 0.49 to 1.58); the difference was not statistically significant, see Analysis 3.2.
See: 'summary of findings Table 3' for this comparison.
4. MI (single) versus HHP
Continuous outcomes
4.1.1 Alcohol use (unpublished) as number of standard drinks consumed per day over the last 30 days
One study, 155 participants (Nyamathi 2010), MD ‐0.10 (95% CI ‐1.89 to 1.69); the difference was not statistically significant, see Analysis 4.1.
4.1.2 Illicit drug use (unpublished) as frequency of drug use (as measured by ASI drug)
One study, 155 participants (Nyamathi 2010), MD 0.00 (95% CI ‐0.03 to 0.03); the difference was not statistically significant, see Analysis 4.1.
4.1.3 Illicit drug use (unpublished) as a composite drug score (frequency*severity for all drugs taken)
One study, 157 participants (Nyamathi 2010), MD ‐0.10 (95% CI ‐0.46 to 0.26); the difference was not statistically significant, see Analysis 4.1.
This study reported an additional outcome as a change score for: daily drug use since baseline (past 30 days and six‐month recall). We do not report this calculated variable here because the authors provided us with unpublished results of two original variables which fed into this aggregate variable.
Dichotomous outcomes
4.2.1 Alcohol use as greater than 50% reduction in number of standard drinks consumed per day over the last 30 days
One study, 177 participants (Nyamathi 2010), RR 0.92 (95% CI 0.68 to 1.26); the difference was not statistically significant, see Analysis 4.1.
4.2.2 Alcohol abstinence as abstinence from alcohol over the last 30 days
One study, 177 participants (Nyamathi 2010), RR 0.97 (95% CI 0.56 to 1.67); the difference was not statistically significant, see Analysis 4.1.
See 'summary of findings Table 4' for this comparison.
5. BMIversus assessment‐only
Continuous outcomes
5.1.1 Alcohol use as number of days in the past 30 days with alcohol use at one month
One study, 187 participants (Stein 2002a), MD ‐0.30 (95% CI ‐3.38 to 2.78); the difference was not statistically significant, see Analysis 5.1.
5.1.2 Alcohol use as number of days in the past 30 days with alcohol use at six months
One study, 187 participants (Stein 2002a), MD ‐1.50 (95% CI ‐4.56 to 1.56); the difference was not statistically significant, see Analysis 5.1.
Dichotomous outcomes
5.2.1 Alcohol use as 25% reduction of drinking days in the past 30 days
One study, 187 participants (Stein 2002a), RR 1.23 (95% CI 0.96 to 1.57); the difference was not statistically significant, see Analysis 5.2.
5.2.2 Alcohol use as 50% reduction of drinking days in the past 30 days
One study, 187 participants (Stein 2002a), RR 1.27 (95% CI 0.96 to 1.68); the difference was not statistically significant, see Analysis 5.2.
5.2.3 Alcohol use as 75% reduction of drinking days in the past 30 days
One study, 187 participants (Stein 2002a), RR 1.21 (95% CI 0.84 to 1.75); the difference was not statistically significant, see Analysis 5.2.
5.2.4 Alcohol use as one or more drinking days' reduction in the past 30 days
One study, 187 participants (Stein 2002a), RR 1.12 (95% CI 0.91 to 1.38); the difference was not statistically significant, see Analysis 5.2.
5.2.5 Alcohol use as seven or more drinking days' reduction in the past 30 days
One study, 187 participants (Stein 2002a), RR 1.67 (95% CI 1.08 to 2.60); the difference was statistically significant in favour of BI (P value = 0.02), see Analysis 5.2.
See 'Summary of findings' table 5 for this comparison
Discussion
Summary of main results
We included four studies involving 594 participants in this review. The studies assessed the effectiveness of six psychosocial interventions: CBT, TSF, BI, HHP, MI and BMI. In the 2014 update of this review, we retrieved only one potentially eligible record (Feldman 2013), which was a 2013 correction of a paper included in our original Cochrane review (Klimas 2012a).
We identified significant clinical and reporting heterogeneity among the included studies, which precluded meta‐analysis. We therefore analysed outcomes from individual studies only. Comparing different psychosocial interventions, we found only one study investigating each comparison. Most of the comparisons did not produce statistically significant findings, with the exception that participants receiving BMI were significantly more likely to reduce their alcohol use by seven or more days in the past 30 days at six months' follow up compared with participants receiving assessment‐only(RR 1.67; 95% CI 1.08 to 2.60; P value = 0.02).
Overall completeness and applicability of evidence
The studies identified are insufficient to address all the objectives of this review. All included studies were conducted either in the USA or Switzerland, which limits their applicability to other contexts. A substantial proportion of participants in the included studies had significant problems with alcohol (e.g. a diagnosis of abuse or dependence), which may have impacted on the effectiveness of the short‐term therapies offered to them. These people may require more intensive interventions, as BIs have been shown to be effective among people with less severe alcohol problems (Raistrick 2006). Only one study examined a longer type of intervention (i.e. 16 sessions); however, it included only 41 participants and reported their outcomes in a way that precluded comparison with other studies (Carroll 1998).
How do the results of this review fit into the context of current practice? This review selected a very narrow clinical question that was limited to a very specific population. Although the size of this population is not negligible, it is highly unlikely that all of the individuals in a treatment service in a real‐life setting will have both of the conditions selected as the eligibility criteria for this review. These stringent eligibility criteria strengthened the internal validity of the review; however, with an inevitable detriment to its external validity. A typical clinician in an actual treatment clinic would normally deal with a mixture of problem drug users who may or may not have other concurrent conditions or comorbidities. To manage this demanding workload, they may want to consider other studies, which did not meet the eligibility criteria of our review (see the 'Characteristics of excluded studies' table).
Quality of the evidence
Key methodological limitations
Overall, we found only low‐quality evidence for the comparisons reported in this review. The methodological quality of studies included in the review was variable.
Half of the studies failed to describe the random sequence generation and allocation concealment satisfactorily, and we judged one trial to have a high risk of allocation concealment bias. We considered two studies to have a low risk of bias for sequence generation. None of the studies were double blind owing to the type of intervention assessed (psychosocial). With regard to the risk of bias related to incomplete outcome data, end‐of‐study outcomes were assessed in one trial only, and this we judged to be at high risk of bias. We judged three studies to be at low risk of bias relating to incomplete outcome data at follow up, and we judged one study to be at unclear risk of bias.
With regard to the risk of bias at an outcome level, we could not assess the objective outcomes (alcohol/drug use measured by breathalysers or urinalysis) because they were used only as an additional measure to check the accuracy of self‐reported alcohol/drug use in two studies; hence, these scores were not reported in the primary studies. Two studies did not use objective measures of outcomes at all. For subjective outcomes (alcohol/drug use measured by self‐reports), we judged two studies to be at unclear or high risk of detection bias. We did not perform sensitivity analyses, including or excluding studies at high risk of bias, owing to the small number of studies identified. Similarly, we were unable to pool the data for illicit drug use outcomes or any other of the anticipated secondary outcomes (e.g. physical or psychological health).
Indirectness of evidence
We did not include studies providing indirect evidence about our research question in this review, for example trials that included illicit drug users with and without a concurrent problem alcohol use. We did not identify other sources of indirectness, for example interventions, outcomes or comparators.
Inconsistency of results
We identified only small unexplained heterogeneity or inconsistency in the results. Most studies did not find significant, or found only a small, differences in effectiveness between the compared interventions on their primary outcomes.
Potential biases in the review process
There is a small chance that we missed some trials during the identification of relevant studies. We did not limit our searches to studies published in English; however, studies in non‐English languages may have been missed because they are commonly less indexed in the selected databases. We may also have missed unpublished studies. Unpublished studies are likely to have negative results, which is why they are not published. None of the authors who were contacted for information about unpublished or ongoing trials provided any information. Owing to the small number of included studies, we did not construct a funnel plot to assess publication bias. The major limitation of the review process was that most trials did not provide enough published data, or data in a form that could be extracted for meta‐analysis. Although we emailed authors from all four studies, only two responded and provided further data. Furthermore, we could not include a number of potentially relevant studies, because they involved drug users without problem alcohol use in their samples.
Agreements and disagreements with other studies or reviews
Comparison of the findings of our review with those of other studies or reviews is complicated by the fact that we did not perform any meta‐analyses and therefore have no aggregated results that would allow this type of comparison. As described in the background section, two narrative literature reviews have to date dealt with our research question (Arias 2008; Bickel 1987). Similarly to our work, these reviews were unable to identify evidence to answer our question or to conduct a meta‐analysis. Subsequently, they based their conclusions on evidence from a mixed type of studies (e.g. case studies, RCTs) or studies that included illicit drug users without a concurrent problem alcohol use. We excluded this type of study in our review (see 'Characteristics of excluded studies'). However, the review by Arias 2008 discussed 14 reports/studies about the treatment of co‐occurring alcohol and cocaine/opioid dependence, two of which were included in our review.
This review is unintentionally tapping into a sensitive controversy regarding the requirement of providing ancillary counselling services to individuals in opioid substitution treatments. The questions are: do additional services provided to individuals receiving MMT improve their outcomes? Does adding any psychosocial support to standard maintenance treatments yield additional benefits?
There are a number of ways to answer these questions. Previous studies (Amato 2011a; Gossop 2006; McLellan 1993; Schwartz 2012) have answered these questions by providing evidence of the effectiveness of psychosocial interventions in general/mixed conditions/outcomes, in studies in mixed populations with or without concurrent alcohol problems, or involving mixed types of interventions (i.e. pharmacological plus psychosocial). In this review, however, we focused on a single type of intervention and a 'pure' population in which all participants had both alcohol and drug problems. This may be one of the reasons why our review found such a small number of studies. Nevertheless, our findings support the weakness of the evidence base to answer this important question, as reported in a previous Cochrane review (Amato 2011a).
Another important question is: what constitutes standard maintenance/outpatient treatment? It appears that all standard treatments contain some type of psychosocial support, which varies considerably, and this makes it difficult to evaluate the added value of additional services. This was apparent in studies included in our review.

Study flow diagram from first publication of this review in 2012.

Study flow diagram for a review update: previous studies incorporated into results of new literature search
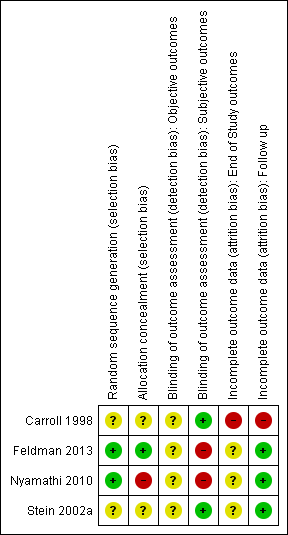
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
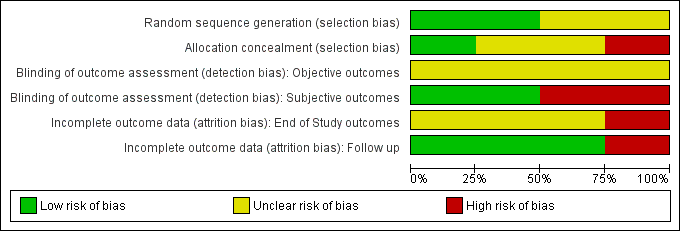
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Cognitive‐behavioural coping skills training (CBT) versus 12‐step facilitation (TSF), Outcome 1 Continuous outcomes.

Comparison 1 Cognitive‐behavioural coping skills training (CBT) versus 12‐step facilitation (TSF), Outcome 2 Dichotomous outcomes.
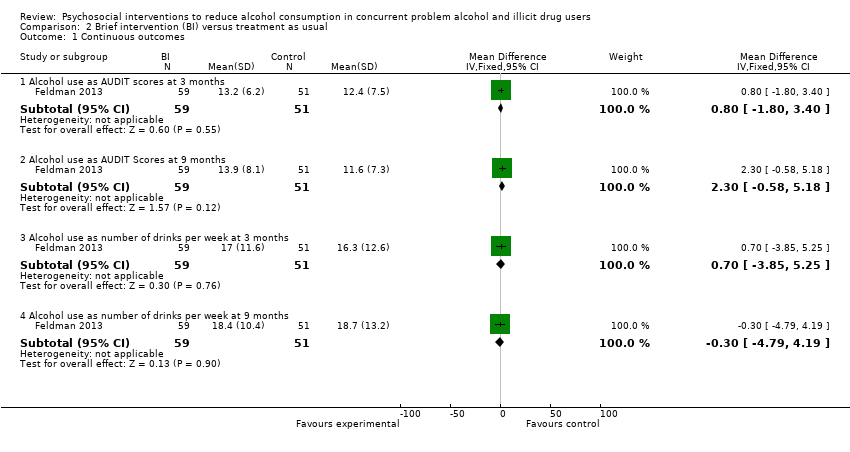
Comparison 2 Brief intervention (BI) versus treatment as usual, Outcome 1 Continuous outcomes.

Comparison 2 Brief intervention (BI) versus treatment as usual, Outcome 2 Dichotomous outcomes.

Comparison 3 Motivational interviewing (group) (MI‐G) versus hepatitis health promotion (HHP), Outcome 1 Continuous outcomes.
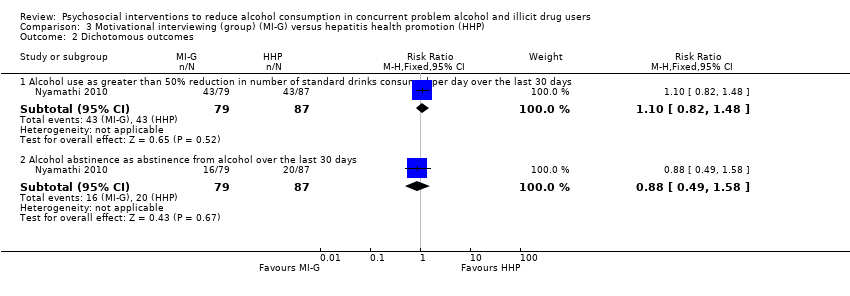
Comparison 3 Motivational interviewing (group) (MI‐G) versus hepatitis health promotion (HHP), Outcome 2 Dichotomous outcomes.
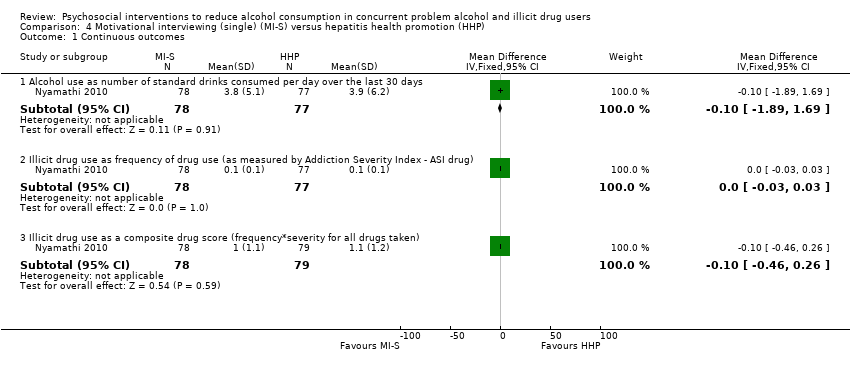
Comparison 4 Motivational interviewing (single) (MI‐S) versus hepatitis health promotion (HHP), Outcome 1 Continuous outcomes.

Comparison 4 Motivational interviewing (single) (MI‐S) versus hepatitis health promotion (HHP), Outcome 2 Dichotomous outcomes.

Comparison 5 Brief motivational intervention (BMI) versus assessment‐only, Outcome 1 Continuous outcomes.

Comparison 5 Brief motivational intervention (BMI) versus assessment‐only, Outcome 2 Dichotomous outcomes.
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | CBT versus TSF | |||||
| Maximum number of weeks of consecutive alcohol abstinence during treatment | The mean maximum number of weeks of consecutive alcohol abstinence during treatment in the control groups was | The mean maximum number of weeks of consecutive alcohol abstinence during treatment in the intervention group was | ‐ | 41 | ⊕⊕⊝⊝ | ‐ |
| Maximum number of weeks of consecutive abstinence from cocaine during treatment | The mean maximum number of weeks of consecutive abstinence from cocaine during treatment in the control groups was | The mean maximum number of weeks of consecutive abstinence from cocaine during treatment in the intervention group was | ‐ | 41 | ⊕⊕⊝⊝ | ‐ |
| Number of people achieving 3 or more weeks of consecutive alcohol abstinence during treatment | Study population | RR 1.96 | 41 | ⊕⊕⊝⊝ | ‐ | |
| 111 per 1000 | 218 per 1000 | |||||
| Moderate | ||||||
| 111 per 1000 | 218 per 1000 | |||||
| Alcohol abstinence | Study population | RR 2.38 | 41 | ⊕⊕⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Incomplete outcome data | ||||||
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | BI versus treatment as usual | |||||
| Number of standard drinks per week | The mean number of standard drinks per week in the control groups was | The mean number of standard drinks per week in the intervention groups was | ‐ | 110 | ⊕⊕⊝⊝ | ‐ |
| Number of standard drinks per week | The mean number of standard drinks per week in the control groups was | The mean number of standard drinks per week in the intervention groups was | ‐ | 110 | ⊕⊕⊝⊝ | ‐ |
| Decreased alcohol use | Study population | RR 1.13 | 110 | ⊕⊕⊝⊝ | ‐ | |
| 314 per 1000 | 355 per 1000 | |||||
| Moderate | ||||||
| 314 per 1000 | 355 per 1000 | |||||
| Decreased alcohol use | Study population | RR 1.34 | 110 | ⊕⊕⊝⊝ | ‐ | |
| 216 per 1000 | 289 per 1000 | |||||
| Moderate | ||||||
| 216 per 1000 | 289 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation and assessment of outcomes weren't blinded | ||||||
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | MI‐G versus HHP | |||||
| Number of standard drinks per day | The mean number of standard drinks per day in the control groups was | The mean number of standard drinks per day in the intervention groups was | ‐ | 147 | ⊕⊕⊝⊝ | ‐ |
| Over 50% less standard drinks per day | Study population | RR 1.1 | 166 | ⊕⊕⊝⊝ | ‐ | |
| 494 per 1000 | 544 per 1000 | |||||
| Moderate | ||||||
| 494 per 1000 | 543 per 1000 | |||||
| Alcohol abstinence | Study population | RR 0.88 | 166 | ⊕⊕⊝⊝ | ‐ | |
| 230 per 1000 | 202 per 1000 | |||||
| Moderate | ||||||
| 230 per 1000 | 202 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Masking: open label. Allocation and assessment of outcomes weren't blinded | ||||||
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | MI‐S versus hepatitis HHP | |||||
| Number of standard drinks consumed per day | The mean number of standard drinks consumed per day in the control groups was | The mean number of standard drinks consumed per day in the intervention groups was | ‐ | 155 | ⊕⊕⊝⊝ | ‐ |
| Over 50% less standard drinks per day | Study population | RR 0.92 | 177 | ⊕⊕⊝⊝ | ‐ | |
| 494 per 1000 | 455 per 1000 | |||||
| Moderate | ||||||
| 494 per 1000 | 454 per 1000 | |||||
| Alcohol abstinence | Study population | RR 0.97 | 177 | ⊕⊕⊝⊝ | ‐ | |
| 230 per 1000 | 223 per 1000 | |||||
| Moderate | ||||||
| 230 per 1000 | 223 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Masking: open label. Allocation and assessment of outcomes weren't blinded | ||||||
| Population: participants with alcohol use in concurrent problem alcohol and illicit drug users | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | BMI) versus assessment‐only | |||||
| Number of days with alcohol use at 6 months | The mean number of days with alcohol use at 6 months in the control groups was | The mean number of days with alcohol use at 6 months in the intervention groups was | ‐ | 187 | ⊕⊕⊕⊝ | ‐ |
| 25% reduction of drinking days in the past 30 days | Study population | RR 1.23 | 187 | ⊕⊕⊕⊝ | ‐ | |
| 522 per 1000 | 642 per 1000 | |||||
| Moderate | ||||||
| 522 per 1000 | 642 per 1000 | |||||
| 50% reduction of drinking days in the past 30 days | Study population | RR 1.27 | 187 | ⊕⊕⊕⊝ | ‐ | |
| 457 per 1000 | 580 per 1000 | |||||
| Moderate | ||||||
| 457 per 1000 | 580 per 1000 | |||||
| Seven or more drinking days' reduction in the past 30 days | Study population | RR 1.67 | 187 | ⊕⊕⊕⊝ | ‐ | |
| 239 per 1000 | 399 per 1000 | |||||
| Moderate | ||||||
| 239 per 1000 | 399 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sparse data: only 1 study with relatively few participants included in comparison | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continuous outcomes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Alcohol abstinence as maximum number of weeks of consecutive alcohol abstinence during treatment | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.14, 1.94] |
| 1.2 Illicit drug abstinence as maximum number of weeks of consecutive abstinence from cocaine during treatment | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.8 [‐0.70, 2.30] |
| 2 Dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Alcohol abstinence as number achieving 3 or more weeks of consecutive alcohol abstinence during treatment | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.43, 8.94] |
| 2.2 Illicit drug abstinence as number achieving 3 or more weeks of consecutive abstinence from cocaine during treatment | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.42, 2.88] |
| 2.3 Alcohol abstinence during follow‐up year | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.10, 55.06] |
| 2.4 Illicit drug abstinence as abstinence from cocaine during follow‐up year | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.04, 3.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continuous outcomes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Alcohol use as AUDIT scores at 3 months | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐1.80, 3.40] |
| 1.2 Alcohol use as AUDIT Scores at 9 months | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [‐0.58, 5.18] |
| 1.3 Alcohol use as number of drinks per week at 3 months | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐3.85, 5.25] |
| 1.4 Alcohol use as number of drinks per week at 9 months | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐4.79, 4.19] |
| 2 Dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Alcohol use as decreased alcohol use at 3 months | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.67, 1.93] |
| 2.2 Alcohol use as decreased alcohol use at 9 months | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.69, 2.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continuous outcomes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Alcohol use as number of standard drinks consumed per day over the last 30 days | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.03, 1.23] |
| 1.2 Illicit drug use as frequency of drug use (as measured by Addiction Severity Index ‐ ASI drug) | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 1.3 Illicit drug use as a composite drug score (frequency*severity for all drugs taken) | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.42, 0.42] |
| 2 Dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Alcohol use as greater than 50% reduction in number of standard drinks consumed per day over the last 30 days | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.82, 1.48] |
| 2.2 Alcohol abstinence as abstinence from alcohol over the last 30 days | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.49, 1.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continuous outcomes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Alcohol use as number of standard drinks consumed per day over the last 30 days | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.89, 1.69] |
| 1.2 Illicit drug use as frequency of drug use (as measured by Addiction Severity Index ‐ ASI drug) | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 1.3 Illicit drug use as a composite drug score (frequency*severity for all drugs taken) | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 2 Dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Alcohol use as greater than 50% reduction in number of standard drinks consumed per day over the last 30 days | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.68, 1.26] |
| 2.2 Alcohol abstinence as abstinence from alcohol over the last 30 days | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.56, 1.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Continuous outcomes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Alcohol use as number of days in the past 30 days with alcohol use at 1 month | 1 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐3.38, 2.78] |
| 1.2 Alcohol use as number of days in the past 30 days with alcohol use at 6 months | 1 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐4.56, 1.56] |
| 2 Dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Alcohol use as 25% reduction of drinking days in the past 30 days | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.96, 1.57] |
| 2.2 Alcohol use as 50% reduction of drinking days in the past 30 days | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.96, 1.68] |
| 2.3 Alcohol use as 75% reduction of drinking days in the past 30 days | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.84, 1.75] |
| 2.4 Alcohol use as 1 or more drinking days' reduction in the past 30 days | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.91, 1.38] |
| 2.5 Alcohol use as 7 or more drinking days' reduction in the past 30 days | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.08, 2.60] |

