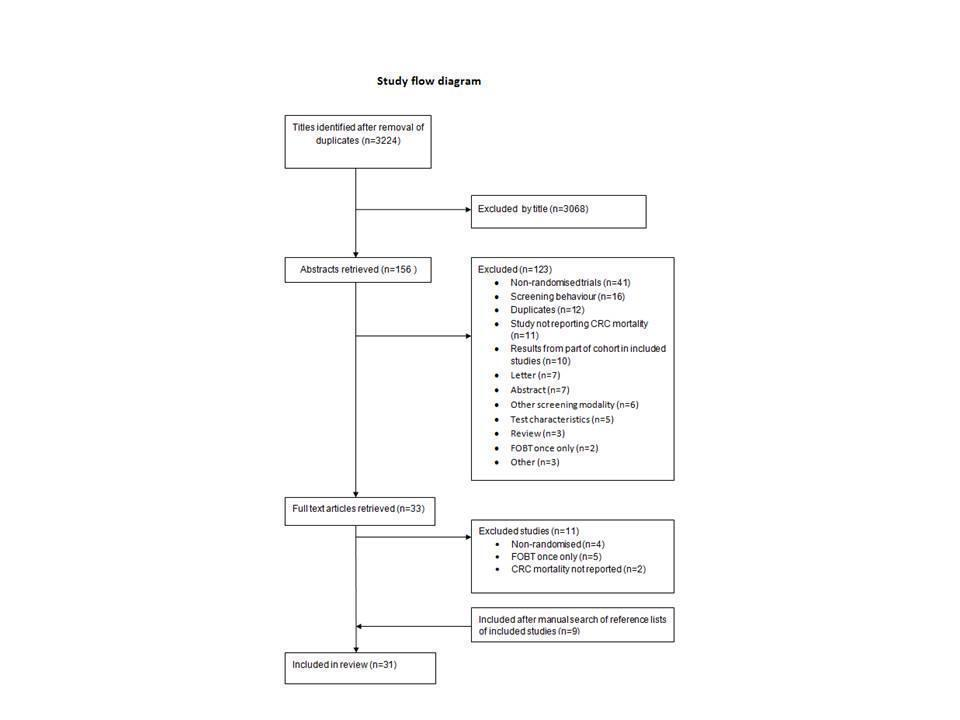
سیگموئیدوسکوپی انعطافپذیر در مقابل تست خون مخفی مدفوع در غربالگری سرطان کولورکتال در افراد بدون علامت
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در حال انجام
منابع اضافی
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | See Atkin 2010 Patient satisfaction assessed by questionnaire completed at baseline (the day after the flexible sigmoidoscopy) and after 3 months | |
| Participants | See Atkin 2010 | |
| Interventions | See Atkin 2010 | |
| Outcomes | Physical complications due to screening by flexible sigmoidoscopy and colonoscopy work‐up Patient satisfaction with the screening procedure | |
| Notes | Numbers of individuals assigned to screening and control group differs from Atkin 2010 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Atkin 2010 |
| Allocation concealment (selection bias) | Low risk | See Atkin 2010 |
| Blinding (performance bias and detection bias) | Low risk | See Atkin 2010 |
| Incomplete outcome data (attrition bias) | Low risk | Baseline questionnaire complete in 98% of screened persons Follow‐up questionnaire complete in 91% of screened persons |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | No other threats to validity detected |
| Methods | 574 general practices around 14 screening‐centres in the UK were invited to participate, and 506 practices accepted the invitation. Recruitment and screening was performed November 1994 ‐ March 1999. All eligible individuals in the participating general practices were screened for predefined exclusion criteria by their general practitioner, and the remaining received an information letter and questionnaire to establish interest in screening. Those who reported interest in screening were randomly assigned to the screening or control group in the ratio 1:2, respectively, by a central randomisation unit in blocks of 12. Randomisation was stratified according to household, trial centre and general practice. Persons in the control group were not contacted further. Follow‐up was by public registries. At follow‐up after median 11.2 years, six people in either group could not be traced. A further 234 persons in the screening group and 451 in the control group had emigrated. | |
| Participants | Individuals aged 55 ‐ 64 who responded with interest in screening and who did not meet any exclusion criteria: history of CRC, adenomas or inflammatory bowel disease; inability to provide informed consent; severe or terminal disease; life expectancy less than 5 years; sigmoidoscopy or colonoscopy within the previous 3 years. Persons reporting a strong family history of CRC or symptoms of CRC were also excluded and managed outside the trial. | |
| Interventions | Flexible sigmoidoscopy once only with removal of small polyps and referring for full colonoscopy if they had polyps 10 mm or larger, three or more adenomas, adenomas with tubulovillous or villous histology, severe dysplasia or malignant disease, or 20 or more hyperplastic polyps above the distal rectum. | |
| Outcomes | Compliance with screening, number referred for colonoscopy work‐up, incidence of CRC (total, proximal and distal), yearly hazard rate, number needed to screen to prevent one colorectal cancer, all‐cause mortality, mortality from CRC (intention‐to‐treat and per protocol), number needed to screen to prevent one death due to CRC. | |
| Notes | Compliance to screening reported as 71% (40674/57237) in the screening population. On the population‐level, compliance will be lower due to the two‐step invitation procedure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequentially numbered randomisation was done centrally in blocks of 12 and with the added constraint of no more than three consecutive allocations to one group within or across blocks. |
| Allocation concealment (selection bias) | Low risk | Central randomisation procedure |
| Blinding (performance bias and detection bias) | Low risk | Outcomes were obtained from or confirmed by public registries. A second analysis as CRCas an underlying cause of death was obtained after blinded verification of death certificates by an independent expert coder who had access to clinical information when available |
| Incomplete outcome data (attrition bias) | Low risk | Six people in each group could not be traced. 658 people had emigrated |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported on |
| Other bias | Low risk | No other threats to validity detected |
| Methods | See Hoff 2009 | |
| Participants | Individuals aged 50‐64 living in city of Oslo and Telemark county, Norway | |
| Interventions | See Hoff 2009 | |
| Outcomes | Physical complications from screening with flexible sigmoidoscopy and colonoscopy work‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Hoff 2009 |
| Allocation concealment (selection bias) | Low risk | See Hoff 2009 |
| Blinding (performance bias and detection bias) | Low risk | See Hoff 2009 |
| Incomplete outcome data (attrition bias) | Low risk | See Hoff 2009 |
| Selective reporting (reporting bias) | Low risk | See Hoff 2009 |
| Other bias | Low risk | See Hoff 2009 |
| Methods | See Scholefield 2012. Median follow‐up 7.8 years | |
| Participants | See Scholefield 2002 | |
| Interventions | See Scholefield 2002 | |
| Outcomes | CRC mortality, CRC incidence, staging | |
| Notes | Number of people included in analyses differ from those reported in Scholefield 2002 and Scholefield 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Scholefield 2002 |
| Allocation concealment (selection bias) | Low risk | See Scholefield 2002 |
| Blinding (performance bias and detection bias) | Low risk | See Scholefield 2002 |
| Incomplete outcome data (attrition bias) | Low risk | See Scholefield 2002 |
| Selective reporting (reporting bias) | Low risk | See Scholefield 2002 |
| Other bias | Low risk | See Scholefield 2002 |
| Methods | See Thiis‐Evensen 1999. Follow‐up 10 years after screening | |
| Participants | See Thiis‐Evensen 1999 | |
| Interventions | See Thiis‐Evensen 1999 | |
| Outcomes | CRC mortality, CRC incidence, all‐cause mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | See Thiis‐Evensen 1999 |
| Allocation concealment (selection bias) | Low risk | See Thiis‐Evensen 1999 |
| Blinding (performance bias and detection bias) | Low risk | See Thiis‐Evensen 1999 |
| Incomplete outcome data (attrition bias) | Low risk | See Thiis‐Evensen 1999 |
| Selective reporting (reporting bias) | Low risk | See Thiis‐Evensen 1999 |
| Other bias | Low risk | See Thiis‐Evensen 1999 |
| Methods | See Thiis‐Evensen 1999. Body Mass Index (BMI) and smoking habits were assessed in attenders at baseline flexible sigmoidoscopy screening in 1983 and at follow‐up after 13 years. Participants were compared according to the findings at screening (any polyp versus no polyps detected). Data were available for all but 3 individuals. | |
| Participants | See Thiis‐Evensen 1999 | |
| Interventions | See Thiis‐Evensen 1999 | |
| Outcomes | BMI, smoking habits, all‐cause mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not applicable |
| Allocation concealment (selection bias) | Unclear risk | Not applicable |
| Blinding (performance bias and detection bias) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Not applicable |
| Other bias | Unclear risk | Not applicable |
| Methods | Eligible participants were individually randomised centrally to the screening group or control group between January 1999 and December 2000. In the screening group, there was a further 1:1 randomisation to either flexible sigmoidoscopy only or FOBT combined with flexible sigmoidoscopy. 459 people allocated to the screening group were excluded from the screening procedure, but included in the intention‐to‐screen analyses, due to pre‐specified exclusion criteria. Follow‐up was purely registry based and participants in the control group were never contacted. Finally, 1196 people were lost to follow‐up due to emigration and 21 people were censored as a result of colorectal malignancy other than colorectal adenocarcinoma. CRC mortality was reported after median 6 years and CRC incidence after median 7 years. | |
| Participants | All residents aged 55‐64 living in the city of Oslo and Telemark County, Norway by November 1998. Individuals with a history of CRC were excluded. | |
| Interventions | Flexible sigmoidoscopy once only or flexible sigmoidoscopy combined with immunologic FOBT. During the endoscopic screening procedure, all detected lesions were biopsied. A positive screening test qualifying for full colonoscopy work‐up and polypectomy was defined as any polyp 10 mm or more in diameter, any histologically verified adenoma irrespective of size, carcinoma or a positive FOBT. | |
| Outcomes | Incidence of CRC, incidence of rectosigmoid CRC, incidence of neoplastic lesions, incidence of high‐risk adenomas, mortality from CRC, mortality from all causes, stage of CRC, compliance with screening, rate and compliance of colonoscopy work‐up. | |
| Notes | In 2000, the trial was expanded for one year (throughout 2001) including persons aged 50‐54 and randomised in the same way as those previously enrolled. This group is not reported on in this paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done independently according to social security number by the National Bureau of Statistics |
| Allocation concealment (selection bias) | Low risk | Central randomisation process |
| Blinding (performance bias and detection bias) | Low risk | Outcomes were obtained from public registries by a person not involved in the trial |
| Incomplete outcome data (attrition bias) | Low risk | 1196 people were lost to follow‐up due to emigration |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | No other threats to validity detected |
| Methods | See Kronborg 2004. Follow‐up 13 years after commencement of screening | |
| Participants | See Kronborg 2004 | |
| Interventions | See Kronborg 2004 | |
| Outcomes | CRC incidence, CRC mortality, all‐cause mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Kronborg 2004 |
| Allocation concealment (selection bias) | Low risk | See Kronborg 2004 |
| Blinding (performance bias and detection bias) | Low risk | See Kronborg 2004 |
| Incomplete outcome data (attrition bias) | Low risk | See Kronborg 2004 |
| Selective reporting (reporting bias) | Low risk | See Kronborg 2004 |
| Other bias | Low risk | See Kronborg 2004 |
| Methods | See Lindholm 2008. Follow‐up 2‐7 years after end of the pre‐screening | |
| Participants | See Lindholm 2008 | |
| Interventions | See Lindholm 2008 | |
| Outcomes | CRC incidence, staging | |
| Notes | CRC mortality not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Linndholm 2008 |
| Allocation concealment (selection bias) | Low risk | See Linndholm 2008 |
| Blinding (performance bias and detection bias) | Low risk | See Linndholm 2008 |
| Incomplete outcome data (attrition bias) | Low risk | See Linndholm 2008 |
| Selective reporting (reporting bias) | Low risk | See Linndholm 2008 |
| Other bias | Low risk | See Linndholm 2008 |
| Methods | See Lindholm 2008. This report addresses endoscopic and surgical complications of work‐up after a positive FOB screening test | |
| Participants | See Lindholm 2008 | |
| Interventions | See Lindholm 2008 | |
| Outcomes | Physical complications due to FOBT screening | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not applicable |
| Allocation concealment (selection bias) | Unclear risk | Not applicable |
| Blinding (performance bias and detection bias) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Not applicable |
| Other bias | Unclear risk | Not applicable |
| Methods | See Kronborg 2004. 10 years follow‐up after start of screening | |
| Participants | See Kronborg 2004 | |
| Interventions | See Kronborg 2004 | |
| Outcomes | CRC incidence, CRC mortality, all‐cause mortality, staging | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Kronborg 2004 |
| Allocation concealment (selection bias) | Low risk | See Kronborg 2004 |
| Blinding (performance bias and detection bias) | Low risk | See Kronborg 2004 |
| Incomplete outcome data (attrition bias) | Low risk | See Kronborg 2004 |
| Selective reporting (reporting bias) | Low risk | See Kronborg 2004 |
| Other bias | Low risk | See Kronborg 2004 |
| Methods | Eligible persons were randomised centrally by block‐randomisation of 14 to the intervention group, control group or not enrolled. Married couples were always randomised to the same group. Controls were never contacted. Follow‐up was based on public registries. Only people participating in the preceding screening round were invited for the next round. Screening started in August 1985 and ended in August 2002. No participants were lost to follow‐up 17 years after study start. | |
| Participants | Subjects aged 45 ‐ 75 years living in Funen, Denmark, in 1985. Exclusion criteria: People with a history of CRC, adenomas, distant spread from all types of malignant disorders or participants in the pilot study preceding the trial. | |
| Interventions | Guaiac‐based FOBT with dietary restrictions every second year. The slides were not rehydrated. A positive screening test was defined as one or more blue slides out of six and qualified for work‐up with colonoscopy. | |
| Outcomes | Incidence of CRC, mortality from CRC by intention‐to‐treat and per protocol, stage of CRC, mortality from all causes, compliance with screening and work‐up | |
| Notes | Separate analysis of CRC mortality which included death due to treatment of CRC (postoperative complications) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central block randomisation procedure based on social security number |
| Allocation concealment (selection bias) | Low risk | Central randomisation procedure |
| Blinding (performance bias and detection bias) | Low risk | The investigators were unaware of the trial allocation during the assessment of death certificates |
| Incomplete outcome data (attrition bias) | Low risk | All participants were followed until death or end of study |
| Selective reporting (reporting bias) | Low risk | All relevant outcome were reported |
| Other bias | Low risk | No other threats to validity detected |
| Methods | See Hoff 2009 Individuals attending screening with flexible sigmoidoscopy between January 1999 and February 2000 were given a questionnaire immediately after the examination to be filled in and returned by mail the following day | |
| Participants | Individuals aged 55‐64 who attended screening with flexible sigmoidoscopy | |
| Interventions | Questionnaire filled in by participants in a flexible sigmoidoscopy screening trial | |
| Outcomes | Participants' satisfaction | |
| Notes | Not randomised study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Hoff 2009. This substudy was not randomised as all screened persons attending the trial between January 1999 and February 2000 were included |
| Methods | See Hoff 2009 Individuals randomised to screening with flexible sigmoidoscopy or to the control group received questionnaires on selected lifestyle indicators at baseline and after 3 years | |
| Participants | People aged 50‐55 in Telemark County and city of Oslo. Exclusion criteria: history of CRC | |
| Interventions | Questionnaire filled in by individuals randomised to screening with flexible sigmoidoscopy or to a control group | |
| Outcomes | Selected lifestyle indicators | |
| Notes | Analyses not by intention to treat | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Hoff 2009 |
| Allocation concealment (selection bias) | Low risk | See Hoff 2009 |
| Blinding (performance bias and detection bias) | Unclear risk | Handling and blinding of questionnaires not stated |
| Incomplete outcome data (attrition bias) | Low risk | Individuals lost to follow‐up and proportion of non‐responders quite similar in the two groups |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other threats to validity detected |
| Methods | See Lindholm 2008. All individuals in the cohort included in 1990 and 1991 and who were randomised to the intervention group received a questionnaire two weeks after the invitation to screening. A sample of these individuals were also chosen for a combined structured/open interview by telephone or personal meeting according to the test results or non‐attendance. | |
| Participants | Individuals aged 60‐64 in Gothenburg, Sweden, who were invited to screening with FOBT | |
| Interventions | Mailed questionnaire received 2 weeks after invitation to screening with FOBT and combined structured/open interview at different occasions according to test results | |
| Outcomes | Worry and interference with daily activities caused by invitation to or results of screening Patient satisfaction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not applicable |
| Allocation concealment (selection bias) | Unclear risk | Not applicable |
| Blinding (performance bias and detection bias) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Not applicable |
| Other bias | Unclear risk | Not applicable |
| Methods | Participants were invited for screening on 2‐3 occasions in 3 cohorts. All 3 cohorts had their own screening scheme. Cohort 1 (recruited August 1982 ‐ June 1983) had rescreening after 21‐24 months and after approximately 11 years. Cohort 2 (recruited January 1987 ‐ March 1988) had one rescreening after 21‐24 months, and cohort 3 (recruited January 1990 ‐ November 1990) had rescreening one and two years after the prevalent screen. Until 1984, a positive screening‐test qualifying for follow‐up was defined as one positive test‐slide out of six. After 1984, those with positive test was re‐tested with FOBT, and only those with at least one positive test slide out of six the second time were referred for work‐up. The work‐up investigation consisted of a flexible sigmoidoscopy and a double‐contrast barium enema. 532 individuals lost to follow‐up due to emigration. Participants were followed through public registries for a median of 15 years and 6 months. | |
| Participants | All inhabitants aged 60‐64 living in Gothenburg, Sweden. Individuals with a history of CRC were excluded | |
| Interventions | Guaiac‐based FOBT with dietary restrictions. Two samples from 3 consecutive stools were collected. FOBT from the first half of cohort 1 was not rehydrated, but all later tests in this cohort and all FOBT in cohort 2 and 3 were rehydrated | |
| Outcomes | Incidence of CRC, death from CRC and all causes by intention‐to‐screen and per protocol analyses, CRC staging, compliance, diagnostic work‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Central randomisation procedure based on the population registry |
| Blinding (performance bias and detection bias) | Low risk | CRC diagnosis and cause of death obtained from public registries. In cases of uncertainty of cause of death, an independent reviewer who was blinded to study group allocation evaluated case records |
| Incomplete outcome data (attrition bias) | Low risk | All individuals could be traced at follow‐up except for 532 emigrants |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | No other threats to validity detected |
| Methods | Eligible individuals were randomly allocated to screening with FOBT (annually or bienially) or to a control group who were not offered any screening. Participants were stratified according to sex, age and place of residence prior to randomisation. Screening started in 1976 and ended in 1982. In February 1986, screening was resumed due to lower than expected mortality from CRC in the control group. This second screening period ended in February 1992. All death certificates along with medical records were reviewed by a blinded review committee. A study pathologist staged all slides from patients with a diagnosis of CRC. All participants in the screening‐ and control groups were mailed a questionnaire annually to ascertain their vital status and occurrence of CRC and polyps. Follow‐up was for 13 years. | |
| Participants | People aged 50‐80 years recruited among volunteers for the American Cancer Society and fraternal, veterans, and employee groups in Minnesota, USA. Exclusion criteria: People with a history of CRC, familial polyposis, chronic ulcerative colitis and persons known to be bedridden or otherwise disabled. | |
| Interventions | Guaiac‐based FOBT with dietary restrictions. Two samples from three consecutive stools were obtained. 82.5% of the test slides were rehydrated. A positive screening test was defined as one or more blue test slides out of six and qualified for work‐up which included colonoscopy. | |
| Outcomes | Compliance with screening, complications due to colonoscopy work‐up, incidence of CRC, mortality from CRC, all‐cause mortality, stage of CRC | |
| Notes | Colonoscopy performed in 38% of participants in the annual screening group, and in 28% of participants in the biennial screening group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Weekly randomisation as participants were enrolled after stratification for age, sex and place of residence |
| Allocation concealment (selection bias) | Low risk | Participants stratified and placed in groups of three who were subsequently randomised to one of six permutations which allocated the three participants to either of the three study groups |
| Blinding (performance bias and detection bias) | Low risk | Outcomes assessed by a death review committee and a study pathologist who were unaware of study allocation |
| Incomplete outcome data (attrition bias) | Low risk | Death certificates obtained for 99.9% of participants |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | Participants recruited among volunteers |
| Methods | See Mandel 1993 Vital status complete for 88.8%, 89.1% and 88.5% and death certificates were obtained for 99.7%, 99.8% and 99.8% for annual, biennial and control group participants, respectively. Follow‐up was for 18 years | |
| Participants | See Mandel 1993 | |
| Interventions | See Mandel 1993 | |
| Outcomes | All‐cause mortality, mortality from CRC | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Mandel 1993 |
| Allocation concealment (selection bias) | Low risk | See Mandel 1993 |
| Blinding (performance bias and detection bias) | Low risk | See Mandel 1993 |
| Incomplete outcome data (attrition bias) | Low risk | See Mandel 1993 |
| Selective reporting (reporting bias) | Low risk | See Mandel 1993 |
| Other bias | Low risk | See Mandel 1993 |
| Methods | See Mandel 1993. Follow‐up for 18 years | |
| Participants | See Mandel 1993 | |
| Interventions | See Mandel 1993 | |
| Outcomes | Incidence of CRC | |
| Notes | See Mandel 1993 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Mandel 1993 |
| Allocation concealment (selection bias) | Low risk | See Mandel 1993 |
| Blinding (performance bias and detection bias) | Low risk | See Mandel 1993 |
| Incomplete outcome data (attrition bias) | Low risk | See Mandel 1993 |
| Selective reporting (reporting bias) | Low risk | See Mandel 1993 |
| Other bias | Low risk | See Mandel 1993 |
| Methods | See Atkin 2010 A sample of individuals invited for screening was included. Individuals were divided into three groups and compared according to the outcome from the flexible sigmoidoscopy screen: 1) Individuals with a negative screening result (no significant pathology), 2) individuals with a low‐risk result (1‐2 adenomas 9 mm or less with a tubular histology and mild to moderate dysplasia or less than 20 hyperplastic polyps) and 3) individuals with a high‐risk result (3 or more adenomas, adenoma 10 mm or larger, adenoma with tubulovillous or villous histology or severe dysplasia or 20 or more hyperplastic polyps) who were recommended colonoscopy work‐up. | |
| Participants | People aged 55‐64 in three selected screening areas in the UK | |
| Interventions | Questionnaire about health attitudes and selected lifestyle indicators before screening with flexible sigmoidoscopy and 3 months post‐screening | |
| Outcomes | Selected health attitudes and lifestyle indicators | |
| Notes | Main analysis not by intention‐to‐treat, but sensitivity analyses by intention‐to‐treat did not change results of the main analysis. There was no no‐screening control group. The no‐risk and low‐risk group only had flexible sigmoidoscopy, while the high‐risk group had both flexible sigmoidoscopy and full colonoscopy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not applicable |
| Allocation concealment (selection bias) | Unclear risk | Not applicable |
| Blinding (performance bias and detection bias) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Not applicable |
| Other bias | Unclear risk | Not applicable |
| Methods | See Hardcastle 1996 2184 individuals assigned to the screening group were randomly chosen to received a questionnaire (the General Health Questionnaire) by mail before the offer of screening and 3 months after screening to asses psychiatric morbidity. Participants were recruited from two general practices. The participants returned the questionnaire by mail. 1693 (70.6%) of the individuals returned the first questionnaire. Of the 1693 subjects offered the questionnaire 3 months after screening,1303 (77%) completed the form. Anxiety levels were measured by another self‐administered questionnaire in all subjects with a positive FOBT. This questionnaire was completed each time the participant attended the hospital, the day after each visit and 1 month after the results of the investigations were known. Data from 100 persons with a false positive FOBT was analysed. | |
| Participants | A sample of participants aged 50 to 75 allocated to screening with FOBT | |
| Interventions | 1): Questionnaire before screening and 3 months after screening with FOBT 2): Questionnaire at each hospital visit, the day after the hospital visit and 1 month after the result of the colonoscopy work‐up examination in participants with a positive FOBT | |
| Outcomes | Psychiatric adverse effects of screening with FOBT | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not applicable |
| Allocation concealment (selection bias) | Unclear risk | Not applicable |
| Blinding (performance bias and detection bias) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Not applicable |
| Other bias | Unclear risk | Not applicable |
| Methods | See Scholefield 2002. This report mainly addresses complications due to work‐up after positive FOB screening tests | |
| Participants | See Scholefield 2002 | |
| Interventions | See Scholefield 2002 | |
| Outcomes | Physical complications related to screening with FOBT | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not applicable |
| Allocation concealment (selection bias) | Unclear risk | Not applicable |
| Blinding (performance bias and detection bias) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Not applicable |
| Other bias | Unclear risk | Not applicable |
| Methods | A total of 1221 individuals (of whom 97% was participating in the PLCO trial) at two screening centre were recruited. A questionnaire was completed on site immediately after the screening intervention or returned by mail a few days after the examination. A random sample completed 2 open‐ended questions about the screening experience and their expectations. | |
| Participants | Ninety‐seven per cent participated in the PLCO trial | |
| Interventions | Questionnaire | |
| Outcomes | Convenience and accessibility, staff interpersonal skills, physical surroundings, perceived technical competence, expectations and beliefs, general satisfaction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not applicable |
| Allocation concealment (selection bias) | Unclear risk | Not applicable |
| Blinding (performance bias and detection bias) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Not applicable |
| Other bias | Unclear risk | Not applicable |
| Methods | Eligilbe individuals were invited to participate in the trial by mass mailing. People who reported interest in screening provided written informed consent and completed a baseline questionnaire before randomisation which was performed in blocks stratified according to screening centre, age and sex. A total of 154,900 people were enrolled from 1993 through 2001; 77,445 to the intervention group and 77,455 to the control group. All cancers and deaths were primarily assessed through a annually mailed questionnaire to all participants and subsequently verified from medical records and through linkage to public registries. Deaths that were potentially related to colorectal cancer were reviewed in a blinded fashion. CRC deaths included deaths due to CRC and its treatment. | |
| Participants | Individuals 55 to 74 years of age with no prior history of prostate, lung, colorectal or ovarian cancer. Other exclusion criteria were: ongoing treatment of any type of cancer except basal‐cell or squamous‐cell skin cancer and, beginning in 1996, flexible sigmoidoscopy, colonoscopy or barium enema in the previous 3 years. | |
| Interventions | Participants in the intervention group were offered a flexible sigmoidoscopy at baseline and at 3‐5 years. Participants in the control group were not offered any screening and continued to receive "care as usual". The screening interventions were conducted at ten screening centres. A positive test result was defined as a finding of a polyp or a mass. Biopsies were not routinely performed, but individuals with a positive test were referred to their general practitioner for decisions regarding diagnostic follow‐up. | |
| Outcomes | CRC mortality, CRC incidence, all‐cause mortality, staging, physical complications due to screening and follow‐up colonoscopy | |
| Notes | Carcinoid tumours were included as colorectal cancers 46.5% of participants in the control group had a flexible sigmoidoscopy or colonoscopy during the screening phase of the study. The rate of routine colonoscopy after the screening phase was 47.7% in the intervention group and 48.0% in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Volunteers who responded to an invitation through mass‐mailing were randomised using a central block‐randomisation process stratified according to screening centre, age and gender |
| Allocation concealment (selection bias) | Low risk | Central randomisation process |
| Blinding (performance bias and detection bias) | Low risk | Death review group unaware of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Vital status was known for 99.9% of participants, and compliance with the annual study update questionnaire was 93.8% |
| Selective reporting (reporting bias) | Unclear risk | No reports of adverse effects due to colonoscopy follow‐up |
| Other bias | Low risk | No other threats to validity detected |
| Methods | See Scholefield 2012. Median follow‐up 11.7 years | |
| Participants | See Scholefield 2012 | |
| Interventions | See Scholefield 2012 | |
| Outcomes | Incidence of CRC, CRC mortality, all cause mortality, number of positive screening tests, work‐up, compliance with screening | |
| Notes | Number of people included in the analyses differ from the previous report of this trial (Hardcastle 1996) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation process by household. More than 50% of households were single persons |
| Allocation concealment (selection bias) | Low risk | Central randomisation procedure |
| Blinding (performance bias and detection bias) | Low risk | Study investigators who assessed cause of death and pathologists were unaware of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | 547 persons could not be traced or had emigrated |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | No other threats to validity detected |
| Methods | Eligible individuals were recruited to the study between February 1981 and June 1983 (pilot study) and February 1985 and January 1991 (main study). People were identified according to the general practice at which they were registered. Family doctors at each practice were asked to exclude any person with serious illness including a diagnosis of CRC the previous 5 years. Randomisation was by household and stratified according to size, sex and average age of eligible members within the household. Housholds were randomly allocated to screening with FOBT or no screening. After randomisation, 547 people could not be traced and were excluded from the mortality analysis. Persons in the control group were not contacted. Follow‐up was based on public registries, histopathologic registers at the local hospitals and family doctors' reports. Strutured case note reviews of certified and registered CRC cases were carried out in order to verify cause of death. Median follow‐up was 19.5 years. | |
| Participants | People aged 45‐75 living in the Nottingham area of the UK | |
| Interventions | Guaiac based FOBT every second year. Two samples of three consecutive stools were collected. The test was taken without dietary restrictions and without rehydration of the test slides. In the pilot study, a positive screening test was defined as one or more blue test slides and individuals with a positive test were referred for flexible sigmoidoscopy and double contrast enema. In the main study, a positive screening test was defined as five or six blue test slides, and these persons were referred for colonoscopy. Individuals with 1‐4 positive test slides in the initial screening test were retested with the FOBT. Dietary restrictions were applied, and two samples from six consecutive stools were collected. Those with one or more positive test slides in the retest were offered colonoscopy. Screening participants with a negative retest were asked to repeat the test again with dietary restrictions after 3 months and were offered colonoscopy if they tested positive. | |
| Outcomes | Incidence of CRC, mortality from CRC, all‐cause mortality | |
| Notes | People excluded from analyses due to emigration or other causes different from the other reports from the same study; Hardcastle 1996 and Scholefield 2002 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation process by household. More than 50% of households were single persons |
| Allocation concealment (selection bias) | Low risk | Central randomisation procedure |
| Blinding (performance bias and detection bias) | Low risk | Study investigators who assessed cause of death and pathologists were unaware of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | 875 people could not be traced or had emigrated after randomisation and were excluded from analyses. Not stated how many people were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | No other threats to validity detected |
| Methods | See Segnan 2011. This report includes the baseline findings and complications to screening in the Italian trial. Patient satisfaction was assessed among screened persons by a questionnaire to be filled out immediately after the flexible sigmoidoscopy | |
| Participants | See Segnan 2011 | |
| Interventions | See Segnan 2011 | |
| Outcomes | Complications to flexible sigmoidoscopy screening and follow‐up colonoscopy Patient satisfaction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Segnan 2011 |
| Allocation concealment (selection bias) | Low risk | See Segnan 2011 |
| Blinding (performance bias and detection bias) | Low risk | See Segnan 2011 |
| Incomplete outcome data (attrition bias) | Low risk | See Segnan 2011 |
| Selective reporting (reporting bias) | Low risk | See Segnan 2011 |
| Other bias | Low risk | See Segnan 2011 |
| Methods | Participants were recruited in a two‐step procedure between June 1995 and May 1999. Eligible individuals first received an interest‐in‐screening questionnaire by mail designed to assess eligibility for and interest in screening. Responders who reported interest in screening, were randomised 1:1 into an intervention group or a control group. The control group was not contacted further. In three regions, randomisation was on an individual basis, and in the other three regions, a cluster randomisation model was adopted with the general practice as the cluster unit. Follow‐up data was obtained from local hospital discharge records, pathology department files, population cancer registries and regional mortality registries. Death certificates were retrieved of all patients diagnosed with CRC during follow‐up and supplemented with clinical information when available. Median follow‐up for incidence was 10.5 years and for mortality 11.4 years. | |
| Participants | Individuals aged 55‐64 in 6 regions in Italy. People were excluded if they reported a history of CRC, colorectal adenomas, inflammatory bowel disease, colorectal endoscopy in the previous two years, had two or more first degree relatives with CRC or had a medical condition that would preclude benefit from screening. | |
| Interventions | Flexible sigmoidoscopy once only and referral for colonoscopy if: Polyp > 5 mm, inadequate bowel preparation and at least one polyp, 3 or more adenomas, adenomas with villous component greater than 20% or high‐grade dysplasia or CRC at the prevalent screening procedure. In addition, attenders were referred for colonoscopy if clinically indicated, judged by the physician who performed the screening procedure. | |
| Outcomes | CRC incidence, CRC mortality, all‐cause mortality | |
| Notes | Compliance with screening reported by the authors was 58.3% (of those who reported interest in screening). On the population‐level, compliance will be lower due to the two‐step invitation procedure. Cluster randomisation was not accounted for in the statistical analyses, and intra‐cluster correlation was not computed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was secured by using a computer‐generated allocation algorithm |
| Blinding (performance bias and detection bias) | Low risk | The independent investigators who assessed outcomes were blinded to group allocation |
| Incomplete outcome data (attrition bias) | Low risk | 280 (1.6%) individuals in the intervention group and 324 (1.9%) in the control group could not be traced |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | No other threats to validity detected |
| Methods | See Atkin 2010. Attenders in the first two screening centres received a questionnaire 3‐6 months after the screening procedure to assess the participants' satisfaction with the screening. Individuals were grouped according to outcome of the flexible sigmoidoscopy screening as described in Miles 2003. In addition, a randomly selected sample of 60 participants, 10 men and 10 women from each of the three outcome groups, had a semi‐structured interview. | |
| Participants | See Atkin 2010 | |
| Interventions | Mailed questionnaire 3 months after screening and semi‐structured interview | |
| Outcomes | Participants' satisfaction | |
| Notes | The no‐risk and low‐risk group only had flexible sigmoidoscopy, while the high‐risk group had both flexible sigmoidoscopy and full colonoscopy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not applicable |
| Allocation concealment (selection bias) | Unclear risk | Not applicable |
| Blinding (performance bias and detection bias) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Not applicable |
| Other bias | Unclear risk | Not applicable |
| Methods | Participants were randomly drawn from the population registry of Telemark county, Norway, to the intervention group or control group. People in the intervention group were born in January or February. Controls were drawn irrespective of month of birth. Intervention with flexible sigmoidoscopy was performed in March and April 1983. Controls were not contacted at this point. Those who accepted invitation for screening in 1983 and all individuals in the control group were invited for colonoscopy in 1996. Two individuals were not invited due to emigration. Outcomes were obtained from public registries. Two, 12 and 73 weeks after the colonoscopy, the attendants received a questionnaire designed to evaluate the experience of taking part in the study. Follow‐up was 13 years. | |
| Participants | Individuals aged 50‐59 years living in Telemark county, Norway, in 1983. Individuals with a history of CRC were not excluded | |
| Interventions | People in the intervention group were offered flexible sigmoidoscopy. All participants with any polyp at the baseline flexible sigmoidoscopy were referred for colonoscopy within two months. Those with polyps 5 mm or larger in diameter during the work‐up colonoscopy had their polyps removed by polypectomy and were offered a repeat colonoscopy in 1989 and 1993. Those with polyps measuring less than 5 mm were not offered polypectomy in 1983, but had a colonoscopy and polypectomy in 1985 and were offered colonoscopy in 1989 and 1993. | |
| Outcomes | CRC incidence, CRC mortality, CRC from all causes, compliance with screening, patients' experience as participants in the trial, complications to the endoscopic examinations | |
| Notes | All‐cause mortality significantly higher in the intervention group than in the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants in the intervention group drawn from eligible individuals born in January and February, while controls were drawn irrespectively of month of birth |
| Allocation concealment (selection bias) | Low risk | Randomisation based on social security number |
| Blinding (performance bias and detection bias) | Low risk | Outcomes were obtained from public registries |
| Incomplete outcome data (attrition bias) | Low risk | Two individuals could not be traced |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | No other threats to validity detected |
| Methods | See Atkin 2010. This study reports two studies. In study 1, individuals who had a flexible sigmoidoscopy screening procedure (participants) in two screening centres received a questionnaire assessing the impact on screening on selected psychological issues by mail 3 months after attendance. In study 2, a random selected sample of participants also received a questionnaire before the screening procedure, making it possible to trace changes in selected psychological issues. Individuals in both studies were grouped and compared according to outcome of the screening procedure as described in Miles 2003. | |
| Participants | See Atkin 2010 | |
| Interventions | Questionnaire before screening with flexible sigmoidoscopy (study 2) and 3 months after screening (study 1 and 2) | |
| Outcomes | Psychological adverse effect of screening with flexible sigmoidoscopy | |
| Notes | No no‐screen control group. Short follow‐up. The no‐risk and low‐risk group only had flexible sigmoidoscopy, while the high‐risk group had both flexible sigmoidoscopy and full colonoscopy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not applicable |
| Allocation concealment (selection bias) | Unclear risk | Not applicable |
| Blinding (performance bias and detection bias) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Not applicable |
| Other bias | Unclear risk | Not applicable |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| FOBT once only | |
| FOBT once only | |
| Non‐randomised study | |
| Non‐randomised study | |
| FOBT once only | |
| FOBT once only | |
| Quasi‐randomised trial | |
| CRC‐mortality not reported | |
| Non‐randomised study | |
| FOBT once only |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | National cancer screening program in Finland |
| Methods | Age‐eligible individuals living in municipalities which volunteered to implement screening were randomised 1:1 to the intervention group or control group. Individuals in the control group were offered the same intervention six years after start of screening in the intervention group (in 2010) in a staged fashion. Follow‐up is passive through public registries. |
| Participants | People aged 60‐64 years living in participating municipalities in Finland. 52,998 subjects were randomised to the intervention group, and 53,002 subjects to the control group. |
| Interventions | Biennial unrehydrated guaiac‐based FOBT until age 69. Dietary and vitamin C restriction applied. 2 samples collected from 3 consecutive stools. Screen‐positive referred for colonoscopy. |
| Outcomes | CRC mortality |
| Starting date | September 2004 |
| Contact information | Dr H.Paimela, e‐mail: [email protected] |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Colorectal cancer mortality Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Screening procedures versus control ‐ all studies, Outcome 1 Colorectal cancer mortality. | ||||
| 1.1 Flexible sigmoidoscopy | 5 | 414754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.79] |
| 1.2 Faecal occult blood testing ‐ all studies | 4 | 329642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.82, 0.92] |
| 1.3 Faecal occult blood testing ‐ biennial screening only | 4 | 305583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.93] |
| 2 Colorectal cancer incidence Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Screening procedures versus control ‐ all studies, Outcome 2 Colorectal cancer incidence. | ||||
| 2.1 Flexible sigmoidoscopy | 5 | 414754 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 2.2 Faecal occult blood testing ‐ all studies | 4 | 329516 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.02] |
| 2.3 Faecal occult blood testing ‐ biennial testing only | 4 | 305515 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.90, 1.07] |
| 3 All‐cause Mortality Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3 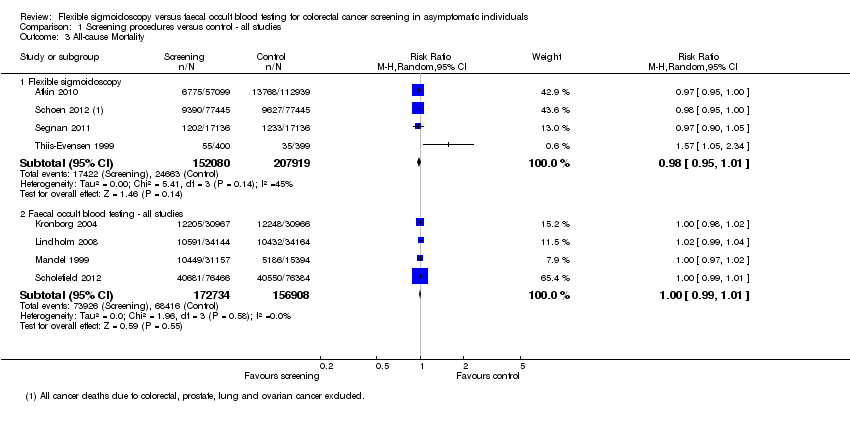 Comparison 1 Screening procedures versus control ‐ all studies, Outcome 3 All‐cause Mortality. | ||||
| 3.1 Flexible sigmoidoscopy | 4 | 359999 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 3.2 Faecal occult blood testing ‐ all studies | 4 | 329642 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.99, 1.01] |
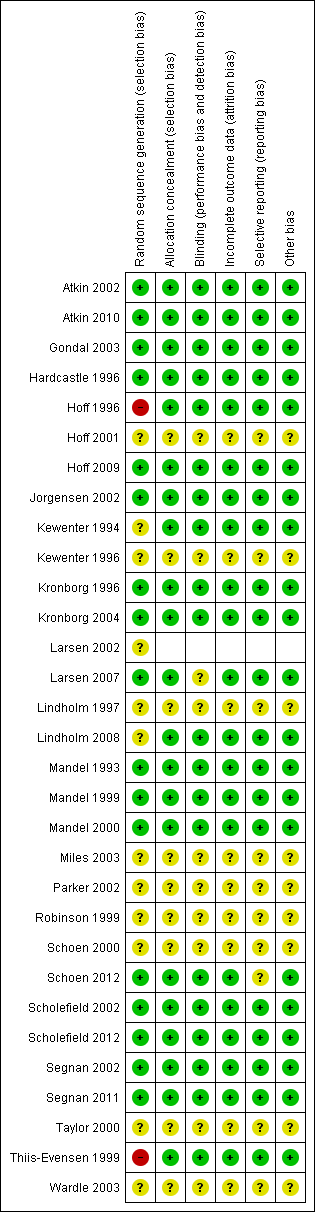
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Screening procedures versus control ‐ all studies, Outcome 1 Colorectal cancer mortality.

Comparison 1 Screening procedures versus control ‐ all studies, Outcome 2 Colorectal cancer incidence.

Comparison 1 Screening procedures versus control ‐ all studies, Outcome 3 All‐cause Mortality.
| Flexible sigmoidoscopy or faecal occult blood testing compared with care as usual for colorectal cancer screening | ||||||
| Patient or population: Asymptomatic individuals Settings: Participants recruited among volunteers or randomly chosen from public registries Intervention: Flexible sigmoidoscopy once only or repeated faecal occult blood testing Comparison: Care as usual | ||||||
| Outcomes | Illustrative comparative risks1 (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No screening | Screening group | |||||
| CRC Mortality ‐ Flexible sigmoidoscopy | 8 per 1000 | 6 per 1000 | RR 0,72 (0.65 to 0.79) | 414,744 | ⊕⊕⊕⊕ | |
| CRC Mortality ‐ Faecal occult blood testing | 8 per 1000 | 7 per 1000 | RR 0,86 (0.80 to 0.92) | 329,642 | ⊕⊕⊕⊕ | |
| CRC incidence ‐ Flexible sigmoidoscopy | 20 per 1000 | 16 per 1000 | RR 0,82 (0.73 to 0.90) | 414,744 | ⊕⊕⊕⊝ | |
| CRC incidence ‐ Faecal occult blood testing | 20 per 1000 | 19 per 1000 | RR 0,95 (0,88 to 1,02) | 329,536 | ⊕⊕⊕⊕ | |
| All‐cause Mortality ‐ Flexible sigmoidoscopy | 254 per 1000 | 249 per 1000 | RR 0,98 (0.95 to 1.01) | 364,827 | ⊕⊕⊕⊕ | |
| All‐cause Mortality ‐ Faecal occult blood testing | 254 per 1000 | 254 per 1000 | RR 1,00 (0,99 to 1,01) | 329,642 | ⊕⊕⊕⊕ | |
| CI: Confidence interval; RR: Risk Ratio; CRC: Colorectal cancer | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assumed risk is computed by combining events and participants in the control groups in all trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 2 Evidence downgraded one level due to heterogeneity between trials. This heterogeneity may be explained by shorter follow‐up of the Norwegian NORCCAP trial, but other explanations like study design cannot be ruled out. | ||||||
| Country | Design | Screening modality | Study period | Age | Control group (n) | Screening group (n) | Men/Women (n) | Compliance+ (%) | Follow‐up (years) |
| United States | Volunteers
| gFOBT | 1975‐1992* | 50‐80 | 15394 | A:15570 B:15587 | 22367/24184 | A: 90 B: 90 | 18 |
| England | Population based
| gFOBT | 1981‐1995 | 45‐74 | 76384 | 76466 | 72172/78079++ | 59 | Median 11.7 |
| Denmark | Population based
| gFOBT | 1985‐2002 | 45‐75 | 30966 | 30967 | 29714/32219 | 67 | 17 |
| Sweden | Population based
| gFOBT | 1982‐1995 | 60‐64 | 34164 | 34144 | NR | 70 | Median 15.5 |
| United Kingdom | Volunteers
| FS | 1994‐1999 | 55‐64 | 112939 | 57099 | 83331/86707 | 71 | Median 11.2 |
| United States | Volunteers | FS | 1993‐2001 | 55‐74 | 77455 | 77445 | 76684/78216 | Single: 87 Dual: 51 | Mortality: median 12.1 Incidence: median 11.9 |
| Italy | Volunteers
| FS | 1995‐1999 | 55‐64 | 17136 | 17136 | 17234/17168 | 58 | Mortality: median 11.4 Incidence: median 10.5 |
| Norway (NORCCAP) | Population based
| FS | 1999‐2000 | 55‐64 | 41092 | 13653 | 50%** | 65 | Mortality: Median 6 Incidence: median 7 |
| Norway (TPS) | Population based
| FS | 1983 | 50‐59 | 399 | 400 | 400/399 | 81 | 13 |
| Characteristics of included studies. *Hiatus in screening 1982‐1986, ** Actual figures not reported, A: Annual screening, B: Biennial screening, NR: Not reported, gFOBT: guaiac faecal occult blood test. FS: Flexible Sigmoidoscopy +At least 1 round in FOBT trials, ++Sum of men and women does not equal sum of screening and control group due to difference in reporting, please see characteristics of included studies for further explanation. | |||||||||
|
| Screening group Duke classification | Control group Duke classification | ||||||
| Country | A | B | C | D | A | B | C | D |
| FOBT trials | ||||||||
| United States (annual screening) | 107/354 (30%) | 101/354 (29%) | 80/354 (23%) | 33/354 (9%) | 88/394 (22%) | 120/394 (30%) | 82/394 (21 %) | 65/394 (17%) |
| United States (biennial screening) | 98/368 (27%) | 95/368 (26%) | 100/368 (27%) | 41/368 (11%) | 88/394 (22%) | 120/394 (30%) | 82/394 (21%) | 65/394 (17%) |
| England | 181/893 (20%) | 286/893 (32%) | 215/893 (24%) | 192/893 (22%) | 95/856 (11%) | 285/856 (33%) | 264/856 (31%) | 179/856 (21%) |
| Denmark | 105/481 (22%) | 164/481 (34%) | 90/481 (19%) | 98/481 (20%) | 54/483 (11%) | 177/483 (37%) | 111/483 (23%) | 114/483 (24%) |
| Sweden | 124/721 (17%) | 261/721 (36%) | 184/721 (26%) | 152/721 (21%) | 112/754 (15%) | 260/721 (35%) | 221/754 (29%) | 161/754 (21%) |
| Flexible sigmoidoscopy trials | ||||||||
| United Kingdom | NR | |||||||
| United States | 574/955 (60%) | 381/955 (40%) | 716/1253 (57%) | 537/1253 (43%) | ||||
| Italy* | 139/251 (55%) | 112/251 (45%) | 154/306 (50%) | 152/306 (50%) | ||||
| Norway+ (NORCCAP) | 33/123 (27%) | 78/123 (63%) | 62/362 (17%) | 262/362 (72%) | ||||
| Norway (TPS) | 1/2 (50%) | 0/2 | 1/2 (50%) | 0/2 | 0/10 | 5/10 (50%) | 3/10 (30%) | 2/10 (20%) |
| Stages of colorectal cancers diagnosed in the screening and control groups. *Cancers classified according to the Union for International Cancer Control as non‐advanced (Stage I and II) or advanced (Stage III and IV). Non‐advanced cancers equals Duke A and B. Advanced cancers equals Duke C and D. +The Norwegian NORCCAP trial classified cancers according to a modified Duke classification system. Duke A and B cancers were classified as “localized”, but Duke B cancers infiltrating neighbouring organs without distant metastasis were classified as “advanced”. NR: Not reported. | ||||||||
| Study | Flexible sigmoidoscopy | Colonoscopy | Bleeding1 | Perforation | Death<30days of procedure2 | Death <30 days of surgery | Major complications3 | Miscellaneous |
| United States (gFOBT) | 12246 | 11 | 4 | NR | NR | NR | NR | |
| United States (FS) | 107236 | NR | 3 | NR | NR | NR | NR | |
| 17672 | NR | 19 | NR | NR | NR | NR | ||
| England (gFOBT) | 1474 | 1 | 5 | 0 | 5 4 | 0 | 15 | |
| Sweden (gFOBT) | 2108 | 0 | 3 | 0 | 0 | 0 | 1415
| |
| 190 | 1 | 2 | 0 | 0 | 0 | |||
| Norway (FS, NORCCAP)6 | 12960 | 0 | 0 | NR | 0 | 0 | 387 | |
| 2524 | 4 | 6 | NR | 0 | 0 | 417 | ||
| United Kingdom (FS) | 403328 | 129 | 1 | 6 10 | 4 11 | 312
| 1313 1727 | |
| 2377 | 9 | 4 | 1 14 | 77 | ||||
| Italy (FS) | 9911 | 0 | 1 | NR | NR | NR | 607 | |
| 775 | 1 | 1 | NR | NR | NR | 307 | ||
| Norway (FS, TPS) | 324 | 0 | 0 | 0 | 0 | 0 | NR | |
| 302 | 0 | 0 | 0 | 0 | 0 | NR | ||
| TOTAL | 172871 | 12 | 5 | 6 | 4 | 3
| 376
| |
| 37560 | 27 | 22 | 1 | 5 | ||||
| 1 Those admitted to hospital due to bleeding 2 Death within 30 days of endoscopic screening or work‐up 3 Bleeding, perforation and death excluded 4 Myocardial infarction, 1 anastomotic leak, 2 pulmonary embolus, 1 carcinomatosis 5 Snare entrapment 6 Includes individuals aged 50‐64 years 7 Minor events not requiring hospitalisation 8 342 individuals had a baseline colonoscopy screening procedure due to strong family history of CRC and is included in the colonoscopy figures 9 Includes 3 individuals with glutaraldehyde colitis 10 3 myocardial infarction, 1 cardiomyopathy, 1 intracerebral haemorrhage, 1 lung cancer 11 2 cardiovascular, 1 respiratory, 1 septicaemia 12 2 myocardial infarction, 1 pulmonary embolus 13 5 cases of definite glutaraldehyde colitis and 8 probable cases 14 Myocardial infarction 15 14 patients who had a laparotomy had complications which prolonged their hospital stay FS: Flexible sigmoidoscopy. gFOBT: Faecal occult blood test. NORCCAP: Norwegian colorectal cancer prevention trial. TPS: Telemark polyp study | ||||||||
|
| Screening group | Control group | Risk ratio (95% CI)
| |||||
| Study | Screening modality | Personyear | Deaths (n) | Deaths/100000py | Personyear | Deaths (n) | Deaths/100000py | |
| US (annual) | gFOBT | 240325 | 121 | 50 | 237420 | 177 | 75 | 0.68 (0.54‐0.96) |
| US (biennial) | gFOBT | 240163 | 148 | 61 | 237420 | 177 | 75 | 0.83 (0.66‐1.03) |
| England | gFOBT | 1296712 | 1176 | 91 | 1296614 | 1300 | 100 | 0.91 (0.84‐0.98) |
| Denmark | gFOBT | 431190 | 362 | 84 | 430755 | 431 | 100 | 0.84 (0.73‐0.96) |
| Sweden | gFOBT | 471072 | 252 | 53 | 471980 | 300 | 64 | 0.84 (0.71‐0.99) |
| UK | FS | 620045 | 189 | 30 | 1224523 | 538 | 44 | 0.69 (0.59‐0.82) |
| US | FS | 868966* | 252 | 29 | 874358* | 341 | 39 | 0.74 (0.63‐0.88) |
| Italy | FS | 186745 | 65 | 35 | 187532 | 83 | 44 | 0.78 (0.57‐1.08) |
| Norway (NORCCAP) | FS | NR | 24 | NR | NR | 99 | NR | 0.73 (0.47‐1.14) |
| Norway (TPS) | FS | NR | 1 | NR | NR | 3 | NR | 0.33 (0.03‐3.18) |
| Mortality rates in screening and control groups, NR: Not reported; gFOBT: guaiac faecal occult blood test; FS: Flexible sigmoidoscopy; py: person year; US: United States; UK: United Kingdom. *Estimated numbers. | ||||||||
|
| Screening group | Control group | Risk ratio (95% CI) | |||||
| Study | Screening modality | Personyear | Cases (n) | Cases/100000py | Personyear | Cases (n) | Cases/100000py | |
| US (annual) | gFOBT | 235584 | 417 | 177 | 232612 | 507 | 218 | 0.81 (0.72‐0.92) |
| US (biennial) | gFOBT | 235513 | 435 | 184 | 232612 | 507 | 218 | 0.85 (0.75‐0.96) |
| England | gFOBT | 1286526 | 2279 | 177 | 1286877 | 2354 | 183 | 0.97 (0.91‐1.03) |
| Denmark | gFOBT | 431190 | 889 | 206 | 430755 | 874 | 203 | 1.02 (0.93‐1.12) |
| Sweden | gFOBT | 471072 | 721 | 153 | 471980 | 754 | 160 | 1.10 (0.99‐1.22) |
| UK | FS | 620045 | 706 | 114 | 1224523 | 1818 | 148 | 0.77 (0.70‐0.84) |
| US | FS | 850420* | 1012 | 119 | 846710* | 1287 | 152 | 0.78 (0.72‐0.85) |
| Italy | FS | 174177 | 251 | 144 | 173437 | 306 | 176 | 0.82 (0.70‐0.97) |
| Norway (NORCCAP) | FS | 91449* | 123 | 135 | 274242* | 362 | 132 | 1.02 (0.83‐1.25) |
| Norway (TPS) | FS | NR | 2 | NR | NR | 10 | NR | 0.20 (0.04‐0.90)** |
| Incidence rates in screening and control groups. *Estimated numbers; **From publication; gFOBT: guaiac faecal occult blood test; FS: Flexible sigmoidoscopy; py:person year; CI: Confidence interval; US: United States; UK: United Kingdom; NR: Not reported. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Colorectal cancer mortality Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Flexible sigmoidoscopy | 5 | 414754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.79] |
| 1.2 Faecal occult blood testing ‐ all studies | 4 | 329642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.82, 0.92] |
| 1.3 Faecal occult blood testing ‐ biennial screening only | 4 | 305583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.93] |
| 2 Colorectal cancer incidence Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Flexible sigmoidoscopy | 5 | 414754 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 2.2 Faecal occult blood testing ‐ all studies | 4 | 329516 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.02] |
| 2.3 Faecal occult blood testing ‐ biennial testing only | 4 | 305515 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.90, 1.07] |
| 3 All‐cause Mortality Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Flexible sigmoidoscopy | 4 | 359999 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 3.2 Faecal occult blood testing ‐ all studies | 4 | 329642 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.99, 1.01] |