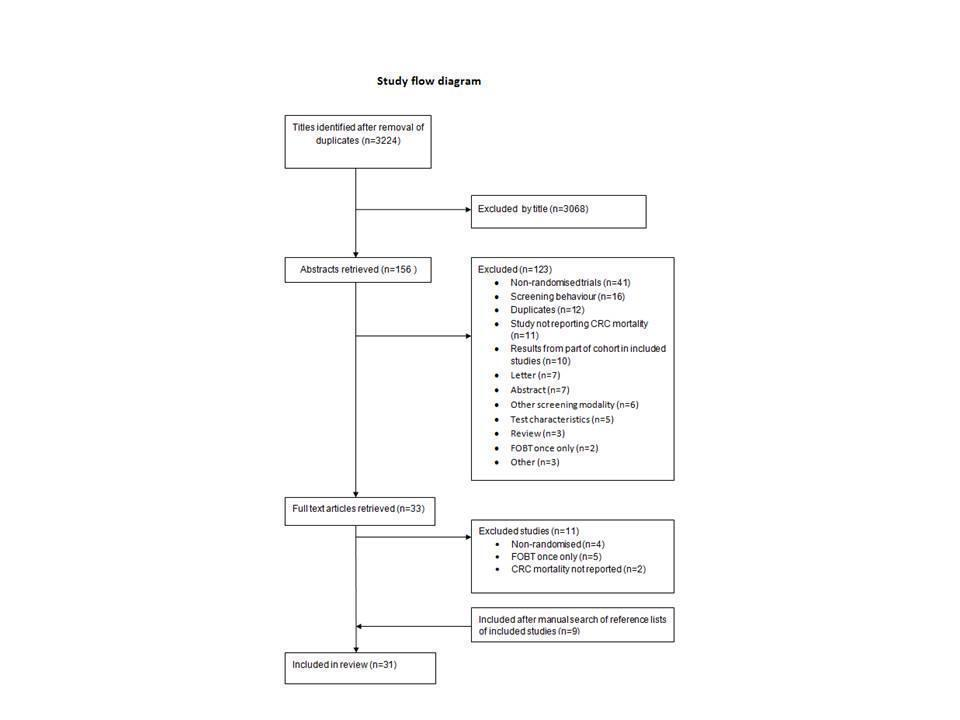
Sigmoidoscopia flexible versus prueba de sangre oculta en materia fecal para la detección del cáncer colorrectal en personas asintomáticas
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009259.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 01 octubre 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Colorrectal
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
AF and MB had the original idea for the review. OH was responsible for drafting the protocol and the first draft of the review. OH and MB performed data extraction and assessed risk of bias of included studies. JOJ was responsible for statistical analyses. All authors participated in writing the manuscript, interpretation of results and approval of the final version of the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Sorlandet Hospital Kristiansand, Norway.
Financial support for OH.
Declarations of interest
GH is the principal investigator of the NORCCAP and TPS trials. MB and OH are co‐investigators of the NORCCAP trial.
Acknowledgements
We thank Marija Barbateskovic at the Cochrane Colorectal Cancer Group for help with the literature search and Torbjorn Wisloeff at the Norwegian Knowledge Centre for the Health Services for valuable comments.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Oct 01 | Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals | Review | Øyvind Holme, Michael Bretthauer, Atle Fretheim, Jan Odgaard‐Jensen, Geir Hoff | |
| 2011 Aug 10 | Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals | Protocol | Oeyvind Holme, Michael Bretthauer, Atle Fretheim, Geir Hoff, Jan Odgaard‐Jensen | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
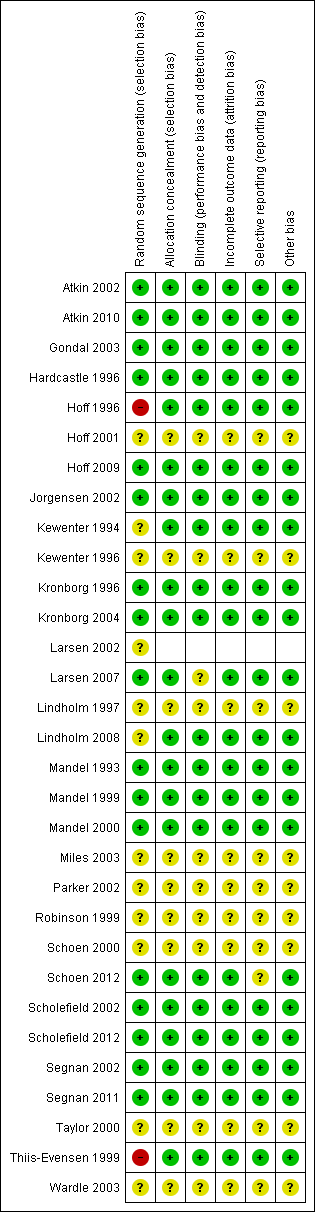
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Screening procedures versus control ‐ all studies, Outcome 1 Colorectal cancer mortality.

Comparison 1 Screening procedures versus control ‐ all studies, Outcome 2 Colorectal cancer incidence.

Comparison 1 Screening procedures versus control ‐ all studies, Outcome 3 All‐cause Mortality.
| Flexible sigmoidoscopy or faecal occult blood testing compared with care as usual for colorectal cancer screening | ||||||
| Patient or population: Asymptomatic individuals Settings: Participants recruited among volunteers or randomly chosen from public registries Intervention: Flexible sigmoidoscopy once only or repeated faecal occult blood testing Comparison: Care as usual | ||||||
| Outcomes | Illustrative comparative risks1 (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No screening | Screening group | |||||
| CRC Mortality ‐ Flexible sigmoidoscopy | 8 per 1000 | 6 per 1000 | RR 0,72 (0.65 to 0.79) | 414,744 | ⊕⊕⊕⊕ | |
| CRC Mortality ‐ Faecal occult blood testing | 8 per 1000 | 7 per 1000 | RR 0,86 (0.80 to 0.92) | 329,642 | ⊕⊕⊕⊕ | |
| CRC incidence ‐ Flexible sigmoidoscopy | 20 per 1000 | 16 per 1000 | RR 0,82 (0.73 to 0.90) | 414,744 | ⊕⊕⊕⊝ | |
| CRC incidence ‐ Faecal occult blood testing | 20 per 1000 | 19 per 1000 | RR 0,95 (0,88 to 1,02) | 329,536 | ⊕⊕⊕⊕ | |
| All‐cause Mortality ‐ Flexible sigmoidoscopy | 254 per 1000 | 249 per 1000 | RR 0,98 (0.95 to 1.01) | 364,827 | ⊕⊕⊕⊕ | |
| All‐cause Mortality ‐ Faecal occult blood testing | 254 per 1000 | 254 per 1000 | RR 1,00 (0,99 to 1,01) | 329,642 | ⊕⊕⊕⊕ | |
| CI: Confidence interval; RR: Risk Ratio; CRC: Colorectal cancer | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assumed risk is computed by combining events and participants in the control groups in all trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 2 Evidence downgraded one level due to heterogeneity between trials. This heterogeneity may be explained by shorter follow‐up of the Norwegian NORCCAP trial, but other explanations like study design cannot be ruled out. | ||||||
| Country | Design | Screening modality | Study period | Age | Control group (n) | Screening group (n) | Men/Women (n) | Compliance+ (%) | Follow‐up (years) |
| United States | Volunteers
| gFOBT | 1975‐1992* | 50‐80 | 15394 | A:15570 B:15587 | 22367/24184 | A: 90 B: 90 | 18 |
| England | Population based
| gFOBT | 1981‐1995 | 45‐74 | 76384 | 76466 | 72172/78079++ | 59 | Median 11.7 |
| Denmark | Population based
| gFOBT | 1985‐2002 | 45‐75 | 30966 | 30967 | 29714/32219 | 67 | 17 |
| Sweden | Population based
| gFOBT | 1982‐1995 | 60‐64 | 34164 | 34144 | NR | 70 | Median 15.5 |
| United Kingdom | Volunteers
| FS | 1994‐1999 | 55‐64 | 112939 | 57099 | 83331/86707 | 71 | Median 11.2 |
| United States | Volunteers | FS | 1993‐2001 | 55‐74 | 77455 | 77445 | 76684/78216 | Single: 87 Dual: 51 | Mortality: median 12.1 Incidence: median 11.9 |
| Italy | Volunteers
| FS | 1995‐1999 | 55‐64 | 17136 | 17136 | 17234/17168 | 58 | Mortality: median 11.4 Incidence: median 10.5 |
| Norway (NORCCAP) | Population based
| FS | 1999‐2000 | 55‐64 | 41092 | 13653 | 50%** | 65 | Mortality: Median 6 Incidence: median 7 |
| Norway (TPS) | Population based
| FS | 1983 | 50‐59 | 399 | 400 | 400/399 | 81 | 13 |
| Characteristics of included studies. *Hiatus in screening 1982‐1986, ** Actual figures not reported, A: Annual screening, B: Biennial screening, NR: Not reported, gFOBT: guaiac faecal occult blood test. FS: Flexible Sigmoidoscopy +At least 1 round in FOBT trials, ++Sum of men and women does not equal sum of screening and control group due to difference in reporting, please see characteristics of included studies for further explanation. | |||||||||
|
| Screening group Duke classification | Control group Duke classification | ||||||
| Country | A | B | C | D | A | B | C | D |
| FOBT trials | ||||||||
| United States (annual screening) | 107/354 (30%) | 101/354 (29%) | 80/354 (23%) | 33/354 (9%) | 88/394 (22%) | 120/394 (30%) | 82/394 (21 %) | 65/394 (17%) |
| United States (biennial screening) | 98/368 (27%) | 95/368 (26%) | 100/368 (27%) | 41/368 (11%) | 88/394 (22%) | 120/394 (30%) | 82/394 (21%) | 65/394 (17%) |
| England | 181/893 (20%) | 286/893 (32%) | 215/893 (24%) | 192/893 (22%) | 95/856 (11%) | 285/856 (33%) | 264/856 (31%) | 179/856 (21%) |
| Denmark | 105/481 (22%) | 164/481 (34%) | 90/481 (19%) | 98/481 (20%) | 54/483 (11%) | 177/483 (37%) | 111/483 (23%) | 114/483 (24%) |
| Sweden | 124/721 (17%) | 261/721 (36%) | 184/721 (26%) | 152/721 (21%) | 112/754 (15%) | 260/721 (35%) | 221/754 (29%) | 161/754 (21%) |
| Flexible sigmoidoscopy trials | ||||||||
| United Kingdom | NR | |||||||
| United States | 574/955 (60%) | 381/955 (40%) | 716/1253 (57%) | 537/1253 (43%) | ||||
| Italy* | 139/251 (55%) | 112/251 (45%) | 154/306 (50%) | 152/306 (50%) | ||||
| Norway+ (NORCCAP) | 33/123 (27%) | 78/123 (63%) | 62/362 (17%) | 262/362 (72%) | ||||
| Norway (TPS) | 1/2 (50%) | 0/2 | 1/2 (50%) | 0/2 | 0/10 | 5/10 (50%) | 3/10 (30%) | 2/10 (20%) |
| Stages of colorectal cancers diagnosed in the screening and control groups. *Cancers classified according to the Union for International Cancer Control as non‐advanced (Stage I and II) or advanced (Stage III and IV). Non‐advanced cancers equals Duke A and B. Advanced cancers equals Duke C and D. +The Norwegian NORCCAP trial classified cancers according to a modified Duke classification system. Duke A and B cancers were classified as “localized”, but Duke B cancers infiltrating neighbouring organs without distant metastasis were classified as “advanced”. NR: Not reported. | ||||||||
| Study | Flexible sigmoidoscopy | Colonoscopy | Bleeding1 | Perforation | Death<30days of procedure2 | Death <30 days of surgery | Major complications3 | Miscellaneous |
| United States (gFOBT) | 12246 | 11 | 4 | NR | NR | NR | NR | |
| United States (FS) | 107236 | NR | 3 | NR | NR | NR | NR | |
| 17672 | NR | 19 | NR | NR | NR | NR | ||
| England (gFOBT) | 1474 | 1 | 5 | 0 | 5 4 | 0 | 15 | |
| Sweden (gFOBT) | 2108 | 0 | 3 | 0 | 0 | 0 | 1415
| |
| 190 | 1 | 2 | 0 | 0 | 0 | |||
| Norway (FS, NORCCAP)6 | 12960 | 0 | 0 | NR | 0 | 0 | 387 | |
| 2524 | 4 | 6 | NR | 0 | 0 | 417 | ||
| United Kingdom (FS) | 403328 | 129 | 1 | 6 10 | 4 11 | 312
| 1313 1727 | |
| 2377 | 9 | 4 | 1 14 | 77 | ||||
| Italy (FS) | 9911 | 0 | 1 | NR | NR | NR | 607 | |
| 775 | 1 | 1 | NR | NR | NR | 307 | ||
| Norway (FS, TPS) | 324 | 0 | 0 | 0 | 0 | 0 | NR | |
| 302 | 0 | 0 | 0 | 0 | 0 | NR | ||
| TOTAL | 172871 | 12 | 5 | 6 | 4 | 3
| 376
| |
| 37560 | 27 | 22 | 1 | 5 | ||||
| 1 Those admitted to hospital due to bleeding 2 Death within 30 days of endoscopic screening or work‐up 3 Bleeding, perforation and death excluded 4 Myocardial infarction, 1 anastomotic leak, 2 pulmonary embolus, 1 carcinomatosis 5 Snare entrapment 6 Includes individuals aged 50‐64 years 7 Minor events not requiring hospitalisation 8 342 individuals had a baseline colonoscopy screening procedure due to strong family history of CRC and is included in the colonoscopy figures 9 Includes 3 individuals with glutaraldehyde colitis 10 3 myocardial infarction, 1 cardiomyopathy, 1 intracerebral haemorrhage, 1 lung cancer 11 2 cardiovascular, 1 respiratory, 1 septicaemia 12 2 myocardial infarction, 1 pulmonary embolus 13 5 cases of definite glutaraldehyde colitis and 8 probable cases 14 Myocardial infarction 15 14 patients who had a laparotomy had complications which prolonged their hospital stay FS: Flexible sigmoidoscopy. gFOBT: Faecal occult blood test. NORCCAP: Norwegian colorectal cancer prevention trial. TPS: Telemark polyp study | ||||||||
|
| Screening group | Control group | Risk ratio (95% CI)
| |||||
| Study | Screening modality | Personyear | Deaths (n) | Deaths/100000py | Personyear | Deaths (n) | Deaths/100000py | |
| US (annual) | gFOBT | 240325 | 121 | 50 | 237420 | 177 | 75 | 0.68 (0.54‐0.96) |
| US (biennial) | gFOBT | 240163 | 148 | 61 | 237420 | 177 | 75 | 0.83 (0.66‐1.03) |
| England | gFOBT | 1296712 | 1176 | 91 | 1296614 | 1300 | 100 | 0.91 (0.84‐0.98) |
| Denmark | gFOBT | 431190 | 362 | 84 | 430755 | 431 | 100 | 0.84 (0.73‐0.96) |
| Sweden | gFOBT | 471072 | 252 | 53 | 471980 | 300 | 64 | 0.84 (0.71‐0.99) |
| UK | FS | 620045 | 189 | 30 | 1224523 | 538 | 44 | 0.69 (0.59‐0.82) |
| US | FS | 868966* | 252 | 29 | 874358* | 341 | 39 | 0.74 (0.63‐0.88) |
| Italy | FS | 186745 | 65 | 35 | 187532 | 83 | 44 | 0.78 (0.57‐1.08) |
| Norway (NORCCAP) | FS | NR | 24 | NR | NR | 99 | NR | 0.73 (0.47‐1.14) |
| Norway (TPS) | FS | NR | 1 | NR | NR | 3 | NR | 0.33 (0.03‐3.18) |
| Mortality rates in screening and control groups, NR: Not reported; gFOBT: guaiac faecal occult blood test; FS: Flexible sigmoidoscopy; py: person year; US: United States; UK: United Kingdom. *Estimated numbers. | ||||||||
|
| Screening group | Control group | Risk ratio (95% CI) | |||||
| Study | Screening modality | Personyear | Cases (n) | Cases/100000py | Personyear | Cases (n) | Cases/100000py | |
| US (annual) | gFOBT | 235584 | 417 | 177 | 232612 | 507 | 218 | 0.81 (0.72‐0.92) |
| US (biennial) | gFOBT | 235513 | 435 | 184 | 232612 | 507 | 218 | 0.85 (0.75‐0.96) |
| England | gFOBT | 1286526 | 2279 | 177 | 1286877 | 2354 | 183 | 0.97 (0.91‐1.03) |
| Denmark | gFOBT | 431190 | 889 | 206 | 430755 | 874 | 203 | 1.02 (0.93‐1.12) |
| Sweden | gFOBT | 471072 | 721 | 153 | 471980 | 754 | 160 | 1.10 (0.99‐1.22) |
| UK | FS | 620045 | 706 | 114 | 1224523 | 1818 | 148 | 0.77 (0.70‐0.84) |
| US | FS | 850420* | 1012 | 119 | 846710* | 1287 | 152 | 0.78 (0.72‐0.85) |
| Italy | FS | 174177 | 251 | 144 | 173437 | 306 | 176 | 0.82 (0.70‐0.97) |
| Norway (NORCCAP) | FS | 91449* | 123 | 135 | 274242* | 362 | 132 | 1.02 (0.83‐1.25) |
| Norway (TPS) | FS | NR | 2 | NR | NR | 10 | NR | 0.20 (0.04‐0.90)** |
| Incidence rates in screening and control groups. *Estimated numbers; **From publication; gFOBT: guaiac faecal occult blood test; FS: Flexible sigmoidoscopy; py:person year; CI: Confidence interval; US: United States; UK: United Kingdom; NR: Not reported. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Colorectal cancer mortality Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Flexible sigmoidoscopy | 5 | 414754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.79] |
| 1.2 Faecal occult blood testing ‐ all studies | 4 | 329642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.82, 0.92] |
| 1.3 Faecal occult blood testing ‐ biennial screening only | 4 | 305583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.93] |
| 2 Colorectal cancer incidence Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Flexible sigmoidoscopy | 5 | 414754 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 2.2 Faecal occult blood testing ‐ all studies | 4 | 329516 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.02] |
| 2.3 Faecal occult blood testing ‐ biennial testing only | 4 | 305515 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.90, 1.07] |
| 3 All‐cause Mortality Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Flexible sigmoidoscopy | 4 | 359999 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 3.2 Faecal occult blood testing ‐ all studies | 4 | 329642 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.99, 1.01] |