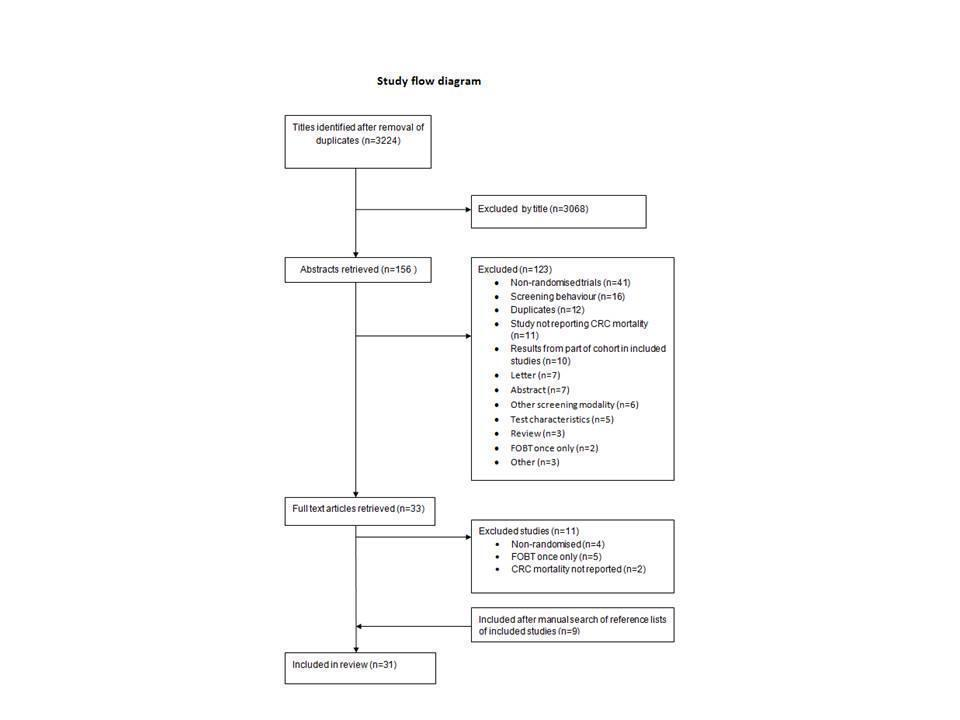
Porównanie giętkiej sigmoidoskopii z badaniem na krew utajoną w stolcu jako badań przesiewowych dla wczesnego wykrywania raka jelita grubego u osób bezobjawowych
Appendices
Appendix 1. MEDLINE (faecal occult blood) search strategy
#1 exp Colorectal Neoplasms/
#2 exp Colonic Neoplasms/
#3 exp Rectal Neoplasms/
#4 ((colorectal* or CRC or colon* or bowel* or rectal or rectum or sigmoid or anal or anus) and (cancer or neoplasm* or tumor* or tumour or carcinom* or sarcom* or adenocarcinom* or adeno?carcinom* or adenom* or lesion*)).mp.
#5 1 or 2 or 3 or 4
#6 exp Occult Blood/
#7 exp Immunochemistry/
#8 (faecal or fecal or feces or faeces or gFOBT or FOBT or FOB or FIT or haemoccult or hemoccult or sensa or heamoccultsensa or hemocare or hema screen or hemascreen or hemacheck or hema check or hemawipe or hema wipe or hemofec or hemofecia or fecatest or fecatwin or coloscreen or seracult or ez?detect or colocare or flexsure or hemmoquant or immocare or hemochaser or bayer detect or hemeselect or immudia or monohaem or insure or hemodia or instant?view or immocare or magstream or guaiac or occult blood or (stool adj3 occult) or (gaiac* adj2 smear*)).mp.
#9 ((((immunochemical* adj3 (test* or screen* or diagn*)) or immunologic*) adj3 (test* or screen* or diagn*)) or enzyme or EIA or assay or RPHA or latex or agglutin* or monocl* or polyclo*).mp.
#10 6 or 7 or 8 or 9
#11 exp Mass Screening/
#12 exp Population Surveillance/
#13 (screen* or test* or (population* adj2 surveillance) or (early adj3 detect*) or (early adj3 prevent*)).mp.
#14 11 or 12 or 13
#15 5 and 10 and 14
#16 randomized controlled trial.pt.
#17 controlled clinical trial.pt.
#18 randomized.ab.
#19 placebo.ab.
#20 clinical trial.sh.
#21 randomly.ab.
#22 trial.ti.
#23 16 or 17 or 18 or 19 or 20 or 21 or 22
#24 humans.sh.
#25 23 and 24
#26 15 and 25
Appendix 2. MEDLINE (flexible sigmoidoscopy) search strategy
#1 exp Colorectal Neoplasms/
#2 exp Colonic Neoplasms/
#3 exp Rectal Neoplasms/
#4 ((colorectal* or CRC or colon* or bowel* or intestine* or large intestine* or rectal or rectum or sigmoid or anal or anus) and (cancer or neoplasm* or malign* or tumor* or tumour* or carcinom* or sarcom* or adenocarcinom* or adeno?carcinom* or adenom* or lesion*)).mp.
#5 1 or 2 or 3 or 4
#6 exp Endoscopy, Gastrointestinal/
#7 exp Colonoscopy/
#8 exp Sigmoidoscopy/
#9 exp Proctoscopy/
#10 (endoscop* or proctoscop* or colonoscop* or sigmoidoscop* or rectosigmoidoscop* or proctosigmoidoscop* or COL or SIG or FSIG or (flex* adj3 sig*)).mp.
#11 6 or 7 or 8 or 9 or 10
#12 exp Mass Screening/
#13 exp Population Surveillance/
#14 (screen* or test* or (population* adj2 surveillance) or (early adj3 detect*) or (early adj3 prevent*)).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier]
#15 12 or 13 or 14
#16 5 and 11 and 15
#17 randomized controlled trial.pt.
#18 controlled clinical trial.pt.
#19 randomized.ab.
#20 placebo.ab.
#21 clinical trial.sh.
#22 randomly.ab.
#23 trial.ti.
#24 17 or 18 or 19 or 20 or 21 or 22 or 23
#25 humans.sh.
#26 24 and 25
#27 16 and 26
Appendix 3. EMBASE (faecal occult blood) search strategy
#1 exp colorectal tumor/
#2 exp colorectal cancer/
#3 exp colorectal carcinoma/
#4 exp colorectal adenoma/
#5 exp colon tumor/
#6 exp colon cancer/
#7 exp colon carcinoma/
#8 exp colon adenoma/
#9 exp colon adenocarcinoma/
#10 exp rectum tumor/
#11 exp rectum cancer/
#12 exp rectum carcinoma/
#13 exp rectum adenoma/
#14 ((colorectal* or CRC or colon or colonic or bowel* or intestine or large intestine or rectal or rectum or sigmoid or anal or anus) and (cancer or neoplasm* or malign* or tumor* or tumour or carcinom* or sarcom* or adenocarcinom* or adeno?carcinom* or adenom* or lesion*)).m_titl.
#15 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
#16 exp occult blood/
#17 exp feces analysis/
#18 exp immunochemistry/
#19 (faecal or fecal or feces or faeces or gFOBT or FOBT or FOB or FIT or haemoccult or hemoccult or sensa or heamoccultsensa or hemocare or hema screen or hemascreen or hemacheck or hema check or hemawipe or hema wipe or hemofec or hemofecia or fecatest or fecatwin or coloscreen or seracult or ez?detect or colocare or flexsure or hemmoquant or immocare or hemochaser or bayer detect or hemeselect or immudia or monohaem or insure or hemodia or instant?view or immocare or magstream or guaiac or occult blood or (stool adj3 occult) or (gaiac* adj2 smear*)).mp.
#20 ((((immunochemical* adj3 (test* or screen* or diagn*)) or immunologic*) adj3 (test* or screen* or diagn*)) or enzyme or EIA or assay or RPHA or latex or agglutin* or monocl* or polyclo*).mp.
#21 16 or 17 or 18 or 19 or 20
#22 exp mass screening/
#23 exp health survey/
#24 (screen* or test* or (population* adj2 surveillance) or (early adj3 detect*) or (early adj3 prevent*)).m_titl.
#25 22 or 23 or 24
#26 15 and 21 and 25
#27 randomized controlled trial/
#28 randomization/
#29 controlled study/
#30 multicenter study/
#31 phase 3 clinical trial/
#32 phase 4 clinical trial/
#33 "human*".ti,ab.
#34 (animal* or nonhuman*).ti,ab.
#35 27 or 28 or 29 or 30 or 31 or 32
#36 33 and 34
#37 34 not 33
#38 35 not 37
#39 26 and 38
#40 (canin* or dog* or rodent* or rat* or mouse or mice* or animal* or mammal* or mice* or bird* or fish* or trout*).m_titl.
#41 39 not 40
Appendix 4. EMBASE (flexible sigmoidoscopy) search strategy
#1 exp colorectal cancer/
#2 exp colorectal tumor/
#3 exp colorectal carcinoma/
#4 exp colorectal adenoma/
#5 exp colon cancer/
#6 exp colon carcinoma/
#7 exp colon cancer/
#8 exp colon adenoma/
#9 exp colon adenocarcinoma/
#10 exp colon tumor/
#11 exp rectum cancer/
#12 exp rectum tumor/
#13 exp rectum carcinoma/
#14 exp rectum adenoma/
#15 ((colorectal* or CRC or colon or colonic or bowel* or intestine or large intestine or rectal or rectum or sigmoid or anal or anus) and (cancer or neoplasm* or malign* or tumor* or tumour* or carcinom* or sarcom* or adenocarcinom* or adeno?carcinom* or adenom* or lesion*)).m_titl.
#16 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
#17 exp gastrointestinal endoscopy/
#18 exp colonoscopy/
#19 exp sigmoidoscopy/
#20 exp rectoscopy/
#21 (endoscop* or proctoscop* or colonoscop* or sigmoidoscop* or rectosigmoidoscop* or proctosigmoidoscop* or COL or SIG or FSIG or (flex* adj3 sig*)).mp.
#22 17 or 18 or 19 or 20 or 21
#23 exp mass screening/
#24 exp health survey/
#25 (screen* or test* or (population* adj2 surveillance) or (early adj3 detect*) or (early adj3 prevent*)).m_titl.
#26 23 or 24 or 25
#27 16 and 22 and 26
#28 randomized controlled trial/
#29 randomization/
#30 controlled study/
#31 multicenter study/
#32 phase 3 clinical trial/
#33 phase 4 clinical trial/
#34 "human*".ti,ab.
#35 (animal* or nonhuman*).ti,ab.
#36 28 or 29 or 30 or 31 or 32 or 33
#37 34 and 35
#38 35 not 37
#39 36 not 38
#40 27 and 39
#41 (canin* or dog* or rodent* or rat* or mouse or mice* or animal* or mammal* or mice* or #bird* or fish* or trout*).m_titl.
#42 40 not 41
Appendix 5. The Cochrane Library (faecal occult blood) search strategy
#1 MeSH descriptor Colorectal Neoplasms explode all trees
#2 MeSH descriptor Colonic Neoplasms explode all trees
#3 MeSH descriptor Rectal Neoplasms explode all trees
#4 (colorectal* or CRC or colon* or bowel* or intestine* or large intestine* or rectal or rectum or sigmoid or anal or anus) and (cancer or neoplasm* or malign* or tumor or tumour* or carcinom* or sarcom* or adenocarcinom* or adeno?carcinom* or adenom* or lesion*
#5 (#1 OR #2 OR #3 OR #4)
#6 MeSH descriptor Occult Blood explode all trees
#7 MeSH descriptor Immunochemistry explode all trees
#8 (faecal or fecal or feces or faeces or gFOBT or FOBT or FOB or FIT or haemoccult or hemoccult or sensa) or (heamoccultsensa or hemocare or hema screen or hemascreen or hemacheck or hema check or hemawipe or hema wipe) or (hemofec or hemofecia or fecatest or fecatwin or coloscreen or seracult or ez?detect or colocare or flexsure) or (hemmoquant or immocare or hemochaser or bayer detect or hemeselect or immudia or monohaem or insure or hemodia or instant?view or magstream or guaiac or occult blood) or (stool near3 occult) or (gaiac* near2 smear*)
#9 (immunochemical* near3 (test* or screen* or diagn*)) or (immunologic* near3 (test* or screen* or diagn*)) or (enzyme or EIA or assay or RPHA or latex or agglutin* or monocl* or polyclo*)
#10 (#6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Mass Screening explode all trees
#12 MeSH descriptor Population Surveillance explode all trees
#13 (screen* or test*) or (population* near2 surveillance) or (early near3 detect*) or (early near3 prevent*)
#14 (#11 OR #12 OR #13)
#15 (#5 AND #10 AND #14)
Appendix 6. The Cochrane Library (flexible sigmoidoscopy) search strategy
#1 MeSH descriptor Colorectal Neoplasms explode all trees
#2 MeSH descriptor Colonic Neoplasms explode all trees
#3 MeSH descriptor Rectal Neoplasms explode all trees
#4 (colorectal* or CRC or colon* or bowel* or intestine* or large intestine* or rectal or rectum or sigmoid or anal or anus) and (cancer or neoplasm* or malign* or tumor* or tumour* or carcinom* or sarcom* or adenocarcinom* or adeno?carcinom* or adenom* or lesion*
#5 (#1 OR #2 OR #3 OR #4)
#6 MeSH descriptor Endoscopy explode all trees
#7 MeSH descriptor Colonoscopy explode all trees
#8 MeSH descriptor Sigmoidoscopy explode all trees
#9 MeSH descriptor Proctoscopy explode all trees
#10 (endoscop* or proctoscop* or colonoscop* or sigmoidoscop* or rectosigmoidoscop* or proctosigmoidoscop* or COL or SIG or FSIG) or (flex* near3 sig*)
#11 (#6 OR #7 OR #8 OR #9 OR #10)
#12 MeSH descriptor Mass Screening explode all trees
#13 MeSH descriptor Population Surveillance explode all trees
#14 (screen* or test*) or (population* near2 surveillance) or (early near3 detect*) or (early near3 prevent*)
#15 (#12 OR #13 OR #14)
#16 (#5 AND #11 AND #15)
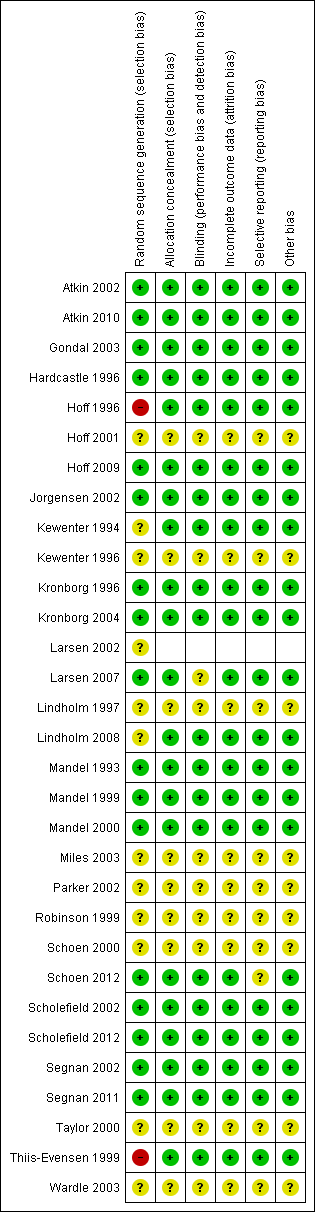
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Screening procedures versus control ‐ all studies, Outcome 1 Colorectal cancer mortality.

Comparison 1 Screening procedures versus control ‐ all studies, Outcome 2 Colorectal cancer incidence.

Comparison 1 Screening procedures versus control ‐ all studies, Outcome 3 All‐cause Mortality.
| Flexible sigmoidoscopy or faecal occult blood testing compared with care as usual for colorectal cancer screening | ||||||
| Patient or population: Asymptomatic individuals Settings: Participants recruited among volunteers or randomly chosen from public registries Intervention: Flexible sigmoidoscopy once only or repeated faecal occult blood testing Comparison: Care as usual | ||||||
| Outcomes | Illustrative comparative risks1 (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No screening | Screening group | |||||
| CRC Mortality ‐ Flexible sigmoidoscopy | 8 per 1000 | 6 per 1000 | RR 0,72 (0.65 to 0.79) | 414,744 | ⊕⊕⊕⊕ | |
| CRC Mortality ‐ Faecal occult blood testing | 8 per 1000 | 7 per 1000 | RR 0,86 (0.80 to 0.92) | 329,642 | ⊕⊕⊕⊕ | |
| CRC incidence ‐ Flexible sigmoidoscopy | 20 per 1000 | 16 per 1000 | RR 0,82 (0.73 to 0.90) | 414,744 | ⊕⊕⊕⊝ | |
| CRC incidence ‐ Faecal occult blood testing | 20 per 1000 | 19 per 1000 | RR 0,95 (0,88 to 1,02) | 329,536 | ⊕⊕⊕⊕ | |
| All‐cause Mortality ‐ Flexible sigmoidoscopy | 254 per 1000 | 249 per 1000 | RR 0,98 (0.95 to 1.01) | 364,827 | ⊕⊕⊕⊕ | |
| All‐cause Mortality ‐ Faecal occult blood testing | 254 per 1000 | 254 per 1000 | RR 1,00 (0,99 to 1,01) | 329,642 | ⊕⊕⊕⊕ | |
| CI: Confidence interval; RR: Risk Ratio; CRC: Colorectal cancer | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assumed risk is computed by combining events and participants in the control groups in all trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 2 Evidence downgraded one level due to heterogeneity between trials. This heterogeneity may be explained by shorter follow‐up of the Norwegian NORCCAP trial, but other explanations like study design cannot be ruled out. | ||||||
| Country | Design | Screening modality | Study period | Age | Control group (n) | Screening group (n) | Men/Women (n) | Compliance+ (%) | Follow‐up (years) |
| United States | Volunteers
| gFOBT | 1975‐1992* | 50‐80 | 15394 | A:15570 B:15587 | 22367/24184 | A: 90 B: 90 | 18 |
| England | Population based
| gFOBT | 1981‐1995 | 45‐74 | 76384 | 76466 | 72172/78079++ | 59 | Median 11.7 |
| Denmark | Population based
| gFOBT | 1985‐2002 | 45‐75 | 30966 | 30967 | 29714/32219 | 67 | 17 |
| Sweden | Population based
| gFOBT | 1982‐1995 | 60‐64 | 34164 | 34144 | NR | 70 | Median 15.5 |
| United Kingdom | Volunteers
| FS | 1994‐1999 | 55‐64 | 112939 | 57099 | 83331/86707 | 71 | Median 11.2 |
| United States | Volunteers | FS | 1993‐2001 | 55‐74 | 77455 | 77445 | 76684/78216 | Single: 87 Dual: 51 | Mortality: median 12.1 Incidence: median 11.9 |
| Italy | Volunteers
| FS | 1995‐1999 | 55‐64 | 17136 | 17136 | 17234/17168 | 58 | Mortality: median 11.4 Incidence: median 10.5 |
| Norway (NORCCAP) | Population based
| FS | 1999‐2000 | 55‐64 | 41092 | 13653 | 50%** | 65 | Mortality: Median 6 Incidence: median 7 |
| Norway (TPS) | Population based
| FS | 1983 | 50‐59 | 399 | 400 | 400/399 | 81 | 13 |
| Characteristics of included studies. *Hiatus in screening 1982‐1986, ** Actual figures not reported, A: Annual screening, B: Biennial screening, NR: Not reported, gFOBT: guaiac faecal occult blood test. FS: Flexible Sigmoidoscopy +At least 1 round in FOBT trials, ++Sum of men and women does not equal sum of screening and control group due to difference in reporting, please see characteristics of included studies for further explanation. | |||||||||
|
| Screening group Duke classification | Control group Duke classification | ||||||
| Country | A | B | C | D | A | B | C | D |
| FOBT trials | ||||||||
| United States (annual screening) | 107/354 (30%) | 101/354 (29%) | 80/354 (23%) | 33/354 (9%) | 88/394 (22%) | 120/394 (30%) | 82/394 (21 %) | 65/394 (17%) |
| United States (biennial screening) | 98/368 (27%) | 95/368 (26%) | 100/368 (27%) | 41/368 (11%) | 88/394 (22%) | 120/394 (30%) | 82/394 (21%) | 65/394 (17%) |
| England | 181/893 (20%) | 286/893 (32%) | 215/893 (24%) | 192/893 (22%) | 95/856 (11%) | 285/856 (33%) | 264/856 (31%) | 179/856 (21%) |
| Denmark | 105/481 (22%) | 164/481 (34%) | 90/481 (19%) | 98/481 (20%) | 54/483 (11%) | 177/483 (37%) | 111/483 (23%) | 114/483 (24%) |
| Sweden | 124/721 (17%) | 261/721 (36%) | 184/721 (26%) | 152/721 (21%) | 112/754 (15%) | 260/721 (35%) | 221/754 (29%) | 161/754 (21%) |
| Flexible sigmoidoscopy trials | ||||||||
| United Kingdom | NR | |||||||
| United States | 574/955 (60%) | 381/955 (40%) | 716/1253 (57%) | 537/1253 (43%) | ||||
| Italy* | 139/251 (55%) | 112/251 (45%) | 154/306 (50%) | 152/306 (50%) | ||||
| Norway+ (NORCCAP) | 33/123 (27%) | 78/123 (63%) | 62/362 (17%) | 262/362 (72%) | ||||
| Norway (TPS) | 1/2 (50%) | 0/2 | 1/2 (50%) | 0/2 | 0/10 | 5/10 (50%) | 3/10 (30%) | 2/10 (20%) |
| Stages of colorectal cancers diagnosed in the screening and control groups. *Cancers classified according to the Union for International Cancer Control as non‐advanced (Stage I and II) or advanced (Stage III and IV). Non‐advanced cancers equals Duke A and B. Advanced cancers equals Duke C and D. +The Norwegian NORCCAP trial classified cancers according to a modified Duke classification system. Duke A and B cancers were classified as “localized”, but Duke B cancers infiltrating neighbouring organs without distant metastasis were classified as “advanced”. NR: Not reported. | ||||||||
| Study | Flexible sigmoidoscopy | Colonoscopy | Bleeding1 | Perforation | Death<30days of procedure2 | Death <30 days of surgery | Major complications3 | Miscellaneous |
| United States (gFOBT) | 12246 | 11 | 4 | NR | NR | NR | NR | |
| United States (FS) | 107236 | NR | 3 | NR | NR | NR | NR | |
| 17672 | NR | 19 | NR | NR | NR | NR | ||
| England (gFOBT) | 1474 | 1 | 5 | 0 | 5 4 | 0 | 15 | |
| Sweden (gFOBT) | 2108 | 0 | 3 | 0 | 0 | 0 | 1415
| |
| 190 | 1 | 2 | 0 | 0 | 0 | |||
| Norway (FS, NORCCAP)6 | 12960 | 0 | 0 | NR | 0 | 0 | 387 | |
| 2524 | 4 | 6 | NR | 0 | 0 | 417 | ||
| United Kingdom (FS) | 403328 | 129 | 1 | 6 10 | 4 11 | 312
| 1313 1727 | |
| 2377 | 9 | 4 | 1 14 | 77 | ||||
| Italy (FS) | 9911 | 0 | 1 | NR | NR | NR | 607 | |
| 775 | 1 | 1 | NR | NR | NR | 307 | ||
| Norway (FS, TPS) | 324 | 0 | 0 | 0 | 0 | 0 | NR | |
| 302 | 0 | 0 | 0 | 0 | 0 | NR | ||
| TOTAL | 172871 | 12 | 5 | 6 | 4 | 3
| 376
| |
| 37560 | 27 | 22 | 1 | 5 | ||||
| 1 Those admitted to hospital due to bleeding 2 Death within 30 days of endoscopic screening or work‐up 3 Bleeding, perforation and death excluded 4 Myocardial infarction, 1 anastomotic leak, 2 pulmonary embolus, 1 carcinomatosis 5 Snare entrapment 6 Includes individuals aged 50‐64 years 7 Minor events not requiring hospitalisation 8 342 individuals had a baseline colonoscopy screening procedure due to strong family history of CRC and is included in the colonoscopy figures 9 Includes 3 individuals with glutaraldehyde colitis 10 3 myocardial infarction, 1 cardiomyopathy, 1 intracerebral haemorrhage, 1 lung cancer 11 2 cardiovascular, 1 respiratory, 1 septicaemia 12 2 myocardial infarction, 1 pulmonary embolus 13 5 cases of definite glutaraldehyde colitis and 8 probable cases 14 Myocardial infarction 15 14 patients who had a laparotomy had complications which prolonged their hospital stay FS: Flexible sigmoidoscopy. gFOBT: Faecal occult blood test. NORCCAP: Norwegian colorectal cancer prevention trial. TPS: Telemark polyp study | ||||||||
|
| Screening group | Control group | Risk ratio (95% CI)
| |||||
| Study | Screening modality | Personyear | Deaths (n) | Deaths/100000py | Personyear | Deaths (n) | Deaths/100000py | |
| US (annual) | gFOBT | 240325 | 121 | 50 | 237420 | 177 | 75 | 0.68 (0.54‐0.96) |
| US (biennial) | gFOBT | 240163 | 148 | 61 | 237420 | 177 | 75 | 0.83 (0.66‐1.03) |
| England | gFOBT | 1296712 | 1176 | 91 | 1296614 | 1300 | 100 | 0.91 (0.84‐0.98) |
| Denmark | gFOBT | 431190 | 362 | 84 | 430755 | 431 | 100 | 0.84 (0.73‐0.96) |
| Sweden | gFOBT | 471072 | 252 | 53 | 471980 | 300 | 64 | 0.84 (0.71‐0.99) |
| UK | FS | 620045 | 189 | 30 | 1224523 | 538 | 44 | 0.69 (0.59‐0.82) |
| US | FS | 868966* | 252 | 29 | 874358* | 341 | 39 | 0.74 (0.63‐0.88) |
| Italy | FS | 186745 | 65 | 35 | 187532 | 83 | 44 | 0.78 (0.57‐1.08) |
| Norway (NORCCAP) | FS | NR | 24 | NR | NR | 99 | NR | 0.73 (0.47‐1.14) |
| Norway (TPS) | FS | NR | 1 | NR | NR | 3 | NR | 0.33 (0.03‐3.18) |
| Mortality rates in screening and control groups, NR: Not reported; gFOBT: guaiac faecal occult blood test; FS: Flexible sigmoidoscopy; py: person year; US: United States; UK: United Kingdom. *Estimated numbers. | ||||||||
|
| Screening group | Control group | Risk ratio (95% CI) | |||||
| Study | Screening modality | Personyear | Cases (n) | Cases/100000py | Personyear | Cases (n) | Cases/100000py | |
| US (annual) | gFOBT | 235584 | 417 | 177 | 232612 | 507 | 218 | 0.81 (0.72‐0.92) |
| US (biennial) | gFOBT | 235513 | 435 | 184 | 232612 | 507 | 218 | 0.85 (0.75‐0.96) |
| England | gFOBT | 1286526 | 2279 | 177 | 1286877 | 2354 | 183 | 0.97 (0.91‐1.03) |
| Denmark | gFOBT | 431190 | 889 | 206 | 430755 | 874 | 203 | 1.02 (0.93‐1.12) |
| Sweden | gFOBT | 471072 | 721 | 153 | 471980 | 754 | 160 | 1.10 (0.99‐1.22) |
| UK | FS | 620045 | 706 | 114 | 1224523 | 1818 | 148 | 0.77 (0.70‐0.84) |
| US | FS | 850420* | 1012 | 119 | 846710* | 1287 | 152 | 0.78 (0.72‐0.85) |
| Italy | FS | 174177 | 251 | 144 | 173437 | 306 | 176 | 0.82 (0.70‐0.97) |
| Norway (NORCCAP) | FS | 91449* | 123 | 135 | 274242* | 362 | 132 | 1.02 (0.83‐1.25) |
| Norway (TPS) | FS | NR | 2 | NR | NR | 10 | NR | 0.20 (0.04‐0.90)** |
| Incidence rates in screening and control groups. *Estimated numbers; **From publication; gFOBT: guaiac faecal occult blood test; FS: Flexible sigmoidoscopy; py:person year; CI: Confidence interval; US: United States; UK: United Kingdom; NR: Not reported. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Colorectal cancer mortality Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Flexible sigmoidoscopy | 5 | 414754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.79] |
| 1.2 Faecal occult blood testing ‐ all studies | 4 | 329642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.82, 0.92] |
| 1.3 Faecal occult blood testing ‐ biennial screening only | 4 | 305583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.93] |
| 2 Colorectal cancer incidence Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Flexible sigmoidoscopy | 5 | 414754 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 2.2 Faecal occult blood testing ‐ all studies | 4 | 329516 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.02] |
| 2.3 Faecal occult blood testing ‐ biennial testing only | 4 | 305515 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.90, 1.07] |
| 3 All‐cause Mortality Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Flexible sigmoidoscopy | 4 | 359999 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 3.2 Faecal occult blood testing ‐ all studies | 4 | 329642 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.99, 1.01] |