氯巴占单药治疗局灶性或全面性癫痫发作
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Open‐label randomized controlled trial | |
| Participants | Inclusion criteria: diagnosis of benign focal epilepsy of childhood with centro‐temporal spikes by ILAE criteria and EEG evidence; 6 or more seizures/month or "considerable parental concern" about seizure(s) Exclusion criteria: people with neurologic disorders other than benign focal epilepsy of childhood with centro‐temporal spikes; history of pseudoseizures; use of psychotropic drugs; metabolic disorders; active infection or malignancy; previous treatment with clobazam or carbamazepine in which therapy stopped because of adverse effects or a diagnosis that prevented randomization or continuation in the study No. randomized: clobazam 18, carbamazepine 25 Duration of trial: 96 weeks Follow‐up: at 1, 2, 3, 4, 5, 6, 20, 14, 18, 22, 26, 30, 34, 40, 48, 56, 64, 72, 80, 88, and 96 weeks Setting: single center, out‐patient clinic, from Cuba | |
| Interventions | 1. Clobazam 1 mg/kg/d for the first week, increased by 0.25 mg/kg/d every week, until a maximum dose of 2 mg/kg/d is reached 2. Carbamazepine 10 mg/kg/d in 3 divided doses for the first week, increased by 5 mg/kg/d every week, until a maximum dose of 30 mg/kg/d is reached | |
| Outcomes | 1. Number of participants that remained in each treatment group 2. Number of participants with more than 50% reduction in seizures after 4 weeks of treatment 3. Number of participants who were free of seizures after week 4, between weeks 4 to 40, and in the last 9 months of treatment 4. Average time in days it took to achieve seizure freedom 5. Number of adverse events 6. Performance on neuropsychological testing; academic performance; behavioral/conduct problems; grade of satisfaction of treatment from parents and teachers | |
| Notes | 1. Retention time (although measured at pre‐specified time points) is mentioned as an outcome in methods section, but not provided in the results 2. Ethics committee approval: yes 3. Informed consent: yes 4. Sample size calculation: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not mentioned in study |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned in study |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “open, controlled and randomized study" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not mentioned in study |
| Incomplete outcome data (attrition bias) | High risk | Quote: “The number of patients that completed the study was 21 (84%) in the group assigned carbamazepine and 17 (94.4%) in the group with clobazam.” |
| Selective reporting (reporting bias) | Unclear risk | Comment: no pre‐published protocol |
| Other bias | Low risk | No baseline imbalance between participants assigned to clobazam vs carbamazepine; no use of imprecise instruments for outcome measurements |
| Methods | Double‐blind randomized controlled trial | |
| Participants | Inclusion criteria: age 6 months‐17 years; diagnosis of epilepsy (≥ 2 unprovoked seizures) by a child neurologist; seizure types of focal, focal with secondary generalization, or primary generalized tonic clonic Exclusion criteria: seizure type: absence, atonic, tonic, or myoclonic seizures; progressive neurological disease; other serious chronic illness; previous history of poor drug compliance with anti‐epileptic drugs No. randomized: clobazam: 63; carbamazepine: 52 Duration of trial: 4 years Follow‐up: 3 and 6 weeks; 3, 6 and 12 months Setting: 15 Canadian pediatric epilepsy centers | |
| Interventions | 1. Clobazam: aim 0.5 mg/kg/d; average maximum dose 0.6 mg/kg/d 2. Carbamazepine: aim 10 mg/kg/d; average maximum dose 14.8 mg/kg/d Study medication was introduced over 1‐3 weeks. Both were given as 2 daily doses | |
| Outcomes | Not classified into primary and secondary. End point was defined as retention on the study medication for 12 months or discontinuation of the medication for any reason, including side effects or inadequate seizure control. | |
| Notes | 1. The study had 3 arms: a. drug‐naive b. previous failure with carbamazepine c. previous failure with other anti‐seizure medications Only the first arm is included for analysis in this review 2. Demographic characteristics Age range: this was not provided; instead the number of participants belonging to arbitrary age groups (< 6 years, 6 to ≤ 12 years and > 12 years) were given for all drug‐naïve participants together and not separately for those receiving clobazam versus carbamazepine Gender: Boys 66, distribution between groups is not stated 3. Ethics committee approval: yes 4. Informed consent: yes 5. Sample size calculation: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Within each study arm, drug assignment was randomized using a permuted block technique with a depth of 6 for each study center" Comment: mentioned in the study |
| Allocation concealment (selection bias) | Unclear risk | Comment: mot mentioned in study |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The study was double blind using a modified 'double dummy' technique" Comment: mentioned in the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not specified in the study |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "For the primary analysis, all data were analyzed according to the intention‐to‐treat principle, including all randomized patients." Comment: mentioned in the study |
| Selective reporting (reporting bias) | Unclear risk | No pre‐published protocol |
| Other bias | Unclear risk | Funding from Hoechst‐Marion‐Roussel Canada, Inc. which also monitored the data collection and entry |
| Methods | Randomized open‐label trial | |
| Participants | Inclusion criteria: age > 12 years; recent onset (< 2 weeks) of generalized seizures or focal seizures with or without secondary generalization; not already on antiepileptic drug treatment; imaging demonstrated a solitary cysticercus granuloma Exclusion criteria: concomitant serious systemic disorder; pregnant women; unwilling to comply with the follow‐up schedule; presenting in status epilepticus or seizure clusters (2 or more seizures in 24 h prior to presentation) No. randomized: clobazam 21, phenytoin 27 (see risk of bias table) Age range: phenytoin: 12‐66 years, clobazam: 12‐46 years Gender: Male participants 17 in phenytoin group, 14 in clobazam group Duration of trial: 14 months Follow‐up: 1, 2, 3 and 6 months Setting: Outpatient Neurology Clinic of an Indian teaching hospital | |
| Interventions | 1. Clobazam 1 mg/kg oral loading followed by 0.5 mg/kg/d 2. Phenytoin 15 mg/kg oral loading in 3 divided doses over 24 h, followed by 0.5 mg/kg/d Both medications for at least 6 months None of the participants received any cysticidal treatment | |
| Outcomes | Primary: total number of treatment failures in each group during 6 months of follow‐up. Treatment failure was defined as breakthrough seizure or adverse effect that required discontinuation or modification of study medication either with increased dose or with additional anti‐seizure medication Secondary: frequency of adverse effects (not explicitly mentioned) | |
| Notes | 1. Ethics committee approval: yes 2. Informed consent: not stated 3. Sample size calculation: yes (see risk of bias table) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized strictly according to computer‐generated random list" Comment: mentioned in the study |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned in study, authors contacted |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open labelled trial" Comment: mentioned in the study |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: " In addition to the treating physician, an independent research team member evaluated seizure control and adverse effects at follow‐up. He was blinded to the treatment arms for the duration of study" Comment: mentioned in the study |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomized participants were analyzed |
| Selective reporting (reporting bias) | Unclear risk | No prepublished protocol |
| Other bias | Unclear risk | 1.Outcome was assessed by the treating physician and a blinded research team member, but they do not report how a consensus was reached in case of disagreement. 2. No report on whether and how informed consent was obtained 3. There is a discrepancy in the number of participants randomized to receive each study treatment. Whereas in the CONSORT flow chart 27 and 21 participants were reported to be respectively randomized to clobazam and phenytoin, these numbers were exactly reversed in the text and subsequent table comparing baseline characteristics and outcome measures 4. Estimated that a sample size of 270 was needed to detect a 10% difference in the primary outcome measure with a 90% confidence, however, study was terminated early with an actual sample of only 48 due to relocation of a study investigator |
EEG: electroencephalogram; ILAE: International League Against Epilepsy
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| It included children with febrile seizures, which are acute symptomatic seizure treated with short‐term intermittent clobazam therapy | |
| The group randomized to receive clobazam also included children who have failed carbamazepine or discontinued one or more other AED | |
| Used clobazam as a short‐term intermittent treatment | |
| Used clobazam as a short‐term intermittent treatment | |
| Uses clobazam as a short‐term intermittent treatment | |
| Case series, not a randomized controlled clinical trial | |
| Used clobazam as add‐on therapy (not monotherapy) | |
| It included children with febrile seizures, which are acute symptomatic seizure treated with short‐term intermittent clobazam therapy | |
| It included participants with refractory epilepsy and used clobazam as add‐on therapy (not monotherapy) | |
| It included children with febrile seizures, which are acute symptomatic seizure treated with short‐term intermittent clobazam therapy | |
| It included children with febrile seizures, which are acute symptomatic seizure treated with short‐term intermittent clobazam therapy | |
| It included participants with refractory epilepsy and used clobazam as add‐on therapy (not monotherapy) | |
| It included participants with refractory epilepsy and used clobazam as add‐on therapy (not monotherapy) | |
| It included participants with refractory epilepsy and used clobazam as add‐on therapy (not monotherapy) |
AED: anti‐epileptic drug
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention at 12 months Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.61, 1.12] |
| Analysis 1.1 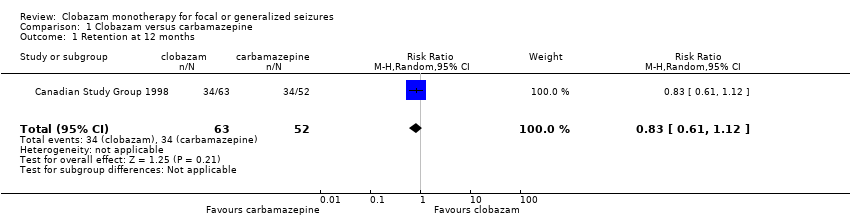 Comparison 1 Clobazam versus carbamazepine, Outcome 1 Retention at 12 months. | ||||
| 2 Seizure freedom at 4 weeks Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.55, 1.32] |
| Analysis 1.2  Comparison 1 Clobazam versus carbamazepine, Outcome 2 Seizure freedom at 4 weeks. | ||||
| 3 Seizure freedom between weeks 4 and 40 Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.36] |
| Analysis 1.3 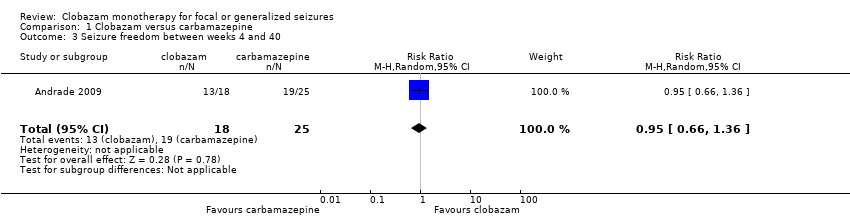 Comparison 1 Clobazam versus carbamazepine, Outcome 3 Seizure freedom between weeks 4 and 40. | ||||
| 4 Terminal remission at 9 months (seizure free for last 9 months) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.87, 1.72] |
| Analysis 1.4  Comparison 1 Clobazam versus carbamazepine, Outcome 4 Terminal remission at 9 months (seizure free for last 9 months). | ||||
| 5 50% responder rate at 4 weeks Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.48] |
| Analysis 1.5  Comparison 1 Clobazam versus carbamazepine, Outcome 5 50% responder rate at 4 weeks. | ||||
| 6 Reported adverse effects (number of participants) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.16, 1.70] |
| Analysis 1.6  Comparison 1 Clobazam versus carbamazepine, Outcome 6 Reported adverse effects (number of participants). | ||||
| 7 Discontinued study medication due to adverse effects (number of participants) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.02, 10.60] |
| Analysis 1.7  Comparison 1 Clobazam versus carbamazepine, Outcome 7 Discontinued study medication due to adverse effects (number of participants). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention at 6 months Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.08, 1.90] |
| Analysis 2.1  Comparison 2 Clobazam versus phenytoin, Outcome 1 Retention at 6 months. | ||||
| 2 Breakthrough seizure(s) during study period Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.05, 3.83] |
| Analysis 2.2 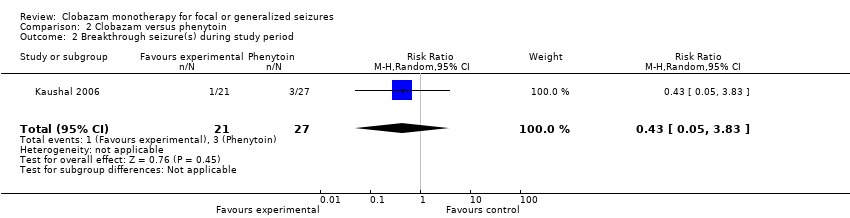 Comparison 2 Clobazam versus phenytoin, Outcome 2 Breakthrough seizure(s) during study period. | ||||
| 3 Discontinued study medication due to adverse effects Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.65] |
| Analysis 2.3 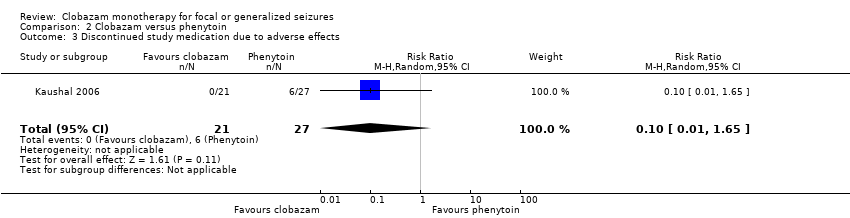 Comparison 2 Clobazam versus phenytoin, Outcome 3 Discontinued study medication due to adverse effects. | ||||
| 4 Reported adverse effects Show forest plot | 1 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.32, 2.14] |
| Analysis 2.4  Comparison 2 Clobazam versus phenytoin, Outcome 4 Reported adverse effects. | ||||
| 4.1 Sedation | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.40, 2.35] |
| 4.2 Skin problems including allergic rash | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.38] |
| 4.3 Tiredness | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.10, 1.91] |
| 4.4 Oral or gingival problems | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.03, 1.65] |
| 4.5 Headache | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.73, 9.09] |
| 4.6 Weight gain | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 11.45 [0.65, 201.60] |

Study flow diagram (results from updated searches)

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
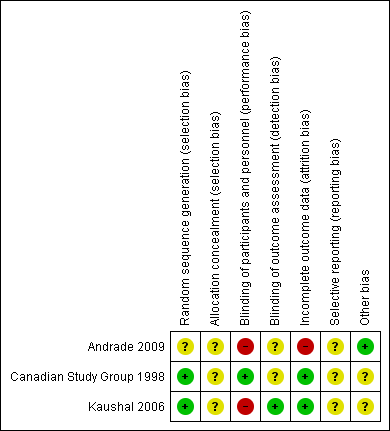
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 2 clobazam vs carbamazepine, outcome: 2.1 retention at 12 months

Forest plot of comparison: 1 clobazam versus phenytoin, outcome: 1.1 retention at 6 months
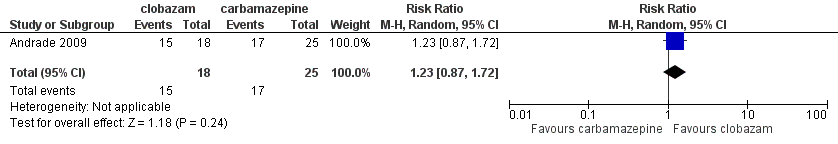
Forest plot of comparison: 1 clobazam versus carbamazepine, outcome: 1.4 terminal remission at 9 months (seizure free for last 9 months)

Comparison 1 Clobazam versus carbamazepine, Outcome 1 Retention at 12 months.
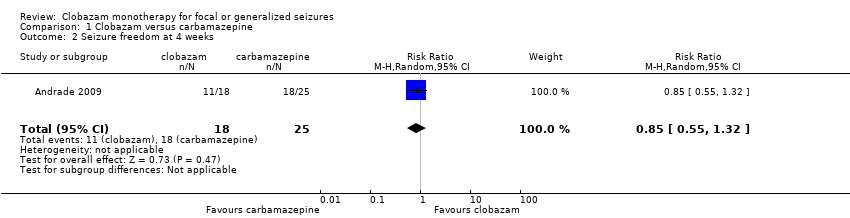
Comparison 1 Clobazam versus carbamazepine, Outcome 2 Seizure freedom at 4 weeks.
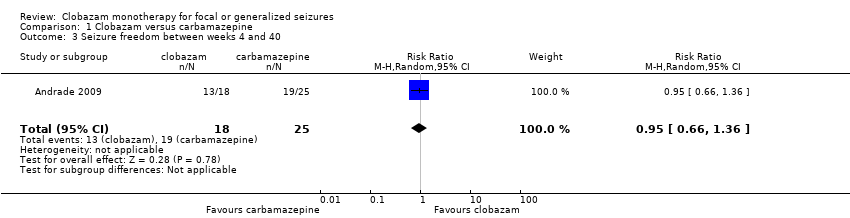
Comparison 1 Clobazam versus carbamazepine, Outcome 3 Seizure freedom between weeks 4 and 40.

Comparison 1 Clobazam versus carbamazepine, Outcome 4 Terminal remission at 9 months (seizure free for last 9 months).

Comparison 1 Clobazam versus carbamazepine, Outcome 5 50% responder rate at 4 weeks.

Comparison 1 Clobazam versus carbamazepine, Outcome 6 Reported adverse effects (number of participants).

Comparison 1 Clobazam versus carbamazepine, Outcome 7 Discontinued study medication due to adverse effects (number of participants).

Comparison 2 Clobazam versus phenytoin, Outcome 1 Retention at 6 months.

Comparison 2 Clobazam versus phenytoin, Outcome 2 Breakthrough seizure(s) during study period.

Comparison 2 Clobazam versus phenytoin, Outcome 3 Discontinued study medication due to adverse effects.

Comparison 2 Clobazam versus phenytoin, Outcome 4 Reported adverse effects.
| Clobazam versus carbamazepine for focal or generalized seizures | ||||||
| Patient or population: people with focal or generalized seizures | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Carbamazepine | Clobazam | |||||
| Time on allocated treatment (retention time) | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Retention at 12 months | Study population | RR 0.83 (0.61 to 1.12) | 115 | ⊕⊕⊝⊝ | ||
| 654 per 1000 | 543 per 1000 (399 to 732) | |||||
| Seizure freedom at 4 weeks | 720 per 1000 | 611 per 1000 (396 to 950) | RR 0.85 (0.55 to 1.32) | 43 (1 study) | low2,3,4 | |
| Seizure freedom 4‐40 weeks | 760 per 1000 | 722 per 1000 (502 to 1034) | RR 0.95 (0.66 to 1.36) | 43 (1 study) | low2,3,4 | |
| 50% responder rate | 800 per 1000 | 944 per 1000 (752 to 1184) | RR 1.18 (0.94 to 1.48) | 43 (1 study) | low2,3,4 | |
| Adverse effects requiring withdrawal/discontinuation of study medication | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Blinding of participants and personnel was not done in this open‐label study. This can potentially affect assessment of this outcome. Downgraded by 1. 4Allocation concealment not stated, which could potentially introduce selection bias. Blinding of participants and personnel was not done in this open‐label study. | ||||||
| Clobazam versus phenytoin for focal or generalized seizures | ||||||
| Patient or population: drug‐naïve people with focal or generalized seizures with solitary cysticercus granuloma Settings: single Indian teaching hospital Intervention: clobazam Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Clobazam versus phenytoin | |||||
| Time on allocated treatment (retention time) | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Retention at 6 months (measured by the number of treatment failures in each group, which was defined as either breakthrough seizure or adverse effects requiring discontinuation or dose modification of study medication) | Study population | RR 1.43 | 48 | ⊕⊕⊝⊝ | ||
| 667 per 1000 | 953 per 1000 (720 to 1267) | |||||
| Seizure freedom at 4 weeks | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Seizure freedom 4‐40 weeks | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| 50% responder rate | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Adverse effects requiring withdrawal/discontinuation of study medication | Study population | RR 0.10 | 48 | ⊕⊕⊝⊝ | ||
| 222 per 1000 | 22 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Allocation concealment not stated, which could potentially introduce selection bias. Blinding of participants and personnel was not done in this open‐label study. This can potentially affect assessment of 'adverse effects requiring withdrawal' and hence 'retention time'. Downgraded by 1. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention at 12 months Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.61, 1.12] |
| 2 Seizure freedom at 4 weeks Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.55, 1.32] |
| 3 Seizure freedom between weeks 4 and 40 Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.36] |
| 4 Terminal remission at 9 months (seizure free for last 9 months) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.87, 1.72] |
| 5 50% responder rate at 4 weeks Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.48] |
| 6 Reported adverse effects (number of participants) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.16, 1.70] |
| 7 Discontinued study medication due to adverse effects (number of participants) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.02, 10.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention at 6 months Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.08, 1.90] |
| 2 Breakthrough seizure(s) during study period Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.05, 3.83] |
| 3 Discontinued study medication due to adverse effects Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.65] |
| 4 Reported adverse effects Show forest plot | 1 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.32, 2.14] |
| 4.1 Sedation | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.40, 2.35] |
| 4.2 Skin problems including allergic rash | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.38] |
| 4.3 Tiredness | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.10, 1.91] |
| 4.4 Oral or gingival problems | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.03, 1.65] |
| 4.5 Headache | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.73, 9.09] |
| 4.6 Weight gain | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 11.45 [0.65, 201.60] |

