Nachtlagerungsschienen für Kinder mit infantiler Zerebralparese
Abstract
Background
Sleep positioning systems can be prescribed for children with cerebral palsy to help reduce or prevent hip migration, provide comfort to ease pain and/or improve sleep. As sleep disturbance is common in children with developmental disabilities, with impact on their carers' sleep, and as sleep positioning systems can be expensive, guidance is needed to support decisions as to their use.
Objectives
To determine whether commercially‐available sleep positioning systems, compared with usual care, reduce or prevent hip migration in children with cerebral palsy. Any negative effect of sleep positioning systems on hip migration will be considered within this objective.
Secondary objectives were to determine the effect of sleep positioning systems on: (1) number or frequency of hip problems; (2) sleep patterns and quality; (3) quality of life of the child and family; (4) pain; and (5) physical functioning. We also sought to identify any adverse effects from using sleep positioning systems.
Search methods
In December 2014, we searched CENTRAL, Ovid MEDLINE, Embase, and 13 other databases. We also searched two trials registers. We applied no restrictions on date of publication, language, publication status or study design. We checked references and contacted manufacturers and authors for potentially relevant literature, and searched the internet using Google.
Selection criteria
We included all randomised controlled trials (RCTs) evaluating whole body sleep positioning systems for children and adolescents (up to 18 years of age) with cerebral palsy.
Data collection and analysis
Two review authors independently screened reports retrieved from the search against pre‐determined inclusion criteria and assessed the quality of eligible studies.
Members of the public (parent carers of children with neurodisability) contributed to this review by suggesting the topic, refining the research objectives, interpreting the findings, and reviewing the plain language summary.
Main results
We did not identify any randomised controlled trials that evaluated the effectiveness of sleep positioning systems on hip migration.
We did find two randomised cross‐over trials that met the inclusion criteria in respect of secondary objectives relating to sleep quality and pain. Neither study reported any important difference between sleeping in sleep positioning systems and not for sleep patterns or sleep quality (two studies, 21 children, very low quality evidence) and pain (one study, 11 children, very low quality evidence). These were small studies with established users of sleep positioning systems and were judged to have high risk of bias.
We found no eligible trials that explored the other secondary objectives (number or frequency of hip problems, quality of life of the child and family, physical functioning, and adverse effects).
Authors' conclusions
We found no randomised trials that evaluated the effectiveness of sleep positioning systems to reduce or prevent hip migration in children with cerebral palsy. Nor did we find any randomised trials that evaluated the effect of sleep positioning systems on the number or frequency of hip problems, quality of life of the child and family or on physical functioning.
Limited data from two randomised trials, which evaluated the effectiveness of sleep positioning systems on sleep quality and pain for children with cerebral palsy, showed no significant differences in these aspects of health when children were using and not using a sleep positioning system.
In order to inform clinical decision‐making and the prescription of sleep positioning systems, more rigorous research is needed to determine effectiveness, cost‐effectiveness, and the likelihood of adverse effects.
PICO
Laienverständliche Zusammenfassung
Nachtlagerungsschienen für Kinder mit infantiler Zerebralparese
Hintergrund
Zahlreiche Kinder mit infantiler Zerebralparese sind von einer Hüftluxation (wenn das obere Ende des Oberschenkelknochens sich langsam vom Beckenknochen wegbewegt und ein Kontaktverlust entsteht) betroffen, die häufig mit Schmerzen einhergeht. Kindern mit infantiler Zerebralparese werden in manchen Fällen, insbesondere bei Gehunfähigkeit, Hilfen empfohlen, die eine Schlafposition herbeiführen, die Hüftluxationen vermindern oder vorbeugen. Diese Lagerungshilfen werden als „Nachtlagerungsschienen“ bezeichnet und können zusammen mit weiteren Hilfsmitteln zur Haltungsstabilisierung tagsüber beim Sitzen und/oder Stehen verschrieben werden. Diese Hilfsmittel werden zusammengefasst als 24‐Stunden‐Programme zur Haltungsstabilisierung bezeichnet.
Angehörige und Fachpersonen im Gesundheitswesen benötigen Informationen über die Wirksamkeit von Nachtlagerungsschienen, um Entscheidungen über deren Einsatz zu erleichtern.
Fragestellung
Ziel dieses Reviews war die Recherche nach stichhaltiger Evidenz aus randomisierten kontrollierten Studien, in denen die Wirksamkeit von Nachtlagerungsschienen für Kinder mit infantiler Zerebralparese bewertet wird. Randomisierte kontrollierte Studien werden mit zwei Personengruppen durchgeführt; die eine Gruppe erhält die Behandlung (experimentelle Gruppe), die andere Gruppe erhält diese nicht (Kontrollgruppe). Anschließend werden die Ergebnisse miteinander verglichen. Um die Vergleichbarkeit zu gewährleisten, erfolgt die Gruppenzuteilung zufällig (randomisiert), sodass etwaige Unterschiede in den Ergebnissen auf die Behandlung zurückzuführen sind (Definition: INVOLVE Jargon Buster).
Studienmerkmale
Wir führten eine umfassende Suche nach Studien durch. Die Evidenz ist auf dem Stand von Dezember 2014.
Hauptergebnisse
Es konnten keine randomisierten kontrollierten Studien zur Bewertung der Alltagswirksamkeit von Nachtlagerungsschienen bei der Verminderung oder Vorbeugung von Hüftluxationen gefunden werden.
In zwei kleinen randomisierten kontrollierten Studien wurde die Schlafqualität von Kindern mit oder ohne Nachtlagerungsschiene verglichen. In einer dieser Studien wurden auch auftretende Schmerzen mit oder ohne Verwendung der Nachtlagerungsschiene untersucht. Diese Studien waren als Cross‐Over‐Studien angelegt (in Cross‐over Studien wird die Wirksamkeit zweier Behandlungsformen verglichen, indem diese zeitlich versetzt den gleichen Probanden verabreicht werden). Die teilnehmenden Kinder verbrachten für diese Studie einige Nächte in ihrer Nachtlagerungsschiene und dann einige Nächte ohne diese, oder in umgekehrter Reihenfolge. Die Reihenfolge, ob zuerst mit oder ohne die Schiene geschlafen wurde, war randomisiert.
An den Studien nahmen 21 Kinder mit infantiler Zerebralparese zwischen 5 und 16 Jahren teil, die daran gewöhnt waren, in Nachtlagerungsschienen zu schlafen. Eine der Studien wurde in einem Schlaflabor durchgeführt, bei der zweiten Studie schliefen die Kinder zu Hause. In keiner der beiden Studien wurden Unterschiede in der Schlafqualität oder im Auftreten von Schmerzen festgestellt, egal ob die Nachtlagerungsschiene verwendet wurde oder nicht. Diese Ergebnisse sind mit Vorsicht zu interpretieren, da in den Studien nur wenige Kinder untersucht wurden und diese bereits an den Gebrauch der Nachtlagerungsschienen gewöhnt waren. Darüber hinaus wiesen die Studien diverse Schwächen im Studienaufbau und ihrer Darstellung auf.
Qualität der Evidenz
Die Qualität der derzeit verfügbaren Evidenz zur Wirksamkeit von Nachtlagerungsschienen bei Kindern mit infantiler Zerebralparese ist sehr niedrig. Es besteht Bedarf an weiteren soliden Forschungsprojekten, um Angehörige und Fachpersonen im Gesundheitswesen dabei zu unterstützen, fundierte Entscheidungen für oder gegen den Einsatz dieser Hilfsmittel zu treffen.
Authors' conclusions
Summary of findings
| Sleeping in a sleep positioning system compared with not sleeping in a sleep positioning system for children with cerebral palsy | |||
| Population: Children with cerebral palsy Settings: United Kingdom (at home or in paediatric research laboratory) Intervention: Sleeping in sleep positioning system Comparison: Not sleeping in sleep positioning system | |||
| Outcomes | Impact | Number of participants | Quality of the evidence |
| Reduce hip migration/hip problems | No RCTs measured effect of sleep positioning systems on hip migration/hip problems | ‐ | ‐ |
| Effect on sleep patterns and quality | Limited data. A small number of established users of sleep positioning systems showed no significant difference in sleep quality indicators | 21 | ⊕⊝⊝⊝ |
| Effect on quality of life of child and family | No RCTs measured effect of sleep positioning systems on child and family quality of life | ‐ | ‐ |
| Effect on pain | Limited data. A small number of established users of sleep positioning systems showed no significant difference in levels of pain | 10 (1 study) | ⊕⊝⊝⊝ |
| Effect on physical functioning | No RCTs measured effect of sleep positioning systems on physical functioning | ‐ | ‐ |
| Adverse effects | No RCTs measured harms or reported adverse events | ‐ | ‐ |
| GRADE Working Group grades of evidence | |||
| GRADE: Grades of Recommendation, Assessment, Development and Evaluation. | |||
Background
Description of the condition
Cerebral palsy affects between 2 and 2.5 per 1000 children and is one of the most common causes of serious childhood physical disability (Stanley 2000). The definition of cerebral palsy as reported by Rosenbaum 2007 is: “Cerebral palsy describes a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non‐progressive disturbances that occurred in the developing fetal or infant brain. The motor disorders of cerebral palsy are often accompanied by disturbances of sensation, perception, cognition, communication, and behaviour, by epilepsy, and by secondary musculoskeletal problems.”
The Surveillance and Cerebral Palsy in Europe group provides a system to classify the types of motor impairment as spastic, dyskinetic (dystonia and choreoathetosis), or ataxic (Cans 2000). Spastic types are further classified according to the distribution as either unilateral or bilateral. However, the terms 'hemiplegia' for unilateral involvement, 'diplegia' when lower limbs are more affected than upper limbs, and 'quadriplegia' for four‐limb 'total body' involvement are also commonly used to describe the topography of spastic cerebral palsy.
The severity of movement disability can be classified into one of five levels using the Gross Motor Function Classification System (GMFCS), which has demonstrated good reliability and validity (Palisano 1997). Children in Level I perform in sitting, standing and walking much as their age‐matched peers, albeit with qualitative differences, while children in Level V have difficulty achieving head and trunk postures, and any voluntary control of movement due to their motor impairment.
Hip displacement is common in children with cerebral palsy and can cause pain and loss of function, and have an effect on personal care (Cornell 1995). Hip displacement refers to the migration of the femoral head laterally from under the acetabulum (Wynter 2008). The amount of displacement is expressed as a migration percentage (hip migration). The risk of hip displacement, defined as an X‐ray measured migration index of greater than 30%, increases linearly by GMFCS Level from negligible in Level I to almost universal in Level V; overall, hip displacement is estimated to affect about a third of children with cerebral palsy (Soo 2006). The risk of scoliosis also appears to increase with greater movement disability (Saito 1998) and spinal deformity is often associated with hip problems (dislocation, dysplasia) and pelvic obliquity.
Hip surveillance is a process using X‐rays and clinical assessment to screen the hips of children to identify and monitor early indicators of progressive hip displacement. It has long been recommended for children with bilateral spastic cerebral palsy who are not walking by 30 months of age (Scrutton 1997). Published guidelines in Australia advocate a more stringent screening programme for all children with cerebral palsy regardless of gross motor functional ability. The guidelines recommend repeated measures at specific intervals and include recommendations to monitor the hip status of a defined group of more severely‐affected children with hemiplegia (Wynter 2008). Surgical management of the hip is considered when the migration index exceeds 30%, and screening combined with early surgical intervention has been proposed to ameliorate hip dislocation (Hägglund 2005).
Non‐surgical interventions that aim to prevent hip migration and displacement include botulinum toxin A or phenol injections to reduce the deforming forces caused by spastic muscles, and also positioning using orthoses and equipment to manage hip and body posture in sitting, standing and lying (NICE 2012).
A consensus statement in 2006 proposed that children in GMFCS Levels IV to V should begin 24‐hour postural management programmes in lying as soon as possible after birth, in sitting from 6 months, and in standing from 12 months (Gericke 2006). Opinions vary though; Gough 2009 queries whether children who are most likely to develop deformity are also those who are least able to adhere to a continuous postural management programme.
Postural management equipment is used to enable and promote symmetry and comfort in sitting, standing and lying. Sometimes, where joint deformity has become established, some level of postural asymmetry can be accommodated (Pountney 2006). Provision of postural management equipment for sitting and standing enables people to experience their environment from a different perspective. In addition, achieving these postures may enable functioning in other ways, for instance using assistive technology to communicate. The use of cushions, pillows, and towels to assist the sleep positioning of children has long been advocated (Bower 2008). However, the provision of commercial sleep positioning systems to support lying postures at night is more controversial.
Reduction or prevention of deformity is a priority for children with cerebral palsy. As hip dislocation cannot be considered an isolated event, and cannot be treated easily, hip migration needs to be considered separately from other deformities (Scrutton 2009). When outcomes are reported to be better when hip surgery is performed as soon as indicated (Morris 2009), delaying surgery to use conservative interventions requires robust evidence. Clinicians and families need to know if sleep positioning systems are effective to reduce or prevent hip migration and which children are most likely to benefit.
Description of the intervention
Sleep positioning systems are commercially‐available, individualised, lying support systems that may contain one or more component parts, which are held in position by a base layer or sheet (Polak 2009). These sleep positioning systems are available in different sizes and are made from a variety of materials, including special foams that conform to body shape or a series of straps that hold the body in a neutral and symmetrical position. The system applies or resists forces to maintain the posture, hence the forces must be applied over maximal surface areas available to minimise pressure, and materials should be such that temperature regulation is not compromised. The idea is that children sleep, positioned in this equipment, with the goal of maintaining one position overnight. While the primary focus of this review was the use of sleep positioning systems to prevent or reduce hip migration, they are also prescribed to improve sleep and provide comfort at night.
Establishing use of sleep positioning systems can be time‐consuming and resource‐intensive. Families and clinicians need to collaborate to identify the particular needs of child and family. Typically, an occupational therapist or physiotherapist will assess the correct support for each individual child before use and regularly during use. An optimum posture is proposed to be supine with symmetrical 30 degrees hip abduction and 30 degrees hip flexion. However, compromises will be necessary to accommodate asymmetry where deformity has already become established or to suit individual preferences. The time it can take to establish a night positioning habit varies, and the length of time that a child can adhere to lying in the equipment (dosage) may need to be increased gradually. Families and carers require training and ongoing support to implement therapeutic positioning at night, including lifting the child in and out safely, correct positioning in the system, and any scope for repositioning within the device. Ongoing review is necessary to ensure the child remains comfortable and to modify the equipment for changing musculoskeletal demands and also for growth. The availability of training, as well as funding for this equipment has been noted to be inconsistent in the National Health Service (NHS) in the United Kingdom (UK) (Humphreys 2012; Pountney 2009). The average purchase price of these commercially‐available sleep positioning systems is £500, but can be around £1500 depending on size and needs (UK prices, Polak 2009). In addition, on‐going costs then include parent carer training, therapist time for monitoring progress and adjustments or renewal to accommodate growth, plus maintenance checks, repairs and cleaning (Polak 2009).
Of all the available postural management equipment prescribed, sleep positioning systems are reported to be the least used after provision, are more often abandoned by families, or both (Humphreys 2012; Pountney 2009). In a survey of UK paediatric chartered physiotherapists, Polak 2007 found that some parent carers would not use sleep positioning systems due to a reluctance to alter the child's sleep pattern, concerns about the child's comfort, lack of immediate tangible benefit, and worries about additional postural control on top of daytime postural management. Families may also be concerned about the appearance and size of the equipment, how portable it is, how easy it is to operate and keep clean, and whether they will receive adequate instructions (Innocente 2014; Polak 2007).
Why it is important to do this review
It is unclear whether the provision of sleep positioning systems helps to prevent hip migration. As sleep positioning systems can create other problems for the child and family, and can be expensive to purchase and maintain, evidence of cost effectiveness is also crucially important.
A study evaluating postural management programmes, including sleep positioning systems, suggested that children using postural management experienced fewer hip problems compared to historical controls (Pountney 2009). These authors identified that sleep positioning systems were the least used item of postural management equipment and suggested that this may have been due to families' reluctance to interfere with a child's sleep routine and increase night‐time disturbance. A case series of children using sleep positioning systems identified that while most children adapted to using the equipment, some children were unable to sleep in the equipment, and a third left the study (Hankinson 2002). Another survey indicated that many families encountered problems using the equipment and needed support and education, particularly in the early stages (Goldsmith 2000). Sleep problems in children with developmental disabilities are much more common and severe than in children who do not have a developmental disability (Keenan 2007), and anything that might interfere with sleep may not be well received by parents.
The evidence for postural management programmes appears limited and it is thought that further research is needed (Pountney 2006). Despite this, and the question of acceptability of sleep positioning systems, the prescription of this equipment is becoming increasingly widespread. It is therefore important to determine whether the provision of sleep positioning systems reduce or prevent hip migration, as well as examining the effects on associated outcomes such as sleep patterns, quality of life, and pain.
Objectives
To determine whether commercially‐available sleep positioning systems, compared with usual care, reduce or prevent further hip migration in children with cerebral palsy. Any negative effect of sleep positioning systems on hip migration was considered within this objective.
Secondary objectives were to determine the effect of sleep positioning systems on: (1) number or frequency of hip problems; (2) sleep patterns and quality; (3) quality of life of the child and family; (4) pain; and (5) physical functioning. We also sought to identify any other adverse effects of sleep positioning systems for children with cerebral palsy.
Public involvement
Parent carers of children with disabilities contributed to this review by identifying the topic at a question‐generation event using the structured Population‐Intervention‐Comparator‐Outcome (PICO) format; subsequently parent carers helped to refine the research objectives, interpret the findings, and write the plain language summary. Parent carers were part of the 'Family Faculty', a group of parent carers who contribute to the research activities of the Peninsula Cerebra Research Unit (PenCRU) (pencru.org), a childhood disability research unit based in Devon, England.
This review is based on a published protocol (Lloyd 2011), and has been prepared following the Cochrane Handbook (Higgins 2011c), and Standards for the reporting of new Cochrane Intervention Reviews (MECIR 2012).
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomised controlled trials (RCTs). Randomised controlled trials are an experimental design where participants are randomly allocated into treatment or control groups. The following trials were eligible for inclusion:
-
RCTs of sleep positioning systems versus usual care (which may include the use of support in seating and standing, botulinum toxin A, and surgery);
-
RCTs comparing one sleep positioning system with another; and
-
RCTs comparing sleep positioning with other postural management systems (e.g. seating or standing support).
Cross‐over trials were considered as RCTs according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Types of participants
Children and adolescents were defined as 18 years of age or younger (where at least 50% of the study population was 18 years of age or under), with a clinical diagnosis of cerebral palsy. We included children with all types (spastic, ataxic, dyskinetic/athetoid) and severity (GMFCS Levels I to V) of cerebral palsy. We only included trials that involved individuals with other diagnoses if the data for individuals with cerebral palsy could be extracted separately.
Types of interventions
Overnight use of any commercially‐manufactured whole body sleep positioning system (such as Chailey Lying SupportTM, DreamaTM, Sleepform®, Moonlite/MoonlightTM, Symmetrisleep®, SnoooooozeTM), applied in any setting (i.e. in the family home or in residential care). We excluded the use of sleep positioning systems in hospital (e.g. neonatal units). We also excluded studies evaluating seating and standing supports without sleep positioning systems, and studies evaluating orthoses worn at night if they were not designed for the whole body.
Types of outcome measures
Primary outcomes
-
Hip migration percentage as determined by frontal anteroposterior, plain film radiograph, which measures the percentage of the femoral head that lies outside the acetabulum (Thomason 2014). Standardised measurement over time enables improvement, deterioration or no change to be determined.
Secondary outcomes
-
Number or frequency of hip problems (e.g. dislocations, dysplasia, surgical interventions).
-
Sleep patterns or sleep quality (e.g. time taken to get to sleep, hours asleep, and number of awakenings as measured by polysomnography, Actigraph, parent‐reported).
-
Quality of life of child and family (as measured by standardised child‐ or parent‐reported outcome measures such as Pediatric Quality of Life Inventory (PedsQL) cerebral palsy module, or Child Health Questionnaire (CHQ)).
-
Pain (as measured by, for example, Paediatric Pain Profile (PPP)).
-
Physical functioning (as measured objectively by a validated scale, such as the Gross Motor Function Measure (GMFM), or through interview‐assessment such as Pediatric Evaluation of Disability Inventory (PEDI)).
-
Adverse events (i.e. intolerability of sleep positioning systems (child‐ or parent‐reported)).
The timing at which outcomes were measured was vital. We expected to see relevant outcomes reported at baseline and an appropriate time at follow‐up. Hip migration might be plotted as trajectory based on annual or six‐monthly X‐rays, or follow‐up after at least one or more years. Pain and sleep outcomes could be measured and compared over shorter periods such as weeks or months.
Search methods for identification of studies
We searched the following databases on 13 June 2012, 13 May 2014 and most recently on 3 December 2014. The searches were not limited by date, language, publication status or study design.
-
Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library), 2014 Issue 12, and which includes the Specialised Register of the Cochrane Developmental, Psychosocial and Learning Problems Group.
-
Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, 1948 to December Week 48 2014.
-
EMBASE (Ovid), 1974 to December Week 48 2014.
-
CINAHL (Cumulative Index to Nursing and Allied Health Literature; EBSCOhost), 1981 to current.
-
Cochrane Database of Systematic Reviews (CDSR; The Cochrane Library), 2014 Issue 12.
-
Database of Abstracts of Reviews of Effects (DARE;The Cochrane Library), 2014 Issue 12.
-
Health Technology Assessment Database (HTA; The Cochrane Library, 2014 Issue 12.
-
NHS Economic Evaluation Database (EED; The Cochrane Library), 2014 Issue 2.
-
BNI (British Nursing Index; ProQuest), 1994 to 3 December 2014.
-
HMIC (Health Management Information Consortium; Ovid), 1979 to 3 December 2014.
-
Conference Proceedings Citation Index ‐ Science (CPCI‐S; Web of Science), 1990 to 3 December 2014.
-
Conference Proceedings Citation Index ‐ Social Sciences & Humanities (CPCI‐SSH; Web of Science), 1990 to 3 December 2014.
-
PEDro (Physiotherapy Evidence Database; pedro.org.au), all available years.
-
OTSeeker (Occupational Therapy Systematic Evaluation of Evidence; otseeker.com), all available years.
-
Cochrane Methodology Register (CMR; The Cochrane Library), 2014 Issue 12.
-
Clinicaltrials.gov (clinicaltrials.gov), all available years.
-
World Health Organisation International Clinical Trials Registry Platform (ICTRP; who.int/ictrp/en), all available years.
-
WorldCat (worldcat.org), all available years.
The search strategy combined terms for cerebral palsy with generic terms for sleep positioning systems or brand name products. The search strategies for each database are reported in Appendix 1.
In addition, we searched the reference lists of all full‐text papers retrieved to identify studies missed by the electronic searches. We contacted the manufacturers of sleep positioning systems and authors of relevant papers to ask if they were aware of any published and unpublished research. We also conducted a general Internet search using the search engine 'Google', using terms from the search strategy to capture any relevant grey literature.
Data collection and analysis
Methods archived for future updates of this review can be found in the Additional Methods Table in Appendix 2 and our protocol for this review (Lloyd 2011).
Selection of studies
Two authors (MR, SB) independently screened titles and abstracts of papers to identify records of potential eligibility. Where necessary, the full‐text article was examined to determine relevance; two papers were translated for this purpose. Two independent review authors (SB and CM or SB and MR) then assessed each record for inclusion in the review using the inclusion criteria set out above. Where a review author was an author of a paper being considered for inclusion, two review authors not involved in that study assessed its eligibility. Disagreements were resolved through discussion and consultation with a third author (JTC or MR).
Data extraction and management
Two review authors (SB, CM) independently extracted the relevant data onto a data extraction form based on examples of forms used by National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care, South West Peninsula (PenCLAHRC) and a Cochrane template. The review authors were not blinded to the study authors or journal when extracting the data. We planned to resolve any disagreements by discussion or with reference to a third person (GH) but no disagreements arose. Relevance of the outcomes assessed was discussed with two parent carers. The following data were extracted from all eligible trials.
-
Study characteristics: Number of participants, inclusion and exclusion criteria, type of intervention and comparison, intervention characteristics (duration, frequency, setting, any training received by parents in the use of the sleep positioning system), and number of withdrawals.
-
Participant characteristics: Gender, age, disease severity, and type of cerebral palsy (e.g. spastic, ataxic, dyskinetic/athetoid).
-
Outcome measures assessed after finishing the intervention and at follow‐up.
Assessment of risk of bias in included studies
Review authors assessed the methodological quality of each trial which met the inclusion criteria using the 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Two review authors (SB, CM) assessed each study independently and resolved disagreements by discussion with each other. Judgements were based on answering a specific question for each domain (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other sources of bias), to which the answer 'yes' indicated a low risk of bias, and the answer 'no' indicated a high risk of bias. If insufficient detail was reported in the paper, the judgement was 'unclear' risk of bias.
Samples of children with cerebral palsy in RCTs are often small and heterogeneous, so randomisation is unlikely to produce equal distributions of known or unknown determinants of outcome (Blair 2004). The review authors considered the possibility of clinically‐significant differences in baseline characteristics as another source of bias when assessing studies, by looking to see whether minimisation for characteristics such as age and hip migration status was applied at baseline to reduce group differences (Altman 2005).
In line with Cochrane guidance, if one or more domains was judged to indicate a high risk of bias, the review authors assessed a study as having an overall high risk of bias (Higgins 2011a). We presented the findings of the 'Risk of bias' assessment in the 'Risk of bias' table (beneath the Characteristics of included studies table) and provided a narrative description in the Risk of bias in included studies subsection.
Measures of treatment effect
Continuous outcomes
Where the reported summary statistics varied (mean and standard deviation; or median and interquartile range), SB requested the raw data from the authors. JM then re‐analysed the data, so that the same summary data could be reported across the studies. For primary outcomes, we reported the mean difference (MD) with 95% confidence intervals (CIs).
Where a study had a large number of variables, we identified a limited set of key variables for each outcome in advance of statistical analysis (Altman 1991; Higgins 2011b). This aimed to control the rate of false positive results and avoid use of adjustments for multiple comparisons, which make statistical tests more conservative. The chosen key variables included those measured by all of the included studies, and those which did not require the performance of laboratory investigations, and so have the potential to be more easily measured in future research. Whether they are the key variables that should be measured for the outcomes under consideration, is not the focus of this review.
Cross‐over trials
For cross‐over trials, we presented the results from paired t‐tests. We considered a P value less than 0.05 to be statistically significant. For more information on additional methods archived for future updates of this review, please see Appendix 2 or Lloyd 2011.
Unit of analysis issues
No unit of analysis issues arose for this review. For more information on methods to overcome unit of analysis issues archived for future updates of this review, please see Appendix 2 or Lloyd 2011.
Dealing with missing data
Study authors provided the raw data from their studies to enable reanalysis. We collected the numbers of participants for whom no outcome data were obtained and reported this information in the 'Risk of bias' assessment.
Assessment of heterogeneity
There were insufficient trials to undertake an assessment of heterogeneity. For more information on methods to assess heterogeneity archived for future updates of this review, please see Appendix 2 or Lloyd 2011.
Assessment of reporting biases
Outcome reporting bias
Where a study met the inclusion criteria, we examined the report of the study to assess for selective outcome reporting. We assessed the study as adequate if it met the following criteria.
-
The study protocol was available and all of the study's pre‐specified (primary and secondary) outcomes that were of interest to the review were reported in the pre‐specified way.
-
The study protocol was not available, but it is clear that the published reports included all expected outcomes, including those that were pre‐specified.
Publication and other reporting bias
We requested the start and end date of studies from the authors to consider time‐lag bias. Where we received this information, we considered it in the 'Risk of Bias' assessment. There were too few included studies to enable other meaningful analysis. Please see Appendix 2 or Lloyd 2011 for additional methods for assessing publication and other reporting biases archived for future updates of this review.
Data synthesis
We planned to perform a meta‐analysis or provide a descriptive review depending on the number of studies and the quality and type of data extracted. The decision of whether or not to carry out a meta‐analysis was reached through consensus of three of the authors (SB, CM, JM). The criteria for our decision was by consideration of the methodological or clinical differences between the included studies (including: measurement tools, experimental location, choice of metric, age of participants, type of motor disorder, position adopted in sleep positioning system, history of seizures, GMFCS Level, and type of sleep positioning system).
We concluded that it would be inappropriate to carry out a fixed‐effect meta‐analysis because the assumption of no between‐study variation is implausible given the heterogeneity, and we rejected a random‐effects meta‐analysis because “[if] there are few studies or if the studies are small, a random‐effects analysis will provide poor estimates of the width of the distribution of intervention effects” (Deeks 2011 Section 9.5.4).
We present a narrative review of the results in the Effects of interventions section. This was produced consistently to include the same elements of information for each study, and presented in the same order. We only discussed patterns in the results in relation to the key variables for the outcomes of this review and only if significant differences were found.
We presented the findings in summary of findings Table for the main comparison in accordance with the recommendations of the Cochrane Handbook and the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach for rating the quality of a body of evidence (Schünemann 2011). Using the GRADE approach, and a form based on a worksheet from the Cochrane Effective Practice and Organisation of Care (EPOC) group (EPOC 2015), we assessed the methodological design, risk of bias, inconsistency, indirectness, and imprecision of the evidence for the outcomes reported in this review.
Subgroup analysis and investigation of heterogeneity
Insufficient trials were available to enable us to consider statistical heterogeneity. Please see Appendix 2 or Lloyd 2011 for methods for conducting subgroup analyses archived for future updates of this review.
Sensitivity analysis
There were too few studies to enable us to perform these analyses. Please see Appendix 2 or Lloyd 2011 for methods for conducting sensitivity analyses archived for future updates of this review.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies.
Results of the search
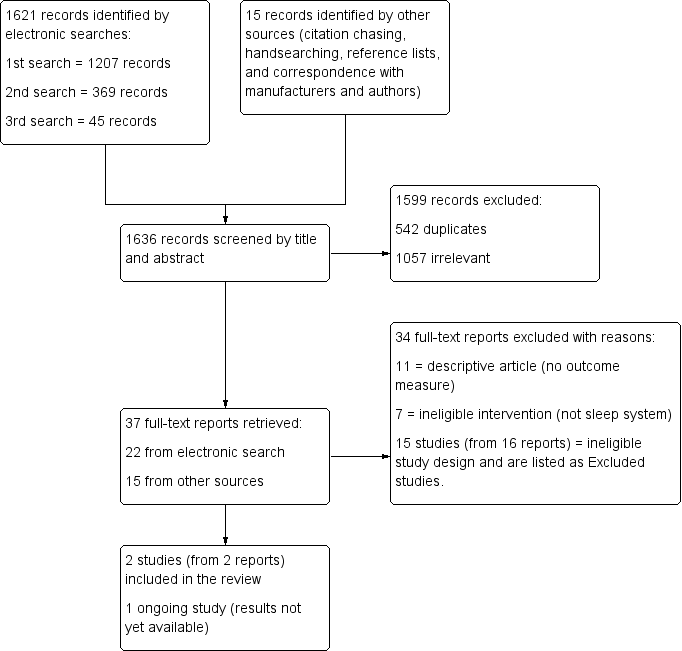
The search results are presented in a PRISMA flow chart in Figure 1. The initial search was carried out in June 2012 and followed by updated searches in May 2014 and December 2014. Our searches identified a total number of 1636 records, 1621 of which were retrieved by the electronic searches. Of the 37 full‐text reports that were retrieved following title and abstract screening, 15 (41%) were found through contact with manufacturers and authors rather than through the electronic search. Although agreement on exclusion was 100%, several full‐text reports could have been excluded for more than one reason (i.e. were both an ineligible intervention and an ineligible study design) (see Excluded studies). In sum, we excluded 34 reports, identified one ongoing study from one report (UKCRN ID 10914), and included two studies (from two reports).
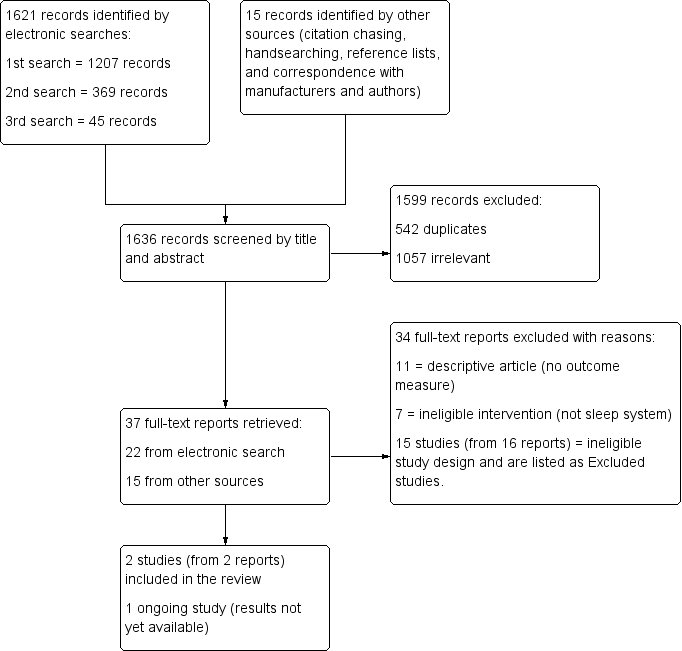
Study flow diagram
Included studies
We found no RCTs that evaluated the effectiveness of sleep positioning systems to reduce or prevent hip migration.
Two cross‐over studies (involving 21 children in total) met our inclusion criteria in respect of two secondary objectives: sleep quality and pain (Hill 2009; Underhill 2012). Both studies, conducted in Southern England, used randomised order of treatment (sleeping in sleep positioning systems or not sleeping in sleep positioning systems). Participants in both trials were children (12 boys, 9 girls in total) with cerebral palsy, graded as Levels III to V according to the GMFCS; aged 5 to 16 years; and established users of sleep positioning systems. The Hill 2009 study, which the authors described as a pilot, was conducted in a laboratory (one night with and one night without sleeping in sleep positioning systems, with at least three nights at home in between). Underhill 2012 was conducted in the child's home (four consecutive nights each sleeping with or without sleep positioning systems).
Neither study reported the effects on hip migration. Hill 2009 measured outcomes in relation to sleep quality using polysomnography and video recording. Underhill 2012 assessed sleep quality by Actigraph and pain by parent‐report using the PPP. While neither study explicitly set out to measure harms or adverse effects, the outcomes measured were capable of showing negative and positive effects. Further details about these studies are provided in the Characteristics of included studies tables.
Excluded studies
Of the 37 full‐text articles retrieved, 11 were descriptive papers, and 7 did not look at either sleep positioning systems, cerebral palsy, or whole body sleep positioning systems (i.e. they focused instead on upper limb splints worn at night). Fifteen studies (from 16 reports), which did assess the interventions of interest but were not RCTs and therefore did not meet our inclusion criteria are listed in the Characteristics of excluded studies. Two of these reports refer to the same study (Polak 2007).
Ongoing studies
We found one ongoing exploratory study, which would meet the criteria for inclusion into this review. No outcome data were available at the time of writing. For further information about this study, please see Characteristics of ongoing studies.
Risk of bias in included studies
Both included studies were judged to have a high risk of bias overall. See Figure 2 and Characteristics of included studies.
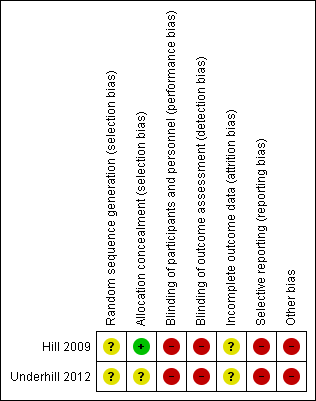
Risk of bias summary: Review authors' judgements about each risk of bias item for each included study.
Random sequence generation
Neither included study reported how the researchers generated the allocation sequence to each treatment condition (Hill 2009; Underhill 2012).
Allocation
Hill 2009 used a sealed‐envelope method and was considered at low risk of bias, while Underhill 2012 did not report how random allocation to the sequence of treatments was concealed.
Blinding
No information is provided as to who did the polysomnography or Actigraph outcomes assessments, nor the training or expertise of those who completed it, in either of the included studies. While blinding of the delivery of this intervention was not possible in the included studies, a lack of blinding may have impacted the subjective outcomes that were not measured by technology (scores on the PPP and the video recording) (Wood 2008). Neither Hill 2009 or Underhill 2012 clearly described how analysts conducting the outcome assessment were blinded to group assignment.
Incomplete outcome data
Twenty‐one children in total were randomised to take part in the included trials, but results are only available for 19 children. Both studies provided details of exclusions from analysis.
Hill 2009 excluded one child due to pyrexial illness on the second study night and only one rapid eye movement (REM) cycle being recorded.
In the Underhill 2012 study, sleep data were not available for one child as the Actiwatch failed. Another child only slept without the sleep positioning system for two of the four intended nights 'due to a severe reaction', but their results are included in the analysis, without explanation as to how the data were managed. Also, no reasons were provided for the lack of pain scores for one of the children.
Selective reporting
Authors of both included studies planned to assess other outcomes specified in their protocol, which were then not reported. Hill 2009 planned to assess day‐time function using the Cambridge Neuropsychological Testing Battery (CANTAB). Underhill 2012 planned to assess number of night awakenings that lasted longer than five minutes and percentage of the sleep period which was motionless. Results for these outcomes were not reported.
Authors of both the included studies provided their start and end dates. The Hill 2009 study was conducted from January to April 2007 and the Underhill 2012 study ran from January 2009 to January 2011.
Other potential sources of bias
Both trials were of very short duration and performed under 'experimental' rather than pragmatic conditions. Hill 2009 had a washout period of a minimum of three nights, but the length of time between the two conditions (sleeping or not sleeping in sleep positioning systems) was not clear in Underhill 2012. Thus, the risk of effects from one condition affecting the other cannot be ruled out.
As the children were all established users of sleep positioning systems, it is also important to consider any possible effect of the order of conditions (whether the children slept in their sleep positioning system first or second). While Hill 2009 tested for a first‐night effect and concluded that randomisation had adequately controlled for period effects, no test was made for these period effects in the Underhill 2012 trial.
In order for the data to be collected from one location only in the Underhill 2012 trial (children in residential settings often go home at the weekends), data were only collected for four nights. However, as the authors report, Actigraph data are recommended to be collected from a minimum of five nights.
Neither trial recruited the number of children needed for statistical power as per their protocol. Both trials recruited existing users of sleep positioning systems, which limited the generalisability of the results to new users of sleep positioning systems, as the children in these studies were likely to have adapted to their use. In the Hill 2009 trial, only 10 of the 22 children initially thought to be eligible actually participated. Children were excluded if they experienced uncontrolled nocturnal seizures, or by their own clinicians on the basis of unsettled domestic situations or extreme behavioural problems. This may have reduced the applicability of the results to those with more complex health conditions or social circumstances, or both.
Effects of interventions
See: Summary of findings for the main comparison
Primary outcomes
Hip migration percentage
No included studies evaluated whether sleep positioning systems reduce or prevent hip migration in children with cerebral palsy.
Secondary outcomes
Number or frequency of hip problems
No included studies evaluated whether sleep positioning systems reduce or prevent hip problems in children with cerebral palsy.
Sleep patterns or sleep quality
Both included studies measured sleep quality. Hill 2009 presented multiple variables (n = 18) for measuring sleep quality and carried out Bonferroni adjustments of the significance level to correct for multiple comparisons. We defined the key variables we thought most closely fit the objectives of this review and would also be comparable to variables measured by Underhill 2012. These were sleep latency and sleep efficiency. We have reported the results of paired t‐tests for treatment effect for these key variables in Table 1. As we included very few key variables, we did not make any adjustments to the significance level.
| Outcome | Variable | Definition | Study ID | Number of participants | Sleeping in sleep positioning system Mean (SD) | Sleeping out of sleep positioning system Mean (SD) | Mean difference (95% CI) | Paired t‐test | |
| t value | P value | ||||||||
| Sleep patterns/sleep quality | Sleep latency | Time (in minutes) to fall asleep | 9* | 69.1† (52.6) | 32.9 (26.0) | 36.2 (‐1.12 to 73.45) | 2.24 | 0.06 | |
| 8£ | 64.1 (54.0) | 37.0 (24.5) | 27.1 (‐8.76 to 62.89) | 1.79 | 0.12 | ||||
| 9 | 68.8 (49.8) | 80.1 (48.1) | ‐11.3 (‐30.70 to 8.03) | ‐1.35 | 0.21 | ||||
| Sleep efficiency | % of time in bed actually asleep | 9 | 80.7 (15.4) | 83.1 (12.0) | ‐2.4 (‐11.77 to 7.04) | ‐0.58 | 0.58 | ||
| 10 | 76.2 (8.3) | 73.8 (11.1) | 2.4 (‐2.98 to 7.73) | 1.00 | 0.34 | ||||
| Pain | Pain | Paediatric Pain Profile (PPP) scale (parent‐reported scores) | 10 | 11.3 (12.1) | 13.0 (14.6) | ‐1.7 (‐4.88 to 0.15) | ‐1.68 | 0.13 | |
| CI: Confidence intervals; ID: Identifier; SD: Standard Deviation | |||||||||
* Includes one participant who fell asleep before recording started (recorded as zero), as reported by Hill 2009.
£ Calculated without the participant who fell asleep before treatment (excluding zero score) to be comparable to Underhill 2012.
† Calculated from data supplied by author; reported as 65.9 in Hill 2009.
Sleep latency
Both included studies measured the time it took for the child to fall asleep once put to bed (latency). In both studies, one child fell asleep before the intervention commenced (bedtime). In the study reported by Underhill 2012, this child's data were excluded from the analysis; in Hill 2009, the nil score (asleep at the point recording began) was included in the analysis on the basis of intention‐to‐treat (ITT). In order to enable comparison, the results for Hill 2009, both including the nil score and without it, are set out in Table 1.
Neither study found a statistically significant difference in the time it takes for a child to fall asleep whether sleeping in the sleep positioning system or not.
Sleep efficiency
Both included studies also measured the percentage of time the child was actually asleep in bed (efficiency). Neither study reported a statistically significant difference whether sleeping in the sleep positioning system or not.
Both included studies reported substantial individual variability. As the Underhill 2012 data provided to us contained a sequence of treatments, we carried out additional t‐tests for a period effect (Altman 1991). We found no significant difference for sleep efficiency (t value = 0.67, P value = 0.52) or sleep latency (t value = 2.01, P value = 0.08). Hill 2009 tested for first‐night effects and also reported no significant differences between the nights studied.
No statistical difference was reported between sleeping in the sleep positioning system or not in respect of any of the other 16 sleep quality variables measured by the included studies. Summary statistics, which we calculated from raw data provided by study authors, are presented in Table 2.
| Study | Variable of sleep quality | Number of participants | Sleeping in sleep positioning systems Mean (SD) | Sleeping out of sleep positioning system Mean (SD) | Sleeping in sleep positioning system Median (IQR) | Sleeping out of sleep positioning system Median (IQR) |
| Total sleep time (in minutes)¹ | 10 | 517.1 (54.4) | 509.1 (72.5) | 511.5 (52.5) | 527.5 (117.5) | |
| Total sleep time (in minutes) | 9 | 349.9 (101.1) | 427.7 (55.0) | 412.5 (143.5)* | 421.0 (89.0)* | |
| Total sleep time that was S1^ (%) | 9 | 2.4 (2.2) | 3.2 (2.4) | 1.7 (1.1)* | 3.6 (3.5)* | |
| Total sleep time that was S4^ (%) | 9 | 33.3 (10.6) | 29.0 (10.6) | 29.2 (11.3) | 28.0 (7.5) | |
| Total sleep time that was S3^ (%) | 9 | 6.4 (1.7) | 6.2 (2.4) | 6.3 (2.2) | 5.5 (3.8) | |
| Total sleep time that was S2^ (%) | 9 | 46.4 (10.0) | 50.5 (11.0) | 48.7 (11.3) | 49.7 (10.8) | |
| REM onset latency (in minutes) | 9 | 159.0 (99.4) | 204.3 (122.4) | 190.0 (18.0) | 187.0 (65.0) | |
| Number of REM cycles | 9 | 3.3 (0.9) | 2.9 (1.1) | 4.0 (1.0)* | 2.0 (2.0) | |
| Total sleep time that was REM^ (%) | 9 | 11.5 (5.1) | 11.0 (4.6) | 10.7 (1.4) | 11.1 (3.9) | |
| Total arousal index | 9 | 11.5 (6.5) | 11.4 (5.0) | 8.5 (6.0)* | 10.8 (8.2)* | |
| Central Apnoea Index (CAI) | 9 | 3.0 (8.0) | 4.0 (9.9) | 0.4 (0.4)* | 0.6 (0.9)* | |
| Respiratory Arousal Index (RAI) | 9 | 2.2 (3.7) | 1.5 (2.5) | 1.4 (1.9)* | 0.6 (1.4)* | |
| Apnoea ‐ Hypopnoea Index (AHI) | 9 | 1.9 (1.8) | 0.9 (1.2) | 2.6 (3.0) | 0.4 (1.5)* | |
| Obstructive Apnoea Index (OAI) | 9 | 0.5 (0.6) | 0.4 (0.9) | 0.3 (0.8)* | 0.1 (0.3)* | |
| % total sleep time with SpO₂ > 95% | 9 | 80.5 (29.0) | 77.2 (28.1) | 98.0 (19.9)* | 87.8 (12.6)* | |
| Average (mean) SpO₂ over total time | 9 | 95.7 (0.9) | 96.2 (1.9) | 95.0 (1.0)* | 97.0 (2.0)* | |
| Minimum SpO₂ (Nidus value) | 9 | 92.7 (1.7) | 90.6 (3.0) | 92.0 (1.0)* | 91.0 (3.0)* | |
| IQR: Interquartile range; REM: Rapid eye movement; SD: Standard deviation; SpO₂: Peripheral capillary oxygen saturation | ||||||
¹ Originally reported in hours and minutes, here given as minutes to be comparable.
^S1, S2, S3, S4 refer to the different stages of sleep; stages one to four.
All values in this table are calculated from data supplied by study authors. For results from Hill 2009, some discrepancies were found between our calculations and the original publication. These are highlighted with an asterisk (*).
Quality of life of child and family
No studies meeting the inclusion criteria examined the impact of sleep positioning systems on the quality of life of the child and family.
Pain
One included study ‐ Underhill 2012 ‐ measured the impact of sleep positioning systems on pain using the PPP. Parent‐reported pain scores for the children were recorded following four nights in a sleep positioning system and compared to parent‐reported pain scores recorded following four nights out of the sleep positioning system. The difference in pain scores of children sleeping in sleep positioning systems compared to those sleeping without was not statistically significant (see Table 1).
Physical functioning
No studies meeting the inclusion criteria explored the impact of sleep positioning systems on physical functioning.
Adverse effects
Neither included study reported on any adverse effects.
Discussion
Summary of main results
We found no RCTs that examined the effectiveness of sleep positioning systems to reduce or prevent hip migration for children with cerebral palsy (see summary of findings Table for the main comparison).
Very low quality evidence (two small randomised cross‐over studies) found no differences in sleep quality or pain between using a sleep positioning system or not, for existing users of sleep positioning systems. These findings need to be interpreted cautiously and are difficult to generalise due to the high risk of bias and low statistical power of these studies.
We found no studies meeting our inclusion criteria that assessed possible adverse effects of sleep positioning systems, or the impact of sleep positioning systems on physical functioning, the number or frequency of hip problems, or the quality of life of the child and family.
Overall completeness and applicability of evidence
We found insufficient high quality evidence to address the objectives of this review. There is currently no robust research evidence to inform clinical decision‐making regarding the prescription of sleep positioning systems to reduce or prevent hip migration for children with cerebral palsy.
The use of sleep positioning systems can be affected by many different factors relating to (1) the equipment (availability, cost, size, appearance, hygiene, temperature control, and transportability); (2) experience, skills, and training of therapists and carers; (3) changing needs of the child; and (4) availability of alternative interventions (Polak 2007). Adverse effects appear under‐reported, perhaps because, in these instances, the equipment is usually abandoned (Humphreys 2012; Pountney 2009); this key outcome should be recorded and reported. Inability to adhere to using sleep positioning systems is often assumed to be due to insufficient skill or confidence on the part of carers, leading to a focus on better training, but there are likely to be many other reasons, including perceived discomfort and temperature regulation (Innocente 2014). Both included and excluded studies reported that children withdrew for reasons such as illness or a need for other treatments. In some of the studies which did not recruit existing users of sleep positioning systems, and were excluded for methodological reasons, authors reported that some children also withdrew, as their carers said the children could not sleep properly when using their sleep positioning system.
The included studies recruited children who were established users of sleep positioning systems and so provided no evidence as to the principles used to identify appropriate children for sleep positioning systems, nor the reasoning for the type of sleep positioning system used by the children. As these factors could have an influence on the outcomes measured, it is important that this is explored and documented in future research.
Despite the prescription of sleep positioning systems, there is little evidence either regarding effectiveness or how best to choose which children are most likely to benefit. We also have no high quality evidence to judge whether commercial sleep positioning systems are more effective at reducing or preventing hip migration than using cushions and pillows to aid positioning at night, which have long been recommended by therapists (Bower 2008).
Quality of the evidence
The overall quality of evidence is very low. This review only found two RCTs, including a total of 21 children, which examined the effects of the intervention on two of the secondary objectives of this review (pain and sleep patterns/quality). Both were judged to have a high risk of bias and inadequate power related to small samples and substantial individual variability in the outcomes assessed. The restriction of the study sample to existing users of sleep positioning systems limits the applicability of the evidence to the general population of children with cerebral palsy. The applicability of the data from the study conducted by Hill 2009 is reduced further as this was carried out under laboratory conditions rather than in the children's homes.
Potential biases in the review process
Assessments for inclusion, risk of bias, and data extraction were carried out independently by two review authors. Authors of the included studies provided raw data and answered questions about how the data had been analysed (on the basis of intention‐to‐treat or not), which enabled more comprehensive appraisal of the methods and findings. Two authors (CM, GH) are part of a current pilot RCT described in the Characteristics of ongoing studies and one of these authors (GH) is also the author of an excluded study. We avoided any potential conflict of interest by ensuring candidate papers were not reviewed by the authors of these papers.
While the searches of the electronic databases were comprehensive, a relatively high proportion of potential references ‐ 15/37 (41%) ‐ were found through contact with manufacturers and authors rather than through the electronic searches. Seven reports were not published and three papers were published in journals not indexed by the electronic databases, which highlights the importance of using supplementary search methods. It is therefore possible that other studies have been conducted but were not found, although it is unlikely that they these would be large RCTs that would change the conclusions of the review.
Agreements and disagreements with other studies or reviews
In line with two other published reviews on sleep positioning systems for children with cerebral palsy (Bush 2013; Wynn 2009), we concluded that there is limited evidence available of effectiveness to prevent deformity. As Montero 2014 reported in their review of studies looking at the technical devices used by children with motor disabilities, the methodological quality of the available evidence is low.
By highlighting the lack of well‐designed RCTs, this review challenges an assumption reported in studies (Dawson 2013; Hill 2009), that sleep positioning systems are a proven, effective treatment to reduce or prevent hip migration.
A consensus statement produced in 2006 proposed that a programme of rigorous research to evaluate sleep positioning systems was needed (Gericke 2006), but we found no evidence of studies likely to provide definitive evidence.
Study flow diagram

Risk of bias summary: Review authors' judgements about each risk of bias item for each included study.
| Sleeping in a sleep positioning system compared with not sleeping in a sleep positioning system for children with cerebral palsy | |||
| Population: Children with cerebral palsy Settings: United Kingdom (at home or in paediatric research laboratory) Intervention: Sleeping in sleep positioning system Comparison: Not sleeping in sleep positioning system | |||
| Outcomes | Impact | Number of participants | Quality of the evidence |
| Reduce hip migration/hip problems | No RCTs measured effect of sleep positioning systems on hip migration/hip problems | ‐ | ‐ |
| Effect on sleep patterns and quality | Limited data. A small number of established users of sleep positioning systems showed no significant difference in sleep quality indicators | 21 | ⊕⊝⊝⊝ |
| Effect on quality of life of child and family | No RCTs measured effect of sleep positioning systems on child and family quality of life | ‐ | ‐ |
| Effect on pain | Limited data. A small number of established users of sleep positioning systems showed no significant difference in levels of pain | 10 (1 study) | ⊕⊝⊝⊝ |
| Effect on physical functioning | No RCTs measured effect of sleep positioning systems on physical functioning | ‐ | ‐ |
| Adverse effects | No RCTs measured harms or reported adverse events | ‐ | ‐ |
| GRADE Working Group grades of evidence | |||
| GRADE: Grades of Recommendation, Assessment, Development and Evaluation. | |||
| Outcome | Variable | Definition | Study ID | Number of participants | Sleeping in sleep positioning system Mean (SD) | Sleeping out of sleep positioning system Mean (SD) | Mean difference (95% CI) | Paired t‐test | |
| t value | P value | ||||||||
| Sleep patterns/sleep quality | Sleep latency | Time (in minutes) to fall asleep | 9* | 69.1† (52.6) | 32.9 (26.0) | 36.2 (‐1.12 to 73.45) | 2.24 | 0.06 | |
| 8£ | 64.1 (54.0) | 37.0 (24.5) | 27.1 (‐8.76 to 62.89) | 1.79 | 0.12 | ||||
| 9 | 68.8 (49.8) | 80.1 (48.1) | ‐11.3 (‐30.70 to 8.03) | ‐1.35 | 0.21 | ||||
| Sleep efficiency | % of time in bed actually asleep | 9 | 80.7 (15.4) | 83.1 (12.0) | ‐2.4 (‐11.77 to 7.04) | ‐0.58 | 0.58 | ||
| 10 | 76.2 (8.3) | 73.8 (11.1) | 2.4 (‐2.98 to 7.73) | 1.00 | 0.34 | ||||
| Pain | Pain | Paediatric Pain Profile (PPP) scale (parent‐reported scores) | 10 | 11.3 (12.1) | 13.0 (14.6) | ‐1.7 (‐4.88 to 0.15) | ‐1.68 | 0.13 | |
| CI: Confidence intervals; ID: Identifier; SD: Standard Deviation | |||||||||
| * Includes one participant who fell asleep before recording started (recorded as zero), as reported by Hill 2009. | |||||||||
| Study | Variable of sleep quality | Number of participants | Sleeping in sleep positioning systems Mean (SD) | Sleeping out of sleep positioning system Mean (SD) | Sleeping in sleep positioning system Median (IQR) | Sleeping out of sleep positioning system Median (IQR) |
| Total sleep time (in minutes)¹ | 10 | 517.1 (54.4) | 509.1 (72.5) | 511.5 (52.5) | 527.5 (117.5) | |
| Total sleep time (in minutes) | 9 | 349.9 (101.1) | 427.7 (55.0) | 412.5 (143.5)* | 421.0 (89.0)* | |
| Total sleep time that was S1^ (%) | 9 | 2.4 (2.2) | 3.2 (2.4) | 1.7 (1.1)* | 3.6 (3.5)* | |
| Total sleep time that was S4^ (%) | 9 | 33.3 (10.6) | 29.0 (10.6) | 29.2 (11.3) | 28.0 (7.5) | |
| Total sleep time that was S3^ (%) | 9 | 6.4 (1.7) | 6.2 (2.4) | 6.3 (2.2) | 5.5 (3.8) | |
| Total sleep time that was S2^ (%) | 9 | 46.4 (10.0) | 50.5 (11.0) | 48.7 (11.3) | 49.7 (10.8) | |
| REM onset latency (in minutes) | 9 | 159.0 (99.4) | 204.3 (122.4) | 190.0 (18.0) | 187.0 (65.0) | |
| Number of REM cycles | 9 | 3.3 (0.9) | 2.9 (1.1) | 4.0 (1.0)* | 2.0 (2.0) | |
| Total sleep time that was REM^ (%) | 9 | 11.5 (5.1) | 11.0 (4.6) | 10.7 (1.4) | 11.1 (3.9) | |
| Total arousal index | 9 | 11.5 (6.5) | 11.4 (5.0) | 8.5 (6.0)* | 10.8 (8.2)* | |
| Central Apnoea Index (CAI) | 9 | 3.0 (8.0) | 4.0 (9.9) | 0.4 (0.4)* | 0.6 (0.9)* | |
| Respiratory Arousal Index (RAI) | 9 | 2.2 (3.7) | 1.5 (2.5) | 1.4 (1.9)* | 0.6 (1.4)* | |
| Apnoea ‐ Hypopnoea Index (AHI) | 9 | 1.9 (1.8) | 0.9 (1.2) | 2.6 (3.0) | 0.4 (1.5)* | |
| Obstructive Apnoea Index (OAI) | 9 | 0.5 (0.6) | 0.4 (0.9) | 0.3 (0.8)* | 0.1 (0.3)* | |
| % total sleep time with SpO₂ > 95% | 9 | 80.5 (29.0) | 77.2 (28.1) | 98.0 (19.9)* | 87.8 (12.6)* | |
| Average (mean) SpO₂ over total time | 9 | 95.7 (0.9) | 96.2 (1.9) | 95.0 (1.0)* | 97.0 (2.0)* | |
| Minimum SpO₂ (Nidus value) | 9 | 92.7 (1.7) | 90.6 (3.0) | 92.0 (1.0)* | 91.0 (3.0)* | |
| IQR: Interquartile range; REM: Rapid eye movement; SD: Standard deviation; SpO₂: Peripheral capillary oxygen saturation | ||||||
| ¹ Originally reported in hours and minutes, here given as minutes to be comparable. ^S1, S2, S3, S4 refer to the different stages of sleep; stages one to four. All values in this table are calculated from data supplied by study authors. For results from Hill 2009, some discrepancies were found between our calculations and the original publication. These are highlighted with an asterisk (*). | ||||||

