Pharmacotherapy for chronic cognitive impairment in traumatic brain injury
Appendices
Appendix 1. Sources searched and search strategies used
| Source
| Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) [last searched 30 December 2014] | Keyword search: traumatic OR TBI OR "brain injury" OR "brain injuries" OR TBIs OR "axonal injury" OR "axonal injuries"
| Dec 2011: 41 Feb 2013: 9 Jan 2014: 1 Dec 2014: 1 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) [last searched 30 December 2014] | 1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. (animals not (humans and animals)).sh. 11. 9 not 10 12. ("drug treatment*" or "pharmacological treatment*" or pharmacotherapy).ti,ab. 13. Cholinergic Agents/ 14. (cholinomimetic or cholinergic*).ti,ab. 15. (arecolin* or arecholin*).ti,ab. 16. (meclofenoxat* or meclophenoxat*).ti,ab. 17. (centrofenoxin* or centrophenoxin*).ti,ab. 18. ("ANP 235" or "EN 1627").ti,ab. 19. (deanol* or demanol* or "CR 121" or "RS 86").ti,ab. 20. (physostigmin* or fysostigmin* or lecithin* or lecitin*).ti,ab. 21. (choline or cholin or coline).ti,ab. 22. (tacrin* or takrin*).ti,ab. 23. (tetrahydroaminoacridin* or tetrahydroaminacrin* or "CI 970" or THA or THAA).ti,ab. 24. ("7‐methoxyacridin*" or "7‐metoxyacridin*" or methoxytacrin* or metoxytacrin* or metoxytakrin* or methoxycrin* or metoxycrin* or MEOTA).ti,ab. 25. (ipidacrin* or amiridin* or NIK247 or "NIK 247").ti,ab. 26. (donepezil* or E2020 or "E 2020" or galanthamin* or galantamin* or "CGP 37267").ti,ab. 27. (rivastigmin* or ENA713 or "ENA 713" or "212 713").ti,ab. 28. (eptastigmin* or heptylstigmin* or heptylphysostigmin* or heptylfysostigmin*).ti,ab. 29. ("L 693 487" or MF201 or "MF 201" or metrifonat* or metriphonat*).ti,ab. 30. (trichlorfon* or trichlorphon* or trichlorfen* or trichlorphen* or "L 1359" or "Bay a 9826" or "Bay 1 1359").ti,ab. 31. (xanomelin* or "LY 246708" or "FG 10232" or cevimelin* or AF102B or "AF 102B'").ti,ab. 32. ("FKS 508" or "SND 5008" or SNK508 or SNI2011).ti,ab. 33. or/12‐32 34. Antidepressive Agents/ 35. Antidepressive Agents, Second‐Generation/ 36. Antidepressive Agents, Tricyclic/ 37. (Amesergide or amineptine hydrochloride or amitriptyline).ti,ab. 38. (amoxapine or "benactyzine hydrochloride" or brofaromine or "bupropion hydrochloride" or "butriptyline hydrochloride").ti,ab. 39. (cianopramine or "citalopram hydrobromide" or clomipramine or "clorgyline hydrochloride" or clovcxamine).ti,ab. 40. ("demexiptiline hydrochloride" or "desipramine hydrochloride" or "dibenzepin hydrochloride" or "dimetacrine tartrate" or "dothiepin hydrochloride" or "doxepin hydrochloride" or "etoperidone hydrochloride").ti,ab. 41. (femoxetine or "fezolamine fumarate" or "fluoxetine hydrochloride" or "fluvoxamine maleate").ti,ab. 42. (ifoxetine or "imipramine hydrochloride" or "iprindole hydrochloride" or "iproniazid phosphate" or isocarboxazid or levoprotiline or "lofepramine hydrochloride" or "maprotiline hydrochloride").ti,ab. 43. (medifoxamine or "melitracen hydrochloride" or "metapramine fumarate" or "mianserin hydrochloride" or milnacipran or "minapri hydrochloride" or mirtazapine).ti,ab. 44. (moclobemide or nefazodone hydrochloride or nialamide or nomifensine maleate or nortriptyline hydrochloride or opipramol hydrochloride or oxaflozane hydrochloride or oxaprotiline hydrochloride or oxitriptan).ti,ab. 45. (paroxetine hydrochloride or phenelzine sulphate or pirlindole or propizepine hydrochloride or protriptyline hydrochloride).ti,ab. 46. (quinupramine or rolipram or rubidium chloride or sertraline hydrochloride or setiptiline or sibutramine or teniloxazine or tianepine sodium or tofenacin hydrochloride).ti,ab. 47. (oloxatone or tranylcypromine sulphate or trazadone hydrochloride or trimipramine or tryptophan or venlafaxine hydrochloride or viloxazine hydrochloride or viqualine or zimelidine hydrochloride).ti,ab. 48. or/34‐47 49. Antipsychotic Agents/ 50. (clozapine or olanzapine or pirenzepine or sertindole or imidazoles or indoles).ti,ab. 51. (risperidone or quetiapine or ziprasidone or piperazines or thiazoles or sulpiride or zotepine or zotepine or amisulpride).ti,ab. 52. (sulpride or deniban or solian or socian or sulamid or DAN or abilify or OPC or clozaril or alemoxan or elcrit or froidir or klozapol).ti,ab. 53. (leponex or olasek or ziprexa or zyprexa or seroquel or risperdal or belivon or risperin).ti,ab. 54. (rispolept or rispolin or neripros or rizodal or serdolect or serlect or zoleptil or aiglonyl or alimoral or ansium).ti,ab. 55. (arminol or betamaks or calmoflorine or championyl or darleton or depex or depreal or desisulpid or digton or dixibon).ti,ab. 56. (dobren or dogmatil or dogmatyl or dolmatil or dresent or eclorion or eglonyl or enimon or equilid or esipride or fardalan).ti,ab. 57. (fidelan or intrasil or lebopride or mariastel or meresa or mirbanil or neogama or neoride or noneston or norestran).ti,ab. 58. (normum or nufarol or nylipark or omaha or omiryl or ozedeprin or paratil or prosulpin or psicocen or quastil).ti,ab. 59. (quiridil or restful or stamoneurol or sulp or sulparex or sulpiphar or sulpir or sulpirid or sulpiryd or sulpitil or sulpivert).ti,ab. 60. (sulpril or suprium or synedil or tepavil or tepazepam or valirem or neogamma or zemorcon or zeprid or zymocomb).ti,ab. 61. (geodon or zoleptil or Benperidol or anquil or benperidols or frenactil or glianimon or psichoben or Chlorpromazine).ti,ab. 62. (aminazins or ancholactil or biscasil or bukatel or chlorazin or chlorpromazin or clordezalin or fenactil or fleksin).ti,ab. 63. (kloproman or klorproman or largactil or largatrex or megaphen or Nevropromazine or Plegomazin or Propaphenin).ti,ab. 64. (prozil or prozin or repazine or solidon or tardyl or thorazine or zuledin or Flupentixol or depixol or fluanxel or fluanxol).ti,ab. 65. (Fluphenazine or anatensol or cardilac or cenilene or dapotum or decafen or decazate or decentan or eutimox).ti,ab. 66. (fludecate or flufenazin or lyogen or lyoridin or lyorodin or mirenil or modecate or moditen or omca or pacinol or permitil).ti,ab. 67. (prolixin or prolongatum or sevinol or siqualone or fludecasin or Haloperidol or alased or aloperidin or avant).ti,ab. 68. (bioperidolo or buteridol or cereen or decaldol or decanoate or dozic or duraperidol or fortunan or haldol or haloneural).ti,ab. 69. (haloper or haloperin or norodol or novo‐peridol or peridol or sedaperidol or serenace or serenelfi or sevium or sigaperidol).ti,ab. 70. (sylador or vesadol or vesalium or zafrionil or levomepromazine or methotrimeprazine or levium or levomepromazin).ti,ab. 71. (levoprome or levozin or neurocil or norzinan or novo‐meprazine or nozinan or sinogan or tisercin or tisercinetta).ti,ab. 72. (veractil or Pericyazine or neulactil or perphenazine or decentan or peratsin or perdenasin or terfluoperazine or trilafon).ti,ab. 73. (trilafan or triptafen or fentazin or Pimozide or antalon or norofren or orap or pirium or Prochlorperazine).ti,ab. 74. (buccastem or bukatel or chlorperazinum or compazine or cotrazine or emetiral or klometil or mitil or nu‐prochlor).ti,ab. 75. (prochlorperazin or scripto‐metic or stemetil or tementil or trinigrin or ultrazac or ultrazinol or vertigon or Promazine).ti,ab. 76. (prazine or promazin or protactyl or prozyl or sinophenin or sparine or talofen or Thioridazine or aldazine or elperil).ti,ab. 77. (flaracantyl or mallorol or mefurine or meleril or mellaril or melleretten or mellerettes or melleril or orsanil or ridazine).ti,ab. 78. (stalleril or thioridazin or tirodil or visergil or Trifluperazine or discimer or eskazine or foille or iremo or jatroneural).ti,ab. 79. (jatrosom or modalina or oxyperazine or parmodalin or parstelin or sedofren or sporalon or stelabid or stelazine).ti,ab. 80. (stelbid or stelium or stilizan or terfluoperazine or terflurazine or terfluzin or terfluzine or trifluoperazin or trisedyl).ti,ab. 81. (Pipotiazine or lonseren or piportil or piportyl or Zuclopenthixol or ciatyl or cisordinol or clopixol or sordinol).ti,ab. 82. or/49‐81 83. (amantadine or bromocriptine or mazindol or pergolide or dopamine agonist).ti,ab. 84. (amphetamine or d‐amphetamine or dexamphetamine or dextroamphetamine or methamphetamine or Methylphenidate or Modafinil or adrafinil or provigil).ti,ab. 85. (melatonin or N‐ACETYL‐5‐METHOXYTRYPTAMINE or atomoxetine or memanthine).ti,ab. 86. or/83‐85 87. Brain Injuries/ 88. Brain Concussion/ 89. Brain Hemorrhage, Traumatic/ 90. Brain Injury, Chronic/ 91. Diffuse Axonal Injury/ 92. "brain injur*".ti,ab. 93. (TBI or TBIs).ti,ab. 94. ("hypoxic brain damage" or "diffuse axonal injur*" or DAI or DAIs).ti,ab. 95. "head injur*".ti,ab. 96. (brain adj2 trauma*).ti,ab. 97. (head adj2 trauma*).ti,ab. 98. concussion.ti,ab. 99. "brain contusion".ti,ab. 100. 33 or 48 or 82 or 86 101. or/87‐99 102. 100 and 101 103. 11 and 102 | Dec 2011: 652 Feb 2013: 95 Jan 2014: 36 Dec 2014: 98 |
| 3. EMBASE 1980‐2011 week 45 (Ovid SP) [last searched 30 December 2014] | 1. "traumatic brain injur*".ti,ab. 2. (TBI or TBIs).ti,ab. 3. ("hypoxic brain damage" or "diffuse axonal injur*" or DAI or DAIs).ti,ab. 4. (brain adj2 trauma*).ti,ab. 5. (head adj2 trauma*).ti,ab. 6. concussion.ti,ab. 7. "brain contusion".ti,ab. 8. exp *traumatic brain injury/ 9. or/1‐8 10. randomly.ab. 11. RCT.ti,ab. 12. clinical trial/ 13. randomi?ed.ab. 14. placebo*.ti,ab. 15. groups.ab. 16. "double‐blind*".ti,ab. 17. or/10‐16 18. 9 and 17
| Dec 2011: 1199 Feb 2013: 367 Jan 2014: 461 Dec 2014: 478 |
| 4. PSYCINFO 1806‐November week 3 2011 (Ovid SP) [last searched 30 December 2014] | 1. traumatic brain injury/ 2. (TBI or TBIs).ti,ab. 3. ("hypoxic brain damage" or "diffuse axonal injur*" or DAI or DAIs).ti,ab. 4. "head injur*".ti,ab. 5. (brain adj2 trauma*).ti,ab. 6. (head adj2 trauma*).ti,ab. 7. concussion.ti,ab. 8. "brain contusion".ti,ab. 9. or/1‐8 10. randomi?ed.ab. 11. placebo*.ti,ab. 12. "double‐blind*".ti,ab. 13. randomly.ab. 14. "single‐blind*".ti,ab. 15. RCT.ti,ab. 16. or/10‐15 17. 9 and 16
| Dec 2011: 430 Feb 2013: 157 Jan 2014: 84 Dec 2014: 117 |
| 5. CINAHL (EBSCOhost) [last searched 30 December 2014] | S1 TX "traumatic brain injur*" S2 (MM "Brain Injuries") S3 TX "hypoxic brain damage" OR "diffuse axonal injur*" S4 TX "brain contusion" S5 TX "brain trauma*" S6 TX "head trauma*" S7 TX TBI OR TBIs S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 S9 (MH "Randomized Controlled Trials") S10 TX placebo* S11 TX randomly S12 TX "double‐blind*" OR "single‐blind*" S13 S9 OR S10 OR S11 OR S12 S8 AND S13 | Dec 2011: 255 Feb 2013: 70 Jan 2014: 46 Dec 2014: 69 |
| 6. Web of Science (1945‐present) (via ISI Web of Science) [last searched 30 December 2014] | Topic=("traumatic brain injury" OR TBI OR TBIs) AND Topic=(randomly OR randomised OR randomized OR RCT) NOT Title=(mice OR mouse OR rat OR rats OR animal OR model) Timespan=All Years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH. Lemmatization=On
| Dec 2011: 1395 Feb 2013: 596 Jan 2014: 411 Dec 2014: 233 |
| 7. LILACS (BIREME) [last searched 30 December 2014] | traumatic brain injury [Words] and random or RCT or randomised OR randomized OR trial OR placebo OR groups [Words] | Dec 2011: 16 Feb 2013: 5 Jan 2014: 0 Dec 2014: 0 |
| 8. CENTRAL (The Cochrane Library) [last searched 30 December 2014] | #1 MeSH descriptor Brain Injuries explode all trees #2 TBI OR TBIs #3 "hypoxic brain damage" OR "diffuse axonal injur*" #4 "head injur*" #5 brain N/2 trauma* #6 head N/2 trauma* #7 "brain contusion" #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) | Dec 2011: 963 Feb 2013: 67 Jan 2014: 15 Dec 2014: 57 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) [last searched 30 December 2014] | Advanced search: Condition: (Traumatic brain injury OR TBI OR TBIs OR brain contusion OR concussion) AND Intervention studies | Dec 2011: 255 Feb 2013: 64 Jan 2014: 8 Dec 2014: 2 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [last searched 30 December 2014] | Advanced search: Condition: (Traumatic brain injury OR TBI OR TBIs OR brain contusion OR concussion) | Dec 2011: 364 Feb 2013: 73 Jan 2014: 0 Dec 2014: 2 |
| TOTAL before de‐duplication and assessment based on title and abstract screening | Dec 2011: 5582 Feb 2013: 1503 Jan 2014: 1062 Dec 2014: 1053 TOTAL: 9200 | |
| TOTAL after de‐duplication and assessment based on title and abstract screening | Dec 2011: 438 Feb 2013: 142 Jan 2014: 71 Dec 2014: 24 TOTAL: 675 | |
Appendix 2. Glossary of abbreviations of cognitive tests
CANTAB: Cambridge Neuropsychological Test Automated Battery. Computerised test battery assessing learning, memory, attention, problem solving, executive function and vigilance.
CANTAB‐PAL: Cambridge Neuropsychological Test Automated Battery Paired Associates Learning.
CANTAB RVIP A': Cambridge Neuropsychological Test Automated Battery Rapid Visual Information Processing. A subtest measuring sensitivity to a stimulus.
CANTAB RVIP mean latency: Cambridge Neuropsychological Test Automated Battery Rapid Visual Information Processing. Measure of time to respond to a stimulus.
CANTAB‐RT: Cambridge Neuropsychological Test Automated Battery Reaction Time.
CANTAB‐SWM: Cambridge Neuropsychological Test Automated Battery Spatial Working Memory.
CDR: Cognitive Drug Research Computerized Cognitive Assessment System. A computer‐controlled battery of tests including reaction time, spatial memory, numeric memory, word and picture recognition.
CCPT‐II: Conners’ Continuous Performance Test II. Computerised assessment of sustained visual attention.
COWA: Controlled Oral Word Association. Assessment of verbal fluency and word finding.
FAS test: F‐A‐S. A subtest of the Neurosensory Center Comprehensive Examination for Aphasia. Assessment of phonemic word fluency.
HVLT: Hopkins Verbal Learning Test. Verbally administered test assessing verbal learning, verbal memory, long‐term recall, and recognition memory.
ImPACT: Immediate Post Concussion Assessment Cognitive Testing. Computerised battery of cognitive tests that provides scores for verbal memory, visual memory, visual motor speed and reaction time.
SF‐12 physical/mental: Medical Outcome Study 12‐Item Short Form Survey. Measure of health‐related quality of life.
Trail A, B, C & D. Assessment of sequencing, mental flexibility and divided attention.
WAIS‐III‐DS: Wechsler Adult Intelligence Scale Digit Span. Assessment of short term verbal and auditory memory.
WAIS‐III Digit Symbol Coding: Wechsler Adult Intelligence Scale Digit Symbol Coding. Assessment of information processing speed.
Appendix 3. Methods for future updates
| Unit of analyses issues |
| In any studies utilising a cross‐over design, only data from the first treatment phase after randomisation will be eligible for inclusion. In the case that numerous eligible studies take repeated observations of outcomes then time frames that reflect short,medium and long term follow‐up will be selected. Time frames will be defined as short term if at six weeks or less, medium term between six and 24 weeks, while long term studies will run for six months or more. If repeated observations are not a significant issue then the longest follow‐up will be selected for each study. |
| Assessment of heterogeneity |
| Heterogeneity to be tested using a standard Chi2 statistic with a P = 0.1 being considered significant and quantified using the I2 statistic. If there is evidence of heterogeneity of the treatment effect between trials then a random‐effects model will be used. If heterogeneity is too great (that is I2 > 75%) then meta‐analysis will not be possible. |
| Assessment of reporting biases |
| To minimize the potential for publication bias, study authors, pharmaceutical companies and experts will be contacted to assist in the identification of unpublished studies. Furthermore, authors of identified studies will be contacted to enquire about duplication of studies and outcome reporting bias. Unpublished studies will be included in the meta‐analysis and the methodology and potential The intervention effect estimates from each study will be plotted against a measure of each study’s size or precision to create a funnel plot. If enough studies are identified (usually > 10) then the asymmetry can be assessed statistically (Begg and Mazumdar 1994), otherwise the funnel plot will be cautiously interpreted with the limitations of this method explicitly stated in the discussion. |
| Data synthesis |
| The review authors expect the studies to employ a wide range of tools assessing the same cognitive function and so meta‐analysis will utilize the standardised mean difference approach, standardising Depending upon whether heterogeneity of results is identified, we will decide whether a fixed‐effect or a random‐effects model is used for meta‐analysis. In either case the inverse variance method The duration of the trials may vary considerably. If the range is considered too great to combine all trials into one meta‐analysis the data can be divided into smaller time frames and a separate meta analysis conducted for each period. Some trials may contribute data to more than one time period if multiple assessments have been made. Pharmacotherapeutic interventions will be combined depending on the general class of drug (for example antidepressants). Subsequent subgroup analyses will focus on the neurotransmitter systems |
| Subgroup analysis and investigation of heterogeneity |
| If statistical heterogeneity is identified then we will adopt the strategies recommended in the Cochrane Handbook, section 9.5.3. We will evaluate possible reasons for the heterogeneity by |
| Sensitivity analysis |
| Issues suitable for sensitivity analysis will be identified during the review process. If a particular decision appears to affect the findings then we will attempt to understand this and perhaps gain further |

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
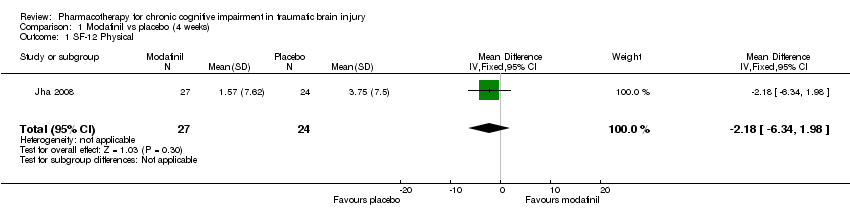
Comparison 1 Modafinil vs placebo (4 weeks), Outcome 1 SF‐12 Physical.

Comparison 1 Modafinil vs placebo (4 weeks), Outcome 2 SF‐12 Mental.

Comparison 1 Modafinil vs placebo (4 weeks), Outcome 3 ImPACT verbal memory composite.
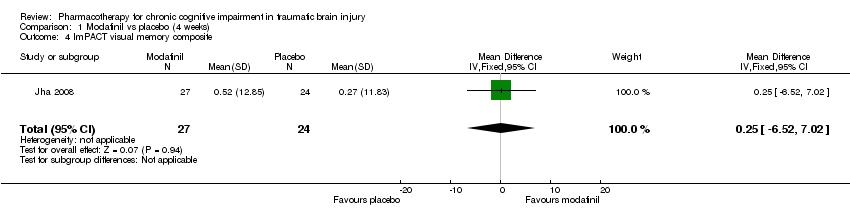
Comparison 1 Modafinil vs placebo (4 weeks), Outcome 4 ImPACT visual memory composite.

Comparison 1 Modafinil vs placebo (4 weeks), Outcome 5 ImPACT visual motor speed composite.
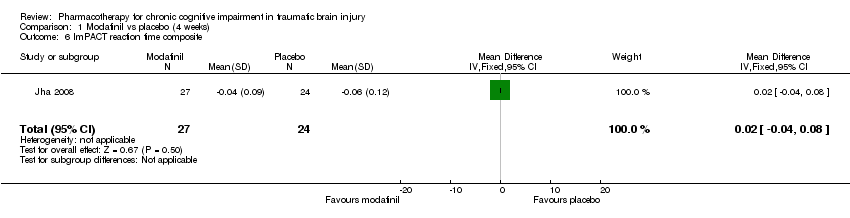
Comparison 1 Modafinil vs placebo (4 weeks), Outcome 6 ImPACT reaction time composite.

Comparison 1 Modafinil vs placebo (4 weeks), Outcome 7 CCPT‐II No. of omissions.
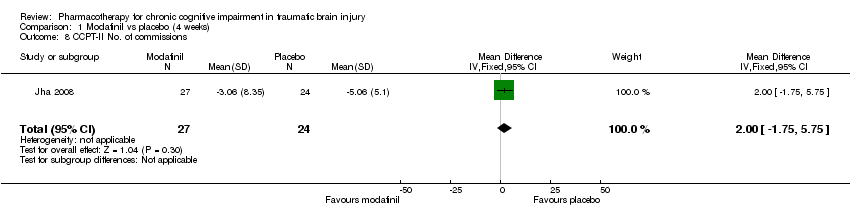
Comparison 1 Modafinil vs placebo (4 weeks), Outcome 8 CCPT‐II No. of commissions.

Comparison 2 Modafinil vs placebo (10 weeks), Outcome 1 SF‐12 Physical.

Comparison 2 Modafinil vs placebo (10 weeks), Outcome 2 SF‐12 Mental.

Comparison 2 Modafinil vs placebo (10 weeks), Outcome 3 ImPACT verbal memory composite.
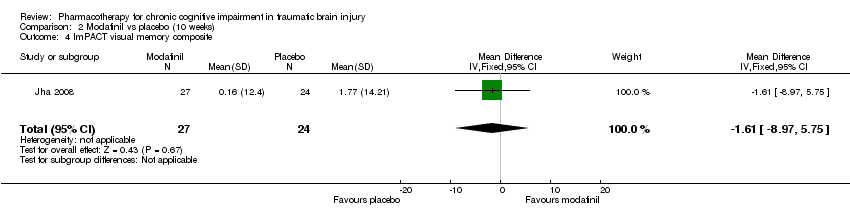
Comparison 2 Modafinil vs placebo (10 weeks), Outcome 4 ImPACT visual memory composite.

Comparison 2 Modafinil vs placebo (10 weeks), Outcome 5 ImPACT visual motor speed composite.

Comparison 2 Modafinil vs placebo (10 weeks), Outcome 6 ImPACT reaction time composite .
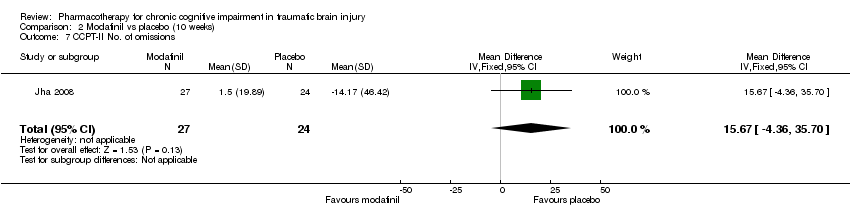
Comparison 2 Modafinil vs placebo (10 weeks), Outcome 7 CCPT‐II No. of omissions.

Comparison 2 Modafinil vs placebo (10 weeks), Outcome 8 CCPT‐II No. of commissions.
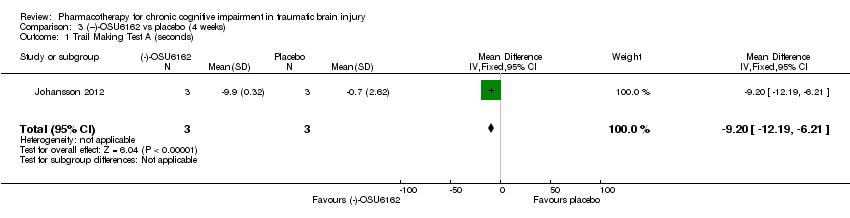
Comparison 3 (−)‐OSU6162 vs placebo (4 weeks), Outcome 1 Trail Making Test A (seconds).

Comparison 3 (−)‐OSU6162 vs placebo (4 weeks), Outcome 2 Trail Making Test B (seconds).
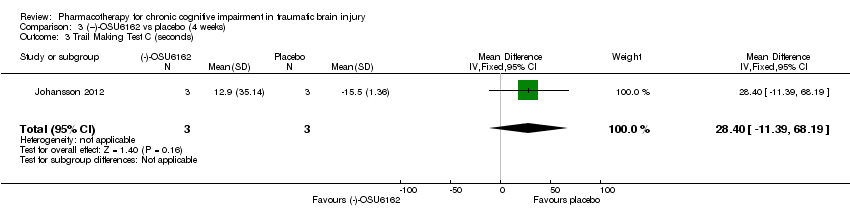
Comparison 3 (−)‐OSU6162 vs placebo (4 weeks), Outcome 3 Trail Making Test C (seconds).

Comparison 3 (−)‐OSU6162 vs placebo (4 weeks), Outcome 4 Trail Making Test D (seconds).

Comparison 3 (−)‐OSU6162 vs placebo (4 weeks), Outcome 5 WAIS‐III Digit Symbol Coding.
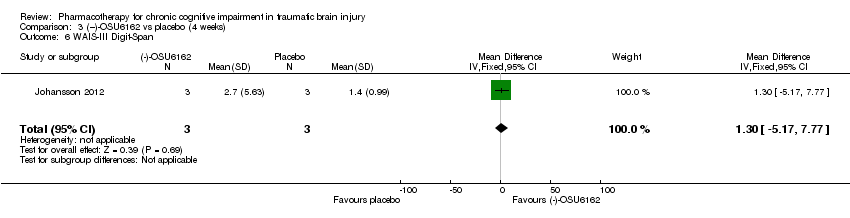
Comparison 3 (−)‐OSU6162 vs placebo (4 weeks), Outcome 6 WAIS‐III Digit‐Span.
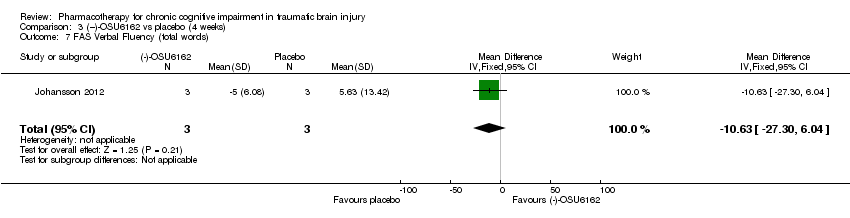
Comparison 3 (−)‐OSU6162 vs placebo (4 weeks), Outcome 7 FAS Verbal Fluency (total words).
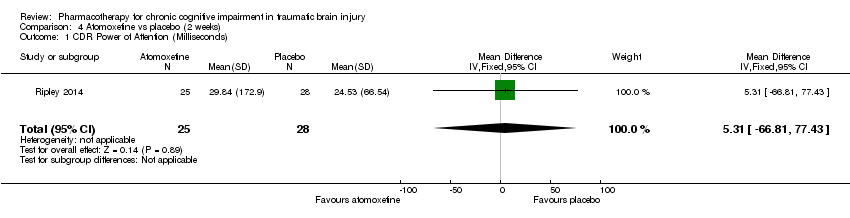
Comparison 4 Atomoxetine vs placebo (2 weeks), Outcome 1 CDR Power of Attention (Milliseconds).
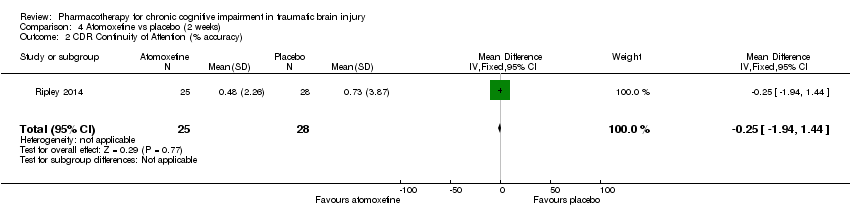
Comparison 4 Atomoxetine vs placebo (2 weeks), Outcome 2 CDR Continuity of Attention (% accuracy).

Comparison 4 Atomoxetine vs placebo (2 weeks), Outcome 3 CDR Efficiency (COA/POA, % accuracy/millisecond).
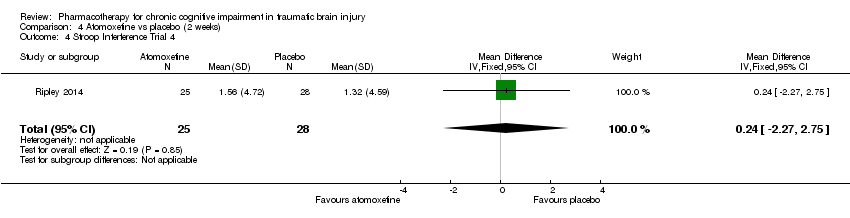
Comparison 4 Atomoxetine vs placebo (2 weeks), Outcome 4 Stroop Interference Trial 4.

Comparison 4 Atomoxetine vs placebo (2 weeks), Outcome 5 Adult ADHD Self‐Report Scale (ASRS‐v1.1.).

Comparison 5 Rivastigmine vs placebo (12 weeks), Outcome 1 HVLT‐total word recall.
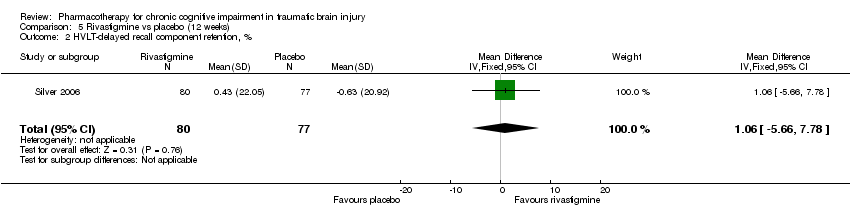
Comparison 5 Rivastigmine vs placebo (12 weeks), Outcome 2 HVLT‐delayed recall component retention, %.

Comparison 5 Rivastigmine vs placebo (12 weeks), Outcome 3 HVLT–recognition discriminant index.

Comparison 5 Rivastigmine vs placebo (12 weeks), Outcome 4 CANTAB RVIP’A.

Comparison 5 Rivastigmine vs placebo (12 weeks), Outcome 5 CANTAB–SWM, total errors.

Comparison 5 Rivastigmine vs placebo (12 weeks), Outcome 6 CANTAB RVIP, mean latency, ms.

Comparison 5 Rivastigmine vs placebo (12 weeks), Outcome 7 CANTAB‐RT, simple reaction time, ms.

Comparison 5 Rivastigmine vs placebo (12 weeks), Outcome 8 CANTAB‐PAL, total errors.
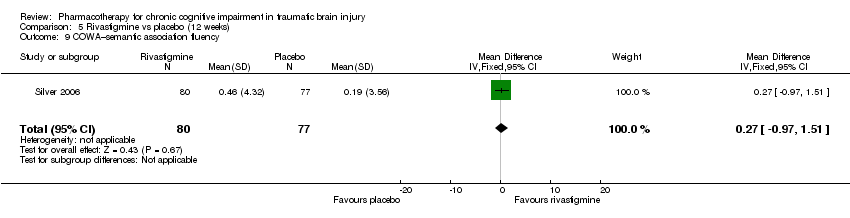
Comparison 5 Rivastigmine vs placebo (12 weeks), Outcome 9 COWA–semantic association fluency.
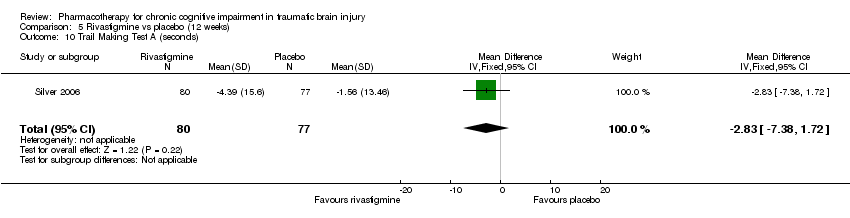
Comparison 5 Rivastigmine vs placebo (12 weeks), Outcome 10 Trail Making Test A (seconds).
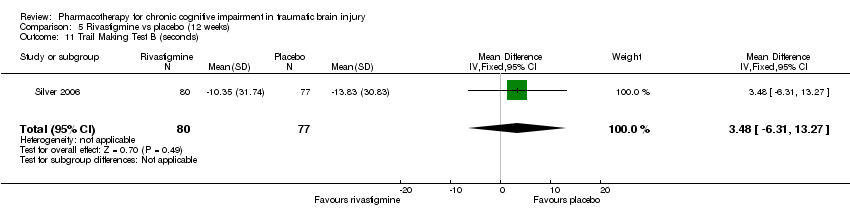
Comparison 5 Rivastigmine vs placebo (12 weeks), Outcome 11 Trail Making Test B (seconds).

Comparison 5 Rivastigmine vs placebo (12 weeks), Outcome 12 WAIS‐III‐DS scaled score.

Comparison 6 Acceptability of treatment, Outcome 1 Modafinil vs placebo.

Comparison 6 Acceptability of treatment, Outcome 2 Rivastigmine vs placebo.

Comparison 6 Acceptability of treatment, Outcome 3 (‐)‐OSU6162 vs placebo.

Comparison 6 Acceptability of treatment, Outcome 4 Atomoxetine vs placebo.

Comparison 7 Modafinil vs placebo adverse events, Outcome 1 Dizziness.

Comparison 7 Modafinil vs placebo adverse events, Outcome 2 Dysgeusia.

Comparison 7 Modafinil vs placebo adverse events, Outcome 3 Dyspepsia.

Comparison 7 Modafinil vs placebo adverse events, Outcome 4 Fatigue.

Comparison 7 Modafinil vs placebo adverse events, Outcome 5 Insomnia.

Comparison 7 Modafinil vs placebo adverse events, Outcome 6 Memory impairment.
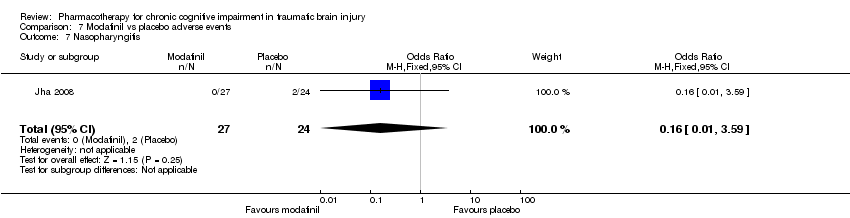
Comparison 7 Modafinil vs placebo adverse events, Outcome 7 Nasopharyngitis.

Comparison 7 Modafinil vs placebo adverse events, Outcome 8 Nausea.

Comparison 7 Modafinil vs placebo adverse events, Outcome 9 Weight loss.
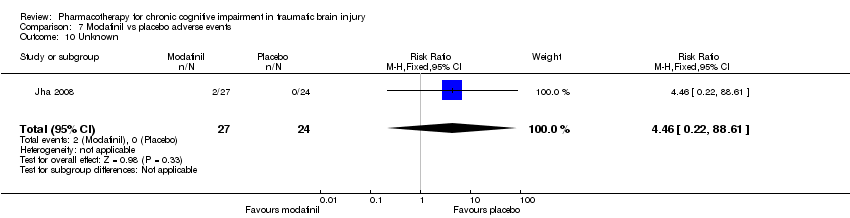
Comparison 7 Modafinil vs placebo adverse events, Outcome 10 Unknown.

Comparison 8 (−)‐OSU6162 vs placebo adverse events, Outcome 1 Nausea.
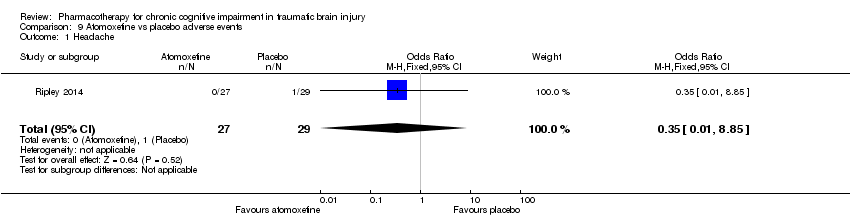
Comparison 9 Atomoxetine vs placebo adverse events, Outcome 1 Headache.
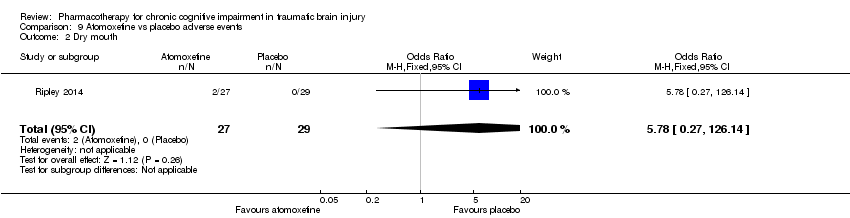
Comparison 9 Atomoxetine vs placebo adverse events, Outcome 2 Dry mouth.

Comparison 9 Atomoxetine vs placebo adverse events, Outcome 3 Globus pharyngeus.

Comparison 9 Atomoxetine vs placebo adverse events, Outcome 4 Hypertension.

Comparison 9 Atomoxetine vs placebo adverse events, Outcome 5 Insomnia.

Comparison 9 Atomoxetine vs placebo adverse events, Outcome 6 Irritable bowl.

Comparison 9 Atomoxetine vs placebo adverse events, Outcome 7 Loss of appetite.

Comparison 9 Atomoxetine vs placebo adverse events, Outcome 8 Nasal congestion.

Comparison 9 Atomoxetine vs placebo adverse events, Outcome 9 Shoulder pain.
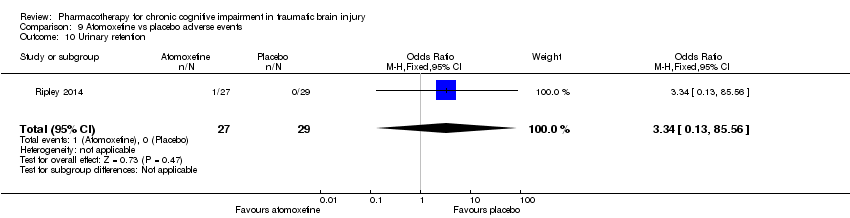
Comparison 9 Atomoxetine vs placebo adverse events, Outcome 10 Urinary retention.
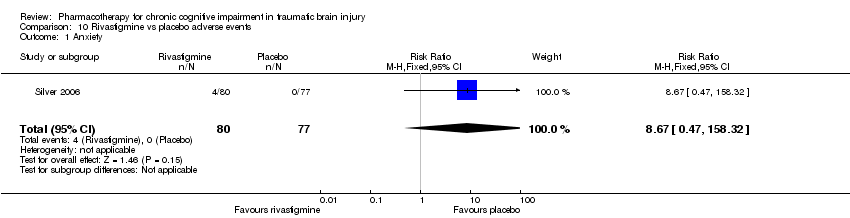
Comparison 10 Rivastigmine vs placebo adverse events, Outcome 1 Anxiety.

Comparison 10 Rivastigmine vs placebo adverse events, Outcome 2 Arthralgia.
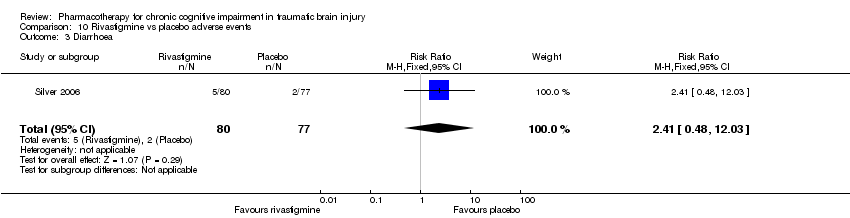
Comparison 10 Rivastigmine vs placebo adverse events, Outcome 3 Diarrhoea.

Comparison 10 Rivastigmine vs placebo adverse events, Outcome 4 Dizziness.

Comparison 10 Rivastigmine vs placebo adverse events, Outcome 5 Headache.

Comparison 10 Rivastigmine vs placebo adverse events, Outcome 6 Nausea.

Comparison 10 Rivastigmine vs placebo adverse events, Outcome 7 Upper respiratory tract infection.
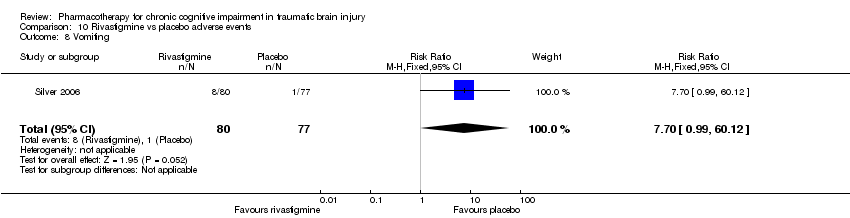
Comparison 10 Rivastigmine vs placebo adverse events, Outcome 8 Vomiting.
| Modafanil, (−)‐OSU6162, atomoxetine or rivastigmine compared to placebo for chronic cognitive impairment in traumatic brain injury | |||||
| Patient or population: Participants with chronic cognitive impairment in traumatic brain injury | |||||
| Outcomes | Effect of drug treatment for people with cognitive impairment in traumatic brain injury | Relative effect | No of Participants | Quality of the evidence | Comments |
| Cognitive performance on psychometric tests | The majority of sub‐tests showed no difference between treatment and placebo. Superiority over placebo was shown in one measure in Silver 2006 and several measures in Johansson 2012 and Johansson 2015. However, interpretation of these findings are cautioned. | See comment. | 274 (4 studies) | ⊕⊝⊝⊝ very low1,2,3 | Data synthesis was not possible due to the heterogeneity of studies. |
| Clinical global improvement | A single study reported no difference between treatment and placebo. | See comment. | 51 (1 study) | ⊕⊕⊝⊝ low1,3 | Data synthesis was not possible as only one study reported a measure on clinical global improvement. |
| Acceptability | No differences between treatment and placebo were found. | See comment. | 274(4 studies) | ⊕⊝⊝⊝ very low1,2,3 | Data synthesis was not possible due to the heterogeneity of studies. |
| Safety | More nausea was reported in participants receiving rivastigmine than placebo (Silver 2006). No other differences were found. | See comment. | 274 (4 studies) | ⊕⊝⊝⊝ very low1,2,3 | Data synthesis was not possible due to the heterogeneity of studies. |
| Mortality | No deaths were reported by any study. | Not estimable. | 274 (4 studies) | ‐ | |
| GRADE Working Group grades of evidence | |||||
| 1 downgraded one level due to serious indirectness, as two of four studies did not investigate cognitive impairment as a primary outcome 2 downgraded one level due to serious inconsistency, due to wide variance of point estimates. 3 downgraded one level due to serious imprecision, as the total population size was less than 400. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SF‐12 Physical Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐2.18 [‐6.34, 1.98] |
| 2 SF‐12 Mental Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 1.95 [‐2.84, 6.74] |
| 3 ImPACT verbal memory composite Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 1.42 [‐4.42, 7.26] |
| 4 ImPACT visual memory composite Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐6.52, 7.02] |
| 5 ImPACT visual motor speed composite Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐3.45 [‐6.48, ‐0.42] |
| 6 ImPACT reaction time composite Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| 7 CCPT‐II No. of omissions Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 16.57 [‐3.59, 36.73] |
| 8 CCPT‐II No. of commissions Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐1.75, 5.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SF‐12 Physical Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐5.46, 4.40] |
| 2 SF‐12 Mental Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐4.28, 5.10] |
| 3 ImPACT verbal memory composite Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 4.11 [‐1.37, 9.59] |
| 4 ImPACT visual memory composite Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐1.61 [‐8.97, 5.75] |
| 5 ImPACT visual motor speed composite Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐1.44 [‐4.61, 1.73] |
| 6 ImPACT reaction time composite Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.08, 0.02] |
| 7 CCPT‐II No. of omissions Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 15.67 [‐4.36, 35.70] |
| 8 CCPT‐II No. of commissions Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 2.96 [‐0.47, 6.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Trail Making Test A (seconds) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐9.20 [‐12.19, ‐6.21] |
| 2 Trail Making Test B (seconds) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐7.81, ‐4.59] |
| 3 Trail Making Test C (seconds) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 28.4 [‐11.39, 68.19] |
| 4 Trail Making Test D (seconds) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 53.5 [36.76, 70.24] |
| 5 WAIS‐III Digit Symbol Coding Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 8.6 [6.47, 10.73] |
| 6 WAIS‐III Digit‐Span Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐5.17, 7.77] |
| 7 FAS Verbal Fluency (total words) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐10.63 [‐27.30, 6.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CDR Power of Attention (Milliseconds) Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 5.31 [‐66.81, 77.43] |
| 2 CDR Continuity of Attention (% accuracy) Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.94, 1.44] |
| 3 CDR Efficiency (COA/POA, % accuracy/millisecond) Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [‐3.10, 4.62] |
| 4 Stroop Interference Trial 4 Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐2.27, 2.75] |
| 5 Adult ADHD Self‐Report Scale (ASRS‐v1.1.) Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐5.63, 3.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HVLT‐total word recall Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐1.14, 1.56] |
| 2 HVLT‐delayed recall component retention, % Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | 1.06 [‐5.66, 7.78] |
| 3 HVLT–recognition discriminant index Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.78, 0.66] |
| 4 CANTAB RVIP’A Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.00] |
| 5 CANTAB–SWM, total errors Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | 1.05 [‐5.85, 7.95] |
| 6 CANTAB RVIP, mean latency, ms Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐44.54 [‐88.62, ‐0.46] |
| 7 CANTAB‐RT, simple reaction time, ms Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐19.81 [‐59.23, 19.61] |
| 8 CANTAB‐PAL, total errors Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐3.16 [‐10.75, 4.43] |
| 9 COWA–semantic association fluency Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.97, 1.51] |
| 10 Trail Making Test A (seconds) Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐2.83 [‐7.38, 1.72] |
| 11 Trail Making Test B (seconds) Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | 3.48 [‐6.31, 13.27] |
| 12 WAIS‐III‐DS scaled score Show forest plot | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.33, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Modafinil vs placebo Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.46 [0.22, 88.61] |
| 2 Rivastigmine vs placebo Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.35, 1.59] |
| 3 (‐)‐OSU6162 vs placebo Show forest plot | 1 | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Atomoxetine vs placebo Show forest plot | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dizziness Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [0.43, 29.66] |
| 2 Dysgeusia Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.46 [0.22, 88.61] |
| 3 Dyspepsia Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.46 [0.22, 88.61] |
| 4 Fatigue Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.44 [0.56, 35.41] |
| 5 Insomnia Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.59, 11.99] |
| 6 Memory impairment Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.46 [0.22, 88.61] |
| 7 Nasopharyngitis Show forest plot | 1 | 51 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.59] |
| 8 Nausea Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.30, 23.96] |
| 9 Weight loss Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.46 [0.22, 88.61] |
| 10 Unknown Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.46 [0.22, 88.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea Show forest plot | 1 | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 8.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache Show forest plot | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.85] |
| 2 Dry mouth Show forest plot | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.78 [0.27, 126.14] |
| 3 Globus pharyngeus Show forest plot | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.13, 85.56] |
| 4 Hypertension Show forest plot | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.13, 85.56] |
| 5 Insomnia Show forest plot | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.78 [0.27, 126.14] |
| 6 Irritable bowl Show forest plot | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.13, 85.56] |
| 7 Loss of appetite Show forest plot | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.13, 85.56] |
| 8 Nasal congestion Show forest plot | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.13, 85.56] |
| 9 Shoulder pain Show forest plot | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.13, 85.56] |
| 10 Urinary retention Show forest plot | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.13, 85.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Anxiety Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.67 [0.47, 158.32] |
| 2 Arthralgia Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.36, 10.21] |
| 3 Diarrhoea Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.48, 12.03] |
| 4 Dizziness Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.7 [0.99, 60.12] |
| 5 Headache Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.35, 2.10] |
| 6 Nausea Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [1.29, 7.22] |
| 7 Upper respiratory tract infection Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.70, 6.74] |
| 8 Vomiting Show forest plot | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.7 [0.99, 60.12] |

