Frecuencia de cambios de apósito para los dispositivos de acceso venoso central en las infecciones relacionadas con el catéter
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: Single centre RCT Sample size calculation: Yes ITT analysis: Yes Ethics and informed consent: Kremlin‐Bicetre, France Registration number and name of registry: Not stated | |
| Participants | Population: Children with a malignancy, who were candidates for high dose chemotherapy and autologous or allogeneic bone marrow transplantation Setting: Paediatric Bone Marrow Transplantation unit at the Gustave Roussy Institute, Villejuif, France Number: A total of 113 patients were randomised, 57 in the 15‐day group and 56 in the 4‐day group. There was 1 post‐randomisation exclusion, results were reported for 112 participants (56 in each group) Age: 15‐day group: median 5 years, range 1‐22 years. 4‐day group: median 7 years, range 2‐19 years Gender (male:female): 15‐day group: 33:23. 4‐day group: 25:31 Skin complexion: 15‐day group: white 43/56; 'mat' 10/56; black 3/56. 4‐day group: white 47/56, 'mat' 6/56, black 3/56 Known allergies to dressings: Not stated Known history of current BSI: Not stated Inclusion criteria: Children with a malignancy, who were candidates for high dose chemotherapy and autologous or allogeneic bone marrow transplant. A qualitative culture of the skin at the catheter entry site was performed before randomisation: only children with a negative culture for Staphyloccus epidermis were eligible Exclusion criteria: Children were only included once in the trial. Those treated with the busulfan‐thiotepa conditioning regimen and those who already had grade ≥ 2 cutaneous toxicity at the catheter dressing site were not eligible | |
| Interventions | Aim: To compare the efficacy of 2 catheter dressing change frequencies (15‐days versus 4‐days) Intervention: Dressing changed every 15 days Control: Dressing changed every 4 days Dressing protocol in both groups: "Three types of dressings were used according to cutaneous toxicity; the adhesive transparent oxygen‐permeable type (Tegaderm) for grade 0 and 1 (48/56; 85% in 15 day group and 32/56; 57% in the 4 day group); the Mefix type for grade 2 and 3 (7/56; 13% in the 15 day group and 23/56; 41% in the 4 day group); and the sterile gauze and tape (American style) dressing (Surgifix, Smith & Nephew, Hull or Velpeau) for grade 4 (1/56; 2% in both the 15 and 4 day groups). Dressings were changed by the nurse in charge of the patient, under sterile conditions: the dressing was cautiously unstuck, the skin was cleaned with a sterile gauze and Hibidil from the catheter entry point towards the periphery. A sterile gauze was then applied under the dressing. The dressing had to cover the catheter entry point as well as the catheter hub, and the upper limit of the extension line, whatever the dressing type." Duration of follow‐up: Daily surveillance of the dressing and its periphery began on the day of randomisation and was continued throughout hospitalisation Numbers lost to follow‐up: 1 child relapsed in the 15‐day arm before HDC Reason for CVAD insertion: HDC for autologous and allogeneic BMT Method of CVAD insertion: "Catheters were all inserted (subclavian site) in the operating room under strict aseptic conditions. Physicians wore a cap, a mask, sterile gloves and a gown. The insertion site was first qualitatively cultured and then prepared with 0.5% alcoholic chlorhexidine (Hibidil). The catheters were then inserted cutaneously using the Seldinger technique, and tunnelled subcutaneously up to 10 cm on average in order to allow rapid removal of the material if severe infectious complications were suspected. In the absence of catheter‐related adverse events, the device was left in place until the patient was discharged from the bone marrow transplant unit." Anatomical location of CVAD: Subclavian site Profession of CVAD inserter: Physician Type of CVAD: Silastic catheters (Vygon) Number of CVAD lumens: Single Dwell time of CVAD: Not stated Study dates: July 1990‐April 1993 | |
| Outcomes | Primary outcomes CRBSI: Not included Suspected CRBSI: Blood cultures were taken in the event of fever above 38.5°C and/or signs of local infection All‐cause mortality: Reported mortality with causes Secondary outcomes Catheter‐site infection: Bacteriological samples were taken from skin around the catheter entry point, using plastic agar‐coated slides (Unipath SA, Dardilly, France). All colonies appearing within 48 h of incubation (37°C) were identified by the usual qualitative bacteriological procedures. Catheter entry site cultures were taken in the event of fever above 38.5°C and/or signs of local infection. Skin damage: Skin toxicity at the catheter dressing site and its periphery. Skin toxicity classified as grade 0: healthy skin; grade 1: slightly inflamed skin; grade 2: minor cutaneous lesions, dressing difficult to remove; grade 3: lesions reaching periphery of the dressing; grade 4: cutaneous lesions to such and extent that the usual dressing could no longer be used Pain: Pain during and between dressing changes. Local pain (classified as none, moderate or severe) during the dressing change and between dressing changes Quality of life: Not included Cost: Not included Other outcomes reported in the trial None Inter‐rater reliability: As dressing changes were performed by many different nurses, the skin toxicity grading scale was tested during the 6 months preceding the trial so that the different nursing teams could familiarise themselves with its use Time points: Daily surveillance of the dressing and its periphery began on the day of randomisation and was continued throughout hospitalisation. Whenever the dressing was changed, the grade of skin toxicity was recorded | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Evidence: "Computer‐generated list was used to allocate patients" Comment: Adequate generation of the randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Evidence: Not stated in the trial report Comment: Unable to judge |
| Blinding of participants and personnel (performance bias) | High risk | Evidence: Not stated in the trial report Comment: Not possible to blind the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Evidence: Not stated Comment: Although it would have been possible to blind outcome assessment, we were unable to ascertain if this was done |
| Incomplete outcome data (attrition bias) | Low risk | Evidence: "One patient relapsed after randomisation and did not receive high dose chemotherapy (15‐day group). The analysis presented here thus concerns 56 patients in each group." Comment: We do not believe that the loss of 1 patient would have affected results |
| Selective reporting (reporting bias) | Unclear risk | Evidence: All planned outcomes reported Comment: No published protocol. We did not request a copy of the protocol from the trialist |
| Other bias | High risk | Evidence: Different dressings according to skin damage Comment: Different dressing protocols may have introduced a bias |
| Methods | Study design: Single centre RCT Sample size calculation: Not stated ITT analysis: Not stated Ethics and informed consent: Local ethics committee Registration number and name of registry: Not stated | |
| Participants | Population: "Patients with haematological malignancies and severe aplastic anaemia, in need of a permanent central venous catheter." Setting: In‐patient unit, Karolinska Hospital, Stockholm, Sweden Number: The abstract states "thirty‐two consecutive patients with haematological disorders . . . were randomly allocated to have their CVC bandages changed once (n=20) or twice (n=19) a week. However, the 'Methods' section of the paper states "Thirty‐one consecutive patients with haematological malignancies and one patient with severe aplastic anaemia, in need of a permanent CVC, were allocated randomly to have their CVC bandages changed, 16 in the once a week group and 16 in the twice a week group." In the results section, tables reported a total of 39 patients. It seems that 32 patients, who had a total of 39 catheters were randomised Age: Once‐weekly group: median 46 years, range 18‐85 years. Twice‐weekly group: median 50 years, range 22‐84 years Gender (male:female): Once‐weekly group: 8:8. Twice‐weekly group: 10:6 Skin complexion: Not stated Known allergies to dressings: Not stated Known history of current BSI: Not stated Inclusion criteria: Not stated Exclusion criteria: Not stated | |
| Interventions | Aim: To determine whether a reduction of dressings from twice to once weekly could be performed safely in neutropenic patients Intervention: Once‐weekly dressing changes Control: Twice‐weekly dressing changes Dressing protocol in both groups: "CVC changes were performed by the nurse responsible for the patient at the ward. The catheter exit site was cleaned with 70% ethanol and a transparent polyurethane dressing Tegaderm (3M) was applied to the area. No other bandages were used, thus allowing the attending nurse to inspect the exit site daily. The presence of erythema or other signs of infection was noted and documented. In the presence of erythema, a gauze dressing moistened with 10% ethanol with aluminium acetotartrate 10% was used. When erythema or other signs of infection had disappeared the patient returned to the allocated changing interval." Patients in the once‐weekly group had more extra dressings due to erythema compared to the twice‐weekly group (3%; 0‐91% once‐weekly group; 0%; 0‐17% twice‐weekly group; P value 0.08 expressed as extra dressings days per CVAD days) Deviation from planned dressing day: Not stated Number of dressing changes during dwell time of CVAD: Not stated Duration of follow‐up: Daily skin assessments until 120 days post CVAD insertion Numbers lost to follow‐up: 12 patients died (Once‐weekly group 6; Twice‐weekly group 6). 2 patients dislocated CVCs. 2 CVC tip cultures not obtained. 23 CVCs (14 Once‐weekly group and 9 Twice‐weekly group) for analysis Reason for CVAD insertion: In need of a permanent CVC Method of CVAD insertion: Inserted under aseptic conditions in an operating theatre Anatomical location of CVAD: 39 catheters were inserted in 32 patients' internal jugular (Once‐weekly group 2; Twice‐weekly group 2); external jugular (Once‐weekly group 5; Twice‐weekly group 4); subclavian (Once‐weekly group 13; Twice‐weekly group 13) and tunnelled subcutaneously for a distance of approximately 15 cm to an exit site at the anterior of the thorax Profession of CVAD inserter: Not stated Type of CVAD: Silicone catheter Number of CVAD lumens: Single Dwell time of CVAD: Once‐weekly group: median 39.5 days (range 8‐114 days); Twice‐weekly group: median 46 days (range 13‐120+ days) Study dates: Not stated | |
| Outcomes | Primary outcomes CRBSI: Not included. Suspected CRBSI: Local catheter infections defined as > 15 CFU at catheter tip culture. Positive blood culture defined as growth of bacteria in at least 1 sample from a peripheral vein, and for coagulase‐negative staphylococci growth in at least 2 of the 3 cultures taken. The CVC was removed aseptically. During granulocytopenia (< 0.5 x 109L‐1) 3 separate cultures were obtained (2 from a peripheral vein and 1 from the central line) for aerobic and anaerobic cultures at start of fever (temperature > 38.0°C on 2 occasions with at least a 4‐h interval, or > 38.5°C on 1 occasion). Additional blood cultures were obtained before change of antibiotic therapy in patients with a persistent fever All‐cause mortality: Reported Secondary outcomes Catheter‐site infection: Skin cultures at exit site graded into 2 categories: < 10 CFU per plate or ≥ 10 CFU per plate. CVC tip cultures Skin damage: Days with erythema at the exit site, temperature > 38°C, antibiotic therapy and the need for extra dressings. Erythema surrounding the exit site was graded into 2 categories: mild erythema, not requiring extra change of dressing or extensive erythema or other signs of local infection requiring extra daily changes Pain: Not included Quality of life: Not included Cost: Not included Other outcomes reported in the trial Number of catheters removed due to complications: The catheters were followed for the first 120 days after insertion Overall catheter survival time Validity of measures: Not stated Inter‐rater reliability: CVC changes were performed by the nurse responsible for the patient at the ward Time points: Skin cultures samples for bacterial culture were obtained from the skin at the exit site and from the skin next to the transparent dressing at the time of changing the bandages | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence: Randomisation envelopes mixed manually Comment: This information was sought from the author; it was not reported in the publication |
| Allocation concealment (selection bias) | Unclear risk | Evidence: Randomisation envelopes mixed manually Comment: This information was sought from the author; it was not reported in the publication |
| Blinding of participants and personnel (performance bias) | High risk | Evidence: Not stated Comment: Not possible to blind the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Evidence: Not stated Comment: Not possible |
| Incomplete outcome data (attrition bias) | Low risk | Evidence: 6 participants died in each group. Results analysed by catheter, not by participant for most outcomes Comment: Equal numbers died in each group. Consequently we judged this element to be at low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Evidence: All planned outcomes reported Comment: Protocol not reviewed |
| Other bias | High risk | Evidence 1: The trial was stopped early, following an interim analysis, when it became clear that differences in the primary outcome would not be found in the time available for the study, this may or may not indicate a potential bias Comment 1: Based on unequal numbers between the number of participant recruited (32) and the numbers reported in the tables (39), it seems as though results were based on the number of catheters, not the number of participants. Consequently, there is, potentially, risk of a 'Unit of analysis' error Evidence 2: Different dressings according to skin damage Comment 2: Different dressing protocols may have introduced a bias |
| Methods | Study design: Multi‐centre RCT Sample size calculation: Not stated ITT analysis: Not stated Ethics and informed consent: Ethical Committee of Azienda Ospedaliera Careggi, Florence, Italy Registration number and name of registry: Not stated | |
| Participants | Population: "Patients undergoing bone marrow transplantation (either autologous, allogeneic from sibling or unrelated donors, or recipients of autologous peripheral blood stem cells)." Setting: 7 Italian BMT centres Number: "399 consecutive patients were enrolled: 230 patients with a tunnelled CVC: 10‐day group 118/230 and 5‐day group 112/230; 169 patients with a non‐tunnelled CVC: 5‐day group 85/169 and 2‐day group 84/169." Age: Not reported Gender (male:female): Not reported Skin complexion: Not reported Known allergies to dressings: Not reported Known history of current BSI: Not reported Inclusion criteria: "Consecutive patients undergoing BMT (either autologous, allogeneic from sibling or unrelated donors, or recipients of autologous peripheral blood stem cells)." Exclusion criteria: "Patients with active cutaneous lesions at the site of CVC insertion at the time of enrolment, patients with known allergy to polyurethane dressings and patients with generalized dermatologic diseases." | |
| Interventions | Aim: To compare 2 different time interval protocols for CVC dressing in order to assess the effects on local infections and toxicity Intervention: Tunnelled CVC 10‐day dressing changes. Non‐tunnelled CVC 5‐day dressing changes Control: Tunnelled CVC 5‐day dressing changes. Non‐tunnelled CVC 2‐day dressing changes Dressing protocol in both groups: "A detailed protocol for CVC dressing under controlled sterile conditions was prepared, and all nurses involved in CVC maintenance were asked to adhere strictly to it for the whole study period; it was the responsibility of each Center’s coordinator to ensure the correct performance of the protocol. Sterile, polyurethane transparent adherent dressings (Tegaderm, 3M) were used for the CVC dressing." Number of dressing changes during dwell time of CVAD: Not stated Duration of follow‐up: Every dressing change until CVAD removal Numbers lost to follow‐up: Tunnelled CVC: 70/230. Non‐tunnelled: 70/169 Reason for CVAD insertion: BMT Method of CVAD insertion: Not stated Anatomical location of CVAD: Not stated Profession of CVAD inserter: Not stated Type of CVAD: Not stated Number of CVAD lumens: Not stated Dwell time of CVAD: Not stated Study dates: March 1996‐October 1997 | |
| Outcomes | Primary outcomes CRBSI: Not included Suspected CRBSI: Not included All‐cause mortality: Not included Secondary outcomes Catheter‐site infection: Cultures for bacterial and fungal agents were set up according to established methodologies used in the microbiology department of each Center’s central hospital laboratory Skin damage: Severity of local skin toxicity directly attributable to the dressing procedure itself. Cutaneous lesions were graded according to the ECOG scale. A specific data form sheet was made available for recording ECOG grading in each patient for each dressing. The following parameters were carefully checked at all dressing changes and at the time of CVC removal: erythema, swelling, tenderness, induration, pain, pruritus, and purulence Pain: Not included Quality of life: Not included Cost: Calculations were made using an exchange rate of USD 1 = ITL 1700. The actual (net) cost of a nurse in an Italian public hospital was about USD 10/hour. Calculations were based on the assumption that the mean hospital stay for an allogeneic patient with a tunnelled CVC was about 40 days (corresponding to a total of 20 dressing changes according to the standard protocol and to 4 changes in the new protocol); the assumption for an autologous BMT recipient with a non‐tunnelled CVC was about 20 days (corresponding to a total of 10 dressing changes in the standard protocol and 4 with the new one). Median time per dressing was calculated from the scheduled time of PNR (10 min), the Clock Survey from Azienda Ospedaliera Careggi, Florence (20 min), and the time measured at the bed‐side in the BMT Unit in Florence (13 min) Other outcomes reported in the trial None Validity of measures: Not stated Inter‐rater reliability: A detailed protocol for CVC dressing under controlled sterile conditions was prepared, and all nurses involved in CVC maintenance were asked to adhere strictly to it for the whole study period; it was the responsibility of each centre's co‐ordinator to ensure the correct performance of the protocol Time points: Skin swabs were taken from the site of CVC insertion in all patients enrolled in the study at the time of admission to the BMT Unit (basal sample) and later on at 10‐day intervals during the BMT procedure for the whole period of the patients’ stay in hospital | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Evidence: Not stated in the trial report Comment: We were unable to judge the adequacy of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Evidence: Not stated Comment: We were unable to judge the adequacy of allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Evidence: Not stated Comment: Not possible to blind the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Evidence: Not stated Comment: It would have been possible to blind assessment of the study outcomes, but this was not stated in the paper |
| Incomplete outcome data (attrition bias) | Low risk | Evidence: All withdrawn patients accounted for Comment: All data complete |
| Selective reporting (reporting bias) | Unclear risk | Evidence: All planned outcomes reported Comment: Protocol not reviewed |
| Other bias | Unclear risk | Evidence: Comment: As no baseline data were published, it was unclear if groups were matched for important risk factors |
| Methods | Study design: Multi‐centre, 2 x 2 factorial, RCT Sample size calculation: Yes ITT analysis: Yes Ethics and informed consent: Grenoble University Hospital Ethics Committee, France Registration number and name of registry: NCT00417235 www.clinicaltrials.gov | |
| Participants | Population: "Patients expected to require an arterial catheter, central‐vein catheter, or both inserted for 48 hours or longer in ICU." Setting: 7 ICUs (2 medical, 2 surgical, 3 medical‐surgical) in 3 university and 2 general hospitals in France. Number: 1653 patients randomised: 416 in the 3‐day standard dressing group; 412 in the 3‐day CHGIS group; 412 in the 7‐day standard dressing group; 413 in the 7‐day CHGIS group Age: Median 63 years (IQR 50‐74) Gender (male:female): 1052:584 Skin complexion: Not stated Known allergies to dressings: Patients with a history of allergy to chlorhexidine or to transparent dressings were excluded Known history of current BSI: Not stated Inclusion criteria: "Patients older than 18 years expected to require an arterial catheter, central‐vein catheter, or both inserted for 48 hours or longer in ICU. CVC inserted in the study ICU or immediately before by the anaesthetist in the emergency unit or in the operating room. CVC inserted under maximal barrier precautions." Exclusion criteria: "Patients with a history of allergy to chlorhexidine or to transparent dressings. Pulmonary arterial, haemodialysis and PICCs were not included. Antiseptic and antibiotic impregnated CVCs were not included. CVC inserted under emergency conditions. CVC not inserted under maximal barrier precautions." | |
| Interventions | Aim: To assess superiority of CHGIS dressings (Biopatch, Ethicon, New Jersey, USA) regarding the rate of major CRIs (clinical sepsis with or without bloodstream infection) and non‐inferiority (less than 3% colonisation‐rate increase) of 7‐day versus 3‐day dressing changes Intervention: 7‐day CHGIS group and 7‐day standard dressing group Control: 3‐day CHGIS group and 3‐day standard dressing group Dressing protocol in both groups: "The same semipermeable transparent dressing (Tegaderm; 3M Inc, St Paul, Minnisota) were used in all 4 treatment groups. The dressing was changed 24 hours after catheter insertion (day 1) and then as randomised. The alcohol‐based povidone‐iodine solution was used for skin antisepsis during dressing changes. In the CHGIS group, the CHGIS dressing was applied to the entire skin surface at and around the insertion site. The semitransparent dressing was then applied. A new CHGIS was used at each dressing change." Deviation from planned dressing day: "Leakage or soiling prompted immediate dressing change." Number of dressing changes during dwell time of CVAD: "Median 3 dressing changes per catheter (IQR 1‐5)." Duration of follow‐up: Until 48 h after ICU discharge Numbers lost to follow‐up: 7‐day CHGIS group: 4 withdrew consent; 52 catheters/19 participants excluded from per protocol analysis 7‐day standard group: 3 withdrew consent; 57 catheters/22 participants excluded from per protocol analysis 3‐day CHGIS group: 4 withdrew consent; 54 catheters/29 participants excluded from per protocol analysis 3‐day standard group: 6 withdrew consent; 83 catheters/41 participants excluded from per protocol analysis Reason for CVAD insertion: ICU admission Method of CVAD insertion: "All study centers followed French recommendations for catheter insertion and care, which are similar to recommendations from the CDC. Maximal sterile barrier precautions (large sterile drape; surgical hand antisepsis; and mask, cap, sterile gloves, and gown) were used at catheter insertion. The insertion site was scrubbed with 4% aqueous povidone iodine solution (Betadine Scrub; Viatris Pharmaceuticals, Merignac, France), rinsed with sterile water, and dried with sterile gauze; an alcohol‐based antiseptic solution (5% povidone‐iodine in 70% ethanol) (Betadine Alcohol‐based Solution, Viatris) was then applied for at least 1 minutes, and sterile drapes were placed around the site." Anatomical location of CVAD: Jugular 560/2051; subclavian 819/2051; femoral 672/2051 Profession of CVAD inserter: Intensivist Type of CVAD: Not stated Number of CVAD lumens: 0 lumens 37/2051; 2 lumens 209/2051; 3 lumens 1805/2051 Dwell time of CVAD: "Median 6 days (IQR 4‐10)" Study dates: 20 December 2006‐20 May 2008 | |
| Outcomes | Primary outcomes CRBSI: Major CRI (defined as catheter‐related sepsis with or without bloodstream infection Catheter‐related clinical sepsis without bloodstream infection defined as fever ≥ 38.5°C or ≤ 36.5°C; catheter tip culture yielding ≥ 10³ CFU/ml; pus at the insertion site or resolution of clinical sepsis after catheter removal; absence of any other infectious focus CRBSI was defined as a combination of ≥ 1 positive peripheral blood cultures sampled immediately before or within 48 h after catheter removal; a quantitative catheter‐tip culture testing positive for the same micro‐organism or a differential time to positivity of blood cultures ≥ 2 h; no other infectious focus explaining the positive blood culture result Suspected CRBSI: Not included All‐cause mortality: Reported Secondary outcomes Catheter‐site infection: Skin colonisation assessed by the semi‐quantitative insertion‐site skin cultures at catheter removal Skin damage: The condition of the skin was described on a standardised form by the nurse in charge of the patients at each dressing change and at catheter removal, using the International Contact Dermatitis Research Group system (0, normal skin; 1, mild erythema; 2, red and slightly thickened skin; 3, intense redness and swelling with coalesced large blisters or spreading reaction) Pain: Not included Quality of life: Not included Cost: Not included Other outcomes reported in the trial None Validity of measures: French (Timsit) and US (Mermel) guidelines Inter‐rater reliability: Not stated Time points: Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Evidence: "The randomization schedule, stratified by ICU, was developed using a Web‐based random‐number generator to select permuted blocks of 8 patients each." Comment: Adequate method for sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Evidence: Not stated in the trial report Comment: We were unable to judge the adequacy of allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Evidence: "The study was not blinded for the investigators or ICU staff. Double‐blinding was not feasible, because visually identical sponges without chlorhexidine were not available and the nurses had to be informed of the dressing change interval." Comment: It was not possible to undertake blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Evidence: "The study was blinded for the microbiologists processing the skin and catheter cultures and for the assessors. A blinded procedure was used for the catheter cultures. Independent assessors conducted blind review of all suspected catheter infections." Comment: Adequate method for blinding outcome assessor used |
| Incomplete outcome data (attrition bias) | Low risk | Evidence: "1653 patients were enrolled, but subsequently 17 withdrew consent to participate, leaving 1636 available for inclusion in the ITT analysis." Comment: Similar numbers were reported in both groups in the ITT analysis |
| Selective reporting (reporting bias) | Low risk | Evidence: Planned outcomes in methods section and in the protocol (clinicaltrials.gov) were reported in the paper Comment: Although we were unable to extract primary outcome data for this review (because of the way it was reported) the planned outcomes were reported in the paper |
| Other bias | Unclear risk | Evidence: "The number needed to treat with CHGIS dressings was 117 catheters (95%CI, 86‐1020). Treatment for 10 days usually requires 3 dressings, each of which costs US$6 (2007 $), and the cost of preventing a single episode of major C‐RI can be estimated at $2106 (95%CI $1518‐$18360). The cost of managing a single case of major C‐RI ranges from $8000 to more than $28000, suggesting the CHGIS dressings may be a cost saving." Comment: Uncertain of the NNTB. All data presented per catheter rather than per patient. Author contacted |
| Methods | Study design: Multicentre, RCT Sample size calculation: Not stated ITT analysis: Not stated Ethics and informed consent: Ethical consent not stated. Informed consent obtained Registration number and name of registry: Not stated | |
| Participants | Population: "Adults with acute myeloid leukaemia treated with intensive chemotherapy containing cytosine‐arabinoside (Ara‐C) and anthracyclines." Setting: Hemato‐Oncology Department, University Hospital Number: Once‐weekly (every 7 days) group: 39 participants. Twice‐weekly (every 3‐4 days) group: 42 participants Age: Once‐weekly group mean age 41.4 years (± 14.9). Twice‐weekly group mean age 49.9 years (± 10.7) Gender (male:female): Once‐weekly group: 19:20. Twice‐weekly group: 16:26 Skin complexion: Not stated Known allergies to dressings: Patients were excluded Known history of current BSI: Not stated Inclusion criteria: "Adults with acute myeloid leukaemia treated with intensive chemotherapy containing cytosine‐arabinoside (ara‐c) and anthracyclines were included in the observation." Exclusion criteria: "Patients with damaged skin at baseline, those allergic to disinfectant, acrylate, or polyurethane, and patients with radiotherapy of the chest in their history were excluded." | |
| Interventions | Aim: To gain experience and to verify whether prolonging the dressing change interval would really be of any benefit and be safe Intervention: Dressings changed once weekly (every 7 days) Control: Dressings changed twice weekly (every 3‐4 days) Dressing protocol in both groups: "Transparent polyurethane semi‐permeable occlusive dressings (Bioclusive, Johnson and Johnson). The dressing could be changed sooner in case of an unstitched, loose, or soiled dressing, insertion‐site inflammation, local cutaneous damage, in‐site bleeding, or other significant (technical) reason." Deviation from planned dressing day: Once‐weekly group: 58% dressing changes as per protocol; Twice‐weekly group: 80% dressing changes as per protocol Number of dressing changes during dwell time of CVAD: "Once‐weekly group: mean number of occlusive dressing changes 4.5 (± 2.4). Twice‐weekly group: mean number of occlusive dressing changes 5.9 (± 2.5)." Duration of follow‐up: "Local cutaneous damage was assessed daily." Numbers lost to follow‐up: All patients accounted for Reason for CVAD insertion: Treatment with intensive chemotherapy Method of CVAD insertion: "Povidone‐iodine was used for skin disinfection at the time of CVC insertion and before any occlusive dressing application." Anatomical location of CVAD: Vena subclavia Profession of CVAD inserter: Not stated Type of CVAD: Non‐tunnelled polyurethane CVCs Number of CVAD lumens: Once‐weekly group: 28 catheters with 1 lumen; 6 catheters with 2 lumens; 8 catheters with 3 lumens. Twice‐weekly group: 19 catheters with 1 lumen; 6 catheters with 2 lumens; 14 catheters with 3 lumens Dwell time of CVAD: Not stated Study dates: August 2003‐August 2005 | |
| Outcomes | Primary outcomes CRBSI: Not included Suspected CRBSI: Not included All‐cause mortality: Not included Secondary outcomes Catheter‐site infection: Infection rate and insertion‐site inflammation. The CVC insertion‐site inflammation was defined as local circular redness accompanied, in case of larger reactions, with swelling and pain or palpitation in the area surrounding the point of percutaneous insertion. Reported across both groups. Skin damage: Local cutaneous damage was assessed daily using local institutional grading (0: healthy skin, 1: erythema, 2: erythema with itching or dry desquamation, 3: moist desquamation, exfoliation, 4: deep ulceration, necrosis) Pain: Any pain or discomfort presented during the dressing change was evaluated by patients using visual analogue scoring (VAS) ranging from 0 to 10 (0: no pain, 5: moderate pain, 10: severe pain) Quality of life: Not included Cost: Not included Other outcomes reported in the trial Highest temperature and blood cultures for microbiological testing Tolerance Validity of measures: Not stated Inter‐rater reliability: Not stated Time points: Daily assessment of skin. Skin swabs for microbiological testing were obtained from the area around the CVC insertion‐site on any dressing change before local disinfection. The highest temperature was recorded on a daily basis and blood cultures for microbiological testing were taken from the CVC on the first occurrence of fever (> 38°C) and thereafter as indicated by the medical staff | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Evidence: "The patients were randomized by GraphPad StatMate (GraphPad Software Inc)" Comment: Computer generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Evidence: "As for our randomized trial allocation, we used a Randomization PC Software to allocate the trial patients into the individual cohorts. We did not used sealed envelopes." Comment: The evidence for this 'bias' element was obtained from the trialist through email contact |
| Blinding of participants and personnel (performance bias) | High risk | Evidence: Not possible Comment: Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Evidence: There was no information about outcome assessor blinding in the report Comment: Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Evidence: All of the enrolled patients were accounted for in the results Comment: An equal number of patients (3) in each group were withdrawn due to intolerance of the dressing Consequently, we judged that this domain was at low risk for bias |
| Selective reporting (reporting bias) | Unclear risk | Evidence: All planned outcomes reported Comment: Protocol not reviewed |
| Other bias | Low risk | Evidence: None reported Comment: As there were no 'other' biases reported, we judged this domain to be at low risk |
Abbreviations
BMT: bone marrow transplant
BSI: blood stream infection
CDC: Centers for Disease Control and Prevention
CFU: colony forming unit
CHGIS: chlorhexidine gluconate‐impregnated sponge
CRBSI: catheter‐related bloodstream infection
CRI: catheter‐related infection
CVAD: central venous access device
CVC: central venous catheter
ECOG: Eastern Cooperative Oncology Group
h: hour(s)
HDC: high dose chemotherapy
ICU: intensive care unit
IQR: inter‐quartile range
ITT: intention‐to‐treat (analysis)
min: minute(s)
NNTB: number needed to treat for an additional beneficial outcome
PICC: peripherally inserted central catheter
PNR: patient nurse ratio
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Study protocol | |
| Comparison of different dressing types not frequencies | |
| Letter to the editor (comment on a study comparing two types of dressings) | |
| Conference abstract related to dressing methods ‐ unrelated to timing | |
| The wards involved were randomly allocated, not the patients | |
| Comparison of different dressing types not frequencies | |
| Co‐interventions (frequency of administration set replacement) were different between different arms of the study | |
| Co‐interventions (frequency of administration set replacement) were different between different arms of the study Quasi‐randomisation | |
| Co‐interventions (frequency of administration set replacement) were different between different arms of the study | |
| Systematic literature review of central venous catheter site care for blood and marrow transplant recipients |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Unknown |
| Participants | Paediatrics |
| Interventions | Frequency of dressing changes |
| Outcomes | Unknown |
| Notes | Prospective randomised trial to study the best time interval between catheter dressing: Study performed by the nurses of paediatric transplantation unit. Title found in a reference list. Awaiting paper from publishers |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter related blood stream infection Show forest plot | 1 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.40, 4.98] |
| Analysis 1.1 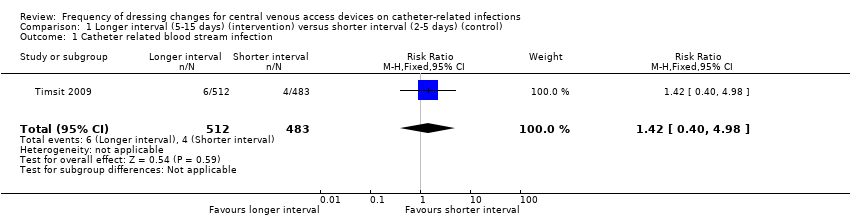 Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 1 Catheter related blood stream infection. | ||||
| 2 Suspected catheter related blood stream infection Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.23, 2.10] |
| Analysis 1.2 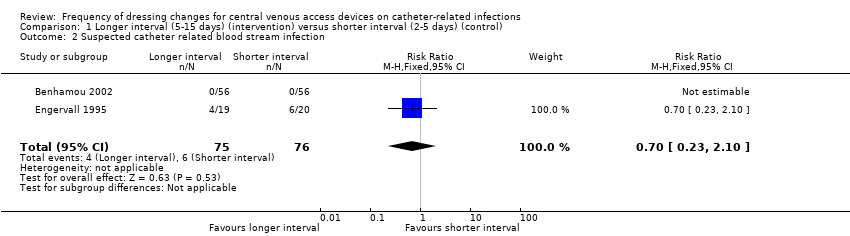 Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 2 Suspected catheter related blood stream infection. | ||||
| 3 All‐cause mortality Show forest plot | 3 | 896 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.25] |
| Analysis 1.3  Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 3 All‐cause mortality. | ||||
| 4 Catheter‐site infection Show forest plot | 2 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.63] |
| Analysis 1.4  Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 4 Catheter‐site infection. | ||||
| 5 Skin damage Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.5 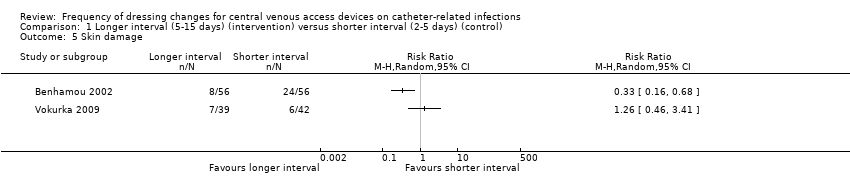 Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 5 Skin damage. | ||||
| 6 Pain Show forest plot | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.46, 1.38] |
| Analysis 1.6 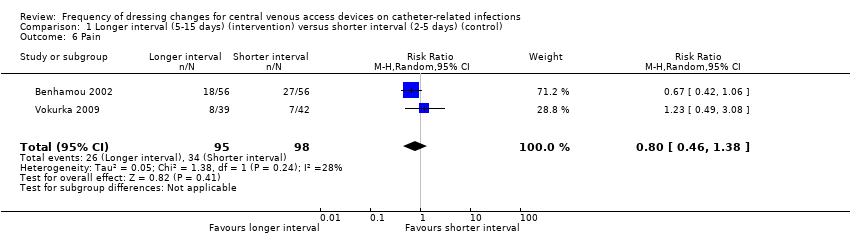 Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 6 Pain. | ||||

Flow diagram of included and excluded studies

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 1 Catheter related blood stream infection.

Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 2 Suspected catheter related blood stream infection.

Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 3 All‐cause mortality.

Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 4 Catheter‐site infection.

Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 5 Skin damage.

Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 6 Pain.
| Patient or population: patients with a central venous access device | ||||||
| Outcomes | Relative effect | Anticipated absolute effects* (95% CI) | Quality of the evidence | What happens | ||
| Without longer interval (5 ‐ 15 days) | With longer interval (5 ‐ 15 days) | Difference | ||||
| Catheter‐related blood stream infection (CRBSI) | RR 1.42 | Study population | ⊕⊕⊝⊝ | Longer intervals between dressing changes may have little or no effect on catheter‐related blood stream infection | ||
| 8 per 1000 | 12 per 1000 | 4 more per 1000 | ||||
| All‐cause mortality | RR 1.06 | Study population | ⊕⊕⊝⊝ | Longer intervals between dressing changes probably have little or no effect on death from any cause | ||
| 354 per 1000 | 375 per 1000 | 21 more per 1000 | ||||
| Skin damage Follow up: unclear | Not estimable | Skin damage was reported in four studies. Two provided data but their results were not combined due to inconsistency of size and direction of the effects. One study in children found less skin damage in the longer interval group (8/56) compared with the shorter interval group (24/56). Rates of skin damage in one study in adults were similar (7/39 in longer interval versus 6/42 in shorter interval).9 | ⊕⊝⊝⊝ | It is uncertain whether longer (compared with shorter) intervals between dressing changes reduce skin damage | ||
| Pain Follow up: unclear | RR 0.80 | Study population | ⊕⊕⊝⊝ | It is uncertain whether longer (compared with shorter) intervals between dressing changes affect pain on dressing removal | ||
| 347 per 1000 | 278 per 1000 | 69 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for risk of bias due to lack of blinding of participants and personnel and for a probable unit of analysis error (individual participants randomised but numbers of infections reported) 2 Downgraded for serious imprecision: result consistent with a reduction in CRBSI or an almost 5 fold increase 3 Downgraded for risk of bias due to lack of blinding of participants and personnel 4 Downgraded for imprecision: result consistent with a 10% reduction in mortality or a 25% increase 5 Downgraded twice for serious risk of bias: risk of performance bias due to lack of blinding of participants and personnel; different dressings were used in response to skin damage 6 Downgraded for inconsistency: experimental and control groups were different between studies and frequency of dressing changes overlapped between longer and shorter groups 7 Downgraded for imprecision 8 Downgraded for risk of bias: blinding of outcome assessment not described 9 Data from two additional RCTs could not be extracted and used within the analysis. One study presented toxicity on a 5‐point scale and reported no differences between groups. We are unable to use the data from the fourth study due to the 2 x 2 factorial design. | ||||||
| CRBSI | Not applicable | Not applicable | Not applicable | High risk | Not applicable |
| Suspected CRBSI | High risk | High risk | Not applicable | Not applicable | Not applicable |
| All‐cause mortality | Low risk | Low risk | Not applicable | Low risk | Not applicable |
| Catheter‐site infection | High risk | High risk | High risk | High risk | High risk |
| Skin damage | High risk | Not applicable | High risk | High risk | High risk |
| Pain | HIgh risk | Not applicable | Not applicable | Not applicable | High risk |
| Quality of life | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Cost | Not applicable | Not applicable | High risk | Not applicable | Not applicable |
| CRBSI | Not applicable | Not applicable | Not applicable | Low risk | Not applicable |
| Suspected CRBSI | Unclear risk | Unclear risk | Not applicable | Not applicable | Not applicable |
| All‐cause mortality | Low risk | Low risk | Not applicable | Low risk | Not applicable |
| Catheter‐site infection | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk |
| Skin damage | Unclear risk | Not applicable | Unclear risk | Unclear risk | Unclear risk |
| Pain | Unclear risk | Not applicable | Not applicable | Not applicable | Unclear risk |
| Quality of life | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Cost | Not applicable | Not applicable | Unclear risk | Not applicable | Not applicable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter related blood stream infection Show forest plot | 1 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.40, 4.98] |
| 2 Suspected catheter related blood stream infection Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.23, 2.10] |
| 3 All‐cause mortality Show forest plot | 3 | 896 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.25] |
| 4 Catheter‐site infection Show forest plot | 2 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.63] |
| 5 Skin damage Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Pain Show forest plot | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.46, 1.38] |

