Administration périopératoire d'Inhibiteurs de l'enzyme de conversion de l'angiotensine ou d'antagonistes des récepteurs de type 1 de l'angiotensine II pour prévenir la mortalité et la morbidité chez les adultes
Résumé scientifique
Contexte
L'hypertension périopératoire nécessite une prise en charge attentive. Les inhibiteurs de l'enzyme de conversion de l'angiotensine (IECA) ou les antagonistes des récepteurs de type 1 de l'angiotensine II (ARA‐II) ont démontré leur efficacité dans le traitement de l'hypertension associée à la chirurgie. Cependant, il n'existe pas de consensus quant à savoir s'ils peuvent prévenir la mortalité et la morbidité.
Objectifs
Evaluer de manière systématique les avantages et les inconvénients de l'administration d'IECA ou d'ARA‐II en périopératoire pour la prévention de la mortalité et de la morbidité chez les adultes (âgés de 18 ans et plus) subissant tout type de chirurgie sous anesthésie générale.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans le numéro actuel du registre Cochrane des essais contrôlés (CENTRAL, 2014, numéro 12), Ovid MEDLINE (de 1966 au 8 décembre 2014), EMBASE (de 1980 au 8 décembre 2014), et dans les références des essais randomisés, méta‐analyses et revues systématiques trouvés. Nous avons réitéré la recherche le 3 février 2017. Trois nouvelles études d'intérêt potentielles ont été ajoutées à une liste d'« Etudes en attente de classification » et seront intégrées aux résultats officiels de la revue au cours de la mise à jour de cette dernière.
Critères de sélection
Nous avons inclus les essais contrôlés randomisés (ECR) comparant l'administration périopératoire des IECA ou des ARA‐II avec un placebo chez les adultes (âgés de 18 ans et plus) subissant tout type de chirurgie sous anesthésie générale. Nous avons exclu les études dans lesquelles les participants avaient subi de procédures qui nécessitaient une anesthésie locale seule, ou les participants à qui on avait déjà administré des IECA ou des ARA‐II.
Recueil et analyse des données
Deux auteurs de la revue ont indépendamment effectué la sélection des études, évalué le risque de biais et extrait les données. Nous avons utilisé les procédures méthodologiques standard prévues par Cochrane.
Résultats principaux
Nous avons inclus sept ECR portant sur un total de 571 participants dans la revue. Deux des sept essais portaient sur 36 participants subissant une chirurgie vasculaire non cardiaque (chirurgie de l'aorte infrarénale), et cinq portaient sur 535 participants subissant une chirurgie cardiaque, y compris des chirurgies valvulaires, des pontages aortocoronariens et des opérations avec circulation extra‐corporelle. L'intervention a été mise en place de 11 jours à 25 minutes avant la chirurgie dans six essais et pendant la chirurgie dans un essai. Nous avons considéré que tous les sept ECR présentaient un risque élevé de biais. Les effets des IECA ou ARA‐II sur la mortalité périopératoire et l'infarctus aigu du myocarde étaient incertains car la qualité des preuves était très faible. Le risque de décès était de 2,7 % dans le groupe des IECA ou ARA‐II et de 1,6 % dans le groupe sous placebo (risque relatif (RR) 1,61 ; intervalle de confiance à 95 % (IC) 0,44 à 5,85). Le risque d'infarctus aigu du myocarde était de 1,7 % dans le groupe des IECA ou ARA‐II et de 3,0 % dans le groupe sous placebo (RR 0,55 ; IC à 95 % 0,14 à 2,26). Les IECA ou ARA‐II peuvent potentiellement améliorer l'insuffisance cardiaque congestive (index cardiaque) en périopératoire (différence moyenne (DM) ‐0,60 ; IC à 95 % ‐0,70 à ‐0,50, preuves de très faible qualité). En termes de taux de complications, il n'y avait aucune différence dans les complications périopératoires cérébrovasculaires (RR 0,48 ; IC à 95 % 0,18 à 1,28, preuves de très faible qualité) et l'hypotension (RR 1,95 ; IC à 95 % 0,86 à 4,41, preuves de très faible qualité). Aucune insuffisance rénale liée à la chirurgie cardiaque n'a été notée. Les IECA ou ARA‐II ont été associés à un raccourcissement de la durée de séjour à l'hôpital (DM ‐0,54 ; IC à 95 % ‐0,93 à ‐0,16, valeur P = 0,005, preuves de très faible qualité). Ces résultats doivent être interprétés avec prudence en raison de risques de confusion dans les antécédents cliniques des patients. Les IECA ou ARA‐II pourraient raccourcir la durée du séjour à l'hôpital (DM ‐0,54 ; IC à 95 % ‐0,93 à ‐0,16, preuves de qualité très médiocre) Deux études ont rapporté des événements indésirables, et il n'y avait aucune preuve d'une différence entre les IECA ou ARA‐II et les groupes témoins.
Conclusions des auteurs
Dans l'ensemble, cette étude n'a pas trouvé de preuves permettant de soutenir que les IECA ou les ARA‐II en périopératoire peuvent prévenir la mortalité, la morbidité et les complications (hypotension, complications périopératoires cérébrovasculaires, insuffisance rénale liée à la chirurgie cardiaque). Nous n'avons trouvé aucune preuve indiquant que l'utilisation de ces médicaments peut réduire le taux de l'infarctus aigu du myocarde. Cependant, les IECA ou les ARA‐II peuvent potentiellement augmenter le débit cardiaque en périopératoire. En raison de la faible et très faible qualité de la méthodologie, d'un risque élevé de biais et du manque de puissance statistique des études incluses, le véritable effet pourrait être sensiblement différent des estimations observées. L'administration périopératoire (principalement en chirurgie cardiaque élective, selon les études incluses) d'un traitement par IECA ou ARA‐II devrait être individualisée.
PICO
Résumé simplifié
Administration de médicaments pour réduire la pression artérielle au moment de l'intervention chirurgicale afin de réduire le risque de décès et de maladie grave chez l'adulte
Question d'analyse
Nous avons examiné les preuves sur deux médicaments utilisés pour diminuer la pression artérielle (inhibiteurs de l'enzyme de conversion de l'angiotensine (IECA) ou antagonistes des récepteurs de type 1 de l'angiotensine II (ARA‐II)) au moment de l'intervention chirurgicale pour réduire le risque de décès et de maladie grave chez les adultes subissant une chirurgie sous anesthésie générale.
Contexte
Les patients ayant une pression artérielle élevée au moment de l'intervention chirurgicale sont traités avec prudence car ils ont un risque plus élevé de complications, telles qu'une réduction du débit sanguin vers le muscle cardiaque (ischémie du myocarde), une crise cardiaque, voire le décès. Les IECA ou ARA‐II détendent les vaisseaux sanguins et sont efficaces dans le traitement de l'hypertension artérielle associée à la chirurgie, mais le résultat est incertain lorsqu'ils sont utilisés pour la prévention des complications liées à la chirurgie.
Caractéristiques de l'étude
Nous avons effectué des recherches dans les bases de données jusqu'au 8 décembre 2014. Nous avons trouvé sept essais contrôlés randomisés (de 1992 à 2014) avec 571 participants qui répondaient à nos critères d'inclusion. Deux des sept essais portaient sur 36 participants subissant une chirurgie vasculaire non cardiaque (chirurgie de l'aorte infrarénale), et cinq portaient sur 535 participants subissant une chirurgie cardiaque, y compris de la chirurgie valvulaire, des pontages aortocoronariens et des opérations avec circulation extra‐corporelle. Les interventions ont commencé de 11 jours à 25 minutes avant la chirurgie dans six essais et pendant l'intervention chirurgicale dans un autre. Les sept études ont été réalisées en Europe et aux États‐Unis. L'une des sept études a été financée par un laboratoire pharmaceutique.
Résultats principaux
Trois essais portant sur 419 participants ont signalé les décès, mais les résultats étaient imprécis et n'apportaient aucune preuve d'une différence entre les groupes d'intervention et les groupes sous placebo (mortalité périopératoire). Deux essais, avec 345 participants, ont rapporté un nombre similaire de participants dans les deux groupes avec des changements dans leur électrocardiogramme qui indiquaient une crise cardiaque (infarctus aigu du myocarde). Le débit cardiaque (index cardiaque) semblait être supérieur dans un seul essai.
Les deux essais qui avaient rendu compte du risque de faible pression artérielle comme complication potentielle de l'intervention n'ont trouvé aucune différence apparente et le risque d'accident vasculaire cérébral (AVC) était similaire avec et sans l'intervention dans trois essais.
Les résultats de trois études ont montré que les IECA ou ARA‐II pourraient réduire la durée de séjour à l'hôpital, mais ces résultats doivent être interprétés avec prudence en raison de l'influence possible des antécédents cliniques des participants étudiés. Deux essais ayant évalué les effets indésirables n'ont trouvé aucune preuve d'une différence entre les IECA ou ARA‐II et un placebo (absence de traitement).
Qualité des preuves
La qualité des preuves pour les résultats était faible ou très faible. Le nombre total de participants était peu élevé. La plupart des participants subissaient une chirurgie cardiaque, ce qui signifie que les résultats ne peuvent pas être généralisés à d'autres types de chirurgie. Nous avons réitéré la recherche le 3 février 2017. Nous traiterons les trois études d'intérêt trouvées lorsque nous mettrons à jour la revue.
Authors' conclusions
Summary of findings
| ACEIs or ARBs compared to placebo for preventing surgery‐related mortality and morbidity in adults | ||||||
| Patient or population: Patients undergoing any type of surgery under general anaesthesia receiving ACEIs or ARBs perioperatively | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | ACEIs or ARBs | |||||
| All‐cause mortality | Study population | RR 1.61 | 419 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total sample size is lower than the calculated. Duration of follow‐up: until discharge from hospital | |
| 16 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 7 per 1000 | 11 per 1000 | |||||
| Risk of acute myocardial ischaemia | Study population | RR 0.55 | 345 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total sample size is lower than the calculated. Duration of follow‐up: until discharge from hospital | |
| 30 per 1000 | 16 per 1000 | |||||
| Moderate | ||||||
| 56 per 1000 | 31 per 1000 | |||||
| Congestive heart failure | The mean cardiac index in the intervention groups was | ‐ | 34 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: not specified | |
| Hypotension | ‐ | RR 1.95 (0.86 to 4.41) | 298 (1 study) | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: not specified | |
| Rate of perioperative cerebrovascular complications | Study population | RR 0.48 | 459 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Duration of follow‐up: until discharge from hospital (Billings 2012; Pretorius 2012); 90 days after surgery (Flesch 2009) | |
| 50 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 71 per 1000 | 34 per 1000 | |||||
| Length of hospital stay | The mean length of hospital stay in the intervention groups was | ‐ | 372 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: until discharge from hospital | |
| Treatment related adverse events | ‐ | ‐ | ‐ | 385 (2 studies) | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Authors did not provided detailed information on adverse events, which made the synthesis of the results less clinically relevant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by three levels due to very serious study limitations (all the trials included were at high risk of bias) and serious imprecision (total population size is less than 400). | ||||||
Background
Hypertension is closely related to cardiac diseases such as myocardial infarction, congestive heart failure, and even sudden death (Lloyd‐Jones 2010). An increasing number of patients scheduled to undergo surgery suffer from hypertension, which is an important contributing factor to perioperative cardiac complications. Patients who undergo non‐cardiac surgery run the risk of myocardial infarction or cardiac death, which also leads to considerably increased costs (Freeman 2009). Perioperative myocardial infarction occurs in 6% of patients, with a mortality rate of 3% in patients with acquired cardiac diseases (Wiesbauer 2007). Due to the high risk of perioperative cardiac complications, strategies for prevention are worth examining (Fleisher 2007).
Description of the condition
As cardiovascular events remain a major threat perioperatively in hypertensive patients undergoing cardiac or non‐cardiac operative procedures, considerable effort has been expended to lower the extent of myocardial ischaemia in these patients. There is also a high economic cost associated with cardiovascular morbidity and mortality. Perioperative medical therapy is one of three categories of interventions intended to reduce the rate of perioperative cardiac complications. Pharmacological therapies include beta‐blockers, alpha 2‐adrenergic agonists, nitrates, diuretics, calcium‐channel blockers, angiotensin‐converting enzyme inhibitors (ACEIs), and angiotensin II receptor blockers (ARBs). Pharmacologic attenuation of sympathetic nervous system activity is thought to improve participant outcomes, and beta‐blockers have been found to reduce perioperative arrhythmias and myocardial ischaemia (Wiesbauer 2007). However, they also seem to be associated with increased mortality and a higher risk of cerebrovascular complications (Devereaux 2008). Several randomized studies showed that alpha 2‐adrenergic agonists did not reduce rates of fatal cardiac events and cardiac death (Ellis 1994; Stuhmeier 1996). Nitrates and calcium‐channel blockers have not shown benefits in reducing the rate of cardiac events (Dodds 1993). Despite the variety of therapeutic interventions available, cardiac complications remain a threat to the safety of patients undergoing surgery.
Description of the intervention
ACEIs and ARBs are two types of effective and widely used antihypertensive drugs targeting the renin‐angiotensin system (RAS). They can be used perioperatively to control hypertension via similar mechanisms. ACEIs prevent the production of angiotensin II from angiotensin I and interfere with the regulation of blood pressure by impairing degradation of bradykinin and inhibit the receptor binding of angiotensin II (Tschope 2002). ARBs exert their vasodilation effect at the receptor level by inhibiting the binding of angiotensin II to the type 1 receptors (AT1R), irrespective of whether angiotensin II is generated by renin‐angiotensin cascade or in local tissues by other means (Zou 2009).
How the intervention might work
Perioperative usage of ACEIs and ARBs is thought to be helpful in controlling high blood pressure. Perioperatively, aggressive and early treatment of hypertensive reactions is suggested to reduce cardio‐cerebral complications. In hypertensive emergencies, intravenous infusion of ACEI enalaprilat lowered blood pressure in more than 60% of participants (Strauss 1984). The use of ACEIs and ARBs may work on multiple foci. Heightened sympathetic nervous system activity contributes greatly to perioperative cardiac complications, and pharmacologic intervention of that pathway improves participant outcomes (Warltier 2000). Research has showed that ACEIs can reduce sympathetic drive and augment vagal tone (Fariello 1989). However, conflicting opinions have been proposed indicating that sympathetic nervous inhibition is not a major component of the blood pressure‐lowering action (Krum 2006). Can RAS inhibitors, apart from decreasing blood pressure, lower hard endpoints like myocardial ischaemia, perioperative mortality, and length of hospitalization?
The RAS are activated during cardiac surgery with cardiopulmonary bypass (CPB), which disturbs the balance of pro‐ and anti‐inflammatory cytokines and modifies regional blood flow, contributing to morbidity (Kwapisz 2004). The systemic vascular resistance index of participants treated with ACEI quinapril was significantly lower compared with those treated with isotonic saline solution, without an increased risk of deleterious haemodynamic episodes (Kwapisz 2004). Thus, administration of RAS inhibitors during cardiac surgery with CPB may lower cardiac complications through modifying regional blood flow during CPB.
In people routinely treated with ACEIs, adrenergic receptor hyporesponsiveness and regression of cardiovascular hypertrophy are thought to be implicated (Licker 1996). Long‐term administration of ACEIs and ARBs has been demonstrated to be beneficial to participants with cardiovascular and renal diseases (Garg 1995; Lee 2004). When taken properly in adequate dosages, ACEIs/ARBs slowed the progression of heart failure and greatly reduced morbidity and mortality for participants with heart failure (Cohn 1991; Hunt 2001). Thus, an increasing number of patients scheduled to undergo cardiac surgery are now chronically treated with ACEIs (Licker 2000). However, some of these patients develop perioperative hypotensive episodes (Coriat 1994; Tuman 1995), which are due to impaired adrenergic vasoconstrictive response in people chronically treated with ACEIs (Licker 2000). In these cases, can perioperative administration of RAS inhibitors for the treatment of hypertensive reactions improve outcomes?
In both diabetic and non‐diabetic proteinuria renal disease, blockade of the RAS is regarded as reno‐protective (Brenner 2003; Nakao 2003). During cardiac surgery with CPB, effective renal plasma flow and glomerular filtration rate decreased in the control group whereas they remained unchanged in the captopril group (Colson 1990). The reno‐protective effect of RAS inhibitors may be beneficial to patients undergoing cardiopulmonary bypass or those with renal diseases.
Why it is important to do this review
The beneficial effects of chronic administration of ACEIs and ARBs have been demonstrated, but the value of the administration of these drugs in the operative setting remains controversial. Different investigators have different opinions (Boeken 1999; Colson 1992; Coriat 1994; Deakin 1998; Di Pasquale 1993; Licker 1996; Pigott 1999; Ryckwaert 2001; Webb 1998). In light of the uncertain evidence for perioperative administration of ACEIs and ARBs, a systematic review of randomized trials appraising the perioperative usage of ACEIs and ARBs is necessary to test whether these two types of RAS inhibitors are suitable for controlling haemodynamic condition and then preventing mortality and morbidity in patients undergoing surgery. Blessberger 2014 conducted a systematic review on perioperative beta‐blockers for preventing surgery‐related mortality and morbidity, but due to the low‐ to moderate‐quality evidence, the authors were uncertain as to whether the intervention had an important effect on these outcomes, which made the present review more interesting.
Objectives
To systematically assess the benefits and harms of administration of ACEIs or ARBs perioperatively for the prevention of mortality and morbidity in adults (aged 18 years and above) undergoing any type of surgery under general anaesthesia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) comparing perioperative administration of ACEIs or ARBs with placebo.
Types of participants
We included adults (aged 18 years and above) undergoing any type of surgery under general anaesthesia receiving ACEIs or ARBs perioperatively, including those participants with pre‐existing hypertension, heart failure, or ventricular dysfunction.
We excluded:
-
participants undergoing procedures that require local anaesthesia only;
-
participants who are already on chronic treatment with ACEIs or ARBs.
Types of interventions
We included trials that compared perioperative administration of any kind of ACEI or ARB via any route versus placebo. ACEIs and ARBs are started preoperatively (after hospital admission), during operation, or up to one day after surgery.
Types of outcome measures
Primary outcomes
-
All‐cause mortality and cardiac mortality (occurring 30 days postoperatively or before hospital discharge).
-
Risk of acute myocardial infarction, defined as the presence of characteristic chest pain, an ST elevation, or increase in myocardial isoenzymes, occurring up to 30 days postoperatively or before discharge from hospital.
-
Risk of myocardial ischaemia (defined as the presence of clinical symptoms and significant ST segment depression, occurring up to 30 days postoperatively or before hospital discharge).
Secondary outcomes
-
Congestive heart failure (occurring 30 days postoperatively or before hospital discharge).
-
Hypotension (defined as hypotension in the individual studies selected for the review).
-
Cerebrovascular complications (diagnosed by clinical symptoms and computed tomography or magnetic resonance imaging).
-
Renal insufficiency (diagnosed by clinical symptoms and laboratory examination, including serum creatinine and urinary diagnostic indices, occurring 30 days postoperatively or before hospital discharge).
-
Length of hospital stay.
-
Treatment related adverse events
Search methods for identification of studies
Electronic searches
We searched the current issue of the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 12), Ovid MEDLINE (1966 to 8 December 2014), and EMBASE (1980 to 8 December 2014).
We reran the search on February 3, 2017. We will deal with the three studies of interest when we update the review.
We retrieved relevant RCTs without language or date restrictions. Please see Appendix 1 for our search terms.
We searched for ongoing clinical trials and unpublished studies via:
Searching other resources
We screened the references of the retrieved randomized trials, meta‐analyses, and systematic reviews for additional trials. We contacted the main authors of studies to ask for missing or unreported data.
Data collection and analysis
Selection of studies
After searching the literature, we reviewed the titles and abstracts of all studies identified, and determined which publications were suitable for further consideration. We then obtained the full records of these publications. Two review authors (ZZ, XYC) independently assessed the eligibility of each trial for inclusion in the review. We conferred with a third review author (XYS) to resolve disagreements. In order to avoid duplication, we only included the data from the latest study if the same group of participants were involved in the different reports.
Data extraction and management
Two review authors (ZZ, XYC) independently extracted data from each identified trial and recorded them on a standardized data extraction form (see Appendix 2). We resolved disagreements by consensus. When additional information was required, we contacted the first author of the relevant trial.
Assessment of risk of bias in included studies
Two review authors (ZZ, XYS) independently appraised the methodological quality of the eligible trials. We resolved any disagreements by discussion. A third review author (HBY) arbitrated when necessary. We assessed each trial according to the quality domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential threats to validity (Higgins 2011; Kjaergard 2001; Moher 1998; Schulz 1995). We considered a trial to be at low risk of bias if all domains were assessed as adequate. We considered a trial to be at high risk of bias if one or more domains were assessed as inadequate or unclear. We reported the 'Risk of bias' table as part of the Characteristics of included studies table and present a 'Risk of bias' summary figure that details all of the judgements made for all included studies in the review (Higgins 2011). We resolved disagreements in a consensus meeting. To avoid selection bias, we did not exclude trials because of low quality or any methodological characteristics. We used study quality assessment to assess the stability of the meta‐analytic results by features of study design such as randomization. We carried out the analysis before and after we excluded certain studies of lower methodological quality. If the effect measures differed significantly between the analyses, with or without the lower methodological quality studies, we reported stratified results according to study quality.
Measures of treatment effect
We summarized dichotomous data as risk ratio and continuous data as mean difference or standardized mean difference. We calculated the number of participants who suffered from treatment‐related adverse effects. In the original studies, "adverse events considered as related with the study medication" or other similar expressions were recognized as the outcomes of interest. We calculated risk ratio and number needed to treat for an additional beneficial outcome with 95% confidence intervals (Cook 1995) if the data allowed.
Unit of analysis issues
We combined different ACEIs or ARBs when trials compared ACEIs or ARBs with placebo. We analysed cardiac surgery and non‐cardiac surgery separately. We ensured that cluster randomized trials were treated appropriately.
Dealing with missing data
We tried to contact the first author of included trials to obtain missing data necessary for the meta‐analysis. We calculated missing standard deviations from the standard errors or confidence intervals (Higgins 2011). When we were unable to calculate standard deviations, we planned to impute these data using the mean value of reported standard deviations of other trials. We addressed the influence of missing data in the Discussion section of the review.
Assessment of heterogeneity
We assessed clinical heterogeneity and statistical heterogeneity. We solved clinical heterogeneity by subgroup analysis and sensitivity analysis. We also examined heterogeneity using the Chi2 statistic with significance set at P value < 0.1. We used the I2 statistic to describe the proportion of any variability due to heterogeneity (Higgins 2002). When P value > 0.1, we carried out the meta‐analysis in a fixed‐effect model; otherwise, we used a random‐effects model. We assumed the corresponding outcomes between groups to be statistically significant when the 95% confidence interval of risk ratio did not include 1.
Assessment of reporting biases
We planned to use a funnel plot to assess publication bias if we included more than 10 trials in the review. We planned to use weighted linear regression to test for funnel plot asymmetry (Egger 1997). However, due to the limited number of trials for each outcome, we did not produce a funnel plot.
Data synthesis
We undertook a meta‐analysis to measure the effect size if the degree of clinical and statistical heterogeneity was not excessive. We performed the meta‐analysis using RevMan 5.3. We used the fixed‐effect and random‐effects model according to the value of P and the I2 statistic. When the data extracted from the original reports did not warrant a quantitative summary measure, we carried out a qualitative description of the outcomes. For trials where continuous data were not given as means, no standard deviations (SDs) or standard errors were presented, or data were difficult to decipher (for instance the results were shown in figures and difficult to quantify accurately), we tried to contact the first authors and corresponding authors as stated in Dealing with missing data. When the attempt failed, we moved these studies to the studies awaiting classification. Only one study, Boldt 1996, should have been classified as awaiting classification since the data reporting in this study was incomplete. However, the lead author of that study has been accused of fraud. Consequently the reliability of the results of that study is questionable, and we have excluded the study.
According to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), when SDchange were unavailable in the original report, we imputed standard deviations for changes from baseline with the following technique: SDchange = (SDbaseline2 + SDfinal2 ‐ 2*Corr*SDbaseline*SDfinal)0.5. Default value (0.8) imputed for the correlation value.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses according to participants and interventions.
Subgroups of participants
We planned to conduct subgroup analyses according to types of surgery, anaesthesia, and other potentially influential factors.
-
Type of surgery: cardiac surgery or non‐cardiac surgery.
-
Type of anaesthesia: general anaesthesia only, or general anaesthesia combined with local anaesthesia. However, since none of the trials contained any information about combination of general anaesthesia and local anaesthesia, we could not perform subgroup analysis to assess the impact of anaesthesia method.
-
Type of potentially influential factors: factors that may increase the perioperative risk (presence of heart failure, recent acute myocardial infarction, diabetes, cerebrovascular insufficiency, lung disease, etc.). However, since individual raw data were not available, we could not perform subgroup analyses to assess the impact of perioperative risk of participants.
Subgroups of interventions
-
Perioperative AECIs versus placebo.
-
Perioperative ARBs versus placebo.
Sensitivity analysis
We performed sensitivity analyses to exclude trials with a high risk of bias. We performed 'trim and fill' sensitivity analysis of the primary outcomes if publication bias existed. To assess the influence of each trial on the result, we excluded them one by one using Stata. When we came across studies where the standard deviation of change from baseline was missing, we imputed the missing standard deviation using an imputed value, Corr, for the correlation coefficient. Besides the default value (0.8), we used different hypothesized values of Corr based on reasoned argument to determine whether the overall result of the analysis was robust to the use of imputed correlation coefficients.
Summary of findings tables and GRADE
We assessed the quality of the body of evidence associated with specific outcomes (all‐cause mortality, risk of acute myocardial ischaemia, risk of myocardial ischaemia, hypotension, cerebrovascular complications, and cardiac mortality) using the principles of the GRADE system (Guyatt 2008). We constructed a 'Summary of findings' table using the GRADE software (GRADEpro). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident an estimate of effect or association reflects the item being assessed. The assessment of the quality of the body of evidence was considered within study risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Our initial electronic search yielded 2258 publications (last searched December 2014). We scanned these publications and identified 46 studies that we could not exclude by scrutiny of titles and abstracts alone. After reading the full texts, we found seven randomized controlled trials (RCTs) that met the inclusion criteria (Billings 2012; Colson 1992; Flesch 2009; Licker 1996; Pretorius 2012; Ryckwaert 2001; Walter 2002):
We included seven trials in this review and excluded 39 studies for the reasons stated in the Characteristics of excluded studies table. One trial, Billings 2012, used more than one eligible drug (candesartan and ramipril), and we combined the two intervention groups numerically using the statistical methods in Chapters 7 and 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), so in effect we compared one intervention group of 46 participants with the placebo group of 28 participants (Figure 1)

Flow diagram of study selection process.
We reran the search on February 3, 2017. The 301 studies yielded were scanned and three studies were found of interest. The three potential new studies of interest were added to a list of 'Studies awaiting Classification' and will be incorporated into the formal review findings during the review update.
Included studies
All the eligible trials were conducted in Europe and the United States. The RCTs were parallel‐group and single‐centre designed. Three trials had more than one ACEIs or ARBs group (Billings 2012; Colson 1992; Pretorius 2012); we discarded the unrelated groups. The seven included studies included 571 enrolled participants, with sample size varying from 14 to 305 participants. The participants in the Colson 1992 and Licker 1996 studies were undergoing non‐cardiac surgery (infrarenal aortic surgery) (n = 36), and the participants in the other RCTs were undergoing cardiac surgery (n = 535), including valvular surgery, coronary artery bypass surgery, and cardiopulmonary bypass surgery. Demographic data in each RCT were specified in a data extraction form. Flesch 2009 and Walter 2002 detailed the disease status of participants preoperatively. Four trials specified prior drug therapies (Billings 2012; Flesch 2009; Licker 1996; Pretorius 2012). We contacted the investigators of the trials for missing data and added this information to the Characteristics of included studies table.
The agents used in the ACEIs or ARBs groups included enalapril (Colson 1992; Licker 1996; Ryckwaert 2001; Walter 2002), ramipril (Billings 2012; Pretorius 2012), and candesartan (Billings 2012; Flesch 2009). In six RCTs pharmaceutical therapy was started preoperatively (11 days to 25 minutes prior to surgery) (Billings 2012; Colson 1992; Flesch 2009; Licker 1996; Pretorius 2012; Walter 2002), and in one RCT it was started intraoperatively (Ryckwaert 2001). Drugs were administered orally or intravenously in the seven included trials.
Three RCTs reported the primary outcomes (Billings 2012; Pretorius 2012; Walter 2002). Of these, acute myocardial infarction was measured in dichotomous data (ST elevation or new Q wave in electrocardiogram test). One trial reported glomerular filtration rate as a measurement of renal function (Colson 1992). Two RCTs reported the rate of hypotension (Pretorius 2012; Walter 2002), but the definitions were different. Pretorius et al defined hypotension as systolic blood pressure less than 90 mmHg or prolonged need for vasopressors, while Walter et al defined hypotension as blood pressure below 80/50 mmHg.
We considered the included studies to be underpowered because only two studies, Flesch 2009 and Pretorius 2012, reported the methods of sample size calculations. Compared with the sample size of these two studies, the other five studies contained fewer participants, which made them underpowered. Besides, in Flesch 2009 and Pretorius 2012, mortality was not included as primary outcome, which means the sample size calculations in these two trials might not be correct for testing statistical significance of mortality.
Excluded studies
We excluded 39 studies for the reasons detailed in Characteristics of excluded studies.
Ongoing studies
No ongoing studies were identified.
Studies awaiting classification
There are three studies awaiting classification (Fan 2016; Fuentes‐Reyes 2016;Tian 2015). For further details see Characteristics of studies awaiting classification.
Risk of bias in included studies
See: Figure 2; Figure 3Characteristics of included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The seven included RCTs did not provide an adequate description of the methods used for generating the allocation sequence; all were described as randomized. We considered that all of the included studies carried an unclear risk of bias. None of the trials specified allocation concealment.
Blinding
One trial had no relevant description (Billings 2012), while the remaining six studies claimed to be double‐blind designs. After contacting the author, we recognized the Billings 2012 study as a double‐blind design. In addition, in Pretorius 2012, the study personnel who assessed the outcomes were blinded.
Incomplete outcome data
Five studies detailed information of dropouts (Billings 2012; Flesch 2009; Licker 1996; Pretorius 2012; Walter 2002). Among these five studies, Flesch 2009, and Pretorius 2012 carried out intention‐to‐treat analysis. In one study all data were included in the analysis, although no information about dropouts was provided (Ryckwaert 2001). Another study provided no relevant description, so we considered this study to be at high risk of bias (Colson 1992).
Selective reporting
We found no protocols for any of the studies, and were therefore unable to assess reporting bias.
Other potential sources of bias
We did not recognize any other potential sources of bias.
Effects of interventions
See: summary of findings Table for the main comparison.
Primary outcomes
All‐cause mortality
Three RCTs involving 419 participants reported perioperative mortality (Billings 2012; Pretorius 2012; Walter 2002). There was no significant difference between the ACEIs or ARBs and control groups (risk ratio (RR) 1.61; 95% confidence interval (CI) 0.44 to 5.85, P value = 0.48) with no statistical heterogeneity across the studies (I2 statistic = 0%) (Figure 4). All of the included trials were at high risk of bias, and the sample sizes were small. For these reasons, we downgraded this outcome to very low quality.

Forest plot of comparison: 1 All‐cause mortality, outcome: 1.1 All‐cause mortality.
Risk of acute myocardial infarction
ST elevation or new Q wave in electrocardiogram test
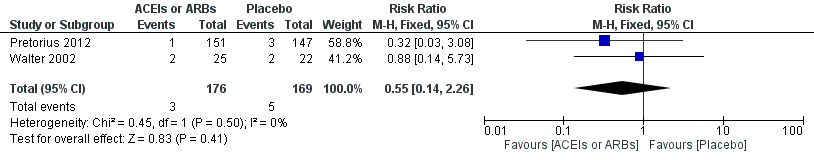
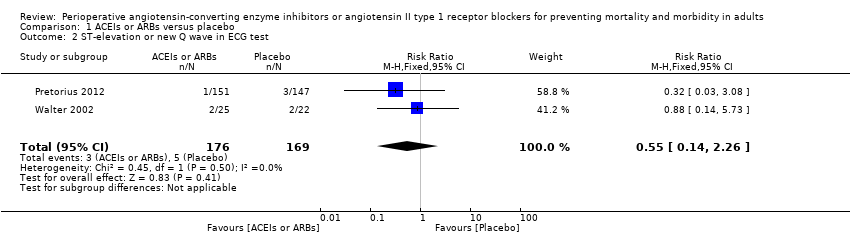
Two RCTs involving 345 participants reported the outcome of perioperative electrocardiogram test (Pretorius 2012; Walter 2002). All of the participants underwent elective cardiac surgery. Positive findings included ST segment elevation or new Q wave, which indicated acute myocardial infarction. The rates of myocardial infarction were similar in both groups without significant difference during the study period (RR 0.55; 95% CI 0.14 to 2.26, P value = 0.41), with no statistical heterogeneity across the studies (I2 statistic = 0%) (Figure 5). However, the direction of the analysis favoured administration of ACEIs or ARBs. All of the included trials were at high risk of bias, and the sample sizes were small. For these reasons, we downgraded the outcome to very low quality.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.2 ST‐elevation or new Q wave in ECG test.
Myocardial ischaemia
None of the trials reported myocardial ischaemia (defined as the presence of clinical symptoms and significant ST segment depression, occurring up to 30 days postoperatively or before hospital discharge).
Secondary outcomes
Congestive heart failure
None of the studies reported the rate of congestive heart failure. Two trials involving 34 participants compared enalapril with placebo on cardiac index (Licker 1996; Ryckwaert 2001). Cardiac index favoured ACEIs (mean difference (MD) ‐0.60; 95% CI ‐0.70 to ‐0.50, P value < 0.00001) (Figure 6), with no significant statistical heterogeneity across the studies (I2 statistic = 0%). When the study containing participants undergoing cardiac surgery, Licker 1996, was removed, we found similar results as follows: cardiac index (MD ‐0.60; 95% CI ‐0.71 to ‐0.49, P value < 0.00001). All of the included trials were at high risk of bias, and the sample sizes were small. For these reasons, we downgraded the outcome to very low quality.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.3 Cardiac index.
Hypotension
Two studies reported the occurrence of hypotension (Pretorius 2012; Walter 2002). As Walter 2002 did not describe the results clearly, we discarded this study when we synthesized the rate of hypotension. There was no statistical difference in the rate of hypotension (RR 1.95; 95% CI 0.86 to 4.41, P value = 0.11) (Table 1).
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
| Rate of hypotension | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.86, 4.41] |
Risk ratio < 1 favours angiotensin‐converting enzyme inhibitors and angiotensin II type 1 receptor blockers group. Risk ratio > 1 favours control group.
Rate of perioperative cerebrovascular complications
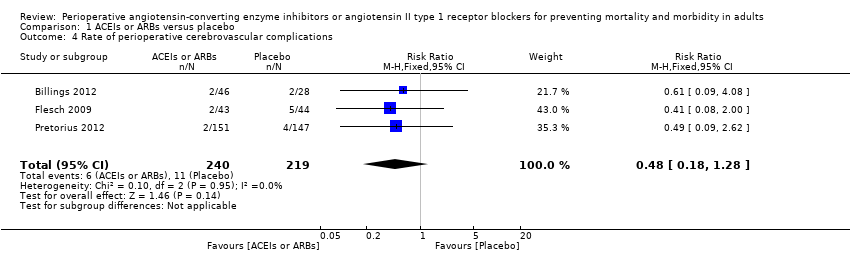
Three trials involving 459 participants compared ACEIs or ARBs with placebo (Billings 2012; Flesch 2009; Pretorius 2012). In total, 19 cerebrovascular events occurred; the rate was similar in both groups without significant difference during the study period (RR 0.48; 95% CI 0.18 to 1.28, P = 0.14), and there was no significant statistical heterogeneity across the studies (I2 statistic = 0%) (Figure 7). The ACEIs or ARBs arm in two studies used ARB (candesartan) as the experimental drug (Billings 2012; Flesch 2009). We conducted subgroup analyses according to the experimental drugs used. We found that when the ACEIs or ARBs group contained ACEIs only (ramipril arm in Billings 2012; Pretorius 2012), the rate of perioperative cerebrovascular complications was similar in both groups (RR 0.51; 95% CI 0.12 to 2.13, P value = 0.35), with no heterogeneity across the studies (I2 statistic = 0%). When the ACEIs or ARBs group contained ARBs only, the rate of perioperative cerebrovascular complications was also similar in both groups (RR 0.45; 95% CI 0.12 to 1.77, P value = 0.26), and there was no heterogeneity across studies (I2 statistic = 0%). All the included trials were at high risk of bias. For these reasons, we downgraded the outcome to very low quality. Subgroup difference was insignificant (P value = 0.80, I2 statistic = 0%).
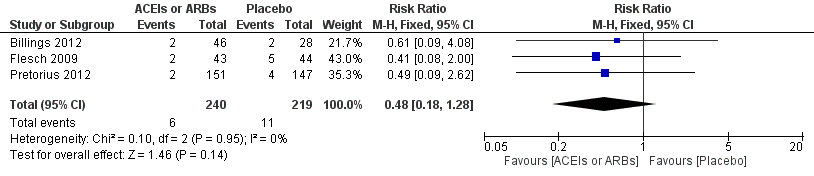
Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.4 Rate of perioperative cerebrovascular complications.
Renal insufficiency
No trial reported the rate of perioperative renal insufficiency, but one trial reported glomerular filtration rate as a measurement of renal function (Colson 1992). There was no significant difference between the ACEIs or ARBs group and control group (MD ‐1.40; 95% CI ‐10.30 to 7.50, P value = 0.76) (Table 2).
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
| Glomerular filtration rate | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐10.30, 7.50] |
IV ‐ inverse variance
IV: intravenous
Length of hospital stay
Two trials involving 372 participants reported the length of hospital stay (Billings 2012; Pretorius 2012). There was a statistical difference between the two groups (MD ‐0.54; 95% CI ‐0.93 to ‐0.16, P value = 0.005), with no significant statistical heterogeneity across the studies (I2 statistic = 0%). However, we found that the clinical backgrounds of the participants varied between trials, which might give rise to confounding factors (Figure 8). All the included trials were at high risk of bias, and the sample sizes were small. For these reasons, we downgraded the outcome to very low quality.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.5 Length of hospital stay.
Treatment related adverse events
Two studies reported adverse events, and there was no evidence of a difference between the ACEIs or ARBs and control groups (Flesch 2009; Pretorius 2012). There were no serious adverse events considered as related to the study drug in Flesch 2009, while Pretorius 2012 reported similar incidence of serious adverse events between the two groups (P value = 0.7) but the authors did not describe what the adverse events were.
Sensitivity analysis
Since all of the included studies were at high risk of bias, we did not perform sensitivity analysis based on the risk of bias. Sensitivity analyses using different hypothesized values of Corr (0.4 to 0.9) yielded stable result. (Corr = 0.4, cardiac index favoured ACEIs (MD ‐0.60; 95% CI ‐0.77 to ‐0.43, P value < 0.00001), with no significant statistical heterogeneity across studies (I2 statistic = 0%); Corr = 0.9, cardiac index favoured ACEIs (MD ‐0.60; 95% CI ‐0.69 to ‐0.51, P value < 0.00001), with no significant statistical heterogeneity across studies (I2 statistic = 0%)).
Discussion
Summary of main results
Seven RCTs with a total of 571 participants, comparing perioperative administration of ACEIs or ARBs with placebo and reporting mortality and morbidity, met the inclusion criteria of this review. As all seven RCTs were underpowered and considered to carry a high risk of bias, no firm conclusion could be drawn. We found insufficient evidence to determine the effects of perioperative administration of ACEIs or ARBs on perioperative mortality and acute myocardial infarction. However, ACEIs or ARBs might improve cardiac output perioperatively. In terms of hypotension, perioperative cerebrovascular complications, and cardiac surgery related renal failure, ACEIs or ARBs might not be helpful in reducing the rate of these complications. Due to the potential confounding factors, the estimation of length of hospital stay should be interpreted cautiously, though the results showed that ACEIs or ARBs might result in an earlier discharge.
Overall completeness and applicability of evidence
The present review suggests that the existing evidence on effectiveness of perioperative ACEIs or ARBs administration is far from sufficient. The generalization of the findings is limited.
All the participants in the eligible RCTs underwent cardiac or vascular surgery, which seriously limited the generalization of the findings in this review. As the necessary raw data such as details of anaesthesia/surgery, disease status, comorbidities, and prior drug therapies were not available in every RCT, we could not perform some of the planned subgroup analyses. Moreover, the male‐dominated population of the studies might also limit the generalization. In summary, we could draw no robust conclusion.
All seven studies provided sufficient information on their interventions. However, it is worth noting that experimental drugs were different among the RCTs. The use of different kinds of ACEIs or ARBs could introduce clinical heterogeneity. In six RCTs pharmacological therapy started preoperatively (11 days to 25 minutes prior to surgery) (Billings 2012; Colson 1992; Flesch 2009; Licker 1996; Pretorius 2012; Walter 2002), and in one RCT it started intraoperatively (Ryckwaert 2001); this difference might also introduce clinical heterogeneity.
Although we included seven studies, there were a limited number of trials for each outcome. None of the seven included studies reported cardiac mortality. Regarding all‐cause mortality, sample sizes were small and total events were few; hence, we were unable to make a firm conclusion. Most of the included RCTs presented their results in table form but failed to detail the diagnostic methods used, which might introduce heterogeneity. For instance, theoretically, cerebrovascular complication should be confirmed by computed tomography or magnetic resonance imaging, but none of the three trials reported this (Billings 2012; Flesch 2009; Pretorius 2012). In addition, none of these RCTs reported the rate of congestive heart failure.
Quality of the evidence
The major weakness of all seven included studies was the high risk of bias. None of the studies strictly abided by the reporting criteria laid down in the Consolidated Standards of Reporting Trials (CONSORT) statement. None of the seven trials reported randomization sequence generation as well as allocation concealment, which might indicate potential selection bias. Only one of the trials attempted to blind the study personnel who assessed the outcome, and Colson 1992 did not report any information of dropouts when not all the participants completed the research. All of these were sources of information bias. Not thoroughly describing the type of potentially influential factors that might increase the perioperative risk of participants (presence of heart failure, recent acute myocardial infarction, diabetes, cerebrovascular insufficiency, and lung disease) could bring about confounding bias. The other weakness for all seven included studies was that the sample sizes were small, which made them susceptible to being underpowered to detect clinically significant differences. None reported a sample size calculation based on mortality, which indicated faulty methodology in these RCTs (Figure 2). Poor methodology was the most common reason for the downgrading of quality of evidence. Since the quality of evidence in each outcome was low or very low, further research is very likely to have an important impact on our confidence in the estimation of effect and is likely to change the estimate.
Potential biases in the review process
Our process for searching for studies was thorough. We followed the review protocol strictly in the process of study selection, data extraction, and analysis. However, there is always the possibility that we missed unreported trials or those that only appeared in unpublished conference abstracts. We also excluded studies whose authors could not provide us required information, which could also introduce bias. Challenges in optimizing search terms/poor indexing of studies were a potential source of bias.
Agreements and disagreements with other studies or reviews
A recently published, well‐designed prospective observational study (Drenger 2012) involving 4224 participants on patterns of perioperative ACEIs usage in coronary artery bypass surgery (CABG) with cardiopulmonary bypass effects suggested that regardless what pattern was adopted (continuation, withdrawal, addition, or no ACEI), no differences in in‐hospital mortality and cerebral events were noted, which is identical with our results. However, another retrospective observational cohort study reported that preoperative therapy with ACEI was associated with an increased risk of mortality, use of inotropic support, postoperative renal dysfunction, and new onset of postoperative atrial fibrillation (Miceli 2009). The IMAGINE trial drew the same conclusion: In participants at low risk of cardiovascular events after CABG, routine early initiation (less than seven days) of ACEI therapy did not appear to improve clinical outcome up to three years after CABG but increased the rate of adverse events, particularly early after CABG (Rouleau 2008). These studies did not meet the criteria of the present review, but our objectives were similar. Their conclusions should be tested with well‐designed RCTs.
A retrospective cohort study with large sample size (n = 1358) suggested that preoperative use of ACEI/ARB was associated with a 27.6% higher risk of acute kidney injury postoperatively (Arora 2008). Stopping ACEIs or ARBs before cardiac surgery might reduce the rate of acute kidney injury. On the other hand, a propensity score‐based analysis of 536 participants undergoing CABG on cardiopulmonary bypass suggested that preoperative ACEIs were associated with a reduced rate of acute kidney injury after on‐pump CABG surgery (Benedetto 2008). Our comprehensive and systematic search did not find any RCT supporting either of these conclusions.
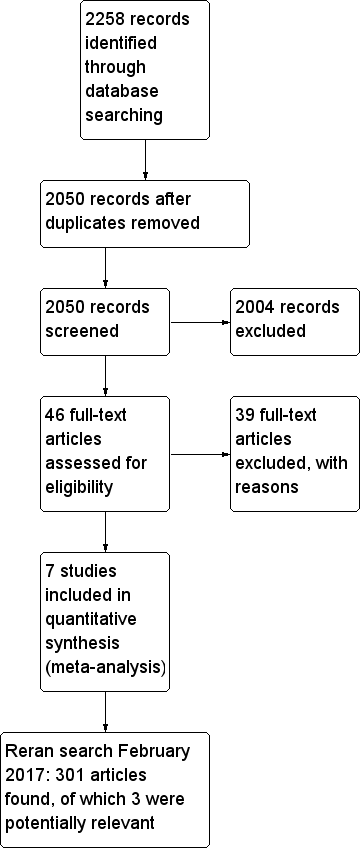
Flow diagram of study selection process.
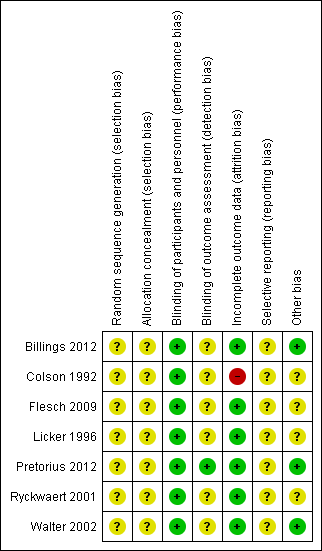
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
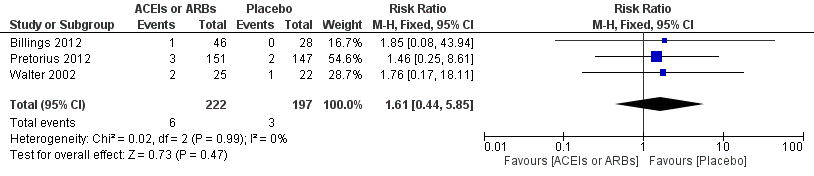
Forest plot of comparison: 1 All‐cause mortality, outcome: 1.1 All‐cause mortality.
Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.2 ST‐elevation or new Q wave in ECG test.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.3 Cardiac index.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.4 Rate of perioperative cerebrovascular complications.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.5 Length of hospital stay.

Comparison 1 ACEIs or ARBs versus placebo, Outcome 1 All cause mortality.
Comparison 1 ACEIs or ARBs versus placebo, Outcome 2 ST‐elevation or new Q wave in ECG test.

Comparison 1 ACEIs or ARBs versus placebo, Outcome 3 Cardiac index.
Comparison 1 ACEIs or ARBs versus placebo, Outcome 4 Rate of perioperative cerebrovascular complications.

Comparison 1 ACEIs or ARBs versus placebo, Outcome 5 Length of hospital stay.
| ACEIs or ARBs compared to placebo for preventing surgery‐related mortality and morbidity in adults | ||||||
| Patient or population: Patients undergoing any type of surgery under general anaesthesia receiving ACEIs or ARBs perioperatively | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | ACEIs or ARBs | |||||
| All‐cause mortality | Study population | RR 1.61 | 419 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total sample size is lower than the calculated. Duration of follow‐up: until discharge from hospital | |
| 16 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 7 per 1000 | 11 per 1000 | |||||
| Risk of acute myocardial ischaemia | Study population | RR 0.55 | 345 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total sample size is lower than the calculated. Duration of follow‐up: until discharge from hospital | |
| 30 per 1000 | 16 per 1000 | |||||
| Moderate | ||||||
| 56 per 1000 | 31 per 1000 | |||||
| Congestive heart failure | The mean cardiac index in the intervention groups was | ‐ | 34 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: not specified | |
| Hypotension | ‐ | RR 1.95 (0.86 to 4.41) | 298 (1 study) | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: not specified | |
| Rate of perioperative cerebrovascular complications | Study population | RR 0.48 | 459 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Duration of follow‐up: until discharge from hospital (Billings 2012; Pretorius 2012); 90 days after surgery (Flesch 2009) | |
| 50 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 71 per 1000 | 34 per 1000 | |||||
| Length of hospital stay | The mean length of hospital stay in the intervention groups was | ‐ | 372 | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: until discharge from hospital | |
| Treatment related adverse events | ‐ | ‐ | ‐ | 385 (2 studies) | ⊕⊝⊝⊝ | All the included trials were at high risk of bias. Total population size is less than 400. Authors did not provided detailed information on adverse events, which made the synthesis of the results less clinically relevant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by three levels due to very serious study limitations (all the trials included were at high risk of bias) and serious imprecision (total population size is less than 400). | ||||||
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
| Rate of hypotension | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.86, 4.41] |
| Risk ratio < 1 favours angiotensin‐converting enzyme inhibitors and angiotensin II type 1 receptor blockers group. Risk ratio > 1 favours control group. | ||||
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
| Glomerular filtration rate | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐10.30, 7.50] |
| IV ‐ inverse variance IV: intravenous | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All cause mortality Show forest plot | 3 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.44, 5.85] |
| 2 ST‐elevation or new Q wave in ECG test Show forest plot | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.14, 2.26] |
| 3 Cardiac index Show forest plot | 2 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.70, ‐0.50] |
| 4 Rate of perioperative cerebrovascular complications Show forest plot | 3 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.18, 1.28] |
| 5 Length of hospital stay Show forest plot | 2 | 372 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.93, ‐0.16] |

