難治性てんかん重積状態の治療におけるプロポフォールとチオペンタールナトリウムの比較
Referencias
References to studies included in this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Pragmatic randomised controlled trial, single‐blind, multicentric | |
| Participants | Participants: adults (> 16 years) with RSE not due to cerebral anoxia, who clinically required coma Sex (female/male): propofol group: 50%/50%; barbiturate group: 66%/34% Age (median (range)): propofol group: 57 years (26 to 87 years); barbiturate group: 64 years (16 to 78 years) Ethnic groups: not reported Duration of epilepsy: not reported Inclusion criteria: patients > 16 years of age suffering from RSE receiving at least 1 first‐line and 1 second‐line drug in adequate doses Exclusion criteria: patients with known pregnancy, known intolerance to the study drugs, mitochondrial disorders, egg allergy, hypertriglyceridaemia (> 5 mmol/L) or significant rhabdomyolysis (creatinine kinase > 1500 U/L) on admission Diagnostic criteria: RSE not due to cerebral anoxia, defined as ongoing clinical or electrographic seizures, or repetitive seizures without return to baseline for at least 30 minutes despite administration of 1 first‐line (benzodiazepine) and 1 second‐line antiepileptic drug (phenytoin, valproate, phenobarbital and levetiracetam) in adequate doses Comorbidities: none Co‐medications: none Total randomised: 24 patients; 14 allocated to propofol group and 10 allocated to barbiturate group (thiopental and pentobarbital); (1 patient in thiopental sodium group did not require treatment and so was excluded, remaining 9 analysed) | |
| Interventions | Number of control centres: 2 Country/location: Switzerland and the US Setting: CHUV et Université de Lausanne, Lausanne and Brigham and Women's Hospital, Harvard School of Medicaine, Boston Intervention I: 2 mg/kg titrated to burst suppression or 4 mg/kg until EEG was available Intervention II: 2 mg/kg iv titrated to burst suppression or 5 mg/kg if no EEG available Treatment before study: first‐ and second‐line antiepileptic drugs Time to treatment since onset of status: not reported Duration of follow‐up: 3 months 2 treatment arms: propofol and barbiturates (thiopental sodium or pentobarbital) | |
| Outcomes | Primary outcomes (as stated in the publication): to assess the effectiveness (RSE control, adverse events) of a first course of propofol versus barbiturates Secondary outcomes (as stated in the publication): none Additional outcomes Outcomes used in our review:
| |
| Notes | Stated aim of study: "This prospective study was undertaken to assess the effectiveness (SE control, adverse events) of a first course of PRO versus barbiturates, the two most commonly used agents according to the aforementioned surveys" Language of publication: English Commercial funding: yes Non‐commercial funding: no Publication status (peer review journal): yes Publication status (journal supplement): no Publication status (abstract): no Funded by AstraZeneca (Switzerland) and UCB (Switzerland) No conflict of interest Clinical Trial.gov ID: NCT00265616 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After written consent was obtained by proxy, randomisation was stratified by institution using sealed envelopes." Comment: The authors do not explain how the randomization was done. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. Comment: authors contacted. No information provided. |
| Blinding (performance bias and detection bias) | High risk | Being a single‐blind study, only the patient was blinded. Assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. All participants randomised completed the study and were included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes that were mentioned in the methodology have been reported. |
| Other bias | Unclear risk | The trial was terminated before completion due to inadequate recruitment. The calculated sample size for this study was 150 patients, 75 in each arm. Funding by pharmaceutical companies. |
EEG: electroencephalography; iv: intravenous; RSE: refractory status epilepticus; SE: status epilepticus.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||
| 1 Total control of seizures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||
| Analysis 1.1  Comparison 1 Propofol versus thiopental sodium, Outcome 1 Total control of seizures. | ||||||||||||||||
| 2 In‐hospital mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||
| Analysis 1.2 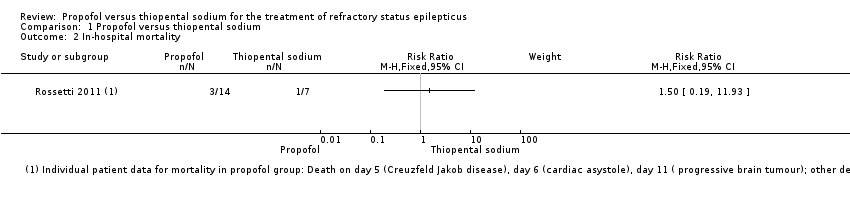 Comparison 1 Propofol versus thiopental sodium, Outcome 2 In‐hospital mortality. | ||||||||||||||||
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||
| Analysis 1.3  Comparison 1 Propofol versus thiopental sodium, Outcome 3 Adverse events. | ||||||||||||||||
| 3.1 Infection | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.41] | ||||||||||||
| 3.2 Hypotension | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.38, 2.00] | ||||||||||||
| 3.3 Other serious complications | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.04, 6.86] | ||||||||||||
| 4 Duration of mechanical ventilation Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 1.4
Comparison 1 Propofol versus thiopental sodium, Outcome 4 Duration of mechanical ventilation. | ||||||||||||||||
| 5 Long‐term outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||
| Analysis 1.5  Comparison 1 Propofol versus thiopental sodium, Outcome 5 Long‐term outcomes. | ||||||||||||||||
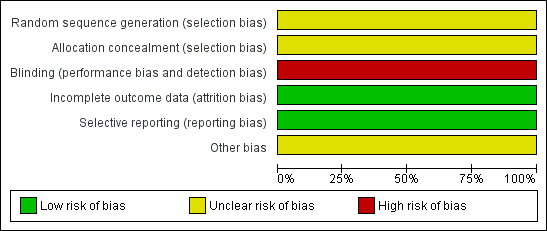
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

Study flow diagram.

Comparison 1 Propofol versus thiopental sodium, Outcome 1 Total control of seizures.

Comparison 1 Propofol versus thiopental sodium, Outcome 2 In‐hospital mortality.

Comparison 1 Propofol versus thiopental sodium, Outcome 3 Adverse events.
| Study | Propofol group | Thiopentone sodium group |
| Rossetti 2011 | Median: 4 days | Median: 17 days |
| Rossetti 2011 | Range: 2 to 28 days | Range: 5 to 70 days |
Comparison 1 Propofol versus thiopental sodium, Outcome 4 Duration of mechanical ventilation.

Comparison 1 Propofol versus thiopental sodium, Outcome 5 Long‐term outcomes.
| Propofol compared to Thiopental sodium for the treatment of refractory status epilepticus | ||||||
| Patient or population: patients with the treatment of refractory status epilepticus | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Thiopental sodium | Propofol | |||||
| Total control of seizures | Study population | RR 1.5 | 21 | ⊕⊕⊝⊝ | ||
| 286 per 1000 | 429 per 1000 | |||||
| In‐hospital mortality | Study population | RR 1.5 | 21 | ⊕⊕⊝⊝ | ||
| 143 per 1000 | 214 per 1000 | |||||
| Length of intensive care unit (ICU) stay | Not reported | Not reported | NA | NA | NA | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Single blinded study: we downgraded one level for risk of bias | ||||||
| Study ID | Interventions | Screened (n) | Randomised (n) | Safety analysis (n) | ITT (n) | Finishing study (n) | [%] of randomised participants |
| I1 Propofol I2 Barbiturate (thiopental (n = 7) and pentobarbital (n = 3)) | I1 14 I2 10 | I1 14 I2 10 | I1 14 I2 10 | I1 14 I2 10 | I1 14 I2 9 | I1 100 I2 90 | |
| I1: intervention 1; I2: intervention 2; ITT: intention‐to‐treat; n: number. | |||||||
| Characteristic | |
| I1 I2 | Propofol Thiopental |
| Participants who died (n) Epilepsy‐related I1 Propofol I2 Thiopental | 0 0 |
| Participants who died (n) All causes I1 Propofol I2 Thiopental | 3 1 |
| Adverse events (n) I1 Propofol I2 Thiopental | 14 11 |
| Serious adverse events (n) I1 Propofol I2 Thiopental | 1 1 |
| Duration of ICU stay | Not reported |
| Duration of mechanical ventilation (median (range)) I1 Propofol I2 Thiopental | 17 days (5 to 70 days) 4 days (2 to 28 days) |
| Duration of hospitalisation | Not reported |
| Neurological deficits | Not reported |
| Cognitive deficits | Not reported |
| Haematological toxicity | Not reported |
| Liver toxicity | Not reported |
| Hypersensitivity or drug allergy | Not reported |
| Bronchopneumonia | Not reported |
| Other side effects | Not reported |
| I1: intervention 1; I2: intervention 2; ICU: intensive care unit; n: number. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total control of seizures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 In‐hospital mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infection | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.41] |
| 3.2 Hypotension | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.38, 2.00] |
| 3.3 Other serious complications | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.04, 6.86] |
| 4 Duration of mechanical ventilation Show forest plot | Other data | No numeric data | ||
| 5 Long‐term outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

