تاثیر وضعیت قرارگیری بدن و تجویز مایعات بر پیشگیری از سردرد متعاقب بیحسی نخاعی
چکیده
پیشینه
سردرد متعاقب بیحسی نخاعی (post‐dural puncture headache; PDPH)، یک عارضه شایع در پونکسیون کمری است. تئوریهای متعددی نشت مایع مغزی نخاعی (cerebrospinal fluid; CSF) را از طریق سوراخی در دورا به عنوان علت بروز این عارضه جانبی تشخیص دادهاند. بنابراین اقدامات پیشگیرانه برای جلوگیری از این عارضه ضروری است. استراحت طولانیمدت در تختخواب برای درمان PDPH از زمانی که شروع شده، استفاده میشود، اما مشخص نیست که استراحت طولانیمدت میتواند از آن پیشگیری هم بکند یا خیر. به طور مشابه، مقدار تجویز مایعات اضافه بر میزان معمول رژیم غذایی برای جایگزینی از دست دادن CSF به دلیل سوراخ دورا مشخص نیست. این مرور، یک نسخه بهروز از مرور قبلا منتشر شده در بانک اطلاعاتی مرورهای سیستماتیک کاکرین (شماره 7؛ 2013) با نام «تاثیر وضعیت قرارگیری بدن و مایعات بر پیشگیری از سردرد متعاقب بیحسی نخاعی» است.
اهداف
بررسی اینکه استراحت بیشتر در تختخواب همراه با حالتهای مختلف بدن و سر، همین طور تجویز مایعات اضافی پس از پونکسیون کمری، از بروز PDPH در افرادی که به دلایل تشخیصی یا درمانی تحت پونکسیون کمری قرار میگیرند، پیشگیری میکند یا خیر.
روشهای جستوجو
ما پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL)؛ MEDLINE؛ EMBASE و LILACS و همین طور پایگاههای ثبت کارآزمایی را تا فوریه 2015 جستوجو کردیم.
معیارهای انتخاب
ما به دنبال کارآزماییهای تصادفیسازی و کنترل شدهای بودیم که استراحت در تختخواب در برابر راه افتادن بلافاصله، حالت قرار گرفتن سر رو به پائین را با حالت خوابیده به پشت، حالت خوابیده به شکم را با حالت خوابیده به پشت در زمان استراحت در تختخواب، و تجویز مایعات مکمل را در برابر عدم تجویز یا تجویز کم مایعات، به عنوان اقدامات پیشگیرانه از PDPH در افرادی که تحت پونکسیون کمری قرار گرفته بودند، مقایسه کرده بودند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم، واجد شرایط بودن مطالعات را با استفاده از نرمافزار EROS مبتنی بر وب (نرمافزار ساماندهی اولیه مرور) ارزیابی کردند. دو نویسنده دیگر این مرور به طور مستقل خطر سوگیری (bias) را به وسیله معیارهای ثبت شده در کتابچه راهنمای کاکرین برای مرورهای سیستماتیک مداخلاتارزیابی کردند. هر گونه اختلافنظری را با اجماع حل کردیم. دادههای مربوط به PDPH؛ PDPH شدید، و هر گونه سردرد پس از پونکسیون را استخراج کردیم و آنالیز قصد درمان (intention‐to‐treat) و آنالیز حساسیت را به وسیله خطر سوگیری انجام دادیم. شواهد را با استفاده از روش درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (Grading of Recommendations Assessment, Development and Evaluation; GRADE) بررسی کردیم و جدول «خلاصهای از یافتهها» را تشکیل دادیم.
نتایج اصلی
ما 24 کارآزمایی را با 2996 شرکتکننده در این نسخه بهروز از مرور گنجاندیم. تعداد شرکتکنندگان در هر کارآزمایی از 39 تا 382 نفر متغیر بود. اکثر مطالعات وارد شده، استراحت در تختخواب را در برابر راه افتادن و تحرک سریع مقایسه کرده بودند، و فقط دو مورد تاثیرات تجویز مایعات مکمل را در برابر عدم تجویز آن بررسی کرده بودند. خطر کلی سوگیری را در مطالعات وارد شده پائین تا نامشخص ارزیابی کردیم. کیفیت کلی شواهد پائین تا متوسط بود، علت این کاهش سطح، عمدتا ارزیابی خطر سوگیری در اغلب موارد بود. پیامد اولیه در مرور ما حضور PDPH بود.
شواهد با کیفیت پائین، مزیتی را به نفع استراحت در تختخواب در مقایسه با راه رفتن و تحرک بلافاصله در بروز PDPH شدید نشان ندادند (خطر نسبی (RR): 0.98؛ 95% فاصله اطمینان (CI): 0.68 تا 1.41؛ 1568 شرکتکننده؛ 9 مطالعه) و شواهد با کیفیت متوسط بروز هر گونه سردرد پس از پونکسیون کمری را نشان دادند (RR: 1.16؛ 95% CI؛ 1.02 تا 1.32؛ 2477 شرکتکننده؛ 18 مطالعه). علاوه بر این، استراحت در تختخواب احتمالا باعث افزایش PDPH در مقایسه با راه رفتن و تحرک بلافاصله میشد (RR: 1.24؛ 95% CI؛ 1.04 تا 1.48؛ 1519 شرکتکننده؛ 12 مطالعه). یک آنالیز، محدود به دقیقترین کارآزماییها از نظر روششناسی (یعنی، مواردی با خطر پائین سوگیری در روش تخصیص، دادههای ازدسترفته و کورسازی در ارزیابی پیامد) نتایج مشابهی داشت. شواهد با کیفیت پائین برای عدم وجود مزایای مرتبط با مایعات مکمل در بروز PDPH شدید (RR: 0.67؛ 95% CI؛ 0.26 تا 1.73؛ 100 شرکتکننده؛ 1 مطالعه) و PDPH (RR: 1؛ 95% CI؛ 0.59 تا 1.69؛ 100 شرکتکننده؛ 1 مطالعه)، و شواهد با کیفیت متوسط در مورد بروز هر گونه سردرد پس از پونکسیون کمری (RR: 0.94؛ 95% CI؛ 0.66 تا 1.34؛ 200 شرکتکننده؛ 2 مطالعه) وجود داشت. انتظار بروز سایر عوارض جانبی را نداشتیم و آنها را در این مرور ارزیابی نکردیم.
نتیجهگیریهای نویسندگان
از زمان انجام نسخه قبلی این مرور، یک مطالعه جدید را برای ورود یافتیم، اما نتیجهگیری کلی تغییری نکرد. کیفیت شواهد را برای اغلب پیامدها در این مرور، در سطح پائین تا متوسط ارزیابی کردیم. از آنجایی که مطالعات شناسایی شده دچار نقصان در موارد مربوط به تصادفیسازی و کورسازی در ارزیابی پیامد بودند، بنابراین سطح کیفیت شواهد را کاهش دادیم. در مجموع، هیچ شواهدی که نشان دهنده سودمندی استراحت در تختخواب به فرم معمول پس از پونکسیون لومبار برای پیشگیری از شروع PDPH باشد، وجود نداشت. نقش مایعات مکمل در پیشگیری از PDPH نامشخص باقی ماند.
PICO
خلاصه به زبان ساده
موقعیت قرارگیری بدن و مصرف مایعات برای پیشگیری از سردرد پس از پونکسیون کمری
پیشینه
پونکسیون کمری (lumbar puncture) یک پروسیجر پزشکی است که با یک سوزن و سرنگ انجام میشود تا یک نمونه از مایع مغزی نخاعی برداشته شود یا تزریق داروها انجام شود. برخی از افراد دچار عارضه جانبی پس از آن، به نام سردرد بعد از سوراخ شدن دورا (post‐dural puncture headache; PDPH)، میشوند. این سردرد با حرکت، نشستن یا ایستادن، ممکن است بدتر شود و میتواند با دراز کشیدن، بهبود یابد. PDPH میزان تحرک افراد و فعالیتهای روزانه آنها را محدود میکند، و همچنین باعث هزینههای پیشبینی نشده، هم برای بیمار و هم سازمانهای خدمات سلامت شود. گاهی اوقات پزشکان به بیماران خود توصیه میکنند پس از پونکسیون کمری در تختخواب باقی بمانند و برای پیشگیری از سردرد مقدار زیادی مایعات بنوشند.
یافتههای کلیدی
این یک نسخه بهروز شده از مرور اصیل منتشر شده در سال 2013 است. در جستوجو برای یافتن منابع علمی منتشر شده در فوریه 2015 یک مطالعه جدید به دست آوردیم. این مرور شامل 24 مطالعه با 2996 شرکتکننده بود. انواع مختلف استراحت در تختخواب و مایعات اضافی را مقایسه کردیم تا ببینیم آنها از PDPH پس از پونکسیون کمری پیشگیری میکنند یا خیر. شواهد با کیفیت پائین تا متوسط یافتیم که نشان میداد استراحت در تختخواب از بروز سردرد پس از پونکسیون کمری، صرف نظر از مدت زمان استراحت یا وضعیت قرارگیری بدن یا سر که توسط بیمار انتخاب میشود، پیشگیری نمیکند. علاوه بر این، استراحت در تختخواب احتمالا شانس داشتن PDPH را افزایش میدهد. دادههای اندکی در مورد سودمندی تجویز مایعات اضافی، که به نظر نمیرسد از بروز سردرد پیشگیری میکند، به دست آوردیم.
از آنجا که هیچ شواهدی برای حمایت از آنها وجود ندارد؛ بر این باور هستیم که این اقدامات نباید بیش از این به طور روتین برای بیماران به منظور پیشگیری از سردرد پس از پونکسیون کمری توصیه شود.
کیفیت شواهد
کیفیت شواهد را برای اغلب پیامدها در این مرور، در سطح پائین تا متوسط ارزیابی کردیم.
Authors' conclusions
Summary of findings
| Bed rest versus ambulation for preventing post‐dural puncture headache | ||||||
| Patient or population: Participants undergoing lumbar puncture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Ambulation | Bed rest | |||||
| Post‐dural puncture headache | 205 per 1000 | 254 per 1000 | RR 1.24 | 1519 | ⊕⊕⊕⊝ | |
| Severe post‐dural puncture headache | 107 per 1000 | 105 per 1000 | RR 0.98 | 1568 | ⊕⊕⊝⊝ | |
| Any headache | 287 per 1000 | 333 per 1000 | RR 1.16 | 2477 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded (‐1) due to unclear risk of bias related to allocation concealment (9 studies), as well as high risk of bias in blinding of outcome assessment (6 studies). | ||||||
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 7, 2013) on "Posture and fluids for preventing post‐dural puncture headache".
Description of the condition
Post‐dural (post‐lumbar or post‐spinal) puncture headache (PDPH) is one of the most common complications of diagnostic, therapeutic, or inadvertent lumbar punctures (Bezov 2010; Davignon 2002). PDPH is defined as any headache after a lumbar puncture that worsens within 15 minutes of sitting or standing and that is relieved within 15 minutes of lying down (IHS 2004). Ninety per cent of PDPHs occur within three days of the procedure, and 66% start within the first 48 hours (Turnbull 2003).
The pathophysiology of PDPH has not been fully described. It is well known that puncture in the dura allows cerebrospinal fluid (CSF) to leak from the subarachnoid space, resulting in a decrease in CSF volume and pressure (Grande 2005). This CSF volume loss may cause a downward pull on pain‐sensitive structures, resulting in a headache (Ahmed 2006; Baumgarten 1987; Davignon 2002; Denny 1987; Harrington 2004). Alternatively, the loss of CSF may cause an increase in blood flow, resulting in arterial and venous vasodilation and PDPH. A third explanation involves the role of substance P (a neurotransmitter/neuromodulator involved in pain perception) and the regulation of neurokinin 1 receptors (NK1R) (Clark 1996). See a glossary of terms in Appendix 1.
Occurrence of PDPH varies from 1% to 40%, according to needle gauge, needle orientation, operator skill level, and presence of risk factors such as patient's age or history of PDPH (Turnbull 2003). During anesthetic procedures (for example epidural anesthesia), PDPH is most commonly caused by an unintentional dural puncture (Thew 2008; Turnbull 2003). In contrast, during diagnostic or therapeutic lumbar punctures, the need for adequate CSF flow requires an intentional lesion that may give rise to PDPH (Kuczkowski 2006). Estimated frequencies vary from less than 10% following spinal anesthesia (Hafer 1997; Vallejo 2000), to 36% following diagnostic lumbar punctures (Lavi 2006; Vallejo 2000), and up to 81% in women with inadvertent dural puncture during active labor. Reported risk of inadvertent dural puncture placement during epidural anesthesia in women ranges from 0.04% to 6% (Berger 1998; Choi 2003). A significant number of mothers cannot provide adequate care for their newborn because of the headache (Sprigge 2008).
The features of PDPH are often variable. PDPH may be accompanied by neck stiffness, tinnitus, hearing loss, photophobia, or nausea. Other features, such as the localization and duration of the headache, are less predictable (Grande 2005). Although PDPH is not a life‐threatening condition, it often restricts physical activity. Likewise, length of hospital stay and medical monitoring increases, especially because patients are usually required to stay in bed for an entire day after the intervention (Angle 2005), as well as direct and indirect costs.
The variability of symptoms makes PDPH a diagnosis of exclusion. Alternative diagnoses, such as viral meningitis, sinus headache, or intracranial hemorrhage should be ruled out first (Turnbull 2003). Once PDPH is diagnosed, the initial treatment involves conservative measures such as bed rest and analgesics. A more specific treatment is indicated if PDPH continues for more than 72 hours (Ahmed 2006). Severe PDPH may respond to some therapeutic drugs and administration of an epidural blood patch (Boonmak 2010; Lavi 2006). There are two Cochrane reviews on drug therapy for the prevention and treatment of PDPH at present (Basurto 2013; Basurto 2015).
Description of the intervention
Many publications and reviews of PDPH have focused on treatment after the onset of symptoms. However, the prevention of PDPH is an equally important topic. Immobilization and fluid intake are the two proposed preventive methods that may foster recovery or even prevent PDPH following lumbar puncture.
Sicard first recommended bed rest after lumbar puncture in 1902. He asserted that patients should rest for 24 hours to prevent onset of PDPH (Armon 2005). Although the effectiveness of resting for symptom relief is well known, it is debatable whether bed rest prevents the development of symptoms (Davignon 2002). In addition, there is disagreement over the appropriate length of bed rest; some authors suggest that around four hours is sufficient, whereas others suggest 24 hours or more (Thoennissen 2001). It is also believed that certain body postures after lumbar puncture, such as a prone position with or without head‐down tilt, may help in the prevention of PDPH onset.
The effectiveness of fluid intake on PDPH prevention has not been investigated thoroughly. Basic characteristics, such as amount of fluid intake and time of treatment, have not been established, although some studies suggest that three additional liters per day for five days is appropriate (Ahmed 2006). Despite lack of evidence, Vanzetta et al. found that hydration is a common recommendation for patients after a dural procedure. Ninety per cent of centers interviewed reported implementing it to prevent the onset of headache (Vanzetta 2005).
How the intervention might work
Prophylactic bed rest may have a mechanism of action similar to the one that has been proposed for therapeutic immobilization after the development of PDPH. As CSF leakage is thought to be fundamental in the development of PDPH, postures such as prone position after a lumbar puncture may reduce hydrostatic pressure. This may in turn reduce pressure in the subarachnoid space and allow a seal to form over the dura, thus enabling CSF leakage repair. As such, this posture may be effective in preventing PDPH onset.
Additional fluid intake may work by replacing lost corporal fluid and increasing CSF production (Ahmed 2006), thus preventing a hydrostatic pull on pain‐sensitive structures and vasodilation (Janssens 2003). By this mechanism, hydration may prevent the development of PDPH.
Why it is important to do this review
Lumbar puncture is a common clinical practice despite its potential adverse effects (Evans 2009; Grande 2005). The morbidity associated with CSF loss, besides PDPH, includes peripartum seizures, cranial subdural hematomas, and subdural fluid collection (Arendt 2009). PDPH may be the first step in a chain of adverse events that could be avoided by following a series of simple recommendations (Janssens 2003). Patient immobilization and oral intake of fluids may be valuable to avoid deleterious complications. Even though most cases of PDPH resolve within a few days, a significant number of people have at least one week of disability, while others require prolonged or recurrent hospitalizations (van Kooten 2008).
A 2002 Cochrane review on strategies to prevent PDPH included published and unpublished literature up to the year 2000 (Sudlow 2002). It is imperative to update these results in order to generate relevant recommendations for consumers, patients, and health practitioners.
Objectives
To assess whether prolonged bed rest combined with different body and head positions, as well as administration of supplementary fluids after lumbar puncture, prevent the onset of PDPH in people undergoing lumbar puncture for diagnostic or therapeutic purposes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) in any clinical/research setting where dural puncture was conducted. We did not include quasi‐RCTs.
Types of participants
Studies that recruited males and females of all ages who had undergone lumbar puncture for medical reasons (therapeutic or diagnostic).
Types of interventions
The studies on participants undergoing lumbar puncture must have assessed one of the following interventions:
-
a period of bed rest after lumbar puncture alone or in combination with a head‐down/up tilt strategy, with or without a specific body position, or even a combination of several postural strategies with immobilization, versus immediate mobilization;
-
head‐down/up tilt versus no head‐down/up tilt in participants prescribed with a period of bed rest;
-
prone versus supine posture in participants assigned to immobilization;
-
administration of supplementary fluids (oral or intravenous) after lumbar puncture versus no/less administration; and
-
any combination of points 1 to 4.
Types of outcome measures
Primary outcomes
We assessed the presence of PDPH defined as each headache occurring within five days of a lumbar puncture, caused by CSF leakage through the dural puncture (IHS 2013). We used the PDPH diagnosis criteria specified by the International Headache Society (IHS) (IHS 2004; IHS 2013), as well as the definition used in each study.
Secondary outcomes
We assessed the presence of severe PDPH using the definition used in each study, which could be based on specific features (for example duration of PDPH), a visual analogue score (VAS), or other criteria, such as need of specialized treatments to relieve the headache (for example epidural blood patch). Likewise, we assessed information on any headache subsequent to the lumbar puncture procedure in order to incorporate any possible data that had not been catalogued as PDPH.
Search methods for identification of studies
Electronic searches
We used the Cochrane Central Register of Controlled Trials (CENTRAL) as the primary source for identifying all relevant RCTs (the Cochrane Library 2013, Issue 6). We used a modified version of the CENTRAL search for our search of MEDLINE (1966 to February 2015), EMBASE (1974 to February 2015), and LILACS (inception to February 2015). The search terms were a combination of thesaurus‐based and free‐text terms, both related to the intervention (lumbar puncture in neurological, anesthesia, or myelography settings) and the outcome. We applied no language restrictions.
See Appendix 2, Appendix 3, Appendix 4, and Appendix 5 for details of the CENTRAL, MEDLINE, EMBASE, and LILACS search strategies.
Searching other resources
We handsearched reference lists from retrieved studies as well as information from the World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch) (up to February 2015). We used unpublished information collected by previous authors of a systematic review that assessed strategies aimed at preventing PDPH to gather information on allocation and blinding of outcomes (Sudlow 2002).
Data collection and analysis
Selection of studies
Two review authors (IA and LM) independently conducted a selection of eligible studies through the web‐based software EROS (Early Review Organizing Software) (Glujovsky 2011). The review authors evaluated titles and abstracts of all identified studies to determine if they fulfilled the inclusion criteria. We assessed full‐text publications of the selected studies to confirm their relevance for inclusion. We resolved any disagreements through discussion with a third review author (AC). Review authors were not blinded to name and affiliation of study authors, journal of publication, or study results at any stage of the review.
Data extraction and management
Two review authors (IA and LM) used predesigned and tested data extraction forms to extract information on participants, methods of randomization, blinding, comparisons of interest, number of participants originally randomized by arm, participants lost to follow‐up, and outcomes. We recorded reasons for exclusion of potential studies in the Characteristics of excluded studies table. We clarified any disagreements by discussion with a third review author (MR). We entered extracted data into Review Manager 5 for analysis (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors (MR and AC) independently assessed risk of bias of the included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered five domains (sequence generation, allocation concealment, blinding in outcome assessment, incomplete outcome data, and selective reporting bias); we classified each one of them as low risk of bias, high risk of bias, or unclear risk of bias. We resolved any disagreements by discussion or by consulting a third review author (XB).
Measures of treatment effect
We presented results as summary risk ratios with 95% confidence intervals. We used the number needed to treat for an additional harmful outcome (NNTH) statistic as an absolute measure of harm. We calculated NNTH as the reciprocal of risk differences (McQuay 1998).
Dealing with missing data
We retrieved levels of attrition data when available. When possible, we carried out analyses on an intention‐to‐treat basis (that is we attempted to include all participants randomized to each group). We assumed that any participant lost to follow‐up had not experienced the respective outcome.
Assessment of heterogeneity
We assessed heterogeneity of effect sizes by means of the I2 statistic. An I2 greater than 30% was indicative of heterogeneity.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). We used a random‐effects model after a full assessment of clinical similarity among the studies (Higgins 2003).
Subgroup analysis and investigation of heterogeneity
For included studies that provided the necessary data, we planned to assess the subgroup analyses detailed below. However, due to scarcity of data, we did not perform analyses 2 to 7:
-
participants undergoing dural puncture for anesthesia only, diagnosis only, or myelography only;
-
subgroup analysis for gender;
-
subgroup analysis for age;
-
subgroup analysis for posture during the lumbar puncture (e.g. lateral or sitting up);
-
subgroup analysis for needle gauge (e.g. 22, 29);
-
subgroup analysis for needle tips (e.g. pencil‐point, diamond, double bevel); and
-
subgroup analysis for amount of CSF aspirated
Sensitivity analysis
We planned to perform sensitivity analyses by excluding any study with high or unclear risk of bias in any of the subgroups detailed below:
-
allocation features;
-
levels of missing data;
-
blinding of outcome assessment.
'Summary of findings' tables
We used the guidelines of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group to assess the quality of the evidence of primary outcomes (Guyatt 2008). We developed a 'Summary of findings' table with the GRADE profiler software (GRADEPro GDT 2015). The GRADE system assesses the quality of evidence based on the extent to which users can be confident that an association reflects the item being evaluated (Guyatt 2008). Assessment of the quality of evidence included risk of bias, heterogeneity, directness of the evidence, risk of publication bias, and precision of effect estimates, among other issues (Guyatt 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g).
Results
Description of studies
Results of the search
Since publication of the previous version of this review, we identified four studies for possible inclusion (Figure 1). At the end of the selection, we added one new study, Afshinmajd 2014, to the original 23 studies included in the previous version of this review. In summary, most of the studies included in this version were published in the 1980s (Andersen 1986; Carbaat 1981; Congia 1985; Cook 1989; Dieterich 1985; Dieterich 1988; Gulati 1981; Handler 1982; Hilton‐Jones 1982; Jensen 1987; Macpherson 1983; Macpherson 1984; Macpherson 1985; Robertson 1980; Smith 1980; Teasdale 1983; Thornberry 1988; Vilming 1988); one was published in 1978 (Eldevik 1978); and 11 were published after 1990 (Afshinmajd 2014; Cucereanu 2010; Ebinger 2004; Fassoulaki 1991; Hafer 1997a; Hallam 1993; Johannsson 1992; Murata 2003; Spriggs 1992; Tejavanija 2006; Vimala 1998).
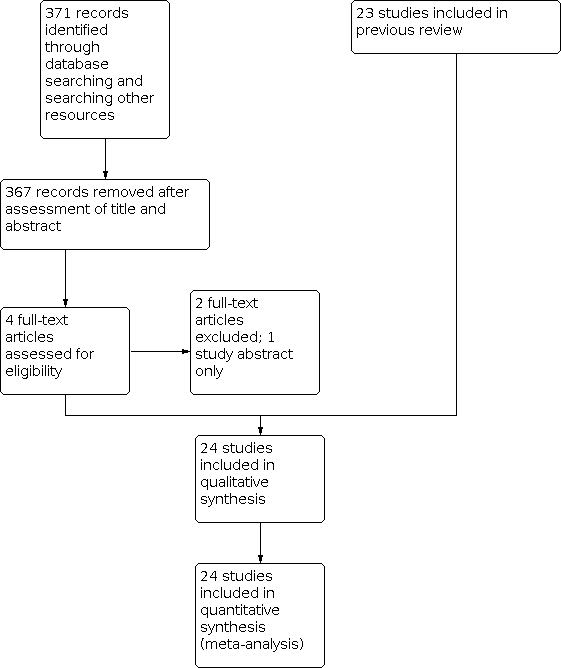
Study flow diagram.
Included studies
At the end of the selection, we added one new study, Afshinmajd 2014, to the original 23 studies included in the previous version of this review, for a total of 24 studies included in this review. We have presented the main features of the studies in the Characteristics of included studies table. Eighteen trials with 2477 participants compared either bed rest versus immediate mobilization or a longer versus a shorter period of bed rest (Andersen 1986; Congia 1985; Cook 1989; Dieterich 1985; Ebinger 2004; Fassoulaki 1991; Jensen 1987; Johannsson 1992; Macpherson 1983; Macpherson 1984; Macpherson 1985; Murata 2003; Robertson 1980; Teasdale 1983; Tejavanija 2006; Thornberry 1988; Vilming 1988; Vimala 1998). Six of these trials involved 723 people undergoing diagnostic lumbar puncture (Congia 1985; Dieterich 1985; Ebinger 2004; Johannsson 1992; Tejavanija 2006; Vilming 1988); four trials involved 381 people undergoing spinal anesthesia for orthopedic, urological, or obstetric procedures (Andersen 1986; Cook 1989; Fassoulaki 1991; Thornberry 1988); and seven involved 1165 people undergoing myelography (Jensen 1987; Macpherson 1983; Macpherson 1984; Macpherson 1985; Murata 2003; Robertson 1980; Teasdale 1983). One trial involved 208 people undergoing lumbar puncture for any reason (Vimala 1998).
Three trials compared the effects of a head‐tilt versus no head‐tilt in addition to bed rest among 106 people undergoing diagnostic lumbar puncture (Hilton‐Jones 1982; Robertson 1980; Smith 1980). Two trials also compared the effects of prone versus supine position during bed rest (Afshinmajd 2014; Hilton‐Jones 1982), while another trial compared prone positioning versus head‐tilt followed by supine positioning (Handler 1982). Hilton‐Jones 1982 study provided four groups of participants (supine versus prone position, with or without head‐tilt), and all were included in further analysis. Two trials assessed the effects of supplementary fluids among 200 people undergoing either diagnostic lumbar puncture or myelography (Dieterich 1988; Eldevik 1978).
Excluded studies
We excluded two studies in this update (Faridi 2014; van Zundert 2013), in addition to five studies excluded in the previous version of this review (Carbaat 1981; Gulati 1981; Hafer 1997a; Hallam 1993; Spriggs 1992). In summary, we excluded five studies because they were not fully randomized (Carbaat 1981; Gulati 1981; Hafer 1997a; Hallam 1993; Spriggs 1992), one study because it was a letter to the editor (van Zundert 2013), and one study because the fluids were supplemented by an intrathecal injection (Faridi 2014).
Two studies were available only in abstract form, which had been submitted for a European Congress (Cucereanu 2010; Ulukaya 2014). We classified these studies as awaiting future full‐text publication. One study was published in two different articles (Andersen 1986). We have presented details of excluded and awaiting classification studies in the Characteristics of excluded studies and Characteristics of studies awaiting classification tables.
Risk of bias in included studies
We have presented summary details of methods used in the studies in the Characteristics of included studies table and illustrated in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All 24 included trials were described as randomized, although only seven contained published or unpublished information about the methodology used to allocate treatments (Andersen 1986; Hilton‐Jones 1982; Tejavanija 2006; Thornberry 1988; Vilming 1988; Vimala 1998). Similarly, only six trials provided published or unpublished information about allocation concealment (Cook 1989; Jensen 1987; Macpherson 1985; Smith 1980; Thornberry 1988; Vilming 1988). Seventeen trials had an unclear risk of bias for allocation concealment (selection bias). Only two trials had low risk of bias for both random sequence generation and allocation concealment (Thornberry 1988; Vilming 1988).
Blinding
We did not evaluate blinding of participants and researchers in our review due to the nature of the intervention (bed rest or supplementary fluids). Outcome assessment was blinded in 13 trials (Eldevik 1978; Fassoulaki 1991; Handler 1982; Hilton‐Jones 1982; Jensen 1987; Macpherson 1983; Macpherson 1985; Murata 2003; Smith 1980; Teasdale 1983; Thornberry 1988; Vilming 1988). These trials reported assessment of headache by another physician or researcher who did not know the results of the randomization scheme. Nine trials did not report information about blinding, or the reported information was classified as high risk of bias (Afshinmajd 2014; Andersen 1986; Congia 1985; Dieterich 1988; Ebinger 2004; Johannsson 1992; Robertson 1980; Tejavanija 2006; Vimala 1998).
Incomplete outcome data
The duration of follow‐up varied between five hours to one month after lumbar puncture. In one trial, 27 of the 129 participants included for randomization were either subsequently excluded due to protocol violations or lost to follow‐up without mention of randomization group (Cook 1989). Two other trials documented minor exclusions (Handler 1982; Murata 2003). No participants were reported as lost to follow‐up in the remaining trials. Thirty‐one participants who were excluded from the different analyses due to protocol violations were included in accordance to intention‐to‐treat analysis.
Selective reporting
We identified high risk of reporting bias in three trials given that no results were found for variables included in the methodology section (Congia 1985; Dieterich 1988; Handler 1982). Fifteen of the 24 included trials did not provide sufficient information to assess risk of bias and were classified as having an unclear risk of bias.
Other potential sources of bias
Trials were generally small. The number of participants in each trial varied from 39 to 382. Ten trials included fewer than 100 people (Congia 1985; Fassoulaki 1991; Handler 1982; Hilton‐Jones 1982; Jensen 1987; Johannsson 1992; Robertson 1980; Smith 1980; Tejavanija 2006; Thornberry 1988). Only one trial used a power calculation to determine the number of people that had to be recruited (Vimala 1998).
Effects of interventions
See: Summary of findings 1 Bed rest versus ambulation for preventing post‐dural puncture headache
See summary of findings Table 1.
Bed rest versus immediate mobilization (Analysis 1 and 2)
Primary outcome
Post‐dural puncture headache
For this outcome, we included 12 studies with 1519 participants (Andersen 1986; Congia 1985; Cook 1989; Dieterich 1985; Ebinger 2004; Fassoulaki 1991; Johannsson 1992; Murata 2003; Tejavanija 2006; Thornberry 1988; Vilming 1988; Vimala 1998). The total incidence of post‐dural puncture headache (PDPH) was 23.5%. Bed rest resulted in more cases of PDPH compared with immediate ambulation (risk ratio (RR 1.24; 95% confidence interval (CI) 1.04 to 1.48; I2 = 0%; Analysis 1.1), corresponding to a number needed to treat for an additional harmful outcome of 17 (95% CI 10 to 60). We downgraded the quality of evidence from high to moderate due to the presence in some studies of unclear risk of bias related to allocation concealment and high risk of bias in blinding of outcome assessment (See summary of findings Table 1). The funnel plot shows an asymmetry since two small studies, Ebinger 2004 and Johannsson 1992, favor immediate mobilization without equivalent studies on the other side of the point estimate (Figure 4). A sensitivity analysis excluding these studies still shows the same effect (RR 1.21; 95% CI 1.02 to 1.45; I2 = 0%).

Funnel plot of comparison: 1 Bed rest versus ambulation, outcome: 1.1 Post‐dural puncture headache.
There was insufficient information on age, gender, postures during lumbar puncture, needle gauge, needle tip, and amount of cerebrospinal fluid (CSF) aspirated to perform all planned subgroup analysis. Information on reason for puncture was available only for trials comparing bed rest versus immediate mobilization. In the subgroup analysis performed for indication of lumbar puncture (12 studies, 1519 participants), we observed some differences in the results for indications of lumbar puncture (Analysis 2.1; I2 subgroup test = 31.9%). There was no difference between bed rest and immediate mobilization in three of the four separate categories of lumbar puncture: diagnostic lumbar puncture (RR 1.11; 95% CI 0.90 to 1.37; 723 participants; 6 studies), myelography (RR 1.48; 95% CI 0.67 to 3.27; 207 participants; 1 study), and mixed (RR 1.27; 95% CI 0.68 to 2.35; 208 participants; 1 study). There was a small difference between bed rest and immediate mobilization on spinal anesthesia data (RR 1.82; 95% CI 1.19 to 2.78; 381 participants; 4 studies), suggesting an increase in the risk of PDPH with bed rest (Figure 5).

Forest plot for reason for puncture: bed rest versus ambulation, outcome: 2.1 Post‐dural puncture headache.
Secondary outcomes
Severe post‐dural puncture headache
For this outcome, we included nine studies with 1568 participants (Cook 1989; Dieterich 1985; Fassoulaki 1991; Johannsson 1992; Macpherson 1984; Macpherson 1985; Thornberry 1988; Vimala 1998). The total incidence of severe PDPH was 10.7%. There were no differences between bed rest and immediate mobilization for severe PDPH (RR 0.98; 95% CI 0.68 to 1.41; I2 = 22%; Analysis 1.2). We downgraded the quality of evidence from high to low due to the presence in some studies of unclear risk of bias related to allocation concealment and high risk of bias in blinding of outcome assessment (See summary of findings Table 1). There was no difference in severe PDPH between bed rest and immediate mobilization with regards to reason of lumbar puncture (Analysis 2.2): diagnostic lumbar puncture (RR 0.93; 95% CI 0.62 to 1.38; 509 participants; 3 studies), myelography (RR 0.97; 95% CI 0.59 to 1.60; 582 participants; 2 studies), anesthesia (RR 2.45; 95% CI 0.89 to 6.72; 269 participants; 3 studies), and mixed (RR 0.25; 95% CI 0.05 to 1.15; 208 participants; 1 study). We observed some subgroup differences in the results for this outcome (I2 subgroup test = 52.5%).
Any headache
For this outcome, we included 18 studies with 2477 participants (Andersen 1986; Congia 1985; Cook 1989; Dieterich 1985; Ebinger 2004; Fassoulaki 1991; Jensen 1987; Johannsson 1992; Macpherson 1983; Macpherson 1984; Macpherson 1985; Murata 2003; Robertson 1980; Teasdale 1983; Tejavanija 2006; Thornberry 1988; Vilming 1988; Vimala 1998). The total incidence of any headache after lumbar puncture was 31.1%. There were small differences between bed rest and immediate mobilization for any headache (RR 1.16; 95% CI 1.02 to 1.32; I2 = 17%; Analysis 1.3). We downgraded the quality of evidence from high to moderate due to the presence in some studies of unclear risk of bias related to allocation concealment and high risk of bias in blinding of outcome assessment (See summary of findings Table 1). In the subgroup analysis performed for indication of lumbar puncture, we observed some differences in the results for indications of lumbar puncture (Analysis 2.3; I2 subgroup test = 57.2%; 2477 participants; 18 studies). There was no difference in any headache after lumbar puncture between bed rest and immediate mobilization in three of four separate categories of lumbar puncture: diagnostic lumbar puncture (RR 1.15; 95% CI 0.94 to 1.40; 723 participants; 6 studies), myelography (RR 1.06; 95% CI 0.89 to 1.26; 1165 participants; 7 studies), and mixed (RR 1.27; 95% CI 0.68 to 2.35; 208 participants; 1 study). There were more headaches following spinal anesthesia after bed rest than after immediate mobilization (RR 1.85; 95% CI 1.27 to 2.71; 381 participants; 4 studies).
Bed rest versus bed rest with head‐down tilt
Primary outcome
Post‐dural puncture headache
No studies reported information for this outcome.
Secondary outcomes
Severe post‐dural puncture headache
No studies reported information for this outcome.
Any headache
For this outcome, we included one study with 60 participants (Robertson 1980). The total incidence of any headache after lumbar puncture was 48.3%. This trial suggested that there was no difference between bed rest with or without head‐down tilt regarding incidence of any headache after lumbar puncture (RR 0.81; 95% CI 0.48 to 1.38). We downgraded the quality of evidence due to the presence in this study of unclear risk of bias related to allocation concealment, high risk of bias in blinding of outcome assessment, as well as insufficient sample size.
Prone versus supine posture (Analysis 3)
Primary outcome
Post‐dural puncture headache
For this outcome, we included one study with 119 participants (Afshinmajd 2014). The estimated risk for PDPH comparing supine versus prone position was 1.09 (95% CI 0.65 to 1.85). The data suggested that there were no differences between positions in incidence of PDPH. We downgraded the quality of evidence due to the presence in this study of unclear risk of bias related to allocation concealment and high risk of bias in blinding of outcome assessment.
Secondary outcomes
Severe post‐dural puncture headache
No studies reported information for this outcome.
Any headache
For this outcome, we included three studies with 239 participants (Afshinmajd 2014; Handler 1982; Hilton‐Jones 1982). Hilton‐Jones 1982 provided four groups of participants (supine versus prone position, with or without head‐tilt), and all were included in this analysis. The total incidence of any headache after lumbar puncture was 34.3%. There was no difference between positions regarding incidence of any headache after lumbar puncture (RR 0.97; 95% CI 0.68 to 1.37; I2 = 0%; Analysis 3.1). We downgraded the quality of evidence due to the presence in the three studies of unclear risk of bias related to allocation concealment, and high risk of bias in blinding of outcome assessment in one study.
Prone or supine posture versus prone or supine posture with head‐down tilt (Analysis 4)
Primary outcome
Post‐dural puncture headache
No studies reported information for this outcome.
Secondary outcomes
Severe post‐dural puncture headache
No studies reported information for this outcome.
Any headache
Regarding supine position, we included two studies with 87 participants (Hilton‐Jones 1982; Smith 1980). The total incidence of any headache after lumbar puncture was 47.1%. There were more headaches associated with the supine posture with head‐down tilt compared with the supine position alone (RR 1.72; 95% CI 1.10 to 2.69; I2 = 0%; Analysis 4.1). We downgraded the quality of evidence due to the unclear risk of bias related either to randomization method or allocation concealment, as well as insufficient sample size.
Regarding prone position, we included one study with 39 participants (Hilton‐Jones 1982). The total incidence of any headache after lumbar puncture was 43.5%. There were no differences between prone with head‐down tilt versus prone position alone regarding incidence of any headache after lumbar puncture (RR 1.18; 95% CI 0.58 to 2.42). We downgraded the quality of evidence due to the study's unclear risk of bias related to allocation concealment, as well as insufficient sample size.
Supplementary fluids (Analysis 5)
Primary outcome
Post‐dural puncture headache
For this outcome, we included one study with 100 participants (Dieterich 1985). The total incidence of PDPH was 36%. The data suggested that there were no differences between fluid supplementation and no supplementation in incidence of PDPH (RR 1; 95% CI 0.59 to 1.69). We downgraded the quality of evidence due to the study's unclear risk of bias related to allocation concealment, high risk of bias in blinding of outcome assessment, as well as insufficient sample size.
Secondary outcomes
Severe post‐dural puncture headache
For this outcome, we included one study with 100 participants (Dieterich 1985). The total incidence of severe PDPH was 15%. The data suggested that there were no differences between fluid supplementation and no supplementation in incidence of severe PDPH (RR 0.67; 95% CI 0.26 to 1.73). We downgraded the quality of evidence due to the study's unclear risk of bias related to allocation concealment, as well as insufficient sample size.
Any headache
For this outcome, we included two studies with 200 participants (Dieterich 1985; Eldevik 1978). The total incidence of any headache after lumbar puncture was 38.5%. There was no difference between fluid supplementation and no supplementation regarding incidence of any headache after lumbar puncture (RR 0.94; 95% CI 0.66 to 1.34; I2 = 0%; Analysis 5.1). We downgraded the quality of evidence due to the presence in some studies of unclear risk of bias related to allocation concealment.
Sensitivity analysis (Analysis 6)
Two trials including 380 participants had low risk of bias with regards to blinding of outcome assessment, losses to follow‐up, adequate randomization, and allocation concealment (Thornberry 1988; Vilming 1988). These trials compared bed rest versus immediate ambulation. Analysis restricted to these trials showed no difference between bed rest and immediate ambulation in incidence of PDPH (RR 1.18; 95% CI 0.90 to 1.54; Analysis 6.1). There was no statistical heterogeneity between studies (I2 = 0%).
Discussion
Summary of main results
Regarding bed rest, this updated systematic review of all available trials found no evidence to suggest that a period of bed rest following dural puncture reduces the risk of PDPH, severe PDPH, or any headache. Furthermore, immobilization could even increase the risk of headache in people undergoing lumbar puncture (low to moderate quality evidence).
A total of 26.4% of participants randomised to the bed rest group in the included studies experienced a postural headache, compared with 20.5% randomised to the immediate‐mobilization groups. These figures show that 49 additional participants out of 1000 receiving bed rest will have a PDPH (with a minimum of eight and a maximum of 98). Sensitivity analysis considering only trials with low risk of bias consistently showed the lack of benefit of bed rest compared with immediate mobilization. Subgroup analyses only showed differences in the anesthesia subgroup (four trials), and only one trial in this analysis had low risk of bias in all categories assessed. It is also important to consider that the low number of participants involved in this analysis (381) may not be sufficient to detect differences between interventions.
These results suggest that there is no role for prolonged immobilization in lumbar puncture practice. Given that bed rest does not provide any benefit in the prevention of headaches after lumbar puncture, it becomes unnecessary to discuss the position that should be adopted during bed rest as well as modification of head postures (head‐down or head‐up tilt). In any case, the results of our updated review do not suggest any benefits related to specific body and head postures on the incidence of headache after lumbar puncture.
Regarding fluid supplements, despite identifying two trials that studied the role of fluid supplementation following lumbar puncture, only one of these trials provided data on incidence of PDPH and severe PDPH, and found no beneficial effect associated with fluid supplementation. We found similar results for incidence of any headache, which both trials assessed. The wide 95% CIs of these comparisons preclude us from making solid conclusions about fluid supplementation in the prevention of PDPH. Sudlow et al. previously estimated that a sample size of 100 to 3000 participants per arm, assuming baseline risks of 20% and 8%, respectively, would be necessary to identify a beneficial effect of this intervention (Sudlow 2002). Recruitment of this number of participants would require the involvement of several centers, as well as a considerable amount of work and resources to conduct the corresponding clinical trial.
Overall completeness and applicability of evidence
Included studies evaluated a wide range of bed rest times in order to determine if extended bed rest had any effect on the prevention of PDPH. Rest periods additional to those indicated as a result of the medical/surgical procedure ranged from four to 24 hours. Several head and body positions were evaluated, taking into account physiological theories about PDPH. Studies that focused on supplementary fluids only assessed additional fluid intake of 1.5 L to 2 L, which does not allow extrapolation to other forms of hydration, such as parenteral supplementation. Considering the nature of the medical problem and its interventions, it is likely that the evidence obtained from the included trials could be applied to similar populations outside of trials. It seems unlikely that publication bias could have influenced the main findings of this review, since such bias usually involves the preferential publication of trials with differences between groups. Applying our findings, both patients and health systems would benefit by avoiding these useless interventions.
Quality of the evidence
Lack of information in published reports was a problem when assessing risk of bias. Many trials did not adequately report the study characteristics that are important in the evaluation of the quality of evidence. Also, it was not possible to blind participants included in these trials to the assigned interventions, which poses a possible source of bias. In most of cases, we downgraded risk of bias due to concerns about allocation concealment and blinding of outcome assessment items. When we only considered trials with low risk of bias in methodological aspects such as randomization methods and blinding of outcome assessment, again there was no evidence for benefit of bed rest on the incidence of PDPH.
Another possible source of bias in these trials arises from the nature of the outcome (headache after lumbar puncture), which depends strongly on the subjective report of participants rather than on an objective assessment. This phenomenon may have influenced the results, especially those related to an excess of PDPH risk in people on bed rest. People with limited mobility may be more susceptible to report minor discomforts or to overrate its seriousness. Some trials implemented a blinded assessment of outcomes to partially avoid this potential source of bias. However, this assessment was misreported and bias was unclear in several cases. The quality of evidence ranged from moderate to low for all comparisons and outcomes assessed.
Potential biases in the review process
We followed the methodology for systematic reviews outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This review was comprehensive in identifying trials addressing the effect of bed rest and supplementation of fluids in the prevention of PDPH. In fact, this version is the second update of the original review published by Sudlow et al. in 2002 (Sudlow 2002). In all this process, after seeing the data collected no marginal decisions about the analysis or investigation of heterogeneity that could have impacted the findings of the review were made.
Agreements and disagreements with other studies or reviews
The previous version of this review highlighted the lack of benefit of both interventions for the prevention of PDPH of any kind (Sudlow 2002). The newly identified evidence does not alter the conclusions of the previous review but provides a warning about the probability of deleterious effects associated with bed rest. One review that included 16 trials with 1083 participants also found that extended rest did not prevent the appearance of headaches after lumbar puncture (Thoennissen 2001). We identified no other reviews on the effectiveness of fluid supplementation in the prevention of headache after lumbar puncture.

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Bed rest versus ambulation, outcome: 1.1 Post‐dural puncture headache.

Forest plot for reason for puncture: bed rest versus ambulation, outcome: 2.1 Post‐dural puncture headache.

Comparison 1: Bed rest versus immediate ambulation, Outcome 1: PDPH

Comparison 1: Bed rest versus immediate ambulation, Outcome 2: Severe PDPH

Comparison 1: Bed rest versus immediate ambulation, Outcome 3: Any headache

Comparison 2: Reason for puncture: bed rest versus immediate ambulation, Outcome 1: PDPH

Comparison 2: Reason for puncture: bed rest versus immediate ambulation, Outcome 2: Severe PDPH

Comparison 2: Reason for puncture: bed rest versus immediate ambulation, Outcome 3: Any headache

Comparison 3: Supine versus prone, Outcome 1: Any headache

Comparison 4: Supine versus supine with head tilt, Outcome 1: Any headache

Comparison 5: Fluids versus less or no fluids, Outcome 1: Any headache

Comparison 6: Sensitivity analysis/low risk of bias: bed rest versus ambulation, Outcome 1: PDPH
| Bed rest versus ambulation for preventing post‐dural puncture headache | ||||||
| Patient or population: Participants undergoing lumbar puncture | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Ambulation | Bed rest | |||||
| Post‐dural puncture headache | 205 per 1000 | 254 per 1000 | RR 1.24 | 1519 | ⊕⊕⊕⊝ | |
| Severe post‐dural puncture headache | 107 per 1000 | 105 per 1000 | RR 0.98 | 1568 | ⊕⊕⊝⊝ | |
| Any headache | 287 per 1000 | 333 per 1000 | RR 1.16 | 2477 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded (‐1) due to unclear risk of bias related to allocation concealment (9 studies), as well as high risk of bias in blinding of outcome assessment (6 studies). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 PDPH Show forest plot | 12 | 1519 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.04, 1.48] |
| 1.2 Severe PDPH Show forest plot | 9 | 1568 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.68, 1.41] |
| 1.3 Any headache Show forest plot | 18 | 2477 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.02, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 PDPH Show forest plot | 12 | 1519 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.04, 1.48] |
| 2.1.1 Diagnostic | 6 | 723 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.90, 1.37] |
| 2.1.2 Myelography | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.67, 3.27] |
| 2.1.3 Anesthesia | 4 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.19, 2.78] |
| 2.1.4 Mixed | 1 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.68, 2.35] |
| 2.2 Severe PDPH Show forest plot | 9 | 1568 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.68, 1.41] |
| 2.2.1 Diagnostic | 3 | 509 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.62, 1.38] |
| 2.2.2 Myelography | 2 | 582 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.59, 1.60] |
| 2.2.3 Anesthesia | 3 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [0.89, 6.72] |
| 2.2.4 Mixed | 1 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.15] |
| 2.3 Any headache Show forest plot | 18 | 2477 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.02, 1.32] |
| 2.3.1 Diagnostic | 6 | 723 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.94, 1.40] |
| 2.3.2 Myelography | 7 | 1165 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.89, 1.26] |
| 2.3.3 Anesthesia | 4 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.27, 2.71] |
| 2.3.4 Mixed | 1 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.68, 2.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Any headache Show forest plot | 3 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.68, 1.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Any headache Show forest plot | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.10, 2.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Any headache Show forest plot | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.66, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 PDPH Show forest plot | 2 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.54] |

