Intervensi untuk meningkatkan kepatuhan pengambilan ubat‐ubatan bagi kebergantungan tembakau
Abstract
Background
Pharmacological treatments for tobacco dependence, such as nicotine replacement therapy (NRT), have been shown to be safe and effective interventions for smoking cessation. Higher levels of adherence to these medications increase the likelihood of sustained smoking cessation, but many smokers use them at a lower dose and for less time than is optimal. It is important to determine the effectiveness of interventions designed specifically to increase medication adherence. Such interventions may address motivation to use medication, such as influencing beliefs about the value of taking medications, or provide support to overcome problems with maintaining adherence.
Objectives
To assess the effectiveness of interventions aiming to increase adherence to medications for smoking cessation on medication adherence and smoking abstinence compared with a control group typically receiving standard care.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialized Register, and clinical trial registries (ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform) to the 3 September 2018. We also conducted forward and backward citation searches.
Selection criteria
Randomised, cluster‐randomised or quasi‐randomised studies in which adults using active pharmacological treatment for smoking cessation were allocated to an intervention arm where there was a principal focus on increasing adherence to medications for tobacco dependence, or a control arm providing standard care. Dependent on setting, standard care may have comprised minimal support or varying degrees of behavioural support. Included studies used a measure that allowed assessment of the degree of medication adherence.
Data collection and analysis
Two authors independently screened studies for eligibility, extracted data for included studies and assessed risk of bias. For continuous outcome measures, we calculated effect sizes as standardised mean differences (SMDs). For dichotomous outcome measures, we calculated effect sizes as risk ratios (RRs). In meta‐analyses for adherence outcomes, we combined dichotomous and continuous data using the generic inverse variance method and reported pooled effect sizes as SMDs; for abstinence outcomes, we reported and pooled dichotomous outcomes. We obtained pooled effect sizes with 95% confidence intervals (CIs) using random‐effects models. We conducted subgroup analyses to assess whether the primary focus of the adherence treatment ('practicalities' versus 'perceptions' versus both), the delivery approach (participant versus clinician‐centred) or the medication type were associated with effectiveness.
Main results
We identified two new studies, giving a total of 10 studies, involving 3655 participants. The medication adherence interventions studied were all provided in addition to standard behavioural support.They typically provided further information on the rationale for, and emphasised the importance of, adherence to medication or supported the development of strategies to overcome problems with maintaining adherence (or both). Seven studies targeted adherence to NRT, two to bupropion and one to varenicline. Most studies were judged to be at high or unclear risk of bias, with four of these studies judged at high risk of attrition or detection bias. Only one study was judged to be at low risk of bias.
Meta‐analysis of all 10 included studies (12 comparisons) provided moderate‐certainty evidence that adherence interventions led to small improvements in adherence (i.e. the mean amount of medication consumed; SMD 0.10, 95% CI 0.03 to 0.18; I² = 6%; n = 3655), limited by risk of bias. Subgroup analyses for the primary outcome identified no significant subgroup effects, with effect sizes for subgroups imprecisely estimated. However, there was a very weak indication that interventions focused on the 'practicalities' of adhering to treatment (i.e. capabilities, resources, levels of support or skills) may be effective (SMD 0.21, 95% CI 0.03 to 0.38; I² = 39%; n = 1752), whereas interventions focused on treatment 'perceptions' (i.e. beliefs, cognitions, concerns and preferences; SMD 0.10, 95% CI –0.03 to 0.24; I² = 0%; n = 839) or on both (SMD 0.04, 95% CI –0.08 to 0.16; I² = 0%; n = 1064), may not be effective. Participant‐centred interventions may be effective (SMD 0.12, 95% CI 0.02 to 0.23; I² = 20%; n = 2791), whereas those that are clinician‐centred may not (SMD 0.09, 95% CI –0.05 to 0.23; I² = 0%; n = 864).
Five studies assessed short‐term smoking abstinence (five comparisons), while an overlapping set of five studies (seven comparisons) assessed long‐term smoking abstinence of six months or more. Meta‐analyses resulted in low‐certainty evidence that adherence interventions may slightly increase short‐term smoking cessation rates (RR 1.08, 95% CI 0.96 to 1.21; I² = 0%; n = 1795) and long‐term smoking cessation rates (RR 1.16, 95% CI 0.96 to 1.40; I² = 48%; n = 3593). In both cases, the evidence was limited by risk of bias and imprecision, with CIs encompassing minimal harm as well as moderate benefit, and a high likelihood that further evidence will change the estimate of the effect. There was no evidence that interventions to increase adherence to medication led to any adverse events. Studies did not report on factors plausibly associated with increases in adherence, such as self‐efficacy, understanding of and attitudes toward treatment, and motivation and intentions to quit.
Authors' conclusions
In people who are stopping smoking and receiving behavioural support, there is moderate‐certainty evidence that enhanced behavioural support focusing on adherence to smoking cessation medications can modestly improve adherence. There is only low‐certainty evidence that this may slightly improve the likelihood of cessation in the shorter or longer‐term. Interventions to increase adherence can aim to address the practicalities of taking medication, change perceptions about medication, such as reasons to take it or concerns about doing so, or both. However, there is currently insufficient evidence to confirm which approach is more effective. There is no evidence on whether such interventions are effective for people who are stopping smoking without standard behavioural support.
PICOs
Ringkasan bahasa mudah
Bolehkah kita membantu para perokok meningkatkan penggunaan ubat‐ubatan berhenti merokok?
Latar belakang
Ubat‐ubatan direka bagi memudahkan para perokok berhenti merokok, seperti terapi gantian nikotin (NRT), Bupropion dan Varenicline, adalah selamat dan berjaya membantu perokok untuk berhenti. Namun begitu, kebiasaannya orang tidak mengikut arahan ubatan dengan betul, menyebabkan kesan ubatan tidak berfungsi sebagus yang sepatutnya. Hal demikian mungkin mengurangkan peluang bagi seseorang untuk terus berhenti merokok. Dalam kajian ini, kami melihat sama ada terdapat cara‐cara bagi membantu orang ramai menggunakan ubat‐ubatan berhenti merokok dengan betul, dan sama ada ini dapat menjadikan seseorang lebih cenderung untuk berhenti merokok.
Ciri‐ciri kajian
Kami mencari kajian‐kajian sehingga September 2018, dan kami mendapati 10 kajian, melibatkan 3655 orang. Mereka semua merupakan perokok, berumur 18 tahun dan ke atas. Kajian‐kajian tersebut mengkaji cara yang berbeza untuk membantu orang menggunakan ubat berhenti merokok dengan betul. Secara amnya ini bermaksud menyediakan maklumat tambahan tentang ubat‐ubatan atau membantu orang ramai untuk mengatasi masalah yang mereka hadapi apabila mengambil ubatan berhenti merokok. Satu kajian memberikan sokongan melalui telefon, dan selebihnya menyediakan sekurang‐kurangnya beberapa sokongan secara bersemuka. Semua kajian yang terlibat mengukur jumlah ubat‐ubatan yang mereka digunakan dan semua kecuali satu kajian mengukur berapa ramai yang berhenti merokok.
Keputusan utama
Mereka yang menerima bantuan penggunaan ubatan berhenti merokok menggunakan ubatan mereka lebih sedikit berbanding mereka yang tidak menerima bantuan. Terdapat beberapa bukti bahawa ini turut menyebabkan agak ramai sedikit orang berhenti merokok.
Kualiti bukti kajian
Bukti menunjukkan bahawa membantu mereka meningkatkan penggunaan ubatan berhenti merokok dapat meningkatkan penggunaan ubatan adalah bukti berkualiti sederhana, bermaksud bukti tambahan dapat menjadikan kita lebih pasti akan kesan ini. Ini adalah kerana terdapat beberapa masalah dengan cara sebahagian kajian yang terlibat. Bukti yang mencadangkan bahawa pendekatan untuk meningkatkan penggunaan ubatan berhenti merokok dapat membantu lebih ramai orang berhenti merokok merupakan bukti berkualiti lemah, ini bererti bahawa kami tidak yakin bahawa sokongan tambahan bagi meningkatkan penggunaan ubatan berhenti merokok dapat membantu lebih ramai orang untuk berhenti dan bukti lanjut mungkin dapat mengukuhkan keyakinan kita terhadap kesan ini. Ini adalah kerana terdapat beberapa masalah dengan sebahagian cara kajian dan kerana ianya tidak jelas sama ada dengan menyediakan sokongan tambahan untuk menggalakkan orang ramai menggunakan ubatan menyebabkan lebih ramai atau lebih sedikit orang yang berjaya berhenti merokok.
Authors' conclusions
Summary of findings
| Interventions to increase adherence compared to standard care for improving adherence to medications for tobacco dependence and abstinence from smoking | |||||
| Patient or population: adult smokers | |||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks (95% CI) | No of participants | Certainty of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Standard care | Intervention to increase adherence | ||||
| Adherence to medications for tobacco dependence | SMD 0.10 (0.03 to 0.18) | Mean proportion of prescribed medication consumed over 28 days was 63.6% | Mean proportion of prescribed medication consumed over 28 days was 3.9% higher (95% CI 1.2% to 7.0% higher) | 3655 | ⊕⊕⊕⊝ |
| Short‐term abstinence from smoking (< 6 months) | RR 1.08 | 357 people per 1000 achieve abstinence) | 386 people per 1000 achieve abstinence (95% CI 343 to 432) | 1795 | ⊕⊕⊝⊝ |
| Long‐term abstinence from smoking (≥ 6 months) | RR 1.16 | 203 people per 1000 achieve abstinence | 236 per 1000 achieve abstinence (95% CI 195 to 284) | 3593 | ⊕⊕⊝⊝ |
| The basis for the illustrative comparative risks is provided in Footnotesd. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High certainty: current evidence provides a very good indication of the likely effect, and the likelihood that the actual effect will be substantially different is low. | |||||
| aMost studies were at high or unclear risk of bias which lowers confidence in estimate of effect (risk of bias). | |||||
Background
Description of the condition
Smoking is one of the largest preventable causes of disease and premature death worldwide, being a key causal factor in heart disease, stroke, chronic lung disease and cancers (GBD 2018). Pharmacological treatments for tobacco dependence, such as nicotine replacement therapy (NRT), are widely considered to be safe and effective interventions for smoking cessation. One Cochrane systematic review found that participants using NRT were over 1.5 times more likely to achieve abstinence than those who did not (Hartmann‐Boyce 2018). Participants using bupropion, nortriptyline and varenicline are also more likely to stop smoking than those using placebo (Cahill 2016; Hughes 2014). However, studies have shown that many smokers who use medications for tobacco dependence do so at a lower dose and for less time than the evidence suggests is optimal (Cheong 2010; Hays 2010; Shiffman 2008; Swan 2010). For example, Burns and Levinson reported that users of NRT, on average, continue medication for less than half the time for which it was prescribed (Burns 2008). Observational evidence controlling for reverse causation (whereby people whose quit attempt was faltering choose not to adhere to their medication) showed that prior adherence to medication promoted later abstinence (Hollands 2013; Shiffman 2007; Shiffman 2008). One review of this relationship, although highlighting the lack of high‐quality studies, suggested that the degree of adherence predicted subsequent abstinence (Raupach 2014). Therefore, it is important to know whether interventions aiming to increase adherence are effective and whether this in turn improves abstinence, the evidence for which we reviewed here.
Description of the intervention
Interventions that specifically aim to increase adherence to prescribed medications vary widely in their content and characteristics (Nieuwlaat 2014). Examples may include, but are not limited to, improved or increased information provision, monitoring and feedback concerning performance, reminders, and psychological therapy or counselling. In the specific context of medications for tobacco dependence, general behavioural support for smoking cessation may include components that target increasing medication adherence. Interventions that are additional to standard behavioural support and that devote special attention to improving adherence may also be delivered, such as addressing individuals' beliefs about the value of taking medications or providing additional support to overcome barriers to adherence.
More‐specific intervention types can be characterised by reference to two key factors informed by the Perceptions and Practicalities Approach (PAPA) (Horne 2013). This approach proposes that non‐adherence can be both intentional and unintentional depending on a person's motivations and capabilities. Perceptual factors ('perceptions'), that is, beliefs, cognitions, concerns and preferences, as well as practical factors ('practicalities'), that is, capabilities, resources, levels of support or skills, can explain non‐adherence and be addressed by interventions to increase adherence. Current guidance in England on medicines adherence emphasises both perceptions and practicalities for improving medication adherence (NICE 2009). PAPA emphasises the importance of tailoring intervention content by eliciting and appreciating the needs, cognitions or behaviours of the patient or participant, and can, therefore, be considered 'participant‐centred'. By contrast, adherence focused interventions that are primarily 'clinician‐centred' tend to be standardised, directive or didactic in nature. We used this approach to categorise interventions in this review.
Why it is important to do this review
To our knowledge, no other published systematic review addresses this question. Reviews of studies of behavioural support interventions (e.g. Hartmann‐Boyce 2019; Lancaster 2017), which may include elements that target medication adherence, are not designed to disentangle the specific effects of those components that focus on increasing adherence. Previous reviews of interventions designed to increase adherence have focused on specific patient groups or treatment contexts, or have not covered smoking cessation treatments (Nieuwlaat 2014). A specific review of the topic is valuable because we cannot be certain that findings relating to adherence to other medications are generalisable to smoking cessation medications, as these provide a unique treatment context with specific issues for adherence. For example, many people see stopping smoking without medication as the best way to stop smoking (Morphett 2015). Additionally, the drawbacks of failing to adhere are less significant than they may be in the treatment of illness. For example, individuals may successfully quit smoking without adhering to therapy, or if they fail to adhere and continue to smoke, they may not feel that they have lost anything or experienced any adverse effects. There is evidence to suggest that it may be more difficult to persuade individuals of the benefits of using smoking cessation medications compared with other health conditions. Hammond 2004 found that over a third of smokers reported that use of pharmacotherapies (NRT or bupropion) would either make no difference or actually reduce the likelihood of quitting smoking. Smokers who perceived cessation assistance methods to be beneficial were more likely to use medication in the future. Finally, some users may perceive risks of harm to their health from the medication that outweigh the potential benefits.
Objectives
To assess the effectiveness of interventions aiming to increase adherence to medications for smoking cessation on medication adherence and smoking abstinence compared with a control group typically receiving standard care.
To assess which intervention approaches are most effective; and determine the impact of interventions on potential precursors of adherence, such as understanding of the treatment and efficacy perceptions.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, cluster‐randomised or quasi‐randomised studies.
Types of participants
Adults (aged 18 years and over) smoking at point of entry into a study.
Types of interventions
All participants across relevant intervention and comparator study arms must have been offered effective pharmacological treatment for smoking cessation. Pharmacological treatments comprised those prescribed to increase cessation rates (e.g. NRT, bupropion, nortriptyline, varenicline and combination regimens).
Interventions to increase adherence may vary widely in their nature (Nieuwlaat 2014), and so the nature of the interventions considered for inclusion in this review were not specified beyond reference to exclusion criteria. Eligible interventions included any intervention that differed from standard care administered to smokers, and where the differing intervention content had a clear principal focus on increasing adherence to medications for tobacco dependence, reflected in described content and stated aims. We did not include interventions that systematically altered the active pharmacological characteristics of a given medication, such as dose strength, length of treatment or means of delivery. Interventions that included the use of financial incentives were not eligible.
Acceptable comparison groups were those that provided standard or usual care. Depending on setting, this could comprise of minimal support or varying degrees of behavioural support.
Types of outcome measures
Primary outcomes
-
Adherence to medication for tobacco dependence.
Studies must have used a quantitative measure of adherence. This could be defined as a continuous measure, such as the amount of medication consumed over a given treatment period, or as a dichotomous outcome, indicating whether the treatment was used to a specific quantified degree (e.g. adherence for x number of days, or x amount of medication consumed). This is in contrast to a binary (i.e. any amount of medication at any time versus non‐use) or categorical checklist measure, which we did not consider an appropriate measure. Adherence could have been measured by electronic measure, tablet counts by a third party or through self‐report (or combinations thereof).
Where studies reported multiple measures of adherence, we used the most stringent available. Where studies assessed treatment periods at multiple time points, we used the longest time point. Where available, we used primary outcome data for only those participants who continued a quit attempt and remained engaged for the duration of a treatment programme rather than dropping out, as opposed to using outcome data from all those randomised to receive a given intervention (i.e. intention‐to‐treat analysis (ITT)) (see Dealing with missing data for further details).
Secondary outcomes
-
Abstinence from smoking measured near or at a time point relevant to the measure of adherence (less than six months, i.e. short‐term abstinence).
Where there were data from multiple time points, we reported data measured near or at a time point closest to the measure of adherence, expected to be less than six months. Where studies reported multiple definitions of abstinence, we used the most stringent.
-
Abstinence at six months or longer (i.e. long‐term abstinence)
We reported abstinence at the longest available time point of six months or longer, in order to assess the long‐term benefit of the intervention on cessation rates. For both abstinence outcomes, we used data as randomised (ITT), assuming people not followed up to be smoking.
Other outcomes
-
Factors plausibly associated with increases in adherence, such as, but not limited to:
-
-
intention or motivation to quit smoking (as measured by the studies, likely using a self‐reported questionnaire measure);
-
attitudes towards treatment, or understanding of the treatment (as measured by the studies, likely using a self‐reported questionnaire measure);
-
self‐efficacy (as measured by the studies, likely using a self‐reported questionnaire measure).
-
-
Adverse events.
Any adverse events or harms reported in included trials, including clinical levels of depression or anxiety.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Tobacco Addiction Group Specialized Register on the 3 September 2018, and two trial registries (ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch/)).
The most recent issues of the databases included in the Register, as searched for the current update of this review, were:
-
Cochrane Central Register of Controlled trials (CENTRAL), issue 8, 2018;
-
MEDLINE (via Ovid) to update 28 August 2018;
-
Embase (via Ovid) to week 36 2018;
-
PsycINFO (via Ovid) to update 20 August 2018.
The search strategy for the Register is given in Appendix 1. For details of the searches used to create the Specialized Register see the Cochrane Tobacco Addiction Group's website.
Searching other resources
We conducted forwards and backwards citation searches from included studies.
Data collection and analysis
Selection of studies
Two review authors independently screened all search results (titles and abstracts) for possible inclusion, and those selected by either or both review authors were subjected to full‐text assessment. Two review authors independently assessed the selected full‐text articles for inclusion. Any discrepancies were resolved by consensus, overseen by a third review author acting as arbiter as necessary. We listed excluded studies after full‐text assessment and gave reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
We developed a data extraction form, which was piloted and amended as necessary. We extracted the following main sets of data from each included study:
-
lead author;
-
date;
-
study participant inclusion criteria;
-
participants (participant condition(s) and demographics: race/ethnicity, gender, religion/culture, socioeconomic status, age);
-
study design and timetable; randomisation; allocation concealment;
-
interventions (content and format of interventions, including details of information provided; intervention setting and delivery provider; delivery of any cointerventions, theoretical basis of intervention if stated; intervention type coded by reference to two factors: 1. focus on perceptions, practicalities, or both; 2. participant‐centred or clinician‐centred);
-
numbers of participants in each trial arm;
-
outcome measures; time(s) at which outcomes assessed;
-
results;
-
balance of baseline characteristics;
-
analysis;
-
additional comments;
-
study funding and authors' declarations of interest
Two review authors independently extracted data. A third review author checked data extraction and resolved any errors or inconsistencies. The first review author entered the data into Review Manager 5, with another review author checking the accuracy of the data entry (Review Manager 2014).
Assessment of risk of bias in included studies
We assessed and reported the risk of bias of included studies by outcome, in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We reported on the following individual domains:
-
random sequence generation (selection bias);
-
allocation concealment (selection bias);
-
blinding of outcome assessment (detection bias) (assessed for each main outcome or class of outcome). We did not assess risk of performance bias pertaining to blinding of participants and personnel due to the difficulty of achieving that in this context, in line with the guidance of the Cochrane Tobacco Addiction Group. It would be impractical to blind those delivering the intervention and attempts to do so could introduce additional limitations, such as reducing potency of the intervention by impairing its delivery and introducing further systematic differences between the intervention exposures by group;
-
incomplete outcome data (attrition bias) (assessed for each main outcome or class of outcome);
-
selective reporting (reporting bias);
-
other sources of bias (consistency in intervention delivery, i.e. was the information standardised/structured; was fidelity to protocol monitored).
Two review authors independently assessed risk of bias of included studies, with any disagreements resolved by discussion and consensus, and with a third review author acting as arbiter as necessary. We present our assessment in Risk of Bias tables for each included study.
A summary risk of bias judgement was derived for each study by applying an algorithm suggested in Section 8.7 (Table 8.7a) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Specifically, if the judgement in at least one of these domains was 'high risk of bias' then summary risk of bias was determined to be high. If there were no judgements of 'high' risk, but the judgement in at least one domain was 'unclear risk of bias', then the summary risk of bias was determined to be unclear. Summary risk of bias was only judged 'low' if judgements in all domains were 'low risk of bias'.
Measures of treatment effect
For continuous outcomes where the precise nature of the measures used differed but the outcomes were regarded as comparable, they were integrated and standardised to have common effect sizes, defined as the standardised mean difference (SMD). The effect measure for comparable dichotomous outcomes was risk ratio (RR). When different studies reported either dichotomous or continuous data for the same outcome, we combined these data using the generic inverse variance method and reported summary effect sizes as SMDs. This followed methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Sections 7.7.7 and 9.4.6; Higgins 2011), whereby standard errors were computed for each study by converting CIs for log odds ratios and SMDs. Log odds ratios were converted to SMDs by multiplying each by the required constant. Where studies provided both dichotomous and continuous measures for the same outcome, a continuous outcome measure was selected. Finally, we accounted for studies that contributed multiple comparisons to the meta‐analysis by reducing their sample sizes in direct relation to how often corresponding data were used.
We obtained a pooled effect size with 95% confidence intervals (CI) using a random‐effects model.
Unit of analysis issues
We included no cluster‐randomised trials and observed no unit of analysis errors. Should we have identified any cluster‐randomised trials, where an analysis was reported accounting for the clustered study design, we would have estimated the effect on this basis. If that had not been possible and the information was not available from authors, then an 'approximately correct' analysis would have been carried out according to current guidelines (Higgins 2011). We would have imputed estimates of the intracluster correlation (ICC) using estimates derived from similar studies or by using general recommendations from empirical research. If it was not possible to implement these procedures, we would have given the effect estimate as presented but reported the unit of analysis error.
Dealing with missing data
In the context of smoking cessation medications, it would be informative for measures of adherence to include only those participants who continue a quit attempt and not all those allocated to receive a given intervention (Hollands 2013). Including those people who abandon a quit attempt is less appropriate because first, treatment such as NRT is not indicated when a person has ceased trying to quit smoking, and second, it potentially confounds adherence with initial uptake (which may be influenced by different factors). As such, we are most interested in adherence to medication in those individuals who continue to engage with a treatment programme and do not dropout from the intervention, and hence remain in the study. Therefore, we intended to analyse data for our primary outcome in this way where available. In practice, primary outcomes for included studies were often presented as ITT, with five instances where it was clear that adherence was assessed and reported only for those who remained engaged with treatment or at least with study follow‐up (Mooney 2005; Nollen 2011; Schlam 2018; Smith 2013; Tucker 2017). For secondary smoking cessation outcomes, we assumed that people not followed up had resumed smoking following Cochrane Tobacco Addiction Group guidance. For such abstinence outcomes, ITT data were reported in all cases.
Assessment of heterogeneity
We tested for heterogeneity by inspecting the overlap of CIs and quantified this using the I² statistic (which describes the percentage of the variability in effect estimates due to heterogeneity rather than sampling error). We considered a value greater than 50% to represent substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
We assessed likelihood of publication bias using funnel plots for the primary adherence outcome as there were at least ten studies within that analysis (Sterne 2011).
Data synthesis
We conducted a narrative synthesis of the included studies, presenting studies' major characteristics and results. As studies were sufficiently similar in terms of setting, population, interventions and outcomes (including the time(s) at which these are assessed), we pooled the data statistically. We used a random‐effects model for meta‐analysis to obtain a pooled effect size with 95% CIs, due to observed clinical heterogeneity in study characteristics, such as differences in the treatment contexts and outcome measures used.
Certainty of the evidence
We used the GRADE framework to rate the certainty of each body of evidence relating to an outcome that was incorporated into a meta‐analysis, to indicate the confidence that may be placed in summary estimates of effect (Guyatt 2011). This is an assessment of the likelihood that the true effect will not be substantially different from what the research found. Within the GRADE approach, the certainty of a body of evidence for intervention effects is assessed based on the design of the underlying studies, with randomised controlled trials (RCTs) initially considered high certainty, and on a number of factors that can decrease or increase certainty. GRADE criteria for downgrading certainty of evidence encompass risk of bias, inconsistency, imprecision, indirectness, publication bias and other considerations. If such a criterion is identified, it is classified either as serious (leading to downgrading by one level) or very serious (downgrading by two levels). The four possible certainty ratings that can be applied are:
-
high certainty (meaning that current evidence provides a very good indication of the likely effect, and the likelihood that the actual effect will be substantially different is low);
-
moderate certainty (current evidence provides a good indication of the likely effect, and the likelihood that the actual effect of the treatment will not be substantially different is moderate);
-
low certainty (current evidence provides some indication of the likely effect, but the likelihood that the actual effect will be substantially different is high); and
-
very low certainty (current evidence does not provide a reliable indication of the likely effect, and the likelihood that the actual effect will be substantially different is very high).
'Summary of findings' tables
The 'Summary of findings' table comprises summaries of the estimated intervention effect and the number of participants and studies for each main outcome, and includes justifications underpinning GRADE assessments. In this case, we completed a 'Summary of findings' table for the primary adherence outcome and the secondary abstinence outcomes: short‐term abstinence and long‐term abstinence. Results of meta‐analyses are presented as SMDs and RRs, with 95% CIs. To facilitate interpretation of effect sizes for the primary outcome that were expressed as SMDs, we re‐expressed these in a more familiar metric (similar to the approach used in other Cochrane Reviews (e.g. Crockett 2018; Hollands 2015a). Because, to our knowledge, there is no larger more definitive survey that uses objective measurement of levels of adherence within standard care, for this translation we used outcome data from Marteau 2012. This was the largest study included within the current review that, first, reported adherence at least partly assessed by tablet counts, and second, used a general population sample in primary care (meaning that its data on adherence is likely to be relatively generalisable). Specifically, we used the standard deviation of the adherence outcome (here assessed as proportion of prescribed NRT that was consumed at 28 days) within the control group (here being the phenotype arm) as this best reflects typical adherence to medication in the absence of an intervention (i.e. within standard care). Such translations have important limitations and are only intended to be broadly illustrative to guide interpretation of the pooled result from the meta‐analysis. For example, what is considered 'standard care' inevitably differs, and in this study involved communicating to smokers that they were being prescribed a higher or lower dose based on their level of nicotine dependence. In addition, NRT may not be representative of all medications used to treat tobacco dependence. More generally, re‐expressed values relate directly to data derived from only one sample with its own context and measurement characteristics and so applying them more widely inevitably extrapolates beyond this.
Subgroup analysis and investigation of heterogeneity
We used subgroup analyses for the primary outcome to examine the specific characteristics or components of adherence interventions that may explain their effectiveness, an understanding of which could inform the design of maximally effective interventions. We coded more specific intervention types using the PAPA approach (Horne 2013). First, we coded whether interventions focused on perceptual factors ('Perceptions'; i.e. beliefs, cognitions, concerns and preferences) or practical factors ('Practicalities'; i.e. capabilities, resources, levels of support or skills), or both. Second, we coded whether the intervention content was shaped by eliciting and appreciating the needs, cognitions or behaviours of the patient or participant ('Participant‐centred') or was primarily standardised, directive or didactic in nature ('Clinician‐centred'). We also looked at these two factors in combination. Finally, we conducted a subgroup analysis looking at differential effects on adherence by the type of prescribed medication, although seven of the 10 studies focused on NRT medication.
Sensitivity analysis
We conducted a sensitivity analysis for the primary and secondary outcome analyses, removing the studies at high risk of bias.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; and Characteristics of studies awaiting classification tables for additional details of studies. Table 1 provides a brief overview of the nature of adherence interventions used in the included studies.
| Study | Brief description of specific intervention components intended to increase adherencea | Additional contact time relative to standard care? | Medication for which adherence was targeted | Intervention focused on perceptions, practicalities or both | Participant‐ or clinician‐centred intervention |
| Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT | Practicalities | Participant | |
| Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT | Practicalities | Participant | |
| Tailored and communicated about NRT dosage using a more potent rationale (genotype vs phenotype) | No | NRT | Perceptions | Clinician | |
| Personalised feedback of questionnaire responses regarding medication | No | NRT | Perceptions | Participant | |
| Personalised feedback of externally validated medication adherence | Yes | Bupropion | Practicalities | Participant | |
| Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | Varenicline | Both | Clinician | |
| Added contact time to standard behavioural support with: 1. medication adherence counselling; 2. automated reminder calls; 3. electronic monitoring counselling | Yes | NRT | 1. Perceptions 2. Both 3. Practicalities | 1. Participant 2. Clinician 3. Participant | |
| Personalised feedback of externally validated medication adherence | Yes | Bupropion | Practicalities | Participant | |
| Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT | Both | Participant | |
| Added contact time to standard behavioural support with module focused on improving adherence to nicotine patch | Yes | NRT | Both | Participant |
aFor further details see Characteristics of included studies table.
NRT: nicotine replacement therapy.
Results of the search
The searches for this update retrieved 294 unique records. 10 articles were identified as potentially eligible for inclusion after title and abstract screening. Of these, six articles were excluded at the full‐text screening stage. Of the remaining four records, one was classified as an ongoing study (NCT02635919), and three contained information on two new studies eligible for inclusion in the review (Schlam 2018; Tucker 2017). The flow of studies through the systematic review process for this update is shown in Figure 1.
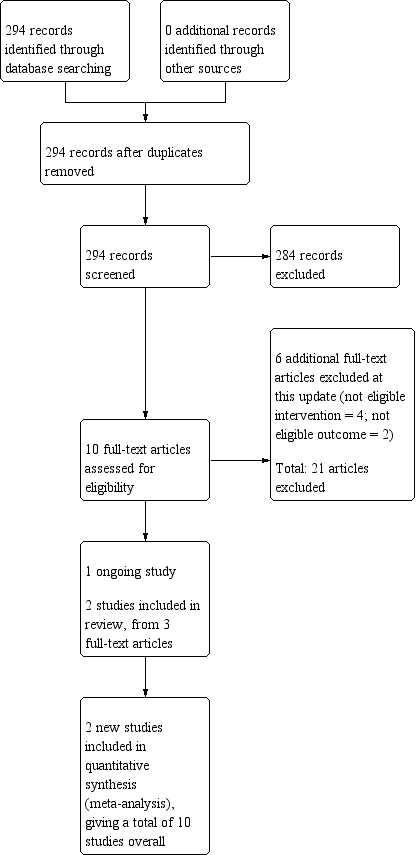
Study flow diagram for the current review update (eight studies were included in the previous version of the review).
Included studies
The review included 10 studies (eight previously included in Hollands 2015b), and two new at this update. These 10 studies included 3655 randomised participants (Chan 2010; Chan 2011; Marteau 2012; Mooney 2005; Mooney 2007; Nollen 2011; Schlam 2018; Schmitz 2005; Smith 2013; Tucker 2017).
Types of studies
All trials were individually randomised controlled trials. Five trials involved randomisation into two groups which were both included in our analysis (Marteau 2012; Mooney 2007; Nollen 2011; Schmitz 2005; Tucker 2017), and three trials involved randomisation into three groups, where only two of these groups were eligible for this review (Chan 2010; Chan 2011; Mooney 2005). One trial involved a 2 × 2 × 2 factorial design with eight randomised groups, but these groups were collapsed into a two‐group comparison, relevant to this review, by the study authors (Smith 2013). One trial involved a 2 × 2 × 2 × 2 × 2 factorial design with 32 randomised groups, with these groups collapsed into three two‐group comparisons relevant to this review (Schlam 2018).
Types of participants and settings
Eight studies included a general population of smokers. Two studies included only participants with a specific clinical condition, namely erectile dysfunction (Chan 2010) and HIV/AIDS (Tucker 2017). The mean ages of participants in trials ranged from 34.6 years (Mooney 2005) to 49 years (Schmitz 2005). In two trials, all participants were women (Mooney 2007; Schmitz 2005). In one trial, all participants were men (Chan 2010). In the remaining trials, percentage women ranged from 7.5% (Tucker 2017) to 62.5% (Nollen 2011). Seven trials took place in the USA (Mooney 2005; Mooney 2007; Nollen 2011; Schlam 2018; Schmitz 2005; Smith 2013; Tucker 2017), two in Hong Kong, China (Chan 2010; Chan 2011), and one in the UK (Marteau 2012). Regarding setting, all but one of the included studies featured interventions that were at least in part delivered in‐person, with the other delivering the intervention by telephone (Smith 2013). The interventions were delivered in clinic (e.g. smoking cessation or outpatient clinics) or social service settings, apart from one that was delivered by telephone (Smith 2013), one where one of the three adherence interventions was delivered by automated telephone call (Schlam 2018), and two where the setting was unclear (Chan 2010; Chan 2011). Those delivering the intervention were trained counsellors or project staff (Chan 2010; Chan 2011; Mooney 2005; Nollen 2011; Schlam 2018; Smith 2013; Tucker 2017), nurses (Marteau 2012; Schmitz 2005), or cognitive behavioural therapy (CBT) practitioners (Mooney 2007).
Types of interventions
The trials all offered pharmacological treatment and some behavioural support, comprising a form of smoking cessation counselling with no particular emphasis on adherence (e.g. providing dosing instructions and weekly checks of adverse effects; Schmitz 2005), to participants in the control arm. Support for the control arm varied from a single support session of 16 minutes (Tucker 2017) or 20 minutes (Mooney 2005) to seven weekly sessions (Marteau 2012; Mooney 2007; Schmitz 2005). In the main, the intervention consisted of an additional component to the standard behavioural support, with eight studies providing additional contact time for those in the intervention arm (Chan 2010; Chan 2011; Mooney 2007; Nollen 2011; Schlam 2018; Schmitz 2005; Smith 2013; Tucker 2017). In the other two studies, the nature of the contact changed but its duration did not significantly differ (Marteau 2012; Mooney 2005). The interventions typically provided information on the rationale for, and emphasised the importance of, adherence to medication, and aided participants in developing strategies to overcome problems and barriers to maintaining adherence. As such, they included a combination of two intervention strategies outlined within a taxonomy of interventions to increase adherence (Haynes 2008), that is included in Appendix 2, namely 1. instruction for participants on medication use or 2. counselling about smoking, and the value of medication in overcoming addiction. Two studies included interventions that involved personalised feedback of medication taking, monitored electronically (Mooney 2007; Schmitz 2005); one study elicited participants' beliefs about medication taking and then provided personalised counselling relating to those beliefs (Mooney 2005); one study tailored medication dose to either genotype or degree of tobacco dependence and explained the rationale for this to participants (Marteau 2012); and five studies added additional counselling contact time to standard behavioural support, with content focusing on medication adherence, including the use of motivational interviewing techniques and the 4/5R approach to increasing motivation (counselling addressing risks, rewards, roadblocks, and repetition, and relevance in the case of the 5Rs; Chan 2010; Chan 2011), a focus on motivation to use the medication and behavioural skills for achieving this (Nollen 2011; Tucker 2017), and targeting medication beliefs with monitoring and feedback on adherence (Smith 2013). One study examined multiple adherence interventions concerning personalised feedback of adherence behaviour, automated medication reminder calls and additional behavioural support content focused on adherence (Schlam 2018).
Seven studies prescribed NRT (Chan 2010; Chan 2011; Marteau 2012; Mooney 2005; Schlam 2018; Smith 2013; Tucker 2017), two studies prescribed bupropion (Mooney 2007; Schmitz 2005), and one study prescribed varenicline (Nollen 2011).
We categorised the content of each intervention by reference to PAPA. Of 12 comparisons included in the review, three comparisons assessed the impact of changing perceptions (Marteau 2012; Mooney 2005; Schlam 2018 (medication adherence counselling intervention)), and five comparisons assessed the impact of interventions aiming to improve the practicalities of medication‐taking (Chan 2010; Chan 2011; Mooney 2007; Schlam 2018 (electronic monitoring feedback intervention); Schmitz 2005). Four comparisons assessed Interventions of perceptions and practicalities (Nollen 2011; Schlam 2018 (automated calls intervention); Smith 2013; Tucker 2017).
We also assessed whether interventions aimed at changing perceptions or practicalities assessed participants' particular beliefs or difficulties (patient‐centred) or provided a standardised intervention (clinician‐centred). Nine comparisons were patient‐centred (Chan 2010; Chan 2011; Mooney 2005; Mooney 2007; Schlam 2018 (medication adherence counselling intervention); Schlam 2018 (electronic monitoring feedback intervention); Schmitz 2005; Smith 2013; Tucker 2017), and three comparisons were clinician‐centred (Marteau 2012; Nollen 2011; Schlam 2018 (automated calls intervention)).
Types of outcome measures
Measures of adherence varied across studies. Five studies reported at least one continuous outcome, measured as the percentage or amount of prescribed medication that was consumed (Marteau 2012; Mooney 2005; Nollen 2011), number of days on which it was used (Smith 2013), or percentage of days on which a person was adherent (Schlam 2018). Five studies used a dichotomous outcome, meaning people were classified as either achieving or not achieving a specified degree of adherence that was deemed adequate (Chan 2010; Chan 2011; Mooney 2007; Schmitz 2005; Tucker 2017). The definitions of adequate adherence naturally varied by medication type and because there may not be agreed standards for what constitutes desirable levels of adherence. Furthermore, the operationalisation of this was not always clear. In assessing adherence, seven studies at least partly used tablet counts (Marteau 2012; Mooney 2005; Nollen 2011; Tucker 2017), or electronic monitoring systems (Mooney 2007; Schlam 2018; Schmitz 2005). One study used self‐report (Smith 2013), while the means of assessing adherence was unclear in two studies (Chan 2010; Chan 2011). The period for which the primary adherence outcome was being assessed ranged from approximately two weeks (Mooney 2005; Smith 2013), to three months (Nollen 2011).
To assess abstinence seven studies used biochemically validated outcomes (Chan 2010; Chan 2011; Marteau 2012; Mooney 2005; Mooney 2007; Nollen 2011; Tucker 2017), but only six of these provided useable data in study reports (Chan 2010; Chan 2011; Marteau 2012; Mooney 2005; Nollen 2011; Tucker 2017). Two studies provided self‐reported abstinence data (Schlam 2018; Smith 2013), and one study did not report abstinence (Schmitz 2005). Time of abstinence outcome measurement ranged from two weeks (Mooney 2005), to six months (Chan 2010; Chan 2011; Marteau 2012; Schlam 2018; Smith 2013), to one year (Schlam 2018).
Excluded studies
We excluded six additional studies at this update. Two did not include an eligible adherence outcome (ISRCTN33423896; McClure 2013), and four did not include an eligible intervention (Cropsey 2017; Gong 2016; McClure 2016; Tseng 2017). Tseng 2017 was previously included in this review as an ongoing study; however, based on information in the published report it was deemed ineligible for inclusion at this update. The detailed description of the intervention made it clear that the content was equally split between standard smoking cessation support and content focused specifically on increasing medication adherence ("Each day participants in the two TM [text message] arms received one adherence‐focused message and one IMB [information‐motivation‐behavioural skills model] smoking cessation‐themed message"). As one of the inclusion criteria for this review stated that differing intervention content should have a clear principal focus on increasing adherence to medications for tobacco dependence, reflected in both described content and stated aims, we decided that this study did not meet the eligibility criteria and would not allow us to assess the effect of the adherence intervention independently.
We excluded 21 studies in the previous version of this review (Hollands 2015b). Our previous searches also identified two studies awaiting classification, which we were still unable to fully assess and include due to a lack of information (Applegate 2007; Yuhongxia 2011). See Characteristics of studies awaiting classification table.
Risk of bias in included studies
It is clear from the risk of bias summary that the included studies were often difficult to assess for bias on our criteria because there was insufficient information in published reports (Figure 2). For summary risk of bias judgements, as described in Assessment of risk of bias in included studies, we were able to judge that these conferred a low summary risk of bias for one study (Marteau 2012). Four studies were assessed at high risk of bias (Mooney 2005; Mooney 2007; Schmitz 2005; Smith 2013), with the remaining studies assessed at unclear risk of bias. Few judgements were made suggesting a high risk of bias for any domain, with the only four examples being risk of bias due to blinding of outcome assessment for Smith 2013 and due to incomplete outcome data for Mooney 2005, Mooney 2007, and Schmitz 2005.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three studies were at low risk of selection bias with details being provided of an adequate sequence generation process and steps to ensure allocation concealment (Chan 2011; Marteau 2012; Schlam 2018). One study provided details of adequate allocation concealment but not sequence generation (Nollen 2011), while one study provided details of adequate sequence generation but not allocation concealment (Smith 2013). In the other studies, there was insufficient detail to judge the risk of selection bias (Chan 2010; Mooney 2005; Mooney 2007; Schmitz 2005; Tucker 2017).
Blinding
We did not assess performance bias, as described in the Assessment of risk of bias in included studies section. We did assess whether outcomes were assessed blind to allocation (detection bias). Six studies were judged to be at low risk of detection bias (Marteau 2012; Mooney 2005; Mooney 2007; Nollen 2011; Schmitz 2005; Tucker 2017), one was judged at high risk of detection bias, as it used self‐report to assess all components of the primary adherence outcome (Smith 2013), and three were judged at unclear risk of bias (Chan 2010; Chan 2011; Schlam 2018). Some studies clearly attempted to blind outcome assessors to the secondary abstinence outcome (Chan 2010; Marteau 2012), although only in one study to the primary adherence outcome (Chan 2011). Elsewhere, attempts to blind outcome assessors were unclear (Mooney 2005; Mooney 2007; Nollen 2011; Schlam 2018; Schmitz 2005; Smith 2013; Tucker 2017). However, the use of objective outcome measures of adherence and biochemical validation of abstinence (for all other than Schlam 2018 and Smith 2013), was evidence that these outcomes were unlikely to be affected by detection bias.
Incomplete outcome data
We deemed five studies at low risk of bias because they had low levels of attrition, or addressed substantial or differential (or both) attrition (Chan 2010; Chan 2011; Marteau 2012; Schlam 2018; Smith 2013). Two studies were judged to be at unclear risk of bias (Nollen 2011; Tucker 2017). Three studies were judged to be at high risk of bias because participant numbers were not fully reported, the overall number of participants lost was 50% or greater, the difference in percentage followed up between groups was 20% or more, or a combination of these (Mooney 2005; Mooney 2007; Schmitz 2005).
Selective reporting
Four trials were preregistered on a clinical trials register enabling us to corroborate that specified outcomes remained consistent and so we assessed risk of bias as low (Chan 2010; Chan 2011; Marteau 2012; Smith 2013). One of these also published a protocol (Marteau 2012). A further study was preregistered on a clinical trials register but the adherence outcomes were not specified (Schlam 2018) and so we assessed risk of bias as unclear. We were unable to find registrations for the other five studies so selective reporting within the final reports could not reasonably be ruled out and risk of bias was considered unclear (Mooney 2005; Mooney 2007; Nollen 2011; Schmitz 2005; Tucker 2017).
Other potential sources of bias
We regarded another potential source of bias that was relevant to this review to be consistency in intervention delivery, judging this by whether it was clear that the information given to participants was standardised or structured to some degree, and fidelity to protocol was systematically monitored. Six studies were judged to be at low risk of other bias (Chan 2011; Marteau 2012; Mooney 2005; Nollen 2011; Schlam 2018; Tucker 2017), with the remaining four assessed at unclear risk of bias (Chan 2010; Mooney 2007; Schmitz 2005; Smith 2013).
Effects of interventions
Primary outcome
Adherence to medication for tobacco dependence
Five studies reported dichotomous adherence measures (Chan 2010; Chan 2011; Mooney 2007; Schmitz 2005; Tucker 2017). Chan 2010 and Chan 2011 assessed whether or not there had been continuous use of NRT, for four weeks (Chan 2010) and eight weeks (Chan 2011). Mooney 2007 and Schmitz 2005 both assessed whether or not participants had taken two daily doses of bupropion as prescribed over the seven‐week treatment period. Tucker 2017 assessed whether participants had used six or more nicotine patches per week, for those participants who provided complete data at baseline and follow‐up. Five studies used continuous adherence measures (Marteau 2012; Mooney 2005; Nollen 2011; Schlam 2018; Smith 2013). Marteau 2012 assessed the proportion of prescribed NRT consumed over the four‐week treatment period and reported the group mean. Mooney 2005 reported the mean pieces of nicotine gum used during the first 15 days of a quit attempt in those who completed the treatment period only. Nollen 2011 assessed the proportion of prescribed varenicline doses taken over three months, for those who remained engaged. Schlam 2018 measured the percentage of days in the first six weeks of the quit attempt where participants were adherent (i.e. where participants used both a nicotine patch and four or more pieces of gum), in those participants who completed the treatment phase. Smith 2013 assessed self‐reported number of days of nicotine patch use in the first two weeks, for those remaining engaged.
Pooled analysis of these data, comprising 12 comparisons from 10 studies, showed that adherence interventions produced a small improvement in adherence, with no significant statistical heterogeneity being observed (SMD 0.10, 95% CI 0.03 to 0.18; I² = 6%; n = 3655; Figure 3). Re‐expressing this effect size produced by the primary random‐effects meta‐analysis in a more familiar metric (see Data synthesis) suggested that interventions to increase adherence could have an effect equivalent to a 3.9% increase (95% CI 1.2% to 7.0%) in the mean proportion of prescribed medications consumed over 28 days.

Forest plot of comparison: 1 Medication adherence intervention plus standard care versus standard care alone, outcome: 1.1 Adherence (combined dichotomous and continuous).
GRADE assessment indicated that the evidence for this outcome was of moderate certainty, meaning that the true effect is probably close to the estimated effect. This judgement was reached through consideration of the following criteria. The current evidence was downgraded once due to risk of bias, because the majority of studies were judged to be at high or unclear risk of bias. We did not downgrade the evidence further based on other GRADE considerations. For imprecision, the number of participants (sample size) incorporated into this meta‐analysis exceeded the optimal information size (i.e. a sufficient sample size for a single adequately powered trial), and the 95% CI ranged from a very small to a small benefit. For inconsistency, there was minimal heterogeneity and considerable overlapping of CIs. There was no clear reason to downgrade certainty of evidence for indirectness (providing it was emphasised that moderate evidence related only to those receiving an adherence intervention in addition to behavioural support for smoking cessation, compared to behavioural support alone). Finally, for other considerations, including publication bias, the certainty of the evidence was not downgraded. Although a funnel plot of the primary outcome data suggested possible asymmetry (Figure 4), only one of 12 included comparisons was statistically significant, and there was not a clearly consistent pattern of smaller studies resulting in greater intervention effect estimates than larger studies. This limited the plausibility of publication bias as an explanation for asymmetry (Sterne 2011).
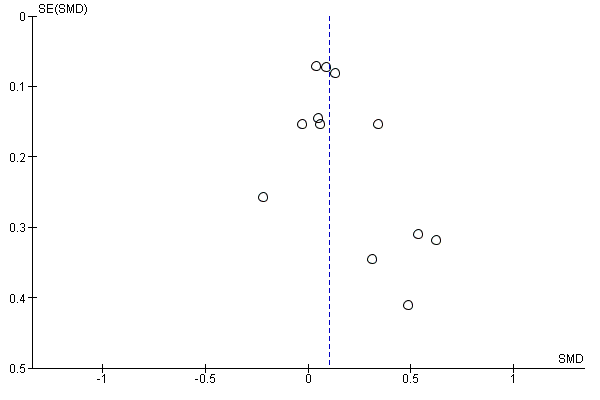
Funnel plot of comparison: 1 Medication adherence intervention plus standard care versus standard care alone, outcome: 1.1 Adherence (combined dichotomous and continuous).
Subgroup analyses
We conducted three subgroup analyses of the primary analysis in order to examine the relative impact of specific intervention types in terms of their focus on 'perceptions'; 'practicalities'; or 'both' (Analysis 1.2); and whether the intervention was participant‐centred or clinician‐centred (Analysis 1.3). The third analysis considered these two factors in combination (Analysis 1.4).
There was no strong evidence that the effect of interventions that focused on perceptions, practicalities, or on both differed in their effect on adherence (I² = 13%, P = 0.32; Analysis 1.2). That said, the effect of interventions focused on practicalities appeared slightly larger than the other two groups, with an SMD of 0.21 (95% CI 0.03 to 0.38; I² = 39%; n = 1752), compared with perceptions (SMD 0.10, 95% CI –0.03 to 0.24; I² = 0%; n = 839), or a combination of both perceptions and practicalities (SMD 0.04, 95% CI –0.08 to 0.16; I² = 0%; n = 1064).
There was no clear evidence that participant‐centred interventions differed in effectiveness from clinician‐centred interventions (I² = 0%, P = 0.71; Analysis 1.3). The SMD for participant‐centred interventions was 0.12 (95% CI 0.02 to 0.23; I² = 20%; n = 2791) and for clinician‐centred interventions was 0.09 (95% CI –0.05 to 0.23; I² = 0%; n = 864).
There was also no strong evidence that combining these two classification systems led to subgroup differences in the effect of interventions on medication adherence (I² = 0%, P = 0.65; Analysis 1.4).
We conducted a further subgroup analysis to examine whether there were differential effects of the intervention depending on which medication was prescribed (Analysis 1.5). In this analysis, there was stronger evidence of subgroup differences (I² = 68%, P = 0.04). The effect of interventions to increase adherence to bupropion (SMD 0.58, 95% CI 0.14 to 1.01; I² = 0%; n = 152) was much larger than that for NRT (SMD 0.09, 95% CI 0.02 to 0.17; I² = 0%; n = 3442) or varenicline (SMD from only one study was –0.22, 95% CI –0.73 to 0.29; n = 61).
Secondary outcomes
We reported assessments measured at the time point that most closely accorded with the assessment of adherence. If this selected abstinence measure assessed short‐term abstinence (less than six months), we additionally report abstinence at the longest available time point of six months or longer in order to assess the long‐term benefit of the intervention on cessation rates.
Short‐term abstinence (less than six months)
Five studies contributed data to the analysis of short‐term abstinence (Marteau 2012; Mooney 2005; Nollen 2011; Smith 2013; Tucker 2017). Marteau 2012 assessed biochemically validated prolonged abstinence at 28 days, Mooney 2005 assessed biochemically validated point‐prevalent abstinence at two weeks and Nollen 2011 assessed biochemically validated point‐prevalent abstinence at three months. Smith 2013 measured self‐reported 30‐day point‐prevalent abstinence at six weeks, while Tucker 2017 assessed biochemically validated continuous abstinence over 90 days.
Random‐effects meta‐analysis pooling these data produced an RR of 1.08 (95% CI 0.96 to 1.21; I² = 0%; n = 1795; Analysis 1.8). This suggested a potential small effect of adherence interventions on short‐term abstinence from smoking but with considerable uncertainty due to CIs overlapping no effect and including the possibility of a very small negative effect on abstinence.
GRADE assessment indicated that the evidence for this outcome was of low certainty, meaning that the true effect might be markedly different from the estimated effect. This judgement was reached through consideration of the following criteria. The current evidence was downgraded by one level due to risk of bias, because the majority of studies were judged to be at high or unclear risk of bias. It was also downgraded by one level due to imprecision, because while the number of participants (sample size) incorporated into this meta‐analysis exceeded the optimal information size (i.e. a sufficient sample size for a single adequately powered trial), the 95% CI overlapped no effect and ranged from a very small harm to a small benefit. We did not downgrade further due to other considerations, namely inconsistency (because there was negligible heterogeneity), indirectness or publication bias (with insufficient studies for formal assessment).
Long‐term abstinence (six months or longer)
Five studies (seven comparisons) contributed data to long‐term abstinence (Chan 2010; Chan 2011; Marteau 2012; Schlam 2018; Smith 2013). All five studies assessed abstinence at six months, which was biochemically validated in three studies (Chan 2010; Chan 2011; Marteau 2012), and based on self‐report in two studies (Schlam 2018; Smith 2013). Random‐effects meta‐analysis pooling these data produced an RR of 1.16 (95% CI 0.96 to 1.40; I² = 48%; n = 3593; Analysis 1.9). This suggested a potential small effect of adherence interventions on long‐term abstinence; however, considerable uncertainty arose due to the lower CI including the possibility of a very small negative effect on abstinence. Participants subject to interventions to improve adherence were between 4% less likely and 16% more likely to be abstinent at six months than those given standard behavioural support.
GRADE assessment indicated that the evidence for this outcome was of low certainty, meaning that the true effect might be markedly different from the estimated effect. This judgement was reached through consideration of the following criteria. The current evidence was downgraded by one level due to risk of bias because the majority of studies were at high or unclear risk of bias. It was also downgraded by one level for imprecision, because while the number of participants (sample size) incorporated into this meta‐analysis exceeded the optimal information size (i.e. a sufficient sample size for a single adequately powered trial), the 95% CI overlapped no effect and ranged from a very small harm to a small benefit. We did not downgrade further for other considerations, namely inconsistency (because heterogeneity was not classed as substantial), indirectness or publication bias (there were insufficient studies for formal assessment).
Sensitivity analyses
In sensitivity analyses, we excluded those studies at high risk of bias to determine if the primary and secondary outcome analyses were affected. Removing the two studies at high risk of bias (Mooney 2005; Smith 2013) did not affect results and interpretation for either the primary outcome (adherence to medication for tobacco dependence: SMD 0.12, 95% CI 0.02 to 0.22) or secondary outcomes (short‐term abstinence: RR 1.05, 95% CI 0.83 to 1.33; long‐term abstinence: RR 1.23, 95% CI 0.99 to 1.54).
Other outcomes
Factors plausibly associated with increases in adherence
No studies reported any relevant outcomes (i.e. factors plausibly associated with increases in adherence, such as intention or motivation, or attitudes towards treatment).
Adverse events
Four studies explicitly reported adverse events (Marteau 2012; Mooney 2005; Schlam 2018; Smith 2013). In Marteau 2012, there were no adverse events that were plausibly related to the intervention or its effect on participants' exposure to medication. There were also no differences between groups in levels of anxiety at either one‐week or six‐month assessment times. In Mooney 2005, there was no difference in adverse events between groups and in Schlam 2018 and Smith 2013 there were no serious adverse events during the study.
Discussion
Summary of main results
There is evidence of moderate certainty that interventions that devote special attention to improving adherence to smoking cessation medications can improve this to a small degree, when added to behavioural support for smoking cessation. Such interventions involve addressing the practicalities of taking medication, including facilitating problem solving, or providing information to address perceptions about the value of taking medication or concerns about doing so. There is low‐certainty evidence that such interventions may slightly improve the likelihood of achieving abstinence. The evidence for these findings was limited in both quality and quantity – characterised by a small number of studies, clinical heterogeneity, impaired study quality and imprecise estimates of effect, incorporating both potential benefit and harm.
Concerning the small improvement seen in adherence, translating the small statistical effect size into a more familiar metric suggests a potential effect equivalent to a 4% increase in the mean proportion of prescribed medication consumed (although see Data synthesis for limitations of such translations). One estimate is that each additional milligram per day of NRT consumed could increase the odds of abstinence by 5% (Hollands 2013), so this would represent a small but appreciable increase, equivalent to consuming one extra milligram of NRT with a prescription for 25 mg. Given evidence that greater adherence improves cessation outcomes for people using NRT, and evidence that higher doses of varenicline are more effective than lower doses (Cahill 2016), it stands to reason that this would apply to other medications too, because medication cannot work if it is not consumed. Characteristics of the treatment could also be shaped to attempt to increase the overall background levels of adherence. For example, characteristics of the medication (Hollands 2013), and its delivery (Hajek 1999), have been shown to impact on adherence. Even if adherence interventions demonstrate effect sizes of the small magnitudes seen here, the potential for aggregate impact is substantial given the extent to which medications for tobacco dependence are currently used, at least in high‐income countries. The degree to which this ultimately applies globally is dependent on increasing the uptake of effective pharmacotherapies, in part via increasing their availability and reducing their cost (van den Brand 2017).
Given that these interventions typically involve relatively minor additions to standard behavioural support, much of the content of the included interventions appeared relatively homogeneous. A detailed assessment of the content specifically concerning adherence did, however, reveal some potential avenues for further investigation. Those interventions that focus on addressing the practical barriers to adherence (as opposed to perceptions of treatment) and respond to participants' needs (as opposed to being governed by a set clinical agenda), and those that combine both of these foci, may be the most effective. However, the evidence suggesting this is very weak, particularly as there was no strong evidence of subgroup differences, and hence should be treated with caution. These tentative findings accord with English guidance on medication adherence (NICE 2009), which itself is based on a review of literature about effective interventions in adherence. This guidance emphasises that practical factors and barriers need to be considered key to explaining non‐adherence, not solely participants' beliefs and preferences about treatment. Furthermore, they reflect evidence suggesting that simply providing information to target cognitions and motivate changes in behaviour is often insufficient without also addressing factors, such as practical actions to overcome structural barriers, that prevent good intentions being realised (Hollands 2012; Webb 2006). It is also possible that a more detailed examination of intervention content would provide clearer insight into effective and ineffective mechanisms. Deriving a more precise understanding of the composition and processes of effective interventions will require a greater depth of evidence, including interventions that assess mediators, and improvements in the science and reporting of behavioural interventions (Sumner 2018).
Overall completeness and applicability of evidence
The review included only 10 trials, which were variable in terms of their context, the components of the intervention and the measures of the primary outcome, medication adherence, which makes summarising the data more difficult and reduces the certainty of the estimates produced. All these studies featured participants who were motivated to quit or reduce smoking, had sought and were receiving some degree of behavioural support – either face‐to‐face or by telephone – to take medication and were not paying for that medication. Furthermore, no studies targeted participants who were more likely to be non‐adherent, such as those who had not adhered to medication previously. Consequently, perhaps, medication adherence was overall reasonably high. For example, Nollen 2011 and Marteau 2012 reported the mean percentage of prescribed doses taken in the intervention arm was over 82% and control arm was over 63%, even though in the latter study, participants who had given up their quit attempt and ceased follow‐up were counted as non‐adherent. In studies using dichotomous measures of adequate adherence, three studies reported over 50% of participants achieving satisfactory levels of adherence (Chan 2011; Mooney 2007; Schmitz 2005). Perhaps in the context of the general population of people seeking support to quit, medication use is relatively high – contrary to perceptions that adherence is commonly suboptimal – and interventions have only limited potential to enhance adherence further. However, most people who stop smoking with the aid of medication do so without behavioural support (Fidler 2011), and typically any medication must be purchased at considerable cost. It is likely that adherence in this context is much lower and that interventions to improve adherence may be particularly helpful, but also that delivering these interventions will be especially challenging. There is currently no evidence on what may be effective in such unsupported contexts, though it seems likely that targeting perceptions or practicalities or both are likely to be relevant. A final point is that there is moderate‐certainty evidence that reimbursing the costs of medication where it is not freely provided improves adherence (van den Brand 2017).
Quality of the evidence
Most studies were judged at unclear risk of bias due to poor reporting of randomisation, even though all of them were published since first publication of the CONSORT statement (Moher 2010). Only three studies reported procedures clearly enough to be classified as having a low risk of bias (Chan 2011; Marteau 2012; Schlam 2018). It is possible, but relatively unlikely, that this led to bias. In the context of smoking cessation clinics, trial participants are usually unknown to the therapists, and this likely decreases, but does not eliminate, the likelihood of therapists assigning particular participants to particular arms and subverting the randomisation. Nonetheless, this should be addressed in reports from future trials. One potential source of bias is that practitioners who provided the adherence intervention also collected data on the degree to which people were adhering. As such they were unblinded, which may also motivate participants to report better adherence. This concern was mitigated substantially by the use of 'tablet counts', common to most of these trials. It is encouraging that the use of more objective measures appears commonplace in these types of trials, meaning that measurement issues were for the majority of studies not considered to confer particular risk of bias. This contrasts with another Cochrane Review focusing on adherence to prescription medications, where most studies used self‐report measures (Nieuwlaat 2014). Furthermore, while in the past, electronic monitoring approaches have been applied primarily to the opening and closing of tablet bottles, making them suitable for certain types of medications only, technology has been developed that will enable this to be used for other types of medication storage.
We assessed the certainty of the evidence for each outcome using the GRADE system. For the primary adherence outcome, GRADE assessment indicated that the evidence for this outcome was of moderate certainty, meaning that the true effect is probably close to the estimated effect. The current evidence was downgraded only once, due to risk of bias, because the majority of studies were judged to be at high or unclear risk of bias. We did not downgrade the evidence further based on other GRADE considerations. Evidence was of low certainty for both secondary outcomes of short‐ and long‐term abstinence, meaning that the true effect might be markedly different from the estimated effect. The current evidence was downgraded twice for each of these outcomes, in both cases being first, for risk of bias, because the majority of studies were judged to be at high or unclear risk of bias, and second, for imprecision, because the 95% CI overlapped no effect and ranged from a very small harm to a small benefit. This suggests that further research on abstinence outcomes will be valuable in increasing the reliability and precision of effect estimates and the certainty we can place in them.
Potential biases in the review process
Key possible limitations of the review are that first, we may have failed to identify all relevant research for inclusion in the review. We did take steps to minimise this possibility, including searching the Tobacco Addiction Group's specialised register in addition to electronic database searches, but this remains possible. Second, it is possible that there was publication bias, given there was asymmetry in the funnel plot, but we did not consider publication bias a likely explanation (see Effects of interventions). Unfortunately, two studies were classified as 'awaiting classification' as there was insufficient available information to confirm inclusion, and we were unable to contact the authors (Applegate 2007; Yuhongxia 2011).
Agreements and disagreements with other studies or reviews
We are not aware of other reviews addressing this topic. Cochrane Reviews show that behavioural support increases smoking cessation and, typically, included studies included people using medication, and receiving adherence advice as part of standard smoking cessation support (Hartmann‐Boyce 2019; Lancaster 2017). However, the studies did not randomise people to receive or not receive a medication adherence component so they do not provide specific evidence on its effect. Nieuwlaat 2014 examined the effect of interventions to improve adherence to a wider range of medication in a general setting. They found that information and counselling approaches improved adherence and patient outcomes but were unable to identify key components of the interventions. Nieuwlaat 2014 excluded tobacco dependence medications, hence the necessity of this current review.

Study flow diagram for the current review update (eight studies were included in the previous version of the review).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Medication adherence intervention plus standard care versus standard care alone, outcome: 1.1 Adherence (combined dichotomous and continuous).
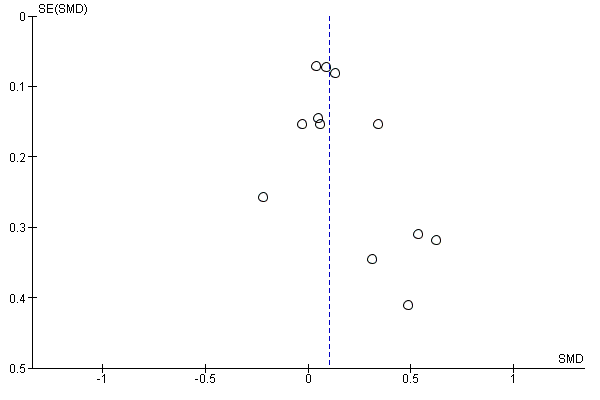
Funnel plot of comparison: 1 Medication adherence intervention plus standard care versus standard care alone, outcome: 1.1 Adherence (combined dichotomous and continuous).

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 1 Adherence (combined dichotomous and continuous).

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 2 Adherence: intervention focus subgroups.

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 3 Adherence: delivery approach subgroups.

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 4 Adherence: combined focus and delivery subgroups.

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 5 Adherence: medication type subgroups.

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 6 Dichotomous adherence data (for calculation purposes).

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 7 Continuous adherence data (for calculation purposes).

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 8 Short‐term smoking abstinence (< 6 months).

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 9 Long‐term smoking abstinence (≥ 6 months).
| Interventions to increase adherence compared to standard care for improving adherence to medications for tobacco dependence and abstinence from smoking | |||||
| Patient or population: adult smokers | |||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks (95% CI) | No of participants | Certainty of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Standard care | Intervention to increase adherence | ||||
| Adherence to medications for tobacco dependence | SMD 0.10 (0.03 to 0.18) | Mean proportion of prescribed medication consumed over 28 days was 63.6% | Mean proportion of prescribed medication consumed over 28 days was 3.9% higher (95% CI 1.2% to 7.0% higher) | 3655 | ⊕⊕⊕⊝ |
| Short‐term abstinence from smoking (< 6 months) | RR 1.08 | 357 people per 1000 achieve abstinence) | 386 people per 1000 achieve abstinence (95% CI 343 to 432) | 1795 | ⊕⊕⊝⊝ |
| Long‐term abstinence from smoking (≥ 6 months) | RR 1.16 | 203 people per 1000 achieve abstinence | 236 per 1000 achieve abstinence (95% CI 195 to 284) | 3593 | ⊕⊕⊝⊝ |
| The basis for the illustrative comparative risks is provided in Footnotesd. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High certainty: current evidence provides a very good indication of the likely effect, and the likelihood that the actual effect will be substantially different is low. | |||||
| aMost studies were at high or unclear risk of bias which lowers confidence in estimate of effect (risk of bias). | |||||
| Study | Brief description of specific intervention components intended to increase adherencea | Additional contact time relative to standard care? | Medication for which adherence was targeted | Intervention focused on perceptions, practicalities or both | Participant‐ or clinician‐centred intervention |
| Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT | Practicalities | Participant | |
| Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT | Practicalities | Participant | |
| Tailored and communicated about NRT dosage using a more potent rationale (genotype vs phenotype) | No | NRT | Perceptions | Clinician | |
| Personalised feedback of questionnaire responses regarding medication | No | NRT | Perceptions | Participant | |
| Personalised feedback of externally validated medication adherence | Yes | Bupropion | Practicalities | Participant | |
| Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | Varenicline | Both | Clinician | |
| Added contact time to standard behavioural support with: 1. medication adherence counselling; 2. automated reminder calls; 3. electronic monitoring counselling | Yes | NRT | 1. Perceptions 2. Both 3. Practicalities | 1. Participant 2. Clinician 3. Participant | |
| Personalised feedback of externally validated medication adherence | Yes | Bupropion | Practicalities | Participant | |
| Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT | Both | Participant | |
| Added contact time to standard behavioural support with module focused on improving adherence to nicotine patch | Yes | NRT | Both | Participant | |
| aFor further details see Characteristics of included studies table. NRT: nicotine replacement therapy. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adherence (combined dichotomous and continuous) Show forest plot | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 2 Adherence: intervention focus subgroups Show forest plot | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 2.1 Perceptions | 3 | 839 | Std. Mean Difference (Random, 95% CI) | 0.10 [‐0.03, 0.24] |
| 2.2 Practicalities | 5 | 1752 | Std. Mean Difference (Random, 95% CI) | 0.21 [0.03, 0.38] |
| 2.3 Both perceptions and practicalities | 4 | 1064 | Std. Mean Difference (Random, 95% CI) | 0.04 [‐0.08, 0.16] |
| 3 Adherence: delivery approach subgroups Show forest plot | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 3.1 Participant‐centred | 8 | 2791 | Std. Mean Difference (Random, 95% CI) | 0.12 [0.02, 0.23] |
| 3.2 Clinician‐centred | 3 | 864 | Std. Mean Difference (Random, 95% CI) | 0.09 [‐0.05, 0.23] |
| 4 Adherence: combined focus and delivery subgroups Show forest plot | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 4.1 Perceptions + participant | 2 | 206 | Std. Mean Difference (Random, 95% CI) | 0.03 [‐0.25, 0.30] |
| 4.2 Perceptions + clinician | 1 | 633 | Std. Mean Difference (Random, 95% CI) | 0.13 [‐0.03, 0.29] |
| 4.3 Practicalities + participant | 5 | 1752 | Std. Mean Difference (Random, 95% CI) | 0.21 [0.03, 0.38] |
| 4.4 Both + participant | 2 | 833 | Std. Mean Difference (Random, 95% CI) | 0.08 [‐0.16, 0.32] |
| 4.5 Both + clinician | 2 | 231 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.27, 0.24] |
| 5 Adherence: medication type subgroups Show forest plot | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 5.1 Nicotine replacement therapy | 7 | 3442 | Std. Mean Difference (Random, 95% CI) | 0.09 [0.02, 0.17] |
| 5.2 Bupropion | 2 | 152 | Std. Mean Difference (Random, 95% CI) | 0.58 [0.14, 1.01] |
| 5.3 Varenicline | 1 | 61 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.73, 0.29] |
| 6 Dichotomous adherence data (for calculation purposes) Show forest plot | 6 | 1664 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [1.05, 2.21] |
| 7 Continuous adherence data (for calculation purposes) Show forest plot | 5 | 4604 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.03, 0.19] |
| 8 Short‐term smoking abstinence (< 6 months) Show forest plot | 5 | 1795 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.96, 1.21] |
| 9 Long‐term smoking abstinence (≥ 6 months) Show forest plot | 5 | 3593 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.96, 1.40] |

