Intervenciones para mejorar la adherencia a los fármacos para la dependencia del tabaco
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009164.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 16 agosto 2019see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Tabaquismo
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Draft the protocol: all authors.
Develop the search strategy: GJH, NL.
Search for trials: GJH, NL, FN.
Obtain copies of trials: GJH, FN.
Select which studies to include: GJH, FN, NL.
Extract data from studies: GJH, FN.
Enter data into Review Manager 5: GJH, FN.
Carry out the analysis: GJH, FN.
Interpret the analysis: all authors.
Draft the final review: all authors.
Update the review: GJH.
Sources of support
Internal sources
-
University of Cambridge, UK.
Computer use, database access
-
NIHR senior investigator, UK.
Paul Aveyard is an NIHR senior investigator.
-
NIHR Oxford Biomedical Research Centre, UK.
Paul Aveyard is supported by the NIHR Oxford Biomedical Research Centre.
-
NIHR Oxford CLAHRC, UK.
Paul Aveyard is supported by NIHR Oxford CLAHRC.
-
NIHR Infrastructure funding, UK.
The Cochrane Tobacco Addiction Group is funded by the NIHR. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
External sources
-
No sources of support supplied
Declarations of interest
GJH is an author of one of the studies included in the review (Marteau 2012).
FN declares no competing interests.
AF declares researcher‐led funding from Johnson and Johnson (Ethicon) being awarded to her institution.
NL is managing editor of the Cochrane Tobacco Addiction Group, although this is not deemed a conflict of interest.
PA is an author of one the studies included in the review (Marteau 2012). PA is also the co‐ordinating editor of the Cochrane Tobacco Addiction Group, although this is not deemed a conflict of interest.
Acknowledgements
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Tobacco Addiction Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health. PA is also part‐funded by the NIHR Oxford Biomedical Research Centre (BRC).
The authors would like to thank Jonathan Livingstone‐Banks who ran searches of the Tobacco Addiction Group Specialized Register, Jamie Hartmann‐Boyce and all at the Cochrane Tobacco Addiction Group. We also greatly appreciate the input of the peer reviewers who have commented on this review throughout its development.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Aug 16 | Interventions to increase adherence to medications for tobacco dependence | Review | Gareth J Hollands, Felix Naughton, Amanda Farley, Nicola Lindson, Paul Aveyard | |
| 2015 Feb 23 | Interventions to increase adherence to medications for tobacco dependence | Review | Gareth J Hollands, Máirtín S McDermott, Nicola Lindson‐Hawley, Florian Vogt, Amanda Farley, Paul Aveyard | |
| 2011 Jun 15 | Interventions to increase adherence to medications for tobacco dependence | Protocol | Gareth J Hollands, Florian Vogt, Máirtín McDermott, Amanda C Parsons, Paul Aveyard | |
Differences between protocol and review
For the 2015 version of the review (Hollands 2015b), the criteria for eligible interventions was refined between the protocol and the review. The original primary intention of the review was to examine the effect of interventions to increase adherence where this was the clearly intended focus of those intervening. However, this primary intention was not adequately reflected in the original criteria. As such, a large number of studies of interventions that could in theory alter adherence but where this was not the researchers' intention would have been relevant for inclusion. Furthermore, this lack of clarity meant that most extant studies that featured any intervention in smokers would have to be examined at the full‐text screening stage because a clear focus on increasing adherence (which can typically be derived from the title and abstract screening process) was not necessary for consideration for inclusion.
For the current update, we made additional changes to the original protocol.
Rather than including separate meta‐analyses combining each of continuous outcome data and dichotomous outcome data for a given outcome, we produced a single meta‐analysis that combined these using generic inverse variance methods. If a study reported both continuous and dichotomous outcomes that were similar in meeting other criteria, continuous data were used. In the previous version of the review, we had applied fixed‐effect models to our pooling of the data. This was in line with the protocol, in which we stated that we intended to group substantially similar studies. In practice, there was considerable methodological and clinical variance between studies, reflecting different characteristics of settings, participant groups, interventions and measures. As such, a random‐effects model was considered more appropriate.
We added formal coding of the focus of the content of the intervention, which while stated as an objective, had not been conducted for the previous version of this review. We coded studies by reference to two key factors: 1. focus on perceptions, practicalities, or both; 2. participant‐centred or clinician‐centred. This was then used as the basis for subgroup analyses to determine which types of interventions were most effective.
In the previous version of this review we proposed to assess the impact of using intention‐to‐treat data for adherence outcomes instead of using data for only those participants who remained engaged with treatment (the latter being our specified preferred approach). This was no longer considered necessary given justification for using the latter data, reflected in the two newly identified studies which both report adherence data for participants who remained engaged with treatment.
For risk of bias assessment, following guidance from the Cochrane Tobacco Addiction Group, we removed three domains (blinding of participants and personnel (performance bias); validity and reliability of outcome measures (other sources of bias); comparability of baseline characteristics (other sources of bias)). We followed guidance in Section 8.7 (Table 8.7a) of the Cochrane Handbook for Systematic Reviews of Interventions for deriving a summary risk of bias judgement from the domains that were assessed (Higgins 2011).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Benzazepines [therapeutic use];
- Bupropion [therapeutic use];
- Drug Therapy, Combination [methods];
- Medication Adherence [*statistics & numerical data];
- Nicotinic Agonists [*therapeutic use];
- Nortriptyline [therapeutic use];
- Quinoxalines [therapeutic use];
- Randomized Controlled Trials as Topic;
- Smoking Cessation [*methods];
- Smoking Prevention;
- *Tobacco Use Cessation Devices;
- Tobacco Use Disorder [*drug therapy];
Medical Subject Headings Check Words
Humans;
PICO
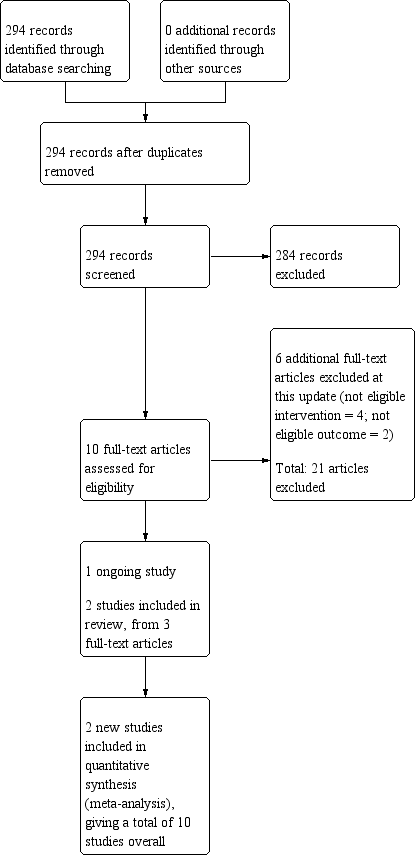
Study flow diagram for the current review update (eight studies were included in the previous version of the review).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Medication adherence intervention plus standard care versus standard care alone, outcome: 1.1 Adherence (combined dichotomous and continuous).

Funnel plot of comparison: 1 Medication adherence intervention plus standard care versus standard care alone, outcome: 1.1 Adherence (combined dichotomous and continuous).

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 1 Adherence (combined dichotomous and continuous).

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 2 Adherence: intervention focus subgroups.
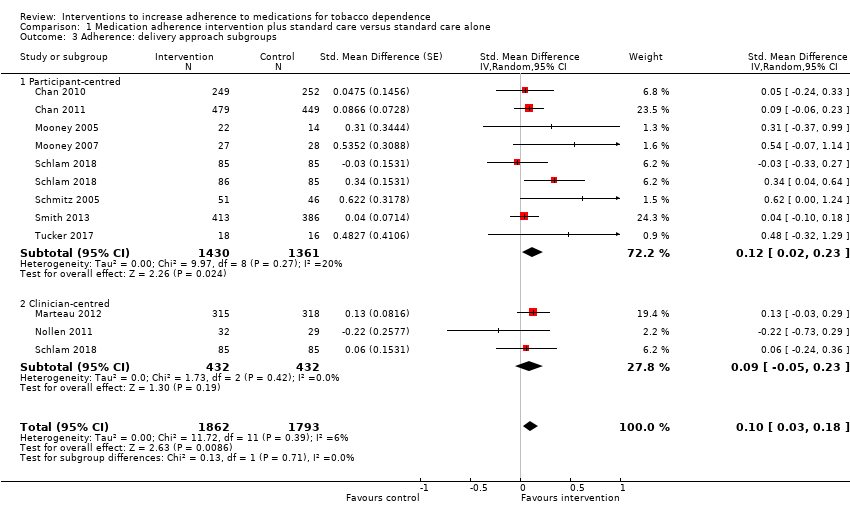
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 3 Adherence: delivery approach subgroups.

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 4 Adherence: combined focus and delivery subgroups.

Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 5 Adherence: medication type subgroups.
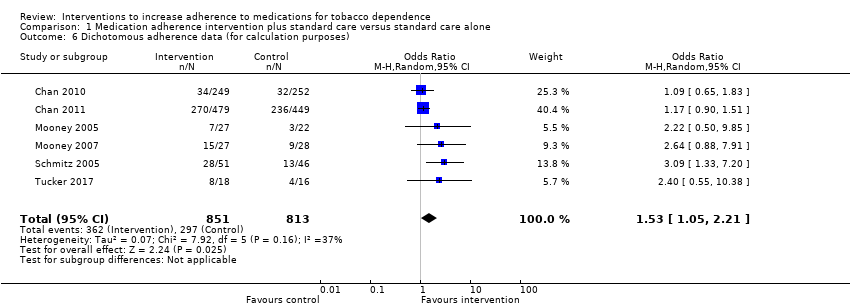
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 6 Dichotomous adherence data (for calculation purposes).
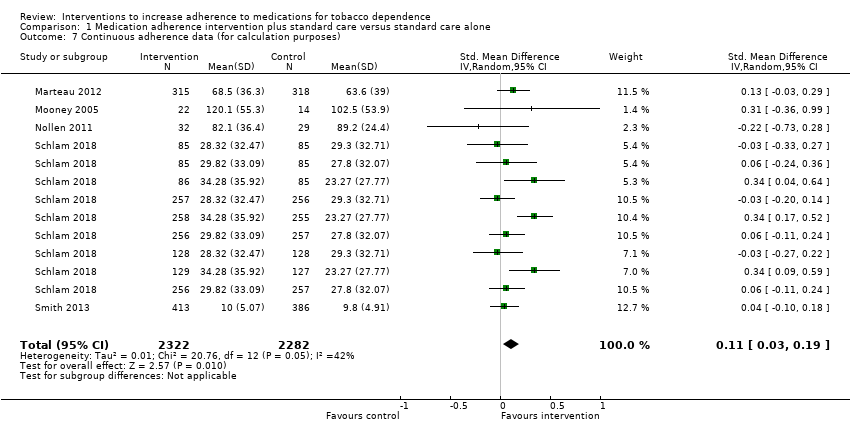
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 7 Continuous adherence data (for calculation purposes).
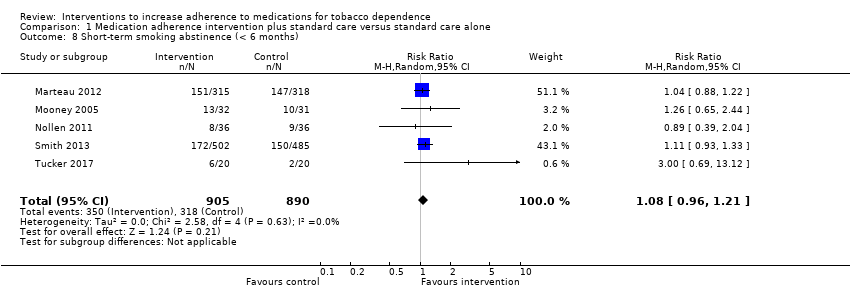
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 8 Short‐term smoking abstinence (< 6 months).
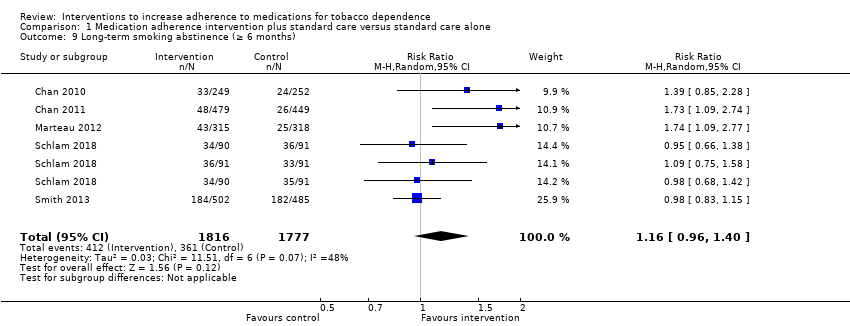
Comparison 1 Medication adherence intervention plus standard care versus standard care alone, Outcome 9 Long‐term smoking abstinence (≥ 6 months).
| Interventions to increase adherence compared to standard care for improving adherence to medications for tobacco dependence and abstinence from smoking | |||||
| Patient or population: adult smokers | |||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks (95% CI) | No of participants | Certainty of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Standard care | Intervention to increase adherence | ||||
| Adherence to medications for tobacco dependence | SMD 0.10 (0.03 to 0.18) | Mean proportion of prescribed medication consumed over 28 days was 63.6% | Mean proportion of prescribed medication consumed over 28 days was 3.9% higher (95% CI 1.2% to 7.0% higher) | 3655 | ⊕⊕⊕⊝ |
| Short‐term abstinence from smoking (< 6 months) | RR 1.08 | 357 people per 1000 achieve abstinence) | 386 people per 1000 achieve abstinence (95% CI 343 to 432) | 1795 | ⊕⊕⊝⊝ |
| Long‐term abstinence from smoking (≥ 6 months) | RR 1.16 | 203 people per 1000 achieve abstinence | 236 per 1000 achieve abstinence (95% CI 195 to 284) | 3593 | ⊕⊕⊝⊝ |
| The basis for the illustrative comparative risks is provided in Footnotesd. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High certainty: current evidence provides a very good indication of the likely effect, and the likelihood that the actual effect will be substantially different is low. | |||||
| aMost studies were at high or unclear risk of bias which lowers confidence in estimate of effect (risk of bias). | |||||
| Study | Brief description of specific intervention components intended to increase adherencea | Additional contact time relative to standard care? | Medication for which adherence was targeted | Intervention focused on perceptions, practicalities or both | Participant‐ or clinician‐centred intervention |
| Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT | Practicalities | Participant | |
| Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT | Practicalities | Participant | |
| Tailored and communicated about NRT dosage using a more potent rationale (genotype vs phenotype) | No | NRT | Perceptions | Clinician | |
| Personalised feedback of questionnaire responses regarding medication | No | NRT | Perceptions | Participant | |
| Personalised feedback of externally validated medication adherence | Yes | Bupropion | Practicalities | Participant | |
| Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | Varenicline | Both | Clinician | |
| Added contact time to standard behavioural support with: 1. medication adherence counselling; 2. automated reminder calls; 3. electronic monitoring counselling | Yes | NRT | 1. Perceptions 2. Both 3. Practicalities | 1. Participant 2. Clinician 3. Participant | |
| Personalised feedback of externally validated medication adherence | Yes | Bupropion | Practicalities | Participant | |
| Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT | Both | Participant | |
| Added contact time to standard behavioural support with module focused on improving adherence to nicotine patch | Yes | NRT | Both | Participant | |
| aFor further details see Characteristics of included studies table. NRT: nicotine replacement therapy. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adherence (combined dichotomous and continuous) Show forest plot | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 2 Adherence: intervention focus subgroups Show forest plot | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 2.1 Perceptions | 3 | 839 | Std. Mean Difference (Random, 95% CI) | 0.10 [‐0.03, 0.24] |
| 2.2 Practicalities | 5 | 1752 | Std. Mean Difference (Random, 95% CI) | 0.21 [0.03, 0.38] |
| 2.3 Both perceptions and practicalities | 4 | 1064 | Std. Mean Difference (Random, 95% CI) | 0.04 [‐0.08, 0.16] |
| 3 Adherence: delivery approach subgroups Show forest plot | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 3.1 Participant‐centred | 8 | 2791 | Std. Mean Difference (Random, 95% CI) | 0.12 [0.02, 0.23] |
| 3.2 Clinician‐centred | 3 | 864 | Std. Mean Difference (Random, 95% CI) | 0.09 [‐0.05, 0.23] |
| 4 Adherence: combined focus and delivery subgroups Show forest plot | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 4.1 Perceptions + participant | 2 | 206 | Std. Mean Difference (Random, 95% CI) | 0.03 [‐0.25, 0.30] |
| 4.2 Perceptions + clinician | 1 | 633 | Std. Mean Difference (Random, 95% CI) | 0.13 [‐0.03, 0.29] |
| 4.3 Practicalities + participant | 5 | 1752 | Std. Mean Difference (Random, 95% CI) | 0.21 [0.03, 0.38] |
| 4.4 Both + participant | 2 | 833 | Std. Mean Difference (Random, 95% CI) | 0.08 [‐0.16, 0.32] |
| 4.5 Both + clinician | 2 | 231 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.27, 0.24] |
| 5 Adherence: medication type subgroups Show forest plot | 10 | 3655 | Std. Mean Difference (Random, 95% CI) | 0.10 [0.03, 0.18] |
| 5.1 Nicotine replacement therapy | 7 | 3442 | Std. Mean Difference (Random, 95% CI) | 0.09 [0.02, 0.17] |
| 5.2 Bupropion | 2 | 152 | Std. Mean Difference (Random, 95% CI) | 0.58 [0.14, 1.01] |
| 5.3 Varenicline | 1 | 61 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.73, 0.29] |
| 6 Dichotomous adherence data (for calculation purposes) Show forest plot | 6 | 1664 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [1.05, 2.21] |
| 7 Continuous adherence data (for calculation purposes) Show forest plot | 5 | 4604 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.03, 0.19] |
| 8 Short‐term smoking abstinence (< 6 months) Show forest plot | 5 | 1795 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.96, 1.21] |
| 9 Long‐term smoking abstinence (≥ 6 months) Show forest plot | 5 | 3593 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.96, 1.40] |

