Inyección de glucocorticoides guiada por imágenes versus no guiada por imágenes para el dolor del hombro
Resumen
Antecedentes
A pesar del uso generalizado, la revisión Cochrane de 2012 no confirmó que el uso de imágenes para guiar la inyección de glucocorticoides en personas con dolor del hombro mejore su eficacia.
Objetivos
Actualizar la revisión y evaluar los efectos beneficiosos y perjudiciales de la inyección de glucocorticoides guiada por imágenes en comparación con la inyección no guiada por imágenes en pacientes con dolor del hombro.
Métodos de búsqueda
Se actualizó la búsqueda en el Registro Cochrane central de ensayos controlados (Cochrane Central Register of Controlled Trials, CENTRAL, por Ovid), MEDLINE (Ovid), Embase (Ovid) y clinicaltrials.gov hasta el 15 de febrero de 2021, y en la Plataforma de registros internacionales de ensayos clínicos de la Organización Mundial de la Salud (http://www.who.int/trialsearch/Default.aspx) hasta el 6 de julio de 2020. Para identificar los estudios potencialmente relevantes, también se examinaron las listas de referencias de los ensayos y artículos de revisión identificados.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados o cuasialeatorizados que compararon la inyección de glucocorticoides guiada por imágenes con la inyección no guiada por imágenes (ya fuera guiada por puntos de referencia o intramuscular) en pacientes con dolor del hombro (enfermedad del manguito de los rotadores, capsulitis adhesiva o dolor mixto o indefinido del hombro). Los desenlaces principales fueron el dolor, la funcionalidad, el porcentaje de participantes con tratamiento exitoso, la calidad de vida, los eventos adversos, los eventos adversos graves y los retiros debido a eventos adversos. Los desenlaces secundarios fueron la amplitud de movimiento del hombro y el porcentaje de participantes que requirieron cirugía o inyecciones adicionales. No hubo restricciones en cuanto al idioma ni la fecha de publicación.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por Cochrane.
Resultados principales
Se incluyeron 19 ensayos (1035 participantes). Catorce ensayos incluyeron a participantes con enfermedad del manguito de los rotadores, cuatro a participantes con capsulitis adhesiva y uno a participantes con dolor indefinido o mixto del hombro. Los tamaños de los ensayos variaron de 28 a 256 participantes, en su mayoría los participantes fueron mujeres, la media de edad varió entre 31 y 60 años y la duración media de los síntomas varió de dos a 23 meses.
Dos ensayos tuvieron bajo riesgo de sesgo en todos los criterios. Las fuentes de sesgo más destacables en el resto de los ensayos fueron el sesgo de realización y el de detección.
La evidencia de certeza moderada (disminuida por el sesgo) indica que la inyección guiada por ecografía probablemente proporciona poco o ningún beneficio clínicamente importante en comparación con la inyección no guiada con respecto al dolor (15 ensayos) o la funcionalidad (14 ensayos) entre las tres y las seis semanas de seguimiento. Podría no mejorar la calidad de vida (dos ensayos, evidencia de certeza baja, disminuida por posible sesgo e imprecisión) y se desconoce el efecto de la inyección guiada por ecografía sobre el éxito del tratamiento calificado por el participante, debido a evidencia de certeza muy baja (disminuida por sesgo, inconsistencia e imprecisión).
La media de dolor (escala de 0 a 10, las puntuaciones más altas indican más dolor) fue de 3,1 puntos en el grupo de inyección no guiada por imágenes y 0,5 puntos mejor (0,2 a 0,8 puntos mejor; 1003 participantes, 15 ensayos) con la inyección guiada por ecografía. Esto representa una ligera diferencia en el dolor (de 0,5 a 1,0 puntos en una escala de 0 a 10). La funcionalidad media (escala de 0 a 100, las puntuaciones más altas indican mejor funcionalidad) fue de 68 puntos en el grupo de inyección no guiada por imágenes y 2,4 puntos mejor (0,2 puntos peor a 5,1 puntos mejor; 895 participantes, 14 ensayos) con la inyección guiada por ecografía. La media de la calidad de vida (escala de 0 a 100, las puntuaciones más altas indican mejor calidad de vida) fue de 65 puntos en el grupo de inyección no guiada por imágenes y 2,8 puntos mejor (0,7 puntos peor a 6,4 puntos mejor; 220 participantes, dos ensayos) con la inyección guiada por ecografía. En cinco ensayos (350 participantes), 101/175 (o 606 por 1000) personas del grupo con guía ecográfica notificaron el éxito del tratamiento en comparación con 68/175 (o 389 por 1000) personas del grupo inyectado sin guía por imágenes (RR 1,56 [IC del 95%: 0,89 a 2,75]), una diferencia absoluta de un 22% más de notificaciones de éxito (4% menos a 62% más).
Evidencia de certeza baja (disminuida por sesgo e imprecisión) indica que las inyecciones guiadas por ecografía podrían no reducir el riesgo de eventos adversos en comparación con las inyecciones sin guía por imágenes. En cinco ensayos (402 participantes), 38/200 (o 181 por 1000) personas del grupo con guía ecográfica notificaron eventos adversos en comparación con 51/202 (o 252 por 1000) personas del grupo inyectado sin guía por imágenes (RR 0,72 [IC del 95%: 0,4 a 1,28]), una diferencia absoluta de un 7% menos de eventos adversos (15% menos a 7% más). Cinco ensayos informaron de que no se produjeron eventos adversos graves. El resto de los ensayos no informaron acerca de los eventos adversos graves. Un ensayo informó que 1/53 (o 19 por 1000) del grupo de inyección no guiada por imágenes y 0/53 del grupo con guía ecográfica se retiraron debido a eventos adversos.
Los análisis de sensibilidad indican que los efectos en el dolor y la funcionalidad podrían haber estado influidos por el sesgo de selección, y los efectos en la funcionalidad podrían haber estado influidos por el sesgo de detección. La evaluación las diferencias entre subgrupos indicó que las diferencias entre las distintas afecciones del hombre fueron improbables para el dolor y la funcionalidad.
Conclusiones de los autores
La revisión actualizada no apoya el uso de la guía por imágenes para las inyecciones en el hombro. Evidencia de certeza moderada indica que la inyección guiada por ecografía en el tratamiento del dolor del hombro probablemente proporciona poco o ningún beneficio frente a la inyección no guiada con respecto al dolor o la funcionalidad y evidencia de certeza baja indica que podría no haber diferencias en la calidad de vida. Se desconoce si la inyección guiada por ecografía mejora el éxito del tratamiento calificado por los participantes, debido a evidencia de certeza muy baja. Evidencia de certeza baja también indica que las inyecciones guiadas por ecografía podrían no reducir el riesgo de eventos adversos comparadas con las inyecciones sin guía por imágenes. Ningún ensayo informó de eventos adversos graves.
La falta de beneficios significativos de la guía por imágenes respecto a la inyección no guiada por imágenes para mejorar los desenlaces relevantes para el paciente o reducir los efectos perjudiciales, indica que cualquier coste añadido de la guía por imágenes no parece estar justificado.
PICO
Resumen en términos sencillos
Inyección de glucocorticoides guiada por imágenes o a ciegas para el dolor del hombro
Antecedentes
El dolor del hombro a menudo está causado por la enfermedad del manguito de los rotadores o la capsulitis adhesiva (“hombro rígido” o “congelado”). El manguito de los rotadores es un grupo de tendones que mantiene la articulación del hombro en su lugar y permite a las personas levantar el brazo. El dolor del hombro también puede estar relacionado con el desgate o la inflamación de los tendones del hombro, y la presión sobre los tendones ejercida por el hueso suprayacente al levantar el brazo hacia arriba (pinzamiento). Ambas afecciones provocan dolor con el movimiento y, a menudo, dolor durante la noche y al dormir sobre el lado afectado; la capsulitis adhesiva también provoca rigidez en el hombro.
Las inyecciones de glucocorticoides pueden aliviar el dolor del hombro aunque su efecto generalmente desaparece después de seis a ocho semanas. Tradicionalmente, las inyecciones se aplican utilizando puntos de referencia anatómicos alrededor del hombro. A veces se utilizan técnicas de diagnóstico por la imagen, como la ecografía, para guiar con mayor precisión las inyecciones en el hombro. No se sabe si la inyección guiada por imágenes alivia el dolor del hombro de forma más eficaz que las inyecciones administradas sin técnicas de imagen.
Características de los estudios
Esta revisión Cochrane está actualizada hasta el 15 de febrero de 2021. Diecinueve ensayos (1035 participantes) compararon la inyección guiada por ecografía con la inyección a ciegas. Catorce ensayos incluyeron a participantes con enfermedad del manguito de los rotadores, cuatro a participantes con capsulitis adhesiva y uno a participantes con dolor mixto del hombro. Los ensayos se realizaron en Corea, Taiwán, Irán, Turquía, Australia, Noruega, España, Irlanda, India y Suiza. La mayoría de los participantes fueron mujeres, con una media de edad de 31 a 60 años y una duración media de los síntomas de dos a 23 meses. Seis estudios informaron la fuente de financiación.
Resultados clave
En comparación con la inyección en el hombro no guiada por imágenes, la inyección guiada por ecografía produjo poco o ningún beneficio a las tres a seis semanas:
Dolor (puntuaciones más bajas significan menos dolor)
Mejoró en 0,5 puntos más (de 0,2 más a 0,8 más) en una escala de 0 a 10 puntos. Las diferencias de 0,5 a 1,0 puntos se consideran leves o pequeñas y es improbable que sean clínicamente importantes.
‐ Las personas que recibieron la inyección guiada por ecografía puntuaron el dolor en 2,6 puntos
‐ Las personas que recibieron la inyección no guiada por imágenes puntuaron el dolor en 3,1 puntos
Funcionalidad (las puntuaciones más altas significan mejor funcionalidad)
Mejoró en 2,4 puntos (de 0,2 menos a 5,1 más) en una escala de 0 a 100 puntos. Las diferencias por debajo de 10 puntos se consideran leves o pequeñas y es improbable que sean clínicamente importantes.
‐ Las personas que recibieron la inyección guiada por ecografía puntuaron la funcionalidad en 70,4 puntos
‐ Las personas que recibieron la inyección no guiada por imágenes puntuaron la funcionalidad en 68 puntos
Calidad de vida (las puntuaciones más altas significan mejor calidad de vida)
Mejoró en 2,8 puntos más (de 0,7 menos a 6,4 más) en una escala de 0 a 100 puntos
‐ Las personas que recibieron la inyección guiada por ecografía puntuaron la calidad de vida en 67,8 puntos
‐ Las personas que recibieron la inyección no guiada por imágenes puntuaron la calidad de vida en 65 puntos
Éxito del tratamiento (definido como dolor moderadamente o mucho mejor)
Un 22% más de personas calificaron el tratamiento como un éxito (4% menos a 62% más), o 22 personas más de cada 100.
‐ 61 de cada 100 personas comunicaron el éxito del tratamiento con la inyección de glucocorticoide guiada por ecografía
‐ 39 de cada 100 personas comunicaron el éxito del tratamiento con la inyección de glucocorticoide no guiada por imágenes
Episodios adversos
Un 7% menos de personas (15% menos a 7% más) tuvo episodios adversos (dolor tras la inyección, enrojecimiento facial y calor) con la inyección guiada por ecografía.
‐ 18 de cada 100 personas comunicaron episodios adversos con la inyección de glucocorticoide guiada por ecografía
‐ 25 de cada 100 personas comunicaron episodios adversos con la inyección de glucocorticoide no guiada por imágenes
Episodios adversos graves
Cinco ensayos informaron de que no se produjeron episodios adversos graves (como infección o lesión del nervio) con o sin guía ecográfica de la inyección.
Retiros debido a episodios adversos
Un ensayo informó que 1/53 (o 19 de cada 1000) del grupo de inyección no guiada por imágenes se retiró del estudio por episodios adversos, mientras que nadie (0/53) del grupo de inyección guiada por ecografía se retiró debido a episodios adversos.
Calidad de la evidencia
Evidencia de certeza baja a moderada muestra que, en las personas con dolor del hombro, las inyecciones guiadas por ecografía no proporcionan beneficios clínicamente importantes en el dolor, la funcionalidad ni la calidad de vida comparadas con las inyecciones no guiadas por imágenes, ni tampoco reducen el riesgo de episodios adversos. Estos hallazgos fueron consistentes entre las diferentes afecciones del hombro. Es poco probable que futuros estudios de investigación de calidad alta cambien las conclusiones de esta revisión.
Authors' conclusions
Summary of findings
| Ultrasound‐guided injection compared to non‐image‐guided (landmark or intramuscular) injection for shoulder pain | ||||||
| Patient or population: Patients with shoulder pain | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Landmark or intramuscular injection | Ultrasound‐guided injection | |||||
| Overall Pain Follow‐up: > 3 weeks up to 6 weeks | 3.1 points1 | 2.6 points (2.9 to 4.9) | 1003 | ⊕⊕⊕⊝ | Image‐guided injection probably results in little to no improvement in pain compared with blind injection. Mean difference in pain 0.5 points better with image‐guided injection (0.2 better to 0.8 points better). | |
| Function various scales 0 to 100 points (higher score indicates better function) Follow‐up: > 3 weeks up to 6 weeks | 68 points1 | 70.4 points (67.8 to 73.1) | 895 | ⊕⊕⊕⊝ | Image‐guided injection may have little or no effect on function compared with blind injection. Mean difference in function 2.4 points better with image guided injection (0.2 worse to 5.1 better). | |
| Participant‐assessed success 50% improvement on numerical rating 0 to 10 scale for pain) Follow‐up: end of study (6 weeks up to 12 weeks) | 389 per 1000 | 606 per 1000 (346 to 1000) | RR 1.56 (0.89 to 2.75) | 350 (5 studies) | ⊕⊝⊝⊝ | We are uncertain if image‐guided injection improves treatment success compared with blind injection. Absolute difference: 22% more reported success (4% fewer to 62% more); relative difference: 56% more reported success (11% fewer to 175% more). |
| Quality of life SF‐36 MCS 0 to 100 points (higher score indicates better quality of life) Follow‐up: > 3 weeks up to 6 weeks | 65 points1 | 67.8 points (64.3 to 71.5) | 220 (2 studies) | ⊕⊕⊝⊝ low2,4 | Image‐guided injection may have little or no effect on quality of life compared with blind injection. Mean difference in quality of life 2.8 points better with image‐guided injection (0.7 worse to 6.4 better). | |
| Number of adverse events Follow‐up: end of study | 252 per 1000 | 181 per 1000 (100 to 323) | RR 0.72 (0.40 to 1.28) | 402 (5 studies) | ⊕⊕⊝⊝ | Image‐guided injection may have little or no effect on adverse events compared with blind injection. Absolute difference of 7% fewer adverse events (15% fewer to 7% more); relative difference was 28% fewer events (60% fewer to 28% more). |
| Serious adverse events Not reported | See comment | See comment | Not estimable | See comment | See comment | Five trials (Ekeberg 2009; Naredo 2004; Saeed 2014; Ucuncu 2009; Zufferey 2012) reported that there were no serious side effects. The remaining trials did not report the incidence of serious adverse events. |
| Number of withdrawals due to adverse events Follow‐up: end of study | See comment | See comment | Not estimable | See comment | See comment | Only one trial reported withdrawals due to adverse events (Ekeberg 2009): 1/53 in the control group withdrew and received an additional local steroid injection at 2 weeks and 0/53 in the image‐guided group withdrew due to adverse events. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Mean pain and function in non‐image‐guided group in Ekeberg 2009 (3.1 points on a 0 to 10 scale for pain; 68 points on a 0 to 100 scale for function); mean QoL in non‐image‐guided group in Hsieh 2013. 2 Downgraded one level for risk of bias. 3Downgraded one level for inconsistency: I2 = 77% for treatment success was difficult to interpret with only five trials but two showed no apparent difference between groups; two favoured image‐guided injection. Although I2 was 79% for pain, Naredo 2004 seemed to drive quite a bit of the inconsistency (I2 = 64% once it was removed); reporting a large benefit compared to other studies. 4Downgraded one level for imprecision due to low event rates, or data from single small study only | ||||||
Background
This review is one in a series of reviews aiming to determine the benefit and safety of common interventions for shoulder pain. This review is an update of a previous Cochrane review (Bloom 2012).
Description of the condition
Shoulder pain is common, with a point
prevalence of 7% to 26% in the general population (Luime 2004). Shoulder pain accounts for 1.3% of all general practice encounters in Australia, being third only to back and knee pain as musculoskeletal reasons for primary care consultation (Britt 2016). Shoulder pain can also substantially reduce a person's quality of life (MacDermid 2004; Taylor 2005).
While shoulder disorders are labelled and defined in diverse and often conflicting ways (Cools 2017), the most common underlying cause of shoulder pain is rotator cuff disorders, estimated to account for between 65% and 85% of cases depending upon the setting and age of the study population (Ostör 2005; Vecchio 1995). Rotator cuff disease is an umbrella term that encompasses all symptomatic disorders of the rotator cuff, regardless of mechanism (inflammatory, degenerative or acute injury), or precise anatomical location (e.g. supraspinatus tendon versus subacromial bursa (Buchbinder 1996)). It includes diagnostic labels such as rotator cuff tendinopathy or tendinitis, impingement or subacromial syndrome, partial and complete rotator cuff tears, calcific tendinitis, painful arc syndrome and subacromial bursitis. People with symptomatic rotator cuff disease present with shoulder pain, often described as pain in the upper outer arm (Karjalainen 2019a). The pain is aggravated by overhead activities and is often worse at night and lying on the affected side, leading to disrupted sleep. It is accompanied by loss of function and often significant disability. A painful arc (as the arm is passively abducted away from the body, pain occurs between 60° and 120°) is nearly always present.
Adhesive capsulitis (also termed frozen shoulder, painful stiff shoulder or periarthritis) is the next most common cause of shoulder pain. Based on presentations to Dutch general practice, its cumulative incidence is reported to be 2.4 per 1000 people per year (95% confidence interval (CI) 1.9 to 2.9) (Van der Windt 1995). It is characterised by spontaneous onset of pain and progressive restriction of movement of the shoulder. Like rotator cuff disease, disability from the condition also results in restriction of activities of daily living, work and leisure (Codman 1934; Neviaser 1945, Reeves 1975). While specific diagnostic criteria for the condition are lacking, clinical trials of adhesive capsulitis have usually indicated that restricted movement must be present (Green 1998, Schellingerhout 2008). Glenohumeral osteoarthritis accounts for about 2 to 5 percent of shoulder pain in the adult population, its prevalence increases with age and it has a female preponderance (Meislin 2005). It is the most frequent site of osteoarthritis after the knees, hands, hips and ankle and subtalar joints. Osteoarthritis in this joint is most commonly primary but can be secondary to other conditions including trauma, massive rotator cuff tears, inflammatory arthritis and avascular necrosis.
Although there are many accepted forms of conservative therapy for shoulder pain, evidence of their efficacy is not well established. Recent systematic reviews of randomised controlled trials investigating conservative (e.g. manual therapy, exercise, electrotherapy) and operative (e.g. subacromial decompression, rotator cuff tear repair) treatments for shoulder pain often conclude that there is very little evidence to support or refute the efficacy of these commonly used interventions (Page 2014a; Page 2014b; Page 2016a; Page 2016b; Karjalainen 2019a; Karjalainen 2019b).
Description of the intervention
Glucocorticoid injections, which act to reduce inflammation, are commonly used in routine care to treat various forms of shoulder pain including both rotator cuff disease and adhesive capsulitis. Glucocorticoid injections are often recommended as standard treatment in clinical practice guidelines for shoulder pain (American Academy of Orthopedic Surgeons 2011). Traditionally, injection has been performed in the doctor's office at the time of the consultation, using anatomical landmarks to guide needle placement into the subacromial space and/or glenohumeral joint (shoulder joint). With the advent of new imaging modalities such as ultrasound, more people are referred for injections using image guidance.
Glucocorticoid injections provide significant short‐term benefits for patients with shoulder pain. For example, a systematic review of placebo‐controlled trials reported a relative risk for improvement in rotator cuff tendonitis following subacromial glucocorticoid injection of 3.08 (95% CI 1.94 to 4.87) and the number of patients needed to treat for an additional beneficial outcome (based on the pooled relative risk) of 3.3 (95% CI 1.8 to 7.7) (Arroll 2005). Benefits were maintained for up to nine months. The same review also found that subacromial glucocorticoid injections are probably more effective than NSAID medication (Arroll 2005).
Between 30 and 80% of subacromial injections given as a blind injection are reported to reach the subacromial bursa or space (Eustace 1997; Henkus 2006; Partington 1998), although in expert hands, blinded injection may not differ in accuracy to ultrasound‐guided injection (Rutten 2007). For glenohumeral injections, the reported accuracy of blinded injections varies from 27% (Porat 2008) to 99% (Sethi 2005), with one study reporting greater accuracy with an anterior approach (95% accuracy compared with 50% accuracy using a posterior approach) (White 1996).
Despite this, the importance of the accuracy of needle placement with respect to patient outcome remains questionable. Based upon moderate evidence from five trials (290 participants), our previous review (Bloom 2012) was unable to establish that ultrasound‐guided glucocorticoid injections for shoulder pain is superior to either landmark‐guided or intramuscular injections for improving pain, function, and shoulder range of motion. Adverse events were also just as likely with either approach.
How the intervention might work
Glucocorticoid injections are potent anti‐inflammatories and have both systemic and local effects (Ekeberg 2009; Pekarek 2011). The onset, duration, and local effect depends upon the anti‐inflammatory potency of the glucocorticoid, its solubility (depot effect), and the dose given. Based on their potency, an injection may be short, intermediate or long‐acting (Pekarek 2011).
A variety of imaging methods have been used to better localise needle placement for glucocorticoid injection into the subacromial bursa, subacromial space or glenohumeral joint. Potential advantages of image guidance might be increased safety (avoidance of important neurovascular structures), decreased discomfort, and diagnostic value in terms of response to accurate anatomic administration. Ultrasound imaging emits no radiation and can be used to visualise subcutaneous body structures including tendons, muscles and joints. Computerised tomography (CT) scans or magnetic resonance imaging (MRI) scans may also be used to visualise the structures around the shoulder. A series of images, usually X‐rays, can be taken after injection (using a small amount of contrast, i.e. an arthrogram) to ensure that a needle is placed in the correct position within the joint.
In contrast to anatomical landmark‐guided injection, which in many instances can be performed by trained general practitioners or specialists such as rheumatologists and orthopaedic surgeons, most image‐guided procedures are performed by radiologists in a radiology service. While still in the minority, some clinical specialists are now learning these techniques and may have the necessary equipment to perform image‐guided injections in their office.
Why it is important to do this review
It is important to know whether image‐guided injection improves outcomes for people with shoulder pain. Any added benefit in patient outcome achieved by the image‐guided approach will also need to be considered in light of any delay in receiving image‐guided treatment and the added expense of the imaging modality used. In Australia, there has been more than a 3.8‐fold increase in the number of image‐guided injections since 2000 from 15,495 services in 2000‐01 (78 per 100,000 population) to 58,116 services in 2017‐8 (231 per 100,00 population) (Medicare Australia 2018). While the site of injection is not specified, most were likely to be for shoulder pain. This has been accompanied by a substantial increase in health care costs. For example, in the 2014/2015 financial year alone, the total benefits paid through the Medical Benefits Scheme for ultrasound‐guided injection at any site was almost AU$27.5 million (Morrisroe 2018). Due to the lack of clinical justification for the use of ultrasound guidance and its significant added cost, the Australian Rheumatology Association recommends against the use of ultrasound guidance to perform injections into the subacromial space (Morrisroe 2018).
Whether image‐guided glucocorticoid injection is an effective therapeutic tool for improving outcomes for people with shoulder pain is yet to be established. Since our previous review (Bloom 2012), several new trials comparing the clinical effects of ultrasound‐guided glucocorticoid injection to injection without image guidance have been published. It is therefore important to update the evidence on the importance of the accuracy of needle placement with respect to outcome.
Objectives
To update our review and assess the benefits and harms of image‐guided glucocorticoid injection (e.g. injection guided by ultrasound or other imaging modality) into the subacromial bursa or space or glenohumeral joint compared with injection given without image guidance (i.e. relies on anatomical landmarks or systemic intramuscular injection) for people with shoulder pain.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and controlled clinical trials (CCTs) (that used quasi‐randomised methods to allocate participants, for example, by date of birth, hospital record number, or alternation). There were no restrictions on language or date of publication.
Types of participants
Trials that included participants with rotator cuff disease, adhesive capsulitis or glenohumeral osteoarthritis of any age were included. We also included trials with participants of unspecified shoulder pain provided that the inclusion/exclusion criteria were compatible with a diagnosis of either rotator cuff disease or adhesive capsulitis. We excluded trials that included participants with a recent history of significant injury or fracture, and systemic inflammatory conditions such as rheumatoid arthritis or polymyalgia rheumatica.
Types of interventions
Trials comparing image‐guided (ultrasound, arthrogram or MRI) subacromial and/or glenohumeral injection to injection given without imaging, i.e. injection relying on anatomical landmarks or systemic intramuscular injection of glucocorticoids were included. We included systemic intramuscular injection as glucorticoid injection is not accurately delivered into the purported site of pathology. Trials that only considered accuracy of needle placement were excluded.
Trials that provided participants with additional care (e.g. physical therapy, exercise, and medication) were included, provided both treatment arms received the same additional care.
Types of outcome measures
Major outcomes
-
Overall pain (mean or mean change) measured by visual analog scales, numerical or categorical rating scales.
-
Function (mean or mean change, as reported in the trials), measured by the Shoulder Pain and Disability Index (SPADI); Constant score; Shoulder Disability Questionnaire; Disabilities of the Arm, Shoulder and Hand (DASH), or any other function scale, as outlined below (Data extraction and management).
-
Participant‐reported global assessment of treatment success (e.g. proportion of participants with significant overall improvement, or proportion reporting treatment success).
-
Quality of life as measured by generic measures (such as components of the SF‐36, EQ‐5D or disease‐specific tools).
-
Number of participants experiencing any adverse events.
-
Number of participants experiencing serious adverse events.
-
Number of participants withdrawing due to adverse events.
Minor outcomes
-
Number of participants requiring additional treatments.
-
Range of motion (active measures preferred over passive measures: shoulder abduction, flexion, and external rotation). External rotation range of motion is only clinically relevant for adhesive capsulitis so was not extracted from studies including participants with rotator cuff disease.
Time points
We extracted outcomes up to 3 weeks; > 3 weeks to 6 weeks (primary time point); > 6 weeks to 3 months; > 3 months to 6 months; and > 6 months. If a trial reported outcomes at more than one time point within these subgroups (e.g. four weeks and five weeks), we extracted the later time point (five weeks).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases, unrestricted by date or language up to 15 February 2021:
-
Cochrane Central Register of Controlled Trials (CENTRAL, via Ovid EBM Reviews) (Appendix 1);
-
MEDLINE (Ovid) (Appendix 2);
-
Embase (Ovid) (Appendix 3).
We searched the following trial registries to identify ongoing trials: ClinicalTrials.gov up to 15 February 2021 and the World Health Organisation International Clinical Trials Registry Platform (http://www.who.int/trialsearch/Default.aspx) up to 06 July 2020 (we could not access the portal after this date as the WHO ICTRP was not available) (Appendix 4).
Although this is an update of the previously published version, we ran the searches from database inception to 15 February 2021 as several MeSH terms were updated in the original search strategy which was run in June 2011.
Searching other resources
We also screened reference lists of retrieved review articles and trials to identify potentially relevant studies.
Data collection and analysis
Selection of studies
Two review authors (JZ, AR) independently selected the trials to be included based on title and abstract screening. Articles selected by at least one of the review authors were retrieved in full text for closer examination. The review authors were not blinded to the journal or authors. Disagreement about inclusion or exclusion of individual studies was resolved by a third review author (RJ or RB).
Data extraction and management
The same two review authors (JZ, AR) independently extracted the following data from the included trials and entered the data in RevMan 5:
1) trial characteristics including size and location of the trial, notable possible author conflicts of interests, and source of funding;
2) characteristics of the study population including age, and characteristics of shoulder pain including diagnosis criteria, and disease duration, baseline measures in treatment and control group for pain, function, quality of life;
3) characteristics of the therapy in all trial arms including type and dose of glucocorticoid therapy, site of injection and the method of anatomic or image‐guided location of the needle;
4) risk of bias domains (as outlined in Assessment of risk of bias in included studies, below);
5) outcome measures ‐ mean and standard deviation (SD) for continuous outcomes, and number of events for dichotomous outcomes (as outlined in Types of outcome measures).
If additional data were required, we contacted the trial authors to obtain this. Where data were imputed or calculated (e.g. standard deviations calculated from standard errors, P values, or confidence intervals, or imputed from graphs, or from standard deviations in other trials) we reported this in the Characteristics of included studies table. Any disagreements were resolved by consensus and/or arbitration by a third review author (RJ).
There may be multiple outcome results available in the trial reports (e.g. from multiple scales, time points and analyses). To prevent selective inclusion of data based on the results (Page 2013), we used the following decision rules to select data from trials:
Where trials did not include a measure of overall pain but included one or more other measures of pain, for the purpose of pooling data for the primary analysis of pain, we combined overall pain with other types of pain in the following hierarchy: unspecified pain, pain at rest or night, pain with activity or daytime pain.
Where trialists reported pain on more than one scale, we extracted data on the scale that was highest on the following list:
-
visual analog scale;
-
numerical rating scale;
-
categorical rating scale;
-
pain subscale of an overall function scale such as the Shoulder Pain and Disability Index (SPADI), Constant score, or other scale;
-
any other measure of pain.
Where trialists reported outcome data for more than one function scale, we extracted data on the scale that was highest on the following list, based on the most commonly used scores used in trials of interventions for shoulder pain (Page 2015):
-
Constant‐Murley Score (Constant 1987);
-
Shoulder Pain and Disability Index (SPADI) (Roach 1991);
-
Oxford Shoulder Score (OSS);
-
American Shoulder and Elbow Surgeons Standardized Form (ASES‐SF);
-
University of California Los Angeles (UCLA) Shoulder Score;
-
Disabilities of the Arm, Shoulder and Hand (DASH);
-
Shoulder Disability Questionnaire (SDQ);
-
Croft Shoulder Disability Questionnaire (Croft 1994); or
-
any other shoulder‐specific function scale.
For continuous outcomes, if trialists reported end of treatment mean scores that were adjusted for baseline scores (e.g. analysis of covariance adjusted for baseline score) along with either final values and change from baseline values for the same continuous outcome, we extracted adjusted mean score preferentially, over final values, and over change scores.
Where trialists reported data analysed based on the intention‐to‐treat (ITT) sample and another sample (e.g. per‐protocol, as‐treated), we extracted ITT‐analysed data.
If we had identified, cross‐over RCTs, we would have extracted data from the first period only.
Assessment of risk of bias in included studies
Two review authors (JZ, AR) independently assessed the risk of bias of each included trial and resolved any disagreements by consensus, or consultation with a third review author (RJ), where necessary.
We assessed the following methodological domains, as recommended by Cochrane (Higgins 2017):
-
sequence generation;
-
allocation sequence concealment;
-
blinding of participants, personnel;
-
blinding of outcome assessors: we considered blinding of assessors of self‐reported subjective outcomes (pain, function, success, quality of life) separately from assessors of more objective outcomes (such as range of motion, number who had additional surgery);
-
incomplete outcome data;
-
selective outcome reporting; and
-
other potential threats to validity, such as unit of analysis issues, inappropriate or unequal application of co‐interventions across treatment groups.
We graded each potential source of bias as high, low or unclear risk, and provided a quote from the study report together with a justification for our judgment in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes, where necessary. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome. We presented the figures generated by the risk of bias tool to provide summary assessments of the risk of bias.
Measures of treatment effect
When possible, the analyses were based on intention‐to‐treat data (outcomes provided for every randomised participant) from the individual trials. For each trial, we presented outcome data as point estimates with mean and standard deviation for continuous outcomes and risk ratios (RRs) with corresponding 95% confidence intervals for dichotomous outcomes. Where possible, for continuous outcomes, we extracted end of treatment scores, rather than change from baseline scores.
Where trials used different measures for the same outcome or concept, we used the most common outcome measure as an index outcome measure (Guyatt 2013). We transformed means and standard deviations (SDs) of other outcome measures to the scale of the index instrument and pooled the data using MD as the summary estimate, according to the methods of Thorlund 2011. For pain, we assumed the zero to 10 numerical rating scale (zero represented no pain) as the index instrument, assumed the 10 cm visual analog scale (VAS) was comparable, and transformed zero to 9 (Ekeberg 2009) to a zero to 10 scale. The trials reported various functional measures (including the Constant score, Shoulder Pain And Disability Index, Shoulder Function Assessment, and others). We assumed these were comparable, and that the zero to 100 Constant score (where 100 is best function) was the index measure of overall shoulder function. We transformed the zero to 70 Shoulder Function Assessment (e.g. Naredo 2004) to a zero to 100 scale, and reversed the direction of the Shoulder Pain And Disability Index shoulder disability score (e.g. used in Ekeberg 2009 and Hsieh 2013) and the Shoulder Disability Questionnaire (e.g. used in Dogu 2012).
For dichotomous outcomes, we calculated the absolute difference from the difference in the risks between the intervention and control group, as calculated in GRADEpro GDT (GRADEpro GDT 2015), and expressed as a percentage. We calculated the relative percent change as the RR minus 1 and expressed this as a percentage.
Rather than using minimal clinically important differences (MCIDs) which are not well defined for shoulder pain, we judged the magnitude of the effect based upon between‐mean group differences for continuous measures based upon a similar approach used in the American College of Physicians 2017 guidelines for low back pain (Chou 2017). For pain measured on a 0 to 10‐point scale, a difference of 0.5 to 1.0 points was considered slight to small, a difference of > 1 to 2 points was considered moderate and a difference of > 2 points was considered large. Similarly, for function and health‐related quality of life measured on a 0 to 100‐point scale, a difference of 5 to 10 points was considered slight/small, a difference of > 10 to 20 points was considered moderate and a difference of > 20 points was considered large.
Unit of analysis issues
The unit of analysis was the participant. For trials that injected both shoulders, we planned to extract data once for each participant, if the trials had adjusted for the unit of analysis, and presented data that way. However, since two trials (randomised by participant) injected both shoulders (Cole 2016; Saeed 2014) in a few participants, and reported the results for the number of shoulders, we had to extract data for the number of shoulders.
Where multiple trial arms were reported in a single trial, we included only the relevant arms for our comparison but have reported that there were multiple trial arms in the Characteristics of included studies table.
Dealing with missing data
We contacted trial authors to obtain data that were missing from the trial reports. For dichotomous outcomes, we used the number randomised as the denominator, making the assumption that any participants missing at the end of treatment did not have a positive outcome. For continuous outcomes with no standard deviation reported, we calculated standard deviations if possible from standard errors, P values, or confidence intervals.
If no measures of variance were reported and standard deviation could not be calculated, we planned to impute standard deviations from other studies in the same meta‐analysis, using the average of the other standard deviations available provided only a small proportion of studies comprising the meta‐analysis had missing data.
Assessment of heterogeneity
We assessed included trials for clinical similarity in terms of participants and interventions and comparators. For studies judged as clinically similar, we quantified the possible magnitude of inconsistency (i.e. heterogeneity) across studies, using the I2 statistic with a rough guide for interpretation as follows: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% may represent considerable heterogeneity (Deeks 2008).
If we had identified cases of considerable heterogeneity (defined as I2 ≥ 75%), we would explore the data further by comparing the characteristics of individual studies and performing subgroup analyses.
Assessment of reporting biases
In order to determine whether outcome reporting bias was present, we checked a priori trial protocols against published reports of trial results (checked if all planned outcomes had results reported).
We compared the fixed‐effect estimate against the random‐effects model to assess the possible presence of small sample bias in the published literature (i.e. in which the intervention effect is more beneficial in smaller studies). In the presence of small sample bias, the random‐effects estimate of the intervention is more beneficial than the fixed‐effect estimate (Sterne 2017). If we were able to pool more than 10 trials, we performed formal statistical tests to investigate funnel plot asymmetry to detect the possibility of publication bias (Sterne 2017).
Data synthesis
For clinically similar studies, we pooled outcomes in a meta analysis using the random‐effects model as a default, based on the assumption that clinical diversity is likely to exist, and that different studies are estimating different intervention effects.
Subgroup analysis and investigation of heterogeneity
To explain the heterogeneity between the results of the included studies, we planned to assess if different underlying shoulder disorders impacted on pain and function. To do this, we presented outcomes separately by diagnosis (rotator cuff disease versus adhesive capsulitis, versus mixed or undefined shoulder pain). We used the formal test for subgroup interactions in Review Manager (RevMan 2014) and exercised caution in the interpretation of subgroup analyses, as advised in section 9.6 of the Cochrane Handbook (Deeks 2017).
Sensitivity analysis
We performed a sensitivity analysis to investigate the robustness of the treatment effect (on pain and function) to the presence of selection and detection biases. To do this, we removed trials that reported inadequate or unclear allocation concealment (at risk of selection bias) and trials that lacked participant blinding (at risk of detection bias) from meta‐analysis to see if this changed the overall treatment effect.
Post hoc, we assessed the robustness of the pain outcome at the primary time point to the inclusion of the two trials with a potential unit of analysis issue. These trials included a small proportion of participants with bilateral shoulder injections that were not adjusted for in their trial analyses.
Summary of findings and assessment of the certainty of the evidence
We presented the major outcomes (pain, function, global assessment of success, quality of life, withdrawals, adverse events, serious adverse events) in a summary of findings (SoF) table which summarised the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes, as recommended by Cochrane (Schünemann 2017a). The summary of findings table includes an overall grading of the evidence related to each of the major outcomes, using the GRADE approach (Schünemann 2017b).
All authors (JZ, AR, RJ, RB) independently assessed the quality of the evidence. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it related to the studies which contributed data to the meta‐analyses for each of the major outcomes. We used methods and recommendations described in sections 8.5, 8.7 and chapters 11 and 13 (section 13.5) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017; Schünemann 2017a ) using GRADEpro software (GRADEpro GDT 2015). We justified all decisions to downgrade the quality of studies using footnotes and made comments to aid the reader's understanding of the review, where necessary.
Results
Description of studies
Results of the search
The search identified 2152 records from the following databases: MEDLINE (633) Embase (592) CENTRAL (757) WHO ICTRP website (49) and Clinicaltrials.gov (121) (see flow chart in Figure 1). There were 1608 records after removal of duplicates, of which 24 were assessed in full text and fourteen new trials were included (Akbari 2020; Azadvari 2020; Bhayana 2018; Cho 2010; Cho 2021; Cole 2016; Dogu 2012; Haghighat 2015; Hsieh 2013; Lee 2015; Raeissadat 2017; Roddy 2020; Saeed 2014; Zufferey 2012) in addition to the five included trials (n = 290 participants) from the previous version of the review (Chen 2006; Ekeberg 2009; Lee 2009; Naredo 2004; Ucuncu 2009). In total, we included 19 trials in this review update.

Study flow diagram.
Four trials are awaiting assessment (Cinar 2018; IRCT2017021524621N6; Moore 2018; Pierce 2018) (see Table of Characteristics of studies awaiting classification). No ongoing trials were identified.
Included studies
We have provided a complete description of the 19 included trials (n = 1035 participants) in the Characteristics of included studies table. Since our previous review (Bloom 2012), 14 additional trials (n = 873 additional participants) were included.
Trial design, setting and characteristics
Three of the five trials included in our previous review were RCTs (Ekeberg 2009; Naredo 2004; Ucuncu 2009). Of the two categorised as CCTs, one (reported to be an RCT) alternatively assigned participants to treatment (Lee 2009), while the other did not specify how participants were allocated to treatment group (Chen 2006). All 14 of the new trials were stated to be RCTs although five failed to report their method of randomisation (Bhayana 2018; Haghighat 2015; Naredo 2004; Roddy 2020; Ucuncu 2009).
Trials were conducted in 11 countries: four trials were conducted in Korea (Cho 2010; Cho 2021; Lee 2009; Lee 2015), two in Taiwan (Chen 2006; Hsieh 2013), three in Iran (Akbari 2020; Haghighat 2015; Raeissadat 2017), three in Turkey (Akbari 2020; Dogu 2012; Ucuncu 2009), and one each in Australia (Cole 2016), Norway (Ekeberg 2009), Spain (Naredo 2004), Ireland (Saeed 2014), India (Bhayana 2018), Switzerland (Zufferey 2012), and the United Kingdom (Roddy 2020).
Twelve trials were conducted in outpatient clinics (Akbari 2020; Azadvari 2020; Bhayana 2018; Cho 2021; Ekeberg 2009; Haghighat 2015; Hsieh 2013; Lee 2009; Roddy 2020; Saeed 2014; Ucuncu 2009; Zufferey 2012), two in a hospital (no further details specified) (Lee 2015; Raeissadat 2017), and one in an orthopaedic research institute (Cole 2016). Four trials did not specify the setting of the trial (Chen 2006; Cho 2010; Dogu 2012; Naredo 2004).
Funding source was reported in six trials (Akbari 2020; Azadvari 2020; Bhayana 2018; Cho 2021; Ekeberg 2009; Hsieh 2013). Akbari 2020 reported funding from the Baskent University Research Fund. Bhayana 2018 reported there was no funding for the study. Ekeberg 2009 declared funding from the University of Oslo. Hsieh 2013 declared funding from Shin Kong Wu Ho‐Su Memorial Hospital. Roddy 2020 reported funding from Arthritis Research UK Primary Care Centre and the National Institute for Health Research.
Trial participants
The mean age of participants in 18 trials varied between 31 (Azadvari 2020) and 60 (Cho 2010) years; the mean age of all participants was 53 years. The percentage of female participants in 17 trials varied between 33% (Chen 2006) and 73% (Ucuncu 2009); the mean percentage of females was 55%. Azadvari 2020 did not report the gender distribution of the sample. Saeed 2014 did not report the mean age or gender distribution of the sample.
Symptom duration was reported in 15 trials. Twelve trials reported mean symptom duration (Azadvari 2020; Cho 2021; Cole 2016; Dogu 2012; Haghighat 2015; Hsieh 2013; Lee 2009; Naredo 2004; Raeissadat 2017; Saeed 2014; Ucuncu 2009; Zufferey 2012); this varied between 1.8 months (Haghighat 2015) and 23 months (Azadvari 2020). Chen 2006 reported that symptom duration ranged from two to 10 months. Ekeberg 2009 and Roddy 2020 reported symptom duration as categories. For Ekeberg 2009, symptom duration was > 24 months for 26% in the ultrasound‐guided group and 23% in the ‘systemic’ group. For Roddy 2020, symptom duration was > 12 months for 41% in the ultrasound‐guided group and 40% in the ‘blind’ group.
Nine studies relied on clinical features alone for confirming eligibility into the trial (Azadvari 2020; Bhayana 2018; Cho 2021; Ekeberg 2009; Haghighat 2015; Hsieh 2013; Naredo 2004; Raeissadat 2017; Roddy 2020), while two relied on ultrasound (Chen 2006; Cho 2010), three relied on ultrasound and radiography (Cole 2016; Lee 2009; Lee 2015), and two relied on MRI (Akbari 2020; Dogu 2012). Three trials did not report how a diagnosis was made (Saeed 2014; Ucuncu 2009; Zufferey 2012).
Fourteen trials included participants that could be categorised as having rotator cuff disease although the diagnostic labels varied (Akbari 2020; Azadvari 2020; Bhayana 2018; Chen 2006; Cho 2010; Cole 2016; Dogu 2012; Ekeberg 2009; Haghighat 2015; Hsieh 2013; Naredo 2004; Roddy 2020; Saeed 2014; Ucuncu 2009). Bhayana 2018 included 60 participants with rotator cuff syndrome. Three trials included participants with 'subacromial bursitis' (Chen 2006: n = 40; Cho 2010: n = 28 with either 'subacromial' or 'subdeltoid bursitis'; and Hsieh 2013: n = 96 with 'chronic subacromial bursitis'). Seven trials included participants with 'subacromial impingement syndrome' (Akbari 2020: n = 29; Azadvari 2020: n = 30; Cole 2016: n = 51 (56 shoulders); Dogu 2012: n = 46; Haghighat 2015: n = 40; Roddy 2020: n = 128; Saeed 2014: n = 100 (125 shoulders)). Ekeberg 2009 included 106 participants with rotator cuff disease; Naredo 2004 included 41 participants with 'periarticular' disorders; and Ucuncu 2009 included 60 participants with 'soft‐tissue lesions' of the shoulder.
Four trials included participants with adhesive capsulitis (Cho 2021; Lee 2009; Lee 2015; Raeissadat 2017). Cho 2021 included 90 participants, Lee 2009 included 43 participants, Lee 2015 included 77 participants and Raeissadat 2017 included 41 participants. One trial (Zufferey 2012) included 70 participants with acute shoulder pain and did not provide a more specific diagnostic label. No trials involving people with glenohumeral osteoarthritis were included in this review but one trial is awaiting assessment as it is currently only available as a pre‐print in bioRxiv and has not yet been published in a peer‐reviewed journal (Moore 2018).
Interventions
Details of the interventions in each trial are presented in the Characteristics of included studies table and Table 1.
| Study ID | Study population | Ultrasound‐guided injection | Type of ultrasound | Landmark‐guided injection | Co‐interventions | Adverse events |
| (Turkey) | Subacromial impingement syndrome confirmed by magnetic resonance imaging (MRI), symptoms for more than three months, increased pain on shoulder abduction, painful restriction of active flexion and/or abduction of the shoulder with more restriction on passive range of motion, and a positive Hawkins‐Kennedy impingement sign | Summary Injection of 1 mL of 40 mg methylprednisolone and 4 mL procaine 2% into the subacromial bursa performed by a radiologist with 10 years of experience in musculoskeletal radiology Procedure The patient was seated and the shoulder was internally rotated with the ipsilateral hand positioned on the hip in the modified Crass position. Methylprednisolone acetate 40 mg in 1 mL and procaine 2% 4 mL were prepared in a 5 mL syringe. The anterolateral aspect of the shoulder was cleaned using 10% povidone iodine solution. The US probe was placed on the anterolateral aspect of the shoulder and the subacromial bursa was visualised. A 21‐gauge needle was used to enter the anteromedial aspect of the shoulder under continuous US guidance. Once the bevel of the needle was visualised in the subacromial bursa, the solution was injected. | 2014 model Siemens Acuson S2000 (Siemens Healthcare, Erlangen, Germany) and a 9 MHz linear probe | Summary Injection of 1 mL of 40 mg methylprednisolone and 4 mL procaine 2% into the subacromial space performed by a physiatrist with more than 10 years of experience in the field Procedure The injection was performed using a standard posterolateral approach and an aseptic technique. The patient was seated upright with the arms resting comfortably at the side. The distal, lateral, and posterior edges of the acromion were palpated and the needle was inserted just inferiorly to the posterolateral edge of the acromion and directed towards the opposite nipple. Aspiration was performed to ensure that the needle was not in a blood vessel prior to administration of the drug. Methylprednisolone acetate 40 mg in 1 mL and procaine 2% 4 mL were injected slowly using a 21‐gauge needle 1 cm into the subacromial space. | Not reported | None in either group |
| (Iran) | Subacromial impingement in paraplegic patients with spinal cord injury (below T6). Shoulder pain visual analog (VAS) score higher than 4 and positive Neer test and Hawkins‐Kennedy test | Summary Injection of 1 mL methylprednisolone and 2 mL lidocaine into the subacromial‐subdeltoid bursa performed by a physiatrist. Procedure The injection was performed with lateral approach. The patients were at sitting position, they put their hand on iliac crest by internal rotation of shoulder joint. The probe was placed on supraspinatus muscle in longitudinal plane. Acromion was found by ultrasound guidance. Then the probe was derived to the inferior part so the subacromial‐subdeltoid bursa was determined. A hypodermic needle (gauge‐22) was entered beneath the probe in in‐plane manner in upward medial direction until the needle tip entered the bursa and its dilatation was observed during injection. Injection in subacromial space was performed by a physiatrist. | Not reported | Summary Injection of 1 mL methylprednisolone and 2 mL lidocaine into the subacromial space performed by a physiatrist Procedure Participants received lateral approached blind glucocorticoid injection guided by anatomic landmarks under sterile condition. The injection site was marked and then sterilised. Lateral edge of the acromion was touched by hand and the needle with gauge of 22–23 was guided 2–3 cm beneath the lateral edge of the acromion toward the medial side (slightly superior). Under resistance‐free condition, 1 cc depo‐medrol (Methylprednisolone, Merck, Germany) was injected along with 2 cc lidocaine. | Exercise therapy was used in both groups after seven days. The exercises included range of motion, posterior capsule stretching and isometric strengthening exercises presented as an instruction. | Not reported |
| Bhayana 2018 (India) | Rotator cuff syndrome with shoulder pain and < 50% reduction in one direction of range of motion | Summary Injection of 2 mL (40 mg/mL) methylprednisolone and 2 mL of 1% lignocaine into subacromial bursa performed by one experienced radiologist Procedure The patient was seated with the affected arm in hyperextension and internal rotation with the elbow bent and back of the hand resting against the lower back. A 10 mL syringe connected to a 5 cm, 21‐gauge needle was prepared with 2 mL of 40 mg/mL methylprednisolone acetate suspension mixed and 2 mL of 1% lignocaine and was inserted parallel to the transducer in a semi‐oblique plane from the anterior side of the shoulder. The bevelled side facing the transducer, the needle was advanced under the real time ultrasound assistance till the tip of the needle appeared in the subacromial bursa. Rotation of the bevelled side of the needle by 180 degrees was done for confirmation of the tip positioning and the bulging of subacromial bursa ascertained in the real time imaging. | Phillips HD 7 US machine with 7–10 MHz multi frequency broad band transducer | Summary Injection of 2 mL (40 mg/mL) methylprednisolone and 2 mL of 1% lignocaine into subacromial space by one orthopaedic surgeon Procedure A 10 mL syringe connected to 5 cm, 21‐gauge needle was prepared with 2 mL of 40 mg/mL methylprednisolone acetate suspension mixed, 2 mL lignocaine and 2 mL of radio opaque non‐ionic contrast media iohexol. Access to the subacromial space was achieved using a lateral approach. The needle was inserted just inferior to the midlateral aspect of the acromion, with the needle angled slightly cephalad, passing through the deltoid muscle, and directed medially and slightly anterior to the subacromial bursa. Care was taken to avoid injection directly into the tendons of the rotator cuff. Correct placement of the drug in the subacromial subdeltoid space was confirmed by the fluoroscopic assessment under the C arm within 10 minutes of the injection. Accuracy of correct instillation of methylprednisolone‐ lignocaine‐iohexol combination was assessed by a senior radiologist. | Both groups received 5‐day course of antibiotics for prevention of injection‐induced subacromial bursitis, and home‐based shoulder rehabilitation programme consisting of shoulder abduction and pendulum exercises. | None in either group |
| (Taiwan) | Ultrasound‐confirmed subacromial bursitis with shoulder pain and a painful arc | Summary Injection of 1 mL betamethasone and 1 mL 1% lignocaine into subacromial bursa by one physician experienced using ultrasound probes Procedure The patient was seated upright, with the back well supported and arms behind the back with elbows bent. The needle was inserted into the subacromial bursa under ultrasound guidance. Aspiration of the effusion was done first before injecting the steroid‐lidocaine suspension into the subacromial bursa. Advancement of the needle to the lesion site was observed as continuous and real‐time images. | LOGIQ 9 machine with 10L probe (4‐10 MHz) (General Electronic Company, Milwaukee, WI) | Summary Injection of 1 mL betamethasone and 1 mL 1% lignocaine into subacromial bursa by one physician experienced in peripheral joint and soft‐tissue injections. Procedure The patient was seated upright, with the back well supported and arms behind the back with elbows bent. The acromion was palpated by the thumb and the needle inserted in a horizontal approach. The needle was adjusted in different depths and angles to try to aspirate the effusion. Exaggerated | Not reported | Not reported |
| Cho 2010 | Ultrasound‐confirmed subacromial or subdeltoid bursitis with shoulder pain and positive findings on Hawkin's test (i.e. reproduction of pain) | Summary Injection of 5 mL of 0.5% lidocaine and 5 mL triamcinolone acetate into subacromial or subdeltoid bursa by a single researcher Procedure After the patient was seated in a chair, the deformed shoulder was extended, the elbow joint was flexed, and the modified crass position was adopted. The transducer was placed in front of the acromion, the needle was inserted in parallel with the probe to position the tip to the bursa and real‐time ultrasound was used to confirm that the tip of the needle was in the bursa and solution was then injected into the bursa. | Philips Newand 13 MHz linear transducer | Summary Injection of 5 mL of 0.5% lidocaine and 5 mL triamcinolone acetate into subacromial or subdeltoid bursa by the same researcher Procedure A posterior approach was used. | Not reported | None in either group |
| (Korea) | Primary frozen shoulder with pain and limitation of passive motion of greater than 30 degrees in two or more planes of movement | Summary Injection of 40 mg of triamcinolone acetonide, 4 mL of 1% lidocaine, 4 mL of normal saline, and 3 mL of water soluble unionised contrast into the glenohumeral joint by a single specialist with 15 years of experience in the field Procedure A posterior approach was used for the injection in both groups, with the patient in the semi‐lateral decubitus position on the unaffected side and 45° anterior tilting of affected side. For the US group, the needle was advanced laterally to medially with visualisation of its shaft using a linear 5‐ to 12‐MHz probe (HD15 ultrasound system; Philips, Bothell, Washington, USA), and reaching the glenohumeral joint space between the posterior aspect of the humeral head and the glenoid labrum. | Linear 5‐ to 12‐MHz probe (HD15 ultrasound system; Philips, Bothell, Washington, USA) | Summary Injection of 40 mg of triamcinolone acetonide, 4 mL of 1% lidocaine, 4 mL of normal saline, and 3 mL of water soluble unionised contrast into the glenohumeral joint by a single specialist with 15 years of experience in the field Procedure A posterior approach was used for the injection in both groups, with the patient in the semi‐lateral decubitus position on the unaffected side and 45° anterior tilting of affected side. For the blind group, the needle was inserted 2 cm inferior to the posterolateral margin of the acromion and directed anteriorly towards the coracoid process. After the needle contacted the humeral head, it was slightly withdrawn and the injection was administered. For the blind group, the US probe was placed just under the acromion without visualisation, to blind the patient to the allocation group. | All patients were instructed to use a home‐based exercise programme to increase ROM and were allowed to perform an exercise three times a day (15 minutes each round). The programme included pendulum exercises, wall‐climbing exercises, and gentle ROM exercises with a bar. During this programme, patients were asked to refrain from provoking post‐mobilisation soreness with self‐feedback. Patients were forbidden from having acupuncture or receiving additional injections from other hospitals. | None in either group |
| (Australia) | Subacromial impingement syndrome with shoulder pain during overhead activities and clinical signs of impingement (either in internal rotation or external rotation) | Summary Injection of 1 mL of 40 mg/mL methylprednisolone acetate and 5 mL of 1% lidocaine hydrochloride into subacromial bursa by one surgeon with over 10 years of experience performing subacromial injections Procedure With the patient in an upright sitting position, a 22‐gauge needle was placed directly underneath the midpoint of the lateral acromion. The needle was directed anteriorly and cephaladly toward the subacromial bursa (as guided by the ultrasound image) until the tip of the needle was seen in the bursa. | General Electric Logiq E9 machine with a 6‐ to 15‐MHz linear transducer and 50 x 10–mm footprint | Summary Injection of 1 mL of 40 mg/mL methylprednisolone acetate and 5 mL of 1% lidocaine hydrochloride into subacromial bursa by the same surgeon that performed the ultrasound‐guided injections Procedure Blind injections were performed with the patient in the same upright sitting position with the ultrasound probe on the acromion to keep the patients blinded to the treatment group. An image could be seen on the screen by the patient, but it was not possible for this to be used by the surgeon to guide the injection, as the way that he was positioned behind the patient meant that he did not have direct vision of the screen. A posterior approach was used, and the needle was inserted 1 cm medially and inferiorly to the posterolateral corner of the acromion and directed cephaladly, anteriorly, and medially toward the subacromial bursa. | Participants in both groups were not restricted from the use of any analgesic or anti‐inflammatory medication or other treatments such as physical therapy. | None in either group |
| (Turkey) | Subacromial impingement syndrome with shoulder pain and at least two positive results in the provocative tests (Neer, Hawkins, and Jobe’s ‘‘Empty Can’’). Diagnosis of subacromial impingement syndrome was confirmed by MRI. | Summary Injection of 1 mL of 5 mg/mL betamethasone dipropionate, 9 mL of 10 mg/mL prilocaine hydrochloride and 0.02 mL of 0.01 mmol gadolinium diethylenetriaminepentaacetic acid directly underneath the mid‐lateral aspect of the acromion, directed anteriorly and cephaladly by a musculoskeletal radiologist Procedure The patient was seated in the upright position. Sterile gel was applied to the probe. The probe was held in one hand, and the syringe with the mixture was held in the other hand. With the patient in an upright sitting position, a 21‐gauge needle was placed under the probe, and a lateral injection was made directly underneath the mid‐lateral aspect of the acromion, directed anteriorly and cephaladly. | Aplio XV machine (Toshiba, Tokyo, Japan) with a 7.5‐ to 14‐MHz linear probe | Summary Injection of 1 mL of 5 mg/mL betamethasone dipropionate, 9 mL of 10 mg/mL prilocaine hydrochloride and 0.02 mL of 0.01 mmol gadolinium diethylenetriaminepentaacetic acid into subacromial space by a physiatrist Procedure Patient seated in upright position. A posterior approach was used. Under the guidance of a Toshiba Power Vision ultrasonograph, gel was applied to a 7.5‐ to 14‐MHz linear probe, and it was placed on the trapezius muscle. A 21‐gauge needle was located 1 cm posteriorly and inferiorly to the border of the acromion and directed cephaladly, anteriorly, and medially toward the subacromial space. | Participants in both groups could use only 2000 mg paracetamol daily; no participant received physical therapy. | None in either group |
| Ekeberg 2009 (Norway) | Rotator cuff disease with shoulder pain, < 50% reduced range of movement in no more than one direction of external rotation, internal rotation or abduction, and a positive Hawkins‐Kennedy impingement sign | Summary Injection of 2 mL (20 mg) triamcinolone and 5 mL (10mg/mL) lidocaine to subacromial bursa and 4 mL (10mg/mL) lidocaine to upper gluteal region by one physician Procedure To improve blinding of participants by inducing a temporary pain relief (impingement test) and mask possible post‐injection pain, participants received an injection of local anaesthetic in the shoulder and the gluteal region. The participant was seated with the arm rotated internally behind the back, elbow bent, and the back of the hand resting against the lower back. The physician used the ultrasound probe to visualise the insertion of the supraspinatus tendon and the subacromial bursa on the longitudinal axis, taking care that the contents of the syringes were never shown to the participants. The physician used the anterior approach with a 0.8 × 50 mm intra‐muscular needle for the subacromial injections, perforating the skin and tracking the needle in real time until it reached the subacromial bursa. Participants received an injection of 2 mL (10 mg/mL) triamcinolone (Kenacort‐T, Bristol‐Myers Squibb) and 5 mL (10 mg/mL) lidocaine hydrochloride (Xylocaine, AstraZeneca) to the subacromial bursa and an intramuscular injection of 4 mL (10 mg/mL) lidocaine hydrochloride to the upper gluteal region. | Commercial ultrasound equipment (Medison 128 BWprime, Medison Co, Seoul, Korea) with a 5‐9 MHz linear transducer | Summary Injection of 5 mL (10mg/mL) lidocaine to subacromial bursa and 2 mL (20 mg) triamcinolone and 2 mL (10 mg/mL) lidocaine to upper gluteal region by same physician Procedure To improve blinding of participants by inducing a temporary pain relief (impingement test) and mask possible post‐injection pain, participants received an injection of local anaesthetic in the shoulder and the gluteal region. The participant was seated with the arm rotated internally behind the back, elbow bent, and the back of the hand resting against the lower back. The physician used the ultrasound probe to visualise the insertion of the supraspinatus tendon and the subacromial bursa on the longitudinal axis, taking care that the contents of the syringes were never shown to the participants. The physician used the anterior approach with a 0.8 × 50 mm intra‐muscular needle for the subacromial injections, perforating the skin and tracking the needle in real time until it reached the subacromial bursa. Participants received an injection of 5 mL (10 mg/mL) lidocaine hydrochloride to the subacromial bursa and an intramuscular injection of 2 mL (10 mg/mL) triamcinolone and 2 mL (10 mg/mL) lidocaine hydrochloride to the upper gluteal region. | Participants in both groups were allowed to use analgesics, and to continue any physiotherapy programme that they were attending at baseline. | There were no serious adverse events in either group. 1/53 participants in the ‘local’ group and 4/53 in the ‘systemic’ group reported post‐injection pain in the shoulder. Nine participants from both groups reported mild adverse effects such as facial redness, dizziness, and feeling of warmth, however, per group data were not given. |
| Haghighat 2015 (Iran) | Subacromial impingement syndrome with shoulder pain, pain in abduction (or painful restriction of glenohumeral mobility) and positive Neer and Hawkins tests | Summary Injection of 40 mg methylprednisolone with 1 cc lidocaine 2% into subacromial bursa; personnel not specified Procedure A lateral approach using the ultrasound equipment with high frequency linear transducer for guidance was adopted. The ultrasound probe was positioned parallel to the long axis of the supraspinatus. The skin was punctured at a distance of about 2–3 cm from the probe in order to avoid contact between the needle and the probe. As soon as the needle had penetrated the subcutaneous tissues, its progress was real‐time monitored on the US image. The needle was visualised as a hyperechoic structure with posterior comet‐tail artefact. Progress of the needle until the tip had entered the subacromial bursa was observed. When the tip of the needle appeared to be inside the subacromial bursa, a small amount of liquid was injected to confirm the correct position (fluid passing from the needle tip into the bursa). The injection could be visualised in real‐time as a spreading "cloud" of hyperechoic echoes inside the bursa. | Ultrasound equipment with high frequency linear transducer. No further details provided | Summary Injection of 40 mg methylprednisolone with 1 cc lidocaine 2% into subacromial bursa; personnel not specified Procedure A posterior approach was used. The needle was placed approximately 2 cm below the posterior‐lateral aspect of the acromion. The needle was guided forward and slightly to the left under the acromion. | Not reported | Not reported |
| (Taiwan) | Subacromial bursitis with shoulder pain, ≥ 4/10 pain during abduction on a VAS, a painful arc, and > 40% reduction in pain during shoulder abduction at end range after injection of 3 mL of 1% lidocaine into the subacromial bursa | Summary Injection of 0.5 mL (5 mg/mL) dexamethasone suspension and 3 mL lidocaine (10mg/ml) into subdeltoid/subacromial bursa by one senior physician who was a board‐certified rheumatologist, physiatrist, and ultrasonographer in musculoskeletal medicine Procedure After the sterilisation of the skin on the lateral side of the affected arm, a 21‐gauge needle was inserted into the subacromial bursa under ultrasound guidance. Any effusion, if present, was aspirated prior to the injection. | LOGIQ P5 machine (General Electronic Company, Milwaukee, WI) with 5‐ to 12‐MHz linear array transducer | Summary Injection of 0.5 mL (5 mg/mL) dexamethasone suspension and 3 mL lidocaine (10 mg/mL) into subacromial bursa by the same senior physician Procedure The physician injected the subacromial bursa according to Cyriax’s method, where he/she first localised the lateral edge of the acromion. He/she then asked the patient to relax the affected arm, and at 1–2 cm beneath the middle point of the lateral edge of the acromion, inserted a 21‐gauge needle medially and in a slightly cranial direction into the bursa. If the needle encountered resistance, either the coracoacromial ligament or the capsulotendinous structures had been contacted, and the needle position was slightly adjusted. | Not reported | Not reported |
| (South Korea) | Adhesive capsulitis with pain and limited range of movement. Ultrasound used to rule out rotator cuff disease. | Summary Injection of 0.5 mL (20 mg) triamcinolone and 1.5 mL 2% lidocaine and 3 mL normal saline into glenohumeral joint by one physician with two years’ experience in ultrasound‐guided shoulder injections Procedure The posterior approach was used. Patients were seated, with the affected shoulder bent and adducted. The ultrasound probe touched the lower part of the acromion sideways, and a long needle (23G, 7 cm) was inserted to the posterior articular surface of the humeral head and positioned inside the articular capsule. The expansion of the articular capsule was checked while the fluid was being injected. | LOGIQ 5a machine with a 10‐MHz linear probe | Summary Injection of 0.5 mL (20 mg) triamcinolone and 1.5 mL 2% lidocaine and 3 mL normal saline into articular capsule by one physician with 7 years experience in anatomic landmark‐guided shoulder injections Procedure The posterior approach was used. Patients were seated, with the affected shoulder bent and adducted. The acromion of the scapula was palpated and the needle inserted 1 cm inferior to the tip of the acromion. The needle was directed toward the coracoid process and advanced into the articular capsule, after which the drug was injected. | Participants in both groups were taught exercises for increasing joint ROM including stretching forward and bending down to a desk, Codman exercise, and a wall‐climbing exercise with the fingers. They were instructed to practice these at home. Participants were checked and encouraged to keep up with the exercises every time they visited the hospital. All participants had 5 weekly injections of 25 mg sodium hyaluronate. | Not reported |
| (South Korea) | Shoulder stiffness on the basis of < 100 degrees flexion or < 30 degrees external rotation or internal rotation at a level below the first lumbar spine junction. Radiography and ultrasonography were used to rule out rotator cuff disease. | Summary Injection of 40 mg triamcinolone acetonide and 2 mL of 2% lidocaine into glenohumeral joint by one experienced senior surgeon with more than 5 years of experience in performance of ultrasound‐guided injection at the shoulder joint Procedure The patient was supine with the arm by the side. After skin and transducer preparation with alcohol and povidone, the 21‐gauge 1.5‐inch needle on a 3 mL syringe with triamcinolone acetonide 40 mg and 2 mL of 2% lidocaine was inserted at the level of the coracoids, from lateral to medial, aimed at the medial border of the humeral head. When the needle made contact with the articular cartilage of the humeral head, the needle was tilted to position the point of the needle in the articular cavity. The intra‐articular position of the needle and fluid infiltration in the shoulder joint was confirmed by ultrasonography. | High‐resolution transducer with 12 MHz linear array | Summary Injection of 40 mg triamcinolone acetonide and 2 mL of 2% lidocaine into glenohumeral joint by the same senior surgeon Procedure With the patient in sitting, the sulcus between the lateral tip of the coracoid and the humeral head was palpated. Then, a 21‐gauge 1.5‐inch needle on a 3 mL syringe with triamcinolone acetonide 40 mg and 2 mL of 2% lidocaine was inserted in a slightly cephalad direction at the anterior‐lateral tip of the coracoid. The needle was slowly advanced while infiltrating the local anaesthetic until resistance was lost, indicating intra‐articular position. The injection was performed slowly. | Both groups received a home‐based exercise programme for rehabilitation involving: pendulum circumduction and passive shoulder exercises for self‐stretching in forward flexion, external rotation and internal rotation, pulley exercise, isometric exercise, thera‐band exercises, scapula stabilisation exercises, and strengthening using dumbbells. | None in either group |
| Naredo 2004 (Spain) | ‘Periarticular disorders’ (impingement syndrome rotator cuff lesions, subacromial‐subdeltoid bursitis and/or biceps tendon abnormalities) diagnosed by clinical history and examination | Summary Injection of 20 mg triamcinolone into either subacromial‐subdeltoid bursa, biceps tendon sheath or rotator cuff calcifications according to ultrasound findings by single rheumatologists experienced in ultrasound Procedure The transducer and the patient’s skin were sterilised with alcohol. Sterile gel was applied to the probe. The transducer was held in one hand and the syringe with glucocorticoid in the other hand. The needle was | Commercial equipment (Sonoline, Prima, Siemens, Seattle, WA, USA) using a 7.5 MHz linear phased array transducer | Summary Injection of 20 mg triamcinolone into subacromial space by same rheumatologist Procedure A 21‐gauge needle was used. The patient’s skin was sterilised with alcohol. Access to the subacromial space was achieved with a lateral approach, inserting the needle under the anterolateral aspect of the acromion process, passing it through the deltoid muscle, and directing it medially and slightly anterior to the subacromial‐subdeltoid bursa, with care taken to avoid injection directly into the tendons of the rotator cuff. Immediately after blind injection, an ultrasound examination was performed to search for steroid deposit as hyperechoic foci or lines, with or without acoustic shadowing. | Participants in both groups with loss of shoulder ROM were instructed to start a home physical therapy programme consisting of pendulum exercises and slow shoulder abduction. No restriction was placed on the participant’s ability to work or to use their shoulder as tolerated, or to take nonsteroidal anti‐inflammatory drugs. Participants did not receive physical therapy during the follow‐up period. | There were no serious adverse events in either group. 1/20 participants in the ‘blind’ injection group had a mild post‐injection adverse effect (not specified). |
| Raeissadat 2017 (Iran) | Adhesive capsulitis on the basis of findings from the history and physical examination | Summary Injection of 1 cm3 lidocaine 1%, then, 3 cm3 water soluble un‐ionised contrast with 1 cm3 distilled water, and finally, 1 cm3 triamcinolone 40 mg/cm3 with 1 cm3 lidocaine 1% into glenohumeral joint by one physical medicine and rehabilitation specialist with 15 years’ experience in his field Procedure The patient was seated and the affected hand was resting on the thigh. A glenohumeral joint injection was given using the posterior short axis approach, and the needle was inserted in the plane relative to the ultrasound probe and medial to the posterior aspect of the head of the humerus. | Alpinion E‐cube 7 ultrasound device with linear 3–12‐MHz probe | Summary Injection of 1 cm3 lidocaine 1%, then, 3 cm3 water soluble un‐ionised contrast with 1 cm3 distilled water, and finally, 1 cm3 triamcinolone 40 mg/cm3 with 1 cm3 lidocaine 1% into glenohumeral joint by the same specialist Procedure A glenohumeral joint injection was given using a posterior approach. A 25‐gauge needle was inserted 2.5 cm lower than the posterolateral aspect of the acromion. If the physician felt that the needle was not accurately inserted, he was not allowed to withdraw and re‐enter again. | After the injection, all participants in both groups received naproxen tablet 500 mg twice daily for 5 days and were told to perform Codman’s exercises. | None in either group |
| (United Kingdom) | Subacromial impingement syndrome with pain in deltoid insertion area, positive Neer and Hawkins Kennedy tests, and pain on shoulder abduction | Summary Injection of methylprednisolone 40 mg and 1 mL 1% lidocaine into the bursa by one of nine clinicians with extensive clinical experience performing ultrasound‐guided injections or completed an accredited course on ultrasound‐guided subacromial injections Procedure The skin and transducer were cleaned with chlorhexidine 0.5% solution and sterile gel applied to the transducer. The participant sat with the shoulder internally rotated and the ipsilateral hand on the buttock to maximise visibility of and access to the subacromial bursa. The transducer was placed anterolaterally, the hypoechoic subacromial bursa visualised, and a 21‐G needle inserted under real‐time ultrasound guidance until the needle‐tip entered the bursa. A commercially available premixed solution of methylprednisolone 40 mg and 1 mL 1% lidocaine was injected into the bursa. | LOGIQ e system with a 12 MHz transducer | Summary Injection of methylprednisolone 40 mg and 1 mL 1% lidocaine into the bursa by one of eight clinicians (different to those performing the ultrasound‐guided injections) with extensive clinical experience performing subacromial injections, and having attended a half‐day injection protocol workshop Procedure The participant sat with the arm hanging with the elbow bent and forearm resting on the lap. The skin was cleaned with chlorhexidine solution 0.5%. A 21‐G needle was inserted through the deltoid under the acromion process laterally. The same premixed solution of methylprednisolone 40 mg and 1 mL 1% lidocaine was injected. | None | Ultrasound‐guided injection and physiotherapist‐led exercise (n = 64): Serious adverse event: No. and nature of events: 1/64 ‐ pyelonephritis Other adverse event: No. and nature of events: 17/64 ‐ shoulder pain > 3 days following injection No. and nature of events: 16/64 ‐ discomfort during the injection or local skin changes, presyncope, nausea or flushing following the injection Ultrasound‐guided injection and exercise leaflet (n = 64): Other adverse event: No. and nature of events: 17/64 ‐ shoulder pain > 3 days following injection No. and nature of events: 16/64 ‐ discomfort during the injection or local skin changes, presyncope, nausea or flushing following the injection Unguided injection and physiotherapist‐led exercise (n = 64): Other adverse event: No. and nature of events: 20/64 ‐ shoulder pain > 3 days following injection No. and nature of events: 17/64 ‐ discomfort during the injection or local skin changes, presyncope, nausea or flushing following the injection Unguided injection and exercise leaflet (n = 64): Other adverse event: No. and nature of events: 20/64 ‐ shoulder pain > 3 days following injection No. and nature of events: 17/64 ‐ discomfort during the injection or local skin changes, presyncope, nausea or flushing following the injection |
| Saeed 2014 (Ireland) | Shoulder impingement pain with shoulder pain | Summary Injection of 40 mg of methylprednisolone acetate with 4 mL of lidocaine hydrochloride into either subacromial‐subdeltoid bursa, acromio‐clavicular joint, biceps tendon or other pathology according to ultrasound findings by one experienced physician. Procedure A two‐handed technique was used with the transducer held in one hand and the syringe with 21‐G needle in the other hand. The needle was directed in real time by ultrasound from the skin to the target (e.g. subdeltoid bursa, acromio‐clavicular joint or biceps pathology). In those shoulders where ultrasound demonstrated more than one pathology, the pathology most consistent with clinical examination was injected. When there was effusion in both the subacromial‐subdeltoid bursa and the biceps tendon sheath, injection was directed into the subacromial‐subdeltoid bursa. For confirmed rotator cuff pathology and inpatients with clinical impingement but normal shoulder, ultrasound subacromial‐subdeltoid bursa injection was performed. When ultrasound confirmed the principal pathology as acromioclavicular joint inflammation or biceps tendon inflammation, ultrasound‐guided injection of the principal pathological structure was performed. | Acuson Sequoia 512 ultrasound systems (Siemens, CA, USA) using 8L‐RS MHz linear phased‐array transducer | Summary Injection of 40 mg of methylprednisolone acetate with 4 mL of lidocaine hydrochloride into subacromial‐subdeltoid bursa by the same physician Procedure A standard technique was performed using a 21‐G needle via a lateral approach to inject the subacromial‐subdeltoid bursa. | Participants in both groups with the loss of shoulder range of movement were given post‐injection instructions in pendulum exercises and slow shoulder abduction. No restriction was placed on participants' ability to work or to use their shoulder as tolerated, or intake of NSAIDs. Participants did not receive physical therapy during the follow‐up period. | Apart from mild shoulder pain in a small number of cases (group not specified) within a few hours of shoulder injection, no serious adverse event was observed. |
| (Turkey) | ‘Soft‐tissue lesions’ (acromioclavicular degeneration, rotator cuff lesions (rupture, partial rupture, tendinosis, impingement, calcification), fluid accumulation or partial rupture of biceps tendon and bursitis (subdeltoid, subacromial)) that had not responded satisfactorily to at least 1 month of nonsteroidal anti‐inflammatory drugs | Summary Injection of 1 mL (40 mg) triamcinolone and 1 mL 1% lidocaine into site of observed pathology. Injections were administered ‘perilesionally’ and ‘intralesionally’; personnel not specified. Procedure The injector was directed to the site of observed pathology beneath the probe by imaging a hyper‐reflective line. | Commercial equipment (Esaote, Mylab 60, Italy) using a 6 to 18MHz linear phased array transducer | Summary Injection of 1 mL (40 mg) triamcinolone and 1 mL 1% lidocaine into subacromial region; personnel not specified Procedure Injections were administered with a lateral entry approach to the sub‐acromial region. | Participants in both groups with loss of shoulder ROM performed a home exercise programme consisting of shoulder abduction and pendulum exercises. No limit was imposed on nonsteroidal anti‐inflammatory consumption or use of the shoulder. Participants did not receive physical therapy during the follow‐up period. | There were no serious adverse events in either group. 1/30 participants in the ultrasound‐guided injection group had mild post‐injection pain. In the ‘blind’ injection group, 5/30 had a slight increase in pain and 1/30 had skin peeling after the injection. |
| Zufferey 2012 (Switzerland) | Acute shoulder pain that had not responded satisfactorily to nonsteroidal anti‐inflammatory drugs or physiotherapy | Summary Injection of 2 mL of Diprophos® (7 mg of a mixture made up of one‐third soluble and two‐thirds long‐acting bethamethasone) into site of observed pathology by two rheumatologists experienced in musculoskeletal ultrasonography Procedure Steroid injection under ultrasound guidance was performed according to the ultrasound diagnosis within two days of the clinical evaluation. The injection was directed into the location assessed by ultrasound to be the cause of shoulder pain. | 5‐9 MHz probe using a Philips HD11 machine in two centres and Easote mylab25 gold machine in one centre | Summary Injection of 2 mL of Diprophos® (7 mg of a mixture made up of one‐third soluble and two‐thirds long‐acting bethamethasone) into subacromial bursa; personnel not specified Procedure Blind injections were directed at the subacromial bursa. | Rescue analgesia was permitted for both groups and participants recorded their intake of nonsteroidal anti‐inflammatory drugs and paracetamol until the end of the study. New infiltrations after two weeks were tolerated but considered a poor result. | There were no serious adverse events in either group. 3/32 in the ultrasound‐guided group and 3/35 in the ‘blind’ injection group required a second local injection after 2 or 6 weeks. Four patients reported flushing and one diabetic patient a transient hyperglycaemia, however, there were no details of which groups these participants belonged to. |
MHz = megahertz; mL = millilitre; mg = milligrams; cm = centimetre; T6 = sixth thoracic vertebrae; cc = cubic centimetre; mmol = millimole; ROM = range of motion; SA‐SD bursa = subacromial‐subdeltoid bursa; 21‐G = 21 gauge; NSAIDs = non‐steroidal anti‐inflammatory drugs
Image guidance in all trials utilised ultrasound. In four trials, ultrasound was used to direct the injection to the site of observed pathology (Naredo 2004; Saeed 2014; Ucuncu 2009; Zufferey 2012), while in the other trials the ultrasound‐guided injection was into either the subacromial bursa (Akbari 2020; Azadvari 2020; Bhayana 2018; Chen 2006; Cho 2010; Cole 2016; Dogu 2012; Ekeberg 2009; Haghighat 2015; Hsieh 2013; Roddy 2020) or glenohumeral joint (Cho 2021; Lee 2009; Lee 2015; Raeissadat 2017).
The landmark‐guided injections in the comparator groups were into the subacromial space (Akbari 2020; Azadvari 2020; Bhayana 2018; Chen 2006; Cho 2010; Cole 2016; Dogu 2012; Haghighat 2015; Hsieh 2013; Naredo 2004; Roddy 2020; Saeed 2014; Ucuncu 2009; Zufferey 2012) or glenohumeral joint (Cho 2021; Lee 2009, Lee 2015 and Raeissadat 2017), except for one trial that compared the ultrasound‐guided injection into the subacromial bursa to an intramuscular injection in the upper gluteal region (Ekeberg 2009).
The dose and type of glucocorticoid and local anaesthetic used varied between studies. Three studies used 20 mg of triamcinolone (Ekeberg 2009; Lee 2009; Naredo 2004) and four trials used 40 mg of triamcinolone (Cho 2021; Lee 2015; Raeissadat 2017; Ucuncu 2009). Five studies used 40 mg of methylprednisolone (Akbari 2020; Cole 2016; Haghighat 2015; Roddy 2020; Saeed 2014) and one study used 80 mg of methylprednisolone (Bhayana 2018). One study used 7 mg of betamethasone (Zufferey 2012), one study used 1 mL of methylprednisolone (Azadvari 2020), one study used 1 mL of betamethasone (dosage not specified) (Chen 2006) and one study used 5 mg of betamethasone with 0.02 mL of 0.01 mmol gadolinium diethylenetriaminepentaacetic (Dogu 2012). One study used 2.5 mg of dexamethasone (Hsieh 2013) and one study used 5 mL of triamcinolone (dosage not specified) (Cho 2010).
Co‐interventions:
Co‐interventions were reported in 12 trials. Nine trials gave all participants a home‐exercise programme (Azadvari 2020; Bhayana 2018; Cho 2021; Lee 2009; Lee 2015; Naredo 2004; Raeissadat 2017; Saeed 2014; Ucuncu 2009), eight allowed participants to have analgesia as required (Bhayana 2018; Cole 2016; Dogu 2012; Ekeberg 2009; Naredo 2004; Saeed 2014; Ucuncu 2009; Zufferey 2012), one trial allowed physical therapy as required (Cole 2016), one allowed participants to continue with physical therapy if they were receiving it at baseline (Ekeberg 2009), one encouraged participants to take a naproxen tablet 500 mg twice daily for five days (Raeissadat 2017), and one provided all participants with antibiotics to prevent infection (Bhayana 2018). All participants in Lee 2009 also received intra‐articular injections of 25 mg low molecular weight hyaluronic acid weekly for five weeks.
Outcomes
Major outcomes
Pain
Seventeen trials assessed pain. Pain was assessed on a 10 cm VAS in 12 trials (Akbari 2020; Azadvari 2020; Cho 2010; Cho 2021; Dogu 2012; Haghighat 2015; Hsieh 2013; Lee 2009; Lee 2015; Raeissadat 2017; Saeed 2014; Ucuncu 2009), 100 cm VAS in three trials (Bhayana 2018; Cole 2016; Naredo 2004), a zero to 10‐point numerical rating scale (NRS) in two trials (Roddy 2020; Zufferey 2012) and one to nine‐point ordinal scale in one trial (Ekeberg 2009). Three trials measured pain at rest and with activity (Dogu 2012; Ekeberg 2009; Zufferey 2012) and three during sleep (Cole 2016; Dogu 2012; Naredo 2004). The following pain measures were only reported in single trials: pain in daytime and just before sleep (Lee 2009), pain in the previous week (Naredo 2004), pain during Hawkin’s impingement test (Cho 2010), pain with overhead activities (Cole 2016), current pain (Roddy 2020) and the frequency of pain during activity, during sleep and extreme pain (Cole 2016). Nine trials did not specify the context in which pain was measured (Akbari 2020; Azadvari 2020; Bhayana 2018;Cho 2021; Haghighat 2015; Hsieh 2013; Lee 2015; Raeissadat 2017; Saeed 2014; Ucuncu 2009).
Function
Eighteen trials assessed function. Six trials measured function using the Constant score (Akbari 2020; Azadvari 2020; Bhayana 2018; Dogu 2012; Ucuncu 2009; Zufferey 2012). Zufferey 2012 excluded the last item on strength and Dogu 2012 reported scores separately for the following domains: range of motion, general pain and activities of daily living. Four studies used the Shoulder Pain And Disability Index (SPADI) (Ekeberg 2009; Haghighat 2015; Hsieh 2013; Roddy 2020), two used the Shoulder Disability Questionnaire (SDQ) (Dogu 2012; Hsieh 2013) and three used the American Shoulder and Elbow Surgeons (ASES) score (Cho 2021; Cole 2016; Lee 2015). The following measures of function were only reported in single studies: Western Ontario Rotator Cuff Index (Ekeberg 2009), Shoulder Function Assessment (SFA) scale (Naredo 2004), Disability of Arm Shoulder and Hand (DASH) scale (Akbari 2020), subjective shoulder value (Cho 2021), functional activities of the shoulder (Lee 2009), Oxford questionnaire (Raeissadat 2017), University of California‐Los Angeles (UCLA) shoulder rating scale (Cho 2010), and Shoulder Function Tests (SFTs) score (Saeed 2014).
Zufferey 2012 did not report data on function at 12 weeks.
Participant‐rated treatment success
Five trials included a dichotomous measure of treatment success (Cole 2016; Naredo 2004; Roddy 2020; Ucuncu 2009; Zufferey 2012). Cole 2016 measured the number of participants with 50% improvement in pain during internal and external rotation impingement tests. Naredo 2004 measured the number of participants with 50% improvement in pain and SFA score. Roddy 2020 measured the number of participants who had completely recovered or felt much better. Ucuncu 2009 and Zufferey 2012 measured the number of participants with 50% improvement in pain scores.
Health‐related quality of life
Two trials measured health‐related quality of life using the Short‐Form Health Survey (SF‐36) Mental Component Score (Hsieh 2013; Roddy 2020). Roddy 2020 also measured the SF‐36 Physical Component Score. Azadvari 2020 used the BREF questionnaire (no further description was reported; we assumed it was the World Health Organization WHOQOL‐BREF quality of life assessment).
Adverse events
Twelve trials reported on adverse events (Akbari 2020; Bhayana 2018; Cho 2021; Dogu 2012; Ekeberg 2009; Lee 2015; Naredo 2004; Raeissadat 2017; Roddy 2020; Saeed 2014; Ucuncu 2009; Zufferey 2012). Of these, five trials did not specify how they assessed adverse events but reported them in the results (Akbari 2020; Bhayana 2018; Cho 2021; Lee 2015; Raeissadat 2017).
Serious adverse events
Five trials (Ekeberg 2009; Naredo 2004; Saeed 2014; Ucuncu 2009; Zufferey 2012) reported that there were no serious side effects. The remaining trials did not report the incidence of serious adverse events.
Withdrawals due to adverse events
One trial reported withdrawals due to adverse events (Ekeberg 2009).
Minor outcomes
Additional treatment or surgery
Three trials reported whether participants received additional injections (Cole 2016; Ekeberg 2009; Zufferey 2012).
One trial reported the number of participants that went on to undergo surgery at the end of the study (Cole 2016).
Range of motion
Sixteen out of 19 trials included at least one measure of shoulder range of motion (excluding Azadvari 2020; Dogu 2012; Roddy 2020). Bhayana 2018, Naredo 2004 and Saeed 2014 measured range of motion but did not report their results. Saeed 2014 did not report what movements were measured. Akbari 2020 did not report SD for their measures of range of motion.
Flexion
Ten trials measured range of shoulder flexion. Active flexion was measured in six trials (Akbari 2020; Cho 2010; Ekeberg 2009; Hsieh 2013; Naredo 2004; Ucuncu 2009) and passive flexion in six (Cho 2021; Cole 2016; Hsieh 2013; Lee 2009; Naredo 2004; Ucuncu 2009). Haghighat 2015, Lee 2015 and Raeissadat 2017 did not specify whether flexion was measured actively or passively.
Abduction
Ten trials measured range of shoulder abduction. Active abduction was measured in six trials (Akbari 2020; Ekeberg 2009; Hsieh 2013; Naredo 2004; Ucuncu 2009; Zufferey 2012) and passive abduction in seven (Cho 2021; Cole 2016; Hsieh 2013; Lee 2009; Naredo 2004; Ucuncu 2009; Zufferey 2012). Chen 2006, Haghighat 2015 and Raeissadat 2017 did not specify whether abduction was measured actively or passively.
External rotation
Nine trials measured shoulder external range of motion. Active external rotation was measured in four trials (Cho 2010; Hsieh 2013; Naredo 2004; Zufferey 2012) and passive external rotation in six (Cho 2021; Cole 2016; Hsieh 2013; Lee 2009; Naredo 2004; Zufferey 2012). Haghighat 2015, Lee 2015 and Raeissadat 2017 did not specify whether external rotation was measured actively or passively. Lee 2015 assessed external rotation with the arm by the side and at 90 degrees of abduction. We only considered shoulder external range of motion measures relevant for the three trials including participants with adhesive capsulitis (Lee 2009; Lee 2015; Raeissadat 2017).
Excluded studies
We excluded 17 studies in our original review after full‐text review and excluded an additional six studies for this update (see Characteristics of excluded studies table). For the original 17 studies, seven studies were excluded because they were not randomised (Alfredson 2006; Bamji 2004; Esenyel 2003; Gutierrez 2004; Koes 2009; McCormack 2009; Yi 2006); two studies did not include the participants of interest (Cohen 2009; Sibbitt 2009); four studies did not include the interventions of interest (Buchbinder 2004; Henkus 2006; Kang 2008; Widiastuti‐Samekto 2004); two studies did not include the comparator of interest (Chavez‐Lopez 2009; Tveita 2008); and one study only considered accuracy of needle placement (Rutten 2007). One study was excluded because data were not presented separately for the treatment groups of interest (Valtonen 1978).
Of the six trials excluded from the update, two were not randomised (Hashiuchi 2010; Micu 2013); two did not compare image‐guided to non‐image‐guided injection (Kim 2016; Kim 2017); one did not include participants of interest (Zhang 2011 included participants with biceps brachii tendinitis); and one did not include the intervention of interest (Graber 1997).
Risk of bias in included studies
The assessment of each domain of risk of bias for the included trials is summarised in the Characteristics of included studies table. The results of the risk of bias assessment are also presented graphically in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Only two trials met all low risk of bias criteria (Cho 2021; Ekeberg 2009). The most notable sources of bias in the remaining trials included lack of blinding of participants and personnel (performance bias) which affected all but three trials (Cole 2016; Dogu 2012; Lee 2009) and lack of blinding of outcome assessment for self‐reported outcomes (detection bias) which affected all but four trials (Chen 2006; Cole 2016; Dogu 2012; Lee 2009).
Allocation
Two trials reported adequate random sequence generation and allocation concealment, and were deemed to have low risk of selection bias (Cho 2021; Ekeberg 2009). Two trials had inadequate random sequence generation and allocation concealment, and were deemed to have high risk of bias (Chen 2006; Lee 2009). Five trials were judged to have unclear risk of selection bias due to failure to report their method of randomisation (Bhayana 2018; Haghighat 2015; Naredo 2004; Roddy 2020; Ucuncu 2009) and thirteen trials were judged to have unclear risk of selection bias due to failure to report their method of allocation concealment (Bhayana 2018; Cho 2010; Cole 2016; Dogu 2012; Haghighat 2015; Hsieh 2013; Lee 2015; Naredo 2004; Raeissadat 2017; Roddy 2020; Saeed 2014; Ucuncu 2009; Zufferey 2012).
Blinding
Only five trials reported adequate blinding of participants and personnel, and were deemed to have low risk of performance bias (Cho 2021; Cole 2016; Dogu 2012; Ekeberg 2009; Lee 2009). Blinding of participants was achieved in three trials by applying the ultrasound probe to the shoulder in both treatment groups but without using it as an aid to injection in the non‐image‐guided groups. In Ekeberg 2009, all participants received an ultrasound‐guided shoulder injection as well as an upper gluteal injection but the interventions could have been either short‐acting local anaesthetic alone or combined with glucocorticoid.
Fourteen trials were judged to have high risk of performance bias due to inadequate blinding (Akbari 2020; Azadvari 2020; Bhayana 2018; Chen 2006; Cho 2010; Haghighat 2015; Hsieh 2013; Lee 2015; Naredo 2004; Raeissadat 2017; Roddy 2020; Saeed 2014; Ucuncu 2009; Zufferey 2012).
Only five trials had adequate blinding for self‐reported outcomes (Cho 2021; Cole 2016; Dogu 2012; Ekeberg 2009; Lee 2009) and were considered to be at low risk of detection bias for these outcomes. An additional trial that did not blind participants did not include self‐reported outcomes so was also considered at low risk (Chen 2006). Six trials were judged to have adequate blinding for assessor‐reported outcomes (Chen 2006; Cho 2021; Cole 2016; Dogu 2012; Ekeberg 2009; Lee 2009) and were deemed to have low risk of detection bias for these outcomes.
Incomplete outcome data
Risk of attrition bias was low in 15 trials (Akbari 2020; Azadvari 2020; Bhayana 2018; Cho 2010; Cho 2021; Cole 2016; Dogu 2012; Ekeberg 2009; Hsieh 2013; Lee 2009; Lee 2015; Raeissadat 2017; Roddy 2020; Saeed 2014; Ucuncu 2009).
In Azadvari 2020, Bhayana 2018, Cho 2010, Cole 2016, Dogu 2012, Ucuncu 2009 and Raeissadat 2017, there was no loss to follow‐up. In Akbari 2020, all 14 participants in the ultrasound‐guided injection group completed follow‐up, while one of 15 (7%) participants in the non‐image‐guided injection group was lost at final follow‐up. In Cho 2021, 4/45 (9%) in the ultrasound‐guided injection group and 6/45 (13%) in the non‐image‐guided injection group were lost at final follow‐up. In Ekeberg 2009, all 53 participants who received ultrasound‐guided injection completed follow‐up, while 2/53 (4%) in the group who received systemic glucocorticoid were lost to follow‐up at six weeks. In Hsieh 2013, 2/48 (4%) in both the ultrasound‐guided and non‐image‐guided injection groups were lost to follow‐up at one month follow‐up. In Lee 2009, 2/22 (9%) in the non‐image‐guided injection group and 1/21 (5%) in the ultrasound‐guided injection group were lost to follow‐up at six weeks, although the number of participants with available data at one week (main end point) was unknown. In Lee 2015, 7/45 (15%) in the ultrasound‐guided injection group and 6/45 (13%) in the non‐image‐guided injection group were lost at final follow‐up. In Roddy 2020, 8/64 (13%) in the ultrasound‐guided injection group and 7/64 (11%) in the non‐image‐guided injection group were lost at 12 months follow‐up. In Saeed 2014, 11/50 (22%) in the non‐image‐guided injection group and 9/50 (18%) in the ultrasound‐guided injection group did not complete the 12‐week assessment and were excluded from the study after the six‐week assessments; all withdrawals were due to the need for a repeat injection or referral for surgery.
Risk of attrition bias was unclear in four trials (Chen 2006; Haghighat 2015; Naredo 2004; Zufferey 2012). Three trials did not provide any information on the number of participants with available data at any time point (Chen 2006; Haghighat 2015; Naredo 2004). In Zufferey 2012, 1/36 (3%) and 7/36 (19%) in the non‐image‐guided injection group and 2/34 (6%) and 7/34 (21%) in the ultrasound‐guided injection group were lost to follow‐up at six weeks (primary end point) and 12 weeks, respectively.
Selective reporting
Risk of reporting bias was low in seven trials (Cho 2021; Cole 2016; Dogu 2012; Ekeberg 2009; Haghighat 2015; Raeissadat 2017; Ucuncu 2009) as all proposed outcomes were reported in the results. Risk of reporting bias was high in two trials (Naredo 2004; Saeed 2014) as not all measured shoulder movements were reported in the results. Further, we could not include data for function from one trial (Saeed 2014) as we were unable to obtain an SD. Risk of reporting bias was unclear in ten trials (Akbari 2020; Azadvari 2020; Bhayana 2018; Chen 2006; Cho 2010; Hsieh 2013; Lee 2009; Lee 2015; Roddy 2020; Zufferey 2012). All of them other than Roddy 2020 were not prospectively registered. Roddy 2020 did not report on all outcomes in the published paper that were to be measured according to the protocol. Lee 2009 stated that means and SDs were reported for continuous outcomes, but the SDs seemed very small in comparison with the reported SDs in other trials, raising the possibility that these were standard errors.
Other potential sources of bias
All but one trial was deemed to be at low risk of other potential sources of bias (Haghighat 2015). In Haghighat 2015, baseline function was higher (worse) in the ultrasound‐guided injection group, which may have biased the result in favour of this intervention.
Effects of interventions
See: summary of findings Table 1 for efficacy of ultrasound‐guided injection versus non‐image‐guided injection for shoulder pain.
Benefits
Overall pain
We pooled data from 18 trials. Nine trials reported pain up to three weeks (Azadvari 2020; Bhayana 2018; Cho 2010; Cho 2021; Ekeberg 2009; Hsieh 2013; Lee 2009; Raeissadat 2017; Zufferey 2012), 15 from > three weeks to six weeks (Akbari 2020; Bhayana 2018; Cho 2021; Cole 2016; Dogu 2012; Ekeberg 2009; Haghighat 2015; Hsieh 2013; Lee 2009; Naredo 2004; Raeissadat 2017; Roddy 2020; Saeed 2014; Ucuncu 2009; Zufferey 2012), seven from > six weeks to three months (Azadvari 2020; Bhayana 2018; Cho 2021; Lee 2009; Lee 2015; Saeed 2014; Zufferey 2012), two trials from > three months to six months (Lee 2015; Roddy 2020) and two trials at > six months (Lee 2015; Roddy 2020). Azadvari reported that mean (SD) pain was 0.0 (0.0) at two months follow‐up, and thus we could not include these data in our > six weeks to three months analysis. Statistical heterogeneity was low to considerable (I2 = 91% up to three weeks, I2 = 74% from > three weeks to six weeks, I2 = 0% from > six weeks to three months, I2= 0% from > three months to six months, and I2 = 0% at > six months).
Moderate‐certainty evidence (downgraded for risk of bias) indicates that injection using ultrasound guidance probably has little to no effect on pain at > three weeks to six weeks when compared to injection performed without image guidance (Analysis 1.1; summary of findings Table 1). Mean pain (0 to 10, lower is better) was 3.1 points with injection not guided by imaging and 0.5 points better (lower) (95% CI 0.2 to 0.8 points better); 15 studies, 1003 participants) with an ultrasound‐guided injection. Repeating this analysis, but excluding Lee 2009, based upon uncertainty about whether SEs rather than SDs were reported, did not appreciably alter the results (mean difference ‐0.6 points lower (95% CI 0.9 points lower to 0.2 points lower)). A funnel plot for data from > three weeks to six weeks did not indicate publication bias (Figure 3).

Funnel plot of comparison: 1 Ultrasound‐guided injection versus non‐image‐guided injection, outcome: 1.1 Overall pain (or daytime, activity‐related or unspecified).
Up to three weeks, mean pain was 2.7 with a non‐image‐guided injection and 0.7 points better (95% CI 0.0 better to 1.3 better; 554 participants) with an ultrasound‐guided injection. From > six weeks to three months, mean pain was 2.8 with a non‐image‐guided injection and 0.0 points better (95% CI 0.1 worse to 0.2 points better; 454 participants) with an ultrasound‐guided injection. From > three months to six months, mean pain was 3.4 with a non‐image‐guided injection and 0.6 points better (95% CI 0.3 points worse to 1.5 points better; 205 participants) with an ultrasound‐guided injection. At > six months, mean pain was 3.2 with a non‐image‐guided injection and 0.2 points better (95% 1.1 points worse to 0.7 points better; 205 participants) with an ultrasound‐guided injection.
Function
We pooled data from 17 trials. Eight trials reported function up to three weeks (Azadvari 2020; Bhayana 2018; Cho 2010; Cho 2021; Ekeberg 2009; Hsieh 2013; Lee 2009; Raeissadat 2017), 14 from > three weeks to six weeks (Akbari 2020; Bhayana 2018; Cho 2021; Cole 2016; Dogu 2012; Ekeberg 2009; Haghighat 2015; Hsieh 2013; Lee 2009; Naredo 2004; Raeissadat 2017; Roddy 2020; Ucuncu 2009; Zufferey 2012), four from > six weeks to three months (Azadvari 2020; Bhayana 2018; Cho 2021; Lee 2015), two from > three months to six months (Lee 2015; Roddy 2020) and two from > six months (Lee 2015; Roddy 2020). Statistical heterogeneity was low to considerable (I2 = 92% up to three weeks, I2 = 61% from three weeks to six weeks, I2 = 97% from six weeks to three months, I2 = 0 from three months to six months, and I2 = 0 at > six months).
Moderate‐certainty evidence (downgraded for risk of bias) indicates that ultrasound‐guided injection probably has little to no effect on function at > three to six weeks when compared to injection that does not use image guidance (Analysis 1.2; summary of findings Table 1). Mean function was 68 points on a 0 to 100‐point scale (a higher score is better) with an injection not guided by imaging and 2.4 points better (95% CI 0.2 points worse to 5.1 points better; 895 participants) with an ultrasound‐guided injection. A funnel plot for data from > three weeks to six weeks did not indicate publication bias (Figure 4).

Funnel plot of comparison: 1 Ultrasound‐guided injection versus non‐image‐guided injection, outcome: 1.2 Function.
Up to three weeks, mean function was 72 points with non‐image‐guided injection and 5.5 points better (95% CI 0.3 points worse to 11.2 points better; 487 participants) with an ultrasound‐guided injection. From > six weeks to three months, mean function was 77.4 points with a non‐image‐guided injection and 9.2 points better (95% CI 5.8 points worse to 24.1 points better; 257 participants) with an ultrasound‐guided injection. Removing data from Azadvari 2020 reduced the I2 to 0 and changed the effect estimate to 3.7 points better (95% CI: 0.5 points better to 6.9 points better; 227 participants). From > three months to six months, mean function was 73.5 points with non‐image‐guided injection and 1.1 points worse (95% 9.3 points worse to 7.2 points better; 205 participants) with an ultrasound‐guided injection. From > six months, mean pain was 72.8 with a non‐image‐guided injection and 3.4 points better (95% CI 4.7 points worse to 11.4 points better; 205 participants) with an ultrasound‐guided injection.
Participant‐rated treatment success
We pooled data from five trials that measured participant‐rated treatment success using a dichotomous outcome (Cole 2016; Naredo 2004; Roddy 2020; Ucuncu 2009; Zufferey 2012) (Analysis 1.3). Statistical heterogeneity was substantial (I2 = 77%). Ultrasound‐guided injection may reduce, increase or have little to no effect on participant‐rated treatment success (at end of study) when compared to non‐image guided injection as the evidence is very uncertain (very low‐quality evidence, downgraded for risk of bias, inconsistency and imprecision), although the 95% CI includes a possible benefit. One hundred and one out of 175 (60.6%) in the ultrasound‐guided group reported treatment success compared with 68/175 (38.9%) in the group who received a non‐image‐guided injection; RR 1.56, 95% CI: 0.89 to 2.75, an absolute difference of 22% (4% fewer to 62% more) and relative difference of 56% (11% fewer to 175% more).
Health‐related quality of life
Three trials measured health‐related quality of life (Azadvari 2020; Hsieh 2013; Roddy 2020).Two trials measured health‐related quality of life up to three weeks (Azadvari 2020; Hsieh 2013), two from > three weeks to six weeks (Hsieh 2013; Roddy 2020), one from > six weeks to three months (Azadvari 2020), and one from > three months to six months and > six months (Roddy 2020). Statistical heterogeneity was low (I2 = 0% from three weeks to six weeks).
Low‐certainty evidence (downgraded for risk of bias and imprecision) indicates that ultrasound‐guided injections may have little to no effect on quality of life at > three weeks to six weeks when compared to injections performed without image guidance (Analysis 1.4; summary of findings Table 1). Mean quality of life was 65.0 points on a 0 to 100 scale (higher is better quality of life) with a non‐image‐guided injection and 2.8 points better (95% CI 0.7 worse to 6.4 points better; 220 participants) with an ultrasound‐guided injection.
Up to three weeks, mean quality of life was 67.0 points with a non‐image‐guided injection and 4.2 points better (95% CI 1.0 point worse to 9.3 points better; 122 participants) with an ultrasound‐guided injection. From > six weeks to three months, mean quality of life was 64.0 points with a non‐image‐guided injection and 4.6 points better (95% CI 1.3 points worse to 10.5 points better; 30 participants) with an ultrasound‐guided injection. From > three months to six months, mean quality of life was 64 points with a non‐image‐guided injection and 1.6 points better (95% CI 2.5 points worse to 5.7 points better; 128 participants) with an ultrasound‐guided injection. From > six months, mean quality of life was 64 points with a non‐image‐guided injection and 0.7 points worse (95% CI 4.9 points worse to 3.5 points better; 128 participants) with an ultrasound‐guided injection.
Harms
Adverse events
Twelve studies reported on adverse events (Akbari 2020; Bhayana 2018; Cho 2021; Dogu 2012; Ekeberg 2009; Lee 2015; Naredo 2004; Raeissadat 2017; Roddy 2020; Saeed 2014; Ucuncu 2009; Zufferey 2012). Seven trials reported that there were no adverse events (Akbari 2020; Bhayana 2018; Cho 2021; Dogu 2012; Lee 2015; Raeissadat 2017; Saeed 2014). We pooled data from the remaining five studies that reported numbers of adverse events (Ekeberg 2009; Naredo 2004; Roddy 2020; Ucuncu 2009; Zufferey 2012). There was low statistical heterogeneity (I2 = 15%). Low‐certainty evidence (downgraded for risk of bias and imprecision ‐ low event rates) indicates that ultrasound‐guided injection may not reduce the risk of adverse events compared to non‐image‐guided injection (Analysis 1.5). Thirty‐eight out of 200 (18%) in the ultrasound‐guided group reported adverse events compared with 51/202 (25%) in the non‐image‐guided injection group; RR 0.72 (95% CI 0.4 to 1.28), an absolute difference of 7% fewer adverse events (15% fewer to 7% more) and a relative difference of 28% fewer events (60% fewer to 28% more).
Withdrawals due to adverse events
Only one trial reported withdrawals due to adverse events (Ekeberg 2009): 1/53 in the systemic injection group withdrew (and received an additional local injection at two weeks), and 0/53 in the ultrasound‐guided group withdrew (Analysis 1.6).
Serious adverse events
Five trials (Ekeberg 2009; Naredo 2004; Saeed 2014; Ucuncu 2009; Zufferey 2012) reported that there were no serious side effects. One trial (Roddy 2020) reported that there was one case of pyelonephritis (1/64; 2%) in the ultrasound‐guided injection group. The remaining trials did not report the incidence of serious adverse events.
Minor outcomes
Additional injections
We pooled data from three trials that reported whether participants received additional injections (Cole 2016; Ekeberg 2009; Zufferey 2012). Statistical heterogeneity was low (I2 = 9%). Low‐certainty evidence (downgraded for risk of bias and imprecision) indicates that ultrasound‐guided injection probably does not reduce the need for additional injections compared to non‐image‐guided injection (Analysis 1.7). Three out of 113 (2.7%) in the ultrasound‐guided group required an additional injection compared with 8/116 (6.9%) in the non‐image‐guided injection group; RR 0.56, 95% CI: 0.14 to 2.15, an absolute difference of 3.2% fewer additional injections with ultrasound‐guided injection (6.0% fewer to 8.0% more) and relative difference of 44% fewer (86% fewer to 115% more).
Surgery
One trial (Cole 2016) reported that 4/28 (14.3%) participants who received an ultrasound‐guided injection needed surgery, compared to 6/28 (21.4%) participants who received a non‐image‐guided injection. Low‐certainty evidence (downgraded for risk of bias and imprecision) indicates that ultrasound‐guided injection probably does not reduce the need for surgery compared to non‐image‐guided injection (Analysis 1.7); RR 0.67, 95% CI: 0.21 to 2.11, an absolute difference of 7.2% fewer surgeries with ultrasound‐guided injection (17.5% fewer to 18.7% more) and relative difference of 33% (79% fewer to 111% more).
Range of shoulder flexion
We pooled data from 10 trials. Five trials reported shoulder flexion up to three weeks (Cho 2010; Cho 2021; Ekeberg 2009; Lee 2009; Raeissadat 2017); eight trials reported this outcome from > three weeks to six weeks (Akbari 2020; Cho 2021; Cole 2016; Ekeberg 2009; Haghighat 2015; Lee 2009; Raeissadat 2017; Ucuncu 2009), two trials reported this outcome from > six weeks to three months (Cho 2021; Lee 2015); one trial reported this outcome from > three months to six months (Lee 2015); and one trial reported this outcome at > six months (Lee 2015). Statistical heterogeneity was substantial to low (I2 = 64% up to three weeks, I2 = 27% from three weeks to six weeks, and I2 = 0% from six weeks to three months).
Low‐certainty evidence (downgraded for risk of bias and indirectness) indicates that ultrasound‐guided injection provides a negligible improvement in shoulder flexion at > three to six weeks when compared to non‐image‐guided injection (Analysis 1.8). Mean shoulder flexion was 140.5 degrees with a non‐image‐guided injection and 1.1 degrees better (95% CI 2.3 degrees worse to 4.5 degrees better; 461 participants) with an ultrasound‐guided injection.
Up to three weeks, mean shoulder flexion was 141.6 degrees with a non‐image‐guided injection and 3.5 degrees better (95% CI 1.6 degrees worse to 8.5 degrees better, 305 participants; normal range of flexion 180 degrees) with an ultrasound‐guided injection. From six weeks to three months, mean shoulder flexion was 136.9 degrees with a non‐image‐guided injection and 3.7 degrees better (95% 1.42 worse to 8.82 better; 167 participants) with an ultrasound‐guided injection. From > three months to six months, mean shoulder flexion was 142.7 degrees with a non‐image‐guided injection and 0.5 degrees better (95% 5.5 degrees worse to 6.5 degrees better; 77 participants) with an ultrasound‐guided injection. At > six months, mean shoulder flexion was 142.2 degrees with a non‐image‐guided injection and 4.0 degrees better (95% 4.5 degrees worse to 12.5 degrees better; 77 participants) with an ultrasound‐guided injection.
Range of shoulder abduction
We pooled data from 10 trials. Five trials reported shoulder abduction range of motion up to three weeks (Chen 2006; Cho 2021; Ekeberg 2009; Lee 2009; Raeissadat 2017), nine from three weeks to six weeks (Akbari 2020; Cho 2021; Cole 2016; Ekeberg 2009; Haghighat 2015; Lee 2009; Raeissadat 2017; Ucuncu 2009; Zufferey 2012), and one from six weeks to three months (Cho 2021). Statistical heterogeneity was substantial (I2 = 87% up to three weeks, I2 = 60% from three weeks to six weeks).
Low‐certainty evidence (downgraded due to risk of bias and indirectness) indicates that ultrasound‐guided injection may provide a slight improvement in shoulder abduction at > three to six weeks when compared to non‐image‐guided injection (Analysis 1.9). From > three to six weeks, mean shoulder abduction was 116.3 degrees with a non‐image‐guided injection and 6.9 degrees better (95% CI 1.5 degrees better to 12.2 degrees better; 528 participants) with an ultrasound‐guided injection.
Up to three weeks, mean shoulder abduction was 122 degrees with a non‐image‐guided injection and 12.6 degrees better (95% CI 2.6 to 22.5 degrees better, 317 participants) with an ultrasound‐guided injection. From > six weeks to three months, mean shoulder abduction was 1.1 degrees better (95% CI 8.5 degrees worse to 10.7 degrees better; 90 participants) with an ultrasound‐guided injection.
Range of shoulder external rotation
We pooled data from eight trials which assessed shoulder external rotation range of motion. Four trials reported shoulder external rotation up to three weeks (Cho 2010; Cho 2021; Lee 2009; Raeissadat 2017), six from > three to six weeks (Cho 2021; Cole 2016; Haghighat 2015; Lee 2009; Raeissadat 2017; Zufferey 2012), two from > six weeks to three months (Cho 2021; Lee 2015), one from > three months to six months (Lee 2015) and one at > six months (Lee 2015). Statistical heterogeneity was substantial to low (I2 = 71% up to three weeks, I2 = 19% from three weeks to six weeks, and I2 = 0% from six weeks to three months).
Low‐certainty evidence (downgraded due to risk of bias and indirectness) indicates that ultrasound‐guided injection may provide no improvement in shoulder external rotation at > three to six weeks when compared to non‐image‐guided injection (Analysis 1.10). From > three weeks to six weeks, mean shoulder abduction was 68.8 degrees with a non‐image‐guided injection and 0.7 degrees worse (95% CI 3.5 degrees worse to 2.0 degree better; 334 participants) with an ultrasound‐guided injection.
Up to three weeks, mean shoulder external rotation was 53.2 degrees with a non‐image‐guided injection and 1.0 degrees better (95% CI 3.9 degrees worse to 5.9 degrees better; 199 participants) with an ultrasound‐guided injection. From > six weeks to three months, mean shoulder external rotation was 77.6 degrees with a non‐image‐guided injection and 3.9 degrees better (95% 0.4 degrees worse to 8.2 degrees better; 167 participants) with an ultrasound‐guided injection. From > three months to six months, mean shoulder external rotation was 79.2 degrees with a non‐image‐guided injection and 3.2 degrees better (95% 8.1 degrees worse to 14.5 degrees better; 77 participants) with an ultrasound‐guided injection. From > six months, mean shoulder external rotation was 87.1 degrees with a non‐image‐guided injection and 2.7 degrees worse (95% 13.3 degrees worse to 7.9 degrees better; 77 participants) with an ultrasound‐guided injection.
Subgroup analysis
See Analysis 2.1 and Analysis 2.2. Fourteen trials included participants that could be categorised as having rotator cuff disease (Akbari 2020; Azadvari 2020; Bhayana 2018; Chen 2006; Cho 2010; Cole 2016; Dogu 2012; Ekeberg 2009; Haghighat 2015; Hsieh 2013; Naredo 2004; Roddy 2020; Saeed 2014; Ucuncu 2009). Four trials included participants with adhesive capsulitis (Cho 2021; Lee 2009; Lee 2015; Raeissadat 2017). One trial (Zufferey 2012) included participants with acute shoulder pain and did not provide a more specific diagnostic label. Stratifying the results for pain and function at > three to six weeks (primary time point) by shoulder condition did not appreciably alter the results; the test for subgroup differences indicated there were unlikely to be differences in pain and function across different shoulder conditions.
Pain
We pooled data from 12 trials of participants with rotator cuff disease (Akbari 2020; Azadvari 2020; Bhayana 2018; Cole 2016; Dogu 2012; Ekeberg 2009; Haghighat 2015; Hsieh 2013; Naredo 2004; Roddy 2020; Saeed 2014; Ucuncu 2009), three trials of participants with adhesive capsulitis (Cho 2021; Lee 2009; Raeissadat 2017) and one trial of participants with mixed or undefined shoulder pain (Zufferey 2012). Statistical heterogeneity was low to substantial (I2 = 79% for rotator cuff disease, I2 = 0% for adhesive capsulitis). In participants with rotator cuff diease, mean pain was 3.1 points with a non‐image‐guided injection and 0.6 points better (95% CI 0.1 to 1.05 points better; 777 participants) with an ultrasound‐guided injection; in participants with adhesive capsulitis, mean function was 1.1 points with a non‐image‐guided injection and 0.2 points better (95% CI 0.0 points to 0.4 points better; 189 participants) with an ultrasound‐guided injection; and in participants with mixed or undefined shoulder pain, mean function was 4.2 points with a non‐image‐guided injection and 1.2 points better (95% CI 0.1 points worse to 2.5 points better; 65 participants) with an ultrasound‐guided injection.
Function
We pooled data from 11 trials of participants with rotator cuff disease (Akbari 2020; Azadvari 2020; Bhayana 2018; Cole 2016; Dogu 2012; Ekeberg 2009; Haghighat 2015; Hsieh 2013; Naredo 2004; Roddy 2020 ; Ucuncu 2009), three trials of participants with adhesive capsulitis (Cho 2021; Lee 2009; Raeissadat 2017) and one trial of participants with mixed or undefined shoulder pain (Zufferey 2012). Statistical heterogeneity was substantial to low (I2 = 94% for rotator cuff disease, I2 = 0% for adhesive capsulitis).
In participants with rotator cuff disease, mean function was 68 points with a non‐image‐guided injection and 5.1 points better (95% CI 3.2 points worse to 13.4 points better; 687 participants) with an ultrasound‐guided injection; in participants with adhesive capsulitis, mean function was 92.8 points with a non‐image‐guided injection and 1.1 points better (95% CI 1.2 points worse to 3.3 points better; 171 participants) with an ultrasound‐guided injection; and in participants with mixed or undefined shoulder pain, mean function was 47 points with a non‐image‐guided injection and 4.0 points better (95% CI 0.1 to 8.0 points better; 65 participants) with an ultrasound‐guided injection.
Sensitivity analysis
We performed a sensitivity analysis to investigate the robustness of pain and function (at > three to six weeks) to the presence of selection and detection biases.
Selection bias
Four trials were judged to be at low risk of selection bias (Akbari 2020; Azadvari 2020; Cho 2021; Ekeberg 2009).
Estimates for pain and function including all trials with eligible data may have been slightly influenced by the presence of selection bias. Mean pain was 3.1 with a non‐image‐guided injection and 0.3 points better (95% CI 0.2 points worse to 0.8 points better, 224 participants) with an ultrasound‐guided injection when pooling the three trials with low risk of selection bias who provided eligible data at this time point (Akbari 2020; Cho 2021; Ekeberg 2009). There was no statistical heterogeneity (I2 = 0%). Mean function was 68 with a non‐image‐guided injection and 0.8 points better (95% CI 7.1 points worse to 8.6 points better, 124 participants) with an ultrasound‐guided injection when pooling the three trials with low risk of selection bias that provided eligible data at this time point (Akbari 2020; Cho 2021; Ekeberg 2009). Statistical heterogeneity was moderate (I2 = 55%).
However, the results of these sensitivity analyses are in keeping with the results obtained by pooling all trials with eligible data irrespective of the risk of selection bias which found that image guidance probably provides little or no benefit in pain or function over injection without imaging.
Detection bias
Five trials were judged to be at low risk of detection bias for participant‐reported outcomes and could be pooled for pain and function at > three to six weeks (Cho 2021; Cole 2016; Dogu 2012; Ekeberg 2009; Lee 2009) . Statistical heterogeneity might not be important for pain (I2 = 19%) and there was no statistical heterogeneity for function (I2 = 0%).
Estimates for pain and function do not appear to have been influenced by the presence of detection bias. Mean pain was 3.1 (on a scale of 0 to 10, higher score indicates more pain) with non‐image‐guided injection and 0.3 points better (95% CI 0.1 to 0.5 points better; 338 participants) with an ultrasound‐guided injection when pooling the five trials with low risk of detection bias. This was not substantively different to the analysis that included all trials.
Mean function was 68 points (on a scale of 0 to 100, higher score indicates better function) with a non‐image‐guided injection and 2.4 points worse (95% CI 5.0 points worse to 0.1 points better; 338 participants) with an ultrasound‐guided injection when pooling the five trials with low risk of detection bias. This was also not substantively different to the analysis that included all trials.
Unit of analysis issues
We performed a post hoc sensitivity analysis to assess the robustness of pain against the potential unit of analysis issue from two trials that randomised by participant, but injected both shoulders (Cole 2016; Saeed 2014).
We included unadjusted data from these trials, as adjusted data were not available. Removal of data from these studies from the analysis of pain at the primary time point confirmed that the analysis was robust to this potential unit of analysis issue: the effect estimates changed little from an MD (95% CI) of ‐0.5 (‐0.2 to ‐0.8) including all trials with eligible data to ‐0.5 (‐0.1 to ‐0.9) excluding these two trials.
Discussion
Summary of main results
Nineteen trials involving 1035 participants compared ultrasound‐guided injections with injections performed without image guidance for shoulder pain. Compared with non‐image‐guided injections, moderate‐certainty evidence (downgraded for risk of bias) indicates that ultrasound‐guided injections probably provides little important improvement in pain and function at six weeks (summary of findings Table 1). Subgroup analyses stratifying results by shoulder condition (i.e. rotator cuff disease, adhesive capsulitis and mixed shoulder pain), did not appreciably alter these estimates. Ultrasound‐guided injection may have little to no effect on quality of life (low‐certainty evidence, downgraded for risk of bias and imprecision), and it is uncertain if there is any difference in participant‐rated treatment success due to very low‐certainty evidence (downgraded for risk of bias, inconsistency and imprecision). There may be little to no difference in the risk of adverse events (low‐certainty evidence, downgraded for risk of bias and imprecision) between ultrasound‐guided and non‐image‐guided injection. Serious adverse events and withdrawals due to adverse events were rarely reported and thus risk estimates could not be calculated.
Furthermore, compared with injections performed without image guidance, ultrasound‐guided injection may provide little to no effect on shoulder flexion, abduction and external rotation (low‐certainty evidence, downgraded for risk of bias and indirectness), and ultrasound‐guided injection may not reduce the need for additional injections or surgery (low‐certainty evidence, downgraded for risk of bias and imprecision).
Overall, current evidence does not confirm an advantage of ultrasound‐guided injections for improving patient‐relevant outcomes, such as pain and function, over injections that do not use image guidance for shoulder pain.
Overall completeness and applicability of evidence
The 19 trials included in this review compared ultrasound‐guided injection with anatomic landmark injection (n = 18 trials) or systemic intramuscular injection (n = 1 trial; Ekeberg 2009) for shoulder pain. Trials were conducted in 11 countries. Fourteen trials included participants with rotator cuff disease (Bhayana 2018; Chen 2006; Cho 2010; Cole 2016; Dogu 2012; Ekeberg 2009; Haghighat 2015; Hsieh 2013; Naredo 2004; Saeed 2014; Ucuncu 2009), four included participants with adhesive capsulitis (Cho 2021; Lee 2009; Lee 2015; Raeissadat 2017), and one did not define their study population beyond 'shoulder pain' (Zufferey 2012). Nine trials relied upon clinical features alone for diagnosis (Bhayana 2018; Ekeberg 2009; Haghighat 2015; Hsieh 2013; Naredo 2004; Raeissadat 2017; Azadvari 2020; Cho 2010; Roddy 2020), while the remaining trials either used MRI or US or did not report how inclusion criteria were determined. Injections typically targeted the subacromial bursa for rotator cuff disease or the glenohumeral joint for adhesive capsulitis and glenohumeral osteoarthritis.
Most trials excluded potential participants who had received previous glucocorticoid injections in the shoulder; five at any time (Bhayana 2018; Cho 2010; Dogu 2012; Naredo 2004; Ucuncu 2009), five within the last three months (Cho 2021; Haghighat 2015; Hsieh 2013; Raeissadat 2017; Zufferey 2012), and two within the preceding month (Ekeberg 2009; Saeed 2014). The other seven trials did not specify whether or not participants had previously received glucocorticoid injections for shoulder pain (Akbari 2020; Azadvari 2020; Chen 2006; Cole 2016; Lee 2009; Lee 2015; Roddy 2020). Most trials also excluded full‐thickness rotator cuff tears and participants who had previous shoulder surgery.
Participants were similar in terms of mean age (ranging from 31 (Azadvari 2020) to 60 (Cho 2010) years), baseline pain and function (except participants in Raeissadat 2017 who reported substantially lower function), and gender ratio (percent female ranged from 22% (Lee 2015) to 73% (Ucuncu 2009)). Although the mean duration of symptoms varied across trials (from 2 (Haghighat 2015) to 23 (Azadvari 2020)) months, we considered the study populations in all trials to be representative of patients seen in routine care. The synthesis of this review can therefore be applied to similar patients in clinical practice.
Measurement of pain varied across trials from pain at rest or sleep, to pain during overhead activities or during a pain provocation test (e.g. Hawkin's impingement test). We preferred to extract overall pain data when available, but some trials only reported pain with activity and others did not specify the framing of the question by which they assessed pain. Measurement of function also varied across trials. Constant score, our primary measure of function, was assessed in five trials (Azadvari 2020; Bhayana 2018; Dogu 2012; Ucuncu 2009; Zufferey 2012), SPADI in four (Ekeberg 2009; Haghighat 2015; Hsieh 2013; Roddy 2020), ASES in three (Cho 2021; Cole 2016; Lee 2015), and SDQ in two (Dogu 2012; Hsieh 2013). Other measures of function were not used in more than one trial.
Seven trials followed participants up to 12 weeks (Akbari 2020; Azadvari 2020; Bhayana 2018; Cho 2021; Lee 2015; Saeed 2014; Zufferey 2012) and one trial up to 6 months (Lee 2015). However, since the benefits of glucocorticoid injection are immediate and generally only last for six to eight weeks (Buchbinder 2003), longer trials are unnecessary to determine the comparative effectiveness of different forms of administration of glucocorticoid injection.
Six trials (Akbari 2020; Ekeberg 2009; Naredo 2004; Saeed 2014; Ucuncu 2009; Zufferey 2012) reported that there were no serious adverse events.
Four unpublished studies (Cinar 2018; IRCT2017021524621N6; Moore 2018; Pierce 2018) were identified. The authors of two studies indicated that they were in the process of publishing the results (IRCT2017021524621N6; Pierce 2018) and we were not able to contact the authors of Cinar 2018. One trial has been published as a pre‐print in bioRxiv but has not yet been published in a peer‐reviewed journal (Moore 2018). It reported that ultrasound‐guided intra‐articular injection into the glenohumeral joint space did not result in greater pain relief at two weeks or six months compared with landmark‐guided injection in participants with painful shoulder due to osteoarthritis.
It is unclear if availability of the results from the other completed trials will substantially change the conclusions of the review, but it may increase the certainty of the effect estimates, by reducing heterogeneity around the pain and function estimates, and reducing imprecision around estimates for treatment success, quality of life and adverse events.
Quality of the evidence
For the major outcomes, evidence for pain and function was downgraded once to moderate‐certainty due to possible selection, performance and detection biases. We considered downgrading evidence for inconsistency; however, the statistical heterogeneity was driven largely by a single trial that appears to show a larger benefit in favour of image‐guided injection. We downgraded twice to low‐certainty evidence for quality of life and adverse events due to bias and imprecision (the 95% CIs around the effect estimate did not rule in or out a clinically important effect around quality of life, and the number of adverse events was low). Participant‐rated treatment success was downgraded to very low‐certainty due to bias, imprecision, and possible inconsistency (although with only four trials contributing to this outcome, it is harder to interpret the I2 value).
The minor outcomes, additional treatments and range of motion were downgraded twice to low‐certainty evidence, due to potential bias, and imprecision (additional treatments) as the 95% CIs around the effect estimate did not rule in or out clinically important effects; and indirectness (range of motion). Evidence for shoulder range of motion was downgraded for indirectness as it is essentially a surrogate for function and presumably a less relevant outcome for participants. Only four studies reported data for range of motion, and it is possible there was reporting bias.
We identified four completed studies with unpublished results, however, as authors of three studies indicated that they planned to publish the results and results for one study is at pre‐publication in a peer‐reviewed journal, we did not downgrade for publication bias. Funnel plots for pain and function at the 6‐week follow‐up time also indicate little evidence of publication bias (Figure 3 and Figure 4). Estimates for pain may have been influenced by the presence of selection bias since the slight/small benefit seen in the main analysis disappeared once we restricted the analysis to studies at low risk of selection bias. Restricting the analysis to studies at low risk of selection bias did not appreciably alter the effect estimate for function and detection bias did not appreciably alter the effect estimates for pain or function.
Low‐certainty evidence shows that ultrasound‐guided injection for shoulder pain probably does not reduce the need for additional treatment, nor reduce the risk of adverse events. Evidence was downgraded for imprecision and risk of performance, detection and attrition bias for these two outcomes. Evidence was not downgraded for indirectness and publication bias for the same reasons listed above.
Potential biases in the review process
We believe that the search likely identified all relevant published studies. A thorough search strategy was devised and all major databases were searched for relevant studies with no language restrictions applied. Two review authors assessed the trials for inclusion in the review and risk of bias, with a third reviewer adjudicating if there was any discrepancy.
Apart from the risk of bias of the included trials, the biggest limitation of the review was that the trials varied in their outcome measures. Measures of both pain and function varied across trials. There were 12 measures of pain (assessed on either 10 cm VAS 100 cm VAS, 0 to 10‐point NRS, or 1‐9 ordinal scale) and 10 measures of function assessed across trials. For 'overall' pain, one of our prespecified primary outcomes, we elected to pool results for pain with activity, daytime pain and unspecified type of pain. For function, we similarly pooled different measures that may not necessarily be measuring the exact same construct.
One trial (Lee 2009) had very small standard deviations. We speculated that the authors had inadvertently published standard errors instead of standard deviations, but this could not be confirmed from the authors.
Another limitation is that we do not know what a meaningful improvement in all outcomes with image‐ versus non‐image‐guided injection for people with shoulder pain might be. Instead of considering the minimal clinically important difference (MCID), we elected to instead describe the magnitude of the mean between‐group differences for pain and function as slight/small, moderate or large. Across all time points, the mean between‐group differences were either slight/small or not different and, in general, the 95% CI also excluded clinically important benefits (large CI were seen when there was substantial statistical heterogeneity).
Agreements and disagreements with other studies or reviews
Another systematic review of image‐guided injection versus non‐image‐guided injection for shoulder pain has been published since the publication of our original review (Aly 2015). Aly 2015 included seven of the 14 trials included in our review (Chen 2006; Dogu 2012; Hsieh 2013; Lee 2009; Naredo 2004; Ucuncu 2009; Zufferey 2012). In contrast to our conclusions, they concluded that patients who received ultrasound‐guided injections had significantly greater improvements in shoulder pain and function at six weeks compared to those who received non‐image‐guided injections. There were, however, several issues with their approach. Notably, the authors did not report the overall quality of the evidence nor acknowledge that the results should be interpreted cautiously in view of the limited number of studies and small sample sizes. The authors' assessment of risk of bias for these trials also differed from our own; they pooled change scores instead of final scores (which appears to have exaggerated their findings for function at six weeks), and they excluded Ekeberg 2009, a high‐quality, adequately powered trial that fulfilled all criteria of low risk of bias in our assessment and failed to demonstrate a difference in efficacy between local ultrasound‐guided injection and intramuscular injection in the upper gluteal buttock region.
Use of ultrasound by clinicians as well as radiologists for both diagnostic and therapeutic purposes for a variety of musculoskeletal indications is expanding rapidly in many settings. There are distinct advantages of ultrasound guidance, for example, when diagnostic aspiration into joints that are deep and difficult to locate using landmark‐guidance are necessary, or for injection of radioisotopes where accuracy is paramount (Iagnocco 2010). There continues to be debate, however, in the literature regarding whether or not accuracy of glucocorticoid injections is a prerequisite for efficacy for the treatment of joint and soft tissue pathology (Hall 2004; Iagnocco 2010), with studies continuing to produce conflicting results (Hartung 2010; Micu 2010; Sibbitt 2009). As outlined by Iagnocco 2010, Naredo 2004 and Ekeberg 2009, the mechanism of local glucocorticoid action is not well understood. Nevertheless, our review provides moderate‐certainty evidence that in patients with shoulder pain, ultrasound‐guided injection does not confer an advantage over landmark or systemic intramuscular injection for improving patient‐relevant outcomes, such as pain and function.
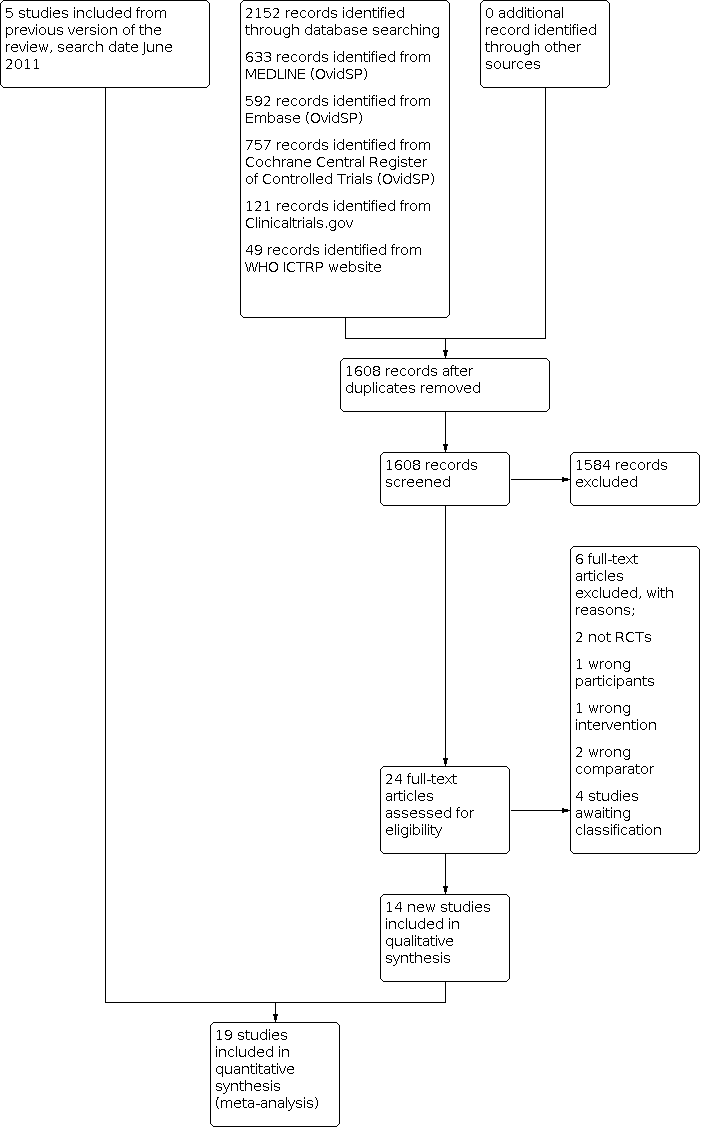
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Ultrasound‐guided injection versus non‐image‐guided injection, outcome: 1.1 Overall pain (or daytime, activity‐related or unspecified).

Funnel plot of comparison: 1 Ultrasound‐guided injection versus non‐image‐guided injection, outcome: 1.2 Function.

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 1: Overall pain (or daytime, activity‐related or unspecified)

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 2: Function

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 3: Treatment success (50% pain improvement on VAS)

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 4: Quality of life

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 5: Number of adverse events

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 6: Withdrawals due to adverse events

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 7: Additional injections or surgery

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 8: Range of flexion

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 9: Range of abduction

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 10: Range of external rotation

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 11: Serious adverse events

Comparison 2: Subgroup analyses (stratified by shoulder condition), Outcome 1: Overall pain

Comparison 2: Subgroup analyses (stratified by shoulder condition), Outcome 2: Function
| Ultrasound‐guided injection compared to non‐image‐guided (landmark or intramuscular) injection for shoulder pain | ||||||
| Patient or population: Patients with shoulder pain | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Landmark or intramuscular injection | Ultrasound‐guided injection | |||||
| Overall Pain Follow‐up: > 3 weeks up to 6 weeks | 3.1 points1 | 2.6 points (2.9 to 4.9) | 1003 | ⊕⊕⊕⊝ | Image‐guided injection probably results in little to no improvement in pain compared with blind injection. Mean difference in pain 0.5 points better with image‐guided injection (0.2 better to 0.8 points better). | |
| Function various scales 0 to 100 points (higher score indicates better function) Follow‐up: > 3 weeks up to 6 weeks | 68 points1 | 70.4 points (67.8 to 73.1) | 895 | ⊕⊕⊕⊝ | Image‐guided injection may have little or no effect on function compared with blind injection. Mean difference in function 2.4 points better with image guided injection (0.2 worse to 5.1 better). | |
| Participant‐assessed success 50% improvement on numerical rating 0 to 10 scale for pain) Follow‐up: end of study (6 weeks up to 12 weeks) | 389 per 1000 | 606 per 1000 (346 to 1000) | RR 1.56 (0.89 to 2.75) | 350 (5 studies) | ⊕⊝⊝⊝ | We are uncertain if image‐guided injection improves treatment success compared with blind injection. Absolute difference: 22% more reported success (4% fewer to 62% more); relative difference: 56% more reported success (11% fewer to 175% more). |
| Quality of life SF‐36 MCS 0 to 100 points (higher score indicates better quality of life) Follow‐up: > 3 weeks up to 6 weeks | 65 points1 | 67.8 points (64.3 to 71.5) | 220 (2 studies) | ⊕⊕⊝⊝ low2,4 | Image‐guided injection may have little or no effect on quality of life compared with blind injection. Mean difference in quality of life 2.8 points better with image‐guided injection (0.7 worse to 6.4 better). | |
| Number of adverse events Follow‐up: end of study | 252 per 1000 | 181 per 1000 (100 to 323) | RR 0.72 (0.40 to 1.28) | 402 (5 studies) | ⊕⊕⊝⊝ | Image‐guided injection may have little or no effect on adverse events compared with blind injection. Absolute difference of 7% fewer adverse events (15% fewer to 7% more); relative difference was 28% fewer events (60% fewer to 28% more). |
| Serious adverse events Not reported | See comment | See comment | Not estimable | See comment | See comment | Five trials (Ekeberg 2009; Naredo 2004; Saeed 2014; Ucuncu 2009; Zufferey 2012) reported that there were no serious side effects. The remaining trials did not report the incidence of serious adverse events. |
| Number of withdrawals due to adverse events Follow‐up: end of study | See comment | See comment | Not estimable | See comment | See comment | Only one trial reported withdrawals due to adverse events (Ekeberg 2009): 1/53 in the control group withdrew and received an additional local steroid injection at 2 weeks and 0/53 in the image‐guided group withdrew due to adverse events. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Mean pain and function in non‐image‐guided group in Ekeberg 2009 (3.1 points on a 0 to 10 scale for pain; 68 points on a 0 to 100 scale for function); mean QoL in non‐image‐guided group in Hsieh 2013. 2 Downgraded one level for risk of bias. 3Downgraded one level for inconsistency: I2 = 77% for treatment success was difficult to interpret with only five trials but two showed no apparent difference between groups; two favoured image‐guided injection. Although I2 was 79% for pain, Naredo 2004 seemed to drive quite a bit of the inconsistency (I2 = 64% once it was removed); reporting a large benefit compared to other studies. 4Downgraded one level for imprecision due to low event rates, or data from single small study only | ||||||
| Study ID | Study population | Ultrasound‐guided injection | Type of ultrasound | Landmark‐guided injection | Co‐interventions | Adverse events |
| (Turkey) | Subacromial impingement syndrome confirmed by magnetic resonance imaging (MRI), symptoms for more than three months, increased pain on shoulder abduction, painful restriction of active flexion and/or abduction of the shoulder with more restriction on passive range of motion, and a positive Hawkins‐Kennedy impingement sign | Summary Injection of 1 mL of 40 mg methylprednisolone and 4 mL procaine 2% into the subacromial bursa performed by a radiologist with 10 years of experience in musculoskeletal radiology Procedure The patient was seated and the shoulder was internally rotated with the ipsilateral hand positioned on the hip in the modified Crass position. Methylprednisolone acetate 40 mg in 1 mL and procaine 2% 4 mL were prepared in a 5 mL syringe. The anterolateral aspect of the shoulder was cleaned using 10% povidone iodine solution. The US probe was placed on the anterolateral aspect of the shoulder and the subacromial bursa was visualised. A 21‐gauge needle was used to enter the anteromedial aspect of the shoulder under continuous US guidance. Once the bevel of the needle was visualised in the subacromial bursa, the solution was injected. | 2014 model Siemens Acuson S2000 (Siemens Healthcare, Erlangen, Germany) and a 9 MHz linear probe | Summary Injection of 1 mL of 40 mg methylprednisolone and 4 mL procaine 2% into the subacromial space performed by a physiatrist with more than 10 years of experience in the field Procedure The injection was performed using a standard posterolateral approach and an aseptic technique. The patient was seated upright with the arms resting comfortably at the side. The distal, lateral, and posterior edges of the acromion were palpated and the needle was inserted just inferiorly to the posterolateral edge of the acromion and directed towards the opposite nipple. Aspiration was performed to ensure that the needle was not in a blood vessel prior to administration of the drug. Methylprednisolone acetate 40 mg in 1 mL and procaine 2% 4 mL were injected slowly using a 21‐gauge needle 1 cm into the subacromial space. | Not reported | None in either group |
| (Iran) | Subacromial impingement in paraplegic patients with spinal cord injury (below T6). Shoulder pain visual analog (VAS) score higher than 4 and positive Neer test and Hawkins‐Kennedy test | Summary Injection of 1 mL methylprednisolone and 2 mL lidocaine into the subacromial‐subdeltoid bursa performed by a physiatrist. Procedure The injection was performed with lateral approach. The patients were at sitting position, they put their hand on iliac crest by internal rotation of shoulder joint. The probe was placed on supraspinatus muscle in longitudinal plane. Acromion was found by ultrasound guidance. Then the probe was derived to the inferior part so the subacromial‐subdeltoid bursa was determined. A hypodermic needle (gauge‐22) was entered beneath the probe in in‐plane manner in upward medial direction until the needle tip entered the bursa and its dilatation was observed during injection. Injection in subacromial space was performed by a physiatrist. | Not reported | Summary Injection of 1 mL methylprednisolone and 2 mL lidocaine into the subacromial space performed by a physiatrist Procedure Participants received lateral approached blind glucocorticoid injection guided by anatomic landmarks under sterile condition. The injection site was marked and then sterilised. Lateral edge of the acromion was touched by hand and the needle with gauge of 22–23 was guided 2–3 cm beneath the lateral edge of the acromion toward the medial side (slightly superior). Under resistance‐free condition, 1 cc depo‐medrol (Methylprednisolone, Merck, Germany) was injected along with 2 cc lidocaine. | Exercise therapy was used in both groups after seven days. The exercises included range of motion, posterior capsule stretching and isometric strengthening exercises presented as an instruction. | Not reported |
| Bhayana 2018 (India) | Rotator cuff syndrome with shoulder pain and < 50% reduction in one direction of range of motion | Summary Injection of 2 mL (40 mg/mL) methylprednisolone and 2 mL of 1% lignocaine into subacromial bursa performed by one experienced radiologist Procedure The patient was seated with the affected arm in hyperextension and internal rotation with the elbow bent and back of the hand resting against the lower back. A 10 mL syringe connected to a 5 cm, 21‐gauge needle was prepared with 2 mL of 40 mg/mL methylprednisolone acetate suspension mixed and 2 mL of 1% lignocaine and was inserted parallel to the transducer in a semi‐oblique plane from the anterior side of the shoulder. The bevelled side facing the transducer, the needle was advanced under the real time ultrasound assistance till the tip of the needle appeared in the subacromial bursa. Rotation of the bevelled side of the needle by 180 degrees was done for confirmation of the tip positioning and the bulging of subacromial bursa ascertained in the real time imaging. | Phillips HD 7 US machine with 7–10 MHz multi frequency broad band transducer | Summary Injection of 2 mL (40 mg/mL) methylprednisolone and 2 mL of 1% lignocaine into subacromial space by one orthopaedic surgeon Procedure A 10 mL syringe connected to 5 cm, 21‐gauge needle was prepared with 2 mL of 40 mg/mL methylprednisolone acetate suspension mixed, 2 mL lignocaine and 2 mL of radio opaque non‐ionic contrast media iohexol. Access to the subacromial space was achieved using a lateral approach. The needle was inserted just inferior to the midlateral aspect of the acromion, with the needle angled slightly cephalad, passing through the deltoid muscle, and directed medially and slightly anterior to the subacromial bursa. Care was taken to avoid injection directly into the tendons of the rotator cuff. Correct placement of the drug in the subacromial subdeltoid space was confirmed by the fluoroscopic assessment under the C arm within 10 minutes of the injection. Accuracy of correct instillation of methylprednisolone‐ lignocaine‐iohexol combination was assessed by a senior radiologist. | Both groups received 5‐day course of antibiotics for prevention of injection‐induced subacromial bursitis, and home‐based shoulder rehabilitation programme consisting of shoulder abduction and pendulum exercises. | None in either group |
| (Taiwan) | Ultrasound‐confirmed subacromial bursitis with shoulder pain and a painful arc | Summary Injection of 1 mL betamethasone and 1 mL 1% lignocaine into subacromial bursa by one physician experienced using ultrasound probes Procedure The patient was seated upright, with the back well supported and arms behind the back with elbows bent. The needle was inserted into the subacromial bursa under ultrasound guidance. Aspiration of the effusion was done first before injecting the steroid‐lidocaine suspension into the subacromial bursa. Advancement of the needle to the lesion site was observed as continuous and real‐time images. | LOGIQ 9 machine with 10L probe (4‐10 MHz) (General Electronic Company, Milwaukee, WI) | Summary Injection of 1 mL betamethasone and 1 mL 1% lignocaine into subacromial bursa by one physician experienced in peripheral joint and soft‐tissue injections. Procedure The patient was seated upright, with the back well supported and arms behind the back with elbows bent. The acromion was palpated by the thumb and the needle inserted in a horizontal approach. The needle was adjusted in different depths and angles to try to aspirate the effusion. Exaggerated | Not reported | Not reported |
| Cho 2010 | Ultrasound‐confirmed subacromial or subdeltoid bursitis with shoulder pain and positive findings on Hawkin's test (i.e. reproduction of pain) | Summary Injection of 5 mL of 0.5% lidocaine and 5 mL triamcinolone acetate into subacromial or subdeltoid bursa by a single researcher Procedure After the patient was seated in a chair, the deformed shoulder was extended, the elbow joint was flexed, and the modified crass position was adopted. The transducer was placed in front of the acromion, the needle was inserted in parallel with the probe to position the tip to the bursa and real‐time ultrasound was used to confirm that the tip of the needle was in the bursa and solution was then injected into the bursa. | Philips Newand 13 MHz linear transducer | Summary Injection of 5 mL of 0.5% lidocaine and 5 mL triamcinolone acetate into subacromial or subdeltoid bursa by the same researcher Procedure A posterior approach was used. | Not reported | None in either group |
| (Korea) | Primary frozen shoulder with pain and limitation of passive motion of greater than 30 degrees in two or more planes of movement | Summary Injection of 40 mg of triamcinolone acetonide, 4 mL of 1% lidocaine, 4 mL of normal saline, and 3 mL of water soluble unionised contrast into the glenohumeral joint by a single specialist with 15 years of experience in the field Procedure A posterior approach was used for the injection in both groups, with the patient in the semi‐lateral decubitus position on the unaffected side and 45° anterior tilting of affected side. For the US group, the needle was advanced laterally to medially with visualisation of its shaft using a linear 5‐ to 12‐MHz probe (HD15 ultrasound system; Philips, Bothell, Washington, USA), and reaching the glenohumeral joint space between the posterior aspect of the humeral head and the glenoid labrum. | Linear 5‐ to 12‐MHz probe (HD15 ultrasound system; Philips, Bothell, Washington, USA) | Summary Injection of 40 mg of triamcinolone acetonide, 4 mL of 1% lidocaine, 4 mL of normal saline, and 3 mL of water soluble unionised contrast into the glenohumeral joint by a single specialist with 15 years of experience in the field Procedure A posterior approach was used for the injection in both groups, with the patient in the semi‐lateral decubitus position on the unaffected side and 45° anterior tilting of affected side. For the blind group, the needle was inserted 2 cm inferior to the posterolateral margin of the acromion and directed anteriorly towards the coracoid process. After the needle contacted the humeral head, it was slightly withdrawn and the injection was administered. For the blind group, the US probe was placed just under the acromion without visualisation, to blind the patient to the allocation group. | All patients were instructed to use a home‐based exercise programme to increase ROM and were allowed to perform an exercise three times a day (15 minutes each round). The programme included pendulum exercises, wall‐climbing exercises, and gentle ROM exercises with a bar. During this programme, patients were asked to refrain from provoking post‐mobilisation soreness with self‐feedback. Patients were forbidden from having acupuncture or receiving additional injections from other hospitals. | None in either group |
| (Australia) | Subacromial impingement syndrome with shoulder pain during overhead activities and clinical signs of impingement (either in internal rotation or external rotation) | Summary Injection of 1 mL of 40 mg/mL methylprednisolone acetate and 5 mL of 1% lidocaine hydrochloride into subacromial bursa by one surgeon with over 10 years of experience performing subacromial injections Procedure With the patient in an upright sitting position, a 22‐gauge needle was placed directly underneath the midpoint of the lateral acromion. The needle was directed anteriorly and cephaladly toward the subacromial bursa (as guided by the ultrasound image) until the tip of the needle was seen in the bursa. | General Electric Logiq E9 machine with a 6‐ to 15‐MHz linear transducer and 50 x 10–mm footprint | Summary Injection of 1 mL of 40 mg/mL methylprednisolone acetate and 5 mL of 1% lidocaine hydrochloride into subacromial bursa by the same surgeon that performed the ultrasound‐guided injections Procedure Blind injections were performed with the patient in the same upright sitting position with the ultrasound probe on the acromion to keep the patients blinded to the treatment group. An image could be seen on the screen by the patient, but it was not possible for this to be used by the surgeon to guide the injection, as the way that he was positioned behind the patient meant that he did not have direct vision of the screen. A posterior approach was used, and the needle was inserted 1 cm medially and inferiorly to the posterolateral corner of the acromion and directed cephaladly, anteriorly, and medially toward the subacromial bursa. | Participants in both groups were not restricted from the use of any analgesic or anti‐inflammatory medication or other treatments such as physical therapy. | None in either group |
| (Turkey) | Subacromial impingement syndrome with shoulder pain and at least two positive results in the provocative tests (Neer, Hawkins, and Jobe’s ‘‘Empty Can’’). Diagnosis of subacromial impingement syndrome was confirmed by MRI. | Summary Injection of 1 mL of 5 mg/mL betamethasone dipropionate, 9 mL of 10 mg/mL prilocaine hydrochloride and 0.02 mL of 0.01 mmol gadolinium diethylenetriaminepentaacetic acid directly underneath the mid‐lateral aspect of the acromion, directed anteriorly and cephaladly by a musculoskeletal radiologist Procedure The patient was seated in the upright position. Sterile gel was applied to the probe. The probe was held in one hand, and the syringe with the mixture was held in the other hand. With the patient in an upright sitting position, a 21‐gauge needle was placed under the probe, and a lateral injection was made directly underneath the mid‐lateral aspect of the acromion, directed anteriorly and cephaladly. | Aplio XV machine (Toshiba, Tokyo, Japan) with a 7.5‐ to 14‐MHz linear probe | Summary Injection of 1 mL of 5 mg/mL betamethasone dipropionate, 9 mL of 10 mg/mL prilocaine hydrochloride and 0.02 mL of 0.01 mmol gadolinium diethylenetriaminepentaacetic acid into subacromial space by a physiatrist Procedure Patient seated in upright position. A posterior approach was used. Under the guidance of a Toshiba Power Vision ultrasonograph, gel was applied to a 7.5‐ to 14‐MHz linear probe, and it was placed on the trapezius muscle. A 21‐gauge needle was located 1 cm posteriorly and inferiorly to the border of the acromion and directed cephaladly, anteriorly, and medially toward the subacromial space. | Participants in both groups could use only 2000 mg paracetamol daily; no participant received physical therapy. | None in either group |
| Ekeberg 2009 (Norway) | Rotator cuff disease with shoulder pain, < 50% reduced range of movement in no more than one direction of external rotation, internal rotation or abduction, and a positive Hawkins‐Kennedy impingement sign | Summary Injection of 2 mL (20 mg) triamcinolone and 5 mL (10mg/mL) lidocaine to subacromial bursa and 4 mL (10mg/mL) lidocaine to upper gluteal region by one physician Procedure To improve blinding of participants by inducing a temporary pain relief (impingement test) and mask possible post‐injection pain, participants received an injection of local anaesthetic in the shoulder and the gluteal region. The participant was seated with the arm rotated internally behind the back, elbow bent, and the back of the hand resting against the lower back. The physician used the ultrasound probe to visualise the insertion of the supraspinatus tendon and the subacromial bursa on the longitudinal axis, taking care that the contents of the syringes were never shown to the participants. The physician used the anterior approach with a 0.8 × 50 mm intra‐muscular needle for the subacromial injections, perforating the skin and tracking the needle in real time until it reached the subacromial bursa. Participants received an injection of 2 mL (10 mg/mL) triamcinolone (Kenacort‐T, Bristol‐Myers Squibb) and 5 mL (10 mg/mL) lidocaine hydrochloride (Xylocaine, AstraZeneca) to the subacromial bursa and an intramuscular injection of 4 mL (10 mg/mL) lidocaine hydrochloride to the upper gluteal region. | Commercial ultrasound equipment (Medison 128 BWprime, Medison Co, Seoul, Korea) with a 5‐9 MHz linear transducer | Summary Injection of 5 mL (10mg/mL) lidocaine to subacromial bursa and 2 mL (20 mg) triamcinolone and 2 mL (10 mg/mL) lidocaine to upper gluteal region by same physician Procedure To improve blinding of participants by inducing a temporary pain relief (impingement test) and mask possible post‐injection pain, participants received an injection of local anaesthetic in the shoulder and the gluteal region. The participant was seated with the arm rotated internally behind the back, elbow bent, and the back of the hand resting against the lower back. The physician used the ultrasound probe to visualise the insertion of the supraspinatus tendon and the subacromial bursa on the longitudinal axis, taking care that the contents of the syringes were never shown to the participants. The physician used the anterior approach with a 0.8 × 50 mm intra‐muscular needle for the subacromial injections, perforating the skin and tracking the needle in real time until it reached the subacromial bursa. Participants received an injection of 5 mL (10 mg/mL) lidocaine hydrochloride to the subacromial bursa and an intramuscular injection of 2 mL (10 mg/mL) triamcinolone and 2 mL (10 mg/mL) lidocaine hydrochloride to the upper gluteal region. | Participants in both groups were allowed to use analgesics, and to continue any physiotherapy programme that they were attending at baseline. | There were no serious adverse events in either group. 1/53 participants in the ‘local’ group and 4/53 in the ‘systemic’ group reported post‐injection pain in the shoulder. Nine participants from both groups reported mild adverse effects such as facial redness, dizziness, and feeling of warmth, however, per group data were not given. |
| Haghighat 2015 (Iran) | Subacromial impingement syndrome with shoulder pain, pain in abduction (or painful restriction of glenohumeral mobility) and positive Neer and Hawkins tests | Summary Injection of 40 mg methylprednisolone with 1 cc lidocaine 2% into subacromial bursa; personnel not specified Procedure A lateral approach using the ultrasound equipment with high frequency linear transducer for guidance was adopted. The ultrasound probe was positioned parallel to the long axis of the supraspinatus. The skin was punctured at a distance of about 2–3 cm from the probe in order to avoid contact between the needle and the probe. As soon as the needle had penetrated the subcutaneous tissues, its progress was real‐time monitored on the US image. The needle was visualised as a hyperechoic structure with posterior comet‐tail artefact. Progress of the needle until the tip had entered the subacromial bursa was observed. When the tip of the needle appeared to be inside the subacromial bursa, a small amount of liquid was injected to confirm the correct position (fluid passing from the needle tip into the bursa). The injection could be visualised in real‐time as a spreading "cloud" of hyperechoic echoes inside the bursa. | Ultrasound equipment with high frequency linear transducer. No further details provided | Summary Injection of 40 mg methylprednisolone with 1 cc lidocaine 2% into subacromial bursa; personnel not specified Procedure A posterior approach was used. The needle was placed approximately 2 cm below the posterior‐lateral aspect of the acromion. The needle was guided forward and slightly to the left under the acromion. | Not reported | Not reported |
| (Taiwan) | Subacromial bursitis with shoulder pain, ≥ 4/10 pain during abduction on a VAS, a painful arc, and > 40% reduction in pain during shoulder abduction at end range after injection of 3 mL of 1% lidocaine into the subacromial bursa | Summary Injection of 0.5 mL (5 mg/mL) dexamethasone suspension and 3 mL lidocaine (10mg/ml) into subdeltoid/subacromial bursa by one senior physician who was a board‐certified rheumatologist, physiatrist, and ultrasonographer in musculoskeletal medicine Procedure After the sterilisation of the skin on the lateral side of the affected arm, a 21‐gauge needle was inserted into the subacromial bursa under ultrasound guidance. Any effusion, if present, was aspirated prior to the injection. | LOGIQ P5 machine (General Electronic Company, Milwaukee, WI) with 5‐ to 12‐MHz linear array transducer | Summary Injection of 0.5 mL (5 mg/mL) dexamethasone suspension and 3 mL lidocaine (10 mg/mL) into subacromial bursa by the same senior physician Procedure The physician injected the subacromial bursa according to Cyriax’s method, where he/she first localised the lateral edge of the acromion. He/she then asked the patient to relax the affected arm, and at 1–2 cm beneath the middle point of the lateral edge of the acromion, inserted a 21‐gauge needle medially and in a slightly cranial direction into the bursa. If the needle encountered resistance, either the coracoacromial ligament or the capsulotendinous structures had been contacted, and the needle position was slightly adjusted. | Not reported | Not reported |
| (South Korea) | Adhesive capsulitis with pain and limited range of movement. Ultrasound used to rule out rotator cuff disease. | Summary Injection of 0.5 mL (20 mg) triamcinolone and 1.5 mL 2% lidocaine and 3 mL normal saline into glenohumeral joint by one physician with two years’ experience in ultrasound‐guided shoulder injections Procedure The posterior approach was used. Patients were seated, with the affected shoulder bent and adducted. The ultrasound probe touched the lower part of the acromion sideways, and a long needle (23G, 7 cm) was inserted to the posterior articular surface of the humeral head and positioned inside the articular capsule. The expansion of the articular capsule was checked while the fluid was being injected. | LOGIQ 5a machine with a 10‐MHz linear probe | Summary Injection of 0.5 mL (20 mg) triamcinolone and 1.5 mL 2% lidocaine and 3 mL normal saline into articular capsule by one physician with 7 years experience in anatomic landmark‐guided shoulder injections Procedure The posterior approach was used. Patients were seated, with the affected shoulder bent and adducted. The acromion of the scapula was palpated and the needle inserted 1 cm inferior to the tip of the acromion. The needle was directed toward the coracoid process and advanced into the articular capsule, after which the drug was injected. | Participants in both groups were taught exercises for increasing joint ROM including stretching forward and bending down to a desk, Codman exercise, and a wall‐climbing exercise with the fingers. They were instructed to practice these at home. Participants were checked and encouraged to keep up with the exercises every time they visited the hospital. All participants had 5 weekly injections of 25 mg sodium hyaluronate. | Not reported |
| (South Korea) | Shoulder stiffness on the basis of < 100 degrees flexion or < 30 degrees external rotation or internal rotation at a level below the first lumbar spine junction. Radiography and ultrasonography were used to rule out rotator cuff disease. | Summary Injection of 40 mg triamcinolone acetonide and 2 mL of 2% lidocaine into glenohumeral joint by one experienced senior surgeon with more than 5 years of experience in performance of ultrasound‐guided injection at the shoulder joint Procedure The patient was supine with the arm by the side. After skin and transducer preparation with alcohol and povidone, the 21‐gauge 1.5‐inch needle on a 3 mL syringe with triamcinolone acetonide 40 mg and 2 mL of 2% lidocaine was inserted at the level of the coracoids, from lateral to medial, aimed at the medial border of the humeral head. When the needle made contact with the articular cartilage of the humeral head, the needle was tilted to position the point of the needle in the articular cavity. The intra‐articular position of the needle and fluid infiltration in the shoulder joint was confirmed by ultrasonography. | High‐resolution transducer with 12 MHz linear array | Summary Injection of 40 mg triamcinolone acetonide and 2 mL of 2% lidocaine into glenohumeral joint by the same senior surgeon Procedure With the patient in sitting, the sulcus between the lateral tip of the coracoid and the humeral head was palpated. Then, a 21‐gauge 1.5‐inch needle on a 3 mL syringe with triamcinolone acetonide 40 mg and 2 mL of 2% lidocaine was inserted in a slightly cephalad direction at the anterior‐lateral tip of the coracoid. The needle was slowly advanced while infiltrating the local anaesthetic until resistance was lost, indicating intra‐articular position. The injection was performed slowly. | Both groups received a home‐based exercise programme for rehabilitation involving: pendulum circumduction and passive shoulder exercises for self‐stretching in forward flexion, external rotation and internal rotation, pulley exercise, isometric exercise, thera‐band exercises, scapula stabilisation exercises, and strengthening using dumbbells. | None in either group |
| Naredo 2004 (Spain) | ‘Periarticular disorders’ (impingement syndrome rotator cuff lesions, subacromial‐subdeltoid bursitis and/or biceps tendon abnormalities) diagnosed by clinical history and examination | Summary Injection of 20 mg triamcinolone into either subacromial‐subdeltoid bursa, biceps tendon sheath or rotator cuff calcifications according to ultrasound findings by single rheumatologists experienced in ultrasound Procedure The transducer and the patient’s skin were sterilised with alcohol. Sterile gel was applied to the probe. The transducer was held in one hand and the syringe with glucocorticoid in the other hand. The needle was | Commercial equipment (Sonoline, Prima, Siemens, Seattle, WA, USA) using a 7.5 MHz linear phased array transducer | Summary Injection of 20 mg triamcinolone into subacromial space by same rheumatologist Procedure A 21‐gauge needle was used. The patient’s skin was sterilised with alcohol. Access to the subacromial space was achieved with a lateral approach, inserting the needle under the anterolateral aspect of the acromion process, passing it through the deltoid muscle, and directing it medially and slightly anterior to the subacromial‐subdeltoid bursa, with care taken to avoid injection directly into the tendons of the rotator cuff. Immediately after blind injection, an ultrasound examination was performed to search for steroid deposit as hyperechoic foci or lines, with or without acoustic shadowing. | Participants in both groups with loss of shoulder ROM were instructed to start a home physical therapy programme consisting of pendulum exercises and slow shoulder abduction. No restriction was placed on the participant’s ability to work or to use their shoulder as tolerated, or to take nonsteroidal anti‐inflammatory drugs. Participants did not receive physical therapy during the follow‐up period. | There were no serious adverse events in either group. 1/20 participants in the ‘blind’ injection group had a mild post‐injection adverse effect (not specified). |
| Raeissadat 2017 (Iran) | Adhesive capsulitis on the basis of findings from the history and physical examination | Summary Injection of 1 cm3 lidocaine 1%, then, 3 cm3 water soluble un‐ionised contrast with 1 cm3 distilled water, and finally, 1 cm3 triamcinolone 40 mg/cm3 with 1 cm3 lidocaine 1% into glenohumeral joint by one physical medicine and rehabilitation specialist with 15 years’ experience in his field Procedure The patient was seated and the affected hand was resting on the thigh. A glenohumeral joint injection was given using the posterior short axis approach, and the needle was inserted in the plane relative to the ultrasound probe and medial to the posterior aspect of the head of the humerus. | Alpinion E‐cube 7 ultrasound device with linear 3–12‐MHz probe | Summary Injection of 1 cm3 lidocaine 1%, then, 3 cm3 water soluble un‐ionised contrast with 1 cm3 distilled water, and finally, 1 cm3 triamcinolone 40 mg/cm3 with 1 cm3 lidocaine 1% into glenohumeral joint by the same specialist Procedure A glenohumeral joint injection was given using a posterior approach. A 25‐gauge needle was inserted 2.5 cm lower than the posterolateral aspect of the acromion. If the physician felt that the needle was not accurately inserted, he was not allowed to withdraw and re‐enter again. | After the injection, all participants in both groups received naproxen tablet 500 mg twice daily for 5 days and were told to perform Codman’s exercises. | None in either group |
| (United Kingdom) | Subacromial impingement syndrome with pain in deltoid insertion area, positive Neer and Hawkins Kennedy tests, and pain on shoulder abduction | Summary Injection of methylprednisolone 40 mg and 1 mL 1% lidocaine into the bursa by one of nine clinicians with extensive clinical experience performing ultrasound‐guided injections or completed an accredited course on ultrasound‐guided subacromial injections Procedure The skin and transducer were cleaned with chlorhexidine 0.5% solution and sterile gel applied to the transducer. The participant sat with the shoulder internally rotated and the ipsilateral hand on the buttock to maximise visibility of and access to the subacromial bursa. The transducer was placed anterolaterally, the hypoechoic subacromial bursa visualised, and a 21‐G needle inserted under real‐time ultrasound guidance until the needle‐tip entered the bursa. A commercially available premixed solution of methylprednisolone 40 mg and 1 mL 1% lidocaine was injected into the bursa. | LOGIQ e system with a 12 MHz transducer | Summary Injection of methylprednisolone 40 mg and 1 mL 1% lidocaine into the bursa by one of eight clinicians (different to those performing the ultrasound‐guided injections) with extensive clinical experience performing subacromial injections, and having attended a half‐day injection protocol workshop Procedure The participant sat with the arm hanging with the elbow bent and forearm resting on the lap. The skin was cleaned with chlorhexidine solution 0.5%. A 21‐G needle was inserted through the deltoid under the acromion process laterally. The same premixed solution of methylprednisolone 40 mg and 1 mL 1% lidocaine was injected. | None | Ultrasound‐guided injection and physiotherapist‐led exercise (n = 64): Serious adverse event: No. and nature of events: 1/64 ‐ pyelonephritis Other adverse event: No. and nature of events: 17/64 ‐ shoulder pain > 3 days following injection No. and nature of events: 16/64 ‐ discomfort during the injection or local skin changes, presyncope, nausea or flushing following the injection Ultrasound‐guided injection and exercise leaflet (n = 64): Other adverse event: No. and nature of events: 17/64 ‐ shoulder pain > 3 days following injection No. and nature of events: 16/64 ‐ discomfort during the injection or local skin changes, presyncope, nausea or flushing following the injection Unguided injection and physiotherapist‐led exercise (n = 64): Other adverse event: No. and nature of events: 20/64 ‐ shoulder pain > 3 days following injection No. and nature of events: 17/64 ‐ discomfort during the injection or local skin changes, presyncope, nausea or flushing following the injection Unguided injection and exercise leaflet (n = 64): Other adverse event: No. and nature of events: 20/64 ‐ shoulder pain > 3 days following injection No. and nature of events: 17/64 ‐ discomfort during the injection or local skin changes, presyncope, nausea or flushing following the injection |
| Saeed 2014 (Ireland) | Shoulder impingement pain with shoulder pain | Summary Injection of 40 mg of methylprednisolone acetate with 4 mL of lidocaine hydrochloride into either subacromial‐subdeltoid bursa, acromio‐clavicular joint, biceps tendon or other pathology according to ultrasound findings by one experienced physician. Procedure A two‐handed technique was used with the transducer held in one hand and the syringe with 21‐G needle in the other hand. The needle was directed in real time by ultrasound from the skin to the target (e.g. subdeltoid bursa, acromio‐clavicular joint or biceps pathology). In those shoulders where ultrasound demonstrated more than one pathology, the pathology most consistent with clinical examination was injected. When there was effusion in both the subacromial‐subdeltoid bursa and the biceps tendon sheath, injection was directed into the subacromial‐subdeltoid bursa. For confirmed rotator cuff pathology and inpatients with clinical impingement but normal shoulder, ultrasound subacromial‐subdeltoid bursa injection was performed. When ultrasound confirmed the principal pathology as acromioclavicular joint inflammation or biceps tendon inflammation, ultrasound‐guided injection of the principal pathological structure was performed. | Acuson Sequoia 512 ultrasound systems (Siemens, CA, USA) using 8L‐RS MHz linear phased‐array transducer | Summary Injection of 40 mg of methylprednisolone acetate with 4 mL of lidocaine hydrochloride into subacromial‐subdeltoid bursa by the same physician Procedure A standard technique was performed using a 21‐G needle via a lateral approach to inject the subacromial‐subdeltoid bursa. | Participants in both groups with the loss of shoulder range of movement were given post‐injection instructions in pendulum exercises and slow shoulder abduction. No restriction was placed on participants' ability to work or to use their shoulder as tolerated, or intake of NSAIDs. Participants did not receive physical therapy during the follow‐up period. | Apart from mild shoulder pain in a small number of cases (group not specified) within a few hours of shoulder injection, no serious adverse event was observed. |
| (Turkey) | ‘Soft‐tissue lesions’ (acromioclavicular degeneration, rotator cuff lesions (rupture, partial rupture, tendinosis, impingement, calcification), fluid accumulation or partial rupture of biceps tendon and bursitis (subdeltoid, subacromial)) that had not responded satisfactorily to at least 1 month of nonsteroidal anti‐inflammatory drugs | Summary Injection of 1 mL (40 mg) triamcinolone and 1 mL 1% lidocaine into site of observed pathology. Injections were administered ‘perilesionally’ and ‘intralesionally’; personnel not specified. Procedure The injector was directed to the site of observed pathology beneath the probe by imaging a hyper‐reflective line. | Commercial equipment (Esaote, Mylab 60, Italy) using a 6 to 18MHz linear phased array transducer | Summary Injection of 1 mL (40 mg) triamcinolone and 1 mL 1% lidocaine into subacromial region; personnel not specified Procedure Injections were administered with a lateral entry approach to the sub‐acromial region. | Participants in both groups with loss of shoulder ROM performed a home exercise programme consisting of shoulder abduction and pendulum exercises. No limit was imposed on nonsteroidal anti‐inflammatory consumption or use of the shoulder. Participants did not receive physical therapy during the follow‐up period. | There were no serious adverse events in either group. 1/30 participants in the ultrasound‐guided injection group had mild post‐injection pain. In the ‘blind’ injection group, 5/30 had a slight increase in pain and 1/30 had skin peeling after the injection. |
| Zufferey 2012 (Switzerland) | Acute shoulder pain that had not responded satisfactorily to nonsteroidal anti‐inflammatory drugs or physiotherapy | Summary Injection of 2 mL of Diprophos® (7 mg of a mixture made up of one‐third soluble and two‐thirds long‐acting bethamethasone) into site of observed pathology by two rheumatologists experienced in musculoskeletal ultrasonography Procedure Steroid injection under ultrasound guidance was performed according to the ultrasound diagnosis within two days of the clinical evaluation. The injection was directed into the location assessed by ultrasound to be the cause of shoulder pain. | 5‐9 MHz probe using a Philips HD11 machine in two centres and Easote mylab25 gold machine in one centre | Summary Injection of 2 mL of Diprophos® (7 mg of a mixture made up of one‐third soluble and two‐thirds long‐acting bethamethasone) into subacromial bursa; personnel not specified Procedure Blind injections were directed at the subacromial bursa. | Rescue analgesia was permitted for both groups and participants recorded their intake of nonsteroidal anti‐inflammatory drugs and paracetamol until the end of the study. New infiltrations after two weeks were tolerated but considered a poor result. | There were no serious adverse events in either group. 3/32 in the ultrasound‐guided group and 3/35 in the ‘blind’ injection group required a second local injection after 2 or 6 weeks. Four patients reported flushing and one diabetic patient a transient hyperglycaemia, however, there were no details of which groups these participants belonged to. |
| MHz = megahertz; mL = millilitre; mg = milligrams; cm = centimetre; T6 = sixth thoracic vertebrae; cc = cubic centimetre; mmol = millimole; ROM = range of motion; SA‐SD bursa = subacromial‐subdeltoid bursa; 21‐G = 21 gauge; NSAIDs = non‐steroidal anti‐inflammatory drugs | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Overall pain (or daytime, activity‐related or unspecified) Show forest plot | 18 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 up to 3 weeks | 9 | 554 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.31, ‐0.03] |
| 1.1.2 > 3 weeks up to 6 weeks | 15 | 1003 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.84, ‐0.20] |
| 1.1.3 > 6 weeks up to 3 months | 6 | 424 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.24, 0.09] |
| 1.1.4 > 3 months to 6 months | 2 | 205 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.53, 0.31] |
| 1.1.5 > 6 months | 2 | 205 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.73, 1.06] |
| 1.2 Function Show forest plot | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 up to 3 weeks | 8 | 487 | Mean Difference (IV, Random, 95% CI) | 5.45 [‐0.29, 11.20] |
| 1.2.2 > 3 weeks up to 6 weeks | 14 | 895 | Mean Difference (IV, Random, 95% CI) | 2.44 [‐0.17, 5.06] |
| 1.2.3 > 6 weeks up to 3 months | 4 | 257 | Mean Difference (IV, Random, 95% CI) | 9.15 [‐5.78, 24.09] |
| 1.2.4 > 3 months to 6 months | 2 | 205 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐9.30, 7.19] |
| 1.2.5 > 6 months | 2 | 205 | Mean Difference (IV, Random, 95% CI) | 3.35 [‐4.69, 11.38] |
| 1.3 Treatment success (50% pain improvement on VAS) Show forest plot | 5 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.89, 2.75] |
| 1.4 Quality of life Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 up to 3 weeks | 2 | 122 | Mean Difference (IV, Random, 95% CI) | 4.17 [‐0.97, 9.31] |
| 1.4.2 > 3 weeks up to 6 weeks | 2 | 220 | Mean Difference (IV, Random, 95% CI) | 2.81 [‐0.73, 6.35] |
| 1.4.3 > 6 weeks to 3 months | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.60 [‐1.27, 10.47] |
| 1.4.4 > 3 months to 6 months | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐2.49, 5.69] |
| 1.4.5 > 6 months | 1 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐4.88, 3.48] |
| 1.5 Number of adverse events Show forest plot | 5 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.40, 1.28] |
| 1.6 Withdrawals due to adverse events Show forest plot | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.00] |
| 1.7 Additional injections or surgery Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7.1 Additional injections | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.14, 2.15] |
| 1.7.2 Surgery | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.11] |
| 1.8 Range of flexion Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 up to 3 weeks | 5 | 305 | Mean Difference (IV, Random, 95% CI) | 3.45 [‐1.60, 8.49] |
| 1.8.2 > 3 weeks up to 6 weeks | 8 | 461 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐2.32, 4.49] |
| 1.8.3 > 6 weeks up to 3 months | 2 | 167 | Mean Difference (IV, Random, 95% CI) | 3.70 [‐1.42, 8.82] |
| 1.8.4 > 3 months to 6 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐5.49, 6.49] |
| 1.8.5 > 6 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 4.00 [‐4.53, 12.53] |
| 1.9 Range of abduction Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 up to 3 weeks | 5 | 317 | Mean Difference (IV, Random, 95% CI) | 12.57 [2.62, 22.53] |
| 1.9.2 > 3 weeks up to 6 weeks | 9 | 528 | Mean Difference (IV, Random, 95% CI) | 6.85 [1.47, 12.22] |
| 1.9.3 > 6 weeks to 3 months | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐8.46, 10.66] |
| 1.10 Range of external rotation Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.10.1 up to 3 weeks | 4 | 199 | Mean Difference (IV, Random, 95% CI) | 1.01 [‐3.88, 5.90] |
| 1.10.2 > 3 weeks up to 6 weeks | 6 | 334 | Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐3.50, 2.04] |
| 1.10.3 > 6 weeks up to 3 months | 2 | 167 | Mean Difference (IV, Random, 95% CI) | 3.87 [‐0.42, 8.17] |
| 1.10.4 > 3 months to 6 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 3.20 [‐8.10, 14.50] |
| 1.10.5 > 6 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐13.33, 7.93] |
| 1.11 Serious adverse events Show forest plot | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.12, 72.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Overall pain Show forest plot | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Subacromial disease | 12 | 777 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.05, ‐0.11] |
| 2.1.2 Adhesive capsulitis | 3 | 189 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.39, ‐0.03] |
| 2.1.3 Mixed or undefined shoulder pain | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.46, 0.06] |
| 2.2 Function Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 Subacromial disease | 11 | 687 | Mean Difference (IV, Random, 95% CI) | 5.06 [‐3.23, 13.35] |
| 2.2.2 Adhesive capsulitis | 3 | 171 | Mean Difference (IV, Random, 95% CI) | 1.05 [‐1.18, 3.29] |
| 2.2.3 Mixed or undefined shoulder pain | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 4.00 [0.05, 7.95] |

