Inyección de glucocorticoides guiada por imágenes versus no guiada por imágenes para el dolor del hombro
Appendices
Appendix 1. CENTRAL search strategy
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <January 2021>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Shoulder/ (550)
2 Rotator Cuff/ (336)
3 1 or 2 (854)
4 Calcium/ (3451)
5 exp bursitis/ (374)
6 4 or 5 (3825)
7 3 and 6 (25)
8 Shoulder Pain/ (919)
9 Shoulder Impingement Syndrome/ (353)
10 (rotator cuff or supraspinatus or infraspinatus or subscapular$ or teres).tw. (2349)
11 ((shoulder$ or subacromial or rotator cuff) adj5 (tendon$ or tendin$ or bursitis or calcium or calcif$ or impinge$ or tear$ or pain$ or periarthritis or stiff$)).tw. (5585)
12 Shoulder Joint/ (748)
13 (glenohumeral adj5 (joint or capsule)).tw. (253)
14 (adhesive capsuliti$ or frozen shoulder$).tw. (640)
15 or/7‐14 (7132)
16 exp Adrenal Cortex Hormones/ (27688)
17 (adrenal hormone$ or steroid$ or corticosteroid$ or corticoid$ or glucocorticoid$ or sub‐acromial or subacromial or hydroxycorticosteroid$).tw. (47844)
18 (triamcinolone or methylprednisolone or hydrocortisone or predniso$ or cortisone or dexamethasone or betamethasone).tw. (33048)
19 or/16‐18 (78875)
20 exp Injections/ (22337)
21 inject$.tw. (90172)
22 20 or 21 (101579)
23 19 and 22 (10869)
24 15 and 23 (757)
Appendix 2. MEDLINE search strategy
Database: Ovid MEDLINE(R) <1946 to February 15, 2021>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Shoulder/ (13264)
2 Rotator Cuff/ (6690)
3 1 or 2 (19195)
4 calcium/ (269579)
5 exp bursitis/ (4832)
6 4 or 5 (274400)
7 3 and 6 (776)
8 shoulder pain/ (5002)
9 shoulder impingement syndrome/ (1798)
10 rotator cuff injuries/ (6083)
11 (rotator cuff or supraspinatus or infraspinatus or subscapular$ or teres).tw. (15951)
12 ((shoulder$ or subacromial or rotator cuff) adj5 (tendon$ or tendin$ or bursitis or calcium or calcif$ or impinge$ or tear$ or pain$ or periarthritis or stiff$)).tw. (17038)
13 shoulder joint/ (19714)
14 (glenohumeral adj5 (joint or capsule)).tw. (2488)
15 (adhesive capsuliti$ or frozen shoulder$).tw. (1482)
16 or/7‐15 (40299)
17 Adrenal Cortex Hormones/ (65507)
18 (adrenal hormone$ or steroid$ or corticosteroid$ or corticoid$ or glucocorticoid$ or sub‐acromial or subacromial or hydroxycorticosteroid$).tw. (352115)
19 (triamcinolone or methylprednisolone or hydrocortisone or predniso$ or cortisone or dexamethasone or betamethasone).tw. (146883)
20 or/17‐19 (477533)
21 exp Injections/ (286306)
22 inject$.tw. (674934)
23 21 or 22 (831792)
24 20 and 23 (41175)
25 randomized controlled trial.pt. (522672)
26 controlled clinical trial.pt. (94047)
27 randomized.ab. (435882)
28 placebo.ab. (193776)
29 drug therapy.fs. (2279437)
30 randomly.ab. (295601)
31 trial.ab. (458427)
32 groups.ab. (1824800)
33 or/25‐32 (4471460)
34 exp animals/ not humans.sh. (4788181)
35 33 not 34 (3828179)
36 16 and 24 and 35 (633)
Appendix 3. Embase search strategy
Database: Embase Classic+Embase <1947 to 2021 February 15>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 shoulder/ (41649)
2 rotator cuff/ (6808)
3 1 or 2 (46178)
4 calcium/ (326795)
5 exp bursitis/ (5666)
6 4 or 5 (332414)
7 3 and 6 (951)
8 shoulder impingement syndrome/ (2943)
9 rotator cuff injury/ (2669)
10 rotator cuff rupture/ (7567)
11 (rotator cuff or supraspinatus or infraspinatus or subscapular$).mp. or teres.tw. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] (28009)
12 ((shoulder$ or subacromial or rotator cuff) adj5 (tendon$ or tendin$ or bursitis or calcium or calcif$ or impinge$ or tear$ or pain or periarthritis or stiff$)).tw. (27117)
13 exp shoulder pain/ (17510)
14 exp frozen shoulder/ (2289)
15 exp humeroscapular periarthritis/ (2365)
16 (glenohumeral adj5 (joint or capsule)).tw. (3612)
17 (adhesive capsuliti$ or frozen shoulder$).tw. (2538)
18 or/7‐17 (54626)
19 corticosteroid/ (267864)
20 corticosteroid therapy/ (51967)
21 (adrenal hormone$ or steroid$ or corticosteroid$ or corticoid$ or glucocorticoid$ or sub‐acromial or subacromial or hydroxycorticosteroid$).tw. (591540)
22 (triamcinolone or methylprednisolone or hydrocortisone or predniso$ or cortisone or dexamethasone or betamethasone).tw. (259780)
23 or/19‐22 (907684)
24 exp injection/ (251615)
25 inject$.tw. (1133111)
26 24 or 25 (1159032)
27 23 and 26 (64060)
28 18 and 27 (1855)
29 random$.tw. (1652459)
30 factorial$.tw. (41088)
31 crossover$.tw. (80422)
32 cross over.tw. (34315)
33 cross‐over.tw. (34315)
34 placebo$.tw. (328887)
35 (doubl$ adj blind$).tw. (224698)
36 (singl$ adj blind$).tw. (26723)
37 assign$.tw. (422103)
38 allocat$.tw. (165987)
39 volunteer$.tw. (273171)
40 crossover procedure/ (66753)
41 double blind procedure/ (185300)
42 randomized controlled trial/ (651777)
43 single blind procedure/ (42092)
44 or/29‐43 (2501102)
45 28 and 44 (592)
Appendix 4. Trial Registry search strategy
WHO International Clinical Trials Search Platform (ICTRP)
Shoulder in condition AND corticosteroid* injection in intervention. Date: 06/07/20 # Hits: 49 trials
ClinicalTrials.gov, using advanced search option:
“Shoulder” in condition AND corticosteroid in intervention. Date: 18/02/21 # Hits: 121 studies
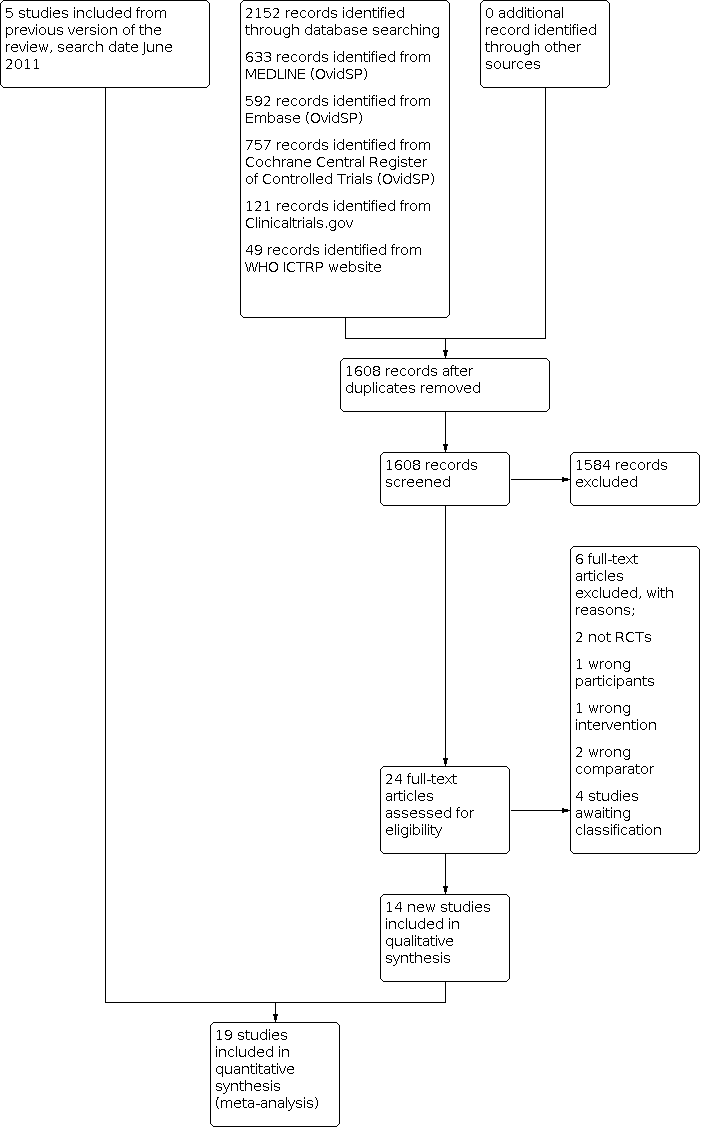
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Ultrasound‐guided injection versus non‐image‐guided injection, outcome: 1.1 Overall pain (or daytime, activity‐related or unspecified).

Funnel plot of comparison: 1 Ultrasound‐guided injection versus non‐image‐guided injection, outcome: 1.2 Function.

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 1: Overall pain (or daytime, activity‐related or unspecified)

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 2: Function

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 3: Treatment success (50% pain improvement on VAS)

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 4: Quality of life

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 5: Number of adverse events

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 6: Withdrawals due to adverse events

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 7: Additional injections or surgery

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 8: Range of flexion

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 9: Range of abduction

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 10: Range of external rotation

Comparison 1: Ultrasound‐guided injection versus anatomic landmark‐guided or systemic injection, Outcome 11: Serious adverse events

Comparison 2: Subgroup analyses (stratified by shoulder condition), Outcome 1: Overall pain

Comparison 2: Subgroup analyses (stratified by shoulder condition), Outcome 2: Function
| Ultrasound‐guided injection compared to non‐image‐guided (landmark or intramuscular) injection for shoulder pain | ||||||
| Patient or population: Patients with shoulder pain | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Landmark or intramuscular injection | Ultrasound‐guided injection | |||||
| Overall Pain Follow‐up: > 3 weeks up to 6 weeks | 3.1 points1 | 2.6 points (2.9 to 4.9) | 1003 | ⊕⊕⊕⊝ | Image‐guided injection probably results in little to no improvement in pain compared with blind injection. Mean difference in pain 0.5 points better with image‐guided injection (0.2 better to 0.8 points better). | |
| Function various scales 0 to 100 points (higher score indicates better function) Follow‐up: > 3 weeks up to 6 weeks | 68 points1 | 70.4 points (67.8 to 73.1) | 895 | ⊕⊕⊕⊝ | Image‐guided injection may have little or no effect on function compared with blind injection. Mean difference in function 2.4 points better with image guided injection (0.2 worse to 5.1 better). | |
| Participant‐assessed success 50% improvement on numerical rating 0 to 10 scale for pain) Follow‐up: end of study (6 weeks up to 12 weeks) | 389 per 1000 | 606 per 1000 (346 to 1000) | RR 1.56 (0.89 to 2.75) | 350 (5 studies) | ⊕⊝⊝⊝ | We are uncertain if image‐guided injection improves treatment success compared with blind injection. Absolute difference: 22% more reported success (4% fewer to 62% more); relative difference: 56% more reported success (11% fewer to 175% more). |
| Quality of life SF‐36 MCS 0 to 100 points (higher score indicates better quality of life) Follow‐up: > 3 weeks up to 6 weeks | 65 points1 | 67.8 points (64.3 to 71.5) | 220 (2 studies) | ⊕⊕⊝⊝ low2,4 | Image‐guided injection may have little or no effect on quality of life compared with blind injection. Mean difference in quality of life 2.8 points better with image‐guided injection (0.7 worse to 6.4 better). | |
| Number of adverse events Follow‐up: end of study | 252 per 1000 | 181 per 1000 (100 to 323) | RR 0.72 (0.40 to 1.28) | 402 (5 studies) | ⊕⊕⊝⊝ | Image‐guided injection may have little or no effect on adverse events compared with blind injection. Absolute difference of 7% fewer adverse events (15% fewer to 7% more); relative difference was 28% fewer events (60% fewer to 28% more). |
| Serious adverse events Not reported | See comment | See comment | Not estimable | See comment | See comment | Five trials (Ekeberg 2009; Naredo 2004; Saeed 2014; Ucuncu 2009; Zufferey 2012) reported that there were no serious side effects. The remaining trials did not report the incidence of serious adverse events. |
| Number of withdrawals due to adverse events Follow‐up: end of study | See comment | See comment | Not estimable | See comment | See comment | Only one trial reported withdrawals due to adverse events (Ekeberg 2009): 1/53 in the control group withdrew and received an additional local steroid injection at 2 weeks and 0/53 in the image‐guided group withdrew due to adverse events. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Mean pain and function in non‐image‐guided group in Ekeberg 2009 (3.1 points on a 0 to 10 scale for pain; 68 points on a 0 to 100 scale for function); mean QoL in non‐image‐guided group in Hsieh 2013. 2 Downgraded one level for risk of bias. 3Downgraded one level for inconsistency: I2 = 77% for treatment success was difficult to interpret with only five trials but two showed no apparent difference between groups; two favoured image‐guided injection. Although I2 was 79% for pain, Naredo 2004 seemed to drive quite a bit of the inconsistency (I2 = 64% once it was removed); reporting a large benefit compared to other studies. 4Downgraded one level for imprecision due to low event rates, or data from single small study only | ||||||
| Study ID | Study population | Ultrasound‐guided injection | Type of ultrasound | Landmark‐guided injection | Co‐interventions | Adverse events |
| (Turkey) | Subacromial impingement syndrome confirmed by magnetic resonance imaging (MRI), symptoms for more than three months, increased pain on shoulder abduction, painful restriction of active flexion and/or abduction of the shoulder with more restriction on passive range of motion, and a positive Hawkins‐Kennedy impingement sign | Summary Injection of 1 mL of 40 mg methylprednisolone and 4 mL procaine 2% into the subacromial bursa performed by a radiologist with 10 years of experience in musculoskeletal radiology Procedure The patient was seated and the shoulder was internally rotated with the ipsilateral hand positioned on the hip in the modified Crass position. Methylprednisolone acetate 40 mg in 1 mL and procaine 2% 4 mL were prepared in a 5 mL syringe. The anterolateral aspect of the shoulder was cleaned using 10% povidone iodine solution. The US probe was placed on the anterolateral aspect of the shoulder and the subacromial bursa was visualised. A 21‐gauge needle was used to enter the anteromedial aspect of the shoulder under continuous US guidance. Once the bevel of the needle was visualised in the subacromial bursa, the solution was injected. | 2014 model Siemens Acuson S2000 (Siemens Healthcare, Erlangen, Germany) and a 9 MHz linear probe | Summary Injection of 1 mL of 40 mg methylprednisolone and 4 mL procaine 2% into the subacromial space performed by a physiatrist with more than 10 years of experience in the field Procedure The injection was performed using a standard posterolateral approach and an aseptic technique. The patient was seated upright with the arms resting comfortably at the side. The distal, lateral, and posterior edges of the acromion were palpated and the needle was inserted just inferiorly to the posterolateral edge of the acromion and directed towards the opposite nipple. Aspiration was performed to ensure that the needle was not in a blood vessel prior to administration of the drug. Methylprednisolone acetate 40 mg in 1 mL and procaine 2% 4 mL were injected slowly using a 21‐gauge needle 1 cm into the subacromial space. | Not reported | None in either group |
| (Iran) | Subacromial impingement in paraplegic patients with spinal cord injury (below T6). Shoulder pain visual analog (VAS) score higher than 4 and positive Neer test and Hawkins‐Kennedy test | Summary Injection of 1 mL methylprednisolone and 2 mL lidocaine into the subacromial‐subdeltoid bursa performed by a physiatrist. Procedure The injection was performed with lateral approach. The patients were at sitting position, they put their hand on iliac crest by internal rotation of shoulder joint. The probe was placed on supraspinatus muscle in longitudinal plane. Acromion was found by ultrasound guidance. Then the probe was derived to the inferior part so the subacromial‐subdeltoid bursa was determined. A hypodermic needle (gauge‐22) was entered beneath the probe in in‐plane manner in upward medial direction until the needle tip entered the bursa and its dilatation was observed during injection. Injection in subacromial space was performed by a physiatrist. | Not reported | Summary Injection of 1 mL methylprednisolone and 2 mL lidocaine into the subacromial space performed by a physiatrist Procedure Participants received lateral approached blind glucocorticoid injection guided by anatomic landmarks under sterile condition. The injection site was marked and then sterilised. Lateral edge of the acromion was touched by hand and the needle with gauge of 22–23 was guided 2–3 cm beneath the lateral edge of the acromion toward the medial side (slightly superior). Under resistance‐free condition, 1 cc depo‐medrol (Methylprednisolone, Merck, Germany) was injected along with 2 cc lidocaine. | Exercise therapy was used in both groups after seven days. The exercises included range of motion, posterior capsule stretching and isometric strengthening exercises presented as an instruction. | Not reported |
| Bhayana 2018 (India) | Rotator cuff syndrome with shoulder pain and < 50% reduction in one direction of range of motion | Summary Injection of 2 mL (40 mg/mL) methylprednisolone and 2 mL of 1% lignocaine into subacromial bursa performed by one experienced radiologist Procedure The patient was seated with the affected arm in hyperextension and internal rotation with the elbow bent and back of the hand resting against the lower back. A 10 mL syringe connected to a 5 cm, 21‐gauge needle was prepared with 2 mL of 40 mg/mL methylprednisolone acetate suspension mixed and 2 mL of 1% lignocaine and was inserted parallel to the transducer in a semi‐oblique plane from the anterior side of the shoulder. The bevelled side facing the transducer, the needle was advanced under the real time ultrasound assistance till the tip of the needle appeared in the subacromial bursa. Rotation of the bevelled side of the needle by 180 degrees was done for confirmation of the tip positioning and the bulging of subacromial bursa ascertained in the real time imaging. | Phillips HD 7 US machine with 7–10 MHz multi frequency broad band transducer | Summary Injection of 2 mL (40 mg/mL) methylprednisolone and 2 mL of 1% lignocaine into subacromial space by one orthopaedic surgeon Procedure A 10 mL syringe connected to 5 cm, 21‐gauge needle was prepared with 2 mL of 40 mg/mL methylprednisolone acetate suspension mixed, 2 mL lignocaine and 2 mL of radio opaque non‐ionic contrast media iohexol. Access to the subacromial space was achieved using a lateral approach. The needle was inserted just inferior to the midlateral aspect of the acromion, with the needle angled slightly cephalad, passing through the deltoid muscle, and directed medially and slightly anterior to the subacromial bursa. Care was taken to avoid injection directly into the tendons of the rotator cuff. Correct placement of the drug in the subacromial subdeltoid space was confirmed by the fluoroscopic assessment under the C arm within 10 minutes of the injection. Accuracy of correct instillation of methylprednisolone‐ lignocaine‐iohexol combination was assessed by a senior radiologist. | Both groups received 5‐day course of antibiotics for prevention of injection‐induced subacromial bursitis, and home‐based shoulder rehabilitation programme consisting of shoulder abduction and pendulum exercises. | None in either group |
| (Taiwan) | Ultrasound‐confirmed subacromial bursitis with shoulder pain and a painful arc | Summary Injection of 1 mL betamethasone and 1 mL 1% lignocaine into subacromial bursa by one physician experienced using ultrasound probes Procedure The patient was seated upright, with the back well supported and arms behind the back with elbows bent. The needle was inserted into the subacromial bursa under ultrasound guidance. Aspiration of the effusion was done first before injecting the steroid‐lidocaine suspension into the subacromial bursa. Advancement of the needle to the lesion site was observed as continuous and real‐time images. | LOGIQ 9 machine with 10L probe (4‐10 MHz) (General Electronic Company, Milwaukee, WI) | Summary Injection of 1 mL betamethasone and 1 mL 1% lignocaine into subacromial bursa by one physician experienced in peripheral joint and soft‐tissue injections. Procedure The patient was seated upright, with the back well supported and arms behind the back with elbows bent. The acromion was palpated by the thumb and the needle inserted in a horizontal approach. The needle was adjusted in different depths and angles to try to aspirate the effusion. Exaggerated | Not reported | Not reported |
| Cho 2010 | Ultrasound‐confirmed subacromial or subdeltoid bursitis with shoulder pain and positive findings on Hawkin's test (i.e. reproduction of pain) | Summary Injection of 5 mL of 0.5% lidocaine and 5 mL triamcinolone acetate into subacromial or subdeltoid bursa by a single researcher Procedure After the patient was seated in a chair, the deformed shoulder was extended, the elbow joint was flexed, and the modified crass position was adopted. The transducer was placed in front of the acromion, the needle was inserted in parallel with the probe to position the tip to the bursa and real‐time ultrasound was used to confirm that the tip of the needle was in the bursa and solution was then injected into the bursa. | Philips Newand 13 MHz linear transducer | Summary Injection of 5 mL of 0.5% lidocaine and 5 mL triamcinolone acetate into subacromial or subdeltoid bursa by the same researcher Procedure A posterior approach was used. | Not reported | None in either group |
| (Korea) | Primary frozen shoulder with pain and limitation of passive motion of greater than 30 degrees in two or more planes of movement | Summary Injection of 40 mg of triamcinolone acetonide, 4 mL of 1% lidocaine, 4 mL of normal saline, and 3 mL of water soluble unionised contrast into the glenohumeral joint by a single specialist with 15 years of experience in the field Procedure A posterior approach was used for the injection in both groups, with the patient in the semi‐lateral decubitus position on the unaffected side and 45° anterior tilting of affected side. For the US group, the needle was advanced laterally to medially with visualisation of its shaft using a linear 5‐ to 12‐MHz probe (HD15 ultrasound system; Philips, Bothell, Washington, USA), and reaching the glenohumeral joint space between the posterior aspect of the humeral head and the glenoid labrum. | Linear 5‐ to 12‐MHz probe (HD15 ultrasound system; Philips, Bothell, Washington, USA) | Summary Injection of 40 mg of triamcinolone acetonide, 4 mL of 1% lidocaine, 4 mL of normal saline, and 3 mL of water soluble unionised contrast into the glenohumeral joint by a single specialist with 15 years of experience in the field Procedure A posterior approach was used for the injection in both groups, with the patient in the semi‐lateral decubitus position on the unaffected side and 45° anterior tilting of affected side. For the blind group, the needle was inserted 2 cm inferior to the posterolateral margin of the acromion and directed anteriorly towards the coracoid process. After the needle contacted the humeral head, it was slightly withdrawn and the injection was administered. For the blind group, the US probe was placed just under the acromion without visualisation, to blind the patient to the allocation group. | All patients were instructed to use a home‐based exercise programme to increase ROM and were allowed to perform an exercise three times a day (15 minutes each round). The programme included pendulum exercises, wall‐climbing exercises, and gentle ROM exercises with a bar. During this programme, patients were asked to refrain from provoking post‐mobilisation soreness with self‐feedback. Patients were forbidden from having acupuncture or receiving additional injections from other hospitals. | None in either group |
| (Australia) | Subacromial impingement syndrome with shoulder pain during overhead activities and clinical signs of impingement (either in internal rotation or external rotation) | Summary Injection of 1 mL of 40 mg/mL methylprednisolone acetate and 5 mL of 1% lidocaine hydrochloride into subacromial bursa by one surgeon with over 10 years of experience performing subacromial injections Procedure With the patient in an upright sitting position, a 22‐gauge needle was placed directly underneath the midpoint of the lateral acromion. The needle was directed anteriorly and cephaladly toward the subacromial bursa (as guided by the ultrasound image) until the tip of the needle was seen in the bursa. | General Electric Logiq E9 machine with a 6‐ to 15‐MHz linear transducer and 50 x 10–mm footprint | Summary Injection of 1 mL of 40 mg/mL methylprednisolone acetate and 5 mL of 1% lidocaine hydrochloride into subacromial bursa by the same surgeon that performed the ultrasound‐guided injections Procedure Blind injections were performed with the patient in the same upright sitting position with the ultrasound probe on the acromion to keep the patients blinded to the treatment group. An image could be seen on the screen by the patient, but it was not possible for this to be used by the surgeon to guide the injection, as the way that he was positioned behind the patient meant that he did not have direct vision of the screen. A posterior approach was used, and the needle was inserted 1 cm medially and inferiorly to the posterolateral corner of the acromion and directed cephaladly, anteriorly, and medially toward the subacromial bursa. | Participants in both groups were not restricted from the use of any analgesic or anti‐inflammatory medication or other treatments such as physical therapy. | None in either group |
| (Turkey) | Subacromial impingement syndrome with shoulder pain and at least two positive results in the provocative tests (Neer, Hawkins, and Jobe’s ‘‘Empty Can’’). Diagnosis of subacromial impingement syndrome was confirmed by MRI. | Summary Injection of 1 mL of 5 mg/mL betamethasone dipropionate, 9 mL of 10 mg/mL prilocaine hydrochloride and 0.02 mL of 0.01 mmol gadolinium diethylenetriaminepentaacetic acid directly underneath the mid‐lateral aspect of the acromion, directed anteriorly and cephaladly by a musculoskeletal radiologist Procedure The patient was seated in the upright position. Sterile gel was applied to the probe. The probe was held in one hand, and the syringe with the mixture was held in the other hand. With the patient in an upright sitting position, a 21‐gauge needle was placed under the probe, and a lateral injection was made directly underneath the mid‐lateral aspect of the acromion, directed anteriorly and cephaladly. | Aplio XV machine (Toshiba, Tokyo, Japan) with a 7.5‐ to 14‐MHz linear probe | Summary Injection of 1 mL of 5 mg/mL betamethasone dipropionate, 9 mL of 10 mg/mL prilocaine hydrochloride and 0.02 mL of 0.01 mmol gadolinium diethylenetriaminepentaacetic acid into subacromial space by a physiatrist Procedure Patient seated in upright position. A posterior approach was used. Under the guidance of a Toshiba Power Vision ultrasonograph, gel was applied to a 7.5‐ to 14‐MHz linear probe, and it was placed on the trapezius muscle. A 21‐gauge needle was located 1 cm posteriorly and inferiorly to the border of the acromion and directed cephaladly, anteriorly, and medially toward the subacromial space. | Participants in both groups could use only 2000 mg paracetamol daily; no participant received physical therapy. | None in either group |
| Ekeberg 2009 (Norway) | Rotator cuff disease with shoulder pain, < 50% reduced range of movement in no more than one direction of external rotation, internal rotation or abduction, and a positive Hawkins‐Kennedy impingement sign | Summary Injection of 2 mL (20 mg) triamcinolone and 5 mL (10mg/mL) lidocaine to subacromial bursa and 4 mL (10mg/mL) lidocaine to upper gluteal region by one physician Procedure To improve blinding of participants by inducing a temporary pain relief (impingement test) and mask possible post‐injection pain, participants received an injection of local anaesthetic in the shoulder and the gluteal region. The participant was seated with the arm rotated internally behind the back, elbow bent, and the back of the hand resting against the lower back. The physician used the ultrasound probe to visualise the insertion of the supraspinatus tendon and the subacromial bursa on the longitudinal axis, taking care that the contents of the syringes were never shown to the participants. The physician used the anterior approach with a 0.8 × 50 mm intra‐muscular needle for the subacromial injections, perforating the skin and tracking the needle in real time until it reached the subacromial bursa. Participants received an injection of 2 mL (10 mg/mL) triamcinolone (Kenacort‐T, Bristol‐Myers Squibb) and 5 mL (10 mg/mL) lidocaine hydrochloride (Xylocaine, AstraZeneca) to the subacromial bursa and an intramuscular injection of 4 mL (10 mg/mL) lidocaine hydrochloride to the upper gluteal region. | Commercial ultrasound equipment (Medison 128 BWprime, Medison Co, Seoul, Korea) with a 5‐9 MHz linear transducer | Summary Injection of 5 mL (10mg/mL) lidocaine to subacromial bursa and 2 mL (20 mg) triamcinolone and 2 mL (10 mg/mL) lidocaine to upper gluteal region by same physician Procedure To improve blinding of participants by inducing a temporary pain relief (impingement test) and mask possible post‐injection pain, participants received an injection of local anaesthetic in the shoulder and the gluteal region. The participant was seated with the arm rotated internally behind the back, elbow bent, and the back of the hand resting against the lower back. The physician used the ultrasound probe to visualise the insertion of the supraspinatus tendon and the subacromial bursa on the longitudinal axis, taking care that the contents of the syringes were never shown to the participants. The physician used the anterior approach with a 0.8 × 50 mm intra‐muscular needle for the subacromial injections, perforating the skin and tracking the needle in real time until it reached the subacromial bursa. Participants received an injection of 5 mL (10 mg/mL) lidocaine hydrochloride to the subacromial bursa and an intramuscular injection of 2 mL (10 mg/mL) triamcinolone and 2 mL (10 mg/mL) lidocaine hydrochloride to the upper gluteal region. | Participants in both groups were allowed to use analgesics, and to continue any physiotherapy programme that they were attending at baseline. | There were no serious adverse events in either group. 1/53 participants in the ‘local’ group and 4/53 in the ‘systemic’ group reported post‐injection pain in the shoulder. Nine participants from both groups reported mild adverse effects such as facial redness, dizziness, and feeling of warmth, however, per group data were not given. |
| Haghighat 2015 (Iran) | Subacromial impingement syndrome with shoulder pain, pain in abduction (or painful restriction of glenohumeral mobility) and positive Neer and Hawkins tests | Summary Injection of 40 mg methylprednisolone with 1 cc lidocaine 2% into subacromial bursa; personnel not specified Procedure A lateral approach using the ultrasound equipment with high frequency linear transducer for guidance was adopted. The ultrasound probe was positioned parallel to the long axis of the supraspinatus. The skin was punctured at a distance of about 2–3 cm from the probe in order to avoid contact between the needle and the probe. As soon as the needle had penetrated the subcutaneous tissues, its progress was real‐time monitored on the US image. The needle was visualised as a hyperechoic structure with posterior comet‐tail artefact. Progress of the needle until the tip had entered the subacromial bursa was observed. When the tip of the needle appeared to be inside the subacromial bursa, a small amount of liquid was injected to confirm the correct position (fluid passing from the needle tip into the bursa). The injection could be visualised in real‐time as a spreading "cloud" of hyperechoic echoes inside the bursa. | Ultrasound equipment with high frequency linear transducer. No further details provided | Summary Injection of 40 mg methylprednisolone with 1 cc lidocaine 2% into subacromial bursa; personnel not specified Procedure A posterior approach was used. The needle was placed approximately 2 cm below the posterior‐lateral aspect of the acromion. The needle was guided forward and slightly to the left under the acromion. | Not reported | Not reported |
| (Taiwan) | Subacromial bursitis with shoulder pain, ≥ 4/10 pain during abduction on a VAS, a painful arc, and > 40% reduction in pain during shoulder abduction at end range after injection of 3 mL of 1% lidocaine into the subacromial bursa | Summary Injection of 0.5 mL (5 mg/mL) dexamethasone suspension and 3 mL lidocaine (10mg/ml) into subdeltoid/subacromial bursa by one senior physician who was a board‐certified rheumatologist, physiatrist, and ultrasonographer in musculoskeletal medicine Procedure After the sterilisation of the skin on the lateral side of the affected arm, a 21‐gauge needle was inserted into the subacromial bursa under ultrasound guidance. Any effusion, if present, was aspirated prior to the injection. | LOGIQ P5 machine (General Electronic Company, Milwaukee, WI) with 5‐ to 12‐MHz linear array transducer | Summary Injection of 0.5 mL (5 mg/mL) dexamethasone suspension and 3 mL lidocaine (10 mg/mL) into subacromial bursa by the same senior physician Procedure The physician injected the subacromial bursa according to Cyriax’s method, where he/she first localised the lateral edge of the acromion. He/she then asked the patient to relax the affected arm, and at 1–2 cm beneath the middle point of the lateral edge of the acromion, inserted a 21‐gauge needle medially and in a slightly cranial direction into the bursa. If the needle encountered resistance, either the coracoacromial ligament or the capsulotendinous structures had been contacted, and the needle position was slightly adjusted. | Not reported | Not reported |
| (South Korea) | Adhesive capsulitis with pain and limited range of movement. Ultrasound used to rule out rotator cuff disease. | Summary Injection of 0.5 mL (20 mg) triamcinolone and 1.5 mL 2% lidocaine and 3 mL normal saline into glenohumeral joint by one physician with two years’ experience in ultrasound‐guided shoulder injections Procedure The posterior approach was used. Patients were seated, with the affected shoulder bent and adducted. The ultrasound probe touched the lower part of the acromion sideways, and a long needle (23G, 7 cm) was inserted to the posterior articular surface of the humeral head and positioned inside the articular capsule. The expansion of the articular capsule was checked while the fluid was being injected. | LOGIQ 5a machine with a 10‐MHz linear probe | Summary Injection of 0.5 mL (20 mg) triamcinolone and 1.5 mL 2% lidocaine and 3 mL normal saline into articular capsule by one physician with 7 years experience in anatomic landmark‐guided shoulder injections Procedure The posterior approach was used. Patients were seated, with the affected shoulder bent and adducted. The acromion of the scapula was palpated and the needle inserted 1 cm inferior to the tip of the acromion. The needle was directed toward the coracoid process and advanced into the articular capsule, after which the drug was injected. | Participants in both groups were taught exercises for increasing joint ROM including stretching forward and bending down to a desk, Codman exercise, and a wall‐climbing exercise with the fingers. They were instructed to practice these at home. Participants were checked and encouraged to keep up with the exercises every time they visited the hospital. All participants had 5 weekly injections of 25 mg sodium hyaluronate. | Not reported |
| (South Korea) | Shoulder stiffness on the basis of < 100 degrees flexion or < 30 degrees external rotation or internal rotation at a level below the first lumbar spine junction. Radiography and ultrasonography were used to rule out rotator cuff disease. | Summary Injection of 40 mg triamcinolone acetonide and 2 mL of 2% lidocaine into glenohumeral joint by one experienced senior surgeon with more than 5 years of experience in performance of ultrasound‐guided injection at the shoulder joint Procedure The patient was supine with the arm by the side. After skin and transducer preparation with alcohol and povidone, the 21‐gauge 1.5‐inch needle on a 3 mL syringe with triamcinolone acetonide 40 mg and 2 mL of 2% lidocaine was inserted at the level of the coracoids, from lateral to medial, aimed at the medial border of the humeral head. When the needle made contact with the articular cartilage of the humeral head, the needle was tilted to position the point of the needle in the articular cavity. The intra‐articular position of the needle and fluid infiltration in the shoulder joint was confirmed by ultrasonography. | High‐resolution transducer with 12 MHz linear array | Summary Injection of 40 mg triamcinolone acetonide and 2 mL of 2% lidocaine into glenohumeral joint by the same senior surgeon Procedure With the patient in sitting, the sulcus between the lateral tip of the coracoid and the humeral head was palpated. Then, a 21‐gauge 1.5‐inch needle on a 3 mL syringe with triamcinolone acetonide 40 mg and 2 mL of 2% lidocaine was inserted in a slightly cephalad direction at the anterior‐lateral tip of the coracoid. The needle was slowly advanced while infiltrating the local anaesthetic until resistance was lost, indicating intra‐articular position. The injection was performed slowly. | Both groups received a home‐based exercise programme for rehabilitation involving: pendulum circumduction and passive shoulder exercises for self‐stretching in forward flexion, external rotation and internal rotation, pulley exercise, isometric exercise, thera‐band exercises, scapula stabilisation exercises, and strengthening using dumbbells. | None in either group |
| Naredo 2004 (Spain) | ‘Periarticular disorders’ (impingement syndrome rotator cuff lesions, subacromial‐subdeltoid bursitis and/or biceps tendon abnormalities) diagnosed by clinical history and examination | Summary Injection of 20 mg triamcinolone into either subacromial‐subdeltoid bursa, biceps tendon sheath or rotator cuff calcifications according to ultrasound findings by single rheumatologists experienced in ultrasound Procedure The transducer and the patient’s skin were sterilised with alcohol. Sterile gel was applied to the probe. The transducer was held in one hand and the syringe with glucocorticoid in the other hand. The needle was | Commercial equipment (Sonoline, Prima, Siemens, Seattle, WA, USA) using a 7.5 MHz linear phased array transducer | Summary Injection of 20 mg triamcinolone into subacromial space by same rheumatologist Procedure A 21‐gauge needle was used. The patient’s skin was sterilised with alcohol. Access to the subacromial space was achieved with a lateral approach, inserting the needle under the anterolateral aspect of the acromion process, passing it through the deltoid muscle, and directing it medially and slightly anterior to the subacromial‐subdeltoid bursa, with care taken to avoid injection directly into the tendons of the rotator cuff. Immediately after blind injection, an ultrasound examination was performed to search for steroid deposit as hyperechoic foci or lines, with or without acoustic shadowing. | Participants in both groups with loss of shoulder ROM were instructed to start a home physical therapy programme consisting of pendulum exercises and slow shoulder abduction. No restriction was placed on the participant’s ability to work or to use their shoulder as tolerated, or to take nonsteroidal anti‐inflammatory drugs. Participants did not receive physical therapy during the follow‐up period. | There were no serious adverse events in either group. 1/20 participants in the ‘blind’ injection group had a mild post‐injection adverse effect (not specified). |
| Raeissadat 2017 (Iran) | Adhesive capsulitis on the basis of findings from the history and physical examination | Summary Injection of 1 cm3 lidocaine 1%, then, 3 cm3 water soluble un‐ionised contrast with 1 cm3 distilled water, and finally, 1 cm3 triamcinolone 40 mg/cm3 with 1 cm3 lidocaine 1% into glenohumeral joint by one physical medicine and rehabilitation specialist with 15 years’ experience in his field Procedure The patient was seated and the affected hand was resting on the thigh. A glenohumeral joint injection was given using the posterior short axis approach, and the needle was inserted in the plane relative to the ultrasound probe and medial to the posterior aspect of the head of the humerus. | Alpinion E‐cube 7 ultrasound device with linear 3–12‐MHz probe | Summary Injection of 1 cm3 lidocaine 1%, then, 3 cm3 water soluble un‐ionised contrast with 1 cm3 distilled water, and finally, 1 cm3 triamcinolone 40 mg/cm3 with 1 cm3 lidocaine 1% into glenohumeral joint by the same specialist Procedure A glenohumeral joint injection was given using a posterior approach. A 25‐gauge needle was inserted 2.5 cm lower than the posterolateral aspect of the acromion. If the physician felt that the needle was not accurately inserted, he was not allowed to withdraw and re‐enter again. | After the injection, all participants in both groups received naproxen tablet 500 mg twice daily for 5 days and were told to perform Codman’s exercises. | None in either group |
| (United Kingdom) | Subacromial impingement syndrome with pain in deltoid insertion area, positive Neer and Hawkins Kennedy tests, and pain on shoulder abduction | Summary Injection of methylprednisolone 40 mg and 1 mL 1% lidocaine into the bursa by one of nine clinicians with extensive clinical experience performing ultrasound‐guided injections or completed an accredited course on ultrasound‐guided subacromial injections Procedure The skin and transducer were cleaned with chlorhexidine 0.5% solution and sterile gel applied to the transducer. The participant sat with the shoulder internally rotated and the ipsilateral hand on the buttock to maximise visibility of and access to the subacromial bursa. The transducer was placed anterolaterally, the hypoechoic subacromial bursa visualised, and a 21‐G needle inserted under real‐time ultrasound guidance until the needle‐tip entered the bursa. A commercially available premixed solution of methylprednisolone 40 mg and 1 mL 1% lidocaine was injected into the bursa. | LOGIQ e system with a 12 MHz transducer | Summary Injection of methylprednisolone 40 mg and 1 mL 1% lidocaine into the bursa by one of eight clinicians (different to those performing the ultrasound‐guided injections) with extensive clinical experience performing subacromial injections, and having attended a half‐day injection protocol workshop Procedure The participant sat with the arm hanging with the elbow bent and forearm resting on the lap. The skin was cleaned with chlorhexidine solution 0.5%. A 21‐G needle was inserted through the deltoid under the acromion process laterally. The same premixed solution of methylprednisolone 40 mg and 1 mL 1% lidocaine was injected. | None | Ultrasound‐guided injection and physiotherapist‐led exercise (n = 64): Serious adverse event: No. and nature of events: 1/64 ‐ pyelonephritis Other adverse event: No. and nature of events: 17/64 ‐ shoulder pain > 3 days following injection No. and nature of events: 16/64 ‐ discomfort during the injection or local skin changes, presyncope, nausea or flushing following the injection Ultrasound‐guided injection and exercise leaflet (n = 64): Other adverse event: No. and nature of events: 17/64 ‐ shoulder pain > 3 days following injection No. and nature of events: 16/64 ‐ discomfort during the injection or local skin changes, presyncope, nausea or flushing following the injection Unguided injection and physiotherapist‐led exercise (n = 64): Other adverse event: No. and nature of events: 20/64 ‐ shoulder pain > 3 days following injection No. and nature of events: 17/64 ‐ discomfort during the injection or local skin changes, presyncope, nausea or flushing following the injection Unguided injection and exercise leaflet (n = 64): Other adverse event: No. and nature of events: 20/64 ‐ shoulder pain > 3 days following injection No. and nature of events: 17/64 ‐ discomfort during the injection or local skin changes, presyncope, nausea or flushing following the injection |
| Saeed 2014 (Ireland) | Shoulder impingement pain with shoulder pain | Summary Injection of 40 mg of methylprednisolone acetate with 4 mL of lidocaine hydrochloride into either subacromial‐subdeltoid bursa, acromio‐clavicular joint, biceps tendon or other pathology according to ultrasound findings by one experienced physician. Procedure A two‐handed technique was used with the transducer held in one hand and the syringe with 21‐G needle in the other hand. The needle was directed in real time by ultrasound from the skin to the target (e.g. subdeltoid bursa, acromio‐clavicular joint or biceps pathology). In those shoulders where ultrasound demonstrated more than one pathology, the pathology most consistent with clinical examination was injected. When there was effusion in both the subacromial‐subdeltoid bursa and the biceps tendon sheath, injection was directed into the subacromial‐subdeltoid bursa. For confirmed rotator cuff pathology and inpatients with clinical impingement but normal shoulder, ultrasound subacromial‐subdeltoid bursa injection was performed. When ultrasound confirmed the principal pathology as acromioclavicular joint inflammation or biceps tendon inflammation, ultrasound‐guided injection of the principal pathological structure was performed. | Acuson Sequoia 512 ultrasound systems (Siemens, CA, USA) using 8L‐RS MHz linear phased‐array transducer | Summary Injection of 40 mg of methylprednisolone acetate with 4 mL of lidocaine hydrochloride into subacromial‐subdeltoid bursa by the same physician Procedure A standard technique was performed using a 21‐G needle via a lateral approach to inject the subacromial‐subdeltoid bursa. | Participants in both groups with the loss of shoulder range of movement were given post‐injection instructions in pendulum exercises and slow shoulder abduction. No restriction was placed on participants' ability to work or to use their shoulder as tolerated, or intake of NSAIDs. Participants did not receive physical therapy during the follow‐up period. | Apart from mild shoulder pain in a small number of cases (group not specified) within a few hours of shoulder injection, no serious adverse event was observed. |
| (Turkey) | ‘Soft‐tissue lesions’ (acromioclavicular degeneration, rotator cuff lesions (rupture, partial rupture, tendinosis, impingement, calcification), fluid accumulation or partial rupture of biceps tendon and bursitis (subdeltoid, subacromial)) that had not responded satisfactorily to at least 1 month of nonsteroidal anti‐inflammatory drugs | Summary Injection of 1 mL (40 mg) triamcinolone and 1 mL 1% lidocaine into site of observed pathology. Injections were administered ‘perilesionally’ and ‘intralesionally’; personnel not specified. Procedure The injector was directed to the site of observed pathology beneath the probe by imaging a hyper‐reflective line. | Commercial equipment (Esaote, Mylab 60, Italy) using a 6 to 18MHz linear phased array transducer | Summary Injection of 1 mL (40 mg) triamcinolone and 1 mL 1% lidocaine into subacromial region; personnel not specified Procedure Injections were administered with a lateral entry approach to the sub‐acromial region. | Participants in both groups with loss of shoulder ROM performed a home exercise programme consisting of shoulder abduction and pendulum exercises. No limit was imposed on nonsteroidal anti‐inflammatory consumption or use of the shoulder. Participants did not receive physical therapy during the follow‐up period. | There were no serious adverse events in either group. 1/30 participants in the ultrasound‐guided injection group had mild post‐injection pain. In the ‘blind’ injection group, 5/30 had a slight increase in pain and 1/30 had skin peeling after the injection. |
| Zufferey 2012 (Switzerland) | Acute shoulder pain that had not responded satisfactorily to nonsteroidal anti‐inflammatory drugs or physiotherapy | Summary Injection of 2 mL of Diprophos® (7 mg of a mixture made up of one‐third soluble and two‐thirds long‐acting bethamethasone) into site of observed pathology by two rheumatologists experienced in musculoskeletal ultrasonography Procedure Steroid injection under ultrasound guidance was performed according to the ultrasound diagnosis within two days of the clinical evaluation. The injection was directed into the location assessed by ultrasound to be the cause of shoulder pain. | 5‐9 MHz probe using a Philips HD11 machine in two centres and Easote mylab25 gold machine in one centre | Summary Injection of 2 mL of Diprophos® (7 mg of a mixture made up of one‐third soluble and two‐thirds long‐acting bethamethasone) into subacromial bursa; personnel not specified Procedure Blind injections were directed at the subacromial bursa. | Rescue analgesia was permitted for both groups and participants recorded their intake of nonsteroidal anti‐inflammatory drugs and paracetamol until the end of the study. New infiltrations after two weeks were tolerated but considered a poor result. | There were no serious adverse events in either group. 3/32 in the ultrasound‐guided group and 3/35 in the ‘blind’ injection group required a second local injection after 2 or 6 weeks. Four patients reported flushing and one diabetic patient a transient hyperglycaemia, however, there were no details of which groups these participants belonged to. |
| MHz = megahertz; mL = millilitre; mg = milligrams; cm = centimetre; T6 = sixth thoracic vertebrae; cc = cubic centimetre; mmol = millimole; ROM = range of motion; SA‐SD bursa = subacromial‐subdeltoid bursa; 21‐G = 21 gauge; NSAIDs = non‐steroidal anti‐inflammatory drugs | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Overall pain (or daytime, activity‐related or unspecified) Show forest plot | 18 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 up to 3 weeks | 9 | 554 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.31, ‐0.03] |
| 1.1.2 > 3 weeks up to 6 weeks | 15 | 1003 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.84, ‐0.20] |
| 1.1.3 > 6 weeks up to 3 months | 6 | 424 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.24, 0.09] |
| 1.1.4 > 3 months to 6 months | 2 | 205 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.53, 0.31] |
| 1.1.5 > 6 months | 2 | 205 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.73, 1.06] |
| 1.2 Function Show forest plot | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 up to 3 weeks | 8 | 487 | Mean Difference (IV, Random, 95% CI) | 5.45 [‐0.29, 11.20] |
| 1.2.2 > 3 weeks up to 6 weeks | 14 | 895 | Mean Difference (IV, Random, 95% CI) | 2.44 [‐0.17, 5.06] |
| 1.2.3 > 6 weeks up to 3 months | 4 | 257 | Mean Difference (IV, Random, 95% CI) | 9.15 [‐5.78, 24.09] |
| 1.2.4 > 3 months to 6 months | 2 | 205 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐9.30, 7.19] |
| 1.2.5 > 6 months | 2 | 205 | Mean Difference (IV, Random, 95% CI) | 3.35 [‐4.69, 11.38] |
| 1.3 Treatment success (50% pain improvement on VAS) Show forest plot | 5 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.89, 2.75] |
| 1.4 Quality of life Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 up to 3 weeks | 2 | 122 | Mean Difference (IV, Random, 95% CI) | 4.17 [‐0.97, 9.31] |
| 1.4.2 > 3 weeks up to 6 weeks | 2 | 220 | Mean Difference (IV, Random, 95% CI) | 2.81 [‐0.73, 6.35] |
| 1.4.3 > 6 weeks to 3 months | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.60 [‐1.27, 10.47] |
| 1.4.4 > 3 months to 6 months | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐2.49, 5.69] |
| 1.4.5 > 6 months | 1 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐4.88, 3.48] |
| 1.5 Number of adverse events Show forest plot | 5 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.40, 1.28] |
| 1.6 Withdrawals due to adverse events Show forest plot | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.00] |
| 1.7 Additional injections or surgery Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7.1 Additional injections | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.14, 2.15] |
| 1.7.2 Surgery | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.11] |
| 1.8 Range of flexion Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 up to 3 weeks | 5 | 305 | Mean Difference (IV, Random, 95% CI) | 3.45 [‐1.60, 8.49] |
| 1.8.2 > 3 weeks up to 6 weeks | 8 | 461 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐2.32, 4.49] |
| 1.8.3 > 6 weeks up to 3 months | 2 | 167 | Mean Difference (IV, Random, 95% CI) | 3.70 [‐1.42, 8.82] |
| 1.8.4 > 3 months to 6 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐5.49, 6.49] |
| 1.8.5 > 6 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 4.00 [‐4.53, 12.53] |
| 1.9 Range of abduction Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 up to 3 weeks | 5 | 317 | Mean Difference (IV, Random, 95% CI) | 12.57 [2.62, 22.53] |
| 1.9.2 > 3 weeks up to 6 weeks | 9 | 528 | Mean Difference (IV, Random, 95% CI) | 6.85 [1.47, 12.22] |
| 1.9.3 > 6 weeks to 3 months | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐8.46, 10.66] |
| 1.10 Range of external rotation Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.10.1 up to 3 weeks | 4 | 199 | Mean Difference (IV, Random, 95% CI) | 1.01 [‐3.88, 5.90] |
| 1.10.2 > 3 weeks up to 6 weeks | 6 | 334 | Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐3.50, 2.04] |
| 1.10.3 > 6 weeks up to 3 months | 2 | 167 | Mean Difference (IV, Random, 95% CI) | 3.87 [‐0.42, 8.17] |
| 1.10.4 > 3 months to 6 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 3.20 [‐8.10, 14.50] |
| 1.10.5 > 6 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐13.33, 7.93] |
| 1.11 Serious adverse events Show forest plot | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.12, 72.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Overall pain Show forest plot | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Subacromial disease | 12 | 777 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.05, ‐0.11] |
| 2.1.2 Adhesive capsulitis | 3 | 189 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.39, ‐0.03] |
| 2.1.3 Mixed or undefined shoulder pain | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.46, 0.06] |
| 2.2 Function Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 Subacromial disease | 11 | 687 | Mean Difference (IV, Random, 95% CI) | 5.06 [‐3.23, 13.35] |
| 2.2.2 Adhesive capsulitis | 3 | 171 | Mean Difference (IV, Random, 95% CI) | 1.05 [‐1.18, 3.29] |
| 2.2.3 Mixed or undefined shoulder pain | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 4.00 [0.05, 7.95] |

