Rituximab bei schubförmig remittierender Multipler Sklerose
Abstract
Background
This is an update of the Cochrane review "Rituximab for relapsing‐remitting multiple sclerosis" (first published in The Cochrane Library 2011, Issue 12).
More than 80% of individuals with multiple sclerosis (MS) experience a relapsing‐remitting disease course. Approximately 10 years after disease onset, an estimated 50% of individuals with relapsing‐remitting MS (RRMS) convert to secondary progressive MS. MS causes a major socioeconomic burden for the individual patient and for society. Effective treatment that reduces relapse frequency and prevents progression could impact both costs and quality of life and help to reduce the socioeconomic burden of MS. Alternative and more effective MS treatments with new modes of action and good safety are needed to expand the current treatment repertoire. It has been shown that B lymphocytes are involved in the pathophysiology of MS and rituximab lyses B‐cells via complement‐dependent cytotoxicity and antibody‐dependent cellular cytotoxicity. Current clinical trials are evaluating the role of rituximab as a B‐cell depletion therapy in the treatment of RRMS.
Objectives
The safety and effectiveness of rituximab, as monotherapy or combination therapy, versus placebo or approved disease‐modifying drugs (DMDs) (interferon‐β (IFN‐β), glatiramer acetate, natalizumab, mitoxantrone, fingolimod, teriflunomide, dimethyl fumarate, alemtuzumab) to reduce disease activity for people with RRMS were assessed.
Search methods
The Trials Search Co‐ordinator searched the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group Specialised Register (9 August 2013). We checked the references in identified trials and manually searched the reports (2004 to August 2013) from neurological associations and MS societies in Europe and America. We also communicated with researchers who were participating in trials on rituximab and contacted Genentech, BiogenIdec and Roche.
Selection criteria
All randomised, double‐blind, controlled parallel group clinical trials with a length of follow‐up equal to or greater than one year evaluating rituximab, as monotherapy or combination therapy, versus placebo or approved DMDs for patients with RRMS without restrictions regarding dosage, administration frequency and duration of treatment.
Data collection and analysis
We used the standard methodological procedures of The Cochrane Collaboration. Two review authors independently assessed trial quality and extracted data. Disagreements were discussed and resolved by consensus among the review authors. Principal investigators of included studies were contacted for additional data or confirmation of data.
Main results
One trial involving 104 adult RRMS patients with an entry score ≤ 5.0 on the Expanded Disability Status Scale (EDSS) and at least one relapse during the preceding year was included. This trial evaluated rituximab as monotherapy versus placebo, with a single course of 1000 mg intravenous rituximab (on day 1 and day 15). A significant attrition bias was found at week 48 (24.0%). Patients receiving rituximab had a significant reduction in total number of gadolinium‐enhancing lesions at week 24 (mean number 0.5 versus 5.5; relative reduction 91%) and in annualised rate of relapse at week 24 (0.37 versus 0.84) but not at week 48 (0.37 versus 0.72). Disability progression was not included as an outcome in this trial. More patients in the rituximab group had adverse events within the 24 hours after the first infusion (78.3% versus 40.0%), such as chills, headache, nausea, pyrexia, pruritus, fatigue, throat irritation, pharyngolaryngeal pain, and most were mild‐to‐moderate events (92.6%). The most common infection‐associated adverse events (> 10% in the rituximab group) were nasopharyngitis, upper respiratory tract infections, urinary tract infections and sinusitis. Among them, only urinary tract infections (14.5% versus 8.6%) and sinusitis (13.0% versus 8.6%) were more common in the rituximab group. One ongoing trial was identified.
Authors' conclusions
There is not sufficient evidence to support the use of rituximab as a disease‐modifying therapy for RRMS because only one RCT was included. The quality of the study was limited due to high attrition bias, the small number of participants, and short follow‐up. The beneficial effects of rituximab for RRMS remain inconclusive. However, short‐term treatment with a single course of rituximab was safe for most patients with RRMS. Mild‐to‐moderate infusion‐associated adverse events were common, as well as nasopharyngitis, upper respiratory tract infections, urinary tract infections and sinusitis. The potential benefits of rituximab for treating RRMS need to be evaluated in large‐scale studies that are of high quality along with long‐term safety.
PICO
Laienverständliche Zusammenfassung
Die Anwendung des monoklonalen Antikörpers Rituximab bei Patienten mit schubförmig remittierender Multipler Sklerose
Es handelt sich hierbei um eine aktualisierte Version des Cochrane Reviews „Rituximab bei schubförmig remittierender Multipler Sklerose“ (erstmalig veröffentlicht in The Cochrane Library 2011, Ausgabe 12).
Neuere Studien zeigen, dass eine Klasse weißer Blutkörperchen (B‐Lymphozyten) an der MS‐Erkrankung beteiligt sein können. Dieses Merkmal hat zu einem neuerlichen Interesse an Therapien geführt, die auf die Kontrolle der Aktivität von B‐Zellen ausgerichtet sind. Rituximab gehört zu dieser Klasse monoklonaler Antikörper und ist in der Lage, die Anzahl an B‐Lymphozyten in der Gehirn‐Rückenmarks‐Flüssigkeit zu verringern. Die Autoren des vorliegenden Reviews bewerteten die Wirksamkeit und Sicherheit bei Patienten mit schubförmig remittierender Multipler Sklerose unter Berücksichtigung von Schüben, Hirnläsionen und der Krankheitsprogression. Von der einschlägigen Literatur wurde nur eine Studie eingeschlossen, die Rituximab im Vergleich zum Placebo bei 104 erwachsenen Patienten mit mindestens einem Schub im vorangegangenen Jahr beurteilte.
Die Autoren fanden keine überzeugende Evidenz, die Rituximab als wirksame Behandlung bei schubförmig remittierender Multipler Sklerose unterstützte, auch deshalb, weil die Qualität der einen identifizierten Studie aufgrund von Bias, der geringen Anzahl an Teilnehmern und der kurzen Nachbeobachtungsdauer eingeschränkt war. Im Hinblick auf die Sicherheit berichteten Patienten unerwünschte Ereignisse in Verbindung mit der Infusion innerhalb von 24 Stunden nach der ersten Infusion, darunter Schüttelfrost, Kopfschmerzen, Übelkeit, Fieber, Juckreiz, Müdigkeit/Erschöpfung, Hustenreiz und Schmerzen im Rachen‐ und Kehlkopfbereich. Von den mit Infektionen verbundenen unerwünschten Ereignissen traten nur Harnwegsinfekte (bei 14,5 % im Vergleich zu 8,6 %) und Sinusitis (13,0 % im Vergleich zu 8,6 %) in der Rituximab‐Gruppe häufiger auf. Der mögliche Nutzen von Rituximab zur Behandlung von schubförmig remittierender Multipler Sklerose muss weiter in größeren Studien, die eine höhere Qualität aufweisen, zusammen mit der Langzeitsicherheit untersucht werden.
Authors' conclusions
Summary of findings
| Rituximab for relapsing‐remitting multiple sclerosis | ||||||
| Patient or population: patients with relapsing‐remitting multiple sclerosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rituximab | |||||
| The annualised rate of relapse | The mean the annualised rate of relapse in the control groups was | The mean the annualised rate of relapse in the intervention groups was 0.35 lower | 104 | ⊕⊕⊝⊝ | ||
| The sum of the number of gadolinium‐enhancing T1‐weighted lesions | The mean the sum of the number of gadolinium‐enhancing t1‐weighted lesions in the control groups was | The mean the sum of the number of gadolinium‐enhancing t1‐weighted lesions in the intervention groups was | 104 | ⊕⊕⊝⊝ | ||
| The number of patients with adverse effects | High | RR 0.99 | 104 | ⊕⊕⊝⊝ | ||
| 100 per 100 | 99 per 100 | |||||
| The number of patients with serious adverse events | Low | RR 0.91 | 104 | ⊕⊕⊝⊝ | ||
| 143 per 1000 | 130 per 1000 | |||||
| The number of patients who withdrew or dropped out from the study because of AEs | Low | RR 0.76 | 104 | ⊕⊕⊝⊝ | ||
| 57 per 1000 | 43 per 1000 | |||||
| The number of patients with disability progression | Study population | Not estimable | 0 | See comment | This outcome was not included as an endpoint in the trial. | |
| See comment | See comment | |||||
| The time to confirmed disease progression | Study population | Not estimable | 0 | See comment | This outcome was not included as an endpoint in the trial. | |
| See comment | See comment | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 This study had a high rate of dropouts (24.0%), and the methodology for random sequence generation and allocation concealment was unclear. | ||||||
Background
Description of the condition
Multiple sclerosis (MS) is a chronic immune‐mediated disease of the central nervous system (CNS) in which autoreactive CD4+ and CD8+ T lymphocytes, B lymphocytes, antibodies, macrophages and cytokines synergize in myelin sheath attack and injury of the underlying axons. It is characterized by recurrent relapses and / or progression that are attributable to multifocal inflammation, demyelination and axonal loss within the CNS, typically striking adults during the primary productive time of their lives and ultimately leading to severe neurological disability.
Initially, more than 80% of individuals with MS experience a relapsing‐remitting disease course (RRMS) characterized by clinical exacerbations of neurologic symptoms followed by complete or incomplete remission (Lublin 1996). After 10 to 20 years, or median age of 39.1 years, about half gradually accumulate irreversible neurologic deficits with or without clinical relapses (Confavreux 2006), which is known as secondary progressive MS (SPMS).
MS causes a major socioeconomic burden for the individual patient and for society. Increasing costs and decreasing quality of life are associated with advancing disease severity, disease progression and existence of relapses (Karampampa 2012a). From a patient's perspective an MS relapse is associated with a significant increase in the economic costs as well as a decline in health related quality of life and functional ability (Oleen‐Burkey 2012).
Effective treatment that reduces relapse frequency and prevents progression could impact both costs and quality of life and may help to reduce the social burden of MS (Karampampa 2012b). On the basis of high quality evidence, natalizumab and interferon β‐1a (IFNβ‐1a) (Rebif) are superior to mitoxantrone, glatiramer acetate, IFNβ‐1b (Betaseron), IFNβ‐1a (Avonex), methotrexate, cyclophosphamide, azathioprine, intravenous immunoglobulins and long‐term corticosteroids for preventing clinical relapses in RRMS in the short‐term (24 months) compared to placebo. However they are associated with long‐term serious adverse events and their benefit‐risk balance might be unfavourable (Filippini 2013). Therefore, alternative MS treatments with new modes of action and good safety are needed to expand the current treatment repertoire, such as B‐cell directed therapy.
Description of the intervention
Evidence is accumulating on the involvement of B lymphocytes in the pathophysiology of MS. The contribution of B‐cells and their secreted products to MS may relate to the abilities of B‐cells to (1) differentiate into plasmocytes that produce antibodies; (2) function as antigen‐presenting cells, contributing to T‐cell activation; (3) produce effector cytokines that may modulate the local immune environment; (4) harbour the Epstein‐Barr virus in a chronically activated state; and (5) play a role in the formation and maintenance of new lymphoid foci within the CNS (Waubant 2008). In people with RRMS, B‐cells are critical for T‐cell trafficking into the CNS and may alter the process by influencing chemokine production within the CNS (Piccio 2010). Interleukin‐6 (IL‐6) secretion is a major mechanism of B‐cell driven pathogenesis in experimental autoimmune encephalomyelitis (EAE) and MS (Barr 2012). B‐cell depletion affects antibody production, cytokine networks, and B‐cell mediated antigen presentation and activation of T‐cells and macrophages in MS (Duddy 2006).
Rituximab is a glycosylated immunoglobulin G1 (IgG1) κ chimeric mouse‐human antibody that binds to the CD20 antigen present on the majority of circulating B‐cells, with the exception of plasma cells and haematopoietic stem cells (Waubant 2008). Rituximab lyses circulating B‐cell populations via complement‐dependent cytotoxicity (CDC) (Di Gaetano 2003), antibody‐dependent cellular cytotoxicity (ADCC), and possibly promotion of apoptosis (Reff 1994). It is indicated for the treatment of patients with non‐Hodgkin's lymphoma (375 mg/m2, intravenously); chronic lymphocytic leukaemia (375 mg/m2 in the first cycle and 500 mg/m2 in cycles 2 to 6, intravenously); rheumatoid arthritis (RA) in combination with methotrexate in adult patients with moderate to severe active RA who have an inadequate response to one or more tumour necrosis factor antagonist therapies (two intravenous infusions of a fixed dosage of 1000 mg separated by 2 weeks (one course) every 24 weeks); granulomatosis with polyangiitis (Wegener's granulomatosis) and microscopic polyangiitis in adult patients in combination with glucocorticoids (375 mg/m2 once weekly for 4 weeks, intravenously) (FDA 2013).
The application of rituximab to the treatment of MS uses intravenous administration of a fixed dosage of 1000 mg rituximab on day 1 and 15 (one course), with subsequent courses of treatment at week 24 (day 169), week 48 (day 337) and week 72 (day 505) and a second infusion 14 ± 1 days after the first one (Taupin 2011). This scheme has been adopted in recent RRMS trials. After two courses of intravenous rituximab infusion, serum drug concentrations exhibit a biphasic profile and the mean terminal half‐life of the drug is 22 days. B‐cell depletion has been shown to be near complete (99.8%) by week two and sustained through week 48 (Bar‐Or 2008). The treatment with rituximab also causes a depletion of cerebrospinal fluid (CSF) B‐cells 24 weeks after the initial treatment in MS patients in association with a reduction in CSF T‐cells (Cross 2006). B lymphocytes were depleted from brain tissue after rituximab therapy in patients with MS (Martin 2009). B‐cell depletion therapy (BCDT) with rituximab normalized the elevated levels of IL‐6 as a result of secretion by B‐cells in RRMS patients (Barr 2012). Current clinical trials are evaluating a role for B‐cell depletion therapy using rituximab in the treatment of MS.
How the intervention might work
Preliminary results of an open‐label study using two courses of intravenous rituximab in people with RRMS suggested that rituximab reduced new gadolinium‐enhancing or T2 lesions and relapses without serious adverse events (Bar‐Or 2008). Very recently, results of a study of rituximab add‐on therapy (375 mg/m2 intravenously at weekly intervals, 4 doses) in patients with RRMS demonstrate that rituximab (added on to INFβ‐1a, INFβ‐1b or glatiramer acetate) reduced gadolinium‐enhancing brain lesions (Naismith 2010). In a randomised, double‐blind, placebo‐controlled, multicentre study with a single course of rituximab in people with RRMS, rituximab (monotherapy versus placebo) demonstrated efficacy in reducing disease activity (relapses and gadolinium‐enhancing lesions) by selectively depleting CD20+ B‐cells (Hauser 2008).
Why it is important to do this review
Although trials on the use of rituximab for relapsing and progressive MS have been systematically reviewed (Castillo‐Trivino 2013), a systematic review of all randomised controlled trials is warranted to evaluate the effectiveness and safety of rituximab for RRMS.
This is an update of the Cochrane review "Rituximab for relapsing‐remitting multiple sclerosis" (first published in The Cochrane Library 2011, Issue 12).
Objectives
The safety and effectiveness of rituximab, as monotherapy or combination therapy, versus placebo or approved disease‐modifying drugs (DMDs) (IFN‐β, glatiramer acetate, natalizumab, mitoxantrone, fingolimod, teriflunomide, dimethyl fumarate, alemtuzumab) to reduce disease activity for people with RRMS were assessed.
Methods
Criteria for considering studies for this review
Types of studies
All randomised, double‐blind, controlled parallel group clinical trials evaluating rituximab, as monotherapy or combination therapy, versus placebo or approved DMDs for people with RRMS. Uncontrolled, non‐randomised or quasi‐randomised trials were excluded. Trials with a length of follow‐up shorter than one year were excluded.
Types of participants
Inclusion criteria
(1) Definite diagnosis of MS according to Poser's (Poser 1983) or McDonald's (McDonald 2001; Polman 2005; Polman 2011) criteria
(2) Male or female, any race, aged > 18 years
(3) Expanded Disability Status Scale (EDSS) (Kurtzke 1983) scores ≤ 6.0
(4) Clinical course characterised by a relapsing‐remitting disease course
(5) Irrespective of duration of disease, frequency of relapse
Exclusion criteria
Diagnosis of other types of MS, such as secondary progressive, primary progressive, or progressive relapsing MS
Types of interventions
Experimental intervention
Treatment with intravenous rituximab, as monotherapy or combination therapy, without restrictions regarding dosage, administration frequency and duration of treatment
Control intervention
Placebo or an approved DMDs
Types of outcome measures
Primary outcomes
We assessed the following primary outcomes measured in the treatment phase and at the completion of follow‐up versus baseline.
Efficacy
(1) The annualised rate of relapse at one year (or later), defined as the mean number of confirmed relapses per patient adjusted for the duration of follow‐up to annualise it. Relapse is defined as the occurrence of new symptoms or worsening of previously stable or improving symptoms and signs that are not associated with fever or infection, occurring at least 30 days after the onset of a preceding relapse and lasting more than 24 hours.
(2) The sum of the number of gadolinium‐enhancing T1‐weighted lesions at one year (or later). Lesions that persisted for more than four weeks were counted more than once.
Safety
The number of patients with adverse effects (AEs), number of patients with serious adverse events (SAEs), and number of patients who withdrew or dropped out from the study because of AEs measured at one year (or later).
Secondary outcomes
We assessed the following secondary outcomes, measured in the treatment phase and at the completion of follow‐up versus baseline.
(1) The number of patients with disability progression at two years (or later). We accepted the definitions of confirmed disability progression reported in the included trials.
(2) The time to confirmed disease progression at two years (or later).
(3) Mean change in quality of life (QoL). The following scales were accepted: Short Form‐36 (SF‐36) (Ware 1992), Multiple Sclerosis Quality of Life‐54 (MSQOL‐54) (Vickrey 1995), Multiple Sclerosis Quality of Life Inventory (MSQLI) (Fischer 1999), or Functional Assessment of MS (FAMS) (Cella 1996) at two years (or later).
Search methods for identification of studies
No language restrictions were applied to the search.
Electronic searches
The Review Group Trials Search Co‐ordinator searched the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group Trials Register (9 August 2013) which, among other sources, contains trials from:
-
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 3);
-
MEDLINE (PubMed) (1966 to 9 August 2013);
-
EMBASE (Embase.com) (1974 to 9 August 2013);
-
CINAHL (EBSCOhost) (1981 to 9 August 2013);
-
LILACS (Bireme) (1982 to 9 August 2013);
-
PEDro (1990 to 9 August 2013);
-
Clinicaltrials.gov (www.clinicaltrials.gov);
-
World Health Organization (WHO) International Clinical Trials Registry Portal (http://apps.who.int/trialsearch/).
Information on the Cochrane Multiple Sclerosis Group Trials Register and details of the search strategies used to identify trials can be found in the 'Specialised Register' section within the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group module.
The keywords used to search for this review are listed in Appendix 1.
Searching other resources
-
We checked references cited in identified trials
-
We handsearched reports (2004 to August 2013) from neurological associations and MS Societies in Europe and America (The Society for Neuroscience, The American Academy of Neurology, The World Federation of Neurology, Federation of European Neuroscience Societies, British Neuroscience Association, National Multiple Sclerosis Society)
-
We communicated with researchers participating in trials on rituximab
-
We contacted Genentech (http://www.gene.com/gene/index.jsp); BiogenIdec (http://www.biogenidec.com); Roche (http://www.roche.com/index.htm) in an effort to identify further studies
Data collection and analysis
Selection of studies
Titles and abstracts of the references identified by the literature search were screened independently for inclusion or exclusion by two review authors (He and Dong). The full texts of potentially relevant studies were selected for further assessment. The eligibility of these studies was evaluated independently. Papers that did not meet the inclusion criteria were listed in the 'Characteristics of excluded studies' table with the reason for omission. Disagreement regarding inclusion was resolved by discussion or by referral to a third assessor (Zhou), if necessary.
Data extraction and management
Two review authors (He and Dong) independently extracted data from the selected trials using standardised forms. Information about study design, participants, the intervention and outcome measures were extracted. Principal investigators of included studies were contacted so that they could provide additional data or confirmation of methodological aspects of the study. Disagreements were discussed and resolved by consensus among the review authors.
Assessment of risk of bias in included studies
The methodological criteria were based on the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (Higgins 2011). Two review authors (He and Dong) independently evaluated the methodological quality of the studies using the risk of bias tool under the domains of random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other biases. Studies with a high risk of bias were excluded. Disagreements among the review authors on the methodological quality of the identified studies were discussed and resolved by consensus.
Measures of treatment effect
We did not enter any data into the RevMan analysis because only one study was included in this review. If data are available in future updates we will analyse them as follows.
The pre‐set outcomes in this review involved counts and rates, dichotomous, ordinal (measurement scales) and time‐to‐event data.
Both the number of relapses and new enhancing lesions are count data. Analyses of counts of rare events (Poisson data) often focus on rates (rates relate the counts to the amount of time during which they could have happened). Rate ratio, which compares the rate of events in the two groups by dividing one by the other, will be used to measure the treatment effect on counts of rare events. When measuring the treatment effect on counts of common events, the mean difference will be used to compare the difference in the mean number of events (possibly standardised to a unit time period) experienced by the participants in the intervention group compared with the participants in the control group. Both MS relapse and gadolinium‐enhancing T1‐weighted lesions are common events, therefore the mean difference will be used as the measure of treatment effect.
For dichotomous outcomes, individual and pooled statistics will be calculated as relative ratio (RR) or odds ratio (OR), the risk difference (RD) (also called the absolute risk reduction) and the number needed to treat (NNT). Both the number of patients with disability progression and the number of patients with adverse effects are dichotomous outcomes, and we will use RRs as the measure of treatment effect.
Where the ordinal rating scales used in the trials have a reasonably large number of categories, the data will be treated as continuous outcomes arising from a normal distribution. Mean change in QoL is ordinal data. For trials that have used the same rating scale to assess outcome the mean difference will be used as the measure of treatment effect. Where different rating scales have been used the measure of the treatment difference is the standardised mean difference.
Time‐to‐event data can be analysed as dichotomous data when the status of all patients in a study is known at a fixed time point. The time to confirmed disability progression provides time‐to‐event data; we will use RRs or hazard ratios as the measure of treatment effect.
For continuous variables, mean values before and after the intervention, the mean change from baseline, standard deviations, and the number of patients for each treatment group of the individual studies will be extracted. The standard deviation will be calculated from the confidence interval or t‐tests when not reported. The standard error of the mean change will be calculated from the standard deviation. For binary outcomes, the number of patients for each treatment group of the individual studies and the number of patients incurring the event in each group will be extracted.
Unit of analysis issues
The pre‐set outcome measures in this review involve events that may reoccur. If data are available in future updates, for the trials with multiple arms we will combine all relevant experimental intervention groups in the study into a single group and combine all relevant control intervention groups into a single control group if there is a study with two control intervention groups treated with the same drug and different doses. Meanwhile, we will perform separate analyses based on the pre‐set outcomes in this review and the different periods of follow‐up.
Dealing with missing data
We did not conduct a meta‐analysis, so trial authors were not contacted for missing data. If sufficient data are not available from published reports in future updates, the authors will be contacted for further details. A last observation carried forward imputation strategy will be used for data not missing at random and the intention‐to‐treat principle will be used for the analyses. For the data assumed to be missing at random, only the available data will be analysed. We will address the potential impact of missing data on the findings of the review in the 'Discussion' section.
Assessment of heterogeneity
The one study included in this review did not permit an assessment of heterogeneity. If more studies are included and further data become available in future updates, we will assess clinical heterogeneity by examining the characteristics of the studies, the similarity between the types of participants, the interventions and the outcomes as specified in the criteria for included studies. The variability in study design and risk of bias (methodological heterogeneity) will be also evaluated. When pooling trials in meta‐analyses, the I2 statistic will be calculated to identify heterogeneity across studies. With I2 > 30% there is some level of heterogeneity (Higgins 2011). If tests for heterogeneity are statistically significant and inspection of the individual results suggests that it still logical to combine results, we will calculate the overall effects using a random‐effects model.
Assessment of reporting biases
We could not perform an assessment of reporting biases due to the lack of adequate studies included in this review. If sufficient RCTs are identified in future updates, potential publication bias will be examined using a funnel plot. For continuous outcomes, the standard error will be used as the vertical axis and the mean difference will be used as the horizontal axis in funnel plots. For dichotomous outcomes, the odds ratio or risk ratio will be plotted on a logarithmic scale as the horizontal axis and the standard error will be used as the vertical axis.
Data synthesis
We were unable to conduct meta‐analysis because only one study was included in this review. We have given a descriptive summary of the results. When clinically and methodologically homogeneous randomised controlled trials (RCTs) are identified in future updates and heterogeneity tests suggest an I2 < 30%, or inspection of the individual results suggests that it still seems logical to combine results even though tests for heterogeneity are statistically significant, formal meta‐analysis will be conducted using Review Manager software (Review Manager 2013). Treatment effect estimates for each study and the weighted average of the treatment effects estimated in the individual studies (as a pooled treatment effect estimate) will be calculated, then a random‐effects model or fixed‐effect model will be selected according to the results of the heterogeneity tests. If it is assumed that each study is estimating exactly the same quantity a fixed‐effect model will be performed, otherwise a random‐effects model will be used. For the outcomes treated as dichotomous data (the number of patients with disability progression, the number of patients with adverse effects, and the time to confirmed disability progression), three fixed‐effect model methods (Mantel‐Haenszel, Peto, or inverse variance) and one random‐effects model method (DerSimonian and Laird) will be selected (the Peto method can only pool odds ratios whilst the other three methods can pool odds ratios, risk ratios, and risk differences). For the outcomes treated as continuous and count data (relapse, the number of gadolinium‐enhancing T1‐weighted lesions, mean change in QoL), the inverse‐variance fixed‐effect model method and the inverse‐variance random‐effects model method will selected.
Subgroup analysis and investigation of heterogeneity
The lack of data did not permit a subgroup analysis, but in future updates and if further data become available we intend to undertake subgroup analyses according to: (1) different therapies (monotherapy, combination therapy); (2) duration of follow‐up (1 year, between 1 and 2 years, more than 2 years); (3) baseline EDSS scores (≤ 3.5, between 3.5 and 6); (4) dosage level (recommended dosage in MS as a fixed dosage of 1000 mg (intravenous), given as a repeated course on day 1 and 15 every 24 weeks with second infusion 14 ± 1 days after the first one, other dosage including 375 mg/m2 given intravenously weekly for 4 doses).
Sensitivity analysis
If a sufficient number of studies had been included, we would have undertaken sensitivity analyses to assess the robustness of our review results. Where possible, we will conduct sensitivity analyses to assess the influence on results of fixed‐effect model versus random‐effects model assumptions, of including trials at high risk of bias, analysing the results by intention to treat, and the effect size (for dichotomous outcomes relative ratios versus odds ratios; and for continuous outcomes the mean difference versus the standardised mean difference).
Results
Description of studies
See: 'Characteristics of included studies', 'Characteristics of excluded studies' and 'Characteristics of ongoing studies'.
Results of the search
A total of 431 articles were retrieved by the search strategy. After screening of titles and abstracts, three articles were provisionally selected. The full papers were obtained for further assessment of eligibility. We excluded two studies: one study (Bar‐Or 2008) was an open‐label trial; one (Naismith 2010) was an uncontrolled trial. Only one study met the inclusion criteria (Hauser 2008). One study is ongoing (NCT01569451). See Figure 1.

Study flow diagram.
Included studies
One study was included. The Hauser 2008 study evaluated the efficacy and safety of rituximab as monotherapy versus placebo for adults with RRMS (n = 104) (Characteristics of included studies). All participants were aged 18 to 55 years and had a diagnosis of RRMS (McDonald 2001), an entry score of 0 to 5.0 on the EDSS, and at least one relapse during the preceding year but without a relapse within 30 days. Patients received intravenous infusions of 1000 mg rituximab (n = 69) or placebo (n = 35) on study days 1 and 15; 30 to 60 minutes before each infusion acetaminophen (at a dose of 1 g) and diphenhydramine hydrochloride (at a dose of 50 mg) were administered orally as premedication. This trial was supported by Biogen Idec and Genentech.
Excluded studies
Two studies were excluded from this review. Reasons for their exclusion are available in the Characteristics of excluded studies table.
Risk of bias in included studies
Further details of this assessment are available in the relevant section of the Characteristics of included studies table and are also presented in the 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
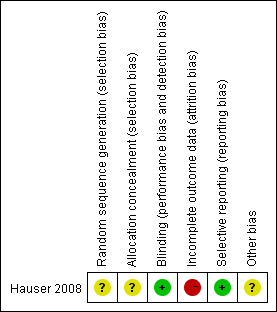
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The specific methods of random sequence generation and allocation concealment were not mentioned. We wrote to the principal author but we did not receive a reply. The risk of selection bias was unclear.
Blinding
This trial was double‐blinded. The risks of performance bias and detection bias were low.
Incomplete outcome data
A high rate of dropouts (24%) over a period of 48 weeks occurred. The reasons for discontinuation were not balanced between the groups. The risk of attrition bias was high.
Selective reporting
All listed outcomes were reported adequately in the study. The risk of reporting bias was low.
Other potential sources of bias
Conflict of interests may exist. Biased results may have resulted from the mismatching of groups at baseline.
Effects of interventions
See: Summary of findings for the main comparison Rituximab for relapsing‐remitting multiple sclerosis
Primary outcomes
Efficacy
(1) Patients in the rituximab group (n = 69), as compared with those in the placebo group (n = 35), had a lower annualised rate of relapse which reached significance at 24 weeks (0.37 versus 0.84, P = 0.04) but not at 48 weeks (0.37 versus 0.72, P = 0.08).
(2) Patients who received rituximab had a reduction in total gadolinium‐enhancing T1‐weighted lesion counts at week 24 as compared with patients who received placebo (mean number 0.5 versus 5.5; relative reduction 91%, P < 0.001). There was a lack of data on the total number of gadolinium‐enhancing T1‐weighted lesions at one year since this study was designed to evaluate this primary endpoint only at 24 weeks.
Safety
(1) More patients in the rituximab group (78.3%) than in the placebo group (40.0%) had infusion‐associated adverse events, within the 24 hours after the first infusion, although acetaminophen (at a dose of 1 g) and diphenhydramine hydrochloride (at a dose of 50 mg) were administered orally 30 to 60 minutes before each infusion as premedication. Most of the infusion‐associated adverse events (92.6%) in the rituximab group were mild to moderate (grade 1 or 2) in severity (the grades correspond to the Common Terminology Criteria for Adverse Events, version 3.0 (Cancer Therapy Evaluation Program 2006)). These included chills, headache, nausea, pyrexia, pruritus, fatigue, throat irritation, and pharyngolaryngeal pain. No grade 4 events associated with infusion were reported. The incidence of any infection was similar in the placebo group (71.4%) and the rituximab group (69.6%). The most common infections (occurring in > 10% of patients) in the rituximab group were nasopharyngitis, upper respiratory tract infections, urinary tract infections and sinusitis. Only urinary tract infections (14.5 versus 8.6%) and sinusitis (13.0% versus 8.6%) were more common in the rituximab group.
(2) Serious adverse events were reported in 14.3% of patients in the placebo group and 13% of patients in the rituximab group. Infection‐associated serious adverse events were reported in 5.7% of patients in the placebo group and 2.9% of patients in the rituximab group. Two serious infection related adverse events (gastroenteritis and bronchitis) occurred in the rituximab group. No clinically significant opportunistic infections were reported.
(3) A total of 5.7% of the patients in the placebo group and 4.3% of the patients in the rituximab group withdrew from the study because of adverse events.
Secondary outcomes
The secondary outcomes (the number of patients with disability progression, the time to confirmed disease progression, and mean change in QoL) in this review were not included as outcomes in the included trial.
Discussion
Summary of main results
This systematic review included only one study (Hauser 2008) involving 104 adult patients with RRMS over a period of 48 weeks. It primarily evaluated a single course of intravenous rituximab at a dose of 1000 mg on days 1 and 15 versus placebo on inflammatory brain lesions, relapse and safety.
We were unable to perform a meta‐analysis. The results of this trial showed that rituximab significantly reduced the total gadolinium‐enhancing T1‐weighted lesion counts (0.5 versus 5.5, a relative reduction of 91%, P < 0.001) and the annualised rate of relapse (0.37 versus 0.84, P = 0.04) at week 24 but that it did not significantly reduce the annualised rate of relapse at week 48 (0.37 versus 0.72, P = 0.08). Mild‐to‐moderate infusion‐associated adverse events such as chills, headache, nausea, pyrexia, pruritus, fatigue, throat irritation and pharyngolaryngeal pain and infection‐associated adverse events such as urinary tract infections and sinusitis were common. Short‐term treatment with rituximab using a single course at a dose of 1000 mg intravenously was safe and well tolerated by most patients with RRMS. However, the high risk of attrition bias that resulted from the high rate of dropouts (40.0% in the placebo group and 15.9% in the rituximab group) at week 48 made the results unconvincing.
Overall completeness and applicability of evidence
In this review, it was difficult to answer the question whether rituximab was effective for MS. We are unable to give any recommendation for clinical practice on the use of rituximab for adult patients with RRMS. Only one study with relatively poor methodological quality was included. Not all primary or secondary outcomes were measured and subgroup and sensitivity analyses could not be performed as planned.
Quality of the evidence
There was not sufficient evidence because only one study with a short‐term efficacy assessment on the primary endpoint was included in the review. The primary aim of the trial was to evaluate the MRI outcome endpoint over 24 weeks with a single course of rituximab (on days 1 and 15). The results were limited because of the high rate of dropouts (24.0%). The methodology used for random sequence generation and allocation concealment were unclear in this trial.
Potential biases in the review process
An extensive, comprehensive search was undertaken to limit bias in the review process, however only one RCT was retrieved. The two authors' independent assessments of the eligibility of studies for inclusion in this review and the extraction of data minimised the potential for additional bias beyond that detailed in the risk of bias tables. The authors of this review had no conflicts of interest.
Agreements and disagreements with other studies or reviews
Recently a systematic review (Castillo‐Trivino 2013) included all available clinical trials irrespective of the types of studies to evaluate the efficacy and safety of rituximab for MS. Four studies in patients with RRMS or primary progressive MS were reviewed. The conclusions were based on qualitative synthesis without fully considering the potential influences of bias on the internal validity of the results.

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Rituximab for relapsing‐remitting multiple sclerosis | ||||||
| Patient or population: patients with relapsing‐remitting multiple sclerosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rituximab | |||||
| The annualised rate of relapse | The mean the annualised rate of relapse in the control groups was | The mean the annualised rate of relapse in the intervention groups was 0.35 lower | 104 | ⊕⊕⊝⊝ | ||
| The sum of the number of gadolinium‐enhancing T1‐weighted lesions | The mean the sum of the number of gadolinium‐enhancing t1‐weighted lesions in the control groups was | The mean the sum of the number of gadolinium‐enhancing t1‐weighted lesions in the intervention groups was | 104 | ⊕⊕⊝⊝ | ||
| The number of patients with adverse effects | High | RR 0.99 | 104 | ⊕⊕⊝⊝ | ||
| 100 per 100 | 99 per 100 | |||||
| The number of patients with serious adverse events | Low | RR 0.91 | 104 | ⊕⊕⊝⊝ | ||
| 143 per 1000 | 130 per 1000 | |||||
| The number of patients who withdrew or dropped out from the study because of AEs | Low | RR 0.76 | 104 | ⊕⊕⊝⊝ | ||
| 57 per 1000 | 43 per 1000 | |||||
| The number of patients with disability progression | Study population | Not estimable | 0 | See comment | This outcome was not included as an endpoint in the trial. | |
| See comment | See comment | |||||
| The time to confirmed disease progression | Study population | Not estimable | 0 | See comment | This outcome was not included as an endpoint in the trial. | |
| See comment | See comment | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 This study had a high rate of dropouts (24.0%), and the methodology for random sequence generation and allocation concealment was unclear. | ||||||

