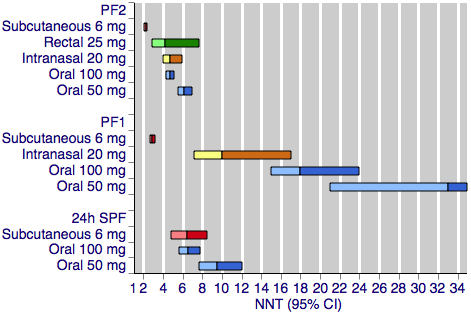
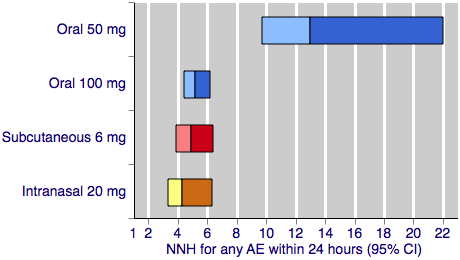
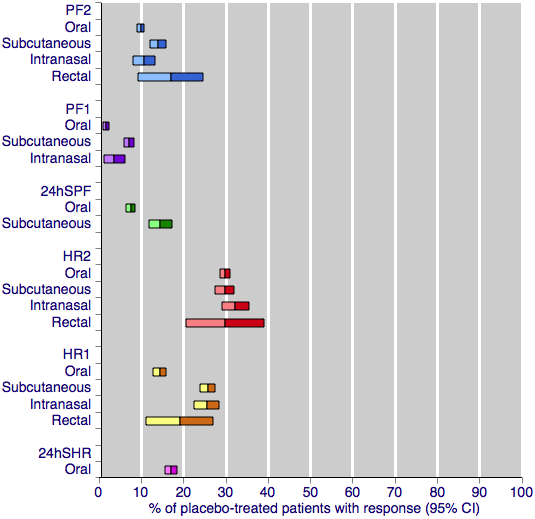
Contenido relacionado
Revisiones y protocolos relacionados
Christopher J Derry, Sheena Derry, R Andrew Moore | 15 febrero 2012
Christopher J Derry, Sheena Derry, R Andrew Moore | 15 febrero 2012
Christopher J Derry, Sheena Derry, R Andrew Moore | 15 febrero 2012
Simon Law, Sheena Derry, R Andrew Moore | 20 abril 2016
Christopher J Derry, Sheena Derry, R Andrew Moore | 15 febrero 2012
Douglas C McCrory, Rebecca N Gray | 15 febrero 2012
Sarah Bird, Sheena Derry, R Andrew Moore | 21 mayo 2014
Lesley A Smith, Anna Oldman, Henry J McQuay, R Andrew Moore, Sheena Derry | 23 julio 2001
Anna Oldman, Lesley A Smith, Henry J McQuay, R Andrew Moore, Sheena Derry | 23 julio 2001
R Andrew Moore, Sheena Derry, Dominic Aldington, Philip J Wiffen | 13 octubre 2015