舒马曲坦(所有给药途径)治疗成人急性偏头痛发作——Cochrane系统综述概述
摘要
研究背景
偏头痛对个人来说是一种高度致残的疾病,对社会、医疗服务和经济也有广泛的影响。舒马曲坦是一种治疗偏头痛发作的顿挫药物,属于曲坦家族。它可通过四种不同的途径给药:口服、皮下、鼻内和直肠。
研究目的
总结来自四项Cochrane干预综述的证据,即舒马曲坦通过四种给药途径(口服、皮下、鼻内和直肠)治疗成人急性偏头痛发作的疗效和耐受性,并将其与安慰剂和活性药物对照组进行比较。
研究方法
所纳入的综述由本概述的作者撰写;没有进行额外的检索。所有纳入的综述均根据标准方案进行,并报告了一组标准结局。从每个单独的综述中,我们提取了关于不同水平的疼痛缓解和不良事件的结果。作为概述的一部分,没有进行更多的统计比较。我们重点研究了北美或欧洲许可的剂量和途径的最重要发现(口服给药25mg、50mg、100mg;皮下给药4mg、6mg;鼻内给药5mg、10mg、20mg;直肠给药25mg)。
主要结果
所纳入的综述为52236名受试者提供了18种不同剂量和给药途径组合的数据。所寻求的主要结局的数据通常都得到了很好的报告,并且包括足够数量的受试者,以对结果给予信心,但直肠给药途径除外,因为接受直肠给药途径的人数较少。
皮下给药是最有效的,服用6mg舒马曲坦的10人中有近6人(59%)在2小时内疼痛从中度或重度减轻至无,而服用安慰剂的人中大约有七分之一(15%);需要治疗的人数 (NNT)为2.3(95% 置信区间为CI [2.1, 2.4]),分析涉及2522名受试者。最常用剂量的口服、直肠和鼻内给药舒马曲坦在临床上也提供了有效的疼痛缓解,口服50mg剂量使得每10人中约3人(28%)的疼痛完全缓解,而约十分之一(11%)的人在服用安慰剂后其疼痛完全缓解(6447名受试者的NNT为6.1(5.5至6.9))。皮下给药较其他途径能够提供更快速的疼痛缓解。在疼痛轻微时尽早服药比等到中度或重度疼痛更有效。
对于头痛缓解(疼痛从中度或重度减轻到无或轻度),舒马曲坦在两小时内的最有效剂量为口服100mg(7811名受试者的NNT为3.5 (3.2至3.7))、皮下给药6mg(2738名受试者的NNT为2.1 (2.0至2.2))、鼻内给药20mg(2020名受试者的NNT为3.5 (3.1至4.1))以及直肠给药25mg(240名受试者的NNT为2.4 (1.9至3.4))。
不良事件通常为轻度或中度严重程度、持续时间短,并且与其他剂量和途径组合相比,皮下给药舒马曲坦和更高剂量的口服和鼻内给药舒马曲坦更常见。
作者结论
舒马曲坦对急性偏头痛发作是一种有效的顿挫治疗,但与安慰剂相比会增加不良事件。给药途径会影响疗效,尤其是在给药后的第一个小时内。皮下给药舒马曲坦在缓解疼痛方面显示出最大的功效,但代价是相对较高的不良事件水平,并且与其他途径相比具有较高的经济成本。关于不同给药途径对不同结局的相对疗效的信息应有助于表明舒马曲坦作为偏头痛治疗的适用性,以及对个体患者进行治疗的最合适的方式。
简语概要
舒马曲坦(所有给药途径)治疗成人急性偏头痛发作
偏头痛是一种复杂的病症,症状种类繁多。对许多人来说,主要特征是痛苦且常常致残的头痛。其他症状包括视力障碍;对光、声音和气味敏感;感到恶心;和呕吐。偏头痛影响约八分之一的人,主要是女性,主要在30至50岁的年龄范围内。
舒马曲坦是用于治疗偏头痛发作的曲坦类药物之一。它可以通过四种不同的途径给药:口服给药(口服)、皮下给药(皮下)、鼻腔喷雾(鼻内)和栓剂(直肠)。Cochrane系统综述对每一种治疗方法的单独综述都提供了舒马曲坦在减轻50000多名偏头痛患者头痛方面的效果的信息。对于口服、皮下和鼻内给药舒马曲坦,有大量来自高质量试验的信息,但关于直肠给药的信息相对较少。
该概述发现,通过这些途径中的任何一种给予单次剂量都可有效缓解偏头痛疼痛。
皮下给药途径提供了最佳的疼痛缓解,服用6mg剂量的10人中有近6人(59%)在2小时内其疼痛从中度或重度减轻到无,而服用安慰剂的人中大约有七分之一(15%)。最常用的口服、直肠和鼻内给药舒马曲坦也能有效缓解疼痛。口服50mg剂量(常用剂量和给药途径组合中最不有效的)使得几乎3/10的人(28%)的疼痛完全缓解,而服用安慰剂后约有1/10的人(11%)的疼痛完全缓解。皮下给药舒马曲坦也是最快的,与任何其他给药途径相比,其在治疗一小时内为更多的人提供了疼痛缓解。
与其他剂量和给药途径组合相比,皮下给药舒马曲坦以及更高剂量的口服和鼻内给药舒马曲坦的不良事件大多为轻度或中度严重程度且持续时间短。
Authors' conclusions
Background
Description of the condition
Migraine is a common, disabling headache disorder, ranked seventh highest among specific causes of disability globally (Steiner 2013), and with considerable social and economic impact (Hazard 2009). Recent reviews found a one‐year prevalence of 15% globally (Vos 2012) and for adults in European countries (Stovner 2010), 13% for all ages in the USA (Victor 2010), 21% in Russia (Ayzenberg 2012) and 9% for adults in China (Yu 2012). Migraine is more prevalent in women than in men (by a factor of two to three), and in the age range 30 to 50 years.
The International Headache Society (IHS) classifies two major subtypes (IHS 2013). Migraine without aura is the most common subtype. It is characterised by attacks lasting 4 to 72 hours that are typically of moderate to severe pain intensity, unilateral, pulsating, aggravated by normal physical activity, and associated with nausea and/or photophobia and phonophobia. Migraine with aura is characterised by reversible focal neurological symptoms that develop over a period of at least five minutes and last for less than 60 minutes, followed by headache with the features of migraine without aura. In some cases the headache may lack migrainous features or be absent altogether (IHS 2013).
A large prevalence study in the USA found that over half of migraineurs had severe impairment or required bed rest during attacks. Despite this high level of disability and a strong desire for successful treatment, only a proportion of migraine sufferers sought professional advice for the treatment of attacks. The majority were not taking any preventive medication, although one‐third met guideline criteria for offering or considering it. Nearly all (98%) migraineurs used acute treatments for attacks, with 49% using over‐the‐counter (OTC) medication only, 20% using prescription medication, and 29% using both. OTC medication included aspirin, other non‐steroidal anti‐inflammatory drugs (NSAIDs), paracetamol (acetaminophen) and paracetamol with caffeine (Bigal 2008; Diamond 2007; Lipton 2007). Similar findings have been reported from other large studies in France and Germany (Lucas 2006; Radtke 2009).
The significant impact of migraine with regard to pain, functional health, and well‐being is well documented (Buse 2011; Leonardi 2005); it is ranked in the top 10 disorders for global years lived with disability (Vos 2012). A cross‐sectional survey of eight European Union (EU) countries (representing 55% of the adult population) has estimated an annual direct and indirect cost of migraine per person of EUR 1222, and a total annual cost for the EU of EUR 111 billion for adults aged 18 to 65 years (Linde 2012). Costs vary between countries, probably due to differences in available therapies and the way they are delivered, and structural differences in healthcare systems (Bloudek 2012). In the USA, the average annual direct cost per person has been estimated at USD 1757 for episodic migraine and USD 7750 for chronic migraine (Munakata 2009). Whatever the exact direct and indirect costs are for each country, it is clear that migraine presents a significant economic burden. Successful treatment of acute migraine attacks not only benefits patients by reducing their disability and improving health‐related quality of life, but also has the potential to reduce the need for healthcare resources and increase economic productivity.
Description of the interventions
The symptomatic treatment of migraine advanced significantly with the development of the triptan class of drugs, of which sumatriptan was the first, in 1991. It is available as a standard oral tablet, nasal spray, subcutaneous injection, and rectal suppository. Different formulations may offer benefits to individuals in terms of speed of onset of relief or adverse events, and non‐oral formulations may be particularly useful for those who experience severe nausea or vomiting with their attacks. Each route of administration has been evaluated in a separate Cochrane intervention review, and this overview summarises evidence from those reviews.
Sumatriptan is available only by prescription in most countries, but in the UK packs of 2 x 50 mg oral tablets are available OTC as Imigran Recovery for individuals with previously diagnosed migraine. Other countries in which sumatriptan is available OTC include Germany and Sweden. Generic (non‐proprietary) formulations are available for the standard tablets and subcutaneous injections in many countries. In primary care in the UK in 2012 there were over 1,150,000 prescriptions for sumatriptan, of which 64% and 23% were for generic 50 mg and 100 mg oral formulations (PCA 2013).
In order to establish whether sumatriptan is effective in reducing pain at specified doses in acute migraine attacks, it is necessary to study its effects in circumstances that permit detection of pain relief. Such studies are carried out in individuals with established pain of moderate to severe intensity, using single doses of the interventions. Participants who experience an inadequate response with either placebo or active treatment are permitted to use rescue medication, and the intervention is considered to have failed in those individuals. In clinical practice, however, individuals would not normally wait until pain is of at least moderate severity, and may take a second dose of medication if the first dose does not provide adequate relief. Once analgesic efficacy is confirmed in studies using single doses in established pain, further studies may investigate different treatment strategies and patient preferences. These are likely to include treating the migraine attack early while pain is mild, and using a low dose initially, with a second dose if response is inadequate.
How the intervention might work
Sumatriptan is a 5‐HT1 agonist, selectively targeting the 5‐HT (serotonin) 1B and 1D receptors. It has three putative mechanisms of therapeutic action (Ferrari 2002; Goadsby 2007).
-
Vasoconstriction of dilated meningeal blood vessels.
-
Inhibition of the release of vasoactive neuropeptides from perivascular trigeminal sensory neurons.
-
Reduction of pain signal transmission in the trigeminal dorsal horn.
Sumatriptan is used for acute treatment, having no efficacy in preventing future attacks.
Why it is important to do this overview
Sumatriptan was the first marketed triptan and is by far the most used triptan worldwide. Since it came off patent, generic formulations have greatly increased its availability, and sumatriptan has become the standard against which new acute migraine treatments are compared. An earlier Cochrane review of oral sumatriptan for acute migraine headaches searched for studies to the end of 2001 (McCrory 2003). Many more studies have been published since that time, and updates were needed to include these new studies and consider the other routes of administration. Owing to the very large amount of information now available, particularly for the oral formulation, we carried out separate reviews for each route of administration (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d).
This overview summarises the main findings from those four reviews so that readers can understand the benefits and harms of sumatriptan, by all routes of administration, without necessarily having to read all four individual reviews. The method of presenting results (in tabular format) is intended to facilitate informal comparison across the various routes of administration, but we did not conduct any formal meta‐analyses of these (indirect) comparisons.
Objectives
The objective of this overview was to summarise evidence from four Cochrane intervention reviews on the efficacy and tolerability of sumatriptan in the treatment of acute migraine attacks in adults by four routes of administration (oral, subcutaneous, intranasal, rectal) compared with both placebo and with active comparators. We limited this overview to doses of sumatriptan licensed in North America or Europe.
Methods
Criteria for considering reviews for inclusion
We included four Cochrane intervention reviews of sumatriptan administered by oral, intranasal, subcutaneous, and rectal routes (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d). Reviews were required to address doses of sumatriptan licensed in North America or Europe. These were:
-
oral sumatriptan 25 mg, 50 mg, 100 mg;
-
subcutaneous sumatriptan 4 mg, 6 mg;
-
intranasal sumatriptan 5 mg, 10 mg, 20 mg;
-
rectal sumatriptan 25 mg.
A fuller listing of results from any dose of sumatriptan for which there was evidence of efficacy or harm, and by any route of administration, can be found in the individual reviews.
Search methods for identification of reviews
Included reviews were known to the authors and published in The Cochrane Library; there was no additional searching.
Data collection and analysis
We used a tabular format to summarise results for a number of IHS‐preferred outcomes (IHS 2000; both efficacy and harm) for sumatriptan administered by different routes, at different doses, and at different levels of baseline pain. These outcomes include:
-
pain‐free outcomes: participants could have either moderate or severe, or mild pain when medication was taken, reduced to no pain at the time of assessment
-
pain‐free at two hours, without the use of rescue medication;
-
pain‐free at one hour, without the use of rescue medication;
-
sustained pain‐free during the 24 hours postdose (pain‐free within two hours, with no use of rescue medication or recurrence of moderate to severe pain within 24 hours).
-
-
headache relief outcomes: participants had moderate or severe pain when medication was taken, reduced to mild or no pain at the time of assessment
-
headache relief at two hours, without the use of rescue medication;
-
headache relief at one hour, without the use of rescue medication;
-
sustained headache relief during the 24 hours postdose (headache relief at two hours, sustained for 24 hours, with no use of rescue medication or a second dose of study medication).
-
-
any adverse event within 24 hours of dosing.
Summaries of other outcomes can be found in Appendix 1. These include:
-
use of rescue medication;
-
relief of headache‐associated symptoms at two hours: nausea, photophobia, and phonophobia;
-
relief of functional disability at two hours: partial relief, complete relief.
As in the individual reviews, we report results for each outcome in three ways in the summary tables under Effects of interventions.
-
First, together with the number of studies and participants, we give the actual number of participants with the outcome, and the total treated, as well as the percentage of participants achieving the outcome. This is done for the active drug and for the comparator (placebo, or a different active drug)
-
Second, we present the relative risk (RR) together with a 95% confidence interval (CI). Where the CI does not include 1, the result is taken to be statistically significant. If the RR is less than 1, then the rate of events is lower with the active drug than with placebo; if the RR is greater than 1, then the rate of events is higher with the active drug than with placebo
-
Third, where the RR is statistically significant, we report the number needed to treat to benefit (NNT), number needed to treat to harm or cause one adverse event (NNH), or number needed to treat to prevent one adverse event (NNTp; Cook 1995)
Comparisons involving fewer than 200 participants or fewer than two studies are highly susceptible to random effects of chance, and were not included in the individual reviews (Moore 1998).
Selection of reviews
Included reviews were carried out by the same authors and covered the four routes of administration available for sumatriptan (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d).
Data extraction and management
One review author collated results from the four reviews, and another checked them.
Assessment of methodological quality of included reviews
Quality of included reviews
All included reviews were carried out according to a standard protocol which satisfied the criteria specified in the 'assessment of multiple systematic reviews' (AMSTAR) measurement tool (Shea 2007) for rigorous methodological quality.
According to these criteria, each review should:
-
provide an a priori design;
-
carry out duplicate study selection and data extraction;
-
carry out a comprehensive literature search;
-
include published and unpublished studies irrespective of language of publication;
-
provide a list of studies (included and excluded);
-
assess and document the scientific quality of the included studies;
-
use the scientific quality of the included studies appropriately in formulating conclusions;
-
use appropriate methods to combine the findings of studies; and
-
state conflicts of interests.
Quality of evidence in included reviews
We assessed the strength of evidence for different outcomes according to the methodological quality of the primary studies as reported in the individual reviews, the total number of participants contributing data, and whether it was sensitive to potential publication bias.
Individual reviews included only randomised, double blind, placebo or active‐controlled trials, with a minimum of 10 participants in each treatment arm. The majority of included studies did not adequately report the methods used to generate the random sequence or to maintain allocation concealment and blinding; this may reflect the age of the studies and reporting deficiencies rather than methodological inadequacy. A minority of studies were judged at high risk of bias because they enrolled fewer than 50 participants per treatment arm (Nuesch 2010).
Individual reviews assessed the potential sensitivity to publication bias of the primary outcomes of pain‐free and headache relief at two hours by examining the number of participants in trials with zero effect (RR 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008). Reviews specified a clinically useful level as an NNT ≤ 8 for pain‐free at two hours, and an NNT ≤ 6 for headache relief at two hours, and judged outcomes to be potentially susceptible to publication bias if fewer than 400 additional participants were required to increase the NNT beyond this clinically useful level. We used this method because statistical tests for presence of publication bias have been shown to be unhelpful (Thornton 2000).
Data synthesis
There was no pooling of data beyond what was reported in the individual reviews. Specifically, we did not conduct any formal statistical analysis of data from indirect comparisons of one route of administration versus another.
Results
We included four Cochrane intervention reviews providing data on 18 different dose and route of administration combinations for sumatriptan administered orally, subcutaneously, intranasally, or rectally, in the treatment of acute migraine attacks in adults (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d). All of the reviews used the same methodological approach and assessed the same efficacy and safety outcomes. The total number of participants included in the 111 individual studies in the four reviews was 52,236.
| Route of administration | Studies | Participants |
| Oral | 61 | 37,250 |
| Subcutaneous | 35 | 9,365 |
| Intranasal | 12 | 4,755 |
| Rectal | 3 | 866 |
This overview summarises the most important findings from the four individual reviews for formulations and doses of sumatriptan licensed in North America or Europe (oral 25 mg, 50 mg, 100 mg; subcutaneous 4 mg, 6 mg; intranasal 5 mg, 10 mg, 20 mg; rectal 25 mg).
Description of included reviews
Included reviews each had the same structure and organisation. They used identical methods that were based on criteria established by extensive analysis and validation, using individual patient data.
All the reviews used the same criteria for inclusion; typically, these were:
-
adult participants with a history of migraine;
-
single dose administration of sumatriptan, active comparator, or placebo (with additional analgesia available, typically after one to two hours);
-
randomised, double blind studies;
-
pain assessed by using standard pain intensity and pain relief scales.
All the reviews used the same process to identify and select studies for inclusion; these were:
-
searching electronic searches (including CENTRAL, MEDLINE, EMBASE, and manufacturers' databases);
-
no language restriction on included reports;
-
assessment of study quality according to established criteria, with minimum criteria for inclusion (randomised, double blind, 10 or more participants per treatment group).
Methodological quality of included reviews
All four reviews satisfied the criteria specified in the AMSTAR measurement tool (Shea 2007) for rigorous methodological quality. Notably, each review used appropriate methods to combine findings of studies, and importantly provided analyses according to drug dose. The scientific quality of the included studies was used appropriately in formulating conclusions, because only studies with minimal risk of bias were included. A particular issue was the number of participants contributing data to some analyses, but conclusions were not drawn from inadequate data sets, based on previously established criteria (Moore 1998).
Effect of interventions
A common set of outcomes has arisen in randomised controlled trials (RCTs) of migraine medication, based around the features migraineurs want from their migraine treatment (Lipton 1999). The four reviews included in this overview addressed this set of outcomes for different routes of administration and doses separately.
In this overview, we focused on outcomes relating to pain relief (pain‐free and headache relief at various time points) and adverse events. All of the studies providing data for the included reviews measured pain intensity on a 4‐point scale, typically 0 = none, 1 = mild, 2 = moderate, and severe = 3, or equivalent terms. The vast majority of data were for comparisons of sumatriptan with placebo; where available we have commented on comparisons with other active treatments.
For each outcome we have reported dose and route of administration combinations for which sufficient data were available to carry out pooled analysis. We have presented the results in the form of a summary of results table to facilitate informal comparison across the various routes of administration and doses, and highlight those for which the results were considered to be robust (not susceptible to publication bias) and clinically relevant (NNT below a defined threshold).
All extracted information for all outcomes is provided in appendices in the individual reviews (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d).
Sumatriptan versus placebo
Pain‐free at two hours
Pooled analyses were performed on 10 dose, route of administration, and baseline pain intensity combinations for which sufficient data were available (Summary of results A). Eight treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results A: Pain‐free at two hours in placebo‐controlled studiesa | ||||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | Susceptibility to publication biasb | |||
| Studies | Participants | Active | Placebo | Active | Placebo | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 25 | 3 | 1108 | 201/809 | 26/299 | 25 | 9 | 2.7 (1.8 to 4.0) | 6.2 (4.9 to 8.5) | 322 |
| Oral | 50 | 13 | 6447 | 1080/3922 | 282/2525 | 28 | 11 | 2.7 (2.4 to 3.1) | 6.1 (5.5 to 6.9) | 2008 |
| Oral | 100 | 16 | 6571 | 1291/4017 | 272/2554 | 32 | 11 | 3.2 (2.8 to 3.6) | 4.7 (4.3 to 5.1) | 4614 |
| Subcutaneous | 4 | 2 | 664 | 201/411 | 23/253 | 49 | 9 | 4.8 (3.2 to 7.2) | 2.5 (2.2 to 3.0) | 1461 |
| Subcutaneous | 6 | 13 | 2522 | 799/1351 | 174/1171 | 59 | 15 | 3.9 (3.3 to 4.5) | 2.3 (2.1 to 2.4) | 6250 |
| Intranasal | 10 | 5 | 1115 | 157/655 | 47/460 | 24 | 10 | 2.5 (1.8 to 3.4) | 7.3 (5.5 to 11) | 107 |
| Intranasal | 20 | 6 | 1379 | 283/891 | 52/488 | 32 | 11 | 3.1 (2.4 to 4.1) | 4.7 (4.0 to 5.9) | 968 |
| Rectal | 25 | 2 | 240 | 60/146 | 16/94 | 41 | 17 | 2.4 (1.5 to 3.9) | 4.2 (2.9 to 7.7) | 217 |
| In participants with mild baseline pain | ||||||||||
| Oral | 50 | 7 | 1514 | 357/783 | 168/731 | 46 | 23 | 2.0 (1.7 to 2.4) | 4.4 (3.7 to 5.6) | 1239 |
| Oral | 100 | 5 | 1240 | 358/618 | 151/622 | 58 | 24 | 2.4 (2.1 to 2.8) | 3.0 (2.6 to 3.5) | 2067 |
| aResults shown in bold font are those considered to be the most robust and clinically relevant (see text for explanation) bNumber of participants in studies with no effect needed to change NNT to >8 | ||||||||||
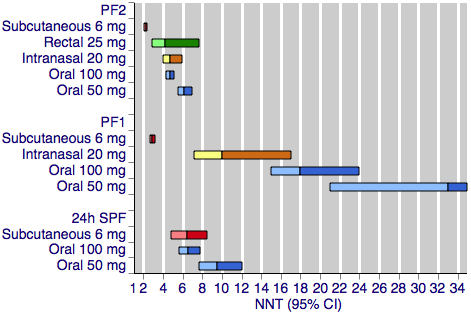
Figure 1 shows the calculated NNTs for pain‐free at two hours for the five most widely used dose and route of administration combinations (oral 50 mg, oral 100 mg, subcutaneous 6 mg, intranasal 20 mg, rectal 25 mg) in patients with moderate or severe baseline pain.
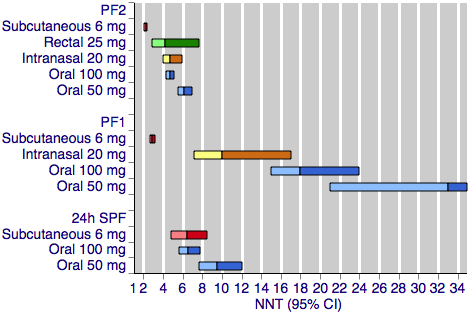
Sumatriptan versus placebo. Calculated NNTs for a pain‐free response after a specified time, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
PF2: pain‐free at two hours; PF1: pain‐free at one hour; 24h SPF: 24‐hour sustained pain‐free.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.
All dose and route combinations provided superior levels of pain relief to placebo. For moderate or severe baseline pain, calculated NNTs for this outcome ranged from 6 to 7 with 10 mg intranasal sumatriptan and 25 mg and 50 mg oral sumatriptan, through 4 to 5 for higher doses of intranasal, oral, and rectal treatments, and 2.3 for high‐dose subcutaneous treatment. The proportion of participants pain‐free two hours after treatment ranged from approximately 25% with low doses of oral and intranasal treatments to 59% with the higher dose of the subcutaneous treatment. The proportion of placebo‐treated participants pain‐free at two hours ranged from 9% to 17%. In general, higher doses of sumatriptan resulted in lower (better) NNTs, but in many cases the differences between NNTs were not statistically significant (overlapping confidence intervals), suggesting a relatively minor dose response relationship. The original reviews showed that the oral 100 mg dose was superior to the 50 mg dose (P = 0.0001), and that the 20 mg intranasal dose was superior to the 10 mg dose (P = 0.015).
The two doses of oral sumatriptan administered to participants with mild baseline pain showed significantly improved efficacy compared with the same doses administered to participants with moderate or severe pain (P = 0.014 for the 50 mg dose, and P < 0.00006 for the 100 mg dose) in indirect comparisons. The calculated NNTs for the 50 mg and 100 mg oral doses were 4.4 and 3.0 after treatment of mild pain, compared with 6.1 and 4.7 after treatment of moderate or severe pain. As with the participants treating moderate or severe headache, the 100 mg dose gave a significantly lower (better) NNT (P = 0.002) than the 50 mg dose. Response with placebo (24%) was greater with mild, compared with moderate or severe, baseline pain.
In the review of subcutaneous sumatriptan (Derry 2012b), three of the included studies provided data on the efficacy of a second dose of sumatriptan in case of an inadequate response to the initial dose. Participants with insufficient pain relief after one hour were offered a second dose of subcutaneous sumatriptan 6 mg and the pain‐free response at two hours recorded. There was no significant difference between the number of participants pain‐free at two hours with this alternative dosing strategy and with the standard single dose strategy.
Pain‐free at one hour
Pooled analyses were performed on seven dose, route of administration, and baseline pain intensity combinations for which sufficient data were available (Summary of results B). Five treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results B: Pain‐free at one hour in placebo‐controlled studies | |||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 50 | 5 | 1735 | 45/902 | 16/833 | 5 | 2 | 2.6 (1.5 to 4.7) | 33 (21 to 73) |
| Oral | 100 | 6 | 3176 | 158/2216 | 15/960 | 7 | 2 | 4.0 (2.3 to 6.8) | 18 (15 to 24) |
| Subcutaneous | 4 | 2 | 664 | 134/411 | 16/253 | 33 | 6 | 4.7 (2.8 to 7.7) | 3.8 (3.2 to 4.8) |
| Subcutaneous | 6 | 16 | 3592 | 905/2198 | 99/1394 | 41 | 7 | 5.6 (4.6 to 6.8) | 2.9 (2.7 to 3.2) |
| Intranasal | 20 | 2 | 499 | 39/320 | 4/179 | 12 | 2 | 6.2 (2.2 to 18) | 10 (7.1 to 17) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 5 | 1246 | 161/624 | 87/622 | 26 | 14 | 1.9 (1.5 to 2.4) | 8.5 (6.2 to 13) |
| Oral | 100 | 5 | 1240 | 189/618 | 87/622 | 31 | 14 | 2.2 (1.8 to 2.8) | 6.0 (4.7 to 8.3) |
Figure 1 shows the calculated NNTs for pain‐free at one hour for four of the five most widely used dose and route of administration combinations in patients with moderate or severe baseline pain (no information was available for rectal 25 mg for this outcome).
All dose and route combinations provided statistically superior levels of pain relief to placebo. When baseline pain was moderate or severe, subcutaneous sumatriptan (4 mg or 6 mg) was far superior to oral or intranasal formulations, with NNTs for this outcome of 3 to 4, compared to 10 to 33. The proportion of participants pain‐free at one hour ranged from 5% to 12% for all oral and intranasal doses, compared to 33% to 41% for the subcutaneous doses. Placebo response rates varied from 2% to 7%. Higher doses of oral sumatriptan resulted in lower (better) NNTs, but the differences between NNTs were not statistically significant (overlapping confidence intervals). The subcutaneous review (Derry 2012b) showed that the subcutaneous 6 mg dose was superior to the 4 mg dose (P = 0.011).
Oral sumatriptan administered to participants with mild baseline pain showed significantly improved efficacy compared with the same doses administered to participants with moderate or severe pain in indirect comparisons, with NNTs of 8.5 and 6.0 compared with 33 and 18 for the 50 mg and 100 mg doses (P = 0.014 for 50 mg, and P < 0.00006 for 100 mg). Response with both sumatriptan and placebo was greater with mild baseline pain than with moderate or severe baseline pain.
Sustained pain‐free during the 24 hours postdose
The 24‐hour sustained pain‐free outcome requires participants to be pain‐free at two hours, with no use of rescue medication and no recurrence of pain within 24 hours.
Pooled analyses were performed on five dose, route of administration, and baseline pain intensity combinations for which sufficient data were available (Summary of results C). Three treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results C: Sustained pain‐free during the 24 hours postdose in placebo‐controlled studies | |||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 50 | 4 | 2526 | 226/1309 | 82/1217 | 17 | 7 | 2.6 (2.1 to 3.4) | 9.5 (7.7 to 12) |
| Oral | 100 | 6 | 2891 | 374/1590 | 106/1301 | 24 | 8 | 2.8 (2.3 to 3.4) | 6.5 (5.6 to 7.8) |
| Subcutaneous | 6 | 5 | 1336 | 222/713 | 91/623 | 31 | 15 | 2.2 (1.8 to 2.8) | 6.1 (4.8 to 8.2) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 4 | 866 | 124/436 | 44/430 | 28 | 10 | 2.8 (2.1 to 3.9) | 5.5 (4.3 to 7.6) |
| Oral | 100 | 3 | 771 | 127/389 | 39/382 | 33 | 10 | 3.2 (2.3 to 4.5) | 4.5 (3.6 to 5.9) |
Figure 1 shows the calculated NNTs for sustained pain‐free during the 24 hours postdose for three of the five most widely used dose and route combinations in patients with moderate or severe baseline pain (no information was available for the intranasal 20 mg or rectal 25 mg formulations for this outcome).
All dose and route combinations provided superior levels of pain relief to placebo. When baseline pain was moderate or severe, calculated NNTs for this outcome ranged from 9.5 with 50 mg oral sumatriptan through to 6.5 and 6.1 with the higher oral dose and subcutaneous treatment, respectively. The proportion of participants with a 24‐hour sustained pain‐free response ranged from approximately 17% with the lower dose oral treatment to 31% with subcutaneous treatment. The proportion of placebo‐treated participants with a 24‐hour sustained pain‐free response ranged from 7% to 15%. Data on more than one dose of sumatriptan were available only for the oral route of administration, for which the 100 mg dose was shown to result in a lower (better) NNT than the 50 mg dose (P = 0.008).
Oral sumatriptan administered to participants with mild baseline pain showed significantly improved efficacy compared with the same doses administered to participants with moderate or severe pain in indirect comparisons, with NNTs of 5.5 and 4.5 compared with 9.5 and 6.5 for the 50 mg and 100 mg doses (P = 0.008 for 50 mg, and P = 0.024 for 100 mg). Response with placebo was similar to that with initially moderate or severe pain.
Headache relief at two hours
Pooled analyses were performed on nine dose and route of administration combinations for which sufficient data were available (Summary of results D).
| Summary of results D: Headache relief at two hours in placebo‐controlled studiesa | ||||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | Susceptibility to publication biasb | |||
| Studies | Participants | Active | Placebo | Active | Placebo | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 25 | 5 | 1580 | 638/1143 | 140/437 | 56 | 32 | 1.7 (1.4 to 1.9) | 4.2 (3.5 to 5.4) | 677 |
| Oral | 50 | 19 | 8102 | 2822/4955 | 1007/3147 | 57 | 32 | 1.8 (1.7 to 1.9) | 4.0 (3.7 to 4.4) | 4051 |
| Oral | 100 | 21 | 7811 | 2877/4751 | 967/3060 | 61 | 32 | 1.9 (1.8 to 2.0) | 3.5 (3.2 to 3.7) | 5579 |
| Subcutaneous | 4 | 2 | 664 | 286/411 | 56/253 | 70 | 22 | 3.1 (2.4 to 4.0) | 2.1 (1.8 to 2.5) | 1233 |
| Subcutaneous | 6 | 14 | 2738 | 1152/1459 | 395/1279 | 79 | 31 | 2.5 (2.3 to 2.7) | 2.1 (2.0 to 2.2) | 5085 |
| Intranasal | 5 | 4 | 830 | 236/496 | 114/334 | 48 | 34 | 1.4 (1.2 to 1.7) | 7.4 (5.0 to 15) | Not calculated (NNT > 6) |
| Intranasal | 10 | 8 | 1755 | 510/1025 | 230/730 | 50 | 32 | 1.6 (1.4 to 1.8) | 5.5 (4.4 to 7.3) | 160 |
| Intranasal | 20 | 9 | 2020 | 767/1262 | 245/758 | 61 | 32 | 1.9 (1.7 to 2.2) | 3.5 (3.1 to 4.1) | 1443 |
| Rectal | 25 | 2 | 240 | 104/146 | 28/94 | 71 | 30 | 2.3 (1.7 to 3.2) | 2.4 (1.9 to 3.4) | 360 |
| aResults shown in bold font are those considered to be the most robust and clinically relevant (see text for explanation) bNumber of participants in studies with no effect needed to change NNT to >6 | ||||||||||
Figure 2 shows the calculated NNTs for headache relief at two hours with the five most widely used dose and route of administration combinations.
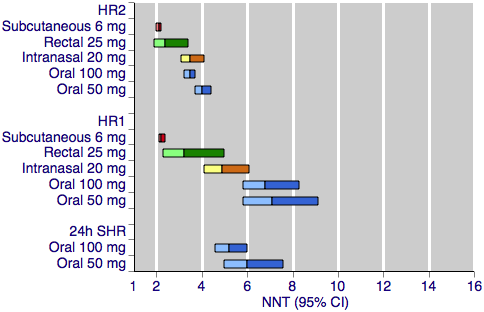
Sumatriptan versus placebo. Calculated NNTs for headache relief after a specified time, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
HR2 (headache relief at two hours); HR1 (headache relief at one hour); 24h SHR (24‐hour sustained headache relief).
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.
All dose and route combinations provided statistically superior levels of headache relief to placebo, with all but one also resulting in clinically useful NNTs for this outcome. The 5 mg intranasal dose gave an NNT of 7.4, which lies outside of the range we consider to be clinically useful for this outcome. Other NNTs ranged from 4.2 with 25 mg oral sumatriptan, through 3.5 to 2.1 for the higher doses of oral, intranasal, rectal, and subcutaneous sumatriptan. The proportion of participants with headache relief at two hours ranged from 48% to 56% with low doses of intranasal and oral treatments, to 79% with the higher dose of the subcutaneous treatment. The proportion of placebo‐treated participants with headache relief after two hours was fairly constant across the doses and routes of administration, ranging from 22% to 34%. In general, higher doses of sumatriptan resulted in lower (better) NNTs, but in many cases the differences between doses were not statistically significant (overlapping confidence intervals), suggesting a relatively minor dose response relationship. The included reviews further showed that the oral 100 mg dose was superior to the 50 mg dose (P = 0.010; Derry 2012a), and that the 20 mg intranasal dose was superior to the 10 mg dose (P = 0.002; Derry 2012c).
In the review of subcutaneous sumatriptan (Derry 2012b), six of the included studies provided data on the efficacy of a second dose of sumatriptan in case of an inadequate response to the initial dose. Participants with insufficient pain relief after one hour were offered a second dose of subcutaneous sumatriptan 6 mg and the number of participants with headache relief at two hours recorded. There was no significant difference between the NNTs for headache relief at two hours with this alternative dosing strategy and with the standard single dose strategy.
Headache relief at one hour
Pooled analyses were performed on nine dose and route of administration combinations for which sufficient data were available (Summary of results E).
| Summary of results E: Headache relief at one hour in placebo‐controlled studies | |||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 25 | 2 | 472 | 95/334 | 16/138 | 28 | 12 | 2.3 (1.4 to 3.7) | 5.9 (4.2 to 10) |
| Oral | 50 | 8 | 2492 | 406/1474 | 137/1018 | 28 | 13 | 1.9 (1.6 to 2.3) | 7.1 (5.8 to 9.1) |
| Oral | 100 | 10 | 3983 | 795/2709 | 187/1274 | 29 | 15 | 1.9 (1.6 to 2.2) | 6.8 (5.8 to 8.3) |
| Subcutaneous | 4 | 2 | 664 | 271/411 | 64/253 | 66 | 25 | 2.6 (2.0 to 3.2) | 2.5 (2.1 to 3.0) |
| Subcutaneous | 6 | 24 | 5177 | 2229/3139 | 532/2038 | 71 | 26 | 2.7 (2.5 to 2.9) | 2.2 (2.1 to 2.4) |
| Intranasal | 5 | 4 | 830 | 193/496 | 95/334 | 39 | 28 | 1.4 (1.1 to 1.7) | 9.6 (5.9 to 25) |
| Intranasal | 10 | 8 | 1755 | 392/1025 | 180/730 | 38 | 25 | 1.6 (1.4 to 1.9) | 7.4 (5.6 to 11) |
| Intranasal | 20 | 9 | 2020 | 579/1262 | 192/758 | 46 | 25 | 1.9 (1.6 to 2.2) | 4.9 (4.1 to 6.1) |
| Rectal | 25 | 2 | 240 | 74/146 | 18/94 | 51 | 19 | 2.7 (1.7 to 4.2) | 3.2 (2.3 to 5.0) |
Figure 2 shows the calculated NNTs for headache relief at one hour for the five most widely used dose and route of administration combinations.
All dose and route combinations provided superior levels of headache relief to placebo. Calculated NNTs for this outcome were highly dependent on the route of administration, ranging from 7.1 to 5.9 for oral administration; 9.6 to 4.9 for intranasal administration; 2.5 to 2.2 for subcutaneous administration,and 3.2 for rectal administration. Similarly, the proportion of participants with headache relief at one hour varied substantially between the different routes of administration. Between 28% and 29% of oral sumatriptan‐treated participants had headache relief by one hour, compared with between 38% and 51% of intranasal or rectal sumatriptan‐treated participants, and 66% to 71% of subcutaneous sumatriptan‐treated participants. The proportion of placebo‐treated participants with headache relief after one hour varied slightly according to the route of administration, at 12% to 15% with oral treatments, 19% with rectal treatment, and 25% to 28% with subcutaneous and intranasal treatments. The general trend for higher doses of sumatriptan giving lower (better) NNTs remains apparent, although again in many cases the differences between NNTs were not statistically significant (overlapping confidence intervals), suggesting a relatively minor dose response relationship.
Sustained headache relief during the 24 hours postdose
The 24‐hour sustained headache relief outcome requires participants to have headache relief at two hours, and then to sustain this relief for a further 22 hours without the use of rescue medication.
Pooled analyses were performed on two dose and route of administration combinations for which sufficient data were available (Summary of results F). There were no data provided for any other combinations for this outcome.
| Summary of results F: Sustained headache relief during the 24 hours postdose in placebo‐controlled studies | |||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 50 | 4 | 2526 | 454/1309 | 220/1217 | 35 | 18 | 1.9 (1.7 to 2.2) | 6.0 (5.0 to 7.6) |
| Oral | 100 | 6 | 4116 | 922/2538 | 270/1578 | 36 | 17 | 2.1 (1.9 to 2.4) | 5.2 (4.6 to 6.0) |
Figure 2 shows the calculated NNTs for 24‐hour sustained headache relief for two of the five most widely used dose and route of administration combinations (no information was available for subcutaneous 6 mg, intranasal 20 mg, or rectal 25 mg for this outcome).
Both doses of oral sumatriptan provided superior levels of sustained headache relief to placebo. Calculated NNTs were 6 with the lower dose and 5 with the higher, with 35% and 36% of participants respectively achieving the outcome with sumatriptan, and 18% and 17% with placebo. There was no significant difference between the NNTs for each of the two doses.
Any adverse event within 24 hours
This outcome captures the number of participants experiencing at least one adverse event during the 24 hours following administration of study medication; it does not attempt to take into consideration the relative severity of different adverse events, the number of individual events experienced, or any relationship between the study medication and the event as judged by the original study investigators.
All four reviews found adverse event reporting in the included studies to be highly variable and often of poor quality. Inconsistencies were found in the duration over which adverse event data were collected, assignment of a causal relationship to the study medication, and the continued collection of adverse data after a second dose of study medication or alternative rescue medication had been administered. Despite these inconsistencies, we included as much data as possible in the analyses in order to be more inclusive and conservative, but analyses of pooled data on adverse events should be interpreted cautiously.
Treatments were generally described as well tolerated, with most adverse events being of mild or moderate severity and self‐limiting.
Pooled analyses were performed on eight dose, route of administration, and baseline pain severity combinations for which sufficient data were available (Summary of results G). Six treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results G: Any adverse event within 24 hours in placebo‐controlled studies | |||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative harm | NNH (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 25 | 4 | 1550 | 371/956 | 220/594 | 39 | 37 | 1.1 (1.0 to 1.3) | Not calculated |
| Oral | 50 | 10 | 3728 | 667/2114 | 389/1614 | 32 | 24 | 1.3 (1.2 to 1.4) | 13 (9.7 to 22) |
| Oral | 100 | 12 | 3257 | 931/2171 | 255/1086 | 43 | 23 | 1.7 (1.5 to 1.9) | 5.2 (4.4 to 6.2) |
| Subcutaneous | 4 | 3 | 720 | 313/442 | 113/278 | 71 | 41 | 1.8 (1.6 to 2.2) | 3.3 (2.7 to 4.4) |
| Subcutaneous | 6 | 9 | 1342 | 341/767 | 137/575 | 44 | 24 | 2.1 (1.8 to 2.5) | 4.9 (3.9 to 6.4) |
| Intranasal | 20 | 2 | 516 | 125/331 | 27/185 | 38 | 15 | 2.9 (2.0 to 4.2) | 4.3 (3.3 to 6.3) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 6 | 1242 | 104/642 | 43/600 | 16 | 7 | 2.3 (1.6 to 3.2) | 11 (8.0 to 18) |
| Oral | 100 | 4 | 941 | 89/471 | 32/470 | 19 | 7 | 2.7 (1.9 to 4.0) | 8.3 (6.1 to 13) |
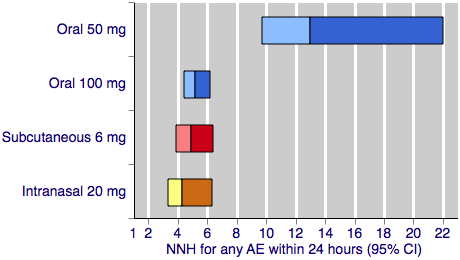
Figure 3 shows the calculated NNHs for any adverse event within 24 hours of dosing for four of the five most widely used dose and route of administration combinations (no information was available for rectal 25 mg for this outcome).

Sumatriptan versus placebo. Calculated NNHs for any adverse event within 24 hours of dosing, in participants treating moderate or severe migraine pain. Results for four of the five most commonly used dose and route of administration combinations (adverse event information for rectal sumatriptan not available), listed in rank order.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, and intranasal doses are shown with yellow bars.
With the exception of the 25 mg oral dose, all of the dose and route combinations resulted in significantly more harm with sumatriptan than placebo. For participants with moderate or severe baseline pain, calculated NNHs for this outcome ranged from 13 with oral 50 mg, to 3.3 with subcutaneous 4 mg. For oral administration, higher doses of sumatriptan resulted in lower (worse) NNHs, with 100 mg significantly worse than 50 mg (P < 0.00006). There was no apparent dose response relationship for the two doses of subcutaneous sumatriptan.
The two doses of oral sumatriptan administered to participants with mild baseline pain also resulted in significantly more participants with at least one adverse event than placebo. Calculated NNHs were 11 and 8.3 for the 50 and 100 mg doses respectively, with about 15% to 20% of participants experiencing an adverse event after sumatriptan, compared with 7% after placebo. Statistical comparison between the treatment effects in mild and moderate or severe baseline pain were not performed for incidence of adverse events due to the inconsistencies described previously in the contributing data.
Other outcomes
The individual reviews also provided information on use of rescue medication, relief of headache‐associated symptoms (nausea, photophobia, and phonophobia), and relief of functional disability in placebo‐controlled studies. Summaries of these outcomes are available in Appendix 1.
Sumatriptan versus active comparators
Only the oral route of administration provided sufficient data to allow pooled analysis of any dose of sumatriptan versus another active migraine treatment. Several individual studies comparing sumatriptan, delivered by other routes, with active treatments were included in the relevant reviews, but the amount of data was insufficient to allow pooled analysis (fewer than two studies or 200 participants, or both, contributing data). Detailed descriptions of all head‐to‐head comparisons can be found in the individual intervention reviews (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d). Using the available information, it is not possible to compare the relative efficacies of the different routes of administration of sumatriptan versus other active treatments, so we have simply summarised the findings from the review of oral sumatriptan. Full summary tables for each individual outcome are provided in Appendix 2.
Of the active comparators tested against oral sumatriptan, only rizatriptan 5 mg and 10 mg, effervescent acetylsalicylic acid 1000 mg, zolmitriptan 2.5 mg and 5 mg, eletriptan 40 mg and 80 mg, almotriptan 12.5 mg, paracetamol 1000 mg plus metoclopramide 10 mg, and acetylsalicylic acid 900 mg plus metoclopramide 10 mg provided sufficient data for analysis of any particular outcome. Results, in brief, were as follows.
-
Rizatriptan 5 mg was superior to sumatriptan 25 mg for pain‐free at two hours and headache relief at two hours, but there was no significant difference between the treatments for headache relief at one hour, and there was no difference between rizatriptan 5 mg and sumatriptan 50 mg for headache relief at two hours.
-
Rizatriptan 10 mg was superior to sumatriptan 25 mg, 50 mg, and 100 mg for all reported outcomes, including pain‐free at two hours and headache relief at one and two hours.
-
Zolmitriptan 2.5 mg and 5 mg showed no significant difference to sumatriptan 50 mg for headache relief at one or two hours.
-
Almotriptan 12.5 mg showed no significant difference to sumatriptan 100 mg for either pain‐free at two hours or 24‐hour sustained pain‐free.
-
Eletriptan in both doses (40 mg and 80 mg) was superior to sumatriptan in both doses (50 mg and 100 mg) for most reported primary outcomes, including pain‐free and headache relief at two hours. However, there was no significant difference between sumatriptan 50 mg and eletriptan 40 mg for headache relief at one hour, or between sumatriptan 100 mg and eletriptan 40 mg for pain‐free at one hour. Eletriptan was also generally superior in terms of the relief of headache‐associated symptoms and need for rescue medication.
-
Effervescent acetylsalicylic acid 1000 mg was more effective than sumatriptan 50 mg for headache relief at one hour, but there was no difference between the treatments for pain‐free at one or two hours, and sumatriptan 50 mg was significantly superior for headache relief at two hours.
-
There was no significant difference between sumatriptan 100 mg and either paracetamol 1000 mg plus metoclopramide 10 mg or acetylsalicylic acid 900 mg plus metoclopramide 10 mg for headache relief at two hours. Sumatriptan 100 mg was, however, significantly superior to acetylsalicylic acid plus metoclopramide for pain‐free at two hours.
-
There was no significant difference in the incidence of adverse events between any of the analysed doses of sumatriptan and their active comparators, with the exception of acetylsalicylic acid 900 mg plus metoclopramide 10 mg, which caused significantly fewer adverse events than sumatriptan 100 mg.
Discussion
A number of features of anti‐migraine treatments come together to determine overall performance and success. Individual patients will prioritise some of these features over others, depending on what aspect of their headache affects them most. The four reviews included in this overview particularly addressed the extent, speed of onset, and maintenance of pain relief, and the incidence of adverse events after treatment with sumatriptan administered via four alternative routes. Other aspects like relief of phonophobia, photophobia, or other symptoms were also reported, and are covered in Appendix 1. In this overview we bring together the information for different doses of sumatriptan and different route of administration to allow indirect comparison. In the context of treating individual patients this information may then be used to inform decisions about the use of sumatriptan to treat acute migraine.
Summary of main results
Extent of pain relief—results two hours after dosing
For the IHS preferred outcome of pain‐free at two hours, the 4 mg and 6 mg doses of subcutaneous sumatriptan, given when pain was moderate or severe, showed the greatest efficacy with 50% to 60% of participants achieving the response, compared with about 13% with placebo. NNTs were 2.5 and 2.3 for the 4 mg and 6 mg doses, respectively. All other routes of administration, at all analysed doses, resulted in reduced efficacy compared with the two subcutaneous doses. NNTs ranged from 4.2 to 7.3, and with overlapping confidence intervals, there was little difference between the these three routes of administration. Efficacy was significantly improved if treatment was taken early, while pain was still mild.
For headache relief at two hours, the two subcutaneous doses analysed (4 mg and 6 mg) again showed the greatest efficacy, with 70% to 80% of participants achieving the response, compared with about 30% of placebo‐treated participants, giving an NNT of 2.1 for both doses. All other routes of administration resulted in reduced efficacy at all analysed doses, with NNTs ranging from 2.4 to 7.4. For the most commonly used doses (oral 50 mg and 100 mg, intranasal 20 mg, and rectal 25 mg), there was very little difference between oral, rectal, and intranasal sumatriptan.
Speed of onset of pain relief—results one hour after dosing
For some migraineurs, rapid pain relief is a priority, and some studies have assessed pain relief outcomes at the earlier time of one hour after administration. There were limited data for the outcome of pain‐free at one hour, and only the subcutaneous route provided clinically useful levels of efficacy. The calculated NNTs for pain‐free at one hour with subcutaneous sumatriptan were 3.8 and 2.9 for 4 mg and 6 mg, respectively, with 33% and 41% of participants achieving the response after sumatriptan compared with 6% and 7% after placebo.
More participants achieved the less stringent outcome of headache relief at one hour, and again the subcutaneous route showed the greatest efficacy. NNTs were 2.5 and 2.2 for subcutaneous sumatriptan 4 mg and 6 mg, respectively, with 66% and 71% of participants achieving the response with sumatriptan compared with 25% and 26% after placebo. Rectal (25 mg) and intranasal (20 mg) treatment had NNTs of 3.2 and 4.9, respectively. A lower intranasal dose, and all analysed doses of orally administered sumatriptan, showed limited efficacy, with NNTs of 5.9 to 9.6, with only about 30% to 40% of participants achieving the response.
Sustained pain relief during the 24 hours postdose
Recurrence of headache following an initial response has been reported as a problem with sumatriptan. Two of the specified outcomes addressed the efficacy of sumatriptan for sustaining initial pain relief (at two hours) over the following 22 hour period, without the use of additional medication. Many studies did not report data for the 24‐hour sustained efficacy measures, so only limited comparison between routes of administration was possible.
For sustained pain‐free response during the 24 hours following the dose of sumatriptan, there was little difference between the subcutaneous dose analysed (6 mg) and the higher of the two oral doses (100 mg). About 25% to 30% of sumatriptan‐treated participants were pain‐free at two hours and sustained this level of pain relief up to 24 hours after administration (compared with about 10% of placebo‐treated participants), giving NNTs of 6.1 and 6.5 for the subcutaneous and oral treatments, respectively. The lower dose of oral sumatriptan (50 mg) showed reduced sustained efficacy, with only 17% of participants achieving this response, and an NNT of 9.5.
Information on sustained headache relief during the 24 hours following the dose of sumatriptan was available only for orally administered sumatriptan, which gave NNTs of about 5 to 6 for the 50 mg and 100 mg doses, respectively (about 35% of participants achieving response after sumatriptan, compared with 17% after placebo).
Safety and tolerability
There was a considerable degree of inconsistency in the reporting of adverse events in all four reviews, and while attempts were made to analyse the available data, results from pooled analyses must be interpreted with caution. A further consideration is that these data are largely obtained from single dose studies and may not represent clinical practice, where single doses may be taken repeatedly at differing time intervals, sometimes over many years.
There was little difference in the number of participants experiencing at least one adverse event within 24 hours of treatment between the subcutaneous dose of 6 mg, the intranasal dose (20 mg), and the highest oral dose analysed (100 mg). Approximately 40% of sumatriptan‐treated participants experienced at least one adverse event within 24 hours, compared with only 15% to 20% of placebo‐treated participants. For the 4 mg subcutaneous dose, there was a high response rate in both the active and placebo treatment arms (71% and 41%, respectively), but the relative benefit was similar. The calculated NNHs for these treatments ranged from 3.3 to 5.2. Fewer participants experienced adverse events with 50 mg (NNH 13) and 25 mg (not significantly different from placebo) oral sumatriptan.
Adverse events were generally described as of mild or moderate severity and self‐limiting. Few events were considered serious, and there was no strong evidence for cardiovascular problems with sumatriptan.
Treating early, when pain is mild
Results discussed up to this point were obtained by treating participants with a single dose of sumatriptan when pain intensity was moderate or severe. Studies have been done in this way, primarily for regulatory approval, to determine whether the drug has efficacy in this condition; the presence of at least moderate pain gives sensitivity to detect a change with treatment. In clinical practice many people are able to recognise the onset of a migraine attack and treat their headache during the initial phase, when pain is usually mild. There is some evidence suggesting that treating attacks in the early stages in this way may be beneficial (Gendolla 2008; Pascual 2002), and recently a number of studies have been carried out to investigate treatment of mild baseline pain.
Data for participants treating mild baseline pain were available only for the oral route of administration. The two doses analysed (50 mg and 100 mg) in these participants provided superior pain relief compared to placebo at all three time points investigated. NNTs for a pain‐free response at one and two hours and for a 24‐hour sustained pain‐free response in comparisons with placebo were all found to be significantly lower (better) in participants who treated attacks early, while pain was still mild, compared with waiting until pain was at least moderate (indirect comparisons). Both doses resulted in similar (or possibly slightly reduced in the case of the 100 mg dose) numbers of participants experiencing at least one adverse event after treatment (NNH values of 11 and 8.3 for the 50 mg and 100 mg doses, respectively). No statistical comparisons were performed due to inconsistencies and uncertainty in the data contributing to pooled analyses of adverse events.
Repeat dosing for inadequate response
Some individuals experience an inadequate response to a single dose of sumatriptan. In clinical practice, it is not uncommon to take a second dose in these circumstances, and a few studies have investigated this as a treatment strategy. Information on the efficacy of repeat dosing strategies was limited to the subcutaneous route of administration. Giving of a second dose of 6 mg subcutaneous sumatriptan if the participant had insufficient relief at one hour did not have a significant effect; the number of participants with either headache relief or a pain‐free response at two hours after two doses was not significantly different to that found after the single 6 mg subcutaneous dose.
Other outcomes
Additional information (provided in Appendix 1) shows that sumatriptan reduces the need for additional medication, relieves headache‐associated symptoms (nausea, photophobia, and phonophobia), and relieves functional disability. Generally, the subcutaneous and intranasal routes of administration, and the 100 mg oral dose gave better results, with clinically useful (< 6) NNTs.
Comparison with other active migraine treatments
Sufficient data were available to perform pooled analyses directly comparing sumatriptan with other active treatments only for the oral route of administration. Oral sumatriptan was compared with rizatriptan, effervescent acetylsalicylic acid, eletriptan, almotriptan, acetylsalicylic acid + metoclopramide, zolmitriptan, and paracetamol + metoclopramide. In general there was little difference between sumatriptan and these active comparators at the doses tested. Only eletriptan (particularly the 80 mg dose) provided consistently superior pain relief and relief of headache‐associated symptoms. Rizatriptan provided better relief when compared with low dose sumatriptan (25 mg), but not when compared with higher doses (50 mg or 100 mg) where the difference was far less substantial and often not significant. More participants experienced the outcome of pain‐free at two hours, but not headache relief at two hours, with sumatriptan 100 mg than with acetylsalicylic acid 900 mg plus metoclopramide 10 mg. There was no difference between sumatriptan 50 mg and acetylsalicylic acid 1000 mg.
Data for other outcomes, such as relief of headache‐associated symptoms, were mostly limited to comparisons with eletriptan. Generally eletriptan 80 mg gave better results than oral sumatriptan 100 mg, but NNTs were of borderline clinical utility (≥ 6). Oral sumatriptan 100 mg gave equivalent relief of nausea to oral acetylsalicylic acid 900 mg plus metoclopramide 10 mg.
Variation in the placebo response
It is possible that route of administration could influence the response to placebo and affect comparisons between different routes of administration of the same drug. It is commonly stated that intravenous or subcutaneous drug administration elicits a greater placebo response than oral administration in the treatment of migraine; however, the evidence is limited and only a few studies have rigorously addressed this question (Bendtsen 2003; de Craen 2000; Diener 2008; Macedo 2006).
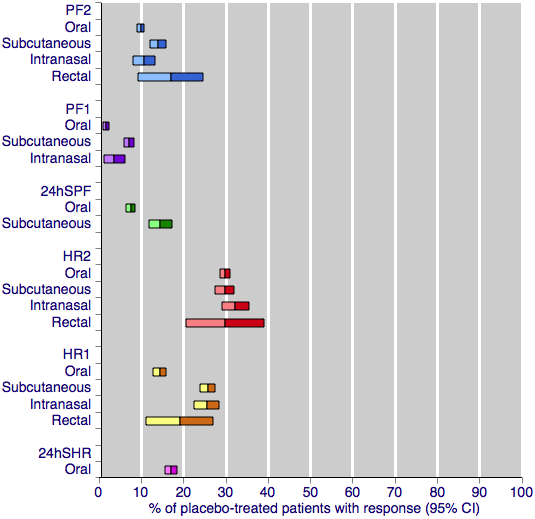
This large and clinically homogeneous data set permits investigation; Figure 4 shows pooled placebo response rates, with 95% confidence intervals, for the four routes of administration and each of the primary efficacy outcomes. Visual comparison between the oral and subcutaneous treatments shows that the placebo response after subcutaneous treatment is, in nearly all cases, higher than after oral treatment. The response after intranasal treatment is slightly more variable, but tends to fall between oral and subcutaneous responses, while the confidence intervals (due to insufficient data) are too large to draw meaningful conclusions about rectal treatment. The only outcome that does not show any variability in placebo response is headache relief at two hours, for which all four routes of administration show a consistent response of around 30%. This outcome, of course, has the largest amount of data for placebo, and it is not unlikely that the similarity in placebo response rates across routes of administration is a refection of limiting random play of chance (Moore 1998).

Placebo response rates for the primary efficacy outcomes, by route of administration. Response rates of each outcome are grouped by colour to facilitate comparison between different routes of administration. Proportion of placebo‐treated participants pain‐free at two hours are shown with blue bars, pain‐free at one hour with purple bars, 24‐h sustained pain‐free with green bars, headache relief at two hours with red bars, headache relief at one hour with yellow bars, and 24‐h sustained headache relief with a pink bar.
While Figure 4 shows a small degree of variation between the placebo responses with different routes of treatment for most outcomes, a much stronger determinant appears to be the outcome measured. There is a substantial difference between different levels of pain relief at the same time point, for example pain‐free and headache relief at two hours, and even greater differences between different levels of pain relief at different time points, for example pain‐free at one hour and headache relief at two hours. These data show clearly that the more stringent or exacting outcomes (those that are harder to achieve) result in lower placebo response rates. This is consistent with previous studies, which have shown the placebo response for pain‐free outcomes to be much lower than for headache relief outcomes Diener 2008; Macedo 2006; Oldman 2002), and parallels what is seen with active treatment. For any given route and outcome, the placebo response rates in this overview of sumatriptan alone are remarkably similar to those in Macedo 2006, which reviewed all acute migraine treatments, and Oldman 2002 which provided an overview across many treatments.
It may be that only the more exacting outcomes (which fewer patients will achieve) can provide the necessary sensitivity to expose small differences in placebo response rates between different routes of administration. This idea that more exacting outcomes provide a greater degree of discrimination and expose relatively small differences between pooled results has been described before (Moore 2011), in the context of active treatments in acute pain trials. Here we show that the same idea can be applied to identifying small differences between placebo responses.
Investigation and discussion of placebo is of academic importance. It could be argued that patients want complete pain relief (Lipton 1999), and that placebo is important relating to complete pain relief. There is a wealth of evidence suggesting that patients generally consider 'no worse than mild pain' (in the present context, the result of achieving 'headache relief' (pain reduced from moderate or severe to none or mild)) a useful outcome (Moore 2013). At two hours after dosing, there is little meaningful difference between placebo response rates using either pain‐free or headache relief (Figure 4).
Overall completeness and applicability of evidence
The four individual reviews involved 52,236 participants, and all used the same methodological approach and assessed the same efficacy and safety outcomes. The outcomes were chosen because they are of known importance to patients who suffer acute migraine attacks (Lipton 1999). Not all of the studies reported results for all of these outcomes, particularly that of sustained pain relief and incidence of adverse events. This inconsistency of reporting limited analysis of these outcomes for some dose and route of administration combinations. For example, only the oral 50 mg and 100 mg doses, and the subcutaneous 6 mg dose provided sufficient data to carry out any analysis of sustained pain relief during the 24 hours postdose.
The vast majority of studies included in each of the four reviews specifically treated participants with moderate or severe baseline pain intensity, and only a small number of studies included in the review of oral sumatriptan provided any efficacy data for sumatriptan in participants with mild baseline pain intensity, which may more closely reflect what happens in clinical practice. Although more participants experienced a pain‐free outcome when treating mild pain, more studies reporting consistently on early treatment and different dosing strategies are needed to inform the best clinical use of sumatriptan.
Several new routes of sumatriptan administration are currently being considered, including needle‐free injection systems, buccal patches, and transdermal patches. Investigations into the possible clinical utility of these new routes are still largely preliminary in nature, and no Phase III RCTs making use of them were found for inclusion in individual reviews. Recently a needle‐free delivery system for subcutaneous sumatriptan has been approved for use. Sumavel DosePro uses compressed gas to create a stream of medication that passes through the skin into the subcutaneous tissue. Bioequivalence with traditional injected subcutaneous sumatriptan has been demonstrated for this novel method of administration (Brandes 2009), but no studies were found specifically addressing its efficacy, safety and tolerability. Similarly, a novel iontophoretic transdermal patch, known as Zelrix, has recently reached Phase III clinical trials, after a phase I study demonstrated good tolerability and equivalent plasma levels to the oral tablet, subcutaneous, and intranasal routes of administration (Pierce 2009). More clinical trial data are required to make adequate assessments of the relative merits of these novel routes for sumatriptan administration.
Quality of the evidence
The quality of the evidence was largely excellent. Each of the included reviews met all of the AMSTAR criteria, including the use of wide searching strategies and no language limitation, and incorporated only studies that were both randomised and double‐blind, and had a low risk of bias from any major source. Where identified, potential sources of bias in the included studies have been discussed in depth in each of the individual reviews, but in each case removal of these data was found to have no significant effect on the calculated results. Perhaps the most important source of potential bias is that of publication bias, where there is a risk that unpublished data not included in the review may be sufficient to overturn any positive effect identified in the review. This is particularly relevant to those doses and routes of administration for which less evidence was available. Susceptibility to publication bias has been assessed for the two most widely reported IHS efficacy outcomes (pain‐free and headache relief at two hours), and taken into consideration when commenting on the robustness of the evidence for a particular dose and route of administration combination. For each route of administration, publication bias was considered unlikely to affect the result for licensed doses, with the exception of 25 mg oral, 10 mg intranasal, and 25 mg rectal for pain‐free at two hours, and of 5 mg intranasal, 10 mg intranasal, and 25 mg rectal for headache relief at two hours.
Potential biases in the overview process
The purpose of this overview was simply to bring together the evidence reported in four separate reviews on the use of sumatriptan to treat acute migraine. Each review used the same methodology to address the same set of outcomes for each of the different routes of administration of sumatriptan currently available. No statistical analyses were performed within this overview, and only informal comparisons were made between the various routes of administration and doses. There was therefore no opportunity to introduce bias through the methods used in the overview process.
Agreements and disagreements with other studies or reviews
The results for each route of administration were found to be consistent with previous studies and reviews using the same route of delivery. The specific agreements and disagreements are described in the appropriate individual reviews.
No previous systematic reviews encompassing or summarising all four routes of administration of sumatriptan could be found to compare with this overview. Several studies (for example, Bigal 2003; Johnston 2010) providing a narrative evaluation of the different routes have reported findings consistent with those reported here. That is, that subcutaneous sumatriptan provides the highest clinical efficacy and the fastest onset of effect, but is associated with a large number of adverse events; and that the oral and intranasal routes provide a similar level of efficacy, albeit with slower onset and for significantly fewer participants than the subcutaneous route. These narrative reports do not provide a systematic assessment with pooled analyses of all the available data, and therefore detailed comparison with this overview is not possible. One review (Oldman 2002) did provide quantitative measures of efficacy for three of the four routes of administration of sumatriptan: subcutaneous, intranasal and oral. Interestingly, despite the fact that the calculations in our up‐to‐date reviews were based on data from many more participants (at least half as many again, and for some dose, route, and outcome combinations, up to six times as many participants), there are no major differences between the NNTs calculated. The table below summarises, for the outcomes that are directly comparable, the NNTs calculated for the four dose and route of administration combinations originally analysed in Oldman 2002.
| Comparison of the calculated NNTs for four different route of administration/dose combinations of sumatriptan in two different reviewsa | |||
| Route of administration/dose combination | Headache relief at 2 hours | Headache relief at 1 hour | Pain‐free at 2 hours |
| Subcutaneous 6 mg | 2.0 (1.8 to 2.2) | 2.1 (1.9 to 2.2) | 2.1 (1.9 to 2.4 |
| 2.1 (2.0 to 2.2) | 2.2 (2.1 to 2.4) | 2.3 (2.1 to 2.4) | |
| Intranasal 20 mg | 3.4 (2.9 to 4.1) | 5.6 (4.3 to 8.0) | 4.6 (3.6 to 6.1) |
| 3.5 (3.1 to 4.1) | 4.9 (4.1 to 6.1) | 4.7 (4.0 to 5.9) | |
| Oral 50 mg | 4.1 (3.4 to 5.2) | 11 (7.1 to 22) | 7.8 (6.1 to 11) |
| 4.0 (3.7 to 4.3) | 7.1 (5.8 to 9.1) | 6.1 (5.5 to 6.9) | |
| Oral 100 mg | 3.3 (3.0 to 3.7) | 7.6 (5.9 to 10) | 4.7 (4.1 to 5.5) |
| 3.5 (3.2 to 3.7) | 6.8 (5.8 to 8.3) | 4.7 (4.3 to 5.1) | |
| aResults in bold are the most up‐to‐date from the four reviews included in this overview. Non‐bold results are from the Oldman 2002 review. | |||
Sumatriptan versus placebo. Calculated NNTs for a pain‐free response after a specified time, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
PF2: pain‐free at two hours; PF1: pain‐free at one hour; 24h SPF: 24‐hour sustained pain‐free.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.

Sumatriptan versus placebo. Calculated NNTs for headache relief after a specified time, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
HR2 (headache relief at two hours); HR1 (headache relief at one hour); 24h SHR (24‐hour sustained headache relief).
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.
Sumatriptan versus placebo. Calculated NNHs for any adverse event within 24 hours of dosing, in participants treating moderate or severe migraine pain. Results for four of the five most commonly used dose and route of administration combinations (adverse event information for rectal sumatriptan not available), listed in rank order.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, and intranasal doses are shown with yellow bars.
Placebo response rates for the primary efficacy outcomes, by route of administration. Response rates of each outcome are grouped by colour to facilitate comparison between different routes of administration. Proportion of placebo‐treated participants pain‐free at two hours are shown with blue bars, pain‐free at one hour with purple bars, 24‐h sustained pain‐free with green bars, headache relief at two hours with red bars, headache relief at one hour with yellow bars, and 24‐h sustained headache relief with a pink bar.

Sumatriptan versus placebo. Calculated NNTs for relief of migraine‐associated symptoms and functional disability after two hours, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.

