Apósitos hidrocoloides para la cicatrización de las úlceras del pie diabético
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009099.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 06 agosto 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Jo Dumville developed the review and co‐ordinated development, completed the first draft of the review, made an intellectual contribution, approved the final version prior to submission and is the guarantor of the review and the update.
Sohan Deshpande completed the first draft of the review, made an intellectual contribution and approved the final version of the review prior to submission.
Susan O’Meara edited the review, made an intellectual contribution and approved the final version of the review and the update prior to submission.
Katharine Speak made an intellectual contribution to the review, advised on the review and approved the final version prior to submission.
Contributions of editorial base:
Nicky Cullum: edited the protocol and review; advised on methodology, interpretation and content. Approved the final review prior to submission.
Joan Webster, Editor: approved the review update prior to submission.
Sally Bell‐Syer: co‐ordinated the editorial process. Advised on methodology, interpretation and content. Edited the review.
Ruth Foxlee: designed the search strategy and edited the search methods section.
Sources of support
Internal sources
-
Department of Health Sciences, University of York, UK.
External sources
-
NIHR Programme Grants for Applied Research, UK.
-
NIHR/Department of Health (England), Cochrane Wounds Group, UK.
Declarations of interest
Susan O'Meara and Jo Dumville receive funding from the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research programme. This study presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research programme (RP‐PG‐0407‐10428). The views expressed in this presentation are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Sohan Deshpande and Katharine Speak: none declared.
Acknowledgements
The authors would like to thank the following people who reviewed the protocol and review for clarity, readability and rigour: Wounds Group editors (Julie Bruce, Andrea Nelson and Gill Worthy) and peer referees (David Armstrong, Duncan Chambers and Janet Yarrow). In addition copy editor Jenny Bellorini; Nikki Stubbs for clinical advice; Xun Li Xun for translation of Chinese language papers. We would like to thank Sally E.M. Bell‐Syer and Ruth Foxlee for all their expertise and support during the review process.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Aug 06 | Hydrocolloid dressings for healing diabetic foot ulcers | Review | Jo C Dumville, Sohan Deshpande, Susan O'Meara, Katharine Speak | |
| 2012 Feb 15 | Hydrocolloid dressings for healing diabetic foot ulcers | Review | Jo C Dumville, Sohan Deshpande, Susan O'Meara, Katharine Speak | |
| 2011 May 11 | Hydrocolloid dressings for healing diabetic foot ulcers | Protocol | Jo C Dumville, Sohan Deshpande, Susan O'Meara, Katharine Speak | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
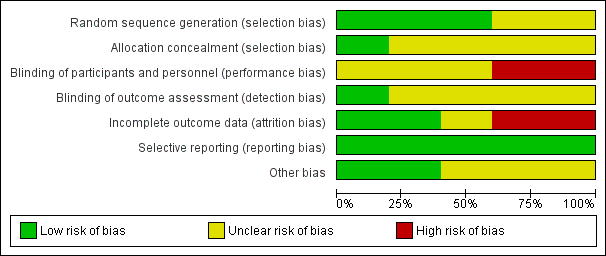
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
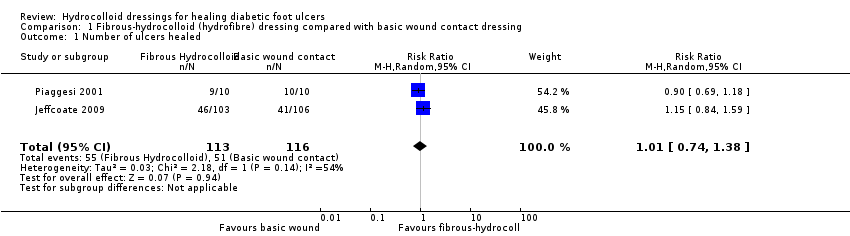
Comparison 1 Fibrous‐hydrocolloid (hydrofibre) dressing compared with basic wound contact dressing, Outcome 1 Number of ulcers healed.

Comparison 2 Hydrocolloid (matrix) dressing compared with foam dressing, Outcome 1 Number of ulcers healed.

Comparison 3 Silver hydrocolloid dressing compared with alginate dressing, Outcome 1 Number of ulcers healed.

Comparison 4 Iodine‐impregnated dressing compared with fibrous‐hydrocolloid (hydrofibre) dressing, Outcome 1 Number of ulcers healed.
| Study | Groups | Primary outcome: ulcer healing | Secondary: health‐related quality of life | Number and level of amputations | Adverse events, including pain | Cost | Ulcer recurrence | Heading 8 |
| Clever 1995 | Group A (n = 20): Hydrocolloid (polyurethane matrix) dressing Group B (n = 20): Foam dressing | Number of ulcers healed: Group B: 14 Group B: 20.43 (14.74) Group B: 16.5 (range 4 to 52) Group B: 33.46 (75.22) | n/r | n/r | (Reasons not reported separately for 2 groups): Group B: 5 | Mean number of dressing changes between clinical visits (SD): Group B: 2.37 (2.18) | n/r | |
| Jeffcoate 2009 | Group A (n = 103): Fibrous‐hydrocolloid (hydrofibre) dressing Group B (n = 108): Iodine‐impregnated dressing | Number of ulcers healed at 24 weeks: Group B: 48 Group B: 127.8 (54.2) | Mean Cardiff Wound Impact Schedule score at 24 weeks (SD) Group A: Physical functioning: 71.4 (19.5). Social functioning: 70.3 (25.4). Well being: 53.1 (19.9) | Minor amputations (Below ankle): | Non‐serious adverse events Group B: 239 Group B: 37 | Cost in GBP per patient for dressing management: Group A: 191.33 (148.41 to 234.25) Group A: 459.87 (354.78 to 564.97) Cost in GBP of generating an additional healed ulcer: Group A: 836 Group B: 848 | At same site | |
| Jude 2007 | Group A (n = 67): Fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver | Number of ulcers healed in 8 weeks | n/r | n/r | Group A: 25 participants experienced one or more events. Death = 1; Infection = 14. 8 participants discontinued treatment due to AE. | Mean number of dressing changes during study: | n/r | |
| Kuo 2012 | Group A (n = 12): fibrous‐hydrocolloid (hydrofibre) dressing | Percent change in wound size (from baseline ‐ assumed to 14 days). Median and (IQR) Group A: ‐22.64 (‐36.90 to ‐3.20) Group B: ‐ 27.18(‐38.86 to 36.10) Reported in paper as not statistically significant from Mann‐Whitney U test. P = 0.673 | n/r | n/r | Number of people with one or more adverse events Group A: 5/12 (41.7%) Group B: 5/12 (41.7%) | |||
| Piaggesi 2001 | Group A (n = 10): Fibrous‐hydrocolloid (hydrofibre) dressing | Number of ulcers healed (during period of study): | n/r | Group A: Amputation of a lesser toe = 3; Amputation of 2 lesser toes = 1; Metatarsal resection = 1 | Group A: Maceration of peri‐lesional skin = 1; Infective complications = 1 | Average number of days between dressings changes (SD) | n/r | |
Comparison 5 Trial data, Outcome 1 Trial data.
| Fibrous‐hydrocolloid (hydrofibre) dressing compared to basic wound contact dressing for healing diabetic foot ulcers | ||||||
| Patient or population: patients with healing diabetic foot ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Basic wound contact dressing | Fibrous‐hydrocolloid (hydrofibre) dressing | |||||
| Number of ulcers healed | Low1 | RR 1.01 | 229 | ⊕⊕⊕⊝ | ||
| 340 per 1000 | 343 per 1000 | |||||
| Moderate1 | ||||||
| 530 per 1000 | 535 per 1000 | |||||
| High1 | ||||||
| 650 per 1000 | 657 per 1000 | |||||
| HRQoL | See comment | See comment | Not estimable | 0 | See comment | One study measured HRQoL at 24 weeks follow‐up. Data from several domains are presented in the report, with no statistically significant difference observed. |
| Adverse events | See comment | See comment | Not estimable | 0 | See comment | AEs for two studies ‐ very similar numbers in each arms. Data not analysed here as not independent ‐ that is one person could have multiple events or due to limited data. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Baseline risk of healing obtained from external source in which data from 27,630 patients with a diabetic neuropathic foot ulcer was used to develop a simple prognostic model to predict likelihood of ulcer healing (Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med. 2003;115:627‐31). It is important to note that given an outcome of ulcer healing, low risk refers to a low risk of healing and thus reflects the most severe patient populations. Conversely high risk refers to a high risk of healing. | ||||||
| First author | Group A | Group B | Group C | Duration of follow up | % healed data |
| Hydrocolloid (polyurethane matrix) dressing (Cutinova Hydro, S&N Hlth) | Foam dressing (Allevyn, S&N Hlth) | 16 weeks | yes | ||
| Fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec) | Iodine‐impregnated dressing (Inadine, Johnson & Johnson) | Non‐adherent dressing (Johnson & Johnson) | 24 weeks | yes | |
| Fibrous‐hydrocolloid (hydrofibre) dressing with 1.2% ionic silver (Aquacel Ag, ConvaTec) | Calcium‐alginate dressing (Algosteril, S&N Hlth) | 8 weeks | yes | ||
| Fibrous‐hydrocolloid (hydrofibre) dressing (Aquacel, ConvaTec) | Cream contained extracts from two botanical raw materials, P. amboinicus and C. asiatica.(Active ingredient 1.25%) | 2 weeks | No | ||
| Fibrous‐hydrocolloid (Hydrofibre) dressing (Aquacel, ConvaTec) | Saline‐moistened gauze | Not reported (maximum follow up was 350 days) | yes |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of ulcers healed Show forest plot | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.74, 1.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of ulcers healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of ulcers healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of ulcers healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Trial data Show forest plot | Other data | No numeric data | ||

