HPV‐Impfung zur Vorbeugung von Gebärmutterhalskrebs und seinen Vorstufen
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [author‐defined order]
Ir a:
| Methods | Phase IIb, randomised, double‐blind, placebo‐controlled trial | |
| Participants | 2392 women (1194 in the vaccine arm and 1198 in the placebo arm) from 16 centres in the USA Age range: 16 to 23 years Inclusion criteria: young women who were HPV16 DNA negative at enrolment and month 7, were HPV16 seronegative at enrolment, had had no other vaccination ±1 month around each dose. Virgins were enrolled if they were seeking contraception Exclusion criteria: pregnancy, history of abnormal Pap smears, more than 5 sexual partners | |
| Interventions | Vaccine: monovalent HPV16 L1 virus‐like particles Placebo: visually indistinguishable aluminium adjuvant placebo | |
| Outcomes | Safety, immunogenicity and efficacy (persistent HPV16 infection and histological lesions of CIN 1+,2+ and 3+) | |
| Notes | Reports: Koutsky 2002; Mao 2006 and Rowhani‐Rahbar 2009 Last report average follow‐up time: 8.5 years (Rowhani‐Rahbar 2009) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study participants were randomised in a 1:1 ratio to receive vaccine or placebo. Permuted blocks were used to ensure similar numbers of participants in each arm |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was generated by computer, allocation numbers were assigned at each centre. No further details were provided regarding the concealment of allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and study staff were blinded to the group assignments |
| Blinding of outcome assessment (detection bias) | Low risk | An independent masked group of 4 pathologists reviewed the slides without knowledge of other clinical or laboratory data |
| Incomplete outcome data (attrition bias) | Low risk | Besides the per‐protocol (PP) analysis (HPV16 DNA negative at enrolment and during vaccination, HPV16 seronegative at enrolment, 3 doses received, no protocol violations) also modified‐intention‐to‐treat (MITT‐1 [HPV16 DNA negative and seronegative at enrolment, at least 1 dose received], MITT‐2 (including also women being HPV16 DNA positive at enrolment) analyses were performed. Unrestricted susceptible population and ITT analysis done. Exclusions and reasons for exclusions were described and were balanced over the trial arms. |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety, immunogenicity and efficacy) were presented |
| Methods | A phase II randomised, double‐blind, controlled multicentre study in Janpan | |
| Participants | Participants: 1040 Japanese women (519 in the vaccine arm and 521 in the placebo arm) Age range: 20 to 25 years Inclusion criteria: women who were not pregnant, had an intact cervix and use adequate contraception over the vaccination period Exclusion criteria: women who had a previous vaccination with HPV vaccine or hepatitis A vaccine, previous 3‐O‐desacy l‐4'‐monophosphoral lipid A administration, hepatitis A infection and various clinically significant diseases, previous colposcopic examination for cervical cytological abnormality | |
| Interventions | Vaccine: bivalent HPV16/18 L1 VLP vaccine Placebo: Hepatitis A vaccine | |
| Outcomes | Safety, immunogenicity, incident & persistent HPV16/18 infection, cytological (ASCUS+) & histopathological abnormalities (CIN1+, CIN2+) associated with vaccine and non‐vaccine oncogenic HPV types | |
| Notes | Main reports: Konno 2010 and Konno 2010a Maximum follow‐up time: 24 months (Konno 2010a) For the outcome high‐grade CIN irrespective of types, the follow‐up results up to 48 months were used (Konno 2014) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomised 1:1 to receive the vaccine or placebo. No further details given |
| Allocation concealment (selection bias) | Unclear risk | Not described in the paper |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding was maintained for all personnel, investigators, study collaborators, and participants |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding can be assumed as covering also the outcome assessment since all investigators including the statisticians were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes are assessed both in the PP group (3 doses received; no protocol violations, were DNA negative for HPV vaccine types at month 0 and 6; had normal or LSIL cytology at month 0) and total vaccination group (at least one dose, were DNA negative for HPV vaccine types at month 0; had normal or LSIL cytology at month 0) |
| Selective reporting (reporting bias) | Low risk | Efficacy, safety and immunogenicity outcomes are reported |
| Methods | Phase II randomised, multicentre, double‐blind placebo‐controlled study | |
| Participants | 1113 women (560 in the vaccine arm and 553 in the placebo arm) from 32 study sites; 433 women were from a Brazilian cohort with longer follow‐up) Age range: 15 to 25 years Inclusion criteria: healthy women who had had no more than 6 sexual partners, no history of an abnormal Pap test, no ablative or excisional treatment of the cervix, and no ongoing treatment for external condylomata; being, at enrolment, cytologically negative, seronegative for HPV16 and HPV18 antibodies by ELISA, and HPV‐DNA negative by PCR for 14 high‐risk HPV types | |
| Interventions | Vaccine: bivalent HPV16/18 L1 VLP vaccine Placebo: Hepatitis A vaccine | |
| Outcomes | Safety, tolerability, immunogenicity, incident & persistent HPV infection, cytological (ASC‐US+, LSIL+) & histopathological abnormalities (CIN1+, CIN2+) associated with vaccine and non‐vaccine oncogenic HPV types | |
| Notes | Main reports: Harper 2004; Harper 2006; The GSK Study Group 2009 and De Carvalho 2010 Last report average follow‐up time: 7.3 years (De Carvalho 2010) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified, block randomisation according to validated algorithms was centralised with an Internet‐based randomisation system |
| Allocation concealment (selection bias) | Low risk | Trial allocation remained concealed from investigators and the women participating throughout initial and follow‐up studies |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded: trial arms were masked for women and medical personal |
| Blinding of outcome assessment (detection bias) | Low risk | A central laboratory, reported cytology results...the central histology laboratory made an initial diagnosis from the formalin‐fixed tissue specimens for clinical management |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes are assessed both in the PP group (3 doses received, seronegative for HPV16/18 at month 0 and negative for hrHPV DNA at month 7) and in the ITT group (at least 1 dose,, seronegative for HPV16/18, negative for hrHPV DNA at month 7, accepting HPV16/18 positive at month 0, including also protocol violations) are shown and reasons for exclusion are presented |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety, immunogenicity and efficacy) are presented |
| Methods | Phase IIIb, double‐blind, randomised, placebo‐controlled, multicentre trial | |
| Participants | Participants: 676 females (450 in the vaccine arm and 226 in the placebo arm) enrolled in Senegal or Tanzania. Age range: 10 to 25 years Inclusion criteria: healthy HIV‐seronegative girls and young women 10 to 25 years old at first vaccination, who were not pregnant and had fewer than 6 lifetime sexual partners | |
| Interventions | Vaccine: HPV16/18 bivalent vaccine Placebo: AI(OH)3 placebo | |
| Outcomes | Immunogenicity and safety outcomes | |
| Notes | Main report: Sow 2013 Last report average follow‐up time: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was computer generated using an Internet‐based randomisation blocking scheme |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed until end of the study |
| Blinding of participants and personnel (performance bias) | Low risk | Investigators, study staff, and participants in each country were blinded to vaccine assignment until all participants in that country had completed the 12‐month visit |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessment was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Safety analyses were based on the total vaccinated cohort, with at least one dose. Immunogenicity analyses were assessed in the PP cohort (3 doses received, no protocol violations). The dropout rates were low and balanced between the vaccine and placebo group |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Methods | Phase II/III randomised, double‐blind, controlled trial | |
| Participants | Participants: 3819 women (3026 in the vaccine arm and 3025 in the placebo arm) enrolled at four sites in JiangSu Province Age range: 18 to 25 years Inclusion criteria: women were agreed to use contraceptive precautions 30 days before the 1st vaccine dose and 2 months after completion of the vaccine series Exclusion criteria: women who were pregnant or breastfeeding, had an immunosuppressive or immunodeficient condition, a history of colposcopy, an allergic disease likely to be exacerbated by any component of the vaccine or previously received HPV vaccination or adjuvant were excluded | |
| Interventions | Vaccine: bivalent vaccine Placebo: aluminium hydroxide placebo | |
| Outcomes | Efficacy (incident and persistent HPV infection, CIN), safety and immunogenicity outcomes | |
| Notes | Report: Zhu 2014. Follow‐up of 15 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised in a 1:1 ratio with an Internet‐based centralised randomisation system |
| Allocation concealment (selection bias) | Low risk | Treatment allocation at the investigation site were using an Internet‐based system |
| Blinding of participants and personnel (performance bias) | Low risk | All participants, investigators and study staff were blinded to individual participant treatment assignments and results |
| Blinding of outcome assessment (detection bias) | Low risk | All participants, investigators and study staff were blinded to individual participants treatment assignments and results |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes (safety and immunogenicity) were reported on the total vaccinated cohort. Reason for exclusion was noted and balanced between vaccine group and placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety and immunogenicity) were presented |
| Methods | Phase IIIb randomised, double‐blind, controlled trial | |
| Participants | Participants: 750 girls (374 in the vaccine arm and 376 in the placebo arm) enrolled in JiangSu Province Age range: 9 to 17 years Inclusion criteria: healthy girls with non‐childbearing potential or who were agreed to use contraceptive precautions 30 days before the 1st vaccine dose and 2 months after completion of the vaccine series; must with written informed consent obtained from the parents Exclusion criteria: girls who had an immunosuppressive or immunodeficient condition, concurrently participating in another clinical study, hypersensitivity to latex, had an allergic disease likely to be exacerbated by any component of the vaccine or previously received HPV vaccination or adjuvant were excluded | |
| Interventions | Vaccine: bivalent vaccine Placebo: aluminium hydroxide placebo | |
| Outcomes | Safety and immunogenicity outcomes | |
| Notes | Report: Zhu 2014a Follow‐up of 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised in a 1:1 ratio to receive HPV vaccine or control, using a central Internet‐based randomisation system (see Zhu 2014) |
| Allocation concealment (selection bias) | Unclear risk | Not described in the paper |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described in the paper |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described in the paper |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes (safety and immunogenicity) were reported on the total vaccinated cohort. Reason for exclusion was noted and balanced between vaccine group and placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety and immunogenicity) were presented |
| Methods | Phase II/III randomised, double‐blind, controlled trial | |
| Participants | Participants: 1212 women (606 in the vaccine arm and 606 in the placebo arm) enrolled in JiangSu Province Age range: 26 to 45 years Inclusion criteria: women were agreed to use contraceptive precautions 30 days before the 1st vaccine dose and 2 months after completion of the vaccine series Exclusion criteria: women who were pregnant or breastfeeding, had an immunosuppressive or immunodeficient condition, a history of colposcopy, an allergic disease likely to be exacerbated by any component of the vaccine or previously received HPV vaccination or adjuvant were excluded | |
| Interventions | Vaccine: bivalent vaccine Placebo: HBV vaccine | |
| Outcomes | Safety and immunogenicity outcomes | |
| Notes | Report: Zhu 2014a Follow‐up of 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised in a 1:1 ratio to receive HPV vaccine or control, using a central internet‐based randomisation system (see Zhu 2014) |
| Allocation concealment (selection bias) | Unclear risk | Not described in the paper |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described in the paper |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described in the paper |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes (safety and immunogenicity) were reported on the total vaccinated cohort. Reason for exclusion was noted and balanced between vaccine group and placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety and immunogenicity) were presented |
| Methods | Randomised, controlled, open, multicentre parallel group study | |
| Participants | Participants: 751 healthy girls and young women were enrolled in France, Germany and Spain. Participants were randomised to receive a) HPV vaccine (n = 248), b) combined Diphtheria‐Tetanus‐Acellular Pertussis–inactivated Poliovirus vaccine (dTpa‐IPV) together with HPV vaccine at month 0 and the HPV vaccine at months 1 and 6 (n = 255) or c) dTpa‐IPV only at month 0 and HPV vaccine at months 1, 2 and 7 (n = 248) Age range: 10 to 18 years Inclusion criteria: healthy girls and young women who had a negative pregnancy test at the time of each vaccination, not breastfeeding, and if of child‐bearing potential, to be abstinent from sexual activity or using adequate contraceptive precautions, should have complete routine childhood vaccinations against diphtheria, tetanus, pertussis, and poliomyelitis Exclusion criteria: girls who had received diphtheria, tetanus, pertussis vaccine, diphtheria‐tetanus booster or dTpa vaccine, and/or oral or inactivated poliovirus vaccine within the previous 5 years; had known exposure to diphtheria or household exposure to pertussis, or diphtheria, tetanus, pertussis, or polio diagnosed within 30 days before vaccination | |
| Interventions | Vaccine: bivalent HPV vaccine Placebo: combined Diphtheria‐Tetanus‐Acellular Pertussis–inactivated Poliovirus vaccine (dTpa‐IPV) | |
| Outcomes | Safety and immunogenicity outcomes | |
| Notes | Report: Garcia‐Sicilia 2010 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was computer generated using a standard SAS program at GSK Biological, Rixensart, Belgium |
| Allocation concealment (selection bias) | High risk | Treatment allocation at the investigator site was performed using a central randomisation system but not blinded |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described in the paper |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described in the paper |
| Incomplete outcome data (attrition bias) | Low risk | All safety outcomes were reported for the total vaccinated cohort. Immunogenicity outcomes were reported for the according‐to‐protocol cohort. Reason for exclusion was noted and balanced between vaccine group and placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety and immunogenicity) were presented |
| Methods | Randomised, controlled, open, multicentre parallel group study | |
| Participants | Participants:741 girls enrolled at seven centres in the Netherlands and Sweden. Participants were randomised to receive HPV vaccine (n = 247), Hepatitis B vaccine (n = 247) or HPV vaccine co‐administrated with Hepatitis B vaccine (n = 247) Age range: 9 to 15 years Inclusion criteria: healthy girls who had a negative pregnancy test at the time of each vaccination and if of child‐bearing potential, to be abstinent from sexual activity or using adequate contraceptive precautions Exclusion criteria: girls with a history of hepatitis B infection or with known exposure to hepatitis B within 6 weeks prior to vaccination, girls with previous vaccination against HPV or hepatitis B | |
| Interventions | Vaccine: bivalent HPV vaccine Placebo: hepatitis B vaccine | |
| Outcomes | Safety and immunogenicity outcomes | |
| Notes | Report: Schmeink 2011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was computer generated at GSK Biological, Rixensart, Belgium |
| Allocation concealment (selection bias) | High risk | This was an open study, the participants and investigators were aware of the group allocated and vaccines given |
| Blinding of participants and personnel (performance bias) | High risk | See above |
| Blinding of outcome assessment (detection bias) | High risk | See above |
| Incomplete outcome data (attrition bias) | Low risk | All safety outcomes were reported for the total vaccinated cohort. Immunogenicity outcomes were reported on the according‐to‐protocol cohort. Reason for exclusion was noted and balanced between vaccine group and placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety and immunogenicity) were presented |
| Methods | Randomised, controlled, open, multicentre parallel group study | |
| Participants | Participants: 813 girls enrolled in Canada, Denmark, Hungary and Sweden. Participants were randomised to receive HPV vaccine (n = 270), Hepatitis A and B vaccine (n = 271) or HPV vaccine co‐administrated with Hepatitis A and B vaccine (n = 272) Age range: 9 to 15 years Inclusion criteria: healthy girls with a negative pregnancy test at the time of each vaccination and if of child‐bearing potential, to be abstinent from sexual activity or using adequate contraceptive precautions Exclusion criteria: girls with a history of hepatitis and or B infection or with known exposure to hepatitis A or B within 6 weeks prior to vaccination, girls with previous vaccination against HPV or hepatitis A or B, or planned administration of HPV, hepatitis A or B or non routine vaccines not foreseen by the study protocol were excluded | |
| Interventions | Vaccine: bivalent vaccine Placebo: GSK combined hepatitis A and B vaccine | |
| Outcomes | Safety and immunogenicity outcomes | |
| Notes | Report: Pedersen 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was computer generated at GSK Biological, Rixensart |
| Allocation concealment (selection bias) | Unclear risk | Not described in the paper |
| Blinding of participants and personnel (performance bias) | Unclear risk | Pesonnel performing serological assays were blinded to group assignment. Not mentioned for safety outcome investigator. |
| Blinding of outcome assessment (detection bias) | Unclear risk | See above |
| Incomplete outcome data (attrition bias) | Low risk | All safety outcomes were reported for the total vaccinated cohort. Immunogenicity outcomes were reported for the according‐to protocol cohort. Reason for exclusion was noted and balanced between vaccine group and placebo group |
| Selective reporting (reporting bias) | Low risk | All safety outcomes were presented |
| Methods | Phase III randomised, double‐blind, controlled trial | |
| Participants | 7466 women (3727 in the vaccine arm and 3739 in the placebo arm) from Guanacaste, Costa Rica Age: 18 to 25 years Inlcusion criteria: healthy women who were not pregnant, not breastfeeding and using contraception during the vaccine period. Women were enrolled regardless of past sexual behavior, HPV status, or cytology. Exclusion criteria: women were excluded if they had history of chronic diseases, history of reactions to vaccines and history of hepatitis A vaccination | |
| Interventions | Vaccine: bivalent HPV16/18 AS04‐adjuvant L1 VLP vaccine Placebo: Hepatitis A vaccine‐licensed Havrix vaccine | |
| Outcomes | Vaccine efficacy (persistent infection 6M & 12M), cross‐protection and pregnancy outcomes | |
| Notes | Main report: Herrero 2011. Last report average follow‐up time: 50.4 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | HPV vaccines and placebo were assigned random vaccine identification numbers at the time of labelling by the manufacturer. These numbers were randomised by the study Data Management Centre with a standard SAS program |
| Allocation concealment (selection bias) | Low risk | Codes were kept at the study's data management centre and GSK under controlled and secured access |
| Blinding of participants and personnel (performance bias) | Low risk | All field workers were blinded to group assignment; as well as investigators from the USA and Costa Rica, participants, and medical monitors |
| Blinding of outcome assessment (detection bias) | Low risk | Analyses were conducted by an external group (Information Management Systems) under the direction of the investigators who remain masked to individuals' randomisation |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes are assessed in the PP cohort (3 doses received, HPV16/18 DNA negative at enrolment, no biopsy or LEEP, no protocol violations) and in the ITT cohort were assessed |
| Selective reporting (reporting bias) | Low risk | Efficacy, cross‐protection pregnancy and other safety outcomes were reported |
| Methods | Phase III, double‐blind, randomised controlled trial | |
| Participants | 294 women (148 in the vaccine arm and 146 in the placebo arm) from Hong Kong Age range: women aged 18 to 35 years. Inclusion criteria: women who were healthy Exclusion criteria: women who were receiving any investigational or non‐registered drug or vaccine, those who had received AS04‐adjuvant or HPV vaccine, those having a chronic disease or were pregnant, breastfeeding or planning to conceive were excluded | |
| Interventions | Vaccine: HPV16/18 AS04‐adjuvant bivalent vaccine Placebo: visually indistinguishable aluminium‐containing placebo | |
| Outcomes | Safety and immunogenicity | |
| Notes | Last report average follow‐up time: 7 months (Hong Kong trial (ph3,2v)) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised in a 1:1 ratio with an Internet‐based centralised randomisation system |
| Allocation concealment (selection bias) | Low risk | A single treatment number was used for each patient uniquely identify the doses administered to the participant |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described in the paper |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described in the paper |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes (safety and immunogenicity) were reported on the total vaccinated cohort. Reason for exclusion was noted and balanced between vaccine group and placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety and immunogenicity) were presented |
| Methods | Phase III, observer‐blind, randomised, controlled and multicentre trial | |
| Participants | 2067 women (1035 in the vaccine arm and 1032 in the placebo arm) recruited from Australia, Colombia, the Czech Republic, France etc. Age range: women aged 10 to 14 years Inclusion criteria: girls who were healthy, were not excluded based on HPV status, Pap smear history or history of sexual activity Exclusion criteria: girls were excluded if they had immunodeficiency, history of allergic disease likely to be exacerbated by a vaccine component, known acute or chronic clinically significant neurologic, hepatic, or renal functional abnormality | |
| Interventions | Vaccine: HPV16/18 AS04‐adjuvant bivalent vaccine Placebo: Hepatitis A vaccine, appearance of the vaccine is different | |
| Outcomes | Safety and immunogenicity | |
| Notes | Last report average follow‐up time: 12 months (Medina 2010) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Girls were randomised in 1:1 ratio based on an algorithm accounted for study centre and age strata |
| Allocation concealment (selection bias) | High risk | Allocation was not blinded since the HPV vaccine and the control vaccine (Hepatitis A) were different in appearance |
| Blinding of participants and personnel (performance bias) | Low risk | Because of differences in vaccines appearance, study staff who administered them were not otherwise involved in study conduct; |
| Blinding of outcome assessment (detection bias) | Low risk | The study staff involved in the assessment of outcomes remained blinded to the administered vaccine |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes (safety and immunogenicity) were reported on the total vaccinated cohort. Reasons for exclusion were noted and balanced between the vaccine group and placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety and immunogenicity) were presented |
| Methods | Phase III, double‐blind, randomised, controlled and multicentre trial | |
| Participants | 354 women (176 in the vaccine arm and 178 in the placebo arm) from Hong Kong Age range: women aged 18 to 35 years Inclusion criteria: healthy women not taking any other investigational products or steroids and not pregnant or planning to become pregnant | |
| Interventions | Vaccine: HPV16/18 AS04‐adjuvant bivalent vaccine Placebo: visually indistinguishable aluminium‐containing placebo | |
| Outcomes | Safety and immunogenicity | |
| Notes | Last report average follow‐up time: 7 months (Indian trial (ph3,2v)) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised in a 1:1 ratio with SAS analysis system |
| Allocation concealment (selection bias) | Low risk | Throughout the study, a single treatment number was used to uniquely identify the vaccine doses to be given to the same participant |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described in the paper |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described in the paper |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes (safety and immunogenicity) were reported on the total vaccinated cohort. Reason for exclusion was noted and balanced between vaccine group and placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety and immunogenicity) were presented |
| Methods | Phase III randomised, double‐blind, placebo‐controlled trial | |
| Participants | Participants: 321 females (160 in the vaccine arm and 161 in the placebo arm) Age range: 10 to 14 years. Inclusion criteria: include healthy Korean women who were using no other investigational products or immune‐modifying drugs, not pregnant or planning to become pregnant, not breastfeeding during the study. Use effective contraception or abstinent from sexual relations Exclusion criteria: women who had received previous HPV vaccination | |
| Interventions | Vaccine: HPV16/18 bivalent vaccine Placebo: hepatitis A vaccine | |
| Outcomes | Immunogenicity and safety outcomes | |
| Notes | Main report: Kim 2010; Last report average follow‐up time: 7 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated to two groups in a 1:1 ratio using an Internet‐based randomisation system |
| Allocation concealment (selection bias) | Low risk | Syringes were prepared and administered by qualified medical personnel not otherwise involved in the study |
| Blinding of participants and personnel (performance bias) | Low risk | See above |
| Blinding of outcome assessment (detection bias) | Low risk | The assessment of symptoms were conducted by personnel not involved in study |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes (safety and immunogenicity) were reported on the total vaccinated cohort. Reason for exclusion was noted and balanced between vaccine group and placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety and immunogenicity) were presented |
| Methods | Phase IIIb randomised, double‐blind, placebo‐controlled, multicentre trial | |
| Participants | Participants: 208 women (149 in the vaccine arm and 76 in the placebo arm) Age range: 15 to 25 years Inclusion criteria: include healthy Korean women who were not pregnant and agreed to use effective contraception during the vaccination period Exclusion criteria: women who were used investigational or non‐registered drug or vaccines, who had a history of HPV vaccination, a history of chronic diseases or cancer were also excluded from the study | |
| Interventions | Vaccine: HPV16/18 bivalent vaccine Placebo: hepatitis A vaccine | |
| Outcomes | Immunogenicity and safety outcomes | |
| Notes | Main report: Kim 2011; Last report average follow‐up time: 7 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised in a 2:1 ratio to vaccine or placebo. Random allocation was done with standard statistical analysis system program applying an Internet‐based 2:1 blocking scheme |
| Allocation concealment (selection bias) | Low risk | A single treatment number was utilised in the entire study to identify the doses to be administered to the participant |
| Blinding of participants and personnel (performance bias) | Low risk | All participants and study personnel involved in the study were blinded throughout the study until the last participant and last visit and the database was frozen |
| Blinding of outcome assessment (detection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) | Low risk | Immunogenecity was assessed in the PP‐cohort (initially seronegative women) and in the TVC (at least one dose, all participants randomised); safety was assessed in the TVC |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported |
| Methods | Phase IIIb, double‐blind, randomised controlled trial | |
| Participants | 271 women (135 in the vaccine arm and 136 in the placebo arm) from Malaysia Age range: women aged 18 to 35 years Inclusion criteria: women who were healthy Exclusion criteria: women who had HPV vaccine, chronic use of immunosuppressants, history of allergy to vaccine compounds, history of chronic conditions of cancer and autoimmune disease, acute disease, pregnant | |
| Interventions | Vaccine: HPV16/18 AS04‐adjuvant bivalent vaccine Placebo: aluminium hydroxide as placebo | |
| Outcomes | Safety and immunogenicity | |
| Notes | Report: Lim 2014 Last report average follow‐up time: 7 months after first dose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised in a 1:1 ratio with an Internet‐based centralised randomisation system |
| Allocation concealment (selection bias) | Low risk | A single treatment number was used for each patient uniquely identify the doses administered to the participant |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described in the paper |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described in the paper |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes (safety and immunogenicity) were reported on the total vaccinated cohort. Reason for exclusion was noted and balanced between vaccine group and placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety and immunogenicity) were presented |
| Methods | Phase III randomised, double‐blind, controlled trial | |
| Participants | 18,644 women (9319 in the vaccine arm and 9325 in the placebo arm) enrolled for the study from 135 centres in 14 countries in Asia, Pacific, Europe, Latin America and North America Age range: 15 to 25 years Inclusion criteria: women who reported no more than six lifetime sexual partners before study enrolment, agreed to using adequate contraception over the vaccination period, and had an intact cervix were eligible. Enrolled irrespective of their HPV DNA status, HPV serostatus or cytology at baseline Exclusion criteria: women were excluded if they had a history of colposcopy, were pregnant or breastfeeding, or had chronic or autoimmune disease or immunodeficiency | |
| Interventions | Vaccine: HPV16/18 AS04‐adjuvant bivalent vaccine Placebo: Hepatitis A vaccine‐licensed Havrix vaccine | |
| Outcomes | Safety, immunogenicity, efficacy (incident infection, persistent infection, CIN1+, CIN2+, CIN3+, AIS associated with HPV16, HPV18, HPV16/18, other oncogenic HPV types, irrespective of HPV DNA) and cross‐protection | |
| Notes | Main reports: Paavonen 2007; Paavonen 2009; Szarewski 2011 and Lehtinen 2012. Last report average follow‐up time: 34.9 months (Lehtinen 2012) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised in a 1:1 ratio with an Internet‐based centralised randomisation system |
| Allocation concealment (selection bias) | Low risk | Allocation of treatment numbers was stratified by study site and by age.The trial remained double‐blinded until all individuals had completed 48 months of follow‐up after the first immunisation |
| Blinding of participants and personnel (performance bias) | Low risk | Enrolled women and study investigators were masked to allocated vaccine |
| Blinding of outcome assessment (detection bias) | Low risk | All CIN cases were reviewed by a panel of three pathologists who were blinded to vaccine allocation. Analysis was done by an independent statistician to maintain the trial blinding |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes are assessed in the PP cohort (received 3 doses, seronegative and DNA negative for the corresponding vaccine type at month 0, normal or low‐grade cytology at month 0, no protocol violations) and in the TVC‐naive cohort (at least one vaccine dose, at baseline normal cytology, DNA negative for hrHPV, seronegative for HPV‐16 and HPV‐18) and in the total vaccinated cohort (all women randomised). Reasons for exclusion were presented |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety, immunogenicity, efficacy and cross‐protection) are presented |
| Methods | Pooled analysis of two phase III randomised, double‐blind, controlled trials | |
| Participants | 26,130 women who enrolled for PATRICIA trial and Costa Rica trial | |
| Interventions | Vaccine: HPV16/18 AS04‐adjuvant bivalent vaccine Placebo: Hepatitis A vaccine‐licensed Havrix vaccine | |
| Outcomes | Pregnancy outcomes | |
| Notes | Main report: Wacholder 2010 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | see PATRICIA & Costa Rica trials |
| Allocation concealment (selection bias) | Low risk | see PATRICIA & Costa Rica trials |
| Blinding of participants and personnel (performance bias) | Low risk | see PATRICIA & Costa Rica trials |
| Blinding of outcome assessment (detection bias) | Low risk | see PATRICIA & Costa Rica trials |
| Incomplete outcome data (attrition bias) | Low risk | see PATRICIA & Costa Rica trials |
| Selective reporting (reporting bias) | Low risk | see PATRICIA & Costa Rica trials |
| Methods | Phase III randomised, double‐blind, controlled trial | |
| Participants | 5752 women (2881 in the vaccine arm and 2871 in the placebo arm) from Australia, Canada, Mexico, the Netherlands, Peru, Philippines, Portugal, Russia, Singapore, Thailand, the UK and the USA Age range: women older than 25 years old, age stratified in 26 to 35, 36 to 45 and older than 46 Inclusion criteria: Women who were older than 25 years old. Each age‐stratum had 15% of women with a history of HPV infection to represent a real‐world setting; no limits on number of lifetime sexual partners Exclusion criteria: women were excluded if they were pregnant, breastfeeding and who had chronic or autoimmune disease or immunodeficiency | |
| Interventions | Vaccine: HPV16/18 AS04‐adjuvant bivalent vaccine; Placebo: visually indistinguishable aluminium‐containing placebo | |
| Outcomes | Safety, immunogenicity, efficacy and cross‐protection | |
| Notes | Main report: Skinner 2014 Last report average follow‐up time: 43.3 months (Skinner 2014) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised in a 1:1 ratio with an Internet‐based centralised randomisation system |
| Allocation concealment (selection bias) | Low risk | The randomisation list was generated by GSK with an algorithm which used a minimisation process that accounted for region, age stratum and HPV history |
| Blinding of participants and personnel (performance bias) | Low risk | All participants, investigators and staff involved were masked to treatment allocation and study results. The interim analysis was done by an external statistician blinded to the allocation of vaccine versus placebo |
| Blinding of outcome assessment (detection bias) | Low risk | All CIN cases were reviewed by a panel of three pathologists who were blinded to vaccine allocation. Analysis was done by an independent statistician to maintain the trial blinding |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes were assessed in the PP cohort (received 3 doses, seronegative and DNA negative for the corresponding vaccine type at month 0, normal or low‐grade cytology at month 0, no protocol violations) and in the TVC‐naive cohort (at least one vaccine dose, at baseline normal cytology, DNA negative for hrHPV, seronegative for HPV‐16 and HPV‐18) and in the total vaccinated cohort (all women randomised). Reasons for exclusion were presented |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety, immunogenicity, efficacy and cross‐protection) are presented |
| Methods | Phase II randomised, double‐blind, controlled trial in Japan | |
| Participants | Participants: 1021 Japanese women (509 in the vaccine arm and 512 in the placebo arm) Age range: 18 to 26 years Inclusion criteria: healthy women who were not pregnant, had no previous abnormal Pap smears and reported a lifetime history of four or fewer male sex partners and agreed to use effective contraception were eligible. Women with previous HPV infection were not excluded | |
| Interventions | Vaccine: quadrivalent HPV 6/11/16/18 L1 VLP vaccine Placebo: visually indistinguishable aluminium‐containing placebo | |
| Outcomes | Efficacy (composite primary endpoint of persistent infection and external genital disease), immunogenicity and safety outcomes | |
| Notes | Main report: Yoshikawa 2013 Last report average follow‐up time: 30 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned in the paper |
| Allocation concealment (selection bias) | Low risk | The prepared randomisation schedule was sealed with other corresponding randomisation listings and retained strictly until un‐blinding by the Center for Patients Allocation |
| Blinding of participants and personnel (performance bias) | Low risk | See above |
| Blinding of outcome assessment (detection bias) | Low risk | Endpoint analysis was done by use of consensus diagnosis from a panel of pathologists who were blinded to the central laboratory diagnosis, vaccination group and HPV status |
| Incomplete outcome data (attrition bias) | Unclear risk | Efficacy result only reported in PP‐cohort (received 3 doses, being naive for the relevant HPV type at enrolment, remained free of infection with the same vaccine HPV type through completion of the vaccination regimen, did not violate the protocol) |
| Selective reporting (reporting bias) | Low risk | All outcomes (efficacy, safety and immunogenicity) reported |
| Methods | Phase II randomised, double‐blind, placebo‐controlled trial | |
| Participants | Participant: 176 Korean participants (117 in the vaccine arm and 59 in the placebo arm) Age range: 9 to 23 years Inclusion criteria: women who were not pregnant, had no fever more than 37.8*C at vaccination, age 9‐15 years must have had no sexual experience before and no plan to have sexual experience during the study period. Participants aged 16 to 23 years must have had history of fewer than 4 sexual partners and use effective contraception during the study period Exclusion criteria: participants who were enrolled in studies of other investigation agents, history of any HPV vaccination, history of allergy to vaccine compound, thrombocytopenia, history of vaccination within 14 days from enrolment (previous 21 days for live vaccine), receipt of blood or blood‐derived products within the 6 months preceding injection, and immunosuppression. Age group 16 to 23 were required to have not had a prior Pap test showing a squamous intraepithelial lesion or worse and/or a biopsy indicating CIN or worse | |
| Interventions | Vaccine: quadrivalent HPV 6/11/16/18 L1 VLP vaccine Placebo: visually indistinguishable aluminium‐containing placebo | |
| Outcomes | Immunogenicity and safety outcomes | |
| Notes | Main report: Kang 2008 Last report average follow‐up time: 7 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the study centres using the block method with decreasing block sizes |
| Allocation concealment (selection bias) | Unclear risk | Not described in the paper |
| Blinding of participants and personnel (performance bias) | Unclear risk | The trial was described as double‐blind but no further details are given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described in the paper |
| Incomplete outcome data (attrition bias) | Low risk | The loss to follow‐up rate is low and well‐balanced for both the vaccine and the placebo groups. Participants being baseline seropositive for the concerned HPV vaccine type were excluded for the immunogenicity outcome |
| Selective reporting (reporting bias) | Low risk | Immunogenicity and safety outcomes reported |
| Methods | Phase II randomised, multicentre, double‐blind, placebo‐controlled trial | |
| Participants | 1158 women enrolled for the study, among them 52 participants were included in study part A which was a dose‐escalation study, and the 1106 remaining women were included study part B which was a dose‐ranging study. In study part B, 554 were in intermediate‐/high‐dose groups and 552 were in low‐dose groups; 277 in low‐dose vaccine group and 275 in the placebo group Age range: 16 to 23 years Inclusion criteria: healthy women who were not pregnant, had no previous abnormal Pap smear and reported a lifetime history of four or fewer male sex partners. The study did not exclude women with previous HPV infection.Virgins were restricted to women of 18 years or older and seeking contraception | |
| Interventions | Vaccine: quadrivalent HPV 6/11/16/18 L1 VLP Placebo: visually indistinguishable aluminium‐containing placebo | |
| Outcomes | Persistent infection (≥ 4 M or at last visit) associated with HPV 6,11,16 or 18, cervical or external genital lesions (CIN 1‐3, condylomata acuminata, vulvar intraepithelial neoplasia and vaginal intraepithelial neoplasia), immunogenicity, safety and tolerability | |
| Notes | Main reports: Villa 2005; Villa 2006 and Villa 2006a Last report average follow‐up time: 36 months and 60 months for a subset of 241 participants (Villa 2006a) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation to the placebo or vaccine arms was applied only on a part of the enrolled women (those included in the low‐dose group). The other women were enrolled in a dose‐escalating or dose‐ranging studies. Only women from the low‐dose group were included were used for the Cochrane Review |
| Allocation concealment (selection bias) | Unclear risk | No further details are provided on allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | The placebo was visually indistinguishable from the vaccine. Both the participants and the investigator and the staff were blinded to who received vaccine and who received placebo |
| Blinding of outcome assessment (detection bias) | Low risk | Biopsies were read for endpoint determination by a blinded panel of four pathologists |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes were assessed in the PP cohort (3 doses and DNA negative for the relevant HPV vaccine type) and the MITTmodified‐intention‐to‐treat cohort (at least 1 dose and DNA negative for the relevant HPV vaccine type) and reasons for exclusion were presented |
| Selective reporting (reporting bias) | Low risk | Alll outcomes (safety, immunogenicity and efficacy) presented |
| Methods | Phase III randomised, partially double‐blind trial | |
| Participants | Participants: 250 women aged 9 to 26 years enrolled in Ghana, Kenya and Senegal. Only 100 women (9 to 12 years) were randomised to receive HPV vaccine or placebo and were considered in this review. Age range: 9 to 12 years Inclusion criteria: healthy, HIV‐uninfected women Exclusion criteria: women who were pregnant, were allergic to any vaccine component, had received any blood product or component in the previous 6 months, had any known immune or coagulation disorder, or had received any inactivated vaccine product within 14 days before enrolment or any live vaccine product within 21 days before enrolment | |
| Interventions | Vaccine: quadrivalent HPV vaccine Placebo: visually indistinguishable aluminium‐containing placebo | |
| Outcomes | Safety and immunogenicity outcomes | |
| Notes | Report: Mugo 2015 Follow‐up: 7 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Girls were randomised in a 4:1 ratio to receive HPV vaccine or placebo |
| Allocation concealment (selection bias) | Unclear risk | Not described in the paper |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described in the paper |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described in the paper |
| Incomplete outcome data (attrition bias) | Low risk | All safety outcomes were reported for the total vaccinated cohort. Immunogenicity outcomes were reported for the according‐to‐protocol cohort. Reason for exclusion was noted and balanced between vaccine group and placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes (immunogenicity and safety) were presented |
| Methods | Phase III randomised, placebo‐controlled, double‐blind trial | |
| Participants | Participants: 5455 women (2723 women in the vaccine arm and 2732 in the placebo arm) from 62 study centres in 16 countries Age range: 16 to 24 years Inclusion criteria: healthy women who were not pregnant and had no history of genital warts or abnormal results on cervical cytologic testing, had a lifetime number of no more than four sex partners and agreed to use contraception during the vaccination period | |
| Interventions | Vaccine: quadrivalent HPV 6/11/16/18 vaccine Placebo: visually indistinguishable aluminium‐containing placebo | |
| Outcomes | Efficacy (CIN of any grade, AIS, cervical cancer, VIN, VaIN, GW, vulvar‐vaginal cancer, Pap abnormalities), immunogenicity and safety | |
| Notes | Main reports: Garland 2007; Last report average follow‐up time: 4.9 years (Munoz 2010) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐based randomised allocation schedule provided by the statistician was used for sequence allocation |
| Allocation concealment (selection bias) | Low risk | An interactive voice response system was used to randomise participants within each study centre |
| Blinding of participants and personnel (performance bias) | Low risk | The participants, investigator and sponsor were blinded to the allocated trial arm |
| Blinding of outcome assessment (detection bias) | Low risk | Central laboratory was unaware of treatment‐group assignment and HPV status. A panel of 4 pathologists was unaware of diagnosis made at the central laboratory, clinical findings, treatment group, and HPV status |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes are assessed in the PP cohort (received 3 doses without protocol violations, being HPV DNA negative for the relevant HPV vaccine type from enrolment to 1 month after dose 3), in the unrestricted susceptible group (all women who were negative on HPV DNA and serology negative for the relevant HPV vaccine type at enrolment) and in the ITT cohort (all participants who had undergone randomisation, regardless of baseline HPV status or presence of HPV‐associated an\anogenital disease). Reasons for exclusion were presented |
| Selective reporting (reporting bias) | Low risk | All outcomes (efficacy, safety and immunogenicity) reported |
| Methods | Phase III randomised, placebo‐controlled, double‐blind trial. | |
| Participants | Participants: 12167 women (6087 women in vaccine arm and 6080 women in the placebo arm) from 90 study centres in 13 countries. Age range: 15 to 26 years. Inclusion criteria: healthy women with an intact uterus, who were not pregnant and had no history of genital warts or abnormal results on cervical cytologic testing, had a lifetime number of no more than four sex partners and agreed to use contraception during the vaccination period | |
| Interventions | Vaccine: Quadrivalent HPV6/11/16/18 vaccine Placebo: visually indistinguishable aluminium‐containing placebo | |
| Outcomes | Efficacy (CIN of any grade, AIS, cervical cancer, VIN, VaIN, GW, vulvar‐vaginal cancer, Pap abnormalities), safety and immunogenicity | |
| Notes | Main reports: FUTURE‐II 2007 and Munoz 2010 Last report average follow‐up time: 4.9 years (Munoz 2010) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐based randomised allocation schedule provided by the statistician.was used for sequence allocation |
| Allocation concealment (selection bias) | Low risk | An interactive voice response system was used to randomise participants within each study centre |
| Blinding of participants and personnel (performance bias) | Low risk | The participants, investigator and sponsor were blinded to the allocated trial arm |
| Blinding of outcome assessment (detection bias) | Low risk | Central laboratory was unaware of treatment‐group assignment and HPV status. A panel of 4 pathologists was unaware of diagnosis made at the central laboratory, clinical findings, treatment group, and HPV status |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes were assessed in the PP cohort (received 3 doses without protocol violations, being HPV DNA negative for the relevant HPV vaccine type from enrolment to 1 month after dose 3), in the unrestricted susceptible group (all women who were negative on HPV DNA and serology negative for the relevant HPV vaccine type at enrolment) and in the ITT cohort (all participants who had undergone randomisation, regardless of baseline HPV status or presence of HPV‐associated anogenital disease). Reasons for exclusion were presented |
| Selective reporting (reporting bias) | Low risk | All outcomes (efficacy, safety and immunogenicity) reported |
| Methods | Pooling of two phase III randomised, placebo‐controlled, double‐blind trials | |
| Participants | Participants: 17,622 women (see FUTURE I and FUTURE II trials for more details) Age range: 16 to 26 years | |
| Interventions | Vaccine: Quadrivalent HPV6/11/16/18 vaccine Placebo: visually indistinguishable aluminium‐containing placebo | |
| Outcomes | Efficacy (CIN of any grade, AIS, cervical cancer, VIN, VaIN, GW, vulvar‐vaginal cancer, Pap abnormalities), safety and immunogenicity | |
| Notes | Main report: Munoz 2010 Last report average follow‐up time: 4.9 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See FUTURE I & II trials |
| Allocation concealment (selection bias) | Low risk | See FUTURE I & II trials |
| Blinding of participants and personnel (performance bias) | Low risk | See FUTURE I & II trials |
| Blinding of outcome assessment (detection bias) | Low risk | See FUTURE I & II trials |
| Incomplete outcome data (attrition bias) | Low risk | See FUTURE I & II trials |
| Selective reporting (reporting bias) | Low risk | All outcomes (efficacy, safety and immunogenicity) reported |
| Methods | Phase III randomised, double‐blind, controlled trial | |
| Participants | Participants: 3819 women (1911 in the vaccine arm and 1908 in the placebo arm) enrolled in 38 international study sites from 7 countries. Age range: 24 to 45 years Inclusion criteria: women were not pregnant, who had not undergone hysterectomy and agreed to use effective contraception until month 7 of the study Exclusion criteria: women were excluded if they have a history of surgical cervical procedure, had biopsy less than 5 years ago, had history of genital warts and cervical disease. Women infected with HIV and those who were immunocompromised were not eligible for enrolment | |
| Interventions | Vaccine: quadrivalent vaccine Placebo: visually indistinguishable aluminium‐containing placebo | |
| Outcomes | Efficacy (persistent HPV infection, CIN, condyloma, VIN or VaIN), safety and immunogenicity outcomes | |
| Notes | Main reports: Munoz 2009 and Castellsagué 2011 Last report average follow‐up time: 48 months ( Castellsagué 2011) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated allocation schedule was generated by the sponsor's Clinical Biostatistics department |
| Allocation concealment (selection bias) | Low risk | Randomised to a vaccination group using an interactive Voice Response System |
| Blinding of participants and personnel (performance bias) | Low risk | All study‐site investigators and personnel, study participants, monitors, and central laboratory personnel were blinded to treatment allocation throughout the study |
| Blinding of outcome assessment (detection bias) | Low risk | Biopsy material was first read for clinical management by pathologists at a central laboratory, and then read for endpoint determination by a blinded panel of 4 pathologists |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes were assessed in the PP cohort (received 3 doses, seronegative at day 1 and HPV DNA negative for the HPV vaccine types from day 0 until month 7, no protocol violations), in the naive to the relevant type (NRT) cohort (at least 1 dose, seronegative at day 1 and HPV DNA negative for the HPV vaccine types on day 1) and in the ITT‐cohort (at least 1 dose, irrespective of initial HPV status, protocol violators included) |
| Selective reporting (reporting bias) | Low risk | All outcomes (safety, immunogenicity and efficacy) are presented |
AIS: adenocarcinoma in situ
ASC: atypical squamous cells
ASC‐US: atypical squamous cells of undetermined significance
CIN: cervical intraepithelial neoplasia
DNA: Desoxyribo‐nucleic acid
ELISA: enzyme‐linked immunosorbent assay
GSK: GlaxoSmithKline
GW: genital wart
HPV: human papillomavirus
ITT: intention‐to‐treat
LEEP: loop electrosurgical excision procedure
LSIL: low‐grade squamous intraepithelial lesion
MITT: modified intention‐to‐treat
PATRICIA: PApiloma TRIal against Cancer In young Adults
PCR: polymerase chain reaction
PP: per‐protocol
TVC: total vaccinated cohort
VAIN: Vaginal intra‐epithelial neoplasia,
VIN: vaginal intraepithelial neoplasia
VLP: virus‐like particles
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Post‐licensure safety surveillance over more than 4 years of routine use of HPV bivalent vaccine. Not a randomised controlled trial. | |
| Randomised trial to evaluate Novartis vaccines co‐administrated with Tdap vaccine and HPV vaccine. No HPV alone group. | |
| Phase I trial. | |
| Pooled analysis of 4 RCTs on both bivalent and quadrivalent vaccine. No new original data were presented. | |
| A review of evidence from phase III trials and national immunisation programs regarding efficacy and safety of HPV vaccines. | |
| Efficacy of the bivalent vaccine against cervical, anal and oral infection in a sub‐cohort nested in the CVT trial. Cervical outcomes already included. | |
| Post hoc analysis using combined data from two Phase I tolerability/immunogenicity trials. | |
| Systemactic review and meta‐analysis of protection of HPV vaccines against CIN, VIN, VAIN, and genital warts in catch‐up populations. No Original data, not a randomised controlled trial. | |
| Systematic review and meta‐analysis of the immunogenicity of the 2‐dose vaccination schedule versus 3‐dose schedule. | |
| A case‐study on HPV vaccination national programme in Australia for future innovation prevention. | |
| Review of the long‐term efficacy and safety of HPV vaccines. No original data. | |
| Assessment of HPV vaccine update and post‐vaccination cervical cancer prevention in Germany. Not a randomised controlled trial. | |
| Randomised trial assessing safety and immunogenicity in HIV‐positive women (N = 120 randomised to bivalent vaccine or placebo). 30 HIV seronegative women all received the bivalent vaccine. | |
| Pooled analysis on safety of the bivalent vaccine including 11 studies. Not all were randomised trials. | |
| Randomised phase III trial assessing immunogenicity after two versus three doses of the quadrivalent vaccine. No vaccine efficacy data. | |
| Non randomised study assessing presence of neutralising antibodies of non‐vaccine HPV types in girls vaccinated with the bivalent vaccine. | |
| Randomised trial comparing generation of cross‐protecting antibodies in serum and vaginal mucus among girls receiving bivalent versus quadrivalent vaccine. No vaccine efficacy data. No placebo group included in the RCT. | |
| Randomised trial comparing safety and generation of antiHPV16/18 antibodies in serum and vaginal mucus among girls receiving bivalent versus quadrivalent vaccine (7 months after 3rd dose). No vaccine efficacy data. No placebo group included in the RCT. | |
| Randomised trial comparing safety and generation of antiHPV16/18 antibodies in serum and vaginal mucus among girls receiving bivalent versus quadrivalent vaccine (12 months after 3rd dose). No vaccine efficacy data. No placebo group included in the RCT. | |
| Dose‐escalation phase I trial addressing immunological response and safety after administration of an L1 HPV11 vaccine. | |
| Systematic review on pregnancy outcomes of bi‐ and quadrivalent vaccines using data from RCTs and post‐marketing surveillance. | |
| Post‐hoc analysis of the bivalent HPV vaccine against the recurrent of the high‐grade CIN after surgical therapy. | |
| Pooled analysis of phase II/III trials assessing immunogenicity according to baseline covariates. No original data. No vaccine‐efficacy or safety data. | |
| Phase III trial assessing safety and efficacy of vaccination with the quadrivalent vaccine in men. | |
| Immunogenicity and safety of Gardasil vaccine among mid‐adult aged men of 27 to 45 years. No data on women. | |
| Quadrivalent HPV vaccine efficacy against disease related to vaccine and non‐vaccine HPV types in men. | |
| Phase I dose‐escalation trial assessing immunogenicity and safety of a mono‐valent HPV16 vaccine. | |
| Pooled analysis of 2 RCTs assessing the incidence of CIN2+/AIS+ related to HPV 16/18 in women who received the quadrivalent vaccine or placebo and who were HPV16/18 DNA and seropositive. The data of the separate studies are already included in the review. | |
| Cohort study on immunogenicity and safety of the bivalent HPV vaccines in female patients with juvenile idiopathic arthritis. Not a randomised controlled trial. | |
| Non randomised trial to evaluate the immunogenicity of the quadrivalent HPV vaccine using 2 versus 3 doses, An observational surveillance study to evaluate alternative vaccination schedules. | |
| Report from the Costa Rica vaccination trial assessing effect of the bivalent vaccine on oral HPV infection. | |
| Report of the Costa Rica trial assessing the effect of the bivalent vaccine on clearance of existing HPV infection. | |
| Phase III randomised trial assessing immunogenicity of the quadrivalent vaccine in men. | |
| Pooled analyses of three randomised trials assessing protection of the quadrivalent vaccine against vulval and vaginal intraepithelial lesions. Protection against cervical lesions was not addressed. | |
| Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV‐infected young women. | |
| Non randomised study assessing effect of the quadrivalent vaccine on the incidence of recurrence of CIN in women treated by excision for high‐grade CIN. | |
| Girls randomised to the experimental arm received the bivalent vaccine, those in the control arm did not receive anything. The trial was not placebo‐controlled. Observation of effects were restricted to participants in the experimental arm. | |
| A pooled analysis of efficacy of quadrivalent HPV vaccines against cervical and genital lesions. No separate data on FUTURE I and FUTURE II trials. The data of the separate studies are already included in the review. | |
| Discussion about conducting a randomised clinical trial to assess the efficacy of a single dose of prophylactic HPV vaccines among adolescent. No original data. | |
| Immunogenicity of quadrivalent HPV vaccine among girls aged 11 to 13 years of age vaccinated using alternative dosing schedules. | |
| A nested analysis of CVT trial on vaccine efficacy against vulvar HPV infection. Cervical outcomes from the trial have been included in the review. | |
| Non‐inferiority of antibody response to human papillomavirus 16/18 vaccine in adolescents vaccinated with alternative dosing schedules. | |
| Phase IV RCT to evaluate the effectiveness, safety and immunogenicity of Cervarix in boys and girls aged 12‐15 years in Finland. | |
| Randomised trial assessing safety and immunogenicity of vaccination with the hepatitis‐B vaccine alone versus co‐administration of the hepatitis‐B vaccine with the bivalent HPV vaccine. No HPV alone group. | |
| Non RCT to compare immunogenicity and safety of 2‐dose bivalent, 2‐dose quadrivalent and 3‐dose quadrivalent vaccination schedule among girls aged 9‐14 years. | |
| Randomised trial assessing safety and immunogenicity of the quadrivalent vaccine in a group of Chinese women and men. Outcomes are presented jointly. Data separated by gender were requested from the authors with no response. | |
| Randomised controlled trial of two dosing schedules for human papillomavirus vaccination among college‐age men. | |
| Systematic review and meta‐analysis of vaccine efficacy and safety of the bivalent vaccine. | |
| Follow‐up report (up to 6 years after dose 1) of the Columbian cohort of the FUTURE III trial, assessing the safety, immunogenicity and protection against the joint ocutome of CIN and extra‐genital lesions combined of the quadrivalent vaccine. No separated data for protection against CIN2+ were reported. | |
| Systematic review and meta‐analysis on cross‐protection of the bi‐ and quadrivalent vaccines. | |
| Review paper on the efficacy of the quadrivalent vaccine. | |
| Review paper on the efficacy of the bivalent vaccine. | |
| Not a randomised trial. Only HIV+ girls or women enrolled. | |
| Randomised trial assessing the safety of vaccination with the quadrivalent vaccine in men | |
| Review of safety, immunogenicity and efficacy of HPV vaccines in low‐ and middle‐income countries. No original data. | |
| Randomised comparison of safety and immunogenicity of the bi‐ and quadrivalent vaccine administered by intra‐muscular versus intradermal injection. No placebo comparison group. | |
| Randomised trial assessing safety and immunogenicity of four alternative schedules of administration of the quadrivalent vaccine in Vietnamese girls. No placebo group. | |
| A pooled analysis of efficacy and safety of quadrivalent HPV vaccines on women with previous HPV infection. No separate data on FUTURE I and FUTURE II trials. The data of the separate studies are already included in the review. | |
| Randomised trial assessing the effect of vaccination with the quadrivalent vaccine on anal HPV infection and AIN in men | |
| Immunobridging study assessing immunogenicity and safety of the bivalent HPV vaccines in women aged 15‐25 years and 10‐14 years. Not a randomised controlled trial and all participants received the bivalent vaccines. | |
| Pooled analysis of RCTs of the efficacy of the quadrivalent vaccine regarding protection against HPV‐related lesions, restricted to the Latin‐American cohorts included in the phase II and III trials (FUTURE II trial (ph3,4v); FUTURE I trial (ph3,4v); Phase2 trial (ph2,4v)). The data of the separate studies are already included in the review. | |
| Randomised trial assessing the immunogenicity and safety of vaccination with the bivalent vaccine in boys. | |
| Trial assessing long‐term (at 48 months) safety and immunogenicity (antibodies in serum and cervicovaginal secretions) of the bivalent vaccine. No placebo group. | |
| Dose‐ranging study assessing safety and immunogenicity of a monovalent HPV16 vaccine. | |
| Randomised open trial to compare 2‐dose versus 3‐dose regimens of the bivalent vaccine in terms of immunogenicity and safety. No placebo group. | |
| Incidence and duration of type‐specific human papillomavirus infection in high‐risk HPV‐naive women. Post study results of Phase2 trial (ph2,2v) trial. | |
| Surveillance of the incidence of genital warts before and after introduction of HPV vaccination in Australia. Not a randomised controlled trial. | |
| Randomised controlled trial assessing the safety and persistent immunogenicity of quadrivalent HPV vaccine in a group of boys and girls. Outcomes were presented jointly. Author was contacted to request data separated by gender. The author responded that separated data were not available. | |
| Randomised open‐label study to assess the safety, tolerability and immunogenicity of quadrivalent vaccine co‐administrated with enactra and Adacel vaccine. No quadrivalent only group and no separate data between girls and boys. | |
| Five‐year sustained immunogenicity of the bivalent vaccine administered as a 2‐dose schedule in girls aged 9‐14 years. No placebo group. | |
| Trial demonstrating immune memory after administration of a dose of quadrivalent vaccine to women enrolled 8.5 years before in a phase II trial assessing the effects of the monovalent HPV16 vaccine. | |
| Cross‐protection efficacy against HPV 31 of bivalent vaccine, results from Costa Rica trials. | |
| Review of the immunological response, including the induction of immune memory after vaccination with the bivalent vaccine. No original data. | |
| Non randomised trial assessing immunogenicity and tolerability of the bivalent vaccine in female participants aged 15‐55 years from Germany and Poland. | |
| Non randomised trial assessing presence of HPV antibodies in serum and cervicovaginal secretions of induced by the bivalent vaccine in female participants aged 15‐55 years from Germany and Poland. | |
| Follow‐up study to the Schwarz 2009 report. | |
| An open follow‐up study of an RCT on safety and immunogenicity of bivalent vaccine in girls aged 10‐13 years. Medina 2010 trial. | |
| Correspondence about HPV vaccine trials in India. No extractable original data. | |
| Correspondence about HPV vaccine trials in India. No extractable original data. | |
| Systematic review on the efficacy of bivalent vaccine summarized from 6 RCTs. No original data. | |
| Short review on pregnancy outcomes after vaccination against HPV. No original data. | |
| Review article. No original data. | |
| RCT to compare of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV‐infected adults. | |
| Surveillance study assessing occurrence of adverse effects reported after HPV vaccination in the Netherlands. | |
| Randomised open‐label study to assess the safety, tolerability and immunogenicity of quadrivalent vaccine co‐administrated with REPEVAX vaccine. No quadrivalent only group and no separate data between girls and boys. | |
| Randomised open‐label study to assess the safety, tolerability and immunogenicity of quadrivalent vaccine co‐administrated with Hepatitis B vaccine. No quadrivalent only group. | |
| Trial assessing reactogenicity and immunogenicity of Tdap (tetanosdiphteria, pertussis) and MCV4 (meningococcal polysaccharide & diphtheria toxoid) vaccines when given alone or co‐administrated with the bivalent HPV vaccine. Not a randomised controlled trial. | |
| Systematic review on vaccine immunogenicity and efficacy in men. | |
| Non randomised phase I trial on safety and immunogenicity of the bivalent vaccine,conducted in China (female participants aged 15‐45 years). | |
| Randomised trial assessing the immunogenicity of the quadrivalent vaccine with two alternative schedules (months 0,2 & 6, versus months 0, 2 & 12). |
AIS: adenocarcinoma in situ
CIN: cervical intraepithelial neoplasia
CVT: Costa Rica Vaccination Trial
HPV: human papillomavirus
RCT: randomised controlled trial
Tdap: tetanosdiphteria, pertussis
VAIN: Vaginal intra‐epithelial neoplasia,
VIN: vaginal intraepithelial neoplasia
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CIN2+ associated with HPV16/18, at least 1 dose Show forest plot | 3 | 23676 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.05] |
| Analysis 1.1  Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 1 CIN2+ associated with HPV16/18, at least 1 dose. | ||||
| 2 CIN2+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.09] |
| Analysis 1.2  Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 2 CIN2+ associated with HPV6/11/16/18, at least 1 dose. | ||||
| 3 CIN3+ associated with HPV16/18, at least 1 dose Show forest plot | 2 | 20214 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.10] |
| Analysis 1.3  Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 3 CIN3+ associated with HPV16/18, at least 1 dose. | ||||
| 4 CIN3+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.18] |
| Analysis 1.4 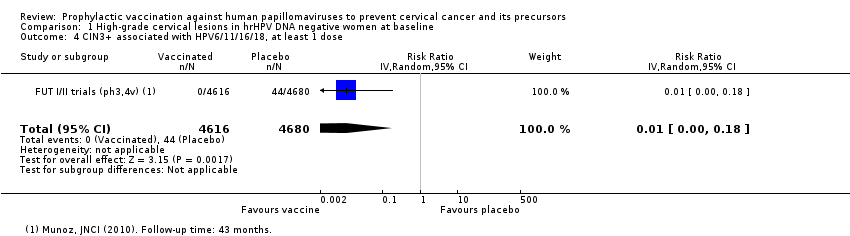 Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 4 CIN3+ associated with HPV6/11/16/18, at least 1 dose. | ||||
| 5 AIS associated with HPV16/18, at least 1 dose Show forest plot | 2 | 20214 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.01, 0.82] |
| Analysis 1.5  Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 5 AIS associated with HPV16/18, at least 1 dose. | ||||
| 6 AIS associated with HPV6/11/16/18, at least 1 dose Show forest plot | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.01, 2.80] |
| Analysis 1.6  Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 6 AIS associated with HPV6/11/16/18, at least 1 dose. | ||||
| 7 Any CIN2+ irrespective of HPV types, at least 1 dose Show forest plot | 5 | 25180 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.25, 0.55] |
| Analysis 1.7  Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 7 Any CIN2+ irrespective of HPV types, at least 1 dose. | ||||
| 7.1 Bivalent vaccine | 4 | 15884 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.25, 0.43] |
| 7.2 Quadrivalent vaccine | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.44, 0.76] |
| 8 Any CIN3+ irrespective of HPV types, at least 1 dose Show forest plot | 3 | 20719 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.04, 1.10] |
| Analysis 1.8  Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 8 Any CIN3+ irrespective of HPV types, at least 1 dose. | ||||
| 8.1 Bivalent vaccine | 2 | 11423 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.03, 0.23] |
| 8.2 Quadrivalent vaccine | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.36, 0.82] |
| 9 Any AIS irrespective of HPV types, at least 1 dose Show forest plot | 2 | 20214 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.01, 0.76] |
| Analysis 1.9  Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 9 Any AIS irrespective of HPV types, at least 1 dose. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CIN2+ associated with HPV16/(18), 3 doses Show forest plot | 8 | 43376 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.04, 0.16] |
| Analysis 2.1  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 1 CIN2+ associated with HPV16/(18), 3 doses. | ||||
| 1.1 Age group 15‐26 years | 6 | 36579 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.03, 0.15] |
| 1.2 Age group 24‐45 years | 2 | 6797 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.04, 0.74] |
| 2 CIN2+ associated with HPV16/(18), at least 1 dose Show forest plot | 8 | 42030 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.05, 0.20] |
| Analysis 2.2  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 2 CIN2+ associated with HPV16/(18), at least 1 dose. | ||||
| 2.1 Age group 15‐26 years | 6 | 34478 | Risk Ratio (IV, Random, 95% CI) | 0.05 [0.03, 0.10] |
| 2.2 Age group 24‐45 years | 2 | 7552 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.11, 0.81] |
| 3 CIN2+ associated with HPV16/(18), 1 or 2 doses (post hoc analysis) Show forest plot | 7 | 3713 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.07, 0.51] |
| Analysis 2.3  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 3 CIN2+ associated with HPV16/(18), 1 or 2 doses (post hoc analysis). | ||||
| 3.1 women age 15‐26 years | 5 | 2958 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.04, 0.26] |
| 3.2 women age 24‐45 years | 2 | 755 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.14, 2.67] |
| 4 CIN2+ associated with HPV6/11/16/18, 3 doses Show forest plot | 2 | 7664 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.01, 0.61] |
| Analysis 2.4  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 4 CIN2+ associated with HPV6/11/16/18, 3 doses. | ||||
| 4.1 Age group 15‐26 years | 1 | 4499 | Risk Ratio (IV, Random, 95% CI) | 0.02 [0.00, 0.25] |
| 4.2 Age group 24‐45 years | 1 | 3165 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.02, 1.39] |
| 5 CIN2+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 2 | 8980 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.00, 2.41] |
| Analysis 2.5  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 5 CIN2+ associated with HPV6/11/16/18, at least 1 dose. | ||||
| 5.1 Age group 15‐26 years | 1 | 5351 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.19] |
| 5.2 Age group 24‐45 years | 1 | 3629 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.10, 1.41] |
| 6 CIN2+ associated with HPV6/11/16/18, 1 or 2 doses (post hoc analysis) Show forest plot | 2 | 1316 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.01, 5.00] |
| Analysis 2.6  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 6 CIN2+ associated with HPV6/11/16/18, 1 or 2 doses (post hoc analysis). | ||||
| 6.1 Age group 15‐26 years | 1 | 852 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.00, 0.74] |
| 6.2 Age group 24‐45 years | 1 | 464 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.14, 6.80] |
| 7 CIN3+ associated with HPV16/18 or HPV6/11/16/18, 3 doses Show forest plot | 3 | 29720 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.02, 0.29] |
| Analysis 2.7  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 7 CIN3+ associated with HPV16/18 or HPV6/11/16/18, 3 doses. | ||||
| 8 CIN3+ associated with HPV 16/18 or HPV6/11/16/18, at least 1 dose Show forest plot | 3 | 33199 | Risk Ratio (IV, Random, 95% CI) | 0.05 [0.02, 0.14] |
| Analysis 2.8  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 8 CIN3+ associated with HPV 16/18 or HPV6/11/16/18, at least 1 dose. | ||||
| 9 CIN3+ associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses (post hoc analysis) Show forest plot | 3 | 3479 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.01, 0.24] |
| Analysis 2.9  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 9 CIN3+ associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses (post hoc analysis). | ||||
| 10 AIS associated with HPV16/18 or HPV6/11/16/18, 3 doses Show forest plot | 3 | 29707 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.02, 0.70] |
| Analysis 2.10  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 10 AIS associated with HPV16/18 or HPV6/11/16/18, 3 doses. | ||||
| 11 AIS associated with HPV16/18 or 6/11/16/18, at least 1 dose Show forest plot | 2 | 17079 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.01, 0.72] |
| Analysis 2.11  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 11 AIS associated with HPV16/18 or 6/11/16/18, at least 1 dose. | ||||
| 12 AIS associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses (post hoc analysis) Show forest plot | 2 | 2015 | Risk Ratio (IV, Random, 95% CI) | 0.15 [0.01, 2.97] |
| Analysis 2.12  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 12 AIS associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses (post hoc analysis). | ||||
| 13 Any CIN2+ irrespective of HPV types, 3 doses Show forest plot | 3 | 7320 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.25, 0.64] |
| Analysis 2.13  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 13 Any CIN2+ irrespective of HPV types, 3 doses. | ||||
| 14 Any CIN2+ irrespective of HPV types, at least 1 dose Show forest plot | 3 | 19143 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.32, 0.52] |
| Analysis 2.14  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 14 Any CIN2+ irrespective of HPV types, at least 1 dose. | ||||
| 15 Any CIN2+ irrespective of HPV types, 1 or 2 doses (post hoc analysis) Show forest plot | 1 | 34 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.15, 3.38] |
| Analysis 2.15  Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 15 Any CIN2+ irrespective of HPV types, 1 or 2 doses (post hoc analysis). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CIN2+ associated with HPV16/18, at least 1 dose Show forest plot | 5 | 44052 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.41, 0.67] |
| Analysis 3.1  Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 1 CIN2+ associated with HPV16/18, at least 1 dose. | ||||
| 1.1 Age group 15‐26 years | 3 | 34852 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.37, 0.57] |
| 1.2 Age group 24‐45 years | 2 | 9200 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.52, 1.05] |
| 2 CIN2+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 2 | 20883 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.38, 0.86] |
| Analysis 3.2  Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 2 CIN2+ associated with HPV6/11/16/18, at least 1 dose. | ||||
| 2.1 Age group 15‐26 years | 1 | 17160 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.42, 0.59] |
| 2.2 Age group 24‐45 years | 1 | 3723 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.44, 1.37] |
| 3 CIN3+ associated with HPV16/18, at least 1 dose Show forest plot | 2 | 34562 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.45, 0.67] |
| Analysis 3.3  Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 3 CIN3+ associated with HPV16/18, at least 1 dose. | ||||
| 4 CIN3+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 1 | 17160 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.43, 0.68] |
| Analysis 3.4  Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 4 CIN3+ associated with HPV6/11/16/18, at least 1 dose. | ||||
| 5 AIS associated with HPV16/18, at least 1 dose Show forest plot | 2 | 34562 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.17, 0.78] |
| Analysis 3.5  Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 5 AIS associated with HPV16/18, at least 1 dose. | ||||
| 6 AIS associated with HPV6/11/16/18, at least 1 dose Show forest plot | 2 | 20830 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.16, 0.98] |
| Analysis 3.6  Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 6 AIS associated with HPV6/11/16/18, at least 1 dose. | ||||
| 7 Any CIN2+ irrespective of HPV types, at least 1 dose Show forest plot | 6 | 45066 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.65, 0.97] |
| Analysis 3.7  Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 7 Any CIN2+ irrespective of HPV types, at least 1 dose. | ||||
| 7.1 Age group 15‐26 years | 4 | 35779 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.58, 0.85] |
| 7.2 Age group 24‐45 years | 2 | 9287 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.83, 1.30] |
| 8 Any CIN3+ HPV type, at least 1 dose Show forest plot | 3 | 35489 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.49, 0.93] |
| Analysis 3.8  Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 8 Any CIN3+ HPV type, at least 1 dose. | ||||
| 8.1 Bivalent vaccine | 2 | 18329 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.43, 0.71] |
| 8.2 Quadrivalent vaccine | 1 | 17160 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.69, 0.96] |
| 9 Any AIS irrespective of HPV types, at least 1 dose Show forest plot | 2 | 34562 | Risk Ratio (IV, Random, 95% CI) | 0.32 [0.15, 0.67] |
| Analysis 3.9  Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 9 Any AIS irrespective of HPV types, at least 1 dose. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident HPV16/18 infection, 3 doses Show forest plot | 1 | 368 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.02, 0.20] |
| Analysis 4.1  Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 1 Incident HPV16/18 infection, 3 doses. | ||||
| 2 Persistent HPV16/18 infection (6M), 3 doses Show forest plot | 1 | 368 | Risk Ratio (IV, Random, 95% CI) | 0.02 [0.00, 0.35] |
| Analysis 4.2  Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 2 Persistent HPV16/18 infection (6M), 3 doses. | ||||
| 3 Persistent HPV16/18 infection (6M), at least 1 dose Show forest plot | 1 | 10826 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.05, 0.09] |
| Analysis 4.3  Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 3 Persistent HPV16/18 infection (6M), at least 1 dose. | ||||
| 4 Persistent HPV16/18 infection(12M), 3 doses Show forest plot | 1 | 368 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.00, 0.73] |
| Analysis 4.4  Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 4 Persistent HPV16/18 infection(12M), 3 doses. | ||||
| 5 Persistent HPV16/18 infection (12M), at least 1 dose Show forest plot | 2 | 14153 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.05, 0.12] |
| Analysis 4.5  Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 5 Persistent HPV16/18 infection (12M), at least 1 dose. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident HPV16/18 infection, 3 doses Show forest plot | 4 | 8034 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.10, 0.31] |
| Analysis 5.1  Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 1 Incident HPV16/18 infection, 3 doses. | ||||
| 2 Incident HPV16/18 infection, at least 1 dose Show forest plot | 5 | 23872 | Risk Ratio (IV, Random, 95% CI) | 0.23 [0.14, 0.37] |
| Analysis 5.2  Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 2 Incident HPV16/18 infection, at least 1 dose. | ||||
| 3 Incident HPV16/18 infection, 1 or 2 doses (post hoc analysis) Show forest plot | 3 | 331 | Risk Ratio (IV, Random, 95% CI) | 0.47 [0.26, 0.84] |
| Analysis 5.3  Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 3 Incident HPV16/18 infection, 1 or 2 doses (post hoc analysis). | ||||
| 4 Persistent HPV16/18 infection (6M), 3 doses Show forest plot | 8 | 34113 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.06, 0.09] |
| Analysis 5.4  Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 4 Persistent HPV16/18 infection (6M), 3 doses. | ||||
| 4.1 Age group 15‐26 years | 6 | 27385 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.05, 0.08] |
| 4.2 Age group 24‐45 years | 2 | 6728 | Risk Ratio (IV, Random, 95% CI) | 0.11 [0.06, 0.20] |
| 5 Persistent HPV16/18 infection (6M), at least 1 dose Show forest plot | 6 | 30323 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.08, 0.17] |
| Analysis 5.5  Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 5 Persistent HPV16/18 infection (6M), at least 1 dose. | ||||
| 5.1 Age group 15‐26 years | 4 | 22803 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.08, 0.12] |
| 5.2 Age group 24‐45 years | 2 | 7520 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.10, 0.29] |
| 6 Persistent HPV16/18 infection (6M), 1 or 2 doses (post hoc analysis) Show forest plot | 4 | 1229 | Risk Ratio (IV, Random, 95% CI) | 0.26 [0.16, 0.44] |
| Analysis 5.6  Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 6 Persistent HPV16/18 infection (6M), 1 or 2 doses (post hoc analysis). | ||||
| 6.1 Age group 15‐26 years | 2 | 437 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.03, 0.42] |
| 6.2 Age group 24‐45 years | 2 | 792 | Risk Ratio (IV, Random, 95% CI) | 0.31 [0.18, 0.54] |
| 7 Persistent HPV6/11/16/18 infection (6M), 3 doses Show forest plot | 2 | 4008 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.06, 0.21] |
| Analysis 5.7  Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 7 Persistent HPV6/11/16/18 infection (6M), 3 doses. | ||||
| 8 Persistent HPV6/11/16/18 infection (6M), at least 1 dose Show forest plot | 2 | 4129 | Risk Ratio (IV, Random, 95% CI) | 0.13 [0.05, 0.37] |
| Analysis 5.8  Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 8 Persistent HPV6/11/16/18 infection (6M), at least 1 dose. | ||||
| 9 Persistent HPV16/18 infection (12M), 3 doses Show forest plot | 4 | 22267 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.06, 0.13] |
| Analysis 5.9 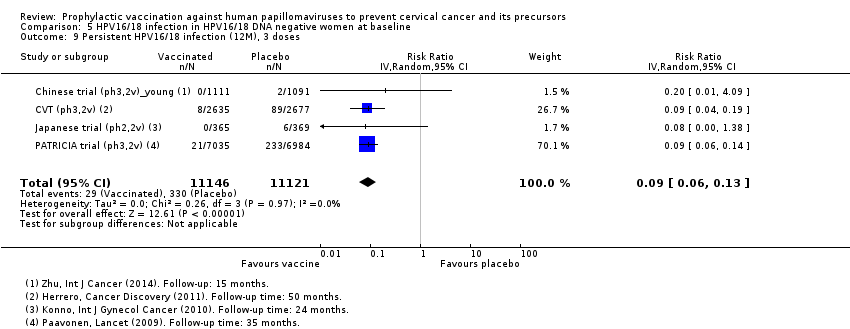 Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 9 Persistent HPV16/18 infection (12M), 3 doses. | ||||
| 10 Persistent HPV16/18 infection (12M), at least 1 dose Show forest plot | 5 | 29464 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.12, 0.20] |
| Analysis 5.10  Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 10 Persistent HPV16/18 infection (12M), at least 1 dose. | ||||
| 11 Persistent HPV16/18 infection (12M), 1 or 2 doses (post hoc analysis) Show forest plot | 3 | 3912 | Risk Ratio (IV, Random, 95% CI) | 0.13 [0.06, 0.33] |
| Analysis 5.11  Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 11 Persistent HPV16/18 infection (12M), 1 or 2 doses (post hoc analysis). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident HPV16/18 infection, at least 1 dose Show forest plot | 1 | 4210 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.17, 0.33] |
| Analysis 6.1 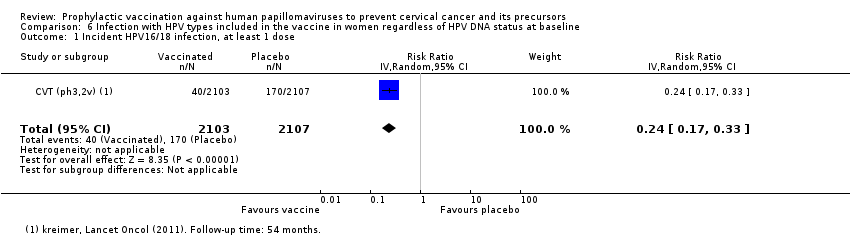 Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 1 Incident HPV16/18 infection, at least 1 dose. | ||||
| 2 Persistent HPV16/18 infection (6M), at least 1 dose Show forest plot | 4 | 33847 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.41, 0.57] |
| Analysis 6.2  Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 2 Persistent HPV16/18 infection (6M), at least 1 dose. | ||||
| 2.1 Age group 15‐26 years | 2 | 25199 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.38, 0.51] |
| 2.2 Age group 24‐45 years | 2 | 8648 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.47, 0.69] |
| 3 Persistent HPV6/11/16/18 infection (6M), at least 1 dose Show forest plot | 1 | 3713 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.42, 0.65] |
| Analysis 6.3  Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 3 Persistent HPV6/11/16/18 infection (6M), at least 1 dose. | ||||
| 4 Persistent HPV16/18 infection (12M), at least 1 dose Show forest plot | 2 | 24785 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.40, 0.54] |
| Analysis 6.4 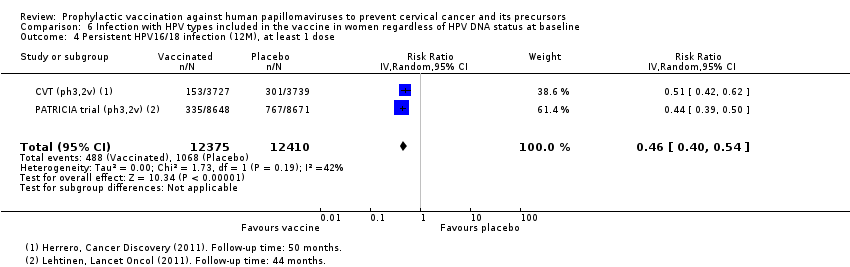 Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 4 Persistent HPV16/18 infection (12M), at least 1 dose. | ||||
| 5 Persistent HPV16/18 infection (12M) by dose (post hoc analysis) Show forest plot | 1 | 7153 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.12, 0.27] |
| Analysis 6.5  Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 5 Persistent HPV16/18 infection (12M) by dose (post hoc analysis). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall local/injection site adverse events Show forest plot | 8 | 18113 | Risk Ratio (IV, Fixed, 95% CI) | 1.18 [1.16, 1.20] |
| Analysis 7.1 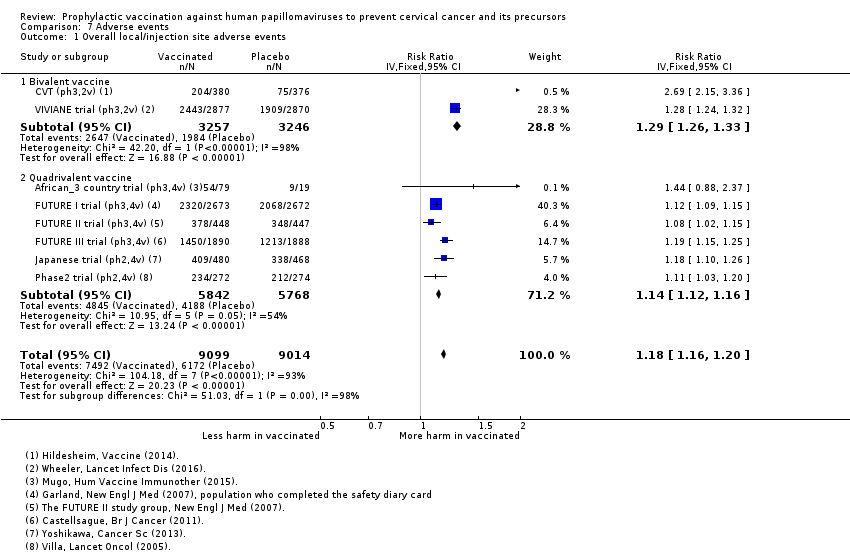 Comparison 7 Adverse events, Outcome 1 Overall local/injection site adverse events. | ||||
| 1.1 Bivalent vaccine | 2 | 6503 | Risk Ratio (IV, Fixed, 95% CI) | 1.29 [1.26, 1.33] |
| 1.2 Quadrivalent vaccine | 6 | 11610 | Risk Ratio (IV, Fixed, 95% CI) | 1.14 [1.12, 1.16] |
| 2 Pain at injection site Show forest plot | 13 | 25691 | Risk Ratio (IV, Random, 95% CI) | 1.35 [1.23, 1.49] |
| Analysis 7.2  Comparison 7 Adverse events, Outcome 2 Pain at injection site. | ||||
| 2.1 Monovalent vaccine | 1 | 2280 | Risk Ratio (IV, Random, 95% CI) | 1.05 [1.01, 1.09] |
| 2.2 Bivalent vaccine | 8 | 16897 | Risk Ratio (IV, Random, 95% CI) | 1.49 [1.26, 1.75] |
| 2.3 Quadrivalent vaccine | 4 | 6514 | Risk Ratio (IV, Random, 95% CI) | 1.13 [1.07, 1.19] |
| 3 Swelling at injection site Show forest plot | 9 | 22106 | Risk Ratio (IV, Random, 95% CI) | 1.73 [1.32, 2.27] |
| Analysis 7.3 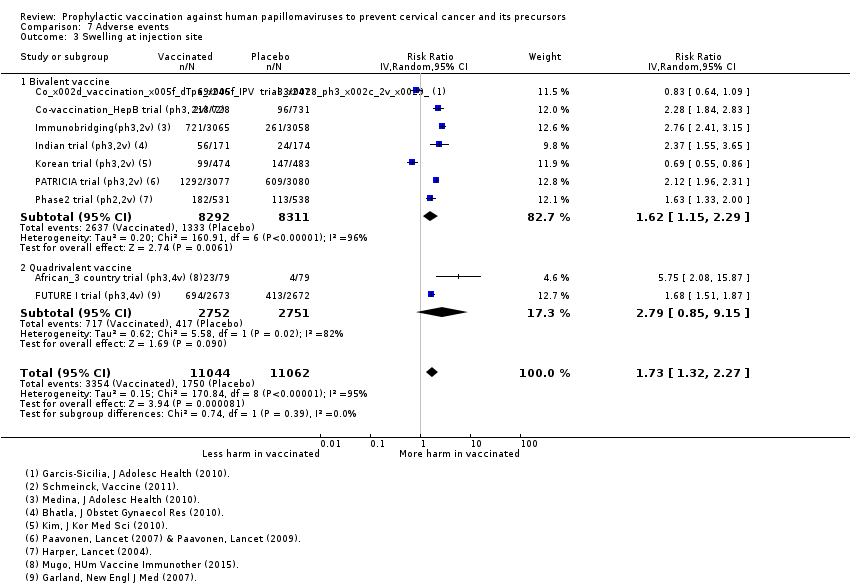 Comparison 7 Adverse events, Outcome 3 Swelling at injection site. | ||||
| 3.1 Bivalent vaccine | 7 | 16603 | Risk Ratio (IV, Random, 95% CI) | 1.62 [1.15, 2.29] |
| 3.2 Quadrivalent vaccine | 2 | 5503 | Risk Ratio (IV, Random, 95% CI) | 2.79 [0.85, 9.15] |
| 4 Redness at injection site Show forest plot | 6 | 19996 | Risk Ratio (IV, Random, 95% CI) | 1.72 [1.50, 1.97] |
| Analysis 7.4  Comparison 7 Adverse events, Outcome 4 Redness at injection site. | ||||
| 4.1 Quadrivalent vaccine | 1 | 5345 | Risk Ratio (IV, Random, 95% CI) | 1.46 [1.32, 1.63] |
| 4.2 Bivalent vaccine | 5 | 14651 | Risk Ratio (IV, Random, 95% CI) | 1.80 [1.53, 2.11] |
| 5 Overall systemic event and general symptoms Show forest plot | 8 | 18191 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.98, 1.07] |
| Analysis 7.5  Comparison 7 Adverse events, Outcome 5 Overall systemic event and general symptoms. | ||||
| 5.1 Bivalent vaccine | 2 | 6503 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.97, 1.19] |
| 5.2 Quadrivalent vaccine | 6 | 11688 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.98, 1.04] |
| 6 Serious adverse events Show forest plot | 23 | 71597 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.92, 1.05] |
| Analysis 7.6  Comparison 7 Adverse events, Outcome 6 Serious adverse events. | ||||
| 6.1 Monovalent vaccine | 1 | 2387 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.51, 1.78] |
| 6.2 Bivalent vaccine | 15 | 46231 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.96, 1.07] |
| 6.3 Quadrivalent vaccine | 7 | 22979 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.65, 1.02] |
| 7 Deaths Show forest plot | 23 | 71176 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.85, 1.98] |
| Analysis 7.7  Comparison 7 Adverse events, Outcome 7 Deaths. | ||||
| 7.1 Monovalent vaccine | 1 | 2280 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Bivalent vaccine | 15 | 46231 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.66, 2.22] |
| 7.3 Quadrivalent vaccine | 7 | 22665 | Risk Ratio (IV, Random, 95% CI) | 1.54 [0.73, 3.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Normal infant Show forest plot | 8 | 8782 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.97, 1.02] |
| Analysis 8.1  Comparison 8 Pregnancy outcomes, Outcome 1 Normal infant. | ||||
| 2 Spontaneous abortion/miscarriage Show forest plot | 9 | 8618 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.68, 1.14] |
| Analysis 8.2  Comparison 8 Pregnancy outcomes, Outcome 2 Spontaneous abortion/miscarriage. | ||||
| 3 Elective termination/induced abortion Show forest plot | 9 | 10909 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.80, 1.02] |
| Analysis 8.3  Comparison 8 Pregnancy outcomes, Outcome 3 Elective termination/induced abortion. | ||||
| 4 Stillbirth Show forest plot | 6 | 8754 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.68, 1.83] |
| Analysis 8.4  Comparison 8 Pregnancy outcomes, Outcome 4 Stillbirth. | ||||
| 5 Abnormal infant Show forest plot | 5 | 9252 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.88, 1.69] |
| Analysis 8.5  Comparison 8 Pregnancy outcomes, Outcome 5 Abnormal infant. | ||||
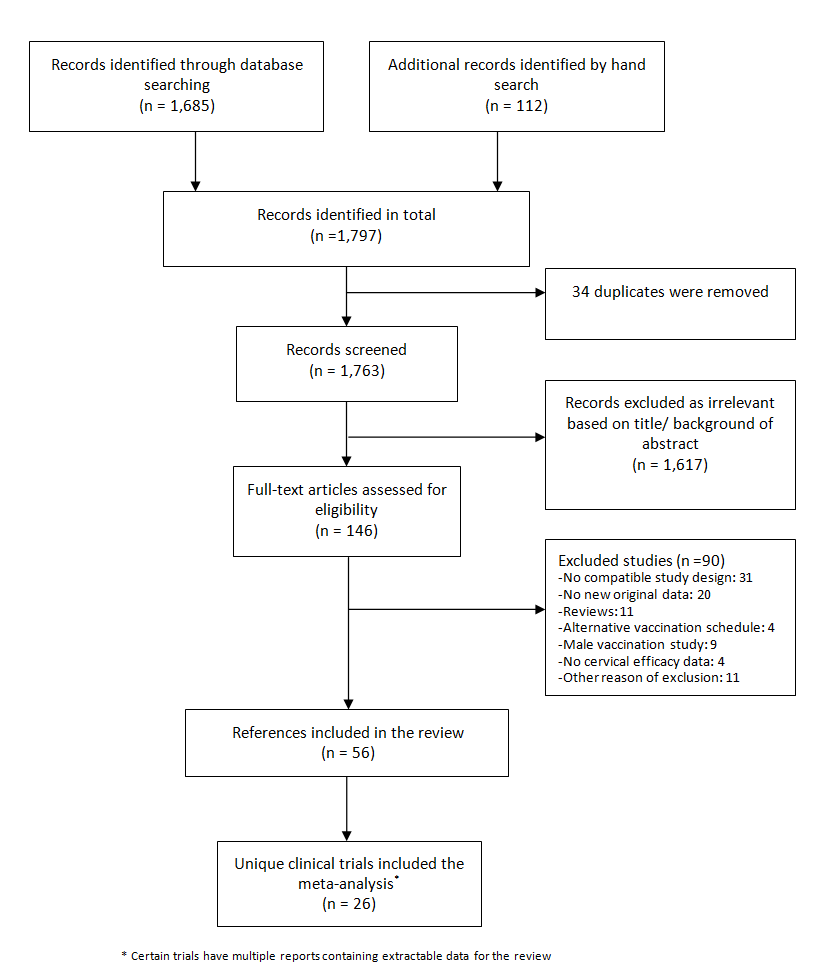
Flow diagram summarising the retrieval, inclusion and exclusion of relevant reports of randomised trials assessing the safety and effects of prophylactic HPV vaccines.
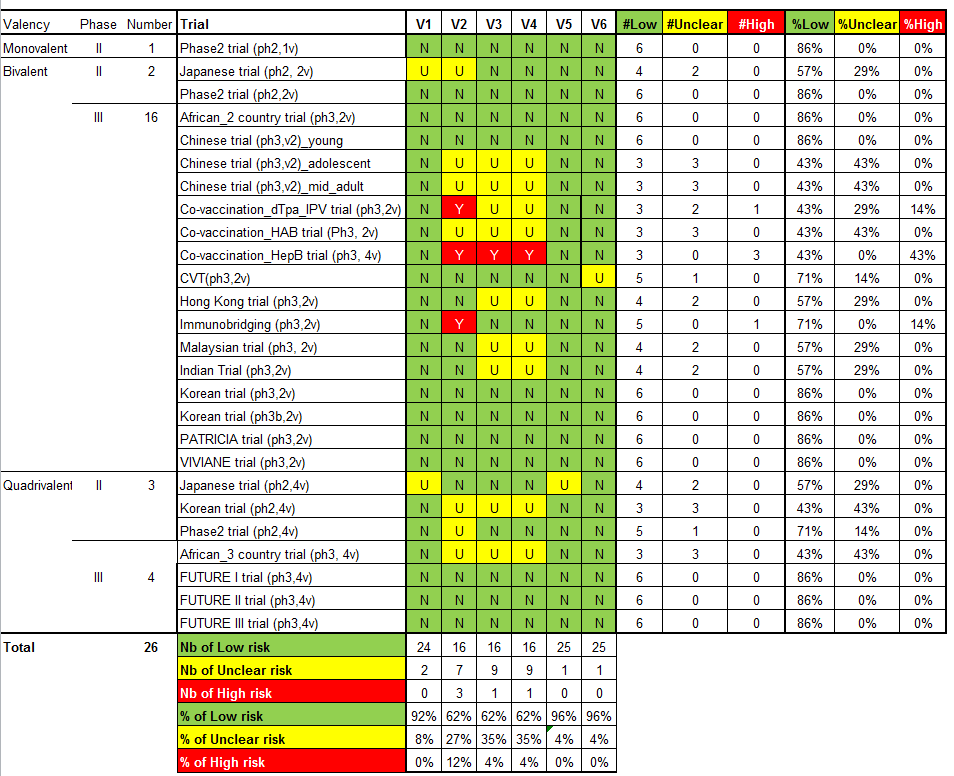
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
V1 = Random sequence generation; V2 = Allocation concealment; V3 = Blinding participants & personnel; V4 = Blinding of outcome assessment; V5 = Incomplete outcomes; V6 = Selective reporting.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
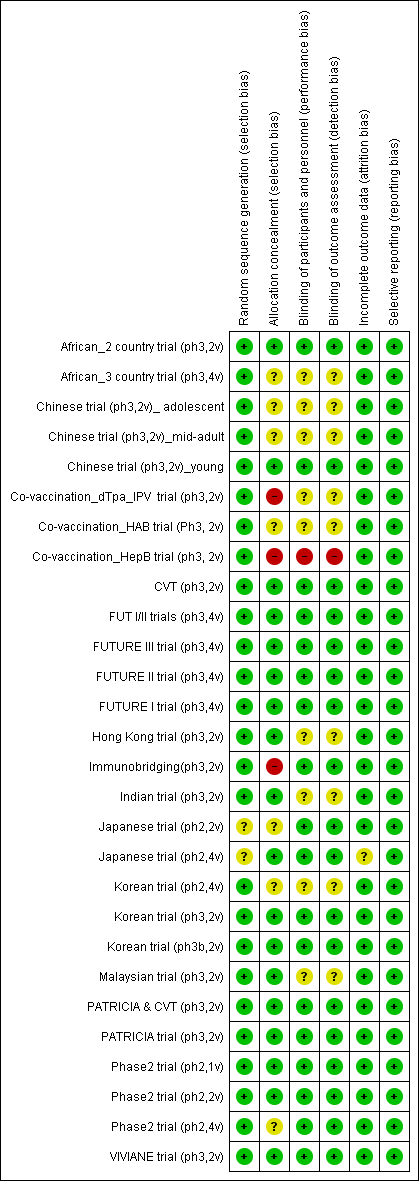
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
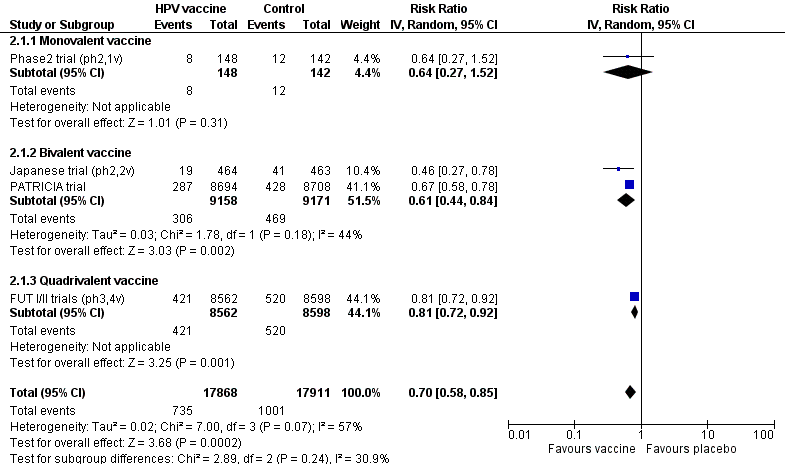
Protection against CIN2+ irrespective of presence of HPV types in women, aged 15‐26 years, regardless of their HPV DNA status at baseline, who received at least one dose.
![Summary of vaccine efficacy estimates, by age group, outcome and HPV DNA status at enrolment (for women who received at least one dose). [REFS BETWEEN SQUARE BRACKETS MUST BE ADAPTED][](/es/cdsr/doi/10.1002/14651858.CD009069.pub3/media/CDSR/CD009069/image_n/nCD009069-AFig-FIG06.png)
Summary of vaccine efficacy estimates, by age group, outcome and HPV DNA status at enrolment (for women who received at least one dose). [REFS BETWEEN SQUARE BRACKETS MUST BE ADAPTED][
![Summary of vaccine efficacy estimates by age group, outcome and number of received doses (for women who were HPV16/18 DNA negative at enrolment). [REFS BETWEEN SQUARE BRACKETS MUST BE ADAPTED][](/es/cdsr/doi/10.1002/14651858.CD009069.pub3/media/CDSR/CD009069/image_n/nCD009069-AFig-FIG07.png)
Summary of vaccine efficacy estimates by age group, outcome and number of received doses (for women who were HPV16/18 DNA negative at enrolment). [REFS BETWEEN SQUARE BRACKETS MUST BE ADAPTED][

Modified Cates plot: Number of cases of CIN2+ associated with HPV16/18 occurring in women who were all hrHPV DNA negative at baseline. 16 out of 1000 non‐vaccinated women developed the lesion (left) whereas fewer than one (0.2) out 1000 vaccinated women developed the lesion (right). Relative risk= 0.01 (95% CI: 0.01 to 0.05).

Modified Cates plot: Number of cases of CIN2+ irrespective of HPV types occurring in women who were all hrHPV DNA negative at baseline. 28 out of 1000 non‐vaccinated women developed the lesion (left) whereas 11 out 1000 vaccinated women developed the lesion (right). Relative risk= 0.37 (95% CI: 0.25 to 0.55).
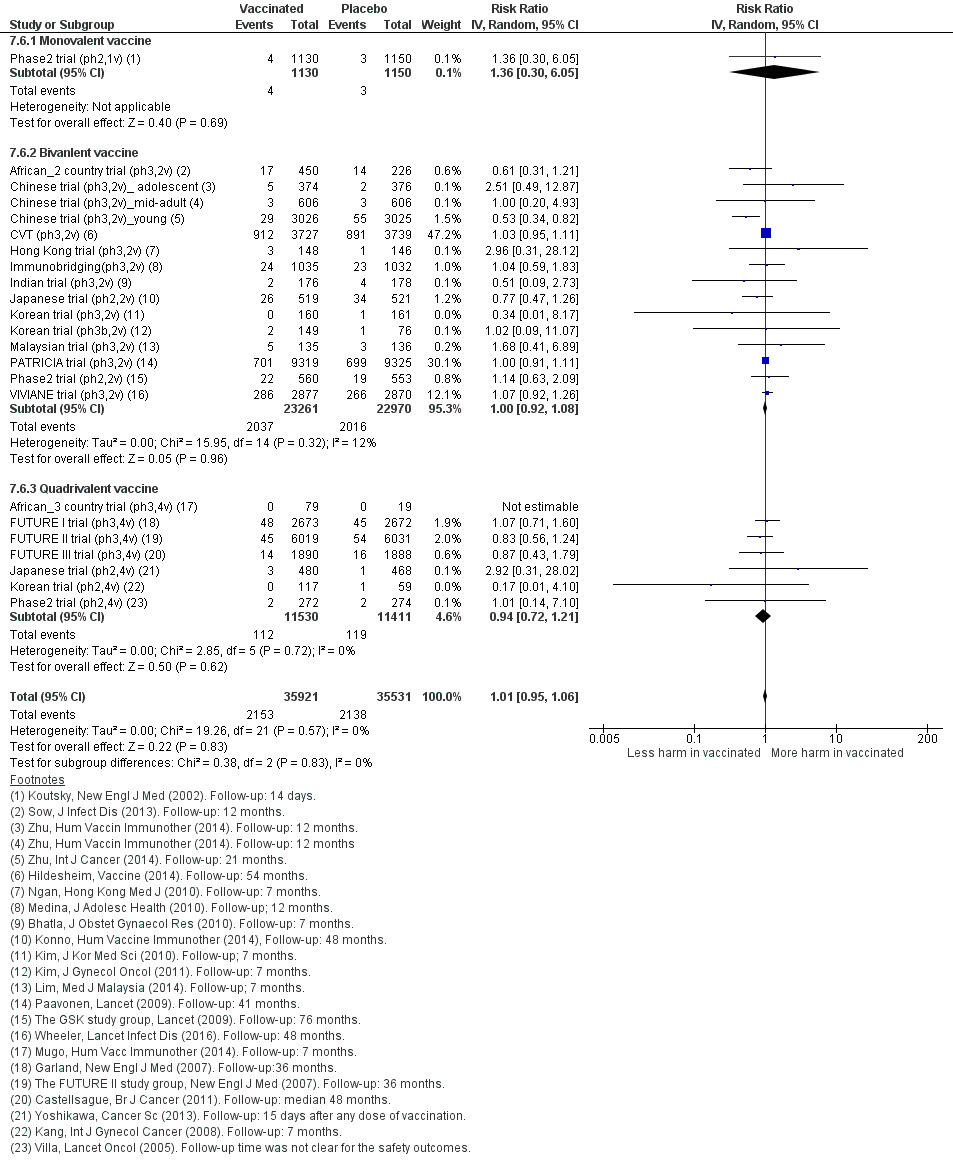
Sensitivity analysis of Analysis 7.6 on severe adverse effects restricting to data extracted from publications in peer‐reviewed journals.

Sensitivity analysis of Analysis 7.7 on deaths restricting to data extracted from publications in peer‐reviewed journals.

Protection against CIN2+ associated with HPV16/18 in women, aged 15‐26 years, who were HPV DNA 16/18 negative at baseline, by number of doses.

Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 1 CIN2+ associated with HPV16/18, at least 1 dose.

Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 2 CIN2+ associated with HPV6/11/16/18, at least 1 dose.

Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 3 CIN3+ associated with HPV16/18, at least 1 dose.
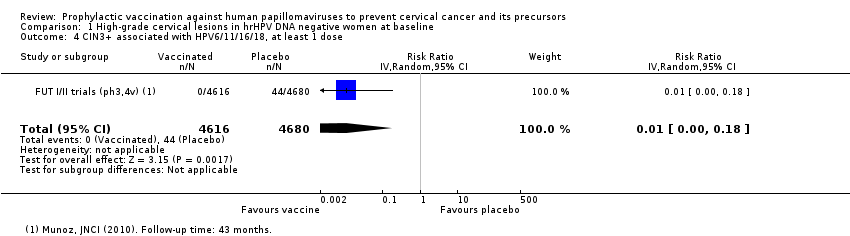
Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 4 CIN3+ associated with HPV6/11/16/18, at least 1 dose.
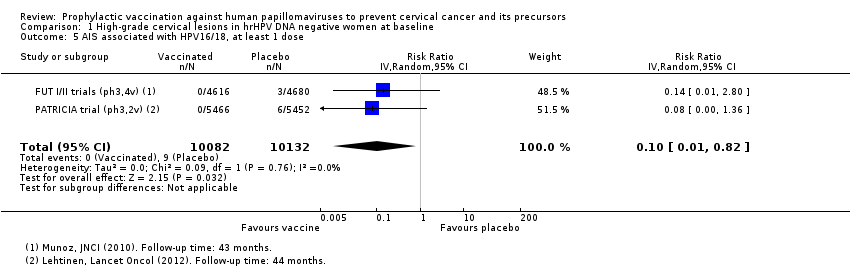
Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 5 AIS associated with HPV16/18, at least 1 dose.
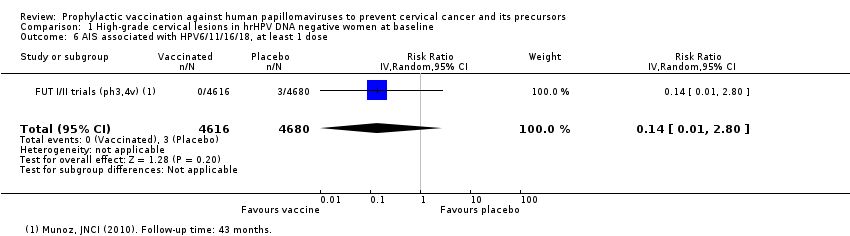
Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 6 AIS associated with HPV6/11/16/18, at least 1 dose.
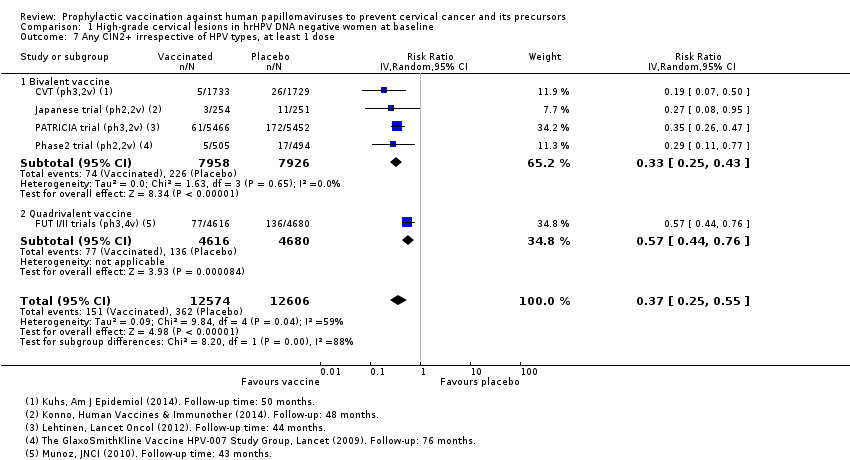
Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 7 Any CIN2+ irrespective of HPV types, at least 1 dose.
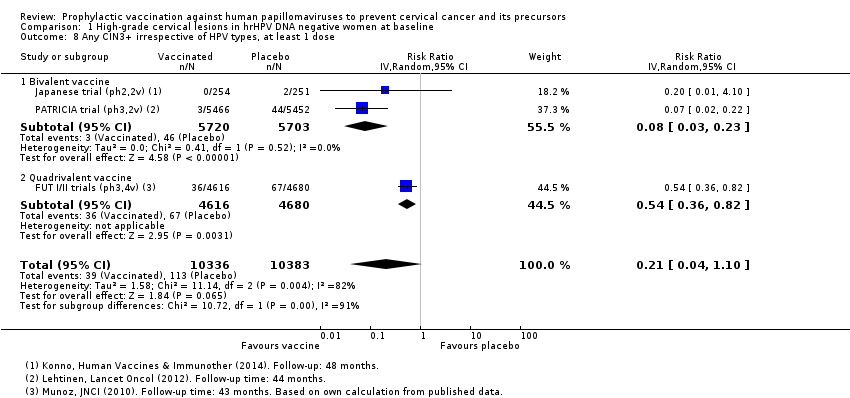
Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 8 Any CIN3+ irrespective of HPV types, at least 1 dose.
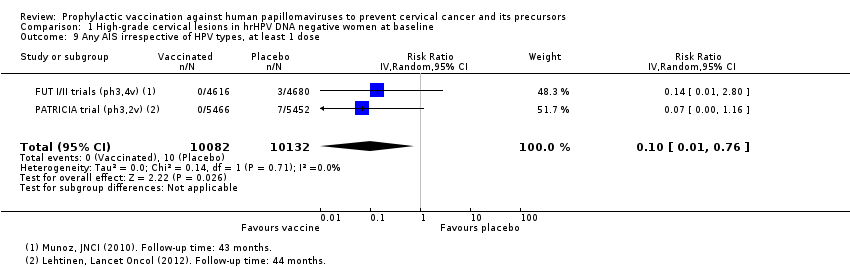
Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 9 Any AIS irrespective of HPV types, at least 1 dose.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 1 CIN2+ associated with HPV16/(18), 3 doses.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 2 CIN2+ associated with HPV16/(18), at least 1 dose.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 3 CIN2+ associated with HPV16/(18), 1 or 2 doses (post hoc analysis).
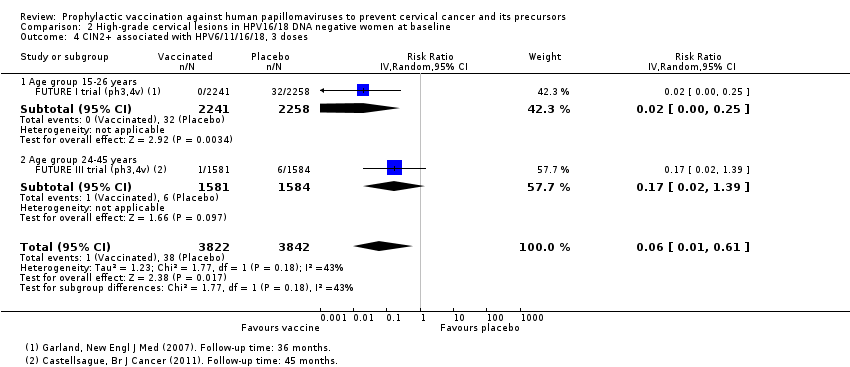
Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 4 CIN2+ associated with HPV6/11/16/18, 3 doses.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 5 CIN2+ associated with HPV6/11/16/18, at least 1 dose.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 6 CIN2+ associated with HPV6/11/16/18, 1 or 2 doses (post hoc analysis).

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 7 CIN3+ associated with HPV16/18 or HPV6/11/16/18, 3 doses.
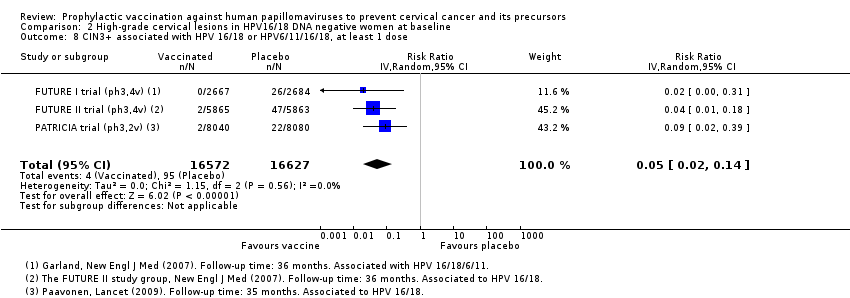
Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 8 CIN3+ associated with HPV 16/18 or HPV6/11/16/18, at least 1 dose.
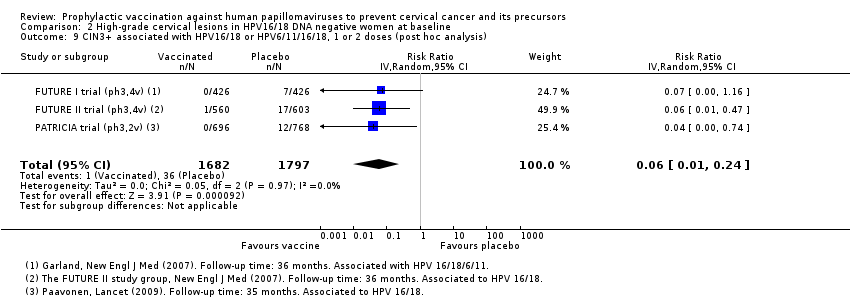
Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 9 CIN3+ associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses (post hoc analysis).

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 10 AIS associated with HPV16/18 or HPV6/11/16/18, 3 doses.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 11 AIS associated with HPV16/18 or 6/11/16/18, at least 1 dose.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 12 AIS associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses (post hoc analysis).

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 13 Any CIN2+ irrespective of HPV types, 3 doses.
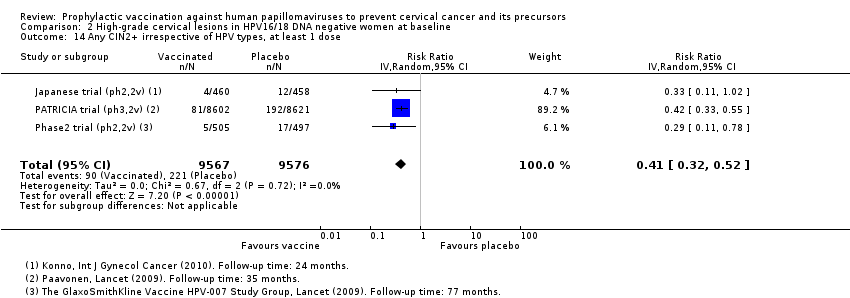
Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 14 Any CIN2+ irrespective of HPV types, at least 1 dose.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 15 Any CIN2+ irrespective of HPV types, 1 or 2 doses (post hoc analysis).
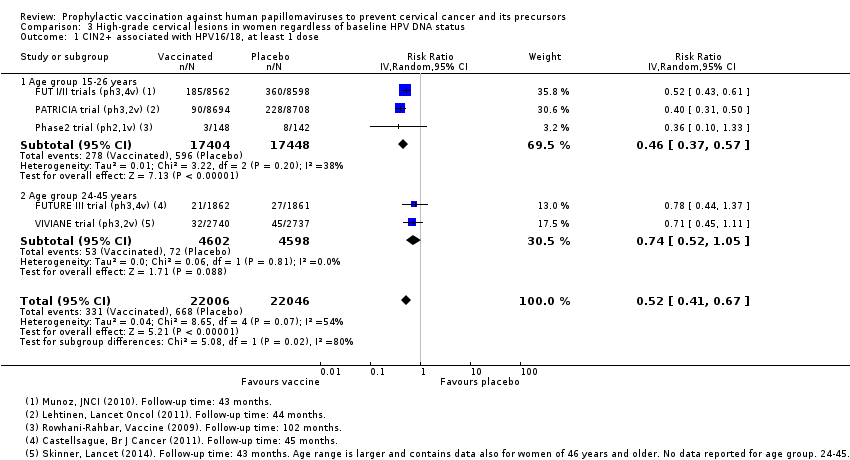
Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 1 CIN2+ associated with HPV16/18, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 2 CIN2+ associated with HPV6/11/16/18, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 3 CIN3+ associated with HPV16/18, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 4 CIN3+ associated with HPV6/11/16/18, at least 1 dose.
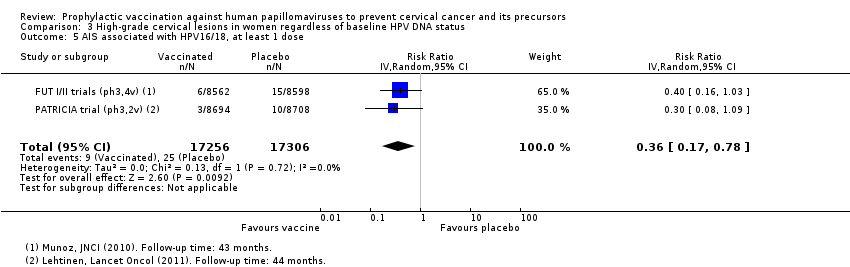
Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 5 AIS associated with HPV16/18, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 6 AIS associated with HPV6/11/16/18, at least 1 dose.
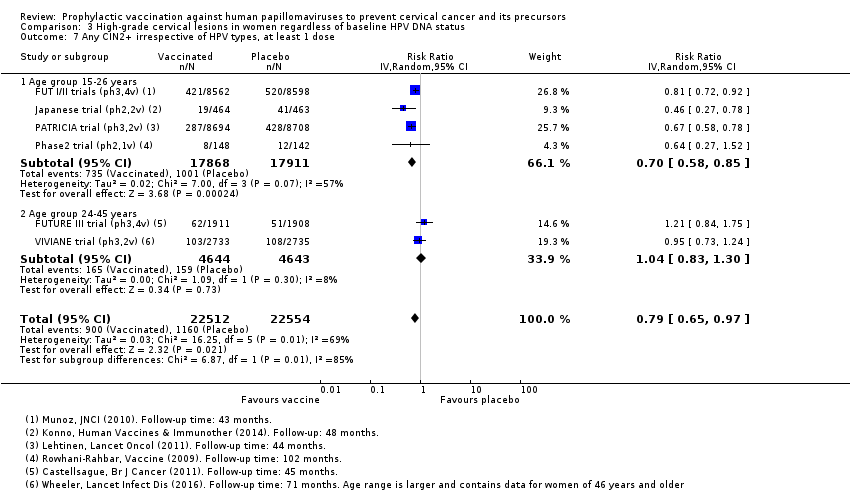
Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 7 Any CIN2+ irrespective of HPV types, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 8 Any CIN3+ HPV type, at least 1 dose.
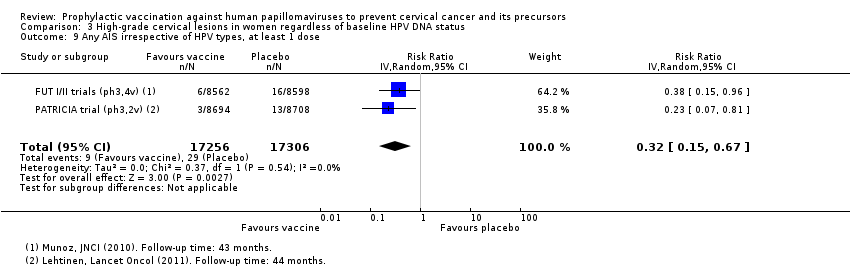
Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 9 Any AIS irrespective of HPV types, at least 1 dose.

Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 1 Incident HPV16/18 infection, 3 doses.

Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 2 Persistent HPV16/18 infection (6M), 3 doses.
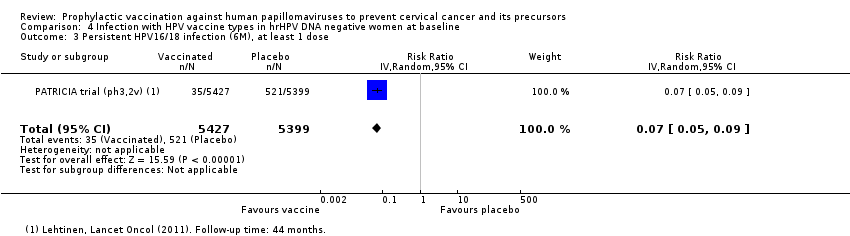
Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 3 Persistent HPV16/18 infection (6M), at least 1 dose.
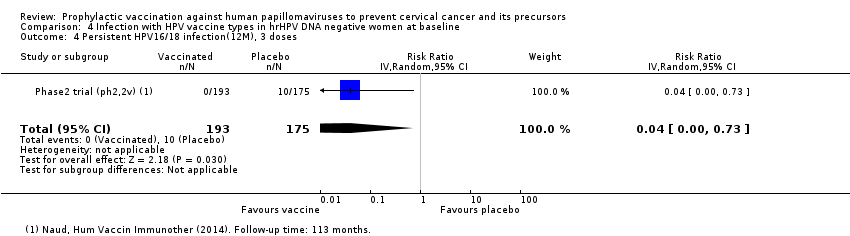
Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 4 Persistent HPV16/18 infection(12M), 3 doses.

Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 5 Persistent HPV16/18 infection (12M), at least 1 dose.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 1 Incident HPV16/18 infection, 3 doses.
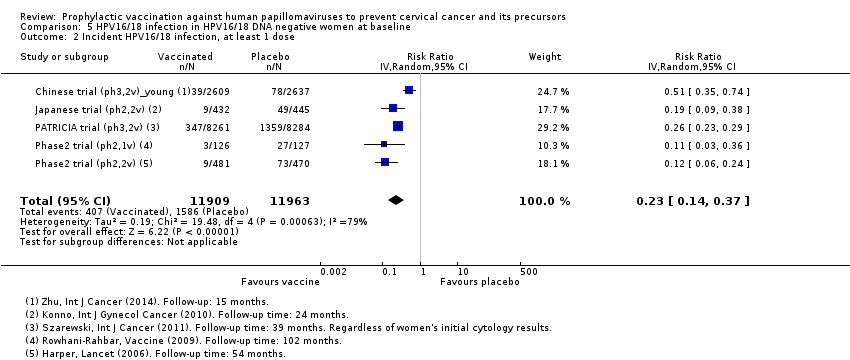
Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 2 Incident HPV16/18 infection, at least 1 dose.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 3 Incident HPV16/18 infection, 1 or 2 doses (post hoc analysis).

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 4 Persistent HPV16/18 infection (6M), 3 doses.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 5 Persistent HPV16/18 infection (6M), at least 1 dose.
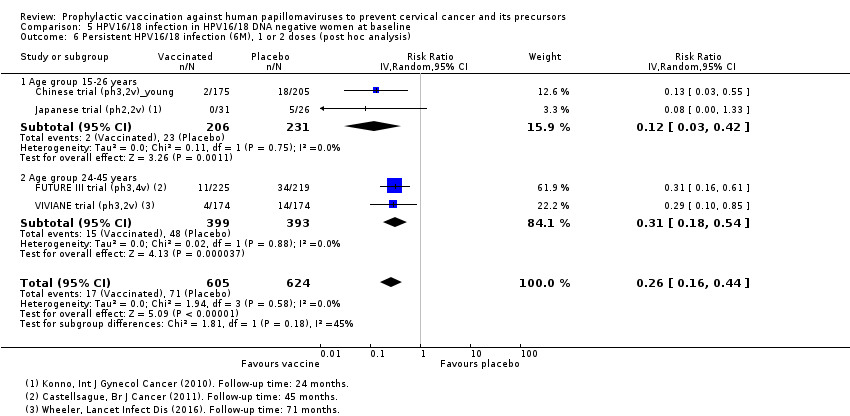
Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 6 Persistent HPV16/18 infection (6M), 1 or 2 doses (post hoc analysis).

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 7 Persistent HPV6/11/16/18 infection (6M), 3 doses.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 8 Persistent HPV6/11/16/18 infection (6M), at least 1 dose.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 9 Persistent HPV16/18 infection (12M), 3 doses.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 10 Persistent HPV16/18 infection (12M), at least 1 dose.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 11 Persistent HPV16/18 infection (12M), 1 or 2 doses (post hoc analysis).

Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 1 Incident HPV16/18 infection, at least 1 dose.

Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 2 Persistent HPV16/18 infection (6M), at least 1 dose.

Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 3 Persistent HPV6/11/16/18 infection (6M), at least 1 dose.
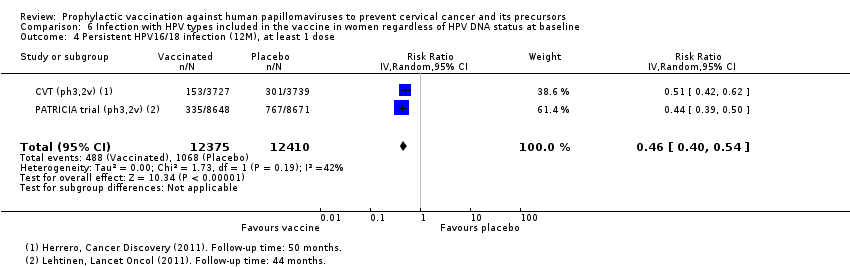
Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 4 Persistent HPV16/18 infection (12M), at least 1 dose.

Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 5 Persistent HPV16/18 infection (12M) by dose (post hoc analysis).
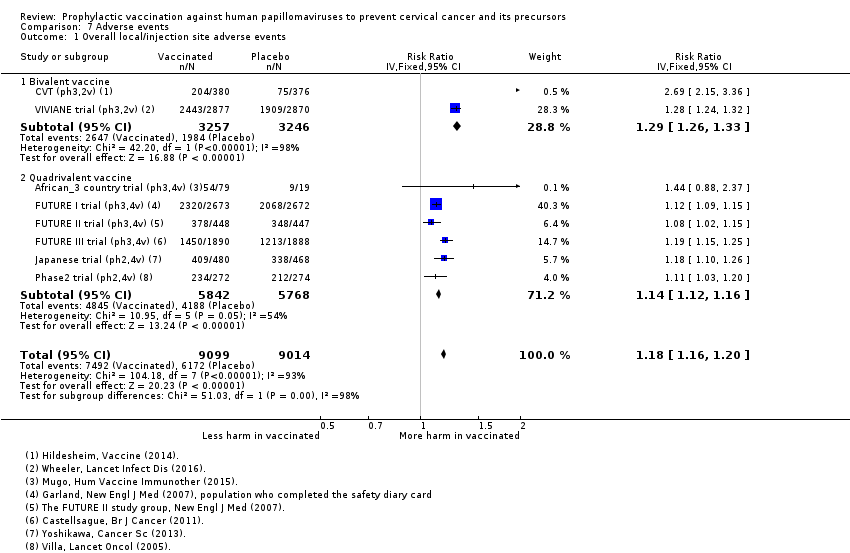
Comparison 7 Adverse events, Outcome 1 Overall local/injection site adverse events.
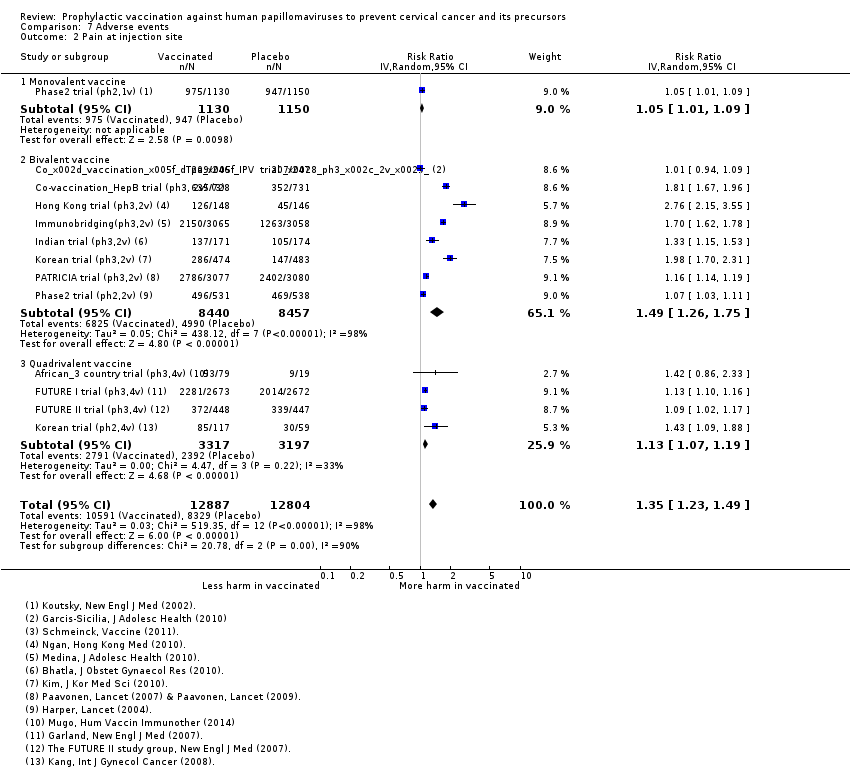
Comparison 7 Adverse events, Outcome 2 Pain at injection site.

Comparison 7 Adverse events, Outcome 3 Swelling at injection site.

Comparison 7 Adverse events, Outcome 4 Redness at injection site.
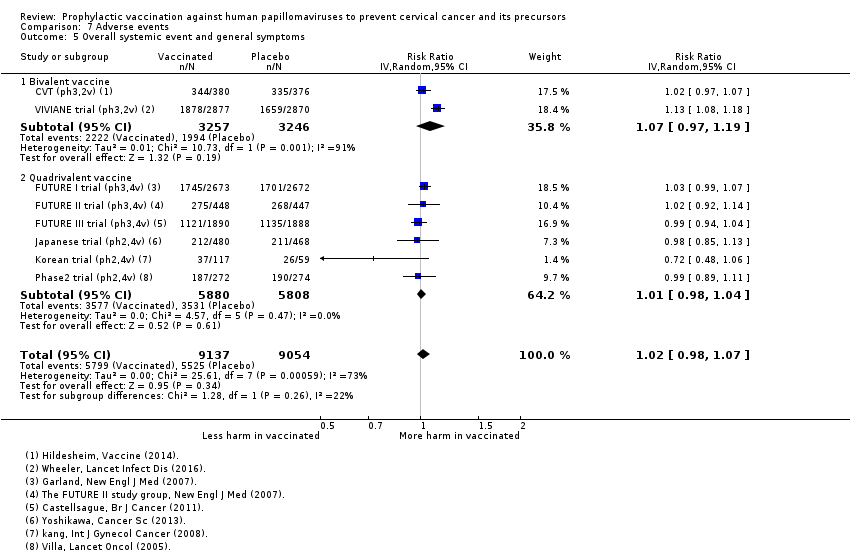
Comparison 7 Adverse events, Outcome 5 Overall systemic event and general symptoms.

Comparison 7 Adverse events, Outcome 6 Serious adverse events.

Comparison 7 Adverse events, Outcome 7 Deaths.
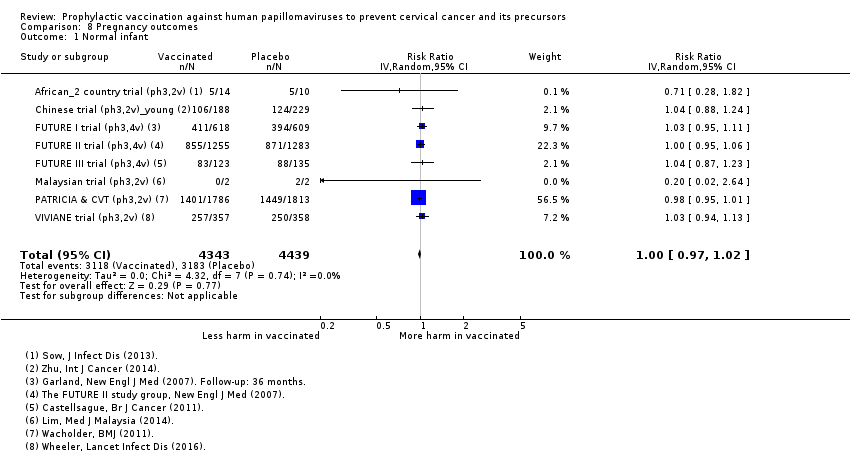
Comparison 8 Pregnancy outcomes, Outcome 1 Normal infant.
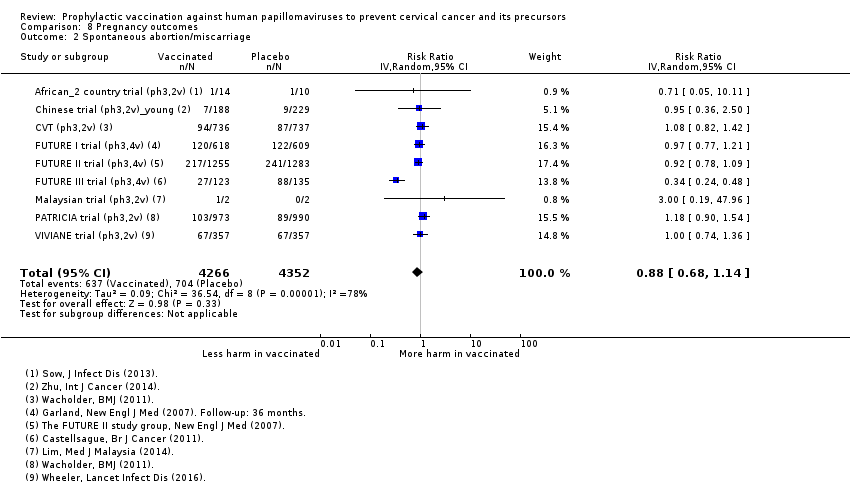
Comparison 8 Pregnancy outcomes, Outcome 2 Spontaneous abortion/miscarriage.

Comparison 8 Pregnancy outcomes, Outcome 3 Elective termination/induced abortion.

Comparison 8 Pregnancy outcomes, Outcome 4 Stillbirth.
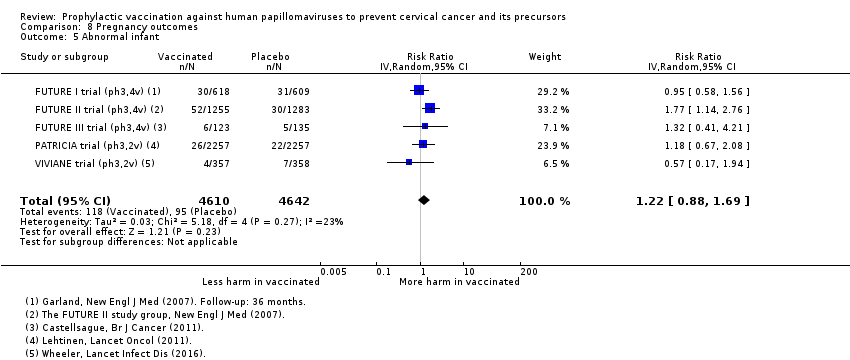
Comparison 8 Pregnancy outcomes, Outcome 5 Abnormal infant.
| HPV vaccine effects on cervical lesions in adolescent girls and women who are hrHPV DNA negative at baseline | ||||||
| Patient or population: adolescent girls and women aged 15 to 26 years who are hrHPV negative before vaccination Setting: Europe, Asia Pacific countries, South & North America Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with HPV vaccination1 | |||||
| Cervical cancer ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| CIN2+ associated with HPV16/18. Follow‐up: 3 to 5 years | 164 per 10,000 | 2 per 10,000 | RR 0.01 | 23,676 | ⊕⊕⊕⊕ | |
| CIN3+ associated with HPV16/18 Follow‐up: 3 to 5 years | 70 per 10,000 | 0 per 10,000 | RR 0.01 | 20,214 | ⊕⊕⊕⊕ | Continuity correction |
| AIS associated with HPV16/18 Follow‐up: 3 to 5 years | 9 per 10,000 | 0 per 10,000 | RR 0.10 | 20,214 | ⊕⊕⊕⊝ | Continuity correction |
| Any CIN2+ irrespective of HPV type, bivalent or quadrivalent vaccine Follow‐up: 2 to 6 years | 287 per 10,000 | 106 per 10,000 | RR 0.37 | 25,180 | ⊕⊕⊕⊕ | Substantial subgroup heterogeneity was observed (I2= 84.3%) for bi‐ and quadrivalent vaccines. So results are reported separately for the 2 vaccines (see next 2 rows). |
| Any CIN2+ irrespective of HPV type Follow‐up (bivalent): 3.5 to 6 years Follow‐up (quadrivalent): 3.5 years | Bivalent vaccine | RR 0.33 (0.25 to 0.43) | 15,884 (4 RCTs) | ⊕⊕⊕⊕ | ||
| 285 per 10,000 | 94 per 10,000 (71 to 122) | |||||
| Quadrivalent vaccine | RR 0.57 (0.44 to 0.76) | 9296 (1 RCT) | ⊕⊕⊕⊝ | |||
| 291 per 10,000 | 166 per 10,000 (128 to 221) | |||||
| Any CIN3+ irrespective of HPV type, bivalent or quadrivalent vaccine Follow‐up: 3.5 to 4 years | 109 per 10,000 | 23 per 10,000 | RR 0.21 | 20,719 | ⊕⊕⊕⊝ | Substantial subgroup heterogeneity was observed (I2 = 84.3%) for bi‐ and quadrivalent vaccines. So results are reported separately for the 2 vaccines (see next 2 rows). |
| Any CIN3+ irrespective of HPV type Follow‐up (bivalent): 4 years Follow‐up (quadrivalent): 3.5 years | Bivalent vaccine | RR 0.08 (0.03 to 0.23) | 11,423 (2 RCTs) | ⊕⊕⊕⊕ | ||
| 81 per 10,000 | 6 per 10,000 (3 to 19) | |||||
| Quadrivalent vaccine | RR 0.54 (0.36 to 0.82) | 9296 (1 RCT) | ⊕⊕⊕⊝ | |||
| 143 per 10,000 | 77 per 10,000 (51 to 117 ) | |||||
| Any AIS irrespective of HPV type | 10 per 10,000 | 0 per 10,000 | RR 0.10 | 20,214 | ⊕⊕⊕⊝ | Continuity correction |
| 1The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). When risk in vaccine group is zero, the 95% CI is computed using an exact binomial method. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Assumed risk calculated from the sum of control group event rates. 2 Downgraded due to serious imprecision in effect estimate (width 95% CI around RR > 0.6). 3 Downgraded one level due to serious imprecision. Few events observed in the two studies (9 in placebo arms and 0 in vaccination arms for the outcome of AIS HPV16/18 and 7 in placebo arms and 0 in vaccination arms for outcome of AIS of any type). | ||||||
| HPV vaccine effects on cervical lesions in adolescent girls and women negative for HPV16/18 DNA at baseline | ||||||
| Patient or population: adolescent girls and women aged 15 to 45 years who were HPV16/18 negative before vaccination | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with HPV vaccination1 | |||||
| Cervical cancer ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| CIN2+ associated with HPV16/18 Follow‐up (age 15 to 26 years): 1 to 8.5 years Follow‐up (age 24 to 45 years): 4 to 6 years | 15 to 26 years | RR 0.05 | 34,478 | ⊕⊕⊕⊕ | ||
| 113 per 10,000 | 6 per 10,000 | |||||
| 24 to 45 years | RR 0.30 (0.11 to 0.81) | 7552 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | |||
| 45 per 10,000 | 14 per 10,000 (5 to 37) | |||||
| CIN3+ associated with HPV16/18 (age 15 to 26 years) Follow‐up: 3 years | 57 per 10,000 | 3 per 10,000 (1 to 8) | RR 0.05 (0.02 to 0.14) | 33,199 (3 studies) | ⊕⊕⊕⊕ | |
| AIS associated with HPV16/18 or 6/11/16/18 (age 15 to 26 years) Follow‐up: 3 years | 12 per 10,000 | 0 per 10,000 | RR 0.09 | 17,079 | ⊕⊕⊕⊝ MODERATE 2 | Continuity correction |
| Any CIN2+ irrespective of HPV type (age 15 to 26 years) Follow‐up: 2 to 6.5 years | 231 per 10,000 | 95 per 10,000 | RR 0.41 | 19,143 | ⊕⊕⊕⊕ | |
| Any CIN3+ irrespective of HPV type ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Any AIS irrespective of HPV type ‐ not measured | ‐ | ‐ | ||||
| 1The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Exception: when risk in vaccine group is zero, the 95% CI is computed using an exact binomial method.. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Assumed risk calculated from the sum of control group event rates. 2 Downgraded due to serious imprecision in effect estimate (width 95% CI around RR > 0.6). | ||||||
| HPV vaccine effects on cervical lesions in adolescent girls and women unselected for HPV DNA status at baseline | ||||||
| Patient or population: adolescent girls and women aged 15 to 45 years regardless of HPV DNA status at baseline | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with HPV vaccination1 | |||||
| Cervical cancer ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| CIN2+ associated with HPV16/18 Follow‐up (age 15 to 26 years): 3.5 to 8.5 years Follow‐up (age 24 to 45 years): 3.5 years | 15 to 26 years | RR 0.46 (0.37 to 0.57 | 34,852 | ⊕⊕⊕⊕ | ||
| 341 per 10,000 | 157 per 10,000 | |||||
| 24 to 45 years | RR 0.74 (0.52 to 1.05) | 9200 (2 studies) | ⊕⊕⊕⊝ | |||
| 145 per 10,000 | 107 per 10,000 (76 to 152) | |||||
| CIN3+ associated with HPV16/18 Follow‐up: 3.5 years | 165 per 10,000 | 91 per 10,000 (74 to 127) | RR 0.55 (0.45 to 0.67) | 34,562 (2 RCTs) | ⊕⊕⊕⊕ | |
| Adeno carcinoma in situ (AIS) associated with HPV16/18 Follow‐up: 3.5 years | 14 per 10,000 | 5 per 10,000 | RR 0.36 | 34,562 | ⊕⊕⊕⊕ | |
| Any CIN2+ irrespective of HPV type Follow‐up (age 15 to 26 years): 3.5 to 8.5 years Follow‐up (age 24 to 45 years): 3.5 to 6 years | 15 to 26 years | RR 0.70 | 35,779 | ⊕⊕⊕⊕ | ||
| 559 per 10,000 | 391 per 10,000 | |||||
| 24 to 45 years | RR 1.04 | 9287 | ⊕⊕⊕⊝ | |||
| 343 per 10,000 | 356 per 10,000 | |||||
| Any CIN3+ irrespective of HPV type (age 15 to 26 years) Follow‐up: 3.5 to 4 years | 266 per 10,000 | 178 per 10,000 (231 to 247) | RR 0.67 (0.49 to 0.93) | 35,489 (3 RCTs) | ⊕⊕⊕⊝ | Substantial subgroup heterogeneity was observed (I2 = 84.3%) for bivalent and quadrivalent vaccines. So results are reported separately for two vaccines. |
| Any CIN3+ irrespective of HPV type (age 15 to 26 years), Follow‐up (bivalent): 3.5 to 4 years Follow‐up (quadrivalent): 3.5 years | Bivalent vaccine | RR 0.55 (0.43 to 0.71) | 18,329 (2 RCTs) | ⊕⊕⊕⊕ | ||
| 188 per 10,000 | 104 per 10,000 (81 to 134) | |||||
| Quadrivalent vaccine | 0.81 (0.69 to 0.96) | 17,160 (1 RCT) | ⊕⊕⊕⊝ | |||
| 349 per 10,000 | 283 per 10,000 (241 to 335) | |||||
| Any AIS irrespective of HPV type (age 15 to 26 years) Follow‐up: 3.5 years | 17 per 10,000 | 5 per 10,000 | RR 0.32 | 34,562 | ⊕⊕⊕⊕ | |
| Serious adverse events Follow‐up: 6 months to 7 years | 669 per 10,000 | 656 per 10,000 | RR 0.98 | 71,597 | ⊕⊕⊕⊕ | |
| Deaths Follow‐up: 7 months to 10 years. Most of the information in the analysis comes from studies with follow‐up ranging from 5‐10 years. | 11 per 10,000 | 14 per 10,000 | RR 1.29 | 71,176 | ⊕⊕⊝⊝ | Older women had higher fatality rate (RR 2.36, 95% CI 1.10 to 5.03). Assessment of the deaths in the studies has not been able to identify a pattern in the cause or timing of death. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Assumed risk calculated from the sum of control group event rates for all outcomes unless otherwise stated. 2 Downgraded due to serious imprecision. Confidence interval is wide and includes large decrease and small increase in lesions with vaccination group in the older age group. 3 Downgraded one level due to serious inconsistency. Reduction in lesions was greater in younger women than in older women (RR 0.46 in 15 to 26 years versus RR 0.74 in 24 to 45 years; P = 0.02 for interaction). 4 Downgraded one level due to serious imprecision. Confidence interval includes potentially meaningful increase in risk of mortality. 5 Downgraded one level due to serious inconsistency. Despite limited evidence of statistical variation, sub grouping studies by age showed higher fatality rate with vaccines in older age group. There is no clear pattern in causes or timing of deaths. | ||||||
| HPV vaccine adverse pregnancy outcomes (regardless of DNA status and age) | ||||||
| Patient or population: adolescent girls and women aged 15 to 45 years who became pregnant during the study | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with HPV vaccines | |||||
| Spontaneous abortion/miscarriage Follow‐up: 1 to 7 years | Study population | RR 0.88 | 8618 | ⊕⊕⊕⊕ | ||
| 1618 per 10,000 | 1,424 per 10,000 | |||||
| Elective termination/induced abortion Follow‐up: 1 to 7 years | Study population | RR 0.90 | 10,909 | ⊕⊕⊕⊕ | ||
| 931 per 10,000 | 838 per 10,000 | |||||
| Stillbirth Follow‐up: 1 to 3.5 years | Study population | RR 1.12 | 8754 | ⊕⊕⊕⊝ | ||
| 70 per 10,000 | 78 per 10,000 | |||||
| Babies born with congenital malformations Follow‐up: 3 to 7 years | Study population | RR 1.22 | 9252 | ⊕⊕⊕⊝ | ||
| 205 per 10,000 | 250 per 10,000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Confidence interval rules out an increased risk of termination so there is no downgrade for imprecision. 2 Downgraded one level due to serious imprecision. Confidence intervals for both outcomes include meaningful increase and reduction in risk of stillbirth or abnormal infants following vaccination. | ||||||
| Valency | Phase | Number of trials | Appelation | N | Outcomes | Main References |
| Monovalent | II | 1 | 2392 | Efficacy, safety | ||
| Bivalent | II | 2 | 1040 | Efficacy, safety | ||
| 1113 | Efficacy, safety | |||||
| III | 16 | 676 | Safety | |||
| 6051 | Efficacy, safety | |||||
| 750 | Safety | |||||
| 1212 | Safety | |||||
| 494 | Safety | |||||
| 494 | Safety | |||||
| 541 | Safety | |||||
| 7466 | Efficacy, safety | |||||
| 294 | Safety | |||||
| 2067 | Safety | |||||
| 354 | Safety | |||||
| 208 | Safety | |||||
| 321 | Safety | |||||
| 271 | Safety | |||||
| 18,644 | Efficacy, safety | |||||
| 5752 | Efficay, safety, | |||||
| Quadrivalent | II | 3 | 1021 | Safety | ||
| 176 | Safety | |||||
| 552 | Efficacy, safety | |||||
| III | 4 | 98 | Safety | |||
| 5455 | Efficacy, safety | |||||
| 12,167 | Efficacy, safety | |||||
| 3819 | Efficacy, safety | |||||
| Total | 26 | 73,428 |
| Outcomes and exposure subgroups | Absolute risk / per 10,000 | Relative risk | Vaccine efficacy (95% CI) | Risk difference/ per 10,000 (95% CI) | No of Participants | Certainty of evidence | |
| Placebo | Vaccinated | ||||||
| 1. High‐grade cervical lesions in women who were hrHPV DNA negative at baseline | |||||||
| Analysis 1.1 CIN2+ associated with HPV16/18, at least 1 dose, age 15‐26 years | 164 | 2 | 0.01 (0.00 to 0.05) | 99% (95% to 100%) | 162 (157 to 164) | 23,676 | ⊕⊕⊕⊕ high |
| Analysis 1.2 CIN2+ associated with HPV6/11/16/18, at least 1 dose, age 15‐26 years | 197 | 2 | 0.01 (0.00 to 0.09) | 99% (91% to 100%) | 195 (179 to 197) | 9296 (1 study) | ⊕⊕⊕⊝ |
| Analysis 1.3 CIN3+ associated with HPV16/18, at least 1 dose, age 15‐26 years | 70 | 0* | 0.01 (0.00 to 0.10) | 99% (90% to 100%) | 70 (63 to 70) | 20,214 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 1.4 CIN3+ associated with HPV6/11/16/18, at least 1 dose, age 15‐26 years | 94 | 0* | 0.01 (0.00 to 0.18) | 99% (82% to 100%) | 94 (77 to 94) | 9296 (1 study) | ⊕⊕⊕⊝ |
| Analysis 1.5 AIS associated with HPV16/18, at least 1 dose, age 15‐26 years | 9 | 0* | 0.10 (0.01 to 0.82) | 90% (18% to 99%) | 9 (2 to 9) | 20,214 | ⊕⊕⊕⊝ |
| Analysis 1.6 AIS associated with HPV6/11/16/18m at least 1 dose, age 15‐26 years | 6 | 0* | 0.14 (0.01 to 2.8) | 86% (‐180% to 99%) | 6 (‐12 to 6) | 9296 (1 study) | ⊕⊕⊕⊝ |
| Analysis 1.7.1 Any CIN2+ irrespective of HPV types, at least 1 dose of the bivalent vaccine, age 15‐26 years | 285 | 94 | 0.33 (0.25 to 0.43) | 67% (57% to 75%) | 191 (163 to 214) | 15,884 (4 studies) | ⊕⊕⊕⊕ high |
| Analysis 1.7.2 Any CIN2+ irrespective of HPV types, at least 1 dose of the quadrivalent vaccine, age 15‐26 years | 291 | 166 | 0.57 (0.44 to 0.76) | 43% (24 to 56%) | 125 (70 to 163) | 9296 (1 study) | ⊕⊕⊕⊝ |
| Analysis 1.8.1 Any CIN3+ irrespective of HPV types, at least 1 dose of the bivalent vaccine, age 15‐26 years | 81 | 6 | 0.08 (0.03 to 0.23) | 92% (77% to 97%) | 74 (62 to 78) | 11,423 (2 studies) | ⊕⊕⊕⊕ |
| Analysis 1.8.2 Any CIN3+ irrespective of HPV types, at least 1 dose of the quadrivalent vaccine, age 15‐26 years | 143 | 77 | 0.54 (0.36 to 0.82) | 46% (17% to 64%) | 66 (26 to 92) | 9296 (1 study) | ⊕⊕⊕⊝ |
| Analysis 1.9 Any AIS irrespective of HPV types, at least 1 dose | 10 | 0* | 0.10 (0.01 to 0.76) | 90% (24% to 99%) | 10 (2 to 10) | 20,214 | ⊕⊕⊕⊝ |
| 2. High‐grade cervical lesions in women who were HPV16/18 negative at baseline | |||||||
| Analysis 2.1.1 CIN2+ associated with HPV16/18, 3 doses, age 15‐26 years | 74 | 5 | 0.07 (0.03 to 0.15) | 93% (85% to 97%) | 69 (63 to 72) | 36,579 (6 studies) | ⊕⊕⊕⊕ high |
| Analysis 2.1.2 CIN2+ associated with HPV16/18, 3 doses, 24‐45 years | 36 | 6 | 0.16 (0.04 to 0.74) | 84% (26% to 96%) | 30 (9 to 34) | 6797 (2 studies) | ⊕⊕⊕⊝ |
| Analysis 2.2.1 CIN2+ associated with HPV16/18, at least 1 dose, 15‐26 years | 113 | 6 | 0.05 (0.03 to 0.10) | 95% (90% to 97%) | 107 (102 to 110) | 34,478 (6 studies) | ⊕⊕⊕⊕ high |
| Analysis 2.2.2 CIN2+ associated with HPV16/18, at least 1 dose, age 24‐45 years | 45 | 14 | 0.30 (0.11 to 0.81) | 70% (19% to 89%) | 32 (9 to 40) | 7552 (2 studies) | ⊕⊕⊕⊝ |
| Analysis 2.3.1 CIN2+ associated with HPV16/18, 1 or 2 doses, 15‐26 years*** | 436 | 44 | 0.10 (0.04 to 0.26) | 90% (74% to 96%) | 392 (323 to 418) | 2958 (5 studies) | ⊕⊕⊝⊝ low1$ |
| Analysis 2.3.2 CIN2+ associated with HPV16/18, 1 or 2 doses, age 24‐45 years*** | 134 | 82 | 0.61 (0.14 to 2.67) | 39% (‐167% to 86%) | 52 (‐2245 to 115) | 755 (2 studies) | ⊕⊝⊝⊝ |
| Analysis 2.4 CIN2+ associated with HPV6/11/16/18, 3 doses, age 15‐45 years | 99 | 6 | 0.06 (0.01 to 0.61) | 94% (39% to 99%) | 93 (39 to 98) | 7664 (2 studies) | ⊕⊕⊕⊝ |
| Analysis 2.4.1 CIN2+ associated with HPV6/11/16/18, 3 doses, age 15‐26 years | 142 | 0* | 0.02 (0.00 to 0.25) | 98% (75% to 100%) | 142 (93 to 190) | 4499 (1 study) | ⊕⊕⊕⊝ |
| Analysis 2.4.2 CIN2+ associated with HPV6/11/16/18, 3 doses, age 24‐45 years | 38 | 6 | 0.17 (0.02 to 1.39) | 83% (‐39% to 98%) | 32 (‐1 to 32) | 3165 (1 study) | ⊕⊕⊝⊝ |
| Analysis 2.5.1 CIN2+ associated with HPV6/11/16/18, at least 1 dose, age 15‐26 years | 160 | 0* | 0.01 (0.00 to 0.19) | 99% (81% to 100%) | 160 (130 to 159) | 5351 (1 study) | ⊕⊕⊕⊝ |
| Analysis 2.5.2 CIN2+ associated with HPV6/11/16/18, at least 1 dose, age 24‐45 years | 44 | 16 | 0.37 (0.10 to 1.41) | 63% (‐41% to 90%) | 28 (‐18 to 40) | 3629 (1 study) | ⊕⊕⊕⊝ |
| Analysis 2.6 CIN2+ associated with HPV6/11/16/18, 1 or 2 doses, age 15‐45 years*** | 199 | 48 | 0.24 (0.01 to 5) | 76% (‐400% to 99%) | 151 (‐795 to 197) | 1316 (2 studies) | ⊕⊝⊝⊝ |
| Analysis 2.6.1 CIN2+ associated with HPV6/11/16/18, 1 or 2 doses, age 15‐26 years*** | 258 | 0* | 0.04 (0.00 to 0.74) | 96% (26% to 100%)) | 258 (108 to 409) | 852 (1 study) | ⊕⊝⊝⊝ |
| Analysis 2.6.2 CIN2+ associated with HPV6/11/16/18, 1 or 2 doses, age 24‐45 years*** | 88 | 85 | 0.97 (0.14 to 6.80) | 3% (‐580% to 86%) | 3 (‐165 to 171) | 464 (1 study) | ⊕⊝⊝⊝ |
| Analysis 2.7 CIN3+ associated with HPV16/18, 3 doses, age 15‐26 years | 40 | 3 | 0.07 (0.02 to 0.29) | 93% (71% to 98%) | 37 (28 to 39) | 29,720 (3 studies) | ⊕⊕⊕⊕ |
| Analysis 2.8 CIN3+ associated with HPV16/18, at least 1 dose, age 15‐26 years | 57 | 3 | 0.05 (0.02 to 0.14) | 95% (86% to 98%) | 54 (49 to 56) | 33,199 (3 studies) | ⊕⊕⊕⊕ high |
| Analysis 2.9 CIN3+ associated with HPV16/18, 1 or 2 doses, age 15‐26 years*** | 200 | 12 | 0.06 (0.01 to 0.24) | 94% (26% to 100%) | 188 (152 to 198) | 3479 (3 studies) | ⊕⊕⊝⊝ low1$ |
| Analysis 2.10 AIS+ associated with HPV16/18, 3 doses, age 15‐26 years | 8 | 0* | 0.12 (0.02 to 0.70) | 88% (36% to 99%) | 8 (2 to 8) | 29,707 (3 studies) | ⊕⊕⊕⊝ |
| Analysis 2.11 AIS+ associated with HPV16/18, at least 1 dose, age 15‐26 years | 12 | 0* | 0.09 (0.01 to 0.72) | 81% (28% to 99%) | 12 (3 to 12) | 17,079 (2 studies) | ⊕⊕⊕⊝ |
| Analysis 2.12 AIS+ associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses, age 15‐26 years*** | 29 | 0* | 0.15 (0.01 to 2.97) | 85% (‐197% to 99%) | 29 (‐57 to 29) | 2015 (2 studies) | ⊕⊝⊝⊝ |
| Analysis 2.13 CIN2+ irrespective of HPV types, 3 doses, age 15‐26 years | 166 | 66 | 0.40 (0.25 to 0.64) | 60% (36% to 75%) | 99 (60 to 124) | 7320 (3 studies) | ⊕⊕⊕⊕ high |
| Analysis 2.14 CIN2+ irrespective of HPV types, at least 1 dose, age 15‐26 years | 231 | 95 | 0.41 (0.32 to 0.52) | 58% (46% to 67%) | 136 (111 to 157) | 19,143 (3 studies) | ⊕⊕⊕⊕ high |
| Analysis 2.15 CIN2+ irrespective of HPV types, 1 or 2 doses, age 20‐25 years*** | 1000 | 710 | 0.71 (0.15 to 3.38) | 29% (‐238% to 85%) | 290 (‐2,380 to 850) | 34 (1 study) | ⊕⊝⊝⊝ |
| 3. High‐grade cervical lesions in all women regardless of HPV DNA status at baseline** | |||||||
| Analysis 3.1.1 CIN2+ associated with HPV16/18, at least 1 dose, age 15‐26 years | 341 | 157 | 0.46 (0.37 to 0.57) | 54% (43% to 63%) | 184 (147 to 215) | 34,852 | ⊕⊕⊕⊕ high |
| Analysis 3.1.2 CIN2+ associated with HPV16/18, at least 1 dose, age 24‐45 years | 157 | 116 | 0.74 (0.52 to 1.05) | 26% (‐5% to 48%) | 41 (‐8 to 75) | 9200 (2 studies) | ⊕⊕⊕⊝ moderate4 |
| Analysis 3.2.1 CIN2+ associated with HPV6/11/16/18, at least 1 dose, age 15‐26 years | 436 | 217 | 0.50 (0.42 to 0.59) | 50% (41% to 58%) | 219 (166 to 272) | 17,160 (1 study) | ⊕⊕⊕⊝ |
| Analysis 3.2.2 CIN2+ associated with HPV6/11/16/18, at least 1 dose, age 24‐45 years | 145 | 113 | 0.78 (0.44 to 1.37) | 22% (‐37% to 56%) | 143 (72 to 204 | 3723 (1 study) | ⊕⊕⊕⊝ |
| Analysis 3.3 CIN3+ associated with HPV16/18, at least 1 dose, age 15‐26 years | 165 | 91 | 0.55 (0.43 to 0.68) | 74% (55% to 91%) | 74 (55 to 91) | 34,562 | ⊕⊕⊕⊕ high |
| Analysis 3.4 CIN3+ associated with HPV16/18, 1 or 2 doses, age 15‐26 years*** | 230 | 124 | 0.54 (0.43 to 0.68) | 46% (32% to 57%) | 106 (74 to 131) | 17,160 (1 study) | ⊕⊕⊝⊝ |
| Analysis 3.5 AIS associated with HPV16/18, at least 1 dose, age 15‐26 years | 14 | 5 | 0.36 (0.17 to 0.78) | 64% (22% to 83%) | 9 (3 to 12) | 34,562 | ⊕⊕⊕⊕ high |
| Analysis 3.6 AIS associated with HPV6/11/16/18, at least 1 dose, age 15‐45 years | 15 | 6 | 0.40 (0.16 to 0.98) | 60% (2% to 84%) | 9 (0 to 13) | 20,830 (1 study) | ⊕⊕⊕⊝ |
| Analysis 3.7.1 Any CIN2+ irrespective of HPV types, at least 1 dose, age 15‐26 years | 559 | 391 | 0.70 (0.58 to 0.85) | 30% (15% to 42%) | 168 (84 to 235) | 35,779 | ⊕⊕⊕⊕ high |
| Analysis 3.7 2 Any CIN2+ irrespective of HPV types, at least 1 dose, age 24‐45 years | 342 | 356 | 1.04 (0.83 to 1.30) | ‐4% (‐30% to 17%) | ‐14 (‐103 to 58) | 9287 (2 studies) | ⊕⊕⊝⊝ |
| Analysis 3.8 Any CIN3+ irrespective of HPV types, at least 1 dose, age 18‐26 years, bivalent vaccine | 188 | 103 | 0.55 (0.43 to 0.71) | 45% (29% to 57%) | 84 (54 to 1107) | 18,329 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 3.8 Any CIN3+ irrespective of HPV types, at least 1 dose, age 15‐26 years, quadrivalent vaccine | 349 | 283 | 0.81 (0.69 to 0.96) | 19% (4% to 31%) | 66 (14 to 108) | 17,160 (1 study) | ⊕⊕⊕⊝ |
| Analysis 3.9 Any AIS irrespective of HPV types, at least 1 dose, age 15‐26 years | 17 | 5 | 0.32 (0.15 to 0.67) | 68% (33% to 0.85%) | 11 (6 to 14) | 34,562 | ⊕⊕⊕⊕ high |
| 4. HPV16/18 infection in women who were hrHPV DNA negative at baseline | |||||||
| Analysis 4.1 Incident HPV16/18 infection, 3 doses, age 18‐26 years | 2,457 | 147 | 0.06 (0.02 to 0.20) | 94% (80% to 98%) | 2,310 (1,966 to 2,408) | 368 (1 study) | ⊕⊕⊕⊝ |
| Analysis 4.2 Persistent HPV16/18 infection(6M), 3 doses, age 15‐26 years | 971 | 29 | 0.02 (0.00 to 0.35) | 97% (57% to 100%) | 942 (554 to 971) | 368 (1 study) | ⊕⊕⊕⊝ |
| Analysis 4.3 Persistent HPV16/18 infection(6M), at least 1 dose, age 18‐25 years | 96 | 7 | 0.07 (0.05 to 0.09) | 93% (81% to 95%) | 90 (88 to 91) | 10,826 (1 study) | ⊕⊕⊕⊝ |
| Analysis 4.4 Persistent HPV16/18 infection(12M), 3 doses, age 15‐26 years | 571 | 23 | 0.04 (0.00 to 0.73) | 96% (27% to 100%) | 549 (154 to 571) | 368 (1 study) | ⊕⊕⊕⊝ |
| Analysis 4.5 Persistent HPV16/18 infection(12M), at least 1 dose, age 15‐26 years | 462 | 37 | 0.08 (0.05 to 0.12) | 92% (88% to 95%) | 425 (406 to 439) | 14,153 ( 2 studies) | ⊕⊕⊕⊕ high |
| 5. HPV16/18 infection in women who were HPV16/18 negative at baseline | |||||||
| Analysis 5.1 Incident HPV16/18 infection, 3 doses, age 15‐26 years | 474 | 81 | 0.17 (0.10 to 0.31) | 87% (78% to 92%) | 412 (369 to 436) | 8,034 (4 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.2 Incident HPV16/18 infection, at least 1 dose, age 15‐26 years | 1,326 | 305 | 0.23 (0.14 to 0.37) | 81% (71% to 88%) | 1,074 (941 to 1,167) | 23,872 (5 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.3 Incident HPV16/18 infection, 1 or 2 dose, age 15‐26 years*** | 2,568 | 1207 | 0.47 (0.26 to 0.84) | 74% (31% to 90%) | 1,901 (796 to 2,311) | 331 (3 studies) | ⊕⊕⊕⊝ moderate1 |
| Analysis 5.4.1 Persistent HPV16/18 infection (6M), 3 doses, age 15‐26 years | 581 | 35 | 0.06 (0.05 to 0.08) | 94% (91% to 95%) | 546 (534 to 552) | 27,385 (6 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.4.2 Persistent HPV16/18 infection (6M), 3 doses, age 24‐45 years | 350 | 38 | 0.11 (0.06 to 0.20) | 89% (80% to 94%) | 311 (280 to 329) | 6728 (2 studies) | ⊕⊕⊕⊝ |
| Analysis 5.5.1 Persistent HPV16/18 infection (6M), at least 1 dose, age 15‐26 years | 657 | 66 | 0.10 (0.08 to 0.13) | 90% (87% to 92%) | 591 (572 to 605) | 22,803 (4 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.5.2 Persistent HPV16/18 infection (6M), at least 1 dose, age 24‐45 years | 441 | 75 | 0.17 (0.10 to 0.29) | 83% (71% to 90%) | 366 (313 to 397) | 7520 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.6.1 Persistent HPV16/18 infection (6M), 1 or 2 doses, age 15‐26 years*** | 996 | 119 | 0.12 (0.03 to 0.42) | 88% (58% to 97%) | 876 (577 to 966) | 437 (2 studies) | ⊕⊕⊝⊝ |
| Analysis 5.6.2 Persistent HPV16/18 infection (6M), 1 or 2 doses, age 24‐45 years*** | 1,221 | 379 | 0.31 (0.18 to 0.54) | 69% (46% to 82%) | 843 (562 to 1002) | 792 (2 studies) | ⊕⊕⊕⊝ moderate1 |
| Analysis 5.7 Persistent HPV6/11/16/18 infection (6M), 3 doses | 518 | 62 | 0.12 (0.06 to 0.21) | 88% (79% to 94%) | 456 (409 to 487) | 4008 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.8 Persistent HPV6/11/16/18 infection (6M), at least 1 dose | 907 | 118 | 0.13 (0.05 to 0.37) | 87% (63% to 95%) | 789 (571 to 862) | 4129 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.9 Persistent HPV16/18 infection (12M), 3 doses | 297 | 27 | 0.09 (0.06 to 0.13) | 91% (87% to 94%) | 270 (258 to 279) | 22,267 (4 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.10 Persistent HPV16/18 infection (12M), at least 1 dose | 365 | 58 | 0.16 (0.01 to 0.13) | 84% (87% to 99%) | 306 (292 to 361) | 29,464 (5 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.11 Persistent HPV16/18 infection (12M), 1 or 2 doses*** | 205 | 27 | 0.13 (0.06 to 0.33) | 87% (67% to 94%) | 178 (137 to 193) | 3912 (3 studies) | ⊕⊕⊕⊝ moderate1 |
| 6. HPV16/18 infection regardless of HPV DNA status at baseline** | |||||||
| Analysis 6.1 Incident HPV16/18 infection, at least 1 dose, age 15‐26 years | 807 | 194 | 0.24 (0.17 to 0.33) | 76% (67% to 83%) | 613 (541 to 670) | 4210 (1 study) | ⊕⊕⊕⊝ |
| Analysis 6.2.1 Persistent HPV16/18 infection (6M), at least 1 dose, age 15‐26 years | 1,359 | 598 | 0.44 (0.38 to 0.51) | 56% (49% to 62%) | 761 (666 to 842) | 25,199 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 6.2.2 Persistent HPV16/18 infection (6M), at least 1 dose, age 24‐45 years | 642 | 366 | 0.57 (0.47 to 0.69) | 43% (31% to 53%) | 276 (199 to 341) | 8648 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 6.3 Persistent HPV6/11/16/18 infection (6M), at least 1 dose, age 24‐45 years | 1,136 | 591 | 0.52 (0.42 to 0.65) | 48% (35% to 58%) | 545 (398 to 659) | 3713 (1 study) | ⊕⊕⊕⊝ |
| Analysis 6.4 Persistent HPV16/18 infection (12M), at least 1 dose, age 15‐26 years | 861 | 396 | 0.46 (0.40 to 0.54) | 54% (46% to 60%) | 465 (396 to 516) | 24,785 (2 studies) | ⊕⊕⊕⊕ high |
| CI: Confidence interval; RR: Risk Ratio; | |||||||
| GRADE Working Group grades of evidence Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | |||||||
| 1In case of study flaws as assessed by the Cochrane Collaboration's tool for assessing risk of bias in randomised trials (Higgins 2011b), not observed but calculated outcome; 2 Substantial heterogeneity defined as I2 >30%, when multiple studies were available for the considered outcome; 3When only one study was retrieved for the outcome; 4Imprecision, when the width of the 95% confidence interval around RR >0.60. 0* When zero events occurred in the vaccine group a continuity correction was applied to compute the RR and its confidence interval. Nevertheless, in this case the absolute risks in the vaccine arms in Table 2 were computed considering an exact binomal distribution. ** Relative and absolute effects in women regardless of HPV DNA status at baseline (headings 3 and 6) must be interpreted with care since influenced by the prevalence of HPV infection at enrolment in the respective trials. *** Post hoc analysis for women who received <3 doses. $ For the precancer endpoints (CIN2/3 and AIS),a higher risk in the placebo arms was observed if <3 doses were received compared to those who received 3 doses Therefore the quality of evidence was downgraded to low or very low. | |||||||
| Outcome | Initial HPV status at enrolment | |
| hrHPV negative | Regardless of HPV status | |
| Lesions associated with HPV16/18 | NNV (95% CI) | NNV (95% CI) |
| CIN2+ | 62 (61 to 64) | 54 (46 to 68) |
| CIN3+ | 204 (149 to 333) | 135 (110 to 263) |
| AIS+ | 1111 (714 to 5000) | 1111 (625 to 3333) |
| Lesions irrespective of HPV types | NNV (95% CI) | NNV (95% CI) |
| CIN2+ | 60 (50 to 76) | 68 (52 to 97) |
| CIN3+ | 141 (106 to 208) | 133 (94 to 227) |
| AIS+ | 1000 (556 to 10,000) | 833 (526 to 2000) |
| AIS: adenocarcinoma in situ, CIN: cervical intraepithelial neoplasia, CIN2+: CIN of degree II or worse, CIN3+: CIN of degree 3 or worse, hrHPV: high‐risk human papillomavirus types, NNV: number needed to vaccinate. | ||
| Outcomes | Absolute risk/ per 10,000 | Relative effect | No of Participants | Quality of the evidence | |
| placebo | vaccinated | ||||
| Analysis 7.1Overall local/injection site adverse events | 6847 | 8080 | 1.18 (1.16 to 1.20) | 18,113 | ⊕⊕⊕⊝ |
| Analysis 7.2Pain at injection site | 6505 | 8782 | 1.35 (1.23 to 1.49) | 25,691 | ⊕⊕⊕⊝ |
| Analysis 7.3Swelling at injection site | 1582 | 2737 | 1.73 (1.32 to 2.27) | 22,106 | ⊕⊕⊕⊝ |
| Analysis 7.4Redness at injection site | 1938 | 3333 | 1.72 (1.50 to 1.97) | 19,996 | ⊕⊕⊕⊝ |
| Analysis 7.5Overall systematic event and general symptoms | 6102 | 6224 | 1.02 (0.98 to 1.07) | 18,191 | ⊕⊕⊕⊝ |
| Analysis 7.6Serious adverse events | 605 | 611 | 1.01 (0.95 to 1.07) | 6978 | ⊕⊕⊕⊕ |
| Analysis 7.7Deaths | 11 | 13 | 1.25 (0.81 to 1.93) | 71,452 (23 studies) | ⊕⊕⊝⊝ |
| Analysis 8.1Normal infant | 7171 | 7171 | 1.00 (0.97 to 1.02) | 8782 | ⊕⊕⊕⊕ |
| Analysis 8.2Spontaneous abortion/miscarriage | 1618 | 1424 | 0.88 (0.68 to 1.14) | 8618 | ⊕⊕⊕⊕ |
| Analysis 8.3Elective termination/induced abortion | 931 | 838 | 0.90 (0.80 to 1.02) | 10.909 | ⊕⊕⊕⊕ |
| Analysis 8.4Stillbirth | 70 | 78 | 1.12 (0.68 to 1.83) | 8754 | ⊕⊕⊕⊝4 |
| Analysis 8.5Abnormal infant | 205 | 250 | 1.22 (0.88 to 1.69) | 9252 | ⊕⊕⊕⊝4 |
| CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | |||||
| 1In case of study flaws as assessed by Cochrane's tool for assessing risk of bias in randomised trials (Higgins 2011b), not observed but calculated outcome 2 Substantial heterogeneity defined as I2 > 30%, when multiple studies were available for the considered outcome 3When only one study was retrieved for the outcome 4Imprecision, when the width of the 95% confidence interval around RR > 0.60 † inter‐age group heterogeneity, absence of pattern in causes of deaths | |||||
| ID | Group | Death causes |
| 1 | C | Pulmonary thromboembolism with background of acute lymphoblastic leukaemia |
| 2 | V | Breast cancer |
| 3 | V | Pulmonary tuberculosis |
| 4 | V | Thyrotoxicosis |
| 5 | V | Cerebral haemorrhage subsequent to hypertension |
| 6 | V | Pericarditis on a background of lupus erythematosus |
| 7 | V | Nasopharyngeal cancer with metastases to brain |
| 8 | V | Pulmonary embolism after intervention for uterine myoma |
| RR of deaths in vaccine vs placebo arm (7 over 1,890 vs 1 over 1888): RR = 6.99 (95% CI 0.86 to 56.78), 2‐sided pexact=0.070. The age at death varied between 29 and 45 years, seven of the deaths occurred in the Philippines and one in Columbia. All participants received three doses of HPV vaccine or placebo except one who received only two doses of vaccine. The time interval between last dose and date at death ranged between 6 and 37 months. | ||
| Group:V = vaccinated against HPV, C = control group. Source: end‐of‐study analysis after a median follow‐up of four years (Castellsagué 2011) and personal communication with Alfred Saah (MSD, 6/05/2016). | ||
| Patient | Cause of death | Group | Age | Country | Source |
| 1 | Breast cancer metastatic | V | 47 | Canada | 1 |
| 2 | Suicide | V | 47 | Mexico | 1 |
| 3 | Lower respiratory tract infection and sepsis* | C | 55 | Mexico | 1 |
| 4 | Cervix cancer metastatic** | V | 45 | Mexico | 1 |
| 5 | Interstitial lung disease | V | 41 | Mexico | 1 |
| 6 | Breast cancer | *** | 32 | Mexico | 1*** |
| 7 | Suicide | V | 41 | Mexico | 1 |
| 8 | Cardiac valve disease and liver disorder* | C | 38 | Mexico | 1 |
| 9 | Drug hypersensitivity and acute renal failure* | V | 46 | Peru | 1 |
| 10 | Cardiorespiratory arrest | C | 44 | Phillipines | 1 |
| 11 | Acute myocardial infarction | V | 31 | Phillipines | 1 |
| 12 | Multiple myeloma and pulmonary embolism* | V | 50 | Phillipines | 1 |
| 13 | Homicide | V | 32 | Phillipines | 1 |
| 14 | Bronchopneumonia | V | 40 | Singapore | 1 |
| 15 | Lung neoplasm malignant | V | 41 | Thailand | 1 |
| 16 | Suicide | V | 28 | USA | 1 |
| 17 | Glioblastoma multiforme | V | 45 | USA | 1 |
| 18 | Anaplastic astrocytoma | C | 43 | **** | 2 |
| 19 | Nasopharyngeal cancer | C | 41 | **** | 2 |
| Remarks | |||||
| * | Multiple death causes | ||||
| ** | This woman had normal cytology but was HPV‐18 DNA‐positive at study entry (May 2006). At the next scheduled cytology testing at Month 12 (April 2007), the cytology finding was atypical squamous cells cannot exclude high‐grade squamous intraepithelial lesion. She was diagnosed with metastatic cervical cancer in May 2007 (approximately 7 months after receiving the third dose of vaccine or control) and died in July 2008 | ||||
| *** | One case of death due to breast cancer reported in the 48 month report (Skinner 2014) had to be excluded from the analysis (Wheeler 2016). | ||||
| **** | Two additional cases of death occurring in the control arm were reported in the 84‐month report (Wheeler 2016). The country for these two cases was not reported. | ||||
| Source: 1) interim analysis after 48 months of follow‐up (Skinner 2014); 2) report at 84 months of follow‐up (Wheeler 2016) The 84‐month follow‐up report revealed 13 deaths in the HPV arm (N = 2877) versus 5 (N = 2870), with death causes allocated to the trial arms (vaccine versus placebo arm) the RR was 2.59 (95% CI 0.93 to 7.27), 2‐sided pexact=0.0957. No pattern was noticed which could indicate a causal role attributed to HPV vaccination. | |||||
| Trial | Target age group | Age category | Reported age sub‐groups |
| 16‐23 | younger | none | |
| 15‐25 | younger | none | |
| 16‐23 | younger | none | |
| 20‐25 | younger | none | |
| 15‐25 | younger | 15‐17, 18‐20, 21‐25 | |
| 18‐25 | younger | 18‐19, 20‐21, 22‐23, 24‐25 | |
| 26+ | older | 26‐35, 36‐45, 46+ | |
| 16‐24 | younger | none | |
| 15‐26 | younger | none | |
| 25‐45 | older | none |
| Outcome | Age | Event/N Vaccine | Event/N Placebo | Relative risk (95% CI) | Vaccine efficacy % (95% CI) | P value for linear effect of age |
| In women with hrHPV DNA negative status at baseline | ||||||
| CIN2+ associated with HPV16/18 | 15‐17 | 1/1997 | 53/2022 | 0.02 (0.00 to 0.14) | 98% (86 to 100%) | 0.995 |
| 18‐20 | 0/1096 | 27/1144 | 0.02 (0.00 to 0.32) | 98% (68 to 100%) | ||
| 21‐25 | 0/2363 | 17/2281 | 0.03 (0.00 to 0.47) | 97% (53 to 100%) | ||
| CIN2+ irrespective of HPV types | 15‐17 | 34/1997 | 101/2022 | 0.34 (0.23 to 0.50) | 66% (50 to 77%) | 0.355 |
| 18‐20 | 10/1096 | 38/1144 | 0.27 (0.14 to 0.55) | 73% (45 to 86%) | ||
| 21‐25 | 17/2363 | 33/2281 | 0.50 (0.28 to 0.89) | 50% (11 to 72%) | ||
| CIN3+ associated with HPV16/18 | 15‐17 | 0/1997 | 14/2022 | 0.04 (0.00 to 0.61) | 96% (39 to100%) | 1.000 |
| 18‐20 | 0/1096 | 8/1144 | 0.07 (0.00 to 1.13) | 93% (‐13 to 100%) | ||
| 21‐25 | 0/2363 | 5/2281 | 0.10 (0.00 to 1.74) | 90%(‐74 to 100%) | ||
| CIN3+ irrespective of HPV types | 15‐17 | 2/1997 | 24/2022 | 0.08 (0.02 to 0.36) | 92% (64 to 98%) | 0.488 |
| 18‐20 | 1/1096 | 11/1144 | 0.09 (0.01 to 0.73) | 91% (27 to 99%) | ||
| 21‐25 | 0/2363 | 9/2281 | 0.05 (0.00 to 0.92) | 95% (8 to 100%) | ||
| Persistent HPV16/18 infection (6M) | 15‐17 | 14/1989 | 303/2020 | 0.05 (0.03 to 0.08) | 95% (92 to 97%) | 0..042 |
| 18‐20 | 9/1090 | 110/1125 | 0.08 (0.04 to 0.17) | 92%(83 to 96%) | ||
| 21‐25 | 12/2338 | 108/2249 | 0.11 (0.06 to 0.19) | 89% (81 to 94%) | ||
| Regardless of women’s baseline HPV DNA status | ||||||
| CIN2+ associated with HPV16/18 | 15‐17 | 21/2882 | 100/2892 | 0.21 (0.13 to 0.24) | 79% (66 to 87%) | 0.000 |
| 18‐20 | 23/1871 | 66/1908 | 0.36 (0.22 to 0.57) | 64% (43 to 78%) | ||
| 21‐25 | 46/3929 | 62/3898 | 0.74 (0.50 to 1.08) | 26% (‐8 to 50%) | ||
| CIN2+ irrespective of HPV types | 15‐17 | 112/2882 | 200/2892 | 0.56 (0.45 to 0.70) | 44% (30 to 55%) | 0.006 |
| 18‐20 | 62/1871 | 105/1908 | 0.60 (0.44 to 0.82) | 40% (18 to 56%) | ||
| 21‐25 | 113/3929 | 123/3898 | 0.91 (0.09 to 1.17) | 9% (‐17 to 29%) | ||
| CIN3+ associated with HPV16/18 | 15‐17 | 7/2882 | 36/2892 | 0.20 (0.09 to 0.44) | 80% (56 to 91%) | 0.000 |
| 18‐20 | 13/1871 | 30/1908 | 0.44 (0.23 to 0.84) | 56% (16 to 77%) | ||
| 21‐25 | 31/3929 | 28/3898 | 1.10 (0.66 to 1.83) | ‐10% (‐83 to 34%) | ||
| CIN3+ irrespective of HPV types | 15‐17 | 21/2882 | 61/2892 | 0.35 (0.21 to 0.57) | 65% (43 to 79%) | 0.008 |
| 18‐20 | 22/1871 | 44/1908 | 0.51 (0.31 to 0.85) | 49% (15 to 69%) | ||
| 21‐25 | 43/3929 | 53/3898 | 0.80 (0.54 to 1.20) | 20% (‐20 to 46%) | ||
| Persistent HPV16/18 infection (6M) | 15‐17 | 167/2916 | 588/2920 | 0.28 (0.24 to 0.34) | 72% (66 to 76%) | 0.000 |
| 18‐20 | 143/1925 | 283/1961 | 0.51 (0.43 to 0.62) | 49% (38 to 57%) | ||
| 21‐25 | 194/4009 | 356/3979 | 0.54 (0.46 to 0.64) | 46% (36 to 54% ) | ||
| Source: Lehtinen 2012. CIN: cervical intraepithelial neoplasia, CIN2+: CIN of degree II or worse, CIN3+: CIN of degree 3 or worse, HPV: human papillomavirus types.. | ||||||
| Outcome | Age | Vaccine | Placebo | Relative risk (95% CI) | Vaccine efficacy (95% CI) | P value for linear effect of age |
| In women with HPV16/18 DNA negative status at baseline cohort | ||||||
| Persistent HPV16/18 infection (6M) | 18‐19 | 1/825 | 51/870 | 0.02 (0.00 to 0.10) | 98% (90% to 100%) | 0.145 |
| 20‐21 | 3/659 | 36/649 | 0.08 (0.02 to 0.24) | 92% (76% to 98%) | ||
| 22‐23 | 2/588 | 36/625 | 0.06 (0.00 to 0.20) | 94% (80% to 100%) | ||
| 24‐25 | 3/563 | 20/533 | 0.14 (0.03 to 0.44) | 86% (56% to 97%) | ||
| Regardless if women’s baseline HPV DNA status | ||||||
| Persistent HPV16/18 infection (6M) | 18‐19 | 47/1193 | 165/1,244 | 0.30 (0.21 to 0.41) | 70% (59% to 79%) | 0.000 |
| 20‐21 | 64/946 | 134/905 | 0.46 (0.34 to 0.61) | 54% (39% to 66%) | ||
| 22‐23 | 59/818 | 112/848 | 0.55 (0.40 to 0.75) | 45% (25% to 60%) | ||
| 24‐25 | 61/770 | 75/742 | 0.78 (0.56 to 1.99) | 22 %(‐9.9 to 44%) | ||
| Source: Herrero 2011. | ||||||
| Outcome | Age | Event/NVaccine | Event/NPlacebo | Relative risk (95% CI) | Vaccine efficacy (95% CI) | P value for linear effect of age |
| In women with HPV16/18 DNA negative status at baseline cohort | ||||||
| Persistent HPV16/18 infection (6M) | 26‐35 | 3/834 | 22/800 | 0.13 (0.04 to 0.44) | 87% (56% to 96%) | 0.532 |
| 36‐45 | 3/816 | 12/809 | 0.25 (0.07 to 0.88) | 75%(12% to 93%) | ||
| 46+ | 0/219 | 0/213 | N.A. | N.A. | ||
| Regardless if women’s baseline HPV DNA status | ||||||
| Persistent HPV16/18 infection (6M) | 26‐35 | 48/1221 | 78/1242 | 0.63 (0.44 to 0.89) | 37% (11% to 56%) | 0.177 |
| 36‐45 | 19/1244 | 43/1228 | 0.44 (0.26 to 0.74) | 56% (26% to 74%) | ||
| 46+ | 4/300 | 11/306 | 0.37 (0.12 to 1.15) | 63% (‐15% to 88%) | ||
| Source: Skinner 2014. | ||||||
| Initial HPV DNA/ status | Serology status | Vaccine | Placebo | Relative Risk (95% CI) | Relative Risk ratio |
| DNA(‐) | Sero‐ | 0/2,241 | 32/2258 | 0.00 (0.02 to 0.26) | 15.93 |
| Sero+ | 0/377 | 2/379 | 0.25 (0.01 to 5.20) | ||
| DNA(+) | Sero‐ | 27/232 | 31/213 | 0.80 (0.49 to 1.29) | 1.50 |
| Sero+ | 41/156 | 30/137 | 1.20 (0.80 to 1.81) | ||
| DNA(‐) | Sero‐ | 0/5,305 | 28/5260 | 0.02(0.00 to 0.14) | 7.41 |
| Sero+ | 0/498 | 4/524 | 0.13 (0.01 to 2.43) | ||
| DNA(+) | Sero‐ | 33/423 | 35/402 | 0.90 (0.57 to 1.41) | 1.12 |
| Sero+ | 47/298 | 52/332 | 1.01 (0.70 to 1.45 | ||
| DNA(‐) | Sero‐ | 5/8709 | 92/8112 | 0.05 (0.02 to 0.12) | 6.16 |
| Sero+ | 3/1710 | 10/1777 | 0.31 (0.09 to 1.13) | ||
| DNA(+) | Sero‐ | 20/309 | 29/293 | 0.65 (0.38 to 1.13) | 1.70 |
| Sero+ | 53/333 | 44/307 | 1.11 (0.77 to 1.61) | ||
| Pooled results for CIN2+ associated with HPV16/18 | |||||
| DNA(‐) | Sero‐ | 5/14,014 | 120/13,372 | 0.03 (0.02 to 0.09) | 5.85 (0.53 to 65.10) |
| Sero+ | 3/2205 | 14/2301 | 0.19 (0.09 t0 o.77) | ||
| DNA(+) | Sero‐ | 53/679 | 64/695 | 0.79 (0.60 to 1.05 | 1.37 (0.97 to 1.93) |
| Sero+ | 100/531 | 96/639 | 1.10 (0.88 to 1.36) | ||
| *RR against HPV 6/11/16/18 related cervical lesions ** RR against HPV16/18 related CIN2+ *** Pooled only for FUTURE II and PATRIACIA, since, in the FUTURE I trial, the endpoints were cervical lesions and not CIN2+ associated with HPV16/18 | |||||
| Outcome | P value | ||||||
| V1 | V2 | V3 | V4 | V5 | V6 | V7 | |
| Persistent HPV16/18 infection (6M), in women being baseline HPV16/18 negative 3 doses | 0.70 | 0.60 | np | np | 0.90 | np | 0.42 |
| Persistent HPV16/18 infection (6M), in women being baseline HPV16/18 negative at least 1 dose | 0.56 | 0.56 | np | np | np | np | np |
| Persistent HPV16/18 infection (12M), in women being baseline HPV16/18 negative 3 doses | 0.94 | 0.94 | np | np | np | np | 0.73 |
| Persistent HPV16/18 infection (12M), in women being baseline HPV16/18 negative at least 1 dose | 0.67 | 0.67 | np | np | np | np | np |
| Influence of study quality (items V1‐V6) and independence of the research team towards the vaccine manufacturer (V7) on protection against persistent HPV16/18 infection assessed by meta‐regression. The P values correspond with the statistical significance of the incorporation of each item in the meta‐regression. V1: Random sequence generation; V2: Allocation concealment; V3: Blinding participants and personnel; V4: Blinding of outcome; V5: Incomplete outcomes; V6: Selective reporting; V7: Involvement of manufacturer, np: meta‐regression not possible because of collinearity. | |||||||
| Outcome | No. of doses | Vaccine arm | Placebo arm | Relative Risk (95%CI) | P value for linear dose‐effect relation |
| 12‐month persistent HPV16/18 infection in women being HPV16/18 negative at baseline | 3 | 84/11,104 | 627/11,203 | 0.135 (0.108 to 0.169) | 0.303 |
| 2 | 3/611 | 26/574 | 0.108 (0.033 to 0.356) | ||
| 1 | 1/292 | 17/249 | 0.050 (0.007 to 0.374) | ||
| 6‐month persistent HPV16/18 infection in women being HPV16/18 negative at baseline | 3 | 114/11,104 | 1000/11,209 | 0.115 (0.095 to 0.139) | 0.269 |
| 2 | 4/611 | 35/574 | 0.107 (0.038 to 0.300) | ||
| 1 | 1/292 | 24/250 | 0.036 (0.005 to 0.261) | ||
| Incident HPV16/18 infection in women being HPV16/18 negative at baseline | 3 | 529/11,110 | 2172/11,217 | 0.246 (0.224 to 0.269) | 0.337 |
| 2 | 22/611 | 82/574 | 0.252 (0.160 to 0.398) | ||
| 1 | 8/292 | 45/251 | 0.153 (0.073 to 0.318) | ||
| 12‐month persistent HPV16/18 infection in women being hrHPV negative at baseline | 3 | 27/6634 | 351/6656 | 0.077 (0.052 to 0.114) | 0.996 |
| 2 | 2/273 | 12/276 | 0.168 (0.038 to 0.746) | ||
| 1 | 0/138 | 5/99 | 0.071 (0.004 to 1.289) | ||
| 6‐month persistent HPV16/18 infection in women being hrHPV negative at baseline | 3 | 38/6634 | 567/6660 | 0.067 (0.049 to 0.093) | 0.809 |
| 2 | 2/273 | 16/276 | 0.126 (0.029 to 0.544) | ||
| 1 | 0/138 | 8/100 | 0.045 (0.003 to 0.774) | ||
| Incident HPV16/18 infection in women being hrHPV negative at baseline | 3 | 38/6634 | 567/6660 | 0.067 (0.049 to 0.093) | 0.809 |
| 2 | 2/273 | 16/276 | 0.126 (0.029 to 0.544) | ||
| 1 | 0/138 | 8/100 | 0.045 (0.003 to 0.774) | ||
| Source: PATRICIA & CVT (ph3,2v) (Kreimer 2015). | |||||
| Outcome | No. of doses | n events | N vaccinated | % (95%CI) | P* for difference with 3 doses |
| Cumulative incidence HPV16/18 infections | 3 | 88 | 2023 | 4.3 (3.5 to 5.3) | ‐ |
| 2 (at months 0 & 6) | 3 | 78 | 3.8 (1.0 to 10.1) | 1.00 | |
| 2 (at months 0 & 1) | 7 | 192 | 3.6 (1.6 to 7.1) | 0.85 | |
| 1 | 2 | 133 | 1.5 (0.3 to 4.9) | 0.17 | |
| Source: Safaeian 2018. * two‐sided exact test for difference between proportions. | |||||
| Outcomes | Age Group (years) | Studies | RR if 3 doses (95% CI) | RR if 1‐2 doses (95% CI) |
| CIN2+ due to HPV16/18 | 15‐26 | 5 (FUTURE II trial (ph3,4v); Japanese trial (ph2,2v); PATRICIA trial (ph3,2v); Phase2 trial (ph2,1v); Chinese trial (ph3,2v)_young) | 0.07 (0.03 to 0.14)* | 0.10 (0.04 to 0.26)* |
| 24‐45 | 0.14 (0.03 to 0.79)* | 0.98 (0.20 to 4.83) | ||
| CIN3+ due to HPV16/18 | 15‐26 | 0.20 (0.04 to 0.91)* | 0.04 (0.01 to 0.74)* | |
| Incident HPV16/18 infection | 15‐26 | 3 (Japanese trial (ph2,2v); Phase2 trial (ph2,1v);Chinese trial (ph3,2v)_young) | 0.20 (0.10 to 0.41)* | 0.47 (0.26 to 0.84)* |
| 6‐month persistent HPV16/18 infection | 15‐26 | 0.05 (0.01 to 0.27)* | 0.12 (0.03 to 0.42)* | |
| 24‐45 | 0.15 (0.09 to 0.27)* | 0.34 (0.19 to 0.61)* | ||
| 12‐month persistent HPV16/18 infection | 15‐26 | 3 (Japanese trial (ph2,2v);CVT (ph3,2v); Chinese trial (ph3,2v)_young) | 0.09 (0.05 to 0.19)* | 0.13 (0.06 to 0.33)* |
| *Vaccine efficacy in women being HPV16/18 DNA negative at enrolment and having received all three or less than three doses (computed from trials where per‐protocol [all doses administered] and intention‐to‐treat analyses [at least one dose administered] are reported). | ||||
| Outcomes | Study | Report (duration of follow‐up) | Vaccine | Placebo | Relative Risk (95%CI) | P value for linear difference of follow‐up time effect |
| CIN2+ associated with HPV16/18 in women being HPV negative at baseline | PATRICIA | Paavonen 2007 14.8 moths | 2/7788 | 21/7838 | 0.096 (0.007 to 0.466) | 0.512 |
| Paavonen 2009 34.9 months | 5/8040 | 91/8080 | 0.054 (0.016 to 0.137) | |||
| Szarewski 2011 39.4 months | 5/8079 | 92/8112 | 0.054 (0.016 to 0.137) | |||
| Lehtinen 2011 43.7 months | 5/7338 | 97/7305 | 0.051 (0.016 to 0.123) | |||
| FUTURE | The FUTURE II study group 2007 36 months | 3/5865 | 87/5836 | 0.039 (0.011 to 0.109) | 0.994 | |
| Munoz 2010* 43 months | 0/4616 | 89/4680 | 0.006 (0.000 to 0.092) | |||
| CIN2+ irrespective of HPV types regardless of women’s initial HPV DNA status | PATRICIA | Paavonen 2009 34.9 months | 224/8667 | 322/8682 | 0.696 (0.579 to 0.8369) | 0.750 |
| Lehtinen 2011 43.7 months | 287/8694 | 428/8708 | 0.669 (0.574 to 0.778) | |||
| FUTURE | The FUTURE II study group 2007 36 months | 281/6087 | 361/6080 | 0.780 (0.668 to 0.905) | 0.665 | |
| Munoz 2010 43 months | 421/8562 | 520/8598 | 0.807 (0.690 to 0.943) | |||
| Assessment of the influence of duration of follow‐up on study outcomes using meta‐regression. p‐values correspond with the statistical significance of incorporating average follow‐up time as a continuous variable. | ||||||
| Number of sex partners | Vaccine | Placebo | Relative Risk (95% CI) | P value of number of sexual partners effect |
| In women being HPV16/18 DNA negative at baseline cohort | ||||
| Virgin | 1/566 | 17/615 | 0.064 (0.003 to 0.352) | 0.7448 |
| 1 partner | 3/904 | 27/915 | 0.112 (0.007 to 0.335) | |
| 2 partners | 1/544 | 17/519 | 0.056 (0.003 to 0.309) | |
| 3+ partners | 3/621 | 28/628 | 0.108 (0.026 to 0.321) | |
| Regardless of women’s baseline HPV DNA status | ||||
| Virgin | 4/733 | 21/819 | 0.202 (0.059 to 0.551) | < 0.0001 |
| 1 partner | 40/1237 | 83/1256 | 0.489 (0.333 to 0.711) | |
| 2 partners | 38/777 | 81/753 | 0.455 (0.307 to 0.665) | |
| 3+ partners | 71/940 | 116/911 | 0.593 (0.440 to 0.796) | |
| The influence of the number of lifetime sexual partners on vaccine efficacy was assessed by Poisson regression. The P value corresponds with the likelihood ratio test comparing a Poisson model with and without inclusion of the sexual history with 3 possible categories. Source: CVT (ph3,2v) (Herrero 2011). | ||||
| Outcomes | Study | Number of participants | Study size | Vaccine | Placebo | Relative Risk (95%CI) | P value |
| CIN2+ associated with HPV16/18 in women being HPV16/18 negative at baseline | Phase2 trial (V1) | 2392 | S | 0/126 | 8/127 | 0.062* (0.004 to 1.071) | 0.598 |
| Phase2 trial (V2) | 1113 | S | 0/219 | 3/212 | 0.161* (0.008 to 3.091) | ||
| Japanese trial (ph2,2v) | 1040 | S | 0/422 | 2/427 | 0.252* (0.012 to 5.241) | ||
| PATRICIA trial (ph3,2v) | 18,644 | L | 5/8040 | 91/8080 | 0.055 (0.022 to 0.136) | ||
| FUTURE II trial (ph3, 4v) | 12,167 | L | 3/5865 | 87/5863 | 0.034 (0.011 to 0.109) | ||
| Chinese trial (ph3,2V) | 6051 | L | 0/2543 | 4/2554 | 0.125 (0.001 to 8.681) | ||
| CIN2+ irrespective of HPV types and regardless of women’s initial HPV DNA status | FUTURE I/II trial (ph3,4v) | 17,622 | L | 421/8562 | 520/8598 | 0.813 (0.718 to 0.921) | 0.703 |
| PATRICIA trial (ph3,2v) | 18,644 | L | 287/8694 | 428/8708 | 0.672 (0.582 to 0.778) | ||
| Phase2 trial (v1) | 2392 | S | 8/148 | 12/142 | 0.640 (0.269 to 1.568) | ||
| Assesment of the influence of the study size on the protection against CIN2+ associated with HPV16/18 according to study size (S = small, < 3000 participants, L = large >= 3000 participants) in women aged 15‐26 years and received at least 1 dose. * P values correspond with the statistical significance of a meta‐regression with vs without study size category. | |||||||
| Analysis | Endpoint | Initial HPV status | Doses | Relative Risk |
| Monovalent vaccine (Rowhani‐Rahbar, 2009): 102 months of follow‐up | ||||
| 3.1 | CIN2+ associated with HPV16 | HPV16‐ | 3 | 0.00 |
| 3.2 | CIN2+ associated with HPV16 | HPV16‐ | >= 1 | 0.00 |
| 3.3 | CIN2+ associated with HPV16 | HPV16‐ | 1‐2 | 0.00 |
| 4.1 | Incident HPV16 infection | HPV16‐ | 3 | 0.05 |
| 4.3 | Incident HPV16 infection | HPV16‐ | >= 1 | 0.11 |
| 4.3 | Incident HPV16 infection | HPV16‐ | 1‐2 | 0.25 |
| 5.1 | CIN2+ associated with HPV16 | regardless of HPV infection | >= 1 | 0.36 |
| 5.3 | CIN2+ irrespective of HPV types | regardless of HPV infection | >= 1 | 0.64 |
| Bivalent vaccine (De Calvaho, 2012): 88 months of follow‐up | ||||
| 2.2 | 6M persistent HPV16/18 infection | hrHPV‐ | 3 | 0.00 |
| 2.4 | 12M persistent HPV16/18 infection | hrHPV‐ | 3 | 0.00 |
| 3.2 | CIN2+ associated with HPV16/18 | HPV16/18‐ | >= 1 | 0.00 |
| Quadrivalent vaccine (Villa, 2006): 60 months of follow‐up | ||||
| 4.8 | Persistent HPV6/11/16/18 infection | HPV16/18‐ | >= 1 | 0.07 |
| Trials | Ref | Endpoint | Relative Risk (95% CI) | P value for difference in VE | |
| Bivalent | Quadrivalent | ||||
| 6‐month persistent HPV31 infection | 0.229 (0.156 to 0.228) | 0.538 (0.336 to 0.847) | 0.003 | ||
| 6‐month persistent HPV45 infection | 0.210 (0.106 to 0.387) | 0.922 (0.507 to 1.670) | 0.0003 | ||
| CIN2+ associated with HPV33 | 0.177 (0.053 to 0.466) | 0.760 (0.328 to 1.712) | 0.02 | ||
| CIN2+ associated with HPV45 | 0.000 (0.000 to 0.583) | 0.481 (0.174 to 1.177) | 0.04 | ||
| CIN2+ associated with other hrHPV | 0.401 (0.192 to 0.793) | ||||
| 6‐month persistent HPV31 infection | 0.209 (0.041 to 0.724) | ||||
| 6‐month persistent HPV45 infection | 0.221 (0.044 to 0.914) | ||||
| Adverse effect | Relative risk | Relative risk ratio | p value | |
| Quadrivalent vs placebo | Bivalent/Quadrivalent | |||
| 1 | Overall adverse effects at injection site | 1.19 (0.89 to 1.59) | 1.69 (0.96 to 2.96) | 0.061 |
| 2 | Pain at injection site | 1.20 (0.78 to 1.85) | 1.19 (0.67 to 2.12) | 0.501 |
| 3 | Swelling at injection site | 2.72 (0.77to 9.61) | 0.62 (0.16 to 2.41) | 0.427 |
| 4 | Redness at injection site | 1.46 (1.23 to 1.74) | 1.08 (0.88 to 1.32) | 0.307 |
| 5 | Overall systemic events | 0.99 (0.91 to 1.07) | 1.06 (0.95 to 1.19) | 0.210 |
| 6 | Serious adverse events | 0.94 (0.70 to 1.26) | 1.08 (0.80 to 1.45) | 0.583 |
| 7 | Deaths | 1.18 (0.25 to 5.62) | 0.84 (0.14 to 4.91) | 0.775 |
| Relative risks of the quadrivalent vaccine versus placebo and the relative risk ratios were computed by meta‐regression including vaccine, age group and type of product injected in the control group (aluminium adjuvants alone or other vaccine such as Hepatitis A vaccine) as covariate. The relative risk ratio reflects how much more an adverse effect is observed after vaccination with the bivalent versus the quadrivalent vaccine. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CIN2+ associated with HPV16/18, at least 1 dose Show forest plot | 3 | 23676 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.05] |
| 2 CIN2+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.09] |
| 3 CIN3+ associated with HPV16/18, at least 1 dose Show forest plot | 2 | 20214 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.10] |
| 4 CIN3+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.18] |
| 5 AIS associated with HPV16/18, at least 1 dose Show forest plot | 2 | 20214 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.01, 0.82] |
| 6 AIS associated with HPV6/11/16/18, at least 1 dose Show forest plot | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.01, 2.80] |
| 7 Any CIN2+ irrespective of HPV types, at least 1 dose Show forest plot | 5 | 25180 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.25, 0.55] |
| 7.1 Bivalent vaccine | 4 | 15884 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.25, 0.43] |
| 7.2 Quadrivalent vaccine | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.44, 0.76] |
| 8 Any CIN3+ irrespective of HPV types, at least 1 dose Show forest plot | 3 | 20719 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.04, 1.10] |
| 8.1 Bivalent vaccine | 2 | 11423 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.03, 0.23] |
| 8.2 Quadrivalent vaccine | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.36, 0.82] |
| 9 Any AIS irrespective of HPV types, at least 1 dose Show forest plot | 2 | 20214 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.01, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CIN2+ associated with HPV16/(18), 3 doses Show forest plot | 8 | 43376 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.04, 0.16] |
| 1.1 Age group 15‐26 years | 6 | 36579 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.03, 0.15] |
| 1.2 Age group 24‐45 years | 2 | 6797 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.04, 0.74] |
| 2 CIN2+ associated with HPV16/(18), at least 1 dose Show forest plot | 8 | 42030 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.05, 0.20] |
| 2.1 Age group 15‐26 years | 6 | 34478 | Risk Ratio (IV, Random, 95% CI) | 0.05 [0.03, 0.10] |
| 2.2 Age group 24‐45 years | 2 | 7552 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.11, 0.81] |
| 3 CIN2+ associated with HPV16/(18), 1 or 2 doses (post hoc analysis) Show forest plot | 7 | 3713 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.07, 0.51] |
| 3.1 women age 15‐26 years | 5 | 2958 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.04, 0.26] |
| 3.2 women age 24‐45 years | 2 | 755 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.14, 2.67] |
| 4 CIN2+ associated with HPV6/11/16/18, 3 doses Show forest plot | 2 | 7664 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.01, 0.61] |
| 4.1 Age group 15‐26 years | 1 | 4499 | Risk Ratio (IV, Random, 95% CI) | 0.02 [0.00, 0.25] |
| 4.2 Age group 24‐45 years | 1 | 3165 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.02, 1.39] |
| 5 CIN2+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 2 | 8980 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.00, 2.41] |
| 5.1 Age group 15‐26 years | 1 | 5351 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.19] |
| 5.2 Age group 24‐45 years | 1 | 3629 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.10, 1.41] |
| 6 CIN2+ associated with HPV6/11/16/18, 1 or 2 doses (post hoc analysis) Show forest plot | 2 | 1316 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.01, 5.00] |
| 6.1 Age group 15‐26 years | 1 | 852 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.00, 0.74] |
| 6.2 Age group 24‐45 years | 1 | 464 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.14, 6.80] |
| 7 CIN3+ associated with HPV16/18 or HPV6/11/16/18, 3 doses Show forest plot | 3 | 29720 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.02, 0.29] |
| 8 CIN3+ associated with HPV 16/18 or HPV6/11/16/18, at least 1 dose Show forest plot | 3 | 33199 | Risk Ratio (IV, Random, 95% CI) | 0.05 [0.02, 0.14] |
| 9 CIN3+ associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses (post hoc analysis) Show forest plot | 3 | 3479 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.01, 0.24] |
| 10 AIS associated with HPV16/18 or HPV6/11/16/18, 3 doses Show forest plot | 3 | 29707 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.02, 0.70] |
| 11 AIS associated with HPV16/18 or 6/11/16/18, at least 1 dose Show forest plot | 2 | 17079 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.01, 0.72] |
| 12 AIS associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses (post hoc analysis) Show forest plot | 2 | 2015 | Risk Ratio (IV, Random, 95% CI) | 0.15 [0.01, 2.97] |
| 13 Any CIN2+ irrespective of HPV types, 3 doses Show forest plot | 3 | 7320 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.25, 0.64] |
| 14 Any CIN2+ irrespective of HPV types, at least 1 dose Show forest plot | 3 | 19143 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.32, 0.52] |
| 15 Any CIN2+ irrespective of HPV types, 1 or 2 doses (post hoc analysis) Show forest plot | 1 | 34 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.15, 3.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CIN2+ associated with HPV16/18, at least 1 dose Show forest plot | 5 | 44052 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.41, 0.67] |
| 1.1 Age group 15‐26 years | 3 | 34852 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.37, 0.57] |
| 1.2 Age group 24‐45 years | 2 | 9200 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.52, 1.05] |
| 2 CIN2+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 2 | 20883 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.38, 0.86] |
| 2.1 Age group 15‐26 years | 1 | 17160 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.42, 0.59] |
| 2.2 Age group 24‐45 years | 1 | 3723 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.44, 1.37] |
| 3 CIN3+ associated with HPV16/18, at least 1 dose Show forest plot | 2 | 34562 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.45, 0.67] |
| 4 CIN3+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 1 | 17160 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.43, 0.68] |
| 5 AIS associated with HPV16/18, at least 1 dose Show forest plot | 2 | 34562 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.17, 0.78] |
| 6 AIS associated with HPV6/11/16/18, at least 1 dose Show forest plot | 2 | 20830 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.16, 0.98] |
| 7 Any CIN2+ irrespective of HPV types, at least 1 dose Show forest plot | 6 | 45066 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.65, 0.97] |
| 7.1 Age group 15‐26 years | 4 | 35779 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.58, 0.85] |
| 7.2 Age group 24‐45 years | 2 | 9287 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.83, 1.30] |
| 8 Any CIN3+ HPV type, at least 1 dose Show forest plot | 3 | 35489 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.49, 0.93] |
| 8.1 Bivalent vaccine | 2 | 18329 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.43, 0.71] |
| 8.2 Quadrivalent vaccine | 1 | 17160 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.69, 0.96] |
| 9 Any AIS irrespective of HPV types, at least 1 dose Show forest plot | 2 | 34562 | Risk Ratio (IV, Random, 95% CI) | 0.32 [0.15, 0.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident HPV16/18 infection, 3 doses Show forest plot | 1 | 368 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.02, 0.20] |
| 2 Persistent HPV16/18 infection (6M), 3 doses Show forest plot | 1 | 368 | Risk Ratio (IV, Random, 95% CI) | 0.02 [0.00, 0.35] |
| 3 Persistent HPV16/18 infection (6M), at least 1 dose Show forest plot | 1 | 10826 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.05, 0.09] |
| 4 Persistent HPV16/18 infection(12M), 3 doses Show forest plot | 1 | 368 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.00, 0.73] |
| 5 Persistent HPV16/18 infection (12M), at least 1 dose Show forest plot | 2 | 14153 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.05, 0.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident HPV16/18 infection, 3 doses Show forest plot | 4 | 8034 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.10, 0.31] |
| 2 Incident HPV16/18 infection, at least 1 dose Show forest plot | 5 | 23872 | Risk Ratio (IV, Random, 95% CI) | 0.23 [0.14, 0.37] |
| 3 Incident HPV16/18 infection, 1 or 2 doses (post hoc analysis) Show forest plot | 3 | 331 | Risk Ratio (IV, Random, 95% CI) | 0.47 [0.26, 0.84] |
| 4 Persistent HPV16/18 infection (6M), 3 doses Show forest plot | 8 | 34113 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.06, 0.09] |
| 4.1 Age group 15‐26 years | 6 | 27385 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.05, 0.08] |
| 4.2 Age group 24‐45 years | 2 | 6728 | Risk Ratio (IV, Random, 95% CI) | 0.11 [0.06, 0.20] |
| 5 Persistent HPV16/18 infection (6M), at least 1 dose Show forest plot | 6 | 30323 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.08, 0.17] |
| 5.1 Age group 15‐26 years | 4 | 22803 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.08, 0.12] |
| 5.2 Age group 24‐45 years | 2 | 7520 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.10, 0.29] |
| 6 Persistent HPV16/18 infection (6M), 1 or 2 doses (post hoc analysis) Show forest plot | 4 | 1229 | Risk Ratio (IV, Random, 95% CI) | 0.26 [0.16, 0.44] |
| 6.1 Age group 15‐26 years | 2 | 437 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.03, 0.42] |
| 6.2 Age group 24‐45 years | 2 | 792 | Risk Ratio (IV, Random, 95% CI) | 0.31 [0.18, 0.54] |
| 7 Persistent HPV6/11/16/18 infection (6M), 3 doses Show forest plot | 2 | 4008 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.06, 0.21] |
| 8 Persistent HPV6/11/16/18 infection (6M), at least 1 dose Show forest plot | 2 | 4129 | Risk Ratio (IV, Random, 95% CI) | 0.13 [0.05, 0.37] |
| 9 Persistent HPV16/18 infection (12M), 3 doses Show forest plot | 4 | 22267 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.06, 0.13] |
| 10 Persistent HPV16/18 infection (12M), at least 1 dose Show forest plot | 5 | 29464 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.12, 0.20] |
| 11 Persistent HPV16/18 infection (12M), 1 or 2 doses (post hoc analysis) Show forest plot | 3 | 3912 | Risk Ratio (IV, Random, 95% CI) | 0.13 [0.06, 0.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident HPV16/18 infection, at least 1 dose Show forest plot | 1 | 4210 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.17, 0.33] |
| 2 Persistent HPV16/18 infection (6M), at least 1 dose Show forest plot | 4 | 33847 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.41, 0.57] |
| 2.1 Age group 15‐26 years | 2 | 25199 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.38, 0.51] |
| 2.2 Age group 24‐45 years | 2 | 8648 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.47, 0.69] |
| 3 Persistent HPV6/11/16/18 infection (6M), at least 1 dose Show forest plot | 1 | 3713 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.42, 0.65] |
| 4 Persistent HPV16/18 infection (12M), at least 1 dose Show forest plot | 2 | 24785 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.40, 0.54] |
| 5 Persistent HPV16/18 infection (12M) by dose (post hoc analysis) Show forest plot | 1 | 7153 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.12, 0.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall local/injection site adverse events Show forest plot | 8 | 18113 | Risk Ratio (IV, Fixed, 95% CI) | 1.18 [1.16, 1.20] |
| 1.1 Bivalent vaccine | 2 | 6503 | Risk Ratio (IV, Fixed, 95% CI) | 1.29 [1.26, 1.33] |
| 1.2 Quadrivalent vaccine | 6 | 11610 | Risk Ratio (IV, Fixed, 95% CI) | 1.14 [1.12, 1.16] |
| 2 Pain at injection site Show forest plot | 13 | 25691 | Risk Ratio (IV, Random, 95% CI) | 1.35 [1.23, 1.49] |
| 2.1 Monovalent vaccine | 1 | 2280 | Risk Ratio (IV, Random, 95% CI) | 1.05 [1.01, 1.09] |
| 2.2 Bivalent vaccine | 8 | 16897 | Risk Ratio (IV, Random, 95% CI) | 1.49 [1.26, 1.75] |
| 2.3 Quadrivalent vaccine | 4 | 6514 | Risk Ratio (IV, Random, 95% CI) | 1.13 [1.07, 1.19] |
| 3 Swelling at injection site Show forest plot | 9 | 22106 | Risk Ratio (IV, Random, 95% CI) | 1.73 [1.32, 2.27] |
| 3.1 Bivalent vaccine | 7 | 16603 | Risk Ratio (IV, Random, 95% CI) | 1.62 [1.15, 2.29] |
| 3.2 Quadrivalent vaccine | 2 | 5503 | Risk Ratio (IV, Random, 95% CI) | 2.79 [0.85, 9.15] |
| 4 Redness at injection site Show forest plot | 6 | 19996 | Risk Ratio (IV, Random, 95% CI) | 1.72 [1.50, 1.97] |
| 4.1 Quadrivalent vaccine | 1 | 5345 | Risk Ratio (IV, Random, 95% CI) | 1.46 [1.32, 1.63] |
| 4.2 Bivalent vaccine | 5 | 14651 | Risk Ratio (IV, Random, 95% CI) | 1.80 [1.53, 2.11] |
| 5 Overall systemic event and general symptoms Show forest plot | 8 | 18191 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.98, 1.07] |
| 5.1 Bivalent vaccine | 2 | 6503 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.97, 1.19] |
| 5.2 Quadrivalent vaccine | 6 | 11688 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.98, 1.04] |
| 6 Serious adverse events Show forest plot | 23 | 71597 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 6.1 Monovalent vaccine | 1 | 2387 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.51, 1.78] |
| 6.2 Bivalent vaccine | 15 | 46231 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.96, 1.07] |
| 6.3 Quadrivalent vaccine | 7 | 22979 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.65, 1.02] |
| 7 Deaths Show forest plot | 23 | 71176 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.85, 1.98] |
| 7.1 Monovalent vaccine | 1 | 2280 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Bivalent vaccine | 15 | 46231 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.66, 2.22] |
| 7.3 Quadrivalent vaccine | 7 | 22665 | Risk Ratio (IV, Random, 95% CI) | 1.54 [0.73, 3.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Normal infant Show forest plot | 8 | 8782 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.97, 1.02] |
| 2 Spontaneous abortion/miscarriage Show forest plot | 9 | 8618 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.68, 1.14] |
| 3 Elective termination/induced abortion Show forest plot | 9 | 10909 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.80, 1.02] |
| 4 Stillbirth Show forest plot | 6 | 8754 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.68, 1.83] |
| 5 Abnormal infant Show forest plot | 5 | 9252 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.88, 1.69] |

