Vacunas para la prevención de la diarrea por Escherichia coli enterotoxigénica (ECET)
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study type: Randomized, natural challenge, efficacy study in an endemic population Trial dates and duration: From January 1985 to May 1986 Surveillance: Surveillance for diarrhoea was done at treatment centres serving the study participants at Matlab for 365 post‐vaccination days | |
| Participants | Number of participants: 49,612 Inclusion criteria: People aged between 2 to 15 years of age and female subjects > 15 years of age residing in Matlab Exclusion criteria: Persons who were absent or refused to participate, pregnant, or suffering from any other illness | |
| Interventions | Vaccine: Cholera toxin B subunit plus killed cholera whole cells (BS‐WC) Control: Killed cholera whole cells (WC) Additional details: In this study participants received 3 doses of vaccine at 6 weeks apart, of BS‐WC vaccine, WC vaccine only, or an E. coli K12 strain placebo. However, protective efficacy was calculated based on WC vaccine as control and BS‐WC as study intervention group, because the killed cholera whole cells, which were identical for the BS‐WC and WC vaccines, were not anticipated to have any protective effects against LT‐ETEC | |
| Outcomes | Included in review:
| |
| Notes | Location: Matlab, Bangladesh Setting: Three different treatment centres at Matlab, a rural setting of Bangladesh Source of funding: US agency for International Development, the Government of Japan, the Swedish agency for Research Cooperation with Developing Countries and the World Health Organization (WHO) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After computerisation of the census, we assigned every person in the eligible age‐gender categories to letters A, B or C, using simple randomisation" (from additional paper describing this study). |
| Allocation concealment (selection bias) | Low risk | "The agents were identified only by the letters A, B and C" (from an additional paper describing this study). Allocation concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | "During the conduct of the study, the identities of these letter...were unknown to all persons connected with the trial in Bangladesh". |
| Blinding of outcome assessment (detection bias) | Low risk | "During the conduct of the study, the identities of these letter...were unknown to all persons connected with the trial in Bangladesh". |
| Incomplete outcome data (attrition bias) | Unclear risk | Losses to follow‐up were not clearly described. |
| Selective reporting (reporting bias) | High risk | This was a three arm study. It was unclear why the group given the cholera WC vaccine was selected as the control arm rather than the group given a placebo. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized safety and immunogenicity study in volunteers Trial dates and duration: Between May 22 and July 10 1995 | |
| Participants | Number of participants: 65 Inclusion criteria: Healthy men and women and were recruited among the School of Military Medicine cadets or the Medical Corps Headquarters staff. Exclusion criteria: Not described. | |
| Interventions | Vaccine: Contained 1.0 mg of rCTB plus a final count of ∼1011 formalin‐inactivated bacteria. Each vaccine dose included the following inactivated ETEC strains: SBL 101 (O78, CFA/I, LT2/ST1), SBL 106 (O6, CS1, LT2/ST2), SBL 107 (OR, CS2, CS3, LT2/ST2), SBL 104 (O25, CS41CS6, LT2/ST2) and SBL 105 (O167, CS51CS6, LT2/ST2). Placebo: Heat‐killed E. coli K12 with an optical density (OD) equivalent to that of the ETEC vaccine, was administered in the same buffered solution as the vaccine. Additional details: Each dose of lot E003 was given in 150 mL of water with a raspberry‐flavoured bicarbonate‐citric acid buffer containing 4 g of sodium bicarbonate per dose (Recip AB, Stockholm, Sweden). | |
| Outcomes | Included in review:
Not included in the review:
| |
| Notes | Location: Israel Setting: Israel Defence Force (IDF), Medical Corps, Army Health Branch Research Unit, and the IDF, Medical Corps, School of Military Medicine Source of funding: US Army Medical Research & Material Command (DAMD 17‐93‐V‐3001) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Group randomization was used so that each group was assigned two letters, and each volunteer was openly allotted to one of the four resulting letter groups". It is unclear if this method was truly random. |
| Allocation concealment (selection bias) | Low risk | "Each volunteer was openly allotted to one of the four resulting letter groups. The association between a letter group and a vaccine/placebo group was determined by a third party and was kept locked from both volunteers and investigators for the duration of the study". |
| Blinding of participants and personnel (performance bias) | Low risk | "The placebo preparation, containing a suspension of heat‐killed E. coli K12 with an optical density (OD) equivalent to that of the ETEC vaccine, was administered in the same buffered solution as the vaccine". |
| Blinding of outcome assessment (detection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up were low: 3 out of 33 (9%) in the vaccine group and 2 out of 31 (6%) in controls either dropped out of the study or were not given the second dose. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Between April 1 and June 18 1997 | |
| Participants | Number of participants: 90 Inclusion criteria: Healthy men and women and were recruited among the School of Military Medicine cadets or the Medical Corps Headquarters staff Exclusion criteria: Not mentioned | |
| Interventions | Vaccine: Contained 1.0 mg of rCTB plus a final count of ∼1011 formalin‐inactivated bacteria. Each vaccine dose included the following inactivated ETEC strains: SBL 101 (O78, CFA/I, LT2/ST1), SBL 106 (O6, CS1, LT2/ST2), SBL 107 (OR, CS2, CS3, LT2/ST2), SBL 104 (O25, CS41CS6, LT2/ST2) and SBL 105 (O167, CS51CS6, LT2/ST2) Placebo: Heat‐killed E. coli K12 with an optical density (OD) equivalent to that of the ETEC vaccine, was administered in the same buffered solution as the vaccine Additional details: Each dose of lot E005 was given in 150 mL of water with a raspberry‐flavoured bicarbonate‐citric acid buffer containing 4 g of sodium bicarbonate per dose (Recip AB, Stockholm, Sweden) | |
| Outcomes | Included in review:
Not included in the review:
| |
| Notes | Location: Israel Setting: IDF, Medical Corps, Army Health Branch Research Unit, and the IDF, Medical Corps, School of Military Medicine Source of funding: US Army Medical Research & Material Command (DAMD 17‐93‐V‐3001) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A blocked randomization scheme was constructed off‐site". |
| Allocation concealment (selection bias) | Low risk | "Subjects were assigned a unique participant identification number (101 to 190) at the time of the first dose and received the correspondingly labelled study agent". |
| Blinding of participants and personnel (performance bias) | Low risk | "The placebo preparation, containing a suspension of heat‐killed E. coli K‐12 with an optical density (OD) equivalent to that of the ETEC vaccine, was administered in the same buffered solution as the vaccine". |
| Blinding of outcome assessment (detection bias) | Low risk | "The investigation team remained blinded until all safety and immunogenicity data were generated, computerized, cleaned, and locked". |
| Incomplete outcome data (attrition bias) | High risk | Losses to follow‐up were moderate: 8 out of 45 (18%) in the vaccine group and 4 out of 45 (9%) in controls either dropped out of the study or were not given the second dose. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, natural challenge, efficacy study in travellers Trial dates and duration: May 2006 to February 2007 Surveillance: Surveillance was conducted on US travellers who visited to Mexico and Guatemala | |
| Participants | Number of participants: 201 Inclusion criteria: Healthy adults aged 18 to 64 years, who planned to travel to Cuernavaca, Guadalajara, San Miguel, or Cancun (Mexico), or Antigua (Guatemala) and who had access to one of the 14 US regional vaccination centres Exclusion criteria: History of travellers' diarrhoea and travelled to an endemic country in the previous 12 months, history of taking cholera, LT or ETEC vaccine, significant illness, immunosuppression or if female, pregnant, nursing, or unwilling to use effective form of any contraceptives | |
| Interventions | Vaccine: LT patch; 37.5 µg of ETEC LT Placebo: All the excipients of LT patch without LT Additional details: Vaccinations with either an LT patch or placebo patch were given to alternate upper arms a minimum of 3 weeks (first vaccination) and 1 week (second vaccination) before departure | |
| Outcomes | Included in review:
| |
| Notes | Location: Mexico and Guatemala Setting: University of Texas Health Science Center at Houston (Houston, TX, USA), Universidad Del Valle De Guatemala (Guatemala City, Guatemala), Inovamed Hospital (Cuernavaca, Mexico) and ViroMed Laboratory, Minnetonka, MN, USA Source of funding: IOMAI corporation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study used a web‐based, audit‐trail enabled, centralised randomisation code and allocation system". |
| Allocation concealment (selection bias) | Low risk | "Vaccination sites accessed a web page, entered participants into the system, and received unique patch numbers for every study participant". |
| Blinding of participants and personnel (performance bias) | Low risk | "Dose information was masked at allocation, as well as on primary and secondary product packaging. Participants and site staff, including those assessing study outcomes, remained masked until database lock". |
| Blinding of outcome assessment (detection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) | High risk | Losses to follow‐up were high: 8 out 67 (12%) in the vaccine group and 23 out of 134 (17%) in the placebo group due to failure to: receive second dose of vaccine, provide diary cards, or attend in‐country visit. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, artifical challenge, efficacy study in volunteers Trial dates and duration: Not given Surveillance: Daily medical rounds were conducted to monitor symptoms during the 7 days of study period. Daily stool samples were taken for bacteriologic examination. Artificial challenge: On day 4 | |
| Participants | Number of participants: 25 Inclusion criteria: Not described Exclusion criteria: Not described | |
| Interventions | Vaccine: Each lyophilized dose containing hyperimmune anti‐E. coli bovine milk IgG dissolved in 150 mL of bicarbonate solution Placebo: A single dose of a lactose‐free infant formula Additional details: Three doses/day for 7 days, vaccine, or placebo were administered 15 minutes after meals Artificial challenge: 109 cfu of H10407 (O78:H11), a CFA/I‐bearing ETEC strain suspended in 1 ounce (30 mL) of water containing sodium bicarbonate | |
| Outcomes | Included in review:
| |
| Notes | Location: Baltimore, MD, USA Setting: Center for Vaccine Development (University of Maryland School of Medicine) Source of funding: ImmuCell | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was generated and secured by ImmuCell’s Quality Assurance Supervisor". |
| Allocation concealment (selection bias) | Low risk | "by assigning subject identification numbers to identically packaged foil pouches containing measured doses of each test article". |
| Blinding of participants and personnel (performance bias) | Low risk | "All investigators and volunteers were blinded to these treatment group assignments throughout the study and during assessment of outcome". |
| Blinding of outcome assessment (detection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs occurred. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Subjects were randomly assigned to receive two doses of vaccine or placebo 2 weeks apart | |
| Participants | Number of participants: 76 Inclusion criteria: Adults aged between 21 and 45 years from Benha, Qalyubia Governorate, Egypt Exclusion criteria: Not mentioned | |
| Interventions | Vaccine: Each 4 mL vaccine dose (lot E003) contained 1 mg of rCTB plus a mixture of ∼2 x 1010 bacteria each of five strains individually expressing CFA/I, CS1, CS2 plus CS3, CS4, and CS5 Placebo: Each 4‐mL placebo dose contained ∼1011 heat‐killed E. coli K12 cells Additional details: Each dose was added to 150 mL of water containing 4 g of sodium bicarbonate plus 1.45 g of citric acid (Recip AB, Stockholm, Sweden) for adult administration. | |
| Outcomes | Included in review:
Not included in the review:
| |
| Notes | Location: Egypt Setting: Benha, Qalyubia Governorate, Egypt Source of funding: Naval Medical Research and Development Command (B69000101. PIX3270), Intragency Agreement Y1‐HD‐0026‐01, the National Institute of Child Health and Human Development and WHO Global Programme for Vaccines and Immunization Research and Development. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After enrollment, subjects were randomized to receive vaccine or placebo in a double‐blind fashion within blocks of 4 sequentially randomized subjects" (Savarino 1998). It is unclear if this was truly random. |
| Allocation concealment (selection bias) | Low risk | "At the time of initial dosing, each subject was assigned a sequential number corresponding to sequentially numbered single‐dose vials of study agent" (Savarino 1998). |
| Blinding of participants and personnel (performance bias) | Low risk | Described as "double blind, placebo controlled", and vaccines labelled only with study code. |
| Blinding of outcome assessment (detection bias) | Low risk | "The code was broken after all clinical and laboratory evaluations were completed". |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up were low: 2/49 (4%) in the vaccine group and 2/48 (4%) in the placebo group. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Subjects were randomly assigned to receive two doses of vaccine or placebo 2 weeks apart. | |
| Participants | Number of participants: 107 Inclusion criteria: School children aged between 6 to 12 years old from Benha, Qalyubia Governorate, Egypt Exclusion criteria: Not mentioned | |
| Interventions | Vaccine: Each 4 mL vaccine dose (lot E003) contained 1 mg of rCTB plus a mixture of ∼2 x 1010 bacteria each of five strains individually expressing CFA/I, CS1, CS2 plus CS3, CS4, and CS5. Placebo: Each 4 mL placebo dose contained ∼1011 heat‐killed E. coli K12 cells. Additional details: Each dose was added to 75 mL of water containing 4 g of sodium bicarbonate plus 1.45 g of citric acid (Recip AB, Stockholm, Sweden) for school children administration. | |
| Outcomes | Included in review:
Not included in the review:
| |
| Notes | Location: Egypt Setting: Benha, Qalyubia Governorate, Egypt Source of funding: Naval Medical Research and Development Command (B69000101. PIX3270), Intragency Agreement Y1‐HD‐0026‐01, the National Institute of Child Health and Human Development and WHO Global Programme for Vaccines and Immunization Research and Development | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After enrollment, subjects were randomized to receive vaccine or placebo in a double‐blind fashion within blocks of 4 sequentially randomized subjects" (Savarino 1998). It is unclear if this was truly random. |
| Allocation concealment (selection bias) | Low risk | "At the time of initial dosing, each subject was assigned a sequential number corresponding to sequentially numbered single‐dose vials of study agent" (Savarino 1998). |
| Blinding of participants and personnel (performance bias) | Low risk | Described as "double blind, placebo controlled", and vaccines labelled only with study code. |
| Blinding of outcome assessment (detection bias) | Low risk | "The code was broken after all clinical and laboratory evaluations were completed". |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up occurred. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Subjects were randomly assigned to receive two doses of vaccine or placebo 2 weeks apart | |
| Participants | Number of participants: 106 Inclusion criteria: Preschool children aged between 2 to 5 years old from Benha, Qalyubia Governorate, Egypt Exclusion criteria: Not mentioned | |
| Interventions | Vaccine: Each 4 mL vaccine dose (lot E003) contained 1 mg of rCTB plus a mixture of ∼2 x 1010 bacteria each of five strains individually expressing CFA/I, CS1, CS2 plus CS3, CS4, and CS5 Placebo: Each 4 mL placebo dose contained ∼1011 heat‐killed E. coli K12 cells Additional details: Each dose was added to 37.5 mL of water containing 4 g of sodium bicarbonate plus 1.45 g of citric acid (Recip AB, Stockholm, Sweden) for adult administration | |
| Outcomes | Included in review:
Not included in the review:
| |
| Notes | Location: Egypt Setting: Benha, Qalyubia Governorate, Egypt Source of funding: Naval Medical Research and Development Command (B69000101. PIX3270), Intragency Agreement Y1‐HD‐0026‐01, the National Institute of Child Health and Human Development and WHO Global Programme for Vaccines and Immunization Research and Development | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After enrollment, subjects were randomized to receive vaccine or placebo in a double‐blind fashion within blocks of 4 sequentially randomized subjects" (Savarino 1998). It is unclear if this was truly random. |
| Allocation concealment (selection bias) | Low risk | 'At the time of initial dosing, each subject was assigned a sequential number corresponding to sequentially numbered single‐dose vials of study agent" (Savarino 1998). |
| Blinding of participants and personnel (performance bias) | Low risk | Described as "double blind, placebo controlled", and vaccines labelled only with study code. |
| Blinding of outcome assessment (detection bias) | Low risk | "The code was broken after all clinical and laboratory evaluations were completed". |
| Incomplete outcome data (attrition bias) | Low risk | Only one participant was lost to follow‐up (from the placebo group). |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, safety study in volunteers Study 1: Single blinded, placebo controlled, randomized study Study 2: Non‐placebo controlled study (data excluded) Study 3: Non‐placebo controlled study (data excluded) Trial dates and duration: Not mentioned in the article Surveillance: Study 1: 5 consecutive post vaccination days | |
| Participants | Number of participants: 20 (vaccine) plus 20 (placebo) Inclusion criteria: Adult Swedish volunteers, aged between 20 to 50 years were recruited, with no history of travel to an endemic country for the past 6 months Exclusion criteria: Not mentioned | |
| Interventions | Vaccine: One single dose of vaccine contained 1.0 mg of rCTB and 1011 formalin‐inactivated enterotoxigenic E. coli bacteria of each of the following strains: O78:H12‐CFA/I ST+, O25:H42‐ CS4+CS6, O167:H5‐CS5+CS6/ST+, O6:H16‐CS2+CS3, and O139:H28‐CS1 Placebo: One single dose of placebo consisted of 150 mL of a sodium bicarbonate solution (Samarin; Cederroths Nordic AB, Upplands Vasby, Sweden) The volunteers were instructed not to eat or drink (except water) for 1 hour before and after intake of the vaccine or placebo preparation | |
| Outcomes | Included in review:
| |
| Notes | Location: Goteborg, Sweden Setting: University of Goteborg, Sweden Source of funding: Swedish Research Council (16X‐09084 and 16X‐3382), the Swedish Agency for Research Cooperation with Developing Countries, the WHO and the Medical Faculty, Goteborg University | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized". No further details. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as "single blind" but no further details. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as "single blind" but no further details. |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up were low: 1 out of 20 in placebo group was excluded due to viral infection. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, immunogenicity study in volunteers Study 1: Open study without any control group (excluded from the review)) Study 2: Double blinded, placebo controlled, randomized study Trial dates and duration: Not mentioned in the article Surveillance: Not described | |
| Participants | Number of participants: Study 1: 36 (excluded from the review) Study 2: 31 Inclusion criteria: Adult Swedish volunteers, aged between 18 to 46 years were recruited, no history of travelling to ETEC endemic areas for 6 months prior to the study Exclusion criteria: Not mentioned | |
| Interventions | Vaccine: Study 1: One 4 mL dose of vaccine (Lot 003) contained 1.0 mg of rCTB and 1011 formalin‐inactivated E. coli bacteria of each of the following strains: SBL101 (O78:H12; CFA/I ST1), SBL104 (O25:H42; CS4), SBL105 (O167:H5; CS5 ST1), SBL 106 (O6:H16; CS1), and SBL 107 (OR:H6; CS21 CS3) (Data not included in the review) Study 2: Different lot (Lot 005) of vaccine with same formulation except the half the amount of CFA/I and three times more CS2 than lot 003 was used in this study Placebo: The 4 mL placebo dose consisted of ∼1 ×1011 heat‐killed E. coli K12 bacteria Additional details: Each dose of a study agent was administered in 150 mL of a sodium bicarbonate solution (Samarin; Cederroths Nordic AB, Upplands Vasby, Sweden). The volunteers were instructed not to eat or drink (except water) for 1 hour before and after intake of the vaccine or placebo preparation. | |
| Outcomes | Included in review:
| |
| Notes | Location: Goteborg, Sweden Setting: University of Goteborg, Sweden Source of funding: Swedish Research Council (16X‐09084), the Swedish Agency for Research Cooperation with Developing Countries, the WHO and the Medical Faculty, Goteborg University | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as ‘randomized’, no further details given. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as ‘double blind, Placebo controlled" study, no further details. |
| Blinding of outcome assessment (detection bias) | Unclear risk | As above. |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs occurred. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, natural challenge, efficacy study in travellers Trial dates and duration: May 1995 to February 1996 Surveillance: All participants kept a diary of their defecation pattern during their stay abroad. On return, they filled out a questionnaire concerning defecation pattern, use of medication and | |
| Participants | Number of participants: 145 Inclusion criteria: Dutch volunteers, travellers from the travellers clinics of the Leiden University Medical Centre (LUMC), the Netherlands, the Municipal Health Centre at Leiden and the Harbour Hospital at Rotterdam. All adults who were intending to travel to Indonesia, Thailand, the Indian subcontinent or West Africa (Gambia or Senegal) for a period of 1 to 4 weeks were invited to take part in the trial. Exclusion criteria: People suffering from acute or chronic inflammatory disease of the intestinal tract, prior recipients of WC‐BS cholera vaccine or CVD 103‐HgR vaccine, subjects receiving immunosuppressive drugs, persons known to be immunodeficient, subjects participating in other clinical trials women who were either pregnant or breast‐feeding | |
| Interventions | Vaccine: CVD 103‐HgR, Single dose of 5 x 108 cfu of lyophilized CVD 103‐HgR live oral cholera vaccine Placebo: 5 x 108 heat‐killed E. coli K12 Additional details: Vaccine has been administered orally. Both vaccine and placebo are identical in appearance. | |
| Outcomes | Included in review:
| |
| Notes | Location: The Netherlands Setting: Leiden University Medical Center (LUMC) Source of funding: Berna Biotech AG, Bern, Switzerland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "For randomisation a computer‐generated randomisation list, was used". |
| Allocation concealment (selection bias) | Low risk | "Sachets and suspensions of vaccine and placebo that were identical in appearance, were labelled by a coded number from 1 to 200". Allocation was concealed through randomization to identical coded vials. |
| Blinding of participants and personnel (performance bias) | Low risk | See above. |
| Blinding of outcome assessment (detection bias) | Low risk | The key to the coded sachets was stored at the hospital pharmacy in a sealed envelope. The envelope was only to be opened by the investigator in case of an emergency that required knowledge of the identity of the trial medication in order to manage the participant’s condition. At the end of the trial the coded envelope was returned to the Berna Biotech AG and checked to ensure that the seal had remained unbroken". |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up were low: in vaccine group 4/73 (5%) and in placebo group 7/72 (10 %). |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, artificial challenge, efficacy study in volunteers Trial dates and duration: September 15 2004 to June 30 2005 Surveillance: Post vaccination follow‐up on day 0, 7, 21, 28, 42, 49, and 77; Artificial challenge: on day 55; Post challenge follow‐up for 5 days | |
| Participants | Number of participants: 59 Inclusion criteria: Healthy adults, between 18 to 45 years of age Exclusion criteria: Clinically significant medical conditions, history of traveller’s diarrhoea in the last 3 years and LT IgG titer > 2000 EU by ELISA | |
| Interventions | Vaccine: 150 μL of saline containing 50 μg of LT Placebo: 150 μL of saline containing no LT Additional details: All participants were randomized to receive transcutaneous application of 3 doses saline containing LT or saline only, at an interval of 21 days Artificial challenge: 120 mL of sodium bicarbonate buffer containing 6 × 108 CFU of the challenge virulent strain of E. coli E24377A | |
| Outcomes | Included in review:
| |
| Notes | Location: USA Setting: Vaccine Testing Unit, the Center for Immunization Research (CIR), Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; IOMAI Corporation, Gaithersburg, MD, USA; Amarex Clinical Research, Germantown, MD, USA Source of funding: IOMAI Corporation, Gaithersburg, MD, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was generated by the statistician". |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as "double blind" but no details given of how this was done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as "double blind" but no details given of how this was done. |
| Incomplete outcome data (attrition bias) | Low risk | 2 out of 30 (7%) subjects from vaccine group and 4 out 29 (14%) of subjects from placebo group subsequently withdrew from the study before the inpatient challenge phase. However, once entered into the challenge phase there were no further drop‐outs. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, artificial challenge, efficacy study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Post vaccination follow‐up for 20 days Artificial challenge: On day 27; post challenge follow‐up for 5 days (passive using diary cards) | |
| Participants | Number of participants: 39 Inclusion criteria: Healthy adults, 18 to 50 years of age Exclusion criteria: Not mentioned in the article | |
| Interventions | Vaccine: PTL‐003 ( PTL‐003 was derived from the spontaneously toxin‐negative, O139:H28 ETEC strain E1392/75‐2A containing 2 × 109 cfu/mL live attenuated bacteria in 200 mL of CeraVacxTM buffer) Placebo: CereVacx buffer (CeraVacx, Cera Products Inc., Jessup, MD: rice solids, 7.0 g; sodium bicarbonate, 2 g; trisodium citrate, 0.5 g in 200 mL of water) Additional details: All participants were randomized to receive 2 doses, at an interval of 10 days Artificial challenge: 30 mL of sodium bicarbonate buffer containing 3 × 109 CFU of the challenge virulent strain of E. coli E24377 | |
| Outcomes | Included in review:
| |
| Notes | Location: USA Setting: Vaccine Testing Unit, the Center for Immunization Research (CIR), Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA Source of funding: Acambis Research Ltd., Cambridge, UK and by Johns Hopkins University School of Medicine General Clinical Research Center grant number M01‐RR00052 from the National Center for Research Resources, NIH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized", no further details given. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as "double blind", no further details given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as "double blind", no further details given. |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up were low (three participants in each group withdrew before the challenge phase), but as the trial was very small this represented 15% of all participants. However once they entered the challenge phase, there was no further drop‐outs. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, natural challenge, efficacy study in travellers Trial dates and duration: October 24 1989 to November 21 1989 Surveillance: Any diarrhoea during the trip period and immediately after returning back was recorded and stool samples were collected | |
| Participants | Number of participants: 615 Inclusion criteria: Travellers from Finland to Morocco Exclusion criteria: Travellers aged less than 15 years and history of taking antimicrobials during the previous 7 days of vaccination days | |
| Interventions | Vaccine: 1 x 1011 heat‐killed whole cells of V. cholerae with 1 mg of the B‐subunit of cholera toxin Placebo: E. coli K12 Additional details: Two doses of vaccine or placebo identical in appearance, 3 and 1 week before the departure were administered | |
| Outcomes | Included in review:
| |
| Notes | Location: Finland and Morocco Setting: Enteric Laboratory, Moroccan Health Authority, Agadir, Morocco; Laboratory of Enteric Pathogen, The National Public Health Institute, Helsinki, Finland Source of funding: National Public Health Authority, Finland, Fintours Company, Sun Tours Company, Moroccan Health Authority, University of Gothenburg, Pohjola Company, and Tapiola Company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized", no further details given. |
| Allocation concealment (selection bias) | Low risk | "The vaccine and placebo were in a similar liquid form, coded blindly and packed in identical 4 mL vials". Allocation was concealed through randomization to identical coded vials. |
| Blinding of participants and personnel (performance bias) | Low risk | See above. |
| Blinding of outcome assessment (detection bias) | Low risk | "The vaccine code was opened after all demographic, clinical, and microbiological data were available". |
| Incomplete outcome data (attrition bias) | High risk | Losses to follow‐up were high (18%) for adverse event data. Losses to follow‐up for clinical outcomes were not clearly stated. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Surveillance for side‐effects was carried out for 3 days after each vaccination. The child was observed for 1 hour in the field clinic and was then allowed to return home. The trained health workers made house to house visits within | |
| Participants | Number of participants: 158 Inclusion criteria: Healthy children aged 18 to 36 months with both sexes Exclusion criteria: History of gastrointestinal disorder, diarrhoeal illness in the past 2 weeks, febrile illness in the preceding week or antibiotic treatment for at least 7 days prior to enrolment as well as children, weight‐for‐height <−2(S.D.) of the median value of the National Centre of Health Statistics (NCHS) were not enrolled in the study. | |
| Interventions | Vaccine: One 6 mL dose of vaccine consisted of 1 mg of rCTB plus ∼2 × 1010 CFU of five strains of formalin‐inactivated ETEC expressing CFA/I, CS1, CS2 + CS3, CS4 and CS5 antigens each. Placebo: ∼1 × 1011 CFU of heat‐killed E. coli K12. Additional details: The children enrolled in the study were received two doses of the oral CF‐BS‐ETEC vaccine (lot E‐009) or the placebo with a 2 week interval in the health station of the field site. | |
| Outcomes | Included in review:
| |
| Notes | Location: Dhaka, Bangladesh Setting: International Centre for Diarrhoeal Disease Research, Bangladesh Source of funding: USAID (HRN‐A‐00‐96‐90005‐00), the Swedish Agency for Research and Economic Cooperation, Sida‐SAREC (1995‐0069) and ICDDR,B. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random code generated by statistician (author communication). |
| Allocation concealment (selection bias) | Low risk | "Each subject was assigned a sequential number at the time of initial dosing, corresponding to numbered and randomized set of two single‐dose vials of study agent". Allocation was concealed through randomization to identical coded vials. |
| Blinding of participants and personnel (performance bias) | Low risk | "The vaccine and placebo formulation appeared similar". |
| Blinding of outcome assessment (detection bias) | Low risk | "The investigators including field staff and laboratory personnel were completely blinded to the identity of the study subjects, whether vaccine or placebo". |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up was short and no losses to follow‐up occurred. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Surveillance for side effects was carried out for 3 days after each vaccination. The children were observed for 1 hour in the field clinic and were then allowed to return home. | |
| Participants | Number of participants: 158 Inclusion criteria: Healthy children aged 6 to 17 months living in same socioeconomic background Exclusion criteria: History of gastrointestinal disorder, diarrhoeal illness in the past 2 weeks, febrile illness in the preceding week or antibiotic treatment at least 7 days prior to enrolment as well as children <−2 S.D. (weight/height) of the National Center of Health Statistics (NCHS) were also not recruited. Children who were found to be asymptomatically positive for any bacterial enteric pathogen including ETEC during the screening and any participant with ETEC infection during the study period was to be excluded. | |
| Interventions | Vaccine: A quarter dose of vaccine composed of total ∼ 2.5 × 1010 CFU of five strains of ETEC. An 1.5 mL dose contained 0.25 mg of recombinant cholera toxin B subunit (BS) plus ∼ 0.5 × 1010 formalin‐inactivated bacteria of each of five different ETEC strains producing CFA/I, CS1, CS2, CS3, CS4, CS5 (lot E 008). Placebo: ∼2.5 × 1010 CFU of heat‐killed E. coli K12 bacteria Additional details: Each two‐dose (quarter dose) of vaccine regimen was given at intervals of 2 weeks intervals | |
| Outcomes | Included in review:
| |
| Notes | Location: Dhaka, Bangladesh Setting: International Centre for Diarrhoeal Disease Research, Bangladesh Source of funding: USAID (HRN‐A‐00‐96‐90005‐00), the Swedish Agency for Research and Economic Cooperation, Sida‐SAREC (2001‐3970) and icddr,b. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random code generated by statistician (author communication). |
| Allocation concealment (selection bias) | Low risk | "Randomized vials of study agents either vaccine or placebo were supplied by the company". The vials were identical in appearance (author communication) |
| Blinding of participants and personnel (performance bias) | Low risk | The blinding of the study code was maintained throughout (author communication). |
| Blinding of outcome assessment (detection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up was short and no losses to follow‐up occurred. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, natural challenge, efficacy study in travellers Trial dates and duration: May 1998 to September 1999 Surveillance: The participants were given vials for collection of faecal samples and daily | |
| Participants | Number of participants: 685 for safety analysis and 669 for efficacy analysis Inclusion criteria: Travellers from USA to Mexico and Guatemala and planned to stay at least 14 days, travellers more than ≥17 years of age, good health condition, US resident with a telephone, willingness to participate and signed consent, females not pregnant and willing to use reliable birth control during the study period Exclusion criteria: Clinically significant acute or chronic gastrointestinal disease, any serious medical condition, immunodeficiency, planned to use antibiotics during the trip and recent exposure to ETEC within the past 1‐year | |
| Interventions | Vaccine: 1 x 1011 formalin‐killed 5 strains of enterotoxigenic E. coli expressing CFA1, CS1, CS2, CS3, CS4 and CS5 plus 1 mg of the recombinant B‐subunit of cholera toxin Placebo: Killed non‐pathogenic E. coli K12 Additional details: The participants fasted for one hour before and after vaccination. The first dose was taken about 3 weeks before travel (acceptable range, 11 to 35 days prior to travel) and the second dose was taken about 8 days before travel (acceptable range: between 4 to 10 days before travel). The two doses were separated by between 7 to 21 days. | |
| Outcomes | Included in review:
| |
| Notes | Location: USA, Mexico, and Guatemala Setting: Vaccine Testing Unit (VTU), Johns HopkinsUniversity, Baltimore, MD, USA; Institute of Nutrition of Central America and Panama (INCAP), Guatemala; Hospital del Nino Morelense, Mexico; University of Gothenburg, Sweden Source of funding: SBL VaccineAB, Stockholm, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation used blocks of 10 (prepared by the CRO Clinical Data Care in Lund, Sweden) to assure similar distribution throughout the study and the blocks were stratified according to destination (Guatemalaor Mexico)". |
| Allocation concealment (selection bias) | Low risk | "The participants were randomised to receive two doses of the vaccine in a bicarbonate citrate buffer, or an identical appearing placebo". "The vials of vaccine had unique study numbers that were then used as the study number to identify that participant. The volunteers were considered as randomised when they had signed the informed consent". |
| Blinding of participants and personnel (performance bias) | Low risk | "The complete randomisation list exists in two sealed and identical copies. One was stored at SBL Vaccin AB, and the other at CDC in Lund". "These envelopes were to be opened only if there was a medical and/or ethical need to know the vaccination code, as requested by the DSMB". |
| Blinding of outcome assessment (detection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up were low (< 10%) in each group. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: Not mentioned in the article. Surveillance: Subjects had oral temperatures taken and underwent a standardized, structured interview on 3 consecutive days after each dose. In addition, subjects were asked to provide 3 venous blood samples, 1 just before the first dose and 1 each 7 days after the first and second doses. | |
| Participants | Number of participants: 76 Inclusion criteria: Healthy men and women aged 21 to 45 years Exclusion criteria: Participants with a history of chronic gastrointestinal illness, diarrhoea, antidiarrhoeal drug usage, febrile illness in the week before dosing or pregnancy. | |
| Interventions | Vaccine: Each 4 mL dose of the ETEC/rCTB vaccine (Lot E003) contained 1 mg rCTB plus 2 x 1010 formalin‐inactivated bacteria of each of the following ETEC strains: SBL101 (O78:H12; CFA/I; ST+); SBL104 (O25:H42; CS4+CS6); SBL105 (O167:H5; CS5+CS6; ST+); SBL106 (O6:H16; CS1); and SBL107 (OR:H6; CS2 CS3) Placebo: ∼1 × 1011 CFU of heat‐killed E. coli K12 bacteria Additional details: Subjects were offered a two dose schedule of study agent in three rounds at intervals of 2 weeks, with fasting for at least 90 minutes before and after dosing | |
| Outcomes | Included in review:
| |
| Notes | Location: Benha, Egypt Setting: Cairo, Egypt. Source of funding: Naval Medical Research and Development Command, the National Institute of Child Health and Human Development Under Interagency Agreement (Y1‐HD‐0026‐01) and WHO Global Programme for Vaccines and Immunization/Vaccine Research and Development. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After enrollment, subjects were randomized to receive vaccine or placebo in a double‐blind fashion within blocks of 4 sequentially randomized subjects". It was unclear if this was truly randomized. |
| Allocation concealment (selection bias) | Low risk | "At the time of initial dosing, each subject was assigned a sequential number corresponding to sequentially numbered single‐dose vials of study agent". |
| Blinding of participants and personnel (performance bias) | Low risk | Described as "double blind, placebo controlled", and vaccines labelled only with study code. |
| Blinding of outcome assessment (detection bias) | Low risk | "The code was broken after all clinical and laboratory evaluations were completed". |
| Incomplete outcome data (attrition bias) | Low risk | Only two subjects were excluded from the 2nd dose from placebo group because of absenteeism or intercurrent diarrhoea. |
| Selective reporting (reporting bias) | Low risk | None. |
| Other bias | Low risk | None. |
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: June 1996 Surveillance: Subjects were observed for 30 minutes after each dose for occurrence of immediate adverse effects. For the next 3 days after each dose, parents were asked to report to the study centre with their child. During each visit, a trained health care provider obtained the child’s temperature and performed a standardized, structured interview for 24 hour recall of symptoms. | |
| Participants | Number of participants: 107 Inclusion criteria: Healthy Egyptian children aged between 6 to 12 years were recruited from Benha, Egypt Exclusion criteria: Children with a history of chronic gastrointestinal disorder, diarrhoea, or febrile illness, or some other serious chronic illness | |
| Interventions | Vaccine: Each 4 mL dose of the ETEC/rCTB vaccine (Lot E003) contained 1 mg rCTB plus 2 x 1010 formalin‐inactivated bacteria of each of the following ETEC strains: SBL101 (O78:H12; CFA/I; ST); SBL104 (O25:H42; CS4); SBL105 (O167:H5; CS5; ST); SBL106 (O6:H16; CS1); and SBL107 (OR:H6; CS2 CS3) Placebo: ∼1 × 1011 CFU of heat‐killed E. coli K12 bacteria Additional details: Subjects received a two‐dose schedule of study agent 2 weeks apart, fasting for at least 90 minutes before and after each dose | |
| Outcomes | Included in review:
| |
| Notes | Location: Benha, Egypt. Setting: US Naval Medical Research Unit No. 3, Cairo, Egypt. Source of funding: Naval Medical Research and Development Command, the National Institute of Child Health and Human Development Under Interagency Agreement (Y1‐HD‐0026‐01) and WHO Global Programme for Vaccines and Immunization/Vaccine Research and Development. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each child was assigned a sequential number corresponding to serially numbered and randomized sets of two single‐dose vials of study agent". |
| Allocation concealment (selection bias) | Low risk | See above. Allocation was concealed through randomization to identical coded vials. |
| Blinding of participants and personnel (performance bias) | Low risk | "Investigators and subjects were kept blinded as to assignments until all clinical and laboratory evaluations were completed and data files were frozen". |
| Blinding of outcome assessment (detection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) | Low risk | Only two subjects were excluded from the 2nd dose because of fever, diarrhoea, or other intercurrent illness. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: November to December 1996, pre‐school children Surveillance: Subjects were observed for 30 minutes after each dose for occurrence of immediate adverse effects. For the next 3 days after each dose, parents were asked to report to the study centre with their child. During each visit, a trained health care provider obtained the child’s temperature and performed a standardized, structured interview for 24 hour recall of symptoms. | |
| Participants | Number of participants: 106 Inclusion criteria: Healthy Egyptian children ages, both boys and girls aged between 2 to 5 years were recruited from Benha, Egypt Exclusion criteria: Children with a history of chronic gastrointestinal disorder, diarrhoea, or febrile illness, or some other serious chronic illness | |
| Interventions | Vaccine: Each 4 mL dose of the ETEC/rCTB vaccine (Lot E003) contained 1 mg rCTB plus 2x1010 formalin‐inactivated bacteria of each of the following ETEC strains: SBL101 (O78:H12; CFA/I; ST); SBL104 (O25:H42; CS4); SBL105 (O167:H5; CS5; ST); SBL106 (O6:H16; CS1); and SBL107 (OR:H6; CS2 CS3). Placebo: ∼1 × 1011 CFU of heat‐killed E. coli K12 bacteria. Additional details: Subjects received a two‐dose schedule of study agent 2 weeks | |
| Outcomes | Included in review:
| |
| Notes | Location: Benha, Egypt Setting: US Naval Medical Research Unit No. 3, Cairo, Egypt Source of funding: Naval Medical Research and Development Command, the National Institute of Child Health and Human Development Under Interagency Agreement (Y1‐HD‐0026‐01) and WHO Global Programme for Vaccines and Immunization/Vaccine Research and Development | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each child was assigned a sequential number corresponding to serially numbered and randomized sets of two single‐dose vials of study agent". |
| Allocation concealment (selection bias) | Low risk | See above. Allocation was concealed through randomization to identical coded vials. |
| Blinding of participants and personnel (performance bias) | Low risk | "Investigators and subjects were kept blinded as to assignments until all clinical and laboratory evaluations were completed and data files were frozen". |
| Blinding of outcome assessment (detection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up were low: Nine subjects were excluded from the 2nd dose because of fever, diarrhoea, or other intercurrent illness. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, safety and immunogenicity study in volunteers Trial dates and duration: September to October 1997 Surveillance: Subjects were directly observed for 30 minutes after each dose for immediate | |
| Participants | Number of participants: 95 Inclusion criteria: Healthy boys and girls aged 6 to 18 months were recruited from Benha, Egypt. Exclusion criteria: Children with a history of chronic gastrointestinal disorder, some other serious chronic illness, or congenital anomaly | |
| Interventions | Vaccine: Each 4 mL dose of the ETEC/rCTB vaccine (Lot E003) contained 1 mg rCTB plus 2 x 1010 formalin‐inactivated bacteria of each of the following ETEC strains: SBL101 (O78:H12; CFA/I; ST); SBL104 (O25:H42; CS4); SBL105 (O167:H5; CS5; ST); SBL106 (O6:H16; CS1); and SBL107 (OR:H6; CS2 CS3) Placebo: ∼1 × 1011 CFU of heat‐killed E. coli K12 bacteria Additional details: Subjects were offered a three dose schedule of study agent in three rounds at intervals of 2 weeks, with fasting for at least 1 hour before and after dosing | |
| Outcomes | Included in review:
| |
| Notes | Location: Benha, Egypt Setting: US Naval Medical Research Unit Number 3, Cairo, Egypt Source of funding: Naval Medical Research and Development Command, the National Institute of Child Health and Human Development Under Interagency Agreement (Y1‐HD‐0026‐01) and WHO Global Programme for Vaccines and Immunization/Vaccine Research and Development | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After enrollment subjects were stratified by age (6‐month bands) and gender and then block randomized to vaccine or control (block size, four) in a 1:1 ratio". |
| Allocation concealment (selection bias) | Low risk | "Within each stratum children were assigned a sequential number corresponding to serially numbered and randomized sets of three single dose vials of study agent". Allocation was concealed through randomization to identical coded vials. |
| Blinding of participants and personnel (performance bias) | Low risk | "Investigators and subjects were kept blinded as to assignments until all clinical and laboratory evaluations were completed, and data files were locked". |
| Blinding of outcome assessment (detection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up was high: 26% of randomized subjects (33/128) dropped out before receiving any dose of study agent, and 33% of subjects who received at least one dose of study agent did not complete the study (31/95). |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, natural challenge, efficacy study in travellers Trial dates and duration: From June 1992 to July 1992 Surveillance: WC/rBS oral cholera vaccine in 502 US college students attending summer educational programs in Mexico | |
| Participants | Number of participants: 502 healthy adults Inclusion criteria: Full‐time US residence, aged 18 and over, willingness to participate in the study and willingness to sign the consent form Exclusion criteria: Failure to understand the nature and plan of the study, inability to receive adequate follow‐up examinations in Mexico, unwillingness to submit serum specimens, use of oral or parenteral antibiotics in the 7 days previous to enrolment, use of more than two doses of anti‐diarrhoeal medications in the 7 days previous to enrolment, significant abnormalities detected by screening of the medical history and physical exam, history of severe allergic reaction to any vaccine, and in women of childbearing age, a positive urine pregnancy test result and nursing mothers | |
| Interventions | Vaccine: WC‐rBS, I mg of purified CTB subunit together with 1X1011 inactivated V. cholerae Placebo: Bicarbonate buffer alone. Additional details: Two doses of oral vaccine given, first dose at day 0 and second days 10 days later. Both vaccine and placebo are identical in appearance. | |
| Outcomes | Included in review:
| |
| Notes | Location: Mexico Setting: Center for Infectious Diseases of the University of Texas Health Science Center in Houston Source of funding: DOD; DAMD 17‐90‐R‐0048 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized", but no further details given. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) | Low risk | "For the placebo group, the 3 rnL dose of vaccine was not added before administration of the buffer solution. In this fashion, study participants were blinded as to which study group they were in". Personnel appear to be unblinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up were low. Data for 492 of 502 (98%) participants were available for analysis. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: Randomized, artificial challenge, efficacy study in volunteers Trial dates and duration: Not mentioned in the article Surveillance: Vaccine or placebo administered 3 times daily for 5 days Artificial challenge: on day 2; post challenge follow‐up for 4 days | |
| Participants | Number of participants: 20 Inclusion criteria: Healthy adults Exclusion criteria: None mentioned | |
| Interventions | Vaccine: All participants were randomized to receive 690 mg of bovine anti‐E. coli CFA milk immunoglobulin capsule Placebo: All participants were randomized to receive 690 mg of placebo capsule Additional details: Vaccine or placebo were administered 3 times daily, 10 minutes after each meal, for 5 days followed by on day 2 with an applesauce containing artificial challenge: 1 × 108 CFU of the challenge virulent strain of E. coli E24377. Schedule dose of vaccine or placebo was also administered 10 minutes after challenge. | |
| Outcomes | Included in review:
| |
| Notes | Location: USA Setting: Research Isolation Ward, Kernan Hospital, University of Maryland, Baltimore, MD, USA Source of funding: ImmuCell Corporation, Portland, ME | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "‘randomized"’, but no further details given. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) | Low risk | The placebo consisted of an identical preparation from non‐immunized cow. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as "‘double blind"’ but no further details given. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up occurred. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
| Methods | Study type: RCT, natural challenge, efficacy study in travellers Trial dates and duration: Not mentioned in the article Surveillance: Post‐vaccination symptoms and adverse events were reported after both doses. All volunteers enrolled in the study were equipped with both a daily record diary to monitor episodes of travellers’ diarrhoea during their stay abroad and with transport media (Portagerm® and Cary Blair tubes) for collection of stool samples in case of diarrhoea. Travelers were instructed to hand over their travel diary and transport media tubes immediately after return. | |
| Participants | Number of participants: 128 travellers (66 placebo group and 62 ETEC vaccine group) Inclusion criteria: Adults and children, who had signed up for a trip to tropical or subtropical destinations (44 different countries in Africa, Asia and Latin‐America) with a duration of stay intended to last 7 to 23 days Exclusion criteria: Not mentioned in the article | |
| Interventions | Vaccine: ETEC vaccine, containing 1 mg of recombinant B‐subunit of cholera toxin plus 1011 formalin‐killed ETEC bacteria of five ETEC strains expressing the most common CFAs such as CFA I, CFA II (CS1, CS2 and CS3) and CFA IV (CS4, CS5 and CS6); a B‐subunit cholera whole cell vaccine (licensed in Sweden since 1992), containing 1 mg recombinant subunit B cholera toxin and 1011 inactivated whole cells (Inaba,Ogawa; classical and El Tor) Placebo: Approximately 1011 inactivated E. coli K12 Additional details: Two consecutive vaccine or placebo doses given at an interval of between 7 to 21 days, not less than 7 days and not more than 30 days before departure | |
| Outcomes | Included in review:
| |
| Notes | Location: Institute for Specific Prophylaxis and Tropical Medicine, University of Vienna, Austria Setting: Institute for Specific Prophylaxis and Tropical Medicine, University of Vienna, Austria; Department of Medical Microbiology and Immunology, Göteborg University, Sweden; Swedish Bacteriological Laboratory, Vaccin, Sweden Source of funding: Not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized" but no further details given. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as "double blind" but no further details given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as "double blind" but no further details given. |
| Incomplete outcome data (attrition bias) | High risk | Seventy‐three recruited participants (29.2%) were excluded from the primary analysis. The reasons for these exclusions were unclear. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Participants did not receive a vaccine to prevent ETEC. | |
| No control group. | |
| Not described as randomized. | |
| Randomized placebo controlled Phase III trial but published the data as a cohort study. Clinical efficacy data are unavailable from the article. | |
| Participants did not receive a vaccine to prevent ETEC. | |
| No clinical efficacy data for this vaccine is available. | |
| Not described as randomized. | |
| No control group. | |
| Not described as randomized. | |
| No control group. | |
| Not described as randomized. | |
| No protective efficacy data against travellers' diarrhoea | |
| No outcomes relevant to this review. | |
| Not described as randomized. | |
| Not described as randomized. | |
| Not described as randomized. | |
| No clinical efficacy data for this vaccine is available. | |
| Not described as randomized. | |
| No true control arm for safety and immunological data. | |
| Participants did not receive a vaccine to prevent ETEC. | |
| Topic unrelated to ETEC vaccination. | |
| Not described as randomized. | |
| No clinical efficacy data for this vaccine is available. | |
| Participants did not receive a vaccine to prevent ETEC. | |
| Not described as randomized. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ETEC diarrhoea Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo, Outcome 1 ETEC diarrhoea. | ||||
| 2 Severe ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo, Outcome 2 Severe ETEC diarrhoea. | ||||
| 3 All‐cause diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo, Outcome 3 All‐cause diarrhoea. | ||||
| 4 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo, Outcome 4 Adverse events. | ||||
| 4.1 Any symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Gastrointestinal symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Respiratory symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Others | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1 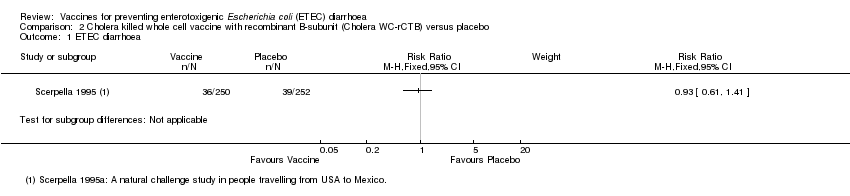 Comparison 2 Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo, Outcome 1 ETEC diarrhoea. | ||||
| 2 All‐cause diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo, Outcome 2 All‐cause diarrhoea. | ||||
| 3 ETEC diarrhoea (Scerpella 1995a subgroup analysis excluding cases of ETEC occurring < 7 days after vaccination) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3 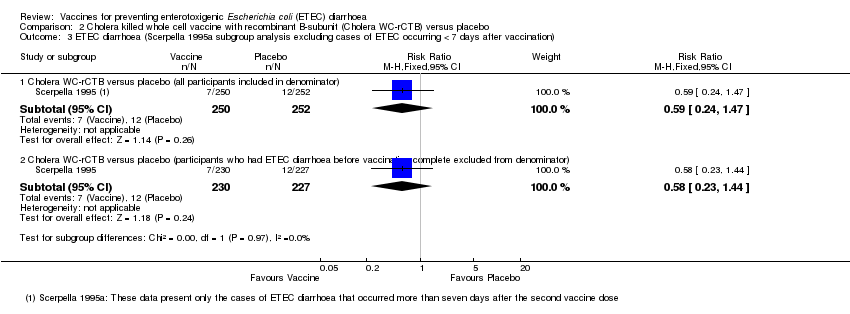 Comparison 2 Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo, Outcome 3 ETEC diarrhoea (Scerpella 1995a subgroup analysis excluding cases of ETEC occurring < 7 days after vaccination). | ||||
| 3.1 Cholera WC‐rCTB versus placebo (all participants included in denominator) | 1 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.47] |
| 3.2 Cholera WC‐rCTB versus placebo (participants who had ETEC diarrhoea before vaccination complete excluded from denominator) | 1 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.44] |
| 4 Immunological response: > 4‐fold increase in toxin‐specific IgG antibody responses in serum/plasma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4 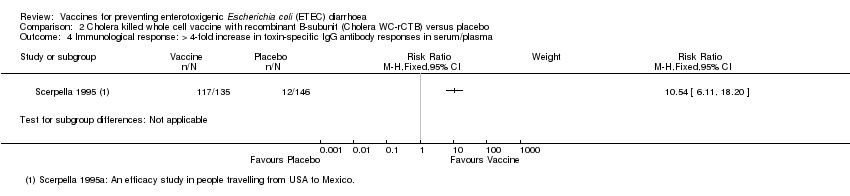 Comparison 2 Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo, Outcome 4 Immunological response: > 4‐fold increase in toxin‐specific IgG antibody responses in serum/plasma. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ETEC diarrhoea Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 1 ETEC diarrhoea. | ||||
| 2 Severe ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 2 Severe ETEC diarrhoea. | ||||
| 3 All‐cause diarrhoea Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 3 All‐cause diarrhoea. | ||||
| 4 Adverse events: ETEC WC‐rCTB versus placebo (after first dose) Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4 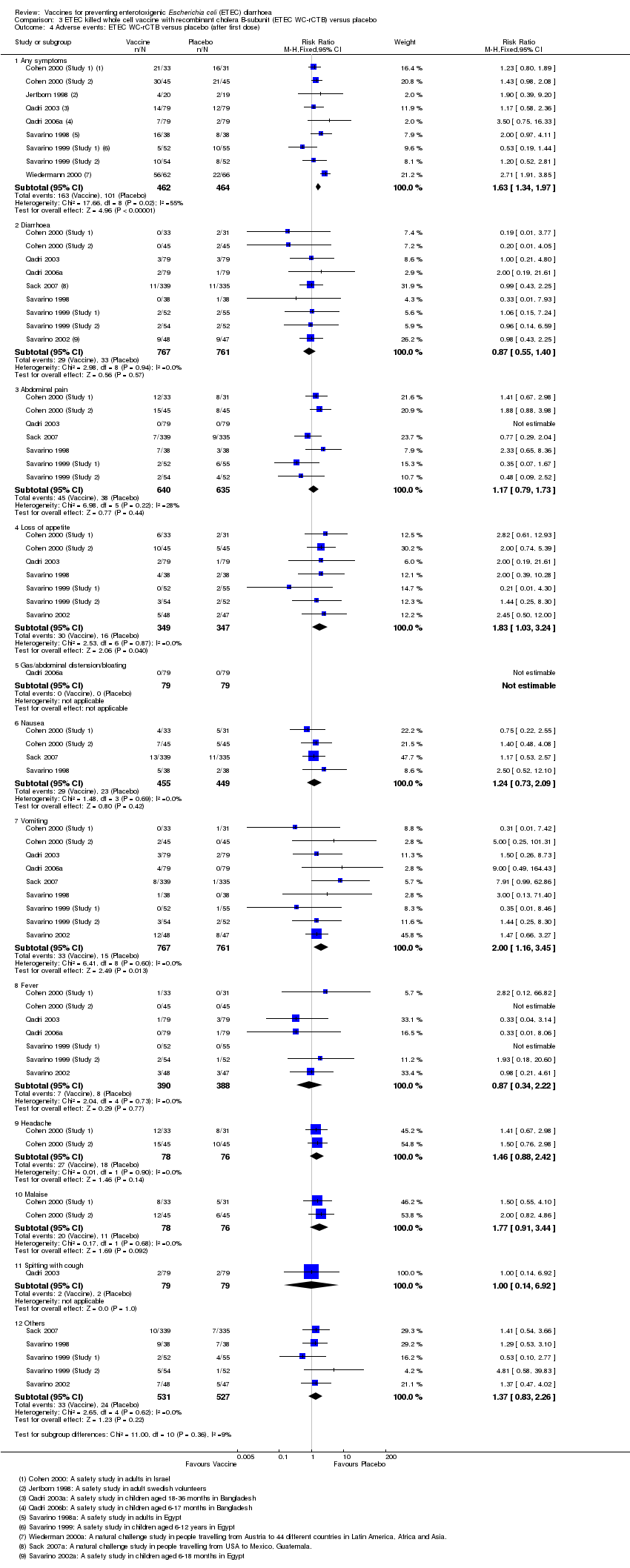 Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 4 Adverse events: ETEC WC‐rCTB versus placebo (after first dose). | ||||
| 4.1 Any symptoms | 9 | 926 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.34, 1.97] |
| 4.2 Diarrhoea | 9 | 1528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.40] |
| 4.3 Abdominal pain | 7 | 1275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.79, 1.73] |
| 4.4 Loss of appetite | 7 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.03, 3.24] |
| 4.5 Gas/abdominal distension/bloating | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Nausea | 4 | 904 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.73, 2.09] |
| 4.7 Vomiting | 9 | 1528 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.16, 3.45] |
| 4.8 Fever | 7 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.34, 2.22] |
| 4.9 Headache | 2 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.88, 2.42] |
| 4.10 Malaise | 2 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.91, 3.44] |
| 4.11 Spitting with cough | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.92] |
| 4.12 Others | 5 | 1058 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.83, 2.26] |
| 5 Immunological response: > 2‐fold increase in CFA/I‐specific IgA antibody response in serum/plasma Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 5 Immunological response: > 2‐fold increase in CFA/I‐specific IgA antibody response in serum/plasma. | ||||
| 6 Immunological response: > 2‐fold increase in toxin‐specific IgA antibody responses in serum/plasma Show forest plot | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 6 Immunological response: > 2‐fold increase in toxin‐specific IgA antibody responses in serum/plasma. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Live attenuated cholera vaccine (CVD 103‐HgR) versus placebo, Outcome 1 ETEC diarrhoea. | ||||
| 2 Moderate to severe ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2 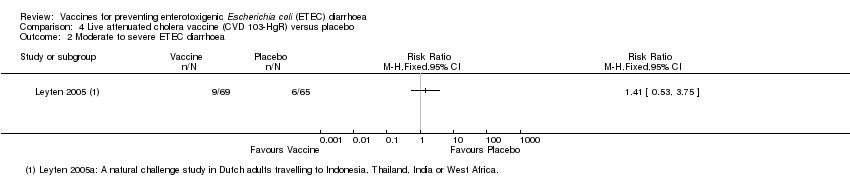 Comparison 4 Live attenuated cholera vaccine (CVD 103‐HgR) versus placebo, Outcome 2 Moderate to severe ETEC diarrhoea. | ||||
| 3 All‐cause diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3 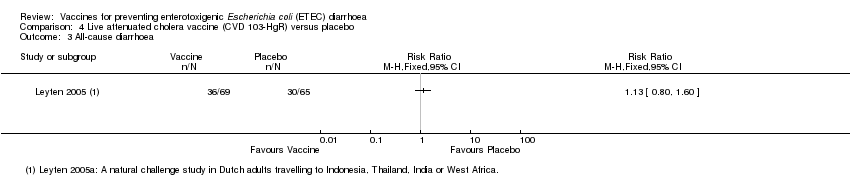 Comparison 4 Live attenuated cholera vaccine (CVD 103‐HgR) versus placebo, Outcome 3 All‐cause diarrhoea. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Live attenuated ETEC vaccine (PTL‐003) versus placebo, Outcome 1 ETEC diarrhoea. | ||||
| 2 Moderate to severe ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Live attenuated ETEC vaccine (PTL‐003) versus placebo, Outcome 2 Moderate to severe ETEC diarrhoea. | ||||
| 3 Adverse events (after first dose) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3 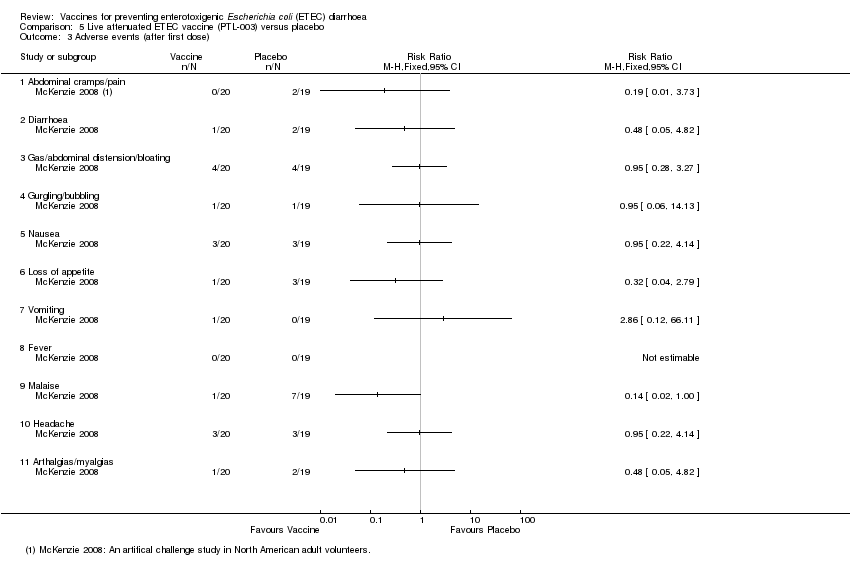 Comparison 5 Live attenuated ETEC vaccine (PTL‐003) versus placebo, Outcome 3 Adverse events (after first dose). | ||||
| 3.1 Abdominal cramps/pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Gas/abdominal distension/bloating | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Gurgling/bubbling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Loss of appetite | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Malaise | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Arthalgias/myalgias | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immunological response: > 2‐fold increase in TSA Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 Live attenuated ETEC vaccine (PTL‐003) versus placebo, Outcome 4 Immunological response: > 2‐fold increase in TSA. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ETEC diarrhoea Show forest plot | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.08] |
| Analysis 6.1  Comparison 6 Transcutaneous LT patch versus placebo, Outcome 1 ETEC diarrhoea. | ||||
| 2 Moderate to severe ETEC diarrhoea Show forest plot | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.25] |
| Analysis 6.2  Comparison 6 Transcutaneous LT patch versus placebo, Outcome 2 Moderate to severe ETEC diarrhoea. | ||||
| 3 All‐cause diarrhoea Show forest plot | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.42] |
| Analysis 6.3  Comparison 6 Transcutaneous LT patch versus placebo, Outcome 3 All‐cause diarrhoea. | ||||
| 4 Adverse events Show forest plot | 2 | 1643 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.87 [3.10, 4.84] |
| Analysis 6.4  Comparison 6 Transcutaneous LT patch versus placebo, Outcome 4 Adverse events. | ||||
| 4.1 Rash | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.80 [3.88, 8.67] |
| 4.2 Pruritus | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.66 [3.25, 6.68] |
| 4.3 Vesicles | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.65 [0.62, 184.25] |
| 4.4 Skin discoloration | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [2.87, 32.92] |
| 4.5 Fever | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.13, 31.48] |
| 4.6 Malaise | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.39, 3.19] |
| 4.7 Headache | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.82] |
| 4.8 Diarrhoea | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.67, 3.38] |
| 5 Immunological response Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.5  Comparison 6 Transcutaneous LT patch versus placebo, Outcome 5 Immunological response. | ||||
| 5.1 > 4‐fold increase in anti‐LT specific IgA responses in serum/plasma | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 43.00 [12.33, 149.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause diarrhoea Show forest plot | 2 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.22, 1.15] |
| Analysis 7.1  Comparison 7 Hyperimmune anti‐E. coli CFA versus placebo, Outcome 1 All‐cause diarrhoea. | ||||
| 2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Hyperimmune anti‐E. coli CFA versus placebo, Outcome 2 Adverse events. | ||||
| 2.1 Anorexia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Malaise | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Gas | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Abdominal pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Study flow diagram.
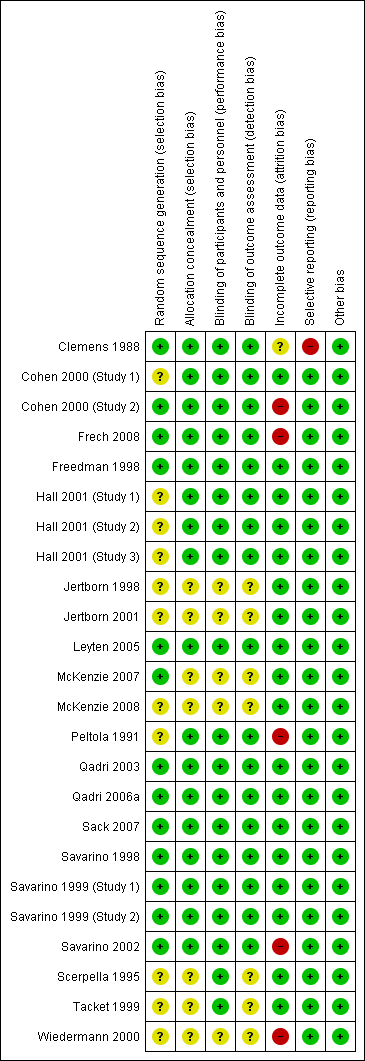
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo, Outcome 1 ETEC diarrhoea.

Comparison 1 Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo, Outcome 2 Severe ETEC diarrhoea.

Comparison 1 Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo, Outcome 3 All‐cause diarrhoea.

Comparison 1 Cholera killed whole cell vaccine (Cholera WC‐BS) versus placebo, Outcome 4 Adverse events.
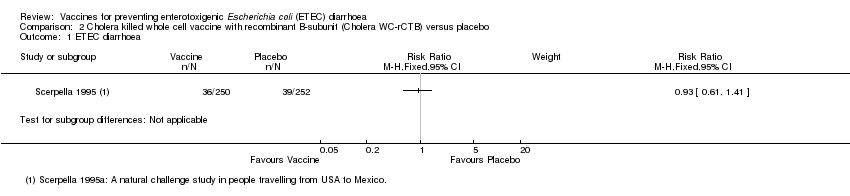
Comparison 2 Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo, Outcome 1 ETEC diarrhoea.
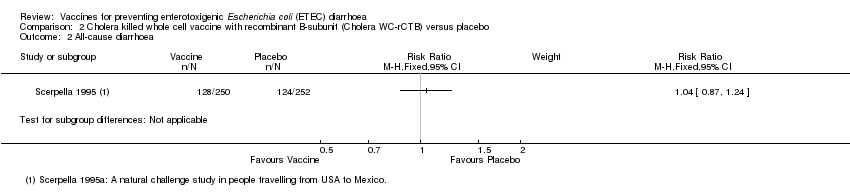
Comparison 2 Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo, Outcome 2 All‐cause diarrhoea.

Comparison 2 Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo, Outcome 3 ETEC diarrhoea (Scerpella 1995a subgroup analysis excluding cases of ETEC occurring < 7 days after vaccination).

Comparison 2 Cholera killed whole cell vaccine with recombinant B‐subunit (Cholera WC‐rCTB) versus placebo, Outcome 4 Immunological response: > 4‐fold increase in toxin‐specific IgG antibody responses in serum/plasma.
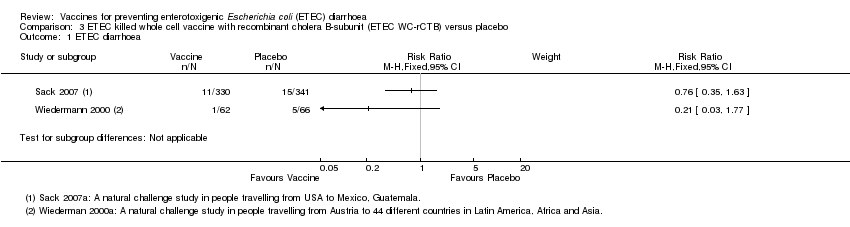
Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 1 ETEC diarrhoea.

Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 2 Severe ETEC diarrhoea.

Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 3 All‐cause diarrhoea.

Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 4 Adverse events: ETEC WC‐rCTB versus placebo (after first dose).

Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 5 Immunological response: > 2‐fold increase in CFA/I‐specific IgA antibody response in serum/plasma.

Comparison 3 ETEC killed whole cell vaccine with recombinant cholera B‐subunit (ETEC WC‐rCTB) versus placebo, Outcome 6 Immunological response: > 2‐fold increase in toxin‐specific IgA antibody responses in serum/plasma.

Comparison 4 Live attenuated cholera vaccine (CVD 103‐HgR) versus placebo, Outcome 1 ETEC diarrhoea.

Comparison 4 Live attenuated cholera vaccine (CVD 103‐HgR) versus placebo, Outcome 2 Moderate to severe ETEC diarrhoea.

Comparison 4 Live attenuated cholera vaccine (CVD 103‐HgR) versus placebo, Outcome 3 All‐cause diarrhoea.

Comparison 5 Live attenuated ETEC vaccine (PTL‐003) versus placebo, Outcome 1 ETEC diarrhoea.
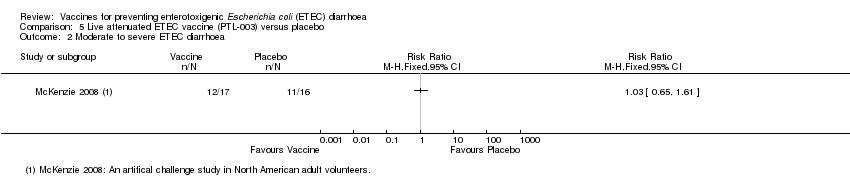
Comparison 5 Live attenuated ETEC vaccine (PTL‐003) versus placebo, Outcome 2 Moderate to severe ETEC diarrhoea.

Comparison 5 Live attenuated ETEC vaccine (PTL‐003) versus placebo, Outcome 3 Adverse events (after first dose).

Comparison 5 Live attenuated ETEC vaccine (PTL‐003) versus placebo, Outcome 4 Immunological response: > 2‐fold increase in TSA.
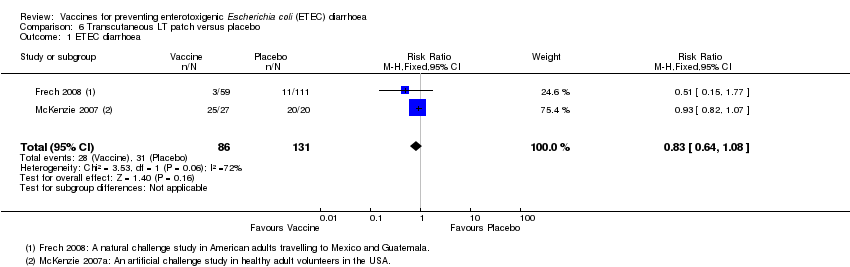
Comparison 6 Transcutaneous LT patch versus placebo, Outcome 1 ETEC diarrhoea.

Comparison 6 Transcutaneous LT patch versus placebo, Outcome 2 Moderate to severe ETEC diarrhoea.
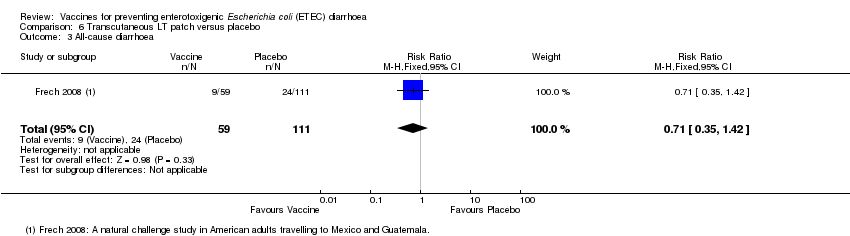
Comparison 6 Transcutaneous LT patch versus placebo, Outcome 3 All‐cause diarrhoea.

Comparison 6 Transcutaneous LT patch versus placebo, Outcome 4 Adverse events.
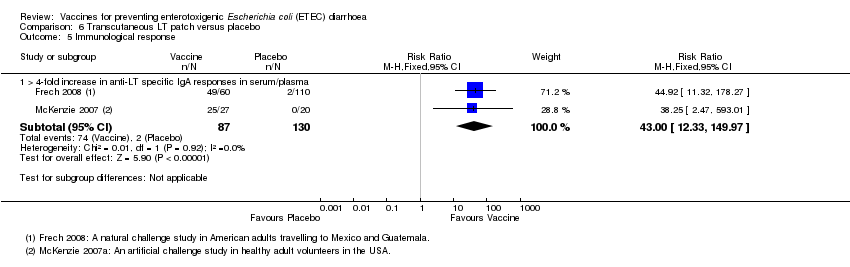
Comparison 6 Transcutaneous LT patch versus placebo, Outcome 5 Immunological response.

Comparison 7 Hyperimmune anti‐E. coli CFA versus placebo, Outcome 1 All‐cause diarrhoea.
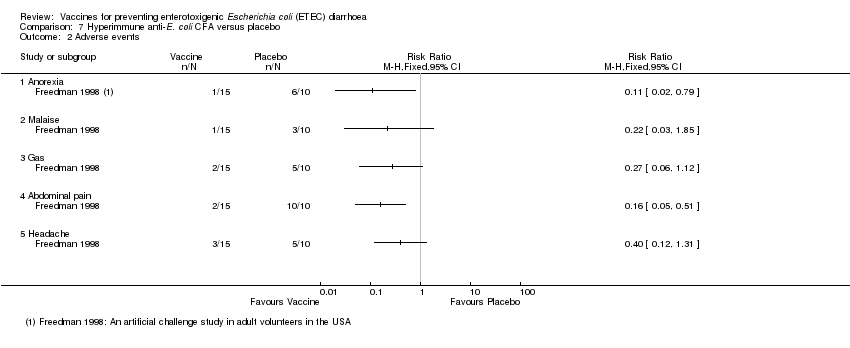
Comparison 7 Hyperimmune anti‐E. coli CFA versus placebo, Outcome 2 Adverse events.
| Cholera killed whole cells plus recombinant B‐subunit vaccine for enterotoxigenic E. coli (ETEC) diarrhoea | ||||||
| Patient or population: People travelling from non‐endemic settings | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vaccine | |||||
| ETEC diarrhoea | 99 per 1000 | 120 per 1000 | RR 0.93 | 502 | ⊕⊕⊝⊝ | |
| Severe ETEC diarrhoea | ‐ | ‐ | ‐ | (0 studies) | ‐ | |
| All‐cause diarrhoea | 492 per 1000 | 512 per 1000 | RR 1.04 | 502 | ⊕⊕⊝⊝ | |
| Adverse events | ‐ | ‐ | ‐ | 502 | 6 | |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 This single study was conducted in adults travelling from the USA to Mexico (Scerpella 1995). Although the paper does not clearly describe the methods used to prevent selection bias, we have not downgraded the evidence as selection bias is probably unlikely in a trial where everyone is healthy at enrolment. | ||||||
| Oral inactivated vaccines
|
| Oral live attenuated vaccines
|
| Other ETEC vaccines under development include
|
| WC/rCTB: whole cell/recombinant cholera toxin B subunit; ETEC: enterotoxigenic E. coli; CFA: colonization factor antigen; CS: E. coli surface antigen; LT: heat labile toxin; CT: cholera toxin; LTB/ST: heat labile toxin B subunit/heat stable toxin. |
| Search set | CIDG SR¹ | CENTRAL | MEDLINE² | EMBASE² | LILACS² |
| 1 | E.coli | Enterotoxigenic Escherichia coli [MeSH] | Enterotoxigenic Escherichia coli [MeSH] | Enterotoxigenic Escherichia coli [Emtree] | E.coli |
| 2 | Enterotoxigenic | ETEC | ETEC ti, ab | ETEC ti, ab | Enterotoxigenic |
| 3 | ETEC | Enterotoxigenic E coli | Enterotoxigenic E coli ti, ab | Enterotoxigenic E coli ti, ab | ETEC |
| 4 | Travel* diarrh* | Travel* diarrh* | Travel* diarrh* ti, ab | Traveller diarrhea [Emtree] | Travel$ diarrh$ |
| 5 | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 |
| 6 | Vaccin* | Vaccin* | Vaccin* ti, ab | Vaccin* ti, ab | Vaccin$ |
| 7 | 5 and 6 | 5 and 6 | 5 and 6 | 5 and 6 | 5 and 6 |
| 8 |
| Escherichia coli vaccines [MeSH] | Escherichia coli vaccines [MeSH] | Escherichia coli vaccine [Emtree] |
|
| 9 |
| 7 or 8 | 7 or 8 | 7 or 8 |
|
| 10 |
|
| Limit 9 to humans | Limit 9 to Human |
|
| ¹Cochrane Infectious Diseases Group Specialized Register. ²Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2008). | |||||
| Vaccine details | Study ID | Population details | Challenge | |||||
| Type | Name | Route | Schedule | Age | Group | Country setting | ||
| Inactivated | Cholera WC‐BS | Oral | Three doses, at 6 week intervals | Children aged 2 to 15 years Women aged > 15 years | Endemic | Bangladesh | Natural | |
| Oral | Two doses two weeks apart | Adults | Travellers | From Finland to Morocco | Natural | |||
| Cholera WC‐rCTB (Dukoral®) | Oral | Two doses, 10 days apart | Adults | Travellers | From USA to Mexico | Natural | ||
| ETEC WC‐rCTB | Oral | Two doses, 7 to 21 days apart | Adults | Travellers | From USA to Mexico or Guatemala | Natural | ||
| Oral | Two doses, 7 to 21 days apart | All ages | Travellers | From Austria to Latin America, Africa, and Asia. | Natural | |||
| Live attenuated | CVD 103‐HgR | Oral | Single dose | Adults | Travellers | From Holland to Indonesia, Thailand, India, or West Africa | Natural | |
| PTL‐003 | Oral | 2 doses, 10 days apart | Adults | Volunteers | USA | Artificial | ||
| Other | Transcutaneous LT‐ETEC patch | Patch | 2 to 3 doses, at 2 to 3 week intervals | Adults | Travellers | From USA to Mexico and Guatemala | Natural | |
| Patch | 2 to 3 doses, at 2 to 3 week intervals | Adults | Volunteers | USA | Artificial | |||
| Hyper immune Anti‐E coli. CFA | Oral | 3 times daily for 5 to 7 days | Adults | Volunteers | USA | Artificial | ||
| Oral | 3 times daily for 5 to 7 days | Adults | Volunteers | USA | Artificial | |||
| WC = killed whole cell, BS = cholera toxin B subunit, rCTB = recombinant cholera toxin B subunit. | ||||||||
| Vaccine | Study ID | Trial definitions | Challenge type | Confirmation of ETEC | |
| DIarrhoea | Moderate or Severe Diarrhoea | ||||
| Cholera WC‐BS | ≥ 3 non‐bloody loose stools in 24 hours, or fewer episodes with signs of dehydration | Severe diarrhoea = absent or feeble pulse plus one other sign of dehydration. | Natural | Faecal culture | |
| Any diarrhoea confirmed by the physician as abnormally loose | Not reported. | Natural | Faecal culture | ||
| Cholera WC‐rCTB (Dukoral®) | ≥ 4 unformed stools in 24 hours, or ≥3 unformed stools in 8 hours, plus an additional symptom (nausea. pain, fever, urgency, tenesmus) | Not reported. | Natural | Faecal culture | |
| ETEC WC‐rCTB | ≥ 3 loose stools in 24 hours, plus at least one other symptom, such as abdominal pain, cramps or nausea. | Severe diarrhoea = ≥ 5 loose stools in 24 hours, or illness episodes with abdominal cramps, pain, or vomiting that interfered with daily activities. | Natural | Faecal culture | |
| ≥ 3 liquid stools | Not reported. | Natural | Faecal culture | ||
| CVD 103‐HgR | ≥ 3 unformed stools in 24 hours, or 2 unformed stools accompanied by vomiting, abdominal cramps or subjective fever, | Moderate diarrhoea = 3 to 6 stools per day Severe diarrhoea = ≥ 6 stools per day | Natural | Faecal culture | |
| PTL‐003 | 1 loose stool weighing ≥ 300 g, or ≥ 2 loose stools in 48 h with a combined weight of ≥200 g, | Moderate diarrhoea = 4 to 5 loose stools, or 401 to 800 g of loose stool, in 24 hours Severe diarrhoea = ≥ 6 loose stools, or > 800 g of loose stools, in 24 hours Or mild diarrhoea plus one of these symptoms rated as moderate or severe: nausea, vomiting, anorexia, abdominal pain, or cramps. | Artificial | Assumed all | |
| Transcutaneous LT‐ETEC patch | ≥ 3 loose stools in 24 hours | Moderate diarrhoea = 4 to 5 loose stools Severe diarrhoea = 6 or more loose stools | Natural | Faecal culture | |
| 1 loose stool weighing ≥ 300 g or ≥ 2 loose stools in 48 hours weighing a total of ≥200 g, within 120 hours after challenge. | Moderate/severe diarrhoea = > 400 g of loose stool in 24 hours, or ≥ 4 loose stools in 24 hours, or ≥ 2 loose stools within a 48 hour period totaling ≥ 200 g, or a single loose stool weighing ≥ 300 g plus one of the following symptoms rated as moderate or severe: nausea, vomiting, abdominal pain, or cramps. | Artificial | Assumed all | ||
| Hyperimmune Anti‐E coli. CFA | 1 liquid stool of ≥ 300 mL or 2 liquid stools totaling > 200 mL during any 48 hour period within 120 hours after challenge. | Not reported. | Artificial | Assumed all | |
| 1 diarrhoeal stool of > 300 mL or 2 diarrhoeal stools totaling > 200 mL passed during a 48 hour period within 96 hours after challenge. | Not reported. | Artificial | Assumed all | ||
| Study ID | Age group | Trial setting | Number of participants with a > 2‐fold increase in immunological response after the second dose of oral ETEC‐rCTB (%) | ||||||||||
| CFA/I | CS1 | CS2 | CS3 | CS4 | Remarks | ||||||||
| V | P | V | P | V | P | V | P | V | P | ||||
| Adults | Egypt | 15/16 (94%) | 4/16 (25%) | 11/16 (69%) | 1/16 (6%) | 13/16 (81%) | 2/16 (13%) | ND | ND | 16/16 (100%) | 6/16 (38%) | Serum | |
| Adults | Israel | 9/35 (26%) | 1/40 (3%) | 11/35 (31%) | 2/40 (5%) | ND | ND | ND | ND | ND | ND | Serum | |
| Adults | Sweden | 16/19 (84%) | 0/12 (0%) | 4/19 (21%) | 1/12 (8%) | 10/19 (53%) | 0/12 (0%) | ND | ND | 12/19 (63%) | 0/12 (0%) | Serum | |
| Adults | Egypt | 26/38 (68%) | 2/35 (6%) | 21/38 (56%) | 0/35 (0%) | 12/38 (31%) | 2/35 (6%) | ND | ND | 26/38 (69%) | 4/35 (12%) | Serum | |
| Children 6‐12 Y | Egypt | 16/16 (100%) | 2/16 (13%) | 3/8 (38%) | 1/9 (11%) | 12/13 (92%) | 2/12 (17%) | ND | ND | 14/15 (93%) | 4/16 (25%) | ASC | |
| Children 6‐12 Y | Egypt | 49/51 (96%) | 6/54 (11%) | 47/51 (92%) | 4/54 (7%) | 40/51 (78%) | 5/54 (9%) | ND | ND | 43/51 (84%) | 3/54 (6%) | Serum | |
| Children 2‐5 Y | Egypt | 18/19 (95%) | 1/10 (10%) | ND | ND | 15/18 (83%) | 1/10 (10%) | ND | ND | ND | ND | ASC | |
| Children 2‐5 Y | Egypt | 41/47 (87%) | 5/46 (11%) | 43/47 (91%) | 1/46 (2%) | 37/47 (79%) | 6/46 (12%) | ND | ND | 33/47 (70%) | 3/46 (7%) | Serum | |
| Children 18‐36 M | Bangladesh | 58/79 (73%) | 13/79 (16%) | 61/79 (77%) | 10/79 (13%) | 69/79 (87%) | 8/79 (10%) | ND | ND | 55/79 (70%) | 2/79 (3%) | Plasma | |
| Children 6‐18 M | Egypt | 22/36 (61%) | 5/28 (18%) | 7/36 (20%) | 1/28 (4%) | 9/36 (26%) | 1/28 (4%) | ND | ND | 14/36 (39%) | 2/28 (7%) | Serum | |
| Children 6‐17 M | Bangladesh | 47/79 (59%) | 4/79 (5%) | 53/79 (67%) | 26/79 (33%) | 37/79 (47%) | 16/79 (20%) | 40/79 (50%) | 12/79 (15%) | ND | ND | Plasma | |
| V = vaccine, P = placebo, CS = colonization surface antigen, ND = not done , ASC = antibody secreting cell, CFA = colonization factor antibody | |||||||||||||
| Study ID | Number of participants with > 2‐fold increase in immunological response (%) | ||||||||
| CS1 | CS2 | CS3 | CS4 | Remarks | |||||
| V | P | V | P | V | P | V | P | ||
| 7/17 (41%) | 1/16 (6%) | ND | ND | 7/17 (41%) | 0/16 (0%) | ND | ND | Serum | |
| V = vaccine, P = placebo, CS = colonization surface antigen, ND = not done | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ETEC diarrhoea Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Severe ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 All‐cause diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Any symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Gastrointestinal symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Respiratory symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Others | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 All‐cause diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 ETEC diarrhoea (Scerpella 1995a subgroup analysis excluding cases of ETEC occurring < 7 days after vaccination) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cholera WC‐rCTB versus placebo (all participants included in denominator) | 1 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.47] |
| 3.2 Cholera WC‐rCTB versus placebo (participants who had ETEC diarrhoea before vaccination complete excluded from denominator) | 1 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.44] |
| 4 Immunological response: > 4‐fold increase in toxin‐specific IgG antibody responses in serum/plasma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ETEC diarrhoea Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Severe ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 All‐cause diarrhoea Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Adverse events: ETEC WC‐rCTB versus placebo (after first dose) Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Any symptoms | 9 | 926 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.34, 1.97] |
| 4.2 Diarrhoea | 9 | 1528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.40] |
| 4.3 Abdominal pain | 7 | 1275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.79, 1.73] |
| 4.4 Loss of appetite | 7 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.03, 3.24] |
| 4.5 Gas/abdominal distension/bloating | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Nausea | 4 | 904 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.73, 2.09] |
| 4.7 Vomiting | 9 | 1528 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.16, 3.45] |
| 4.8 Fever | 7 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.34, 2.22] |
| 4.9 Headache | 2 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.88, 2.42] |
| 4.10 Malaise | 2 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.91, 3.44] |
| 4.11 Spitting with cough | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.92] |
| 4.12 Others | 5 | 1058 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.83, 2.26] |
| 5 Immunological response: > 2‐fold increase in CFA/I‐specific IgA antibody response in serum/plasma Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Immunological response: > 2‐fold increase in toxin‐specific IgA antibody responses in serum/plasma Show forest plot | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Moderate to severe ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 All‐cause diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Moderate to severe ETEC diarrhoea Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events (after first dose) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Abdominal cramps/pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Gas/abdominal distension/bloating | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Gurgling/bubbling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Loss of appetite | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Malaise | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Arthalgias/myalgias | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immunological response: > 2‐fold increase in TSA Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ETEC diarrhoea Show forest plot | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.08] |
| 2 Moderate to severe ETEC diarrhoea Show forest plot | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.25] |
| 3 All‐cause diarrhoea Show forest plot | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.42] |
| 4 Adverse events Show forest plot | 2 | 1643 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.87 [3.10, 4.84] |
| 4.1 Rash | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.80 [3.88, 8.67] |
| 4.2 Pruritus | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.66 [3.25, 6.68] |
| 4.3 Vesicles | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.65 [0.62, 184.25] |
| 4.4 Skin discoloration | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [2.87, 32.92] |
| 4.5 Fever | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.13, 31.48] |
| 4.6 Malaise | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.39, 3.19] |
| 4.7 Headache | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.59, 2.82] |
| 4.8 Diarrhoea | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.67, 3.38] |
| 5 Immunological response Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 > 4‐fold increase in anti‐LT specific IgA responses in serum/plasma | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 43.00 [12.33, 149.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause diarrhoea Show forest plot | 2 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.22, 1.15] |
| 2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Anorexia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Malaise | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Gas | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Abdominal pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

