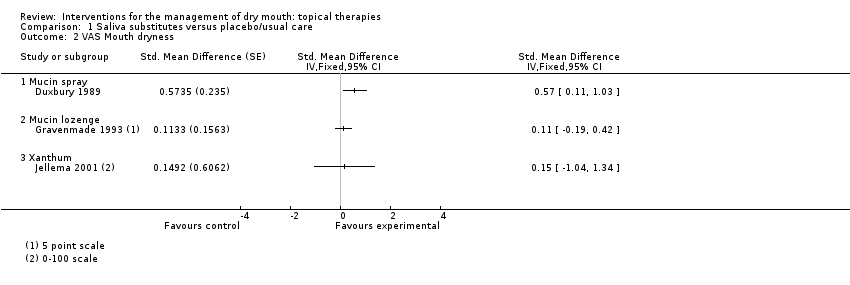
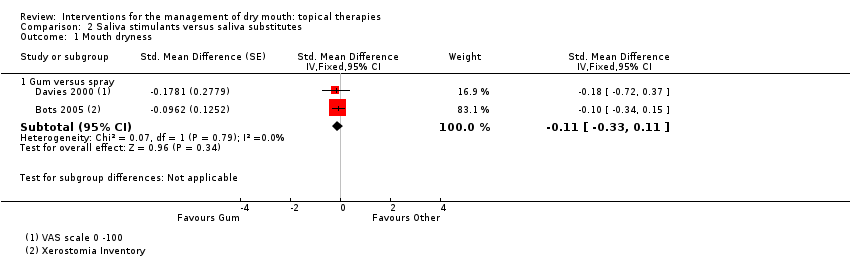
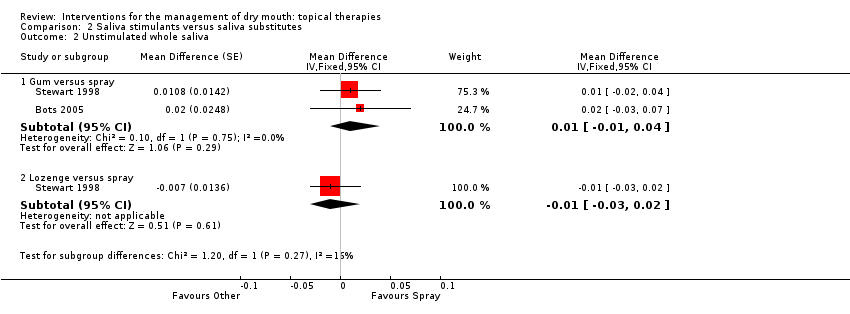
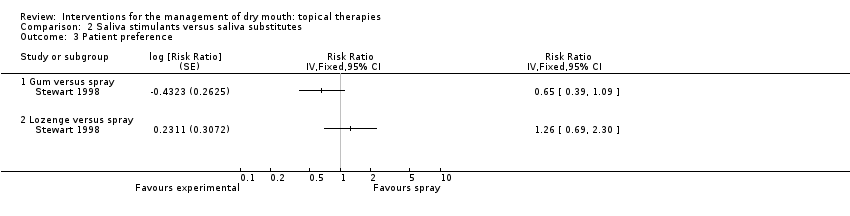
| Trial | Interventions | Trial Design | Outcomes |
| Andersson 1995 | CMC versus Salinum linseed oil spray | Crossover | Data did not account for crossover design. Text states that more patients experienced reduction in oral dryness when using Salinum linseed spray than when the CMC spray was used. More participants preferred the linseed oil spray. Paper states that both mean plaque and gingival indices were reduced during the Salinum period but gives incomplete comparative data. No adverse effects reported. |
| Epstein 1999 | Biotene oral Balance Gel & toothpaste versus placebo (CMC gel and regular toothpaste) | Crossover trial with some period effect so only first period data reported | Based on first period data authors report Biotene products "more effective than control (P = 0.04) and reduced dry mouth on waking (P = 0.05)" |
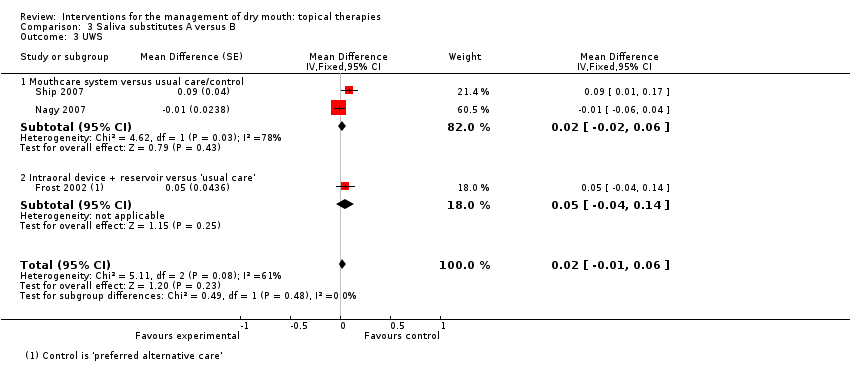
| Frost 2002 | IntraOral device releasing Biotene Oral Balance gel versus usual care | Crossover | Median (IQR) treatment versus control. Dryness 4 (2 to 4) versus 3 (2 to 4) (P = 0.056) Speech 4 (2 to 5) versus 2 (2 to 5) (P = 0.003) Swallowing 4 (2 to 5) versus 2.5 (2 to 4) (P = 0.031) Patient preference was greater in the device group (66%) compared to control but data available were not suitable for meta‐analysis. |
| Furumoto 1998 | CMC spray versus HCC spray versus HCC gel versus margarine | Crossover. Appropriate paired analysis and medians (IQR) reported as data distribution skewed | Median oral dryness score after treatment (low score = less dry) on day 10 (IQR). CMC spray 28.00 (3.06) HCC spray 28.75 (2.23) HCC gel 27.75 (3.62) Margarine 29.25 (5.06) Median Duration of Relief ‐ minutes (IQR). CMC spray 1.33 (0.61) HCC spray 1.33 (1.25) HCC gel 2.44 (0.90) Margarine 1.39 (0.68) Only HCC gel showed statistically significant improvement in oral dryness compared to baseline over 10 days of use (P < 0.05). HCC gel "rated better than margarine for convenience, perceived value, minutes of relief & overall rating. No other significant between group differences." |
| Gil‐Montoya 2008 | Biotene Oral Balance mouthwash & gel versus mint flavoured water and CMC cream, used 3 to 4 x daily for 4 weeks | Pilot crossover | Results: "improvement in the need to drink liquids in order to swallow was greater in the study group during the first period, but smaller in the same group during the second period.....improvement in dry floor of mouth was greater in the placebo group during the first period, but smaller in the same group during the second period.....the number of patients that improved in their OHIP scores was paradoxically somewhat greater in the placebo group in both interventional periods." |
| Johansson 2001 | Salinum (linseed) mouthwash versus Salinum + chlorhexidine | Crossover | Paired nature of data not accounted for in analysis. Both products resulted in improved oral dryness & chewing/swallowing & Salinum reduced speaking problems & burning mouth. |
| McMillan 2006 | Oral Balance gel by oral device versus Oral Balance gel bolus | Crossover | Trial presented median ± IQR for the outcome of change in oral dryness (X Inventory & GOHAI) & UWS after treatment and found "no difference in oral dryness or flow rates between the 2 treatments". Likewise "there was no significant difference in change in oral microbial profile or generalized inflammation between the two treatments". |
| Momm 2005 | Aloe vera gel versus CMC spray versus canola oil spray versus mucin spray | Crossover | At baseline mean xerostomia score (scale 1 to 6) was 4.5 ± 0.11. Post‐treatment mean xerostomia scores were 3.7 ± 0.11, 3.8 ± 0.12, 3.8 ± 0.12, 3.8 ± 0.11 for aloe vera, CMC, Canola oil and mucin respectively. P < 0.001 for comparison between baseline and post‐treatment mean, for all groups. |
| Mouly 2007b | Oxygenated glycerol triester (OGT) spray versus Saliveeze | Parallel group | Patient satisfaction concerning taste favoured OGT, mean difference of VAS score for taste 1.4 ± 0.6 P = 0.04. Overall mouth condition showed improvement in both groups & "no significant differences between the treatment groups were found". |
| Poland 1987 | CMC/sorbitol swabs versus lemon glycerin swabs | Crossover | CMC sorbitol reported to be better than lemon glycerin. Incomplete data presented could not be included in meta‐analysis and the authors noted a period effect. CMC/sorbitol also improved "dentition and gingival scores" though no data to support this were presented in the report. Patient preference favoured CMC/sorbitol (Chi² 10.89 (df = 1); P = 0.001). |
| Rantanen 2003 | 1% sodium lauryl sulphate (SLS) toothpaste versus 4% betaine (BET) toothpaste versus combined 1% SLS+4% BET toothpaste | Crossover trial with 6‐week washout periods between treatment phases when a reference toothpaste containing neither SLS nor betaine was used | Relief of oral dryness in xerostomic patients. BET 44% BET + SLS 22% (P = 0.002 compared to BET) SLS 18% (P = 0.022 compared to BET) Ref 7% (P = 0.000 compared to BET) No adverse effects reported. |
| Shahdad 2005 | Biotene mouthcare system versus BioXtra mouthcare system | Crossover | Participants were asked 5 questions concerning the 'pleasance' of the products. BioXtra was rated statistically significantly better for pleasant taste of toothpaste, mouthwash and gel and there was no difference between the systems for mouth 'feel'. Xerostomia‐related QoL was assessed with a 15 item survey, and overall there was no difference between the 2 systems. This trial is likely to have inadequate statistical power to detect a difference. |
| Ship 2007 | Xerostom system (betaine, olive oil and xylitol) versus usual care | Crossover | Evaluated 8 aspects of xerostomia and found statistically significant differences between control and Xerostom in overall mouth dryness (P = 0.038), overall tongue dryness (P = 0.002) and level of thirst (P = 0.0001) favouring Xerostom group. No data presented. |
| Shirodaria 2006 | OASIS moisturising mouthwash versus experimental mouthwash | Crossover design: 3 days acclimatisation, 3 day treatment A, 3 day washout usual care, 3 day treatment B | Paper reports OASIS showed "statistically significant improvement over subject's normal remedies for managing dry mouth for strength of flavour, taste, freshening breath, immediate and long‐lasting lubrication and moisturization" based on 5 point rating scales, but no data were presented. |