Maßnahmen zur Behandlung von Spastizität der Skelettmuskeln nach Schädel‐Hirn‐Trauma
Abstract
Background
Skeletal muscle spasticity is a major physical complication resulting from traumatic brain injury (TBI), which can lead to muscle contracture, joint stiffness, reduced range of movement, broken skin and pain. Treatments for spasticity include a range of pharmacological and non‐pharmacological interventions, often used in combination. Management of spasticity following TBI varies from other clinical populations because of the added complexity of behavioural and cognitive issues associated with TBI.
Objectives
To assess the effects of interventions for managing skeletal muscle spasticity in people with TBI.
Search methods
In June 2017, we searched key databases including the Cochrane Injuries Group Specialised Register, CENTRAL, MEDLINE (Ovid), Embase (Ovid) and others, in addition to clinical trials registries and the reference lists of included studies.
Selection criteria
We included randomised controlled trials (RCTs) and cross‐over RCTs evaluating any intervention for the management of spasticity in TBI. Only studies where at least 50% of participants had a TBI (or for whom separate data for participants with TBI were available) were included. The primary outcomes were spasticity and adverse effects. Secondary outcome measures were classified according to the World Health Organization International Classification of Functioning, Disability and Health including body functions (sensory, pain, neuromusculoskeletal and movement‐related functions) and activities and participation (general tasks and demands; mobility; self‐care; domestic life; major life areas; community, social and civic life).
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Data were synthesised narratively; meta‐analysis was precluded due to the paucity and heterogeneity of data.
Main results
We included nine studies in this review which involved 134 participants with TBI. Only five studies reported between‐group differences, yielding outcome data for 105 participants with TBI. These five studies assessed the effects of a range of pharmacological (baclofen, botulinum toxin A) and non‐pharmacological (casting, physiotherapy, splints, tilt table standing and electrical stimulation) interventions, often in combination. The studies which tested the effect of baclofen and tizanidine did not report their results adequately. Where outcome data were available, spasticity and adverse events were reported, in addition to some secondary outcome measures.
Of the five studies with results, three were funded by governments, charities or health services and two were funded by a pharmaceutical or medical technology company. The four studies without useable results were funded by pharmaceutical or medical technology companies.
It was difficult to draw conclusions about the effectiveness of these interventions due to poor reporting, small study size and the fact that participants with TBI were usually only a proportion of the overall total. Meta‐analysis was not feasible due to the paucity of data and heterogeneity of interventions and comparator groups. Some studies concluded that the intervention they tested had beneficial effects on spasticity, and others found no difference between certain treatments. The most common adverse event was minor skin damage in people who received casting. We believe it would be misleading to provide any further description of study results given the quality of the evidence was very low for all outcomes.
Authors' conclusions
The very low quality and limited amount of evidence about the management of spasticity in people with TBI means that we are uncertain about the effectiveness or harms of these interventions. Well‐designed and adequately powered studies using functional outcome measures to test the interventions used in clinical practice are needed.
PICO
Laienverständliche Zusammenfassung
Behandlungsmethoden für Spastizität (überaktive Muskelkontraktionen) nach Schädel‐Hirn‐Trauma
Fragestellung
Wir untersuchten die Evidenz bezüglich der Wirkung von Behandlungsmaßnahmen (medikamentös und nicht‐medikamentös) für Spastizität nach einer, durch einen Schlag gegen den Kopf bedingten, Verletzung des Gehirns (Schädel‐Hirn‐Trauma, SHT).
Hintergrund
Viele Menschen mit SHT weisen eine muskuläre Spastizität auf, wenn sich ihre Muskeln unwillkürlich zusammenziehen oder verkrampfen. Das kann sich negativ auf die Fähigkeit eines Menschen auswirken, tägliche Aktivitäten auszuführen, indem es zu Schmerzen, Steifheit und Hautschädigungen kommt. Es gibt viele Behandlungsmethoden, um die Spastizität zu beeinflussen, beispielsweise Medikamente, Gipsfixation, Schienen und Dehnungsübungen. Häufig werden diese Behandlungsmethoden in Kombination benutzt.
Studienmerkmale
Wir schlossen neun Studien mit 134 Teilnehmern mit SHT in diesen Review ein. Nur fünf Studien mit 105 Teilnehmern lieferten brauchbare Ergebnisse. Diese Studien untersuchten die Wirkungen einer Reihe von Behandlungsmassnahmen, wie Medikamente (Baclofen oder Botulinumtoxin A), Gipsfixation, Physiotherapie, Schienen, einen Tisch, der die Menschen von der liegenden in die stehende Position bringt und Elektrostimulation (hier werden den Muskeln elektrische Impulse zugeführt). Die Studien, die die Ergebnisse unzureichend berichteten, hatten die Wirkung von Medikamenten (Baclofen oder Tizanidin) untersucht.
Quellen der Studienfinanzierung
Von den fünf Studien mit Ergebnissen wurden drei von Regierungen, Wohltätigkeitsorganisationen oder Institutionen des Gesundheitswesens finanziert. Zwei wurden von einem Arzneimittelhersteller und einem Medizintechnik‐Unternehmen finanziert. Die anderen vier Studien ohne brauchbare Ergebnisse wurden von einem Arzneimittelhersteller oder Medizintechnik‐Unternehmen finanziert.
Hauptergebnisse
Die Evidenz ist auf dem Stand von Juni 2017.
Es war schwierig, die Ergebnisse dieser Studien zu interpretieren, da Informationen fehlten und Bedenken bezüglich der Qualität der Evidenz vorlagen. Spastizität betreffend kamen einige Studien zum Schluss, dass die untersuchte Behandlungsmaßnahme zu einer Verbesserung führte, während andere keinen Unterschied zwischen den Behandlungsmethoden fanden. Die häufigste Nebenwirkung bestand in einer leichten Hautschädigung bei Menschen, die mit Gipsfixation behandelt wurden. Wir glauben, dass es irreführend wäre, weitere Beschreibungen der Studienresultate bereitzustellen, da die Qualität der Evidenz sehr niedrig für alle Messungen war.
Qualität der Evidenz
Die Qualität der Evidenz war sehr niedrig; wir hatten nur fünf Studien mit Ergebnissen und keine dieser Studien war groß oder mit den anderen vergleichbar. Wir hatten ebenfalls Bedenken, wie diese Studien durchgeführt oder ausgewertet wurden. Aus diesen Gründen können wir keine verlässlichen Schlussfolgerungen über Nutzen und Schaden der verschiedenen Behandlungsmethoden der Spastizität bei Menschen mit SHT ziehen.
Authors' conclusions
Summary of findings
| Baclofen compared with placebo for spasticity in people with traumatic brain injury | |||
| Patient or population: adults with traumatic brain injury with spasticity in their arms and legs Settings: outpatient rehabilitation clinic (US) Intervention: intrathecal baclofen 50 μg (injected into the lumbar spine) Comparison: saline placebo | |||
| Outcomes | Results and conclusions | No of participants | Quality of the evidence |
| Spasticity at up to 6 hours after treatment (measured by the Ashworth Scale, 0‐, with a higher score indicating greater spasticity) | We are uncertain about the effect of baclofen on spasticity compared with placebo.1 | 11 | ⊕⊝⊝⊝ |
| Adverse events | We are uncertain about the effect of baclofen on adverse events compared with placebo.4 | 11 | ⊕⊝⊝⊝ |
| Sensory functions and pain | No study measured this outcome. | ||
| Neuromusculoskeletal and movement‐related functions up to 6 hours after treatment (Measured by spasm and deep tendon reflex scores, 0‐5, with 0 being no reflexes and 5 being clonus, or repeated involuntary muscle contractions) | We are uncertain about the effect of baclofen on neuromusculoskeletal and movement‐related functions compared with placebo.6 | 11 (1)2 | ⊕⊝⊝⊝ |
| General tasks and demands | No study measured this outcome. | ||
| Mobility | No study measured this outcome. | ||
| Self‐care | No study measured this outcome. | ||
| GRADE Working Group grades of evidence | |||
| 1One study of baclofen reported an improvement in spasticity in the upper and lower limbs, compared to placebo, several hours after the injections but it was unclear how meaningful this improvement was due to reporting of P values only (Meythaler 1996). 2Three additional studies, with 35 participants, measured this outcome but had no useable results (Meythaler 1997; Meythaler 1999a; Meythaler 1999b). 3Downgraded four times due to risk of bias limitations (this study provided insufficient information about random sequence generation or allocation concealment), our concerns about indirectness of the Ashworth Score, an inability to assess imprecision relating to an absence of confidence intervals and a further downgrade for there only being one study for this outcome and the likelihood of publication bias in this area. 4No adverse events or changes in alertness level were observed in the baclofen or placebo group. 5Downgraded three times due to risk of bias limitations (no study provided sufficient information about random sequence generation or allocation concealment), the fact that there was only one study for this outcome and the likelihood of publication bias in this area. 6One study reported improvement in upper and lower limb spasm and reflexes compared to placebo several hours after treatment but it was unclear how meaningful this improvement was due to reporting of P values only (Meythaler 1996). 7Downgraded four times due to risk of bias limitations (no study provided sufficient information about random sequence generation or allocation concealment), an inability to assess imprecision relating to an absence of confidence intervals, the fact that there was only one study for this outcome and the likelihood of publication bias in this area. | |||
| Botulinum toxin A (with and without casting) compared with placebo (with and without casting) for spasticity in people with traumatic brain injury | |||
| Patient or population: adults with traumatic brain injury with spasticity in their arms (1 study) or calves (1 study) Settings: rehabilitation/neurology clinics or acute general hospital, in Europe or the UK Intervention: botulinum toxin A × 1 dose (500/1000 U) or botulinum toxin A × 1 dose of 200 U + serial casting Comparison: placebo (± casting) | |||
| Outcomes | Results and conclusions | No of participants | Quality of the evidence |
| Spasticity at 4‐12 weeks (measured by both Modified Ashworth Scale, 0‐5, at 12 weeks and Tardieu Scale, 0‐5, at 4 weeks) | We are uncertain about the effect of botulinum toxin A (± casting) vs placebo (± casting) on spasticity.1 | 47 (2)2 | ⊕⊝⊝⊝ |
| Adverse events | We are uncertain about the effect of botulinum toxin A (± casting) vs placebo (± casting) on adverse events.4 | 47 (2)2 | ⊕⊝⊝⊝ |
| Sensory functions and pain | No study measured this outcome. | ||
| Neuromusculoskeletal and movement‐related functions at 12 weeks (measured by ankle dorsiflexion) | We are uncertain about the effect of botulinum toxin A (± casting) vs placebo (± casting) on adverse events.6 | 47 (2)2 | ⊕⊝⊝⊝ |
| General tasks and demands | No study measured this outcome. | ||
| Mobility | No study measured this outcome. | ||
| Self‐care | No study measured this outcome. | ||
| GRADE Working Group grades of evidence | |||
| 1Gracies 2015 reported that "with abobotulinumtoxinA, the angle of catch (XV3 of the Tardieu Scale) improved in finger (+35 degree), elbow (+22 degree) and wrist (+12 degree) flexors" but no further outcome data were provided. For Verplancke 2005, we calculated the between‐group difference in spasticity (as measured by the Modified Ashworth Scale) as mean difference 0.30 (95% confidence interval ‐0.87 to 1.47). 2Included studies: Gracies 2015; Verplancke 2005. 3Downgraded four times due to: risk of bias concerns for both studies (downgraded twice, because either insufficient information about random sequence generation and allocation concealment, in one study, and potential selective outcome reporting in both studies), indirectness (one study included mixed traumatic brain injury and stroke populations, and measured spasticity using the Modified Ashworth Scale) and a high likelihood of publication bias in this area. 4In the main trial of Gracies 2015 (in which the traumatic brain injury population was a part (9.5%)) the most common botulinum toxin A‐related adverse event was 'mild muscle weakness' and investigators reported that all adverse events were mild or moderate only. In Verplancke 2005, botulinum toxin A was reported to be well tolerated, with only one participant with 'flu‐like' symptoms (i.e. shivering, sweating and fever). In groups who received casting (either alone, or in addition to botulinum toxin A), 41% to 50% developed 'minor' skin damage. Overall, 90.9% of those resolved spontaneously or with therapeutic dressing. 5Downgraded three times due to: risk of bias concerns for both studies (downgraded twice, because in one study there was insufficient information about random sequence generation and allocation concealment, and in both studies the adverse events data was reporting in percentages only) and a high likelihood of publication bias in this area. 6Verplancke 2005 reported between‐group differences in ankle dorsiflexion, finding no differences between groups in a one‐way ANOVA (casting + placebo versus casting + botulinum toxin A: P = 0.11). However, they did not report any summary statistics for this, or any baseline scores. 7Downgraded four times due to: risk of bias concerns for both studies (downgraded twice, because either insufficient information about random sequence generation and allocation concealment, in one study, and potential selective outcome reporting in both studies), indirectness (one study included mixed traumatic brain injury and stroke populations) and a high likelihood of publication bias in this area. | |||
| Pseudoelastic orthosis versus traditional (static) splint for spasticity in people with traumatic brain injury | |||
| Patient or population: children/young people aged 4‐18 years with traumatic brain injury and with 'mild to severe spastic tetraparesis' (weakness) in all limbs Settings: Istituro Eugenio Media (Italy) Intervention: repositioning splints equipped with participant‐specific pseudoelastic hinges Comparison: traditional splints with fixed angle braces | |||
| Outcomes | Results and conclusions | No of participants | Quality of the evidence |
| Spasticity at up to 6 hours after treatment (measured by the Modified Ashworth Scale, 0‐4, with a higher score indicating greater spasticity) | We are uncertain about the effect of pseudoelastic splints compared with traditional splints on spasticity.1 | 25 | ⊕⊝⊝⊝ |
| Adverse events | We are uncertain about the effect of pseudoelastic splints compared with traditional splints on adverse events.3 | 25 | ⊕⊝⊝⊝ |
| Sensory functions and pain | The included study did not report this outcome. | ||
| Neuromusculoskeletal and movement‐related functions post treatment (measured by range of movement) | We are uncertain about the effect of pseudoelastic splints compared with traditional splints on range of movement.5 | 25 (1) | ⊕⊝⊝⊝ |
| General tasks and demands | The included study did not report this outcome. | ||
| Mobility | The included study did not report this outcome. | ||
| Self‐care | The included study did not report this outcome. | ||
| GRADE Working Group grades of evidence | |||
| 1One study comparing novel pseudoelastic orthoses to traditional fixed angle splints reported no improvement in spasticity in the upper and lower limbs, over a period of one month of intervention. and that results of the two steps were not significantly different (Pittaccio 2013). 2Downgraded four times due to risk of bias limitations (study provided no information about sequence generation and allocation concealment; blinding was impossible for participants or personnel and not reported for outcome assessors; selective outcome reporting bias was high); our concerns about indirectness of the Ashworth Score and indirectness due to 36% of participants not having traumatic brain injury and one participant was of dubious eligibility; an inability to assess imprecision relating to an absence of meaningful outcome data (no numerical data were provided for spasticity; investigators reported only that there were no significant differences), and there was only one study for this comparison/outcome and that publication bias was possible in this area. 3No adverse events were reported for pseudoelastic orthoses neither did any require adjustments after fitting. Adjustments were required for 30% of traditional splints to reduce skin rash, haematomas and oedema. 4Downgraded four times due to risk of bias limitations (study provided insufficient information about sequence generation and allocation concealment, blinding was impossible for participants and personnel and not reported for outcome assessors, and selective reporting bias was high). We had concerns about indirectness given that 36% of participants did not have traumatic brain injury and one participant was of dubious eligibility. Furthermore, there was only one study for this comparison/outcome and publication bias was possible in this area. 5One study reported no improvement in range of movement in the upper and lower limbs, over a period of one month of intervention (Pittaccio 2013). 6Downgraded five times due to risk of bias limitations (this study provided insufficient information about sequence generation and allocation concealment, blinding was impossible for participants and personnel and not reported for outcome assessors, and selective reporting bias was high). We had concerns about indirectness due to 36% of participants not having traumatic brain injury and one participant was of dubious eligibility; our inability to assess imprecision given that means and standard deviations were only presented within a small box and whiskers plot, and a further downgrade for there only being one study for this comparison/outcome and the likelihood of publication bias in this area. | |||
Background
Traumatic brain injury (TBI) is the result of an external force to the head, that can lead to permanent damage to the brain. There are many causes of TBI including motor vehicle accidents, falls, violent assaults or blast injuries (Maas 2008). In 2005, The US Centre for Disease Control and Prevention estimated at least 3.17 million Americans, approximately 1.1% of the US population, are living with long‐term disability as a result of TBI (Summers 2009). In Europe, the incidence of TBI in studies published between 1983 and 2013 has been estimated to be between 47.3 to 849 per 100,000 population per year (Brazinova 2016). There are limited data available for low‐ to middle‐income countries. The impact of TBI to a person can be far‐reaching and may result in ongoing physical, cognitive and behavioural issues (Khan 2003). Skeletal muscle spasticity is one of the major physical complications following TBI (Brashear 2016).
Description of the condition
Spasticity is defined as an ongoing contraction of a muscle caused by an increase in muscle tone and deep tendon reflexes that is partly due to a reduction of the skeletal stretch reflex threshold (Lance 1980). It is often described as muscle overactivity. Spasticity occurs due to damage of upper motor neurons (UMN) of the corticoreticular pathways in the brain cortex or internal capsule, or damage to the UMNs in the reticulospinal or vestibulospinal tracts in the spinal cord (Pandyan 2005). Spasticity may occur sporadically or continuously, for periods of short and long duration.
Spasticity tends to affect the antigravity muscle groups in the upper and lower limbs (Nair 2014). In the upper limbs, this can commonly include the shoulder adductors, elbow, wrist and finger flexors, forearm pronators and thumb adductors. In the lower limbs, spasticity often affects the hip adductors, knee flexors, ankle plantarflexors and invertors, and big toe extensors (Nair 2014). Spasticity can also affect muscles in the neck.
There are limited epidemiological data regarding the prevalence of spasticity following TBI (Martin 2014; McGuire 2016). In one systematic review of the epidemiology of lower limb spasticity, Martin 2014 identified only one study (Singer 2004), conducted in 105 people with TBI that found the prevalence of ankle spasticity was 13%. Similarly, McGuire 2016 was only able to find one study of spasticity prevalence following TBI (Wedekind 2005), which was a study of 32 people with TBI, in which the prevalence of spasticity (location unclear) was 32%. While both the definition and measurement of spasticity is inconsistent, and often poorly defined (Malhotra 2009), McGuire 2016 suggests that by extrapolating data from studies in other populations (including people with stroke, spinal cord injury and multiple sclerosis), 'problematic' spasticity may occur in between 30% and 50% of people with TBI.
Spasticity can lead to a range of musculoskeletal issues such as muscle contracture, involuntary and uncontrollable shaking, joint stiffness, reduced range of movement, broken skin and pain (Ada 2006a; Ada 2006b). The debilitating nature of the condition can directly impact a person's ability to carry out normal activities of daily living (ADL), such as self‐care and household tasks, and is likely to lead to dependency on carers or family members for assistance. Participation in daily life and opportunities for community integration can prove difficult and may ultimately impact the person's quality of life (Kwakkel 1999). Management of spasticity in people with TBI varies from other clinical populations primarily due to behavioural and cognitive issues that affect their ability to participate in, or tolerate, treatment (e.g. their ability to follow instructions, monitor use of a spastic limb or tolerate a cast). These factors are likely to impact on whether or not a treatment is effective in this population and limit the applicability of findings from other populations where behavioural and cognitive issues are not a primary concern (Manchester 1997; Wood 1999). Furthermore, some studies have shown that mobility limitations can be improved over time and that the presence, distribution and severity of spasticity may not necessarily be the best determinant in recovery and mobility outcomes (Williams 2015a; Williams 2015b).
The measurement of spasticity in clinical practice, and in research, is challenging (Malhotra 2009). The most common scales to measure spasticity are the (Modified) Ashworth Scale (Pandyan 1999) and the (Modified) Tardieu Scale (Haugh 2006). The (Modified) Ashworth Scale is commonly used by clinicians as it is the easier scale to complete (Pandyan 1999), however it only measures the resistance in a muscle, which may or may not be caused by spasticity (Patrick 2006). This is in contrast to the (Modified) Tardieu Scale which measures spasticity by the spasticity catch angle as well as resistance in the muscle (Haugh 2006). The Tardieu has also demonstrated greater test‐retest and inter‐rater reliability compared with the Modified Ashworth Scale (Mehrholz 2005). The (Modified) Tardieu includes a continuous and nominal (or sometimes considered ordinal (Haugh 2006)) component, and the (Modified) Ashworth is ordinal, but both are commonly treated as continuous scales by trialists. While there does not appear to be a clear consensus about the most appropriate way to analyse these scales, we note that for another five‐point ordinal scale used in TBI (the Glasgow Outcome Scale (Jennett 1975)), a sliding dichotomy or proportional odds methodology is recommended for analysis (Maas 2010).
Description of the intervention
Interventions for managing skeletal muscle spasticity can be broadly categorised as either pharmacological or non‐pharmacological. Examples of pharmacological interventions include baclofen (Becker 1997), botulinum toxin A, clonidine, dantrolene sodium, tizanidine and phenol injection (Meythaler 2001a; Yelnik 2009). Examples of non‐pharmacological interventions include casting, splinting, stretching, strengthening, transcutaneous electric nerve stimulation (TENS) (Aydin 2005), Bobath treatment, weight bearing gait training and seating. In practice, a combination of both pharmacological and non‐pharmacological interventions is used to manage spasticity. Interventions can be either focal or systemic in their action. Focal interventions involve treatment of one or two muscle groups whereas systemic interventions are used to treat generalised spasticity.
How the intervention might work
Interventions used to manage spasticity all aim to reduce overactivity within the muscle so that it can be lengthened (Esquenazi 2006).
Pharmacological interventions can act locally at the muscle or systemically through the central nervous system. For example, botulinum toxin A and phenol are both injected locally at the site of the spastic muscle whereas other interventions such as tizanidine and clonidine are administered orally. Oral medications act systemically and can induce unwanted adverse effects, such as drowsiness. For those with severe spasticity in multiple areas, adverse effects associated with oral medications can sometimes outweigh the potential benefits of reduced spasticity. An alternative approach, as seen with baclofen, is to administer the treatment through a pump into the space around the spinal cord, thus reducing the impact of adverse effects whilst maintaining improved outcomes for spasticity (Becker 1997).
Non‐pharmacological interventions involve the use of physical modalities such as stretching and strengthening to promote elongation and control of spastic muscles, or external devices such as casting or splinting to modify and maintain correct positioning of a spastic muscle. Table 1 provides a summary of the range of different interventions and their mode of action.
Table 1: Examples of the range of interventions to manage spasticity
| Pharmacological intervention | Mode of action | Non‐pharmacological intervention | Mode of action |
| Baclofen | Administered orally or via intrathecal pump to limit the release of excitatory neurotransmitters in the spinal cord. | Casting | Applied directly to the limb to maintain the muscle in an extended position. |
| Botulinum toxin A | A neurotoxin injected directly into the muscle to block the release of the neurotransmitter acetylcholine. | Splinting | Thermoplastic or fabric material that is customised to provide support to a person's limb and maintain the limb in the corrected position. |
| Clonidine | Administered orally or by transdermal patch to act on the central nervous system by reducing the excitability of alpha motor neurons. | Seating | Custom made seating for people to provide maximal support and reduce the impact of spasticity. |
| Dantrolene sodium | Administered orally to reduce the excitation‐contraction coupling within the skeletal muscle and decrease the strength of muscle contraction. | Stretching | Promotes elongation of a muscle for varying lengths of time causing viscous deformation changes. |
| Phenol | Injected into specific nerves to induce neurolysis to permanently block nerve transmission. | Transcutaneous electric nerve stimulation | Portable electric stimulator placed on the skin over a spastic muscle to reduce pain. |
| Tizanidine | Administered orally to act on the central nervous system and reduce the excitability of alpha motor neurons. | Surgery | Surgical techniques primarily aim to alter the structure of a muscle or nerve or relocate a tendon to change its function. |
Why it is important to do this review
A range of interventions are currently used to manage skeletal muscle spasticity for people with TBI. Clinical management often involves a combination of pharmacological and non‐pharmacological interventions. Management of spasticity following TBI varies from other clinical populations because of the added complexity of behavioural and cognitive issues associated with TBI. Furthermore, the current management for spasticity in other conditions may not be applicable to TBI as a result of global UMN damage that can occur such as axonal shearing, haemorrhage and hypoxia. A comprehensive systematic review of interventions assessed in the TBI population is needed to identify those likely to have the greatest impact for managing spasticity as well as determining whether the severity of TBI or the timing of an intervention is relevant to the outcome.
Reviews of interventions for spasticity in people with TBI are focused solely on the effect of one intervention (botulinum toxin A), and include people with spasticity due to other conditions, predominantly stroke (Baker 2015; Dashtipour 2016; Dong 2017). None of these reviews allow conclusions to be drawn specific to people with TBI. Systematic reviews that consider the effects of a broader range of interventions for managing spasticity have done so in populations other than TBI such as stroke (Taricco 2000) and spinal cord injury (Demetrios 2013; Hsieh 2012). To our knowledge, there are no systematic reviews that consider all potential interventions used to manage skeletal muscle spasticity specifically for people with TBI.
Objectives
To assess the effects of interventions for managing skeletal muscle spasticity in people with TBI.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCT). Cross‐over trials were included as long as the sequence of treatments was randomly allocated.
Types of participants
We included people with TBI of any age (i.e. children and adults), who had skeletal muscle spasticity experienced at any time after injury. We made a post‐hoc decision to include only studies that either:
-
included at least 50% of people with TBI amongst their participants;
-
provided disaggregated data for participants with TBI if the proportion of participants within the trial was less than 50%.
This decision was made in response to the identification of a large number of studies with mixed populations (e.g. stroke and TBI) that were found during screening to ensure the evidence would be applicable to people with TBI.
Types of interventions
We included studies that compared any of the following: pharmacological or non‐pharmacological intervention (or a combination of both) or placebo/no treatment.
Types of outcome measures
Primary outcomes
-
Spasticity, measured using common tools such as the Tardieu or Modified Tardieu Scale (Haugh 2006), or the Ashworth Scale or Modified Ashworth (Pandyan 1999).
-
Adverse events.
As a post‐hoc decision, we took Tardieu or Modified Tardieu, in preference to the (Modified) Ashworth Scale, in the instance that an included study used both these measures. This decision was made given the (Modified) Ashworth poorly differentiates between spasticity and contracture (Patrick 2006), and as the Tardieu is considered to have greater test‐retest and inter‐rater reliability compared with the Modified Ashworth Scale (Mehrholz 2005).
Secondary outcomes
A range of secondary outcomes were included, classified according to the World Health Organization International Classification of Functioning, Disability and Health (WHO 2010) using the following domains.
Body functions
-
Sensory functions and pain (e.g. pain intensity).
-
Neuromusculoskeletal and movement‐related functions (e.g. goniometric measurement).
Activities and participation
-
General tasks and demands (e.g. Canadian Occupational Performance Measure (Law 2000)).
-
Mobility (e.g. gait and balance measures).
-
Self‐care (e.g. Functional Independence Measure (Keith 1987)).
-
Domestic life (e.g. Goal Attainment Scale (Kiresuk 1994)).
-
Major life areas (e.g. Functional Assessment Measure (Hall 1993)).
-
Community, social and civic life (e.g. quality of life measures).
Information size calculation
In line with Cochrane Injuries policy, we undertook a post‐hoc information size calculation, to determine the sample size required in a meta‐analysis for the primary outcome, spasticity. Determining the parameters for this calculation was difficult, as the most commonly used measure of spasticity (the Ashworth or Modified Ashworth Scale) was not necessarily the most clinically meaningful, or appropriate (Haugh 2006), and it is also an ordinal scale that is frequently treated as continuous. Considering we could be combining more than one spasticity scale, we applied the 'rule of thumb' for determining a 'small' difference between two groups as a standardised mean difference (SMD) of 0.2 standard deviations (Guyatt 2011). Assuming 90% power at the 5% significance level, this means that we require 526 participants in both the intervention and control groups within a meta‐analysis to be able to detect a difference between groups, if such a difference exists.
Search methods for identification of studies
To reduce publication and retrieval bias, we did not restrict our search by language, date or publication status.
Electronic searches
The information in this review is current to June 2017. Studies were identified through searches run in 2013, 2016 and 2017.
In November 2013, we searched the following databases:
-
Cochrane Injuries Group specialised register (1 November 2013);
-
the Cochrane Library (2013, Issue 10);
-
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) (1946 to 1 November 2013);
-
Embase Classic + Embase (OvidSP) (1947 to 1 November 2013);
-
PubMed (www.ncbi.nlm.nih.gov/pubmed/) (1 November 2013);
-
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to 01 November 2013);
-
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to 1 November 2013);
-
CINAHL Plus (1939 to 1 November 2013);
-
PsycINFO (1806 to November 2013);
-
PEDro (Physiotherapy Evidence Database) (1929 to November 2013);
-
OTSeeker (Occupational Therapy Systematic Evaluation of Evidence) www.otseeker.com (1955 to November 2013);
-
Database of Abstracts of Reviews of Effects (DARE) (November 2013).
In May 2016, the Cochrane Injuries Group Information Specialist developed and ran a new search. This was because of changes in the author team that meant we were unable to locate the exact search strategies used in the original search (beyond the MEDLINE strategy). The database list was reduced and included:
-
Cochrane Injuries Group Specialised Register (16 May 2016);
-
MEDLINE (OvidSP) (16 May 2016);
-
Embase Classic and Embase (OvidSP) (16 May 2016).
The search strategies for the 2016 search are listed in Appendix 1.
The Cochrane Injuries Information Specialist conducted a final prepublication search in June 2017. Given these searches were revised again by the Cochrane Injuries Information Specialist, we have listed the databases below. Some of the original databases were not searched in 2017 as the databases did not yield any unique studies in earlier searches.
We searched the following databases in June 2017 (and deduplicated against earlier yields):
-
Cochrane Injuries Group Specialised Register (SR‐INJ) (22 June 2017);
-
the Cochrane Library (2017, Issue 6);
-
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) (2013 to 22 June 2017);
-
Ovid Embase (1974 to 22 June 2017);
-
PubMed (not MEDLINE) (www.ncbi.nlm.nih.gov/pubmed/) (22 June 2017);
-
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to 22 June 2017);
-
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to 22 June 2017);
-
ClinicalTrials.gov (www.clinicaltrials.gov) (22 June 2017);
-
WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch/) (22 June 2017).
These search strategies are listed in Appendix 2 so they can be used in future updates.
Searching other resources
We screened the reference lists of all included articles and relevant reviews to identify additional studies for inclusion in the review.
Data collection and analysis
Selection of studies
Two review authors (KP, MC or AS) independently screened citations on abstract and title against the selection criteria. We obtained potentially eligible citations in full text and repeated the process. The two review authors discussed disagreements regarding study eligibility until consensus was reached or consulted a third review author for a final decision. For trials with mixed populations, we contacted study authors to obtain data or clarification (or both) to inform inclusion and exclusion decisions.
Data extraction and management
Two review authors (KP, MC or AS) independently extracted the data from included studies using a standardised data collection form, that was first piloted by two authors. One review author (KP) made a final check. We contacted the primary authors of included studies to provide data and clarification where adequate data were not reported.
Assessment of risk of bias in included studies
Two review authors (KP, MC or AS) independently assessed risk of bias of the included studies using the Cochrane 'Risk of bias' tool (Higgins 2011). We assessed sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; selective outcome reporting; baseline imbalances and other bias issues. Additional domains for cross‐over trials included appropriate study design and adequate washout period. Studies were rated as low, high or unclear risk of bias for each domain, according to the criteria used in the Cochrane 'Risk of bias' tool.
Measures of treatment effect
We extracted raw data (means and standard deviations for continuous outcomes and number of events for binary outcomes) for the primary and secondary outcomes. Where these were not provided, we extracted additional data such as sum scores and P values. We extracted postintervention scores over change scores.
Where possible, we calculated summary data as risk ratio (RR) with 95% confidence intervals (CI) (dichotomous outcomes) and mean difference (MD) with 95% CI (continuous data). Had we been able to pool continuous outcomes where the outcome was measured using different tools across studies, we would have used standardised mean difference (SMD) with 95% CI. We analysed data in Review Manager 5 (RevMan 2011).
Unit of analysis issues
We sought to explore unit of analysis issues in the cross‐over trials, but were unable to do so due to insufficient reporting.
Dealing with missing data
We contacted the primary authors of potentially eligible studies to provide data and clarification, where required. While several authors provided additional information to inform eligibility decisions, we did not receive any additional outcome data (some authors did not respond to our contact and others no longer had access to study data). We describe missing data and dropouts/attrition for each included study in the 'Risk of bias' table, and discussed the extent to which the missing data could have altered the results and conclusions of the review.
Assessment of heterogeneity
We considered the clinical heterogeneity of studies, but statistical assessment of heterogeneity was not possible due to the absence of meta‐analysis. If meta‐analysis is possible in future updates, we will consider the magnitude and direction of effect and make a visual inspection of forest plots to assess the degree of overlap of CIs across separate studies and statistical heterogeneity quantified using the I2 statistic (Higgins 2011).
Assessment of reporting biases
Due to insufficient studies, formal assessment of reporting bias (via funnel plots) could not be carried out. In future updates, if there are more than 10 studies assessing the same outcome(s), we will construct a funnel plot to investigate small study effects (Higgins 2011).
Data synthesis
We had planned to pool outcome data for studies that were considered sufficiently similar. The diversity of interventions and comparator groups as well as paucity of data available meant that a pooled analysis was not possible, therefore the results for each study are presented narratively. We grouped and assessed studies based on the type of intervention (pharmacological or non‐pharmacological or a combination of both) and the comparison group (no treatment, placebo studies or an alternative intervention).
We assessed and reported the quality of the body of evidence contributing each outcome using the GRADE criteria (Schünemann 2011). These five criteria are: risk of bias, inconsistency, imprecision, indirectness and publication bias. Two review authors (AS, MC) independently assessed the quality of the evidence using the GRADE approach (Ryan 2016; Schünemann 2011).
Subgroup analysis and investigation of heterogeneity
We were unable to conduct planned subgroup analyses due to the lack of data. Should there be sufficient data in future updates, we will undertake subgroup analyses to investigate the effect of:
-
timing of intervention post injury (i.e. early: within two years, versus late: at least two years' postinjury);
-
severity of TBI defined by the Glasgow Coma Scale (Teasdale 1974);
-
severity of spasticity as defined by the Ashworth Scale for muscle spasticity;
-
single intervention versus combined interventions;
-
mixed population studies versus studies with TBI participants only;
-
adults versus children.
Sensitivity analysis
We were unable to conduct the planned sensitivity analysis due to the lack of available data. Should there be sufficient studies in future updates, we will investigate the effect of removing studies with inadequate or unclear allocation concealment from meta‐analyses. This domain was selected as the allocation sequence can be concealed in all RCTs (unlike some domains, e.g. like blinding), and there is empirical evidence demonstrating the increased risk of selection bias that can be introduced by unconcealed allocation in studies with more subjective outcomes (such as spasticity and others assessed in this review) (Wood 2008).
'Summary of findings' tables
We made a post‐hoc decision to prepare 'Summary of findings' tables to present the meta‐analysed or narrative results along with the GRADE ratings of our main outcomes. These outcomes were selected on the basis of their clinical importance, rather than the study results. The outcomes included in the 'Summary of findings' table are: spasticity, adverse events, sensory functions and pain, neuromusculoskeletal and movement‐related functions, general tasks and demands, mobility and self‐care.
Results
Description of studies
Results of the search
Combining the yields of all searches up to June 2017, we identified 1743 citations after deduplication, excluding 1527 of these on title and abstract (see Figure 1). This left 216 citations assessed in full text, of which 201 were excluded (see Figure 1; Excluded studies; Characteristics of excluded studies table and Table 1 for more information and reasons).
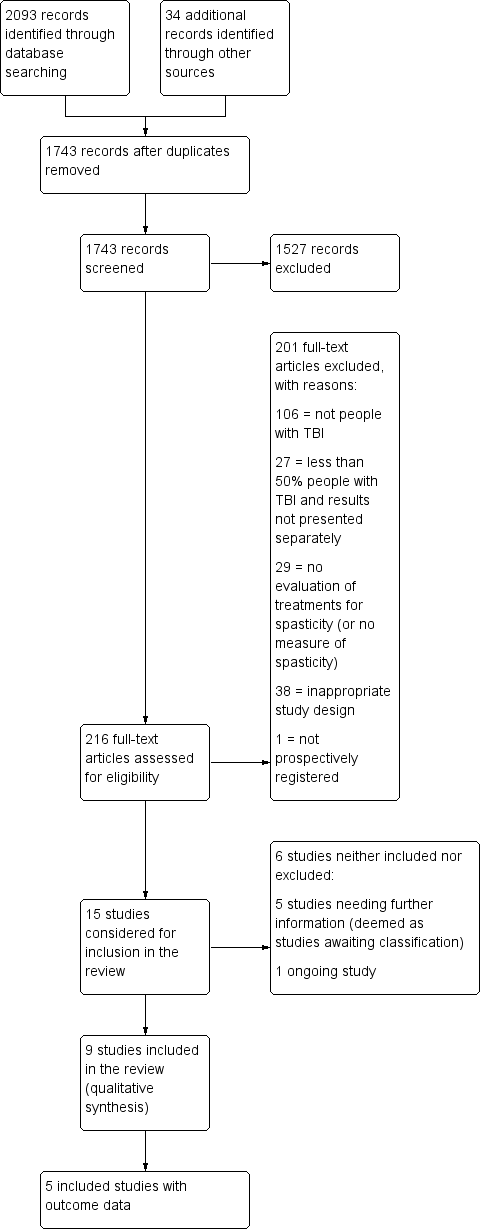
Study flow diagram for searches up until June 2017. TBA: traumatic brain injury.
| Author (year) | n total | n TBI | % TBI | Intervention | Comparator | %TBI Intervention | %TBI comparator |
| Botulinum toxin A vs placebo | |||||||
| 19 | 7 | 37 | Botulinum toxin A | Placebo | NR | NR | |
| 23 | 4 | 17 | Botulinum toxin A | Placebo | NR | NR | |
| 52 | 6 | 12 | Botulinum toxin A | Placebo | 12 | 12 | |
| 20 | 1 | 5 | Botulinum toxin A | Placebo | Cross‐over trial | ‐ | |
| 60 | 11 | 18 | Botulinum toxin A | Tizanidine or placebo | 15 | 14 and 26 placebo | |
| 21 | 2 | 10 | Botulinum toxin A | Placebo | 10 | 16 | |
| Botulinum toxin A vs therapy | |||||||
| 60 | 17 | 28 | Botulinum toxin A with rehab | Rehab only | 26 | 30 | |
| Botulinum toxin A vs botulinum toxin A (dosage) | |||||||
| 21 | 6 | 29 | High dilution botulinum toxin A with endplate target | Low dilution botulinum toxin A with end plate target | NR | NR | |
| Botulinum toxin A vs botulinum toxin A (volume) | |||||||
| 13 | 3 | 23 | High volume botulinum toxin A | Low volume botulinum toxin A | 16 | 28 | |
| 192 | 11 | 6 | High volume botulinum toxin A | Low volume botulinum toxin A | 5 | 6 | |
| Botulinum toxin A vs botulinum toxin A (location) | |||||||
| 17 | 2 | 12 | Botulinum toxin A injections towards mid belly | Botulinum toxin A injections away from mid belly | 0 | 25 | |
| 21 | 6 | 29 | High dilution botulinum toxin A with endplate target | Low dilution botulinum toxin A with end plate target | NR | NR | |
| Baclofen vs placebo | |||||||
| 19 | 2 | 11 | Intrathecal dose of baclofen | Saline | 16 | 0 | |
| 11 | 1 | 9 | Intrathecal baclofen | Placebo | 16 | 0 | |
| Cyclobenzaprine vs placebo | |||||||
| 15 | 4 | 27 | Cyclobenzaprine | Placebo | Cross‐over trial | ‐ | |
| Phenothiazine vs placebo | |||||||
| 9 | 2 | 22 | Phenothiazine | Placebo | Cross‐over trial | ‐ | |
| Tizanidine vs placebo | |||||||
| 17 | 8 | 47 | Tizanidine | Placebo | Cross‐over trial | ‐ | |
| Tizanidine vs botulinum toxin A | |||||||
| 60 | 11 | 18 | Botulinum toxin A | Tizanidine or placebo | 15 | 14 and 26 placebo | |
| Tizanidine vs diazepam | |||||||
| 105 | 16 | 15 | Tizanidine | Diazepam | 10 | 20 | |
| Casting vs control | |||||||
| 44 | 7 | 16 | Splint | No splint | 13 | 21 | |
| Casting vs therapy | |||||||
| 44 | 7 | 16 | Splint | No splint | 13 | 21 | |
| Splinting vs control | |||||||
| 10 | 2 | 20 | Individualised hand splint | No splint | 33 | 0 | |
| 28 | 2 | 7 | Stretching and hand splint | Stretching only | NR | NR | |
| 17 | 7 | 41 | Soft splints | No treatment | NR | NR | |
| Splinting vs therapy | |||||||
| 28 | 2 | 7 | Stretching and hand splint | Stretching only | NR | NR | |
| 17 | 7 | 41 | Soft splints | Stretching | NR | NR | |
| Functional electrical stimulation vs control | |||||||
| 14 | 3 | 21 | Upper limb botulinum toxin A injections for higher hand function | Upper limb botulinum toxin A injections for lower hand function | 33 | 0 | |
| Electrical stimulation + splinting vs splinting | |||||||
| 36 | 5 | 14 | Electrical stimulation to the wrist and finger extensor muscles for 1 hour a day + wrist splint for 12 hours a day, over 4 weeks | Wrist splint for 12 hours a day, over 4 weeks | 6 | 22 | |
| Repetitive peripheral magnetic stimulation vs sham | |||||||
| 66 | 3 | 5 | Repetitive peripheral magnetic stimulation | Sham stimulation | 10 | 0 | |
| Transcutaneous electrical acupoint stimulation vs another dose | |||||||
| 60 | 1 | 2 | Transcutaneous electrical acupoint stimulation (100 Hz) | Transcutaneous electrical acupoint stimulation (2 Hz) | 0 | 5 | |
| Transcutaneous electrical acupoint stimulation vs sham | |||||||
| 60 | 1 | 2 | Transcutaneous electrical acupoint stimulation (100 Hz) | Sham stimulation | 0 | 0 | |
| Ultrasound vs infrared | |||||||
| 21 | 1 | 5 | Infrared | Therapeutic ultrasound | NR | NR | |
| Robot vs bobath | |||||||
| 30 | 8 | 27 | Robot‐mediated therapy with bobath therapy | Bobath therapy | 13 | 40 | |
n: number of participants; NR: not reported; TBI: traumatic brain injury.
Some studies are listed in the table twice, given their multiple comparisons.
Of the remaining 15 studies that were considered for inclusion, we require further information about five studies (see the Characteristics of studies awaiting classification table) and one study is ongoing (see the Characteristics of ongoing studies table). The anticipated end date for the ongoing study investigating the combined effect of serial casting and botulinum toxin A on ankle contractures is September 2018 (ACTRN12615000821594).
We therefore included nine completed trials (Gracies 2015; Leung 2014; Meythaler 1996; Meythaler 1997; Meythaler 1999a; Meythaler 1999b; NCT00287157; Pittaccio 2013; Verplancke 2005).
Included studies
Design
We included nine studies involving data from 385 participants (134 with TBI) (Gracies 2015; Leung 2014; Meythaler 1996; Meythaler 1997; Meythaler 1999a; Meythaler 1999b; NCT00287157; Pittaccio 2013; Verplancke 2005; see Characteristics of included studies table). Of these, three were parallel group RCTs, with two (Leung 2014) or three comparator groups (Gracies 2015; Verplancke 2005). Six were randomised cross‐over trials comparing treatment with placebo or standard care (Meythaler 1996; Meythaler 1997; Meythaler 1999a; Meythaler 1999b; NCT00287157; Pittaccio 2013).
Of the nine trials, three did not identify between‐group differences in outcome data, neither did they report sufficient information about the results to allow us to calculate these differences (Meythaler 1997; Meythaler 1999a; Meythaler 1999b). Results from one trial remain unpublished and unavailable (NCT00287157). As such, only the data from five studies (combined 350 participants, of whom 105 had TBI) contributed outcome data to the results of this review (Gracies 2015; Leung 2014; Meythaler 1996; Pittaccio 2013; Verplancke 2005).
Number of participants
Within each study, the number of participants (TBI and non‐TBI) ranged from six (Meythaler 1999a) to 243 (Gracies 2015). Most of these trials included mixed populations (TBI and non‐TBI). Two studies solely recruited people with TBI (51 combined participants) (Meythaler 1999b; Leung 2014), while Gracies 2015 provided disaggregated data for their subgroup of 23 participants with TBI (in the form of a conference abstract; O'Dell 2015). In the remaining six studies with mixed populations, the number of participants with TBI ranged from three (Meythaler 1999a) to 20 (Verplancke 2005), and the proportions of participants with TBI ranged from 50% (Meythaler 1999a) to 91% (Meythaler 1996).
Setting
All the trials were conducted in high‐income settings, including the US (four trials), Australia (one trial), the UK (one trial), Italy (one trial) and Israel (one trial). One trial drew data from sites in nine high‐income countries (Belgium, Czech Republic, France, Hungary, Italy, Poland, Russia, Slovakia, USA) (Gracies 2015). Participants were recruited from either tertiary care (outpatient or inpatient, or both) rehabilitation clinics or acute general hospitals.
Of the five studies with results, three were funded by governments, charities or health services (Meythaler 1996; Leung 2014; Pittaccio 2013), and two were funded by pharmaceutical/medical technology companies (Gracies 2015; Verplancke 2005). The four studies without useable results were funded by pharmaceutical/medical technology companies (Meythaler 1997; Meythaler 1999a; Meythaler 1999b; NCT00287157).
Participants
Age and sex
With the exception of Gracies 2015, data for age and gender were not reported separately for TBI participants when they formed a proportion of 'mixed' populations. For this reason, the following information relates to the whole sample (including non‐TBI participants) within trials except for Gracies 2015, along with Leung 2014 and Meythaler 1999b, which were the only trials in which all participants had a diagnosis of TBI.
Seven studies included adults, with a mean (or median) age of participants between 24 years (Meythaler 1996) and 41.5 years (Verplancke 2005). Two studies included children (NCT00287157; Pittaccio 2013); only Pittaccio 2013 reported the mean age of participants, being 7.75 years. Where reported, all studies include more males than females, ranging from 60% (Meythaler 1999a) to 92% (Meythaler 1997).
Body part treated
Four studies treated spasticity anywhere in the lower limbs, but also treated upper limb spasticity, if present (Meythaler 1996; Meythaler 1997; Meythaler 1999a; Meythaler 1999b). Two studies treated spasticity in the calf muscles (gastrocnemius, or gastrocnemius and soleus) (Leung 2014; Verplancke 2005), while Gracies 2015 treated spasticity in the upper limbs only (specifically elbow flexors, wrist flexors or finger flexors) and Pittaccio 2013 treated spasticity in the ankle or elbow. One study did not report the location of the spasticity (NCT00287157).
Interventions and comparators
The nine studies tested a varied range of pharmacological and non‐pharmacological interventions. Pharmacological interventions that were tested against saline/placebo included intrathecal baclofen (Meythaler 1996; Meythaler 1997; Meythaler 1999a; Meythaler 1999b) and tizanidine (NCT00287157). Two additional, three‐armed trials tested botulinum toxin A; Gracies 2015 tested two different doses of botulinum toxin A against placebo, and Verplancke 2005 assessed botulinum toxin A plus casting against placebo plus casting and against physiotherapy.
Non‐pharmacological interventions and comparisons tested include casting and physiotherapy (Verplancke 2005), pseudoelastic orthosis versus a traditional splint (Pittaccio 2013), and a combination of tilt table standing, electrical stimulation and ankle splinting versus tilt table standing alone (Leung 2014).
More details are provided about the interventions tested, grouped under different comparisons.
Baclofen versus placebo
Meythaler 1996 examined the effect of intrathecal baclofen for spastic hypertonia in the lower limbs. Eleven participants were allocated to receive a bolus intrathecal injection of either normal saline or baclofen 50 μg. Cross‐over of participants occurred 48 hours after the initial administration. This study was in two parts: use of a bolus dose and then those participants with an adequate response were progressed to implantation of an intrathecal baclofen pump. This would be considered the usual method for evaluation for implantation of a baclofen pump.
The three other baclofen studies (Meythaler 1997; Meythaler 1999a; Meythaler 1999b) also assessed intrathecal baclofen infusion at the same dosage (50 μg), but provided no useable results.
Botulinum toxin A (with or without casting) versus placebo (with or without casting)
Gracies 2015 assessed the effect of botulinum toxin A versus placebo for upper limb spasticity in 23 participants with TBI. In the initial treatment cycle, 14 participants received either abobotulinumtoxinA (500 U or 1000 U) and nine received placebo. The mode of administration was intramuscular injection. However, for participants with TBI, results were given in a binary method (only intervention versus placebo, without regard to dosage of abobotulinumtoxinA).
Verplancke 2005 compared the effects of three different interventions in 25 adults with lower limb spasticity, which we split into three, two‐arm comparisons. The comparison included here is botulinum toxin A injections plus casting (12 participants) (200 U/leg) versus placebo plus casting (12 participants) (total of 4 mL saline injections).
Physiotherapy versus placebo plus casting or botulinum toxin A plus casting
The remaining comparisons for Verplancke 2005 were physiotherapy alone (11 participants) compared with casting plus botulinum toxin A injections (12 participants) (200 U/leg) or casting with placebo (12 participants) (total of 4 mL saline injections) in adults with lower limb spasticity.
Tizanidine versus placebo
NCT00287157 assessed the effect of tizanidine versus placebo on spasticity, cognition and daily function. The results were unpublished and no data were available.
Pseudoelastic orthosis versus traditional splint
In Pittaccio 2013, 25 children with elbow or ankle spasticity wore a spring‐loaded orthosis comprised of two parts that could rotate relative to one another around a common axis that was individually customised and worn for one month. A traditional static splint was fitted and worn for one month. These treatments were delivered in a randomised cross‐over manner.
Tilt table standing, electrical stimulation and ankle splinting versus tilt table standing alone
Leung 2014 (36 participants) evaluated a multi‐modal treatment including 30 minutes of tilt table standing with electrical stimulation to the ankle dorsiflexors five times a week, with 12 hours per day of ankle splinting, at least five days a week. This was compared to the control group, who received 30 minutes of tilt table standing, three times a week. The total programme duration for intervention and control groups was six weeks.
Outcomes
For the five studies in which outcome data were provided, results were available for the primary outcomes of spasticity (assessed using the Tardieu Scale (Gracies 2015; Leung 2014), the Ashworth Scale (Meythaler 1996), or the Modified Ashworth Scale (Gracies 2015; Pittaccio 2013; Verplancke 2005)) and adverse events. All authors treated their measures of spasticity as continuous and numerical data provided were often sparse. Secondary outcomes measured included neuromusculoskeletal and movement‐related functions (i.e. deep tendon reflexes, ankle range of movement), mobility, major life areas and community, social and civic life. The remaining secondary outcomes of interest to this review (sensory function and pain, general tasks and demands, self‐care and domestic life) were not measured. Detailed information about the domains and scores used for these outcomes and the timing of their measurement in the studies is described in Table 2 and Table 3.
| Outcome measure | Domains with score | Studies referring to this outcome | Time point analysis |
| Ashworth Scale (0‐4, lower score = better; Pandyan 1999) | 0: no increase in muscle tone. 1: slight increase in muscle tone, manifested by a catch and release or by minimum resistance through remainder of range of motion 2: more marked increase in muscle tone through most of the range of motion; limb easily moved. 3: considerable increase in muscle tone; passive movement is difficult. 4: rigid limb. | Baseline, and 1, 2, 4, 6 hours | |
| Baseline, and 4, 8 weeks | |||
| Modified Ashworth Scale (0‐5, lower score = better; Pandyan 1999) | 0: no increase in muscle tone. 1: slight increase in muscle tone, manifested by a catch and release or is moved in flexion, extension/abduction, adduction, etc. 1+: slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the range of motion. 2: More marked increase in muscle tone through most of the range of motion, but the affected part is easily moved. 3: considerable increase in muscle tone, passive movement is difficult. 4: affected part is rigid flexion or extension/abduction or adduction. | Baseline, 12 weeks | |
| Baseline, 4 weeks | |||
| Tardieu Scale (TS; 2 measurements: Quality of Muscle Reaction 0‐4, lower score better, and Angle of muscle reaction, R2 ‐ R1; Haugh 2006) | Quality of muscle reaction 0: no resistance throughout the course of the passive movement. 1: slight resistance throughout the course of the passive movement, with no clear catch at precise angle. 2: clear catch at precise angle, interrupting the passive movement, followed by release. 3: fatigable clonus (< 10 seconds when maintaining pressure) occurring at precise angle. 4: infatigable clonus (> 10 seconds when maintaining pressure) occurring at precise angle. Angle of muscle reaction (also referred to as R2 ‐ R1) Measured relative to the position of minimal stretch of the muscle (corresponding to angle) where it is relative to the resting anatomic position. R2: first measure (the maximum passive range of movement of the muscle group). R1: second measure (the angle at which the initial 'catch' or muscle resistance is felt when the muscle is moved from its shortest to longest position using a 'rapid velocity stretch'). | Baseline, 4 weeks | |
| Baseline, and 6 and 10 weeks |
| Outcome measure | Domains with score | Studies referring to this outcome | ICF classification |
| Glasgow Coma Scale (Teasdale 1974) (3‐15, higher score = better) | Eye opening (E) 4: spontaneous 3: to voice 2: to pain 1: none Verbal response (V) 5: normal conversation 4: disoriented conversation 3: words, but not coherent 2: no words, only sounds 1: none Motor response (M) 6: normal 5: localised to pain 4: withdraws to pain 3: decorticate posture (an abnormal posture that can include rigidity, clenched fists, legs held straight out, and arms bent inward towards the body with the wrists and fingers bend and held on the chest) 2: decerebrate (an abnormal posture that can include rigidity, arms and legs held straight out, toes pointed downward, head and neck arched backwards) 1: none The final GCS score or grade is the sum of these numbers. Severe: GCS 3‐8 (minimum possible score is 3) Moderate: GCS 9‐12 Mild: GCS 13‐15 | b. Body functions b1‐b8 | |
| Glasgow Outcome Scale (Jennett 1975) (1‐5, higher score = better) | To generalise and categorise the outcomes of people with TBI. 1: dead 2: vegetative state (meaning the person is unresponsive, but alive; a "vegetable" in lay language) 3: severely disabled (conscious but the person requires others for daily support due to disability) 4: moderately disabled (the person is independent but disabled) 5: good recovery (the person has resumed most normal activities but may have minor residual problems) | Body function b1‐b8 | |
| Range of movement | The joint is taken through the total arc of movement from flexion to extension | Verplancke 2005 (ankle) | Body function b7 |
| Deep tendon reflexes | 0: reflexes absent 1: hyporeflexia 2: normal 3: mild hyperreflexia 4: 3 or 4 beats clonus only 5: clonus | Body function b750 | |
| Disability Assessment Scale (DAS; Brashear 2002; 0‐3, lower score better) | People are interviewed to determine the extent of functional impairment in: hygiene, dressing, limb position and pain, according to the following scale: 0: no disability 1: mild disability (noticeable but does not interfere significantly with normal activities) 2: moderate disability (normal activities require increased effort or assistance, or both) 3: severe disability (normal activities limited) | Activities and participation |
ICF: International Classification of Functioning.
Excluded studies
In total, we excluded 169 studies on reading the full text, for reasons including no TBI participants (96 studies), less than 50% of participants had TBI and results not presented separately (27 studies), no evaluation of treatments for spasticity (28 studies) or ineligible study design (e.g. review article; 18 references; see Characteristics of excluded studies for a list of 58 key studies excluded on full text).
Given the relatively large number of studies (27) that were excluded as less than 50% of study participants had a TBI (and the data for these participants were not presented separately), we provided more information in Table 1. Across these studies, there were 1000 participants, of whom 142 (14%) had TBI. The percentage of people within each study who had TBI ranged from 2% to 47%.
Risk of bias in included studies
The risk of bias for all included studies varied as detailed in the Characteristics of included studies table, and presented in Figure 2 and Figure 3. Many items were scored as 'unclear' due to poor reporting.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Nine studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study (note: only Meythaler 1996 and Verplancke 2005 contributed outcome data to the review).
Allocation
Of the nine included studies, investigators involved in only two studies clearly reported the methods of both randomisation and allocation concealment, and these were assessed at low risk of bias for these domains (Gracies 2015; Leung 2014). None of the other study authors provided details of either randomisation or allocation concealment methods, making it difficult to determine any associated risks of bias. Seven studies were thus rated at 'unclear' risk of bias for both these domains.
Blinding
Five studies reported adequate blinding of participants and personnel (Gracies 2015; Meythaler 1996; Meythaler 1997; Meythaler 1999a; Meythaler 1999b). In three studies neither participants nor personnel could be blinded due to the obvious differences between interventions and controls (e.g. serial casting, different orthoses/splints), leading to ratings of high risk (Leung 2014; Pittaccio 2013; Verplancke 2005). The registry entry for NCT00287157 provided insufficient information and as such this study was rated as unclear.
Four studies explicitly reported that outcome assessors were blinded (Gracies 2015; Leung 2014; Meythaler 1996; Verplancke 2005). It is noteworthy that these were amongst the only studies with outcome data included in the review. In four studies, it was unclear if outcome assessors were blinded (Meythaler 1997; Meythaler 1999a; Meythaler 1999b; NCT00287157), and in one study outcome assessors could not be blinded, hence it was rated at high risk (Pittaccio 2013).
Incomplete outcome data
Six studies reported no losses to follow‐up and were at low risk of attrition bias (Gracies 2015; Meythaler 1996; Meythaler 1997; Meythaler 1999a; Meythaler 1999b; Pittaccio 2013). Verplancke 2005 reported four withdrawals and three deaths; however, their final measurements for these participants were those taken before withdrawal from the study (rated as unclear as they did not report which group they were randomised to). Leung 2014 reported four losses to follow‐up, with reasons that were unrelated to the trial; however, they were not balanced between groups. Both studies were rated as unclear risk of bias. The rating for NCT00287157 was 'unclear' due to lack of information.
Selective reporting
One study was at low risk of bias given the availability of a published protocol, which aligned with the subsequently published trial (Leung 2014). Three studies were at unclear risk of bias for selective reporting, as there were no published protocols (Meythaler 1996; Meythaler 1997; Meythaler 1999b). One study was also rated at unclear risk of bias as, while it did have a published protocol for the main study, the TBI subset outcome data for different doses of the active intervention were conflated and results were given in the form of percentages only (Gracies 2015). Three studies did not reference published protocols (neither could we identify any) and were at high risk due to additional incomplete reporting of their results (Meythaler 1999a; Pittaccio 2013; Verplancke 2005). NCT00287157 was at high risk of bias as the authors advised that the trial data were "negative," and was subsequently not published.
Other potential sources of bias
Baseline imbalances
With regards to baseline imbalances, five studies were at low risk of bias (Meythaler 1996; Meythaler 1997; Meythaler 1999a; Meythaler 1999b; Pittaccio 2013). Four studies were judged as unclear for this domain (Gracies 2015; Leung 2014; NCT00287157; Verplancke 2005). NCT00287157 was judged as unclear due to lack of information, whereas the other two studies described their groups as being 'similar' at baseline, but subsequently outlined some differences between the groups.
Appropriate study design
For the six cross‐over trials, five used an appropriate study design (Meythaler 1996; Meythaler 1997; Meythaler 1999a; Meythaler 1999b; Pittaccio 2013). The remaining trial was at unclear risk of bias.
Adequate washout period
Three cross‐over trials provided enough information to be judged as having an adequate washout period (low risk of bias) (Meythaler 1996; Meythaler 1997; Meythaler 1999a). Investigators in one trial judged it unethical to have a washout period (Pittaccio 2013). Statistical tests were conducted and presented demonstrating that sequential order did not affect treatment response, so this trial was also assessed as having a low risk of bias. The remaining two cross‐over trials were at unclear risk of bias for this domain (Meythaler 1999b; NCT00287157).
Other bias
With regards to 'other bias', three studies (Meythaler 1997; Meythaler 1999a; Meythaler 1999b) were at high risk of bias due to unit of analysis issues (outcomes were analysed by looking at upper versus lower extremities, rather than considering each muscle separately; scores for muscle tone, spasms and reflexes were averaged for the upper or lower extremities in each participant). Four studies were at unclear risk of bias due to insufficient information (NCT00287157); the close role of the funder (a pharmaceutical company) in the study design, conduct and analysis (Gracies 2015); the inclusion of a participant who did not meet the study inclusion criteria (Pittaccio 2013); and marked differences in the number of tilt table sessions between the intevention and control groups (Leung 2014). We did not identify any other bias concerns with the remaining two studies (Meythaler 1996; Verplancke 2005).
Effects of interventions
See: Summary of findings for the main comparison Baclofen compared with placebo for spasticity in people with traumatic brain injury; Summary of findings 2 Botulinum toxin A (with and without casting) compared with placebo (with and without casting) for spasticity in people with traumatic brain injury; Summary of findings 3 Pseudoelastic orthosis versus traditional (static) splint for spasticity in people with traumatic brain injury
Baclofen versus placebo
One study with usable results compared baclofen versus placebo (Meythaler 1996). (Three other studies compared baclofen to placebo but had no useable results; Meythaler 1997; Meythaler 1999a; Meythaler 1999b.) A summary of the results of the main outcomes for this comparison is provided in summary of findings Table for the main comparison.
Spasticity
Meythaler 1996 (11 participants, all included in the analysis) reported between‐group differences in both the lower and upper extremity Ashworth scores using P values, but with no effect sizes or CIs. It was unclear which statistical tests were used. As the study authors did not report any raw data for the placebo group results, we could do no further analysis. The authors reported a 'significant' improvement with baclofen compared to placebo at four hours (P = 0.0084) and six hours (P = 0.0163) after administration. Baclofen was also associated with 'significant' improvements compared to placebo for upper extremity Ashworth score (P = 0.0097) at four hours after administration, however this effect was not sustained at six hours (P value not reported).
The quality of the evidence on the effect of baclofen versus placebo for spasticity was very low (downgraded four times). This was due to risk of bias limitations (no study provided sufficient information about the random sequence generation or allocation concealment), our concerns about indirectness of the Ashworth score, an inability to assess imprecision relating to an absence of CIs and a further downgrade for there only being a single study for this outcome and the likelihood of publication bias in this area. Considered together with the fact that we could not judge how clinically relevant the improvements were in this study, we were very uncertain about the effect of baclofen on spasticity and unable to draw any conclusions.
Adverse events
There were no adverse events or changes in alertness level in the baclofen or placebo groups (see Table 4).
| Adverse effect | Number of participants affected | Studies |
| Deep vein thrombosis | 1 participants withdrawn from physiotherapy group. | |
| Contracture at subtalar joint | 1 participants withdrawn from the casting + placebo group. | |
| No adverse events or changes in alertness level were observed in the baclofen group or placebo arm | Not applicable. | |
| Unspecified "treatment emergent AE [adverse effects]": "none were unexpected" | 7/23 participants, no other information given. | |
| Skin rashes/oedema/tolerability issues | No pseudoelastic device required adjustment for comfort; 30% of traditional devices did. Families reported novel treatment tolerated for 40% longer than traditional (Pittaccio 2013). 2 participants adherence to splinting was affected by 'skin problems' and 'poor tolerance' (Leung 2014). 50% of participants in casting group and 41.7% of participants in casting + botulinum toxin A group developed 'minor skin damage'. Overall, 90% of those resolved spontaneously or with therapeutic dressing (Verplancke 2005). | |
| Fainting, fatigue, storming1 | Several participants' adherence to the tilt table was affected due to fainting, fatigue and storming. |
1When someone with a head injury responds to a sensation with a tonic posture or sympathetic response.
The quality of the evidence on the effect of baclofen versus placebo for spasticity was very low (downgraded three times). This was due to the same risk of bias concerns as for spasticity and that there was only one study for this outcome and the likelihood of publication bias in this area. As such, we were very uncertain about the effect of baclofen on adverse events compared to placebo and were unable to draw any conclusions.
Neuromusculoskeletal and movement‐related functions
Meythaler 1996 (11 participants, all included in the analysis) reported that lower extremity spasm scores were significantly improved for baclofen versus placebo at four hours (P = 0.0073) and six hours (P = 0.0049) as were lower extremity reflex scores (P = 0.0086 at four hours and P = 0.0085 at six hours). This effect was also observed for the upper extremity spasm scores (P = 0.0117) and reflex scores (P = 0.0272) at four hours after administration when baclofen was compared to placebo. This effect was not sustained at six hours (P value not reported).
The quality of this evidence was very low (downgraded four times, for all the reasons outlined for spasticity) and we are very uncertain about the effect of baclofen on neuromusculoskeletal and movement‐related functions.
Outcomes not measured
The study did not report: sensory functions and pain; general tasks and demands; mobility; self‐care; domestic life; major life areas; and community, social and civic life.
Botulinum toxin A (with and without casting) versus placebo (with and without casting)
Two studies compared botulinum toxin A (with and without casting) versus placebo (with and without casting) (Gracies 2015; Verplancke 2005). A summary of the results of the main outcomes for this comparison is provided in summary of findings Table 2.
Spasticity
Gracies 2015 (23 participants) report that four weeks after injection, "with abobotulinumtoxinA, the angle of catch (XV3 of the TS) improved in finger (+35 degree), elbow (+22 degree) and wrist (+12 degree) flexors, resulting in a gain in active muscle extension of at least 5 degrees active range of movement." No further outcome data were provided, and they did not comment on the 'statistical significance' of this result. We note that the dosage that produced this result (500 U or 1000 U) was unclear as data from both groups (total 14 participants) were pooled against placebo (nine participants).
Verplancke 2005 (35 participants) compared three different interventions, which we report as three two‐arm comparisons (the authors analysed pre‐ and post‐treatment differences in the Modified Ashworth Scale within groups, but not between groups). As such, we calculated the between‐group difference in spasticity (as measured by the Modified Ashworth Scale at 12 weeks) for botulinum toxin A plus casting versus placebo plus casting as MD 0.30 points (95% CI ‐0.87 to 1.47; Analysis 1.1).
The quality of the evidence for this outcome was very low (downgraded four times). This was due to risk of bias concerns for both studies (downgraded twice, because either insufficient information about random sequence generation and allocation concealment, in one study, and potential selective outcome reporting in both studies), indirectness (one study measured spasticity using the Modified Ashworth Scale) and a high likelihood of publication bias in this area. As such, we were very uncertain about the effect of botulinum toxin A with and without casting versus placebo with and without casting on spasticity and were unable to draw any conclusions.
Adverse events
In Gracies 2015, the authors reported that "7 of 23 patients experienced a treatment emergent AE [adverse event]" and that of these, "none were unexpected." There was no further detail given in the short conference proceeding reporting results for the TBI population. In the main trial publication (of which people with TBI only made up 9%), the most common treatment‐related adverse event was 'mild muscle weakness' and investigators reported that all adverse events were mild or moderate only (see Table 4).
In Verplancke 2005, botulinum toxin A was to be well tolerated, with only one participant with 'flu‐like' symptoms (i.e. shivering, sweating and fever). For the casting group, 50% developed minor skin damage (66.6% of those was partial thickness skin loss involving epidermis). Finally, in the botulinum toxin A plus casting group, 41.7% of participants developed skin damage (60% of those was discolouration, but skin still intact). Overall, 90.9% of those resolved spontaneously or with therapeutic dressing (see Table 4).
The quality of the evidence for adverse events was very low (downgraded three times). This was due to risk of bias concerns for both studies (downgraded twice, because in one study there was insufficient information about random sequence generation and allocation concealment, and in both studies the adverse events data were reporting in percentages only) and a high likelihood of publication bias in this area. As such, we were very uncertain about the effect of botulinum toxin A with and without casting versus placebo with and without casting on adverse events and were unable to draw any conclusions.
Neuromusculoskeletal and movement‐related functions
Verplancke 2005 reported between‐group differences in ankle dorsiflexion, and found no differences between groups in a one‐way ANOVA (placebo plus casting versus botulinum toxin A plus casting: P = 0.11). However, they did not report any summary statistics for this, or any baseline scores.
The quality of the evidence for neuromusculoskeletal and movement‐related functions was very low for the reasons outlined for spasticity (downgraded four times). As such, we were very uncertain about the effect of botulinum toxin A with and without casting versus placebo with and without casting on neuromusculoskeletal and movement‐related functions and were unable to draw any conclusions.
Major life areas
Both Gracies 2015 and Verplancke 2005 measured outcomes we classified under major life area outcomes. Gracies 2015 reported an "improvement in subjective function for the treated group (>1 grade decrease from baseline for the principal target of treatment on the Disability Assessment Scale (DAS)): 71% versus 22% for placebo." They did not provide this in absolute numbers, or comment on the 'statistical significance' of the result. Verplancke 2005 reported Glasgow Outcome Scale (GOS) and Glasgow Coma Scale scores at follow‐up in each of the three groups, but reported mean values and did not report between‐group differences.
The quality of the evidence for major life areas was very low (downgraded three times). This was due to risk of bias concerns for both studies (downgraded twice, because in one study there was insufficient information about random sequence generation and allocation concealment, and in both studies the outcome data were inappropriately analysed or reported in percentages only) and a high likelihood of publication bias in this area. As such, we were uncertain about the effect of botulinum toxin A with and without casting versus placebo with and without casting on major life areas and were unable to draw any conclusions.
Community, social and civic life
In Gracies 2015, the authors reported two quality of life measures (subscores of the 36‐item Short Form Health (SF‐36) and the EuroQol (EQ‐5D); however, these outcomes were not reported separately for the subset of participants with TBI.
Outcomes not measured
The studies did not report: sensory functions and pain, general tasks and demands, mobility, self‐care and domestic life.
Physiotherapy versus placebo plus casting or botulinum toxin A plus casting
One study compared physiotherapy versus placebo plus casting or botulinum toxin A plus casting (Verplancke 2005).
Spasticity
Verplancke 2005 compared three treatment groups: physiotherapy, placebo plus casting and botulinum toxin A plus casting. The authors analysed pre‐ and post‐treatment differences in the Modified Ashworth Scale within but not between groups. As such, we calculated the following between‐group differences in spasticity (as measured by the Modified Ashworth Scale at 12 weeks): botulinum toxin A plus casting versus physiotherapy (MD ‐0.50 points, 95% CI ‐1.82 to 0.82; Analysis 2.1) and placebo plus casting versus physiotherapy (MD ‐0.80 points, 95% CI ‐2.00 to 0.40; Analysis 3.1).
The quality of the evidence for physiotherapy compared to casting or botulinum toxin A plus casting on spasticity was very low. This was due to concerns about risk of bias related to insufficient information provided about allocation concealment and blinding, concerns about indirectness of the Modified Ashworth Scale, an inability to assess imprecision relating to an absence of CIs and a further downgrade for there only being one study for this outcome and the likelihood of publication bias in this area. As such, we are very uncertain about the effect of casting versus botulinum toxin A plus casting versus physiotherapy on spasticity and were unable to draw any conclusions.
Adverse events
Verplancke 2005 withdrew two participants due to an adverse event (see Table 4). One was due to deep vein thrombosis (DVT) in the physiotherapy group and one was due to soft tissue contracture at the subtalar joint in the placebo plus casting group. For the adverse events related to botulinum toxin A, see the adverse events listed under the comparison above, botulinum toxin A with and without casting versus placebo with and without casting.
The quality of the evidence for physiotherapy versus placebo plus casting or botulinum toxin A plus casting on adverse events was very low. This was due to concerns about risk of bias related to insufficient information provided about allocation concealment and blinding, and a further downgrade for there only being one study for this outcome and the likelihood of publication bias in this area. As such, we were very uncertain about the effect of casting versus botulinum toxin A plus casting versus physiotherapy on adverse events and were unable to draw any conclusions.
Neuromusculoskeletal and movement‐related functions
Verplancke 2005 reported between‐group differences in ankle dorsiflexion and found no differences between groups in a one‐way ANOVA (physiotherapy versus placebo plus casting: P value not significant, physiotherapy versus botulinum toxin A plus casting: P = 0.07). However, they did not report any summary statistics for this, or any baseline scores.
The quality of the evidence for physiotherapy versus placebo plus casting or botulinum toxin A plus casting on neuromuscular and movement‐related outcomes was very low (downgraded three times). This was due to concerns about risk of bias (related to insufficient information provided about randomisation schedule generation and allocation concealment, a lack of blinding and selective outcome reporting), an inability to assess imprecision relating to an absence of data and a further downgrade for there only being one study for this outcome and the likelihood of publication bias in this area. As such, we were very uncertain about the effect of casting versus botulinum toxin A plus casting on neuromuscular and movement‐related functions and were unable to draw any conclusions.
Major life areas (e.g. Functional Assessment Measure)
Verplancke 2005 reported GOS and GCS scores at follow‐up in each of the three groups, but reported mean values and did not report between‐group differences.
Outcomes not measured
The study did not report: sensory functions and pain; general tasks and demands; mobility; self‐care; domestic life; and community, social and civic life.
Pseudoelastic orthosis versus traditional (static) splint
One study compared pseudoelastic orthosis versus traditional (static) splint in children (Pittaccio 2013). A summary of the results of the main outcomes for this comparison is provided in summary of findings Table 3.
Spasticity
Pittaccio 2013 measured spasticity using the Ashworth Scale, but reporting for clinical outcomes in this paper was scant. The authors provided no outcome data for spasticity but stated that, "there were no significant differences on means by paired 2‐tailed Student's t test" between the two types of splint.
The quality of the evidence was very low. The rating was downgraded four times due to risk of bias limitations (the study provided no information about sequence generation and allocation concealment; blinding was impossible for participants or personnel and not reported for outcome assessors; selective outcome reporting bias was high); our concerns about indirectness of the Ashworth score and indirectness due to 36% of participants not having TBI and one participant was of dubious eligibility; an inability to assess imprecision relating to an absence of meaningful outcome data (no numerical data were provided for spasticity; investigators reported only that there were no significant differences), there was only one study for this comparison/outcome and publication bias was possible in this area. As such, we were very uncertain about the effect of pseudoelastic orthoses versus a traditional (static) splint on spasticity and were unable to draw any conclusions.
Adverse events
Pittaccio 2013 reported that both participants and families 'reacted positively' to the pseudoelastic orthoses, which were described as well‐tolerated for several hours a day, and never caused problems familiar to those using more traditional devices including a skin rash, haematomas or pain (see Table 4). None of the pseudoelastic orthoses had to be modified to improve comfort, while some adjustments were required for approximately 30% of traditional splints, in order to reduce skin rash, haematomas and oedema. Tolerability was assessed by questionnaire. The investigators conceded that true knowledge of comfort/discomfort experienced by children in the trial was impossible to assess as most participants were unable to communicate; nevertheless, questionnaire data were reported to be encouraging and on average, the pseudoelastic devices were tolerated 40% longer than traditional splints.
The quality of the evidence was very low (downgraded four times). The quality was downgraded due to risk of bias limitations (this study provided insufficient information about sequence generation and allocation concealment, blinding was impossible for participants and personnel and not reported for outcome assessors, and selective reporting bias was high). We had concerns about indirectness given that 36% of participants did not have TBI and one participant was of dubious eligibility. Furthermore, there was only one study for this comparison/outcome and publication bias was possible in this area. As such, we were very uncertain about the effect of pseudoelastic orthoses versus a traditional (static) splint on adverse events and were unable to draw any conclusions.
Neuromusculoskeletal and movement‐related functions
Pittaccio 2013 reported results for range of motion as non‐significant ("no significant differences on means by paired 2‐tailed Student's t tests") and no further numerical data were given.
The quality of the evidence was very low. The quality of the evidence was downgraded five times due to risk of bias limitations (this study provided insufficient information about sequence generation and allocation concealment, blinding was impossible for participants and personnel and not reported for outcome assessors, and selective reporting bias was high). We had concerns about indirectness due to 36% of participants not having TBI and one participant was of dubious eligibility; our inability to assess imprecision given that means and standard deviations were only presented within a small box and whiskers plot, and a further downgrade for there only being one study for this comparison/outcome and the likelihood of publication bias in this area. As such, we were very uncertain about the effect of pseudoelastic orthoses versus a traditional (static) splint on neuromusculoskeletal and movement‐related functions and were unable to draw any conclusions.
Outcomes not measured
The study did not report: sensory functions and pain; general tasks and demands; mobility; self‐care; domestic life; major life areas; and community, social and civic life.
Tilt table standing, electrical stimulation and ankle splinting versus tilt table standing alone
One study compared tilt table standing, electrical stimulation and ankle splinting versus tilt table standing alone (Leung 2014).
Spasticity
Leung 2014 measured spasticity at six weeks (end of programme) and 10 weeks (end of trial) using the 5‐point 'reaction to passive stretch at high speed' component of the Tardieu Scale. Leung 2014 treated this as a continuous scale and reported the difference between groups using change scores, which we plotted using postintervention scores only (see Analysis 4.1). This resulted in an MD of ‐1.00 points (95% CI ‐1.66 to ‐0.34) at week six (in favour of tilt table standing, electrical stimulation and ankle splinting) and an MD of 1.00 (95% CI 0.31 to 1.69) at week 10 (in favour of tilt table standing alone). This is broadly consistent with the conclusions of the authors that "there was a small mean reduction of 1 point in spasticity at Week 6 (95% CI 0.1 to 1.8) in favour of the experimental group, but this effect disappeared at Week 10."
The quality of the evidence for tilt table standing, electrical stimulation and ankle splinting versus tilt table standing alone on spasticity was very low (downgraded three times). This was due to concerns about indirectness (downgraded once for potentially inappropriate analysis of the Tardieu Scale as a continuous measure), imprecision (downgraded once as there was only one small study for this outcome) and the likelihood of publication bias in this area (downgraded once). As such, we were very uncertain about the effect of tilt table standing, electrical stimulation and ankle splinting versus tilt table standing alone on spasticity and were unable to draw any conclusions.
Adverse events
While Leung 2014 did not explicitly measure adverse events, they provided a description of a number of factors that influenced several participants' adherence to tilt table standing, including fainting, fatigue and storming (when someone with a head injury responds to a sensation with a tonic posture or sympathetic response). Additionally, two participants' adherence to splinting was affected by "skin problems" and "poor tolerance."
Neuromusculoskeletal and movement‐related functions
Leung 2014 measured passive ankle dorsiflexion at 12 Nm at six weeks (end of programme) and 10 weeks (end of trial). Dorsiflexion was measured relative to a neutral ankle position (0 degrees), with a positive score indicating dorsiflexion (the desired direction of movement) and a negative score indicating plantarflexion. They explored the differential effects using change scores, but we recalculated this using postintervention scores (see Analysis 4.2). This yielded an MD of ‐2.00 degrees (95% CI ‐7.19 to 3.19) at six weeks and an MD of 1.00 degree (95% CI ‐3.31 to 5.31) at 10 weeks.
The quality of the evidence for tilt table standing, electrical stimulation and ankle splinting versus tilt table standing alone on neuromuscular and movement‐related functions was very low (downgraded three times). This was due to imprecision (downgraded once) related to the CI including both a meaningful benefit and harm, using Leung 2014's prespecified 'minimum worthwhile treatment effect' of 5 degrees, there was only one study for this outcome (downgraded once) and the likelihood of publication bias in this area (downgraded once). As such, we were very uncertain about the effect of tilt table standing, electrical stimulation and ankle splinting versus tilt table standing alone on neuromusculoskeletal and movement‐related functions and were unable to draw any conclusions.
Mobility
Leung 2014 measured mobility using walking speed (in metres/second) at six weeks (end of programme) and 10 weeks (end of trial). They assessed between‐group differences using change scores, which we recalculated using postintervention scores (see Analysis 4.3). This yielded a MD of ‐0.10 m/second (95% CI ‐0.43 to 0.23) at six weeks and 0.00 (95% CI ‐0.38 to 0.38) at 10 weeks.
The quality of the evidence for tilt table standing, electrical stimulation and ankle splinting versus tilt table standing alone on mobility was very low. The quality of the evidence was downgraded four times due to risk of bias concerns (participants and personnel could not be blinded and there were some imbalances between groups in the length of time since injury and the number of tilt table sessions received), for imprecision (single study and the CI included a potential benefit and harm) and the likelihood of publication bias in this area. As such, we were uncertain about the effect of tilt table standing, electrical stimulation and ankle splinting versus tilt table standing alone on mobility and were unable to draw any conclusions.
The study did not report: sensory functions and pain; general tasks and demands; self‐care; domestic life; major life areas; and community, social and civic life.
Tizanidine versus placebo
One study compared tizanidine versus placebo (NCT00287157) in children. However, we have no information regarding participants and no information about the effect of tizanidine versus placebo on spasticity or any other outcomes as the results of this study are unavailable and unpublished.
Discussion
Summary of main results
We found nine RCTs, including 385 participants (of whom 134 had TBI), that assessed the effect of a wide range of pharmacological and non‐pharmacological treatments for spasticity as a result of TBI. Only five of these studies reported results in sufficient detail to allow synthesis in the review. As such, we included data from studies with 350 participants (105 of whom had TBI) in which baclofen, botulinum toxin A, casting, physiotherapy, splints, tilt table standing and electrical stimulation were tested, either alone or in combination.
Given such heterogeneity of interventions, trials for any given intervention were few, and sample sizes were small. Added to this, reporting of methods and results for trials was patchy, we had concerns about indirectness given the number of studies in this review with mixed populations, and publication bias given the known (and potentially unknown) unpublished studies in this area. These factors combined to mean there was a paucity of evidence about the effect of interventions for the management of spasticity in people with TBI. The quality of the evidence for all outcomes was very low.
In the interests of completeness, we provided a rudimentary summary of the results for the primary outcomes of spasticity and adverse events. Results were mixed for spasticity, but we cautioned against drawing conclusion given the very low quality evidence. For pharmacological interventions, baclofen had a greater effect than placebo after six hours of treatment in the lower limbs but not upper limbs (Meythaler 1996), and botulinum toxin A had a greater effect than placebo at four weeks in the upper limbs (Gracies 2015), but when combined with casting it had no greater effect than casting alone in the lower limbs (Verplancke 2005). For non‐pharmacological studies, physiotherapy compared with casting in the lower limbs (Verplancke 2005), and novel pseudoelastic splints with traditional splints at the ankle or elbow (Pittaccio 2013), demonstrated no differences between treatments.
For adverse events, the quality of the evidence was similarly very low, but the results were a little more consistent. For pharmacological treatments, there were no adverse events reported in the baclofen study (Meythaler 1996), or the group which received botulinum toxin A in Verplancke 2005, but 7/23 participants in the botulinum toxin A study by Gracies 2015 experienced a "treatment emergent adverse event" (no further information provided). Many of the non‐pharmacological treatments were associated tolerability/adherence issues, including traditional splints (Leung 2014; Pittaccio 2013) and tilt table standing (Verplancke 2005). In Verplancke 2005, between 41.7% and 50% of participants who received casting developed minor skin damage, of which, 90% resolved spontaneously. In Verplancke 2005, two more serious adverse events developed in the physiotherapy group (DVT) and the casting plus placebo group (joint contracture).
Due to the very low quality of evidence, we were uncertain about the effects of any of these interventions for managing spasticity.
Overall completeness and applicability of evidence
This review indicated that the evidence for existing interventions for managing spasticity in TBI is limited. Given the paucity of well‐designed and reported trials, the applicability of this review to the TBI population was limited as there were many factors that needed to be considered which none of the studies addressed.
Clinicians can find the management of TBI challenging due to the varying extent of the UMN damage causing spasticity affecting many muscles and joints, trunk, limbs, head and neck leading to complex physical disabilities and often coexisting cognitive and behavioural impairments. These confounding factors can impact on spasticity management as people with TBI may have a lower tolerance for treatments, as well as an inability to monitor or participate in the learning and practice required for rehabilitation. Consideration for a greater research base to be developed in this area is extremely important.
There are varying levels of evidence for the management of spasticity in other neurological populations (Ade‐Hall 2000; Amatya 2013; Duarte 2016; Demetrios 2013; Lindsay 2013; RCP 2009; Shakespeare 2003; Taricco 2000). There is an assumption made that the effects of pharmacological and non‐pharmacological interventions in other conditions (e.g. multiple sclerosis, stroke) are the same for TBI. While at an impairment level the outcome may potentially be comparable, there can be a difference at a functional level. There is little evidence for the effect of these interventions at a functional level (Francis 2004).
Quality of the evidence
The quality of the evidence for all outcomes was very low due to risk of bias concerns (a combination of poor reporting and poor conduct), indirectness related to mixed populations or the outcome measures used (or both), imprecision and the likelihood of publication/reporting biases in this area. We did not formally downgrade the quality of the evidence for the analysis approach taken in studies that measured spasticity (where all the authors treated the ordinal scales as continuous) given the lack of consensus about how best to analyse these data, but this may introduce a further flaw in the interpretation.
Potential biases in the review process
The decision to limit inclusion of studies with a mixed clinical population to those with greater than 50% of participants with TBI had a major impact on the number of included studies in the review. Had we reduced or removed the threshold, we could have increased the number of included studies by a further 27. Had we increased the threshold to 100% (similar to the approach taken in one TBI trial overview (Bragge 2016)) we would have reduced the number of included studies to three. As such, the level of the threshold used in the review may have affected the results and conclusions. This threshold was set during the screening stages of the review (i.e. before extraction of results data) and was determined using clinical judgement.
Agreements and disagreements with other studies or reviews
As far as we are aware, this is the only systematic review evaluating any intervention for skeletal muscle spasticity, specifically in people with TBI. Three non‐Cochrane systematic reviews of RCTs have each investigated the efficacy of botulinum toxin A for spasticity (due to TBI and other causes) in the upper limbs (Dong 2017), lower limbs (Dashtipour 2016), and upper and lower limbs (Baker 2015). Each review included a majority of studies in people with stroke, but all included some studies with mixed stroke and TBI populations. Dashtipour 2016 was the only review to include any study (Gracies 2015) that was common to our review. These reviews concluded that there was favourable evidence to support the use of botulinum toxin A in the upper limb (both Baker 2015 and Dong 2017 found improvements in global benefit or "ease of care" measures) and either an absence of evidence (Baker 2015) or 'the beginnings of an evidence base' showing spasticity reductions (Dashtipour 2016) in the lower limb. Given the included studies in each of these reviews included predominantly people with stroke, we would question the applicability of these results to people with TBI. One other non‐Cochrane systematic review looked at the use of casting in people with brain injury, the majority of whom had TBI (Mortenson 2003). Only five out of the 13 included studies measured spasticity as an outcome and reported improvement to some extent following casting. However, it was noted by the author that the quality of this evidence was low and that none of these studies used a true randomised design.
There are several Cochrane Reviews that investigated a broader range of treatments for spasticity in other conditions such as stroke (Demetrios 2013), multiple sclerosis (Amatya 2013; Shakespeare 2003), amyotrophic lateral sclerosis/motor neuron disease (Ashworth 2012), cerebral palsy (Ade‐Hall 2000; Hoare 2010), and spinal cord injury (Taricco 2000). Interventions ranged from multidisciplinary rehabilitation following botulinum toxin A (and other intramuscular treatment), pharmacological (e.g. botulinum toxin A, baclofen, tizanidine) and non‐pharmacological interventions (e.g. physical activity), or a combination of interventions. It is notable that with the exception of one review (Hoare 2010), all reviews reported low or insufficient evidence for any of these interventions as a result of heterogeneity, high risk of bias and lack of information. Hoare 2010 found strong evidence to support botulinum toxin A as an adjunct to occupational therapy in managing upper limb spasticity in children with cerebral palsy.

Study flow diagram for searches up until June 2017. TBA: traumatic brain injury.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Nine studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study (note: only Meythaler 1996 and Verplancke 2005 contributed outcome data to the review).

Comparison 1 Casting plus botulinum toxin A versus casting plus placebo, Outcome 1 Spasticity at 12 weeks.
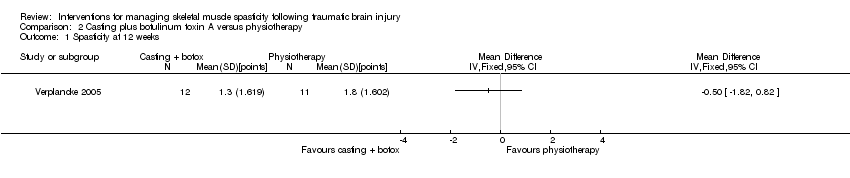
Comparison 2 Casting plus botulinum toxin A versus physiotherapy, Outcome 1 Spasticity at 12 weeks.

Comparison 3 Casting plus placebo versus physiotherapy, Outcome 1 Spasticity at 12 weeks.
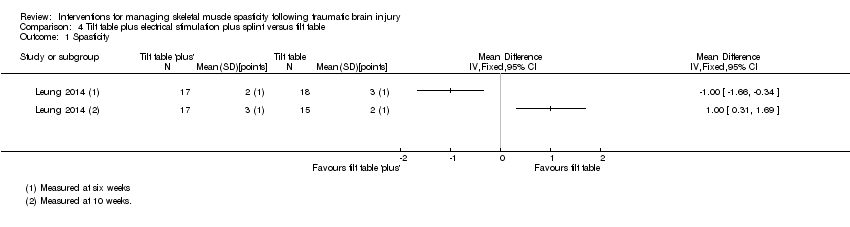
Comparison 4 Tilt table plus electrical stimulation plus splint versus tilt table, Outcome 1 Spasticity.
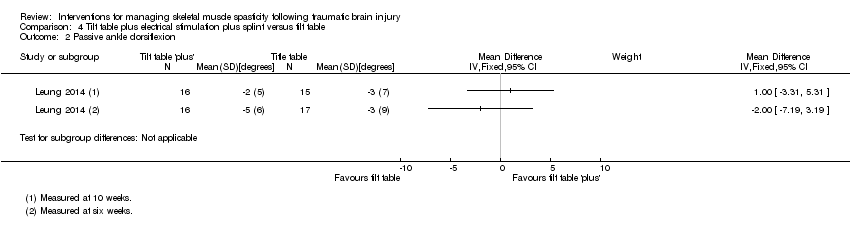
Comparison 4 Tilt table plus electrical stimulation plus splint versus tilt table, Outcome 2 Passive ankle dorsiflexion.

Comparison 4 Tilt table plus electrical stimulation plus splint versus tilt table, Outcome 3 Walking speed.
| Baclofen compared with placebo for spasticity in people with traumatic brain injury | |||
| Patient or population: adults with traumatic brain injury with spasticity in their arms and legs Settings: outpatient rehabilitation clinic (US) Intervention: intrathecal baclofen 50 μg (injected into the lumbar spine) Comparison: saline placebo | |||
| Outcomes | Results and conclusions | No of participants | Quality of the evidence |
| Spasticity at up to 6 hours after treatment (measured by the Ashworth Scale, 0‐, with a higher score indicating greater spasticity) | We are uncertain about the effect of baclofen on spasticity compared with placebo.1 | 11 | ⊕⊝⊝⊝ |
| Adverse events | We are uncertain about the effect of baclofen on adverse events compared with placebo.4 | 11 | ⊕⊝⊝⊝ |
| Sensory functions and pain | No study measured this outcome. | ||
| Neuromusculoskeletal and movement‐related functions up to 6 hours after treatment (Measured by spasm and deep tendon reflex scores, 0‐5, with 0 being no reflexes and 5 being clonus, or repeated involuntary muscle contractions) | We are uncertain about the effect of baclofen on neuromusculoskeletal and movement‐related functions compared with placebo.6 | 11 (1)2 | ⊕⊝⊝⊝ |
| General tasks and demands | No study measured this outcome. | ||
| Mobility | No study measured this outcome. | ||
| Self‐care | No study measured this outcome. | ||
| GRADE Working Group grades of evidence | |||
| 1One study of baclofen reported an improvement in spasticity in the upper and lower limbs, compared to placebo, several hours after the injections but it was unclear how meaningful this improvement was due to reporting of P values only (Meythaler 1996). 2Three additional studies, with 35 participants, measured this outcome but had no useable results (Meythaler 1997; Meythaler 1999a; Meythaler 1999b). 3Downgraded four times due to risk of bias limitations (this study provided insufficient information about random sequence generation or allocation concealment), our concerns about indirectness of the Ashworth Score, an inability to assess imprecision relating to an absence of confidence intervals and a further downgrade for there only being one study for this outcome and the likelihood of publication bias in this area. 4No adverse events or changes in alertness level were observed in the baclofen or placebo group. 5Downgraded three times due to risk of bias limitations (no study provided sufficient information about random sequence generation or allocation concealment), the fact that there was only one study for this outcome and the likelihood of publication bias in this area. 6One study reported improvement in upper and lower limb spasm and reflexes compared to placebo several hours after treatment but it was unclear how meaningful this improvement was due to reporting of P values only (Meythaler 1996). 7Downgraded four times due to risk of bias limitations (no study provided sufficient information about random sequence generation or allocation concealment), an inability to assess imprecision relating to an absence of confidence intervals, the fact that there was only one study for this outcome and the likelihood of publication bias in this area. | |||
| Botulinum toxin A (with and without casting) compared with placebo (with and without casting) for spasticity in people with traumatic brain injury | |||
| Patient or population: adults with traumatic brain injury with spasticity in their arms (1 study) or calves (1 study) Settings: rehabilitation/neurology clinics or acute general hospital, in Europe or the UK Intervention: botulinum toxin A × 1 dose (500/1000 U) or botulinum toxin A × 1 dose of 200 U + serial casting Comparison: placebo (± casting) | |||
| Outcomes | Results and conclusions | No of participants | Quality of the evidence |
| Spasticity at 4‐12 weeks (measured by both Modified Ashworth Scale, 0‐5, at 12 weeks and Tardieu Scale, 0‐5, at 4 weeks) | We are uncertain about the effect of botulinum toxin A (± casting) vs placebo (± casting) on spasticity.1 | 47 (2)2 | ⊕⊝⊝⊝ |
| Adverse events | We are uncertain about the effect of botulinum toxin A (± casting) vs placebo (± casting) on adverse events.4 | 47 (2)2 | ⊕⊝⊝⊝ |
| Sensory functions and pain | No study measured this outcome. | ||
| Neuromusculoskeletal and movement‐related functions at 12 weeks (measured by ankle dorsiflexion) | We are uncertain about the effect of botulinum toxin A (± casting) vs placebo (± casting) on adverse events.6 | 47 (2)2 | ⊕⊝⊝⊝ |
| General tasks and demands | No study measured this outcome. | ||
| Mobility | No study measured this outcome. | ||
| Self‐care | No study measured this outcome. | ||
| GRADE Working Group grades of evidence | |||
| 1Gracies 2015 reported that "with abobotulinumtoxinA, the angle of catch (XV3 of the Tardieu Scale) improved in finger (+35 degree), elbow (+22 degree) and wrist (+12 degree) flexors" but no further outcome data were provided. For Verplancke 2005, we calculated the between‐group difference in spasticity (as measured by the Modified Ashworth Scale) as mean difference 0.30 (95% confidence interval ‐0.87 to 1.47). 2Included studies: Gracies 2015; Verplancke 2005. 3Downgraded four times due to: risk of bias concerns for both studies (downgraded twice, because either insufficient information about random sequence generation and allocation concealment, in one study, and potential selective outcome reporting in both studies), indirectness (one study included mixed traumatic brain injury and stroke populations, and measured spasticity using the Modified Ashworth Scale) and a high likelihood of publication bias in this area. 4In the main trial of Gracies 2015 (in which the traumatic brain injury population was a part (9.5%)) the most common botulinum toxin A‐related adverse event was 'mild muscle weakness' and investigators reported that all adverse events were mild or moderate only. In Verplancke 2005, botulinum toxin A was reported to be well tolerated, with only one participant with 'flu‐like' symptoms (i.e. shivering, sweating and fever). In groups who received casting (either alone, or in addition to botulinum toxin A), 41% to 50% developed 'minor' skin damage. Overall, 90.9% of those resolved spontaneously or with therapeutic dressing. 5Downgraded three times due to: risk of bias concerns for both studies (downgraded twice, because in one study there was insufficient information about random sequence generation and allocation concealment, and in both studies the adverse events data was reporting in percentages only) and a high likelihood of publication bias in this area. 6Verplancke 2005 reported between‐group differences in ankle dorsiflexion, finding no differences between groups in a one‐way ANOVA (casting + placebo versus casting + botulinum toxin A: P = 0.11). However, they did not report any summary statistics for this, or any baseline scores. 7Downgraded four times due to: risk of bias concerns for both studies (downgraded twice, because either insufficient information about random sequence generation and allocation concealment, in one study, and potential selective outcome reporting in both studies), indirectness (one study included mixed traumatic brain injury and stroke populations) and a high likelihood of publication bias in this area. | |||
| Pseudoelastic orthosis versus traditional (static) splint for spasticity in people with traumatic brain injury | |||
| Patient or population: children/young people aged 4‐18 years with traumatic brain injury and with 'mild to severe spastic tetraparesis' (weakness) in all limbs Settings: Istituro Eugenio Media (Italy) Intervention: repositioning splints equipped with participant‐specific pseudoelastic hinges Comparison: traditional splints with fixed angle braces | |||
| Outcomes | Results and conclusions | No of participants | Quality of the evidence |
| Spasticity at up to 6 hours after treatment (measured by the Modified Ashworth Scale, 0‐4, with a higher score indicating greater spasticity) | We are uncertain about the effect of pseudoelastic splints compared with traditional splints on spasticity.1 | 25 | ⊕⊝⊝⊝ |
| Adverse events | We are uncertain about the effect of pseudoelastic splints compared with traditional splints on adverse events.3 | 25 | ⊕⊝⊝⊝ |
| Sensory functions and pain | The included study did not report this outcome. | ||
| Neuromusculoskeletal and movement‐related functions post treatment (measured by range of movement) | We are uncertain about the effect of pseudoelastic splints compared with traditional splints on range of movement.5 | 25 (1) | ⊕⊝⊝⊝ |
| General tasks and demands | The included study did not report this outcome. | ||
| Mobility | The included study did not report this outcome. | ||
| Self‐care | The included study did not report this outcome. | ||
| GRADE Working Group grades of evidence | |||
| 1One study comparing novel pseudoelastic orthoses to traditional fixed angle splints reported no improvement in spasticity in the upper and lower limbs, over a period of one month of intervention. and that results of the two steps were not significantly different (Pittaccio 2013). 2Downgraded four times due to risk of bias limitations (study provided no information about sequence generation and allocation concealment; blinding was impossible for participants or personnel and not reported for outcome assessors; selective outcome reporting bias was high); our concerns about indirectness of the Ashworth Score and indirectness due to 36% of participants not having traumatic brain injury and one participant was of dubious eligibility; an inability to assess imprecision relating to an absence of meaningful outcome data (no numerical data were provided for spasticity; investigators reported only that there were no significant differences), and there was only one study for this comparison/outcome and that publication bias was possible in this area. 3No adverse events were reported for pseudoelastic orthoses neither did any require adjustments after fitting. Adjustments were required for 30% of traditional splints to reduce skin rash, haematomas and oedema. 4Downgraded four times due to risk of bias limitations (study provided insufficient information about sequence generation and allocation concealment, blinding was impossible for participants and personnel and not reported for outcome assessors, and selective reporting bias was high). We had concerns about indirectness given that 36% of participants did not have traumatic brain injury and one participant was of dubious eligibility. Furthermore, there was only one study for this comparison/outcome and publication bias was possible in this area. 5One study reported no improvement in range of movement in the upper and lower limbs, over a period of one month of intervention (Pittaccio 2013). 6Downgraded five times due to risk of bias limitations (this study provided insufficient information about sequence generation and allocation concealment, blinding was impossible for participants and personnel and not reported for outcome assessors, and selective reporting bias was high). We had concerns about indirectness due to 36% of participants not having traumatic brain injury and one participant was of dubious eligibility; our inability to assess imprecision given that means and standard deviations were only presented within a small box and whiskers plot, and a further downgrade for there only being one study for this comparison/outcome and the likelihood of publication bias in this area. | |||
| Author (year) | n total | n TBI | % TBI | Intervention | Comparator | %TBI Intervention | %TBI comparator |
| Botulinum toxin A vs placebo | |||||||
| 19 | 7 | 37 | Botulinum toxin A | Placebo | NR | NR | |
| 23 | 4 | 17 | Botulinum toxin A | Placebo | NR | NR | |
| 52 | 6 | 12 | Botulinum toxin A | Placebo | 12 | 12 | |
| 20 | 1 | 5 | Botulinum toxin A | Placebo | Cross‐over trial | ‐ | |
| 60 | 11 | 18 | Botulinum toxin A | Tizanidine or placebo | 15 | 14 and 26 placebo | |
| 21 | 2 | 10 | Botulinum toxin A | Placebo | 10 | 16 | |
| Botulinum toxin A vs therapy | |||||||
| 60 | 17 | 28 | Botulinum toxin A with rehab | Rehab only | 26 | 30 | |
| Botulinum toxin A vs botulinum toxin A (dosage) | |||||||
| 21 | 6 | 29 | High dilution botulinum toxin A with endplate target | Low dilution botulinum toxin A with end plate target | NR | NR | |
| Botulinum toxin A vs botulinum toxin A (volume) | |||||||
| 13 | 3 | 23 | High volume botulinum toxin A | Low volume botulinum toxin A | 16 | 28 | |
| 192 | 11 | 6 | High volume botulinum toxin A | Low volume botulinum toxin A | 5 | 6 | |
| Botulinum toxin A vs botulinum toxin A (location) | |||||||
| 17 | 2 | 12 | Botulinum toxin A injections towards mid belly | Botulinum toxin A injections away from mid belly | 0 | 25 | |
| 21 | 6 | 29 | High dilution botulinum toxin A with endplate target | Low dilution botulinum toxin A with end plate target | NR | NR | |
| Baclofen vs placebo | |||||||
| 19 | 2 | 11 | Intrathecal dose of baclofen | Saline | 16 | 0 | |
| 11 | 1 | 9 | Intrathecal baclofen | Placebo | 16 | 0 | |
| Cyclobenzaprine vs placebo | |||||||
| 15 | 4 | 27 | Cyclobenzaprine | Placebo | Cross‐over trial | ‐ | |
| Phenothiazine vs placebo | |||||||
| 9 | 2 | 22 | Phenothiazine | Placebo | Cross‐over trial | ‐ | |
| Tizanidine vs placebo | |||||||
| 17 | 8 | 47 | Tizanidine | Placebo | Cross‐over trial | ‐ | |
| Tizanidine vs botulinum toxin A | |||||||
| 60 | 11 | 18 | Botulinum toxin A | Tizanidine or placebo | 15 | 14 and 26 placebo | |
| Tizanidine vs diazepam | |||||||
| 105 | 16 | 15 | Tizanidine | Diazepam | 10 | 20 | |
| Casting vs control | |||||||
| 44 | 7 | 16 | Splint | No splint | 13 | 21 | |
| Casting vs therapy | |||||||
| 44 | 7 | 16 | Splint | No splint | 13 | 21 | |
| Splinting vs control | |||||||
| 10 | 2 | 20 | Individualised hand splint | No splint | 33 | 0 | |
| 28 | 2 | 7 | Stretching and hand splint | Stretching only | NR | NR | |
| 17 | 7 | 41 | Soft splints | No treatment | NR | NR | |
| Splinting vs therapy | |||||||
| 28 | 2 | 7 | Stretching and hand splint | Stretching only | NR | NR | |
| 17 | 7 | 41 | Soft splints | Stretching | NR | NR | |
| Functional electrical stimulation vs control | |||||||
| 14 | 3 | 21 | Upper limb botulinum toxin A injections for higher hand function | Upper limb botulinum toxin A injections for lower hand function | 33 | 0 | |
| Electrical stimulation + splinting vs splinting | |||||||
| 36 | 5 | 14 | Electrical stimulation to the wrist and finger extensor muscles for 1 hour a day + wrist splint for 12 hours a day, over 4 weeks | Wrist splint for 12 hours a day, over 4 weeks | 6 | 22 | |
| Repetitive peripheral magnetic stimulation vs sham | |||||||
| 66 | 3 | 5 | Repetitive peripheral magnetic stimulation | Sham stimulation | 10 | 0 | |
| Transcutaneous electrical acupoint stimulation vs another dose | |||||||
| 60 | 1 | 2 | Transcutaneous electrical acupoint stimulation (100 Hz) | Transcutaneous electrical acupoint stimulation (2 Hz) | 0 | 5 | |
| Transcutaneous electrical acupoint stimulation vs sham | |||||||
| 60 | 1 | 2 | Transcutaneous electrical acupoint stimulation (100 Hz) | Sham stimulation | 0 | 0 | |
| Ultrasound vs infrared | |||||||
| 21 | 1 | 5 | Infrared | Therapeutic ultrasound | NR | NR | |
| Robot vs bobath | |||||||
| 30 | 8 | 27 | Robot‐mediated therapy with bobath therapy | Bobath therapy | 13 | 40 | |
| n: number of participants; NR: not reported; TBI: traumatic brain injury. Some studies are listed in the table twice, given their multiple comparisons. | |||||||
| Outcome measure | Domains with score | Studies referring to this outcome | Time point analysis |
| Ashworth Scale (0‐4, lower score = better; Pandyan 1999) | 0: no increase in muscle tone. 1: slight increase in muscle tone, manifested by a catch and release or by minimum resistance through remainder of range of motion 2: more marked increase in muscle tone through most of the range of motion; limb easily moved. 3: considerable increase in muscle tone; passive movement is difficult. 4: rigid limb. | Baseline, and 1, 2, 4, 6 hours | |
| Baseline, and 4, 8 weeks | |||
| Modified Ashworth Scale (0‐5, lower score = better; Pandyan 1999) | 0: no increase in muscle tone. 1: slight increase in muscle tone, manifested by a catch and release or is moved in flexion, extension/abduction, adduction, etc. 1+: slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the range of motion. 2: More marked increase in muscle tone through most of the range of motion, but the affected part is easily moved. 3: considerable increase in muscle tone, passive movement is difficult. 4: affected part is rigid flexion or extension/abduction or adduction. | Baseline, 12 weeks | |
| Baseline, 4 weeks | |||
| Tardieu Scale (TS; 2 measurements: Quality of Muscle Reaction 0‐4, lower score better, and Angle of muscle reaction, R2 ‐ R1; Haugh 2006) | Quality of muscle reaction 0: no resistance throughout the course of the passive movement. 1: slight resistance throughout the course of the passive movement, with no clear catch at precise angle. 2: clear catch at precise angle, interrupting the passive movement, followed by release. 3: fatigable clonus (< 10 seconds when maintaining pressure) occurring at precise angle. 4: infatigable clonus (> 10 seconds when maintaining pressure) occurring at precise angle. Angle of muscle reaction (also referred to as R2 ‐ R1) Measured relative to the position of minimal stretch of the muscle (corresponding to angle) where it is relative to the resting anatomic position. R2: first measure (the maximum passive range of movement of the muscle group). R1: second measure (the angle at which the initial 'catch' or muscle resistance is felt when the muscle is moved from its shortest to longest position using a 'rapid velocity stretch'). | Baseline, 4 weeks | |
| Baseline, and 6 and 10 weeks |
| Outcome measure | Domains with score | Studies referring to this outcome | ICF classification |
| Glasgow Coma Scale (Teasdale 1974) (3‐15, higher score = better) | Eye opening (E) 4: spontaneous 3: to voice 2: to pain 1: none Verbal response (V) 5: normal conversation 4: disoriented conversation 3: words, but not coherent 2: no words, only sounds 1: none Motor response (M) 6: normal 5: localised to pain 4: withdraws to pain 3: decorticate posture (an abnormal posture that can include rigidity, clenched fists, legs held straight out, and arms bent inward towards the body with the wrists and fingers bend and held on the chest) 2: decerebrate (an abnormal posture that can include rigidity, arms and legs held straight out, toes pointed downward, head and neck arched backwards) 1: none The final GCS score or grade is the sum of these numbers. Severe: GCS 3‐8 (minimum possible score is 3) Moderate: GCS 9‐12 Mild: GCS 13‐15 | b. Body functions b1‐b8 | |
| Glasgow Outcome Scale (Jennett 1975) (1‐5, higher score = better) | To generalise and categorise the outcomes of people with TBI. 1: dead 2: vegetative state (meaning the person is unresponsive, but alive; a "vegetable" in lay language) 3: severely disabled (conscious but the person requires others for daily support due to disability) 4: moderately disabled (the person is independent but disabled) 5: good recovery (the person has resumed most normal activities but may have minor residual problems) | Body function b1‐b8 | |
| Range of movement | The joint is taken through the total arc of movement from flexion to extension | Verplancke 2005 (ankle) | Body function b7 |
| Deep tendon reflexes | 0: reflexes absent 1: hyporeflexia 2: normal 3: mild hyperreflexia 4: 3 or 4 beats clonus only 5: clonus | Body function b750 | |
| Disability Assessment Scale (DAS; Brashear 2002; 0‐3, lower score better) | People are interviewed to determine the extent of functional impairment in: hygiene, dressing, limb position and pain, according to the following scale: 0: no disability 1: mild disability (noticeable but does not interfere significantly with normal activities) 2: moderate disability (normal activities require increased effort or assistance, or both) 3: severe disability (normal activities limited) | Activities and participation | |
| ICF: International Classification of Functioning. | |||
| Adverse effect | Number of participants affected | Studies |
| Deep vein thrombosis | 1 participants withdrawn from physiotherapy group. | |
| Contracture at subtalar joint | 1 participants withdrawn from the casting + placebo group. | |
| No adverse events or changes in alertness level were observed in the baclofen group or placebo arm | Not applicable. | |
| Unspecified "treatment emergent AE [adverse effects]": "none were unexpected" | 7/23 participants, no other information given. | |
| Skin rashes/oedema/tolerability issues | No pseudoelastic device required adjustment for comfort; 30% of traditional devices did. Families reported novel treatment tolerated for 40% longer than traditional (Pittaccio 2013). 2 participants adherence to splinting was affected by 'skin problems' and 'poor tolerance' (Leung 2014). 50% of participants in casting group and 41.7% of participants in casting + botulinum toxin A group developed 'minor skin damage'. Overall, 90% of those resolved spontaneously or with therapeutic dressing (Verplancke 2005). | |
| Fainting, fatigue, storming1 | Several participants' adherence to the tilt table was affected due to fainting, fatigue and storming. | |
| 1When someone with a head injury responds to a sensation with a tonic posture or sympathetic response. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spasticity at 12 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spasticity at 12 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spasticity at 12 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spasticity Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Passive ankle dorsiflexion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Walking speed Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |

