Neuromoduladores para el tratamiento del dolor en la artritis reumatoide
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, double‐blind, multi‐centre parallel group study | |
| Participants | 58 patients with RA 12 male, 46 female Mean age 62.8yrs Inclusion: active RA (ACR criteria), stable NSAIDs or steroids for one month and stable DMARDs for 3 months Exclusion: history of psychiatric disorders or substance misuse, severe cardiovascular, renal or hepatic disorder, or a history of epilepsy Sample size calculation: not reported | |
| Interventions | Sativex oromucosal spray (2.7 mg Tetrahydrocannabinol (THC) and 2.5 mg cannabidiol (CBD) with each activation (dose)) vs Placebo Single dose nights 1 and 2, increased by one dose every 2 days to a maximum of six doses (according to individual response). Stable dosing was then maintained for a further 3 weeks Dosing was restricted to the evening to minimize possible intoxication‐type reactions | |
| Outcomes | Baseline, day 14, 28, 49 and 57‐59 Primary: pain on movement (0–10 numerical rating scale (NRS)) Secondary: 1) Pain at rest (0‐10 NRS), Short Form McGill Pain Questionnaire (SF‐MPQ) 2) Sleep quality (0‐10 NRS) 3) Morning stiffness 4) 28‐joint disease activity score (DAS28) | |
| Notes | Conclusion: statistically significant improvements in pain on movement, pain at rest, quality of sleep, DAS28 and the SF‐MPQ pain at present component were seen following CBM in comparison with placebo. Data was skewed as median differences were reported for most measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised treatment allocation using permuted blocks of four" Comment: adequate |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "versus placebo" Comment: no further information specified. Sativex has a mint flavour and it is likely the placebo did not replicate this |
| Blinding (performance bias and detection bias) | Unclear risk | No information specified |
| Incomplete outcome data (attrition bias) | Unclear risk | 4 patients withdrew from the study and were accounted for, however it was unclear at what time point they withdrew and how their data were treated Comment: it is likely they were excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Compliance? | Unclear risk | No information specified |
| Co‐interventions? | Unclear risk | No information specified |
| Baseline characteristics? | Low risk | Quote: "There were no significant differences in demographics between groups" Comment: baseline characteristics were similar |
| intention to treat analysis? | Unclear risk | 4 patients withdrew from the study. It is not clear how their data were dealt with Comment: no information specified |
| Drop Outs? | Low risk | 4/58 (8.3%) patients dropped out, 3 from the placebo group and 1 active treatment |
| Summary Assessment of Bias? | High risk | High risk of bias |
| Methods | Double‐blind randomised controlled trial in patients wit RA and OA (excluded) | |
| Participants | 31 patients with ACR criteria for RA 6 male, 25 female Mean age 20‐79, mean pain 55‐57 (VAS 100mm) 84% taking NSAIDs, 32% corticosteroids, 13% gold, 10% other immunosuppressive agent Inclusion: >18yrs, moderate to severe unilateral or bilateral knee pain Exclusion: intrarticular corticosteroid injection ≤3 weeks or application of topical corticosteroids ≤7 days prior to study onset, skin disorder in affected knee Sample size calculation: not reported | |
| Interventions | Topical 0.025% capsaicin versus placebo (vehicle cream) applied to the front, back, and both sides of the selected knee four times per day | |
| Outcomes | Baseline, 1, 2 and 4 weeks Primary: 1) Pain Intensity (VAS 100mm; no pain to worst imaginable pain) 2) Pain Relief (VAS 100mm; no pain relied to complete pain relief) 3) Physician Global Response:
| |
| Notes | Capsaicin was significantly superior to placebo after one week of treatment in RA patients and after two weeks in OA patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were then randomly assigned..." Comment: no information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | High risk | A placebo vehicle cream was used, however topical capsaicin gives a characteristic burning sensation which was not blinded Comment: patients likely to have known they were receiving the active treatment |
| Blinding (performance bias and detection bias) | Unclear risk | No information specified Comment: likely to know that patients reporting burning were receiving the active treatment, however authors performed a repeated‐measures analysis (two‐tailed) of the physician's global evaluation, comparing the response of capsaicin patients with burning versus those without burning and found no significant difference. The numbers in this analysis were small however and may not have been able to detect a significant effect |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 patients in the capsaicin group dropped out. It was unclear whether this was due to an adverse event or because of protocol violation. These patients were excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Compliance? | Low risk | Quote: "Patients were interviewed at each visit to assess side effects, compliance..." Comment: no further information specified. It is likely that participants will have over reported compliance which would lead to a more conservative estimate. |
| Co‐interventions? | Unclear risk | Quote: "Patients were allowed to take standard oral arthritis medications during the study provided that the doses were stabilized before study start and the medications continued without interruption during the study." Quote: "Patients were interviewed at each visit to assess.....use of concomitant medications" Comment: co‐analgesics not specifically reported, but is likely they remained stable |
| Baseline characteristics? | Low risk | Baseline characteristics were similar |
| intention to treat analysis? | High risk | Completers only analysis |
| Drop Outs? | Low risk | 2/31 patients dropped out and were excluded |
| Summary Assessment of Bias? | High risk | High risk of bias |
| Methods | Double‐blind cross‐over trial | |
| Participants | 27 patients with RA 2 male, 25 female Mean age 59 years, disease 4‐30 years, 51% receiving gold, penicillamine, chloroquine or prednisone, "All were receiving maximal doses of one or more nonsteroidal anti‐inflammatory drugs, but had persistent pain" Inclusion: not specified Exclusion: not specified Sample size calculation: not reported | |
| Interventions | Nefopam 60mg tds or placebo for 4 weeks, followed by one week washout then further 4 weeks on the alternative preparation | |
| Outcomes | Baseline, 2 weeks and 4 weeks for each cross‐over period Primary 1) Pain (VAS 100mm) 2) EMS (VAS 100mm) 3) Joint tenderness 4) Grip strength 5) Proximal interphalangeal joint (PIP) size | |
| Notes | Conclusion: nefopam was a more effective analgesic than placebo when given as a supplement to anti‐inflammatory drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Placebo tablets were identical in appearance" Comment: it is likely the patients remained blinded |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "Neither doctor nor patient was aware of the treatment order" Comment: no information specified |
| Incomplete outcome data (attrition bias) | Low risk | 5 patients dropped out and were accounted for. They were excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Compliance? | Unclear risk | Information not specified |
| Co‐interventions? | Unclear risk | Quote: "Patients were asked to discontinue pure analgesics one week prior to the study" Comment: no further information on co‐interventions during the trial were supplied |
| Baseline characteristics? | Low risk | Cross‐over trial. Quote: "Baseline measurements in the first and second periods were not significantly different for any variable", "Evidence for an order of treatment or carry‐over drug effect was sought by applying the approach described by Hill and Armitage" Comment: patients underwent a one week washout period and there was no evidence of a carry over effect |
| intention to treat analysis? | High risk | Completers only analysis |
| Drop Outs? | Low risk | 5/27 (18.5%) patients withdrew due to adverse events |
| Summary Assessment of Bias? | High risk | High risk of bias |
| Methods | Double‐blind randomised cross‐over trial | |
| Participants | 25 patients with RA 7 male, 18 female Mean age 58yrs (35‐71), mean duration RA12.6 years (2‐30), most patients taking NSAIDs which were continued Inclusion: classical or definite RA Exclusion: information not specified Sample size calculation: not reported | |
| Interventions | Nefopam was 60 mg tds versus placebo for 2 weeks then cross‐over | |
| Outcomes | Baseline weeks 2 and 4 1) Pain: resting, standing, exercise and night pain (VAS100mm) 2) EMS (minutes) 3) Grip strength 4) Ring size | |
| Notes | Conclusion: overall there was no statistically significant improvement in pain in patients receiving nefopam compared to placebo. Recruitment into the study was stopped because it was felt unethical to continue given the high dropout rate. High risk of bias as a consequence of dropouts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) | Low risk | Quote: "placebo tablets were identical" Comment: it is likely that patients remained blind |
| Blinding (performance bias and detection bias) | Unclear risk | No further information specified |
| Incomplete outcome data (attrition bias) | Low risk | 12 patients dropped out and were accounted for |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Compliance? | Unclear risk | No information specified |
| Co‐interventions? | Low risk | 24/25 patient were taking analgesics before the trial and these were ceased for the trial period. Any use of corticosteroids or DMARDs was not described |
| Baseline characteristics? | Unclear risk | Baseline characteristics not described. No washout period before or during the trial |
| intention to treat analysis? | High risk | Completers only analysis |
| Drop Outs? | High risk | 12/25 (48%) patients dropped out and were excluded |
| Summary Assessment of Bias? | High risk | High risk of bias |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Wrong patient population (OA) | |
| Case series | |
| Mixed population, unable to extract RA data topical capsaicin in temporomandibular joint (TMJ) pain | |
| Mixed population, unable to extract RA data | |
| Review article | |
| Unable to extract RA data from mixed patient population (OA) | |
| Comparator was DMARD | |
| Comparator was DMARD | |
| Case series | |
| Mixed population, unable to extract RA data |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 VAS Pain 2 weeks Show forest plot | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐21.16 [‐35.61, ‐6.71] |
| Analysis 1.1  Comparison 1 Nefopam 60 mg tds versus placebo, Outcome 1 VAS Pain 2 weeks. | ||||
| 2 VAS Pain at 4 weeks Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐25.0 [‐42.22, ‐7.78] |
| Analysis 1.2  Comparison 1 Nefopam 60 mg tds versus placebo, Outcome 2 VAS Pain at 4 weeks. | ||||
| 3 Withdrawal Due to adverse events Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.64, 189.65] |
| Analysis 1.3  Comparison 1 Nefopam 60 mg tds versus placebo, Outcome 3 Withdrawal Due to adverse events. | ||||
| 4 Total adverse events Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.11 [1.58, 10.69] |
| Analysis 1.4  Comparison 1 Nefopam 60 mg tds versus placebo, Outcome 4 Total adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS (% reduction from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Capsaicin 0.025% versus placebo, Outcome 1 Pain VAS (% reduction from baseline). | ||||
| 1.1 Week 1 | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐23.8 [‐44.81, ‐2.79] |
| 1.2 Week 2 | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐34.4 [‐54.66, ‐14.14] |
| 1.3 Week 4 | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐25.0 [‐51.76, 1.76] |
| 2 Pain Categorical pain score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Capsaicin 0.025% versus placebo, Outcome 2 Pain Categorical pain score (change from baseline). | ||||
| 2.1 Week 1 | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.77, 0.03] |
| 2.2 Week 2 | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐0.99, ‐0.21] |
| 2.3 Week 4 | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.08, 0.14] |
| 3 Physician Global Evaluation *Global evaluation ( ‐1 to 3, worse to completely gone) Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Capsaicin 0.025% versus placebo, Outcome 3 Physician Global Evaluation *Global evaluation ( ‐1 to 3, worse to completely gone). | ||||
| 3.1 Week 1 | 1 | 31 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐0.11, 1.33] |
| 3.2 Week 2 | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.08, 1.58] |
| 3.3 Week 4 | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.16 [0.36, 1.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short Form McGill Pain Questionnaire (SF‐MPQ) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Cannabis (Setivax) versus placebo, Outcome 1 Short Form McGill Pain Questionnaire (SF‐MPQ). | ||||
| 1.1 Pain at present | 1 | Mean Difference (Fixed, 95% CI) | ‐0.72 [‐1.31, ‐0.13] | |
| 2 Sleep Numerical Rating Score (0‐10) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 1.17 [0.13, 2.21] | |
| Analysis 3.2  Comparison 3 Cannabis (Setivax) versus placebo, Outcome 2 Sleep Numerical Rating Score (0‐10). | ||||
| 3 Withdrawal due to adverse events Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.39] |
| Analysis 3.3  Comparison 3 Cannabis (Setivax) versus placebo, Outcome 3 Withdrawal due to adverse events. | ||||
| 4 Total adverse events Show forest plot | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.10, 3.00] |
| Analysis 3.4  Comparison 3 Cannabis (Setivax) versus placebo, Outcome 4 Total adverse events. | ||||
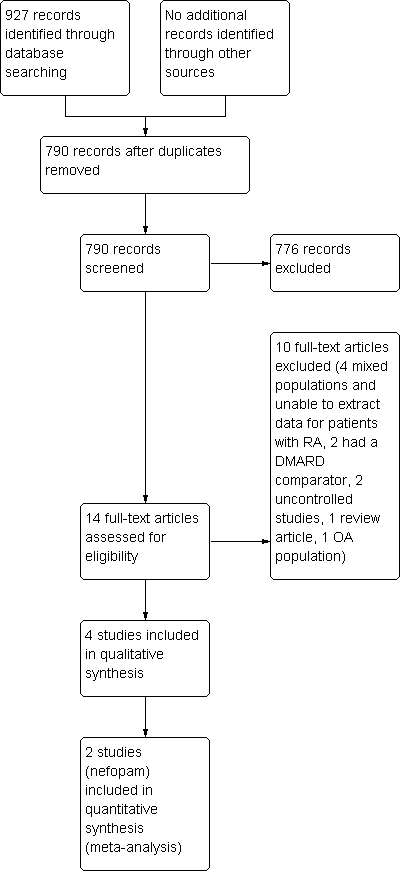
Study flow diagram.
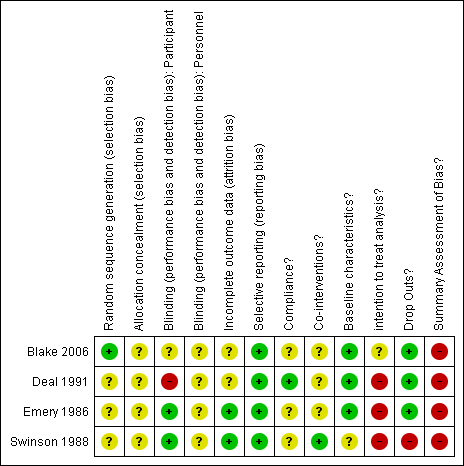
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Nefopam 60 mg tds versus placebo, outcome: 1.1 VAS Pain 2 weeks.
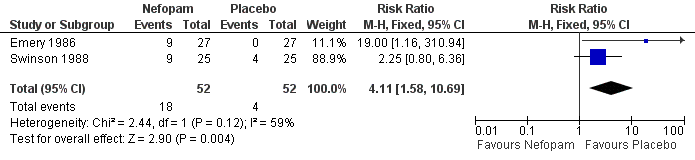
Forest plot of comparison: 1 Nefopam 60 mg tds versus placebo, outcome: 1.4 Total adverse events.

Forest plot of comparison: 2 Capsaicin 0.025% versus placebo, outcome: 2.1 Pain VAS (% reduction from baseline).

Comparison 1 Nefopam 60 mg tds versus placebo, Outcome 1 VAS Pain 2 weeks.
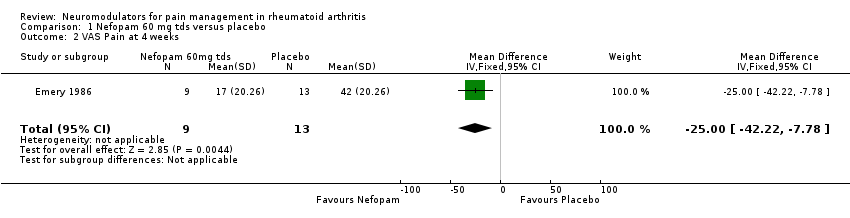
Comparison 1 Nefopam 60 mg tds versus placebo, Outcome 2 VAS Pain at 4 weeks.
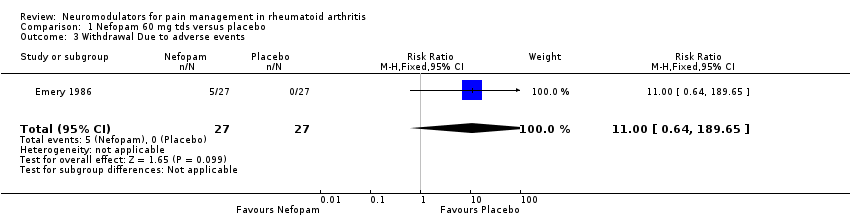
Comparison 1 Nefopam 60 mg tds versus placebo, Outcome 3 Withdrawal Due to adverse events.

Comparison 1 Nefopam 60 mg tds versus placebo, Outcome 4 Total adverse events.

Comparison 2 Capsaicin 0.025% versus placebo, Outcome 1 Pain VAS (% reduction from baseline).
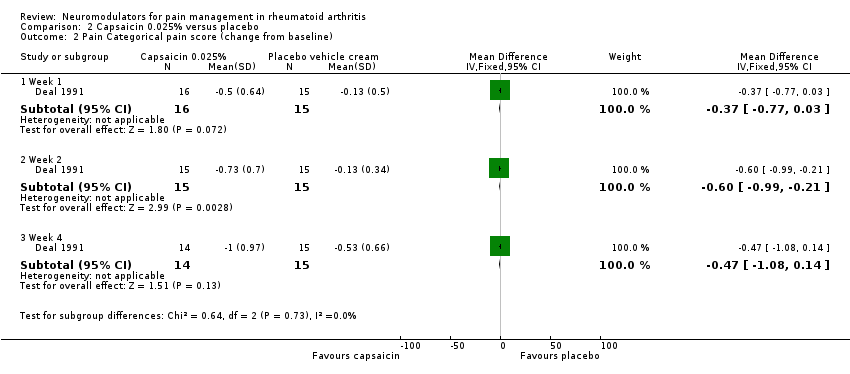
Comparison 2 Capsaicin 0.025% versus placebo, Outcome 2 Pain Categorical pain score (change from baseline).
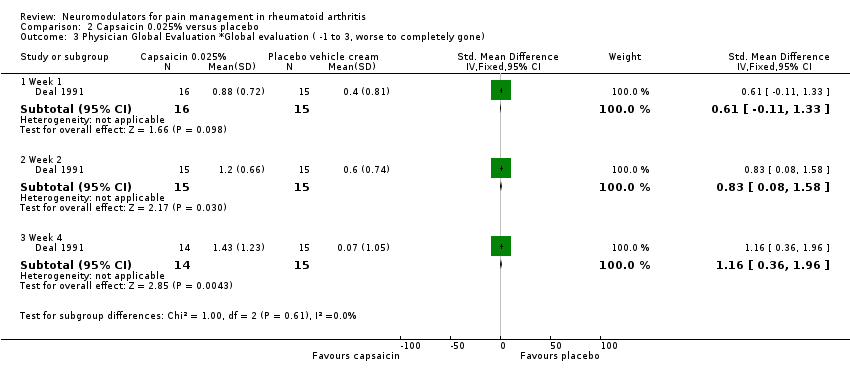
Comparison 2 Capsaicin 0.025% versus placebo, Outcome 3 Physician Global Evaluation *Global evaluation ( ‐1 to 3, worse to completely gone).

Comparison 3 Cannabis (Setivax) versus placebo, Outcome 1 Short Form McGill Pain Questionnaire (SF‐MPQ).

Comparison 3 Cannabis (Setivax) versus placebo, Outcome 2 Sleep Numerical Rating Score (0‐10).
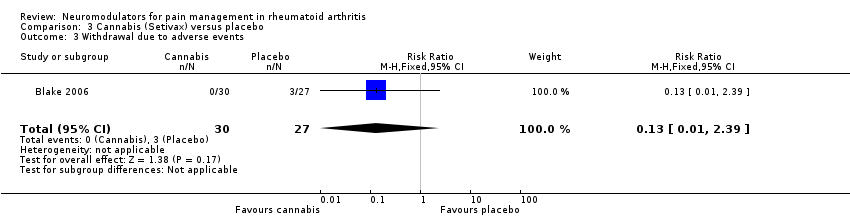
Comparison 3 Cannabis (Setivax) versus placebo, Outcome 3 Withdrawal due to adverse events.

Comparison 3 Cannabis (Setivax) versus placebo, Outcome 4 Total adverse events.
| Nefopam 60 mg tds compared to placebo for pain management in rheumatoid arthritis | ||||||
| Patient or population: patients with pain management in rheumatoid arthritis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Nefopam 60mg tds | |||||
| Pain | The mean Pain in the intervention groups was | 48 | ⊕⊕⊝⊝ | Absolute risk difference 21% (7% to 36%), relative percent change 54% (17% to 91%). Number needed to treat (NNT) was 2 (95%CI 1.4‐9.5) | ||
| Withdrawal Due to adverse events | 0 per 1000 | 0 per 1000 | RR 11 | 54 | ⊕⊕⊕⊝ | Not statistically significant. Absolute risk difference 19% (3% to 34%), relative percent change 1000% (‐36% to 18865%) |
| Total adverse events | 77 per 1000 | 316 per 1000 | RR 4.11 | 104 | ⊕⊕⊝⊝ | Absolute risk difference 27% (12% to 42%), relative percent change 311% (58% to 969%). Number needed to harm (NNTH) 9 (95% CI 2 to 367). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only small number of participants | ||||||
| Capsaicin 0.025% compared to placebo for knee pain in patients with rheumatoid arthritis | ||||||
| Patient or population: patients with Knee pain in patients with rheumatoid arthritis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Capsaicin 0.025% | |||||
| Pain (% reduction from baseline) | The mean Pain (% reduction from baseline) in the intervention groups was | 30 | ⊕⊕⊝⊝ | Absolute risk difference 34% (14% to 55%), relative percent change 63% (26% to 99%). Number needed to treat (NNT) 2 (95% CI 1.4 to 6). | ||
| Pain (% reduction from baseline) | The mean Pain (% reduction from baseline) in the intervention groups was | 29 | ⊕⊕⊝⊝ | Not statistically significant. Absolute risk difference ‐25% (‐52% to 2%), relative percentage change ‐46% (‐94% to 3%). | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Patients were not adequately blinded, short duration trial and inadequately powered . | ||||||
| Oromucosal cannabis (Sativex) compared to placebo for pain management in patients with rheumatoid arthritis | ||||||
| Patient or population: patients with pain management in patients with rheumatoid arthritis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oromucosal Cannabis (Sativex) | |||||
| Pain at present | The mean Pain at present in the intervention groups was | 58 | ⊕⊕⊝⊝ | Number needed to treat (NNT) 3 (2‐18), absolute risk difference 14% (3%% to 26%), relative percentage change 23% (4% to 41%). | ||
| Quality of Sleep | The mean Quality of Sleep in the intervention groups was | 58 | ⊕⊕⊝⊝ | NNT 2 (1 to 18), absolute risk difference 12% (1% to 22%), relative percentage change 20% (2% to 38%). | ||
| Withdrawal due to adverse events | 111 per 1000 | 14 per 1000 | RR 0.13 | 58 | ⊕⊕⊝⊝ | Not significant difference. Absolute risk difference 13% (1% to 239%), relative percentage change |
| Total adverse events | 407 per 1000 | 741 per 1000 | RR 1.82 | 58 | ⊕⊕⊝⊝ | Number needed to harm (NNTH) of 3 (95% CI 3‐13), absolute risk difference 33% (9% to 58%), relative percentage change 82% (10% to 200%). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Indaquate blinding and skewed data | ||||||
| Outcome | Median Difference | 95% Confidence Intervals | p value |
| Morning pain on movement | ‐0.95 | 1.83, 0.02 | 0.044 |
| Morning pain at rest | ‐1.04 | 1.90, 0.18 | 0.018 |
| SF‐MPQ, total intensity of pain | 3.00 | ‐3.00. 9.00 | 0.302 |
| SF‐MPQ, intensity of pain at present | ‐3.00 | 18.0, 9.00 | 0.572 |
| These scores were not normally distributed and were analysed non‐parametrically (Wilcoxon rank‐sum test, Hodges–Lehmann median difference and 95% CI). SF‐MPQ, short form McGill Pain Questionnaire. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 VAS Pain 2 weeks Show forest plot | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐21.16 [‐35.61, ‐6.71] |
| 2 VAS Pain at 4 weeks Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐25.0 [‐42.22, ‐7.78] |
| 3 Withdrawal Due to adverse events Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.64, 189.65] |
| 4 Total adverse events Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.11 [1.58, 10.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS (% reduction from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Week 1 | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐23.8 [‐44.81, ‐2.79] |
| 1.2 Week 2 | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐34.4 [‐54.66, ‐14.14] |
| 1.3 Week 4 | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐25.0 [‐51.76, 1.76] |
| 2 Pain Categorical pain score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Week 1 | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.77, 0.03] |
| 2.2 Week 2 | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐0.99, ‐0.21] |
| 2.3 Week 4 | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.08, 0.14] |
| 3 Physician Global Evaluation *Global evaluation ( ‐1 to 3, worse to completely gone) Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Week 1 | 1 | 31 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐0.11, 1.33] |
| 3.2 Week 2 | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.08, 1.58] |
| 3.3 Week 4 | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.16 [0.36, 1.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short Form McGill Pain Questionnaire (SF‐MPQ) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 Pain at present | 1 | Mean Difference (Fixed, 95% CI) | ‐0.72 [‐1.31, ‐0.13] | |
| 2 Sleep Numerical Rating Score (0‐10) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | 1.17 [0.13, 2.21] | |
| 3 Withdrawal due to adverse events Show forest plot | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.39] |
| 4 Total adverse events Show forest plot | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.10, 3.00] |

