Acetato de eslicarbazepina como tratamiento complementario para la epilepsia focal farmacorresistente
Resumen
Antecedentes
Esta es una actualización de una revisión publicada por primera vez en 2011 y actualizada por última vez en 2017.
La mayoría de las personas con epilepsia tienen buen pronóstico, pero hasta un 30% continúa con las crisis a pesar del uso de varios regímenes de fármacos antiepilépticos. En esta revisión, se resumió la evidencia actual sobre el acetato de eslicarbazepina (ESL) cuando se utiliza como tratamiento complementario para la epilepsia focal farmacorresistente.
Objetivos
Evaluar la eficacia y la tolerabilidad del ESL como tratamiento complementario en personas con epilepsia focal farmacorresistente.
Métodos de búsqueda
Para esta actualización se realizaron búsquedas en las siguientes bases de datos el 10 de septiembre de 2020: Registro Cochrane de estudios (Cochrane Register of Studies) (CRS Web) y en MEDLINE (Ovid). El CRS Web incluye ensayos controlados aleatorizados o cuasialeatorizados de los Registros especializados de los Grupos Cochrane de Revisión, incluido el de Epilepsia, CENTRAL, PubMed, Embase, ClinicalTrials.gov y la ICTRP de la OMS. No hubo restricciones de idioma. Se examinaron las listas de referencias de los estudios identificados y se estableció contacto con los fabricantes de ESL y con expertos en la materia para obtener información sobre cualquier estudio no publicado o en curso.
Criterios de selección
Ensayos aleatorizados doble ciego controlados con placebo de ESL en personas con epilepsia focal farmacorresistente.
Obtención y análisis de los datos
Dos autores de la revisión seleccionaron de forma independiente los ensayos para la inclusión y extrajeron los datos. Los desenlaces investigados incluyeron una reducción del 50% o más en la frecuencia de las crisis epilépticas, el cese de las crisis epilépticas, el retiro del tratamiento, los efectos adversos y las interacciones con otros fármacos. Los análisis primarios fueron por intención de tratar (ITT). La relación dosis‐respuesta se evaluó en los modelos de regresión.
Resultados principales
Se incluyeron siete ensayos (2185 participantes, con edades comprendidas entre los dos y los 77 años), que tenían un riesgo de sesgo bajo o incierto, aparte de un alto riesgo de sesgo de desgaste; todos los estudios estuvieron financiados por la compañía farmacéutica BIAL.
La razón de riesgos (RR) general para una reducción del 50% o más en la frecuencia de las crisis fue 1,57 (intervalo de confianza [IC] del 95%: 1,34 a 1,83). Para los adultos, el RR fue 1,71 (IC del 95%: 1,42 a 2,05; cinco estudios, 1799 participantes; evidencia de certeza moderada); para los niños de seis a 18 años, el RR fue 1,35 (IC del 95%: 0,98 a 1,87; dos estudios, 322 participantes; evidencia de certeza moderada). El análisis de regresión de dosis demostró evidencia de que el ESL redujo la frecuencia de las crisis convulsivas con un aumento de la eficacia tras aumentar las dosis del ESL. El ESL se asoció con el cese de las crisis epilépticas (RR 3,16; IC del 95%: 1,73 a 5,78; seis estudios, 1922 participantes; evidencia de certeza moderada). Los participantes fueron más propensos a que se les retirara el tratamiento con ESL por los efectos adversos (RR 2,72; IC del 95%: 1,66 a 4,46; siete estudios, 2185 participantes; evidencia de certeza moderada), pero no por cualquier motivo (RR 1,25; IC del 95%: 0,93 a 1,70; siete estudios, 2185 participantes; evidencia de certeza moderada). Los siguientes efectos adversos se asociaron con el ESL: mareos (RR 2,77; IC del 99%: 1,85 a 4,15); náuseas (RR 2,55; IC del 99%: 1,39 a 4,67); somnolencia (RR 1,75; IC del 99%: 1,18 a 2,61); diplopía (RR 4,07; IC del 99%: 1,86 a 8,89) y vómitos (RR 2,37; IC del 99%: 1,19 a 4,74). En general, la certeza de la evidencia fue moderada debido a la alta tasa de interrupción en los estudios con adultos.
Conclusiones de los autores
El ESL reduce la frecuencia de las crisis epilépticas cuando se utiliza como tratamiento complementario en los adultos con epilepsia focal farmacorresistente. Los ensayos incluidos en esta revisión fueron de corta duración. Además, esta actualización encontró que el ESL podría reducir la frecuencia de las crisis epilépticas en niños de seis a 18 años de edad; sin embargo, los resultados no son concluyentes.
PICO
Resumen en términos sencillos
Acetato de eslicarbazepina como tratamiento complementario para la epilepsia focal resistente a los medicamentos
Pregunta de la revisión
Esta es una actualización de una revisión publicada por primera vez en 2011 y actualizada por última vez en 2017.
Se revisó la evidencia sobre la efectividad y los efectos secundarios del acetato de eslicarbazepina cuando se usa como tratamiento complementario para la epilepsia focal resistente a los medicamentos.
Antecedentes
El acetato de eslicarbazepina es un medicamento antiepiléptico que se puede agregar (lo que se conoce como tratamiento "complementario") para tratar a las personas que toman otros medicamentos antiepilépticos pero que continúan teniendo crisis (llamada epilepsia resistente a los medicamentos). Esta revisión analizó los efectos beneficiosos del acetato de eslicarbazepina cuando se utiliza como tratamiento complementario y algunos de los efectos secundarios del medicamento.
Características de los estudios
La evidencia está actualizada hasta septiembre de 2020. Se incluyeron siete ensayos clínicos con 2185 participantes de entre dos y 77 años de edad. Los estudios incluidos tuvieron diferentes períodos de tratamiento de 12 a 18 semanas. Los cinco ensayos fueron ensayos controlados aleatorizados, lo que significa que las personas se asignaron de forma aleatoria en grupos y se compararon.
Resultados clave
Esta revisión encontró que el acetato de eslicarbazepina fue efectivo cuando se administró en combinación con otros medicamentos para reducir el número de crisis epilépticas en adultos con epilepsia focal resistente a los medicamentos. El acetato de eslicarbazepina también podría ser efectivo para reducir la frecuencia de las crisis en niños con epilepsia focal resistente a los medicamentos. Las personas que tomaron acetato de eslicarbazepina tuvieron más probabilidades de no tener convulsiones en comparación con las que tomaron un placebo (un comprimido falso), pero tuvieron más probabilidades de dejar de tomar el acetato de eslicarbazepina debido a los efectos secundarios. Entre ellos se encuentran los mareos, las náuseas (sensación de malestar), la somnolencia (sensación de sueño), los vómitos y la diplopía (visión doble).
Calidad de la evidencia
En conjunto, los siete ensayos utilizaron buenas metodologías, pero en cinco ensayos con adultos faltó información de entre el 10% y el 45% de las personas que recibieron el medicamento. Esta información faltante podría haber introducido incertidumbre en los resultados, por lo que la evidencia de esta revisión es de calidad moderada. Se necesitan más estudios de investigación para analizar los efectos a largo plazo del acetato de eslicarbazepina y para explorar su eficacia en los niños con epilepsia.
Authors' conclusions
Summary of findings
| Eslicarbazepine acetate add‐on for drug‐resistant focal epilepsy | ||||||
| Patient or population: people with drug‐resistant focal epilepsy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo | Risk with ESL | |||||
| ≥ 50% reduction in seizure frequency (adults) Follow‐up: up to 18 weeks | Study population | RR 1.71 | 1799 | ⊕⊕⊕⊝ | Results presented for ITT population. | |
| 203 per 1000 | 347 per 1000 | |||||
| ≥ 50% reduction in seizure frequency (children aged 6–18 years) Follow‐up: up to 18 weeks | Study population | RR 1.35 (0.98 to 1.87) | 322 (2 RCTs) | ⊕⊕⊕⊝ | ESL may reduce seizures in children aged 6–18 years with drug‐resistant focal epilepsy. | |
| 294 per 1000 | 397 per 1000 | |||||
| Freedom from seizures Follow‐up: up to 18 weeks | Study population | RR 3.16 | 1922 | ⊕⊕⊕⊝ | — | |
| 20 per 1000 | 63 per 1000 | |||||
| Treatment withdrawal (any reason) Follow‐up: up to 18 weeks | Study population | RR 1.25 | 2185 | ⊕⊕⊕⊝ | — | |
| 164 per 1000 | 205 per 1000 | |||||
| Treatment withdrawal (adverse effect) Follow‐up: up to 18 weeks | Study population | RR 2.72 | 2185 | ⊕⊕⊕⊝ | — | |
| 38 per 1000 | 104 per 1000 | |||||
| Adverse effects Follow‐up: up to 18 weeks | Study population | See comment | See comment | ⊕⊕⊕⊝ | ESL was associated with dizziness, nausea, somnolence, diplopia and vomiting. | |
| See comment | See comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ESL: eslicarbazepine acetate; ITT: intention to treat; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aThese studies were funded by BIAL – Portela & Ca, SA. There was no indication that the source of funding introduced bias. | ||||||
Background
This review is an update of a Cochrane review first published in 2011 (Chang 2011), and last updated in 2017 (Chang 2017).
Description of the condition
Epilepsy is a common neurological condition. The incidence of epilepsy is estimated at about 50 (range 40 to 70) per 100,000 per year in high‐income countries, and it seems to be higher in low‐income countries. The prevalence is around 5 to 10 per 1000 in most settings (Sander 2003). Focal seizures are seizures which primarily affect one hemisphere of the brain (Hussein 2018).
Most people with epilepsy have a good prognosis but up to 30% of people continue to have seizures despite several regimens of antiepileptic drugs (AEDs) (Walker 1997).
People with drug‐resistant epilepsy often experience psychosocial, psychiatric and medical comorbidities, which are due to recurrent seizures, long‐term drug effects and employment restrictions (Schuele 2008). Eslicarbazepine acetate (ESL) may help to alleviate some of these problems by reducing seizure frequency.
Description of the intervention
Since the late‐1990s, many AEDs have been licensed worldwide. ESL is a novel, once‐daily AED for the adjunctive treatment of drug‐resistant epilepsy. It shares a similar structure with carbamazepine and oxcarbazepine but does not inhibit most cytochrome P450 enzymes (CYP450) and has a low potential for drug interaction (Almeida 2007). Eslicarbazepine is the main active component of ESL and its pharmacokinetics are not affected by age, gender, food or moderate hepatic impairment, but clearance of ESL is dependent on renal function (McCormack 2009; Mestre 2009).
How the intervention might work
The main mechanism of action of ESL is thought to be by blocking voltage‐gated sodium channels (Almeida 2007).
Why it is important to do this review
ESL is an anticonvulsant medication which was approved for use in Europe in 2009 and in the US in 2013 as a monotherapy or add‐on treatment for focal‐onset seizures with or without secondary generalization. In this review, we assessed the efficacy and tolerability of ESL when used as an add‐on treatment in adults and children with drug‐resistant focal epilepsy.
Objectives
To evaluate the efficacy and tolerability of ESL when used as an add‐on treatment for people with drug‐resistant focal epilepsy.
Methods
Criteria for considering studies for this review
Types of studies
-
Randomized controlled trials (RCTs) using an adequate method of concealment of randomization (e.g. sequential allocation of sealed packages of medication, sealed opaque envelopes, telephone allocation).
-
Double‐blind trials in which both participants and clinicians treating or assessing the outcome were blinded to the treatment allocated.
-
Placebo‐controlled studies.
-
Parallel group or cross‐over studies (see Unit of analysis issues).
Types of participants
People of any age with drug‐resistant focal epilepsy (i.e. experiencing simple focal, complex focal or secondary generalized tonic‐clonic seizures).
In this review, we defined drug resistance as continued seizures despite treatment with one or more AEDs.
Types of interventions
-
Active treatment group received ESL in addition to an existing AED regimen at the time of randomization.
-
Control group received a matched placebo in addition to an existing AED regimen at the time of randomization.
Types of outcome measures
Primary outcomes
-
A 50% or greater reduction in seizure frequency – the proportion of people with a 50% or greater reduction in seizure frequency within the treatment period compared to the prerandomization baseline period. This was chosen as a primary outcome because it is a commonly reported and can be calculated for studies that did not report this outcome provided that baseline seizure data were recorded.
-
Freedom from seizures – the proportion of participants with complete cessation of seizures from the time of randomization to the trial conclusion.
Secondary outcomes
-
Treatment withdrawal – the proportion of participants withdrawn from treatment, for any reason, during the treatment period as a measure of global effectiveness. Treatment was likely to be withdrawn due to adverse effects, lack of efficacy, or a combination of both, and this is an outcome to which the individual makes a direct contribution. In trials of short duration, it was likely that adverse effects were the most common reason for withdrawal. We also assessed the proportion of people having treatment withdrawn because of adverse effects.
-
Adverse effects
-
Proportion of participants experiencing any of the following five adverse effects, which we considered to be common, important adverse effects of AEDs:
-
ataxia;
-
dizziness;
-
fatigue;
-
nausea;
-
somnolence.
-
-
Proportion of participants experiencing rash and the five most common adverse effects that were different from those listed above.
-
-
Drug interactions – any drug interactions reported in the included studies.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for RCTs and imposed no language restrictions. The searches for the original review were run in November 2011. Subsequent searches were run in November 2013, April 2016, December 2016 and March 2019. For the latest update, we searched the following databases on 10 September 2020:
-
Cochrane Register of Studies (CRS Web), using the strategy outlined in Appendix 1;
-
MEDLINE (Ovid, 1946 to 9 September 2020), using the strategy outlined in Appendix 2.
CRS Web includes randomized or quasi‐randomized, controlled trials from Specialized Registers of Cochrane Review Groups including Epilepsy, the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase, ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (ICTRP).
Searching other resources
We reviewed the reference lists of retrieved studies to search for additional reports of relevant studies. We also contacted the manufacturers of ESL and experts in the field for information about any unpublished or ongoing studies.
Data collection and analysis
Two review authors (XCC and HY) independently assessed trials for inclusion. We compared results and resolved any disagreements by discussion. If a disagreement was not resolved, a third review author arbitrated.
Selection of studies
The process for selecting studies for inclusion in the review involved merging search results using reference management software and removing duplicates of the same report. We examined titles and abstracts to remove obviously irrelevant reports. We retrieved the full texts of the remaining reports and examined studies for compliance with our eligibility criteria. The review authors agreed on trials for inclusion before proceeding to data collection.
Data extraction and management
Two review authors (XCC and HY) extracted the following information from included trials. Disagreements were resolved by discussion. If a disagreement was not resolved, a third review (RYZ) author arbitrated.
Methodology and trial design
-
Method of randomization concealment.
-
Method of blinding.
-
Whether any participants were excluded from the reported analyses.
-
Duration of baseline period.
-
Duration of treatment period.
-
Dose(s) of ESL tested.
Participant and demographic information
-
Total number of participants allocated to each treatment group.
-
Age and sex.
-
Numbers with focal and generalized epilepsy.
-
Seizure types.
-
Number of background drugs.
-
Seizure frequency during the baseline period.
Outcomes
-
Number of participants experiencing each outcome (see Types of outcome measures) per randomized group.
Other information
All trials found so far have been sponsored by BIAL (Portela & Ca, S.A.) who confirmed the following information (Sperling 2015 was verified by Professor Sperling and Sunovion Pharmaceuticals Inc).
-
Method of randomization.
-
Total number of participants randomized to each group.
-
Number of participants in each group achieving a 50% or greater reduction in seizures.
-
Number of participants having treatment withdrawn postrandomization per treatment group.
-
For those excluded, the reason for exclusion, whether any of those excluded completed the treatment phase, and whether any of those excluded had a 50% or greater reduction in seizure frequency during the treatment phase.
Assessment of risk of bias in included studies
We evaluated methodological quality of the studies according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
For dichotomous data, we determined the risk ratio (RR) and 95% confidence interval (CI).
Unit of analysis issues
For cross‐over studies, we planned to use the first treatment period as a parallel group trial. For multiple dose trials, we planned to add analysis by dose.
Dealing with missing data
We contacted the manufacturers and original investigators of relevant trials to identify any additional published or unpublished data.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the distribution of important participant factors between trials (age, seizure type, duration of epilepsy, number of AEDs taken at the time of randomization) and trial factors (randomization concealment, blinding, losses to follow‐up). We assessed statistical heterogeneity using the Chi2 test, where a P value lower than 0.1 indicated substantial heterogeneity. We also used the I2 statistic to quantify inconsistency across studies. An approximate guide to the interpretation of the I2 statistic was as follows (Higgins 2019):
-
0% to 40%: might not be important;
-
30% to 60%: may represent moderate heterogeneity;
-
50% to 90%: may represent substantial heterogeneity;
-
75% to 100%: considerable heterogeneity.
If statistical heterogeneity existed, we explored potential causes. If statistical heterogeneity was below 30%, we synthesized data using a fixed‐effect model. If substantial heterogeneity could not readily be explained, we adopted a random‐effects model.
Assessment of reporting biases
The possibility of publication bias was not explored using funnel plots because there was an insufficient number of trials for the relevant comparisons.
Data synthesis
We performed data synthesis and analysis using Review Manager 5 (Review Manager 2014). We calculated the RR. For the outcome measures of 50% reduction in seizure frequency, seizure freedom and treatment withdrawal, we quoted 95% CIs. For individual adverse effects, we quoted 99% CIs to make an allowance for multiple testing. Analyses included all participants in the treatment groups to which they were allocated (ITT).
For the efficacy outcome (50% or greater reduction in seizure frequency), we undertook three analyses.
-
Primary (ITT) analysis: participants not completing follow‐up or with inadequate seizure data were assumed to be non‐responders.
-
Worst‐case scenario analysis: participants not completing follow‐up or with inadequate seizure data were assumed to be non‐responders in the ESL group and responders in the placebo group.
-
Best‐case scenario analysis: participants not completing follow‐up or with inadequate seizure data were assumed to be responders in the ESL group and non‐responders in the placebo group.
Dose regression analysis: we also examined dose‐response relationships for the primary outcome using logistic regression in the framework of generalized linear models (McCullagh 1989). Probabilities for the following were calculated for differing doses: the percentage of participants having a 50% response and the difference in the percentage of participants responding to each dose compared to placebo. A binary variable was defined with value of 0 if the response was less than 50% and a value of 1 if otherwise.
Subgroup analysis and investigation of heterogeneity
We undertook a subgroup analysis according to different doses of ESL and age (children versus adults).
Sensitivity analysis
We intended to carry out sensitivity analyses if there were peculiarities between study quality, characteristics of participants, interventions and outcomes.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to interpret findings (Schünemann 2019). We used GRADE Profiler software (GRADEpro GDT), and imported data from Review Manager 5 (Review Manager 2014), to create summary of findings tables for each comparison included in the review for 50% or greater reduction in seizure frequency, freedom from seizures, treatment withdrawal (any reason or adverse effects) and adverse effects. Considering the different effects of ESL on 50% or greater reduction in seizure frequency in children and adults, we reported this outcome in children and adults separately.
The summary of findings table for each comparison included information on the overall certainty of the evidence from the trials and information of importance for healthcare decision making. The GRADE approach determined the certainty of evidence on the basis of an evaluation of eight criteria (risk of bias, inconsistency, indirectness, imprecision, publication bias, effect size, presence of plausible confounding that will change effect and dose‐response gradient). We used these to guide our conclusions.
Results
Description of studies
We included two new trials in this update: one investigating the use of ESL in children aged six to 16 years with drug‐resistant focal epilepsy (Jóźwiak 2018), and the other investigating the use of ESL in children aged two to 18 years with focal epilepsy (Kirkham 2020). Full details of the individual studies are provided in the Characteristics of included studies table.
Results of the search
The latest search in September 2020 found one eligible study (Kirkham 2020). This study was also identified via the additional records identified through other sources in the search undertaken in March 2019.
The search carried out in March 2019 identified 77 records, and we also found one additional record identified through other sources (Kirkham 2020). Twenty‐seven were duplicates and removed. We excluded 47 of the remaining records due to irrelevance leaving four full‐text articles to be assessed for eligibility. After this, we excluded two studies (see Figure 1 and Characteristics of excluded studies table for reasons for exclusion). Therefore, we added two studies (Jóźwiak 2018; Kirkham 2020), to the five studies already included in the previous version of this review (Ben‐Menachem 2010; Elger 2007; Elger 2009; Gil‐Nagel 2009; Sperling 2015).
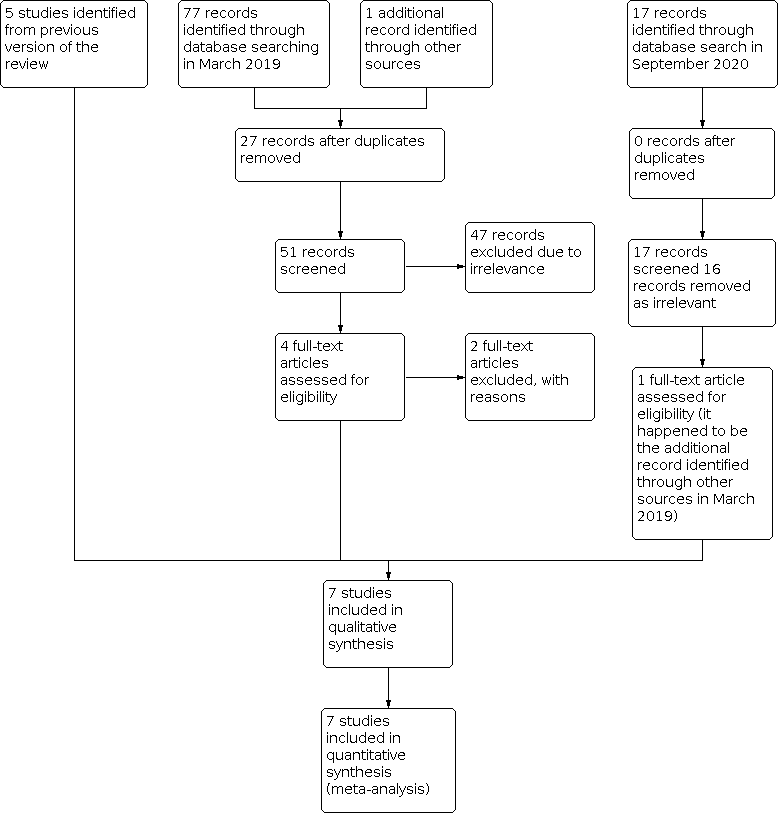
Study flow diagram for update.
Included studies
Ben‐Menachem and colleagues published a randomized, double‐blind, placebo‐controlled, parallel group, multi‐center (45 sites in 13 countries) study in 2010 with 395 participants aged between 18 and 69 years (Ben‐Menachem 2010). After a baseline period of eight weeks, participants were randomized to one of the four treatment arms for a treatment period of 14 weeks. The treatment arms included ESL 400 mg, 800 mg and 1200 mg once‐daily and a placebo arm. A total of 115 participants dropped out of the study and two participants had no postbaseline data.
Elger and colleagues reported a multi‐center (19 sites in five countries) parallel group trial of 144 participants aged 18 to 65 years (Elger 2007). The treatment groups included an ESL once‐daily group, an ESL twice‐daily group and a placebo group. The daily doses of ESL were increased from 400 mg to 800 mg to 1200 mg at four‐week intervals. The study consisted of a two‐month retrospective baseline period, 12‐week maintenance period and four‐week tapering‐off period. A total of 113 participants completed the study and one participant did not take any study medication. In this review, we included only the data in the once‐daily group and the placebo group in the analysis for clinical heterogeneity as the other studies have all used such a titration method of once‐daily.
Elger and colleagues published a multi‐center (40 sites in 11 countries) parallel group trial including 402 participants aged 18 to 76 years (Elger 2009). Participants were randomized into one of four treatment arms: ESL 400 mg (100 participants), 800 mg (98 participants) and 1200 mg (102 participants) once‐daily; while 102 participants were in the placebo group. The trial duration was 18 weeks including a two‐week titration period, a 12‐week maintenance period and a four‐week tapering‐off period. A total of 72 participants discontinued the study and five participants lacked postbaseline efficacy data.
Gil‐Nagel and colleagues described a multi‐center (39 sites in three countries) trial including 252 participants aged 17 to 77 years (Gil‐Nagel 2009). Parallel groups included 85 participants taking ESL 800 mg once‐daily, 80 participants taking ESL 1200 mg once‐daily and 87 participants taking placebo. After a baseline assessment period of eight weeks, the trial was conducted over 18 weeks (including a two‐week titration period, 12‐week maintenance period and four‐week tapering‐off period). A total of seven participants had no postbaseline efficacy data and 58 participants dropped out of the study.
Jóźwiak and colleagues published a multi‐center (four countries) parallel group trial including 123 participants aged six to 16 years (Jóźwiak 2018). Participants were randomized (2:1) to ESL (83 participants) and placebo (40 participants). The ESL daily doses were increased from 10 mg/kg/day to 30 mg/kg/day (or to a maximum of 1200 mg/day). After a baseline assessment period of four weeks, the trial was conducted over 12 double‐blind weeks (including a four‐week titration period, eight‐week maintenance period) and a tapering‐off period. A total 11 participants discontinued the study.
Kirkham and colleagues reported a multi‐center (20 countries) parallel group trial including 304 participants aged two to 18 years (Kirkham 2020). A total of 304 children were randomized (1:1) and stratified by age (2 to 6 years; 7 to 11 years; 12 to 18 years) to receive either ESL or placebo. The trial included an eight‐week observational baseline period followed by a six‐week double‐blind titration period, a 12‐week double‐blind maintenance period and a tapering‐off period. Parallel groups included 155 participants taking ESL 20 mg/kg/day to 30 mg/kg/day (or to a maximum of 1200 mg/day), and 149 participants taking placebo. Forty‐one participants aged two to six years (21 in ESL group, 20 on placebo group) were excluded from the ITT analysis due to the investigational medicinal product (IMP) recall. There were 25 participants who discontinued the study from the remaining participants (263 participants: 134 in ESL group, 129 in placebo).
Sperling and colleagues described a multi‐center (173 sites in 19 countries) trial including 653 participants aged 16 to 71 years (Sperling 2015). Parallel groups included 216 participants taking ESL 800 mg once‐daily, 211 participants taking ESL 1200 mg once‐daily and 226 participants taking placebo. After a baseline assessment period of eight weeks, the trial was conducted over 14 weeks (including a two‐week titration period, a 12‐week maintenance period and a two‐week tapering‐off period). A total of 13 participants had no postbaseline efficacy data and 149 participants dropped out of the study.
Excluded studies
We excluded two studies from this review update, as they were not RCTs (Chaves 2017; Losch 2016).
Risk of bias in included studies
Detailed assessments of each risk item for each included study can be found in the risk of bias tables in the Characteristics of included studies table. A summary of the review authors' judgements is shown in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For all trials, we rated the methods by which allocation was concealed at low risk of bias, because four used sequentially numbered drug containers (Ben‐Menachem 2010; Elger 2007; Elger 2009; Gil‐Nagel 2009) and three used central randomization (Jóźwiak 2018; Kirkham 2020; Sperling 2015). For sequence generation, we rated all seven studies at low risk of bias due to the use of a computer‐generated randomization schedule.
Blinding
We rated all seven trials at low risk of performance and detection bias because participants, personnel and outcome assessors were blinded.
Incomplete outcome data
All seven trials reported missing data and performed an ITT analysis. However, the proportions of missing data were relatively high in five studies, ranging from 10% to 45% missing data per treatment arm (Ben‐Menachem 2010; Elger 2007; Elger 2009; Gil‐Nagel 2009; Sperling 2015). In particular, in the four trials with an ESL 1200 mg dose arm, the proportion of missing data was high, ranging from 26% to 45%. Therefore, we judged the risk of attrition bias to be high in these five studies and performed best‐case scenario and worst‐case scenario analyses to examine the impact of the missing data (see Data synthesis). We rated the other two studies at low risk of attrition bias due to the ITT analyses and relatively low dropout rate (Jóźwiak 2018; Kirkham 2020).
Selective reporting
We rated one study at unclear risk of reporting bias due to the lack of the outcome of freedom from seizures (Kirkham 2020), and the other six studies at low risk in this domain because there was no suspicion of selective outcome reporting bias.
Other potential sources of bias
We rated one study at high risk of bias on this domain because 41 participants aged two to six years were involved with the IMP recall (Kirkham 2020). The other studies were at low risk of bias as there was no evidence of further bias in any of the included studies.
Effects of interventions
See: Summary of findings 1 Eslicarbazepine acetate add‐on for drug‐resistant focal epilepsy
Primary outcomes
Intention‐to‐treat analysis: 50% or greater reduction in seizure frequency
All seven trials (2185 adults and children) contributed to this outcome. A Chi2 testfor responses to ESL indicated moderate heterogeneity between trials (Chi2 = 9.71, degrees of freedom (df) = 6, P = 0.14; I2 = 38%). Considering that heterogeneity came from a trial only recruiting children (Kirkham 2020), a fixed‐effect model was used. Those participants allocated to receive ESL were significantly more likely to achieve a 50% or greater reduction in seizure frequency (RR 1.57, 95% CI 1.34 to 1.83, Analysis 1.1). For adults, the RR was 1.71 (95% CI 1.42 to 2.05). For children, the RR was 1.19 (95% CI 0.88 to 1.61). However, due to the IMP in the two‐ to six‐year age group (Kirkham 2020), we excluded the data from this age group from analysis. This analysis found that the RR was 1.35 (95% CI 0.98 to 1.87), which means that participants receiving ESL may be more likely to have 50% or greater reductions in seizure frequency in the 6‐ to 18‐year age group though results are inconclusive. Subgroup analyses assessing the effects of individual doses showed that higher doses of ESL were associated with higher 50% or greater reductions in seizure frequency (400 mg/day: RR 1.22, 95% CI 0.80 to 1.85; 800 mg/day: RR 1.66, 95% CI 1.34 to 2.07; 1200 mg/day: RR 1.92, 95% CI 1.56 to 2.37; Analysis 1.1). Subgroup analysis stratified by the dose of ESL did not include the data from the two studies that only recruited children, since the dose they took was given by weight (Jóźwiak 2018; Kirkham 2020).
Best‐case and worse‐case scenarios: 50% or greater reduction in seizure frequency
The Chi2 tests for heterogeneity for a response to ESL indicated significant heterogeneity between trials in best‐case scenarios (Chi2 = 22.38, df = 6, P = 0.001; I2 = 73%), not in worst‐case scenarios (Chi2 = 6.40, df = 6, P = 0.38; I2 = 0%). The heterogeneity in best‐case scenarios results from inconsistent results in children. Therefore, the random‐effects model was used to obtain the pooled effect size. For the best‐case scenario, the overall RR was 2.47 (95% CI 1.84 to 3.31; Analysis 1.2) and for the worst‐case scenario, it was 0.89 (95% CI 0.79 to 1.00; Analysis 1.3). These results were not consistent with the result of the ITT analysis, which may be due to the high dropout rate of the included studies.
Dose regression analysis for 50% or greater reduction in seizure frequency
Four trials studied the dose‐response regression with aggregate data, as a result of the similar ways of titration (Ben‐Menachem 2010; Elger 2009; Gil‐Nagel 2009; Sperling 2015). As one trial tested different doses in each group and each dose period lasted only four weeks, the data from Elger 2007 were not included in this analysis. Also the data from Jóźwiak 2018 and Kirkham 2020 were not included because children received different doses of ESL based on weight. The results from the four studies indicated an increase in effect with increasing dose, with no clear plateau at the doses tested. The difference between ESL 400 mg and placebo was not significant. Table 1 shows the estimated percentage of participants responding to each dose with 95% CIs. Table 2 shows the percentage difference in participants responding to each dose compared to placebo with 95% CIs.
| Dose (mg/day) | Responders (%) | 95% Confidence intervals |
|---|---|---|
| 0 | 19.0 | 15.9 to 22.7 |
| 400 | 24.5 | 19.0 to 30.0 |
| 800 | 30.9 | 27.1 to 35.2 |
| 1200 | 38.2 | 34.1 to 42.7 |
| Dose (mg/day) | Difference | 95% Confidence intervals |
|---|---|---|
| 400 | 6 | –1 to 12 |
| 800 | 13 | 7 to 17 |
| 1200 | 21 | 14 to 25 |
Freedom from seizures
An analysis pooling the data from six studies (adults and children) showed no evidence of heterogeneity (Chi2 = 0.64, df = 5, P = 0.99; I2 = 0%).
Subgroup analysis showed that ESL 400 mg was not associated with freedom from seizures (RR 1.03, 95% CI 0.21 to 5.02; Analysis 2.1), whereas higher doses of ESL were associated with a seizure‐free state (800 mg: RR 3.42, 95% CI 1.38 to 8.46; Analysis 2.2; 1200 mg: RR 3.46, 95% CI 1.40 to 8.54; Analysis 2.3). For children, including only one study, the RR was 4.34 (95% CI 1.06 to 17.79; Analysis 2.4). For adults, the RR was 2.90 (95% CI 1.49 to 5.68; Analysis 2.5). Participants taking ESL were significantly more likely to achieve a seizure free state (RR 3.16, 95% CI 1.73 to 5.78; Analysis 2.6).
Secondary outcomes
Treatment withdrawal for any reason
Given that the test for overall heterogeneity among the included studies showed a statistical significance (Chi2 = 12.33, df = 6, P = 0.06; I2 = 51%), we used the random‐effects model. Although the cause of heterogeneity was not found, we found that with the increase of the participating countries and center, the discontinuation rate also increased. Participants allocated ESL were no more likely to have treatment withdrawn compared with participants allocated placebo (RR 1.25, 95% CI 0.93 to 1.70; Analysis 3.1). For adults, the RR was 1.19 (95% CI 0.86 to 1.64; Analysis 3.1); for children, the RR was 2.01 (95% CI 0.58 to 6.93; Analysis 3.1). The only dose associated with significant withdrawal for all reasons was 1200 mg once‐daily (RR 1.75, 95% CI 1.26 to 2.41; Analysis 3.1).
Treatment withdrawal for adverse effects
The random‐effect model was also selected to analysis the data, due to significant heterogeneity in subgroup analysis of the adult data (Chi2 = 7.73, df = 4, P = 0.10; I2 = 48%). The overall RR across any dose was 2.72 (95% CI 1.66 to 4.46; Analysis 3.2). For adults, the RR was 2.66 (95% CI 1.42 to 4.96; Analysis 3.2); for children, the RR was 2.62 (95% CI 0.78 to 8.76; Analysis 3.2). Subgroup analyses assessing treatment withdrawal with different doses of ESL suggested a higher withdrawal rate with higher doses (400 mg: RR 2.12, 95% CI 0.53 to 8.48; 800 mg: RR 2.52, 95% CI 1.47 to 4.35; 1200 mg: RR 4.66, 95% CI 2.68 to 8.09; Analysis 3.2).
Adverse effects
The following adverse effects were associated with ESL: dizziness (RR 2.77, 99% CI 1.85 to 4.15; Analysis 4.2); nausea (RR 2.55, 99% CI 1.39 to 4.67; Analysis 4.4); somnolence (RR 1.75, 99% CI 1.18 to 2.61; Analysis 4.5); vomiting (RR 2.37, 99% CI 1.19 to 4.74; Analysis 4.8); and diplopia (RR 4.07, 99% CI 1.86 to 8.89; Analysis 4.9). Results for other adverse effects were: ataxia (RR 2.14, 99% CI 0.71 to 6.48; Analysis 4.1); fatigue (RR 1.42, 99% CI 0.65 to 3.13; Analysis 4.3); rash (RR 1.24, 99% CI 0.38 to 4.01; Analysis 4.6); and headache (RR 1.32, 99% CI 0.90 to 1.94; Analysis 4.7).
Drug interactions
The included studies did not assess outcomes related to drug interactions.
Discussion
Summary of main results
The seven trials included in this review recruited adults and children and were sponsored by BIAL. They used adequate methods of concealment of randomization and were double‐blind. The included studies enrolling adults had a high dropout rate.
The included studies tested ESL at doses of 400 mg, 800 mg and 1200 mg in adults and 10 mg/kg/day to 30 mg/kg/day in children once‐daily. The results of this review show that ESL, when used as an add‐on treatment, reduces seizure frequency in adults with drug‐resistant focal epilepsy. For children, ESL may be more effective than placebo in the 6‐ to 18‐year age group (RR 1.35, 95% CI 0.98 to 1.87), although this was not statistically significant. There is a discrepancy between the results of the best‐case scenario, worst‐case scenario and ITT analyses, which is likely to result from of a higher discontinuation rate of included studies. There is a tendency that the discontinuation rate increases as the participating countries and subcenters increase across studies. A dose‐regression analysis showed evidence of a dose effect, with no evidence of the plateau of the effect for the doses tested.
For freedom from seizures, results show that ESL was more likely to result in a complete seizure‐free state than placebo in trials of relatively short duration in adults. Only one study provided data on freedom from seizures in included trials recruiting children, so we cannot draw a reliable conclusion. Participants receiving ESL seemed more likely to discontinue ESL than placebo due to adverse effects in adults. With respect to adverse effects, dizziness, nausea and diplopia were significantly more likely to occur in the ESL‐treated group, especially at higher doses. No individual adverse effect was significantly associated with ESL over placebo in children, and this may be partly because there were insufficient studies included involving children.
Overall completeness and applicability of evidence
The trials included in this review focused on the use of ESL in people with drug‐resistant focal epilepsy, and the results cannot be generalized to add‐on treatment for adults or children with generalized epilepsies. This review also does not indicate if ESL is effective in the long term and how ESL compares with other AEDs in the same scenario.
Quality of the evidence
The trials included in our review were of generally high methodological quality. We were able to obtain further details of outcomes from trial sponsors. Each study was given a rating of low risk for selection bias, performance bias and detection bias. Reporting bias was at low risk in most studies, except one study recruiting children where it was at unclear risk (Kirkham 2020). All five adult trials were at high risk of attrition bias due to large proportions of missing data, ranging between 10% and 45% of data missing per arm of the trial. For the efficacy outcome, 50% or greater reduction in seizure frequency, best‐case and worst‐case scenario analyses were inconsistent with results of ITT analyses. When the GRADE approach was used within summary of findings Table 1, the certainty of evidence overall was moderate.
Potential biases in the review process
The main problems highlighted by our review were limitations on the lack of enough research on children at present. There were no major potential biases in the review process.
Agreements and disagreements with other studies or reviews
Another review has similarly reported that ESL (800 mg and 1200 mg) is efficacious at reducing seizure frequency for adults with drug‐resistant partial epilepsy (Biton 2017). However, it is important to note that Biton 2017 did not assess the risk of bias of included studies and did not consider the high proportions of missing data (10% to 45% per treatment arm).

Study flow diagram for update.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: 50% or greater reduction in seizure frequency, Outcome 1: Primary analysis

Comparison 1: 50% or greater reduction in seizure frequency, Outcome 2: Best‐case scenario

Comparison 1: 50% or greater reduction in seizure frequency, Outcome 3: Worst‐case scenario

Comparison 2: Freedom from seizures, Outcome 1: Eslicarbazepine acetate 400 mg/day vs placebo

Comparison 2: Freedom from seizures, Outcome 2: Eslicarbazepine acetate 800 mg/day vs placebo

Comparison 2: Freedom from seizures, Outcome 3: Eslicarbazepine acetate 1200 mg/day vs placebo

Comparison 2: Freedom from seizures, Outcome 4: Eslicarbazepine acetate any dose vs placebo in children

Comparison 2: Freedom from seizures, Outcome 5: Eslicarbazepine acetate any dose vs placebo in adults

Comparison 2: Freedom from seizures, Outcome 6: Eslicarbazepine acetate any dose vs placebo, adults and children

Comparison 3: Treatment withdrawal, Outcome 1: Treatment withdrawal (any reason)

Comparison 3: Treatment withdrawal, Outcome 2: Treatment withdrawal (adverse effect)

Comparison 4: Adverse effects, Outcome 1: Ataxia

Comparison 4: Adverse effects, Outcome 2: Dizziness

Comparison 4: Adverse effects, Outcome 3: Fatigue

Comparison 4: Adverse effects, Outcome 4: Nausea

Comparison 4: Adverse effects, Outcome 5: Somnolence

Comparison 4: Adverse effects, Outcome 6: Rash

Comparison 4: Adverse effects, Outcome 7: Headache

Comparison 4: Adverse effects, Outcome 8: Vomiting

Comparison 4: Adverse effects, Outcome 9: Diplopia
| Eslicarbazepine acetate add‐on for drug‐resistant focal epilepsy | ||||||
| Patient or population: people with drug‐resistant focal epilepsy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo | Risk with ESL | |||||
| ≥ 50% reduction in seizure frequency (adults) Follow‐up: up to 18 weeks | Study population | RR 1.71 | 1799 | ⊕⊕⊕⊝ | Results presented for ITT population. | |
| 203 per 1000 | 347 per 1000 | |||||
| ≥ 50% reduction in seizure frequency (children aged 6–18 years) Follow‐up: up to 18 weeks | Study population | RR 1.35 (0.98 to 1.87) | 322 (2 RCTs) | ⊕⊕⊕⊝ | ESL may reduce seizures in children aged 6–18 years with drug‐resistant focal epilepsy. | |
| 294 per 1000 | 397 per 1000 | |||||
| Freedom from seizures Follow‐up: up to 18 weeks | Study population | RR 3.16 | 1922 | ⊕⊕⊕⊝ | — | |
| 20 per 1000 | 63 per 1000 | |||||
| Treatment withdrawal (any reason) Follow‐up: up to 18 weeks | Study population | RR 1.25 | 2185 | ⊕⊕⊕⊝ | — | |
| 164 per 1000 | 205 per 1000 | |||||
| Treatment withdrawal (adverse effect) Follow‐up: up to 18 weeks | Study population | RR 2.72 | 2185 | ⊕⊕⊕⊝ | — | |
| 38 per 1000 | 104 per 1000 | |||||
| Adverse effects Follow‐up: up to 18 weeks | Study population | See comment | See comment | ⊕⊕⊕⊝ | ESL was associated with dizziness, nausea, somnolence, diplopia and vomiting. | |
| See comment | See comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ESL: eslicarbazepine acetate; ITT: intention to treat; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aThese studies were funded by BIAL – Portela & Ca, SA. There was no indication that the source of funding introduced bias. | ||||||
| Dose (mg/day) | Responders (%) | 95% Confidence intervals |
|---|---|---|
| 0 | 19.0 | 15.9 to 22.7 |
| 400 | 24.5 | 19.0 to 30.0 |
| 800 | 30.9 | 27.1 to 35.2 |
| 1200 | 38.2 | 34.1 to 42.7 |
| Dose (mg/day) | Difference | 95% Confidence intervals |
|---|---|---|
| 400 | 6 | –1 to 12 |
| 800 | 13 | 7 to 17 |
| 1200 | 21 | 14 to 25 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Primary analysis Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Eslicarbazepine acetate 400 mg/day vs placebo | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.80, 1.85] |
| 1.1.2 Eslicarbazepine acetate 800 mg/day vs placebo | 4 | 1015 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.34, 2.07] |
| 1.1.3 Eslicarbazepine acetate 1200 mg/day vs placebo | 4 | 1006 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.56, 2.37] |
| 1.1.4 Eslicarbazepine acetate any dose vs placebo in children (aged 2–18 years) | 2 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.88, 1.61] |
| 1.1.5 Eslicarbazepine acetate any dose vs placebo in children (aged 6–18 years) | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.98, 1.87] |
| 1.1.6 Eslicarbazepine acetate any dose vs placebo in adults | 5 | 1799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.42, 2.05] |
| 1.1.7 Eslicarbazepine acetate any dose vs placebo, adults and children | 7 | 2185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.34, 1.83] |
| 1.2 Best‐case scenario Show forest plot | 7 | 2185 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [1.84, 3.31] |
| 1.3 Worst‐case scenario Show forest plot | 7 | 2185 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Eslicarbazepine acetate 400 mg/day vs placebo Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.21, 5.02] |
| 2.2 Eslicarbazepine acetate 800 mg/day vs placebo Show forest plot | 4 | 1015 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.42 [1.38, 8.46] |
| 2.3 Eslicarbazepine acetate 1200 mg/day vs placebo Show forest plot | 4 | 1006 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [1.40, 8.54] |
| 2.4 Eslicarbazepine acetate any dose vs placebo in children Show forest plot | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.34 [1.06, 17.79] |
| 2.5 Eslicarbazepine acetate any dose vs placebo in adults Show forest plot | 5 | 1799 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [1.49, 5.68] |
| 2.6 Eslicarbazepine acetate any dose vs placebo, adults and children Show forest plot | 6 | 1922 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [1.73, 5.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Treatment withdrawal (any reason) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1.1 Eslicarbazepine acetate 400 mg/day vs placebo | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.38, 2.30] |
| 3.1.2 Eslicarbazepine acetate 800 mg/day vs placebo | 4 | 1015 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.74, 1.39] |
| 3.1.3 Eslicarbazepine acetate 1200 mg/day vs placebo | 4 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.26, 2.41] |
| 3.1.4 Eslicarbazepine acetate any dose vs placebo in children | 2 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.58, 6.93] |
| 3.1.5 Eslicarbazepine acetate any dose vs placebo in adults | 5 | 1799 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.86, 1.64] |
| 3.1.6 Eslicarbazepine acetate any dose vs placebo, adults and children | 7 | 2185 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.93, 1.70] |
| 3.2 Treatment withdrawal (adverse effect) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.2.1 Eslicarbazepine acetate 400 mg/day vs placebo | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [0.53, 8.48] |
| 3.2.2 Eslicarbazepine acetate 800 mg/day vs placebo | 4 | 1015 | Risk Ratio (M‐H, Random, 95% CI) | 2.52 [1.47, 4.35] |
| 3.2.3 Eslicarbazepine acetate 1200 mg/day vs placebo | 4 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 4.66 [2.68, 8.09] |
| 3.2.4 Eslicarbazepine acetate any dose vs placebo in children | 2 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 2.62 [0.78, 8.76] |
| 3.2.5 Eslicarbazepine acetate any dose vs placebo in adults | 5 | 1799 | Risk Ratio (M‐H, Random, 95% CI) | 2.66 [1.42, 4.96] |
| 3.2.6 Eslicarbazepine acetate any dose vs placebo, adults and children | 7 | 2185 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [1.66, 4.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Ataxia Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 4.1.1 Eslicarbazepine acetate 400 mg/day vs placebo | 1 | 196 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.04 [0.21, 5.09] |
| 4.1.2 Eslicarbazepine acetate 800 mg/day vs placebo | 2 | 373 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.49 [0.75, 8.29] |
| 4.1.3 Eslicarbazepine acetate 1200 mg/day vs placebo | 2 | 365 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.59 [0.77, 8.64] |
| 4.1.4 Eslicarbazepine acetate any dose vs placebo in children | 0 | 0 | Risk Ratio (M‐H, Fixed, 99% CI) | Not estimable |
| 4.1.5 Eslicarbazepine acetate any dose vs placebo in adults | 2 | 647 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.14 [0.71, 6.48] |
| 4.1.6 Eslicarbazepine acetate any dose vs placebo, adults and children | 2 | 647 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.14 [0.71, 6.48] |
| 4.2 Dizziness Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 4.2.1 Eslicarbazepine acetate 400 mg/day vs placebo | 2 | 398 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.25 [0.97, 5.22] |
| 4.2.2 Eslicarbazepine acetate 800 mg/day vs placebo | 4 | 1015 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.41 [1.52, 3.80] |
| 4.2.3 Eslicarbazepine acetate 1200 mg/day vs placebo | 4 | 1006 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.58 [2.33, 5.52] |
| 4.2.4 Eslicarbazepine acetate any dose vs placebo in children | 2 | 386 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.02 [0.37, 11.21] |
| 4.2.5 Eslicarbazepine acetate any dose vs placebo in adults | 5 | 1799 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.81 [1.86, 4.27] |
| 4.2.6 Eslicarbazepine acetate any dose vs placebo, adults and children | 7 | 2185 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.77 [1.85, 4.15] |
| 4.3 Fatigue Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 4.3.1 Eslicarbazepine acetate 400 mg/day vs placebo | 1 | 196 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.83 [0.15, 4.51] |
| 4.3.2 Eslicarbazepine acetate 800 mg/day vs placebo | 2 | 643 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.21 [0.43, 3.40] |
| 4.3.3 Eslicarbazepine acetate 1200 mg/day vs placebo | 2 | 635 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.72 [0.66, 4.50] |
| 4.3.4 Eslicarbazepine acetate any dose vs placebo in children | 1 | 263 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.60 [0.25, 10.25] |
| 4.3.5 Eslicarbazepine acetate any dose vs placebo in adults | 2 | 1048 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.39 [0.58, 3.31] |
| 4.3.6 Eslicarbazepine acetate any dose vs placebo, adults and children | 3 | 1352 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.42 [0.65, 3.13] |
| 4.4 Nausea Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 4.4.1 Eslicarbazepine acetate 400 mg/day vs placebo | 1 | 196 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.08 [0.45, 9.66] |
| 4.4.2 Eslicarbazepine acetate 800 mg/day vs placebo | 3 | 815 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.12 [0.99, 4.55] |
| 4.4.3 Eslicarbazepine acetate 1200 mg/day vs placebo | 3 | 802 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.64 [1.80, 7.39] |
| 4.4.4 Eslicarbazepine acetate any dose vs placebo in children | 2 | 386 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.20 [0.46, 10.55] |
| 4.4.5 Eslicarbazepine acetate any dose vs placebo in adults | 4 | 1397 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.61 [1.36, 5.01] |
| 4.4.6 Eslicarbazepine acetate any dose vs placebo, adults and children | 6 | 1824 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.55 [1.39, 4.67] |
| 4.5 Somnolence Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 4.5.1 Eslicarbazepine acetate 400 mg/day vs placebo | 2 | 398 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.15 [0.54, 2.45] |
| 4.5.2 Eslicarbazepine acetate 800 mg/day vs placebo | 4 | 1015 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.39 [0.83, 2.31] |
| 4.5.3 Eslicarbazepine acetate 1200 mg/day vs placebo | 4 | 1006 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.10 [1.31, 3.37] |
| 4.5.4 Eslicarbazepine acetate any dose vs placebo in children | 2 | 386 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.04 [0.73, 5.72] |
| 4.5.5 Eslicarbazepine acetate any dose vs placebo in adults | 5 | 1799 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.71 [1.11, 2.63] |
| 4.5.6 Eslicarbazepine acetate any dose vs placebo, adults and children | 7 | 2185 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.75 [1.18, 2.61] |
| 4.6 Rash Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 4.6.1 Eslicarbazepine acetate any dose vs placebo | 4 | 1702 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.24 [0.38, 4.01] |
| 4.7 Headache Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 4.7.1 Eslicarbazepine acetate 400 mg/day vs placebo | 2 | 398 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.17 [0.49, 2.80] |
| 4.7.2 Eslicarbazepine acetate 800 mg/day vs placebo | 4 | 1015 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.20 [0.71, 2.01] |
| 4.7.3 Eslicarbazepine acetate 1200 mg/day vs placebo | 4 | 1006 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.55 [0.95, 2.53] |
| 4.7.4 Eslicarbazepine acetate any dose vs placebo in children | 2 | 386 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.41 [0.64, 3.09] |
| 4.7.5 Eslicarbazepine acetate any dose vs placebo in adults | 5 | 1799 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.30 [0.84, 2.02] |
| 4.7.6 Eslicarbazepine acetate any dose vs placebo, adults and children | 7 | 2185 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.32 [0.90, 1.94] |
| 4.8 Vomiting Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 4.8.1 Eslicarbazepine acetate 400 mg/day vs placebo | 1 | 196 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.39 [0.20, 9.59] |
| 4.8.2 Eslicarbazepine acetate 800 mg/day vs placebo | 3 | 815 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.59 [0.96, 7.02] |
| 4.8.3 Eslicarbazepine acetate 1200 mg/day vs placebo | 3 | 802 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.59 [1.80, 11.70] |
| 4.8.4 Eslicarbazepine acetate any dose vs placebo in children | 2 | 386 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.17 [0.38, 3.60] |
| 4.8.5 Eslicarbazepine acetate any dose vs placebo in adults | 4 | 1397 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.30 [1.34, 8.13] |
| 4.8.6 Eslicarbazepine acetate any dose vs placebo, adults and children | 6 | 1783 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.37 [1.19, 4.74] |
| 4.9 Diplopia Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 4.9.1 Eslicarbazepine acetate 400 mg/day vs placebo | 2 | 398 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.42 [0.59, 10.01] |
| 4.9.2 Eslicarbazepine acetate 800 mg/day vs placebo | 4 | 1015 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.03 [1.61, 10.08] |
| 4.9.3 Eslicarbazepine acetate 1200 mg/day vs placebo | 4 | 1006 | Risk Ratio (M‐H, Fixed, 99% CI) | 5.12 [2.07, 12.62] |
| 4.9.4 Eslicarbazepine acetate any dose vs placebo in children | 2 | 386 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.74 [0.63, 22.34] |
| 4.9.5 Eslicarbazepine acetate any dose vs placebo in adults | 4 | 1702 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.14 [1.74, 9.84] |
| 4.9.6 Eslicarbazepine acetate any dose vs placebo, adults and children | 6 | 2088 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.07 [1.86, 8.89] |

