Kakao za snižavanje krvnog tlaka
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [author‐defined order]
| Methods | P DB | |
| Participants | Community setting, Melbourne, Australia Eligibility: healthy N = 28 Age: 43.5 Male: 53% Normotensive (mean baseline BP = 117/77 mmHg) | |
| Interventions | 1. Cocoa tablets (234 mg flavanols/procyanidins) Duration: 28 days | |
| Outcomes | SBP and DBP measured after 28 days. (No description of position of participant or which arm) Secondary outcome measure | |
| Notes | Supported in part by Mars Inc, USA who supplied active tablets (CocoaPro; Mars Inc, Hackettstown, NJ) and placebo tablets | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were separated into 2 groups that were sex‐matched and randomly assigned to consume either treatment. Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) | Low risk | 12.5% (4/32) loss to follow‐up: 1 did not to meet inclusion criteria, 2 withdrew because of family illnesses, and 1 failed to consume the specified number of tablets during the final week of the intervention. No other missing outcome data reported. |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | industry‐supported |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded (active and placebo tablets) |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | C SB | |
| Participants | Community setting, Cologne, Germany Eligibility: healthy N = 13 Age: 55 ‐ 64 Male: 54% Hypertensive (Mean baseline BP = 153/84 mgHg) | |
| Interventions | 1. 100 g dark chocolate (500 mg flavanols) Duration: 2 weeks | |
| Outcomes | Seated SBP and DBP (left upper arm) measured daily Primary outcome measure | |
| Notes | Sponsor not involved in data collection or analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to receive 14 consecutive daily doses of either treatment. Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. No missing outcome data reported |
| Selective reporting (reporting bias) | Low risk | BP data were provided for all time points |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants |
| Blinding of outcome assessment (detection bias) | Low risk | BP was recorded "in a blinded fashion" |
| Methods | P DB | |
| Participants | Community setting, San Francisco, USA Eligibility: healthy N = 21 Age: 38 (21 ‐ 55) Male: 52% Normotensive (Mean baseline BP = 116/67 mmHg) | |
| Interventions | 1. 46 g dark high flavanoid (213 mg procyanidin/46 mg epicatechin) chocolate Duration: 2 weeks | |
| Outcomes | Resting supine SBP and DBP after 2 weeks Secondary outcome measure | |
| Notes | Funded by the University of California, San Francisco. Chocolate sourced from American Cocoa Research Institute, Vienna, VA. Sponsor not involved in data collection or analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized. Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. Excellent compliance in all participants was documented by the return of all empty sample wrappers and by plasma epicatechin concentrations at 2 weeks |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | Low risk | Each chocolate sample was provided in coded foil wrapped containers. Both high‐ and low‐flavonol chocolate bars were similar in physical appearance and taste. |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | C SB | |
| Participants | Study dates: 10/00‐11/00 Community setting, Buenos Aires, Argentina Eligibility: young male active soccer players N = 28 Age: 18 (18 ‐ 21) Male: 100% Normotensive (mean baseline BP = 123/72 mmHg) | |
| Interventions | 1. 105 g (168 mg flavanols) containing milk chocolate (M&M's) Duration: 2 weeks | |
| Outcomes | SBP and DBP measured daily. No description of position of participant or which arm Primary outcome measure | |
| Notes | 3 authors from Mars. Funding supplied by the University of Buenos Aires and Argentinian government (ANPCYT). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised Sequence generation not described |
| Allocation concealment (selection bias) | Low risk | 2 treatments were provided in 105 g‐coded bags (1 daily dose) for 7‐day periods |
| Incomplete outcome data (attrition bias) | Low risk | 3.6% (1/28) loss to follow‐up; reason not reported |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | High risk | Industry‐funded and authored |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinding of participants (dark/white chocolate) |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | C SB | |
| Participants | Community setting, L'Aquila, Italy Eligibility: hypertensive N = 15 Age: 34 (SD = 7.6) Male: 47% Normotensive (mean baseline BP = 113/74 mgHg) | |
| Interventions | 1. 100 g dark chocolate (500 mg flavanols) Duration: 15 days | |
| Outcomes | Seated resting SBP and DBP after 15 days Primary outcome measure | |
| Notes | Normotensive group; Influence of funding body unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP at start and end of study reported |
| Other bias | Unclear risk | Influence of funding body unclear |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants |
| Blinding of outcome assessment (detection bias) | Low risk | BP was measured always by the same physician who was unaware of the study design, results, and purpose |
| Methods | C SB | |
| Participants | Community setting, L'Aquila, Italy Eligibility: hypertensive N = 15 Age: 34 (SD = 7.6) Male: 47% Normotensive (mean baseline BP = 113/74 mgHg) | |
| Interventions | 1. 100 g dark chocolate (500 mg flavanols) Duration: 15 days | |
| Outcomes | Seated resting SBP and DBP after 15 days Primary outcome measure | |
| Notes | Hypertensive subgroup; Influence of funding body unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP at start and end of study reported |
| Other bias | Unclear risk | Influence of funding body unclear |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants |
| Blinding of outcome assessment (detection bias) | Low risk | BP was measured always by the same physician who was unaware of the study design, results, and purpose |
| Methods | C SB | |
| Participants | Study dates: 1/05‐12/16 Community setting, Cologne, Germany Eligibility: (pre‐)hypertensive N = 44 Age: 55 ‐ 75 Male: 45% Hypertensive (mean baseline BP = 148/86 mmHg) | |
| Interventions | 1. 6.3 g dark chocolate (30 mg flavanols) Duration: 18 weeks | |
| Outcomes | Seated resting SBP and DBP (left upper arm) after 6, 12, and 18 weeks Primary outcome | |
| Notes | Funded by the University Hospital of Cologne, Germany. Funding body not involved in study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted randomisation in sex‐stratified blocks of 4 persons each, sequentially allocated to dark chocolate and white chocolate using a computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | To conceal allocation from investigators, instructed trained staff at a separate site not involved with the trial generated and maintained the randomization list and prepared the chocolate |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP data at start, during and end of study. |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants (dark/white chocolate) All clinical investigations, dietary assessments, laboratory tests, data collection, and data analysis were performed by physicians and trained staff who were blinded to group assignment. |
| Blinding of outcome assessment (detection bias) | Low risk | "Participants received no information about their examination data and the exact objective of the study until trial completion. Participants were instructed that disclosing their group assignment to investigators would result in exclusion from the study. To further minimize the confounding influence of alerting reactions on BP, measurements were performed at a separate location outside the physician’s office and not associated with usual patient care." |
| Methods | P SB | |
| Participants | Community setting, Riyadh University for girls, Saudi Arabia Eligibility: healthy Intervention: N = 30; age: 21 (SD = 2.0); male: 0% Control: N = 30; age: 22 (SD = 1.8); male: 0% Normotensive (mean baseline BP = 115.5/73 mmHg) | |
| Interventions | 1. 100 g dark chocolate (50%; 500 mg flavanols) Duration: 15 days | |
| Outcomes | Resting SBP and DBP (position not stated) after 15 days; | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Influence of funding body unclear |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Methods | P DB | |
| Participants | Community setting, Virginia, USA Eligibility: healthy N = 90 Age: 69 (SD = 8.3) Male: 42% Normotensive (mean baseline BP = 127.5/74.5 mmHg) | |
| Interventions | 1. High‐flavanol dark chocolate bars (37.0 g; containing 60% cacao; 755 mg flavanols) and cocoa beverage (12 g cocoa) Duration: 6 weeks | |
| Outcomes | Seated resting SBP and DBP (left upper arm) after 3 and 6 weeks | |
| Notes | Industry research grant. The authors declared no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation of the products was conducted by an independent researcher |
| Allocation concealment (selection bias) | Low risk | "The boxes and containers containing the products (and their randomization numbers, 1–101) were subsequently issued to participants in an ascending and sequential order as they entered the study (at the time of their pretreatment baseline assessments)." |
| Incomplete outcome data (attrition bias) | Low risk | 11% (11 of 101) lost to follow‐up. 10 withdrew, 1 was excluded from analysis due to non‐compliance |
| Selective reporting (reporting bias) | Low risk | BP reported at start, middle, and end of study |
| Other bias | Unclear risk | Industry‐funded |
| Blinding of participants and personnel (performance bias) | Low risk | Placebos were matched for appearance (e.g. colour and quantity), smell, taste, and caloric content |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | P DB | |
| Participants | Study dates: 9/05‐12/16 Community setting, Adelaide, Australia Eligibility: sedentary, overweight Intervention: N = 12; age: 45 (SD = 4.4); male: 33% Control: N = 11; Age: 44 (SD = 4.4); male: 27% Normotensive (mean baseline BP = 124/76.5 mmHg) | |
| Interventions | 1. HiFl drink (902 mg flavanols) Duration: 12 weeks | |
| Outcomes | Resting supine SBP and DBP at 6 and 12 weeks Primary outcome measure | |
| Notes | no exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Volunteers were block‐matched into 2 groups according to BMI, gender, age and BP. The groups were then randomised to the daily consumption. Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) | High risk | 21% (14/65) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Change of BP from baseline reported |
| Other bias | Unclear risk | Manufacturer (Mars Inc.) provided the cocoa drinks and financial support |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind. Cocoa beverages were matched for taste and appearance |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | P DB | |
| Participants | Study dates: 9/05‐12/16 Community setting, Adelaide, Australia Eligibility: sedentary, overweight Intervention: N = 13; age: 45 (SD = 3.0); male: 31% Control: N = 13; age: 46 (SD = 4.0); male: 46% Normotensive (mean baseline BP = 124/76 mmHg) | |
| Interventions | 1. HiFl drinks (902 mg flavanols); in addition to physical exercise Duration: 12 weeks | |
| Outcomes | Resting supine SBP and DBP at 6 and 12 weeks Primary outcome measure | |
| Notes | Intervention in addition to physical exercise; Manufacturer (Mars Inc.) provided the cocoa drinks and financial support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Volunteers were block‐matched into 2 groups according to BMI, gender, age and BP. The groups were then randomised to the daily consumption Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) | High risk | 21% (14/65) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Change of BP from baseline reported |
| Other bias | Unclear risk | Industry‐funded |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind. Cocoa beverages were matched for taste and appearance |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | C SB | |
| Participants | Hospital outpatients setting, L'Aquila, Italy Eligibility: hypertensive, impaired glucose tolerance N = 19 Age: 45 (SD = 8) Male: 58% Hypertensive (Mean baseline BP = 141/91 mmHg) | |
| Interventions | 1. 100 g flavanol‐rich dark chocolate bars (1080 mg flavanols) Duration: 15 days | |
| Outcomes | 24‐hour automated ambulatory SBP and DBP, in addition to seated SBP and DBP; after 15 days. Primary outcome measure | |
| Notes | Supported by the Italian government (Ministero della Universita´e della Ricerca Scientifica) and the US government (USDA Agricultural Research Service). The dark chocolate bars were donated by the manufacturer. The authors declared no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. Sequence generation not described |
| Allocation concealment (selection bias) | Low risk | "Chocolate doses for each subject were rolled in aluminium foil and administered in dated, sequentially numbered, nontransparent boxes not labelled with regard to content. Involved physicians and staff were unaware of the group assignment." |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants, only of personnel. Participants did not receive information regarding the chocolate and were instructed not to disclose their assigned group to investigators |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | C DB | |
| Participants | Community setting, Bethesda, USA Eligibility: hypertensive N = 20 Age: 51 (SEM = 1.5) Male: 40% Hypertensive (mean baseline BP = 141/91 mmHg) | |
| Interventions | 1. 31 g cocoa drink powder mixed in 150 mL warm water (902 mg flavanols) Duration: 2 weeks | |
| Outcomes | Resting (seated) SBP and DBP (on nondominant arm) measured 3 times a week Primary outcome measure | |
| Notes | Supported by the US government (Intramural Research Program, NCCAM, NIH, and Office of Dietary Supplements, NIH). Cocoa and placebo preparations provided by manufacturer (Mars Inc.), not involved in research.The authors declared no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation by NIH Clinical Center Pharmacy |
| Allocation concealment (selection bias) | Low risk | Assignment codes were not available to investigators until 20 participants completed the entire study and the database had been completed and secured |
| Incomplete outcome data (attrition bias) | High risk | 31% (9/29) participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | BP measured 3 times a week, but only outcomes at baseline and after 2 weeks treatment reported |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | Low risk | "The cocoa and placebo drinks were similar in colour, taste, and packaging and participants were blinded to treatment assignment. Participant blinding was assessed by a questionnaire administered at the end of 6 wks that asked participants to indicate which treatment they believed they received during each of the 2 phases (cocoa, placebo, or uncertain)." |
| Blinding of outcome assessment (detection bias) | Unclear risk | In addition to monitoring BP in the outpatient clinic, participants were required to self‐monitor their blood pressure at home using a portable BP device |
| Methods | C SB | |
| Participants | Hospital outpatients setting, Barcelona, Spain Eligibility: diabetes, or >=3 CVD risk factors N = 25 Age: 70 Male: 45% Prehypertensive (mean baseline BP = 138/84 mmHg) | |
| Interventions | 1. 40 g cocoa powder (495 mg flavanols) in milk Duration: 4 weeks | |
| Outcomes | Resting SBP and DBP (position not stated) after 4 weeks, | |
| Notes | Supported by grants from the Spanish Ministries of Education and Science and Innovation. Funding body not involved in the study. No conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised Sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Allocation concealment achieved by using closed envelopes with correlative numbers by prespecified subgroups of sex and age |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention. |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding of participants, but blinding of personnel: The clinical investigators and laboratory technicians were blinded to the interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | P SB | |
| Participants | Study dates: 6/07‐12/07 Community setting, Adelaide, Australia Eligibility: (pre‐)hypertensive Intervention: N = 11; age: 49 (SD = 12.2); male: 64% Control: N = 10; age: 58 (SD = 13.4); male: 50% Prehypertensive (mean baseline BP = 135.5/81 mmHg) | |
| Interventions | 1. 50 g dark chocolate (70%) (750 mg flavanols) Duration: 8 weeks | |
| Outcomes | Resting supine SBP and DBP at 4 and 8 weeks Primary outcome measure | |
| Notes | Chocolate provided by manufacturer (Haigh's Chocolates, Adelaide). Manufacturer did not provide funding and were not involved in study design, data collection, analysis or preparation of the manuscript. The authors stated that they had no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated by permuted block randomisation using the SAS 9.1 software package. |
| Allocation concealment (selection bias) | Low risk | To conceal allocation from investigators, trained staff not involved in trial design and analysis handed out intervention packs to participants |
| Incomplete outcome data (attrition bias) | Low risk | 8% (4/39) lost to follow‐up/ withdrawal |
| Selective reporting (reporting bias) | Low risk | BP data reported comprehensively |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants to chocolate was impractical, however blinding of participants in the capsule groups was achieved by identical packaging of active tomato extract and placebo capsules. Control group and personnel blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | P SB | |
| Participants | Community setting, Chiba, Japan Eligibility: males Intervention: N = 20; age: 29 (SD = 3.4); male: 100% Control: N = 19; age: 30 (SD = 4.5); male: 100% Normotensive (Mean baseline BP = 119/68.5 mm Hg) | |
| Interventions | 1. 45 g dark chocolate (80%) (550 mg flavanols) Duration: 2 weeks | |
| Outcomes | Resting SBP and DBP (position not stated) after 2 weeks; | |
| Notes | Sponsor not involved in data collection and analysis. No conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | High risk | Participants were not blinded (dark/white chocolate) |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | C DB | |
| Participants | Study dates: 11/08‐10/09 Community setting, Amsterdam, Netherlands Eligibility: (pre‐)hypertensive n=41 Age: 62 (SD = 4.5) Male: 76% Hypertensive (mean baseline BP = 142/84 mmHg) | |
| Interventions | 1. High flavanol drink (529 mg flavanols) Duration: 3 weeks | |
| Outcomes | Resting (seated) SBP and DBP (on nondominant arm) after 3 weeks; 24‐hour automated ambulatory SBP and DBP (on nondominant arm) after 3 weeks; | |
| Notes | Mean of theobromine‐enriched chocolate group (TEC) + natural dose theobromine chocolate group (NTC); Sponsored by manufacturer (Unilever); one co‐author (but none of the investigators) employed by Unilever; The contractual agreement between the Academic Medical Center and Unilever allowed the sponsor to review and comment on the article, but the investigators remained responsible for its contents and decision to submit the results for publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Test product allocation and order of treatment were determined by a computer‐ generated randomised schedule |
| Allocation concealment (selection bias) | Low risk | Test products were provided in sequentially‐numbered sealed bottles |
| Incomplete outcome data (attrition bias) | Low risk | 4% (2/42) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry‐funded and co‐authored |
| Blinding of participants and personnel (performance bias) | Low risk | The different test products all had similar taste and appearance |
| Blinding of outcome assessment (detection bias) | Low risk | All of the haemodynamic measurements were performed by a single investigator, blinded for treatment allocation |
| Methods | P DB | |
| Participants | Community setting, San Franscisco, USA Eligibility: coronary artery disease Group 1 (33 mg flavanol): N = 14; age: 53 (SD = 6.7); male: 71% Group 2 (372 mg flavanol): N = 12; age: 56 (SD = 14.2); male: 58% Group 3 (712 mg flavanol) N = 13; age: 60 (SD = 13.7); male: 62% Group 4 (1052 mg flavanol): N = 13; sage: 57 (SD = 9.7); male: 54% Hypertensive (mean baseline BP = 144/85.5 mmHg) | |
| Interventions | Cocoa drink containing 33 mg/372 mg flavanol/712 mg flavanol/1052 mg flavanol; daily Duration: 6 weeks | |
| Outcomes | Seated clinic DBP and SBP (non‐dominant arm) after 3 and 6 weeks; 24‐hour automated ambulatory SBP and DBP (non‐dominant arm) after 3 and 6 weeks Primary outcome measure | |
| Notes | Trial received funding from industry. The authors declared no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of groups was undertaken independently of group minimisation procedure by separate staff members of the research centre not otherwise involved with the trial |
| Allocation concealment (selection bias) | Low risk | Trial investigators remained blinded to treatment allocation until after the completion of data analysis |
| Incomplete outcome data (attrition bias) | Low risk | 12% (7/59) lost to follow‐up: 5 withdrawals, 1 exclusion due to non‐compliance (deliberate weight loss), 1 exclusion due to gastric complaints |
| Selective reporting (reporting bias) | Low risk | BP reported for each assessment point (baseline, week 3, week 6) |
| Other bias | Unclear risk | Industry‐funded |
| Blinding of participants and personnel (performance bias) | Low risk | The reconstituted cocoa beverages were matched for appearance and taste |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | C | |
| Participants | Community setting, San Franscisco, USA Eligibility: coronary artery disease N = 16 Age: 64 (SD = 3) Male: 19% Prehypertensive (mean baseline SBP = 131.5 mmHg; no DBP given) | |
| Interventions | 1. High flavanol drink (750 mg flavanols) Duration: 4 weeks | |
| Outcomes | Resting supine SBP and DBP after 30 days Tertiary outcome measure | |
| Notes | This study was supported by a grant from the American Heart Association, and an unrestricted research grant from Mars, Inc. Two authors received funding from industry, and one author is employed by Mars. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation and dispensing of cocoa drinks were performed by the Department of Pharmacology. Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | 6% (1/17) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | High risk | Industry‐funded and co‐authored |
| Blinding of participants and personnel (performance bias) | Low risk | All drinks were similar in taste. Participants and investigators were masked throughout the study with regard to flavanol content of the test drinks |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | C DB | |
| Participants | Study dates: 08/05‐06/06 Community setting, Derby, USA Eligibility: overweight N = 38 Age = 52.5 (SD = 10.4) Male: 15% Normotensive (mean baseline BP = 123/68 mmHg) | |
| Interventions | 1. High flavanol drink (805 mg flavanols) Duration: 6 weeks | |
| Outcomes | Resting supine SBP and DBP after 6 weeks; | |
| Notes | Grant funding from manufacturer Hershey. One author received speaker's fee. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 44 participants were randomly assigned using a computer‐generated random number sequence |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) | Low risk | 16% (7/44) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry‐funded |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Adequate |
| Methods | C SB | |
| Participants | Community setting, Cambridge, UK N=21 Age: not provided Male: 0% Normotensive (Mean baseline BP: 107/70 mm Hg) | |
| Interventions | Polyphenol‐rich dark chocolate (500 mg polyphenol) The placebo was a dark chocolate matched for taste, texture, colour and macronutrient composition to the polyphenol‐rich DC, but which contained no polyphenols. Duration: 8 weeks | |
| Outcomes | A validated automated A&D Medical UA‐767 BP monitor (A&D medical, San Jose, CA, USA) was used to measure BP after a rest of 10 min. Three values were taken at 2 min intervals Secondary | |
| Notes | BMI < 25 (Subgroup 1); The authors declare no conflicts of interest. Funding source not given, except for a manufacturer supplying the chocolate products. The authors declare no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Following a 1‐week run‐in phase, eligible people were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment given |
| Incomplete outcome data (attrition bias) | Low risk | 1/22 (5%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Funding unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Single‐blinded", but unclear who was blinded. Judging from the elaborate placebo, the investigators appear to have been unblinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Single‐blinded", but unclear who was blinded. Judging from the elaborate placebo, the investigators appear to have been unblinded |
| Methods | C SB | |
| Participants | Community setting, Cambridge, UK N = 21 Age: not provided Male: 0% Normotensive (mean baseline BP = 119/76 mmHg) | |
| Interventions | 1. Polyphenol‐rich dark chocolate (500 mg polyphenol) The placebo was a dark chocolate Duration: 8 weeks | |
| Outcomes | As in Almoosawi 2012a | |
| Notes | BMI > 25 (Subgroup 2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Following a 1‐week run‐in phase, eligible people were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment given |
| Incomplete outcome data (attrition bias) | Low risk | 1/22 (5%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Funding unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Single‐blinded", but unclear who was blinded. Judging from the elaborate placebo, the investigators appear to have been unblinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Single‐blinded", but unclear who was blinded. Judging from the elaborate placebo, the investigators appear to have been unblinded |
| Methods | P DB | |
| Participants | Hospital setting: Alzheimer unit, L'Aquila, Italy Eligibility criteria: Mild cognitive impairment, Petersen criteria Intervention: N = 30; age: 71.2 (SD = 4.9); male: 47% Control: N = 30; age: 71.0 (SD = 4.5); male: 53% Hypertensive (mean baseline BP = 141/85 mmHg) | |
| Interventions | 1. High flavanol drink (990 mg flavanols) Duration: 8 weeks | |
| Outcomes | Seated rested SBP and DBP after 8 weeks; Secondary outcome measure | |
| Notes | Study was supported by industry grant (Mars Inc), who supplied high/low flavanol powder. One of the authors is employed by Mars Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation of the products was conducted by an independent researcher |
| Allocation concealment (selection bias) | Low risk | Personnel not involved in the trial labelled identical boxes containing individual anonymised sachets. The boxes were subsequently issued to participants in an ascending and sequential order as they entered the study (at the time of their pre‐treatment baseline assessments). Neither the treating physicians, nor the participants were aware of treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 participant (1.1%) discontinued due to side effects |
| Selective reporting (reporting bias) | Low risk | BP reported at baseline and end of study |
| Other bias | High risk | Industry‐funded and co‐authored |
| Blinding of participants and personnel (performance bias) | Low risk | Research staff, treating physicians, and the participants were blinded to treatment allocation. Drink powder was indistinguishable in taste and appearance |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given |
| Methods | C Open‐label, unblinded | |
| Participants | Hospital setting, Barcelona, Spain Eligibility criteria: >= 3 risk factors CVD N = 42 Age: 69.7 (SD = 11.5) Male: 45% 78% hypertensive; mean baseline BP = 138/84 mmHg (pre‐hypertensive) | |
| Interventions | 1. 40 cocoa powder (495 mg polyphenol incl. 56.5 mg epicatechin) in 500 ml skimmed milk Duration: 4 weeks | |
| Outcomes | BP after 4 weeks Secondary outcome measure | |
| Notes | Study was supported by grants from the Spanish Ministries of Education and Science and Innovation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information given |
| Allocation concealment (selection bias) | Unclear risk | No further information given |
| Incomplete outcome data (attrition bias) | Low risk | No loss to ‐follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at baseline and end of study periods |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | High risk | Participants unblinded. No information of blinding of research staff given |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given |
| Methods | P DB | |
| Participants | Study dates: 7/08‐4/09 Hospital setting, Quebec, Canada Eligibility: pregnancy Intervention: N = 22; age: 28.7 (SD = 3.2); male: 0%, all pregnant women Control: N = 20 ; age: 29.8 (SD = 3.6); male: 0%, all pregnant women Normotensive (mean baseline BP = 109/69 mmHg) | |
| Interventions | 1. High‐flavanol chocolate (400 mg flavanols) Duration: 12 weeks | |
| Outcomes | BP was measured by a trained, certified nurse blinded to treatment allocation, with an electronic monitor (Microlife 3 BTO‐A) after 15 mins of rest, back supported, arm supported at the heart level, and cuff placed on the left upper arm Primary outcome measure | |
| Notes | All other authors declare that they have no conflicts of interest. Hospital employees | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed randomisation was generated using computer‐aided block randomisation (block size was kept secret), under the responsibility of an independent statistician |
| Allocation concealment (selection bias) | Low risk | Statistician undertook treatment allocation independently of the trial team. All clinical investigations, laboratory analyses, data collection and assessment were blinded to the randomisation allocation |
| Incomplete outcome data (attrition bias) | Low risk | 2 women dropped out of the study for reasons not related to the intervention |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | Low risk | Chocolate placebo was identical to the experimental chocolate in its content for all other nutrients except for flavanols (including theobromine and caffeine contents), similar in taste and supplied in individual, opaque packaging |
| Blinding of outcome assessment (detection bias) | Low risk | All clinical investigations, laboratory analyses, data collection and assessment were blinded to the randomisation allocation |
| Methods | P DB | |
| Participants | Study dates: 12/10‐2/11 Community setting, Grenoble and Lyon, France Eligibility: <10% CVD risk on European risk chart Intervention: N = 10; age: 55.2 (SD = 8.2); male: 50% Control: N = 10; age: 55.4 (SD = 8.7); male: 50% Normotensive (mean baseline BP: 118/75 mmHg) | |
| Interventions | 1. 6 g cocoa as chocolate‐flavoured (325 mg flavanoids) pasteurised acidified milk drink Duration: 4 weeks | |
| Outcomes | 24‐hour ambulatory Mean BP | |
| Notes | 4‐group study, only cocoa and placebo group considered here, additional groups: theobromine only (850 mg), n = 10 and cocoa + theobromine (C+T) group, n = 10 (total theobromine 1000 mg); adverse events in n = 6 (C+T), N = 1 (T): nausea, vomiting, headache, diarrhoea, potentially related to high dose of theobromine. All authors were employed by Unilever R&D Vlaardingen at the time the research was conducted. Unilever has no products enriched with theobromine under development or on the market; however, it markets food products enriched with plant sterols to lower LDL cholesterol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pre‐established blockwise randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Sequentially allocated by clinical investigator |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | BP reported at baseline and end of study |
| Other bias | Unclear risk | Industry‐supported |
| Blinding of participants and personnel (performance bias) | Low risk | Drinks supplied in identical tinted bottles that were packed individually for each participant in a neutral box and labelled with the participant code; participants were instructed not to pour the drink into a glass but to consume it directly out of the tinted bottle. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given |
| Methods | P DB | |
| Participants | Hospital setting, Neurology Research Unit, Boston, USA Eligibility: Hypertension N = 60 Age: 72.9 (SD = 5.4) yrs Male: 48% Normotensive (mean baseline BP = 125.5/69 mmHg) | |
| Interventions | 1. Flavanol‐rich cocoa 1218 mg Duration: 4 weeks | |
| Outcomes | BP mean of 3 measurements with automated cuff | |
| Notes | Controlled hypertensives (on BP medication); Supported by government grants from the National Institite on Aging and National Heart Lung and Blood Institute. Cocoa was provided by Mars Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided, unclear whether randomised |
| Allocation concealment (selection bias) | High risk | No details provided |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up: n = 2 (3%) |
| Selective reporting (reporting bias) | Low risk | BP at baseline, day 1 and end of the study |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, but no further details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Methods | C DB | |
| Participants | Community setting, Wageningen, Netherlands Eligibility: overweight N = 41 Age: 63 (SD = 5) Male: 100% Normotensive (mean baseline BP = 128/79 mmHg) | |
| Interventions | 1. High flavanol chocolate (1078mg flavanols) Duration: 4 weeks | |
| Outcomes | Brachial SBP, DBP, and heart rate (HR) were assessed automatically (Dinamap Pro 100; GE Healthcare, Little Chalfont, UK) for 10 mins with a 3‐min interval; Secondary outcome measure | |
| Notes | Study was funded by Top Institute Food and Nutrition (Wageningen, The Netherlands). The chocolate used in this study was donated by Barry Callebaut (Lebbeke, Belgium). The authors declare no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed by an independent research assistant using a computer‐generated table. We constructed 25 blocks with a size of 2." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment given |
| Incomplete outcome data (attrition bias) | Low risk | 3/44 (7%) participants dropped out or were excluded, 1 due to medical reasons not related to the study, 1 due to dislike of the chocolate and 1 due to failure to adhere to the treatment |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | Low risk | Researchers as well as participants were blinded to randomisation until after data analysis |
| Blinding of outcome assessment (detection bias) | Low risk | Researchers as well as participants were blinded to randomisation until after data analysis. |
| Methods | P DB | |
| Participants | Study dates: 3/12‐6/12 Community setting, Navarra, Spain Eligibility: overweight N = 47 Age: 57.3 (SD = 5.2) Male: 46% Normotensive (mean baseline BP: 120/80 mmHg) | |
| Interventions | 1. Meals supplemented with 1.4 g/day cocoa extract (645 mg total polyphenols/414mg total flavanols) Duration: 4 weeks | |
| Outcomes | BP was taken 3 times with automatic monitor (Intelli Sense. M6, OMRON Healthcare, Hoofddorp, Netherlands), to use the average value obtained from the last 2 measurements Secondary outcome measure | |
| Notes | Co‐funded by food industry and government. Conducted at seemingly independent research institutions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was performed using the “random between 1 and 2” function in the Microsoft Office Excel (Microsoft Iberica, Spain) |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment given |
| Incomplete outcome data (attrition bias) | Low risk | 3/50 (6%) participants dropped out or were excluded, 1 due to personal reasons and 2 due to failure to adhere to the treatment |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry co‐funded |
| Blinding of participants and personnel (performance bias) | Low risk | Boxes in which the meals were provided had the same appearance and differed only on the code label, ensuring the double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment given |
| Methods | P Unblinded? | |
| Participants | Study dates: 7/09‐7/10 Community setting, Pennsylvannia, USA Eligibility: overweight N = 60 Age: 35.9 (SEM = 0.8) Male: 0% Normotensive (mean baseline BP = 118/73 mmHg) | |
| Interventions | 1. 236 mL natural cocoa beverage and 2.9 oz dark chocolate (270 mg flavanols) Duration: 18 weeks | |
| Outcomes | Seated systolic and diastolic BP; Primary outcome measure | |
| Notes | Co‐funded by food industry and public sources | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no further information given |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment given |
| Incomplete outcome data (attrition bias) | Unclear risk | 85% of the women completed the intervention with no difference between DC and NC groups in discontinuation rate |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry co‐funded |
| Blinding of participants and personnel (performance bias) | High risk | Participants not blinded; no information on blinding of personnel given |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment given |
| Methods | C unblinded | |
| Participants | Community setting, Madrid, Spain N = 24 Age: 27 (SD = 8.4) Male: 46% Normotensive (Mean baseline BP: 116/72 mmHg) | |
| Interventions | 1. Milk with cocoa (416 mg flavanols) Duration: 4 weeks | |
| Outcomes | Seated systolic and diastolic BP Secondary outcome measure | |
| Notes | Subgroup: Normal cholesterol; Funded by food industry. The authors stated that they had no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No further information on allocation concealment given |
| Incomplete outcome data (attrition bias) | Unclear risk | 6/50 withdrew due to personal, health or professional reasons (numbers not provided by intervention groups) |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry funded |
| Blinding of participants and personnel (performance bias) | High risk | Lack of blinding of participants and investigators |
| Blinding of outcome assessment (detection bias) | High risk | Lack of blinding of participants and investigators |
| Methods | C Unblinded | |
| Participants | Community setting, Madrid, Spain N = 20 Age: 30 (SD = 9) Male: 45% Normotensive (mean baseline BP = 121/76 mmHg) | |
| Interventions | As in Sarria 2014a | |
| Outcomes | As in Sarria 2014a | |
| Notes | Subgroup: High cholesterol; Funded by food industry. The authors stated that they had no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No further information on allocation concealment given |
| Incomplete outcome data (attrition bias) | Unclear risk | 6/50 withdrew due to personal, health or professional reasons (numbers not provided by intervention groups) |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry funded |
| Blinding of participants and personnel (performance bias) | High risk | Lack of blinding of participants and investigators |
| Blinding of outcome assessment (detection bias) | High risk | Lack of blinding of participants and investigators |
| Methods | P DB | |
| Participants | Community setting, Duesseldorf, Germany Eligibility: healthy male N = 22 Age: 26 (SEM = 1) Male: 100% Normotensive (mean baseline BP: 120/77 mmHg) | |
| Interventions | 1. Cocoa extract powder (900 mg flavanols) dissolved in water Duration: 2 weeks | |
| Outcomes | Office blood pressure was measured 3 times after 10 mins of rest using an automated clinical digital sphygmomanometer (Dynamap, Tampa, FL, USA) with appropriately sized cuff placed around the upper arm at heart level Primary outcome measure | |
| Notes | Young subgroup; Co‐funded by food industry and public sources. One author employed by the company that manufactures and markets the specific cocoa powder used in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned, no further information |
| Allocation concealment (selection bias) | Low risk | Anonymised sachets in alphanumeric order |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study and all data were included in the analysis |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | High risk | Industry funded and co‐authored |
| Blinding of participants and personnel (performance bias) | Low risk | The beverage mixes were provided in sachets labelled with an alphanumeric identifier to enable a double‐masked study design |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment given |
| Methods | P DB | |
| Participants | Community setting, Duesseldorf, Germany Eligibility: healthy male N = 20 Age: 60 (SEM = 2) Male: 100% Prehypertensive (mean baseline BP = 131/82 mmHg) | |
| Interventions | 1. Cocoa extract powder (900 mg flavanols) dissolved in water Duration: 2 weeks | |
| Outcomes | as in Heiss 2015a | |
| Notes | Elderly subgroup; Co‐funded by food industry and public sources. One author employed by the company that manufactures and markets the specific cocoa powder used in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned, no further information |
| Allocation concealment (selection bias) | Low risk | Anonymised sachets in alphanumeric order |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study and all data were included in the analysis |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | High risk | Industry funded and co‐authored |
| Blinding of participants and personnel (performance bias) | Low risk | The beverage mixes were provided in sachets labelled with an alphanumeric identifier to enable a double‐masked study design |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment given |
| Methods | C Unblinded (no placebo, but reduced snack intake during study period) | |
| Participants | Community setting, Helsinki, Finnland Eligibility: hypertensive N = 22 Age: 45.8 (SD = 8.3) Male: 64% Hypertensive (mean baseline BP = 142/89 mmHg) | |
| Interventions | 1. 49 g dark chocolate (70% cacao, 603 mg flavanols) Duration: 8 weeks | |
| Outcomes | Clinical blood pressure and 24‐hr ambulatory BP monitor measured, no details given on assessment of clinical BP; Ambulatory 24‐hour blood pressure was monitored on a day of standard physical activity, with an adequate cuff for the size of the participant’s arm. Welch Allyn ABPM 6100 (Welch Allyn Inc, USA) validated according to the protocol of the Finnish Hypertension Society Primary outcome measure | |
| Notes | Funded by Finnish chocolate manufacturer Oy Karl Fazer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The participants were randomly assigned to 1 of the 2 groups (denoting order of interventions) after stratification by sex and BMI. No details on random sequence generation provided |
| Allocation concealment (selection bias) | High risk | Participants knew which group they were in before/after cross‐over, not stated if researchers knew as well |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study and all data were included in the analysis |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry funded |
| Blinding of participants and personnel (performance bias) | High risk | Participants were unblinded, no placebo; unclear if investigators were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment given |
| Methods | P DB | |
| Participants | Study dates: 8/13‐9/14 Community setting, Melbourne, Australia Eligibility: healthy N = 38 Age: 24 (SD = 4.5) Male: 33% Normotensive (mean baseline BP = 119/71 mmHg) | |
| Interventions | 1. Active cocoa tablet (3058 mg cacao seed extract, 250 mg catechin polyphenols) Duration: 4 weeks | |
| Outcomes | BP was assessed in a quiet, dedicated university laboratory following a 5‐min rest period completed by participants in the supine position on an examination bed; Secondary outcome measure | |
| Notes | Funded from public or charitable sources. Cocoa and placebo tablets provided by supplement company, not involved in study design, data collection, analysis and publication. Authors declare no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to receive either active or placebo tablets using a computer‐generated permuted block randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Identical bottles in alphanumerical order, packaged offsite by staff not involved in participant recruitment and testing |
| Incomplete outcome data (attrition bias) | Low risk | 5% (2/40) lost to follow‐up, 1 each from intervention and control groups |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo tablet (Identical in appearance, size, texture and colour to cocoa tablet, containing inert cellulose powder). |
| Blinding of outcome assessment (detection bias) | Low risk | The blinding code was only revealed after analysis of the main study |
| Methods | P DB | |
| Participants | Study dates: 12/06‐7/08 Community setting, L'Aquila, Italy Eligibility: cognitively intact, Mini‐Mental‐State‐Examination Score < 27 N = 30 (high flavanol group), N = 30 (low flavanol group = control); (N = 30 intermediate flavanol group not included in this meta‐analysis) Age: 70 (SE = 0.8) Male: 43% Prehypertensive (mean baseline BP = 135/80 mmHg), incl. about 50% hypertensive | |
| Interventions | 1. Dry dairy‐based beverage mixes with flavanol‐rich cocoa powder (993 mg flavanols, Cocoapro processed cocoa powder; Mars Inc) Duration: 8 weeks | |
| Outcomes | "Seated systolic and diastolic BP recorded in the morning with a validated oscillometric device with appropriately sized cuffs (Omron 705 CP; Omron Matsusaka) on the nondominant upper arm. These evaluations were performed by staff blinded to the study protocol. At each visit, participants rested 15 mins in a seated position, the first blood pressure measurement was taken but discarded, and the subsequent 3 consecutive blood pressure readings, taken at 3‐min intervals, were recorded. The average of these latter measures was considered for statistical analysis." Secondary outcome measure | |
| Notes | One of the authors is employed by Mars Inc., a company with long‐term research and commercial interests in cocoa flavanols. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on random sequence generation given |
| Allocation concealment (selection bias) | Unclear risk | Neither the treating physicians nor the participants were aware of treatment allocation. No further details provided |
| Incomplete outcome data (attrition bias) | Low risk | 1 discontinued trial, 0 lost to follow‐up per group |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | High risk | Industry funded and co‐authored |
| Blinding of participants and personnel (performance bias) | Low risk | Food products were indistinguishable in appearance and had a flavanol content that was not obvious on the basis of flavour. Staff were blinded to the study protocol |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment given |
| Methods | P SB | |
| Participants | Study dates: 3/11‐2/12 Hospital setting, Tehran, Iran Eligibility: type‐2‐diabetes, hypertension Intervention: N = 32; age: 59 (SD = 9); male: 37.5% Control: N = 28; age: 57 (SD = 8); male: 42.9% Prehypertensive (Mean baseline BP = 137/86 mmHg) | |
| Interventions | 1. 25 g chocolate containing 83% cocoa solids no flavanol content given Duration: 8 weeks | |
| Outcomes | Systolic and diastolic blood pressure was reported on average of 2 properly measured in the right or left arm supported at the heart level of seated position after 10 mins of rest by a trained nurse using a mercury sphygmomanometer; Primary outcome measure | |
| Notes | Funded by University. The authors stated that they had no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation method |
| Allocation concealment (selection bias) | Low risk | The participants were given chocolate bars containing either dark chocolate or white chocolate in the same package by blinded person to the same colour and shape |
| Incomplete outcome data (attrition bias) | Unclear risk | 13% (8/60) lost to follow‐up: intervention group: n = 2; control group: n = 6 |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Low risk | none |
| Blinding of participants and personnel (performance bias) | High risk | SB only personnel‐blinded. The participants were given chocolate bars containing either dark chocolate or white chocolate in the same package by blinded person to the same colour and shape. Participants were aware unblinded to the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment given |
| Methods | C DB | |
| Participants | Community setting, London, UK Eligibility: hypertension N = 21 Age: 55 (SEM = 1.5) Male: 100% Prehypertensive (mean baseline BP = 135/85 mmHg) | |
| Interventions | 1. 50 g high flavanol (1064 mg) dark chocolate bars Duration: 12 weeks | |
| Outcomes | Ambulatory blood pressure measurements (24‐hour) were made during participant screening and at 6 and 12 weeks using a Spacelabs ABP monitor 90207 (Dolby UK, Stirling) | |
| Notes | This study was supported by a grant from Barry Callebaut Belgium NV to one of the authors (R. Corder). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule was sent as a password‐protected file to Barry Callebaut, who prepared separate participant coded boxes for each phase of the study |
| Allocation concealment (selection bias) | Unclear risk | All interventions were provided in anonymised sachets |
| Incomplete outcome data (attrition bias) | High risk | High loss to follow‐up; 11/32 participants (34%) due to failure to attend the clinic on the required day, or BP monitor recording failure at either 6 or 12 weeks |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | Unclear risk | Industry funded and co‐authored |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐control chocolate specifically manufactured, suggested to be similar in appearance to intervention, both plain foil wrapped. The investigators were blinded to the randomisation schedule |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment given |
| Methods | P | |
| Participants | Study dates: 2/13‐8/14 Community / Hospital setting, Duesseldorf, Germany Eligibility: healthy N = 100 Age: 44.5 (SD = 8.5) M: 52.4% Normotensive (mean baseline BP = 123/77 mmHg) | |
| Interventions | 1. High flavanol (450 mg) drink Duration: 4 weeks | |
| Outcomes | Office BP was measured using an automated clinical digital sphygmomanometer (Dynamap) at the upper left arm in supine position, after 10 mins of rest in a quiet room with the arm at the heart level. 3 measurements were taken, the first discarded and the second and third averaged for further analysis. Secondary outcome measure | |
| Notes | One of the authors is employed by Mars Inc., a company engaged in flavanol research and flavanol‐related commercial activities. None of the other authors has a conflict of interest to declare other than stated above. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to 1 of 2 parallel groups by block randomisation |
| Allocation concealment (selection bias) | Low risk | All interventions were provided as drink powder in sachets to be freshly prepared by mixing with approximately 500 ml of water. The beverage mixes were provided in sachets (7 g = 1 serving) labelled with an alphanumeric identifier to enable a double‐masked study design |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on compliance or dropouts reported |
| Selective reporting (reporting bias) | Low risk | BP reported at beginning and end of intervention |
| Other bias | High risk | Industry funded and co‐authored |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and investigators were masked throughout the study for flavanol content of the test drinks |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment given |
BMI: body mass index
BP: blood pressure
C: cross‐over
CVD: cardiovascular disease
DB: double‐blind
DBP: diastolic blood pressure
P: parallel
SB: single‐blind
SBP: systolic blood pressure
SD: standard deviation
SEM: standard error of the mean
Characteristics of excluded studies [author‐defined order]
| Study | Reason for exclusion |
| Data for meta‐analysis not available (mean SBP/DBP, SD) | |
| Low quality (50% lost to follow‐up, small sample size) | |
| Duration < 2 weeks, acute effects of cocoa, (heart transplant patients) | |
| Data for meta‐analysis not available (mean SBP/DBP, SD) | |
| High cocoa dosage in control group, cocoa+plant sterols vs cocoa; same study as Allen 2008 | |
| Duration < 2 weeks, acute effects of cocoa | |
| High cocoa dosage in control group | |
| Duration < 2 weeks, acute effects of cocoa | |
| Control group 25% flavanol content (6 g dark chocolate) vs intervention group (25 g dark chocolate) | |
| No true control group: dark chocolate bar versus dark chocolate bar plus lycopene or dark chocolate bar plus lycosome | |
| Combination treatment of chocolate (850 mg flavanols) plus 100 mg isoflavones daily for 1 year in active group | |
| Combination treatment of cocoa (30 mg) + isoflavanols (80 mg) + myo‐inositol (2g) in active group | |
| No intervention in control group | |
| No true control group: flavanol/polyphenol content in active group intervention not provided; dietary polyphenol intake similar in active and control groups | |
| Acute BP after 2 hours | |
| Acute BP | |
| 5‐week cross‐over trial of different cocoa dosages and placebo, each taken 1 week | |
| conference abstract only, insufficient information | |
| conference abstract only, insufficient information | |
| cohort study, not randomized, only conference abstract, insufficient information | |
| Duration < 2 weeks | |
| Duration < 2 weeks | |
| Duration < 2 weeks | |
| Duration < 2 weeks, no separate chocolate intervention |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | 6‐week clinical trial |
| Participants | nine panelists (age: 22.6 ± 1.7; BMI: 22.3 ± 2.1) |
| Interventions | chocolate‐protein beverages once per week, including placebo, whey protein isolate (WPI), low polyphenolic cocoa (LP), high polyphenolic cocoa (HP), LP‐WPI, and HP‐WPI |
| Outcomes | blood glucose and adiponectin levels, and hunger ratings at baseline and 0.5–4.0 h following beverage consumption |
| Notes |
| Methods | single‐centre randomized double‐blind placebo‐controlled investigation with a crossover design |
| Participants | Thirty‐two patients with chronic HF, stable on guideline‐directed medical therapy, were randomized. Twenty‐four patients completed the study |
| Interventions | 50 g/day of high‐flavanol dark chocolate (HFDC; 1064 mg of flavanols/day) or low‐flavanol dark chocolate (LFDC; 88 mg of flavanols/day) for 4 weeks and then crossed over to consume the alternative dark chocolate for a further 4 weeks |
| Outcomes | reductions in N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) as an index of improved cardiac function. Changes in blood pressure. Effect on platelet function. |
| Notes | supported by a grant from Barry Callebaut Belgium NV |
| Methods | 4 week double‐blind, randomized placebo‐controlled trial |
| Participants | Twenty‐two patients with stable CHF (NYHA ≥ II) and ejection fraction <50% have been randomized. Two patients dropped out during follow‐up. Twenty patients were included into the final analysis. |
| Interventions | two chocolate bars/day commercially available flavanol‐rich chocolate compared with cocoa‐liquor‐free control chocolate |
| Outcomes | endothelial function; platelet function; blood pressure; heart rate |
| Notes |
| Methods | 12‐week randomised controlled, single‐blinded dietary intervention design |
| Participants | 92 participants aged 40–65 years, with documented grade I (140–159/90–99 mm Hg) or grade II (160–179/100–109 mm Hg) hypertension |
| Interventions | The study commenced with a four‐week ‘run‐in phase’ for all participants, during which they were asked to consume two portions or less of F&V, and to exclude berries and dark chocolate (low‐polyphenol diet). At the end of this period, subjects were randomised to continue with the above low‐polyphenol diet for a further 8‐week ‘intervention period’ or to consume a high‐polyphenol diet of six portions F&V (including one portion of berries per day) and 50 g of dark chocolate per day |
| Outcomes | The primary endpoint was between‐group change in maximum FBF response to the endothelium‐dependent vasodilator, ACh. Secondary endpoints included between‐group change in self‐reported polyphenol‐rich food intake, between‐group change in biochemical markers of nutritional status and between‐group change in SBP and lipid profile |
| Notes | NCT01319786 |
| Methods | Part 1 was an open‐label, intake‐amount escalation study. Part 2 was a controlled, randomized, double‐masked, 2‐parallel‐arm dietary intervention study |
| Participants | 34 healthy adults aged 35‐55 years |
| Interventions | Part 1: consume escalating amounts of cocoa flavanol, ranging from 1000 to 2000 mg/d over 6 wk Part 2: consume for 12 consecutive weeks up to 2000 mg cocoa flavanol per day (n = 46) or a CF‐free control (n = 28) |
| Outcomes | Primary outcomes were blood pressure and platelet function, select metabolic variables, and the occurrence and severity of AEs. Secondary outcomes included plasma concentrations of CF‐derived metabolites and methylxanthines |
| Notes |
| Methods | 12‐week randomised, controlled, parallel study |
| Participants | 102 non‐obese participants |
| Interventions | 4 arms: ˜1100 kJ/day for each of hazelnuts (42 g), chocolate (50 g), potato crisps (50 g), or no added snack food |
| Outcomes | Diet records, body composition, and physical activity were measured at baseline and week 12 |
| Notes |
| Methods | cross‐over, placebo‐controlled, double‐blind, randomized clinical trial |
| Participants | 92 individuals on antiretroviral therapy for at least six months and at viral suppression |
| Interventions | 65 g of chocolate or chocolate‐placebo or 3 g of yerba mate or mate‐placebo for 15 days each, alternating by a washout period of 15 days |
| Outcomes | data regarding anthropometry, inflammatory, oxidative and immunological parameters were collected at baseline, and at the end of each intervention regimen. High‐sensitivity C‐reactive protein, fibrinogen, lipid profile, white blood cell profile and thiobarbituric acid reactive substances were assessed |
| Notes |
| Methods | randomized, double‐blind, placebo‐controlled trial |
| Participants | Fifty‐seven participants with ESRD |
| Interventions | ingested CF‐rich beverages (900 mg CF per study day), compared with those ingesting CF‐free placebo |
| Outcomes | changes in flow–mediated dilation and hemodynamics |
| Notes | independent investigator–initiated trial without any commercial interest |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The effect of long term intervention with cocoa flavanols on metabolic control and cardiovascular parameters in subjects with and without type 2 diabetes |
| Methods | Randomised controlled trial |
| Participants | Randomisation among groups with and without diabetes |
| Interventions | High flavanol supplement:low flavanol supplement |
| Outcomes | Systolic and diastolic blood pressure |
| Starting date | 2007 |
| Contact information | Dr Anne Reutens, Baker IDI Heart and Diabetes Institute, 250 Kooyong Road Caulfield VIC 3162, [email protected] |
| Notes | Sponsor: Mars Symbioscience, a division of Mars Incorporated |
| Trial name or title | Effect of Polyphenol‐rich Dark Chocolate on Insulin Sensitivity in Normal Weight and Overweight Adults |
| Methods | Duration: 4 weeks Allocation: Randomized Intervention Model: Parallel Assignment Masking: Single Blind (Participant) |
| Participants | 61 Adults with no history of hypertension, diabetes and cardiovascular diseases
|
| Interventions | Experimental: Polyphenol‐rich Dark chocolate: Participants will be asked to consume 20g of dark chocolate containing 500mg of polyphenols daily for a period of 4 weeks Placebo Comparator: Placebo Dark chocolate: Participants will be asked to consume 20g of dark chocolate containing little or no polyphenols for a period of 4 weeks |
| Outcomes | Primary Outcome Measures: Determine if the consumption of DC rich in polyphenols can induce a change in insulin sensitivity [ Time Frame: Baseline and week 4 ]Insulin sensitivity will be determined by determined by HOMA‐IR (Homeostasis Model of Assessment ‐ Insulin Resistance) |
| Starting date | March 2012 |
| Contact information | Grace Farhat, PhD research student, Queen Margaret University, Musselburgh, East Lothian, United Kingdom, EH21 6UU |
| Notes |
| Trial name or title | Impact of High Energy Nutritional Supplement Drink (HENSD) consumed for five consecutive days on appetite, energy intake and cardiometabolic risk factors in underweight females |
| Methods | Single‐blinded randomised controlled crossover study |
| Participants | 22 Healthy women with body mass index of 17‐ 20 kg/m2 |
| Interventions | 1. HENSD (Scandishake, Chocolate, Nutricia) made up with 240 g of full fat milk, according to the manufacturer instructions (Nutricia, 2009) 2. Placebo (a low calorie drink prepared with 240 g of skimmed milk, 4 g of cocoa and two sweeteners) |
| Outcomes | Primary: 1. Fasting lipids, postprandial lipaemia, insulin resistance 2. Energy intake and body mass Secondary: 1. Appetite measures 2. Metabolic rate |
| Starting date | 12/02/2014 |
| Contact information | Dr Sadia Fatima Human Nutrition Section |
| Notes |
| Trial name or title | An investigation into the effects of chronic consumption of cocoa flavonoids on vascular function: a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | 16 Non‐smoking postmenopausal women aged between 48 and 65 years |
| Interventions | cocoa powder |
| Outcomes | Primary: Blood pressure taken at the beginning and end of each intervention period. Secondary: Arterial stiffness, flow mediated dilatation, plasma ICAM‐1, VCAM‐1, C‐reactive protein, P‐selectin, 8‐isoprostane F2 α, lipids and urinary 8‐isoprostane F2 |
| Starting date | 24/08/2006 |
| Contact information | Dr Ummezeinab Mulla Professor Thomas Sanders |
| Notes |
| Trial name or title | The effect of cocoa flavanoids on blood pressure |
| Methods | RCT double‐blind parallel |
| Participants | Children, adults, elderly people with hypertension, n = 50 |
| Interventions | Flavonoid‐rich cocoa drink vs low‐flavanoid drink daily for 12 weeks |
| Outcomes | Primary: mean diff 24‐hour AMBP; |
| Starting date | Sep 2005 |
| Contact information | Neil R Poulter, Imperial College London, Paddington, IK W21PG |
| Notes | Sponsor: MasterFoods |
| Trial name or title | Controlled clinical trial to determine the effective dose of cocoa in lowering blood pressure |
| Methods | RCT, double‐blind, parallel |
| Participants | Adults 18 ‐ 65 yrs, I‐II hypertension |
| Interventions | 6.5 g, 12 g, 25 g, or 50 g (change of groups every 2 weeks) of chocolate for 18 weeks |
| Outcomes | Primary: blood pressure inpatient |
| Starting date | 12/2008 |
| Contact information | Monica Lucia Giraldo Restrepo, Universidad de Antioquia, Colombia |
| Notes | Sponsor: Universidad de Antioquia |
| Trial name or title | A Pilot Study Investigating the Effects of the Combined Effects of Cocoa and Soy Polyphenols in a Soy Protein Matrix on Insulin Resistance and Cardiovascular Disease Risk in Type 2 Diabetes |
| Methods | 8‐week Randomised Placebo‐Controlled Double‐Blind Parallel Study |
| Participants | 84 Patients with type 2 diabetes controlled by diet or metformin only, Stable medication history for 3 months prior to screening visit, Age 45‐80 |
| Interventions | Soy protein with isoflavones and cocoa Soy protein alone with cocoa Soy protein with soy isoflavones Soy protein alone Placebo bar without soy protein, isoflavones or cocoa polyphenols |
| Outcomes | Primary: Insulin resistance, lipid profile Secondary: Cardiovascular risk, Isoflavones, Endothelial function |
| Starting date | October 2011 |
| Contact information | Stephen L Atkin, University of Hull |
| Notes |
| Trial name or title | Effects of Polyphenolic‐rich Dark Chocolate/Cocoa and Almonds on Cardiovascular Disease Risk Factors |
| Methods | Allocation: Randomized Intervention Model: Crossover Assignment Masking: Investigator Primary Purpose: Prevention |
| Participants | 48 Overweight and obese adults (BMI ≥25, ≤40 kg/m2) with moderately elevated LDL‐C between the 25‐95th percentile from NHANES: 105‐194 mg/dL for males; 98‐190 mg/dL for females |
| Interventions | Experimental: Dark Chocolate/Cocoa + Almond Diet Experimental: Almond Diet Experimental: Dark Chocolate/Cocoa Diet Active Comparator: Healthy American Control Diet |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
Other Outcome Measures:
|
| Starting date | March 2012 |
| Contact information | Penny Kris‐Etherton, Penn State University |
| Notes |
| Trial name or title | The Vascular and Cognitive Effects of Chronic High‐flavanol Intake in Healthy Males |
| Methods | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Double Blind (Participant, Investigator) Primary Purpose: Prevention |
| Participants | 34 male adults (18 to 40 years) Body Mass Index 18.5‐27.5 kg/m2 Normal Blood pressure (< 150/90) Non‐smoker Regular exercise routine |
| Interventions | Active Comparator: High‐flavanol milk chocolate Placebo Comparator: Low‐flavanol milk chocolate |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | January 2016 |
| Contact information | Jeremy Paul Edward Spencer, University of Reading |
| Notes |
| Trial name or title | Multicountry Studies on the Effect of Positional Distribution of Fatty Acids at Triglyceride Backbone on Serum Lipids, Lipoprotein(a) and LDL‐subclasses in Healthy Malaysian Volunteers |
| Methods | 4 weeks Allocation: Randomized Intervention Model: Crossover Assignment Masking: Single Blind (Participant) |
| Participants | 42 Healthy adult male or female, aged 20‐50 years, BMI 18.5‐ 24.9 kg/m2 as per WHO Classification (1998) |
| Interventions | Experimental: Palm olein IV 64 Experimental: Cocoa butter Experimental: Virgin olive oil |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | January 2016 |
| Contact information | Malaysia Palm Oil Board |
| Notes |
| Trial name or title | Effects of Hazelnuts and Cocoa on Metabolic Parameters and Vascular Reactivity |
| Methods | 2 weeks Allocation: Randomized Intervention Model: Parallel Assignment Masking: Open Label Primary Purpose: Health Services Research |
| Participants | 61 adults (18 to 40 years) with BMI 18.5‐24.9 kg/m2 |
| Interventions | 1. Experimental: 30g peeled hazelnuts cream 2. Experimental: 30g unpeeled hazelnuts cream 3. Experimental: snack w/ 30g peeled hazelnuts 4. Experimental: snack w/ 2.5g cocoa powder 5. Experimental: snack w/ 30g peeled hazelnuts+2.5g cocoa 6. Placebo Comparator: empty snack |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | June 2014 |
| Contact information | Anna Ferrulli, Ospedale San Donato, Italy |
| Notes |
AMBP: ambulatory measurement of blood pressure
PWV: pulse wave velocity
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| Analysis 1.1  Comparison 1 Effect of cocoa on BP, Outcome 1 SBP. | ||||
| 2 DBP Show forest plot | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| Analysis 1.2  Comparison 1 Effect of cocoa on BP, Outcome 2 DBP. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| Analysis 2.1 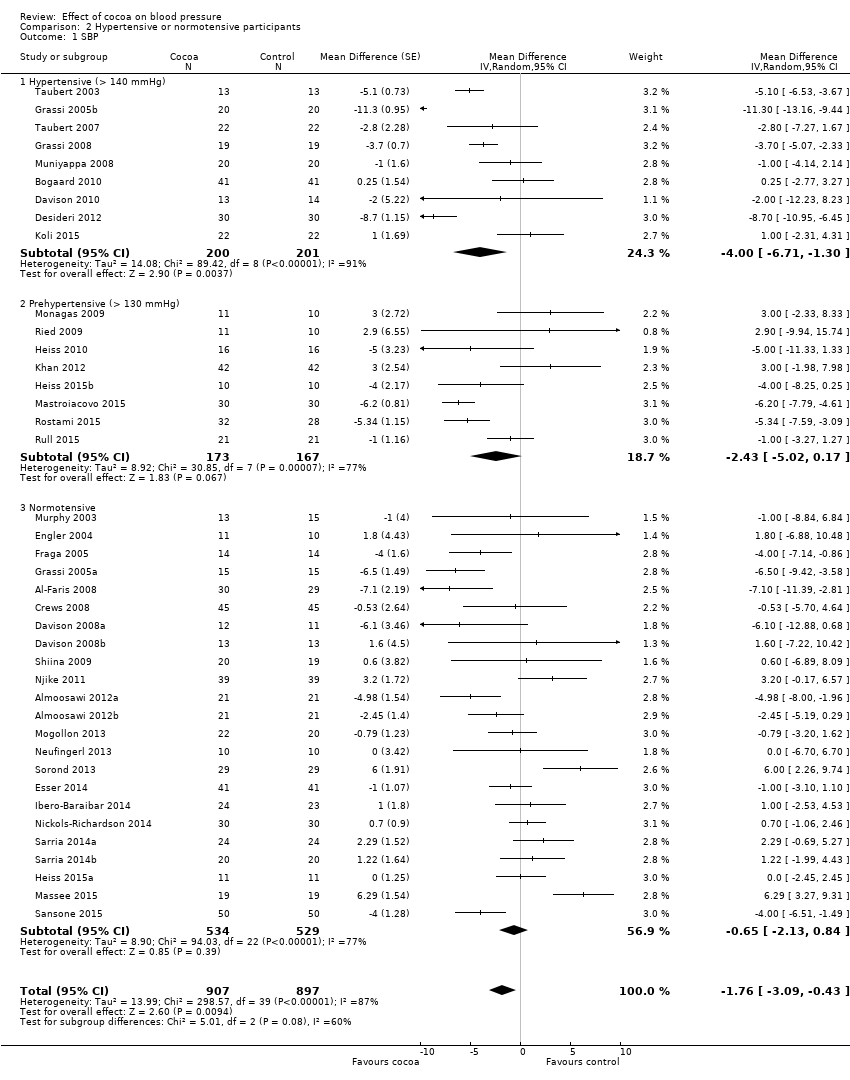 Comparison 2 Hypertensive or normotensive participants, Outcome 1 SBP. | ||||
| 1.1 Hypertensive (> 140 mmHg) | 9 | 401 | Mean Difference (Random, 95% CI) | ‐4.00 [‐6.71, ‐1.30] |
| 1.2 Prehypertensive (> 130 mmHg) | 8 | 340 | Mean Difference (Random, 95% CI) | ‐2.43 [‐5.02, 0.17] |
| 1.3 Normotensive | 23 | 1063 | Mean Difference (Random, 95% CI) | ‐0.65 [‐2.13, 0.84] |
| 2 DBP Show forest plot | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| Analysis 2.2 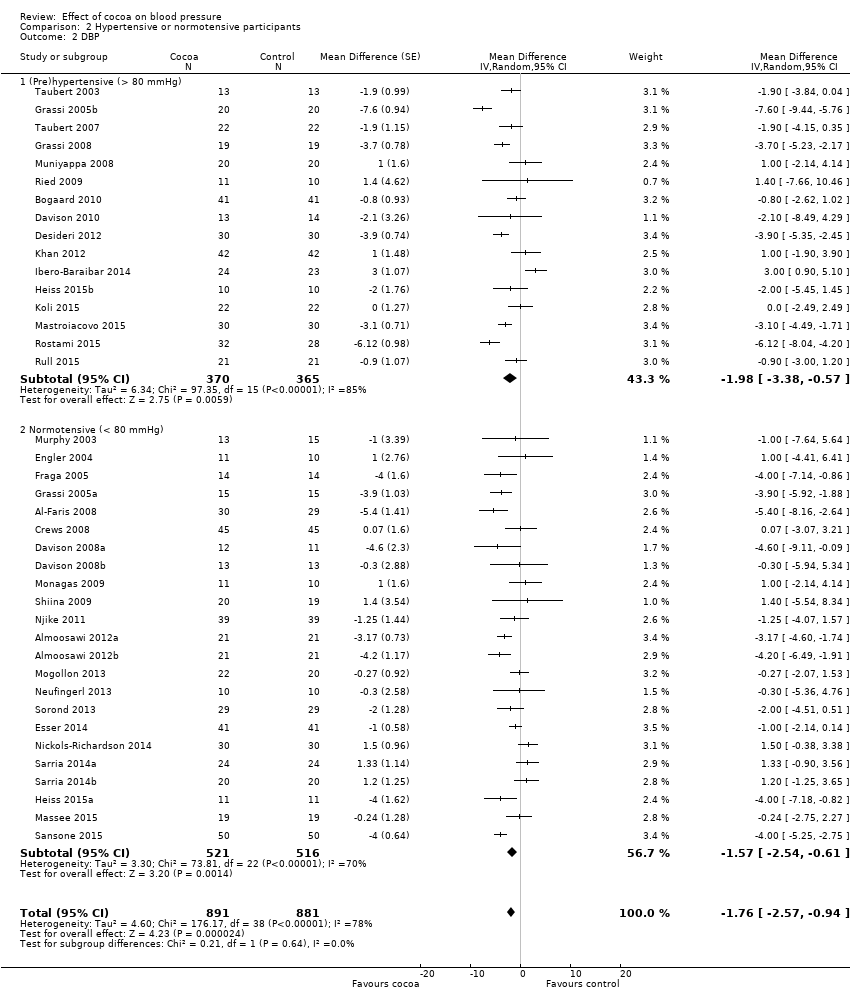 Comparison 2 Hypertensive or normotensive participants, Outcome 2 DBP. | ||||
| 2.1 (Pre)hypertensive (> 80 mmHg) | 16 | 735 | Mean Difference (Random, 95% CI) | ‐1.98 [‐3.38, ‐0.57] |
| 2.2 Normotensive (< 80 mmHg) | 23 | 1037 | Mean Difference (Random, 95% CI) | ‐1.57 [‐2.54, ‐0.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| Analysis 3.1  Comparison 3 Flavanol‐free or low flavanol control, Outcome 1 SBP. | ||||
| 1.1 Flavanol‐free control | 26 | 1116 | Mean Difference (Random, 95% CI) | ‐1.80 [‐3.46, ‐0.13] |
| 1.2 Low flavanol control | 14 | 688 | Mean Difference (Random, 95% CI) | ‐1.67 [‐4.03, 0.69] |
| 2 DBP Show forest plot | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| Analysis 3.2 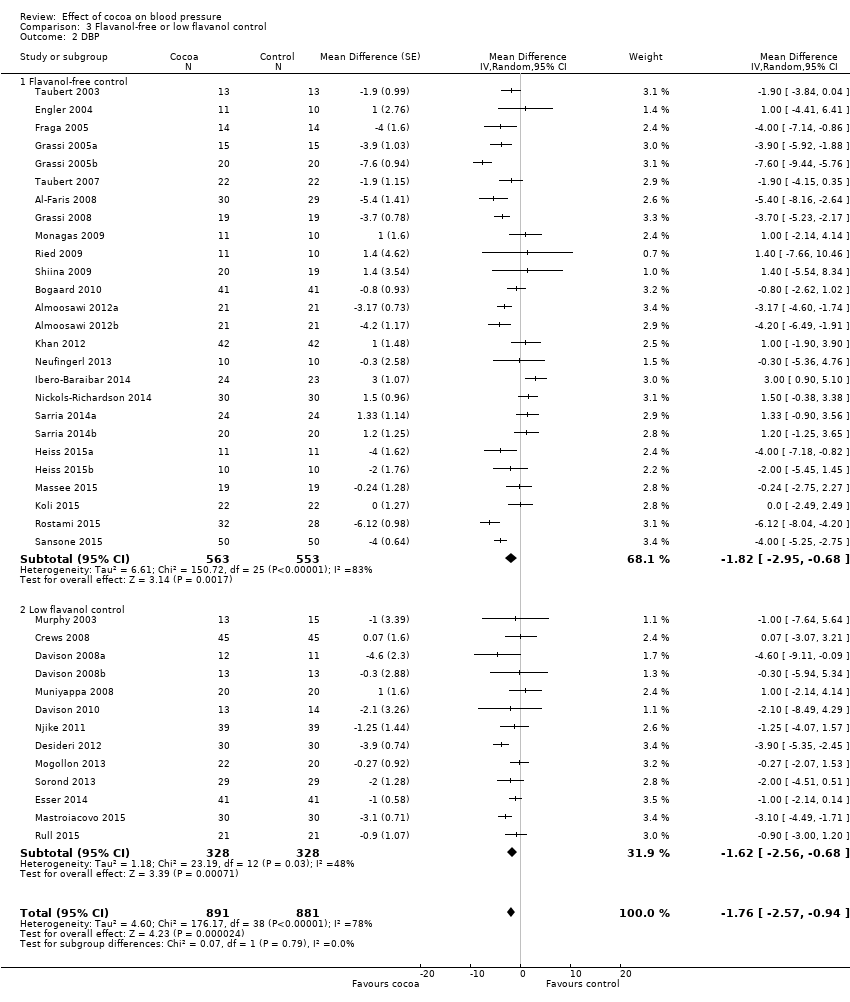 Comparison 3 Flavanol‐free or low flavanol control, Outcome 2 DBP. | ||||
| 2.1 Flavanol‐free control | 26 | 1116 | Mean Difference (Random, 95% CI) | ‐1.82 [‐2.95, ‐0.68] |
| 2.2 Low flavanol control | 13 | 656 | Mean Difference (Random, 95% CI) | ‐1.62 [‐2.56, ‐0.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| Analysis 4.1  Comparison 4 Double‐blinded or unblinded/single‐blinded, Outcome 1 SBP. | ||||
| 1.1 Double‐blind | 23 | 1059 | Mean Difference (Random, 95% CI) | ‐0.95 [‐2.77, 0.86] |
| 1.2 Unblinded, single‐blinded | 17 | 745 | Mean Difference (Random, 95% CI) | ‐2.71 [‐4.66, ‐0.76] |
| 2 DBP Show forest plot | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| Analysis 4.2  Comparison 4 Double‐blinded or unblinded/single‐blinded, Outcome 2 DBP. | ||||
| 2.1 Double‐blind | 21 | 927 | Mean Difference (Random, 95% CI) | ‐1.16 [‐2.05, ‐0.27] |
| 2.2 Unblinded, single‐blinded | 18 | 845 | Mean Difference (Random, 95% CI) | ‐2.33 [‐3.62, ‐1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 38 | 1762 | Mean Difference (Random, 95% CI) | ‐1.36 [‐2.79, 0.06] |
| Analysis 5.1 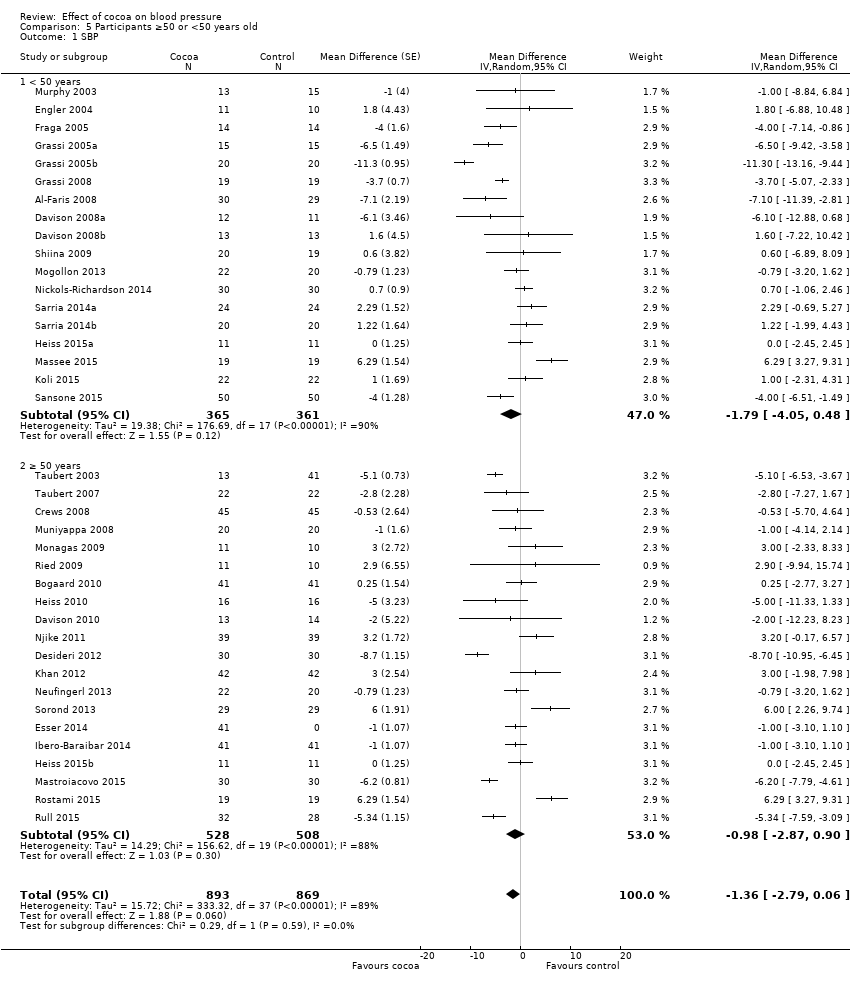 Comparison 5 Participants ≥50 or <50 years old, Outcome 1 SBP. | ||||
| 1.1 < 50 years | 18 | 726 | Mean Difference (Random, 95% CI) | ‐1.79 [‐4.05, 0.48] |
| 1.2 ≥ 50 years | 20 | 1036 | Mean Difference (Random, 95% CI) | ‐0.98 [‐2.87, 0.90] |
| 2 DBP Show forest plot | 37 | 1688 | Mean Difference (Random, 95% CI) | ‐1.62 [‐2.49, ‐0.76] |
| Analysis 5.2 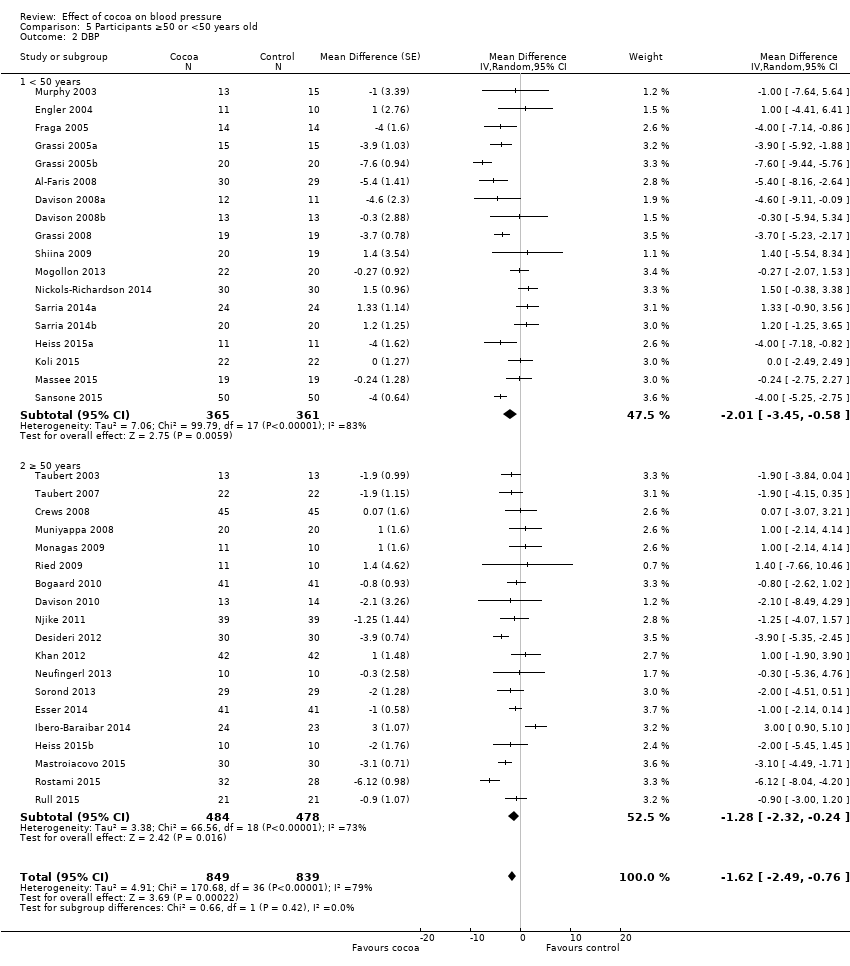 Comparison 5 Participants ≥50 or <50 years old, Outcome 2 DBP. | ||||
| 2.1 < 50 years | 18 | 726 | Mean Difference (Random, 95% CI) | ‐2.01 [‐3.45, ‐0.58] |
| 2.2 ≥ 50 years | 19 | 962 | Mean Difference (Random, 95% CI) | ‐1.28 [‐2.32, ‐0.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| Analysis 6.1 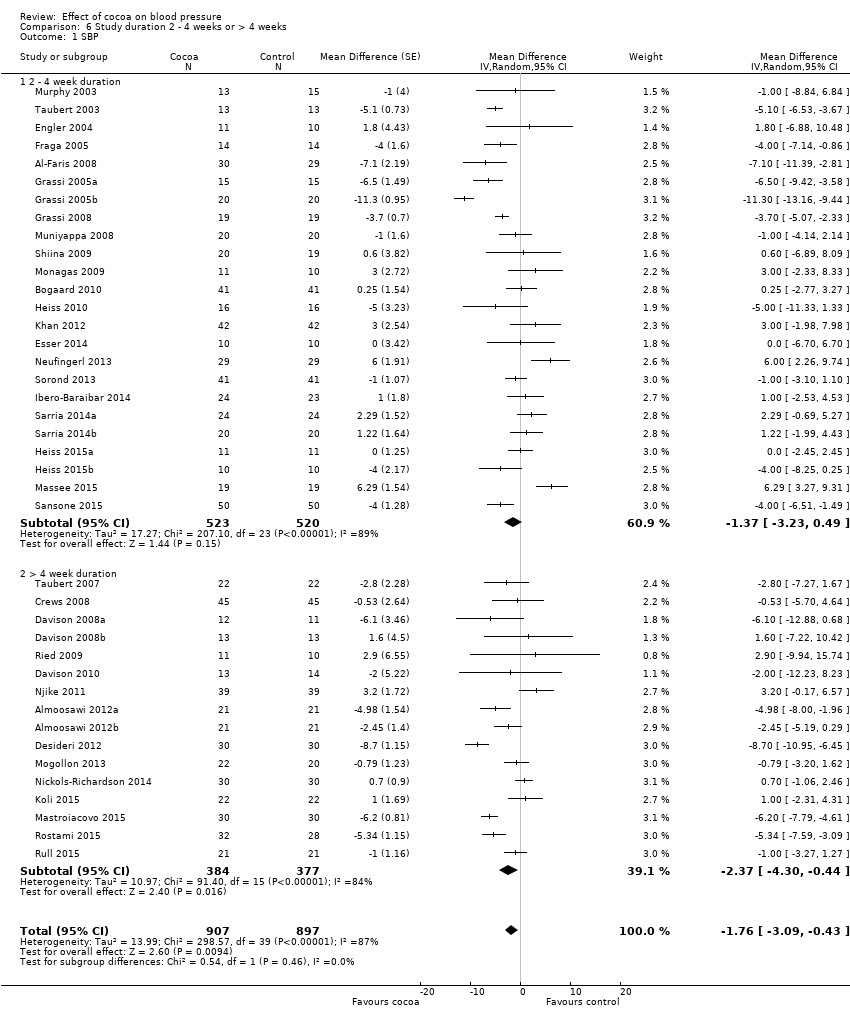 Comparison 6 Study duration 2 ‐ 4 weeks or > 4 weeks, Outcome 1 SBP. | ||||
| 1.1 2 ‐ 4 week duration | 24 | 1043 | Mean Difference (Random, 95% CI) | ‐1.37 [‐3.23, 0.49] |
| 1.2 > 4 week duration | 16 | 761 | Mean Difference (Random, 95% CI) | ‐2.37 [‐4.30, ‐0.44] |
| 2 DBP Show forest plot | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| Analysis 6.2  Comparison 6 Study duration 2 ‐ 4 weeks or > 4 weeks, Outcome 2 DBP. | ||||
| 2.1 2 ‐ 4 week duration | 23 | 1011 | Mean Difference (Random, 95% CI) | ‐1.55 [‐2.71, ‐0.39] |
| 2.2 > 4 week duration | 16 | 761 | Mean Difference (Random, 95% CI) | ‐2.04 [‐3.18, ‐0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 33 | 1482 | Mean Difference (Random, 95% CI) | ‐1.08 [‐2.60, 0.43] |
| Analysis 7.1  Comparison 7 Sensitivity analysis: excl studies with industry employed authors, Outcome 1 SBP. | ||||
| 2 DBP Show forest plot | 33 | 1482 | Mean Difference (Random, 95% CI) | ‐1.37 [‐2.31, ‐0.43] |
| Analysis 7.2 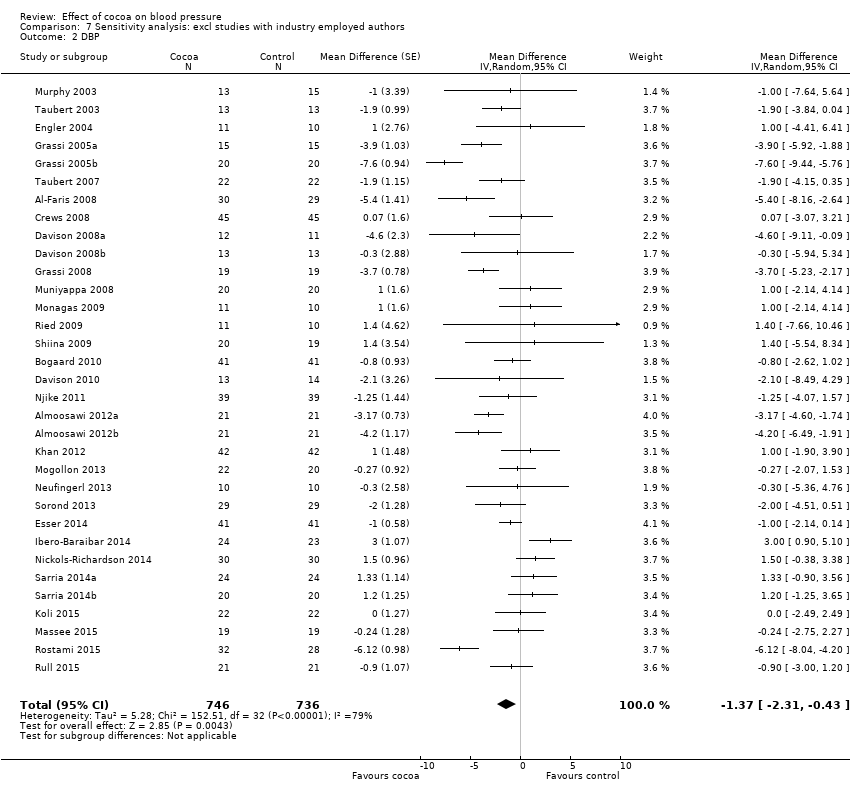 Comparison 7 Sensitivity analysis: excl studies with industry employed authors, Outcome 2 DBP. | ||||

PRISMA Flow diagram
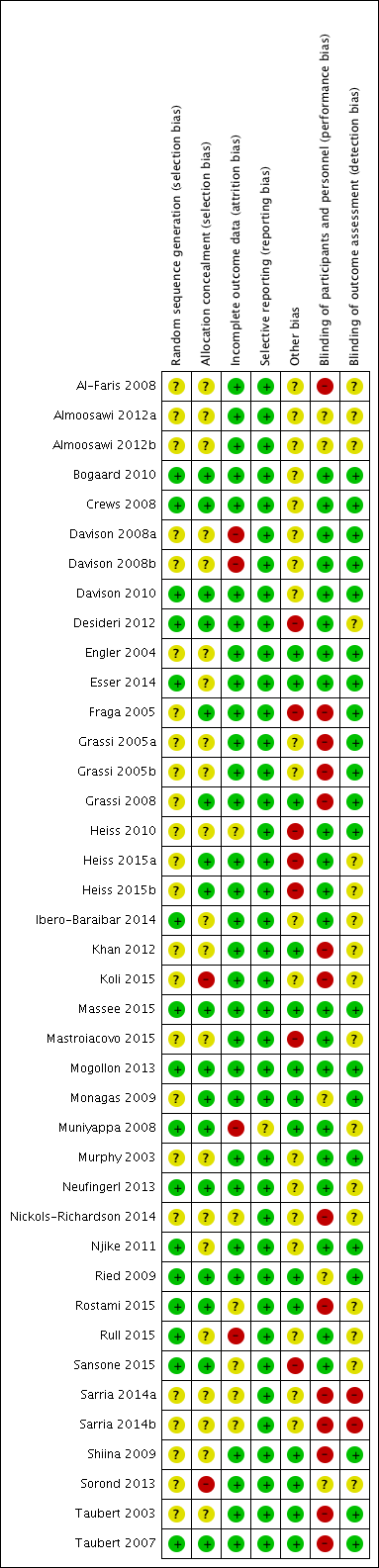
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Effect of cocoa on BP, outcome: 1.1 SBP.
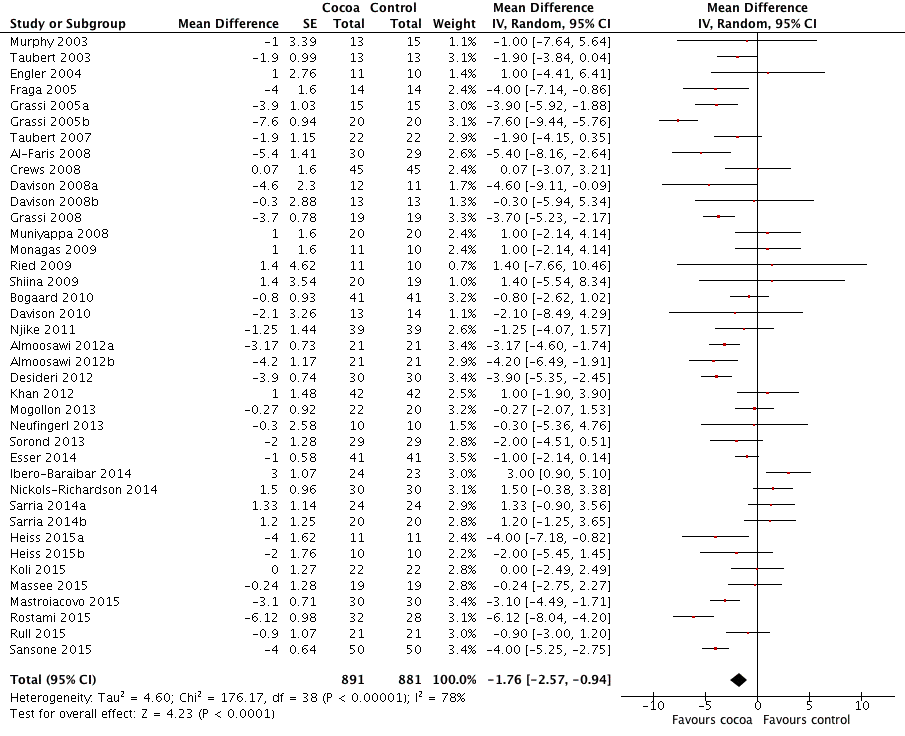
Forest plot of comparison: 1 Effect of cocoa on BP, outcome: 1.2 DBP.

Forest plot of comparison: 2 Hypertensive or normotensive subjects, outcome: 2.1 SBP.

Forest plot of comparison: 2 Hypertensive or normotensive subjects, outcome: 2.2 DBP.

Comparison 1 Effect of cocoa on BP, Outcome 1 SBP.
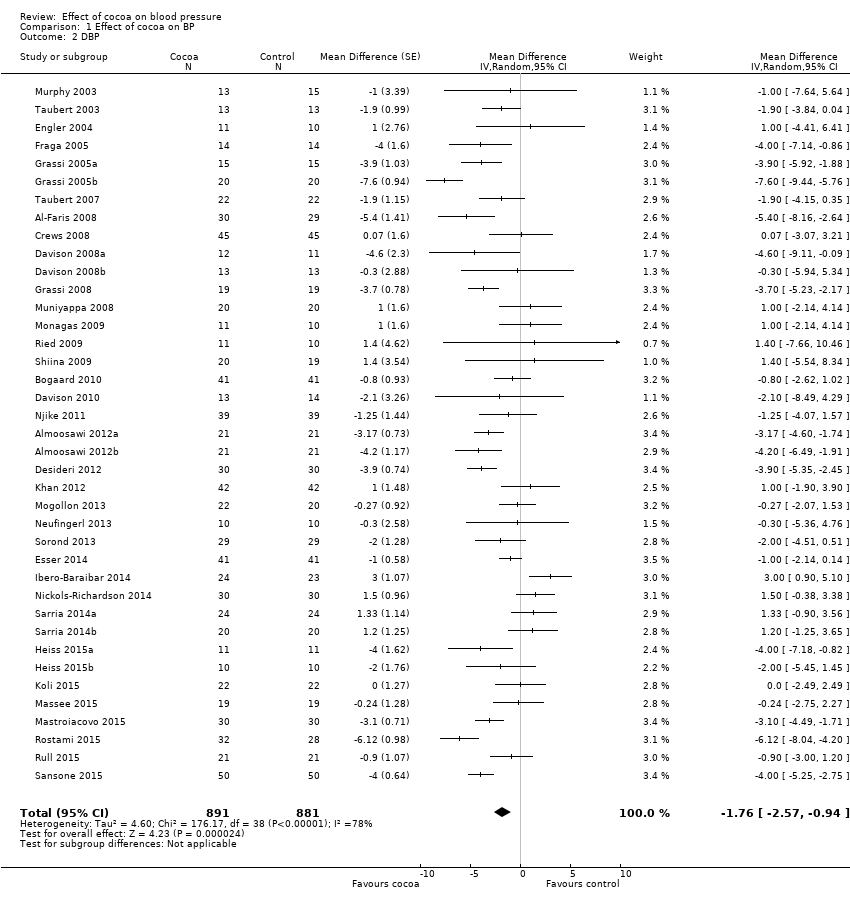
Comparison 1 Effect of cocoa on BP, Outcome 2 DBP.

Comparison 2 Hypertensive or normotensive participants, Outcome 1 SBP.
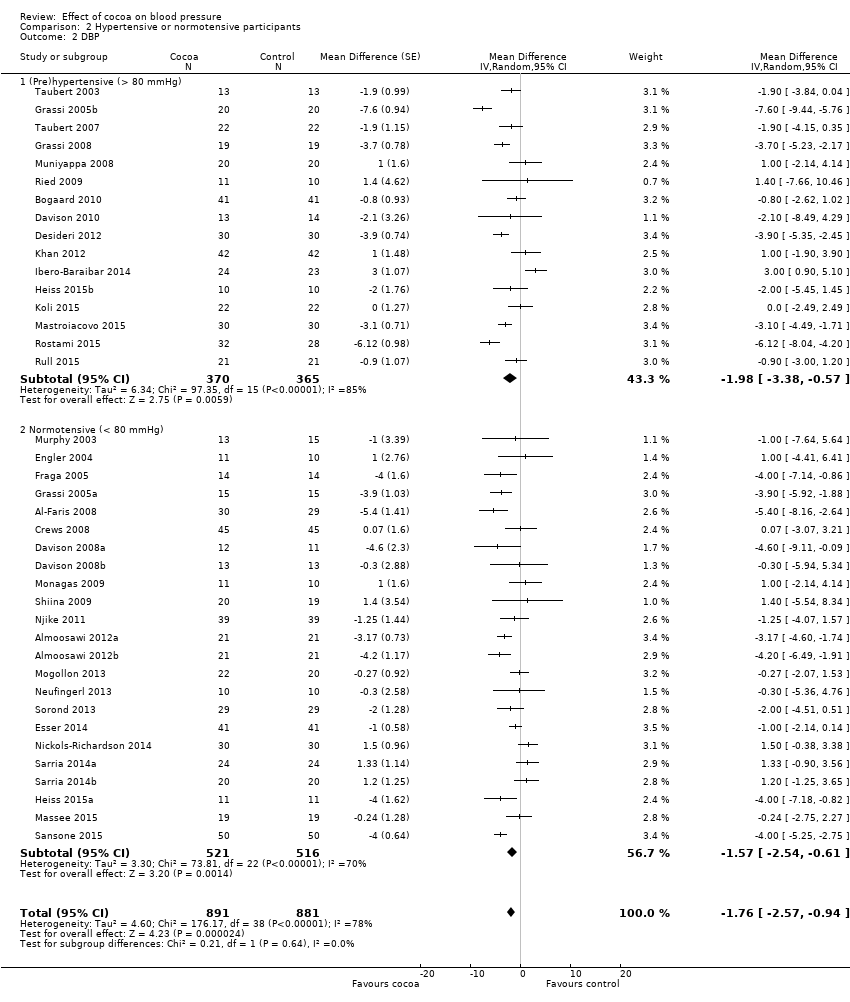
Comparison 2 Hypertensive or normotensive participants, Outcome 2 DBP.

Comparison 3 Flavanol‐free or low flavanol control, Outcome 1 SBP.
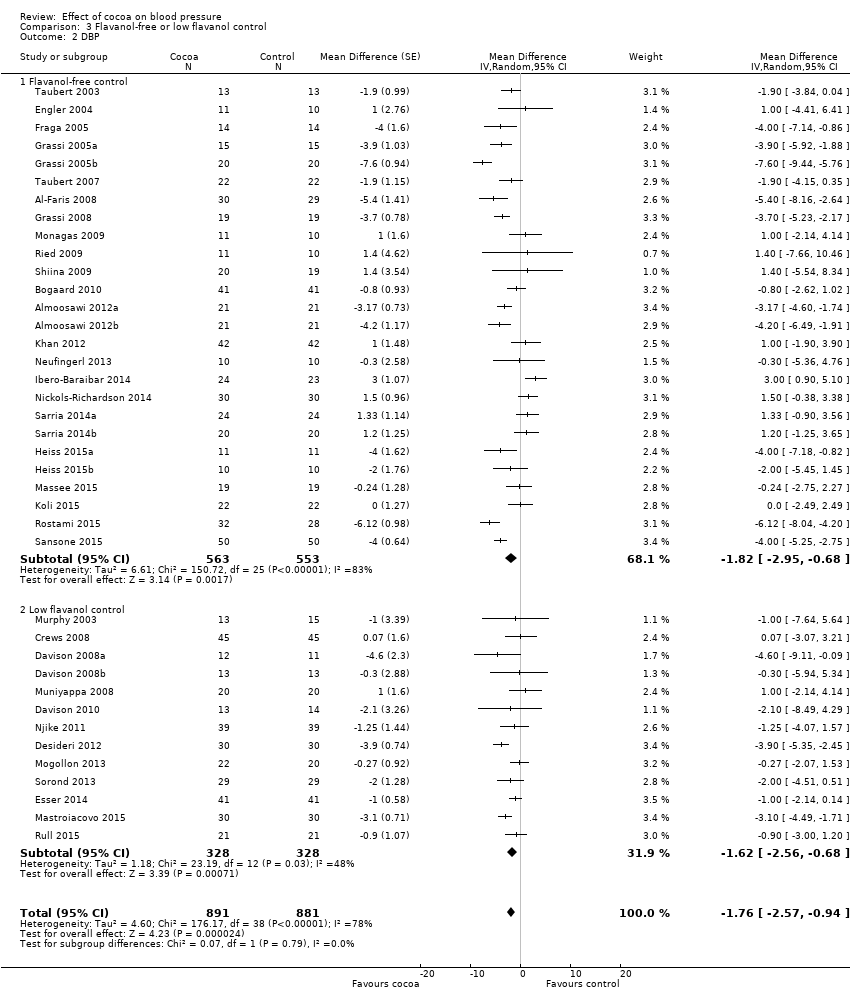
Comparison 3 Flavanol‐free or low flavanol control, Outcome 2 DBP.

Comparison 4 Double‐blinded or unblinded/single‐blinded, Outcome 1 SBP.
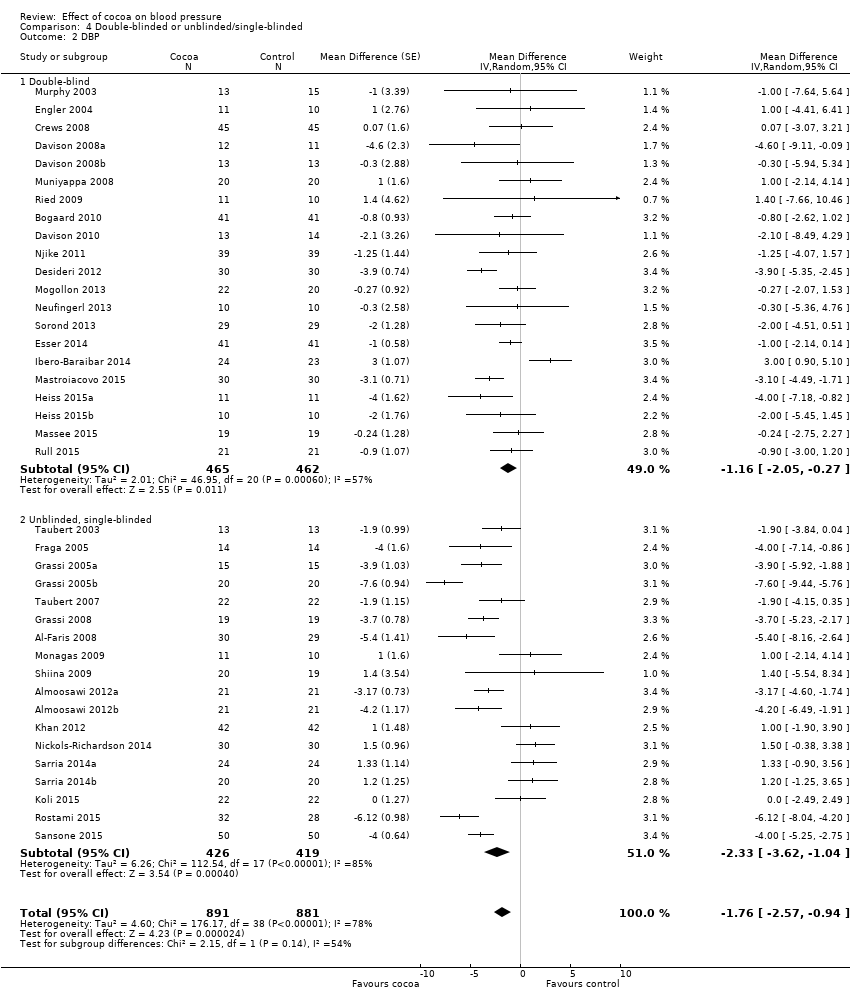
Comparison 4 Double‐blinded or unblinded/single‐blinded, Outcome 2 DBP.

Comparison 5 Participants ≥50 or <50 years old, Outcome 1 SBP.

Comparison 5 Participants ≥50 or <50 years old, Outcome 2 DBP.
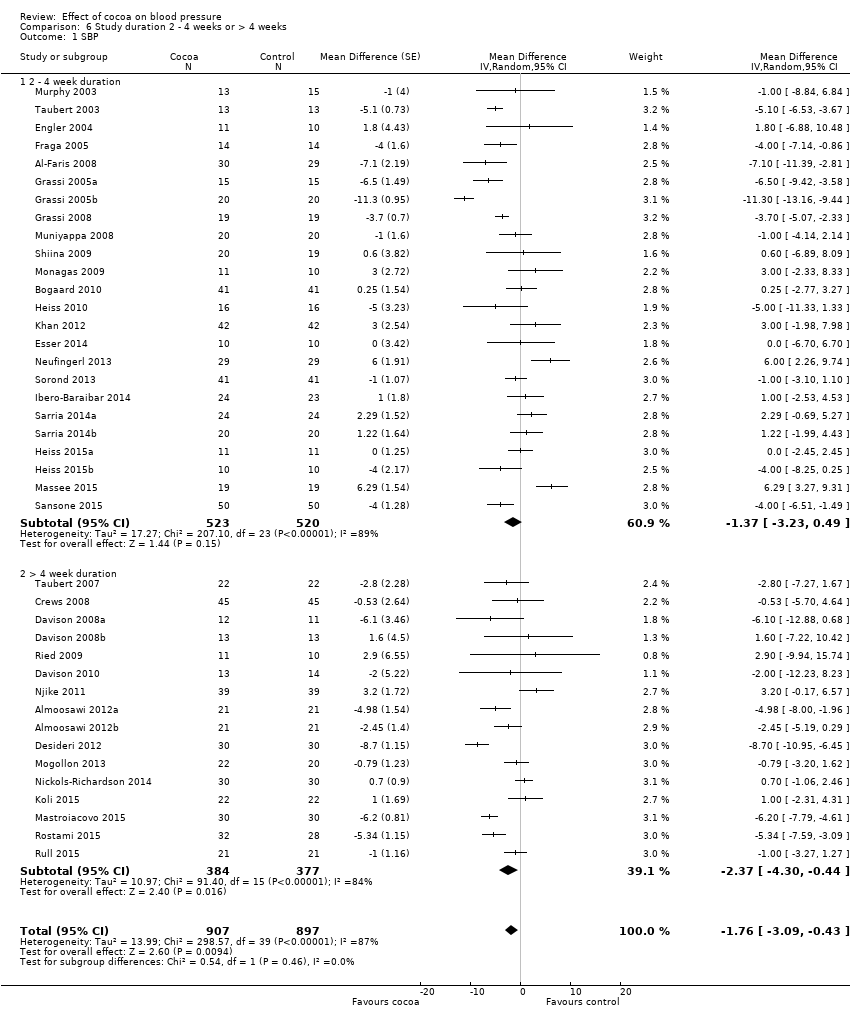
Comparison 6 Study duration 2 ‐ 4 weeks or > 4 weeks, Outcome 1 SBP.
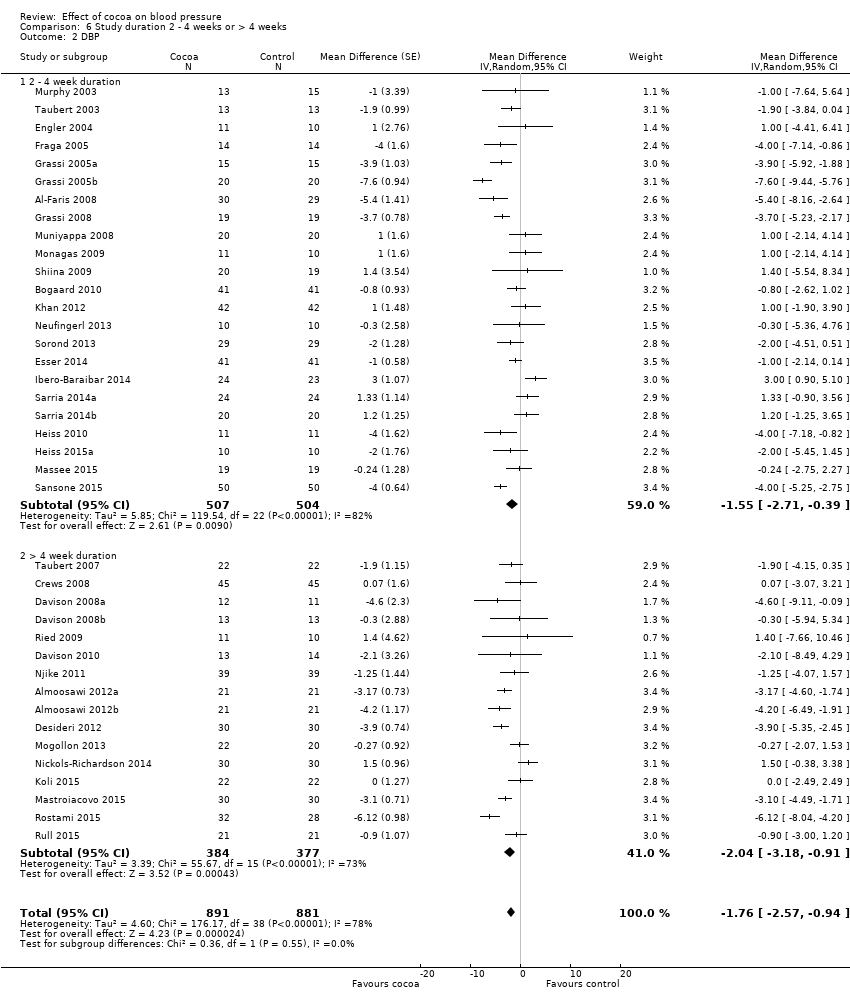
Comparison 6 Study duration 2 ‐ 4 weeks or > 4 weeks, Outcome 2 DBP.
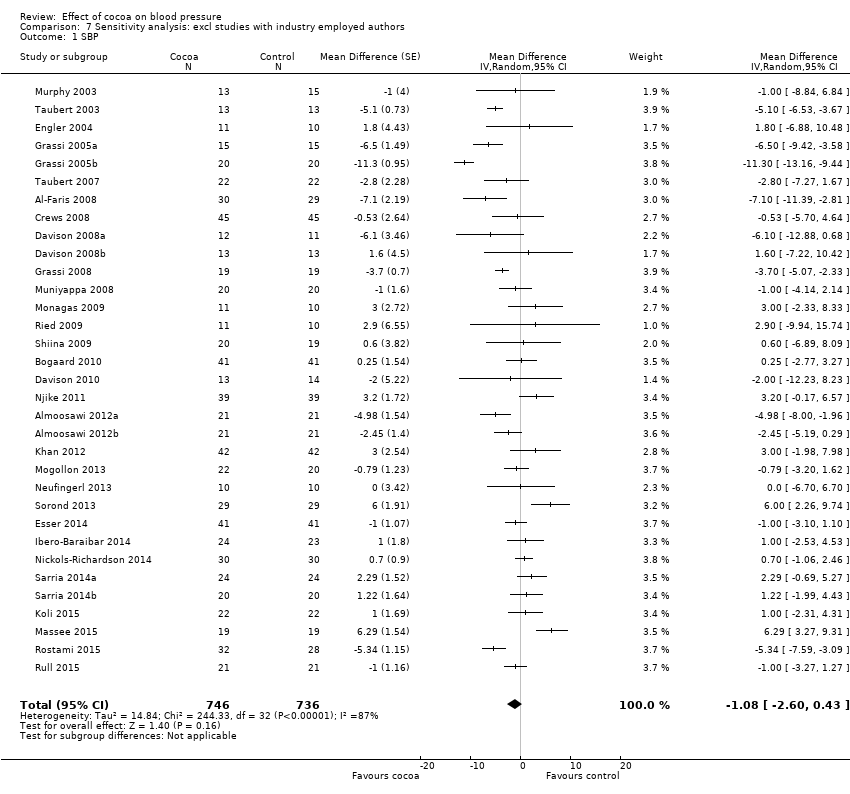
Comparison 7 Sensitivity analysis: excl studies with industry employed authors, Outcome 1 SBP.

Comparison 7 Sensitivity analysis: excl studies with industry employed authors, Outcome 2 DBP.
| Flavanol‐rich cocoa products for blood pressure | ||||||
| Patient or population: adults with or without hypertension | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Flavanol‐rich cocoa products | |||||
| Systolic blood pressure | The mean systolic blood pressure ranged across control groups from 107 to 154 mm Hg | The mean systolic blood pressure in the intervention groups was | 1804 | ⊕⊕⊕⊕ | ||
| Diastolic blood pressure | The mean diastolic blood pressure ranged across control groups from 66 to 92 mm Hg | The mean diastolic blood pressure in the intervention groups was | 1772 | ⊕⊕⊕⊕ | ||
| Withdrawals due to adverse effects | 8 trials reported no withdrawals and no adverse effects. 9 trials reported adverse effects, including gastrointestinal complaints (cocoa groups: n = 8/760 (1%), control groups: n = 3/754 (0.4%)); dislike of the trial product (cocoa: n = 4/760; control: n = 1/754), headache (cocoa: n = 2/760; control: n = 1/754), and jitteriness (cocoa: n = 1/760, control: n = 0/754). | 1514 (31 trials) reported on withdrawals and adverse effects | ⊕⊕⊕⊕ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1.Downgraded to moderate quality due to high heterogeneity which cannot be explained by subgroup analyses. SBP/DBP: I2 = 87%/78%. 2.Good quality across 40 treatment comparisons. Only 5 trials (12.5%) had 2 items at high risk of bias, 19 trials (47.5%) had 1 item at high risk of bias, and 16 trials (40%) had no items at high risk of bias. 17 trials were unblinded or single‐blinded. 7 industry‐sponsored trials had authors employed by industry. Only 4 trials (10%) had more than 20% attrition. We explored influence of trials with items at high risk of bias by subgroup and sensitivity analysis. | ||||||
| Study | Study design
| Participants Cocoa/ Control | Withdrawn Cocoa/Control | Reasons for withdrawal including adverse effects Cocoa/Control |
| Taubert 2003 | C | 13/13 | 0/0 | ‐ |
| Murphy 2003 | P | 13/15 | 3 in total | Family illness (2) Non‐compliance in final week (1) |
| Engler 2004 | P | 11/10 | 0/0 | ‐ |
| Fraga 2005 | C | 14/14 | 1/0 | No reason given |
| Grassi 2005a | C | 15/15 | 0/0 | ‐ |
| Grassi 2005b | C | 20/20 | 0/0 | ‐ |
| Taubert 2007 | P | 22/22 | 0/0 | ‐ |
| Crews 2008 | P | 45/45 | 6/5 | Gastrointestinal upset/headache/cold sweat (2/1) Bronchitis (1/0) Jitteriness/increased energy (1/0) Atrial arrhythmia/medication change (1/0) Dislike of study product (1/1) Family illness (0/1) Unspecified reason (0/1) No adherence to trial regimen (0/1) |
| Grassi 2008 | C | 19/19 | 0/0 | ‐ |
| Muniyappa 2008 | C | 20/20 | 5/4 | Lost to follow‐up (0/1) Discontinued intervention (4/2) due to Intolerance to treatment, family emergencies, personal problems excluded from analysis (1/1) |
| Davison 2008a | P | 12/11 | 7 in total | Time restrictions, personal circumstances (14) Non‐compliance (exercise or diet) (2)
|
| Davison 2008b | P | 13/13 | 5 in total | |
| Al‐Faris 2008 | P | 30/29 | 0/0 | ‐ |
| Shiina 2009 | P | 20/19 | 0/0 | ‐ |
| Ried 2009 | P | 11/10 | 2/2 | Study product unpalatable (2/0) Gastrointestinal upset (0/1) Illness unrelated to study (0/1) |
| Monagas 2009 | C | 42/42 | 0/0 | Constipation (resolved with fibre intake) |
| Bogaard 2010 | C | 41/41 | 3 in total | Nausea (1) Headache (1) Arrythmia unrelated (1)
|
| Heiss 2010 | C | 16/16 | 3 in total | Did not come to first visit |
| Davison 2010 | P | 13/14 | 7 in total | Mild gastric symptoms (1) Non‐compliance with study protocol (1) Withdrew due to personal circumstances (5) |
| Njike 2011 | C | 38/38 | 7 in total | Non‐compliance with study protocol (1) Withdrew for personal reasons (6)
|
| Almoosawi 2012a | C | 21/21 | 1/1 | Personal reasons unrelated to study |
| Desideri 2012 | P | 30/30 | 0/1 | Gastric discomfort (1) |
| Khan 2012 | C | 42/42 | 1/0 | Constipation |
| Mogollon 2013 | P | 22/20 | 1/1 | Unrelated to study (1)/headache (1) |
| Neufingerl 2013 | P | 10/10 | 1/1 | Nausea (1)/unrelated (1) |
| Sorond 2013 | P | 29/29 | 1/1 | No details provided |
| Esser 2014 | C | 41/41 | 3 in total | Medical reasons (1), disliked chocolate (1), poor compliance (1) |
| Ibero‐Baraibar 2014 | P | 24/23 | 2/1 | Personal reason (2), poor compliance (1) |
| Nickols‐Richardson 2014 | P | 30/30 | 0/0 | None |
| Sarria 2014 (a) | C | 24/24 20/20 | ? | No information given |
| Heiss 2015 (a) | P | 11/11 10/10 | 0/0 | None |
| Massee 2015 | P | 19/19 | 1/1 | Personal reasons (1) |
| Rostami 2015 | P | 32/28 | 2/6 | No information given |
| Koli 2015 | C | 22/22 | 0/0 | No side effects reported |
| Mastroiacovo 2015 | P | 30/30 | 1/0 | Personal reasons (1) No side effects reported |
| Rull 2015 | C | 21/21 | 11 | No details provided |
| Sansone 2015 | P | 50/50 | ? | No information given |
| C:Cross‐over | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| 2 DBP Show forest plot | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| 1.1 Hypertensive (> 140 mmHg) | 9 | 401 | Mean Difference (Random, 95% CI) | ‐4.00 [‐6.71, ‐1.30] |
| 1.2 Prehypertensive (> 130 mmHg) | 8 | 340 | Mean Difference (Random, 95% CI) | ‐2.43 [‐5.02, 0.17] |
| 1.3 Normotensive | 23 | 1063 | Mean Difference (Random, 95% CI) | ‐0.65 [‐2.13, 0.84] |
| 2 DBP Show forest plot | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| 2.1 (Pre)hypertensive (> 80 mmHg) | 16 | 735 | Mean Difference (Random, 95% CI) | ‐1.98 [‐3.38, ‐0.57] |
| 2.2 Normotensive (< 80 mmHg) | 23 | 1037 | Mean Difference (Random, 95% CI) | ‐1.57 [‐2.54, ‐0.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| 1.1 Flavanol‐free control | 26 | 1116 | Mean Difference (Random, 95% CI) | ‐1.80 [‐3.46, ‐0.13] |
| 1.2 Low flavanol control | 14 | 688 | Mean Difference (Random, 95% CI) | ‐1.67 [‐4.03, 0.69] |
| 2 DBP Show forest plot | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| 2.1 Flavanol‐free control | 26 | 1116 | Mean Difference (Random, 95% CI) | ‐1.82 [‐2.95, ‐0.68] |
| 2.2 Low flavanol control | 13 | 656 | Mean Difference (Random, 95% CI) | ‐1.62 [‐2.56, ‐0.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| 1.1 Double‐blind | 23 | 1059 | Mean Difference (Random, 95% CI) | ‐0.95 [‐2.77, 0.86] |
| 1.2 Unblinded, single‐blinded | 17 | 745 | Mean Difference (Random, 95% CI) | ‐2.71 [‐4.66, ‐0.76] |
| 2 DBP Show forest plot | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| 2.1 Double‐blind | 21 | 927 | Mean Difference (Random, 95% CI) | ‐1.16 [‐2.05, ‐0.27] |
| 2.2 Unblinded, single‐blinded | 18 | 845 | Mean Difference (Random, 95% CI) | ‐2.33 [‐3.62, ‐1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 38 | 1762 | Mean Difference (Random, 95% CI) | ‐1.36 [‐2.79, 0.06] |
| 1.1 < 50 years | 18 | 726 | Mean Difference (Random, 95% CI) | ‐1.79 [‐4.05, 0.48] |
| 1.2 ≥ 50 years | 20 | 1036 | Mean Difference (Random, 95% CI) | ‐0.98 [‐2.87, 0.90] |
| 2 DBP Show forest plot | 37 | 1688 | Mean Difference (Random, 95% CI) | ‐1.62 [‐2.49, ‐0.76] |
| 2.1 < 50 years | 18 | 726 | Mean Difference (Random, 95% CI) | ‐2.01 [‐3.45, ‐0.58] |
| 2.2 ≥ 50 years | 19 | 962 | Mean Difference (Random, 95% CI) | ‐1.28 [‐2.32, ‐0.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 40 | 1804 | Mean Difference (Random, 95% CI) | ‐1.76 [‐3.09, ‐0.43] |
| 1.1 2 ‐ 4 week duration | 24 | 1043 | Mean Difference (Random, 95% CI) | ‐1.37 [‐3.23, 0.49] |
| 1.2 > 4 week duration | 16 | 761 | Mean Difference (Random, 95% CI) | ‐2.37 [‐4.30, ‐0.44] |
| 2 DBP Show forest plot | 39 | 1772 | Mean Difference (Random, 95% CI) | ‐1.76 [‐2.57, ‐0.94] |
| 2.1 2 ‐ 4 week duration | 23 | 1011 | Mean Difference (Random, 95% CI) | ‐1.55 [‐2.71, ‐0.39] |
| 2.2 > 4 week duration | 16 | 761 | Mean Difference (Random, 95% CI) | ‐2.04 [‐3.18, ‐0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 33 | 1482 | Mean Difference (Random, 95% CI) | ‐1.08 [‐2.60, 0.43] |
| 2 DBP Show forest plot | 33 | 1482 | Mean Difference (Random, 95% CI) | ‐1.37 [‐2.31, ‐0.43] |

