伤寒和副伤寒(肠)发热的快速诊断检测
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | |||
| Patient sampling | Prospective multi‐centre study Healthcare setting: primary, secondary, and tertiary healthcare centres Point of recruitment: inpatients and outpatients | ||
| Patient characteristics and setting | Countries: Indonesia Level of typhoid endemicity (Crump 2004): high Age: both adults and children Gender distribution: not stated Entry criteria: clinical suspicion of typhoid Sample size: 425 | ||
| Index tests | Name: latex agglutination assay, Royal Tropical Institute (KIT), Netherlands Biological sample: venous blood | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Retrospective analysis. Index tests performed on stored serum samples. Time interval not stated. | ||
| Comparative | |||
| Notes | The study authors report that two raters evaluated the reproducibility of 123 of the index tests. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: secondary Point of recruitment: not specified whether inpatient or outpatient | ||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: not specified Gender distribution: not specified Entry criteria: fever > 4 days and clinical suspicion of typhoid Sample size: 83 | ||
| Index tests | Enterocheck WB | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Prospective analysis.Time interval not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary paediatric hospital. Point of recruitment: not specified whether inpatients or outpatients | ||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: mean age 6.25 years, SD 3.86 years Gender distribution: male 52% female 48% Entry criteria: children between 6 months and 18 years of age, and fever ≥ 3 days, and clinical features of typhoid Sample size: 450 | ||
| Index tests | Enterocheck WB | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Prospective study. | ||
| Comparative | |||
| Notes | Index tests were used on whole blood or serum, but the study authors did not specify the numbers of each. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: not specified whether inpatient or outpatient | ||
| Patient characteristics and setting | Countries: Bangladesh Level of typhoid endemicity (Crump 2004): high Age: not specified Gender distribution: not specified Entry criteria: clinical suspicion of typhoid fever, and febrile non‐typhoid controls, and healthy controls Data extraction was based on febrile non‐typhoid controls only Sample size: 100 | ||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Prospective study. Timing not stated. | ||
| Comparative | |||
| Notes | Healthy (afebrile) controls also recruited. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: paediatric inpatient | ||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: children (not formally stated) Gender distribution: not stated Entry criteria: 6 months to 12 years, and fever > 4 days, and clinical suspicion of typhoid Sample size: 145 | ||
| Index tests | Typhidot‐M | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Prospective study. Timing not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: paediatric inpatients | ||
| Patient characteristics and setting | Countries: Pakistan Level of typhoid endemicity (Crump 2004): high Age: children (not formally stated) Gender distribution: male 41% female 49% Entry criteria: clinical suspicion of typhoid fever Sample size: 97 | ||
| Index tests | Typhidot and Typhidot‐M | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: Peripheral blood culture and/or bone marrow culture | ||
| Flow and timing | Prospective study. Timing unclear. | ||
| Comparative | |||
| Notes | Malaysian Biodiagnostic Research (Kuala Lumpur, Malaysia) donated rapid diagnostic tests (RDTs) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study as part of a vaccine surveillance programme Healthcare settings: primary, secondary, and tertiary centres (85 in total) Point of recruitment: inpatient and outpatient | ||
| Patient characteristics and setting | Countries: China Level of typhoid endemicity (Crump 2004): medium Age: not specified Gender distribution: not specified Entry criteria: aged between 5 and 60 years with a history of fever ≥ 3 days Sample size: 1874 | ||
| Index tests | Typhidot‐M TUBEX | ||
| Target condition and reference standard(s) | Target condition: both Salmonella Typhi and Salmonella Paratyphi A Reference standard: peripheral blood culture (8 mL) | ||
| Flow and timing | Prospective multicentre study as part of a vaccine surveillance programme. Index tests performed in real time during patient recruitment. | ||
| Comparative | |||
| Notes | Reported diagnostic test accuracy for detecting cases of Salmonella Paratyphi A as well as Salmonella Typhi. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study as part of a community‐based typhoid surveillance study and mass vaccination programme Healthcare setting: primary, secondary, and tertiary (7 health outposts in total) Point of recruitment: inpatient and outpatient | ||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: not specified Gender distribution: not specified Entry criteria: fever ≥ 3 days Sample size: 6697 plus 172 healthy controls. Only a subset of participants had TUBEX or Typhidot testing. Control participants for 2x2 were based on febrile participants and did not include healthy controls. | ||
| Index tests | TUBEX Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Community‐based typhoid surveillance study and mass vaccination programme. Timing of sample testing unclear. | ||
| Comparative | |||
| Notes | Not all patients received the same index test. If Salmonella Paratyphi was isolated, study authors classified this as a true negative. If a participant was both blood culture‐positive and malaria film‐positive, the study authors excluded them from the analysis (n = 1). Study authors only included a small number or participants in the analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: secondary and tertiary (5 fever hospitals) Point of recruitment: inpatients | ||
| Patient characteristics and setting | Countries: Egypt Level of typhoid endemicity (Crump 2004): medium Age: over the age of 4 years Gender distribution: not stated Entry criteria: fever lasting for at least 2 days, or febrile ≥ 38.5°C on admission, with a clinical suspicion of typhoid fever or brucellosis Sample size: 2897 | ||
| Index tests | TUBEX Typhidot‐M | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standards: peripheral blood culture | ||
| Flow and timing | Divided into 3 main groups of 'typhoid' (cases), 'febrile non‐typhoid' (controls), and healthy controls. Timing unclear. | ||
| Comparative | |||
| Notes | Case: control design. Excluded febrile cases of diarrhoea and pneumonia. Study authors classified a Widal Test titre of > 320 as a typhoid case | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: secondary (3) and tertiary (1) Point of recruitment: inpatient | ||
| Patient characteristics and setting | Countries: Indonesia Level of typhoid endemicity (Crump 2004): high Age: not stated Gender distribution: not stated Entry criteria: clinical suspicion of typhoid (127) and 80 febrile 'non‐typhoids' Sample size: 207 | ||
| Index tests | Dipstick assay from the Royal Tropical Institute, Netherlands (KIT) | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standards: peripheral blood culture or bone marrow culture, or both | ||
| Flow and timing | Prospective multi‐centre study. Timing unclear. | ||
| Comparative | |||
| Notes | Not all patients had both bone marrow culture and blood culture. Study authors classified Isolation of Salmonella Paratyphi as a non‐typhoid case. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Retrospective single‐centre study Healthcare setting: tertiary Point of recruitment: not specified whether inpatient or outpatient | ||
| Patient characteristics and setting | Countries: Malaysia Level of typhoid endemicity (Crump 2004): medium Age: not specified Gender distribution: not specified Entry criteria: Widal test titres greater than 640 Sample size: 144 | ||
| Index tests | Typhidot PanBio | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standards: peripheral blood culture or stool culture, or both | ||
| Flow and timing | Retrospective analysis of stored samples. Timing unclear. | ||
| Comparative | |||
| Notes | Inclusion criteria based on Widal Test titres ‐ limiting. Reference standard included isolation of Salmonella Typhi from stool Index tests were performed retrospectively on stored samples. Typhidot‐M performed on only small subset of samples. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: primary, secondary, and tertiary Point of recruitment: inpatient and outpatient | ||
| Patient characteristics and setting | Countries: Indonesia and Kenya Level of typhoid endemicity (Crump 2004): high Age: not specified Gender distribution: not specified Entry criteria: clinical suspicion of typhoid, and other febrile illnesses (controls), and healthy afebrile controls Sample size: 504 | ||
| Index tests | Dipstick Assay, Royal Tropical Institute (KIT), Netherlands | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Prospective recruitment at multiple sites. Timing unclear. | ||
| Comparative | |||
| Notes | Case‐control study design from 2 geographical locations, including controls from a non‐endemic area (Netherlands). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Propspective multicentre study Healthcare setting: Primary, secondary, and tertiary Point of recruitment: inpatient and outpatient | ||
| Patient characteristics and setting | Countries: Indonesia Level of typhoid endemicity (Crump 2004): high Age: not specified Gender distribution: not specified Entry criteria: clinical suspicion of typhoid Sample size: 473 | ||
| Index tests | Dipstick assay, Royal Tropical Institute (KIT) Netherlands | ||
| Target condition and reference standard(s) | Target condition:Salmonella Typhi Reference standard: peripheral blood culture (5 mL) | ||
| Flow and timing | Prospective multi‐centre study. Timing unclear. | ||
| Comparative | |||
| Notes | There is a potential overlap of patients/data between the paper by Hatta 2002a. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: not stated | ||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: mixed Gender distribution: 58% Male 42% Female Entry criteria: history of fever more than 2 to 3 days duration and a clinical diagnosis of enteric fever | ||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (volume not stated) | ||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | No sources of funding declared. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: secondary and tertiary Point of recruitment: inpatients | ||
| Patient characteristics and setting | Countries: Vietnam Level of typhoid endemicity (Crump 2004): high Age: adults and children Gender distribution: not specified Entry criteria: Salmonella Typhi on blood culture, and febrile controls, and healthy controls Sample size: 290 | ||
| Index tests | TUBEX Dipstick Assay, Royal Tropical Institute (KIT), Netherlands | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Prospective multicentre study. Timing unclear. | ||
| Comparative | |||
| Notes | Mostly children recruited. Sample size 290 but only 127 analysed. Case control design. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary international reference centre Point of recruitment: not stated | ||
| Patient characteristics and setting | Countries: Bangladesh Level of typhoid endemicity (Crump 2004): high Age: mixed Gender distribution: 52% male 48% female Entry criteria: non‐pregnant, 1 to 59 years of age, fever ≥ 39.0°C for 3 to 7 days duration, lacking obvious alternative diagnosis | ||
| Index tests | TUBEX Typhidot TPTest | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (3 to 5 mL) | ||
| Flow and timing | Prospective study at a tertiary reference centre. Timing unclear. | ||
| Comparative | |||
| Notes | Unable to clarify whether patients in Group VI (visceral leishmaniasis/tuberculosis) also received a blood culture. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: tertiary (5 infectious diseases hospitals) Point of recruitment: inpatients | ||
| Patient characteristics and setting | Countries: Egypt Level of typhoid endemicity (Crump 2004): medium Age: not specified Gender distribution: not specified Entry criteria: febrile in‐patients meeting pre‐determined case definitions Sample size: 85 | ||
| Index tests | Dipstick assay, Royal Tropical Institute (KIT), Netherlands | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Prospective multicentre study. Samples tested retrospectively 2 to 3 months after recruitment. | ||
| Comparative | |||
| Notes | Part of a brucellosis diagnostic study. Samples tested retrospectively 2 to 3 months later. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Tertiary healthcare setting Point of recruitment: unclear whether inpatient, outpatient, or both | ||
| Patient characteristics and setting | Country: India Level of typhoid endemicity (Crump 2004): high Age(s): unclear Gender distribution: unclear Four pre‐determined groups for entry into the study:
Sample size: 150 recruited (60 analysed) | ||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | Indian Association Medical Microbiology External Quality Assurance Scheme laboratory accreditation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: both inpatients and outpatients | ||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Ages: unclear Gender distribution: unclear Entry criteria: clinical suspicion of typhoid fever Sample size: 563 | ||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | Study authors excluded one case of Salmonella paratyphi A. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary infectious diseases hospital Point of recruitment: inpatients | ||
| Patient characteristics and setting | Countries: Philippines Level of typhoid endemicity (Crump 2004): high Age: both adults and children Gender distribution: 53.6% (male) 46.4% (female) Entry criteria: febrile patients with a clinical suspicion of typhoid fever Sample size: 177 | ||
| Index tests | TUBEX Typhidot SD Bioline Mega Salmonella | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: secondary and tertiary hospitals Point of recruitment: inpatient | ||
| Patient characteristics and setting | Countries: South Africa and Tanzania Level of typhoid endemicity (Crump 2004): medium Age: both adults and children Gender distribution: 54.3% (male) 45.7% (female) Entry criteria: South Africa ‐ clinically suspected typhoid fever with no pre‐treatment with antibiotics Tanzania ‐ unselected febrile illnesses, but only those with clinical suspicion of typhoid fever were recruited Sample size: 92 | ||
| Index tests | TUBEX Typhidot | ||
| Target condition and reference standard(s) | Target condition:Salmonella Typhi Reference standard: peripheral blood culture | ||
| Flow and timing | Prospective multicentre study | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Retrospective single centre study Healthcare setting: tertiary hospital Point of recruitment: both inpatient and outpatient | ||
| Patient characteristics and setting | Countries: Pakistan Level of typhoid endemicity (Crump 2004): high Age: unclear Gender distribution: unclear Entry criteria: patients with clinical suspicion of typhoid who went on to have the index RDT Sample size: 1760 (128 analysed) | ||
| Index tests | Typhidot‐M | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture, or bone marrow culture, or both | ||
| Flow and timing | Retrospective analysis on stored samples. Timing unclear. | ||
| Comparative | |||
| Notes | Unable to distinguish which cases were bone marrow positive. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: unclear | ||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: mixed Gender distribution: not stated Entry criteria: cases were febrile patients with a positive blood culture for Salmonella Typhi. Healthy afebrile controls | ||
| Index tests | TUBEX Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (5 mL) | ||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | Case control study | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single‐centre study Healthcare setting: tertiary Point of recruitment: not stated | ||
| Patient characteristics and setting | Countries: Pakistan Level of typhoid endemicity (Crump 2004): high Age: adults (> 18 years) Gender distribution: not stated Entry criteria: aged 18 to 40 years; fever < 14 days; clinical features suggesting typhoid fever; no history of antimicrobial therapy or typhoid immunization in the recent past | ||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (volume not stated) | ||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | No declaration of funding. Entry criteria could exclude numerous cases of typhoid. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Retrospective multi‐centre study Healthcare settings: secondary Point of recruitment: both inpatient and outpatient | ||
| Patient characteristics and setting | Countries: Tanzania Level of typhoid endemicity (Crump 2004): medium Age: children between the ages of 2 months and 14 years Gender distribution: unclear Entry criteria: selected samples from a fever surveillance study Surveillance study entry criteria: fever > 3 days or those matching set clinical severity criteria | ||
| Index tests | TUBEX | ||
| Target condition and reference standard(s) | Target condition:Salmonella Typhi Reference standard(s): peripheral blood culture | ||
| Flow and timing | Retrospective analysis on stored samples. Timing unclear. | ||
| Comparative | |||
| Notes | Only blood culture positive patients included. Samples from 2 different patient populations | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study (3 hospitals within a single province) Healthcare setting: secondary Point of recruitment: both inpatients and outpatients | ||
| Patient characteristics and setting | Countries: Thailand Level of typhoid endemicity (Crump 2004): high Age: children under 15 years of age Gender distribution: not recorded Entry criteria: any febrile illness in children under 15 years of age | ||
| Index tests | SD Bioline | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (volume not stated) | ||
| Flow and timing | Prospective recruitment with a retrospective analysis of stored samples. | ||
| Comparative | |||
| Notes | Outbreak situation in Songkhla Province. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single‐centre study Healthcare setting: tertiary Point of recruitment: inpatient | ||
| Patient characteristics and setting | Countries: Bangladesh Level of typhoid endemicity (Crump 2004): high Age: mixed Gender distribution: 173 males; 127 females Entry criteria: > 6 months of age with < 2 weeks fever and a documented fever > 38 | ||
| Index tests | Test‐It‐Typhoid (KIT immunochromatographic lateral flow assay) SD Bioline CTK Biotech Onsite | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (1 to 12 mL in children, 5 to 12mL in adults) or blood nucleic acid amplification (polymerase chain reaction (PCR)), or both | ||
| Flow and timing | Prospective recruitment with retrospective testing of stored samples. | ||
| Comparative | |||
| Notes | Two review authors (LW and CMP) are authors on this study. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Retrospective single centre analysis study Healthcare setting: tertiary Point of recruitment: not stated | ||
| Patient characteristics and setting | Countries: Pakistan Level of typhoid endemicity (Crump 2004): high Age: mixed Gender distribution: 59 males/86 females Entry criteria: unselected fever of greater than 3 days | ||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (volume not specified) | ||
| Flow and timing | Retrospective analysis of stored samples. Timing unclear. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: inpatient | ||
| Patient characteristics and setting | Countries: Cambodia Level of typhoid endemicity (Crump 2004): high Age: children over 6 months and under 16 years Gender distribution: unclear Entry criteria: documented fever of > 38°C Sample size: 500 | ||
| Index tests | Immunochromatographic lateral flow assay, KIT (Test‐It‐Typhoid prototype) | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture | ||
| Flow and timing | Prospective single centre study. Retrospective testing of stored samples. | ||
| Comparative | |||
| Notes | Score of 2+ or more considered positive. We contacted the study authors for further details based on the abstract. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: primary community clinics Point of recruitment: outpatients | ||
| Patient characteristics and setting | Countries: Bangladesh Level of typhoid endemicity (Crump 2004): high Age: both adults and children Gender distribution: 51% (male) 49% (female) Entry criteria: fever for any duration in < 5 years / > 3 days in > 5years and a documented fever of 38.0°C Sample size: 867 | ||
| Index tests | TUBEX Typhidot | ||
| Target condition and reference standard(s) | Target condition:Salmonella Typhi Reference standard(s): peripheral blood culture | ||
| Flow and timing | Prospective multicentre study. Timing unclear. | ||
| Comparative | |||
| Notes | Study authors classified 139 results that were indeterminate as negative. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: secondary and tertiary Point of recruitment: inpatients | ||
| Patient characteristics and setting | Countries: Vietnam Level of typhoid endemicity (Crump 2004): high Age: both adults and children Gender distribution: 56.9% (male) 43.1% (female) Entry criteria: > 4 days of fever, and greater than 3 years old and controls with other febrile illnesses Sample size: 79 (59 patients and 20 controls) | ||
| Index tests | TUBEX Typhidot Multi‐Test Dip‐S‐Tick | ||
| Target condition and reference standard(s) | Target condition:Salmonella Typhi Reference standard(s): peripheral blood culture | ||
| Flow and timing | Prospective multicentre study. Samples processed at a different site. Timing unclear. | ||
| Comparative | |||
| Notes | Different processing sites for blood culture, that is not in the same laboratory | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: inpatient | ||
| Patient characteristics and setting | Countries: Indonesia Level of typhoid endemicity (Crump 2004): high Age: unclear Gender distribution: unclear Entry criteria: clinical suspicion of typhoid fever Sample size: 209 | ||
| Index tests | Immunochromatographic lateral flow assay, Royal Tropical Institute (KIT), Netherlands | ||
| Target condition and reference standard(s) | Target condition:Salmonella Typhi Reference standard(s): peripheral blood culture and Widal Test | ||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | Study authors compared diagnostic test results of the ICT with both blood culture and the Widal Test. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Single centre retrospective analysis study Healthcare setting: tertiary Point of recruitment: both inpatients and outpatients | ||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: unclear Gender distribution: unclear Entry criteria: clinical suspicion of enteric fever | ||
| Index tests | Typhidot‐M Enteroscreen‐IgM | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (volume not stated) | ||
| Flow and timing | Retrospective analysis of stored samples. Timing unclear. | ||
| Comparative | |||
| Notes | Study authors classified Salmonella Paratyphi blood culture positive cases as disease‐negative. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: outpatients | ||
| Patient characteristics and setting | Countries: Bangladesh Level of typhoid endemicity (Crump 2004): high Age: children Gender distribution: unclear Entry criteria: fever > 3 days but < 7 days Sample size: 243 | ||
| Index tests | TUBEX | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture | ||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: not stated | ||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: not clear Gender distribution: not stated Entry criteria: clinical suspicion of typhoid fever | ||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (volume not specified) | ||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: secondary and tertiary hospitals Point of recruitment: outpatients | ||
| Patient characteristics and setting | Country: Papua New Guinea Level of typhoid endemicity (Crump 2004): high Age: adults and children Gender distribution: 51% (male) 49% (female) Entry criteria: febrile patients with axillary temp > 37.5°C and > 2 days of fever (or clinical suspicion of typhoid fever) Sample size: 530 (500 analysed) | ||
| Index tests | TUBEX Typhidot TyphiRapid‐Tr02 | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture and PCR | ||
| Flow and timing | Prospective multicentre study. Timing unclear. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multi‐centre study Healthcare setting: primary Point of recruitment: outpatient | ||
| Patient characteristics and setting | Countries: Zimbabwe Level of typhoid endemicity (Crump 2004): medium Age: mixed Gender distribution: not stated Entry criteria: 'typical signs and symptoms of typhoid' | ||
| Index tests | TUBEX On‐Site Typhoid IgG/IgM Combo | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (3 to 5 mL) | ||
| Flow and timing | Prospective multicentre study. Timing unclear. | ||
| Comparative | |||
| Notes | Diagnostic test accuracy data not provided in published paper but supplied separately by the corresponding authors. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Abbreviations: PCR: polymerase chain reaction; RDT: rapid diagnostic test.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Meta‐analysis from an International Congress on Infectious Diseases (ICID) poster abstract | |
| 4 different types of Widal Test used, that is, not a new rapid diagnostic test (RDT) | |
| Antigen detection was neither a commercially‐available rapid diagnostic test or a prototype. | |
| We were unable to extract specificity and sensitivity data | |
| We were unable to extract sensitivity and specificity data | |
| Not a commercially available test ('Dot Blot' Test from Bio‐Rad Laboratories, Richmond, CA) | |
| We could only extract data for patients with Gram‐negative rod positive blood cultures. The study authors did not present data on RDT performance on culture negative patients, therefore we could not perform analyses. | |
| Not a commercially available test (an unspecified Indirect dot blot ELISA) | |
| Not a commercially available RDT. A range of Salmonella serodiagnostic tests were performed at a UK reference laboratory on sera from UK residents returning from travelling abroad. | |
| Not a commercially available test. "COAG" co‐agglutination test produced in‐house by Indian tertiary hospital laboratory. | |
| We were unable to extract data about performance of test in blood culture positive patients. DOT EIA (early Typhidot‐M). | |
| We were unable to extract relevant sensitivity and specificity data. DOT‐EIA (early Typhidot‐M). | |
| Evaluates a test for detecting chronic carriage rather than acute typhoid (enteric) fever | |
| Not a commercially available test (passive haemagglutination). | |
| Not a commercially available test ‐ candidate created by SPAN Diagnostics (India) | |
| We were unable to determine which blood culture positive patients were also positive on the urinary COAG tests. | |
| We were unable to extract sensitivity and specificity data as no cut‐offs mentioned for haemagglutination. | |
| Not a commercial test: ELISA antibody detection from urine | |
| Letter outlining use of TUBEX to detect non‐typoidal Salmonella infections (e.g. S. enteritidis) | |
| Comparison of two types of Widal Test | |
| Evaluation of a Widal slide agglutination test, a variant of an existing diagnostic test. | |
| Evaluation of a slide agglutination Widal Test | |
| Paired serum samples rather than a single use RDT | |
| Dot Enzyme Immmunoassay (EIA) ‐ early Typhidot‐M. We were unable to extract sensitivity or specificity data. | |
| Not a commercial test: passive bacterial agglutination | |
| Not a commercial test: reverse passive haemagglutination assay (possible RDT candidate) | |
| Not a commercial test: Latex Agglutination Test | |
| No actual RDT evaluated. Study compared blood culture with the Widal Test. | |
| Not commercially‐available rapid diagnostic tests. In‐house latex agglutination (LAT) and coagglutination (COAG) tests which are not prototypes. | |
| The serodiagnostic tests evaluated were not commercially available point‐of‐care tests. | |
| The TPTest is not a commercially‐available RDT | |
| The study detailed the assessment of the human immune response rather than diagnostic test accuracy | |
| Letter to the editor about a single case ‐ not a diagnostic study | |
| Not a commercial test: Indirect ELISA IgM antibody detection | |
| No commercial RDTs were used in the febrile illness study, only laboratory serology for Salmonella Typhi | |
| Reference standard inadequately described, and not all patients received any form of reference standard. TUBEX. | |
| Use of TUBEX to determine cases as part of active surveillance during an outbreak. We were unable to extract any data regarding diagnostic test accuracy. | |
| No data of index test (Typhidot) positivity in non‐culture positive patients. | |
| Not a commercial test. In‐house co‐agglutination test | |
| This study only included clinical typhoid or conifrmed typhoid cases. Study authors excluded patients currently receiving or who had recently received antimicrobials. We were unable to extract data related to diagnostic test accuracy. | |
| We were unable to extract data index test data (Typhidot‐M) from control (non‐typhoid fever) group | |
| Variety of serological diagnostic tests used during investigation of an acute outbreak in Uganda. No specific RDT used. | |
| The monoclonal antibody‐based dot‐blot ELISA evaluated is not a commercially‐available rapid diagnostic test. | |
| Test based on adherence IgM "capture" ‐ not commercially available. Confirmed typhoid case was blood or stool culture positive, or both. | |
| Not a commercially available RDT: latex agglutination to a) Typhi Vi; and b) Barber protein | |
| Not a commercial test: passive haemagglutination test (PHA) We were unable to extract sensitivity and specificity data | |
| Evaluation of general bacterial microarray/genetics rather than point‐of‐care testing | |
| RDT development rather than evaluation of test accuracy | |
| Non‐commercial tests. We were unable to extract sensitivity and specificity data. | |
| Repeat publication of data published by Olsen 2004 from Vietnam. | |
| TUBEX in asthmatics. We were unable to extract diagnostic test data. | |
| Not a commercial test: latex agglutination. | |
| Widal Test evaluation, not a commercial RDT | |
| We were unable to extract sensitivity and specificity data | |
| Not a commercial test: urinary co‐agglutination technique | |
| Data from this study had already been included in Moore 2014 | |
| We were unable to extract specificity data | |
| Not a commercial test: Salivary IgA to lipopolysaccharide (LPS) |
Abbreviations: RDT: rapid diagnostic test.
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 Typhidot. Antibody: IgM or as reported. 1 result per study Show forest plot | 17 | 3691 |
| Test 1 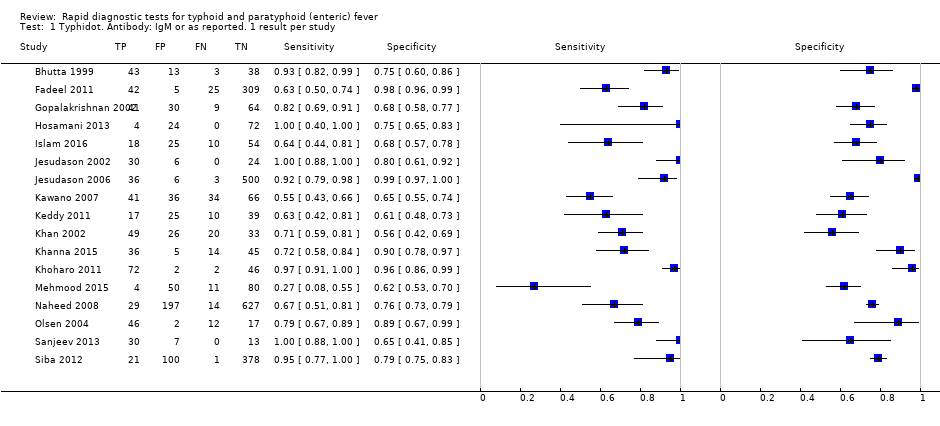 Typhidot. Antibody: IgM or as reported. 1 result per study. | ||
| 2 Typhidot. Antibody: IgM or as reported. Reference: BC Show forest plot | 15 | 3466 |
| Test 2  Typhidot. Antibody: IgM or as reported. Reference: BC. | ||
| 3 Typhidot. Antibody: IgM or as reported. Reference: BC and BM Show forest plot | 2 | 225 |
| Test 3  Typhidot. Antibody: IgM or as reported. Reference: BC and BM. | ||
| 4 Typhidot. Antibody: IgM or as reported. Reference: BC and PCR Show forest plot | 1 | 500 |
| Test 4 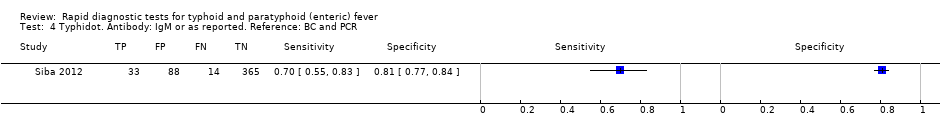 Typhidot. Antibody: IgM or as reported. Reference: BC and PCR. | ||
| 5 Typhidot. Antibody: IgM or as reported. Indeterminates reported Show forest plot | 6 | 1721 |
| Test 5  Typhidot. Antibody: IgM or as reported. Indeterminates reported. | ||
| 6 Typhidot. Antibody: IgM or as reported. Indeterminates not reported Show forest plot | 11 | 1970 |
| Test 6  Typhidot. Antibody: IgM or as reported. Indeterminates not reported. | ||
| 7 Typhidot‐M. Antibody: IgM Show forest plot | 6 | 3334 |
| Test 7 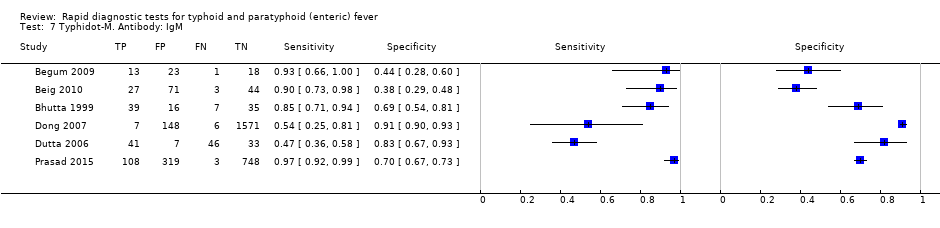 Typhidot‐M. Antibody: IgM. | ||
| 8 Typhi rapid Tr‐02. Reference: BC. Antibody: IgM Show forest plot | 1 | 500 |
| Test 8  Typhi rapid Tr‐02. Reference: BC. Antibody: IgM. | ||
| 9 Typhi rapid Tr‐02. Reference: BC & PCR. Antibody: IgM Show forest plot | 1 | 500 |
| Test 9 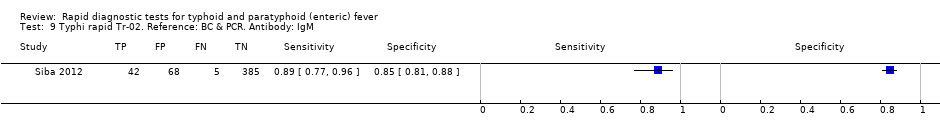 Typhi rapid Tr‐02. Reference: BC & PCR. Antibody: IgM. | ||
| 10 Typhidot all tests 1 result per study Show forest plot | 22 | 6928 |
| Test 10  Typhidot all tests 1 result per study. | ||
| 11 TUBEX. Reference:BC Show forest plot | 14 | 4885 |
| Test 11  TUBEX. Reference:BC. | ||
| 12 TUBEX. Reference: BC & PCR Show forest plot | 1 | 500 |
| Test 12  TUBEX. Reference: BC & PCR. | ||
| 13 TUBEX 1 result per study Show forest plot | 14 | 4885 |
| Test 13  TUBEX 1 result per study. | ||
| 14 KIT ICT. Reference:BC. Threshold > 1+ Show forest plot | 2 | 709 |
| Test 14  KIT ICT. Reference:BC. Threshold > 1+. | ||
| 15 KIT ICT. Reference: BC & PCR. Threshold > 1+ Show forest plot | 2 | 800 |
| Test 15  KIT ICT. Reference: BC & PCR. Threshold > 1+. | ||
| 16 KIT latex agglutination. Threshold > 1+ Show forest plot | 1 | 425 |
| Test 16  KIT latex agglutination. Threshold > 1+. | ||
| 17 KIT Dipstick. Threshold > 1+ Show forest plot | 5 | 1394 |
| Test 17  KIT Dipstick. Threshold > 1+. | ||
| 18 KIT ICT. Threshold > 1+ Show forest plot | 3 | 1009 |
| Test 18  KIT ICT. Threshold > 1+. | ||
| 19 KIT all tests. Threshold > 1+. One result per study. Show forest plot | 9 | 2828 |
| Test 19  KIT all tests. Threshold > 1+. One result per study.. | ||
| 20 KIT all tests. Threshold > 2+ studies only Show forest plot | 5 | 1607 |
| Test 20  KIT all tests. Threshold > 2+ studies only. | ||
| 21 Enterocheck WB Show forest plot | 2 | 533 |
| Test 21  Enterocheck WB. | ||
| 22 PanBio Show forest plot | 1 | 144 |
| Test 22 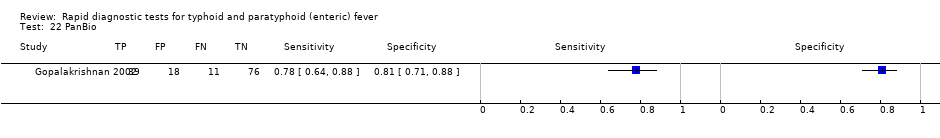 PanBio. | ||
| 23 SD Bioline. Antibody: IgG Show forest plot | 3 | 1669 |
| Test 23  SD Bioline. Antibody: IgG. | ||
| 24 SD Bioline. Antibody: IgM Show forest plot | 3 | 1590 |
| Test 24 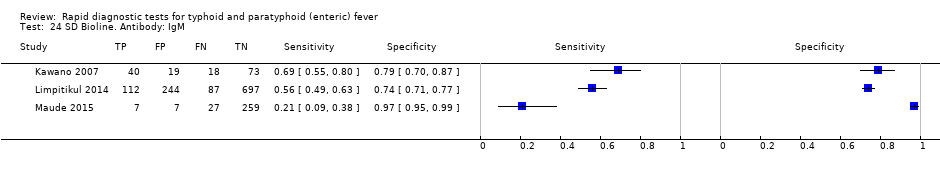 SD Bioline. Antibody: IgM. | ||
| 25 SD Bioline Antibody: IgM and IgG Show forest plot | 1 | 300 |
| Test 25  SD Bioline Antibody: IgM and IgG. | ||
| 26 Mega Salmonella. Antibody: IgG Show forest plot | 1 | 177 |
| Test 26  Mega Salmonella. Antibody: IgG. | ||
| 27 Mega Salmonella. Antibody: IgM Show forest plot | 1 | 177 |
| Test 27  Mega Salmonella. Antibody: IgM. | ||
| 28 Multi‐Test Dip‐S‐Tick Show forest plot | 1 | 75 |
| Test 28  Multi‐Test Dip‐S‐Tick. | ||
| 29 Enteroscreen Show forest plot | 1 | 1521 |
| Test 29  Enteroscreen. | ||
| 30 Onsite Typhoid Combo CTK Biotech Show forest plot | 2 | 436 |
| Test 30 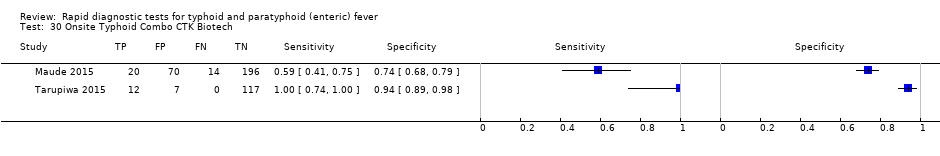 Onsite Typhoid Combo CTK Biotech. | ||
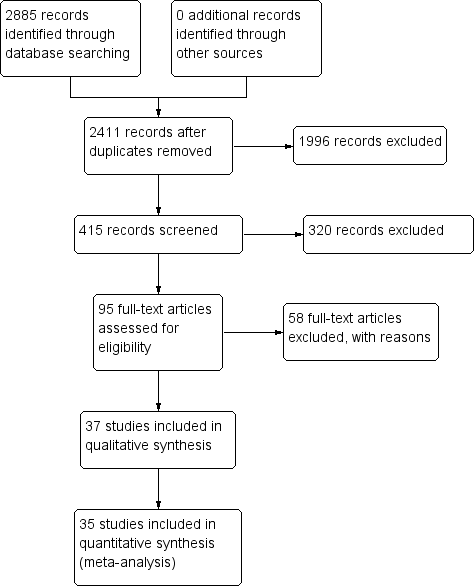
PRISMA flow diagram.

Summary receiver operating characteristic plot: Enterocheck WB, PanBio, SD Bioline, Mega Salmonella, Multi‐Test Dip‐S‐Tick.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
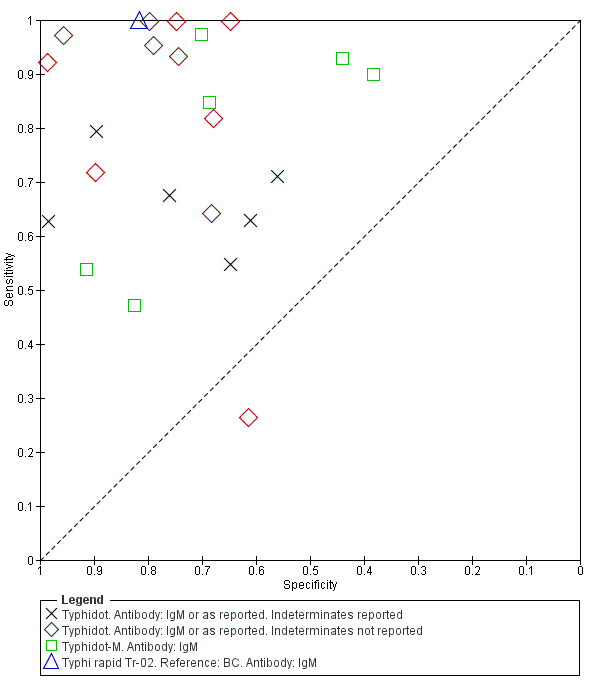
Summary ROC Typhidot all test types.
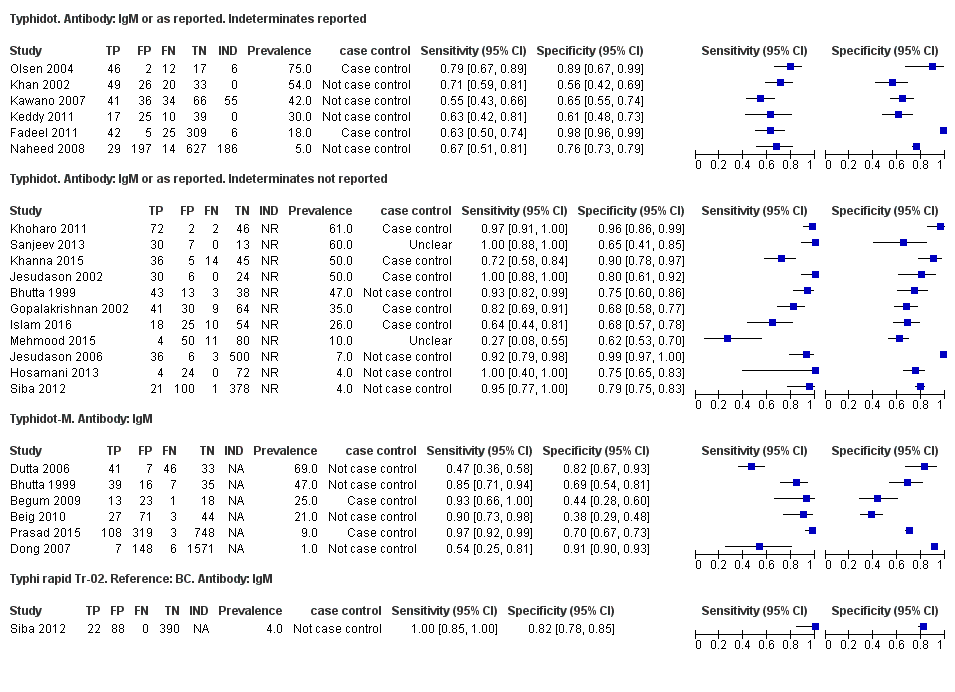
Forest plots for Typhidot all test types.

Summary receiver operating characteristic plot of tests: Typhidot and Typhidot‐M by reference test.
Abbreviations: BC: blood culture; BM: bone marrow; BC & PCR: blood culture and polymerase chain reaction.

Summary receiver operating characteristic plot of test: TUBEX. Reference test: Blood culture. One result per study.

Forest plot of TUBEX. Reference test blood culture.

Summary receiver operating characteristic plot: TUBEX by case control design.
Abbreviation: BC: blood culture.

Summary receiver operating characteristic plot: TUBEX by reference test
Abbreviations: BC: blood culture; BC & PCR: blood culture and polymerase chain reaction.
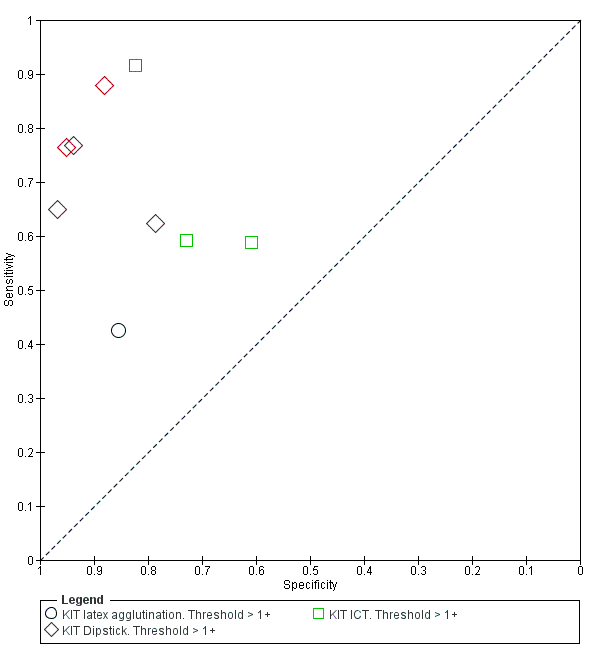
Summary receiver operating characteristic plot: KIT all test types. Threshold > 1+.
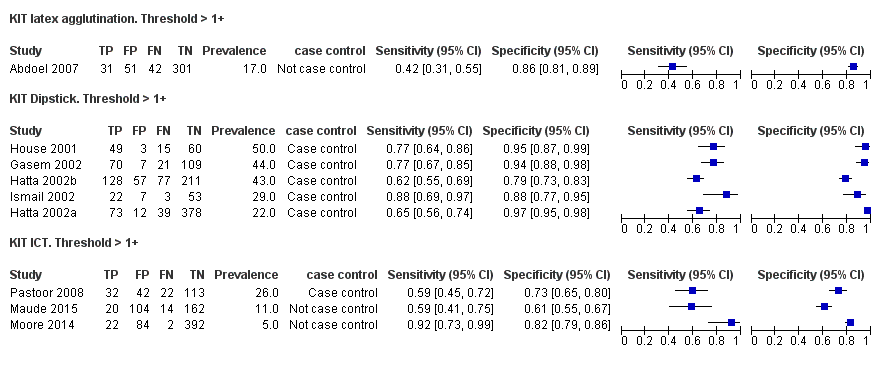
Forest plot of tests: KIT Threshold > 1+ by test type. Reference test: blood culture.

Summary receiver operating characteristic plot: KIT test by threshold > 1+ and > 2+.

Summary receiver operating characteristic plot: KIT ICT by reference test.
Abbreviations: BC: blood culture; BC & PCR: blood culture and polymerase chain reaction.
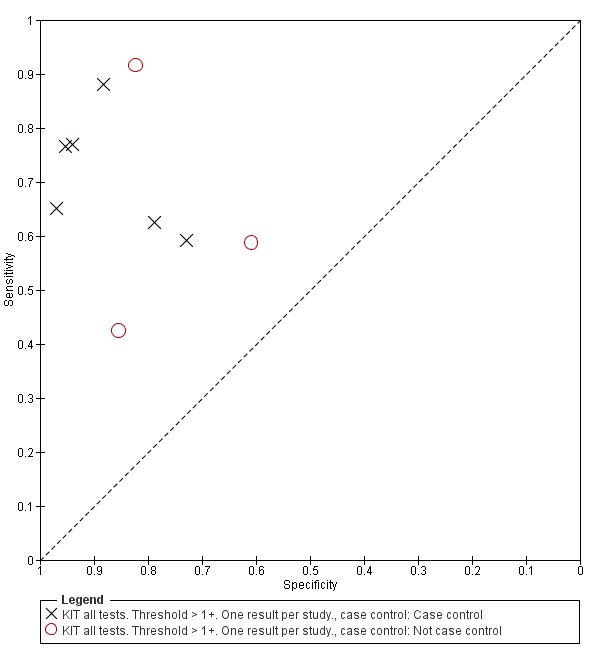
Summary receiver operating characteristic plot: KIT by case control (All test types. Threshold >1+).
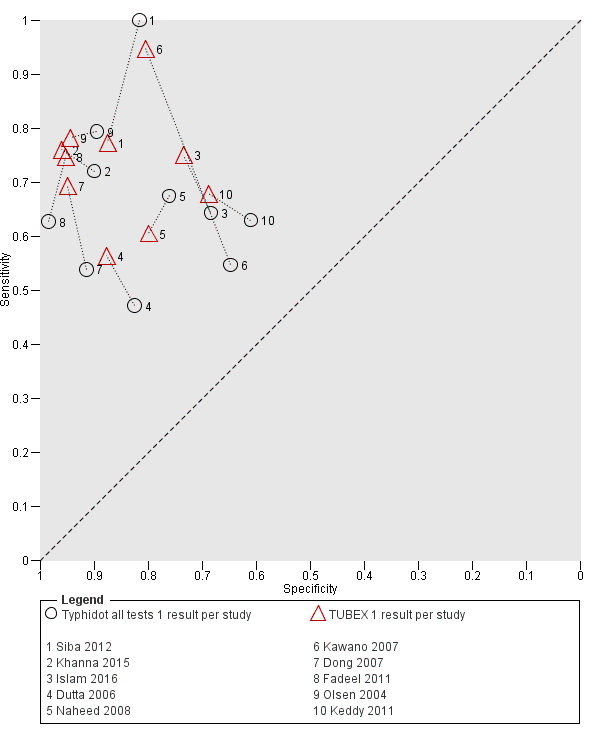
Summary receiver operating characteristic plot: Typhidot versus TUBEX. Paired studies only. One result per index test per study.

Summary receiver operating characteristic: TUBEX versus KIT. Paired results. One result per index per study.

Summary receiver operating characteristic plot: Typhidot versus TUBEX tests. One result per index test per study.
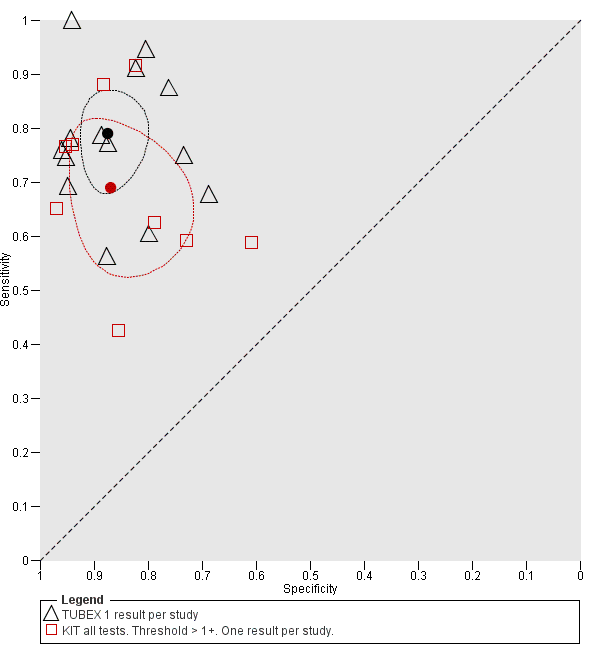
Summary receiver operating characteristic plot: TUBEX versus Test‐it Typhoid (KIT) tests. One result per index test per study.

Summary receiver operating characteristic: Typhidot versus KIT. No paired studies. One result per index per study.

Summary receiver operating characteristic plot: Typhidot tests by case control design.

Typhidot. Antibody: IgM or as reported. 1 result per study.
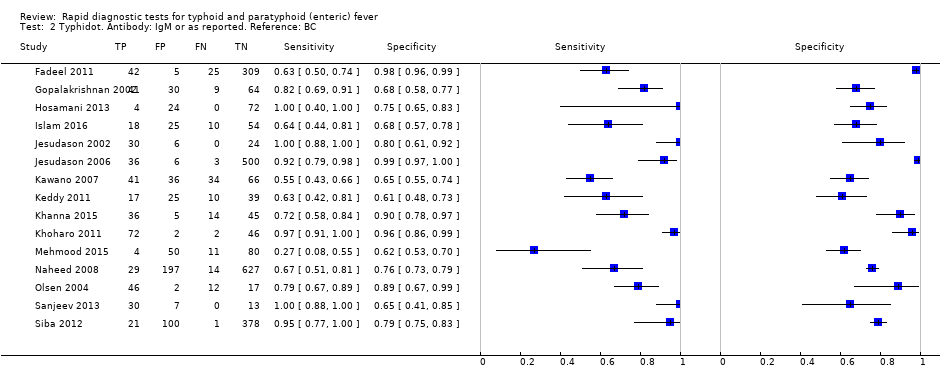
Typhidot. Antibody: IgM or as reported. Reference: BC.

Typhidot. Antibody: IgM or as reported. Reference: BC and BM.

Typhidot. Antibody: IgM or as reported. Reference: BC and PCR.

Typhidot. Antibody: IgM or as reported. Indeterminates reported.

Typhidot. Antibody: IgM or as reported. Indeterminates not reported.

Typhidot‐M. Antibody: IgM.

Typhi rapid Tr‐02. Reference: BC. Antibody: IgM.

Typhi rapid Tr‐02. Reference: BC & PCR. Antibody: IgM.

Typhidot all tests 1 result per study.

TUBEX. Reference:BC.

TUBEX. Reference: BC & PCR.
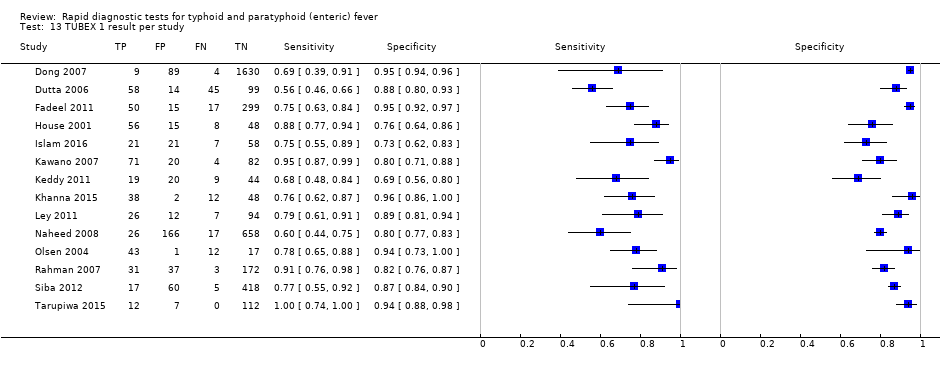
TUBEX 1 result per study.

KIT ICT. Reference:BC. Threshold > 1+.

KIT ICT. Reference: BC & PCR. Threshold > 1+.
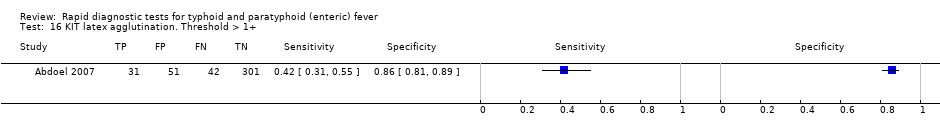
KIT latex agglutination. Threshold > 1+.

KIT Dipstick. Threshold > 1+.

KIT ICT. Threshold > 1+.

KIT all tests. Threshold > 1+. One result per study..
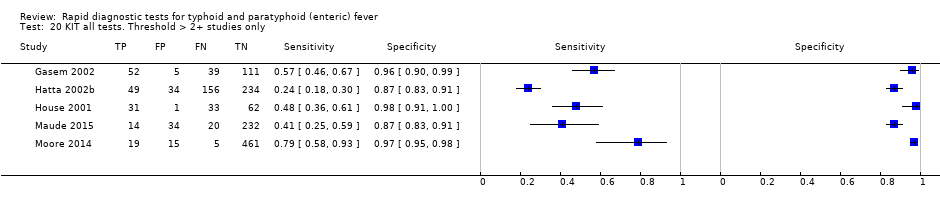
KIT all tests. Threshold > 2+ studies only.

SD Bioline. Antibody: IgG.

SD Bioline. Antibody: IgM.
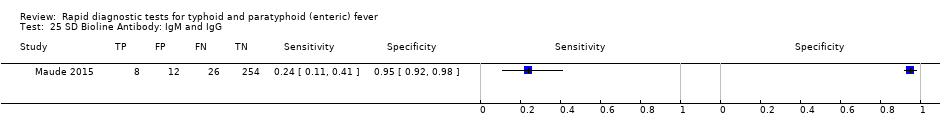
SD Bioline Antibody: IgM and IgG.

Mega Salmonella. Antibody: IgG.

Mega Salmonella. Antibody: IgM.

Multi‐Test Dip‐S‐Tick.
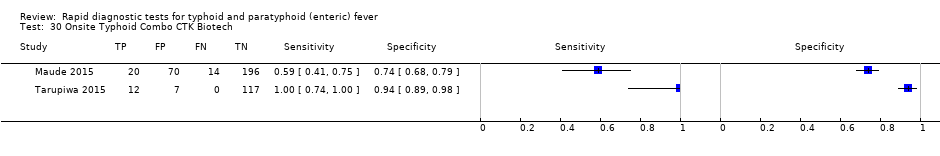
Onsite Typhoid Combo CTK Biotech.
| Review question: to assess the diagnostic accuracy of rapid diagnostic tests (RDTs) for detecting enteric fever in persons living in endemic areas presenting to a healthcare facility with fever Patients/population: clinically‐suspected enteric fever patients or unselected febrile patients Role: first test for enteric fever in patients presenting to a healthcare facility with fever in endemic areas Index tests: all RDTs specifically designed to enteric fever cases applied to patient blood or urine samples Reference standards: bone marrow culture, peripheral blood culture, peripheral blood culture, and polymerase chain reaction (PCR) on blood Studies: prospective cohort, retrospective case control Setting: healthcare facility in enteric fever endemic areas | ||||||
| Index test | Effect (95% confidence interval (CI)) | Participants Total number, number with disease, (number of studies) | Test result | Number of results per 1000 participants tested 1 (95% CI) | ||
| Prevalence 1% | Prevalence 10% | Prevalence 30% | ||||
| Typhidot (all types) | Sensitivity 84 (73 to 91) Specificity 79 (70 to 87) | 6928, 982 (22) | TP FN FP TN | 8 (7 to 9) 2 (1 to 3) 208 (129 to 297) 782 (693 to 861) | 84 (73 to 91) 16 (9 to 27) 189 (117 to 270) 711 (630 to 783) | 252 (219 to 273) 48 (27 to 81) 147 (91 to 210) 553 (490 to 609) |
| Typhidot indeterminants reported or not applicable | Sensitivity 78 (65 to 87) Specificity 77 (66 to 86) | 5555, 662 (13) | TP FN FP TN | 8 (7 to 9) 2 (1 to 3) 228 (139 to 337) 762 (653 to 851) | 78 (65 to 87) 22 (13 to 35) 207 (126 to 306) 693 (594 to 774) | 234 (195 to 261) 66 (39 to 105) 161 (98 to 238) 539 (462 to 602) |
| Typhidot indeterminate results reported | Sensitivity 66 (59 to 73) Specificity 81 (58 to 93) | 1721, 339 (6) | TP FN FP TN | 7 (6 to 7) 3 (3 to 4) 188 (69 to 416) 802 (574 to 921) | 66 (59 to 73) 34 (27 to 41) 171 (63 to 378) 729 (522 to 837) | 198 (177 to 219) 102 (81 to 123 ) 133 (49 to 294) 567 (406 to 651) |
| TUBEX | Sensitivity 78 (71 to 85) Specificity 87 (82 to 91) | 4885, 627 (14) | TP FN FP TN | 8 (7 to 9) 2 (2 to 3) 129 (89 to 178) 861 (812 to 901) | 78 (71 to 85) 22 (15 to 29) 117 (81 to 162) 783 (738 to 819) | 234 (213 to 255) 66 (45 to 87) 91 (63 to 126) 609 (574 to 637) |
| Test‐it Typhoid and KIT prototypes (threshold > 1+) | Sensitivity 69 (59 to 78) Specificity 90 (78 to 93) | 2828, 682 (9) | TP FN FP TN | 7 (6 to 8) 3 (2 to 4) 99 (69 to 218) 891 (772 to 921) | 69 (59 to 78) 31 (22 to 41) 90 (63 to 198) 810 (702 to 837) | 207 (177 to 234) 93 (66 to 123) 70 (49 to 154) 630 (546 to 651) |
| Attributes of tests contributing to benefits and risks | ||||||
| Rapid diagnostic tests (RDTs)2 | RDTs are designed to provide test results typically in less than 1 hour, whereas currently used blood culture tests require 48 hours. The technical ability needed to conduct these rapid tests is designed to be lower than typical laboratory based tests, meaning they have the potential to be delivered nearer to the patient, further reducing time to diagnosis. However, some variants of the Typhidot test requires additional laboratory equipment, whereas the TUBEX and Test‐it Typhoid test do not. The TUBEX tests and some variants of Typhidot require cold chain storage. The Test‐it Typhoid test does not. In this Cochrane Review all included rapid tests were used on blood samples. None of the included studies conducted tests on urine samples. | |||||
| Overall certainty of evidence | ||||||
| Indeterminate results: for the Typhidot index test, there are concerns about studies which do not report indeterminate results (IgM negative and IgG positive). These results can frequently occur and if these results are not included in the analysis this biases study results to be overly‐optimistic. Case control studies: many of these studies use a case control design. This study design is at risk of overestimating both sensitivity and specificity. Reference standard: the highest grade of reference standard includes either bone marrow culture or PCR using blood, in addition to blood culture. However using bone marrow as a reference standard is invasive and more severe patients may be selected into these studies. Most included studies use only blood culture, and studies using more than 1 reference standard for example, PCR showed a reduction in RDT sensitivity by 20% to 25%. Precision: average estimates of both sensitivity and specificity have low precision, due to the heterogeneity between studies. Paired studies: there are few paired studies, where more than 1 test is used in the same patients. These studies provide the most direct evidence for comparing tests. Typhidot paired with TUBEX: Total 4245, 484 patients with disease. Typhidot paired with Test‐it Typhoid and KIT prototypes: no paired studies. Test‐it Typhoid and KIT prototypes paired with TUBEX: total 127, 64 patients with disease. It remains unclear if the tests were used in the same cohort of patients. | ||||||
| Abbreviations: False Negatives (FN); False Positives (FP); immunoglobulin‐G (IgG); immunoglobulin‐M (IgM); Royal Tropical Institute, Amsterdam (KIT); polymerase chain reaction (PCR); True Negatives (TN); True Positives (TP). 1We used 2 systematic reviews of bacteraemia in Asia and Africa to inform prevalences of 30% (Asia); 10% (Africa: adults and children) and 1% (Africa: children) (Reddy 2010; Deen 2012). | ||||||
| Index Test Name | Manufacturer | Methods | Formats | Biological specimen | Threshold for positivity values | Number of evaluations |
| TUBEX® TF | IDL Biotech, Bromma, Sweden | Inhibition Binding Magnetic Immunoassay. Detects IgM to S. Typhi O9 antigen. Semi‐quantitative colorimetric. | Mix buffer/reagent into plastic well with patient specimen. 3 minutes for result. | Whole blood, plasma, or serum | Semi‐quantitative colour change scale (0 to 10) provided by manufacturer. Positive if colour change scale ≥ 3. | 14 |
| Typhidot® | Malaysian Bio‐Diagnostics Research, Selangor, Malaysia | Dot‐enzyme immunoassay. Detects IgG and IgM to 50 kdA S. Typhi Outer Membrane Protein (OMP) antigen. | Mix serum/whole blood plus reagent incubating commercially‐prepared pre‐dotted antigen filter paper strips. 60 minutes for result. | Whole blood, plasma, or serum | Qualitative: either positive or negative. A positive result is a visible reaction (IgG or IgM) of an intensity equal to or greater than that of the control reaction on the commercially prepared filter paper. | 17 |
| Typhidot‐M® | Malaysian Bio‐Diagnostics Research, Selangor, Malaysia | Dot‐enzyme immunoassay. Detects IgM to 50 kdA S. Typhi OMP antigen. | Mix serum/whole blood plus reagent incubating commercially‐prepared pre‐dotted antigen filter paper strips. 60 minutes for result. | Whole blood, plasma, or serum | Qualitative: either positive or negative. Positive as per Typhidot. The absence of any visible spot indicated a negative test result. | 6 |
| TyphiRapid Tr‐02 (Typhidot) | Reszon Diagnostics International, Malaysia | Prototype of Typhidot. Immunochromatography | Mix serum/whole blood plus buffer/reagent into a well. | Whole blood, plasma, or serum | We were unable to get hold of the manufacturer and are awaiting a response from the study author | 1 |
| KIT ICT Test‐It TyphoidTM | LifeAssay Diagnostics, Cape Town, South Africa | Lateral flow immunochromatographic (ICT) assay. Detects IgM to S. Typhi lipopolysaccharide (LPS) antigen. Semi‐quantitative. | Mix serum/whole blood plus buffer/reagent into lateral flow cassette. Two‐site (test and control) immunoassay on a porous nitrocellulose membrane. 15 minutes for result. | Whole blood, plasma, or serum | Semi‐quantitative result line intensity scale (negative to +4) provided by manufacturer. A positive result is ≥ +1 | 3 |
| KIT Dipstick Assay | Royal Tropical Institute (KIT), Amsterdam | Detects IgM to S. Typhi LPS antigen. Simplified version of ELISA technique. | Strip of nitrocellulose membrane with immobilized antigen detection band. Serum plus reagent incubated on dipstick for 3 hours at room temperature. Dipsticks rinses with water and dried. >3 hours for result. | Serum | Semi‐quantitative result line intensity scale (negative to +4) provided by manufacturer. A positive result is ≥ +1 | 5 |
| KIT Dri‐Dot Assay (latex agglutination) | Royal Tropical Institute (KIT), Amsterdam | Detects IgM to S. Typhi LPS antigen. White agglutination card. | Dot of dried detection reagent conjugated to blue latex reagent. Antigen‐activated latex stabilized by drying a drop of latex reagent onto card suspended in serum. Card rotated by hand in near‐horizontal position to further induce agglutination. 30 seconds for result. | Serum | Qualitative: positive or negative. Positive when agglutination was observed within 30 seconds. Negative when no agglutination was observed. | 1 |
| SD Bioline Salmonella typhi IgG/IgM Fast | Standard Diagnostics Inc., Gyeonggi, Korea | ICT flow method. Detects IgM and IgG antibodies to unspecified S. Typhi antigens. | 4 drops of reagent mixed well with patient specimen. Nitrocellulose strip suspended into with 3 sites (IgM, IgG, and control). 30 minutes for result. | Serum, plasma, or whole blood | Qualitative: positive or negative. Positive if line appears in both control and 1 or both of IgM or IgG test zones. | 3 |
| Enterocheck WB® | Zephyr Biologicals, Goa, India | ICT Detects IgM antibodies to S. Typhi LPS antigen. | Two‐site (IgM test, and control) immunoassay cassette on a porous nitrocellulose membrane. 15 minutes for result. | Whole blood, plasma, or serum | Qualitative: positive or negative. Presence of a line in both the test and control zones indicates a positive result. | 2 |
| Enteroscreen ® | Zephyr Biologicals, Goa, India | ICT Detects IgM and IgG antibodies to S. Typhi LPS antigen. | Three‐site (IgG, IgM, and control) immunoassay cassette on a porous nitrocellulose membrane. 15 minutes for result. | Whole blood, plasma, or serum | Qualitative: positive or negative. Presence of a line in both the test (IgG, IgM, or both) and control zones indicates a positive result. | 1 |
| Multi‐test Dip‐S‐Tick | PanBio Inc., Columbia, Maryland, USA | Tests for five pathogens, including S. Typhi. Dipstick format that detects anti‐O, anti‐H,anti‐Vi, IgM, or IgG antibodies. | Detailed information not available | Heparinized whole blood, serum, or plasma | Detailed information not available | 1 |
| Mega Salmonella | Mega Diagnostics, Los Angeles, California, USA | Detect IgG and IgM antibodies to unspecified Salmonella antigens. Quantitatively detected by ELISA with peroxidase‐labelled reagents. | Results read in a microplate ELISA reader. | Whole blood, serum, or plasma | Detailed information not available | 1 |
| OnSite Typhoid IgG/IgM Combo | CTK Biotech Inc., San Diego, California, USA | Lateral flow immunoassay. Detects IgG and IgM antibodies against recombinant O and H S. Typhi antigens. | Three‐site (IgG, IgM, and control) immunoassay cassette on a porous nitrocellulose membrane. 15 minutes for result. | Whole blood, serum, or plasma | Qualitative: positive or negative. Presence of a line in both the test (IgG, IgM, or both) and control zones indicates a positive result. | 2 |
| Abbreviations: immunochromatographic (ICT); immunoglobulin‐G (IgG); immunoglobulin‐M (IgM); Tropical Institute, Amsterdam (KIT); lipopolysaccharide (LPS); outer membrane protein (OMP). | ||||||
| Test | No. of studies | No. of participants |
| 1 Typhidot. Antibody: IgM or as reported. 1 result per study Show forest plot | 17 | 3691 |
| 2 Typhidot. Antibody: IgM or as reported. Reference: BC Show forest plot | 15 | 3466 |
| 3 Typhidot. Antibody: IgM or as reported. Reference: BC and BM Show forest plot | 2 | 225 |
| 4 Typhidot. Antibody: IgM or as reported. Reference: BC and PCR Show forest plot | 1 | 500 |
| 5 Typhidot. Antibody: IgM or as reported. Indeterminates reported Show forest plot | 6 | 1721 |
| 6 Typhidot. Antibody: IgM or as reported. Indeterminates not reported Show forest plot | 11 | 1970 |
| 7 Typhidot‐M. Antibody: IgM Show forest plot | 6 | 3334 |
| 8 Typhi rapid Tr‐02. Reference: BC. Antibody: IgM Show forest plot | 1 | 500 |
| 9 Typhi rapid Tr‐02. Reference: BC & PCR. Antibody: IgM Show forest plot | 1 | 500 |
| 10 Typhidot all tests 1 result per study Show forest plot | 22 | 6928 |
| 11 TUBEX. Reference:BC Show forest plot | 14 | 4885 |
| 12 TUBEX. Reference: BC & PCR Show forest plot | 1 | 500 |
| 13 TUBEX 1 result per study Show forest plot | 14 | 4885 |
| 14 KIT ICT. Reference:BC. Threshold > 1+ Show forest plot | 2 | 709 |
| 15 KIT ICT. Reference: BC & PCR. Threshold > 1+ Show forest plot | 2 | 800 |
| 16 KIT latex agglutination. Threshold > 1+ Show forest plot | 1 | 425 |
| 17 KIT Dipstick. Threshold > 1+ Show forest plot | 5 | 1394 |
| 18 KIT ICT. Threshold > 1+ Show forest plot | 3 | 1009 |
| 19 KIT all tests. Threshold > 1+. One result per study. Show forest plot | 9 | 2828 |
| 20 KIT all tests. Threshold > 2+ studies only Show forest plot | 5 | 1607 |
| 21 Enterocheck WB Show forest plot | 2 | 533 |
| 22 PanBio Show forest plot | 1 | 144 |
| 23 SD Bioline. Antibody: IgG Show forest plot | 3 | 1669 |
| 24 SD Bioline. Antibody: IgM Show forest plot | 3 | 1590 |
| 25 SD Bioline Antibody: IgM and IgG Show forest plot | 1 | 300 |
| 26 Mega Salmonella. Antibody: IgG Show forest plot | 1 | 177 |
| 27 Mega Salmonella. Antibody: IgM Show forest plot | 1 | 177 |
| 28 Multi‐Test Dip‐S‐Tick Show forest plot | 1 | 75 |
| 29 Enteroscreen Show forest plot | 1 | 1521 |
| 30 Onsite Typhoid Combo CTK Biotech Show forest plot | 2 | 436 |