Pruebas de diagnóstico rápido para la fiebre tifoidea y fiebre paratifoidea (entérica)
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008892.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 25 mayo 2017see what's new
- Tipo:
-
- Diagnostic
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Enfermedades infecciosas
- Clasificada:
-
- Actualizada
All studies incorporated from most recent search
All eligible published studies found in the last search (4 Mar, 2016) were includedEvaluada: 2 April 2019
- Actualizada
- Copyright:
-
- Copyright © 2017 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
- This is an open access article under the terms of the Creative Commons Attribution‐Non‐Commercial Licence, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
LW and CMP conceived the review. LW wrote the protocol and SD and CMP edited the protocol (Wijedoru 2010). LW and CMP assessed abstracts, selected studies for inclusion, extracted data, and assessed methodological quality. Susan Mallett (SM) led the statistical analysis and interpretation of statistical results. LW and CMP led clinical interpretation of results. LW wrote the report with editing by CMP and SM. All review authors have seen and approved the final version of this Cochrane Review.
Sources of support
Internal sources
-
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development (DFID), UK.
Grant: 5242
Declarations of interest
LW and CMP are authors of Moore 2014 and Maude 2015.
SM has no known conflicts of interest.
Acknowledgements
The editorial base for the Cochrane Infectious Diseases Group is funded by the UK Department for International Development (DFID) for the benefit of low‐ and middle‐income countries (Grant: 5242). The views expressed in this review do not necessarily reflect UK government policy. We thank Sarah Donegan (SD) who contributed to the statistical integrity of the protocol and review planning; Vittoria Lutje (VL) who was responsible for the strategy and conduct of the literature search; Christianne Esparza (CE) who co‐ordinated the retrieval of papers for the review; and Anne‐Marie Stephani (AMS) who co‐ordinated administrative and technical support.
The Contact Editors for this review were Dr Karen Steingart and Dr Olalekan Uthman.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 May 25 | Rapid diagnostic tests for typhoid and paratyphoid (enteric) fever | Review | Lalith Wijedoru, Sue Mallett, Christopher M Parry | |
| 2010 Dec 08 | Rapid Diagnostic Tests for Typhoid and Paratyphoid (Enteric) Fever | Protocol | Lalith Wijedoru, Sarah Donegan, Christopher Parry | |
Differences between protocol and review
We amended the reference test definition when it became apparent that some studies had used a PCR test to detect Salmonella Typhi or Salmonella Paratyphi A DNA in blood samples. We included peripheral blood PCR in addition to peripheral blood culture as a Grade 2 reference standard. In the studies that used a blood PCR in addition to blood culture, a positive blood culture or blood PCR represented a positive reference test.
During the interval between protocol and full review publication, a modified tool assessment of methodological quality was ratified and released (QUADAS‐2). We used this newer tool for the full review instead of QUADAS‐1 as originally intended in the protocol (Appendix 3).
The major differences between the protocol and the review relate to the intended statistical analysis. Some of the studies of the Test‐it Typhoid test and its KIT prototypes used two test thresholds. We were able to use bivariate analysis to focus on test operating points instead of hierarchical summary receiver operating characteristic (HSROC) analysis. Typhidot and TUBEX tests results did not use different test thresholds. A number of the planned statistical analyses of subgroups were underpowered due to the low number of available studies. The main subgroup analysis performed was by test manufacturer (Typhidot/Typhidot‐M, TUBEX and Test‐it Typhoid and KIT prototype RDTs) as there were sufficient available studies to potentially allow robust comparisons. We did not perform the following planned subanalyses: Salmonella enterica serovars (Typhi, Paratyphi A, or both); reference standard test applied (bone marrow and blood culture [Grade 1] versus blood culture alone [Grade 2]); study design (case control, prospective cohort, randomized controlled trial, paired comparative trial); test population (clinically‐suspected enteric fever versus unselected febrile patients); and index test biological sample type (blood versus urine). Where possible we have replaced these subanalyses with graphical presentation of subgroups in SROC plots.
For the Typhidot test and its variants we decided to extract the IgM data alone from each study. Typhidot detects both IgG and IgM antibodies, while Typhidot‐M detects IgM antibodies only. A detectable IgG result may indicate current or recent acute but also previous infection whereas IgM indicates current or recent acute infection. In order to compare the data of Typhidot with the data of Typhidot‐M, if the IgM data was not recorded separately from the IgG data, we excluded the results.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Child; Humans;
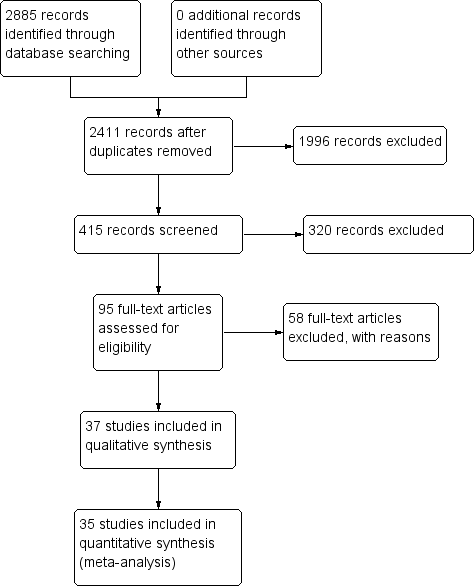
PRISMA flow diagram.

Summary receiver operating characteristic plot: Enterocheck WB, PanBio, SD Bioline, Mega Salmonella, Multi‐Test Dip‐S‐Tick.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
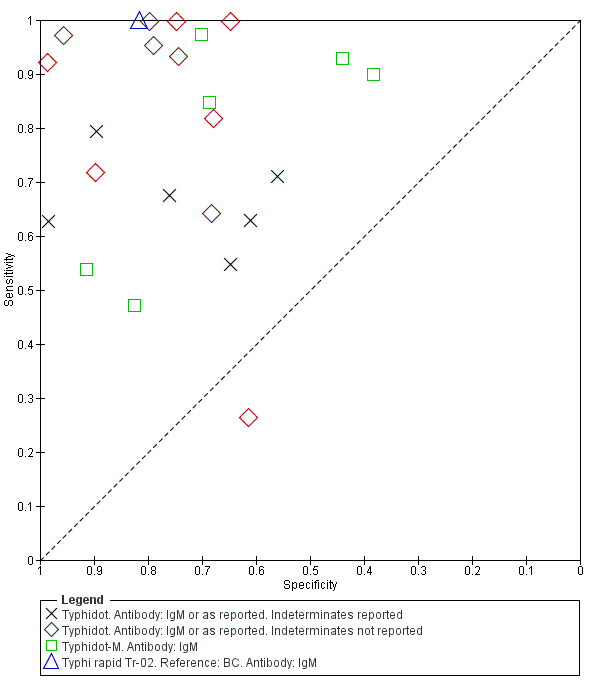
Summary ROC Typhidot all test types.
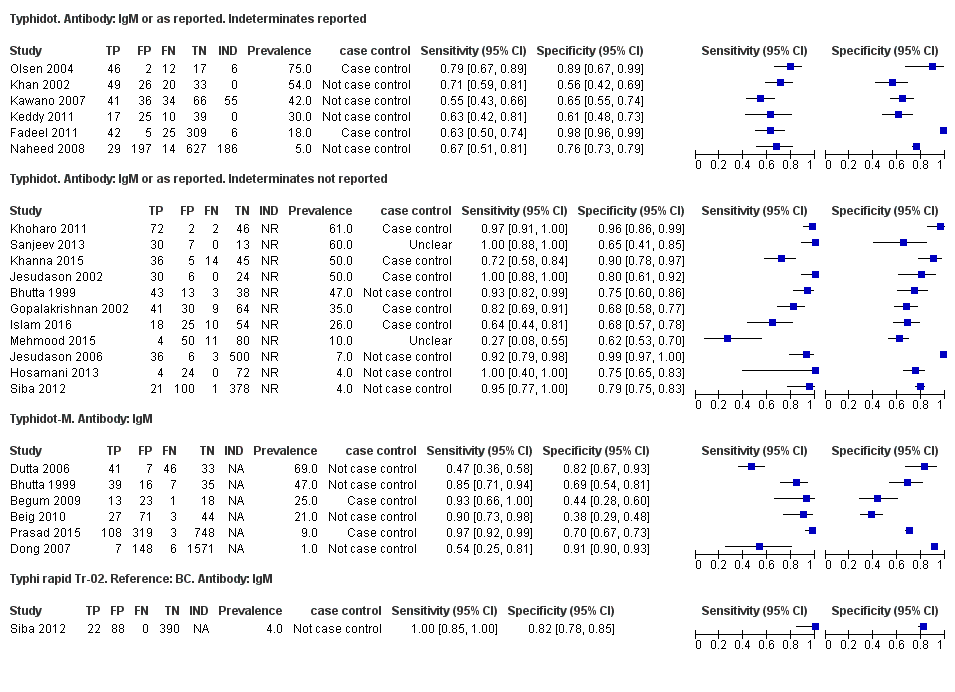
Forest plots for Typhidot all test types.

Summary receiver operating characteristic plot of tests: Typhidot and Typhidot‐M by reference test.
Abbreviations: BC: blood culture; BM: bone marrow; BC & PCR: blood culture and polymerase chain reaction.

Summary receiver operating characteristic plot of test: TUBEX. Reference test: Blood culture. One result per study.

Forest plot of TUBEX. Reference test blood culture.

Summary receiver operating characteristic plot: TUBEX by case control design.
Abbreviation: BC: blood culture.

Summary receiver operating characteristic plot: TUBEX by reference test
Abbreviations: BC: blood culture; BC & PCR: blood culture and polymerase chain reaction.
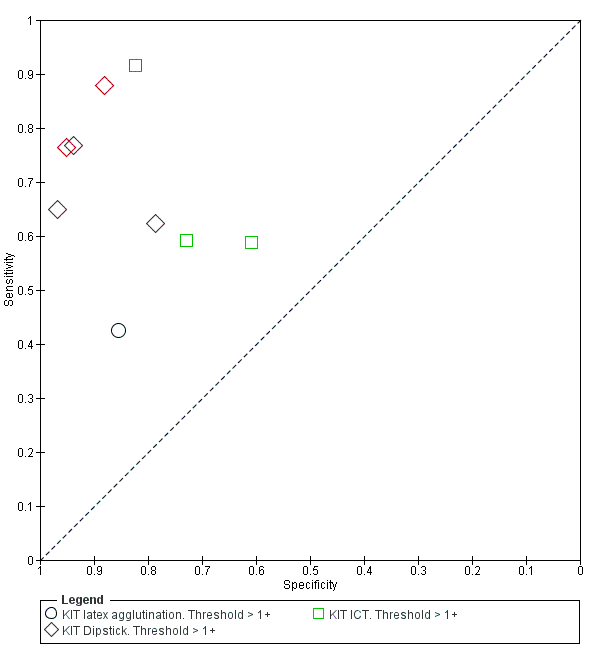
Summary receiver operating characteristic plot: KIT all test types. Threshold > 1+.
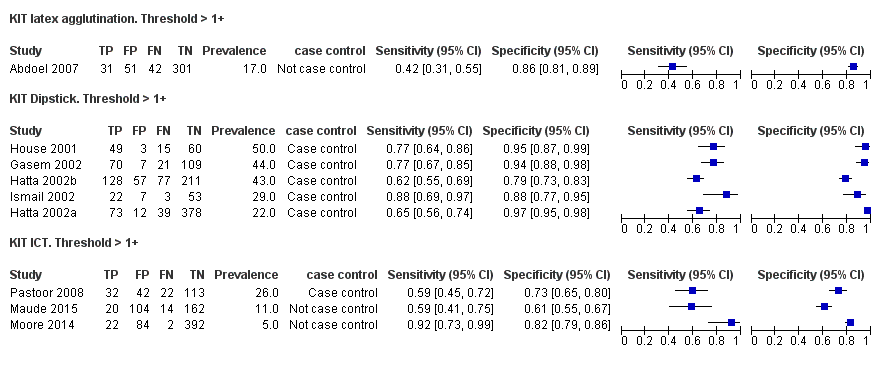
Forest plot of tests: KIT Threshold > 1+ by test type. Reference test: blood culture.

Summary receiver operating characteristic plot: KIT test by threshold > 1+ and > 2+.

Summary receiver operating characteristic plot: KIT ICT by reference test.
Abbreviations: BC: blood culture; BC & PCR: blood culture and polymerase chain reaction.
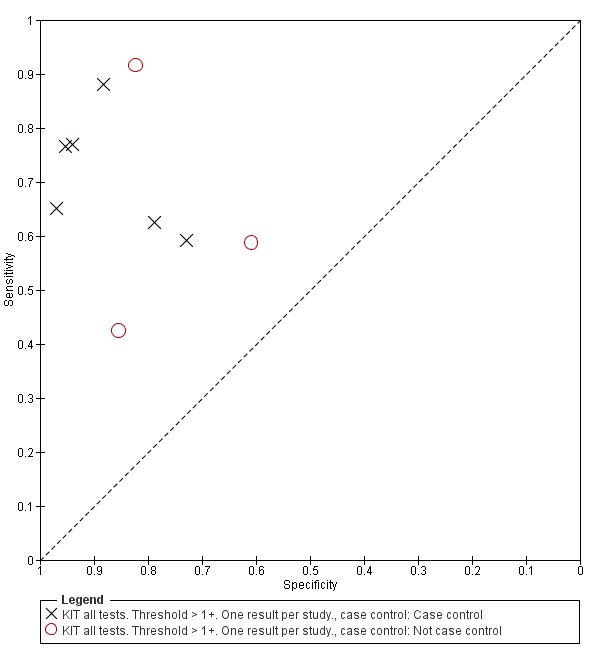
Summary receiver operating characteristic plot: KIT by case control (All test types. Threshold >1+).
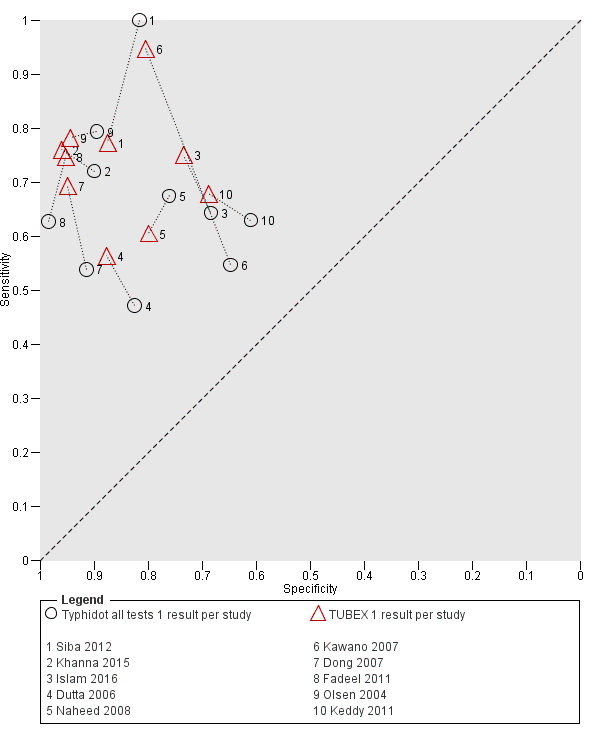
Summary receiver operating characteristic plot: Typhidot versus TUBEX. Paired studies only. One result per index test per study.

Summary receiver operating characteristic: TUBEX versus KIT. Paired results. One result per index per study.

Summary receiver operating characteristic plot: Typhidot versus TUBEX tests. One result per index test per study.
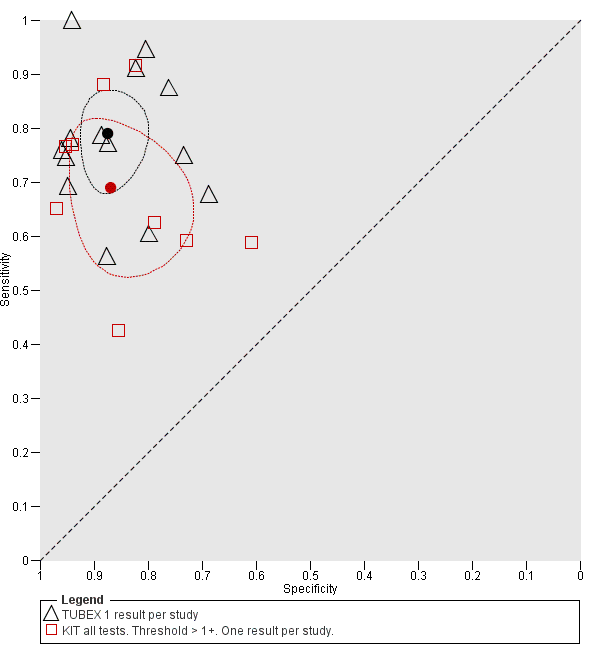
Summary receiver operating characteristic plot: TUBEX versus Test‐it Typhoid (KIT) tests. One result per index test per study.

Summary receiver operating characteristic: Typhidot versus KIT. No paired studies. One result per index per study.

Summary receiver operating characteristic plot: Typhidot tests by case control design.

Typhidot. Antibody: IgM or as reported. 1 result per study.
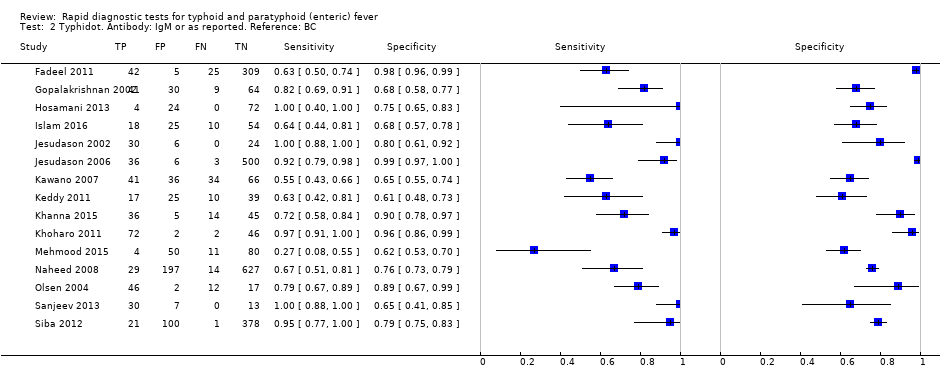
Typhidot. Antibody: IgM or as reported. Reference: BC.

Typhidot. Antibody: IgM or as reported. Reference: BC and BM.

Typhidot. Antibody: IgM or as reported. Reference: BC and PCR.

Typhidot. Antibody: IgM or as reported. Indeterminates reported.

Typhidot. Antibody: IgM or as reported. Indeterminates not reported.

Typhidot‐M. Antibody: IgM.

Typhi rapid Tr‐02. Reference: BC. Antibody: IgM.

Typhi rapid Tr‐02. Reference: BC & PCR. Antibody: IgM.

Typhidot all tests 1 result per study.

TUBEX. Reference:BC.

TUBEX. Reference: BC & PCR.
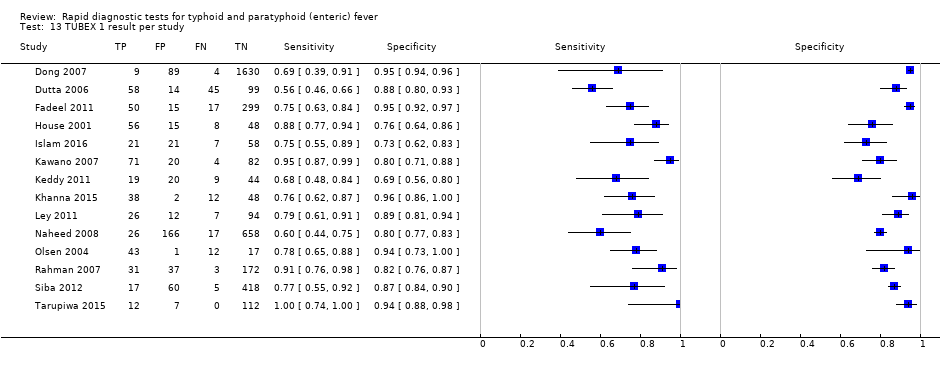
TUBEX 1 result per study.

KIT ICT. Reference:BC. Threshold > 1+.

KIT ICT. Reference: BC & PCR. Threshold > 1+.
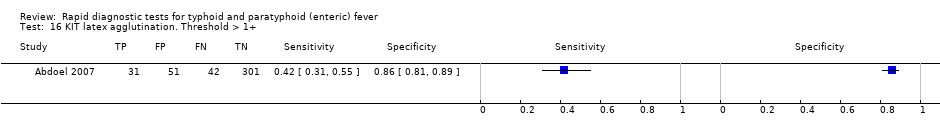
KIT latex agglutination. Threshold > 1+.

KIT Dipstick. Threshold > 1+.

KIT ICT. Threshold > 1+.

KIT all tests. Threshold > 1+. One result per study..
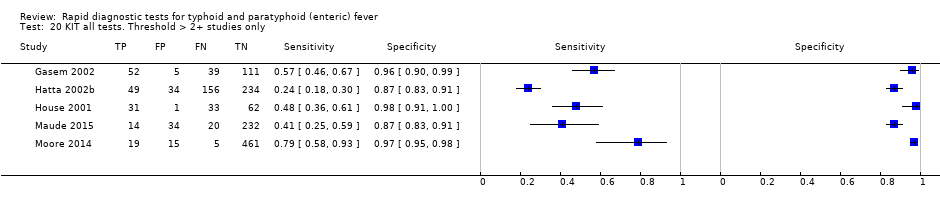
KIT all tests. Threshold > 2+ studies only.

SD Bioline. Antibody: IgG.

SD Bioline. Antibody: IgM.
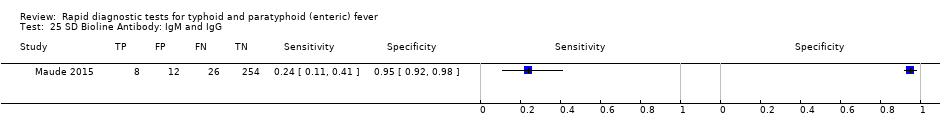
SD Bioline Antibody: IgM and IgG.

Mega Salmonella. Antibody: IgG.

Mega Salmonella. Antibody: IgM.

Multi‐Test Dip‐S‐Tick.
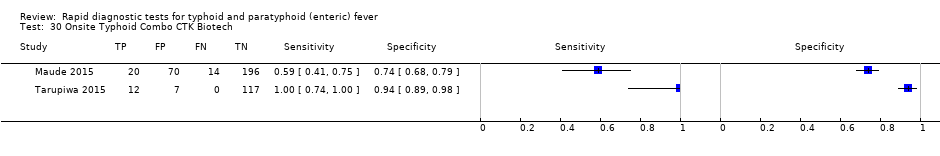
Onsite Typhoid Combo CTK Biotech.
| Review question: to assess the diagnostic accuracy of rapid diagnostic tests (RDTs) for detecting enteric fever in persons living in endemic areas presenting to a healthcare facility with fever Patients/population: clinically‐suspected enteric fever patients or unselected febrile patients Role: first test for enteric fever in patients presenting to a healthcare facility with fever in endemic areas Index tests: all RDTs specifically designed to enteric fever cases applied to patient blood or urine samples Reference standards: bone marrow culture, peripheral blood culture, peripheral blood culture, and polymerase chain reaction (PCR) on blood Studies: prospective cohort, retrospective case control Setting: healthcare facility in enteric fever endemic areas | ||||||
| Index test | Effect (95% confidence interval (CI)) | Participants Total number, number with disease, (number of studies) | Test result | Number of results per 1000 participants tested 1 (95% CI) | ||
| Prevalence 1% | Prevalence 10% | Prevalence 30% | ||||
| Typhidot (all types) | Sensitivity 84 (73 to 91) Specificity 79 (70 to 87) | 6928, 982 (22) | TP FN FP TN | 8 (7 to 9) 2 (1 to 3) 208 (129 to 297) 782 (693 to 861) | 84 (73 to 91) 16 (9 to 27) 189 (117 to 270) 711 (630 to 783) | 252 (219 to 273) 48 (27 to 81) 147 (91 to 210) 553 (490 to 609) |
| Typhidot indeterminants reported or not applicable | Sensitivity 78 (65 to 87) Specificity 77 (66 to 86) | 5555, 662 (13) | TP FN FP TN | 8 (7 to 9) 2 (1 to 3) 228 (139 to 337) 762 (653 to 851) | 78 (65 to 87) 22 (13 to 35) 207 (126 to 306) 693 (594 to 774) | 234 (195 to 261) 66 (39 to 105) 161 (98 to 238) 539 (462 to 602) |
| Typhidot indeterminate results reported | Sensitivity 66 (59 to 73) Specificity 81 (58 to 93) | 1721, 339 (6) | TP FN FP TN | 7 (6 to 7) 3 (3 to 4) 188 (69 to 416) 802 (574 to 921) | 66 (59 to 73) 34 (27 to 41) 171 (63 to 378) 729 (522 to 837) | 198 (177 to 219) 102 (81 to 123 ) 133 (49 to 294) 567 (406 to 651) |
| TUBEX | Sensitivity 78 (71 to 85) Specificity 87 (82 to 91) | 4885, 627 (14) | TP FN FP TN | 8 (7 to 9) 2 (2 to 3) 129 (89 to 178) 861 (812 to 901) | 78 (71 to 85) 22 (15 to 29) 117 (81 to 162) 783 (738 to 819) | 234 (213 to 255) 66 (45 to 87) 91 (63 to 126) 609 (574 to 637) |
| Test‐it Typhoid and KIT prototypes (threshold > 1+) | Sensitivity 69 (59 to 78) Specificity 90 (78 to 93) | 2828, 682 (9) | TP FN FP TN | 7 (6 to 8) 3 (2 to 4) 99 (69 to 218) 891 (772 to 921) | 69 (59 to 78) 31 (22 to 41) 90 (63 to 198) 810 (702 to 837) | 207 (177 to 234) 93 (66 to 123) 70 (49 to 154) 630 (546 to 651) |
| Attributes of tests contributing to benefits and risks | ||||||
| Rapid diagnostic tests (RDTs)2 | RDTs are designed to provide test results typically in less than 1 hour, whereas currently used blood culture tests require 48 hours. The technical ability needed to conduct these rapid tests is designed to be lower than typical laboratory based tests, meaning they have the potential to be delivered nearer to the patient, further reducing time to diagnosis. However, some variants of the Typhidot test requires additional laboratory equipment, whereas the TUBEX and Test‐it Typhoid test do not. The TUBEX tests and some variants of Typhidot require cold chain storage. The Test‐it Typhoid test does not. In this Cochrane Review all included rapid tests were used on blood samples. None of the included studies conducted tests on urine samples. | |||||
| Overall certainty of evidence | ||||||
| Indeterminate results: for the Typhidot index test, there are concerns about studies which do not report indeterminate results (IgM negative and IgG positive). These results can frequently occur and if these results are not included in the analysis this biases study results to be overly‐optimistic. Case control studies: many of these studies use a case control design. This study design is at risk of overestimating both sensitivity and specificity. Reference standard: the highest grade of reference standard includes either bone marrow culture or PCR using blood, in addition to blood culture. However using bone marrow as a reference standard is invasive and more severe patients may be selected into these studies. Most included studies use only blood culture, and studies using more than 1 reference standard for example, PCR showed a reduction in RDT sensitivity by 20% to 25%. Precision: average estimates of both sensitivity and specificity have low precision, due to the heterogeneity between studies. Paired studies: there are few paired studies, where more than 1 test is used in the same patients. These studies provide the most direct evidence for comparing tests. Typhidot paired with TUBEX: Total 4245, 484 patients with disease. Typhidot paired with Test‐it Typhoid and KIT prototypes: no paired studies. Test‐it Typhoid and KIT prototypes paired with TUBEX: total 127, 64 patients with disease. It remains unclear if the tests were used in the same cohort of patients. | ||||||
| Abbreviations: False Negatives (FN); False Positives (FP); immunoglobulin‐G (IgG); immunoglobulin‐M (IgM); Royal Tropical Institute, Amsterdam (KIT); polymerase chain reaction (PCR); True Negatives (TN); True Positives (TP). 1We used 2 systematic reviews of bacteraemia in Asia and Africa to inform prevalences of 30% (Asia); 10% (Africa: adults and children) and 1% (Africa: children) (Reddy 2010; Deen 2012). | ||||||
| Index Test Name | Manufacturer | Methods | Formats | Biological specimen | Threshold for positivity values | Number of evaluations |
| TUBEX® TF | IDL Biotech, Bromma, Sweden | Inhibition Binding Magnetic Immunoassay. Detects IgM to S. Typhi O9 antigen. Semi‐quantitative colorimetric. | Mix buffer/reagent into plastic well with patient specimen. 3 minutes for result. | Whole blood, plasma, or serum | Semi‐quantitative colour change scale (0 to 10) provided by manufacturer. Positive if colour change scale ≥ 3. | 14 |
| Typhidot® | Malaysian Bio‐Diagnostics Research, Selangor, Malaysia | Dot‐enzyme immunoassay. Detects IgG and IgM to 50 kdA S. Typhi Outer Membrane Protein (OMP) antigen. | Mix serum/whole blood plus reagent incubating commercially‐prepared pre‐dotted antigen filter paper strips. 60 minutes for result. | Whole blood, plasma, or serum | Qualitative: either positive or negative. A positive result is a visible reaction (IgG or IgM) of an intensity equal to or greater than that of the control reaction on the commercially prepared filter paper. | 17 |
| Typhidot‐M® | Malaysian Bio‐Diagnostics Research, Selangor, Malaysia | Dot‐enzyme immunoassay. Detects IgM to 50 kdA S. Typhi OMP antigen. | Mix serum/whole blood plus reagent incubating commercially‐prepared pre‐dotted antigen filter paper strips. 60 minutes for result. | Whole blood, plasma, or serum | Qualitative: either positive or negative. Positive as per Typhidot. The absence of any visible spot indicated a negative test result. | 6 |
| TyphiRapid Tr‐02 (Typhidot) | Reszon Diagnostics International, Malaysia | Prototype of Typhidot. Immunochromatography | Mix serum/whole blood plus buffer/reagent into a well. | Whole blood, plasma, or serum | We were unable to get hold of the manufacturer and are awaiting a response from the study author | 1 |
| KIT ICT Test‐It TyphoidTM | LifeAssay Diagnostics, Cape Town, South Africa | Lateral flow immunochromatographic (ICT) assay. Detects IgM to S. Typhi lipopolysaccharide (LPS) antigen. Semi‐quantitative. | Mix serum/whole blood plus buffer/reagent into lateral flow cassette. Two‐site (test and control) immunoassay on a porous nitrocellulose membrane. 15 minutes for result. | Whole blood, plasma, or serum | Semi‐quantitative result line intensity scale (negative to +4) provided by manufacturer. A positive result is ≥ +1 | 3 |
| KIT Dipstick Assay | Royal Tropical Institute (KIT), Amsterdam | Detects IgM to S. Typhi LPS antigen. Simplified version of ELISA technique. | Strip of nitrocellulose membrane with immobilized antigen detection band. Serum plus reagent incubated on dipstick for 3 hours at room temperature. Dipsticks rinses with water and dried. >3 hours for result. | Serum | Semi‐quantitative result line intensity scale (negative to +4) provided by manufacturer. A positive result is ≥ +1 | 5 |
| KIT Dri‐Dot Assay (latex agglutination) | Royal Tropical Institute (KIT), Amsterdam | Detects IgM to S. Typhi LPS antigen. White agglutination card. | Dot of dried detection reagent conjugated to blue latex reagent. Antigen‐activated latex stabilized by drying a drop of latex reagent onto card suspended in serum. Card rotated by hand in near‐horizontal position to further induce agglutination. 30 seconds for result. | Serum | Qualitative: positive or negative. Positive when agglutination was observed within 30 seconds. Negative when no agglutination was observed. | 1 |
| SD Bioline Salmonella typhi IgG/IgM Fast | Standard Diagnostics Inc., Gyeonggi, Korea | ICT flow method. Detects IgM and IgG antibodies to unspecified S. Typhi antigens. | 4 drops of reagent mixed well with patient specimen. Nitrocellulose strip suspended into with 3 sites (IgM, IgG, and control). 30 minutes for result. | Serum, plasma, or whole blood | Qualitative: positive or negative. Positive if line appears in both control and 1 or both of IgM or IgG test zones. | 3 |
| Enterocheck WB® | Zephyr Biologicals, Goa, India | ICT Detects IgM antibodies to S. Typhi LPS antigen. | Two‐site (IgM test, and control) immunoassay cassette on a porous nitrocellulose membrane. 15 minutes for result. | Whole blood, plasma, or serum | Qualitative: positive or negative. Presence of a line in both the test and control zones indicates a positive result. | 2 |
| Enteroscreen ® | Zephyr Biologicals, Goa, India | ICT Detects IgM and IgG antibodies to S. Typhi LPS antigen. | Three‐site (IgG, IgM, and control) immunoassay cassette on a porous nitrocellulose membrane. 15 minutes for result. | Whole blood, plasma, or serum | Qualitative: positive or negative. Presence of a line in both the test (IgG, IgM, or both) and control zones indicates a positive result. | 1 |
| Multi‐test Dip‐S‐Tick | PanBio Inc., Columbia, Maryland, USA | Tests for five pathogens, including S. Typhi. Dipstick format that detects anti‐O, anti‐H,anti‐Vi, IgM, or IgG antibodies. | Detailed information not available | Heparinized whole blood, serum, or plasma | Detailed information not available | 1 |
| Mega Salmonella | Mega Diagnostics, Los Angeles, California, USA | Detect IgG and IgM antibodies to unspecified Salmonella antigens. Quantitatively detected by ELISA with peroxidase‐labelled reagents. | Results read in a microplate ELISA reader. | Whole blood, serum, or plasma | Detailed information not available | 1 |
| OnSite Typhoid IgG/IgM Combo | CTK Biotech Inc., San Diego, California, USA | Lateral flow immunoassay. Detects IgG and IgM antibodies against recombinant O and H S. Typhi antigens. | Three‐site (IgG, IgM, and control) immunoassay cassette on a porous nitrocellulose membrane. 15 minutes for result. | Whole blood, serum, or plasma | Qualitative: positive or negative. Presence of a line in both the test (IgG, IgM, or both) and control zones indicates a positive result. | 2 |
| Abbreviations: immunochromatographic (ICT); immunoglobulin‐G (IgG); immunoglobulin‐M (IgM); Tropical Institute, Amsterdam (KIT); lipopolysaccharide (LPS); outer membrane protein (OMP). | ||||||
| Test | No. of studies | No. of participants |
| 1 Typhidot. Antibody: IgM or as reported. 1 result per study Show forest plot | 17 | 3691 |
| 2 Typhidot. Antibody: IgM or as reported. Reference: BC Show forest plot | 15 | 3466 |
| 3 Typhidot. Antibody: IgM or as reported. Reference: BC and BM Show forest plot | 2 | 225 |
| 4 Typhidot. Antibody: IgM or as reported. Reference: BC and PCR Show forest plot | 1 | 500 |
| 5 Typhidot. Antibody: IgM or as reported. Indeterminates reported Show forest plot | 6 | 1721 |
| 6 Typhidot. Antibody: IgM or as reported. Indeterminates not reported Show forest plot | 11 | 1970 |
| 7 Typhidot‐M. Antibody: IgM Show forest plot | 6 | 3334 |
| 8 Typhi rapid Tr‐02. Reference: BC. Antibody: IgM Show forest plot | 1 | 500 |
| 9 Typhi rapid Tr‐02. Reference: BC & PCR. Antibody: IgM Show forest plot | 1 | 500 |
| 10 Typhidot all tests 1 result per study Show forest plot | 22 | 6928 |
| 11 TUBEX. Reference:BC Show forest plot | 14 | 4885 |
| 12 TUBEX. Reference: BC & PCR Show forest plot | 1 | 500 |
| 13 TUBEX 1 result per study Show forest plot | 14 | 4885 |
| 14 KIT ICT. Reference:BC. Threshold > 1+ Show forest plot | 2 | 709 |
| 15 KIT ICT. Reference: BC & PCR. Threshold > 1+ Show forest plot | 2 | 800 |
| 16 KIT latex agglutination. Threshold > 1+ Show forest plot | 1 | 425 |
| 17 KIT Dipstick. Threshold > 1+ Show forest plot | 5 | 1394 |
| 18 KIT ICT. Threshold > 1+ Show forest plot | 3 | 1009 |
| 19 KIT all tests. Threshold > 1+. One result per study. Show forest plot | 9 | 2828 |
| 20 KIT all tests. Threshold > 2+ studies only Show forest plot | 5 | 1607 |
| 21 Enterocheck WB Show forest plot | 2 | 533 |
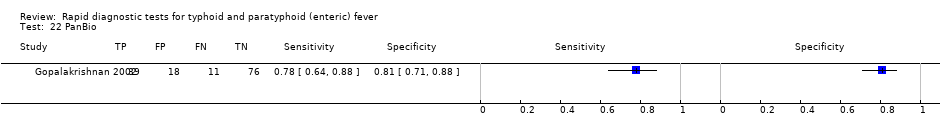
| 22 PanBio Show forest plot | 1 | 144 |
| 23 SD Bioline. Antibody: IgG Show forest plot | 3 | 1669 |
| 24 SD Bioline. Antibody: IgM Show forest plot | 3 | 1590 |
| 25 SD Bioline Antibody: IgM and IgG Show forest plot | 1 | 300 |
| 26 Mega Salmonella. Antibody: IgG Show forest plot | 1 | 177 |
| 27 Mega Salmonella. Antibody: IgM Show forest plot | 1 | 177 |
| 28 Multi‐Test Dip‐S‐Tick Show forest plot | 1 | 75 |
| 29 Enteroscreen Show forest plot | 1 | 1521 |
| 30 Onsite Typhoid Combo CTK Biotech Show forest plot | 2 | 436 |