Esteroides anabolizantes para reabilitação após fratura de quadril em idosos.
Resumo
Introdução
A fratura do quadril ocorre predominantemente em pessoas mais velhas, que geralmente são mais frágeis e desnutridas. Após a cirurgia de fratura de quadril e o período de reabilitação, ocorre um declínio da mobilidade e da função da maioria dos pacientes. Os atletas usam esteroides anabolizantes (derivados sintéticos do hormônio masculino testosterona) junto com exercícios para aumentar sua massa muscular e sua força. Essa intervenção pode ter efeitos similares nos idosos que estão se recuperando de uma fratura de quadril.
Objetivos
Avaliar os efeitos (desfechos funcionais e eventos adversos) do uso de esteroides anabolizantes em idosos que passaram por cirurgia para fratura de quadril.
Métodos de busca
Fizemos buscas nas seguintes base de dados: Cochrane Bone, Joint and Muscle Trauma Group Specialized Register (10 setembro de 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, issue 8), MEDLINE (1946 até a 4a semana de agosto de 2013), EMBASE (1974 a setembro de 2013, semana 36) e plataformas de registros de ensaios clínicos. Também fizemos buscas manuais em anais de congressos, e nas listas de referências de artigos relevantes. A busca foi realizada em setembro de 2013.
Critério de seleção
Incluímos ensaios clínicos randomizados (ECR) que avaliaram o uso de esteroides anabolizantes em pacientes idosos internados ou ambulatoriais que haviam passado por cirurgia de fratura de quadril. Os desfechos deveriam incluir melhora da função física.
Coleta dos dados e análises
Dois revisores, trabalhando de forma independente, selecionaram os estudos (baseado em critérios de inclusão pré‐definidos), extraíram os dados, e avaliaram o risco de viés de cada estudo incluído. Um terceiro revisor resolveu as divergências. Pudemos combinar poucos dados. Os desfechos primários foram função (como independência para mobilidade e atividades da vida diária) e eventos adversos, incluindo morte.
Principais resultados
Avaliamos 1290 referências e incluímos apenas três estudos envolvendo 154 participantes. Todos eram mulheres, com mais de 65 anos, que haviam passado por cirurgia para fratura de quadril. Todos estudos tinham deficiências metodológicas e foram portanto classificados como tendo risco de viés alto ou incerto. Devido ao alto risco de viés dos estudos, à imprecisão dos resultados, e à probabilidade de viés de publicação, classificamos a qualidade da evidência para todos os desfechos primários como muito baixa.
Os estudos testaram duas comparações. Um estudo tinha três grupos e contribuiu com dados para as duas comparações. Nenhum dos estudos avaliou a aceitabilidade da intervenção pelos pacientes.
Dois estudos bastante heterogêneos compararam esteroides anabolizantes versus controle (não usar anabolizantes ou placebo). Um ECR comparou injeções semanais de esteroides anabolizantes até a alta hospitalar ou por quatro semanas (o que viesse primeiro) versus injeções de placebo em 29 "mulheres idosas frágeis". Não houve diferença entre os dois grupos (evidência de qualidade muito baixa) quanto ao número de mortes (1 pessoa do grupo controle morreu), ou de pacientes transferidos para uma unidade de cuidados mais intensivos (8/15 versus 10/14, RR 0,75, IC 95% 0,42 a 1,33, P = 0,32), ou no tempo até o paciente se movimentar com independência, ou quanto à frequência de eventos adversos. O segundo ECR comparou injeções de esteroides anabolizantes (a cada três semanas durante seis meses) mais suplementos proteicos diários versus suplementos proteicos diários em 40 "idosas magras" que foram acompanhadas no primeiro ano após a cirurgia. Segundo este estudo, o uso de esteroides anabólicos pode reduzir o número de pacientes dependentes em pelo menos duas funções, ou o número de mortes (1 caso no grupo controle morreu) na avaliação realizada entre 6 e 12 meses após a cirurgia. Porém, os resultados também indicam que a intervenção pode produzir piora da função ou não ter nenhum efeito sobre esse desfecho: 1/17 versus 5/19, RR: 0,22, IC 95% 0,03 a 1,73, P = 0,15. O estudo não encontrou diferença na frequência de eventos adversos.
Dois estudos compararam o uso de esteroides anabolizantes combinados com outra intervenção nutricional versus não usar esteroides. Um deles comparou injeções de esteroides anabolizantes a cada três semanas durante 12 meses em combinação com suplementação diária de vitamina D e cálcio versus apenas cálcio em 63 mulheres que eram independentes e viviam em suas casas. O outro estudo envolveu 40 "idosas magras" e comparou injeções de esteroides anabolizantes a cada três semanas durante seis meses mais suplementos proteicos diários versus um grupo controle. Os dois estudos encontraram alguma evidência de melhora da função no grupo que usou esteroides. Um estudo relatou maior independência, maior escore de Harris, e maior velocidade de marcha nos participantes do grupo esteroide aos 12 meses. O outro estudo relatou que o grupo dos esteroides teve menor número de participantes dependentes em pelo menos duas funções ou mortes, incluindo banhar‐se (1/17 versus 7/18, RR: 0,15, IC 95% 0,02‐1,10, P = 0,06) com 6 e 12 meses. Ocorreu um óbito no grupo controle. A metanálise dos dois estudos não mostrou diferença entre os grupos na mortalidade aos 12 meses (2/51 versus 3/51). Também não houve diferença na taxa de eventos adversos individuais entre os grupos. Em um dos estudos, três participantes do grupo esteroide relataram eventos adversos (voz grossa e aumento dos pelos faciais). O outro estudo relatou melhora da qualidade de vida no grupo com esteroide.
Conclusão dos autores
As evidências disponíveis são insuficientes para permitir conclusões sobre os efeitos dos esteroides anabolizantes (isolados ou combinados com suplementos nutricionais) sobre desfechos funcionais e eventos adversos em pacientes idosos que passaram por cirurgia para fratura de quadril. Como os dados disponíveis sugerem um efeito promissor dos esteroides anabolizantes combinados com suplementos nutricionais, recomendamos que as futuras pesquisas avaliem esta combinação.
PICOs
Resumo para leigos
Esteroides anabolizantes para reabilitação após fratura de quadril em idosos.
Porque os esteroides anabolizantes podem ajudar a pessoa que teve uma fratura do quadril a se recuperar
As fraturas de quadril ocorrem principalmente nos idosos, pois muitas pessoas mais velhas são mais frágeis. Após a cirurgia para fratura do quadril, a maioria dos pacientes perde massa muscular e força. Apesar da reabilitação, a maioria dos pacientes tem uma perda progressiva da sua mobilidade e função motora no longo prazo. Os atletas usam esteroides anabolizantes (derivados sintéticos do hormônio masculino testosterona) junto com exercícios para aumentar sua massa muscular e sua força. Esta revisão avaliou as evidências sobre o uso de esteroides anabolizantes para ajudar na recuperação dos idosos que tiveram uma fratura de quadril.
Descrição dos estudos incluídos nesta revisão
Fizemos buscas para encontrar estudos médicos que houvessem sido realizados até setembro de 2013. Identificamos três estudos relevantes com um total de 154 mulheres com mais de 65 anos que haviam passado por cirurgia de quadril. Dois estudos foram conduzidos na Suíça e um no Canadá. Os estudos avaliaram duas comparações. Um estudo teve três grupos e contribuiu com dados para as duas comparações.
Qualidade da evidência
Encontramos apenas três estudos. Todos eram pequenos (poucos participantes) e tinham alto risco de viés. Portanto, consideramos a qualidade da evidência como muito baixa. Isso significa que não temos certeza quanto à confiabilidade desta evidência.
Resumo da evidência
Dois estudos muito diferentes compararam esteroides anabolizantes versus controle (placebo ou não usar esteroide anabolizante). Um estudo envolveu 29 “senhoras frágeis" internadas em um hospital e comparou injeções semanais de esteroides anabolizantes versus injeções de placebo. Este estudo não encontrou evidência que o uso dos esteroides anabolizantes melhorasse a função das pacientes. Isso foi avaliado pelo número de pacientes que foram encaminhadas para um centro mais especializado, ou que morreram, ou o tempo até que a paciente pudesse se locomover. O segundo estudo envolveu 40 idosas magras e comparou o uso de injeções de esteroides a cada três semanas por seis meses mais tomar um suplemento proteico diário versus tomar um suplemento proteico diário. Este estudo concluiu que o uso dos esteroides anabolizantes pode tanto melhorar como piorar ou não produzir nenhuma diferença na função das pacientes. Nenhum estudo encontrou diferenças na incidência de eventos adversos individuais nos dois grupos.
Dois estudos compararam um grupo que recebeu esteroides anabolizantes mais uma intervenção nutricional versus um grupo controle que não recebeu esteroides. Um estudo comparou duas intervenções diferentes em 63 mulheres independentes e que moravam sozinhas. Um grupo recebeu injeções de esteroides anabolizantes a cada três semanas por 12 meses mais suplementos diários de vitamina D e cálcio enquanto o outro grupo recebeu apenas suplementos diários de cálcio. O outro estudo envolveu 40 idosas magras e comparou injeções de esteroides anabolizantes a cada três semanas por seis meses mais suplementos diários de proteína versus um grupo controle. Os dois estudos encontraram alguma evidência de melhora da função no grupo que recebeu esteroide. A combinação dos dados dos dois estudos mostrou não haver nenhuma diferença entre os grupos quanto ao risco de morte dentro de um ano. Também não houve diferença na taxa de eventos adversos individuais entre os grupos. Em um dos estudos, três participantes do grupo esteroide relataram engrossamento da voz e crescimento de pelos no rosto, como eventos adversos. O outro estudo relatou melhora da qualidade de vida no grupo dos esteroides mais suplementos. Nenhum dos estudos avaliou a aceitabilidade da intervenção pelas participantes.
Conclusões
A qualidade da evidência foi muito baixa. Isso quer dizer que não temos muita certeza quanto ao tamanho ou à direção do efeito da intervenção. Portanto, não sabemos se o uso de esteroides anabolizantes, (isoladamente ou junto com suplementos alimentares) melhora a recuperação de idosos que passaram por cirurgia de fratura do quadril. Como os dados disponíveis sugerem resultados mais promissores quando os esteroides anabolizantes são usados junto com suplementos alimentares, recomendamos que os futuros estudos testem essa combinação.
Authors' conclusions
Background
Description of the condition
Fracture of the proximal femur (known widely as hip fracture) is a common cause of morbidity and mortality in older people. About 40% to 50% of women and 13% to 22% of men are at risk of having an osteoporotic fracture in their lifetime (Dennison 2006). With the rise in life expectancy, the prevalence of hip fracture is expected to increase (Cooper 1992; Gullberg 1997). There is worldwide variation in the incidence of hip fracture. While it is already an established health problem in the West, it is increasingly recognised as a growing problem in Asia (Mithal 2009).
Surgical management is the mainstay of the treatment for hip fracture. This is generally followed by inpatient rehabilitation, with or without extension to an outpatient rehabilitation programme (Marks 2003). Despite treatment, functional recovery after hip fracture is often incomplete, with many people who were walking independently before their hip fracture losing their independence afterwards (Osnes 2004). This negatively impacts on their quality of life (Adachi 2001). By six to 12 months after a hip fracture, between 22% and 75% of people have not recovered their pre‐fracture ambulatory or functional status (Cummings 1988; Koval 1995). People sustaining hip fracture use extensive health system resources (Braithwaite 2003), and many patients require continued support and care services. After their initial treatment, people who have had a hip fracture are at high risk for re‐hospitalisation (Wolinsky 1997), refracture (Johnell 1985) and institutionalisation (Rosell 2003; Tajeu 2013). Older adults have five‐ to eight‐fold increased risk of dying during the first three months after hip fracture (Haentjens 2010). There is also some evidence that functional recovery after hip fracture is the main determinant of long‐term mortality (Dubljanin‐Raspopović 2013).
Description of the intervention
Following surgical treatment of hip fracture, a wide range of therapies are used to promote functional recovery (SIGN 2009). Some of these have specific goals such as restoration of mobility and independence in basic activities of daily living. This review focuses on the use of anabolic steroids for restoring and maintaining function after hip fracture surgery.
Anabolic steroids are a group of synthetic hormones, related to the male hormone testosterone, that promote the storage of protein and the growth of tissue (anabolism) (Dorland 2007). Their use has been demonstrated to have a positive effect in the treatment of diverse clinical conditions, including the treatment of anaemia in renal disease patients (Navarro 2002;Teruel 1996), osteoporosis (specifically bone density), cachexia (muscle wasting) in people with chronic illness (Johns 2009), and improving muscle mass and strength in older people (Snyder 1999). Women show an age‐related decline in endogenous androgen levels, which might influence the development of osteoporosis (Zofkova 2000). A double‐blind randomised controlled trial showed better mobility and less pain in people with vertebral fractures after treatment with anabolic steroids compared with a vitamin D analogue, alphacalcidol (Lyritis 1994).
Anabolic steroids come in different preparations, which can be given various ways (e.g. orally, skin patches, intramuscular injections), started at different times (prior to, or at any stage of recovery after hip fracture surgery) and can be administered for different lengths of time.
How the intervention might work
Patients with hip fractures are often elderly, frail and undernourished (Bachrach‐Lindström 2000; Lumbers 2001). They may undergo a catabolic state (Patterson 1992), which leads to chronic muscle wasting and reduced muscle strength. This can affect mobility and result in falls. Loss of muscle mass and lean body weight contribute to generalised weakness, an impaired immune response and slower wound healing. Anabolic steroids provide some benefit in other conditions associated with increased catabolic rates such as burns, chronic obstructive airway disease and acquired immune deficiency syndrome (AIDS) (Berger 1996).
There is also good reason to combine the use of anabolic steroids with nutritional supplementation. Protein energy malnutrition occurs in 30% to 50% of people who sustain a hip fracture (Lumbers 1996; Ponzer 1999). Postoperative hip fracture rehabilitation is facilitated by improving the nutritional intake of the patient (Delmi 1990). A Cochrane review concluded that some evidence exists for the beneficial effects of nutritional supplementation after hip fracture (Avenell 2006). There is some evidence that combining testosterone and nutritional supplementation for undernourished older people reduces both the number of people hospitalised and the duration of hospital admissions (Chapman 2009).
Adverse effects, often dose‐related, from anabolic steroids include changes in voice, growth of facial hair in women, hair loss, acne, oedema, thromboembolic events and liver damage.
Why it is important to do this review
Despite advances in surgical treatments, hip fractures continue to have a large impact on older people and society because they often result in disability and institutionalisation (Fierens 2006; Osnes 2004). Anabolic steroids may have a role in improving outcomes and recovery, and allowing greater independence in these patients. It is important to assess the evidence for the use of these drugs in this predominantly elderly and frail population.
Objectives
To examine the effects (primarily in terms of functional outcome and adverse events) of anabolic steroids after surgical treatment of hip fracture in older people.
The following main comparisons were intended, set in the context of usual or conventional care.
-
Anabolic steroid versus no anabolic steroid or a placebo control.
-
Anabolic steroid in conjunction with other intervention (either nutrition or exercise or both) versus no anabolic steroid plus same other intervention or a placebo control.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials of anabolic steroid treatment following surgery for hip fracture. We planned also to include trials that used quasi‐randomisation (e.g. allocation by date of birth or hospital record number) or cluster randomisation (e.g. by hospital ward). The failure to use an intention‐to‐treat approach to analysis was not a reason for exclusion.
Types of participants
We considered older people with any type of hip fracture that was surgically treated. It was anticipated that a large proportion of these people would be older than 65 years of age. We did not exclude trials that included younger participants if the mean age minus one standard deviation was greater than 65 years. Participants younger than 65 years, or with multi‐trauma or with pathological fractures, would have been included provided they made up less than 25% of the total sample size, and there was adequate randomisation of these participants to intervention and control groups.
Types of interventions
The intervention assessed was anabolic steroids, administered enterally (orally, nasogastric or via percutaneous gastrostomy tubes), parenterally (intramuscular routes) or via alternative routes such as transdermal. The intervention could start prior to or at any stage of recovery after hip fracture surgery, but interventions that were pre‐surgical only were excluded. The duration of administration could vary and it was acceptable for it to continue until the end of the rehabilitation phase. We compared the administration of anabolic steroids with the provision of no intervention or a placebo intervention. It was envisaged that usual or conventional care would be provided to all trial participants. Such care, as described below, could comprise nutrition or exercise or both.
We included the following comparisons, set in the context of usual or conventional care.
-
Anabolic steroid versus no anabolic steroid or a placebo control. This included studies where nutrition or exercise or both were provided to both groups in the comparison.
-
Anabolic steroid plus 'other intervention', where this was either nutrition or exercise or both, versus no anabolic steroid plus same other intervention or a placebo control.
Types of outcome measures
Primary outcomes
The primary outcome was function: for example, independence in mobility and activities of daily living. We gave preference to validated, patient‐reported outcome measures. We also sought data on adverse events including mortality, hospital readmission and complications from the use of anabolic steroids.
Secondary outcomes
Secondary outcomes were patients' perceived quality of life, adherence and acceptability of the intervention, objective assessments of body composition, nutritional indices, muscle strength and the use of resources such as length of hospital stay.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (10 September 2013), the Cochrane Central Register of Controlled Trials (The Cochrane Library, 2013 Issue 8), MEDLINE (1946 to August Week 4 2013), MEDLINE In‐Process & Other Non‐Indexed Citations (9 September 2013) and EMBASE (1974 to 2013 Week 36). We also searched Current Controlled Trials and the WHO international Clinical Trials Registry Platform for ongoing and recently completed trials (September 2013). We did not impose any restrictions based on language or publication status.
In MEDLINE (OvidSP), the subject specific search was combined with the sensitivity‐maximizing version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). Search strategies for the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE are shown in Appendix 1.
Searching other resources
We assessed the American Orthopaedic Trauma Association's annual meetings (1996 to 2012) by handsearching the table of contents of the meeting proceedings. We also searched reference lists of relevant articles.
Data collection and analysis
The intended methodology for data collection and analysis was described in our published protocol (Farooqi 2010).
Selection of studies
Two review authors (VF and MvdB) independently screened titles and abstracts of records identified from database searches for possible inclusion. We retrieved the full text of potentially relevant citations. From the full text, we selected trials that met the selection criteria for inclusion. We sought further information from trial authors where necessary. A third author (MC) moderated any disagreement. We documented the reasons for exclusion.
Data extraction and management
Two review authors (VF and MvdB) independently performed data extraction using a piloted form. The data collected included study design characteristics, study population, interventions, outcome measures, and length of follow‐up. We attempted to contact trial authors for clarification when necessary. Disagreements were resolved by a third review author (MC).
Assessment of risk of bias in included studies
Two review authors (VF and MvdB) independently assessed risk of bias using a piloted version of The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011). Disagreements were resolved by discussion. We assessed random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other biases, including those associated with major baseline imbalance and early stopping. We rated each domain as either 'low risk'; 'unclear risk' or 'high risk' of bias.
Measures of treatment effect
We analysed results both at short‐term (six months or less) and longer‐term (more than six months) intervals. We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes. We calculated mean differences with 95% CIs for continuous outcomes. We had planned to use standardised mean differences for pooling continuous outcomes based on differences in scores or scales.
Unit of analysis issues
The unit of randomisation in these trials was the individual patient. However, we would have considered randomised trials where the unit of randomisation was another entity, such as a hospital ward. If possible, appropriate adjustments would have been made before presenting data from such trials if the trial authors had not adjusted for clustering. We planned to seek advice on the interpretation and presentation of the results from such trials from the statistical editors of the Cochrane Bone, Joint and Muscle Trauma Review Group.
Dealing with missing data
We attempted to contact the authors of included trials for missing data. Where appropriate, we intended to perform intention‐to‐treat analysis to include all people randomised. However, where drop‐outs were identified, the actual denominators of participants contributing data at the relevant outcome assessment were used. When possible, in future updates, we will investigate the effect of drop‐outs and exclusions by conducting worst‐ and best‐case scenario sensitivity analyses. The 'best‐case' scenario is when all participants with missing outcomes in the experimental intervention group are assigned a good outcome, and all those with missing outcomes in the control intervention group a bad outcome; the 'worst‐case' scenario is the converse. We will continue to be alert to potential mislabelling or non‐identification of standard errors and standard deviations. Additionally, unless missing standard deviations can be derived from CIs, P values or standard errors, we will not assume values in order to present these in the analyses.
Assessment of heterogeneity
We planned to assess heterogeneity by visual inspection of the forest plot (analysis) and consideration of the Chi² test for heterogeneity and the I² statistic (Higgins 2003). We interpreted I² values as follows: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
If at least 10 trials had contributed data to a forest plot, we would have considered preparing a funnel plot to assess publication bias. We investigated selective outcome reporting by comparing the study outcomes with those routinely presented for similar studies and also by comparing the methods section with the results reported in the trials.
Data synthesis
Where considered appropriate, we pooled results of comparable groups of trials. We used the fixed‐effect model and 95% CIs. We would have considered using the random‐effects model, especially where there was unexplained heterogeneity. It was anticipated that we would pool data even if heterogeneity was high. For continuous outcomes, where outcomes were reported using different scales or instruments but assessing the same dimension, the results were to be pooled using the standardised mean difference. Mindful of unit of analysis issues, we intended to pool the data from cluster‐randomised trials using the generic inverse variance.
Subgroup analysis and investigation of heterogeneity
If sufficient data are available in future, we will conduct subgroup analyses to determine whether the primary outcomes vary according to gender and route of steroid administration. We will use the test for subgroup differences available in Review Manager (RevMan 2014) to determine if the results for subgroups are statistically significantly different.
Sensitivity analysis
Had sufficient data been available, we would have performed sensitivity analyses to explore the effects of important sources of bias, such as whether allocation was concealed, in the included studies.
Assessment of the quality of the evidence
We used the GRADE approach to assess the quality of evidence related to the primary outcomes listed in the Types of outcome measures (Schunemann 2011, section 12.2). Where there is sufficient evidence in future to merit the preparation of 'Summary of findings' tables, we will develop these for the main comparisons.
Results
Description of studies
Results of the search
The search was completed in September 2013. We screened a total of 1290 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (8 records); Cochrane Central Register of Controlled Trials (CENTRAL) (113), MEDLINE (598), EMBASE (480), the WHO International Clinical Trials Registry Platform (27) and Current Controlled Trials (64).
We identified six potentially eligible studies, published in 10 reports. On full review of their texts, we included three trials (Hedstrom 2002 (two reports); Sloan 1992;Tidermark 2004 (four reports)) and excluded two (Beringer 1986; Naessen 2008). One trial (NCT00280267), while completed, has yet to be published (see the Characteristics of ongoing studies).
A flow diagram summarising the study selection process is shown in Figure 1.
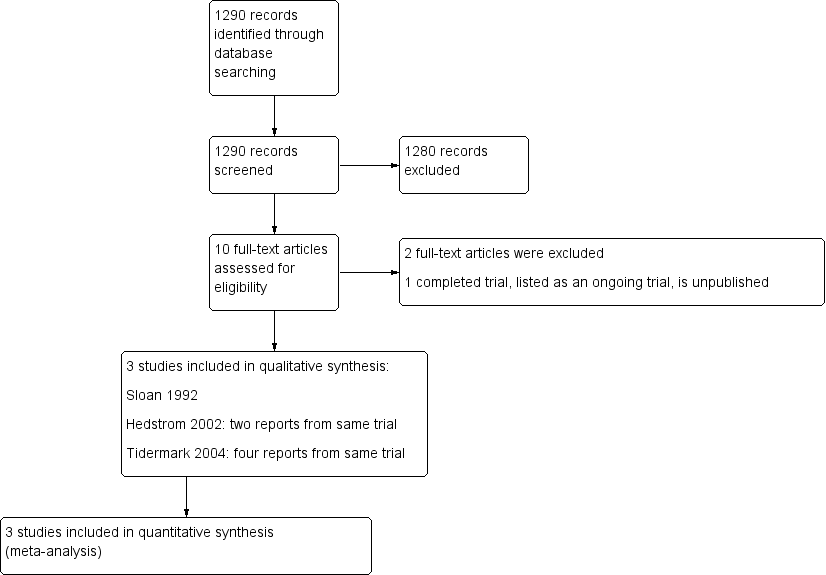
Study flow diagram
Included studies
We included three trials (Hedstrom 2002; Sloan 1992; Tidermark 2004), all of which investigated the effects of anabolic steroids on recovery from hip fracture surgery in older people aged 65 years or over. The three trials included a total of 154 female participants (Table 1). Sloan 1992 also included eight males but provided only a brief summary of the results of this small subgroup. For completeness, we present the results for adverse events for this subgroup in this review.
| Study | Number of participants | Mean (SD) age (years) | Mean (SD) weight (kg) | Cognitive status | Pre‐injury function | Nutritional status |
| 63 | 80.5 (6.3) | 60.8 (11.0) | Dementia excluded | Independent living status | BMI < 24 | |
| 29 (of 31) | 82 (6.5) | 57.8 (9.9) | Severe dementia excluded | Extended care home residents excluded only | No information | |
| 60 | 82.9 (5.4) | 53.3 (8.8) | Severe cognitive impairment excluded | Independent living status | BMI < 24 |
All study participants described above were females. However, no other details and very limited results were provided for eight male participants in Sloan 1992
BMI: body mass index
SD: standard deviation
Summaries of the three trials are given below. For further details, please see the Characteristics of included studies.
Hedstrom 2002 compared nandrolone decanoate (steroid) at the dose of 25 mg intramuscularly every third week for one year and a daily supplement of 1alpha‐hydroxylated vitamin D3 (0.25 g) plus calcium (500 mg) versus calcium (500 mg) alone (control group). This study, conducted in Sweden, included 63 women (mean age 80.5 years) who were living independently at home after hip fracture surgery. Follow‐up assessments were at 3, 6, 9 and 12 months.
Sloan 1992 included 31 women (mean age 82 years) who had been treated surgically for hip fracture. They compared weekly nandrolone decanoate (steroid) intramuscular injections 2 mg/kg versus a placebo group. The steroid injections were given weekly until discharge from hospital or four weeks, whichever came first. Follow‐up assessments were weekly until four weeks or discharge, whichever came first.
Tidermark 2004 was conducted in Sweden and involved 60 women (mean age 83 years) who had hip fracture surgery. The purpose was to evaluate the effects of a protein‐rich liquid supplementation, alone or in combination with the anabolic steroid nandrolone decanoate. The participants were randomised to the following groups.
-
Steroid plus group: nandrolone decanoate (Deca‐Durabol 25 mg every three weeks) + protein‐rich formula (Fortimel 200 ml/day, 20 g protein/day) + daily calcium (1 g) and vitamin D (CalchichewD3 800 IE).
-
Control group (protein supplementation): protein‐rich formula (Fortimel) + daily calcium + vitamin D (doses as above).
-
Control group: daily calcium and vitamin D (doses as above).
Follow‐up assessments were at six and 12 months. The Tengstrand 2007 publication was a post hoc analysis of Tidermark 2004 and thus not a separate trial.
Excluded studies
We excluded two studies. The primary reason for excluding Beringer 1986, which compared the relative effects on calcitonin secretion of anabolic steroid (Stanozolol) versus oral calcium in 20 women with hip fracture, was the lack of a no treatment control. We excluded Naessen 2008 because it included only healthy women without hip fracture. For more details, please see the Characteristics of excluded studies.
Risk of bias in included studies
Summaries of our risk of bias assessment of the included studies are presented in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
None of the trials gave details of their method of random sequence generation and we therefore judged all three trials as being at unclear risk of bias for this item.
Two trials (Hedstrom 2002; Tidermark 2004) used sealed envelopes; Tidermark 2004 further described these as opaque and was thus considered to be at low risk of selection bias relating to successful concealment of allocation. The risk of selection bias in Hedstrom 2002 was judged as unclear. The same applied for Sloan 1992, which did not disclose their method of allocation concealment.
Blinding
Hedstrom 2002 did not describe blinding of either participants, investigators or outcome assessors and we judged this trial to be at high risk of both performance and detection bias. Sloan 1992 did not provide details of blinding but we considered this trial to be at low risk of performance and detection bias given that it was clearly reported to be a double‐blind, placebo‐controlled study. Tidermark 2004 was 'open label' and at high risk of performance bias; however, although it did not describe blinding, there was independent outcome assessment, which we judged put it at 'unclear' rather than high risk of detection bias.
Incomplete outcome data
Only Tidermark 2004 was at low risk of attrition bias as it used an intention‐to‐treat analysis and gave a participant flow diagram, illustrating losses at 12 months follow‐up. While both Hedstrom 2002 and Sloan 1992 documented the reasons for drop‐outs in their trials, the reporting of losses was incomplete and unclear in Hedstrom 2002. The added failure to add in the denominators in the tables makes this trial at high risk of attrition bias. Similarly, we judged Sloan 1992 as being at high risk of attrition bias, reflecting both the large percentage of participants with missing data at four weeks (39% = 12/31) (patients being discharged from their inpatient stay) and an imbalance between the two groups in those available at four weeks (12/16 versus 7/15). This is a consequence of a flawed study design where no efforts were made to obtain post‐discharge outcomes.
Selective reporting
Published trial registration information or protocols were not available for any of the three trials. However, all the outcomes that were listed in the methods sections were reported in the results. All three trial reports had aspects that appeared to indicate post hoc reporting decisions and thus we judged them as being unclear for reporting bias. Hedstrom 2002 reported functional outcomes using the Katz Index but provided the median and range and it was not clear if they intended to report the three‐month results. Tidermark 2004 used the same scale (the Katz Index) as Hedstrom 2002 but did not report means and standard deviations. Instead they chose to present the results in a graph format without giving actual values. They also reported significant differences among groups in terms of the quality of life scores using EQ‐5D index but again presented the results as a graph. We judged Sloan 1992 to be at unclear risk of bias because of the incomplete reporting of the results of the male participants and the lack of clarity about whether they intended to report males and females separately from the outset.
Other potential sources of bias
We judged Hedstrom 2002 and Tidermark 2004 to be at low risk of other bias, specifically bias relating to major imbalances in baseline characteristics and early stopping. Baseline characteristic data for Sloan 1992 provided for 29 of the 31 female participants (none were provided for the male participants) showed the participants in the steroid group had lower functional ability and were more dependent in activities of daily living than in the placebo group. We judged this trial as being at high risk of other bias.
Effects of interventions
Anabolic steroid versus no anabolic steroid or a placebo intervention
Sloan 1992 compared anabolic steroid with placebo intervention, reporting results for 17 participants at four weeks follow‐up. Tidermark 2004 compared anabolic steroid and protein supplementation versus protein supplementation alone, reporting results for 35 participants at 6 and 12 months follow‐up. The large difference in the timing of follow‐up of these two trials meant that pooling data for common outcomes was inappropriate.
Primary outcomes
Functional independence
One person in the control group of Sloan 1992 died in hospital. Similar numbers of participants in the two groups of Sloan 1992 were discharged to a higher level of care. The results of the combined outcome of death or discharge to a higher level of care ('more dependent or dead at hospital discharge') are shown in Analysis 1.1 (8/15 versus 10/14, risk ratio (RR) 0.75, 95% CI (confidence interval) 0.42 to 1.33; P = 0.32).
One person in the control group (nutrition only) of Tidermark 2004 died within one year. Tidermark 2004 based their functional assessment on the results of the Katz Index and used graphs to display the results grouped by independence in five or all six activities of daily living functions versus dependence in bathing and at least one other function. Fewer participants in the anabolic steroid group were either dependent in at least two functions or dead at six months (0/17 versus 3/19; RR 0.16, 95% CI 0.01 to 2.87; P = 0.21) and at 12 months (1/17 versus 5/19; RR 0.22, 95% CI 0.03 to 1.73; P = 0.15) (Analysis 1.2); neither result was statistically significant.
Although the anabolic steroid group in Sloan 1992 took a longer time to stand with support (steroid: 3.1 days versus control: 2.3 days; MD 0.80 days, 95% CI ‐0.59 to 2.19 days; P = 0.26), and five days longer on average to mobilise independently (steroid: 14.5 days versus control: 9.3 days; MD 5.20 days, 95% CI ‐1.68 to 12.08 days; P = 0.14), neither of the differences between the two groups was statistically significant (Analysis 1.3).
Adverse events
One participant in the control group of each trial died within the follow‐up period (Analysis 1.4). Since the number of participants experiencing an adverse event was not available, details of the individual events are described below and displayed in Analysis 1.5. This is restricted to female participants only.
Sloan 1992 withdrew one participant in the steroid group because of the doubling of the serum liver enzymes after their second injection (liver function tests were normal within the next week) and one participant in the placebo group because of nightmares. Of the 15 remaining in the steroid group, there was one case of pulmonary embolism and two cases of delirium within one week post‐surgery. In the 14 participants in the control group, there was one case of deep venous thrombosis, one gastrointestinal bleed and two participants developed post‐operative depression. There was a total of 10 units of blood transfused to participants in the steroid group and seven units to participants in the placebo group.
Of the eight male participants in Sloan 1992, one of the three steroid group participants required a transurethral prostatectomy after developing a urinary obstruction. One participant in each group became depressed. In the control group, one participant had a deep venous thrombosis and one had a syncopal event.
In Tidermark 2004, fracture healing complications were recorded in six participants of the steroid group versus four of the control group; and urinary tract infection was reported for five versus three participants. A slight and transient elevation of serum calcium was noted in six participants of the steroid group.
Secondary outcomes
Patients' perceived quality of life
Tidermark 2004 captured patient‐rated health‐related quality of life (HRQoL) during the week preceding the injury using the EuroQol (EQ‐5D). They performed a logistic regression analysis in order to evaluate factors of importance for changes in the HRQoL. Randomisation to the combined anabolic steroid and protein supplement group (steroid group) was reported to be associated with an increased odds ratio for improved HRQoL at the end of the six month intervention period (P < 0.05). These results were presented as a graph only and no baseline values, changes in scores or means or standard deviations were given in textual format or a table.
Adherence and acceptability of the intervention
All but one participant in the steroid group of Sloan 1992 received at least four doses of the anabolic steroid. In Tidermark 2004, one patient withdrew consent following randomisation, but prior to receiving the intervention. The intramuscular nandrolone injections were administered by a research nurse in patients' own homes while the compliance regarding the protein‐rich formula and calcium/vitamin D was verified with interviews and logbooks. The adherence for steroid administration was 100%.
Neither trial recorded patient acceptability of the intervention nor, in Sloan 1992, of the weekly injection.
Objective assessments of body composition
On average, participants in the steroid group in Sloan 1992 were lighter and had lower lean body mass (measured in three ways) and smaller mid‐arm muscle circumference than participants on placebo group at baseline; a difference that persisted in the follow‐up data (Analysis 1.6).
Tidermark 2004 showed smaller declines in body weight and lean body mass (measured using dual energy X‐ray absorptiometry) at 12 months in the steroid group; the differences between the two groups did not reach statistical significance (Analysis 1.7).
Nutritional indices
Both Sloan 1992 and Tidermark 2004 reported changes in albumin as a marker for nutritional improvement during recovery from hip fracture. Neither trial found a difference between the two groups (Analysis 1.8). None of the differences in three other nutritional indices reached statistical significance in Sloan 1992.
Muscle strength
Sloan 1992 and Tidermark 2004 both reported on hand grip strength as a measure of muscle strength. Neither study found a statistically significant difference between the groups at final follow‐up (four weeks: Sloan 1992; one year: Tidermark 2004) (Analysis 1.9).
Use of resources such as length of hospital stay
With the exception of length of hospital stay data, neither trial reported resource use or cost outcomes. In Sloan 1992, the steroid group stayed in hospital an average of five days longer than those in the placebo group (steroid: 45 days versus control: 40 days; MD 5.00 days, 95% CI ‐11.46 to 21.46 days; P = 0.55); the difference between the two groups was not statistically significant (Analysis 1.10). Tidermark 2004 reported that the difference in length of hospital stay in the first year after surgery was not significant (median (range): 18 (8 to 51) days versus 20 (5 to 356) days).
Anabolic steroid plus other intervention ('steroid plus') versus no anabolic steroid plus some other intervention or a placebo control
The two trials (Hedstrom 2002; Tidermark 2004) in this comparison differed in their co‐interventions. Hedstrom 2002 compared anabolic steroid plus vitamin D plus calcium (steroid plus group) with calcium alone (control group); and reported results for an estimated 44 participants at 12 months follow‐up. Tidermark 2004 compared anabolic steroid and protein supplementation (steroid plus group) versus no steroid or protein supplementation (control group); and reported results for 34 patients at follow‐up.
Primary outcomes
Functional independence
Hedstrom 2002 used the Katz index to assess the level of independence in activities of daily living. Both the steroid plus group and the control group had equal medians at both six and 12 months (median = 6); however, the range was narrower and towards improved independence at 12 months in the steroid plus group (reported P = 0.05; Analysis 2.1).
Tidermark 2004 based their functional assessment on the results of the Katz Index and used graphs to display the results grouped by independence in five or all six activities of daily living functions versus dependence in bathing and at least one more function. Fewer participants in the anabolic steroid group were either dependent in at least two functions, including bathing, or dead at six months (0/17 versus 8/16; RR 0.06, 95% CI 0.00 to 0.89; P = 0.04) and 12 months (1/17 versus 7/18; RR 0.15, 95% CI 0.02 to 1.10; P = 0.06) (Analysis 2.2). One person in the control group had died within 12 months.
Hedstrom 2002 reported significantly higher Harris hip scores in the steroid plus group at six months (median 86 versus 72; reported P = 0.006) and 12 months (median 88.5 versus 79; reported P = 0.04) (Analysis 2.3), suggesting a better outcome for this group.
Gait speed
Hedstrom 2002 reported significantly higher gait speeds in the steroid plus group at both six months (P = 0.007) and 12 months (P = 0.009) post‐intervention (Analysis 2.4).
Adverse events
Hedstrom 2002 reported four deaths, two in each group, up to 12 months follow‐up (Analysis 2.5). Seven participants, four in the steroid plus group and three in the control group developed pseudarthrosis or avascular necrosis and underwent arthroplasty subsequently (Analysis 2.6). Three participants of the steroid group had side effects such as hoarseness or increased facial hair.
In Tidermark 2004, one person died in the control group. Fracture healing complications were recorded in six participants of the steroid plus group versus seven of the control group; and urinary tract infection was reported for five versus three participants (Analysis 2.6). A slight and transient elevation of serum calcium was noted in six participants of the steroid group. Two participants in the steroid group developed deep infection.
Secondary outcomes
Patients' perceived quality of life
Tidermark 2004 captured patient‐rated HRQoL during the week preceding the injury using the EuroQol (EQ‐5D). A logistic regression analysis was performed in order to further evaluate factors of importance for changes in the HRQoL. Randomisation to the steroid plus group was associated with an increased odds ratio for improved HRQoL at the end of the six month intervention period (P < 0.05). These results were presented as a graph only with no presentation of actual values, means or standard deviations.
Adherence and acceptability of the intervention
Hedstrom 2002 stated that participants who discontinued treatment "were analysed by intention‐to‐treat", but gave only limited information relating to adherence. Four people, two in each group, declined to participate in the study after their hip surgery and two others (group not stated) appeared to have stopped due to ill health at seven and eight months. There were several others lost to follow‐up, including four deaths. Aside from one person allocated in the steroid plus group who withdrew her consent, Tidermark 2004 reported 100% compliance for the anabolic steroid administration and that, based on interviews and a logbook, there appeared to be "no indications of inadequate compliance" regarding the protein supplementation or vitamin D and calcium interventions.
Neither trial reported directly on the acceptability of the intervention to the participants.
Objective assessments of body composition
The data for the various measures relating to body composition presented in Hedstrom 2002 are shown in Analysis 2.7. No measures of variability were reported. The differences between the two groups in the changes in the body mass index or skinfold thickness between groups at six and 12 months were generally statistically not significant. There was a significantly greater increase in muscle volume in the steroid plus group at six months (P = 0.004) and at 12 months (P = 0.002) in the non‐operated leg. The operated leg results also favoured the steroid plus group (P = 0.01 at both follow‐ups). Hedstrom 2002 calculated relative muscle mass to demonstrate that these differences were not due to intracellular fluid accumulation, a known side‐effect of anabolic steroid treatment.
Tidermark 2004 found no statistically significant differences between the two groups in the decline in body weight and lean body mass (measured using dual energy X‐ray absorptiometry) at 12 months (Analysis 2.8).
Nutritional indices
Tidermark 2004 found no statistically significant difference between the two groups in changes in albumin at 12 months (Analysis 2.9).
Muscle strength
Tidermark 2004 reported on hand grip strength as a measure of muscle strength. Although the results favoured the steroid plus group, the difference between the two groups in grip strength at one year did not reach statistical significance (P = 0.16) (Analysis 2.10).
Use of resources such as length of hospital stay
With the exception of length of hospital stay data for Tidermark 2004, neither trial reported resource use or costs outcomes. Tidermark 2004 reported that the difference in length of hospital stay in the first year after surgery was not significant (median (range): 18 (8 to 51) days versus 27 (5 to 197) days).
Discussion
Summary of main results
The aim of this review was to evaluate the effect of anabolic steroids on functional outcome after surgical treatment of hip fracture in older people. We included three heterogeneous randomised controlled trials, involving a total of 154 female patients after hip fracture surgery. As well as being small trials, all three had methodological shortcomings that placed them at high or unclear risk of bias. One trial had three groups and contributed data to the two main comparisons listed in our Objectives. Reflecting the high risk of bias of the included trials, the imprecision of the results and the risk of publication bias, we concluded that the evidence for all primary outcomes is of very low quality, which means that we are very uncertain about the results.
Anabolic steroid versus control (no anabolic steroid or a placebo intervention)
The two trials that compared anabolic steroid with control tested this in very different settings and patient populations. Sloan 1992, a 'pilot' trial, compared anabolic steroid injections versus placebo injections given weekly until discharge from hospital or four weeks, whichever came first, in 31 "frail elderly females" (mean age 82 years). Tidermark 2004 compared anabolic steroid injections every three weeks for six months and daily protein supplementation versus daily protein supplementation alone, in 40 "lean elderly women" (mean age 82 years) who were followed up for one year post‐surgery. These differences and the incompatible outcome measurement, including timing of follow‐up, precluded pooling of data. Sloan 1992 provided very low quality evidence of little difference between the two groups in the combined outcome of discharge to a higher level of care or dead, in the restoration of mobility (time to weight‐bear; time to mobilise independently) or in individual adverse events. It is plausible that the tendency in the steroid group to take longer to weight‐bear and walk independently could relate to the poorer pre‐fracture function of this group. One participant in this group withdrew after a doubling of serum liver enzymes. Tidermark 2004 provided very low quality evidence that anabolic steroid may result in less dependency, assessed in terms of being either dependent in at least two functions or dead at six and 12 months, but the result was also compatible with no difference or an increase in dependency. Tidermark 2004 found no evidence of between‐group differences in adverse events (fracture healing complications; urinary tract infection). Both trials reported nearly complete compliance with the administration of the steroid injection. Neither trial reported on patient acceptability of the intervention.
Anabolic steroid plus nutritional co‐intervention (steroid plus) versus control (no anabolic steroid plus nutritional co‐intervention)
Although the study design and patient population of two trials that compared anabolic steroid plus a nutritional co‐intervention versus control were sufficiently similar to warrant pooling, this was possible only for one‐year mortality. Hedstrom 2002 compared anabolic steroid injections every three weeks for 12 months and a daily supplement of vitamin D versus control in 63 women (mean age 80.5 years) who were living independently at home after hip fracture surgery. Tidermark 2004 compared anabolic steroid injections every three weeks for six months and daily protein supplementation versus control in 40 "lean elderly women" (mean age 83 years) who were followed up for one year post‐surgery.
Although data could not be pooled, both trials found some very low quality evidence of better function in the steroid plus group. One trial reported a narrower distribution of Katz scores towards greater independence in the steroid plus group at 12 months. The same trial reported significantly higher Harris hip scores and gait speeds for both six months and 12 months follow‐up times in the steroid plus group. The second trial found fewer participants in the anabolic steroid group were either dependent in at least two functions, including bathing, or dead at six months and 12 months (one person in the control group had died within 12 months). Pooled mortality data from the two trials showed no evidence of a difference between the two groups at one year. Similarly, there was no evidence of between‐group differences in adverse events. However, Hedstrom 2002 reported that three participants (9.4%) of the steroid group had side effects (hoarseness or increased facial hair) of anabolic steroids while Tidermark 2004 reported better quality of life in the steroid plus group. Tidermark 2004 also reported nearly complete compliance with the administration of the steroid injection. Neither trial reported on patient acceptability of the intervention.
Overall completeness and applicability of evidence
All three trials were small and pooling of data was undertaken for one outcome (mortality) from two trials only.
Since hip fractures occur predominantly in older females, the restriction to older females in the three trials is acceptable although it limits applicability. A further limitation is that all three trials excluded patients with severe cognitive impairment or dementia, which is present in around 25% to 33% of the typical hip fracture population. Other considerations include that Sloan 1992 was an inpatient study, with a more representative population in the hospital setting, whereas Hedstrom 2002 was an outpatient trial that included only people who were able to live independently in their own home after recovery from hip fracture surgery.
As well as differing in their populations, the trials differed in their interventions, co‐interventions, control groups, durations and outcome assessment. Sloan 1992 used weekly nandrolone decanoate intramuscular injections at a dose of 2 mg/kg until discharge or four weeks, whichever came first. In contrast, Hedstrom 2002 and Tidermark 2004 used 25 mg intramuscular nandrolone injections every three weeks for 12 and six months, respectively. These two trials also used steroids in combination with either protein supplement or Vitamin D. Combining interventions adds complexity to the interpretation as interaction between interventions could be responsible for the effects, or the effect could be solely attributed to the nutritional intervention other than the anabolic steroid. The variability in the duration and dosages of anabolic steroids makes it difficult to establish what doses should be tested in future studies. Cummings 1988 and Koval 1995 have documented the deterioration in function following hip fracture and the deterioration in the control group in Tidermark 2004 at six months is not unexpected.
There was a lack of information on what constituted "standard rehabilitation" in Sloan 1992, but the average length of hospital stay in this trial is noticeably longer than that reported in a recent study carried out in a nearby hospital (43 versus 24 days) in Vancouver (Lefaivre 2009). Similarly, the prolonged treatment regimen in both Hedstrom 2002 and Tidermark 2004 is not customary in many countries.
The follow‐up until discharge in Sloan 1992 resulted in bias because of the missing data for participants at the four‐week time point. The presentation of outcome data relating to dependence was inadequate in all three trials. In Sloan 1992, this was reported as a change in the level of care but it is unclear how permanent this change was and what exactly this meant. Hedstrom 2002 provided inadequate data to assess their conclusions, while Tidermark 2004 provided dichotomised data that may hide more subtle variation. Side effects of anabolic steroids were reported in the three trials, the most serious one occurring in a male patient in Sloan 1992. These trials were too small to get any meaningful measure of the harms of anabolic steroid treatment. However, Hedstrom 2002 reported three participants with well known side effects (hoarseness and facial hair) of anabolic steroids in females, and this points to the importance of recording patient acceptability of this intervention, which was not done in these trials.
Quality of the evidence
Overall, this review presents very low quality evidence for the effects of anabolic steroids for rehabilitation of hip fractures in older people. Each of three small trials were at high risk of bias in one or more domains evaluated in our assessment. Despite the use of a placebo control group in Sloan 1992, there was a major baseline imbalance in participant characteristics that could have affected the findings of this trial. Post‐randomisation exclusions and failure to follow‐up patients after hospital discharge also reduces the quality of the evidence on the use of anabolic steroids in the inpatient setting. The two studies (Tidermark 2004 and Hedstrom 2002) that reported positive results, favouring the use of anabolic steroid in combination with nutritional interventions, were not adequately blinded as no placebo injections were given to the control group. The interaction between interventions could be responsible for the effects, or the effect could be solely attributed to the nutritional intervention other than the anabolic steroid. As well as downgrading for limitations in design and implementation (risk of bias), we downgraded the evidence for imprecision (all three trials) and publication bias (all three trials), reflecting the very small numbers of published trials on this topic.
Potential biases in the review process
Although we conducted extensive searches and were careful and systematic in our screening processes, it is possible that we may have failed to identify studies, especially those that are unpublished. Additionally, we may have missed published trials that are only listed in other databases such as CINAHL and LILACS. We were unsuccessful in our attempts to get further information and data on the included trials. Deviations and changes from protocol described in Differences between protocol and review were few and unlikely to bias the review findings.
Agreements and disagreements with other studies or reviews
To our knowledge there have been no previous systematic reviews on the use of anabolic steroids for recovery after hip fracture surgery. There is some moderate quality evidence showing improved outcomes in patients with hip fractures when nutritional supplementation has been used (Avenell 2010), which points to the importance of considering anabolic steroids as part of a package of interventions aimed at improving recovery. We have not identified cohort studies monitoring the short‐term or long‐term adverse effects of anabolic steroids in older people.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Anabolic steroids versus control, Outcome 1 More dependent or dead at hospital discharge.

Comparison 1 Anabolic steroids versus control, Outcome 2 Dependence in bathing and one more function (Katz ADL index) or dead.

Comparison 1 Anabolic steroids versus control, Outcome 3 Mobility (inpatient).

Comparison 1 Anabolic steroids versus control, Outcome 4 Mortality.

Comparison 1 Anabolic steroids versus control, Outcome 5 Adverse events.

Comparison 1 Anabolic steroids versus control, Outcome 6 Body composition at 4 weeks follow‐up.

Comparison 1 Anabolic steroids versus control, Outcome 7 Body composition at 12 months follow‐up.

Comparison 1 Anabolic steroids versus control, Outcome 8 Nutritional indices.

Comparison 1 Anabolic steroids versus control, Outcome 9 Muscle strength.

Comparison 1 Anabolic steroids versus control, Outcome 10 Length of hospital stay (days).
| Study | Assessment | Steroid plus nutrition group | Control group | Reported P value |
| Hedstrom 2002 | 6 months: median (range); N | 6 (5 to 6); N = 26 | 6 (5 to 6); N = 24 | 0.7 |
| Hedstrom 2002 | 12 months: median (range); N | 6 (5 to 6); N = 21 | 6 (2 to 6); N = 23 | 0.05 |
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 1 Katz index of ADL (Score 0 to 6; 6 = completely independent, 0 = totally dependent).

Comparison 2 Anabolic steroids with other intervention versus control, Outcome 2 Dependence in bathing and one more function (Katz ADL index) or dead.
| Study | Assessment | Steroid plus nutrition group | Control group | Reported P value |
| Hedstrom 2002 | 6 months: median (range); N | 86 (59 to 100); N = 26 | 72 (42 to 91); N = 24 | 0.006 |
| Hedstrom 2002 | 12 months: median (range); N | 88.5 (64 to 100); N = 21 | 79 (48 to 98); N = 23 | 0.04 |
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 3 Harris hip score (100 point scale; higher score = better outcome).
| Study | Assessment | Steroid plus nutrition group | Control group | Reported P value |
| Hedstrom 2002 | 6 months: median (range); N | 34 (20 to 70); N = 26 | 50 (20 to 145); N = 24 | 0.007 |
| Hedstrom 2002 | 12 months: median (range); N | 30 (20 to 90); N = 21 | 46 (25 to 250); N = 23 | 0.009 |
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 4 Gait speed (s/30m).

Comparison 2 Anabolic steroids with other intervention versus control, Outcome 5 Mortality (12 months).

Comparison 2 Anabolic steroids with other intervention versus control, Outcome 6 Adverse events.
| Study | Assessment | Steroid plus nutrition | Control group | Reported P value |
| BMI (kg/m²) | ||||
| Hedstrom 2002 | 6 months: mean change | ‐1.3 | ‐1.0 | "NS" (> 0.05) |
| Hedstrom 2002 | 12 months: mean change | ‐1.6 | ‐0.5 | "NS" (> 0.05) |
| Muscle volume operated leg (cm²) | ||||
| Hedstrom 2002 | 6 months: mean change | ‐0.1 | ‐7.1 | 0.01 |
| Hedstrom 2002 | 12 months: mean change | 3 | ‐6.3 | 0.01 |
| Muscle volume non‐operated leg (cm²) | ||||
| Hedstrom 2002 | 6 months: mean change | 12.6 | 8.6 | 0.004 |
| Hedstrom 2002 | 12 months: mean change | 14.1 | 8.1 | 0.002 |
| Relative muscle mass operated leg (cm²) | ||||
| Hedstrom 2002 | 6 months: mean change | 339 | ‐25 | 0.03 |
| Hedstrom 2002 | 12 months: mean change | 617 | ‐161 | 0.002 |
| Relative muscle mass non‐operated leg (cm²) | ||||
| Hedstrom 2002 | 6 months: mean change | 836 | 573 | 0.02 |
| Hedstrom 2002 | 12 months: mean change | 1049 | 434 | 0.0005 |
| Triceps skinfold (mm) | ||||
| Hedstrom 2002 | 6 months: mean change | ‐1.6 | 0.5 | 0.04 |
| Hedstrom 2002 | 12 months: mean change | ‐1.6 | 0.6 | "NS" (> 0.05) |
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 7 Body composition.

Comparison 2 Anabolic steroids with other intervention versus control, Outcome 8 Body composition at 12 months follow‐up.

Comparison 2 Anabolic steroids with other intervention versus control, Outcome 9 Nutritional indices: albumin (g/L) change from baseline at 12 months (higher values suggest better outcome).

Comparison 2 Anabolic steroids with other intervention versus control, Outcome 10 Muscle strength: grip strength (hand grip dynamometer (kg)); difference from baseline at 12 months.
| Study | Number of participants | Mean (SD) age (years) | Mean (SD) weight (kg) | Cognitive status | Pre‐injury function | Nutritional status |
| 63 | 80.5 (6.3) | 60.8 (11.0) | Dementia excluded | Independent living status | BMI < 24 | |
| 29 (of 31) | 82 (6.5) | 57.8 (9.9) | Severe dementia excluded | Extended care home residents excluded only | No information | |
| 60 | 82.9 (5.4) | 53.3 (8.8) | Severe cognitive impairment excluded | Independent living status | BMI < 24 | |
| All study participants described above were females. However, no other details and very limited results were provided for eight male participants in Sloan 1992 BMI: body mass index SD: standard deviation | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 More dependent or dead at hospital discharge Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Dependence in bathing and one more function (Katz ADL index) or dead Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mobility (inpatient) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Days to weight‐bear with support | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Days to mobilise independently | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mortality Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 In hospital | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 At 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Fracture healing complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Urinary track infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Thrombotic complication (deep vein thrombosis or pulmonary embolism) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Gastrointestinal bleed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Postoperative depression | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Postoperative delirium | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Body composition at 4 weeks follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Weight (kg) (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Midarm muscle circumference (cm²) (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Lean body mass measured by subscapular skinfold (kg) (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Lean body mass via bioelectric impedence (kg) (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Body composition at 12 months follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Weight (kg): difference from baseline (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Lean body mass by dual energy X‐ray absorptiometry (kg) difference from baseline (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Nutritional indices Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Albumin (g/L) at 4 weeks (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Albumin (g/L) change from baseline at 12 months (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Haemoglobin (g/L) at 4 weeks (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Muscle strength Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Grip strength (modified sphygmomanometer (kPa)) at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Grip strength (hand grip dynamometer (kg)); difference from baseline at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Length of hospital stay (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Katz index of ADL (Score 0 to 6; 6 = completely independent, 0 = totally dependent) Show forest plot | Other data | No numeric data | ||
| 2 Dependence in bathing and one more function (Katz ADL index) or dead Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Harris hip score (100 point scale; higher score = better outcome) Show forest plot | Other data | No numeric data | ||
| 4 Gait speed (s/30m) Show forest plot | Other data | No numeric data | ||
| 5 Mortality (12 months) Show forest plot | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.15, 3.46] |
| 6 Adverse events Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Arthroplasty for avascular necrosis or pseudarthrosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Side effects of anabolic steroids | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Fracture healing complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Deep infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Urinary track infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Body composition Show forest plot | Other data | No numeric data | ||
| 7.1 BMI (kg/m²) | Other data | No numeric data | ||
| 7.2 Muscle volume operated leg (cm²) | Other data | No numeric data | ||
| 7.3 Muscle volume non‐operated leg (cm²) | Other data | No numeric data | ||
| 7.4 Relative muscle mass operated leg (cm²) | Other data | No numeric data | ||
| 7.5 Relative muscle mass non‐operated leg (cm²) | Other data | No numeric data | ||
| 7.6 Triceps skinfold (mm) | Other data | No numeric data | ||
| 8 Body composition at 12 months follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Weight (kg): difference from baseline (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Lean body mass by dual energy X‐ray absorptiometry (kg) difference from baseline (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Nutritional indices: albumin (g/L) change from baseline at 12 months (higher values suggest better outcome) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Muscle strength: grip strength (hand grip dynamometer (kg)); difference from baseline at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

