Analgesia para el parto con fórceps
Resumen
Antecedentes
El parto con fórceps puede estar indicado cuando el feto no logra progresar hasta el nacimiento o cuando es necesario acelerar el parto en el período expulsivo. Es necesaria una analgesia eficaz que asegure que la paciente esté cómoda durante el parto para permitir que el obstetra realice con seguridad el procedimiento. Actualmente no están claros el agente o el método más efectivos y seguros para proporcionar alivio del dolor durante el parto con fórceps.
Objetivos
Evaluar la efectividad y la seguridad de diferentes agentes y métodos analgésicos disponibles para el parto con fórceps para las pacientes y los fetos.
Métodos de búsqueda
Se hicieron búsquedas en el Registro de Ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (31 de julio de 2013) se revisaron las guías publicadas y se buscó en las listas de referencias de los artículos de revisión.
Criterios de selección
Ensayos controlados aleatorios que compararan un agente o método analgésico utilizado para el parto con fórceps con placebo / ningún tratamiento o un agente o método alternativo.
Obtención y análisis de los datos
Dos revisores de forma independiente evaluaron la elegibilidad de los estudios, extrajeron los datos y evaluaron el riesgo de sesgo de los estudios incluidos.
Resultados principales
Se incluyeron cuatro ensayos con 388 mujeres, los que se consideraron con un riesgo de sesgo general de incierto a alto. En los ensayos se evaluaron diversos agentes para proporcionar analgesia y se usaron varios métodos diferentes para medir el alivio del dolor, por lo que no fue posible combinarlos en un metanálisis. Tres ensayos compararon diazepam con un agente alternativo (ketamina; vinydan‐éter; "otro" agente anestésico) para proporcionar anestesia general y un ensayo comparó analgesia espinal con bloqueo nervioso pudendo (en ambos grupos se administró lidocaína).
Con respecto a los resultados primarios, las pacientes que recibieron diazepam para el parto con fórceps en un ensayo pequeño tuvieron mayores probabilidades de considerar eficaz el alivio del dolor en comparación con las pacientes que recibieron vinydan‐éter (cociente de riesgos [CR] 1,13; intervalo de confianza [IC] del 95%: 1,02 a 1,25; 101 pacientes). En un ensayo pequeño adicional no se observaron diferencias significativas en el número de pacientes que consideraron eficaz el alivio del dolor cuando se comparó diazepam con ketamina (CR 1,42; IC del 95%: 0,98 a 2,07; 26 pacientes). En el ensayo que comparó analgesia espinal con bloqueo nervioso pudendo fue significativamente más probable que las pacientes que recibieron analgesia espinal consideraran adecuada la analgesia (CR 3,36; IC del 95%: 2,46 a 4,60; 183 pacientes) y fue menos probable que informaran dolor intenso durante el parto con fórceps (CR 0,02; IC del 95%: 0,00 a 0,27; 183 pacientes). Ningún ensayo informó sobre los otros dos resultados primarios de la revisión (efectos adversos o complicaciones maternas graves, y morbilidad grave o mortalidad neonatal).
En cuanto a los resultados secundarios, las pacientes que recibieron diazepam en comparación con las que recibieron vinydan‐éter tuvieron significativamente menos probabilidades de presentar vómitos (CR 0,04; IC del 95%: 0,00 a 0,62; 101 pacientes). No se observaron diferencias significativas en los pocos resultados neonatales que se informaron a través de cualquiera de las comparaciones (que incluyeron puntuación de Apgar menor de 7 a los cinco minutos y acidosis definida como pH arterial de la sangre del cordón menor de 7,2).
Conclusiones de los autores
No hay pruebas suficientes para apoyar cualquier agente o método analgésico particular como más eficaz para proporcionar alivio del dolor en el parto con fórceps. La mayoría de los resultados neonatales no se ha evaluado.
PICO
Resumen en términos sencillos
Analgesia para el parto con fórceps
Los fórceps son instrumentos diseñados para agarrar la cabeza del feto y ayudar en su nacimiento. Se han creado muchos tipos diferentes de fórceps. Los fórceps se pueden utilizar cuando el feto no logra progresar hasta el nacimiento o para ayudar a acortar el trabajo de parto de la madre cuando es necesario, por ejemplo, cuando la madre está exhausta en el período expulsivo, si se sospecha sufrimiento fetal o cuando la madre presenta un trastorno médico como una afección cardíaca, respiratoria o neurológica que le pueda impedir pujar. Cuando una paciente requiere el uso de fórceps para ayudarle al parto del feto necesita un alivio eficaz del dolor (analgesia) para que permanezca cómoda y ayude al médico a realizar el procedimiento con seguridad.
Esta revisión encontró que no hay pruebas suficientes a partir de los cuatro ensayos controlados aleatorios incluidos, con 388 mujeres y fetos, para determinar la técnica o el agente analgésico más eficaz y seguro para las pacientes que tienen un parto con fórceps. Tres de los cuatro ensayos compararon diazepam con agentes alternativos (ketamina, vinydan‐éter, u "otro" agente anestésico) para proporcionar anestesia general durante el parto con fórceps. Se utilizaron diferentes métodos para medir el alivio del dolor y los resultados no pudieron combinarse. Los datos de un ensayo no se pudieron incluir en la revisión. En un ensayo pequeño las pacientes que recibieron diazepam tuvieron mayores probabilidades de considerar eficaz el alivio del dolor en comparación con las pacientes que recibieron vinydan‐éter. Sin embargo, en otro ensayo pequeño no hubo diferencias en el alivio del dolor cuando el diazepam se comparó con la ketamina. En el ensayo que comparó analgesia espinal con bloqueo nervioso pudendo, las pacientes que recibieron analgesia espinal tuvieron mayores probabilidades de informar un alivio adecuado del dolor y tuvieron menores probabilidades de informar dolor intenso. Ninguno de los cuatro ensayos informó complicaciones graves o muerte de la madre o el feto.
Los ensayos incluidos tuvieron un riesgo alto o incierto de sesgo y no fueron de gran calidad. Cada uno de los cuatro ensayos incluidos se realizó antes de 1980 y evaluaron agentes o métodos que no se utilizan habitualmente en la práctica clínica actual. Por lo tanto, se necesitan más estudios para determinar qué fármaco o técnica es más eficaz y seguro para reducir el dolor de la madre. Estos estudios también deben evaluar la seguridad del feto.
Authors' conclusions
Background
Forceps have been used since the 17th century to help deliver babies by applying traction to the fetal head (Ross 2012). In those times, it was common for women to be heavily sedated during labour and childbirth. Around the middle of the last century, women undergoing forceps deliveries were often given a general anaesthetic, but it soon became clear that the use of a general anaesthetic for this indication was associated with significant maternal morbidity and mortality. Use of general anaesthesia for forceps delivery is now rare, with Li 2011 reporting that in Australia in 2009, only three in 1000 women undergoing an instrumental delivery (vacuum or forceps) were administered a general anaesthetic.
In the 1950s Gate 1955, trialled the use of local analgesia for forceps delivery in 65 women, finding improvements in maternal and perinatal morbidity, as well as greater maternal satisfaction. A short time later, O'Sullivan 1962 described the use of pethilorfan (pethidine, levallorphan and promethazine) administered as a slow intravenous injection for forceps delivery, causing the woman to fall asleep but wake with each contraction. Since then, various forms of local and regional anaesthesia have become the mainstay of analgesia for forceps delivery.
The type of forceps to be used may depend on the specific indications and conditions. Clinical guidelines have however acknowledged that the choice may often be subjective, with over 700 different models of forceps in existence, and with no randomised controlled trial evidence to support one model over another (RCOG 2011). The most commonly used forceps are Simpson forceps, which are used to deliver a moulded fetal head, as is commonly seen in nulliparous women. Also commonly used are Tucker‐McLane forceps, which have a more rounded cephalic curve, more suitable for the unmoulded fetal head commonly seen in multiparous women (Ross 2012).
Description of the condition
Typically, forceps are used when a singleton fetus in the cephalic position fails to progress to delivery or when delivery needs to be expedited in the second stage of labour because of fetal distress. Indications for forceps delivery include delay (prolonged second stage) or maternal exhaustion in the second stage of labour; analgesic drug‐related diminished urge to push (associated with epidural or spinal anaesthesia); suspected fetal distress (for example, in the presence of non‐reassuring fetal heart tracing); after‐coming head in breech delivery; and maternal medical conditions (e.g. cardiac, respiratory or neurologic conditions) that preclude pushing (Patel 2004; RCOG 2011; SOGC 2004).
While instrumental vaginal birth has been a frequently and widely practiced obstetric intervention, declining rates have been reported (Bailey 2005), along with great variation in practice worldwide particularly when considering high‐ and low‐resource settings (Ameh 2009). In high‐resource settings, reported rates of instrumental delivery vary from 10% to 15% in the United Kingdom (NHS 2012; RCOG 2011), to 14.8% in Canada (Public Health Agency of Canada 2008), 12% in Australia (Li 2011), and as low as 4.5% in the United States (where the rate has reportedly halved over the last two decades) (Martin 2009). In low‐resource settings, rates of less than 1% have been reported (such as for sub‐Saharan Africa) (Bailey 2005). Instrumental vaginal delivery has been identified as an under‐utilised evidence‐based intervention particularly in low‐resource settings, such as in Africa, Asia, Latin America and the Caribbean, with the potential to prevent maternal deaths associated with prolonged and obstructed labour (Ameh 2009).
As obstetrics forceps preceded the development of the ventouse (vacuum extraction device), forceps were for a number of decades the primary instrument for assisted vaginal births. While in some (particularly low‐resource) settings, this may still be the case. More recently there has been an increase in the use of ventouse compared to forceps for instrumental births, with forceps deliveries now comprising, for example only 4.6% of births in Canada (Public Health Agency of Canada 2008), 3.7% of births in Australia (Li 2011), and less than 1% of all births in the United States (Martin 2009; Ross 2012).
Description of the intervention
Regional analgesia (especially epidural) is commonly used in forceps deliveries (Li 2011; NHS 2012; Osterman 2011); women may for example request an epidural during their labour, which may be 'topped up' if a forceps delivery is indicated. In 2009 in Australia, approximately 50.6% of all instrumental births (vacuum extraction or forceps) used epidural or caudal methods (Li 2011); while in 2011 to 2012 in England approximately 37.3% and 51.5% of instrumental spontaneous and induced births, used epidural or caudal methods (NHS 2012). Comparatively, spinal anaesthetic was used in only 2.7% of instrumental births in Australia in 2009; and in 9.5% and 6.3% of instrumental spontaneous and induced births in England in 2011 to 2012. In the United States, rates of epidural/spinal anaesthesia use during forceps delivery and vacuum extraction have been estimated as 83.8% and 77.3% respectively (Osterman 2011).
Local anaesthetics (such as pudendal block or local infiltration) are also commonly used during instrumental births (5.2% and 28.4% respectively in Australia in 2009) (Li 2011), although regional anaesthesia is often preferred for forceps delivery (Gibbs 2008). As previously detailed, the rate of use of general anaesthetic during instrumental vaginal births is now considered extremely low; estimated as 0.5% in Australia in 2009 (Li 2011) and between 0.4% to 0.5% in England in 2011 to 2012 (NHS 2012).
How the intervention might work
Effective analgesia may help to ensure that the woman remains as comfortable as possible throughout the forceps procedure and subsequently, which should also help the obstetrician perform the procedure safely. While the aim of analgesia is to give sufficient coverage with the least amount of pain and fewest adverse effects, different analgesic agents and methods will vary in their capacity to balance anaesthetic coverage, pain relief and the avoidance of adverse effects.
Why it is important to do this review
It is important to assess the effects of different types/methods of analgesia for forceps delivery in order to inform women and obstetricians of the most effective and safe methods, associated with fewest adverse consequences for women and their babies.
Objectives
To assess the effectiveness and safety of different analgesic agents and methods available for forceps delivery for women and their babies.
Methods
Criteria for considering studies for this review
Types of studies
All identified randomised and quasi‐randomised trials assessing and comparing the effects of different analgesics (or methods/techniques for providing analgesia) for forceps delivery. We planned to exclude cluster‐randomised and cross‐over trials. We planned to include studies presented as abstracts.
Types of participants
Pregnant women in the second stage of labour undergoing forceps delivery for any indication, including all singleton and twin deliveries with cephalic and breech presentation.
Types of interventions
Different methods, any mode or combination of analgesics compared with placebo or no treatment, or compared with an alternative method or pharmacological agent.
Types of outcome measures
Primary outcomes
Effects of intervention
-
Pain relief, however measured by the authors
Safety of intervention
-
Serious maternal adverse effects or complications associated with the intervention (as defined by trial authors) (e.g. in relation to regional analgesia: local anaesthetic toxicity (seizures, cardiac rhythm abnormality with cardiac arrest, unconsciousness, death), nerve/spinal cord damage, epidural/intraspinal haematoma, infective complications (meningitis, epidural abscess)
-
Neonatal mortality or serious morbidity (as defined by trial authors) (e.g. fetal distress, low Apgar score less than seven at five minutes, need for neonatal intensive care unit (NICU) or special care neonatal admission)
Secondary outcomes
Maternal
Effects of intervention
-
Request for additional analgesia
-
Maternal satisfaction with childbirth experience (as defined by trial authors)
Safety of intervention
-
Mother‐baby bonding (as defined by trial authors)
-
Breastfeeding success and duration (as defined by trial authors)
-
Side effects for the mother (as defined by trial authors), including:
-
Postnatal depression (treatment for depression or self‐reported)
-
Maternal hypotension
-
Motor blockade
-
Respiratory depression requiring oxygen administration
-
Headache
-
Headache requiring blood patch
-
Vomiting
-
Itching
-
Fever
-
Shivers
-
Drowsiness
-
Urinary retention
-
Other outcomes relating to use of health services
-
Duration of postpartum hospital stay
-
Postpartum hospital admission within six weeks of discharge
Neonatal
Safety of intervention
-
Side effects for the baby, including:
-
Acidosis as defined by cord blood arterial pH less than 7.2
-
Acidosis as defined by cord blood arterial pH less than 7.15
-
Naloxone administration
-
Neonatal hypoglycaemia (less than or equal to 1.67 mmol/L)
-
-
Neonatal intensive care unit admission
-
Apgar score less than seven at five minutes
-
Long‐term neonatal complication (as defined by trialists e.g. seizures, disability in childhood)
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (31 July 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
weekly searches of Embase;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searched the register for each review using the topic list rather than keywords.
Searching other resources
We reviewed published guidelines and searched the reference lists of review articles.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2012) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at a low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study, we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐included missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. high attrition (greater than 20%) or numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials and the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
We considered cluster‐randomised trials inappropriate for inclusion in this review.
Dealing with missing data
We noted levels of attrition for the included study. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis (however, we were unable to do this due to the paucity of data, with no two trials included together in a meta‐analysis).
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We planned to assess statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We planned to regard heterogeneity as substantial if the I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analyses using the Review Manager software (RevMan 2012). We planned to use a fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. We planned to treat the random‐effects summary as the average range of possible treatment effects and we planned to discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we would not have combined trials.
If we had used random‐effects analyses, we planned to present the results would as the average treatment effect with 95% confidence intervals, with the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We planned to consider whether an overall summary was meaningful, and if it was, use random‐effects analysis to produce it.
If possible, we planned to carry out the following subgroup analyses.
-
Types of analgesia, e.g. continuation of the existing analgesia through labour versus newly administered analgesia
-
Mode of analgesia, e.g. regional anaesthesia versus local analgesia
-
Analgesic agent used, e.g. systemic opioids versus nitrous oxide
We intended to use only the primary outcomes in subgroup analyses.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2012). We would have reported the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates of this review, we plan to carry out sensitivity analysis to explore the effects of trial quality assessed by allocation concealment and other risk of bias components, by omitting studies rated as 'high risk of bias' for these components. Sensitivity analysis will be restricted to the primary outcomes.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register found five trial reports (Ellingson 1977; Hutchins 1980; Mundow 1974; Sagen 1973; Pingsuthiwong 1992). We included four trials (Ellingson 1977; Hutchins 1980; Mundow 1974; Sagen 1973) involving 388 women, and excluded one trial (Pingsuthiwong 1992).
Included studies
We included four trials in this review (Ellingson 1977; Hutchins 1980; Mundow 1974; Sagen 1973). Two trials were conducted in Norway (Ellingson 1977; Sagen 1973), one in Ireland (Mundow 1974) and one in New Zealand (Hutchins 1980); all trials were conducted prior to 1980.
All trials included women requiring forceps delivery, however, the specific inclusions and exclusions of the trials varied. Ellingson 1977 included women for whom forceps delivery was indicated due to a second stage of labour exceeding 60 minutes, and excluded women with complications including: hypertension, pre‐eclampsia, epilepsy, premature labour and intrauterine asphyxia. In Hutchins 1980, all women who had not received regional analgesia and required an instrumental (forceps) delivery were included; women for whom the 'presenting part' was more than 2 cm below the ischial spines were excluded. Mundow 1974 included all forceps deliveries performed by registrars with no listed exclusions, and similarly Sagen 1973, included all women where there was fetal/maternal indication for a forceps delivery, with no specified exclusions.
Three trials compared the use of diazepam for providing general anaesthesia with an alternative agent. In Ellingson 1977 the general anaesthesia induced by diazepam (30 mg administered rapidly, with the use of nitrous oxide (N2O2) in a semi‐closed system on a mask), was compared with that induced by ketamine (2 mg/kg body weight given over 30 seconds intravenously; with no N2O2 given). Sagen 1973 similarly utilised 30 mg diazepam (dissolved in 9 mLl saline, administered intravenously over 30 seconds), however, it was compared with vinydan‐ether for general anaesthesia. In Mundow 1974 a lower dose of diazepam (10 mg administered intravenously) was compared with "other" anaesthesia (either general, local, or "other").
In the remaining trial (Hutchins 1980), spinal analgesia (lignocaine 1.5 mL 5% in 10% dextrose injected slowly after aspiration) was compared with pudendal nerve block anaesthesia (20 mL 1% lignocaine).
See Characteristics of included studies for further details.
Excluded studies
One study was excluded (Pingsuthiwong 1992) as it included all pregnant women (recruitment was not restricted to women undergoing forceps delivery) and data for forceps deliveries were not reported separately. For further details, see Characteristics of excluded studies.
Risk of bias in included studies
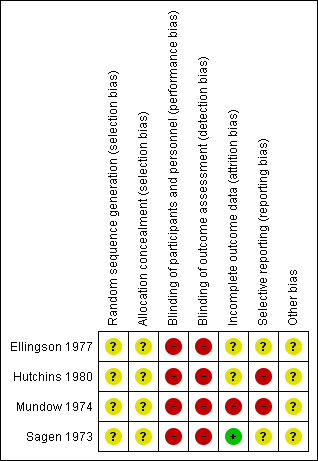
Overall, the trials were judged to be at an unclear to high risk of bias. Summaries for the risk of bias of the included studies are given in Figure 1 and Figure 2.
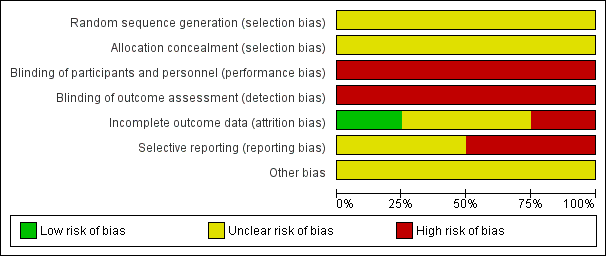
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All four included trials (Ellingson 1977; Hutchins 1980; Mundow 1974; Sagen 1973) were at an unclear risk of selection bias, with their methods for allocation concealment and for generation of the random number sequence being unclear (not detailed).
Blinding
The four included trials were at a high risk of bias due to a lack of (or believed ineffective) blinding (Ellingson 1977; Hutchins 1980; Mundow 1974; Sagen 1973). No trial detailed whether participants, personnel or outcome assessors were blinded; however, in all cases, effective blinding was considered unlikely due to the nature of the interventions being compared.
Incomplete outcome data
One trial was judged to be at a high risk of bias due to incomplete reporting; with maternal outcome data reported in Mundow 1974 for the diazepam group only (no maternal outcomes reported for the comparison group). Two further trials were judged to be at an unclear risk of bias due to incomplete outcome data (Ellingson 1977; Hutchins 1980), and one trial was judged to be at a low risk of bias, with no losses, withdrawals, exclusions or missing data evident (Sagen 1973).
Selective reporting
Two trials were judged to be at a high risk of selective reporting. In Hutchins 1980, outcomes were not pre‐specified, and a number of outcomes were reported incompletely, for example: "Mean Apgar scores were similar". In Mundow 1974, in addition to the lack of maternal outcomes reported for the comparison group, the outcomes reported were not clearly pre‐specified. The two remaining trials were judged to be at an unclear risk of bias, with outcomes not clearly pre‐specified, and/or incomplete reporting (including data reported in such a way that it could not be used in meta‐analysis if it had been applicable) (Ellingson 1977; Sagen 1973).
Other potential sources of bias
All four trials were judged to be at an unclear risk of other sources of potential bias (Ellingson 1977; Hutchins 1980; Mundow 1974; Sagen 1973); the absence of detailed trial methods for all studies made it difficult to make clear judgements.
For further details of the risk of bias components across each trial, see Characteristics of included studies.
Effects of interventions
A variety of different agents for providing analgesia were assessed in the four included trials, and a number of different methods to measure pain relief were utilised, and thus results could not be combined in meta‐analysis. The trials are therefore assessed in four separate comparisons.
Comparison 1: Diazepam versus ketamine
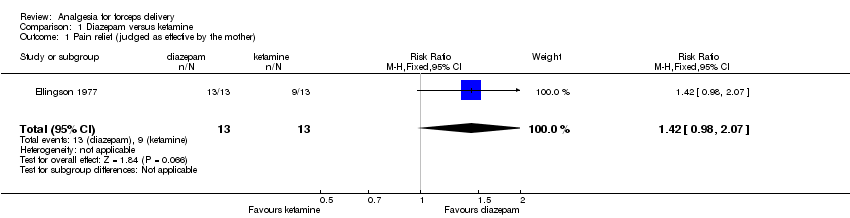
One small study was included in this comparison (Ellingson 1977), which compared the rapid intravenous administration of 30 mg diazepam (and N2O2 by mask), with the administration of 2 mg/kg ketamine over 30 seconds (no N2O2 was given).
Primary outcomes
Pain relief
In Ellingson 1977 women were asked to judge their pain relief as effective where they experienced no pain. Women receiving diazepam as compared with ketamine were not significantly more likely to judge their pain relief as effective (P = 0.07) (risk ratio (RR) 1.42; 95% confidence interval (CI) 0.98 to 2.07; 26 women) (Analysis 1.1).
Serious maternal adverse effects/complications and neonatal mortality or serious morbidity
No data on the other primary outcomes of serious maternal adverse effects or complications, or neonatal mortality or serious morbidity were reported in this trial.
Secondary outcomes
Maternal
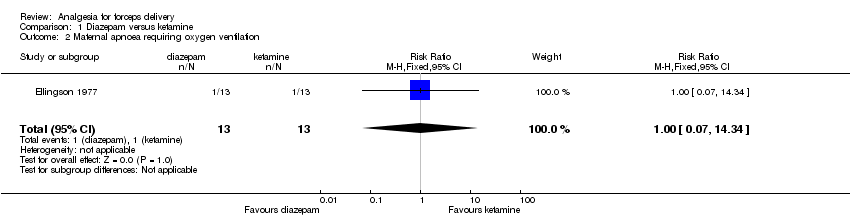
In this trial, one woman in each group experienced respiratory depression requiring oxygen ventilation (RR 1.00; 95% CI 0.07 to 14.34; 26 women) (Analysis 1.2) (Ellingson 1977).
No data on the review's other secondary maternal outcomes were reported by this trial including: maternal satisfaction with childbirth experience; request for additional analgesia; mother‐baby bonding; maternal hypotension as a result of regional anaesthesia; postnatal depression; breastfeeding success and duration; motor blockage; headache; headache requiring blood patch; vomiting; itching; fever; shivers; drowsiness; urinary retention; duration of postpartum hospital stay; and postpartum hospital admission within six weeks of discharge.
Neonatal
No significant differences were seen between the diazepam and ketamine groups for the two neonatal outcomes reported by this trial: Apgar score of less than seven at five minutes (no cases in either group) (Analysis 1.3), and acidosis as defined by cord blood arterial pH less than 7.2 (RR 1.10; 95% CI 0.08 to 15.36; 21 infants) (Analysis 1.4).
No data were reported for any of the other neonatal secondary review outcomes in this trial, including: acidosis defined by cord blood arterial pH less than 7.15; naloxone administration, NICU admission; neonatal hypoglycaemia; and long‐term complications.
Non pre‐specified outcomes
Ellingson 1977 reported on additional outcomes relating to pain relief and maternal satisfaction with the childbirth experience (that were not pre‐specified in the review protocol, but were thought to be important). Whilst women receiving diazepam were found to be significantly less likely to have good anaesthesia (judged by the obstetrician as when the woman was quiet) (RR 0.63; 95% CI 0.41 to 0.97; 26 women) (Analysis 1.5), they were significantly more likely to report a pleasant recovery (RR 2.08; 95% CI 1.17 to 3.68; 26 women) (Analysis 1.6). No significant difference was shown between diazepam and ketamine for the outcome maternal awareness ("when the patient claimed to have sensed the operation") (RR 0.11; 95% CI 0.01 to 1.88; 26 women) (Analysis 1.7).
Comparison 2: Diazepam versus vinydan‐ether
One trial was included in this comparison (Sagen 1973), which compared 30 mg diazepam given over 30 seconds, with vinydan‐ether (given by an anaesthetic nurse).
Primary outcomes
Pain relief
As in Ellingson 1977, women in Sagen 1973 were asked to judge their pain relief as effective where they experienced no pain. In this trial, women receiving diazepam were significantly more likely to judge the pain relief as effective (RR 1.13; 95% CI 1.02 to 1.25; 101 women) (Analysis 2.1).
Serious maternal adverse effects/complications and neonatal mortality or serious morbidity
No data on the other primary outcomes of serious maternal adverse effects or complications, or neonatal mortality or serious morbidity were reported in this trial.
Secondary outcomes
Maternal
In the Sagen 1973 trial, women receiving diazepam were significantly less likely to experience vomiting than those receiving vinydan‐ether (RR 0.04; 95% CI 0.00 to 0.62; 101 women) (Analysis 2.2).
No data on the review's other secondary maternal outcomes were reported by this trial including: maternal satisfaction with childbirth experience; request for additional analgesia; mother‐baby bonding; maternal hypotension as a result of regional anaesthesia; postnatal depression; breastfeeding success and duration; motor blockage; respiratory depression requiring oxygen administration; headache; headache requiring blood patch; itching; fever; shivers; drowsiness; urinary retention; duration of postpartum hospital stay; and postpartum hospital admission within six weeks of discharge.
Neonatal
No significant difference was seen between groups for the one neonatal outcome that the trial reported: Apgar score of less than seven at five minutes (RR 1.26; 95% CI 0.45 to 3.50; 104 infants) (Analysis 2.3).
No data were reported for any of the other neonatal secondary review outcomes in this trial, including: acidosis defined by cord blood arterial pH less than 7.15 and less than 7.2; naloxone administration, NICU admission; neonatal hypoglycaemia; and long‐term complications.
Non pre‐specified outcomes
Sagen 1973, like Ellingson 1977, reported on further outcomes relating to pain relief and maternal satisfaction with childbirth (that were not pre‐specified in the review protocol, but thought to be important). Women receiving diazepam were found to be significantly more likely to have good anaesthesia (judged by the obstetrician as when the woman was quiet) (RR 1.56; 95% CI 1.11 to 2.21; 101 women) (Analysis 2.4). Women receiving diazepam were also significantly more likely to report feeling comfortable during induction and recovery than women receiving the vinydan‐ether (RR 3.45; 95% CI 2.26 to 5.26; 101 women) (Analysis 2.5).
Comparison 3: Diazepam versus other
One small trial was included in this comparison; this trial compared 10 mg diazepam with "other" anaesthesia (including general, local or other) during forceps delivery (Mundow 1974).
Primary outcomes
Pain relief
The trial reported no data on pain relief,
Serious maternal adverse effects/complications and neonatal mortality or serious morbidity
The trial reported no data on serious maternal adverse effects or complications, or neonatal mortality or serious morbidity .
Secondary outcomes
Maternal
Mundow 1974 did not report of any of the review's secondary outcomes for the mother.
Neonatal
Mundow 1974 did not report of any of the review's secondary outcomes for the neonate.
Non pre‐specified outcomes
Mundow 1974 reported data on "amnesic effect" and "women's behaviour", but only for the group of women receiving diazepam (see Characteristics of included studies).
The trial reported on Apgar score of less than eight at two minutes (not the review's pre‐specified outcome of Apgar score of less than seven at five minutes) (Mundow 1974); no significant difference between groups was shown for this outcome (RR 1.10; 95% CI 0.51 to 2.38; 78 infants) (Analysis 3.1).
Comparison 4: Spinal analgesia versus pudendal block
One trial was included in this comparison, comparing spinal analgesia (lignocaine 1.5 mL 5% injected slowly) with pudendal nerve block (infiltration with 20 mL 1% lignocaine) (Hutchins 1980).
Primary outcomes
Pain relief
In regards to pain relief, the trial reported on "analgesia achieved", and found that women receiving spinal analgesia were significantly more likely to regard their analgesia as adequate (RR 3.36; 95% CI 2.46 to 4.60; 183 women) (Analysis 4.1). Hutchins 1980 also reported on severe pain during delivery; women receiving spinal analgesia were found to be significantly less likely to report severe pain, compared to women receiving pudendal block (RR 0.02; 95% CI 0.00 to 0.27; 183 women) (Analysis 4.2).
Serious maternal adverse effects/complications and neonatal mortality or serious morbidity
No data on any of the review's other primary outcomes of serious maternal adverse effects or complications, or neonatal mortality or serious morbidity were reported by this trial, though the manuscript reported that no "serious complications" were reported for women in either group in (Analysis 4.3) (Hutchins 1980).
Secondary outcomes
Maternal
In Hutchins 1980, no women in either group requested additional analgesia (Analysis 4.4), or experienced maternal hypotension (Analysis 4.5). There was no significant difference found between groups in this trial for the outcome maternal headache (mild or moderate) (RR 0.91; 95% CI 0.53 to 1.58; 183 women) (Analysis 4.6).
No data on any of the other maternal secondary outcomes were reported in this trial, including: maternal satisfaction with childbirth experience; mother‐baby bonding; postnatal depression; breastfeeding success and duration; motor blockage; respiratory depression requiring oxygen administration; headache requiring blood patch; vomiting; itching; fever; shivers; drowsiness; urinary retention; duration of postpartum hospital stay; and postpartum hospital admission within six weeks of discharge.
Neonatal
The trial reported no data on the pre‐specified secondary neonatal review outcomes (Hutchins 1980).
Discussion
Summary of main results
We included four randomised controlled trials (involving 388 women) in this review, all of which were conducted prior to 1980, and assessed a variety of different agents and techniques for achieving pain relief during forceps delivery (Ellingson 1977; Hutchins 1980; Mundow 1974; Sagen 1973). Three of the trials compared diazepam with alternative agents (ketamine, vinydan‐ether, "other") for the provision of general anaesthesia during forceps delivery, and the fourth trial compared the use of spinal analgesia versus pudendal block (using lignocaine in both groups). No trials assessed the use of epidural analgesia.
Considering the review's primary outcome of pain relief, no significant difference was found when diazepam was compared with ketamine in one small trial (Ellingson 1977). A further trial suggested possible benefit of diazepam compared with vinydan‐ether, with women receiving diazepam being significantly more likely to judge pain relief as effective, than women receiving vinydan‐ether (Sagen 1973). In a trial comparing spinal analgesia with pudendal nerve block, women receiving spinal analgesia were shown to be significantly more likely to regard the analgesia as adequate and less likely to report severe pain (Hutchins 1980).
None of the trials reported on the review's other two primary outcomes of serious maternal adverse effects or complications, and neonatal mortality and serious morbidity.
No differences were shown between groups in trials assessing the outcomes maternal hypotension (Hutchins 1980), and maternal apnoea requiring oxygen ventilation (Ellingson 1977). In Sagen 1973, women receiving diazepam compared with vinydan‐ether, were however significantly less likely to experience vomiting.
No significant differences between groups were seen for the neonatal outcomes Apgar score of less than seven at five minutes (Ellingson 1977; Sagen 1973), and acidosis defined by cord blood arterial pH less than 7.2 (Ellingson 1977) in any of the trials. No further secondary maternal and neonatal outcomes were reported in any of the four included trials.
Some support for diazepam, as compared with vinydan‐ether and ketamine, was provided by two of the included trials in relation to non pre‐specified review outcomes (Ellingson 1977; Sagen 1973). In the trial comparing diazepam with vinydan‐ether, women receiving diazepam were more likely to report feeling comfortable during induction and recovery, and were more likely to have good anaesthesia as judged by the obstetrician (Sagen 1973). As compared with ketamine, women receiving diazepam were more likely to report a pleasant recovery in one trial (Ellingson 1977); interestingly however, in this trial, women receiving diazepam were less likely to have good anaesthesia as judged by the obstetrician.
Three of the four included trials compared diazepam with alternative agents for the provision of general anaesthesia during operative delivery (Ellingson 1977; Mundow 1974; Sagen 1973). The fourth trial compared spinal anaesthesia with pudendal nerve block (Hutchins 1980). The risks, including maternal death, associated with obstetric general anaesthesia have however lead to its use now being restricted predominately to true emergency cases, where there is insufficient time for a regional technique (Djabatey 2009). Accordingly, estimates of use of general anaesthesia during forceps deliveries from current clinical practice are extremely low (estimated to be used in only 0.5% of instrumental births in Australia in 2009 (Li 2011) and in England in 2011 to 2012 (NHS 2012)), and rather regional anaesthesia (particularly epidural or caudal, accounting for over 50%), is the most commonly used method, followed by local anaesthesia to the perineum. While both spinal and pudendal block anaesthesia are used in current clinical practice, they too are used comparatively infrequently (in 2.7% and 5.2% of instrumental birth respectively).
The possible benefits of diazepam shown in two of the included trials when compared with vinydan‐ether (Sagen 1973) and ketamine (Ellingson 1977), should be interpreted with caution, and not without acknowledgement of the now known potential dangers of diazepam for obstetric patients (FDA 2008; Grant 2011). While use throughout pregnancy (such when indicated for anxiety) has been suggested to be associated with an increased risk of congential malformations and other developmental abnormalities for the fetus, single high doses during labour and delivery (as used in Ellingson 1977 and Sagen 1973) have been associated with irregularities in fetal heart rate tracing, along with respiratory depression, hypotonia, poor sucking and hypothermia in the neonates (FDA 2008). Indeed, the dose used in both Ellingson 1977 and Sagen 1973 (30 mg intravenously, administered rapidly), was notably high (with recent cited dosing regimens for diazepam analgesia during labour and delivery including 2‐5 mg intravenously, and 10 mg intramuscularly (Grant 2011)). For the mother, the risk of aspiration (due to obtunded (dulled/reduced) airway reflexes) is also increased with the use of diazepam during labour and delivery; and as a potent amnesic, the risk of an impaired memory of delivery for the mother is also considered high (Grant 2011).
Forceps deliveries (indicated when the fetus fails to progress to delivery, or when delivery needs to be expedited in the second stage) are no longer considered common; comprising approximately 1% to 4.6% of deliveries in high‐resource settings (Li 2011; Martin 2009; Public Health Agency of Canada 2008) and comprising a significantly lower proportion of deliveries in low‐resource settings (with for example, an estimation of less than 1% of all births being assisted/instrumental in sub‐Saharan Africa) (Bailey 2005). Clinical practice guidelines for instrumental delivery recommend that in preparation of the mother for delivery "appropriate analgesia" should be administered (RANZCOG 2009a; RCOG 2011; SOGC 2004), however, no further guidance as to the particular agent or method to use is provided. For rotational forceps deliveries, such guidelines suggest that regional anaesthesia (either epidural or spinal) should be used (RANZCOG 2009b); yet pudendal block may be appropriate in the context of urgent delivery (RCOG 2011). In Australia and the United Kingdom, it has been estimated that approximately 50% of women undergoing an instrumental delivery will receive regional anaesthesia; and in Australia, approximately 28.4% of women will receive a local anaesthetic to the perineum, and 5.2%, a pudendal block (Li 2011).
Overall completeness and applicability of evidence
There is a significant lack of randomised trials in this area, particularly assessing the techniques and agents commonly used in current clinical practice for the provision of pain relief during forceps delivery.
This review is limited with the inclusion of only four small trials (Ellingson 1977; Hutchins 1980; Mundow 1974; Sagen 1973), that were all conducted prior to 1980, and did not report on many of the review's pre‐specified maternal and neonatal primary and secondary outcomes. The variety of analgesic agents/methods used in the four included trials meant that no data could be pooled in meta‐analysis, making interpretation difficult. The different methods of measuring pain relief and maternal satisfaction/comfort also made comparisons between trials difficult. One trial (Mundow 1974), reported no data in way that could be included in the review (outcome data were reported for one group only).
Three of the four trials compared diazepam with alternative agents for the provision of general anaesthesia during forceps delivery; this method for providing analgesia during instrumental delivery is however, now infrequently used in clinical practice. The fourth trial compared the use of lignocaine for spinal and pudendal block anaesthesia; while both methods are currently used in practice, they too are employed much less frequently than regional anaesthesia (epidural and caudal), and local anaesthesia to the perineum, which have not been evaluated in any randomised trials of forceps delivery to date.
An important consideration to note, further limiting the applicability of the current evidence, is the now common use of regional, particularly epidural analgesia in modern practice; not a feature of practice at the time the included trials were conducted. Since the introduction of epidural for pain relief approximately four decades ago, the rates of use have increased substantially, with approximately a third of women in labour in the United Kingdom and Australia (Li 2011; NHS 2012), and approximately two thirds of women in labour in the United States now receiving epidural analgesia (McGrady 2004; Osterman 2011). Consideration of this clinical context will be important during the design of any future clinical trials ‐ for example in the setting of high rates of epidural use for pregnant women in labour, an appropriate trial intervention for analgesia for forceps delivery, might be the use of a 'top up' epidural.
Quality of the evidence
All trials were judged to be at a unclear to high risk of bias overall. The four trials were judged at an unclear risk of selection bias with unclear methods for allocation concealment and random number sequence generation (Ellingson 1977; Hutchins 1980; Mundow 1974; Sagen 1973). All four trials were at a high risk of performance and detection bias, with no blinding detailed. One trial was judged at a high risk of bias due to incomplete reporting (Mundow 1974), and two at an unclear risk (Ellingson 1977; Hutchins 1980); only one trial was judged at a low risk of attrition bias (Sagen 1973). Two trials were judged at an unclear risk of reporting bias (Ellingson 1977; Sagen 1973), and two at a high risk (Hutchins 1980; Mundow 1974).
Potential biases in the review process
The evidence for this review is derived from trials identified through a detailed search process. It is possible (but unlikely) that additional trials assessing analgesia for forceps delivery, have been published but not identified. It is also possible that other studies have been conducted but not published. Should such studies be identified we will include them in future updates of this review.
Agreements and disagreements with other studies or reviews
This review confirms that there is currently insufficient evidence to support a particular analgesic agent or method as most effective and safe for providing pain relief during forceps delivery. There have not been other systematic reviews on the use of analgesia for this indication.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Diazepam versus ketamine, Outcome 1 Pain relief (judged as effective by the mother).
Comparison 1 Diazepam versus ketamine, Outcome 2 Maternal apnoea requiring oxygen ventilation.

Comparison 1 Diazepam versus ketamine, Outcome 3 Apgar score of less than seven at five minutes.
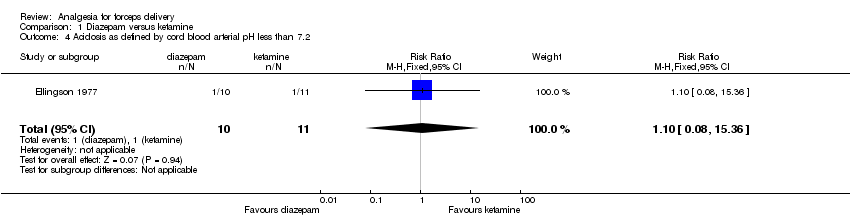
Comparison 1 Diazepam versus ketamine, Outcome 4 Acidosis as defined by cord blood arterial pH less than 7.2.

Comparison 1 Diazepam versus ketamine, Outcome 5 Good anaesthesia (judged by the obstetrician).

Comparison 1 Diazepam versus ketamine, Outcome 6 Pleasant recovery (judged by the mother).

Comparison 1 Diazepam versus ketamine, Outcome 7 Awareness (mother sensed the operation).
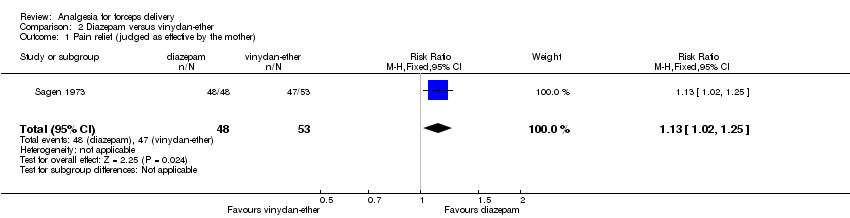
Comparison 2 Diazepam versus vinydan‐ether, Outcome 1 Pain relief (judged as effective by the mother).

Comparison 2 Diazepam versus vinydan‐ether, Outcome 2 Vomiting.

Comparison 2 Diazepam versus vinydan‐ether, Outcome 3 Apgar score of less than seven at five minutes.

Comparison 2 Diazepam versus vinydan‐ether, Outcome 4 Good anaesthesia (judged by the obstetrician).

Comparison 2 Diazepam versus vinydan‐ether, Outcome 5 Comfortable induction and recovery (judged by the mother).

Comparison 3 Diazepam versus other (general, local, other anaesthetic), Outcome 1 Apgar score of less than eight at two minutes.

Comparison 4 Spinal analgesia versus pudendal block anaesthesia, Outcome 1 Pain relief (analgesia achieved).

Comparison 4 Spinal analgesia versus pudendal block anaesthesia, Outcome 2 Severe pain during delivery.
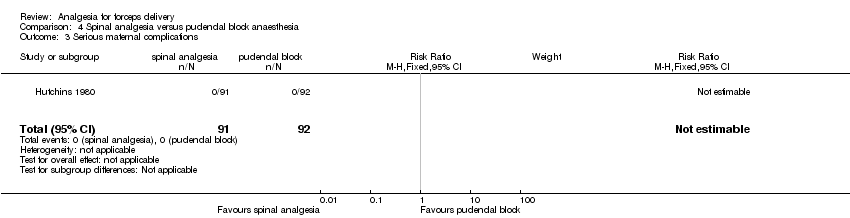
Comparison 4 Spinal analgesia versus pudendal block anaesthesia, Outcome 3 Serious maternal complications.

Comparison 4 Spinal analgesia versus pudendal block anaesthesia, Outcome 4 Request for additional anaesthesia.

Comparison 4 Spinal analgesia versus pudendal block anaesthesia, Outcome 5 Maternal hypotension (defined as a decrease in diastolic or systolic blood pressure of more than 10 mmHg).

Comparison 4 Spinal analgesia versus pudendal block anaesthesia, Outcome 6 Headache (mild or moderate).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain relief (judged as effective by the mother) Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.98, 2.07] |
| 2 Maternal apnoea requiring oxygen ventilation Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.34] |
| 3 Apgar score of less than seven at five minutes Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Acidosis as defined by cord blood arterial pH less than 7.2 Show forest plot | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.08, 15.36] |
| 5 Good anaesthesia (judged by the obstetrician) Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.41, 0.97] |
| 6 Pleasant recovery (judged by the mother) Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.17, 3.68] |
| 7 Awareness (mother sensed the operation) Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain relief (judged as effective by the mother) Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.02, 1.25] |
| 2 Vomiting Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.62] |
| 3 Apgar score of less than seven at five minutes Show forest plot | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.45, 3.50] |
| 4 Good anaesthesia (judged by the obstetrician) Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.11, 2.21] |
| 5 Comfortable induction and recovery (judged by the mother) Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [2.26, 5.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar score of less than eight at two minutes Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.51, 2.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain relief (analgesia achieved) Show forest plot | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.36 [2.46, 4.60] |
| 2 Severe pain during delivery Show forest plot | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.27] |
| 3 Serious maternal complications Show forest plot | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Request for additional anaesthesia Show forest plot | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal hypotension (defined as a decrease in diastolic or systolic blood pressure of more than 10 mmHg) Show forest plot | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Headache (mild or moderate) Show forest plot | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.58] |

